Abstract
The effects of CB1 antagonist/inverse agonists on the acquisition and consolidation of conditioned fear remain uncertain. Recent studies suggest that the CB1 antagonist/inverse agonist AM251 affects acquisition or consolidation of both contextual and discretely cued fear memories. AM251 is frequently referred to as a CB1 antagonist; however in vitro signal transduction assays indicate that this drug also elicits inverse agonist activity at CB1 receptors. The present studies were undertaken to compare the effects of AM251 on conditioned fear with those produced by AM4113, a novel CB1 antagonist with minimal inverse agonist activity. All drugs were administered prior to conditioning. In retention tests conducted two weeks after conditioning, both AM251 (4.0 mg/kg) and AM4113 (6.0 mg/kg)-treated animals exhibited reduced freezing during a conditioned tone cue played within a novel context. In contextual fear retention tests, animals previously treated with 4.0 or 8.0 mg/kg AM251 exhibited enhanced freezing. By contrast, no dose of AM4113 had any significant effect on contextual fear memory, which is consistent with the lower signal transduction activity of AM4113 at CB1 receptors compared to AM251. These results suggest that CB1 neutral antagonists may be less likely than CB1 inverse agonists to facilitate the acquisition or consolidation of contextual fear that may contribute to some clinical disorders.
Keywords: conditioned fear, CB1 antagonist, anxiety, rimonabant
Introduction
Drugs that interfere with cannabinoid CB1 transmission have been studied as potential treatments for obesity as well as other disorders. CB1 inverse agonists such as SR141716 (rimonabant) and AM251 have been shown to reduce food intake under a variety of conditions in animal models (Arnone et al. 1997; Colombo et al. 1998; Williams and Kirkham 1999; Wiley et al. 2005; McLaughlin et al. 2003, 2005; Gardner and Mallet 2006; Salamone et al. 2007). Clinical trials with the CB1 inverse agonists rimonabant and taranabant demonstrated that these drug were effective at reducing body weight in humans (Curioni and Andre, 2006; Despres et al., 2005; Pi-Sunyer et al., 2006; Van Gaal et al., 2005; Addy et al., 2008). However, the high incidence of adverse emotional effects observed in clinical trials with these drugs has caused researchers to question their clinical usefulness (Le Foll et al. 2009). For example, CB1 inverse agonists have been shown to increase the incidence of nausea, anxiety and depression (Pi-Sunyer et al., 2006; Van Gaal et al., 2005; US Food and Drug Administration Advisory Committee, 2007; Addy et al., 2008). In view of these problems with CB1 inverse agonists, recent studies have begun to focus on the effects of CB1 receptor neutral antagonists such as AM4113, which is a pyrazole-3-carboxamide analog of rimonabant. (Salamone et al. 2007; Le Foll et al. 2009). In contrast to the inverse agonists AM251 and rimonabant, AM4113 demonstrated no significant inverse agonism at CB1 receptors as assessed with in vitro cAMP accumulation assays (Chambers et al. 2007; Sink et al. 2008a). Although AM4113 is able to suppress food intake and food-reinforced behavior, it does not induce nausea or malaise at comparable doses (Chambers et al. 2007; Sink et al. 2008a,b; Sink et al. 2009a). Furthermore, we recently found that, unlike AM251, AM4113 did not appear to evoke a pattern of anxiety-like behaviors in the elevated plus maze, a rodent anxiety test (Sink et al. 2010). Also, a study of c-Fos immunoreactivity showed that AM4113 induced less neural activation than AM251 in a number of brain structures, including the amygdala, a structure that is important for anxiety as well as fear conditioning (Sink et al. 2010).
In addition to studies of anxiety, it is also important to investigate how these compounds may affect the acquisition of conditioned fear. Classical fear conditioning in animals is thought to share many similarities with the acquisition and expression of memory-associated fears that characterize post-traumatic stress disorder (PTSD) and phobias in humans (Mineka and Oehlberg, 2008). Thus, a more thorough understanding of how CB1 antagonists and inverse agonists influence the acquisition of fear conditioning has important implications for understanding the clinical significance of these compounds. Numerous studies have implicated CB1 signaling in behaviors related to conditioned fear. Several brain areas important for fear conditioning (Barad et al. 2006; LeDoux, 2000; Rodrigues et al. 2009) including prefrontal cortex, hippocampus and basolateral amygdala, express a moderate to high density of CB1 receptor protein (Chhatwal et al. 2008; Herkenham et al. 1991; Katona et al. 2001; McDonald and Mascagni 2001). Both enhancement of CB1 transmission (Chhatwal et al. 2005; Mikics et al. 2006; Pamplona et al. 2008), and administration of CB1 antagonist/inverse agonists SR141716 (N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichloroph nyl)-4-methyl-1H-pyrazole-3-carboxamide; rimonabant) and AM251 (N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophen yl)-4-methyl-1H-pyrazole-3-carboxamide; Arenos et al. 2006; Chhatwal et al. 2005; Finn et al. 2004; Marsicano et al. 2002; Mikics et al. 2006; Reich et al. 2008; Roche et al. 2007; Suzuki et al. 2004) can affect certain aspects of both cued and contextual classically conditioned fear. Particularly, modification of CB1 signaling consistently produces changes in fear extinction when given just prior to extinction training (Chhatwal et al. 2005; Chhatwal et al. 2008; Marsicano et al. 2002; Niyuhire et al. 2007; Pamplona et al. 2008). However, pre-conditioning administration of CB1 antagonist/inverse agonists has produced equivocal effects on conditioned fear. One study showed no effect of a CB1 antagonist/inverse agonist on fear conditioning in mice (Marsicano et al. 2002), but did report an effect of CB1 receptor knockout and injection of rimonabant on extinction of fear conditioning. Arenos et al. (2006) observed that CB1 antagonism impaired the expression of conditioned fear (Arenos et al. 2006). In contrast, Reich et al. (2008) showed that AM251 enhanced acquisition of freezing for both trace and delay forms of tone-cued fear conditioning.
In the present paper, we examined the effects of a CB1 antagonist (AM4113) and an inverse agonist (AM251) given prior to training on acquisition of classically conditioned fear, employing a two-week period between conditioning and testing. This interval, which is substantially longer than the typical one to four day period used in similar studies, was chosen because the longer delay between conditioning and test might make the procedure more sensitive to any differences in the strength of the associations, as stronger associations tend to take longer to forget (Annau and Kamin, 1961). In view of the differential effects of AM4113 and AM251 on anxiety-related behavior and neural activation (Sink et al. 2010), we hypothesized that the CB1 antagonist/inverse agonist, AM251, given prior to conditioning, would produce stronger effects on contextual or discretely cued fear memory than the neutral CB1 antagonist, AM4113. The same doses of AM251 and AM4113 that were used in the previous study of anxiety-related behavioral also were used in the present study.
Methods
Subjects
A total of 95 animals were used for these experiments. Adult male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) were pair-housed in a colony maintained at 23°C, with a 12-h light/dark cycle (lights on 07:00). Food and water was available ad libitum in the home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee, and the studies were conducted according to NIH guidelines for animal care and use.
Drugs
AM251 and AM4113 (synthesized at the Center for Drug Discovery, Northeastern University) were dissolved in a vehicle of dimethylsulfoxide (DMSO; Fisher, Waltham, MA, USA), Tween-80 (Fisher), and 0.9% saline in a 1:1:8 ratio. This mixture also served as vehicle for these experiments. Doses and pretreatment times for AM251 and AM4113 were chosen based upon previous research demonstrating these doses to be efficacious for suppression of food intake (McLaughlin et al. 2003; Sink et al. 2008a, 2008b, 2009a). Both drugs were administered IP in a volume of 1.0 mL/kg 30 min prior to conditioning.
Locomotor assessment
For assessment of locomotion, rats were placed in small activity chambers (28 × 28 × 28 cm) inside soundproof shells. These were different chambers than the fear conditioning boxes described below. The floor of each chamber consisted of two wire mesh panels (27 × 13 cm) connected through the center by a metal rod, which serves as a fulcrum for the floor panels. Locomotion by the subjects produced a slight deflection of one or more floor panels, which closed one or more of four microswitches mounted on the exterior of the chamber. Microswitch closure sent a signal to an external computer running a custom program, by means of an interface (Med Associates). Each microswitch closure was processed as a single activity count.
Fear Conditioning Procedures
Within the two weeks prior to conditioning, each animal received four adaptation sessions, which consisted of placing the rat in a novel chamber within a novel room for 5 min. The last of these adaptations took place in the locomotion assessment chambers described above during a 5-min baseline activity assessment session. Rats also received habituation injections of 0.3 mL 0.9% saline for four days prior to conditioning. On the day of conditioning, each rat was injected with drug and 30 min later carried by hand and placed in the shock chamber (28 × 21 × 21 cm, Med Associates, East Fairfield, VT) for a 95-s exploratory period. This period was followed by four tone-shock pairings. These pairings consisted of 35-second 70 dB tones co-terminating with 2-second 0.4 mA shocks generated by a scrambler (Lafayette Instruments, Lafayette, IN). The tone-shock pairings were interleaved with 95-second inter-tone intervals. The conditioning chamber was cleaned with dilute PineSol (The Clorox Co.; 1% solution, sprayed on the walls of the chamber with a spray bottle after which it was wiped off with a dry paper towel) between rats. The rats were given four more adaptation sessions as described above in the 14 days between conditioning and testing sessions. For context retention testing each animal was carried by hand into the room in which conditioning occurred and placed in the conditioning chamber for 95 seconds. The rat received neither conditioned tone nor shock during this test session. The next day, the rat was subject to an additional adaptation session as described above. The following day, each rat was carried into a novel test room inside a plastic mouse cage and placed in a novel chamber containing aspen shavings and smelling of a different odor (isopropyl alcohol; 30% solution of alcohol was sprayed on the walls of the chamber) than the conditioned context. After 95 seconds had elapsed, the conditioned tone sounded for 95 seconds. As with context testing, no shock was delivered at any time during the session. The novel chamber was cleaned with isopropyl alcohol and the shavings changed between rats. The same experimenter handled the rats during all conditioning and retention sessions. Conditioning and test sessions were observed from a video monitor in an adjacent room. The amount of time spent freezing, defined as the absence of movement, was determined by deducting the time in motion as detected using microwave activity monitors (RadioShack) and recorded on a PC running DOS (Oler and Markus, 1998).
Experiments
For each experiment, rats were randomly assigned to one of the drug treatment conditions, and between-subjects designs were used. In experiment 1, rats received IP injections of either vehicle (n=10), 2.0 mg/kg AM251 (n=14), 4.0 mg/kg AM251 (n=11), or 8.0 mg/kg AM251 (n=13). In experiment 2, rats received IP injections of either vehicle (n=9), 3.0 mg/kg AM4113 (n=13), 6.0 mg/kg AM4113 (n=14), or 12.0 mg/kg AM4113 (n=11).
Data Analysis
To insure that baseline activity was similar across dose treatment groups, pre-injection locomotor activity was examined using one-way ANOVA with dose as the independent variable. For freezing data, the first five seconds of each test session and the five seconds following onset of the tone cue were removed prior to data analysis. The first five seconds of data for each segment (context, novel context, and tone) were dropped to eliminate motion data unrelated to the fear response such as movement or postural changes in response to placement in the chamber or receiving the shock. This methodology was based upon previous studies (e.g. Ward et al. 1999). The percentage of time in motion for each test period was calculated and then subtracted from 100 in order to determine the percent of time the animal spent motionless (freezing). To assess the change in the percent time freezing over the course of the four tone-cued intervals during conditioning session, a dose by tone interval factorial ANOVA with repeated measures on the tone interval was performed for each experiment. For analysis of retention test data, separate one-way ANOVAs were used to analyze the effect of dose on percent time spent freezing within the conditioned context, within the novel context, and in response to the conditioned tone. If the overall ANOVA term was significant, Tukey post-hoc comparisons were employed to compare each dose with vehicle. If, however, the overall ANOVA was not significant, a linear regression analysis with dose as the independent variable was calculated. In order to determine the specificity of the freezing response to contextual and tone-cued conditioned fear, paired t-tests compared freezing data obtained from the vehicle-treated animals between the novel context and the conditioned context and between novel context and conditioned tone cue test.
Results
Pre-treatment locomotor activity
For each of the three experiments, one-way ANOVA analyzing locomotor activity prior to treatment showed no significant differences among the treatment groups (data not shown).
Fear conditioning
Experiment 1: AM251
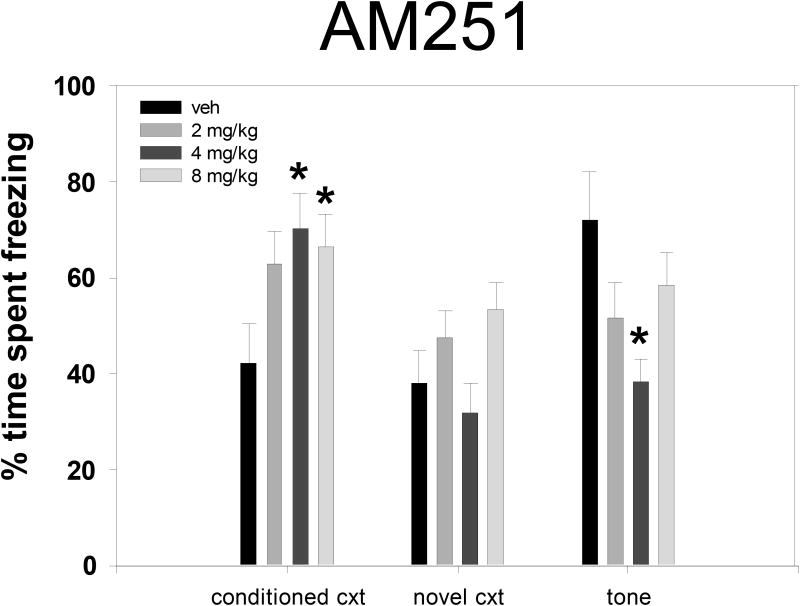
Repeated measures ANOVA showed that freezing for all dose groups increased significantly over the course of the four tone-shock pairings during the conditioning session [F(3,132) = 269.6, p < 0.001]. Tukey post-hoc comparisons did not reveal any significant differences among the treatment groups during the final tone cue of the conditioning session (see Table I). Figure 1 shows freezing levels during the fear retention test sessions that took place two weeks after conditioning. Vehicle-treated animals were not significantly different between conditioned context and novel context tests (42.2 +/- 17.0% vs. 38.1 +/-9.6%, p = 0.62, n.s.); however, these vehicle-treated animals froze significantly more in response to the conditioned tone played within the novel context compared to the novel context alone (72.1 +/- 20.0% vs. 38.1 +/- 9.6%, p < 0.001). One-way ANOVAs comparing all dose treatments and vehicle revealed that AM251 given prior to conditioning produced overall dose-related effects on freezing during the conditioned context test [F(3,44) = 4.022, p = 0.013] and the conditioned tone test [F(3,44) = 3.308, p = 0.029], but there were no significant differences in freezing during the novel context test [F(3,44) = 2.379, p = 0.56, n.s.]. Tukey post-hoc analysis showed that both 4.0 and 8.0 mg/kg AM251 increased contextual freezing (p = 0.01, p = 0.04, respectively), and 4.0 mg/kg AM251 significantly suppressed freezing during a conditioned tone cue compared to vehicle controls (p = 0.02).
Table I. Percent time spent freezing during four consecutive tone periods of conditioning session.
| Dose AM251 | Tone 1 | Tone 2 | Tone 3 | Tone 4 |
|---|---|---|---|---|
| Vehicle | 4.1 +/- 1.0 | 83.9 +/- 4.4 | 92.7 +/- 2.5 | 93.6 +/- 2.8b |
| 2.0 mg/kg | 15.6 +/- 5.1 | 61.2 +/- 7.8 | 93.2 +/- 1.5 | 84.9 +/- 5.0b |
| 4.0 mg/kg | 9.9 +/- 4.0 | 66.7 +/- 6.8 | 91.4 +/- 3.5 | 91.8 +/- 3.0b |
| 8.0 mg/kg | 10.9 +/-3.4 | 57.5 +/- 6.3a | 84.9 +/- 5.0 | 77.4 +/- 5.4b |
significantly different from vehicle within the same tone period (p<0.05)
significantly different from freezing level during 1st tone period (p<0.05)
Figure 1.
Effects of AM251 in tests of conditioned fear: percent of time spent motionless during retention test sessions in the conditioned context, in a novel context, or in the novel context during the sounding of the conditioned tone. * Significantly different from vehicle within a single epoch at p < 0.05 as revealed by Tukey post-hoc comparisons.
Experiment 2: AM4113
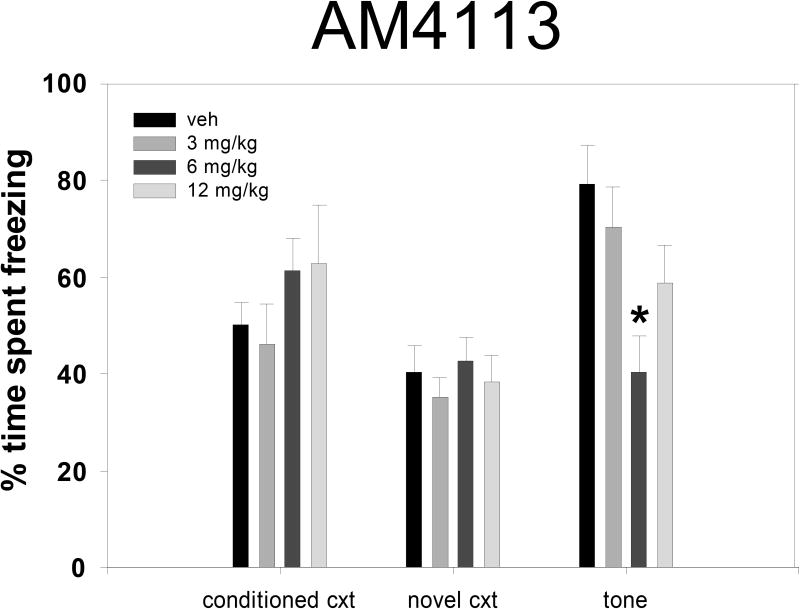
Similar to results from experiment 1, repeated measures ANOVA showed that freezing for all dose groups increased significantly over the course of the four tone-shock pairings during the conditioning session [F(3,129) = 203.2, p < 0.001]. Tukey post-hoc comparisons did not reveal any significant differences among the treatment groups during the final tone cue of the conditioning session (see Table II). During the fear retention test sessions that took place two weeks after conditioning (figure 2), freezing levels from vehicle-treated animals were not significantly different between conditioned context and novel context tests (50.1 +/- 9.6% vs. 40.4 +/- 10.6%, p = 0.15, n.s.); however, as in the AM251 experiment, these vehicle-treated animals froze significantly more in response to the conditioned tone played within the novel context compared to the novel context alone (40.4 +/- 10.6% vs. 79.1 +/- 16.2%, p <0.001). Overall ANOVA terms indicated that preconditioning treatment with AM4113 did not significantly affect freezing in fear retention tests conducted within the conditioned context or the novel context, and regression analyses also showed that AM4113 did not produce any dose-related trends with these measures. However, AM4113 did produce a significant overall treatment effect on freezing during the conditioned tone cue test [F(3,43) = 4.072, p = 0.012]. Post hoc analysis comparing each dose of AM4113 with vehicle showed that 6.0 mg/kg significantly suppressed freezing during the conditioned tone (p = 0.015).
Table II. Percent time spent freezing during four consecutive tone periods of conditioning session.
| Dose AM4113 | Tone 1 | Tone 2 | Tone 3 | Tone 4 |
|---|---|---|---|---|
| Vehicle | 2.3 +/- 6.5 | 76.0 +/- 6.0 | 80.0 +/- 8.8 | 90.1 +/- 3.6b |
| 3.0 mg/kg | 21.5 +/- 5.6a | 77.3 +/- 6.1 | 90.9 +/- 3.1 | 92.0 +/- 2.8b |
| 6.0 mg/kg | 13.0 +/- 2.3a | 69.3 +/- 5.7 | 88.6 +/- 4.0 | 84.8 +/- 3.5b |
| 12.0 mg/kg | 33.2 +/- 9.8a | 75.7 +/- 6.5 | 95.9 +/- 2.0 | 94.1 +/- 1.3b |
significantly different from vehicle within the same tone period (p<0.05)
significantly different from freezing level during 1st tone period (p<0.05)
Figure 2.
Effects of AM4113 in tests of conditioned fear: percent of time spent motionless during retention test sessions in the conditioned context, in a novel context, or in the novel context during the sounding of the conditioned tone. * Significantly different from vehicle within a single epoch at p < 0.05 as revealed by Tukey post-hoc comparisons.
Discussion
This set of experiments was designed to compare the effects of the CB1 inverse agonist AM251 and the CB1 antagonist AM4113 on the retention of classically conditioned fear memory. Animals were treated with AM251, AM4113, or vehicle 30 min prior to conditioning. Two weeks later, the amount of freezing was measured in the conditioned context, in a novel context, and in response to the conditioned tone while in the novel context. The use of a two week period between conditioning and testing was intended to make the procedure more sensitive to any differences in the strength of the associations, as stronger associations tend to take longer to forget (Annau and Kamin, 1961).
Rats treated with the two highest doses of AM251 (4.0 and 8.0 mg/kg) prior to conditioning exhibited increased freezing in the familiar context retention test. However, neither one-way ANOVA nor regression analysis revealed any significant effects of AM4113 in the conditioned context retention test. These data are in agreement with previous studies comparing the effects of AM251 and AM4113. Although AM251 significantly enhanced conditioned gaping (McLaughlin et al. 2005), which is a marker of nausea in rats, AM4113 did not (Sink et al. 2008a). Doses of AM251 that suppress feeding also were shown to produce anxiogenic effects in the elevated plus maze, while AM4113 failed to do so (Sink et al. 2010). Furthermore, AM4113 produced a weaker pattern of c-Fos activation in striatal and limbic areas compared to AM251 (Sink et al. 2010). Thus, across several behavioral and neural markers, the CB1 antagonist AM4113 often has produced weaker effects than the CB1 inverse agonist AM251, which is consistent with reports demonstrating that AM4113 produced little or no intrinsic biological activity at CB1 receptors compared to AM251 (Chambers et al. 2007; Sink et al. 2008a). Nevertheless, unlike the dissimilar effects these drugs produced in tests of contextual conditioning, both the CB1 antagonist and the inverse agonist suppressed the freezing response to the conditioned tone cue. For both drugs, the effects on tone retention occurred within a narrow dose range, and were only significant at the middle dose, which suggests that both drugs had biphasic dose/response curves. The effective dose of AM4113 was slightly higher than that for AM251 (4.0 mg/kg AM251 vs. 6.0 mg/kg AM4113), which is consistent with previous studies of food-related behaviors (McLaughlin et al. 2003; Sink et al. 2008a). Furthermore, the fact that AM4113 was effective in the conditioned tone test indicates that there is greater endogenous cannabinoid tone in regions specifically involved in fear conditioning to discrete auditory cues, which would allow a neutral antagonist to exert behavioral effects by blocking this endogenous tone.
AM251 enhanced freezing during the contextual retention test, but decreased freezing to the conditioned tone, which suggests the opposite influences upon these two different aspects of fear memory. Thus, despite the fact that AM251 can produce anxiogenic effects in the elevated plus maze (Sink et al. 2010), it does not appear that this drug was altering fear conditioning by producing a general aversive effect; instead, it appeared to exert dissociable actions on distinct aspects of fear conditioning. It could be argued that these differential effects on contextual and discretely cued fear indicate that endocannabinoids may have different effects in the brain systems mediating contextual vs. tone-cued conditioned fear. Although the hippocampus is thought to be primarily involved in contextual fear, the amygdala appears to be important for both tone-cued and contextual fear, as is evident from the fact that lesions of the amygdala impair both discretely cued and contextual fear (Phillips and LeDoux, 1992). The partial overlap in the brain circuits involved in tone-cued and contextually conditioned fear could make it difficult to explain how cannabinoid actions within different structures could produce this particular pattern of effects, i.e., CB1 inverse agonist-induced decrease in tone-cued fear but increase in contextual fear. Nevertheless, it also is true that lesions are relatively nonspecific in terms of their effects on particular brain area, whereas drugs act on specific receptor mechanisms that are located on particular populations of cells. CB1 receptors that are located on presynaptic terminals generally act to modulate release of whatever transmitter is used by those terminals (Piomelli, 2003); thus, AM251 could be having differential effects on contextual vs. tone-cued conditioned fear because it is modulating the release of different neurotransmitters within multiple brain areas, which differentially mediate these two distinct behavioral processes. Some evidence from local infusion studies appears to support this hypothesis. AM251 administered into the basolateral amygdala immediately after conditioning impaired contextually conditioned fear memory (Bucherelli et al. 2006) and inhibitory avoidance learning (Campolongo et al. 2009). In contrast, an intra-hippocampal dose of CB1 antagonist/inverse agonist (SR141716) given prior to training enhanced spatial learning in the Morris water maze (Robinson et al. 2008), a result that is in accord with the enhanced contextual freezing in present experiments and the enhanced spatial memory observed in many studies of systemic CB1 antagonist/inverse agonist effects (Lichtman, 2000; Takahashi et al. 2005; Wise et al. 2008, but see also de Oliviera Alvares et al. 2008). Finally, one might wonder whether the order of testing could have influenced the results of the second (tone-cued) retention test to produce the dissimilar outcomes between retention tests for contextual and discretely cued conditioned fear. In fact, this second test session was conducted in a novel context very different from the conditioned context in order to minimize the influence of contextual fear and extinction effects. As expected, all treatment groups exhibited higher levels of freezing in response to the tone in the second test compared to the conditioned context in the first test. Also, the fact that the treatment effect in the second test was specific to the conditioned cue, in that no treatment group differed from vehicle during the pre-tone period, suggests that the enhanced tone-cued freezing observed in AM4113 and AM251 treatment groups was not a result of general fear sensitization. It therefore appears unlikely that either extinction or sensitization due to test order can account for the differential pattern of effects observed between tests of contextual and discretely cued fear.
In all experiments, freezing significantly increased over the course of the conditioning session. AM4113 also produced a small but significant increase in freezing during the initial tone-shock presentation, which could reflect an action on pain or locomotor mechanisms. Nevertheless, during the last tone cue all dose groups were freezing at similar levels that were not significantly different from vehicle groups. These results indicate that all treatment groups in all experiments acquired a comparably strong fear response during conditioning. Vehicle-treated rats exhibited similar levels of freezing in the conditioned context compared to a novel context, despite several measures employed to prevent generalization. This indicates that the animals were not discriminating between the conditioned context and the novel context, and in fact were generalizing. Such generalization in retention testing is reported to be more likely to occur when the conditioning procedure produces very strong fear associations (Baldi et al. 2004). These observations suggest that very strong fear associations were produced in the present experiments. Nevertheless, despite the contextual generalization observed in vehicle-treated animals, AM251 increased freezing in the conditioned context retention, but not in the novel context test. This finding indicates that the effects observed in retention tests were indeed the result of pre-conditioning drug influence on fear conditioning processes and not enduring motor deficits or other more general impairments produced by the drug treatments.
The present study was designed to investigate the effects of CB1 antagonists and inverse agonists on retention of fear conditioning, under conditions in which both drugs were administered prior to conditioning and a long retention interval was used. Admittedly, administration prior to conditioning does not allow discrimination of drug effects on acquisition versus consolidation of fear memory, since the influence of AM4113 and AM251 continues for a considerable time after the 8.7-minute conditioning session (McLaughlin et al. 2003; Sink et al. 2009b). Thus, the observed effects may be the result of influence on acquisition, consolidation, or some combination of these processes. Findings from other studies of fear conditioning using various CB1 antagonists suggest that both acquisition and consolidation-related processes may contribute to the changes in fear memory retention produced by AM251 and AM4113 (Arenos et al. 2006; Bucherelli et al. 2006; Reich et al. 2008).
It should be noted that the present results obtained with AM251 on discretely cued conditioned fear are apparently discordant with those of a similar study conducted by Marsicano et al. (2002). In that experiment, 3.0 mg/kg of the CB1 antagonist/inverse agonist SR141716, administered prior to conditioning, had no effect on expression of tone-cued fear during the initial re-exposure to the tone 24 hours after conditioning, but did impair extinction. By contrast, we found that both AM251 and AM4113 suppressed conditioned fear of a discrete tone cue. It is possible that differences in experimental parameters such as species (mice vs. rats), specific drugs used, or retention interval (1 vs. 14 days) may have contributed to the disparities between these studies. Interestingly, however, Marsicano et al. (2002) also found that re-exposure to the tone cue 24 hours after conditioning produced elevated levels of endocannabinoids within the basolateral amygdala, a structure implicated in acquisition as well as extinction of conditioned fear (LeDoux, 2000; Phelps and LeDoux, 2005). This finding suggests that drugs blocking CB1 receptors within the basolateral amygdala could hypothetically affect the strength of fear acquisition, as was found in the present studies. With this in mind, the contrast between the present results and those of Marsicano et al. (2002) may reflect differences in the susceptibility of fear acquisition and extinction processes to perturbations in CB1 signaling.
In conclusion, these results seem to indicate a complex influence of CB1 inverse agonists and antagonists on defensive responses to conditioned fear. The different effects of AM251 on contextual versus tone-cued freezing during retention tests imply a multifaceted influence of the CB1 system on different forms of associative fear. Complementary studies using different measures of conditioned fear, such as fear-potentiated startle or fear-conditioned analgesia, could further clarify CB1 antagonist and inverse agonist effects on conditioned fear acquisition and consolidation. While it is not clear why AM251 produced opposite effects in contextual and tone-cued fear memory tests, it does seem apparent that the CB1 antagonist AM4113 produced a weaker effect than AM251 in tests of contextual fear, which may correspond to the low signal transduction activity of AM4113 at CB1 receptors compared to AM251. These results suggest that CB1 neutral antagonists may be less likely to facilitate acquisition of contextual fears that can contribute to stress-related disorders. Since some effects of cannabinergic drugs are known to habituate or sensitize after a period of chronic administration, future studies should investigate the influence of chronic CB1 antagonist administration on emotion-related behaviors including conditioned fear.
Acknowledgments
This research was supported by grants to JDS and AM from the United States NIH/NIDA. Many thanks to James Chrobak and David Walker for their valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addy C, Rothenberg P, Li S, Majumdar A, Agrawal N, Li H, Zhong L, Yuan J, Maes A, Dunbar S, Cote J, Rosko K, Van Dyck K, De Lepeleire I, de Hoon J, Van Hecken A, Depré M, Knops A, Gottesdiener K, Stoch A, Wagner J. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, in healthy male volunteers. J Clin Pharmacol. 2008;48:734–44. doi: 10.1177/0091270008317591. [DOI] [PubMed] [Google Scholar]
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–32. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Arenos JD, Musty RE, Bucci DJ. Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur J Pharmacol. 2006;539:177–83. doi: 10.1016/j.ejphar.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81:162–6. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–8. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn Mem. 2006;13:426–30. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–93. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–24. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2008;34:509–21. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–7. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira Alvares L, Genro BP, Diehl F, Quillfeldt JA. Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem. 2008;90:1–9. doi: 10.1016/j.nlm.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L Rimonabant in Obesity-Lipids Study Group, 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Richardson D, Kendall DA, Marsden CA, Chapman V. Evidence for differential modulation of conditioned aversion and fear-conditioned analgesia by CB1 receptors. Eur J Neurosci. 2004;20:848–52. doi: 10.1111/j.1460-9568.2004.03509.x. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–6. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–4. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–9. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–52. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–8. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Mikics E, Dombi T, Barsvari B, Varga B, Ledent C, Freund TF, Haller J. The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav Pharmacol. 2006;17:223–30. doi: 10.1097/00008877-200605000-00003. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–80. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 2007;191:223–31. doi: 10.1007/s00213-006-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus. 1998;8:402–15. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–3. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group, 2006 Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-north america: A randomized controlled trial. JAMA. 2006;295:761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Reich C, Mohammadi M, Alger B. Endocannabinoid modulation of fear responses: Learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–77. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, McKillop-Smith S, Ross NL, Pertwee RG, Hampson RE, Platt B, Riedel G. Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 2008;198:551–63. doi: 10.1007/s00213-007-1012-8. [DOI] [PubMed] [Google Scholar]
- Roche M, O'Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci. 2007;26:2643–53. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Ledoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–8. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, et al. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008a;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008b;196:565–74. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: Effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009a;91:303–6. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Nunes EJ, Collins LE, Vemuri VK, Thakur G, Makriyannis A, Salamone JD. Intracerebroventricular administration of cannabinoid CB1 receptor antagonists AM251 and AM4113 fails to alter food-reinforced behavior in rats. Psychopharmacology (Berl) 2009b doi: 10.1007/s00213-009-1602-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Collins LE, Correa M, Vemuri VK, Thakur G, Makriyannis A, Salamone JD. Potential anxiogenic effects of cannabinoid CB1 receptor antagonist/inverse agonists in rats: comparisons between AM251, AM4113, and the benzodiazepine inverse agonist, FG-7142. Eur Neuropsychopharmacol. 2010;20:112–22. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–95. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–5. doi: 10.1016/j.neulet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Advisory Committee. Rockville: 2007. [accessed September 2008]. FDA briefing document: zimulti (rimonabant) Tablets, 20 mg, FDA. WWW document. URL ( http://www.fda.gov/OHRMS/DOCKETS/AC/07/briefing/2007-4306b1-00-index.htm) [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, RIO-Europe Study Group, 2005 Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Ward MT, Oler JA, Markus EJ. Hippocampal dysfunction during aging I: Aged rats do not show retrograde amnesia of contextual fear conditioning. Neurobiology of Aging. 1999;20:363–372. doi: 10.1016/s0197-4580(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–7. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Wise LE, Iredale PA, Lichtman AH. The cannabinoid CB(1) receptor antagonist CE prolongs spatial memory duration in a rat delayed radial arm maze memory task. Eur J Pharmacol. 2008;590:246–9. doi: 10.1016/j.ejphar.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]