Abstract
Purpose
It is unclear whether women with a history of postpartum depression (PPD) have residual, abnormal hypothalamic-pituitary-adrenal (HPA) axis reactivity, as has been reported in major depression (MDD). Further unclear is whether the abnormalities in HPA axis reactivity associated with MDD represent a stable, underlying predisposition or a state-dependent phenomenon. This study sought the following: 1) to determine if euthymic postpartum women with a history of depression have an abnormal HPA axis reactivity to pharmacologic and psychological challenges and 2) to compare HPA reactivity in women with histories of PPD versus MDD. As a secondary objective, we wanted to determine the influence of trauma history on HPA axis function.
Methods
Forty-five parous (12–24 months postpartum), euthymic women with history of MDD (n=15), PPD (n=15) and controls (n=15) completed pharmacologic (dexamethasone/CRH test [DEX/CRH]) and psychological (Trier Social Stress Test [TSST]) challenges during the luteal phase. Outcome measures were cortisol and adrenocorticotropic hormone (ACTH) response after DEX/CRH, and blood pressure, heart rate, epinephrine, norepinephrine, and cortisol response during the TSST.
Results
All groups had robust cortisol and ACTH response to DEX/CRH and cortisol response to TSST. Groups did not differ significantly in cortisol or ACTH response to DEX/CRH or in blood pressure, heart rate, epinephrine, norepinephrine, or cortisol response to TSST. Cortisol/ACTH ratio did not differ significantly between groups. Trauma history was associated with decreased cortisol response to DEX/CRH in women with histories of MDD, which was not significant after correction (F8,125, p=0.02, Greenhouse-Geisser corrected p= 0.11).
Conclusions
Currently euthymic women with histories of MDD or PPD did not demonstrate residual abnormal stress responsivity following administration of either a pharmacologic or psychological stressor.
Keywords: postpartum depression, major depressive disorder, HPA axis, cortisol, Trier social stress test, dexamethasone/corticotropin-releasing hormone test
Introduction
Over the past five decades, many studies evaluating hypothalamic-pituitary-adrenal (HPA) axis reactivity in individuals with major depressive disorder (MDD) have demonstrated abnormal reactivity of the HPA axis in response to both pharmacologic and psychological stressors (Burke, Davis, Otte, & Mohr, 2005; Gold, Gabry, Yasuda, & Chrousos, 2002; Pariante & Lightman, 2008). This body of work has led to the hypothesis that abnormalities in HPA axis functioning may play a role in the pathogenesis of depression (Pariante & Lightman, 2008). The current study sought to better understand what role HPA axis abnormalities may play in the pathogenesis of postpartum depression (PPD), an important subtype of major depression that is associated with childbirth (Dietz et al., 2007; Glynn, Davis, & Sandman, 2013), through characterization of HPA axis reactivity to pharmacologic and psychological stressors in the postpartum period in euthymic women with histories of PPD compared to both women with histories of non-puerperal depression and healthy controls.
It remains unclear whether abnormalities in HPA axis function in depression represent a stable, underlying predisposition or a state-dependent phenomenon of the disorder. More detailed investigations of HPA axis function in individuals with depression across time have led to conflicting results, with some studies finding a return to normal functioning of the HPA axis after successful antidepressant treatment (Anacker, Zunszain, Carvalho, & Pariante, 2011; Pariante, 2009; Ruhe et al., 2015; Schule, 2007) or during periods of euthymia (Lange et al., 2013) and others reporting abnormalities of HPA axis function even during periods of remission from depression, including both hyper-reactivity (Hohne et al., 2014; Holsen et al., 2013; Keating, Dawood, Barton, Lambert, & Tilbrook, 2013; Lok et al., 2012) and hypo-reactivity (Ahrens et al., 2008; Bagley, Weaver, & Buchanan, 2011) of the HPA axis. Studies evaluating HPA axis reactivity in individuals with histories of early life stress have more consistently demonstrated persistent hyperreactivity of the HPA axis over time irrespective of current psychopathology/symptoms (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), leading to the hypothesis that the finding of HPA axis hyperreactivity in individuals with depression represents an underlying biological predisposition to depression shaped by early life events rather than a transient biomarker of depressive symptoms (Pariante & Lightman, 2008).
Significantly fewer studies have evaluated HPA axis reactivity in women with PPD. There are great alterations in the HPA axis during pregnancy, due in large part to the presence of the placenta, a highly active endocrine organ which produces its own CRH, as well as increases in gonadal hormones contributing to puerperal hypertrophy of both the pituitary and adrenal glands resulting in major increases in levels of ACTH and cortisol. During the postpartum period, after delivery of the placenta, there is an abrupt withdrawal of placentally-derived CRH followed by slow decline in cortisol levels over the following months as the HPA axis returns to pre-pregnancy level of functioning (Glynn et al., 2013). Studies have consistently demonstrated hyporesponsivity of the HPA axis during the first few months postpartum, with some hypothesizing that this shift in HPA axis reactivity may play a role in the pathogenesis of PPD (Magiakou et al., 1996) along with shifts in reproductive hormones and changes in thyroid function, immune function, and genetic expression (Schiller, Meltzer-Brody, & Rubinow, 2015) observed during this period. Indeed, several studies have demonstrated an exaggeration of this postpartum hyporeactivity of the HPA axis in women with PPD with lower levels of morning cortisol (Groer & Morgan, 2007; Parry et al., 2003; Taylor, Glover, Marks, & Kammerer, 2009) as well as more severe and prolonged blunting of ACTH response to ovine CRH (Magiakou et al., 1996) compared to postpartum women without depression.
Even fewer studies have been conducted to evaluate HPA reactivity in women with histories of PPD during periods of euthymia. One notable study evaluated the effects of administration followed by withdrawal of supraphysiologic doses of gonadal steroids (estrogen and progesterone), a scaled-down model of pregnancy and the puerperium, on cortisol response to ovine CRH in women who were currently euthymic with histories of PPD and found that these women demonstrated exaggerated cortisol response compared with controls, suggesting possible HPA axis hyperreactivity during pregnancy in women who develop PPD (Bloch et al., 2005). It is not known whether this abnormality in HPA axis function exists in response to physiologic elevations in gonadal steroids outside of pregnancy, such as during the luteal phase of the menstrual cycle in euthymic women with histories of PPD.
Similar to MDD outside of the perinatal period, early life stress appears to play a role in HPA axis reactivity in women with PPD, with studies demonstrating pronounced hyporeactivity of the HPA axis postpartum (Brand et al., 2010). This association with early life stress again led some to question if HPA axis dysfunction represents an underlying predisposition to depression rather than an associated phenomenon of the disorder, with this vulnerability being unmasked during the great alterations in HPA axis function in the perinatal period.
Given the conflicting prior reports examining HPA axis dysregulation in MDD and PPD, in the present study, we sought to address one of the gaps in this body of literature. Specifically, we wanted to take the next logical step and examine HPA axis reactivity in euthymic women with histories of PPD under normal physiologic conditions in the luteal phase (as opposed to conditions of manipulated supraphysiologic levels of gonadal steroids). Therefore, the current study sought the following: 1) to determine if euthymic postpartum women with histories of depression have abnormalities in stimulated HPA axis reactivity to pharmacologic (dexamethasone/corticotropin-releasing hormone test [DEX/CRH test]) and psychological (Trier Social Stress Test [TSST]) challenges while exposed to normal physiologic elevations in gonadal steroids (the luteal phase of their menstrual cycle); and 2) to compare HPA reactivity in women with histories of PPD versus non-puerperal MDD. As a secondary objective, given the influence of early life stress on HPA axis reactivity in previous studies of MDD and PPD, we wanted to determine the influence of trauma history on HPA axis function in our sample of currently euthymic women. We hypothesized that women with a history of PPD would show increased HPA axis reactivity (higher plasma ACTH and cortisol levels) when given DEX/CRH and administered the TSST compared with women without a history of PPD (including both non-puerperal MDD and controls).
Materials and Methods
Participants
The study was approved by the Biomedical Institutional Review Board, and all participants provided informed consent. Postpartum women were recruited from central North Carolina using fliers, advertisements in local printed media sources, and mass emails distributed at local universities from 2008 to 2014. A phone screening was conducted, and eligible participants were then invited to participate. Following informed consent, the participants were interviewed using the Structured Clinical Interview for DSM-IV (SCID) performed by a board-certified psychiatrist with extensive training in conducting structured clinical interviews (SMB) to assess for history of mood disorders, anxiety disorders (including trauma history elicited during assessment of the post-traumatic stress disorder module), substance use disorders, and psychotic disorders. Inclusion criteria included being 12–24 months postpartum and one of the following: 1) history of at least two weeks of post-partum related mood disturbance meeting criteria for major depressive episode (per SCID) occurring within three months of an uncomplicated vaginal delivery or Caesarean section (PPD group); 2) history of at least two weeks of mood disturbance meeting criteria for major depressive episode (per SCID) but without a major depressive episode in the perinatal period (MDD group); or 3) no history of a mood disorder (controls). Participants were excluded if they were taking any medications (including oral contraceptives), taking dietary or herbal supplements on a regular basis (with exception of a multivitamin), if they were currently pregnant or lactating, if they had a history of irregular menstrual cycles, if they had a serious medical illness (cardiac, pulmonary, hepatic, renal, cancer, diabetes) or abnormality on routine labwork performed at screening (complete blood count, chemistry, and thyroid stimulating hormone), if they currently met criteria for major depressive episode, or if they had a history of moderate to severe lifetime psychiatric illness (other than that described above) including psychotic disorder, alcohol or other substance dependence, Bipolar I Disorder, or postpartum psychosis at any time in the past.
Pharmacologic challenge: Dexamethasone/Corticotropin-releasing hormone test
For the DEX/CRH test, participants presented 6–10 days following the LH surge as determined by home urinary testing (corresponding to luteal phase days 19–23 of an idealized 28 day menstrual cycle), underwent a serum pregnancy test (participants were excluded if test was positive), and received 1.5 mg dexamethasone tablet, which they were instructed to take at 23:00 that night before they returned for CRH testing. The next day, testing began uniformly at 12:00 (with exception of one PPD participant and one MDD participant starting at 09:30) with participants having an intravenous (IV) catheter placed followed by a forty minute rest period. At the end of the rest period, participants were given 1 ug/kg ovine CRH by IV push. Participants were instructed to then rest with IV in place for the next three and a half hours. Blood samples for ACTH and cortisol were drawn 15 minutes before CRH administration, concurrent with CRH administration (time 0), and at 5, 15, 30, 60, 90, 120, 150, and 180 minutes after CRH administration.
Psychological challenge: Trier social stress test
Within forty-eight hours of the pharmacologic challenge, participants underwent a psychological challenge with the TSST, performed during days 6–10 of the luteal phase of the menstrual cycle (as confirmed by home urinary testing). The TSST is a well validated stress test that includes a standardized speech task and a paced auditory serial subtraction task, and has been shown to induce large and consistent HPA axis and cardiovascular responses (Kirschbaum, Pirke, & Hellhammer, 1993). Testing was conducted beginning at 13:00. Participants had an IV catheter placed followed by a thirty minute rest period. The TSST was then conducted with the following components: Pre-stress baseline (10 minutes): Participants continued to rest for ten additional minutes, classified as pre-task baseline period, during which time blood pressure and heart rate were measured every two minutes; blood pressure and heart rate continued to be measured every two minutes throughout the remainder of the TSST (but not recovery period). Blood was drawn to measure baseline epinephrine (EPI), norepinephrine (NE), and cortisol at the end of this ten minute baseline period. Pre-Task Instructions (5 minutes): Participants were introduced to the ‘selection committee’, comprised of three individuals dressed in professional clothing, after which the participant was instructed to envision that she has been invited for a personnel interview with the company’s staff managers (the selection committee). The participant was informed that following a five minute quiet preparation period, she would be asked to give a free speech of five minutes in duration convincing the managers that she would be the perfect candidate for the position. All study participants were instructed that they would be audiorecorded for later performance review and that managers were specially trained to analyze their nonverbal behavior. Anticipation Period (5 minutes): Participants were left alone to prepare their speech. Speech Task (5 minutes): Participants then delivered their speech to the selection committee for the following five minutes; if a participant finished her speech before five minutes, managers responded with prepared questions to ensure participants spoke for the entire five minutes. Blood was drawn for EPI and NE one minute into speech delivery. Paced Auditory Serial Addition Task (8 minutes): Immediately following the speech task, participants underwent a paced auditory serial addition task guided via audiotape. Blood was drawn for EPI and NE two minutes into math task. Stress Recovery (60 minutes): Participants then underwent a stress recovery period for the following 60 minutes with blood being drawn to measure cortisol at 10, 20, 30, and 60 minutes into recovery.
Neuroendocrine marker assessment
To summarize time-points of neuroendocrine assessment from detailed protocols above, during DEX/CRH testing, ACTH and cortisol were measured at time -15, 0, 5, 15, 30, 60, 90, 120, 150, and 180 minutes relative to CRH administration. During the TSST, EPI and NE were measured after ten-minute baseline rest period, one minute into speech task, and two minutes into math task, and cortisol was measured after ten-minute baseline rest period and 10, 20, 30, and 60 minutes into recovery period (since peak cortisol is reliably found 10 minutes after cessation of the TSST). Blood samples were collected into pre-chilled vacutainer tubes, immediately centrifuged, and plasma was pipetted into pre-chilled cryotubes and stored at −80°C until assay. We used commercial enzyme immunoassay kits to measure ACTH, cortisol, EPI, and NE. For ACTH, the level of detection was 5.7 pg/mL, with intra- and inter-assay variability of 4.1% and 3.9%, respectively. For cortisol, the level of detection was .07 ug/dL, with intra- and inter-assay variability of 4.7% and 7.6%, respectively. For EPI, the level of detection was 10 pg/ml, with intra- and inter-assay variability of 4.6% and 6.1%, respectively, and for NE, the level of detection was 50 pg/ml, with intra- and inter-assay variability of 4.0% and 10.1%, respectively.
Statistical analysis
All analyses were performed with SAS version 9.4. For demographic variables, differences between groups were evaluated using student’s t-tests for continuous variables and Fisher’s exact tests for categorical variables. Repeated measures analyses of variance (ANOVA) of log-transformed values [with one between subject factor (group) and one within subject factor (time)] were used to evaluate the effect of group on outcomes of cortisol and ACTH response to DEX/CRH, and on blood pressure, heart rate, EPI, NE, and cortisol response to TSST. ANOVAs were followed by area under the curve analyses for further characterization of influence of group on neuroendocrine markers of interest. To assess influence of trauma history on neuroendocrine responses to DEX/CRH and TSST, the entire sample and then groups (MDD, PPD, and controls) were analyzed separately using repeated measures ANOVA evaluating effect of trauma history on log-transformed outcome variables of ACTH and cortisol response to DEX/CRH and cortisol response to TSST. Cortisol/ACTH ratios from DEX/CRH were compared between groups using ANOVA across time. All significance tests were two- tailed, and a p-value of <0.05 was considered significant. P values reported in this study were not corrected for multiple comparisons.
Due to a shortage of commercially-available CRH in 2012, six participants did not undergo DEX/CRH testing when they participated in the study (one participant in the MDD group, three in the PPD group, and two controls). Data are missing from the first two participants recruited in each group for cortisol after TSST, as the protocol was adjusted early in the study to include more timepoints for measurement of cortisol after TSST (changed from one timepoint during TSST recovery to four timepoints; those with only one timepoint for cortisol were not included in analyses).
A sample size of 15 women per group was chosen in order to have 90% power to detect differences in cortisol between groups over time in response to DEX/CRH test (α=0.05) based on previous work evaluating response to DEX/CRH test in women with PPD compared with controls (Bloch et al., 2005).
Results
Participant Characteristics
Participants’ mean age was 31 years, mean education was 17 years, mean months postpartum was 18, and most participants were white (76%) and married (76%). Groups did not differ significantly on any of the baseline characteristics (Table 1). Mean number of lifetime major depressive episodes (MDEs) was 1.9 for women in the MDD group and 1.3 for women in the PPD group. No women in the PPD group reported history of a MDE outside of the peripartum period (four women reported histories of MDEs with multiple pregnancies). Three women (20%) in the PPD group had a lifetime history of trauma reported on the SCID compared with eight women (53%) in the MDD group and four women (27%) in the control group. Five women (33%) in the PPD group, five women (33%) in the MDD group, and no controls had a lifetime history of an anxiety disorder (including generalized anxiety disorder, post-traumatic stress disorder, panic disorder, social anxiety disorder, specific phobia, obsessive-compulsive disorder, and anxiety disorder not otherwise specified). One woman (7%) in the MDD group and four women (27%) in the PPD group currently met criteria for anxiety disorder at the time of testing.
Table 1.
Demographic characteristics of participants
| Controls (n=15) | MDD (n=15) | PPD (n=15) | |
|---|---|---|---|
| Age (y)* | 30.5 (4.2) | 31.9 (4.9) | 31.5 (4.3) |
| Education (y) | 17.3 (2.4) | 16.7 (2.1) | 16.9 (2.7) |
| Months Postpartum | 17.3 (3.0) | 17.9 (3.5) | 18.2 (3.4) |
| Ethnicity** | |||
| White | 11 (73%) | 11 (73%) | 12 (80%) |
| Hispanic | 1 (7%) | 0 | 1 (7%) |
| Black/African American | 3 (20%) | 3 (20%) | 2 (13%) |
| Asian | 0 | 0 | 0 |
| Native Hawaiian/Other Pacific | 0 | 0 | 0 |
| Other | 0 | 1 (7%) | 0 |
| Marital Status | |||
| Single, never married | 4 (27%) | 3 (20%) | 1 (7%) |
| Living with a partner unmarried | 0 | 0 | 1 (7%) |
| Married | 11 (73%) | 12 (80%) | 11 (73%) |
| Divorced/Separated | 0 | 0 | 2 (13%) |
| Widowed | 0 | 0 | 0 |
Age education, and months postpartum expressed as mean(SD)
Ethnicity and marital status expressed as number(percentage) Abbreviations: MDD: major depressive disorder; PPD: postpartum depression
Neuroendocrine Responses to DEX/CRH and TSST
All groups demonstrated robust ACTH response to DEX/CRH (Figure 1A; average peak at 30 minutes post-CRH push) and robust cortisol response to DEX/CRH (Figure 2A; average peak at 180 minutes post-CRH push). There was a significant change in cortisol over time during recovery to TSST in all groups (Figure 3A, average peak at 10 minutes post-TSST; all p-values for effect of time <0.0001). Groups did not differ significantly in ACTH (Figure 1B) or cortisol (Figure 2B) response to DEX/CRH or in blood pressure, heart rate, epinephrine, norepinephrine, or cortisol (Figure 3B) response to TSST (non-significant p-values for group x time interaction in all ANOVAs and AUC analyses comparing groups). Furthermore, cortisol/ACTH ratio did not differ significantly between groups (F2,36=0.58, p=0.56).
Figure 1.
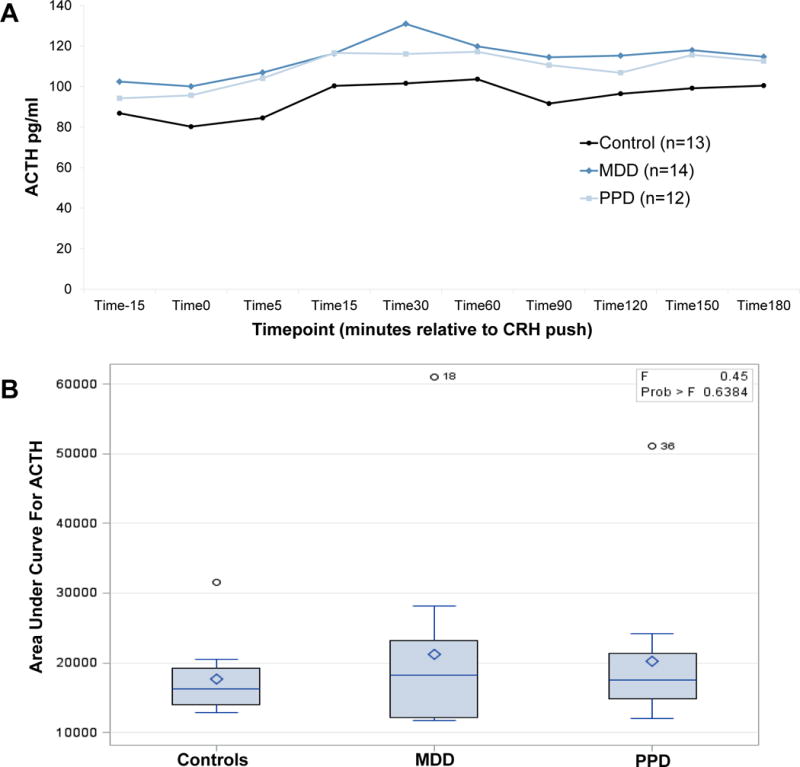
All groups demonstrate rise in ACTH (A) after DEX/CRH with area under curve analysis demonstrating no significant difference between groups (B)
Figure 2.
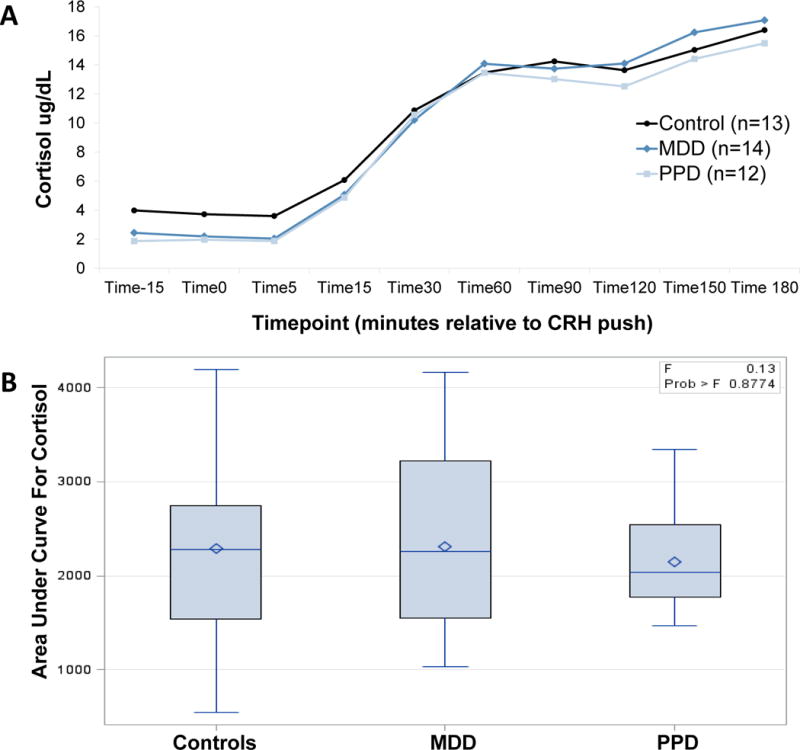
All groups demonstrate rise in cortisol (A) after DEX/CRH with area under curve analysis demonstrating no significant difference between groups (B)
Figure 3.
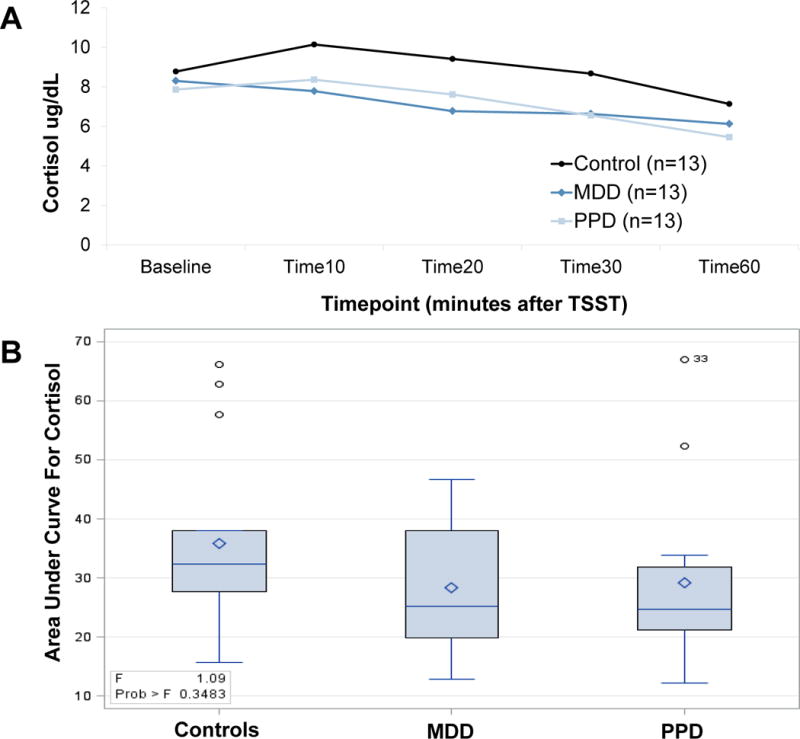
All groups demonstrate cortisol response (A) after TSST with area under curve analysis demonstrating no significant difference between groups (B)
Influence of Trauma History on Neuroendocrine Response
Secondary analyses explored the effects of trauma history on neuroendocrine responses to stress in our sample. Trauma history was not significantly associated with differences in cortisol or ACTH responses to DEX/CRH or cortisol response to TSST in the full sample or in PPD or control groups separately, though sample sizes were small with only three women having a history of trauma in the PPD group and four women having a history of trauma in the control group.
In the MDD group analysis, there were seven women with history of trauma (one of the eight women with MDD and history of trauma was missing DEX/CRH data and thus not included in the analysis) and seven women without history of trauma. In evaluation of response to DEX/CRH, main effects for trauma were F1,12=3.21 p=0.098. Trauma by time interaction effects were significant (F8,125= 2.37 p=0.02), with women with histories of trauma having decreased cortisol response to DEX/CRH at time 0, 15, and 30 minutes post-CRH push; however, Mauchly’s test statistic indicated the assumption of sphericity was violated (x2 143.1, p<.001). The degrees of freedom were corrected using the Greenhouse-Geisser estimates of sphericity (ε= .24), and the adjusted interaction effects supported a trauma*time trend (p=0.11) that was no longer statistically significant. Trauma history was not significantly associated with differences in ACTH response to DEX/CRH or cortisol response to TSST in the MDD group. The small sample of fourteen women in the MDD group had 38% power to detect main level differences at the 0.05 level.
Discussion
We sought to study HPA axis reactivity in response to both a pharmacologic and psychological challenge in euthymic women with a history of PPD compared with postpartum women with a history of non-puerperal depression or control women. As a secondary objective, we wanted to determine the influence of trauma history on HPA axis reactivity in our sample of currently euthymic women. We found that neither women with history of PPD nor MDD showed significant residual stress axis abnormality following either a pharmacologic or psychological stressor, with the exception of a trend toward blunting of cortisol response to DEX/CRH in women with histories of MDD who have also experienced past trauma.
These findings differ from previous studies showing abnormalities in the HPA axis exist even during periods of euthymia in MDD (Hohne et al., 2014; Holsen et al., 2013; Keating et al., 2013; Lok et al., 2012). It is possible that we were unable to detect this effect in our small sample powered only to detect large effect sizes. Other possible explanations for the discrepancy in findings include differences in samples in terms of severity of depressive history (average of only 1.9 and 1.3 lifetime depressive episodes in women with MDD and PPD, respectively, in our sample), timing of onset of previous depressive episodes relative to the time of study enrollment, and comorbid trauma history, all of which are known to influence HPA axis reactivity (Heim et al., 2008; Kunzel et al., 2003; Lok et al., 2012; Sher et al., 2004). Indeed, trauma history in our sample of women with MDD was associated with a trend toward hyporeactivity of cortisol response to DEX/CRH. Our findings, in line with previous work evaluating early life stress and the HPA axis, suggest that history of trauma may be the greater predictor of lasting HPA axis dysregulation rather than history of depression (Pariante & Lightman, 2008). Similar to our study, others have reported normal HPA axis reactivity after resolution of a major depressive episode (Lange et al., 2013) and with successful antidepressant treatment (Anacker et al., 2011; Pariante, 2009; Ruhe et al., 2015; Schule, 2007). It is also possible that divergent HPA axis responses within our sample - with some individuals displaying hyperreactivity of the HPA axis commonly reported in MDD and others displaying the hyporeactivity described in individuals with severe and repeated early life stress (Carpenter et al., 2009; Lee et al., 2012; MacMillan et al., 2009) - may have offset each other, resulting in no observed effect. To address this question, we conducted a post-hoc analysis dividing cortisol and ACTH responses to DEX/CRH and cortisol responses to TSST into normal and abnormal (upper and lower 10% of responses) categories for each group and conducted chi-square analyses, the results of which were not significant.
These findings also differ from the study by Bloch et. al (2005) demonstrating abnormalities in HPA axis reactivity in euthymic women with histories of PPD when exposed to supraphysiologic doses of gonadal steroids mimicking pregnancy. In our study, we evaluated women during the luteal phase of their menstrual cycle at the peak of physiologic levels of gonadal steroids. Taken together, these results suggest that women with histories of PPD may have a vulnerability in HPA axis reactivity that is unmasked when exposed to the large shift in levels of gonadal steroids during the transition from pregnancy to the postpartum period but not in response to the smaller changes in gonadal steroid levels accompanying the normal menstrual cycle. This emphasizes the potential pathophysiologic distinction between PPD and PMDD; although both are reproductive mood disorders, PMDD is a luteal phase disorder that is distinct from PPD. Further, the literature demonstrates that having one type of reproductive mood disorder is not necessarily associated with onset of other reproductive mood disorders and that there are likely some differences in underlying pathophysiology (Buttner et al., 2013; Kepple et al., 2016).
Trauma history was not significantly associated with HPA axis abnormalities in our sample of euthymic postpartum women as a whole or in PPD, MDD, or control groups when analyzed individually (an effect seen in MDD group was not significant after correcting for violation of sphericity). This did represent an exploratory analysis for a secondary objective; sample sizes were exceedingly small, resulting in limited power to detect anything but very large effect sizes, so further investigation with a larger sample is needed. A further limitation in our study was the use of the SCID to assess trauma history rather than the use of a more detailed, validated trauma-specific instrument, which could be used in future studies. If our initial finding of HPA axis hyporeactivity (prior to statistical correction) in women with histories of both MDD and trauma is found to be significant in larger samples, this would help confirm earlier suggestions that trauma has an enduring influence on HPA axis function that may be a mediating variable in the increased risk of depression observed in those with a history of trauma.
Several limitations should be considered when interpreting our results. First, the sample size was selected to have the power to detect only large effect sizes in an effort to account for the difficulty of recruiting women during the second year post-partum. Given our limited power to detect smaller effects, failure to detect a significant effect does not imply that an effect does not exist. This is particularly true in analyses for the secondary objective of evaluating the effect of trauma history on HPA axis reactivity in which the sample sizes were increasingly small. Second, while all women in the present study were currently euthymic, there were a number of women in the MDD and PPD groups with current symptoms of anxiety, which may have influenced results, masking any underlying differences between these groups due to history of puerperal versus non-puerperal depression. Previous work has demonstrated an association of concurrent anxiety disorder in MDD (unlike our study with comorbidity but not concurrent symptoms) with hyperreactivity of the HPA axis (Young, Abelson, & Cameron, 2004). Lastly, although we intentionally studied women during the luteal phase of their menstrual cycle to assure peak physiologic levels of gonadal steroids, the luteal phase is also known to enhance HPA axis activity (Roca et al., 2003), which may have masked any underlying HPA axis hyporeactivity present in our sample.
Conclusion
Currently euthymic women with histories of PPD or MDD did not demonstrate residual abnormal stress responsivity following administration of either a pharmacologic or psychological stressor, with the exception of a trend toward blunting of cortisol response to DEX/CRH in women with history of both MDD and past trauma. This suggests that the appearance of residual abnormal HPA axis stress response in prior studies in those with MDD histories may reflect the following: 1) ongoing dysphoric symptoms at the time of testing, 2) greater severity/duration or more episodes of prior depression, or 3) the presence of comorbid trauma history. Differences between groups may have been blunted in our study due to known enhancement of HPA axis performance in the luteal phase. Future work should further investigate the contribution of trauma history to persistent HPA axis dysregulation and other biological systems that may have a lasting impact on mental and physical health, particularly, during the vulnerable perinatal period.
Figure 4.
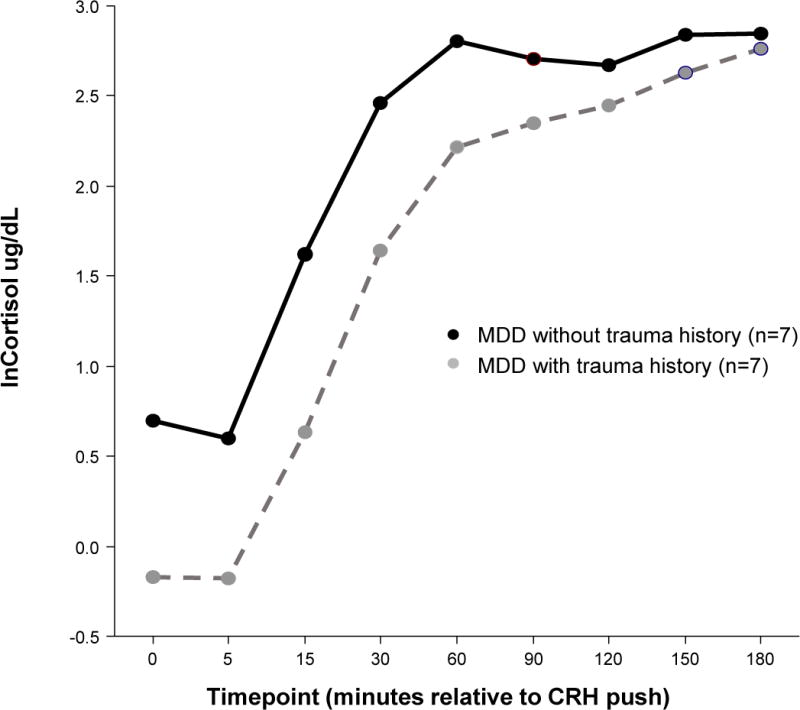
Women with MDD and a history of trauma exhibit decreased cortisol response to DEX/CRH at 0, 15, and 30 minutes post-CRH push compared with women with MDD and no history of trauma (trauma*time F=2.37, p=0.02, Greenhouse-Geisser corrected p=0.11).
Acknowledgments
This research was supported by the National Institutes of Health Grant K23MH085165 (PI: Meltzer-Brody). We would like to thank the study participants who generously gave of their time. We would also like to acknowledge the late Dr. Robert M. Hamer, biostatistician, for his contribution to the study design, his thoughtful guidance, and the invaluable role he played in the training of Dr. Ferguson and so many others at The University of North Carolina at Chapel Hill.
Funding: This research was supported by the National Institutes of Health Grant K23MH085165 (PI: Meltzer-Brody).
Footnotes
Conflict of Interest: All authors of this manuscript declare that they have no conflict of interest.
Ethical Approval: All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosomatic Medicine. 2008;70(4):461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34(9):1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley SL, Weaver TL, Buchanan TW. Sex differences in physiological and affective responses to stress in remitted depression. Physiology and Behavior. 2011;104(2):180–186. doi: 10.1016/j.physbeh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kunzel HE, Nickel T, Kern N, Pfennig A, Majer M, Holsboer F. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34(1):99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):695–699. doi: 10.1210/jc.2004-1388. doi: jc.2004-1388[pii] [DOI] [PubMed] [Google Scholar]
- Brand SR, Brennan PA, Newport, D. J. Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35(5):686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. doi: S0306-4530(05)00083-1 [pii] [DOI] [PubMed] [Google Scholar]
- Buttner MM, Mott SL, Pearlstein T, Stuart S, Zlotnick C, O’Hara MW. Examination of premenstrual symptoms as a risk factor for depression in postpartum women. Archives of Women’s Mental Health. 2013;16(3):219–225. doi: 10.1007/s00737-012-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biological Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S. Glucocorticoid receptor polymorphisms in major depression. Annals of the New York Academy of Sciences. 2009;1179:216–228. doi: 10.1111/j.1749-6632.2009.05012.x. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. American Journal of Psychiatry. 2007;164(10):1515–1520. doi: 10.1176/appi.ajp.2007.06111893. doi: 164/10/1515 [pii] [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47(6):363–370. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinology and metabolism clinics of North America. 2002;31(1):37–62. vi. doi: 10.1016/s0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32(2):133–139. doi: 10.1016/j.psyneuen.2006.11.007. doi: S0306-4530(06)00202-2 [pii] [DOI] [PubMed] [Google Scholar]
- Haefner S, Baghai TC, Schule C, Eser D, Spraul M, Zill P, Bondy B. Impact of gene-gender effects of adrenergic polymorphisms on hypothalamic-pituitary-adrenal axis activity in depressed patients. Neuropsychobiology. 2008;58(3–4):154–162. doi: 10.1159/000182891. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport, D. J. Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hohne N, Poidinger M, Merz F, Pfister H, Bruckl T, Zimmermann P, Ising M. Increased HPA axis response to psychosocial stress in remitted depression: the influence of coping style. Biological Psychology. 2014;103:267–275. doi: 10.1016/j.biopsycho.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:733–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C, Dawood T, Barton DA, Lambert GW, Tilbrook AJ. Effects of selective serotonin reuptake inhibitor treatment on plasma oxytocin and cortisol in major depressive disorder. BMC Psychiatry. 2013;13:124. doi: 10.1186/1471-244X-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepple AL, Lee EE, Haq N, Rubinow DR, Schmidt PJ. History of post-partum depression in a clinic-based sample of women with premenstrual dysphoric disorder. Journal of Clinical Psychiatry. 2016;77(4):e415–e420. doi: 10.4088/JCP.15m09779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Binder EB, Nickel T, Ising M, Fuchs B, Majer M, Modell S. Pharmacological and nonpharmacological factors influencing hypothalamic-pituitary-adrenocortical axis reactivity in acutely depressed psychiatric in-patients, measured by the Dex-CRH test. Neuropsychopharmacology. 2003;28(12):2169–2178. doi: 10.1038/sj.npp.1300280. [DOI] [PubMed] [Google Scholar]
- Lange C, Zschucke E, Ising M, Uhr M, Bermpohl F, Adli M. Evidence for a normal HPA axis response to psychosocial stress in patients remitted from depression. Psychoneuroendocrinology. 2013;38(11):2729–2736. doi: 10.1016/j.psyneuen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Hempel J, Tenharmsel A, Liu T, Mathe AA, Klock A. The neuroendocrinology of childhood trauma in personality disorder. Psychoneuroendocrinology. 2012;37(1):78–86. doi: 10.1016/j.psyneuen.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok A, Mocking RJ, Ruhe HG, Visser I, Koeter MW, Assies J, Schene AH. Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology. 2012;37(7):892–902. doi: 10.1016/j.psyneuen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. Journal of Clinical Endocrinology and Metabolism. 1996;81(5):1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Zerwas S, Leserman J, Holle AV, Regis T, Bulik C. Eating disorders and trauma history in women with perinatal depression. Journal of women’s health. 2011;20(6):863–870. doi: 10.1089/jwh.2010.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Frodl T, Dinan TG. A review of Atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology. 2012;37(10):1589–1599. doi: 10.1016/j.psyneuen.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Annals of the New York Academy of Sciences. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, Hauger R. Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep. 2003;3(3):230–235. [PubMed] [Google Scholar]
- Robertson-Blackmore E, Putnam FW, Rubinow DR, Matthieu M, Hunn JE, Putnam KT, O’Connor TG. Antecedent trauma exposure and risk of depression in the perinatal period. The Journal of clinical psychiatry. 2013;74(10):e942–948. doi: 10.4088/JCP.13m08364. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. Journal of Clinical Endocrinology and Metabolism. 2003;88(7):3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Khoenkhoen SJ, Ottenhof KW, Koeter MW, Mocking RJ, Schene AH. Longitudinal effects of the SSRI paroxetine on salivary cortisol in Major Depressive Disorder. Psychoneuroendocrinology. 2015;52:261–271. doi: 10.1016/j.psyneuen.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS spectrums. 2015;20(1):48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule C. Neuroendocrinological mechanisms of actions of antidepressant drugs. Journal of Neuroendocrinology. 2007;19(3):213–226. doi: 10.1111/j.1365-2826.2006.01516.x. doi: JNE1516 [pii] [DOI] [PubMed] [Google Scholar]
- Sher L, Oquendo MA, Galfalvy HC, Cooper TB, Mann JJ. The number of previous depressive episodes is positively associated with cortisol response to fenfluramine administration. Annals of the New York Academy of Sciences. 2004;1032:283–286. doi: 10.1196/annals.1314.039. doi: 1032/1/283 [pii] [DOI] [PubMed] [Google Scholar]
- Sit D, Luther J, Buysse D, Dills JL, Eng H, Okun M, Wisner KL. Suicidal ideation in depressed postpartum women: Associations with childhood trauma, sleep disturbance and anxiety. Journal of psychiatric research. 2015:66–67. doi: 10.1016/j.jpsychires.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Poon L, Papadopoulos AS, Kumari V, Cleare AJ. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology. 2014;50:289–299. doi: 10.1016/j.psyneuen.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology. 2009;34(8):1184–1188. doi: 10.1016/j.psyneuen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, Bader K. Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology. 2015;51:58–67. doi: 10.1016/j.psyneuen.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Wardenaar KJ, Vreeburg SA, van Veen T, Giltay EJ, Veen G, Penninx BW, Zitman FG. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biological Psychiatry. 2011;69(4):366–373. doi: 10.1016/j.biopsych.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biological Psychiatry. 2004;56(2):113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]


