Abstract
Since Emil Kraepelin’s conceptualization of endogenous psychoses as dementia praecox and manic depression, the separation between primary psychotic disorders and primary affective disorders has been much debated. We conducted a systematic review of case–control studies contrasting magnetic resonance imaging studies in schizophrenia and bipolar disorder. A literature search in PubMed of studies published between January 2005 and December 2016 was conducted, and 50 structural, 29 functional, 7 magnetic resonance spectroscopy, and 8 combined imaging and genetic studies were deemed eligible for systematic review. Structural neuroimaging studies suggest white matter integrity deficits that are consistent across the illnesses, while gray matter reductions appear more widespread in schizophrenia compared to bipolar disorder. Spectroscopy studies in cortical gray matter report evidence of decreased neuronal integrity in both disorders. Functional neuroimaging studies typically report similar functional architecture of brain networks in healthy controls and patients across the psychosis spectrum, but find differential extent of alterations in task related activation and resting state connectivity between illnesses. The very limited imaging-genetic literature suggests a relationship between psychosis risk genes and brain structure, and possible gene by diagnosis interaction effects on functional imaging markers. While the existing literature suggests some shared and some distinct neural markers in schizophrenia and bipolar disorder, it will be imperative to conduct large, well designed, multi-modal neuroimaging studies in medication-naïve first episode patients that will be followed longitudinally over the course of their illness in an effort to advance our understanding of disease mechanisms.
Introduction
Since Emil Kraepelin’s conceptualization of endogenous psychoses as two categories, dementia praecox and manic depression, the separation between primary psychotic disorders and primary affective disorders has been much debated.1–4 There is substantial evidence that these illnesses share genetic risk factors and overlap in clinical presentation and treatments, suggesting these clinical phenotypes to be on a disease continuum or even differential expressions of the same illness.5 Contemporary models attempt to move towards classification of psychiatric disorders based on etiologic and pathophysiologic processes, integrating complex relationships between genetics, physiology, and behavior.6, 7
Since the initial description of the principles of magnetic resonance imaging (MRI) by Paul Lauterbur in 19738 and the first human MRI scan in 1977,9 much progress has been made in the field of neuroimaging. A variety of contemporary non-invasive sequences are now available to aid structural, functional, and neurochemical characterization of the human brain, providing new opportunities to shed light on biological and pathological processes in vivo.10
Conventional, T1-weighted, structural MRI images provide static anatomical information with excellent detail and strong gray/white matter contrast. Manual tracing methods examine brain morphology of pre-defined regions of interest (ROI) such as the hippocampus, but are quite labor intensive. Computational advances offer semi-automated and automated alternatives to manual tracings. One of the most popular automated techniques is voxel-based morphometry (VBM11), which quantifies brain structure at the voxel level, with a typical resolution of 1 mm3. Another frequently used automated structural imaging analysis technique is Free Surfer,12 which also examines cortical thickness, cortical gyrification, and shapes of sub-cortical structures. Diffusion weighted imaging techniques, such as diffusion tensor imaging (DTI), map three dimensional motion of water as a function of spatial location to describe anatomy of anisotropic tissues such as white mater.13 The most widely reported diffusion tensor measures are fractional anisotropy (FA) and mean diffusivity (MD) which describe complementary information on structural white matter integrity. Tract based spatial statistics (TBSS) is an automated method describing diffusion metrics from white matter in the entire brain,14 whereas fiber tracking quantitatively assess the microstructure of a specific white matter tract.15 Fiber reconstruction methods are either deterministic or probabilistic; deterministic methods follow the primary eigenvector from voxel to voxel in three dimensions, whereas probabilistic methods incorporate expected uncertainty into the algorithm.16
Functional MRI (fMRI) imaging provides dynamic physiological information by measuring the blood oxygen level dependent (BOLD) signal.17 Task fMRI characterizes brain activity while subjects perform cognitive tasks by measuring changes in the BOLD signal in different areas of the brain. In block designs different conditions are alternated in blocks, where the condition assessing the cognitive process of interest is alternated with a control condition, which makes in the change in the fMRI signal in response to the stimulus additive.18 Block designs offer considerable statistical power, but are prone to signal drift and run the risk of having subjects become aware of the order of events. On the other hand, event related designs are more reflective of the real world, but come at the expense of statistical power.19 Alternatively, resting state fMRI measures the temporal covariance of low frequency fluctuations of the BOLD signal across spatially disparate areas while no explicit task is performed, in an effort to assess the brain’s intrinsic functional organization.20 Seed based analysis is a popular hypothesis-driven method to visualize resting state networks.21 In this case, an area of interest is defined from which correlations in BOLD fluctuations with all other voxels in the brain are calculated, allowing to examine connectivity of a specific ROI. In contrast, independent component analysis (ICA) is a data-driven method that decomposes the multivariate signal across the brain into statistically independent components (either spatially or temporally) reflecting resting state networks.22, 23
Magnetic resonance spectroscopy (MRS) measures chemical composition of tissues, energy metabolism, and neurotransmitter levels in vivo.24 The most common type of spectroscopy leverages the signal produced by protons located in the molecules of living tissue to quantify different metabolites (1H-MRS). Metabolites that can be measured include N-acetyl-aspartate (NAA), a putative marker of neuronal integrity, choline (Cho), a marker of cellular turnover and cell membrane breakdown, creatine (Cr), a signal that is related to phosphate metabolism, and the amino acids glutamate (Glu), glutamine (Gln), often expressed as Glx (glutamate + glutamine), and ɣ-amino-butyric acid (GABA).25 Other spectroscopy techniques include 31Phosphorus spectroscopy, which provides a wide range of information on energy metabolism, and 13Carbon spectroscopy which use cerebral glucose metabolism to assess glutamine synthesis and glutamatergic transmission.
These complementary imaging techniques have been applied to better delineate the neurobiology of psychiatric disorders in vivo. Structural gray and white matter deficits, as well as neurometabolite alterations, and BOLD signal abnormalities during task and at rest have been found in schizophrenia and bipolar disorder (BD). However, it remains unclear to what extent neural signatures are converging or distinct.
Here, we conducted a systematic review of case–control studies contrasting structural, functional, and neurochemical abnormalities in BD, schizoaffective disorder, schizophrenia, and healthy controls conducted in the past 10 years with the objective to summarize progress made in the quest to better delineate pathophysiological patterns across the psychosis spectrum. Where relevant, selected older publications considered key developments within the field and meta-analyses using quantitative methods to synthesize the literature are also included.
Results
Study identification
Figure 1 describes outcomes at each level of our study identification process. Of the 394 potentially relevant articles, we included 50 structural, 29 functional, seven MRS, and eight combined imaging and genetic studies in this systematic review.
Fig. 1.
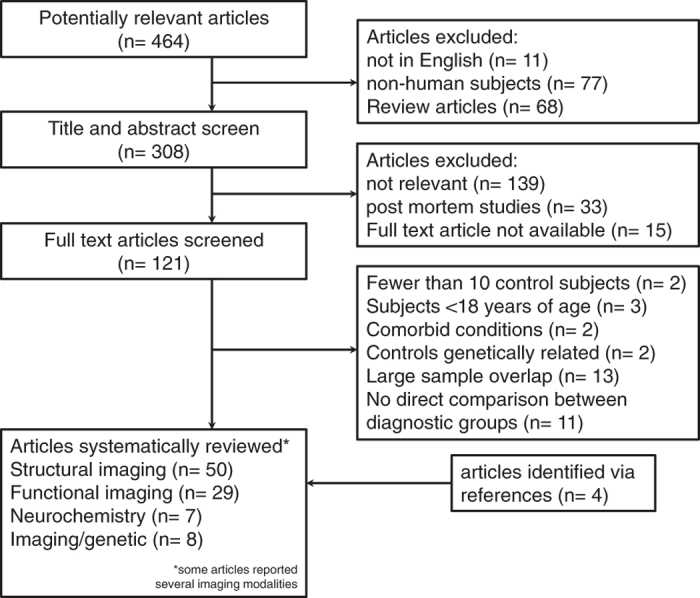
Process of study selection
Structural MRI studies
Gray matter structural MRI
Gray matter has been an early focus of neuroimaging research in psychiatric illnesses. Using anatomical likelihood estimation (ALE), a meta-analysis of 37 studies demonstrated extensive gray matter deficits in frontal, limbic and subcortical deficits in schizophrenia when compared to healthy controls.26 Similarly gray matter reductions were found in anterior cingulate and insula in an ALE meta- analysis of patients with BD when compared to healthy controls.27 A recent analyses attempting to quantitatively, albeit not directly, contrast gray matter deficits in a number of psychiatric disorders, including schizophrenia and BD, using meta-analytic techniques found shared decrease in the dorsal anterior cingulate and anterior insula, areas of the brain that are attributed to the salience networks.28
Forty studies explicitly examined differences in gray matter across the psychosis spectrum since 2005, but only seventeen of those were conducted at magnetic field strengths of 3T. All but one study included subjects who were medicated at the time of scanning, and only a minority of studies focused on first episode patients (Table 1). Cortical gray matter volume loss appears widespread in schizophrenia, but less extensive29–31 or even absent32, 33 in BD. It has been suggested as an intermediate phenotype across disease categories, possibly reflecting lifetime psychosis burden, with patients diagnosed with schizophrenia and schizoaffective disorder demonstrating extensive neocortical and subcortical gray matter reductions, and smaller reductions limited to frontotemporal regions in BD.29 Further supporting this concept is a recent study by Song et al. who examined gray matter volumes in unmedicated patients with schizophrenia and BD and reported a negative correlation between severity of delusions and frontal gray matter volumes as well as extent of hallucinations and right uncus gray matter volume across diagnostic groups.34 Examining subcortical areas of the brain, studies consistently suggest that hippocampal volume reduction may be a feature that is shared across the psychosis spectrum,35–37, with a majority of studies reporting that volume loss is greater in schizophrenia compared to BD,38 even when examining individual hippocampal subfields.35, 39 Similarly, early studies suggest thalamus volume reductions to be present across disease categories,36, 40 but later reports with larger sample sizes detected this feature only in schizophrenia.29, 41 Findings are more inconsistent in regards to amygdala volume, which has been reported to be unaffected in both diagnostic groups,42, 43 decreased only in schizophrenia,44 more prominently decreased in schizophrenia compared than in BD45 and vice versa.37 Reports on basal ganglia volumes are also conflicting where some find volume increase46, 47 or decrease48, 49 that is shared across illnesses, abnormalities in schizophrenia but not BD,34, 36 or lack of abnormalities in either diagnostic group (except for the nucleus accumbens).50 Those contrasting reports likely reflect the heterogeneity across studies in regards to patient characteristics, medication exposure, data acquisition, and data analysis methods.
Table 1.
Studies examining gray matter
| Author | Year | n (HC/SZ/SAD/BD) | Bipolar subtype | Illness duration | Medication status | Areas of interest | Analysis method | Tesla | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Farrow et al. 113 | 2005 | 22/25/0/8 | P | FE | medicated | Whole brain | VBM | 1.5 | HC > SZ in lateral and medial frontal and posterior temporal regions. HC > BD inferior temporal gyri and ACC |
| Strasser et al. 114 | 2005 | 44/33/0/38 | P | Chronic | Medicated | Cerebral volume, hippocampus | MEASURE, ROI (manual tracing) | 1.5 | HC = BD = SZ in cerebral volume and hippocampus volume |
| Mc Donald et al. 115 | 2006 | 54/42 SZ/38 | P | Chronic | Medicated | Whole brain | MEASURE | 1.5 | HC = BD = SZ in cerebral volume. HC = BD > SZ in hippocampus |
| Salisbury et al. 116 | 2007 | 32/20/0/21 | P | FE | Medicated | Heschl gyri | ROI (manual tracing) | 1.5 | SZ < HC = BD in left heschl gyrus gray matter volumes |
| Nakamura et al. 117 | 2007 | 36/29/0/34 | P | FE | Medicated | Neocortex | ROI (manual tracing) | 1.5 | HC > SZ = BD neocortical gray matter volumes |
| Morgan et al. 118 | 2007 | 58/44/0/29 | P | FE | Medicated | Whole brain | VBM | 1.5 | No group differences |
| Frazier et al. 42 | 2008 | 29/20/0/54 | P/NP | Chronic | Medicated | Subcortical volumes | ROI (manual tracing) | 1.5 | No group differences in hippocampus and amygdala volumes |
| Koo et al. 52 | 2008 | 40/39/0/41 | P | FE | Medicated | Cingulate gyrus | ROI (manual tracing) | 1.5 | HC > SZ in subgenual, anterostratal, anterodorsal, and posterior subregions. HC > BD in the subgenual subregion |
| Killgore et al. 43 | 2009 | 20/19/0/11 | Acute psychosis | Medicated | Cerebrum, amygdala, hippocampus | ROI (manual tracing) | 1.5 | SZ < BD = HC total brain volume, SCZ = HC = BD in amygdala and hippocampus volume | |
| Reite et al. 119 | 2010 | 89/58/26/51 | Chronic | Medicated | Whole brain | manual tracing and semiautomated | 1.5/3 | No group differences | |
| Molina et al. 48 | 2011 | 24/38/0/19 | P/NP | Chronic | Medicated | Whole brain | VBM | 1.5 | No differences in SZ vs BD. SZ < HC in medial frontal lobe and basal ganglia. BD < HC in medial frontal lobe and caudate |
| Rimol et al. 33 | 2011 | 207/142/31/139 | Chronic | Medicated | Cortical volume | Free Surfer | 1.5 | SZ < BD in temporal lobe, fusiform and parahippocampal gyrus. BD = HC in cortical volume. Cortical thinning seen in SZ only | |
| Hartberg et al. 36 | 2011 | 192/101/16/121 | Chronic | Medicated | Subcortical volumes | Free Surfer | 1.5 | HC > SZ = BD hippocampus, left thalamus, SZ > BD = HC putamen; HC > BD in cerebellum | |
| Brown et al. 37 | 2011 | 21/17/0/15 | Chronic | Medicated | Whole brain, hippocampus, amygdala | VBM | 1.5 | HC > SZ and BD in frontotemporal regions. HC > SZ > BD in amygdala and right hippocampus | |
| Radonic et al. 120 | 2011 | 15/15/15/15 | Chronic | Medicated | Hippocampus | ROI (manual tracing) | 2 | SZ = SAD < HC = BD in hippocampal volume | |
| de Castro-Manglano et al. 51 | 2011 | 20/10/0/14 | P | FE | Medicated | Whole brain | VBM | 1.5 | HC > BD in frontal lobe, thalamus, superior temporal gyrus, cerebellum; HC > SZ frontal lobe, thalamus, hippocampus; BD > SZ hippocampus |
| Cui et al. 47 | 2011 | 36/24/0/24 | P | Acutely ill FE and chronic | Medicated | Whole brain | VBM | 3 | HC > SZ in superior temporal and inferior parietal lobe, HC < SZ in putamen; HC > BD in superior temporal, inferior parietal lobe, and caudate; HC < BD in putamen |
| Ivleva et al. 49 | 2012 | 10/19/16/17 | P | Chronic | Medicated | Whole brain | VBM, Free Surfer | 3 | HC > SZ and SAD in frontal, temporal and insular cortices, HC > SZ across neocortex, thalamus, and basal ganglia, BD = HC in cortical and subcortical gray matter volume |
| Yuksel et al. 32 | 2012 | 43/37/21/28 | P | Chronic | Medicated | Whole brain | VBM | 3 | HC > SZ in prefrontal and temporal cortices, HC < SZ in cerebellum, BD > SZ in subgenual cortex |
| Watson et al. 40 | 2012 | 59/25/0/24 | P | FE | Medicated | Whole brain | VBM | 1.5 | HC > SZ in hippocampus, thalamus, striatum and cerebellum. HC > BD in precuneus; BD > HC in cerebellum |
| Mahon et al. 44 | 2012 | 27/31/0/36 | P | Chronic | Medicated | Amygdala | Free Surfer | 1.5 | Amygdala volume SZ < BD = HC |
| Ivleva et al. 29 | 2013 | 200/146/90/115 | P | Chronic | Medicated | Whole brain | VBM | 3 | HC > SZ and SAD in frontal, temporal and insular cortices, thalamus, basal ganglia, and cerebellum. HC > BD frontal, insular, temporal and parietal cortex |
| Ratnanather et al. 121 | 2013 | 27/31/0/36 | Chronic | Medicated | STG and planum temporale | ROI | 1.5 | HC = BD > SZ in planum temporale | |
| Womer et al. 50 | 2014 | 27/28/4/33 | P/NP | Chronic | Medicated | Subcortical gray matter | Free Surfer | 3 | HC = SZ = BP in caudate, putamen, nucleus accumbens, thalamus. BP < HC < SZ in globus pallidus |
| Haukvik et al. 39 | 2014 | 300/182/28/192 | Chronic | Medicated | Hippocampus | Free Surfer | 1.5 | HC > SZ in all hippocampal subfields, HC > BD in all subfields except for the presubiculum. BD > SZ in presubiculum and subiculum | |
| Mathew et al. 35 | 2014 | 337/219/142/188 | P | Chronic | Medicated | Medial temporal lobe | Free Surfer | 3 | BD = HC > SZ and SAD in medial temporal cortex volume, SZ < BD < HC in hippocampal subfields |
| Knochel et al. 38 | 2014 | 21/21/0/21 | Chronic | Medicated | Hippocampus | VBM | 3 | Hippocampal volume HC > BD > SZ | |
| Findikili et al. 122 | 2015 | 30/17/0/17 | Chronic | Medicated | Pineal gland | ROI (manual tracing) | 1.5 | Mean pineal gland volume SZ < BD < HC | |
| Nenandic et al. 41 | 2015 | 34/34/0/17 | P | Chronic | Medicated | Whole brain | VBM | 3 | HC > SZ prefrontal cortex and insula, temporal cortex, thalamus, and cerebellum. SZ < BD in hippocampus, DLPFC, and cerebellum |
| Kittel-Schneider et al. 45 | 2015 | 18/23/0/30 | P/NP | FE | Medicated | Amygdala | ROI (manual tracing) | 1.5 | HC > BD > SZ in amygdala volume |
| Laidi et al. 123 | 2015 | 52/32/0/115 | P/NP | Chronic | Medicated | Cerebellum | Free Surfer | 3 | SZ < HC = BD in cerebellar volume |
| Song et al. 34 | 2015 | 35/71/0/44 | P | Chronic | Off medication | Whole brain | VBM | 3 | HC > SZ precentral gyrus, caudate, and cerebellum; HC > BD middle frontal gyrus, fusiform gyrus; BD > SZ cerebellum, temporal lobe, basal ganglia |
| Pina-Camacho et al. 53 | 2015 | 157/92/0/32/72 | P | FE | Medicated | Whole brain | VBM, Free Surfer | 1.5/3 | HC > SZ in frontal and temporal lobe, SZ > HC in basal ganglia. HC > BD ACC, BD > HC in caudate and temporal lobe thicker temporal cortex |
| Shepherd et al. 124 | 2015 | 34/28/12/30 | Chronic | Medicated | Whole brain | VBM | 3 | HC > SZ in hippocampus and frontal cortex. HC > BD in precuneus, superior parietal and postcentral gyrus. SZ = BD in GM volume | |
| Amann et al. 125 | 2015 | 45/45/45 /45 | P/NP | Mixed | Medicated | Whole brain | VBM | 1.5 | HC > SZ and SAD in anterior cingulate, insula, temporal lobe, cerebellum; HC = BD |
| Royer et al. 126 | 2015 | 63/31/0/20 | Chronic | Medicated | Whole brain gray matter asymmetry | In house processing | 3 | No group differences | |
| Poletti et al. 31 | 2016 | 136/96/0/206 | Chronic | Medicated | Whole brain | VBM | 3 | HC > SZ in inferior frontal gyrus, thalamus, insula and superior temporal gyrus; HC > BD inferior frontal gyrus; BD > SZ thalamus | |
| Reavis et al. 127 | 2016 | 30/33/0/31 | NP | Chronic | Medicated | Lateral occipital complex and retinotopic visual cortex | ROI | 3 | HC >BD>SZ in retinotopic cortex and lateral occipital complex |
| Knoechel et al. 128 | 2016 | 38/32/0/34 | NP | Chronic | Medicated | Whole brain | Free Surfer | 3 | HC > BD = SZ in cortical thickness in inferior frontal gyrus, ACC, PCC. HC > SZ in dorsal frontal and temporal areas. HC > BD in orbitofrontal cortex |
| Nenadic et al. 129 | 2016 | 38/32/0/34 | P | Chronic | Medicated | Whole brain | Free Surfer | 3 | BD > HC in local gyrification in ACC and DLPFC, SZ > HC in local gyrification in MPFC and orbitofrontal cortex, BD > SZ in ACC gyrification |
HC healthy control, BD bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, FE first episode, VBM voxel based morphometry, ROI region of interest analysis, ACC anterior cingulate cortex, PCC posterior cingulate cortex, DLPFC dorsolateral prefrontal cortex, MPFC medial prefrontal cortex, STG superior temporal gyrus
Significant cortical and subcortical volume loss that resembles the chronic illness stage is already reported in first episode patients. While many of the areas with gray matter loss appear to overlap across diagnostic groups, several reports suggest greater abnormalities in first episode schizophrenia compared to first episode BD, both in terms of volume loss45, 51 and spatial extent.40, 52 It is perhaps not surprising that Pina-Camacho et al. reported that age at first onset of psychosis modulated structural abnormalities in a nonlinear and diagnosis dependent manner. Specifically, they report that patients with an earlier onset of a schizophrenia spectrum disorder had the most significant ventricular and basal ganglia enlargement along with the greatest fronto-temporal cortical volume and thickness deficits among diagnostic groups, with affective disorder patients having less extensive cortical deficits that were again more prominent in those with younger age of onset of psychosis.53 However, none of the studies examined medication naïve patients, making it impossible to definitively conclude that observed group differences in gray matter abnormalities are due to intrinsic differences across the diagnostic spectrum rather than secondary to differential exposure to psychotropic medications.
White matter structural MRI
White matter abnormalities have been reported to be widespread in both schizophrenia and BD. An activation likelihood estimation meta-analysis showed decreased FA in first episode schizophrenia compared to healthy controls across the commissural, association, and projection tracts, with main involvement of the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, cingulum bundle, and corpus callosum.54 Similarly, a meta-analysis of fifteen DTI studies in BD reported decreased FA in all types of tracts when compared to healthy controls, with the most robust decreases in the inferior fronto-occipital fasciculus.55 Findings suggest a shared spatial distribution of white matter integrity deficits across the illness spectrum, but allow no inference on the comparability in magnitude of abnormalities.
Eleven studies contrasted white matter integrity in schizophrenia and BD; all but one included patients who were medicated at the time of assessment, and only two focused on first episode patients (Table 2). It is striking that the majority of studies conducted in chronic patients on psychotropic medications show decreases in white matter integrity that do not appear different across diagnostic groups. Region of interest analyses show shared FA decreases in the uncinate fasciculus, corona radiata, anterior limb of the internal capsule, anterior and posterior thalamic radiation, and corpus callosum.56–60 This is also corroborated by Skudlarski and colleagues who conducted the largest study thus far and found a close agreement between spatial distributions and magnitudes in FA reductions assessed with whole brain TBSS across diagnostic groups. Interestingly, they reported higher variance in patients with psychotic BD, suggestive of greater heterogeneity in white matter integrity abnormalities compared to patients with schizophrenia.61 Heterogeneity may also explain discrepancies with Anderson and colleagues who found FA reductions in temporal and occipital white matter in schizophrenia but not bipolar I disorder,62 and Knoechel and colleagues who report that the magnitude of white matter integrity abnormalities in the left cingulum and right uncinate fasciculus is greater in schizophrenia than BD.38
Table 2.
Studies examining white matter
| Author | Year | n (HC/SZ/ SAD/BD) | Bipolar subtype | Illness duration | Medication status | Area of interest | Tesla | Direct-ions | Analysis type | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| McIntosh | 2008 | 49/25/0/40 | Chronic | Medicated | Uncinate fasciculus and anterior thalamic radiation | 1.5 | 51 | Tractography | HC > BD and SZ in FA in the uncinate fasciculus and anterior thalamic radiation | |
| Sussmann et al. 59 | 2009 | 38/28/0/42 | Chronic | Medicated | Uncinate fasciculus and internal capsule | 1.5 | 51 | ROI analyses | HC > BD = SZ in FA in internal capsule, HC > BD in anterior limb of internal capsule | |
| Cui et al. 130 | 2011 | 30/25/0/18 | P | Chronic | Medicated | Whole brain | 3 | 15 | Voxel based analysis | HC > SZ = BD in FA in posterior corona radiata, HC > BD in FA in fronto-parietal white matter |
| Lu et al. 64 | 2011 | 18/21/0/13 | P | FE | Minimally treated/ medication naïve | Whole brain | 3 | 27 | VBM | HC > BP = SZ in MD in cingulum, corpus callosum, corona radiata, internal capsule, and occipital WM including posterior thalamic radiation, inferior longitudinal fasciculus/inferior fronto-occipital fasciculus. HC = SZ > BD in FA in multiple commissural, projection, and association tracts |
| Sui et al. 57 | 2011 | 62/54/0/48 | Chronic | Medicated | Whole brain | 3 | 12 | Tractography | HC > BD and SZ in FA in occipital and frontal lobes | |
| da Cunha Colombo et al. 63 | 2012 | 94/55/7/26 | P | FE | Medicated | Whole brain | 1.5 | VBM | HC = SZ = BD in regional WM volume | |
| Anderson et al. 62 | 2013 | 56/35/0/20 | P/NP | Chronic | Medicated | 21 regions | 1.5 | 25 | ROI analyses | HC = BD > SZ in FA in superior temporal, parahippocampal, and occipital white matter; HC < BD = SZ in MD in superior temporal, parahippocampal, inferior frontal, fusiform, angular white matter; BD > SZ in MD precentral and middle frontal cortex |
| Skudlarski et al. 61 | 2013 | 104/109/35/63 | P | Chronic | Medicated | Whole brain | 3 | 32 | TBSS | HC > SZ = BD in FA in 29 of 76 regions analyzed |
| Knochel et al. 38 | 2014 | 21/21/0/21 | Chronic | Medicated | Uncinate fasciculus, cingulum, fornix | 3 | 60 | TBSS | HC = BD < SZ in MD in the cingulum; HC > SZ = BD in MD in the fornix | |
| Li et al. 60 | 2014 | 24/19/0/16 | Chronic | Medicated | Corpus Callosum | 3 | 25 | ROI analyses | HC > SZ in entire corpus callosum, HC > BD in all subregions except the middle genu | |
| Kumar et al. 56 | 2015 | 41/34/6/22 | P | Chronic | Medicated | Whole brain | 3 | 32 | TBSS/ Tractography | HC > SZ = BD in FA in corpus callosum, corona radiata |
HC healthy control, BD bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, FE first episode, TBSS tract based spatial statistics, ROI region of interest, FA fractional anisotropy, MD mean diffusivity, WM white matter
In first episode patients, no white matter volume abnormalities or no differences across diagnostic groups were observed63. The only study to date examining white matter microstructure in medication-naïve and minimally treated patients found a shared increase in MD in a large number of white matter tracts across diagnostic categories, but showed that patients with first episode BD had decreased FA in the cingulum, internal capsule, and posterior brain regions that was not evident in first episode schizophrenia.64
Functional MRI studies
Task functional MRI
A total of thirteen studies, all in chronically medicated patients, have directly contrasted functional activation patterns in BD and schizophrenia using various tasks (Table 3). In working memory tasks, studies largely observe engagement of the same brain networks in healthy controls and patients across the psychosis spectrum, but find alterations in activation patterns within those networks. A graded pattern of group differences in the amplitude of the BOLD signal has been reported in several studies, with the greatest alteration typically reported in schizophrenia, and more subtle or lack of abnormalities in BD.65–67 Findings are more inconsistent, with both hypo- and hyper-activation reported, which may be explained by different, but overlapping, inverted u-shaped curves of activation depending on task difficulty68 across the psychosis spectrum, with less abnormal patterns of activation in BD compared to schizophrenia. Alternatively, it is possible that these patterns of differential activation could represent compensatory processes or secondary effects of primary changes in signal processing. Greater task related alterations in schizophrenia compared to BD were also reported in a verbal fluency task,69 but not a sentence completion task,70 or emotionally salient memory tasks,71, 72 the latter appearing more altered in BD than schizophrenia. Furthermore, activation during reward anticipation in the ventral striatum appears decreased in schizophrenia, but not patients with BD in a manic state.73 The authors speculated that striatal dopamine dysfunction, which could be clinically expressed as anhedonia, may be underlying their finding.
Table 3.
Studies examining task activation
| Author | Year | n (HC/SZ/SAD/BD) | Bipolar subtype | Illness duration | Medication status | Tesla | Task | Design | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| McIntosh et al. 70 | 2008 | 37/27/0/42 | Chronic | Medicated | 1.5 | Hayling sentence completion test | Block | BD > HC activation in VLPFC, striatum and caudate. SZ < HC in the DLPFC | |
| Hamilton et al. 131 | 2009 | 38/20/0/21 | Chronic | Medicated | 3 | Visual working memory | Block | HC > SZ in activation in inferior frontal gyrus and DLPFC. HC > BD in activation in occipital regions, SZ > BD in precentral and postcentral gyrus, medial frontal gyrus and parietal regions | |
| Costrafreda et al. 132 | 2009 | 48/39/0/28 | Chronic | Medicated | 1.5 | Phonological letter fluency | Block | SZ > HC = BD in activation in inferior frontal cortex | |
| Whalley et al. 71 | 2009 | 14/15/0/14 | Chronic | Medicated | 1.5 | Emotionally salient memory | Block | BD > HC > SZ in left hippocampus, BD > HC in posterior cingulate, STG and precentral gyrus. BD > SZ in posterior cingulate, superior temporal sulcus, precentral gyrus. SZ < HC in amygdala | |
| Hall et al. 133 | 2009 | 14/15/0/14 | Chronic | Medicated | 1.5 | Face/name encode and retrieve | Block | HC = BD > SZ in activation of anterior right hippocampus during encode, BD < SZ = HC in activation of left DLPFC during encode. SZ > BD in DMPFC activation during retrieval, SZ > HC in DLPFC activation during retrieval | |
| Milanovic et al. 66 | 2011 | 19/10/0/12 | P | Chronic | Medicated | 1.5 | n-Back | Block | SZ > HC = BD activation in the MPFC region of interest, but no group differences in the DLPFC region of interest |
| Costrafreda et al. 69 | 2011 | 40/32/0/32 | Chronic | Medicated | 1.5 | Phonological letter fluency | Block | SZ > BD > HC activation in anterior cingulate, middle frontal gyrus, and putamen. SZ > BD = HC superior, middle, and inferior frontal gyrus; SZ = BD > HC in precuneus, posterior cingulate, and angular gyrus | |
| Morris et al. 72 | 2012 | 15/12/0/13 | Chronic | Medicated | 3 | Emotionally salient memory | Block | BD > SZ > HC in emotion downregulation in the prefrontal cortex; BD = SZ > HC in emotion upregulation in prefrontal cortex | |
| Palaniyappan et al. 134 | 2013 | 34/34/0/20 | P | Chronic | Medicated | 3 | n-Back | Block | HC > SZ in degree centrality in frontal lobe, STG and insula, HC > BD in degree centrality in insula, SZ > HC in hippocampus, thalamus, and fusiform gyrus, BD > HC hippocampus, thalamus, and caudate |
| Brandt et al. 65 | 2014 | 100/86/14/100 | P/NP | Chronic | Medicated | 1.5 | n-Back | Block | SZ > BD > HC in activation in the dorsolateral and ventrolateral PFC, premotor cortex and parietal cortex; HC > BD > SZ in inferior frontal gyrus, inferior parietal cortex, STG, and precuneus |
| Wu et al. 67 | 2014 | 29/36/0/20 | Chronic | Medicated | 3 | n-Back | Block | SZ > BD > HC in hyperactivity in the PCC and MPFC | |
| Zhang et al. 135 | 2015 | 21/17/0/17 | P | Chronic | Medicated | 3 | self-reflection task | Block | HC > BD in PCC precuneus activation during other vs. self-contrast |
| Hagele et al. 73 | 2015 | 54/44/0/13 | Chronic | Medicated | 1.5 | Monetary incentive delay | SZ showed less ventral striatal activation compared to HC = BD during reward anticipation. No group differences in loss anticipation |
HC healthy control, BD bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, PFC prefrontal cortex, DLPFC dorsolateral prefrontal cortex, DMPFC dorsomedial prefrontal cortex, MPFC medial prefrontal cortex, VLPFC ventrolateral prefrontal cortex, STG superior temporal gyrus, PCC posterior cingulate cortex
Resting state functional MRI
Investigation of functional connectivity at rest has become increasingly popular, in part because task performance differences between groups need not be accounted for in this paradigm.74 An activation likelihood estimation meta-analysis of whole brain resting state studies in schizophrenia suggests decreased activity in the medial prefrontal cortex, left hippocampus, posterior cingulate cortex and precuneus (all areas of the brain that are typically conceptualized as part of the default mode network75), as well as increased activity in the lingual gyrus.76 A recent review attempting to reconcile methodological differences in schizophrenia studies suggested increased functional connectivity to be a replicated finding.77 In a qualitative systematic review Vargas and colleagues reported aberrant resting state connectivity in between frontal and meso-limibic areas in BD when compared to controls.78
All of the sixteen resting state studies comparing connectivity across diagnostic groups included here were conducted in patients who were medicated at the time of scanning (Table 4). The default mode network, a large scale brain network that is more active at rest and has been implicated in self-referential thinking, is perhaps the most widely studied. An early report in acutely ill patients with schizophrenia and BD identified the medial prefrontal cortex as major locus of shared abnormality, with BD being characterized by reduced default mode network connectivity to the hippocampus and fusiform gyrus as well as increased connectivity with the primary visual cortex, and schizophrenia being characterized by abnormal recruitment of the frontal polar cortex and the basal ganglia.79 The largest study to date examining default mode network connectivity with ICA reported connectivity reductions in the medial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex and precuneus across the psychosis spectrum, but also found that selective nodes within the network appear to be differentially affected in schizophrenia and BD.80 The same group also reported aberrant connectivity between the default mode and a fronto-occipital network as shared illness feature in schizophrenia and BD, whereas increased connectivity between fronto-temporal and mesolimbic regions was only evident in BD, and decreased connectivity between sensory-motor and mesolimbic areas was limited to schizophrenia.81 Others report within and between network connectivity decreases within a cingulo-opercular network and between a cingulo-opercular and cerebellar network that are shared across illnesses, decreased connectivity between the cingulo-opercular and salience network in BD only, and decreased connectivity between cingulo-opercular and fronto-parietal network in schizophrenia only. Notably, default mode network connectivity was not reported abnormal.82 Similarly, Baker and colleagues reported resting state connectivity disruptions of cortical association networks, preferentially the frontoparietal control network, but not default mode network abnormalities, in schizophrenia and BD.83
Table 4.
Resting state studies
| Author | Year | n (HC/SZ/SAD/BD) | Bipolar subtype | Illness duration | Medication status | Areas of interest | Tesla | Scan (min) | eyes open/closed | Analysis | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ongur et al. 79 | 2010 | 15/7/7/17 | Acutely ill | Medicated | Default mode network | 3 | 10 | Open | ICA | Mixed increase and decrease in DMN connectivity in both SZ and BD compared to HC | |
| Chai et al. 136 | 2011 | 15/16/0/14 | Chronic | Medicated | Medial prefrontal cortex | 3 | 10 | Open | Seed | BD > SZ > HC in ventrolateral prefrontal cortex and insula connectivity; HC > SZ > BD in DLPFC connectivity | |
| Meda et al. 81 | 2012 | 118/70/0/64 | P | Chronic | Medicated | 16 Large scale networks | 3 | 5.25 | Open | ICA | HC > SZ = BD in fronto-occipital and anterior DMN, HC = BD > SZ in meso/paralimbic and sensory-motor network, BD > HC in Fronto-temporal/paralimbic and meso/paralimbic |
| Mamah et al. 82 | 2013 | 33/25/0/35 | Chronic | Medicated | Five large scale networks | 3 | 13.7 | Closed | Seed | HC > BD = SZ in cingulo-opercular network | |
| Liu et al. 85 | 2014 | 18/18/0/18 | Chronic | Medicated | Prefrontal cortex, amygdala | 3 | Closed | Seed | BD = SZ mixed increase and decrease in connectivity between amygdala subregions and the prefrontal cortex compared to HC | ||
| Meda et al. 80 | 2014 | 324/269/0/300 | P | Chronic | Medicated | Default mode network | ICA | HC > BD > SZ | |||
| Anticevic et al. 84 | 2014 | 146/90/0/73 | P/NP | Chronic | Medicated | Thalamus | 3 | 5.25 | Open | Seed | Mixed increase and decrease in connectivity in SZ and BD compared to HC; BD show less cerebellar dysconnectivity than SZ |
| Knochel et al. 38 | 2014 | 21/21/0/21 | Chronic | Medicated | Hippocampus | 3 | 13.3 | Seed | No significant differences when all three groups are compared | ||
| Rashid et al. 137 | 2014 | 61/60(SZ/ SAD)/0/38 | P/NP | Chronic | Medicated | 49 Large scale networks | 3 | 5.25 | Open | Dynamic ICA | HC > BD = SZ in number of transitions between states |
| Argyelan et al. 87 | 2014 | 32/18/0/19 | P/NP | Chronic | Medicated | Whole brain connectivity, caudate | 3 | 5 | Closed | Seed | SZ < BD < HC in global connectivity, specifically thalamus, paracingulate, caudate, fusiform and lingual gyrus |
| Baker et al.JT83 | 2014 | 100/28/32/40 | P | Chronic | Medicated | 17 Large scale networks | 3 | 6.2 | Open | Seed | HC > BD = SZ in frontoparietal network |
| Meda et al. 138 | 2015 | 242/220/147/180 | P | Chronic | Medicated | Whole brain | 3 | 5 | Open | ALFF | Mixed increase and decrease in ALFF in all diagnostic groups |
| Du et al. 139 | 2015 | 20/20/33/20 | Chronic | Medicated | 12 Large scale networks | 3 | 5 | Open | ICA | Discriminative regions included frontal, parietal, precuneus, cingulate, supplementary motor, cerebellar, insula and supramarginal cortices | |
| Anticevic et al. 140 | 2015 | 56/73/0/73 | P/NP | Chronic | Medicated | Ventral ACC | 3 | 5.25 | Open | Seed | HC > SZ = BD |
| Hager et al. 141 | 2016 | 156/107/98/125 | P | Chronic | Medicated | Whole brain | 3 | 5 | Multiple time scales | SZ < HC in complexity in hypothalamus, BD < HC in complexity in left inferior occipital, right precentral and left superior parietal. HC > SZ = SAD = in complexity PFC | |
| Skatun et al. 142 | 2016 | 196/60/11/43 | Chronic | Medicated | 3 | 5.3 | Open | Eigenvector centrality mapping | HC > SZ in global connectivity in sensory regions, HC > SZ = BD in subcortical regions; SZ > BD > HC in connectivity in frontal and parietal areas |
HC healthy control, BD bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, ICA independent components analysis, ALFF amplitude of low frequency fluctuations, ACC anterior cingulate cortex, PFC prefrontal cortex, DLPFC dorsolateral prefrontal cortex, DMN default mode network
Resting state studies of non-neocortical structures revealed distinct patterns of thalamic84 and amygdalar85 dysconnectivity in schizophrenia and BD, but no differences across diagnostic groups in hippocampal38, 86 connectivity. Examining connectivity across the entire brain with global brain connectivity, a measure that computes connectivity strength of every region of the brain with every other region of the brain, was reported to be lower in schizophrenia than in healthy controls, whereas patients with BD had intermediate global connectivity strength that was significantly different from both patients with schizophrenia and healthy controls.87
Magnetic resonance spectroscopy studies
There is substantial evidence that neurometabolite levels are altered in both schizophrenia and BD. A meta-analysis pooling data from 146 studies suggests decreases in NAA in the frontal lobe, hippocampus, thalamus, and basal ganglia in schizophrenia, but only in the basal ganglia and frontal lobe in BD.88 Another meta-analysis summarizing findings of glutamatergic abnormalities across 28 studies in schizophrenia revealed a decrease in medial frontal glutamate compared with healthy controls,89 but the majority of studies were conducted in medicated patients. Contrastingly, several reports do suggest an elevation of glutamatergic indices in unmedicated patients with schizophrenia in the medial prefrontal cortex, striatum, and hippocampus.90–93 A smaller meta-analysis in BD including nine studies measuring Glx (a combination of glutamate and glutamine) across different areas of the brain, suggested that this metabolite may be higher in patients with BD compared to controls, irrespective of medication status.94 Taken together, it appears that some of the neurometabolite alterations, specifically decreased NAA in the frontal cortex and basal ganglia may be shared across the illness spectrum, whereas others may not.
However, studies directly contrasting neurometabolites in BD and schizophrenia are sparse. All of these studies used single voxel1 H-MRS placed in cortical areas of the brain and were conducted in chronically ill patients who were medicated at the time of assessment, most commonly with very small sample sizes (Table 5). Molina and colleagues were the first to report that NAA/Cr decreases in the left, but not the right dorsolateral prefrontal cortex were greater in schizophrenia than in BD, with no Cho/Cr abnormalities appreciated in either group.95 Findings were partially replicated by Kalayci and colleagues, who reported a universal decrease in left and right dorsolateral prefrontal cortex NAA/Cr across diagnostic groups, but a decrease in Cho/Cr only in BD and schizoaffective disorder, but not schizophrenia when compared to controls.96 Anterior cingulate cortex metabolite measurements at 1.5 Tesla were suggestive of elevations of Cho/Cr in schizophrenia only without detectable abnormalities in NAA/Cr across groups.97 However, because of a later report of decreased Cr levels in acutely ill patients with schizophrenia but not BD, it is unclear if findings are attributable to Cr alterations rather than Cho or NAA changes.98 In the left Heschl’s gyrus, Glu, NAA, and inositol levels were found to be decreased in BD, but not in schizophrenia when compared to healthy controls, while no metabolite abnormalities in either diagnostic group were detected in the right Heschl’s gyrus, suggesting a lateralized abnormality in the dominant hemisphere.99 Only one study to date has examined GABA, with reports of perisylvian GABA elevations in patients with schizophrenia, but not those with BD, when compared to healthy controls.100
Table 5.
Studies examining magnetic resonance spectroscopy (MRS)
| Author | Year | n (HC/SZ/ SAD/ BD) | Bipolar subtype | Illness duration | Medication status | Area of interest | MRS | Sequence | Tesla | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Molina et al. 95 | 2007 | 10/11/0/13 | P/NP | Chronic | Medicated | DLPFC | SV | PRESS | 1.5 | NAA/Cr decreased in SZ and BD No abnormalities in Cho/Cr in either group compared to HC |
| Crespo et al. 97 | 2008 | 15/14/0/17 | NP | Chronic | Medicated | dACC | SV | PRESS | 1.5 | NAA/Cho SZ < HC and BD; Cho/Cr SZ > HC and BD; NAA/Cr no group differences |
| Ongur et al. 98 | 2009 | 22/8/7/15 | P | Chronic | Medicated | ACC/POC | SV | PRESS | 4 | Cr HC = BD > SZ in both voxels |
| Ongur et al. 143 | 2010 | 20/15/0/15 | P | Chronic | Medicated | ACC/ POC | SV | PRESS | 4 | Numerically shortened T2 relaxation times in BD and SZ |
| Kalayci et al. 96 | 2012 | 15/15/15/15 | Chronic | Medicated | DLPFC | SV | PRESS | 1.5 | NAA HC > BD, SZ, SAD; Cho HC > BD, SAD; Cr C > BD, SAD, SZ | |
| Atagun et al. 99 | 2015 | 30/30/0/28 | Chronic | Medicated | Heschl’s gyrus | SV | PRESS | 3 | Glu, NAA, and Cr HC > BD in left hemisphere; no group differences in right hemisphere | |
| Atagun et al. 100 | 2016 | 30/25/0/48 | Chronic | Medicated | Perisylvian structures | SV | MEGA-PRESS | 3 | GABA SZ > BD = HC |
HC healthy control, BD, bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, DLPFC dorsolateral prefrontal cortex, ACC anterior cingulate cortex, dACC dorsal anterior cingulate cortex, POC parieto-occipital cortex, SV single voxel, PRESS point resolved spectroscopy sequence, NAA N-acetyl-aspartate, Cr creatine, Cho choline, Glu glutamate, GABA gamma-aminobutyric acid
Imaging-genetic studies
Eight studies, all in medicated patients, have examined relationships between imaging and genetic markers across the illness spectrum (Table 6). In verbal fluency tasks, significant diagnosis by genotype interactions with task related activations were observed for Neuregulin 1,101 disrupted in Schizophrenia Gene 1 (DISC 1102), and the d-amino acid oxidase (See ref. 103) gene. Interestingly, a modest relationship between white matter volume and a number of schizophrenia risk genes was found across the psychosis spectrum and in healthy controls, suggesting that cumulative genetic risks may help explain the extent of observed white matter alterations,104 but other risk genes did not show such relationships.105
Table 6.
Imaging genetic studies
| Author | Year | n (HC/SZ/SAD/BD) | Bipolar subtype | Illness duration | Medication status | Genes | Imaging modality | Design | Analysis type | Tesla | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechelli et al. 101 | 2008 | 45/41/0/29 | Chronic | Medicated | Neuregulin 1 | Verbal fluency task fMRI | Block design | Full factorial ANOVA | 1.5 | HC > BD = SZ in activation in the angular gyrus. Significant diagnosis x genotype interaction in prefrontal cortex activation | |
| Prata et al. 102 | 2011 | 53/44/0/35 | P/NP | Chronic | Medicated | DISC 1 | Verbal fluency task fMRI | Block design | Full factorial ANOVA | 1.5 | HC < SZ in activation in middle frontal gyrus, no differences between HC and BD. Group × genotype interaction (HC and SZ) in left superior frontal gyrus activation |
| Papagni et al. 103 | 2011 | 48/40/0/33 | Chronic | Medicated | DAAO | Verbal fluency task fMRI | Block design | Full factorial ANOVA | 1.5 | HC > SZ in activation of the PCC, no group differences between SZ and BD. Diagnosis x genotype interaction in precuneus and PCC | |
| Tesli et al. 144 | 2013 | 123/52/9/66 | P | Chronic | Medicated | CACNAC1C | Emotionally salient fMRI | Block design | Full factorial ANOVA | 1.5 | No significant diagnosis x genotype interaction in all groups |
| Kittel-Schneider et al. 45 | 2015 | 18/23/0/30 | P/NP | FE | Medicated | DGKH | Structural MRI | ROI (manual tracing) | ANOVA | 1.5 | HC > BD > SZ in left amygdala volume. Significant gene × volume effect in right amygdala |
| Oertel-Knochel et al. 104 | 2015 | 50/24/0/20 | Chronic | Medicated | 7 SZ risk SNPs | Structural MRI | VBM | Hierarchal regression analyses | 3 | HC > SZ = BD in white matter volume, association between risk genes and white matter volume found only in HC, not SZ or BD | |
| Mallas et al. 105 | 2016 | 124/63/0/42 | NP | Chronic | Medicated | CACNAA1C | Diffusion tensor imaging | TBSS | ANOVA | 1.5 | No genotype main effect, no group by genotype interaction |
| Tandon et al. 145 | 2016 | 123/139/90/160 | P | Chronic | Medicated | Large number of SNPs | Structural MRI | Free surfer | Para-ICA | 3 | Four structural and three genetic components that showed overlapping relationships with the disease risk genes across illnesses |
HC healthy control, BD bipolar disorder, SZ schizophrenia, SAD schizoaffective disorder, P psychotic bipolar disorder, NP non-psychotic bipolar disorder, FE first episode, ROI region of interest, TBSS tract based spatial statistics, ANOVA analysis of variance, ICA independent components analysis, PCC posterior cingulate cortex
Discussion
In this systematic review, we sought to summarize converging and distinct neural signatures in schizophrenia and BD. Structural neuroimaging studies suggest white matter integrity deficits that are consistent, both in magnitude and spatial extent, across the psychosis spectrum, while gray matter reductions, especially those that are cortical, appear more widespread in schizophrenia compared to BD. Similarly, spectroscopy studies in cortical gray matter report evidence of decreased neuronal integrity in both disorders, but not enough data exists to draw firm conclusions as to differences in magnitudes and spatial distribution between illnesses. On a functional level, findings are inconsistent, possibly because of small sample sizes in many of the studies. Functional MRI studies using task paradigms typically report engagement of the same brain networks in healthy controls and patients across the psychosis spectrum, but find differential extent of alterations in magnitude of task related activation between illnesses depending on the task paradigm. The larger resting state connectivity studies are inconsistent as to abnormalities in the default mode network, but it appears that decreased fronto-parietal network connectivity may be a shared feature across the psychosis spectrum. The very limited imaging-genetic literature suggests a relationship between psychosis risk genes and brain structure, and possible gene by diagnosis interaction effects on functional imaging markers.
Our work needs to be considered in context of several limitations. We performed a systematic review, but did not perform quantitative assessments using meta-analytic techniques which could be informative in future work. We did not do separate analyses in schizophreniform disorder or schizoaffective disorder due to a lack of studies investigating these as a distinct disease category (schizoaffective disorder is most commonly grouped under schizophrenia). We decided not to include ultra-high risk subjects as conversion rates to psychotic illness is reported to be 30% or less,106 and psychotic depression due to the paucity of studies including this disease category.
It is important to note that the vast majority of studies have been conducted in chronically ill, medicated patients, which precludes us from being able to disentangle intrinsic illness characteristics from changes attributable to disease progression and exposure to psychotropic medications. While the existing literature suggests some shared and some distinct neural markers in across the psychosis spectrum, it will be imperative to conduct large, well designed, multi-modal neuroimaging studies in medication-naïve first episode patients that will be followed longitudinally over the course of their illness in an effort to advance our understanding of disease mechanisms and to resolve the illness dichotomy vs. illness spectrum debate. But because this type of studies are notoriously difficult to conduct, and sample sizes are typically fewer than 50 subjects,107–112 a potential alternative strategy may be to obtain very large, multi-site datasets and attempt to mitigate medication confounds with statistical adjustments.
Methods
Eligibility criteria
Studies were included if they presented original data published between January 2005 and December 2016 (last search December 9th 2016), compared individuals with BD and schizophrenia/schizoaffective disorder and healthy controls. Studies were not included when the healthy control group was genetically related to the patient groups. Studies published in languages other than English, post mortem studies, non-human studies, and review articles were excluded. We only included trials with ten or more healthy subjects, aged 18 or older. Studies expressively including subjects with comorbid substance use disorders, neurological or genetic diseases, or intellectual disabilities were not considered. When a single study was published in several articles, the article reporting the largest group was used. Articles that did not explicitly compare imaging findings between diagnostic groups were excluded as well.
Literature search
BB and NVK performed a literature search in PubMed including subjects across the psychosis spectrum using the following key words: (Schizophrenia AND bipolar disorder) AND (gray matter OR morphometry OR VBM OR volume OR white matter OR DTI OR magnetic resonance spectroscopy OR MRS OR functional MRI OR resting state). The reference lists of included studies, as well as relevant meta-analyses were inspected for additional eligible publications.
Study selection
After removal of duplicate articles, BB and NVK screened titles and abstracts retrieved from the search and selected potentially eligible studies for full text review. Both authors applied eligibility criteria, and a list of eligible full text articles was developed through consensus. Full text articles were then downloaded or requested from the university library and assessed for eligibility. Figure 1 describes the study selection process and outcome.
Data extraction
We extracted the following data from each study: name of first author, year of publication, number of participants per diagnostic category, illness duration, mood state, use of psychotropic medications, data acquisition parameters, magnetic field strength, data processing parameters, main study outcomes.
Acknowledgement
This work was supported by the National Institute of Mental Health (R01MH102951, ACL; K23MH106683, NVK).
Author contributions
A.C.L. designed the project. B.B. and N.V.K. collected the data, all authors contributed to interpretation of the data. B.B. and N.V.K. wrote the first draft of the manuscript. All authors critically revised and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Moller HJ. Bipolar disorder and schizophrenia: distinct illnesses or a continuum? J. Clin. Psychiatry. 2003;64:23–27. [PubMed] [Google Scholar]
- 2.Lake CR, Hurwitz N. Schizoaffective disorder merges schizophrenia and bipolar disorders as one disease—there is no schizoaffective disorder. Curr. Opin. Psychiatry. 2007;20:365–379. doi: 10.1097/YCO.0b013e3281a305ab. [DOI] [PubMed] [Google Scholar]
- 3.Lawrie SM, Hall J, McIntosh AM, Owens DG, Johnstone EC. The ‘continuum of psychosis’: scientifically unproven and clinically impractical. Br. J. Psychiatry. 2010;197:423–425. doi: 10.1192/bjp.bp.109.072827. [DOI] [PubMed] [Google Scholar]
- 4.Keshavan MS, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the schizo-bipolar scale. Schizophr. Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br. J. Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am. J. Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 8.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. doi: 10.1038/242190a0. [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw WS, Bottomley PA, Holland GN. Radiographic thin-section image of the human wrist by nuclear magnetic resonance. Nature. 1977;270:722–723. doi: 10.1038/270722a0. [DOI] [PubMed] [Google Scholar]
- 10.Viallon M, et al. State-of-the-art MRI techniques in neuroradiology: principles, pitfalls, and clinical applications. Neuroradiology. 2015;57:441–467. doi: 10.1007/s00234-015-1500-1. [DOI] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 12.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Bihan D, et al. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am. J. Neuroradiol. 2008;29:632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc. Natl Acad. Sci. USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwong KK, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl Acad. Sci. USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen BR, Buckner RL, Dale AM. Event-related functional MRI: past, present, and future. Proc. Natl Acad. Sci. USA. 1998;95:773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 21.Van Dijk KR, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc. Natl Acad. Sci. USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Barker PB. MR spectroscopy and spectroscopic imaging of the brain. Methods Mol. Biol. 2011;711:203–226. doi: 10.1007/978-1-61737-992-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci. Biobehav. Rev. 2015;51:276–295. doi: 10.1016/j.neubiorev.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr. Res. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Goodkind M, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivleva EI, et al. Gray matter volume as an intermediate phenotype for psychosis: bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) Am. J. Psychiatry. 2013;170:1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intravenous Ketamine for the Treatment of Mental Health Disorders: a Review of Clinical Effectiveness and Guidelines. Ottawa (ON). (2014). [PubMed]
- 31.Poletti S, et al. Adverse childhood experiences influence the detrimental effect of bipolar disorder and schizophrenia on cortico-limbic grey matter volumes. J. Affect. Disord. 2016;189:290–297. doi: 10.1016/j.jad.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 32.Yuksel C, et al. Gray matter volume in schizophrenia and bipolar disorder with psychotic features. Schizophr. Res. 2012;138:177–182. doi: 10.1016/j.schres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimol LM, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry. 2012;71:552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Song J, et al. Differences in gray matter volume corresponding to delusion and hallucination in patients with schizophrenia compared with patients who have bipolar disorder. Neuropsychiatr. Dis. Treat. 2015;11:1211–1219. doi: 10.2147/NDT.S80438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew I, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- 36.Hartberg CB, et al. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1122–1130. doi: 10.1016/j.pnpbp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Brown GG, et al. Voxel-based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatry Res. 2011;194:149–156. doi: 10.1016/j.pscychresns.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knochel C, et al. Multimodal assessments of the hippocampal formation in schizophrenia and bipolar disorder: evidences from neurobehavioral measures and functional and structural MRI. Neuroimage Clin. 2014;6:134–144. doi: 10.1016/j.nicl.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haukvik UK, et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol. Psychiatry. 2015;77:581–588. doi: 10.1016/j.biopsych.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Watson DR, et al. A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav. Brain Res. 2012;227:91–99. doi: 10.1016/j.bbr.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Nenadic I, et al. Brain structure in schizophrenia vs. psychotic bipolar I disorder: a VBM study. Schizophr Res. 2015;165:212–219. doi: 10.1016/j.schres.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Frazier JA, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34:37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Killgore WD, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cogn. Behav. Neurol. 2009;22:28–37. doi: 10.1097/WNN.0b013e318192cc67. [DOI] [PubMed] [Google Scholar]
- 44.Mahon PB, et al. An MRI study of amygdala in schizophrenia and psychotic bipolar disorder. Schizophr. Res. 2012;138:188–191. doi: 10.1016/j.schres.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kittel-Schneider S, et al. Influence of DGKH variants on amygdala volume in patients with bipolar affective disorder and schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265:127–136. doi: 10.1007/s00406-014-0513-9. [DOI] [PubMed] [Google Scholar]
- 46.Pina-Camacho, L. et al. Age at first episode modulates diagnosis-related structural brain abnormalities in psychosis. Schizophr Bull.43, 344–357 (2016). [DOI] [PMC free article] [PubMed]
- 47.Cui L, et al. Overlapping clusters of gray matter deficits in paranoid schizophrenia and psychotic bipolar mania with family history. Neurosci. Lett. 2011;489:94–98. doi: 10.1016/j.neulet.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 48.Molina V, et al. Different gray matter patterns in chronic schizophrenia and chronic bipolar disorder patients identified using voxel-based morphometry. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261:313–322. doi: 10.1007/s00406-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 49.Ivleva EI, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Womer FY, et al. Basal ganglia and thalamic morphology in schizophrenia and bipolar disorder. Psychiatry Res. 2014;223:75–83. doi: 10.1016/j.pscychresns.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Castro-Manglano P, et al. Structural brain abnormalities in first-episode psychosis: differences between affective psychoses and schizophrenia and relationship to clinical outcome. Bipolar Disord. 2011;13:545–555. doi: 10.1111/j.1399-5618.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- 52.Koo MS, et al. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch. Gen Psychiatry. 2008;65:746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pina-Camacho L, et al. Age at first episode modulates diagnosis-related structural brain abnormalities in psychosis. Schizophr. Bull. 2016;42:344–357. doi: 10.1093/schbul/sbv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao L, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:100–106. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 56.Kumar J, et al. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol. Med. 2015;45:759–770. doi: 10.1017/S0033291714001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui J, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011;57:839–855. doi: 10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McIntosh AM, et al. White matter tractography in bipolar disorder and schizophrenia. Biol. Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 59.Sussmann JE, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 60.Li J, et al. A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Res. 2014;221:58–62. doi: 10.1016/j.pscychresns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Skudlarski P, et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry. 2013;170:886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- 62.Anderson D, et al. Overlapping and distinct gray and white matter abnormalities in schizophrenia and bipolar I disorder. Bipolar Disord. 2013;15:680–693. doi: 10.1111/bdi.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colombo RR, et al. Voxelwise evaluation of white matter volumes in first-episode psychosis. Psychiatry Res. 2012;202:198–205. doi: 10.1016/j.pscychresns.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Lu LH, Zhou XJ, Keedy SK, Reilly JL, Sweeney JA. White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord. 2011;13:604–613. doi: 10.1111/j.1399-5618.2011.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandt CL, et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br. J. Psychiatry. 2014;204:290–298. doi: 10.1192/bjp.bp.113.129254. [DOI] [PubMed] [Google Scholar]
- 66.Milanovic SM, et al. Medial prefrontal cortical activation during working memory differentiates schizophrenia and bipolar psychotic patients: a pilot FMRI study. Schizophr. Res. 2011;129:208–210. doi: 10.1016/j.schres.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu G, et al. Effective connectivity of the posterior cingulate and medial prefrontal cortices relates to working memory impairment in schizophrenic and bipolar patients. Schizophr. Res. 2014;158:85–90. doi: 10.1016/j.schres.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 68.Kraguljac NV, Srivastava A, Lahti AC. Memory deficits in schizophrenia: a selective review of functional magnetic resonance imaging (FMRI) studies. Behav. Sci. 2013;3:330–347. doi: 10.3390/bs3030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costafreda SG, et al. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McIntosh AM, et al. Prefrontal function and activation in bipolar disorder and schizophrenia. Am. J. Psychiatry. 2008;165:378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- 71.Whalley HC, et al. Functional imaging of emotional memory in bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:840–856. doi: 10.1111/j.1399-5618.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 72.Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Transl. Psychiatry. 2012;2:e90. doi: 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagele C, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232:331–341. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 75.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 76.Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fornito A, Bullmore ET. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr. Opin. Neurobiol. 2015;30:44–50. doi: 10.1016/j.conb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network—functional MRI in bipolar disorder. J. Affect. Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 79.Ongur D, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meda SA, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl Acad. Sci. USA. 2014;111:E2066–2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meda SA, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol. Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mamah D, Barch DM, Repovs G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J. Affect. Disord. 2013;150:601–609. doi: 10.1016/j.jad.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker JT, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anticevic A, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr. Bull. 2014;40:1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu H, et al. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40:469–477. doi: 10.1093/schbul/sbt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samudra N, et al. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 2015;233:148–157. doi: 10.1016/j.pscychresns.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Argyelan M, et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40:100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kraguljac NV, et al. Neurometabolites in schizophrenia and bipolar disorder—a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marsman A, et al. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr. Bull. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kegeles LS, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate–glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 91.de la Fuente-Sandoval C, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014;24:1524–1532. doi: 10.1002/hipo.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gigante AD, et al. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 95.Molina V, et al. Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with chronic bipolar disorder. Eur. Psychiatry. 2007;22:505–512. doi: 10.1016/j.eurpsy.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Kalayci D, Ozdel O, Sozeri-Varma G, Kiroglu Y, Tumkaya S. A proton magnetic resonance spectroscopy study in schizoaffective disorder: comparison of bipolar disorder and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;37:176–181. doi: 10.1016/j.pnpbp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Sarramea Crespo F, et al. Biochemical changes in the cingulum in patients with schizophrenia and chronic bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258:394–401. doi: 10.1007/s00406-008-0808-9. [DOI] [PubMed] [Google Scholar]
- 98.Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atagun MI, et al. Investigation of Heschl’s gyrus and planum temporale in patients with schizophrenia and bipolar disorder: a proton magnetic resonance spectroscopy study. Schizophr. Res. 2015;161:202–209. doi: 10.1016/j.schres.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atagun, M. I. et al. Perisylvian GABA levels in schizophrenia and bipolar disorder. Neurosci. Lett.637, 70–74 (2017). [DOI] [PMC free article] [PubMed]
- 101.Mechelli A, et al. The effects of neuregulin1 on brain function in controls and patients with schizophrenia and bipolar disorder. Neuroimage. 2008;42:817–826. doi: 10.1016/j.neuroimage.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 102.Prata DP, et al. No association of disrupted-in-schizophrenia-1 variation with prefrontal function in patients with schizophrenia and bipolar disorder. Genes Brain Behav. 2011;10:276–285. doi: 10.1111/j.1601-183X.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 103.Papagni SA, et al. Differential effects of DAAO on regional activation and functional connectivity in schizophrenia, bipolar disorder and controls. Neuroimage. 2011;56:2283–2291. doi: 10.1016/j.neuroimage.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 104.Oertel-Knochel V, et al. Schizophrenia risk variants modulate white matter volume across the psychosis spectrum: evidence from two independent cohorts. Neuroimage Clin. 2015;7:764–770. doi: 10.1016/j.nicl.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mallas, E. et al. The impact of CACNA1C gene, and its epistasis with ZNF804A, on white matter microstructure in health, schizophrenia and bipolar disorder1. Genes Brain Behav. doi:10.1111/gbb.12355 [epub ahead of print] (2016). [DOI] [PubMed]
- 106.Simon AE, Umbricht D, Lang UE, Borgwardt S. Declining transition rates to psychosis: the role of diagnostic spectra and symptom overlaps in individuals with attenuated psychosis syndrome. Schizophr. Res. 2014;159:292–298. doi: 10.1016/j.schres.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 107.Kraguljac NV, et al. Abnormalities in large scale functional networks in patients with schizophrenia and effects of risperidone. Neuroimage Clin. 2016;10:146–158. doi: 10.1016/j.nicl.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kraguljac NV, et al. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr. Bull. 2016;42:1046–1055. doi: 10.1093/schbul/sbv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hadley JA, et al. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. NPJ Schizophr. 2016;2:16014. doi: 10.1038/npjschz.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarpal, D. K. et al. Baseline striatal functional connectivity as a predictorof response to antipsychotic drug treatment. Am. J. Psychiatry. 173, 69–77 (2016). [DOI] [PMC free article] [PubMed]
- 111.Sarpal DK, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hadley JA, et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39:1020–1030. doi: 10.1038/npp.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol. Psychiatry. 2005;58:713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 114.Strasser HC, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol. Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 115.McDonald C, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am. J. Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 116.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch. Gen. Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakamura M, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol. Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morgan KD, et al. Grey matter abnormalities in first-episode schizophrenia and affective psychosis. Br. J. Psychiatry Suppl. 2007;51:s111–116. doi: 10.1192/bjp.191.51.s111. [DOI] [PubMed] [Google Scholar]
- 119.Reite M, et al. Brain size and brain/intracranial volume ratio in major mental illness. BMC Psychiatry. 2010;10:79. doi: 10.1186/1471-244X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radonic E, et al. Comparison of hippocampal volumes in schizophrenia, schizoaffective and bipolar disorder. Coll. Antropol. 2011;1:249–252. [PubMed] [Google Scholar]
- 121.Ratnanather JT, et al. Morphometry of superior temporal gyrus and planum temporale in schizophrenia and psychotic bipolar disorder. Schizophr. Res. 2013;150:476–483. doi: 10.1016/j.schres.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Findikli E, et al. Pineal gland volume in schizophrenia and mood disorders. Psychiatr. Danub. 2015;27:153–158. [PubMed] [Google Scholar]
- 123.Laidi C, et al. Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatr. Scand. 2015;131:223–233. doi: 10.1111/acps.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shepherd AM, et al. Shared intermediate phenotypes for schizophrenia and bipolar disorder: neuroanatomical features of subtypes distinguished by executive dysfunction. J. Psychiatry Neurosci. 2015;40:58–68. doi: 10.1503/jpn.130283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amann, B. L. et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr. Scand. 133, 23–33 (2016). [DOI] [PMC free article] [PubMed]
- 126.Royer C, et al. Functional and structural brain asymmetries in patients with schizophrenia and bipolar disorders. Schizophr. Res. 2015;161:210–214. doi: 10.1016/j.schres.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 127.Reavis, E. A. et al. Cortical thickness of functionally defined visual areas in schizophrenia and bipolar disorder. Cereb. Cortex. pii:bhw151 [epub ahead of print] (2016). [DOI] [PMC free article] [PubMed]
- 128.Knochel C, et al. Cortical thinning in bipolar disorder and schizophrenia. Schizophr. Res. 2016;172:78–85. doi: 10.1016/j.schres.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 129.Nenadic I, et al. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J. Affect. Disord. 2015;185:104–107. doi: 10.1016/j.jad.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 130.Cui L, et al. Assessment of white matter abnormalities in paranoid schizophrenia and bipolar mania patients. Psychiatry Res. 2011;194:347–353. doi: 10.1016/j.pscychresns.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 131.Hamilton LS, et al. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Hum. Brain Mapp. 2009;30:3958–3969. doi: 10.1002/hbm.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Costafreda SG, et al. Increased inferior frontal activation during word generation: a marker of genetic risk for schizophrenia but not bipolar disorder? Hum. Brain Mapp. 2009;30:3287–3298. doi: 10.1002/hbm.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hall J, et al. Hippocampal function in schizophrenia and bipolar disorder. Psychol. Med. 2010;40:761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 134.Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr. Bull. 2014;40:675–684. doi: 10.1093/schbul/sbt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L, Opmeer EM, Ruhe HG, Aleman A, van der Meer L. Brain activation during self- and other-reflection in bipolar disorder with a history of psychosis: comparison to schizophrenia. Neuroimage Clin. 2015;8:202–209. doi: 10.1016/j.nicl.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chai XJ, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front. Hum. Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meda SA, et al. Frequency-specific neural signatures of spontaneous low-frequency resting state fluctuations in psychosis: evidence from bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) consortium. Schizophr. Bull. 2015;41:1336–1348. doi: 10.1093/schbul/sbv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Du Y, et al. A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. Neuroimage. 2015;122:272–280. doi: 10.1016/j.neuroimage.2015.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anticevic A, et al. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr. Bull. 2015;41:133–143. doi: 10.1093/schbul/sbu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hager, B. et al. Neural complexity as a potential translational biomarker for psychosis. J. Affect. Disord. doi:10.1016/j.jad.2016.10.016 [epub ahead of print] (2016). [DOI] [PMC free article] [PubMed]
- 142.Skatun KC, et al. Global brain connectivity alterations in patients with schizophrenia and bipolar spectrum disorders. J. Psychiatry Neurosci. 2016;41:331–341. doi: 10.1503/jpn.150159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ongur D, et al. T2 relaxation time abnormalities in bipolar disorder and schizophrenia. Magn. Reson. Med. 2010;63:1–8. doi: 10.1002/mrm.22148. [DOI] [PubMed] [Google Scholar]
- 144.Tesli M, et al. CACNA1C risk variant and amygdala activity in bipolar disorder, schizophrenia and healthy controls. PLoS One. 2013;8:e56970. doi: 10.1371/journal.pone.0056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tandon, N. et al. Novel gene-brain structure relationships in psychoticdisorder revealed using parallel independent component analyses. Schizophr. Res. doi:10.1016/j.scres.2016.10.026 [epub ahead of print] (2016). [DOI] [PubMed]


