Abstract
Objective
To identify factors associated with methicillin‐resistant Staphylococcus aureus (MRSA) bloodstream infections at the level of the hospital organization.
Data Sources
Data from all 173 acute trusts in the English National Health Service (NHS).
Study Design
A longitudinal study based on trust‐level panel data for the 5‐year period from April 2004 to March 2009. Fixed effects negative binominal and system generalized method of moment models were used to examine the effect of (i) patient mix characteristics, (ii) resource endowments, and (iii) infection control practices on yearly MRSA counts.
Data Collection
Archival and staff survey data from multiple sources, including Public Health England, the English Department of Health, and the Healthcare Commission, were merged to form a balanced panel dataset.
Principal Findings
MRSA infections decrease with increases in general cleaning (−3.52 MRSA incidents per 1 standard deviation increase; 95 percent confidence interval: −6.61 to −0.44), infection control training (−3.29; −5.22 to −1.36), hand hygiene (−2.72; −4.76 to −0.68), and error reporting climate (−2.06; −4.09 to −0.04).
Conclusions
Intensified general cleaning, improved hand hygiene, additional infection control training, and a climate conducive to error reporting emerged as the factors most closely associated with trust‐level reductions in MRSA infections over time.
Keywords: Health care–associated infections, MRSA, National Health Service, panel data
According to estimates by the European Centre for Disease Prevention and Control (European Centre for Disease Prevention and Control 2013), each year, roughly 3.2 million patients admitted to hospitals in the European Union (EU) acquire an infection that is associated with health care delivery. The most prevalent gram‐positive organism was Staphylococcus aureus, where 41 percent of patient isolates were antibiotic‐resistant. Drug‐resistant organisms are particularly problematic, with methicillin–resistant S. aureus (MRSA) identified as the predominant cause of health care‐associated infection in the European Union (Köck et al. 2010). In the United States, MRSA‐related hospitalizations doubled between 1999 and 2005, reaching an estimated annual number of 278,200 (Klein, Smith, and Laxminarayan 2007). Despite the notable decreases in MRSA infection rates that have been observed since then (Kallen et al. 2010; Dantes et al. 2013), fighting MRSA remains a national priority in the United States, and elsewhere, as reflected in the ambitious MRSA reduction targets formulated by the U.S. Department of Health and Human Services (2015) for 2020. The continued focus on MRSA is all but surprising given the associated morbidity and mortality risks (Lambert et al. 2011; Porto et al. 2013; Kalil et al. 2014), the estimated financial costs of $42,300 per case (Zimlichman et al. 2013), and the increasing recognition that health care–associated MRSA infections are largely preventable (Umscheid et al. 2011).
Often associated with the inappropriate use of antibiotics (Porto et al. 2013; Wooten and Winston 2013; Couderc et al. 2014), MRSA and its transmission have been linked to a range of salient patient‐ and organization‐level factors. The patient factors that have been identified with health care–associated MRSA infection include colonization of the nares and skin on admission (Ridgway et al. 2013; Popoola et al. 2014), surgical wounds (Murray et al. 2014), invasive devices, comorbid disease, recent and repeated hospitalizations, and residing in an assisted living facility (McHugh and Riley 2004; Tehrani et al. 2014; Loftus et al. 2015). Organizational factors identified in previous research include contaminated environmental surfaces (Coia et al. 2006; Plipat et al. 2013; Loftus et al. 2015) and poor hand hygiene practices (Pittet et al. 2000; Stegenga, Bell, and Matlow 2002; Clements et al. 2008).
In this paper, we add to this growing body of research by providing new longitudinal evidence regarding important factors associated with the prevalence of MRSA bloodstream infections at the level of the hospital organization. We estimate the extent to which patient mix characteristics, organizational resource endowments, and infection control practices can explain variance not only between hospital organizations at one point in time but also—and arguably more important—within hospital organizations over time. We draw on data from all 173 acute trusts operating in the English National Health Service (NHS) covering the 5‐year period from April 2004 to March 2009 to examine the effects of the October 2005 implementation of an enhanced mandatory nation‐wide MRSA surveillance program (Pearson, Chronias, and Murray 2009). MRSA bloodstream infections decreased sharply by 58.62 percent in our core study period to a new mean of 17 MRSA bloodstream infections per trust in the period from April 2008 to March 2009, down from 41 in 2005–2006. Our study identified some of those factors that have allowed English acute trusts to contain MRSA infections, when many organizations in other countries have failed to do so. Our findings from fixed effects negative binominal and generalized method of moment (GMM) analyses suggest the potential for acute trust administrators to contribute to the containment of MRSA in their trust by intensifying general cleaning activities, fostering inflection control training, and establishing effective hand hygiene practices as well as a climate in which staff members feel comfortable reporting, discussing, and learning from errors, near misses, and incidents.
Conceptual Background
Based on extant literature, we identified and examined a set of trust‐level factors expected to be associated with MRSA infections. These included (i) patient mix characteristics, (ii) organizational resource endowments, and (iii) infection control practices.
Patient Mix Characteristics
While S. aureus colonization affects around 30 percent of the healthy population and is generally considered harmless to healthy humans (EARSS 2008), hospitalized patients differ notably in terms of their diagnosis and associated treatments, and their individual risk for MRSA infection (Kluytmans, Van Belkum, and Verbrugh 1997). Severity of illness is likely to increase the risk of MRSA infection, especially in patients receiving treatment for renal, oncologic, or hematologic conditions (Wyllie, Peto, and Crook 2005). Treating these patients often involves the use of intravascular devices, which are known to facilitate bacterial infections. Intravenous catheters, open wounds, and more intense nursing contact are also assumed to contribute to MRSA incidents (Coello et al. 1997). Studies also suggest that intensive and emergency care increases the risk of MRSA infection (Frazee et al. 2005; Gopal Rao et al. 2007). Moreover, MRSA infection risks among patients colonized at admission were found to increase with patients' length of stay and age at admission (Davis et al. 2004).
Organizational Resource Endowments
The resource‐based view (Barney 1991) highlights the critical role of an organization's tangible and intangible resources in achieving sustained superior performance. In the context of this study, this perspective points to the potential for acute trust administrators to contribute to the containment of MRSA by providing physicians and nurses with adequate working conditions, suitable physical premises, and an organizational climate conducive to safety and learning from failure. In contrast, understaffing and stress attributed to excessive workload levels can jeopardize the effectiveness of MRSA control measures (Vicca 1999; Borg 2003; Dancer et al. 2006). Similarly, overcrowding was found to trigger lower compliance with hand hygiene practices (Beggs et al. 2006) and to contribute to the spread of MRSA (National Audit Office 2004; Clements et al. 2008). This calls for adequate space per patient and more single patient rooms to contain MRSA. Going beyond human and physical resources, research shows that intangible resources such as organizational climate can help to prevent and learn from health service failures (Edmondson 2004; Salge and Vera 2012). In particular, employees need to be encouraged to report errors, near misses, and incidents, and feel protected from interpersonal risks when doing so (Zhao and Olivera 2006; Singer et al. 2009). When endowed with a favorable error reporting climate, organizations are best positioned to identify lapses in the care process that increase the risk of MRSA infection.
Infection Control Practices
Infection control and hygiene practices are vitally important if acute trust administrators expect to control MRSA infection. MRSA possess high environmental resistance and can be found on a wide range of surfaces (Coia et al. 2006; Grundmann et al. 2006). Therefore, general cleaning and cleanliness are essential to containment. A number of reports suggest that poor cleaning and disinfection of surfaces increase the risk of MRSA colonization and transmission (Rampling et al. 2001; French et al. 2004; Jeanes et al. 2005; Boswell and Fox 2006; Hardy et al. 2006). In addition to environmental surfaces, the contaminated hands of health care providers contribute to transmission of MRSA. Yet poor compliance with hand hygiene standards remains common among physicians and nurses (Pittet et al. 2000; Stegenga, Bell, and Matlow 2002; Clements et al. 2008). Pittet et al. (2000) found hand hygiene compliance to increase with the availability of disinfectant rub, and after participation in a dedicated infection control training program that stressed the importance of hand disinfection.
Methods
Setting and Data
Given its mandatory MRSA reporting system introduced in 2001 and the substantial improvements in MRSA infections realized since then, the English NHS constituted a particularly suitable setting to inform our study. Since 2005, all NHS trusts are required to submit MRSA reports, signed off by their Chief Executive Officer, to the Health Protection Agency (now Public Health England) using a web‐enabled reporting system. The resulting mandatory MRSA reporting figures were found to exceed those of the decades‐old voluntary laboratory reporting system in England by as much as 40 percent in 2001 and 30 percent in 2008 (Pearson, Chronias, and Murray 2009). Our study period covered the launch of the enhanced mandatory MRSA surveillance program in 2005, which allowed us to draw on robust MRSA data less affected by underreporting biases. Our sample included all of English NHS acute trusts (n = 173) during the 5‐year period between April 2004 and March 2009. These trusts are public organizations that provide acute care hospital services under the auspice of the NHS. Each acute trust is an independent legal entity and on average manages 2.3 hospitals. Our panel dataset included archival and staff survey data compiled from several sources, including Public Health England, the Department of Health, the NHS, and the Healthcare Commission. For incomplete records, we contacted the individual trusts to request the missing data. Given the inclusion of a lagged dependent variable, all our models were based on 692 (684 for fixed effects negative binominal model due to lack of within‐trust variance in MRSA infection counts in two acute trusts) trust‐year observations. While previous studies were conducted primarily at the level of the individual patient, our study examined the organization‐level factors associated with between‐ and within‐trust differences in MRSA bloodstream infection prevalence.
Measures
The measures employed in this study are described in Table 1 and further explained below.
Table 1.
Variable Description and Measurement
| Name | Description | Scale |
|---|---|---|
| Dependent variable | ||
| MRSA incident count | Yearly number of MRSA bloodstream infections reported by each acute trust as part of the mandatory MRSA surveillance system | Nonnegative, integer count |
| Control variables | ||
| Lagged MRSA incident count | Dependent variable with a 1‐year time lag | Nonnegative, integer count |
| Trust size | Number of hospital beds available for use in each acute trust | Nonnegative, integer count |
| Foundation trust | Acute trust with foundation trust status associated with greater autonomy and higher local accountability | Dummy variable |
| Specialist trust | Acute trust with single medical specialty (e.g., oncology) | Dummy variable |
| University affiliation | Acute trust with a university affiliation | Dummy variable |
| Trust location dummies | Set of indicators to reflect the English region the acute trust is located in | Dummy set |
| Time dummies | Set of indicators for each of the years in our study period | Dummy set |
| Patient mix characteristics | ||
| Severity of illness | Share of all admitted patients not expected to survive their hospital stay given, among others, their primary diagnosis, comorbidities, age, gender, and socioeconomic status | Percentage |
| Emergency admission rate | Share of all admitted patients classified as emergencies | Percentage |
| Length of stay | Mean length of stay in days of all patients | Ratio scale |
| Age at admission | Mean age of all patients in years at the time of admission | Ratio scale |
| Resource endowments | ||
| Medical workload | Yearly number of admissions per medical full‐time equivalent (FTE) | Ratio scale |
| Nursing workload | Yearly number of admissions per nursing FTE | Ratio scale |
| Single room ratio | Share of single rooms compared to all patient rooms | Percentage |
| Error reporting climate | Four items from annual staff survey with approximately 400 usable responses per year and acute trust aggregated to the acute trust level | 5‐point Likert‐type items from 1 (strongly disagree) to 5 (strongly agree) |
| Infection control practices | ||
| General cleaning | Number of cleaning staff (in FTE) undertaking cleaning work for acute trust including directly employed and contracted out staff, but excluding managers, administrative and supervisory staff not physically carrying out the cleaning function | Count |
| Hand hygiene | Four items from annual staff survey with approximately 400 usable responses per year and acute trust aggregated to the acute trust level | 5‐point Likert‐type items from 1 (never) to 5 (always) |
| Infection control training | Share of staff members having received infection control training | Percentage |
MRSA, methicillin‐resistant Staphylococcus aureus; FTE, full‐time equivalent.
Dependent Variable
The dependent variable in all our models was the number of MRSA bloodstream infections detected within each trust in a given year and reported to Public Health England under the nation‐wide mandatory MRSA surveillance system. The MRSA infection counts reported by an acute trust were first detected, but not necessarily acquired, in that trust. It was possible that MRSA infections reported by the acute trusts could have originated in another trust or the community. As part of our post hoc analyses, however, we replicated our analyses based on cross‐sectional data from a subsample of 160 acute trusts, for which trust‐acquired infection data were available. Here, MRSA infections were identified as trust‐acquired if they met the following criteria: the blood culture was taken in that trust; the patient was an inpatient, day‐patient, or in emergency assessment in that trust; and the blood culture date was the third day of admission or later.
Control Variables
To account for factors that might confound the effect of patient mix characteristics, organizational resource endowments, and infection control practices, we included a number of additional control variables. These included lagged MRSA incident count, which allows for a conservative estimation of the other regressors and helped control for unobserved heterogeneity (Jacobson 1990), trust size measured as the number of beds, and a set of dummy variables to capture observable heterogeneity with regard to acute trusts' foundation trust status granting trusts greater autonomy from central government, specialist trust status, university affiliation, and trust location. We also included a full set of time dummies.
Patient Mix Characteristics
We captured differences in trust‐level severity of illness as the percentage of all admitted patients in a trust‐year expected not to survive their hospital stay given their primary diagnosis, comorbidities, age, gender, and socioeconomic status (Bottle, Jarman, and Aylin 2011). We also computed for each acute trust and year the emergency admission rate as the percentage of admitted patients classified as emergencies, the mean patient length of stay in days, and the mean patient age in years.
Organizational Resource Endowments
Trust‐level resource endowments included measures of medical workload and nursing workload computed as the ratio between the number of admissions in a given year and the number of full‐time equivalents (FTE) for each group. We also computed the single room ratio in percent and relied on extensive data from four successive waves of the annual NHS staff survey to measure a trust's overall error reporting climate. For this purpose, we aggregated approximately 400 individual employee responses per acute trust and year (response rates above 50 percent) to the trust level. Staff members indicated their agreement with four items: “My trust treats fairly staff who are involved in an error, near miss or incident,” “My trust encourages us to report errors, near misses or incidents,” “My trust treats reports of errors, near misses or incidents confidentially,” and “My trust blames or punishes people who make errors, near misses or incidents” (reverse coded). Each item was measured on a 5‐point Likert‐type scale ranging from 1 (strongly disagree) to 5 (strongly agree). We then employ the overall mean of these four items as assessed by all responding staff members of an acute trust in a given year as our indicator of error reporting climate.
Infection Control Practices
Trust‐level infection control practices included the number of FTE staff undertaking cleaning work in a trust and given year as a proxy for the intensity of general cleaning. To measure hand hygiene, we also relied on annual staff survey data to extract staff responses to the following three 5‐point Likert‐type items with response options ranging from 1 (never) to 5 (always): “Hot water, soap and paper towels, or alcohol rubs, are available when they are needed … by staff,” “ … by patients/service users,” and “ … by visitors to the Trust.” Individual‐level perceptions were then aggregated to the acute trust level. Finally, we measured infection control training as the percentage of staff members who received infection control training.
Analysis
The dependent variable in this study, the MRSA incident count per trust per year, was a nonnegative integer count variable. This called for specific estimation procedures for count data. Likelihood‐ratio tests yielded statistically significant alpha values, indicating overdispersion. As part of our main analyses, we hence report estimates from negative binominal regression instead of more restrictive Poisson estimates. Our exposure variable, the number of bed days per trust per year was appropriate, as it is commonly used as a denominator in studies computing MRSA rates (e.g., Borg, Suda, and Scicluna 2008). We report fixed effects estimates, as they exploit only within‐trust variance and hence provide insights into the factors associated with within‐trust changes in MRSA infection counts over time.
Importantly, most independent variables of interest were potentially endogenous in that they might be affected by unobserved time‐variant heterogeneity and simultaneous causality. Consider the case of infection control training, which we assume to be important for containing MRSA. It appears plausible, however, that trust administrators will intensify infection control training when faced with high MRSA infection counts—an assumption that is supported by Granger (1988) causality analyses we conducted. Similar arguments can be developed for all choice variables in our model that can be influenced at least to some extent by trust administrators in response to changes in MRSA prevalence. This clearly applied to all variables capturing distinct infection control practices and organizational resource endowments. Similarly, patients' mean length of stay in a trust and the emergency admission ratio might be affected by changes in MRSA. All other variables were treated as exogenous. These endogeneity concerns called for additional analyses using either a quasi‐experimental or instrumental variable approach. Given the multiple and nonbinary “treatments,” popular techniques such as two‐stage least squares (2SLS) or propensity score matching proved impractical. We hence relied on an alternative instrumental variable approach explicitly developed for dynamic panel data models with multiple potentially endogenous variables known as system GMM estimation (Blundell and Bond 1998). In contrast to 2SLS, system GMM does not rely on external instrumental variables, but instead uses the lagged differences and lagged levels of the model variables as instruments. System GMM estimation is increasingly common for panel data analysis in health services research (e.g., Brown et al. 2006), health economics (e.g., Baltagi, Bratberg, and Holmås 2005), and beyond. To account for the potential endogeneity issue, we therefore report not only fixed effects negative binominal estimates but also one‐step system GMM estimates. All analyses were conducted using STATA/SE 13.1 (Stata Corp, College Station, TX, USA).
Results
Results from Descriptive Analyses
Tables 2 and 3 contain descriptive statistics and linear time trends for all time‐variant variables. Most notably, these analyses revealed a steep decrease in MRSA infection counts from a trust mean of 41 MRSA bloodstream infection reports in 2005–2006 (SD: 30.12; min: 0; max: 165) to 17 in 2008–2009 (SD: 13.80; min: 0; max: 127). This corresponds to a mean decrease of 8.27 MRSA bloodstream infections per trust per year and an overall reduction of 4,158 MRSA bloodstream infections in English acute trusts within just 3 years (−58.62 percent). We also detected statistically significant decreases in patients' length of stay (−0.16 days per year) and increases in the share of single patient rooms (+1.01 percentage points per year), error reporting climate (+0.01 units per year), hand hygiene practices (+0.04 units per year), and the prevalence of infection control training (+2.48 percentage points per year). We also observed an increase in nursing workload (+1.34 admissions per nurse per year) and a decrease in general cleaning staff (−13.89 cleaning staff members (FTE) per trust per year).
Table 2.
Descriptive Statistics
| Variable | Unit | Mean | SD Overall | SD Between | SD Within | Min | Max |
|---|---|---|---|---|---|---|---|
| MRSA incident count | Cases | 30.012 | 25.298 | 21.559 | 13.312 | 0.000 | 179.000 |
| Trust size | Beds | 719.540 | 400.291 | 397.974 | 50.367 | 13.540 | 2,355.184 |
| Foundation trust | Dummy | 0.345 | 0.476 | 0.407 | 0.249 | 0.000 | 1.000 |
| Severity of illness | % | 5.448 | 1.704 | 1.663 | 0.389 | 0.230 | 13.199 |
| Emergency admission ratio | % | 35.052 | 10.001 | 9.822 | 1.994 | 1.556 | 61.763 |
| Length of stay | Days | 4.802 | 1.526 | 1.487 | 0.355 | 0.600 | 21.700 |
| Age at admission | Years | 48.865 | 8.393 | 8.376 | 0.762 | 5.000 | 66.000 |
| Medical workload | Ratio | 180.402 | 51.132 | 47.764 | 18.522 | 39.909 | 462.972 |
| Nursing workload | Ratio | 65.108 | 25.298 | 21.559 | 13.312 | 19.087 | 97.457 |
| Single room ratio | % | 19.359 | 8.898 | 8.083 | 3.758 | 0.000 | 93.000 |
| Error reporting climate | Scale | 3.538 | 0.077 | 0.068 | 0.038 | 3.275 | 3.819 |
| General cleaning | FTE | 183.999 | 125.277 | 116.185 | 47.477 | 16.000 | 1,272.000 |
| Hand hygiene | Scale | 3.622 | 0.112 | 0.084 | 0.074 | 3.255 | 3.928 |
| Infection control training | % | 75.629 | 8.476 | 6.739 | 5.160 | 46.601 | 97.402 |
Descriptive statistics for pooled panel data reported. Time‐invariant dummy variables are not reported. Sample contains 32 acute trusts with a university affiliation and 20 acute specialist trusts. Thirty‐two trusts are located in London, 18 in South West, 11 in South Central, 13 in South East Coast, 18 in the East of England, 20 in the West Midlands, 9 in the East Midlands, 29 in the North West, 8 in the North East, and 15 in Yorkshire and the Humber.
Table 3.
Linear Time Trends
| Variable | Mean | Time Trenda | |||
|---|---|---|---|---|---|
| 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | ||
| MRSA incident count | 41.000 | 36.358 | 25.723 | 16.965 | −8.274*** |
| Trust size | 752.021 | 720.581 | 700.758 | 704.641 | −16.165 |
| Foundation trust | 0.185 | 0.312 | 0.399 | 0.486 | 0.099*** |
| Severity of illness | 5.814 | 5.401 | 5.279 | 5.300 | −0.167** |
| Emergency admission ratio | 35.736 | 35.280 | 34.489 | 34.703 | −0.389 |
| Length of stay | 5.104 | 4.811 | 4.673 | 4.620 | −0.159** |
| Age at admission | 48.480 | 48.593 | 48.867 | 49.520 | 0.339 |
| Medical workload | 182.840 | 178.627 | 180.191 | 179.951 | −0.710 |
| Nursing workload | 63.233 | 64.148 | 65.978 | 67.074 | 1.335* |
| Single room ratio | 17.907 | 18.903 | 19.600 | 21.028 | 1.006*** |
| Error reporting climate | 3.524 | 3.535 | 3.519 | 3.576 | 0.014*** |
| General cleaning | 218.704 | 169.528 | 168.447 | 174.148 | −13.890** |
| Hand hygiene | 3.585 | 3.592 | 3.582 | 3.731 | 0.043*** |
| Infection control training | 73.42563 | 72.99586 | 75.11489 | 80.9798 | 2.478*** |
***p < .001; **p < .01; *p < .05.
Unstandardized coefficient estimate for linear time trend during study period reported.
Time‐invariant dummy variables are not reported.
Figure 1 shows considerable variance among acute trusts in terms of both their baseline MRSA infection count in 2005–2006 and their ability to reduce MRSA infections between 2005–2006 and 2008–2009. It is in particular, this variance in within‐trust changes in MRSA infections over time that we seek to explain in this study.
Figure 1.
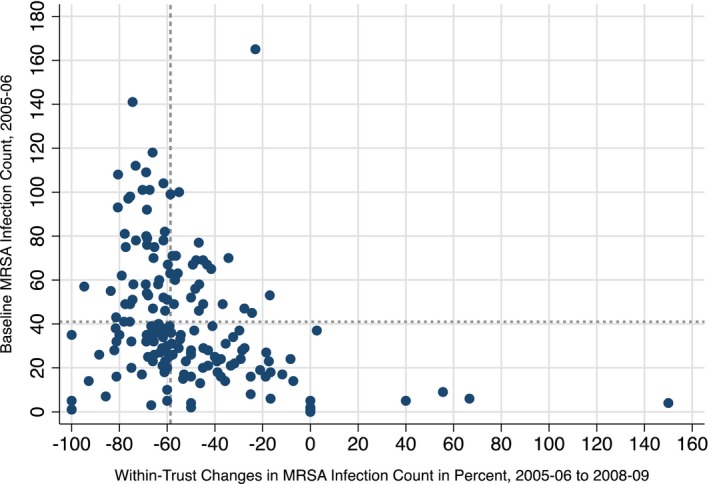
MRSA Infection Baseline and Changes by NHS Acute Trust
Note: Dashed reference lines illustrate the respective sample mean.
Results from Panel Data Analyses
Table 4 presents the results from our panel data analyses. Model 1 shows incidence rate ratios (IRR) based on standardized negative binominal estimates with 95 percent confidence intervals (CI) reported in parentheses. IRRs above (below) 1 indicate a positive (negative) link with within‐trust changes in MRSA infection counts. This model exploits only the within‐trust variance in MRSA infection counts over time. All time‐invariant control variables have thus been omitted from this model. Model 2, in turn, accounts for the potential endogeneity of our main variables and shows one‐step system GMM estimates based on standardized independent variables. Again, we report the 95 percent CI in parentheses. Variance inflation factors well below 10 in both models indicate that multicollinearity is unlikely to be an issue.
Table 4.
Negative Binominal and System GMM Models Explaining MRSA Incident Counts
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Fixed Effects Negative Binominal Estimates | System GMM Estimates | |||
| IRR | 95% CI | Coeff. | 95% CI | |
| Control variables | ||||
| Lagged MRSA incident count | 1.0004 | [0.9983, 1.0024] | 0.6765*** | [0.5409, 0.8121] |
| Trust size | 0.8401* | [0.7356, 0.9595] | 10.3952* | [2.0964, 18.6941] |
| Foundation trust | 0.9694 | [0.8844, 1.0626] | 3.0843 | [−1.0350, 7.2036] |
| Specialist trust | – | 16.9482 | [−10.4058, 44.3023] | |
| University affiliation | – | 9.0002 | [−11.1853, 29.1858] | |
| Trust location dummies | – | Yes | ||
| Times dummies | Yes*** | Yes*** | ||
| Patient mix characteristics | ||||
| Severity of illness | 0.9875 | [0.8758, 1.1134] | −2.7412 | [−6.4184, 0.9361] |
| Emergency admission ratio | 0.8994 † | [0.8072, 1.0021] | −0.9520 | [−5.6264, 3.7223] |
| Length of stay | 0.8908 | [0.7684, 1.0327] | 1.7419 | [−3.3311, 6.8149] |
| Age at admission | 1.1368 | [0.8996, 1.4367] | 0.7886 | [−5.8281, 7.4052] |
| Resource endowments | ||||
| Medical workload | 0.9194 † | [0.8444, 1.0011] | 0.9541 | [−1.7401, 3.6484] |
| Nursing workload | 1.0217 | [0.9377, 1.1132] | 0.5980 | [−2.6846, 3.8807] |
| Single room ratio | 0.9468* | [0.8997, 0.9963] | −0.0608 | [−2.2308, 2.1092] |
| Error reporting climate | 0.9455* | [0.8956, 0.9981] | −2.0607* | [−4.0864, −0.0350] |
| Infection control practices | ||||
| General cleaning | 0.9535* | [0.9116, 0.9973] | −3.5265* | [−6.6120, −0.4409] |
| Hand hygiene | 0.9135** | [0.8617, 0.9684] | −2.7206** | [−4.7616, −0.6796] |
| Infection control training | 1.0296 | [0.9868, 1.0742] | −3.2910*** | [−5.2175, −1.3645] |
| Trusts | 171 | 173 | ||
| Years | 4 | 4 | ||
| Total observations | 684 | 692 | ||
| Log likelihood | −1,522.0523*** | – | ||
| Wald chi‐squared | – | 827.46*** | ||
***p < .001; **p < .01; *p < .05; † p < .10.
Model 1: IRR based on standardized negative binominal estimates for panel data reported. Yearly number of bed days used as exposure variable. 95% confidence interval in parentheses.
Model 2: Coefficient estimates for standardized independent variables reported. All variables for resource endowments and infection control practices as well as emergency admission ratio and length of stay are treated as endogenous. All other variables are treated as exogenous. The Hansen J‐test of overidentifying restrictions and the Arellano–Bond test for zero second‐order autocorrelation remain insignificant. The dependent variable in both models is the unstandardized yearly MRSA incident count in each organization.
GMM, generalized method of moments; IRR, incidence rate ratios; MRSA, methicillin‐resistant Staphylococcus aureus.
Control Variables
Model 1 reveals a negative association between changes in trust size and the expected change in the MRSA incident count, which decreases by a factor of 0.84 (16 percent) for every 1 standard deviation within‐trust increase in trust size, that is in hospital beds. At first glance surprisingly, the GMM estimates presented in Model 2 indicate that every 1 standard deviation increase in trust size will be associated with 10.39 additional MRSA infections. This divergence can be attributed to the fact that the natural relationship between patient volume and MRSA incidents is explicitly accounted for in Model 1, which uses bed days as the exposure variable. In Model 2, in contrast, the effects of patient volume and bed capacity are conflated. The only other noteworthy finding with regard to our control variables pertains to the statistically significant time dummies, indicating systematic temporal variation in MRSA infection counts.
Patient Mix Characteristics
We find surprisingly little evidence for patient mix characteristics contributing to trust‐level differences in MRSA incidents, with none of the coefficient estimates reaching statistical significance at the 5 percent level in either Model 1 or Model 2.
Organizational Resource Endowments
Similarly, our expectation that increases in medical and nursing workload are likely to be associated with increases in MRSA infection counts is not supported. In line with our expectations, though, Model 1 shows increases in the single room ratio (−5.32 percent) and error reporting climate (−5.45 percent) to be associated with significant within‐trust decreases in MRSA infections. The single room effect, however, appears to be subject to endogeneity bias and loses its significance in Model 2. The effect of error reporting climate, in contrast, persisted after accounting for its potential endogeneity in Model 2. This effect also remains practically relevant given the estimated reduction of 2.06 MRSA bloodstream infections for every one standard deviation increase in error reporting climate.
Infection Control Practices
Models 1 and 2 yielded consistent results for the effect of changes in hand hygiene (−8.65 percent) and in general cleaning (−4.65 percent). It is only with regard to infection control training that our two estimation approaches yield diverging results. This was not surprising in light of the strong evidence of reverse causality we detected. It was precisely this increase in infection control training in response to higher MRSA incident counts that remained unaccounted for in Model 1 and biased the effect of infection control training. It was hence only the endogenous specification reported in Model 2 that revealed what was arguably the true effect of infection control training. This effect was not only statistically but also practically significant as evidenced by the fact that a 1 standard deviation increase in infection control training was estimated to be associated with a reduction in MRSA infections by 3.29 cases per acute trust and year.
Results from Post Hoc Analyses
We conducted a set of post hoc analyses to test the robustness of our main findings and to shed light on open issues that emerged as part of our analyses. We found consistent results for Model 1, when (i) using the number of admissions or finished treatment episodes as exposure variables, (ii) computing Poisson instead of negative binominal estimates, (iii) including a linear time trend instead of time dummies, (iv) using cleaning expenditures and cleaning staff per square foot of patient occupied floor area instead of the overall number of cleaning staff as a proxy for general cleaning, (v) calculating workload figures on the basis of head counts rather than FTEs, and (vi) excluding the number of doctors in training from medical workload. As for Model 2, consistent estimates also emerged when (i) replacing the simple MRSA infection count by an MRSA infection rate irrespective of whether we used the number of bed days, admissions, or finished treatment episodes as denominator, and (ii) employing the less efficient Arellano–Bond difference GMM estimator. Our main results also remained robust to the inclusion of additional control variables such as the ethnic diversity of an acute trust's patient mix or the geographic rurality of its headquarter location. Moreover, we tested the robustness of our findings to changes in sample composition and found a comparable pattern of results for subsamples excluding specialist trusts or trusts with university affiliation. Finally, we collected cross‐sectional data on the number of MRSA infections classified as trust‐acquired for a subsample of 160 acute trusts in 2008–2009. Analyses revealed that 58.32 percent of MRSA infections reported in that year were classified as trust‐acquired, were strongly correlated with the overall MRSA infection count (0.912, p < .001), and exhibited a consistent pattern of results for that year.
Discussion
To the best of our knowledge, this study is among the first to draw on large‐scale longitudinal data to identify trust‐level factors related to (i) patient mix characteristics, (ii) organizational resource endowments, and (iii) infection control practices to explain in particular within‐trust variance in MRSA bloodstream infection counts. The findings presented complement the wealth of patient‐level studies on this topic (e.g., Forster et al. 2013). Such research has been highly useful for providing evidence on the individual patient and treatment characteristics associated with patients' MRSA infection risk. However, patient‐level research is less suited to uncover how hospital organizations can achieve systematic improvements in the overall prevalence of MRSA bloodstream infections over time. Our study is unique in this regard in that it covers the entire population of English NHS acute trusts over five consecutive years. Located in a setting that achieved dramatic reductions in MRSA prevalence by as much as 8.27 MRSA cases per trust per year during the study period, it provides new evidence on both the preventability of most MRSA bloodstream infections and specific trust‐level infection control measures.
Our study revealed several organization‐level factors that were associated with meaningful within‐trust reductions in MRSA infection counts between 2005–2006 and 2008–2009 and that can be influenced directly by managerial and clinical staff in acute trusts and other health care organizations. The most salient of these factors comprise not only increases in general cleaning staff, greater availability of hand hygiene products to staff members, visitors, and patients, and more infection control training but also an organizational climate conducive to learning from errors, near misses, and incidents. These findings support decades of research on the importance of antisepsis. Several researchers have reported on the importance of environmental decontamination and hand hygiene as a mechanism to control infection (Larson 1995; Dancer et al. 2006; Allegranzi and Pittet 2009; Datta et al. 2011; Boyce 2013). Yet despite these findings, many studies report that hand hygiene compliance is poor (FitzGerald, Moore, and Wilsom 2013; Lebovic, Siddiqui, and Muller 2013; Song et al. 2013; Rowlands et al. 2014). Our findings also complement previous studies that examined the role of hand hygiene in containing MRSA (Rampling et al. 2001) and the effectiveness of a hospital‐wide program for greater compliance with hand hygiene standards (Pittet et al. 2000). We strengthen this existing body of research by providing novel longitudinal evidence from English acute trusts. We hope that the lessons that can be extracted from our analysis will be of interest and value to hospital administrators and policy makers around the globe. Informed by this evidence, campaigns to fight health care‐associated MRSA bloodstream infection might wish to target in particular those hospital organizations—or indeed wards—suffering from insufficient cleaning staff, poor hand hygiene standards, and little infection control training to make the best possible use of available resources.
Our findings also point to the value of building a favorable error reporting climate, where employees feel comfortable reporting and discussing errors, near misses and incidents. The significant MRSA‐reducing effect we detect is in line with previous research that highlights that nurses and physicians are well positioned to help their organization recognize and learn from the causes of system failures (Edmondson 2004; Singer et al. 2009). In order to reduce MRSA infections, hospital administrators might thus wish to attempt to create a general working environment and specific forums where nurses and physicians do not conceal errors for fear of ridicule or punishment, but instead feel safe to take the interpersonal risks that reporting and discussing errors and failures entail. This requires not only inviting others to express their concerns and admitting own errors but also responding to reported errors and following through on suggestions for improvement (Tucker and Edmondson 2003).
Limitations
This study has several limitations. First, we provide evidence from a field study based on longitudinal archival and survey data. Although such a design is well suited to disentangle the concurrent effects of multiple organization‐level interventions and trace them over time, it has disadvantages relative to randomized control trials and other experimental designs in terms of the strength of the causal claims that can be derived. Even though we attempted to account for unobserved heterogeneity and endogeneity, management and policy implications should be drawn with caution. We hence call for replication studies that use organization‐ or ward‐level data from field or lab experiments, whenever feasible and ethically appropriate. Second, the acute trust served as the unit of analysis in this study, with patient and employee data being aggregated to the trust level. This enabled us to examine the MRSA‐reducing effects of organizational factors such as establishing and maintaining a favorable error reporting climate. It also helped us add to the limited body of evidence on the factors associated with within‐trust changes in MRSA infections over time. However, it precluded us from examining effects at lower levels of aggregation such as the individual hospital site or clinical ward. We hence welcome multilevel research that spans units of analysis in an effort to further improve our understanding of the determinants of MRSA. Third, it would have been desirable to measure some variables with greater precision. This applies to medical and nursing workload, for which we had to rely on aggregate trust‐level admission data ignoring possible differences in workload across types of admissions and clinical wards of the same trust. Similarly, in our longitudinal analyses, we were unable to distinguish between community‐ and trust‐acquired MRSA bloodstream infections. Although our post hoc analyses found trust‐acquired MRSA counts to strongly correlate with overall MRSA counts for a subsample of acute trusts in 2008–2009, we could only perform cross‐sectional and not longitudinal robustness checks. A more granular dynamic analysis of MRSA infections thus remains an important avenue for future research. Fourth, our findings are based on data from acute trusts that are part of a single public health system, the English NHS. Importantly, our study period coincides with the first 4 years of an enhanced mandatory MRSA surveillance system and ambitious national targets and action plans for the containment of MRSA. Although this has notable advantages in terms of data availability and internal validity, it might limit the generalizability of our findings to other health systems and health care providers, calling for comparative replication studies. Finally, our analytical approach based on yearly time intervals revealed all salient MRSA‐reducing effects to materialize within the first 12 months. More granular monthly data for all model variables, however, is needed to estimate the time lag and duration of these effects. This is an important avenue for future research considering the rich additional insights that can be gained by trust‐level, longitudinal analyses of infection control mechanisms.
Conclusion
This study relied on panel data from all 173 English acute trusts for the 5‐year period from April 2004 to March 2009 to identify trust‐level factors associated with decreases in MRSA bloodstream infections over time. Mean MRSA incident counts per trust dropped by 58.84 percent from 41 in 2005–2006 to 17 in 2008–2009. At a broad level, this suggests that a substantial share of MRSA bloodstream infections can indeed be prevented if made a national priority. The enhanced mandatory MRSA reporting system implemented in the NHS might have played an important facilitating role in this improvement process. At a more granular level, our panel data analyses allowed us to identify several relevant factors associated with substantial within‐trust reductions in MRSA infections during our study period. Those with the most substantial and robust effect included intensified general cleaning, improved hand hygiene practices, additional infection control training, and a climate conducive to talking about—and learning from—errors, near misses, and incidents.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This article benefited from valuable feedback provided by reviewers and participants of the Public Management Research Association (PMRA) Conference (2013) as well as the Health Care Management Division of the Academy of Management Meeting (2015). We also thank all organizations in the NHS and beyond that supported our study by providing the data our analyses are based on. The project was fully funded by the authors' home universities. No external funding was received. Finally, we acknowledge the excellent research assistance of Patrick Cichy, Eduard Grünwald, Ina Meixner, and Sebastian Schäfer.
Disclosures: None.
Disclaimers: None.
References
- Allegranzi, B. , and Pittet D.. 2009. “Role of Hand Hygiene in Healthcare‐Associated Infection Prevention.” Journal of Hospital Infection 73: 305–15. [DOI] [PubMed] [Google Scholar]
- Baltagi, B. H. , Bratberg E., and Holmås T. H.. 2005. “A Panel Data Study of Physicians' Labor Supply: The Case of Norway.” Health Economics 14 (10): 1035–45. [DOI] [PubMed] [Google Scholar]
- Barney, J. B. 1991. “Firm Resources and Sustained Competitive Advantage.” Journal of Management 17 (1): 99–120. [Google Scholar]
- Beggs, C. B. , Noakes C. J., Shepherd S. J., Kerr K. G., Sleigh P. A., and Banfield K.. 2006. “The Influence of Nurse Cohorting on Hand Hygiene Effectiveness.” American Journal of Infection Control 34 (10): 621–6. [DOI] [PubMed] [Google Scholar]
- Blundell, R. , and Bond S.. 1998. “Initial Conditions and Moment Restrictions in Dynamic Panel Data Models.” Journal of Econometrics 87 (1): 115–43. [Google Scholar]
- Borg, M. A. 2003. “Bed Occupancy and Overcrowding as Determinant Factors in the Incidence of MRSA Infections within General Ward Settings.” Journal of Hospital Infection 54 (4): 316–8. [DOI] [PubMed] [Google Scholar]
- Borg, M. A. , Suda D., and Scicluna E.. 2008. “Time‐Series Analysis of the Impact of Bed Occupancy Rates on the Incidence of Methicillin‐Resistant Staphylococcus aureus Infection in Overcrowded General Wards.” Infection Control 29 (06): 496–502. [DOI] [PubMed] [Google Scholar]
- Boswell, T. C. , and Fox P. C.. 2006. “Reduction in MRSA Environmental Contamination with a Portable HEPA‐Filtration Unit.” Journal of Hospital Infection 63 (1): 47–54. [DOI] [PubMed] [Google Scholar]
- Bottle, A. , Jarman B., and Aylin P.. 2011. “Hospital Standardized Mortality Ratios: Sensitivity Analyses on the Impact of Coding.” Health Services Research 46 (6): 1741–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce, J. M. 2013. “Update on Hand Hygiene.” American Journal of Infection Control 41: S94–6. [DOI] [PubMed] [Google Scholar]
- Brown, T. T. , Coffman J. M., Quinn B. C., Scheffler R. M., and Schwalm D. D.. 2006. “Do Physicians Always Flee from HMOs? New Results Using Dynamic Panel Estimation Methods.” Health Services Research 41 (2): 357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, A. , Halton K., Graves N., Pettitt A., Morton A., Looke D., and Whitby M.. 2008. “Overcrowding and Understaffing in Modern Health‐Care Systems: Key Determinants in Meticillin‐Resistant Staphylococcus aureus Transmission.” The Lancet Infectious Diseases 8 (7): 427–34. [DOI] [PubMed] [Google Scholar]
- Coello, R. , Glynn J. R., Gaspar C., Picazo J. J., and Fereres J.. 1997. “Risk Factors for Developing Clinical Infection with Methicillin‐Resistant Staphylococcus aureus (MRSA) amongst Hospital Patients Initially Only Colonized with MRSA.” Journal of Hospital Infection 37 (1): 39–46. [DOI] [PubMed] [Google Scholar]
- Coia, J. E. , Duckworth G. J., Edwards D. I., Farrington M., Fry C., Humphreys H., Mallaghan C., and Tucker D. R.. 2006. “Guidelines for the Control and Prevention of Meticillin‐Resistant Staphylococcus aureus (MRSA) in Healthcare Facilities.” Journal of Hospital Infection 63 (1): 1–44. [DOI] [PubMed] [Google Scholar]
- Couderc, C. , Jolivet S., Thiébaut A. C., Ligier C., Remy L., Alvarez A. S., Lawrence C., Salomon J., Herrmann J. L., and Guillemot D.. 2014. “Antibiotic Use and Staphylococcus aureus Resistant to Antibiotics (ASAR) Study Group. Fluoroquinolone Use is a Risk Factor for Methicillin‐Resistant Staphylococcus aureus Acquisition in Long‐Term Care Facilities: A Nested Case‐Case‐Control Study.” Clinical Infectious Diseases 59 (2): 206–15. [DOI] [PubMed] [Google Scholar]
- Dancer, S. J. , Coyne M., Speekenbrink A., Samavedam S., Kennedy J., and Wallace P. G.. 2006. “MRSA Acquisition in an Intensive Care Unit.” American Journal of Infection Control 34 (1): 10–7. [DOI] [PubMed] [Google Scholar]
- Dantes, R. , Mu Y., Belflower R., Aragon D., Dumyati G., Harrison L. H., Lessa F. C., Lynfield R., Nadle J., Petit S., Ray S. M., Schaffner W., Townes J. and Fridkin S.. 2013. “National Burden of Invasive Methicillin‐Resistant Staphylococcus aureus Infections, United States, 2011.” Journal of the American Medical Association Internal Medicine 173 (21): 1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, R. , Platt R., Yokoe D. S., and Huang S. S.. 2011. “Environmental Cleaning Intervention and Risk of Acquiring Multidrug‐Resistant Organisms from Prior Room Occupants.” Archives of Internal Medicine 171 (6): 491–4. [DOI] [PubMed] [Google Scholar]
- Davis, K. A. , Stewart J. J., Crouch H. K., Florez C. E., and Hospenthal D. R.. 2004. “Methicillin‐Resistant Staphylococcus aureus (MRSA) Nares Colonization at Hospital Admission and Its Effect on Subsequent MRSA Infection.” Clinical Infectious Diseases 39 (6): 776–82. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services . 2015. “National Action Plan to Prevent Health Care‐Associated Infections: Road Map to Elimination” [accessed on January 1, 2016]. Available at http://health.gov/hcq/pdfs/HAI-Targets.pdf
- EARSS . 2008. “EARSS Annual Report 2008—On‐Going Surveillance of S. pneumoniae, S. aureus, E. coli, E. faecium, E. faecalis, K. pneumoniae, P. aeruginosa” [accessed on April 26, 2013]. Available at http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Documents/2008_EARSS_Annual_Report.pdf
- Edmondson, A. C. 2004. “Learning from Failure in Health Care: Frequent Opportunities, Pervasive Barriers.” Quality and Safety in Health Care 13 (2): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . 2013. Point Prevalence Survey of Healthcare‐Associated Infections and Antimicrobial use in European Acute Care Hospitals 2011–2012. Stockholm: ECDC. [Google Scholar]
- FitzGerald, G. , Moore G., and Wilsom A. P. R.. 2013. “Hand Hygiene after Touching a Patient's Surroundings: The Opportunities Most Commonly Missed.” Journal of Hospital Infection 84: 27–31. [DOI] [PubMed] [Google Scholar]
- Forster, A. J. , Oake N., Roth V., Suh K. N., Majewski J., Leeder C., and van Walraven C.. 2013. “Patient‐Level Factors Associated with Methicillin‐Resistant Staphylococcus aureus Carriage at Hospital Admission: A Systematic Review.” American Journal of Infection Control 41 (3): 214–20. [DOI] [PubMed] [Google Scholar]
- Frazee, B. W. , Lynn J., Charlebois E. D., Lambert L., Lowery D., and Perdreau‐Remington F.. 2005. “High Prevalence of Methicillin‐Resistant Staphylococcus aureus in Emergency Department Skin and Soft Tissue Infections.” Annals of Emergency Medicine 45 (3): 311–20. [DOI] [PubMed] [Google Scholar]
- French, G. L. , Otter J. A., Shannon K. P., Adams N. M. T., Watling D., and Parks M. J.. 2004. “Tackling Contamination of the Hospital Environment by Methicillin‐Resistant Staphylococcus aureus (MRSA): A Comparison between Conventional Terminal Cleaning and Hydrogen Peroxide Vapour Decontamination.” Journal of Hospital Infection 57 (1): 31–7. [DOI] [PubMed] [Google Scholar]
- Gopal Rao, G. , Michalczyk P., Nayeem N., Walker G., and Wigmore L.. 2007. “Prevalence and Risk Factors for Meticillin‐Resistant Staphylococcus aureus in Adult Emergency Admissions – A Case for Screening All Patients?” Journal of Hospital Infection 66 (1): 15–21. [DOI] [PubMed] [Google Scholar]
- Granger, C. W. 1988. “Some Recent Development in a Concept of Causality.” Journal of Econometrics 39 (1): 199–211. [Google Scholar]
- Grundmann, H. , Aires‐de‐Sousa M., Boyce J., and Tiemersma E.. 2006. “Emergence and Resurgence of Meticillin‐Resistant Staphylococcus aureus as a Public‐Health Threat.” The Lancet 368 (9538): 874–85. [DOI] [PubMed] [Google Scholar]
- Hardy, K. J. , Oppenheim B. A., Gossain S., Gao F., and Hawkey P. M.. 2006. “A Study of the Relationship between Environmental Contamination with Methicillin‐Resistant Staphylococcus aureus (MRSA) and Patients' Acquisition of MRSA.” Infection Control and Hospital Epidemiology 27 (2): 127–32. [DOI] [PubMed] [Google Scholar]
- Jacobson, R. 1990. “Unobservable Effects and Business Performance.” Marketing Science 9 (1): 74–85. [Google Scholar]
- Jeanes, A. , Rao G., Osman M., and Merrick P.. 2005. “Eradication of Persistent Environmental MRSA.” Journal of Hospital Infection 61 (1): 85–6. [DOI] [PubMed] [Google Scholar]
- Kalil, A. C. , Van Schooneveld T. C., Fey P. D., and Rupp M. E.. 2014. “Association between Vancomycin Minimum Inhibitory Concentration and Mortality among Patients with Staphylococcus aureus Bloodstream Infections: A Systematic Review and Meta‐Analysis.” Journal of the American Medical Association 312 (15): 1552–64. [DOI] [PubMed] [Google Scholar]
- Kallen, A. J. , Mu Y., Bulens S., et al. 2010. “Health Care‐Associated Invasive MRSA Infections, 2005–2008.” Journal of the American Medical Association 304 (6): 641–7. [DOI] [PubMed] [Google Scholar]
- Klein, E. , Smith D. L., and Laxminarayan R.. 2007. “Hospitalizations and Deaths Caused by Methicillin‐Resistant Staphylococcus aureus, United States, 1999–2005.” Emerging Infectious Diseases 13 (12): 1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans, J. , Van Belkum A., and Verbrugh H.. 1997. “Nasal Carriage of Staphylococcus aureus: Epidemiology, Underlying Mechanisms, and Associated Risks.” Clinical Microbiology Reviews 10 (3): 505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck, R. , Becker K., Cookson B., van Gemert‐Pijnen J. E., Harbarth S., Kluytmans J., Mielke M., Peters G., Skov R. L., and Struelens M. J.. 2010. “Methicillin‐Resistant Staphylococcus aureus (MRSA): Burden of Disease and Control Challenges in Europe.” Euro Surveillance 15 (41): 1–9. [DOI] [PubMed] [Google Scholar]
- Lambert, M.‐L. , Suetens C., Savey A., Palomar M., Hiesmayr M., Morales I., Agodi A., Frank U., Mertens K., Schumacher M., and Wolkewitz M.. 2011. “Clinical Outcomes of Health‐Care‐Associated Infections and Antimicrobial Resistance in Patients Admitted to European Intensive‐Care Units: A Cohort Study.” The Lancet Infectious Diseases 11 (1): 30–8. [DOI] [PubMed] [Google Scholar]
- Larson, E. L. 1995. “APIC Guidelines for Handwashing and Hand Antisepsis in Health Care Settings.” American Journal of Infection Control 23 (4): 251–69. [DOI] [PubMed] [Google Scholar]
- Lebovic, G. , Siddiqui N., and Muller M. P.. 2013. “Predictors of Hand Hygiene Compliance in the Era of Alcohol‐Based Hand Rinse.” Journal of Hospital Infection 83 (4): 276–83. [DOI] [PubMed] [Google Scholar]
- Loftus, R. W. , Koff M. D., Brown J. R., Patel H. M., Jensen J. T., Reddy S., Ruoff K. L., Heard S. O., Yeager M. P., and Dodds T. M.. 2015. “The Epidemiology of Staphylococcus aureus Transmission in the Anesthesia Work Area.” Anesthesia and Analgesia 120 (4): 807–18. [DOI] [PubMed] [Google Scholar]
- McHugh, C. G. , and Riley L. W.. 2004. “Risk Factors and Costs Associated with Methicillin‐Resistant Staphylococcus aureus Bloodstream Infections.” Infection Control 25 (5): 425–30. [DOI] [PubMed] [Google Scholar]
- Murray, M. T. , Krishnamurthy G., Corda R., Turcotte R. F., Jia H., Bacha E., and L. Saiman . 2014. “Surgical Site Infections and Bloodstream Infections in Infants after Cardiac Surgery.” Journal of Thoracic and Cardiovascular Surgery 148 (1): 259–65. [DOI] [PubMed] [Google Scholar]
- National Audit Office . 2004. Improving Patient Care by Reducing the Risk of Hospital Acquired Infection: A Progress Report. London: The Stationery Office. [Google Scholar]
- Pearson, A. , Chronias A., and Murray M.. 2009. “Voluntary and Mandatory Surveillance for Methicillin‐Resistant Staphylococcus aureus (MRSA) and Methicillin‐Susceptible S. aureus (MSSA) Bacteraemia in England.” Journal of Antimicrobial Chemotherapy 64 (suppl 1): i11–7. [DOI] [PubMed] [Google Scholar]
- Pittet, D. , Hugonnet S., Harbarth S., Mourouga P., Sauvan V., Touveneau S., and T. V. Perneger . 2000. “Effectiveness of a Hospital‐Wide Programme to Improve Compliance with Hand Hygiene.” The Lancet 356 (9238): 1307–12. [DOI] [PubMed] [Google Scholar]
- Plipat, N. , Spicknall I. H., Koopman J. S., and Eisenberg J. N.. 2013. “The Dynamics of Methicillin‐Resistant Staphylococcus aureus Exposure in a Hospital Model and the Potential for Environmental Intervention.” BMC Infectious Diseases 13 (595): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoola, V. O. , Budd A., Wittig S. M., Ross T., Aucott S. W., Perl T. M., Carroll K. C., and Milstone A. M.. 2014. “Methicillin‐Resistant Staphylococcus aureus Transmission and Infections in a Neonatal Intensive Care Unit Despite Active Surveillance Cultures and Decolonization: Challenges for Infection Prevention.” Infection Control and Hospital Epidemiology 35 (4): 412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto, J. P. , Santos R. O., Gontijo Filho P. P., and Ribas R. M.. 2013. “Active Surveillance to Determine the Impact of Methicillin Resistance on Mortality in Patients with Bacteremia and Influences of the Use of Antibiotics on the Development of MRSA Infection.” Revista da Sociedade Brasileira de Medicina Tropical 46 (6): 713–8. [DOI] [PubMed] [Google Scholar]
- Rampling, A. , Wiseman S., Davis L., Hyett A. P., Walbridge A. N., Payne G. C., and Cornaby A. J.. 2001. “Evidence That Hospital Hygiene Is Important in the Control of Methicillin‐Resistant Staphylococcus aureus .” Journal of Hospital Infection 49 (2): 109–16. [DOI] [PubMed] [Google Scholar]
- Ridgway, J. P. , Peterson L. R., Brown E. C., Du H., Hebert C., Thomson R. B. Jr, Kaul K. L., and Robicsek A.. 2013. “Clinical Significance of Methicillin‐Resistant Staphylococcus aureus Colonization on Hospital Admission: One‐Year Infection Risk.” PLoS ONE 8 (11): e79716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands, J. , Yeager M. P., Beach M., Patel H. M., Huysman B. C., and Loftus R. W.. 2014. “Video Observation to Map Hand Contact and Bacterial Transmission in Operating Rooms.” American Journal of Infection Control 42 (7): 698–701. [DOI] [PubMed] [Google Scholar]
- Salge, T. O. , and Vera A.. 2012. “Benefiting from Public Sector Innovation: The Moderating Role of Customer and Learning Orientation.” Public Administration Review 72 (4): 550–9. [Google Scholar]
- Singer, S. , Lin S., Falwell A., Gaba D., and Baker L.. 2009. “Relationship of Safety Climate and Safety Performance in Hospitals.” Health Services Research 44: 399–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. , Stockwell D. C., Floyd T., Short B. L., and Singh N.. 2013. “Improving Hand Hygiene Compliance in Health Care Workers: Strategies and Impact on Patient Outcomes.” American Journal of Infection Control 41: e101–5. [DOI] [PubMed] [Google Scholar]
- Stegenga, J. , Bell E., and Matlow A.. 2002. “The Role of Nurse Understaffing in Nosocomial Viral Gastrointestinal Infections on a General Pediatrics Ward.” Infection Control and Hospital Epidemiology 23 (3): 133–6. [DOI] [PubMed] [Google Scholar]
- Tehrani, D. M. , Phelan M. J., Cao C., Billimek J., Datta R., Nguyen H., Kwark H., and Huang S. S.. 2014. “Substantial Shifts in Ranking of California Hospitals by Hospital‐Associated Methicillin‐Resistant Staphylococcus aureus Infection Following Adjustment for Hospital Characteristics and Case Mix.” Infection Control and Hospital Epidemiology 10: 1263–70. [DOI] [PubMed] [Google Scholar]
- Tucker, A. L. , and Edmondson A. C.. 2003. “Why Hospitals Don't Learn from Failures: Organizational and Psychological Dynamics That Inhibit System Change.” California Management Review 45 (2): 55–72. [Google Scholar]
- Umscheid, C. A. , Mitchell M. D., Doshi J. A., Agarwal R., Williams K., and Brennan P. J.. 2011. “Estimating the Proportion of Healthcare‐Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs.” Infection Control & Hospital Epidemiology 32: 101–14. [DOI] [PubMed] [Google Scholar]
- Vicca, A. F. 1999. “Nursing Staff Workload as a Determinant of Methicillin‐Resistant Staphylococcus aureus Spread in an Adult Intensive Therapy Unit.” Journal of Hospital Infection 43 (2): 109–13. [DOI] [PubMed] [Google Scholar]
- Wooten, D. A. , and Winston L. G.. 2013. “Risk Factors for Methicillin‐Resistant Staphylococcus aureus in Patients with Community‐Onset and Hospital‐Onset Pneumonia.” Respiratory Medicine 107 (8): 1266–70. [DOI] [PubMed] [Google Scholar]
- Wyllie, D. H. , Peto T. E., and Crook D.. 2005. “MRSA Bacteraemia in Patients on Arrival in Hospital: A Cohort Study in Oxfordshire 1997–2003.” British Medical Journal 331 (7523): 992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B. , and Olivera F.. 2006. “Error Reporting in Organizations.” Academy of Management Review 31 (4): 1012–30. [Google Scholar]
- Zimlichman, E. , Henderson D., Tamir O., et al. 2013. “Health Care‐Associated Infections: A Meta‐analysis of Costs and Financial Impact on the US Health Care System.” Journal of the American Medical Association Internal Medicine 173 (22): 2039–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


