Abstract
Background
This study aimed to compare the efficacy and prognostic value of partial sensory rhizotomy (PSR) and microvascular decompression (MVD) for primary trigeminal neuralgia (PTN).
Material/Methods
From June 2010 to June 2012, 117 patients with PTN were recruited for the study, of which 52 cases were treated with MVD (the MVD group) and 65 cases were treated with PSR (the PSR group). Visual Analog Scoring (VAS) was performed at 1 and 2 weeks, and at 1, 3, and 6 month after surgery. The overall response rate (ORR) was determined 1 month after surgery. Barrow Neurological Institute score was adopted to value the reoccurrence at 6, 12, 24, and 36 months after surgery. A 3-year follow-up was conducted and the complications were recorded.
Results
The ORR 2 weeks after surgery in the MVD and PSR groups was 98.08% and 84.62%, respectively. One and 2 weeks after surgery, the VAS was lower in the MVD group than in the PSR group, but there was no significant difference in VAS between the 2 groups at 1, 3, and 6 months after surgery. Three years after surgery, the recurrence rate was significantly lower in the MVD group than in the PSR group. The recurrence-free survival time was longer in the MVD group than in the PSR group. The occurrence rates of herpes and total postoperative complications were significantly higher in the PSR group than in the MVD group.
Conclusions
Compared with PSR, MVD is more suitable for PTN treatment, with less disturbance, lower recurrence rate, and better efficacy.
MeSH Keywords: Comparative Study, Microvascular Decompression Surgery, Neuralgia, Rhizotomy, Treatment Outcome
Background
Primary trigeminal neuralgia (PTN) is a common disease in the elderly, and causes temporary intense pain, mainly in the trigeminal nerve area [1]. This disease is characterized by unbearable and progressive pain in the face [2]. Currently, the pathogenesis of PTN remains unclear, but most scholars believe that vascular compression of the dorsal root of the trigeminal nerve is involved [3]. Pharmacotherapy is preferred, but fails to cure the disease completely, with a large possibility of adverse effects, which may result in recurrence [4]. In patients with PTN, small vessel loops of trigeminal nerve root entry zone are compressed and the nerve root is twisted, angulated, and adhered. Therefore, surgery has been a main method of treatment [5–7].
Recently, microvascular decompression (MVD) has been widely applied in the treatment of PTN because of its high efficacy [8]. Although MVD is a very mature and safe operation, facial paralysis and facial and auditory nerve damage often occur during the surgery due to stretching the cerebellum and related vasa nervorum [9]. Clinical studies have found that some surgical treatments are invalid and some patients relapse because of the unclear pathogenesis of PTN, and some doctors lack knowledge about decompression therapy or have difficulty in identifying responsible vessels and understanding the different implementations of MVD [10,11]. Partial sensory rhizotomy (PSR) is conducted after MVD fails or when microvascular compression of the trigeminal nerve root and other compression lesions are excluded in the first operation [12]. PSR causes slightly abnormal facial feeling without obvious discomfort in most patients [13]. Moreover, complications like bleeding, intracranial infection, cerebrospinal rhinorrhea, oral herpes simplex, postoperative headache, facial numbness, postoperative numbness, and nerve damage occurred after surgery but these symptoms often disappear in the short term [14].
This research compares the therapeutic effects ot these 2 surgical treatments. By long-term follow-up observation of 117 PTN patients receiving PSR and MVD treatments, we aimed to obtain clinical data on PTN treatment and discuss related mechanisms to develop new treatments.
Material and Methods
General data
This study included 117 PTN patients (49 males and 68 females; age ranging from 29 to 65 years and the mean age 49.30±5.58 years) in the China-Japan Union Hospital of Jilin University between June 2010 and June 2012. The course of disease was 0.5~15 years and the average course of disease was 6.32±2.93 years. The diagnostic criteria were based on the criteria for trigeminal neuralgia in Intel Certified Hadoop Developer (ICHD-II) published in 2004 [15]. The inclusion criteria were: the patients who conformed to PTN diagnostic criteria were diagnosed by more than 3 chief physicians or associate chief physicians of neurology and neurosurgery; the patients had medical history of more than 6 months; the patients underwent pharmacotherapy before surgery for more than 3 months with recurrence or no efficacy; the patients had no operation history or history of other surgical treatment; the patients had complete follow-up data. The exclusion criteria were: the patients who received MRI skull examination before operation showed secondary trigeminal neuralgia caused by intracranial neoplasm; the female patients in gestation or suckling period; the patients with cerebral vascular malformation or intracranial tumor; the patients with age more than 65 years old; the patients with severe liver, kidney, or cardio-pulmonary dysfunction; the patients unwilling to accept surgical treatment or follow-up. The 117 patients were randomly divided by use of a random numbers table into the MVD group (n=52) and PSR group (n=65). This study was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University and all patients in this study signed the informed consent before surgery.
Surgical methods
The PSR operation was performed in the patients of the PSR group under general anesthesia. The patients were in lateral prone posture with the affected side upward and fixed with a head frame. Sub-occipital retrosigmoid sinus approach was chosen for surgery under a microscope. A straight incision was made 2 cm from the posterior mastoid and a bone window was made outside to the edge of the sigmoid sinus and up to the transverse sinus. The dura mater cerebralis was incised flap-shaped to fully expose the upper external posterior cranial fossa until trigeminal nerve roots were seen. The arachnoid membrane between facial and auditory nerves and trigeminal nerves was opened to expose trigeminal nerves near the brain. We severed 2/3 of the sensory nerve of external posterior trigeminal nerve roots 0.5~1.0 cm from the pons. If the arteriole was approached or the sensory roots were coiled or compressed, they were separated in case of injury and bleeding difficult to stop or damage to the brain stem in the process of hemostasis. After surgery, normal saline was used to flush the blood, and low-voltage bipolar electrocoagulation was used to burn the broken ends. Next, the dura mater cerebralis was sutured tightly, followed by the muscle, subcutaneous tissues, and skin. Thick dressing was used to cover the cut and pressure dressing was applied.
The MVD operation was performed in patients of the MVD group under general anesthesia. The patients were in lateral prone posture and fixed with a head frame. A straight incision was made within the hairline and behind the ear. A milling cutter was used to make a small (3×3 cm) bone flap in the sub-occipital area and the transverse sinus and sigmoid sinus were exposed in the upper external bone flap. The upper external corner of the cerebellar hemisphere was retracted inwards and downwards, and the full length of trigeminal nerve was exposed under the microscope. Then, a 360° detailed exploration was conducted where nerves enter the brain stem. If explicit vascular compression existed, especially with nerve deformation, a Teflon ball of proper size was used to separate them. If typical arterial oppressor was not found and the artery was contacted with the nerve, but nerves were not compressed or discolored or there was just single arterial oppressor, after the Teflon ball was used to separate vessels and nerves, hepatic vascular exclusion and PSR were performed. The surface of blood vessels of the pons and large petrosal vein needed to be carefully protected. When surgery under the dura mater cerebralis was finished, the dura mater cerebralis was sutured and bone flaps were fixed and sutured layer by layer.
Visual analog score (VAS)
At 1 and 2 weeks and 1, 3, and 6 months after surgery, all patients were scored by VAS with a VAS ruler (10 cm in length) with 0~10 moving rod on the front and numbers 0~10 on the back [16]. The length from the starting point to the mark represented the intensity of pain. Patients were asked to use the moving rod to mark the intensity of pain and the doctors recorded the intensity of pain. The VAS value was 0~10, in which 0 represented no pain, 1~3 represented mild pain, 4~6 represented moderate pain, 7~9 represented severe pain, and 10 represented unbearable pain.
Efficacy and recurrence criteria
One month after surgery, all patients were evaluated on the efficacy of surgery according to the efficacy criteria proposed by Brisman et al. [17]: “cured” indicated no pain and no need to take medicine; “markedly effective” indicated pain was relieved by more than 90% and there was need to take medicine sometimes; “effective” indicated pain was relieved or dose was decreased by more than 50%; and “ineffective” indicated pain was slightly or not relieved, or the dose was decreased by less than 50%. Overall response rate (ORR)=the accumulated cases of cured, markedly effective and effective)/the total cases ×100%. The Barrow Neurological Institute (BNI) scoring system was used to evaluate degrees of pain relief [18]. Grade I meant no pain and there was no need to take medicine; grade II meant occasional pain and there was no need to take medicine; grade IIIa meant no pain and there was need to take medicine; grade IIIb meant that the pain could be controlled after taking medicine; grade IV meant that the pain was relieved a little but could not be controlled after taking medicine; and grade V meant the pain could not be relieved. Grade IV and grade V were considered recurrence. Recurrence was assessed at 6, 12, 24, and 36 months after surgery.
Follow-up
All patients received 3-year follow-up. Independent observers (who were not doctors or nurses-in-charge were involved in the surgery) carried out qualitative and quantitative follow-up to record complications and recurrence through outpatient services, telephone, letters, or e-mails. Facial nerve test, hearing monitoring, and inquiry were performed during the follow-up to record postoperative complications.
Statistical analysis
SPSS 21.0 was used to conduct data analysis and quantitative data are represented by mean ± standard deviation. Comparisons between 2 groups were conducted using the independent-sample t test. The method of repetitive measure was applied to analyze values at different time points. The enumeration data are expressed in percentage. Chi-square analysis or Fisher exact test was applied in the pair-wised comparison. The Wilcoxon test was applied to analyze ranked data and the Kaplan-Meier test was applied to draw relapse-free survival curves. P<0.05 was considered as statistically significant.
Results
General data of PTN patients in the PSR and MVD groups
As shown in Table 1, the differences were not statistically significant between the 2 groups in terms of sex, age, course of disease, pain distribution, location of pain, and clinical classification (all P>0.05). The locations of pain in the 2 groups were mainly on the right side; the MVD group accounted for 65.4% (34/52) and the PSR group accounted for 60.0% (39/65). The pain distributions of the 2 groups were mainly in maxillary and mandibular division of the trigeminal nerve.
Table 1.
Comparison of baseline characteristics of patients with primary trigeminal neuralgia between the PSR and MVD groups.
| Characteristic | MVD group (n=52) | PSR group (n=65) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 22/30 | 27/38 | 0.01 | 0.933 |
| Age (years) | 48.37±5.71 | 50.05±5.41 | 1.63 | 0.106 |
| Course of disease (year) | 5.87±2.63 | 6.68±3.11 | 1.50 | 0.137 |
| Pain distribution | 0.36 | 0.550 | ||
| V1 | 3 (5.77) | 1 (1.54) | ||
| V2 | 19 (36.54) | 16 (24.62) | ||
| V3 | 10 (19.23) | 12 (18.46) | ||
| V1, 2 | 5 (9.62) | 9 (13.85) | ||
| V2, 3 | 14 (26.92) | 25 (38.46) | ||
| V1, 2, 3 | 1 (1.92) | 2 (3.08) | ||
| Location of pain | 4.63 | 0.463 | ||
| Left side | 18 (34.62) | 26 (40.00) | ||
| Right side | 34 (65.38) | 39 (60.00) | ||
| Clinical classification | 0.58 | 0.446 | ||
| Typical | 43 (82.69) | 57 (87.69) | ||
| Atypical | 9 (17.31) | 8 (12.31) |
V1 – ophthalmic division of trigeminal nerve; V2 – maxillary division of trigeminal nerve; V3 – mandibular division of trigeminal nerve; MVD – micro-vascular decompression; PSR – partial sensory rhizotomy.
Efficacy of PTN patients in the PSR and MVD groups after operation
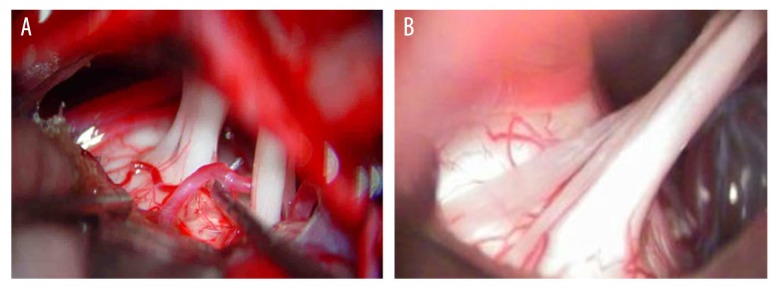
One month after operation, in the MVD group (n=52), 26 cases were cured, 21 cases markedly effective, 4 cases effective, and 1 case ineffective; the ORR was 98.08%. In the PSR group (n=65), 38 cases were cured, 14 cases were markedly effective, 3 cases effective, and 10 cases ineffective; the ORR was 84.62%. The ORR of the MVD group was significantly higher than that of the PSR group, but there was no significant difference between the 2 groups in terms of postoperative efficacy (P>0.05) (Table 2). The intraoperative images of MVD and PSR are shown in Figure 1.
Table 2.
Comparison of the efficacy of patients with primary trigeminal neuralgia between the PSR and MVD groups.
| Efficacy | MVD group (n=52) | PSR group (n=65) | Z | P |
|---|---|---|---|---|
| Cured | 26 (50.00) | 38 (58.46) | 0.72 | 0.672 |
| Markedly effective | 21 (40.38) | 14 (21.54) | ||
| Effective | 4 (7.69) | 3 (4.62) | ||
| Ineffective | 1 (1.92) | 10 (15.38) |
MVD – micro-vascular decompression; PSR – partial sensory rhizotomy.
Figure 1.
Intraoperative images of MVD (A) and PSR (B). MVD – microvascular decompression; PSR – partial sensory rhizotomy.
VAS score of PTN patients in the PSR and MVD groups
As shown in Table 3, at 1 week and 2 weeks after surgery, the VAS score of the MVD group was significantly lower than that of the PSR group (all P<0.05). Compared with 1 week and 2 weeks after surgery, the VAS scores had decreased at 1, 3, and 6 months after surgery, but the difference between the 2 groups was not significant (all P>0.05).
Table 3.
Comparison of VAS scores of patients with primary trigeminal neuralgia after operation between the PSR and MVD groups.
| Time point | MVD group (n=52) | PSR group (n=65) | t | P |
|---|---|---|---|---|
| 1 week after operation | 2.23±1.58 | 3.78±2.04 | 4.51 | <0.001 |
| 2 weeks after operation | 0.87±0.95 | 1.31±1.26 | 2.10 | 0.038 |
| 1 month after operation | 0.38±0.49 | 0.57±0.68 | 1.64 | 0.104 |
| 3 months after operation | 0.12±0.32 | 0.18±0.43 | 0.97 | 0.337 |
| 6 months after operation | 0.10±0.30 | 0.11±0.31 | 0.56 | 0.575 |
MVD – micro-vascular decompression; PSR – partial sensory rhizotomy.
The recurrence rate and recurrence-free survival time of PTN patients in the PSR and MVD groups
All patients received 36-month follow-up. At 6 and 12 months after surgery, the recurrence rates in the MVD group were 3.85% and 5.77%, respectively; the recurrence rates in the PSR group were 7.69% and 12.31%, respectively; the recurrence rate in the PSR group was higher than that in the MVD group, but the difference was not significant (both P>0.05). At 24 and 36 months after surgery, the recurrence rates in the MVD group were 9.62% and 15.38%, respectively; the recurrence rates in the PSR group were 27.69% and 32.31%; and the recurrence rate in the PSR group was significantly higher than that in the MVD group (both P<0.05). At 36 months after surgery, the recurrence-free survival time in the MVD group was 34.81±0.67 months, which was significantly longer than the 32.12±1.07 months in the PSR group (P<0.05) (Table 4, Figure 2).
Table 4.
Comparison of the recurrence rate and recurrence-free survival time of patients with primary trigeminal neuralgia after operation between the PSR and MVD groups.
| Time point | MVD group (n=52) | PSR group (n=65) | ||
|---|---|---|---|---|
| I~IIIb | IV + V | I~IIIb | IV + V | |
| 6 months after operation | 50 (96.15) | 2 (3.85) | 60 (92.31) | 5 (7.69) |
| 12 months after operation | 49 (94.23) | 3 (5.77) | 57 (87.69) | 8 (12.31) |
| 24 months after operation | 47 (90.38) | 5 (9.62) | 49 (72.31) | 16 (27.69) |
| 36 months after operation | 44 (84.62) | 8 (15.38) | 43 (67.69) | 21 (32.31) |
I~IIIb, no reoccurrence; IV + V, reoccurrence; MVD, micro-vascular decompression; PSR, Partial Sensory Rhizotomy.
Figure 2.
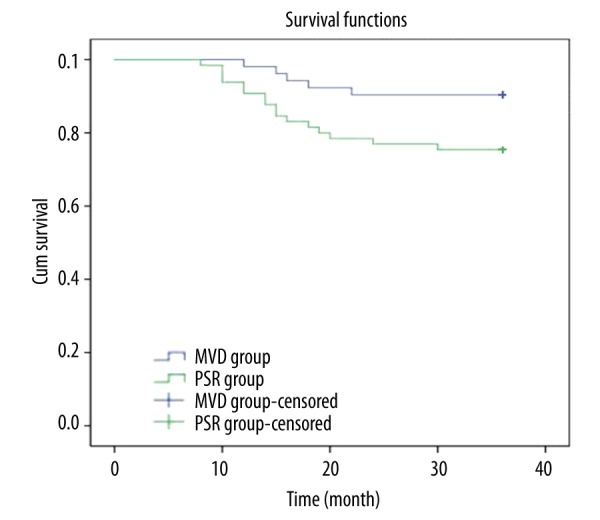
Kaplan-Meier survival curves of patients with primary trigeminal neuralgia in the MVD and PSR groups. MVD – microvascular decompression; PSR – partial sensory rhizotomy.
Postoperative complications of PTN patients in the PSR and MVD groups
There were 34 cases with postoperative complications in the PSR group, including 18 cases of abnormal facial sensation, 9 cases of oral herpes, 2 cases of paralytic corneal ulcer, 2 cases of contralateral ataxia, and 1 case of subcutaneous hydrops at the incision; the postoperative complication rate was 52.31%. There were 17 cases with complications after surgery in the MVD group, including 7 cases of abnormal facial sensation, 1 case of oral herpes, 2 cases of hearing loss, 3 cases of paralytic corneal ulcer, 2 cases of masticatory atonia, and 1 case of subcutaneous effusion; the postoperative complication rate was 36.54%. No significant differences were noted regarding abnormal facial sensation, hearing loss, corneal ulcer, masticatory atonia, contralateral ataxia, and subcutaneous hydrops between the MVD and PSR groups (all P>0.05). However, the occurrence of herpes and total postoperative complications in the PSR group were significantly higher than those in the MVD group (both P<0.05) (Table 5).
Table 5.
Comparison of postoperative complications of patients with primary trigeminal neuralgia between the PSR and MVD groups.
| Complication | MVD group (n=52) | PSR group (n=65) | χ2 | P |
|---|---|---|---|---|
| Abnormal facial sensation | 7 (7.69) | 18 (21.54) | 3.482 | 0.062 |
| Herpes | 1 (1.92) | 9 (13.85) | 5.254 | 0.022 |
| Hearing loss | 2 (3.85) | 1 (1.54) | 0.616 | 0.433 |
| Corneal ulcer | 3 (5.77) | 2 (3.08) | 0.512 | 0.474 |
| Masticatory atonia | 2 (3.85) | 1 (1.54) | 0.616 | 0.433 |
| Contralateral ataxia | 1 (1.92) | 2 (3.08) | 0.154 | 0.695 |
| Subcutaneous hydrops | 1 (1.92) | 1 (1.54) | 0.025 | 0.873 |
| Total | 17 (32.69) | 34 (52.31) | 4.520 | 0.034 |
MVD – micro-vascular decompression; PSR – partial sensory rhizotomy.
Discussion
Fully endoscopic microvascular decompression (E-MVD) of the trigeminal nerve was initially described more than 1 decade ago, but has not yet gained wide acceptance [19]. Currently, it is widely considered that abnormal trigeminal nerve roots or sensory neurons of trigeminal ganglion causes PTN and neurites are excited by the damage, then paroxysmal discharge occurs [20]. Common treatment mainly includes pharmacotherapy, radiofrequency thermocoagulation of the gasserian ganglion, block technique of trigeminal nerve and Gasser’s ganglion, gamma knife radiosurgery, and PSR [21]. However, there is no unified standard for every method on indication choice. The clinical efficacy, complications, and recurrence rate are different in different methods.
In the results of this study, the ORR in the MVD group was significantly higher than that in the PSR group. The efficacy of MVD was outstanding because sensory roots were severed more than 3/4 and as long as they were connected, severe facial disorder and corneal sensory disturbance would not happen and there was no discomfort in patients and pain could be totally relieved [13]. However, after PSR surgery, patients often had complications, including incision infection, cerebral spinal fluid leakage, intracranial hematoma, nausea and vomiting, dizziness, hearing loss, tinnitus, and facial nerve dysfunction [21,22]. The main reason was that when the trigeminal nerve was exposed during operation, stretching the cerebellum would cause damages to facial and auditory nerves, or stretching and stimulating related vasa nervorum would cause vasospasm [9]. Furthermore, about 15% of blood vessels of trigeminal nerves cannot be found during surgery. At that time, patients need to receive MVD, so they not only have to accept several risks of craniotomy but also have to accept general anesthesia, which is a major challenge [23]. During surgical procedures, facial nerve vascular compressions were exposed and Gore-Tex grafts were successfully placed as virtually planned [24]. This study suggests that 3 years after surgery, the recurrence rate of the PSR group is significantly higher than that of the MVD group, and the recurrence-free survival time is significantly longer. The possible reasons may include slipped or displaced decompression equipment, inadequate decompression, new compression, fibrotic adhesions occur between nerves and micrangium, or compression from the arachnoid membrane. The combination of HFS-TN-GPN is extremely rare and is often associated with a looped VBA and a smaller posterior fossa. However, MVD is still a good choice for treatment [25]. Therefore, PTN patients who received MVD method and recurred should be treated with reasonable surgery according to individual conditions, and this can significantly improve efficacy.
This study shows that at 1 and 2 weeks after surgery, the VAS score of the PSR group was significantly higher than that of the MVD group. It is believed that PSR method should be performed or nerve roots should be combed when vessels are not compressed [26]. MVD is an ideal surgical method which can effectively relieve pain. Previous studies have shown that VAS scores before and after MVD surgery are high, while 78.7% of patients are painless and 14.9% of moderate pain is relieved after PSR [27,28]. In addition, this study also shows that at 1, 3, and 6 months after surgery, there was no significant difference between VAS scores of the 2 groups and they are lower than those before surgery. The possible explanation may be that in the short term after decompression, short circuits among fibers still work or there are not enough cases; this often happens when VAS scoring is applied to represent degree of pain [13].
Additionally, no significant differences regarding abnormal facial sensation, hearing loss, corneal ulcer, masticatory atonia, contralateral ataxia, and subcutaneous hydrops were noted between the MVD and PSR groups. However, the occurrence rates of herpes and total postoperative complications in the PSR group were significantly higher than those in the MVD group. A surgical method should be selected according to individual conditions and comprehensive illness evaluation chose a safe and effective surgical method.
Conclusions
Compared with the PSR group, the MVD group was characterized by fewer disturbances, lower recurrence rate, and more reliable efficacy, providing a clinical basis for the choice of treatment for PTN. Our study has some shortcomings; for example, the sample size was not large enough to conduct a precise analysis, so more comprehensive and precise research is needed to find a more effective treatment for PTN.
Footnotes
Competing interests
None.
Source of support: Departmental sources
References
- 1.Chen GQ, Wang XS, Wang L, et al. Arterial compression of nerve is the primary cause of trigeminal neuralgia. Neurol Sci. 2014;35:61–66. doi: 10.1007/s10072-013-1518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan GY, Liang HF, Zhang JH. Current gamma knife treatment for ophthalmic branch of primary trigeminal neuralgia. Int J Ophthalmol. 2011;4:121–24. doi: 10.3980/j.issn.2222-3959.2011.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eugene AR. Trigeminal neuralgia and radiofrequency lesioning. Brain (Bacau) 2015;6:91–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oomens MA, Forouzanfar T. Pharmaceutical management of trigeminal neuralgia in the elderly. Drugs Aging. 2015;32:717–26. doi: 10.1007/s40266-015-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punyani SR, Jasuja VR. Trigeminal neuralgia: An insight into the current treatment modalities. J Oral Biol Craniofac Res. 2012;2:188–97. doi: 10.1016/j.jobcr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodaie M, Chen DQ, Quan J, et al. Tractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgia. PLoS One. 2012;7:e32745. doi: 10.1371/journal.pone.0032745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toda H, Goto M, Iwasaki K. Patterns and variations in microvascular decompression for trigeminal neuralgia. Neurol Med Chir (Tokyo) 2015;55:432–41. doi: 10.2176/nmc.ra.2014-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Yuan Y, Fang Y, et al. Microvascular decompression for atypical hemifacial spasm: lessons learned from a retrospective study of 12 cases. J Neurosurg. 2016;124:397–402. doi: 10.3171/2015.3.JNS142501. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H, Kondo A, Shimano H, et al. Recurrent trigeminal neuralgia at 20 years after surgery: Case report. Neurol Med Chir (Tokyo) 2013;53:37–79. doi: 10.2176/nmc.53.37. [DOI] [PubMed] [Google Scholar]
- 11.Capelle HH, Brandis A, Tschan CA, et al. Treatment of recurrent trigeminal neuralgia due to Teflon granuloma. J Headache Pain. 2010;11:339–44. doi: 10.1007/s10194-010-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trojnik T, Smigoc T. Percutaneous trigeminal ganglion balloon compression rhizotomy: Experience in 27 patients. ScientificWorldJournal. 2012;2012:328936. doi: 10.1100/2012/328936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koopman JS, de Vries LM, Dieleman JP, et al. A nationwide study of three invasive treatments for trigeminal neuralgia. Pain. 2011;152:507–13. doi: 10.1016/j.pain.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Zakrzewska JM, Lopez BC, Kim SE, et al. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery. 2005;56:1304–11. doi: 10.1227/01.neu.0000159883.35957.e0. discussion 1311–12. [DOI] [PubMed] [Google Scholar]
- 15.Viana M, Tassorelli C, Allena M, et al. Diagnostic and therapeutic errors in trigeminal autonomic cephalalgias and hemicrania continua: A systematic review. J Headache Pain. 2013;14:14. doi: 10.1186/1129-2377-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frighetto L, De Salles AA, Smith ZA, et al. Noninvasive linear accelerator radiosurgery as the primary treatment for trigeminal neuralgia. Neurology. 2004;62:660–62. doi: 10.1212/wnl.62.4.660. [DOI] [PubMed] [Google Scholar]
- 17.Brisman R. Gamma knife radiosurgery for primary management for trigeminal neuralgia. J Neurosurg. 2000;93(Suppl 3):159–61. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 18.Salama H, Ben-Khayal H, Mohamed MA, et al. Outcome of medical and surgical management in intractable idiopathic trigeminal neuralgia. Ann Indian Acad Neurol. 2009;12:173–78. doi: 10.4103/0972-2327.56317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohman LE, Pierce J, Stephen JH, et al. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurg Focus. 2014;37:E18. doi: 10.3171/2014.7.FOCUS14318. [DOI] [PubMed] [Google Scholar]
- 20.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Oesman C, Mooij JJ. Long-term follow-up of microvascular decompression for trigeminal neuralgia. Skull Base. 2011;21:313–22. doi: 10.1055/s-0031-1284213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JS, Lee JA, Kong DS, et al. Delayed cranial nerve palsy after microvascular decompression for hemifacial spasm. J Korean Neurosurg Soc. 2012;52:288–92. doi: 10.3340/jkns.2012.52.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatli M, Satici O, Kanpolat Y, et al. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien) 2008;150:243–55. doi: 10.1007/s00701-007-1488-3. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez Sanchez JJ, Ensenat Nora J, Candela Canto S, et al. New stereoscopic virtual reality system application to cranial nerve microvascular decompression. Acta Neurochir (Wien) 2010;152:355–60. doi: 10.1007/s00701-009-0569-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang YN, Zhong J, Zhu J, et al. Microvascular decompression in patients with coexistent trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Acta Neurochir (Wien) 2014;156:1167–71. doi: 10.1007/s00701-014-2034-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhang Y, Li C, et al. Surgical treatment of primary trigeminal neuralgia: comparison of the effectiveness between MVD and MVD+PSR in a series of 210 patients. Turk Neurosurg. 2012;22:32–38. doi: 10.5137/1019-5149.JTN.4447-11.2. [DOI] [PubMed] [Google Scholar]
- 27.Abhinav K, Love S, Kalantzis G, et al. Clinicopathological review of patients with and without multiple sclerosis treated by partial sensory rhizotomy for medically refractory trigeminal neuralgia: A 12-year retrospective study. Clin Neurol Neurosurg. 2012;114:361–65. doi: 10.1016/j.clineuro.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Montano N, Conforti G, Di Bonaventura R, et al. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–99. doi: 10.2147/TCRM.S37592. [DOI] [PMC free article] [PubMed] [Google Scholar]