Abstract
Background
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report provides a framework for classifying COPD reflecting the impacts of disease on patients and for targeting treatment recommendations. The GOLD 2017 introduced a new classification with 16 subgroups based on a composite of spirometry and symptoms/exacerbations.
Methods
Data from the population-based PLATINO study, collected at baseline and at follow-up, in three sites in Latin America were analyzed to compare the following: 1) the distribution of COPD patients according to GOLD 2007, 2013, and 2017; 2) the stability of the 2007 and 2013 classifications; and 3) the mortality rate over time stratified by GOLD 2007, 2013, and 2017.
Results
Of the 524 COPD patients evaluated, most of them were classified as Grade I or II (GOLD 2007) and Group A or B (GOLD 2013), with ≈70% of those classified as Group A in GOLD 2013 also classified as Grade I in GOLD 2007 and the highest percentage (41%) in Group D (2013) classified as Grade III (2007). According to GOLD 2017, among patients with Grade I airflow limitation, 69% of them were categorized into Group A, whereas Grade IV patients were more evenly distributed among Groups A–D. Most of the patients classified by GOLD 2007 remained in the same airflow limitation group at the follow-up; a greater temporal variability was observed with GOLD 2013 classification. Incidence-mortality rate in patients classified by GOLD 2007 was positively associated with increasing severity of airflow obstruction; for GOLD 2013 and GOLD 2017 (Groups A–D), highest mortality rates were observed in Groups C and D. No clear pattern was observed for mortality across the GOLD 2017 subgroups.
Conclusion
The PLATINO study data suggest that GOLD 2007 classification shows more stability over time compared with GOLD 2013. No clear patterns with respect to the distribution of patients or incidence-mortality rates were observed according to GOLD 2013/2017 classification.
Keywords: chronic obstructive lung diseases, Latin America, GOLD classification
Introduction
COPD has been classified, in most of the literature, by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, that first was launched in 1997, and has been regularly updated to the current version in 2017.1 Although these criteria have been modified in the different versions of the GOLD recommendations, it has been an important tool in the management of this disease worldwide and has raised the awareness of the burden of COPD.
In GOLD Version 2007, airflow limitation measured using spirometry was standardly used to grade the severity of COPD (Grades I–IV),2 and several studies have validated this categorization in terms of prognosis and patient-related outcomes.3–5 GOLD Version 2011, followed by a revision in 2013, introduced the combined COPD assessment to acknowledge the consensus that factors other than airflow limitation alone should be considered for optimal COPD assessment, including symptoms, measured either by the modified Medical Research Council (mMRC) dyspnea score or by the COPD Assessment Test (CAT) score, and the risk of exacerbations based on the history of exacerbations and the grade of spirometric obstruction.6 The result was a 2×2 table and the A/B/C/D cells classification. Although the idea of considering symptoms measured with spirometry seemed to be more comprehensive, the A/B/C/D three-way categorization was complicated and the evidence on the use of therapy based on this classification has been limited.7 In addition, in terms of mortality prediction, this classification has not been consistently better than the 2007 classification that is based only on spirometry results.8,9 Most recently, the fourth document of the GOLD report, GOLD 2017,1 has been released describing a refined composite classification, including the components of the GOLD 2007 and the GOLD 2013 parameters, and taking into account the severity of airflow limitation (spirometric Grades 1–4) and combined symptoms/exacerbation risk (Groups A–D), resulting in 16 subgroups. GOLD 2017 classification also proposes a pharmacologic treatment algorithm by GOLD “A/B/C/D” group, based only on symptoms and exacerbations among those who have been classified as having COPD according to the forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7 criterion.1
Some previous studies comparing the GOLD 2007 and 2013 classifications have reported that the A/B/C/D classification identified more patients at risk of exacerbations and poorer outcomes compared with FEV1 alone, identified a temporal stability in Groups A and D versus temporal variability in Groups B and C, but showed poor differentiation in the prognostic validity for mortality.8–12 To the authors’ knowledge, no studies have been available to date comparing GOLD 2017 with the previous GOLD classifications.
The PLATINO study, a population-based study, evaluated subjects aged ≥40 years at two different time points, in three sites in Latin America,13–15 allowing to describe in this paper: 1) the distribution of patients according to GOLD 2007, 2013, and 2017 classifications; 2) the stability of the different GOLD classifications over time (2007 and 2013); and 3) the incidence-mortality rate ratio comparing the three GOLD classifications.
Methods
Study design and population
The PLATINO study was a population-based study carried out in five centers in Latin America (namely Montevideo, Santiago, São Paulo, Mexico City, and Caracas) from 2002 to 2004, among adults aged ≥40 years.13 The study was conducted at two phases: 1) the baseline survey that occurred in all the five centers14 and 2) the follow-up visit that occurred in three of the five centers, namely Montevideo (conducted at 5 years after baseline), Santiago (6 years after baseline), and São Paulo (9 years after baseline).15 The PLATINO baseline study sample consisted of 5,315 subjects (Montevideo: 885; Santiago: 1173; São Paulo: 963; Mexico City: 1000, and Caracas: 1294), and the prevalence of COPD (postbronchodilator FEV1/FVC ratio <0.70) ranged from 7.8% to 19.7% across the five centers. At follow-up, 687 (77.6%) subjects in Montevideo, 898 (76.6%) subjects in Santiago, and 613 (63.7%) subjects in São Paulo from the original baseline sample were located and reinterviewed. In addition, mortality data were prospectively collected from the time of the baseline visit to the follow-up visit. The study population included in the current analysis was comprised of 524 COPD patients identified in the PLATINO baseline in Montevideo, Santiago, and São Paulo.
The current analysis was performed by using the data accessed from the PLATINO study.16 Written consent from the subjects and ethical approvals were not required for this analysis but were obtained previously: detailed methods of the PLATINO baseline14 and follow-up15 studies are available elsewhere.
Measurements
The same core questionnaire was used at the PLATINO baseline and follow-up visits. This was a combined version of the American Thoracic Society (ATS) – Division of Lung Disease,17 the European Community Respiratory Health Survey II,18 and the Lung Health Study.19
Spirometry was undertaken in 99% of the PLATINO sample by using a portable, battery-operated, EasyOne spirometer (ndd Medical Technologies, Zurich, Switzerland).14 Tests were conducted pre- and post-200 µg of salbutamol according to the ATS criteria of acceptability and reproducibility,20 and >90% of the spirometry measurements fulfilled the ATS criteria of quality.20 The COPD diagnosis at the baseline and in the follow-up considered the fixed ratio criteria (FEV1/FVC <0.7). The reference equations for lung function parameters (FEV1% of predicted) used in this analysis were those derived by using the PLATINO data published by Perez-Padilla et al.21
For the present analysis, COPD was stratified according to the GOLD 2007, 2013, and 2017 definitions as shown in Table 1. For symptom assessment according to the GOLD A/B/C/D classification, the PLATINO study used mMRC scale as the CAT was not routinely applied in all the centers.
Table 1.
COPD classifications according to GOLD 2007, 2013, and 2017
| GOLD 20072 | GOLD 20136 | GOLD 20171 |
|---|---|---|
| Spirometric classification based on FEV1/FVC <0.70 and postbronchodilator FEV1% of predicted | GOLD A/B/C/D classification based on symptoms (mMRC or CAT) and exacerbation risk (measured by airflow limitation or history of exacerbations) | Combination of spirometric classification (1 [mild] to 4 [very severe] A/L) and A/B/C/D classification based on symptoms (mMRC or CAT) and exacerbation risk (history of exacerbations) |
| Grade I: Mild (≥80%) Grade II: Moderate (≥50% and <80%) Grade III: Severe (≥30% and <50%) Grade IV: Very severe (<30% or <50% plus chronic respiratory failure) |
A: Less symptoms (mMRC 0 or 1, CAT <10), low risk (mild/moderate airflow limitation or 0–1 exacerbations per year) B: More symptoms (mMRC ≥2, CAT ≥10), low risk (mild/moderate airflow limitation or 0–1 exacerbations per year) C: Less symptoms (mMRC 0 or 1, CAT <10), high risk (severe or very severe airflow limitation or ≥2 exacerbations per year or ≥1 hospitalized exacerbation per year) D: More symptoms (mMRC ≥2, CAT ≥10), high risk (severe or very severe airflow limitation or ≥2 exacerbations per year or ≥1 hospitalized exacerbation per year) |
1A: Mild A/L, less symptoms, low risk 1B: Mild A/L, more symptoms, low risk 1C: Mild A/L, less symptoms, high risk 1D: Mild A/L, more symptoms, high risk 2A: Moderate A/L, less symptoms, low risk 2B: Moderate A/L, more symptoms, low risk 2C: Moderate A/L, less symptoms, high risk 2D: Moderate A/L, more symptoms, high risk 3A: Severe A/L, less symptoms, low risk 3B: Severe A/L, more symptoms, low risk 3C: Severe A/L, less symptoms, high risk 3D: Severe A/L, more symptoms, high risk 4A: Very severe A/L, less symptoms, low risk 4B: Very severe A/L, more symptoms, low risk 4C: Very severe A/L, less symptoms, high risk 4D: Very severe A/L, more symptoms, high risk |
Abbreviations: A/L, airflow limitation; CAT, COPD Assessment Test; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council.
Mortality data were collected from death certificates from the National Death Registry from each country, and the quality of the death certificate was evaluated by an expert.22
Data analysis
Descriptive statistics with absolute (n) and relative (%) frequencies were considered as categorical variables, and the mean and standard deviation were considered as continuous variables. The COPD classification group based on GOLD 2017 was described according to sex (male/female), age (completed years), skin color (white, mulatto/black, and others), schooling (completed years of formal education), body mass index (BMI) calculated as the ratio of weight (kg) to height (m2) and categorized as <25.0, 25.0–29.9, and ≥30 kg/m2, smoking (pack-years <10, 10–20, and >20), mMRC dyspnea score (0, 1, 2, 3, and 4), chronic cough (no/yes), chronic phlegm (no/yes), wheezing in the last 12 months (no/yes), use of any respiratory medicine in the last 12 months (no/yes), and medical diagnosis of COPD or chronic bronchitis or emphysema (no/yes). A mean symptom score was calculated based on wheezing, cough, or phlegm (ranging from 0 to 3 per patient).
For the baseline questionnaire, an imputation technique (Stata command: mi impute for multiple imputation at random assumption, see Table S1) for ~35% missing information in the dyspnea variable (mMRC scale). The variables were considered as dichotomous (one variable for each item), and logistic regression models were used taking into account sex, wheezing in the past year (yes/no), medicine use in the past year (yes/no), BMI (kg/m2), comorbidity score, and the site (namely São Paulo, Santiago, and Montevideo).
Incidence-mortality rate was calculated for all-cause mortality due to the bigger sample size for this outcome compared with specific causes of death such as cardiovascular, respiratory, or cancer, among others.22 The log rank test was used to compare the mortality rates between the GOLD classifications (2007, 2013, and 2017).
Statistical analysis was performed by using the software Stata Version 12.2 (Stata Corp., College Station, TX, USA).
Results
Table 2 shows the demographic and clinical characteristics of the 524 patients who fulfilled the criteria for COPD (post-bronchodilator FEV1/FVC ratio <0.70) at the baseline. In the whole sample, slightly more number of individuals were male (53.6%); approximately two thirds were aged ≥60 years (61.6%) and had completed ≥5 years of school education (62.8%); the majority had a BMI of <29.9 kg/m2 (75%); and almost 40% had a smoking history of >20 pack-years, the highest category. Nearly half of all the patients had an mMRC dyspnea scale score of 1, 2, or 3 (49.4%) and had wheezing in the last 12 months, whereas approximately one third of them complained of cough (34.3%) and phlegm (27.7%). Only a quarter of the patients reported the use of a respiratory medicine in the past year, and 11% of them had a medical diagnosis of COPD.
Table 2.
Demographic and clinical characteristics of the subjects who fulfilled COPD criterion (postbronchodilator FEV1/FVC ratio <0.70) at baseline
| Variables | São Paulo (n=152)
|
Montevideo (n=174)
|
Santiago (n=198)
|
Total sample (n=524)
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Sex (male, %) | 77 (50.7) | 98 (56.3) | 106 (53.5) | 281 (53.6) |
| Age (years) | ||||
| 40–49 | 32 (21.1) | 12 (6.9) | 28 (14.1) | 72 (13.7) |
| 50–59 | 50 (32.9) | 30 (17.2) | 49 (24.8) | 129 (24.6) |
| ≥60 | 70 (46.0) | 132 (75.9) | 121 (61.1) | 323 (61.6) |
| Skin color | ||||
| White | 90 (59.2) | 161 (92.5) | 144 (72.7) | 395 (75.4) |
| Mulatto/black | 52 (34.2) | 8 (4.6) | 41 (20.7) | 101 (19.3) |
| Others | 10 (6.6) | 5 (2.9) | 13 (6.6) | 28 (5.3) |
| Schooling (completed years of formal education) | ||||
| 0–2 | 49 (32.2) | 15 (8.6) | 26 (13.1) | 90 (17.2) |
| 3–4 | 48 (31.6) | 32 (18.4) | 25 (12.6) | 105 (20.0) |
| 5–8 | 32 (21.1) | 74 (42.5) | 62 (31.3) | 168 (32.1) |
| ≥9 | 23 (15.1) | 53 (30.5) | 85 (42.9) | 161 (30.7) |
| BMI (kg/m2)* | ||||
| <25.0 | 70 (46.1) | 61 (35.1) | 61 (31.0) | 192 (36.7) |
| 25.0–29.9 | 50 (32.9) | 68 (39.1) | 82 (41.6) | 200 (38.2) |
| ≥30 | 32 (21.0) | 45 (25.8) | 54 (27.4) | 131 (25.1) |
| Smoking (pack-years*) | ||||
| <10 | 78 (51.3) | 74 (42.5) | 110 (55.8) | 262 (50.1) |
| 10–20 | 19 (12.5) | 15 (8.6) | 30 (15.2) | 64 (12.2) |
| >20 | 55 (36.2) | 85 (48.9) | 57 (28.9) | 197 (37.7) |
| mMRC dyspnea scale score* | ||||
| 0 | 88 (57.9) | 80 (46.5) | 86 (45.0) | 254 (49.3) |
| 1 | 25 (16.5) | 47 (27.3) | 38 (19.9) | 110 (21.4) |
| 2 | 19 (12.5) | 20 (11.6) | 31 (16.2) | 70 (13.6) |
| 3 | 16 (10.5) | 23 (13.4) | 35 (18.3) | 74 (14.4) |
| 4 | 4 (2.6) | 2 (1.2) | 1 (0.5) | 7 (1.4) |
| Cough (yes, %) | 52 (34.2) | 61 (25.1) | 67 (33.8) | 180 (34.3) |
| Phlegm (yes, %) | 41 (27.0) | 46 (26.4) | 58 (29.3) | 145 (27.7) |
| Wheezing (in the last 12 months; yes, %) | 59 (38.8) | 70 (40.2) | 98 (49.5) | 227 (43.3) |
| Any respiratory medicine use (in the last 12 months; yes, %) | 28 (18.4) | 37 (21.3) | 71 (35.9) | 136 (25.9) |
| Medical diagnosis of COPD (yes, %) | 19 (12.5) | 13 (7.5) | 25 (12.6) | 57 (10.9) |
Note:
Variables with missing values (smoking and BMI with one missing value and mMRC scale with nine missing values).
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council.
The majority of 524 PLATINO baseline patients were classified as Grade I or II according to GOLD 2007 and Group A or B according to GOLD 2013. The cross-sectional comparison of patients classified by GOLD 2013 with those classified by GOLD 2007 (Table 3) shows that more symptomatic subjects also tended to have more severe airflow obstruction: ~70% of those classified as Group A in GOLD 2013 were in Grade I in GOLD 2007; those in Group B according to GOLD 2013 were distributed in Grades I or II in GOLD 2007; most of those in Group C according to GOLD 2013 were distributed in Grades III or IV in GOLD 2007; and the highest percentage of those classified as Group D in GOLD 2013 were in Grade III in GOLD 2007 classification.
Table 3.
COPD classification in 524 subjects, according to GOLD 20072 and GOLD 20136 criteria at the baseline assessment
| GOLD 2007 criteria | GOLD 2013 criteria
|
|||
|---|---|---|---|---|
| A, n (%) | B, n (%) | C, n (%) | D, n (%) | |
| I | 262 (69.7) | 53 (57.6) | 3 (11.1) | 5 (17.2) |
| II | 114 (30.3) | 39 (42.4) | 5 (18.5) | 9 (31.0) |
| III | – | – | 15 (55.6) | 12 (41.4) |
| IV | – | – | 4 (14.8) | 3 (10.3) |
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
When considering GOLD 2017, among patients with mild airflow limitation (Grade I by GOLD 2007), the majority of them were categorized as Group A (few symptoms and few exacerbations; Table 4), and 21.7% of them presented with moderate-to-severe dyspnea (Groups B–D). On the other hand, those with more severe airflow limitation (Grade IV by GOLD 2007) were more evenly distributed among Groups A–D, although it should be noted that the number of patients was small.
Table 4.
Distribution of GOLD 20171 A/B/C/D groups at the baseline according to airflow limitation severity (Grades I–IV) in 524 COPD subjects
| Airflow obstruction (grade) | Symptoms classification | n (%) |
|---|---|---|
| I (n=323) | A | 253 (78.3) |
| B | 62 (19.2) | |
| C | 3 (0.9) | |
| D | 5 (1.6) | |
| II (n=167) | A | 98 (58.7) |
| B | 58 (34.7) | |
| C | 4 (2.4) | |
| D | 7 (4.2) | |
| III (n=27) | A | 11 (40.7) |
| B | 11 (40.7) | |
| C | 1 (3.7) | |
| D | 4 (14.8) | |
| IV (n=7) | A | 1 (14.3) |
| B | 3 (42.9) | |
| C | 2 (28.6) | |
| D | 1 (14.3) |
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 5 shows the distribution of patient sociodemographic and clinical characteristics according to GOLD 2017 subgroups. Almost half of the patients were classified as GOLD Group IA (48.3%), ~20% as Group IIA, and ~10% as Groups IB and IIB each. The distribution of patients in the remaining subgroups was <3% per group. The variations observed in some sociodemographic and clinical variables may reflect the small number of patients in the majority of subgroups.
Table 5.
Demographic and clinical characteristics of 524 subjects who fulfilled COPD criteria at the baseline, according to GOLD 20171 classification at the baseline assessment
| Variables | I
|
II
|
III
|
IV
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A
|
B
|
C
|
D
|
A
|
B
|
C
|
D
|
A
|
B
|
C
|
D
|
A
|
B
|
C
|
D
|
|
| n=253 (48.3%) |
n=62 (11.8%) |
n=3 (<1%) |
n=5 (1%) |
n=98 (18.7%) |
n=58 (11.1%) |
n=4 (<1%) |
n=7 (1.3%) |
n=11 (2.1%) |
n=11 (2.1%) |
n=1 (<1%) |
n=4 (<1%) |
n=1 (<1%) |
n=3 (<1%) |
n=2 (<1%) |
n=1 (<1%) |
|
| Sex (male, %) | 64.0 | 21.0 | 66.7 | 40.0 | 61.2 | 36.2 | 75.0 | 14.3 | 54.6 | 27.3 | 100.0 | 50.0 | 100.0 | 66.7 | 50.0 | 100.0 |
| Age (completed years, mean) | 64.7 | 66.9 | 63.2 | 77.0 | 62.4 | 63.8 | 48.3 | 54.4 | 67.7 | 62.4 | 51.9 | 65.0 | 74.5 | 56.8 | 65.7 | 81.0 |
| Schooling (completed years of formal education, mean) | 6.9 | 6.4 | 6.7 | 3.4 | 7.4 | 6.6 | 12.5 | 5.7 | 5.8 | 6.3 | 2.0 | 3.5 | 4.0 | 7.0 | 8.0 | 4.0 |
| BMI (kg/m2, mean) | 26.7 | 29.3 | 31.0 | 27.0 | 26.5 | 29.0 | 27.4 | 23.6 | 23.9 | 25.2 | 29.5 | 27.2 | 21.6 | 27.8 | 23.5 | 25.5 |
| Smoking (pack-years, mean) | 15.6 | 12.1 | 0.5 | 0.4 | 23.6 | 24.0 | 12.7 | 16.7 | 19.4 | 17.5 | 75.0 | 9.6 | 2.3 | 27.1 | 0.0 | 55.0 |
| mMRC scale score (mean) | 0.3 | 2.6 | 0.7 | 2.8 | 0.4 | 2.6 | 0.5 | 2.6 | 0.2 | 2.5 | 1.0 | 2.3 | 1.0 | 2.7 | 1.0 | 2.0 |
| Cough (yes, %) | 27.3 | 37.1 | 33.3 | 40.0 | 35.7 | 48.3 | 25.0 | 42.9 | 27.3 | 72.7 | 0.0 | 75.0 | 100.0 | 66.7 | 50.0 | 0.0 |
| Phlegm (yes, %) | 20.2 | 32.3 | 33.3 | 20.0 | 20.6 | 36.2 | 25.0 | 71.4 | 18.2 | 63.6 | 100.0 | 75.0 | 100.0 | 66.7 | 0.0 | 0.0 |
| Wheezing (yes, %) | 26.9 | 58.1 | 66.7 | 40.0 | 44.9 | 72.4 | 100.0 | 100.0 | 36.4 | 81.8 | 0.0 | 75.0 | 100.0 | 100.0 | 50.0 | 100.0 |
| Symptoms score (wheezing + cough + phlegm, mean) | 0.7 | 1.3 | 1.3 | 1.0 | 1.1 | 1.6 | 1.5 | 2.1 | 0.8 | 2.2 | 1.0 | 2.3 | 3.0 | 2.3 | 1.0 | 1.0 |
| Any respiratory medicine use in the past year (%) | 10.3 | 33.9 | 100.0 | 80.0 | 28.6 | 41.4 | 100.0 | 71.4 | 36.4 | 72.7 | 100.0 | 100.0 | 0.0 | 33.3 | 100.0 | 100.0 |
| Medical diagnosis of COPD (%) | 2.4 | 16.1 | 0.0 | 40.0 | 12.2 | 15.5 | 25.0 | 28.6 | 18.2 | 54.6 | 0.0 | 75.0 | 100.0 | 66.7 | 50.0 | 0.0 |
Abbreviations: BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council.
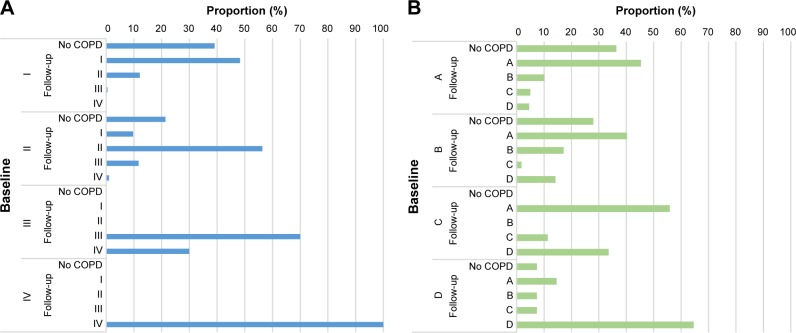
Among the 524 COPD patients identified in the PLATINO baseline, 321 patients were reassessed in the PLATINO follow-up, 106 patients had died, and 97 patients were lost to follow up. Figure 1 shows the distribution of the four GOLD groups at the baseline visit and at the follow-up visit for those who were alive and who were reassessed in both phases of the survey. For GOLD 2007, the majority of the patients in each category did not change their grade of severity during the follow-up period (Figure 1A). It might also be noticed that subjects classified as GOLD III at baseline were classified as III or IV at follow-up, with some 30% progressing to GOLD IV. For GOLD 2013, the stability of the classification at the baseline and follow-up visits was more inconsistent: ~36% and 28% of patients who were categorized as Group A and B, respectively, at the baseline, and 7% categorized as Group D, moved to the no COPD category at the follow-up (Figure 1B). Most of those categorized as Group C at the baseline migrated to Group A in the follow-up, and ~64% of those who were in Group D remained in the same classification in both phases of the study. It was not possible to evaluate the stability of the new 2017 GOLD classification, due to the number of subgroups in the new version and the small sample sizes for some groups.
Figure 1.
Change in COPD classification from baseline to follow-up using (A) GOLD 20072 criteria and (B) 2013 criteria.6
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The incidence-mortality rate in patients classified according to GOLD 2007 was positively associated with the severity of airflow obstruction; the highest mortality rate was among those COPD patients categorized as GOLD Grade IV (Table 6). In patients classified by GOLD 2013 criteria, the highest incidence-mortality rate was observed in Group C; there was no clear association between the incidence-mortality rate and the presence of symptoms and exacerbations. Similarly, when patients were classified according to GOLD 2017, the mortality rates varied considerably across the subgroups.
Table 6.
Number of deaths, time at risk, and crude incidence-mortality rates according to COPD classification (GOLD 20072, 20136, and 20171 criteria)
| COPD severity | Number of deaths | Total time at risk (years) | Mortality rate (per 1,000 person-year) | P-value |
|---|---|---|---|---|
| GOLD 2007 | <0.001 | |||
| I | 54 | 1,540.98 | 35.0 | |
| II | 35 | 788.93 | 44.4 | |
| III | 13 | 123.18 | 105.5 | |
| IV | 4 | 25.90 | 154.4 | |
| GOLD 2013 | <0.001 | |||
| A | 66 | 1,735.18 | 38.04 | |
| B | 16 | 492.90 | 32.46 | |
| C | 14 | 119.27 | 117.38 | |
| D | 10 | 131.65 | 75.96 | |
| GOLD 2017 | <0.001 | |||
| I-A | 40 | 1,155.42 | 34.62 | |
| I-B | 10 | 349.01 | 28.65 | |
| I-C | 1 | 18.04 | 55.43 | |
| I-D | 3 | 18.51 | 162.12 | |
| II-A | 22 | 455.28 | 49.31 | |
| II-B | 11 | 279.87 | 39.30 | |
| II-C | 1 | 19.95 | 50.13 | |
| II-D | 1 | 33.84 | 29.55 | |
| III-A | 6 | 52.27 | 114.79 | |
| III-B | 4 | 48.09 | 83.18 | |
| III-C | 1 | 0.00 | – | |
| III-D | 2 | 22.82 | 87.66 | |
| IV-A | 1 | 1.03 | 966.27 | |
| IV-B | 1 | 11.42 | 87.58 | |
| IV-C | 1 | 9.82 | 101.80 | |
| IV-D | 1 | 3.63 | 275.66 |
Note: P-values were obtained using the log rank test.
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 7 shows the distribution and the incidence-mortality rate for patients according to the GOLD 2017 classification as Groups A/B/C/D based on symptoms only (as recommended for the purpose of the pharmacotherapeutic management of the disease). Approximately 95% of patients were classified into Group A or B and ~5% into Group C or D. The incidence-mortality rate was similar for Groups A and B and for Groups C and D.
Table 7.
Number of deaths, time at risk, and incidence-mortality rate according to COPD severity using only symptoms without obstruction severity
| COPD severity | Distribution at the baseline n (%) | Number of deaths | Total time at risk (person-year) | Mortality rate (per 1,000 person-year) |
|---|---|---|---|---|
| GOLD 20171 based only on symptoms | P=0.093 | |||
| A – Low symptoms/low exacerbations | 363 (69.3) | 69 | 1,664.01 | 41.47 |
| B – High symptoms/low exacerbation | 134 (25.6) | 26 | 688.40 | 37.77 |
| C – Low symptoms/high exacerbations | 10 (1.9) | 4 | 47.81 | 83.67 |
| D – High symptoms/high exacerbation | 17 (3.2) | 7 | 78.79 | 88.85 |
Note: P-values were obtained using the log rank test.
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Discussion
The main objective for proposing several GOLD classifications over the years has been to improve the understanding and management of COPD, a known heterogeneous and complex disease. Evaluating the distribution of patients according to GOLD classification is important for the assessment of disease stratification and for the determination of appropriate pharmacotherapy. However, due to the changing definitions associated with the iteration of the classification, the distribution of patients in each category also differs over time, making the comparison of groups defined by different classification criteria, and the subsequent management of patients, more challenging.
The present analysis aimed at showing the distribution of COPD patients according to three different GOLD classifications (2007, 2013, and 2017),1,2,6 among the PLATINO cohort of COPD patients, over a period of time. The comparison between GOLD 2007 and GOLD 2013 classifications showed that the highest proportion of patients had mild obstruction and few symptoms; that is, they were predominantly classified into Groups A and B (GOLD 2013) and Grades I and II (GOLD 2007). Similarly, the most symptomatic patients tended to have more severe obstruction: individuals in Group C (GOLD 2013) were predominantly classified as Grades III and IV (GOLD 2007); although those in Group D (GOLD 2013) were unevenly distributed, the majority of them were classified as Grade III (GOLD 2007). The new GOLD 2017 classification, using spirometry, symptoms, and exacerbations, did not show a clear independent pattern among the subgroups, but it is important to highlight that the PLATINO study was a population-based study with a high proportion of COPD patients in mild or moderate categories and with the limited numbers of subjects in some of the more severe subgroup categories.
The data from the PLATINO cohort showed that the majority of patients in each group classified by GOLD 2007 at the baseline remained in the same group at the follow-up. Greater temporal variability was observed when patients were classified by GOLD 2013. A possibility to be considered is that the elimination of symptoms in individuals with COPD could be due to an effective treatment and/or stopping tobacco smoking or that the original symptoms (mainly dyspnea) could have been related to nonrespiratory system factors. A variety of diseases, other than respiratory diseases, could be the cause of symptoms such as dyspnea and “respiratory exacerbations,” mainly heart failure, other cardiovascular conditions, and obesity. In the PLATINO population, ~40% of the non-COPD sample complained of dyspnea (mMRC Grades 1–4) with 1.8% of dyspnea Grade 4, compared with 51.8% and 1.4%, respectively, among those who had COPD defined by the fixed ratio criteria, and 2.3% of non-COPD patients reported “exacerbations” compared with 5.2% in the COPD patients (data not shown). One interesting finding from this analysis is that 36.1%, 27.7%, and 7.1% of patients who were categorized into Groups A, B, and D, respectively, at the baseline became “free” of the disease in the follow-up, when classified according to GOLD 2013 criteria. It would be expected that after taking into account the variability in the spirometry testing,23 a diagnosis of COPD, with relevant health connotations, would not disappear, and it is likely that these cases represent asthma or COPD misdiagnosis.
Another explanation for obtaining different responses in two evaluations taken at different time points may be the limitation of the method of diagnosis.23–25 The stability of a COPD diagnosis will be impacted by variations in spirometry and symptom assessments, the reliability of these instruments frequently falling short of the gold standard. Due to their limited reliability, very specific instruments and spirometric cutoffs should be used to diagnose and classify patients, on which the best management of the disease will later rely. A strength of the PLATINO study is the good quality of spirometry performed in the field with most readings undertaken by using the same spirometer and >90% fulfilling the ATS criteria for quality.15 In addition, the same questionnaire was used at both the baseline and follow-up.
It is also important to discuss the potential pharmacotherapy implication associated with the distribution of patients according to GOLD 2017 classification. The new GOLD document indicates that all the patients in Group A should be offered a bronchodilator treatment based on its effects on breathlessness. In the present analysis, the patients were observed with mild (Grade 1) to very severe (Grade 4) airflow limitation in Group A, suggesting that patients with very severe airflow limitation may be at risk of undertreatment as these patients, even when not exacerbating, are more at risk of future events.
A wide variation was also observed in airflow limitation in patients classified as Groups C and D (finding patients in Groups C and D with mild obstruction such as GOLD 1D), with the recommendation to start therapy preferably with a LAMA/LABA combination, with escalation to triple therapy in patients who experience further exacerbations. Currently, the diagnosis of an exacerbation relies exclusively on the clinical presentation of the patient complaining of an acute change of symptoms, often confounded by symptoms associated with comorbidities, and there is no biomarker or panel of biomarkers that allows a more precise diagnosis. This may represent a situation where there is a risk of overtreating patients using high-cost drugs for the most developing countries. However, it is acknowledged that these observations need to be confirmed with other studies due to the limitation of the population-based study design of the PLATINO and the small number of subjects in the most severe categories.
The evaluation of incidence-mortality rates for the GOLD 2007 showed a clear dose–response gradient according to the severity of airflow limitation from Grades I to IV. According to GOLD 2013 classification, the highest incidence-mortality rate was observed in Groups C and D. A similar incidence-mortality rate in Groups B and C was not observed in the present study, as has been reported by other authors.9,11 For the 2017 GOLD, although a statistically significant difference was observed across groups, similar to that noted with the other two GOLD classifications, the incidence-mortality rate across the 16 subgroups did not reveal any clear pattern. The incidence-mortality rate according to the GOLD 2017 A/B/C/D classification (based only on symptoms and exacerbation and not on spirometry) showed lower rates in Groups A and B than in Groups C and D.
It has been pointed out that the A/B/C/D assessment is not intended to predict mortality, and it is acknowledged that the 2013 classification (considering spirometry and exacerbation history for exacerbation risk) was not a better predictor of mortality than the spirometric grades.1 This was one of the reasons for eliminating the level of FEV1 from the GOLD 2017 classification (for guiding treatment), but a potential risk of the new A/B/C/D GOLD 2017 classification could be the use of expensive medications for false-positive COPD individuals (because of the recommended fixed ratio criteria), possibly in the presence of few symptoms (mMRC 0–1 and on the 0–1 exacerbation history). Perhaps a COPD diagnosis based on the lower limit of normal (LLN) values for FEV1/FVC26,27 could be a more specific diagnosis than that based on the fixed ratio criteria and could reduce potential errors in treatment. It has been recognized in the GOLD 2017 report that the fixed ratio criteria may result in more frequent diagnosis of COPD in the elderly and less frequent diagnosis among those aged <45 years,28,29 compared with the LLN, but also emphasizes the dependence of the LLN on the choice of valid reference equations using postbronchodilator FEV1 and the lack of longitudinal studies validating its use.1 In fact, long-acting bronchodilators are recommended for all the patients, except for those in Group A initially,1 despite being more expensive and unavailable in many countries as they are not included on the World Health Organization’s essential medication list.30,31
Two important learnings described in the new GOLD 2017 are, first, a model for the escalation or de-escalation of treatment (not just treatment initiation as described in earlier versions) and, second, the advice to be cautious on the use of inhaled corticosteroids due to the risk of pneumonia.
An important limitation of the present analysis is the small sample size in many of the 16 categories of the new GOLD assessment, as the PLATINO study was a population-based study with a few subjects in the most severe groups and therefore not representative of all COPD patients. However, these data do reflect a real-life population, not subject to selection criteria bias often observed in typical clinical studies. Many observational studies with additional evidence or pooled analysis will contribute to the better understanding of the 2017 GOLD version and for its validation and stability over time. Another limitation of this study was the reliance of patient recall for the accurate reporting of some types of data, including exacerbations episodes. An advantage of this analysis was the high follow-up rates in the three centers evaluated and the good standards of spirometry achieved.
Conclusion
The GOLD strategy documents have been very successful in raising the awareness of the burden of COPD worldwide, and GOLD committee members have been diligent in developing new approaches to aid and improve the diagnosis, grading, and management of COPD patients. The new classification (GOLD 2017) attempts to assess COPD patients more comprehensibly by separating the spirometric grades from the A/B/C/D groups and considering only the latter for pharmacological assessments. A disadvantage of this approach is that symptoms, exacerbations, and obstruction are not completely independent, with patients clustering according to various combinations of these factors. Another disadvantage of the new classification is that it demands very large sample sizes due to the number of subgroups; for epidemiological studies, this can be a challenge. The PLATINO data have not shown any clear patterns with respect to the distribution of patients or incidence-mortality rates in the new 2017 classification subgroups; hence, more research is required.
Supplementary material
Table S1.
Distribution of some characteristics according to imputation for the variable mMRC
| Variables | Walking slower than people of same age
|
Stop walking to breath
|
Stop walking after 100 m
|
Cannot change the clothes
|
||||
|---|---|---|---|---|---|---|---|---|
| Not imputed | Imputed | Not imputed | Imputed | Not imputed | Imputed | Not imputed | Imputed | |
|
|
|
|
|
|||||
| n=1,752 | n=1,318 | n=1,721 | n=1,349 | n=1,705 | n=1,365 | n=1,694 | n=1,376 | |
| Sex, male, n (%) | 866 (49.9) | 395 (30.0) | 856 (49.7) | 405 (30.0) | 851 (49.9) | 410 (30.0) | 849 (50.1) | 412 (29.9) |
| Age, years, mean (SD) | 56.9 (12.1) | 57.8 (12.0) | 56.8 (12.1) | 57.9 (12.0) | 56.8 (12.1) | 57.9 (12.0) | 56.8 (12.7) | 57.9 (12.1) |
| Schooling, years, mean (SD) | 8.2 (5.1) | 7.3 (4.5) | 8.1 (5.2) | 7.2 (4.4) | 8.2 (5.2) | 7.2 (4.4) | 8.3 (5.2) | 7.2 (4.4) |
| BMI, kg/m2, mean (SD) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) |
| FEV1, L, mean (SD) | 2.8 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) |
| FVC, L, mean (SD) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council; SD, standard deviation.
Acknowledgments
The authors wish to acknowledge the Asociación Latinoamericana de Torax (ALAT) for its support for the PLATINO study; the following individuals for their contributions in the data collection of the PLATINO study: Mariana Gazzotti, Adriana Muino, and Carnem Lisboa; and editorial support in the form of copyediting, which was provided by Kate Hollingworth of Continuous Improvement Ltd.
The present analysis was funded by GlaxoSmithKline (GSK). The PLATINO study has been sponsored by The ALAT, Boehringer Ingelheim GmbH, GSK, and Novartis for the collection of the data during the field work.
Footnotes
Disclosure
All the authors met the authorship criteria set forth by the International Committee for Medical Journal Editors. AMM received fees from GSK for the analysis and reporting of this study and from AstraZeneca for project analyses. KPV, CS, and HM are employees of GSK, and CS and HM hold GSK stocks. FCW, RP-P, GV, JRJ, and MMdeO report no conflicts of interest in this work.
References
- 1.Global Strategy for the Diagnosis, Management and prevention of Chronic, Obstructive Pulmonary Disease, 2017 Report. 2016. [Accessed February 27, 2017]. [cited November, 2016]. Available from: http://goldcopd.org/
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 4.Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13(3):291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 7.Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Molken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163. doi: 10.1186/1471-2466-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano JB, Alfageme I, Almagro P, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013;143(3):694–702. doi: 10.1378/chest.12-1053. [DOI] [PubMed] [Google Scholar]
- 9.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 10.Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42(3):636–646. doi: 10.1183/09031936.00195212. [DOI] [PubMed] [Google Scholar]
- 12.Montes de Oca M, López Varela MV, Laucho-Contreras ME, et al. Clasificación de los pacientes con enfermedad pulmonar obstructiva crónica según los sistemas de estadificación de la Asociación Latino-americana de Tórax (ALAT) y la iniciativa global para la enfermedad pulmonar obstructiva crónica (GOLD) [Classification of patients with chronic obstructive pulmonary disease according to the Latin American Thoracic Association (ALAT) staging systems and the global initiative for chronic obstructive pulmonary disease (GOLD)] Arch Bronconeu-mol. 2017;53(3):98–106. doi: 10.1016/j.arbres.2016.08.015. English, Spanish. [DOI] [PubMed] [Google Scholar]
- 13.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 14.Menezes AM, Victora CG, Perez-Padilla R, PLATINO Team The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15. doi: 10.1186/1471-2288-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes AM, Muino A, Lopez-Varela MV, et al. Estudio de cohorte de base poblacional sobre la enfermedad pulmonarobstructiva crónica en Latinoamérica: métodos y resultados preliminares. Fase II del estudio PLATINO [A population-based cohort study on chronic obstructive pulmonary disease in Latin America: methods and preliminary results. The PLATINO Study Phase II] Arch Bronconeumol. 2014;50(1):10–17. doi: 10.1016/j.arbres.2013.07.014. English, Spanish. [DOI] [PubMed] [Google Scholar]
- 16.The PLATINO Study [homepage on the Internet] Associacion Latino Americana del Tórax. 2011. [Accessed May 3, 2017]. Available from: www.platino-alat.org/datasets.html.
- 17.Ferris BG. Epidemiology standardization project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 18.European Community Respiratory Health Survey II Steering Committee The European Community Respiratory Health Survey II. Eur Respir J. 2002;20:1071–1079. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 19.BC Cancer Research Centre . Lung Health Study Questionnaire. Vancouver: BC Cancer Research Centre; 2004. [Google Scholar]
- 20.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. No authors listed. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Padilla R, Valdivia G, Muino A, et al. Valores de referencia espriométrica en 5 grandes ciudades de Latinoamérica para sujetos de 40 anos o más años de edad. [Spirometric reference values in 5 large Latin American cities for subjects aged 40 years or over] Arch Bronconeumol. 2006;42(7):317–325. doi: 10.1016/s1579-2129(06)60540-5. Spanish. [DOI] [PubMed] [Google Scholar]
- 22.Menezes AM, Perez-Padilla R, Wehrmeister FC, et al. FEV1 is a better predictor of mortality than FVC: the PLATINO cohort study. PLoS One. 2014;9(10):e109732. doi: 10.1371/journal.pone.0109732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Padilla R, Wehrmeister FC, Montes de Oca M, et al. Instability in the COPD diagnosis upon repeat testing vary with the definition of COPD. PLoS One. 2015;10(3):e0121832. doi: 10.1371/journal.pone.0121832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer WM, Gislason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34(3):588–597. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montes de Oca M, Talamo C, Halbert RJ, et al. Health status perception and airflow obstruction in five Latin American cities: the PLATINO study. Respir Med. 2009;103(9):1376–1382. doi: 10.1016/j.rmed.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD. 2006;3(2):95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 27.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13(1):41–48. doi: 10.1370/afm.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guder G, Brenner S, Angermann CE, et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir Res. 2012;13(1):13. doi: 10.1186/1465-9921-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. 2013. [Accessed February 27, 2017]. [cited November, 2016]. Available from: http://www.who.int/
- 31.World Health Organization Model List of Essential Medicines. [Accessed February 27, 2017]. [cited April 2015]. Available from: http://www.who.int/medicines/publications/essentialmedicines/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Distribution of some characteristics according to imputation for the variable mMRC
| Variables | Walking slower than people of same age
|
Stop walking to breath
|
Stop walking after 100 m
|
Cannot change the clothes
|
||||
|---|---|---|---|---|---|---|---|---|
| Not imputed | Imputed | Not imputed | Imputed | Not imputed | Imputed | Not imputed | Imputed | |
|
|
|
|
|
|||||
| n=1,752 | n=1,318 | n=1,721 | n=1,349 | n=1,705 | n=1,365 | n=1,694 | n=1,376 | |
| Sex, male, n (%) | 866 (49.9) | 395 (30.0) | 856 (49.7) | 405 (30.0) | 851 (49.9) | 410 (30.0) | 849 (50.1) | 412 (29.9) |
| Age, years, mean (SD) | 56.9 (12.1) | 57.8 (12.0) | 56.8 (12.1) | 57.9 (12.0) | 56.8 (12.1) | 57.9 (12.0) | 56.8 (12.7) | 57.9 (12.1) |
| Schooling, years, mean (SD) | 8.2 (5.1) | 7.3 (4.5) | 8.1 (5.2) | 7.2 (4.4) | 8.2 (5.2) | 7.2 (4.4) | 8.3 (5.2) | 7.2 (4.4) |
| BMI, kg/m2, mean (SD) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) | 27.1 (4.7) | 29.2 (6.0) |
| FEV1, L, mean (SD) | 2.8 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) | 2.9 (0.8) | 2.4 (0.7) |
| FVC, L, mean (SD) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) | 3.7 (1.0) | 3.2 (0.9) |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council; SD, standard deviation.