Abstract
The products of the UL16 and UL21 genes represent tegument proteins which are conserved throughout the mammalian herpesviruses. To identify and functionally characterize the respective proteins in the alphaherpesvirus pseudorabies virus, monospecific antisera against bacterially expressed fusion proteins were generated. In immunoblots the UL16 antiserum detected a ca. 40-kDa protein in infected cells and purified virion preparations, whereas the anti-UL21 serum recognized a protein of approximately 60 kDa. Interestingly, in immunoprecipitations using either antiserum, both proteins were coprecipitated, demonstrating the formation of a physical complex. To investigate protein function, viruses lacking either UL16, UL21, or both were constructed. Mutant viruses could be propagated on noncomplementing cells, indicating that these proteins, either alone or in combination, are not required for viral replication in cell culture. However, plaque sizes and viral titers were reduced. Electron microscopy showed only slight alterations in cytoplasmic virion morphogenesis, whereas intranuclear maturation stages were not affected. Similar results were obtained with a triple mutant simultaneously lacking the three conserved tegument proteins UL11, UL16, and UL21. In summary, our results uncover a novel interaction between conserved herpesvirus tegument proteins that increases the complexity of the intricate network of protein-protein interactions involved in herpesvirus morphogenesis.
During herpesvirus virion formation, more than 30 proteins assemble to form the nucleocapsid, the tegument, and the virion envelope (50, 53). Nucleocapsid assembly takes place in the nuclei of infected cells, where in an autocatalytic process capsids are formed and DNA is packaged (51). For capsid formation of the prototypic alphaherpesvirus, herpes simplex virus type 1 (HSV-1), coexpression of six genes, namely UL18, UL19, UL35, and UL38, whose products are components of the capsid shell, and UL26 and UL26.5, which encode proteins that are involved in scaffold formation, is sufficient (46, 55). DNA cleavage and packaging involve at least seven essential gene products (6), including the products of the UL15 and UL28 genes, which are thought to constitute subunits of the terminase, and the UL17, UL32, UL33, and UL6 proteins, of which the latter has been shown to act as a portal protein (47). The UL25 protein is required for stable DNA packaging but not for cleavage (41). In the related alphaherpesvirus pseudorabies virus (PrV), the UL21 gene product has been described as a capsid protein involved in capsid maturation (15).
The DNA-containing capsids leave the nucleus by budding at the inner nuclear membrane, a process which requires two conserved essential gene products: the UL31 protein, which may represent a primary tegument protein, and the UL34 protein, a primary envelope protein (reviewed in reference 43). Both proteins are components of primary enveloped particles but are lost during fusion of the primary envelope with the outer nuclear membrane and thus are not detectable in mature extracellular virus particles (19, 28, 49). Recently, an alphaherpesvirus-specific protein kinase, the product of the US3 gene, has been shown to be involved in, but not required for, efficient fusion of the primary envelope with the outer nuclear membrane (29, 49).
After transfer to the cytosol, nucleocapsids mature by the acquisition of at least 15 different tegument proteins and a lipid envelope containing more than 10 different virally encoded membrane (glyco)proteins. This assembly process follows an intricate network of protein-protein interactions whose complexity is only incompletely understood (43). The product of the conserved UL36 gene, the largest protein found among herpesviruses, is thought to form the innermost part of the tegument (30, 59). It physically interacts with the UL37 protein, which presumably forms a second layer of tegument (30, 31). On the other side, one of the most abundant alphaherpesvirus tegument proteins, the UL49 gene product, has been shown to interact with the carboxy termini of PrV envelope glycoproteins gE and gM, indicating that it is part of the outer tegument shell (18) which connects the envelope with the tegument. Recently, the product of the conserved UL11 gene, a small myristoylated membrane protein (39), has also been implicated in virion formation by HSV-1, PrV, and human cytomegalovirus (HCMV) (4, 8, 33, 52). In PrV, deletion of gM together with either gE and gI (9), or with the cytoplasmic tail of gE (10), or in combination with UL11 (34), led to drastic impairment of secondary envelopment, resulting in the accumulation of large capsid-tegument clusters in the cytoplasm that failed to become enveloped. This finding indicates that these proteins are required for linking tegumented capsids with the virion envelope. After budding into vesicles in the trans-Golgi network, mature enveloped virions are released by fusion of the vesicle membrane with the plasma membrane (reviewed in reference 43). Although a rough outline of the process of virion formation has thus been obtained, the detailed interactions, as well as the roles several other conserved and nonconserved tegument proteins play, remain unclear.
We were interested in analyzing the roles of the UL16 and UL21 proteins. Both represent virion components which are conserved throughout the herpesvirus subfamilies (see e.g., references 1, 11, 14, 16, 27, 40, and 56). The UL16 open reading frame is located in a conserved gene cluster within the intron of the spliced UL15 gene adjacent to the conserved UL17 open reading frame in all mammalian and avian herpesviruses studied so far. The only exception is infectious laryngotracheitis virus, which lacks a UL16 open reading frame at the corresponding location (17). Amino acid comparisons of members of the UL16 family revealed a possible zinc-binding motif in the conserved C terminus and a putative nuclear localization signal in the N terminus (58). The HSV-1 UL16 protein has been identified as a virion component that is dispensable for viral replication in cell culture (3, 45). A role in capsid assembly and/or maturation had been suggested, because the protein was found in the nucleus early in infection partly colocalizing with assemblons, i.e., nuclear areas containing high concentrations of capsid proteins. However, it was also detectable in the cytoplasm (45). In contrast, the UL16 protein of HSV-2 was not detectable in purified virions, whereas weak binding to C-type capsids and DNA suggested a possible role in genome packaging (48). The UL16 homolog of HCMV, the UL94 protein, is a structural component of the capsid or tegument which was detected in the nuclear fraction of infected-cell lysates (58). Interestingly, the HSV-1 UL16 protein has recently been identified as a binding partner of UL11 (37), and it has been speculated, based on the evidence that UL16 associates with nucleocapsids and that UL11 binds to cytoplasmic membranes in the trans-Golgi network (5, 36), that interaction between these two proteins may target cytoplasmic nucleocapsids to the site of secondary envelopment (37).
Although the molecular interactions during PrV virion morphogenesis are under intense study (reviewed in reference 43), the PrV UL16 protein had not been identified and functionally analyzed yet. As deduced from the genomic sequence (27), the gene product is composed of 328 amino acids (aa) and has a calculated molecular mass of 34.8 kDa. Computer predictions indicate a putative N-glycosylation signal at aa 211 and a putative nuclear localization signal between aa 261 and 267 (PSORT II; http://psort.nibb.ac.jp) (23).
In contrast to the UL16 product, the PrV UL21 protein has already been studied in some detail. It has been described as a 61-kDa capsid-associated protein which is involved in capsid maturation (15, 57). A UL21 mutant generated by linker insertion mutagenesis with stop codons inserted after codon 291 of the UL21 open reading frame showed a 10-fold reduction in virus titer but produced wild-type-size plaques, while a mutant truncated after codon 4 replicated only poorly and formed small plaques in cultured cells. Moreover, the cleavage of concatemeric DNA was impaired (15). Electron microscopy showed that UL21-negative virions produced on porcine SK-6 cells or lung alveolar macrophages contained little or no DNA. In contrast, on nasal mucosa explants this defect was not observed, which could indicate that UL21 is involved in packaging of newly replicated DNA in a cell type-specific manner (57).
Previously, we isolated a mutant (PrV-UL21β) in which the UL21 gene had been interrupted by insertion of a lacZ gene expression cassette (26). This mutant replicated to only slightly decreased titers on Vero cells but produced smaller plaques than wild-type virus. In PrV strain Bartha, which is widely used as a live-attenuated vaccine for control of Aujeszky's disease in pigs (42), point mutations within the UL21 gene were found to contribute to loss of virulence. Moreover, pigs infected with PrV-UL21β showed no symptoms after intranasal infection, while wild-type-infected controls died within 8 days (26), indicating that UL21 plays an important role in viral replication in the host animal. In infected cells as well as in purified virions, the HSV-1 UL21 gene product was detected as a 62- to 64-kDa protein (2), a size which matches that of the homologous PrV protein. However, deletion of UL21 from the HSV-1 genome had no effect on DNA cleavage, and replication in cell culture was only marginally affected, with three- to fivefold-reduced titers in human embryonic lung cells (2). Immunofluorescence studies localized the UL21 protein mainly in the cytoplasm in brightly staining granules (2), a finding that argues against a role in the nuclear phase of viral replication. Recently, the HSV-1 UL21 protein has been shown to promote the outgrowth of long cellular processes when overexpressed in nonneuronal cells by interaction with microtubules. This finding could suggest a role for UL21 in intracellular virus transport (54).
To further elucidate the PrV assembly process, we isolated mutant viruses lacking UL16, UL21, or both and analyzed them in cell culture. Moreover, since the HSV-1 UL16 protein binds the membrane-associated UL11 protein (37), we also constructed and analyzed a triple mutant simultaneously lacking the UL11, UL16, and UL21 proteins.
MATERIALS AND METHODS
Viruses and cells.
All PrV mutants were derived from the laboratory strain Kaplan (PrV-Ka) (24). Viruses were grown in rabbit kidney (RK13) or porcine kidney (PSEK) cells in Eagle's minimum essential medium supplemented with 10 or 5% fetal calf serum, respectively. Mutant PrV-ΔUL28 (332-31) was propagated on complementing African green monkey (Vero) cells as described elsewhere (44). Cloning of PrV as a bacterial artificial chromosome (BAC) has been described elsewhere (33). For generation of UL16- or UL21-expressing cells, the open reading frames were cloned into the eukaryotic expression vector pcDNA3 (Invitrogen, Karlsruhe, Germany). For constitutive expression of UL16, the 16,099-bb BamHI fragment 3 cloned into pUC19 (pUC19-Bam3) was first digested with Tth111I and KpnI and then religated after blunt ending. The insert of this plasmid was then further shortened to 1.1 kb by double digestion with BamHI and NcoI, resulting in plasmid pUC-UL16. The KpnI site was provided by the vector, whereas BamHI, NcoI, and Tth111I sites were located in the viral DNA insert (see Fig. 1). The UL16 open reading frame was excised by BamHI and EcoRI cleavage using the flanking vector-specific restriction sites and was cloned into appropriately digested vector pcDNA3, giving rise to plasmid pcDNA-UL16. The complete UL21 open reading frame was excised from plasmid pGEX1-UL21 (see below) by cleavage with EcoRI and XhoI and was inserted into pcDNA3, yielding plasmid pcDNA-UL21. Cell lines constitutively expressing UL16 or UL21 were selected after transfection of RK13 cells using the Superfect transfection reagent (QIAGEN, Hilden, Germany) with the corresponding plasmids followed by selection with 500 μg of G418 (Invitrogen) per ml. G418-resistant cell clones were assayed by immunofluorescence with the monospecific anti-UL16 and anti-UL21 sera (see below). One positive cell clone each, designated RK13-UL16 and RK13-UL21, respectively, was used for further studies. Correct expression was also verified by Western blot analysis (data not shown).
FIG. 1.
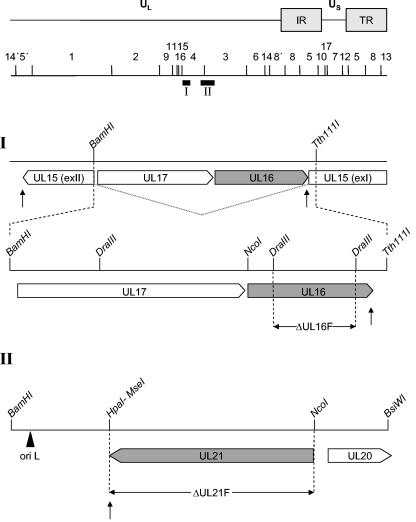
Construction of virus mutants. Schematic map of the PrV genome shows the unique long (UL) and unique short (US) regions, the inverted repeat regions (IR, TR), and the positions of BamHI restriction fragments. Black bars labeled I and II indicate locations of the relevant genomic regions. (I) Enlargement of the PrV UL15-UL17-UL16 gene region (27). The UL17 and UL16 genes are located within the intron of the spliced UL15 gene (dotted line), which is flanked by exons I (exI) and II (exII) of the UL15 gene. UL17 and UL16 are transcribed into 3′-coterminal mRNAs which share a common polyadenylation signal (arrow pointing up). Relevant restriction sites are indicated. (II) Enlargement of the UL20-UL21 gene region (32). The left part of BamHI fragment 4 is shown. The UL21 open reading frame is located adjacent to an origin of replication (ori L) and is transcribed antiparallel to the UL20 gene. Pointed rectangles show locations of the respective open reading frames and also indicate transcriptional orientation. Relevant restriction site are given, and the location of the UL21 polyadenylation signal is indicated by an arrow pointing up.
Preparation of monospecific antisera.
For generation of a UL16-specific antiserum, the complete UL16 open reading frame was cloned into the prokaryotic expression vector pGEX-4T-1. To this end, pUC-UL16 (see above) was cleaved by using flanking vector-specific BamHI and EcoRI sites, and the excised fragment was cloned into appropriately cleaved pGEX-4T-1 (Amersham Biosciences, Freiburg, Germany). Correct in-frame cloning was verified by sequencing using plasmid-specific primers (pGEX-5′; Amersham Biosciences). After induction with isopropyl-β-d-thiogalactopyranoside (IPTG), the resulting 66-kDa glutathione S-transferase (GST)-UL16 fusion protein was purified by gel electrophoresis and used for immunization of a rabbit as described earlier (28).
For generation of a UL21-specific antiserum, plasmid pBS-B4-B/S3.8, containing the cloned BamHI/SalI subfragment of BamHI fragment 4 (32), was digested with NcoI and MseI, releasing a fragment corresponding to nucleotides 64488 to 66062 of the complete PrV sequence (GenBank accession no. BK001744) (27). This fragment, which contained the entire UL21 open reading frame, was cloned into the SmaI-cleaved expression vector pGEX-4T-1 after Klenow fill in of the noncompatible ends. The 81-kDa GST-UL21 fusion protein was purified and used for immunization of a rabbit.
The generation of a PrV UL17-specific serum will be described elsewhere (B. G. Klupp et al., unpublished data).
Isolation of PrV-ΔUL16F, PrV-ΔUL21F, PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, and PrV-ΔUL16F/21K/11G.
Virus mutants and marker genes are described in Table 1. For deletion of a major part of the UL16 open reading frame, plasmid pUC-UL16 (see above) was digested with DraIII (see Fig. 1), thereby deleting 648 bp corresponding to UL16 codons 68 to 282. They were then replaced with a 1,256-bp BstBI fragment carrying a kanamycin resistance gene flanked by Flp recombinase recognition target (FRT) sites derived from plasmid pKD13 (12), giving rise to plasmid pUC-ΔUL16kanF. The 1.7-kb insert of this plasmid was amplified by PCR using vector-specific primers M13 and M13 reverse (New England Biolabs, Frankfurt, Germany) and Pfx DNA polymerase (Invitrogen). The PCR product was excised after gel electrophoresis and used for mutagenesis of the BAC clone pPrV-ΔgB (33) by utilizing the Red recombinase of bacteriophage λ (13). After subsequent deletion of the kanamycin resistance gene by Flp recombinase (12) and cotransfection by calcium phosphate coprecipitation (20) with plasmid pUC-BclI (33), which contains the authentic gB gene of PrV-Ka and restores gB expression, PrV-ΔUL16F could be isolated on RK13 cells.
TABLE 1.
Summary of virus mutants and marker genes
| Mutant | UL11 | UL16 | UL21 |
|---|---|---|---|
| PrV-Ka | WTa | WT | WT |
| PrV-ΔUL11F | FRT site | WT | WT |
| PrV-ΔUL16F | WT | FRT site | WT |
| PrV-ΔUL21F | WT | WT | FRT site |
| PrV-ΔUL16F/21K | WT | FRT site | Kanamycin |
| PrV-ΔUL16F/11G | GFP | FRT site | WT |
| PrV-ΔUL16F/21K/11G | GFP | FRT site | Kanamycin |
WT, wild type.
For generation of PrV-ΔUL21F, the complete UL21 open reading frame was deleted from pBS-B4-B/S3.8 by digestion with NcoI and HpaI (see Fig. 1). Instead, a 1,256-bp BstBI fragment containing the kanamycin resistance gene was initially inserted to facilitate selection but was deleted thereafter by the same procedure described for generation of PrV-ΔUL16F (see above).
Mutant PrV-ΔUL16F/21K was isolated based on the BAC clone pPrV-ΔUL16F. The UL21 open reading frame was removed by using the same restriction sites as for generation of PrV-ΔUL21F but was replaced with a kanamycin resistance gene without flanking FRT sites, since insertion of a second FRT site might result in loss of sequences located between the two sites. Thus, this mutant stably carries the kanamycin resistance gene in its genome. The double mutant PrV-ΔUL16F/11G was isolated after cotransfection into RK13-UL16 cells of PrV-ΔUL16F DNA with plasmid pUC-ΔUL11gfp, which contains a green fluorescent protein (GFP) expression cassette within the UL11 gene (B. G. Klupp et al., unpublished data). Green fluorescent plaques were picked and purified to homogeneity. PrV-ΔUL16F/21K/11G was isolated after cotransfection of genomic PrV-ΔUL16F/21K DNA with pUC-ΔUL11gfp and repeated purification of green fluorescent plaques. Correct recombination was verified by Southern blotting using standard procedures (data not shown).
Indirect immunofluorescence and confocal laser scanning microscopy.
RK13 cells grown on coverslips were infected with PrV-Ka or transfected with expression plasmids for UL16 or UL21 for approximately 18 h. They were then fixed for 20 min with 3% paraformaldehyde, followed by a 10-min incubation with 3% paraformaldehyde plus 0.3% Triton X-100. Subsequently, cells were incubated for 1 h at room temperature with antisera against the UL16 or UL21 proteins followed by Alexa 488-conjugated secondary antibodies (Molecular Probes, Leiden, The Netherlands). Coverslips were washed repeatedly with phosphate-buffered saline (PBS) after each step, and fluorescence was preserved with a 9:1 mixture of glycerol and PBS containing 2.5 mg of 1,4-diazabicyclooctane per ml and 1 μg of propidium iodide per ml for chromatin counterstaining. The slides were analyzed with a confocal laser scanning microscope (LSM510; Zeiss, Göttingen, Germany).
Virus purification and immunoblotting.
Virus purification and preparation of infected-cell lysates were performed as described elsewhere (33). Parallel blots were incubated with monospecific antisera against the UL16 (1:50,000) (this study), UL21 (1:20,000) (this study), UL11 (1:10,000) (33), UL17 (1:10,000) (unpublished data), UL19 (major capsid protein; 1:1,000,000) (28), UL37 (1:50,000) (30), gH (1:50,000) (25), and UL49 (1:50,000) (9) proteins. Bound antibody was visualized after incubation with horseradish peroxidase-conjugated secondary antibodies by enhanced chemiluminescence (Supersignal; Pierce, Bonn, Germany) recorded on X-ray film.
In vitro radiolabeling.
For metabolic labeling, PSEK cells were infected at a multiplicity of infection (MOI) of 10 with either PrV-Ka or the respective mutant in a medium containing [35S]methionine-cysteine (Tran35S-label; ICN, Eschwege, Germany). Radioimmunoprecipitations were performed as described elsewhere (28, 31, 38) and were analyzed after electrophoretic separation on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels (35) in a phosphorimager (FLA-3000; Raytest, Straubenhardt, Germany).
Electron microscopy.
For ultrathin sectioning, RK13, RK13-UL16, or RK13-UL21 cells were infected at an MOI of 1 and were fixed 14 h postinfection (p.i.). Fixation, dehydration, and embedding for electron microscopy were performed as described previously (21, 22). Sections were examined with an electron microscope (Tecnai 12; Philips, Eindhoven, The Netherlands).
RESULTS
Identification of the PrV UL16 gene product.
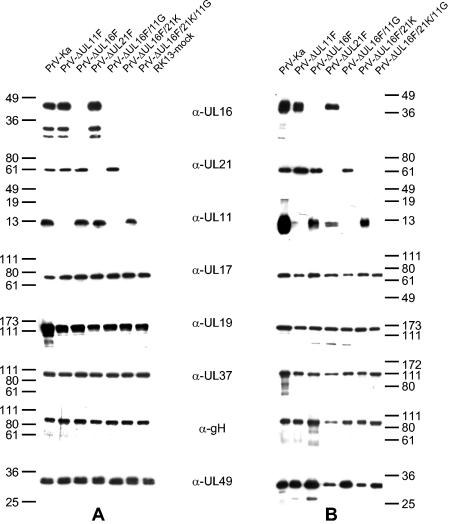
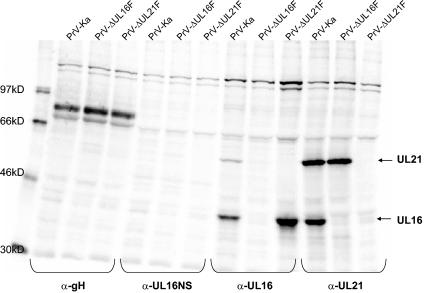
For identification of the PrV UL16 protein, the complete open reading frame was cloned into the prokaryotic expression vector pGEX-4T-1, expressed as a GST fusion protein in Escherichia coli, and used for immunization of a rabbit. Serum obtained after the fifth immunization was used throughout this study. In Western blot analyses on infected-cell lysates, the antiserum specifically detected a prominent 40-kDa protein (Fig. 2A), which was absent in mock-infected cell lysates and in cell lysates infected with the UL16 deletion mutant PrV-ΔUL16F, PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, or PrV-ΔUL16F/21K/11G (Fig. 2A). The smaller proteins of 32 and 30 kDa labeled by the antiserum in infected-cell lysates may represent degradation products which are not present in purified virion preparations (Fig. 2B). We found no evidence for expression of the N-terminal 67 aa of the UL16 protein, whose coding region was retained in the UL16 deletion mutants. The size of the detected protein is in good correlation with the predicted molecular mass of 35 kDa (27). Probable dimerization as described for the homologous HCMV UL94 protein (58) was not obvious either in cell lysates (Fig. 2A) or in purified virion preparations (Fig. 2B). Although a possible N-glycosylation consensus site is present at aa 210 to 212 of the UL16 open reading frame, N-glycosidase F treatment did not result in a decrease in the apparent size of the UL16 protein (data not shown), indicating that it is not N-glycosylated. However, the UL16 protein may be phosphorylated, since computer analysis (www.cbs.dtu.dk/services/NetPhos) (7) predicted eight possible phosphorylation sites. As a control, parallel blots were probed with sera against the UL21 (see below), UL11, UL17, UL19, UL37, gH, and UL49 proteins (Fig. 2).
FIG. 2.
Identification of the PrV UL16 protein and characterization of mutant viruses. Infected-cell lysates (A) or purified virions (B) of PrV-Ka, PrV-ΔUL11F, PrV-ΔUL16F, PrV-ΔUL21F, PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, PrV-ΔUL16F/21K/11G, or mock-infected RK13 cells were separated on SDS-10 or 15% (for demonstration of UL11) polyacrylamide gels and incubated with monospecific antisera against the UL16, UL21, UL11, UL17, UL19, UL37, gH, or UL49 protein. Locations of molecular mass markers (in kilodaltons) are shown to the left (A) and right (B) of the gels.
Subcellular localization of the PrV UL16 protein.
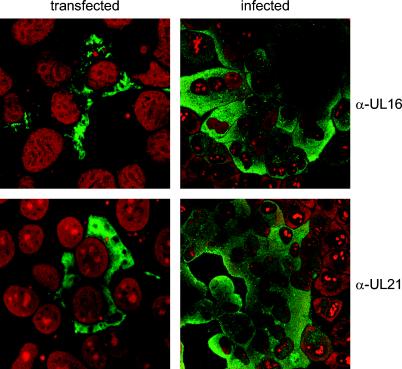
To analyze the subcellular localization of PrV UL16, confocal laser scanning microscopy was performed on PrV-Ka-infected and transiently transfected RK13 cells. As shown in Fig. 3, UL16 specific fluorescence was detected mainly in the cytoplasm of infected cells, and only weak nuclear staining was observed. In cells transiently transfected with pcDNA-UL16, specific staining was found exclusively in the cytoplasm (Fig. 3). Adjacent noninfected or nontransfected cells, which served as internal controls, exhibited no specific staining.
FIG. 3.
Intracellular localization of UL16 and UL21 proteins. RK13 cells were either transiently transfected with expression plasmids for UL16 (upper left panel) or UL21 (lower left panel) or infected with PrV-Ka (right panels). Immunofluorescence analysis was performed by confocal laser scanning microscopy using monospecific anti-UL16 or anti-UL21 serum and Alexa 488-conjugated secondary antibodies (green). Chromatin was counterstained with propidium iodide (red).
In vitro growth properties of PrV-ΔUL16.
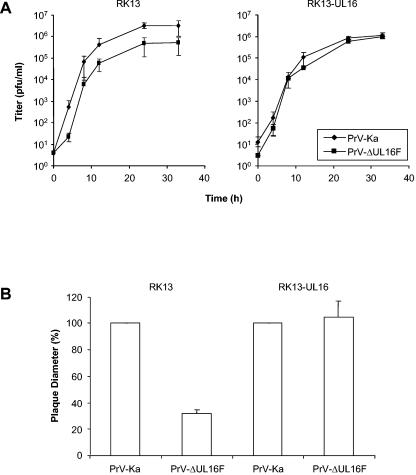
Since PrV-ΔUL16F could be propagated on noncomplementing cells, UL16 is not essential for PrV replication in cultured cells. To monitor for possible replication deficiencies, RK13 or RK13-UL16 cells were infected with PrV-Ka or PrV-ΔUL16F at an MOI of 10 and were harvested at the indicated time points (Fig. 4A). When propagated on noncomplementing RK13 cells, PrV-ΔUL16F showed a decrease in viral titers at all time points tested (Fig. 4A, left panel) that resulted in ca. 10-fold-reduced final titers. This defect was fully compensated for on RK13-UL16 cells (Fig. 4A, right panel). In contrast to viral titers, plaque size was more drastically affected (Fig. 4B). The mean diameter of PrV-ΔUL16F plaques reached only 35% of the corresponding PrV-Ka plaque diameters on RK13 cells. This defect was also compensated for on RK13-UL16 cells, indicating that the observed impairment was due to deletion of UL16 and not to other, unwanted mutations elsewhere in the genome.
FIG. 4.
Growth properties of PrV-ΔUL16F. (A) RK13 or RK13-UL16 cells were infected with PrV-Ka or PrV-ΔUL16F at an MOI of 10 and were harvested immediately or after 4, 8, 12, 24, or 36 h p.i. Virus progeny was titrated on RK13 cells. Mean virus titers and standard deviations from three independent experiments are shown. (B) RK13 or RK13-UL16 cells were infected with PrV-Ka or PrV-ΔUL16F under plaque assay conditions, and the diameters of 30 plaques each were measured microscopically after 48 h. The mean value for PrV-Ka was set at 100%, and the average plaque size of PrV-ΔUL16 was calculated accordingly. Means and standard deviations are shown for three independent experiments.
In electron microscopic analyses, nucleocapsid assembly in the nucleus appeared unaffected and no major impairment of cytoplasmic virion maturation was observed, although minor effects on virion formation could not be excluded (data not shown).
Tegument proteins UL16 and UL21 form a complex.
In immunoprecipitations of PrV-Ka-infected cell lysates with the anti-UL16 serum, besides the 40-kDa UL16 protein, a 60-kDa protein which was not precipitated by the preimmune serum was consistently detected (Fig. 5). No specific protein was precipitated by using lysates of PrV-ΔUL16F-infected cells (Fig. 5). The size of the coprecipitated protein matched that of the UL21 protein (15). By using an anti-UL21 serum, coprecipitation of an additional protein of ca. 40 kDa had been reported (15) but suggested to represent a processed form of UL21. To test whether the 40-kDa protein is the UL16 gene product, an antiserum was raised against the PrV-Ka UL21 protein. The specificity of the antiserum was verified by Western blot analysis of PrV-infected cell lysates (Fig. 2A) and purified virions (Fig. 2B). The PrV-Ka UL21 gene product was detectable as an approximately 60-kDa protein in infected cells which was also present in purified virions. No specific reactivity was found on cell lysates of RK13 cells that were infected with the UL21 deletion mutant PrV-ΔUL21F, PrV-ΔUL16F/21K, or PrV-ΔUL16F/21K/11G (Fig. 2A) or were mock infected, or in the corresponding virion preparations (Fig. 2B). In indirect immunofluorescence analysis, UL21-specific staining was prominent in the cytoplasm in infected and transiently transfected cells (Fig. 3).
FIG. 5.
Coimmunoprecipitation of UL16 and UL21 proteins. Lysates from metabolically labeled PSEK cells infected with PrV-Ka, PrV-ΔUL16F, or PrV-ΔUL21F were precipitated with the UL16-specific antiserum (α-UL16) or the corresponding preimmune serum (α-UL16NS), the UL21-specific antiserum (α-UL21), or an anti-gH (α-gH) monospecific serum. Precipitates were separated on an SDS-10% polyacrylamide gel and analyzed with a phosphorimager. Labeled marker proteins are shown in the left lane, and their relative molecular masses are indicated.
By using metabolically labeled lysates of PrV-Ka-, PrV-ΔUL16F-, and PrV-ΔUL21F-infected cells and the UL16- and UL21-specific antisera for immunoprecipitation, coprecipitation of the UL16 and UL21 proteins could clearly be shown (Fig. 5). From lysates of PrV-Ka-infected cells, proteins of 40 and 60 kDa were coprecipitated by using either the UL16- or the UL21-specific antiserum. In contrast, only the 60-kDa UL21 protein was precipitated from lysates of PrV-ΔUL16F-infected cells by using the anti-UL21 serum (Fig. 2A). Conversely, only the 40-kDa UL16 protein was found in precipitates of PrV-ΔUL21-infected cell lysates incubated with the anti-UL16 serum. Control immunoprecipitations using anti-gH serum showed that similar amounts of labeled infected-cell proteins had been used. We note that coprecipitation of the UL16 and UL21 proteins was most prominent with the UL21-specific antiserum and less so with the anti-UL16 serum. Taken together, these results demonstrate that PrV proteins UL16 and UL21 form a physical complex.
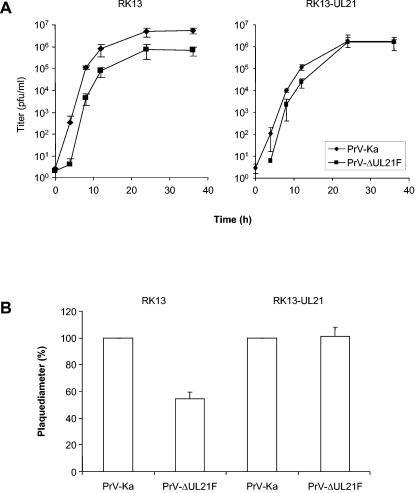
Growth properties of PrV-ΔUL21F.
In earlier studies we have shown that UL21 is not essential for viral replication in cultured cells and that its deletion resulted in only marginally reduced titers but clearly decreased plaque sizes (26). The mutant used for these studies contained a small deletion of coding sequences in the middle of the UL21 open reading frame and carried a lacZ expression cassette instead. Thus, we could not exclude the possibility that the N terminus of UL21 might still be expressed and provide some function. In contrast, a PrV UL21 mutant in which expression was blocked after codon 4 exhibited a strong defect and replicated only poorly in cell culture (15). To clarify the role of UL21 in the replication of PrV, we isolated PrV-ΔUL21F, in which the complete open reading frame has been deleted by BAC mutagenesis. This method allows for engineering of the mutated virus in E. coli, thus minimizing the possibility of second-site mutations, and results in the generation of mutant viruses with only a minimum of foreign sequences inserted. PrV-ΔUL21F (Fig. 1) showed only a slight decrease in viral titers in one-step growth analysis on noncomplementing RK13 cells (Fig. 6A, left panel) but a more significant reduction in plaque size, which amounted to approximately 50% of the size of wild-type plaques on RK13 cells (Fig. 6B). Both defects were compensated for on RK13-UL21 cells (Fig. 6A, right panel, and 6B). In ultrathin sections, extracellular virions as well as L-particles were found in large amounts on the cell surface. Moreover, capsid maturation appeared unaffected, and all stages of virus maturation were detectable in the nucleus and in the cytoplasm (data not shown).
FIG. 6.
Growth properties of PrV-ΔUL21F. RK13 and RK13-UL21 cells were infected in parallel with PrV-Ka or PrV-ΔUL21F. One-step growth kinetics (A) and plaque sizes (B) were analyzed as described in the legend to Fig. 4.
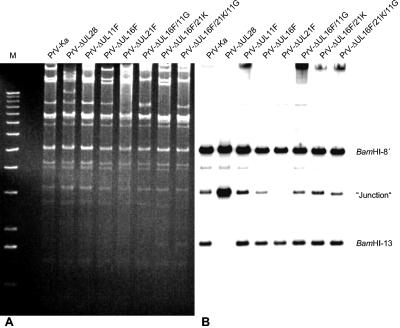
Although electron microscopy revealed no defect in capsid maturation in the absence of UL21, we aimed to verify earlier data (15). Therefore, whole-cell DNA of infected RK13 cells was investigated for the presence of the terminal fragments BamHI-13 (Fig. 7) and BamHI-14′ (data not shown) (for locations of fragments, see Fig. 1). Hybridization with the radiolabeled cloned fragment BamHI-13 revealed signals corresponding to BamHI fragments 8′ and 13, which share homologous sequences because both contain sequences from the inverted-repeat regions (see Fig. 1), as well as the junction fragment composed of BamHI-13 and -14′, which is derived from head-to-tail concatemeric or circular DNA. As is evident in Fig. 7, all mutants tested, lacking either UL16, UL21, or UL11 singly or in different combinations specified the terminal fragment BamHI-13. Moreover, it was detectable in similar amounts, indicating uninhibited cleavage of concatemeric DNA into unit-length molecules. In cells infected with a PrV mutant unable to express functional UL28 protein, which is known to be necessary for cleavage and encapsidation (44), no terminal fragments were detectable. Instead, a larger amount of the junction fragment was observed (Fig. 7B). These results indicate that UL21 is not involved in capsid maturation or processing of viral DNA.
FIG. 7.
Processing of viral DNA. RK13 cells were infected with PrV-Ka, PrV-ΔUL28, PrV-ΔUL11F, PrV-ΔUL16F, PrV-ΔUL21F, PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, or PrV-ΔUL16F/21K/11G at an MOI of 1, and cells were harvested when the cytopathic effect approached 100%. Whole-cell DNA was digested with BamHI and separated on an 0.8% agarose gel. (A) Ethidium bromide-stained gel. (B) After transfer to a nylon membrane, the filter was hybridized with labeled terminal BamHI fragment 13. Hybridization reveals signals corresponding to fragments 8′ and 13, which share homologous sequences because both originate from the inverted repeat regions (see Fig. 1), as well as the junction fragment composed of BamHI fragments 13 and 14′, which is derived from head-to-tail concatemeric or circular DNA.
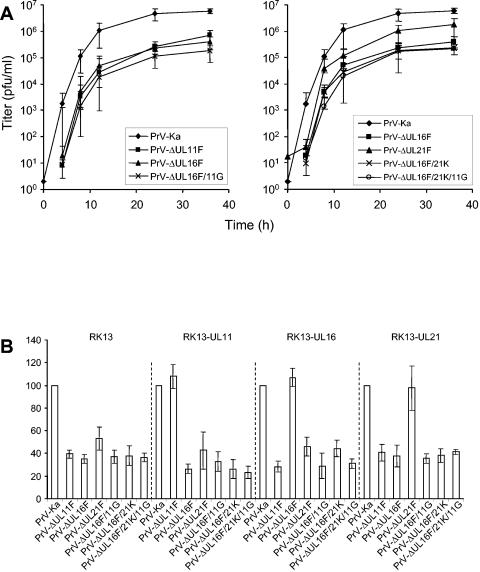
Growth properties of mutants PrV-ΔUL16F/21K, PrV-ΔUL16F/11G, and PrV-ΔUL16F/21K/11G.
We demonstrated the formation of a physical complex between the UL16 and UL21 proteins of PrV (this study). In a recent paper, an interaction between the HSV-1 UL11 and UL16 proteins has been reported, and results suggested a similar interaction between the PrV homologs (37). Thus, we aimed to study the functional role of the bi- or possibly tripartite complex by the isolation and analysis of mutant viruses simultaneously lacking UL16 and UL21, UL16 and UL11, or UL16, UL21, and UL11. Mutants lacking the corresponding proteins in the different combinations were characterized by Western blot analyses (Fig. 2), one-step growth kinetics (Fig. 8A), plaque size measurements (Fig. 8B), and electron microscopy (Fig. 9). Western blot analyses verified the absence of the respective proteins in mutant virus-infected cell lysates (Fig. 2A) as well as in purified virion preparations (Fig. 2B). Incorporation or expression of other virally encoded proteins seemed largely unaffected in the absence of either UL16, UL21, or UL11 (Fig. 2). Interestingly, the deletion of UL16, UL21, or UL11 did not appreciably influence the packaging of the remaining components of the hypothetical tripartite complex, although it appeared that the UL11 single-deletion mutant might carry less UL16 and the UL16 mutant might carry less UL11. However, quantitation by Western blot analysis is difficult, and these data should be interpreted with care. Generally, the detection of the UL11 protein was more variable than that of the other proteins tested.
FIG. 8.
Growth properties of mutants PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, and PrV-ΔUL16F/21K/11G. (A) One-step growth kinetics. RK13 cells were infected with the indicated viruses at an MOI of 5 and harvested as described in the legend to Fig. 4. Progeny virus titers were determined on RK13 cells. Shown are means and standard deviations from three independent experiments. (B) Plaque size determination. RK13, RK13-UL11, RK13-UL16, or RK13-UL21 cells were infected with PrV-Ka, PrV-ΔUL11F, PrV-ΔUL16F, PrV-ΔUL21F, PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, or PrV-ΔUL16F/21K/11G under plaque assay conditions. Plaque size was determined 2 days p.i. Mean relative values and standard deviations from three different experiments are shown, with the PrV-Ka plaque size set at 100%.
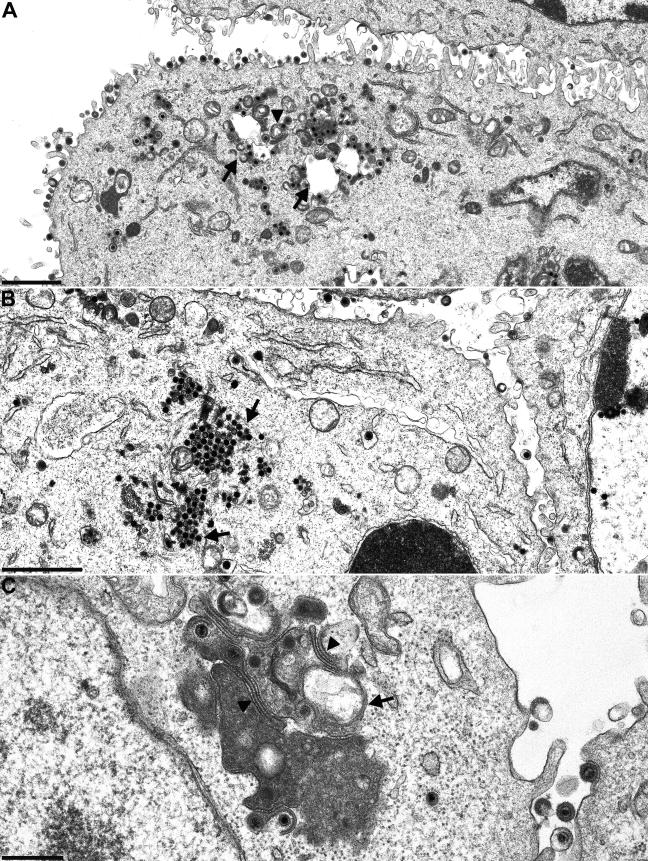
FIG.9.
Electron microscopy of PrV-ΔUL16F/11G, PrV-ΔUL16F/21K, and PrV-ΔUL16F/21K/11G. RK13 cells were infected with PrV-ΔUL16F/11G (A), PrV-ΔUL16F/21K (B), or PrV-ΔUL16F/21K/11G (C) at an MOI of 1 and were processed for electron microscopy at 14 h p.i. Arrows in panel A point to capsids in association with cytoplasmic vesicles, and the arrowhead indicates distorted, tightly connected membranes. Arrows in panel B show capsid accumulations. Arrowheads in panel C again point to distorted membranes, whereas the arrow indicates a large cytoplasmic vesicle. Bars in panels A and B, 1.5 μm; bar in panel C, 500 nm.
The double mutants PrV-ΔUL16F/21K and PrV-ΔUL16F/11G and the triple mutant PrV-ΔUL16F/21K/11G replicated on noncomplementing RK13 cells with kinetics similar to those of the UL16 and UL11 single-deletion mutants (Fig. 8A) and produced comparable plaque sizes (Fig. 8B), indicating that none of the proteins alone or in the different combinations is required for productive replication in cultured cells.
In electron microscopic analyses, PrV-ΔUL16F/11G (Fig. 9A) revealed a phenotype similar to that of PrV-ΔUL11 (33), with capsid aggregations in association with the tegument, sometimes juxtaposed to intracytoplasmic vesicles, as well as distorted membranes. Extracellular virions were found lining the cell surface. In the simultaneous absence of UL16 and UL21, capsid aggregations were found in the cytoplasm (Fig. 9B), suggesting that UL16 and UL21 are involved, either directly or indirectly, in virion formation. In the simultaneous absence of UL11, UL16, and UL21, distorted membranes, intracytoplasmic vesicles, and capsids undergoing secondary envelopment were observed (Fig. 9C). Extracellular virus particles were also present, indicating that virion morphogenesis proceeds in the absence of all three conserved tegument proteins.
DISCUSSION
The assembly of the mature herpesvirus particle, which consists of more than 30 different virus-encoded proteins, is a complex process. Whereas the molecular interactions resulting in capsid formation in the nucleus are known to some extent (59), nuclear egress, tegumentation, and envelopment of cytoplasmic nucleocapsids to form infectious mature enveloped virus particles are still not understood well at the molecular level. Recent data indicate that tegumentation is not a random but a highly structured event following a complicated network of protein-protein interactions (reviewed in reference 43). Whereas several of the proteins involved are conserved throughout the herpesviruses, which may be indicative of important functions these proteins fulfill during the basic herpesvirus replication cycle, others are not.
In this study we concentrated on the conserved products of the UL11, UL16, and UL21 genes, which have been shown to be virion components in PrV (15, 33; this study), HSV-1 (2, 4, 45), and HCMV (52, 58), respectively. The salient findings can be summarized as follows: (i) the PrV UL16 protein has been identified as a 40-kDa virion protein which is not required for productive replication in cultured rabbit kidney cells; (ii) the PrV UL16 protein physically interacts with the 60-kDa PrV UL21 gene product; (iii) neither the UL21, the UL16, nor the UL11 protein either alone or in combination is required for productive viral replication; (iv) neither the UL16 nor the UL21 protein of PrV is relevant for nuclear processes of viral replication.
Genes homologous to the UL16 open reading frames of HSV-1 and PrV include varicella-zoster virus ORF44 (14), HCMV UL94 (11), and BGLF2 of Epstein-Barr virus (1). Here we identified the PrV UL16 protein as a 40-kDa virion component. The PrV UL16 DNA sequence has been published recently, and the calculated molecular mass of 35 kDa is in good agreement with the experimental data (27). Although an N-glycosylation consensus sequence is present in the UL16 gene product, the PrV UL16 protein is not modified by N-glycosylation, as evidenced by the fact that its electrophoretic mobility was unchanged after treatment with PNGase F (data not shown). However, when isolated from purified virions, the protein migrates as one broad band, which may result from differential phosphorylation. Modification by phosphorylation has been shown for several herpesvirus tegument proteins (reviewed in reference 43). By indirect immunofluorescence analyses, the UL16 protein was found predominantly in the cytoplasm of infected cells, although we cannot exclude a minor nuclear localization. Computer analysis predicts a nuclear localization signal between aa 261 and 267. Further studies are under way to test whether this signal is functional. However, it is of interest that in transfected cells UL16 is found only in the cytoplasm, which argues against a nuclear localization function of the predicted signal.
Despite its conservation UL16 has been shown to be dispensable for replication of HSV-1 in cultured cells (3), but a role in capsid assembly and/or maturation has been suggested (45, 48). In the absence of UL16, PrV replicated with only 10-fold-reduced virus titers. In contrast, plaque size was significantly decreased, amounting to only 35% that of wild-type PrV. This finding could be indicative of a role of PrV UL16 in direct cell-to-cell spread. No defect in the nuclear phase of viral replication was observed, and capsid egress from the nucleus to the cytoplasm appeared unaffected, arguing against a role of UL16 in intranuclear capsid maturation or primary envelopment of PrV.
Recently, the HSV-1 UL16 protein has been demonstrated to interact with the UL11 gene product (37). These authors also showed that GST-tagged PrV UL11 protein interacted with the HSV-1 UL16 protein. In addition, a GST-tagged PrV UL11 protein pulled down a protein with a molecular mass of 36 kDa from PrV-infected cells, which most likely represents the PrV UL16 protein (37). Although we did not observe coprecipitation of the UL11 protein in our assays, a tripartite UL11-UL16-UL21 complex, though hypothetical to date, may nevertheless exist.
While we could not confirm a physical interaction between the PrV UL16 and UL11 proteins, the anti-UL16 serum reproducibly coprecipitated a 60-kDa protein which was identified as the product of the UL21 open reading frame. Like UL11 and UL16, UL21 homologs are found in all herpesvirus subfamilies. Previously, the PrV UL21 protein had been described as a capsid protein involved in DNA cleavage and packaging (15). In the absence of UL21, formation of DNA-deprived capsids was observed in a cell type-dependent manner (57). In contrast, a UL21 mutant virus we isolated earlier exhibited only a slight effect on replication in cell culture but was strongly attenuated in the natural host (26). Since only a small part of the UL21 open reading frame was deleted in this mutant, we could not exclude the possibility that the N-terminal part of the protein was expressed and at least partially functional. Therefore, we constructed a novel mutant lacking the complete UL21 open reading frame as well as a double-mutant virus unable to express UL16 and UL21. Paralleling our earlier results, the UL21 deletion mutant grew to only slightly lower viral titers than wild-type PrV-Ka but formed small plaques, which were about half the size of PrV-Ka plaques on RK13 cells. Electron microscopy showed normal virus assembly: maturation of nucleocapsids was unaffected, and mature extracellular virus particles were present in abundance. We did not observe any defect in the cleavage of concatemeric DNA into unit-length DNA in the absence of UL21. The differences in replication between our mutants (this study) and those isolated by others (15) might be due either to the different virus strains or cell lines used or to the accidental disturbance of additional genes in the linker insertion mutants.
We noted in our immunoprecipitations that the anti-UL21 serum efficiently coprecipitated the UL16 protein, whereas the anti-UL16 serum coprecipitated only limited amounts of UL21 protein. Moreover, in the absence of UL21, precipitation of the UL16 protein by the UL16 antiserum was stronger than in the presence of this protein (see Fig. 5). It is conceivable that complex formation between the UL16 and UL21 proteins results in masking of antigenic epitopes on the UL16 protein, which consequently is less well recognized.
The UL16-UL21 double mutant replicated with properties similar to those of the UL16 single-deletion mutant. This finding is in line with the idea that the two proteins form a functional unit. Moreover, a mutant virus lacking all three conserved tegument proteins, UL16, UL21, and UL11, was comparable in its replication to the UL11 and UL16 single mutants, which were very similar in their growth properties. Although this would correlate with the notion of a functional tripartite complex, so far we were unable to show that it actually exists. It will be of interest to determine whether one of the proteins coprecipitated by the HSV-1 UL11 antiserum (37) will be identified as the UL21 protein product. The PrV and HSV-1 UL11 gene products are membrane associated, while the PrV and HSV-1 UL21 proteins appear to be located adjacent to the capsid (15, 54). This could suggest that the hypothetical UL21-UL16-UL11 complex may connect the nucleocapsid and the envelope.
As already mentioned, ultrastructurally no strong defects in virus replication were detected in any of the mutant virus-infected cells, a finding that correlates with the only moderate impairment of one-step growth. However, in the simultaneous absence of UL16 and UL21, capsids were observed to form small clusters in the cytoplasm. The additional absence of the UL11 protein produced distortion of cytoplasmic membranes, as has been described before (33). Thus, slight but specific defects in cytoplasmic virion formation are associated with absence of these proteins.
It is somewhat puzzling that the concomitant absence of three conserved tegument proteins results in only slight impairment of viral replication. However, the in vitro growth phenotype does not necessarily reflect the situation in the animal host. It has already been shown that a PrV UL21 mutant, despite exhibiting only slightly impaired replication in cell culture, is strongly attenuated in PrV's natural host, the pig (26). Thus, animal experiments are under way to assess the roles of these proteins in vivo.
Acknowledgments
This study was in part supported by the Deutsche Forschungsgemeinschaft (Me 854/5-2).
We thank Uta Hartwig, Mandy Jörn, Petra Meyer, and Diana Werner for expert technical assistance.
REFERENCES
- 1.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. F. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, P. Tuffnell, and B. G. Barrell. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 2.Baines, J. D., A. H. Koyama, T. Huang, and B. Roizman. 1994. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J. Virol. 68:2929-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines, J. D., and B. Roizman. 1991. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 65:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines, J. D., R. J. Jacob, L. Simmerman, and B. Roizman. 1995. The herpes simplex virus UL11 proteins are associated with cytoplasmic and nuclear membranes and with nuclear bodies of infected cells. J. Virol. 69:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines, J. D., and S. K. Weller. 2004. Cleavage and packaging of herpes simplex virus 1 DNA, in press. In C. E. Catalano (ed.), Viral genome packaging machines. Landes Press, Georgetown, Tex.
- 7.Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. [DOI] [PubMed] [Google Scholar]
- 8.Bowzard, J. B., R. J. Visalli, C. B. Wilson, J. S. Loomis, E. M. Callahan, R. J. Courtney, and J. W. Wills. 2000. Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera. J. Virol. 74:8692-8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brack, A. R., J. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchinson, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 12.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 13.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 15.de Wind, N., F. Wagenaar, J. Pol, T. Kimman, and A. Berns. 1992. The pseudorabies virus homolog of the herpes simplex virus UL21 gene product is a capsid protein which is involved in capsid maturation. J. Virol. 66:7096-7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs, W., and T. C. Mettenleiter. 1999. DNA sequence of the UL6 to UL20 gene of infectious laryngotracheitis virus and characterization of the UL10 gene product as a nonglycosylated and nonessential virion protein. J. Gen. Virol. 80:2173-2182. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 21.Granzow, H., F. Weiland, A. Jöns, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: a comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, P., and K. Nakai. 1997. Better prediction of protein cellular localization sites with the k nearest neighbors classifier. Proc. Int. Conf. Intell. Syst. Mol. Biol. 5:147-152. [PubMed] [Google Scholar]
- 24.Kaplan, A. S., and A. E. Vatter. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394-407. [DOI] [PubMed] [Google Scholar]
- 25.Klupp, B. G., and T. C. Mettenleiter. 1999. Glycoprotein gL-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J. Virol. 73:3014-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klupp, B. G., B. Lomniczi, N. Visser, W. Fuchs, and T. C. Mettenleiter. 1995. Mutations affecting the UL21 gene contribute to avirulence of pseudorabies virus vaccine strain Bartha. Virology 212:466-473. [DOI] [PubMed] [Google Scholar]
- 27.Klupp, B. G., C. J. Hengartner, T. C. Mettenleiter, and L. W. Enquist. 2004. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 78:424-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 30.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. The pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klupp, B. G., H. Kern, and T. C. Mettenleiter. 1992. The virulence determining genomic BamHI fragment 4 of pseudorabies virus contains genes corresponding to the UL15 (partial), UL18, UL19, UL20, and UL21 genes of herpes simplex virus and a putative origin of replication. Virology 191:900-908. [DOI] [PubMed] [Google Scholar]
- 33.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 77:5339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopp, M., H. Granzow, W. Fuchs, B. Klupp, and T. C. Mettenleiter. 2004. Simultaneous deletion of pseudorabies virus tegument protein UL11 and glycoprotein M severely impairs secondary envelopment. J. Virol. 78:3024-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Loomis, J. S., J. B. Bowzard, R. J. Courtney, and J. W. Wills. 2001. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 75:12209-12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomis, J. S., R. J. Courtney, and J. W. Wills. 2003. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 77:11417-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukács, N., H.-J. Thiel, T. C. Mettenleiter, and H.-J. Rziha. 1985. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J. Virol. 53:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLean, C., B. Clark, and D. J. McGeoch. 1989. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 70:3147-3157. [DOI] [PubMed] [Google Scholar]
- 40.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses type 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 41.McNab, A. R., P. Desai, S. Person, L. Roof, D. R. Thompson, W. W. Newcomb, J. C. Brown, and F. L. Homa. 1998. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J. Virol. 72:1060-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 43.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mettenleiter, T. C., A. Saalmüller, and F. Weiland. 1993. Pseudorabies virus protein homologous to herpes simplex virus type 1 ICP18.5 is necessary for capsid maturation. J. Virol. 67:1236-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nalwanga, D., S. Rempel, B. Roizman, and J. D. Baines. 1996. The UL16 gene product of herpes simplex virus is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226:236-242. [DOI] [PubMed] [Google Scholar]
- 46.Newcomb, W. W., F. L. Homa, D. R. Thomsen, Z. Ye, and J. C. Brown. 1994. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 68:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpesvirus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshima, S., T. Daikoku, S. Shibata, H. Yamada, F. Goshima, and Y. Nishiyama. 1998. Characterization of the UL16 gene product of herpes simplex virus type 2. Arch. Virol. 143:863-880. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roizman, B., and D. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 51.Roizman, B., and P. Pellet. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 52.Silva, M. C., Q.-C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. M. Burnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 54.Takakuwa, H., F. Goshima, T. Koshizuka, T. Murata, T. Daikoku, and Y. Nishiyama. 2001. Herpes simplex virus encodes a virion-associated protein which promotes long cellular processes in over-expressing cells. Genes Cells 6:955-966. [DOI] [PubMed] [Google Scholar]
- 55.Tatman, J. D., V. G. Preston, P. Nicholson, R. M. Elliott, and F. J. Rixon. 1994. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J. Gen. Virol. 75:1101-1113. [DOI] [PubMed] [Google Scholar]
- 56.Telford, E. A. R., M. S. Watson, K. McBride, and A. J. Davison. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304-316. [DOI] [PubMed] [Google Scholar]
- 57.Wagenaar, F., J. M. A. Pol, N. deWind, and T. G. Kimman. 2001. Deletion of the UL21 gene in pseudorabies virus results in the formation of DNA deprived capsids. Vet. Res. 32:47-54. [DOI] [PubMed] [Google Scholar]
- 58.Wing, B., G. C. Y. Lee, and E.-S. Huang. 1996. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J. Virol. 70:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, Z., D. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]