Abstract
Behavioral and neuroplastic changes occurring in the development of addiction parallel those that occur in social bonding. This has led to speculation that drugs of abuse co-opt systems that subserve social attachment to shift attachment to drugs of abuse. Oxytocin, a neuropeptide that is important in social bonding, has been shown in rodents to decrease psychostimulant self-administration, locomotor activity, and conditioned place preference, it is unclear what role it may play in human drug addiction. In this double-blind, placebo-controlled crossover study, 23 cocaine-dependent inpatients in court-ordered treatment completed 4 task sessions measuring desire to use cocaine, cue-induced craving, monetary reward decisions and social cognition. Before each session, subjects administered 24 IU of intranasal oxytocin or placebo. Oxytocin increased desire to use cocaine and cue-induced excitability with no effect on cue-induced desire to use. Oxytocin also removed the effect of state anger on several measures of cue reactivity. Response to monetary reward increased under oxytocin and measures of social cognition worsened. The significant increase in the desire for drug and monetary reward as well as the significant decrease in measures of social cognition was small but warrant further study of the effect of oxytocin’s effect in cocaine dependent subjects. The effect of oxytocin to modulate the relationship between state anger and cue reactivity should be explored further for potential therapeutic use of oxytocin in cocaine dependent patients. These findings are discussed in light of the human and rodent oxytocin literature.
Keywords: Oxytocin, Addiction, Cocaine, Reward, Social cognition, Cue reactivity
1. Introduction
Behavioral and neuroplastic changes occurring in the development of addiction parallel those that occur in social bonding (Burkett and Young, 2012). This has led to speculation that drugs of abuse co-opt systems that subserve social attachment to shift attachment to drugs of abuse at the expense of social affiliation (Insel, 2003; Burkett and Young, 2012). The peptide oxytocin (OT) has been shown to facilitate pair-bond formation in animal models of social affiliation, such as prairie voles (Liu and Wang, 2003). Pair-bonded animals display a preference for their mate and are less likely to form a new pair bond (Young and Wang, 2004). OT and dopamine signaling in the striatum are both necessary for pair bond formation (Young and Wang, 2004), with OT appearing to facilitate dopamine effects (Insel, 2003). Further, in prairie voles, pair-bonding has been shown to decrease the rewarding properties of psychostimulants (Liu et al., 2011); conversely, drugs of abuse, have been shown to reduce affiliative behavior (Liu et al., 2010), suggesting that drugs may elicit a pair-bond-like response to drug that inhibits new pair bonding.
The direct effects of exogenous OT on drug related behaviors in affiliative species such as the prairie vole have not been examined. However, in non-affiliative rodents, OT has been shown to decrease psychostimulant self-administration (Carson et al., 2010;Carson et al., 2013), locomotor activity (Sarnyai and Kovacs, 1994), conditioned place preference (Qi et al., 2009), alcohol consumption (Bowen et al., 2011) and mitigate withdrawal symptoms from morphine and alcohol (Kovacs et al., 1998; Sarnyai and Kovacs, 1994). It is not clear what effects exogenous OT may have in affiliative animals that are already drug dependent.
Human addiction studies have shown that OT decreases symptoms of alcohol withdrawal and reduces the dose of benzodiazepine needed for detoxification, perhaps by rapidly reversing tolerance (Pedersen et al., 2013). OT has also been shown to reduce stress-induced craving in current cannabis dependent individuals (McRae-Clark et al., 2013). However, no studies have looked at its effects in humans apart from the contexts of withdrawal and stress provocation. Given the human literature in non-drug users, OT’s effect to increase measures of prosocial functioning such as trust (Kosfeld et al., 2005) and the prominent role of social rehabilitation in successful drug addiction treatment programs (McGregor and Bowen, 2012), OT has been proposed as a potential treatment that may promote improved social functioning that would be beneficial in the treatment of addiction, beyond its effects on acute withdrawal (McGregor and Bowen, 2012).
However, there are no human studies aimed to look at the effects of OT in cocaine addiction. Of special note, there are reasons for some caution in developing OT for treatment in drug addiction, especially cocaine dependence. As OT may facilitate the effects of dopamine (Insel, 2003), which is involved in the reinforcing properties of and cue reactivity to drugs (Volkow et al., 2006;Wong et al., 2006), exogenous OT could exacerbate drug-related behaviors in dependent individuals. Further, chronicity of drug dependence independently impacts human affiliative mechanisms (Flores, 2001) so that chronic drug dependent subjects may not exhibit the prosocial responses to exogenous OT reported in control subjects (Meyer-Lindenberg et al., 2011). In addition, recent studies have extended previous findings of the prosocial, antistress, and anxiolytic effects (Meyer-Lindenberg et al., 2011) of OT to underscore the demonstrated person and context specific effect of the peptide (Bartz et al., 2011). OT has been shown to increase memory of negative events (Bartz et al., 2010) in persons with insecure attachment patterns, startle response to aversive social stimuli (Striepens et al., 2012) and, in a nonsocial context, anxiety to unpredictable threat (Striepens et al., 2012), perhaps an adaptive response to uncertain circumstances.
We therefore sought to examine, in this pilot study, the effect of exogenously administered OT on chronic cocaine-dependent patients in treatment to investigate, primarily, the effect of exogenously administered OT on desire to use and on drug cue reactivity. Also, given recent reports on the pro-anxiety effect of OT in certain contexts (Grillon et al., 2013; Striepens et al., 2012; Macdonald et al., 2013), and the known relationship of anxiety and anger to drug use (Marlatt GA, 85 A.D.; Walfish et al., 1990), we investigated how trait and state anxiety and anger affects the desire to use and drug cue reactivity following oxytocin compared to placebo administration. Secondarily, since money is a cue for drugs in dependent individuals and OT has been shown to enhance measures of social cognition in normal adults, we sought to explore the effect of OT in cocaine dependent individuals on response to tasks involving decisions resulting in monetary reward and measures of social cognition.
2. Experimental procedures
2.1. Participants
Data were collected from 23 cocaine dependent patients (22 men) who were in inpatient treatment for cocaine dependence (Table 1), with smoked crack cocaine as their drug of choice. All subjects participated in a jail-based diversion program, i.e., they were court-ordered to 6 months inpatient treatment following incarceration. Their last use of cocaine immediately preceded their placement into a controlled environment; hence, they met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for current cocaine dependence, in a controlled environment. After complete description of the study to the subjects, written informed consent was obtained on this protocol approved by the National Institute on Drug Abuse-Intramural Research Program (NIDA-IRP) Institutional Review Board with approval for research with prisoners. Screening procedures included a history and physical exam, a comprehensive laboratory panel and an EKG. Participants were excluded if they had any current major medical or neurologic illnesses or current major psychiatric disorders other than substance abuse or dependence such as psychosis, bipolar disorder or major depression. Cognitive function was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI), vocabulary subscore. Psychiatric diagnoses were confirmed by the Structured Clinical Interview for DSM-IV-TR (SCID).
Table 1.
Subject characteristics (mean±SD (range).
| N | 23 |
| Male/female | 22/1 |
| Age (years) | 38.5±11.4 (21–52) |
| (WASI*, vocabulary subscore) | 53.0±9.2 (32–68) |
| Years cocaine use | 19.7±9.5 (4–32) |
| Years drug use | 24.6±11.9 (8–40) |
| Longest lifetime period of | 30.9±26.7 |
| abstinence (months) | (2–117) |
| Time since last use (months) | 28.0±27.8 (0.5–117) |
| BDI** | 8.4±6.6 (0–24) |
Wechsler abbreviated scale of intelligence.
Beck depression inventory.
All experimental procedures took place at a residential treatment center. Patients were able to leave the treatment center only under staff supervision. Random urine drug and breath alcohol testing was done at the center and patients were discharged from treatment in the event of a positive urine drug screen or breath alcohol test. Individuals were not able to be in the study if they tested positive for illicit drugs or alcohol (smoking was allowed). Six of the 23 subjects were on one or more psychoactive medications for mood symptoms: atomoxetine (n=2), valproate (n=3), buproprion (n=1), sertraline (n=1). These subjects, however, did not meet DSM-IV-TR criteria for current or past bipolar or major depressive disorders. Subjects were taking the medications before study entry and were on a stable dose that did not change through the course of the study.
2.2. Procedure
In this randomized, double-blind, placebo-controlled crossover study, subjects completed 2 days of tasks (2 sessions/day; AM and PM; each session lasted approximately 60 min) separated by 3–7 days. AM and PM task sessions (details below) were done in order to accommodate administration of multiple tasks employed and the expected duration of action as assessed by cerebrospinal fluid levels of peptides administered intranasally [vasopressin (Born et al., 2002) and OT (Striepens et al., 2013)] and behavioral effects (Macdonald et al., 2011). Before each daily task session, subjects administered 24 IU of intranasal OT (Syntocinon; Novartis) or placebo (PL) approximately 45 min before beginning the tasks in accordance with standard practice (Macdonald et al., 2011). The tasks were completed in fixed order to preclude residual effect of one task on another or time since drug administration. Both drug administrations on a study day were the same, but the order of OT and placebo days was randomized. State and trait anxiety and anger were assessed by the Spielberger State-Trait Anxiety and Anger Inventory (STAI) (Spielberger, 1983) at study screening (trait) and immediately before and approximately 30 min after each drug administration (state). After participation in this study, subjects entered a treatment study (NCT 00975416) involving cognitive behavioral therapy and OT, which was subsequently terminated due to subject drop out and budget limitations.
2.3. Behavioral tasks and performance order
AM session
Delayed discounting questionnaire (Kirby et al., 1999) consisted of 27 items where subjects choose between small, immediate and larger, delayed rewards. The questionnaire is scored yielding a discounting constant, k, for small, medium and large rewards that describes how quickly a person devalues rewards that are not immediately received.
Charitable donation (modified from Moll et al. (2006)): Subjects are given an endowment of money, which they can use to give or oppose monetary contributions to charitable organizations related to major societal causes. The task yields 5 conditions: costly donation or opposition, (a measure of altruism); non-costly donation or opposition (a measure of generosity); and pure monetary reward given to the subject with no contribution or denial of contribution to the organization.
-
Urge to use: subjects were asked to rate on a 7 point visual analog scale (VAS) their urge to use drugs by answering: “If your drug of choice was in front of you right now, what’s the likelihood that you would use?” In addition, 5 questions related to arousal were also posed to serve as baseline measures for the later cue reactivity session:
How excited do you feel right now?
How irritated do you feel right now?
Is your heart pounding right now?
How upset are you that you can’t use right now?
How worked up do you feel right now?
During the cue reactivity task, these 6 questions were repeated immediately after subjects viewed each of 6 unique videoclips of cocaine-related scenes from films and 6 unique videoclips of neutral scenes matched for visual and auditory content. Each videoclip was 30 s long and presented in 3 blocks of 4 videos each (cocaine alternating with neutral).
PM session
Social exchange (King-Casas et al., personal communication) task: a 30-round modified dictator game, in which players compete for control of an endowment. Within each round, players are designated as either Alpha or Beta. At the start of each round Alpha is endowed with $10 and may transfer between $0 and $4 to Beta. Beta is endowed with $10 and may choose to challenge Alpha (or not) with up to $10 to gain the Alpha position in the next round and Alpha may defend his position with up to $10. The person who spends more during the challenge assumes the Alpha role in the next round. Overall rates of challenge as well as average amounts of challenge and defense were calculated. As it is costly for both Alpha and Beta to defend and challenge respectively, rates of challenge and defend should be lower following more equitable transfers resulting in enhanced cooperation and maximized winnings.
Reading the mind in the eyes (RMET) (Baron-Cohen et al., 1999): Subjects infer the internal emotional state of another person by evaluating photos of the eye region of 36 different human faces. Outcome is percent correct identification of the emotional state. Intranasal oxytocin has been shown to improve performance on this task in healthy control subjects (Domes et al., 2007).
Reinforcement learning (Pizzagalli et al., 2005): A signal detection task where one of two barely discernible stimuli is reinforced with monetary reward. Three hundred trials are presented across each of three blocks of 100 trials. A reward bias and discriminability score is calculated for each block. Reward learning on this task has been shown to be sensitive to dopaminergic agonists (Santesso et al., 2009) and correlated with measures of anhedonia (Pizzagalli et al., 2005).
2.4. Analysis
2.4.1. State measures of anxiety and anger
The drug effect on symptom measures of anxiety and anger for AM and PM sessions was calculated with repeated measures ANOVA with drug (OT/PL) and time (pre/post-drug administration) as within subject factors.
2.4.2. Baseline drug craving
Repeated measures ANOVA was used to measure the effect of OT on urge to use with study drug (OT/PL) as a within-subject factor.
2.4.3. Cue-induced drug craving
The within-session differences in response to drug and neutral cues were used as outcome variables (response after drug cue video minus response after neutral video) for each of the six outcome measures. To test the effect of OT on difference in response to drug vs. neutral cues, while controlling for any differences in baseline response level, repeated-measures regression was applied with OT or PL as an independent variable and the response observed during the corresponding baseline session as a time-varying covariate for each of the 6 change scores.
2.4.4. Correlations between trait anger/anxiety and baseline desire to use and cue reactivity
Trait and state measures of anger and anxiety were correlated with (1) baseline desire to use (gathered after OT or PL administration; Spearman’s correlation) and (2) cocaine minus neutral cue difference scores for all six questions in the cue reactivity task (Pearson’s partial correlation). Separate correlation coefficients were calculated for each study drug condition (OT and PL). Repeated measures regression was used to determine if the correlations between drug conditions were different. Each regression model consisted of a cocaine craving measure as the dependent variable, with study drug condition and state and trait anger/anxiety as independent variables. The baseline level of the cocaine craving measure for each study drug condition was used as the covariate for the corresponding change score to control for any differences in baseline cocaine craving level.
2.4.5. Monetary decision making and social cognition
A secondary aim in this pilot study was to explore, overall, the differential effect of OT on decision making in the context of pure monetary reward compared to that in the context of socially salient stimuli, rather than the effect of oxytocin on any one individual task outcome. Further, in this small pilot study we lacked the power to look at the drug effect on individual task outcomes. As such, we grouped task outcomes into two domains: pure monetary reward (MR) and social cognition (SC) decision making. Repeated measures ANOVA was used to determine within-subject factors of TASK DOMAIN (MR/SC decision making) and DRUG (OT/PL) and a TASK DOMAIN × DRUG interaction, that is, whether OT affected performance on monetary decision making tasks differently from social cognition tasks. Performance on each task was the dependent variable. Separate task performance measures for monetary decision making included delayed discounting constants for small, medium and large rewards, pure reward condition of the charitable donation task, and reward bias scores. Social cognition outcomes included costly and non-costly opposition and donation, performance on the RMET and performance on the social exchange task (amount of challenge/defend and rate of challenge). Social exchange outcomes were reversed scored to be consistent with scoring on the other social cognition tasks as lower scores on the social exchange task represent higher levels of social cooperation. Since tasks were not assessed on the same measurement scales, standardized scores were created by dividing each individual score by the within-group SD (i.e., for each drug condition and task). We followed up on the significant DRUG × TASK DOMAIN interaction with paired t-tests for the SC vs. MR measures and further followed up with paired t-tests to determine which individual task outcomes contributed to the results. Statistical analyses were conducted using SPSS and SAS with alpha levels set at p<0.05 throughout.
3. Results
3.1. Anxiety/anger symptoms
There was no effect of drug on state measures of anxiety or anger for any of the sessions (anxiety: AM session: df=1,21; F=0.098; p=0.757; PM session: df=1,22; F=0.189; p=0.668. Anger: AM session: df=1,21; F=0.195; p=0.664; PM session: df=1,21; F=1.279; p=0.271).
3.2. Urge to use
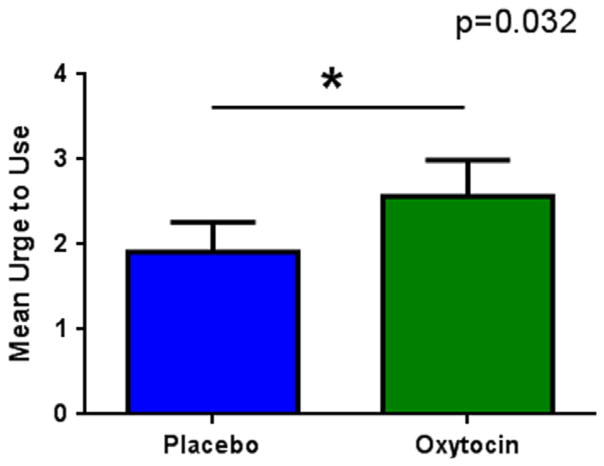
The effect of OT on desire to use before exposure to cues was small (mean±SE: OT=2.57±0.39; PL=1.91±0.39) but significant (df=1,22; F=5.22, p=0.032), Figure 1, where desire to use was augmented under OT.
Figure 1.
Oxytocin effect on urge to use (mean±SE).
3.3. Cue reactivity
There was a small (mean±SE: OT=0.71±0.28; PL=0.19± 0.27) but significant (df=1,16; F=5.28; p=0.035) positive association between OT and excitability rating after exposure to drug cues, where under OT, exposure to drug vs. neutral cues increased the rating of excitement. There was no significant drug effect for the other 5 craving questions posed.
3.4. Correlations
Trait anger/anxiety (Table 2): While there were some significant correlations between trait anger or anxiety and baseline desire to use or cue reactivity measures, none was significantly different across study drug conditions.
Table 2.
Correlations between trait anger and anxiety, baseline desire to use and cue reactivity. (p<0.05) correlations are highlighted in bold type.
| BL Desire to Use | Placebo | Oxytocin | ||
|---|---|---|---|---|
|
|
|
|||
| Anger R=0.56, p=0.006 |
Anxiety R=0.29, p=0.17 |
Anger R=0.66, p=0.0006 |
Anxiety R=0.42, p=0.049 |
|
| Drug – neutral cue difference scores | ||||
| Excitability | R=0.42, p=0.06 | R=0.44, p=0.052 | R=0.53, p=0.02 | R=0.33, p=0.17 |
| Irritability | R= −0.13, p=0.56 | R=0.21, p=0.35 | R=0.51, p=0.02 | R=0.53, p=0.01 |
| Worked up | R=0.33, p=0.13 | R=0.48, p=0.03 | R=0.44, p=0.04 | R=0.30, p=0.18 |
| Heart pound | R=0.37, p=0.09 | R=0.48, p=0.03 | R=0.39, p=0.07 | R=0.03, p=0.91 |
| Upset | R=0.39, p=0.08 | R=0.61, p=0.003 | R=0.40, p=0.07 | R=0.45, p=0.04 |
| Desire to use | R=0.46, p=0.03 | R=0.31, p=0.16 | R=0.14, p=0.52 | R=0.26, p=0.24 |
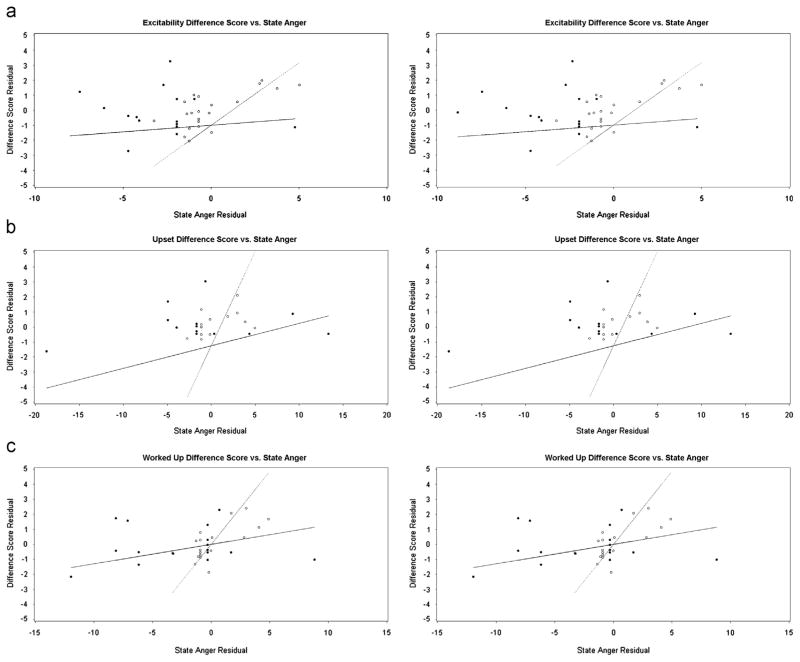
State anger/anxiety (Table 3): Regression models assessing the association between state anger and three cue reactivity variables were significantly different across drug conditions after adjusting for baseline craving level (Figure 2): excitability change score (df=2,22; F=7.62; p=0.0032), worked-up change score (df=2,22F=7.77; p=0.0030), and upset change score (df=2,22; F=27.62; p<0.0001). For each of these cue reactivity measures, OT disrupted the association between state anger and cue reactivity. There were no significant differences in the relationship of state anxiety to cue reactivity measures between the study drug conditions.
Table 3.
Correlations between State Anger and Anxiety, baseline desire to use and cue reactivity measures. (p<0.05) correlations are highlighted in bold type.
| BL desire to use | Placebo | Oxytocin | ||
|---|---|---|---|---|
|
|
|
|||
| Anger R=0.24, p=0.28 |
Anxiety R=0.55, p=0.0087 |
Anger R=0.28, p=0.20 |
Anxiety R=0.32, p=0.15 |
|
| Drug – neutral cue difference scores | ||||
| Excitability | R=0.71, p=0.0005* | R= −0.11, p=0.64 | R=0.31, p=0.21* | R=0.28, p=0.26 |
| Irritability | R=0.33, p=0.15 | R=−0.16, p=0.49 | R=0.32, p=0.16 | R=0.72, p=0.0003 |
| Worked up | R=0.72, p=0.0002* | R=−0.05, p=0.82 | R=0.37, p=0.099* | R=0.35, p=0.12 |
| Heart pound | R=0.78, p<0.0001 | R=0.06, p=0.80 | R=0.56, p=0.008 | R=0.34, p=0.14 |
| Upset | R=0.55, p=0.011* | R=0.10, p=0.69 | R=0.21, p=0.36* | R=0.45, p=0.04 |
| Desire to use | R=0.42, p=0.057 | R=0.003, p=0.99 | R= −0.06, p=0.79 | R=0.31, p=0.18 |
Significant difference between drug conditions.
Figure 2.
Residual plots by drug condition of cue reactivity measures vs. state anger partialing out baseline response on cue reactivity measures: (a) excitability, (b) upset, (c) worked up. [Closed circles, solid line: oxytocin; open circles, dotted line: placebo; evaluable data from (a) n=18, (b) n=21, (c) n=22 subjects].
3.5. Monetary decision making (MR) and social cognition (SC)
There was a significant DRUG × TASK domain interaction (df=1, 159; F=20.341; p<0.001), where performance for MR increased under OT (mean±SE: PL=0.644±0.080; OT=0.875±0.079) and decreased for SC (PL=3.061± 0.150; OT=2.602±0.875). Post-hoc paired t-tests for each domain showed a significant drug effect for SC tasks (OT–PL: mean difference= −0.28, SE=0.09, t= −3.17, p=0.002), and for MR tasks (OT–PL mean difference=0.30, SE=0.08, t= −3.94, p<0.001), indicating OT enhanced responding for monetary rewards and impaired social cognition. Paired t-tests for individual task outcomes revealed a significant drug effect only for delayed discounting for small rewards (p=0.05), pure monetary reward condition on the charitable donation task (p=0.04) and reward bias for block 1 of the reward bias task (p=0.01). For each of these results, OT increased response for monetary reward compared to placebo.
4. Discussion
We report here the effect of intranasal OT on desire to use and on aspects of cue reactivity in cocaine dependent subjects in a controlled environment. This was the first human study that investigated the effects of OT in cocaine addiction. In response to drug cues, OT disrupted the relationship between state anger and several measures of cue reactivity while increasing the baseline desire to use and excitement in response to drug cues. OT also differentially impacted performance on composite measures of monetary reward and social cognition, increasing the former and decreasing the latter.
In rodent models, acute administration of OT decreases psychostimulant self-administration, stress induced reinstatement and decreases dopamine release after ICV cocaine administration (Yang et al., 2010). However, it should be noted that these preclinical OT studies were carried out using non-affiliative rodent models of self-administration that may be of limited relevance to human addiction (Belin et al., 2008) and fail to model important aspects of human social interactions (Young and Wang, 2004).
Two recent human studies with alcohol and marijuana dependent subjects have shown that intranasal OT reduces anxiety and craving in the context of alcohol withdrawal (Pedersen et al., 2013) and reduces laboratory stress-induced marijuana craving, anxiety and DHEA levels (McRae-Clark et al., 2013). This apparently contrasts with our results in long-term abstinent, cocaine-dependent individuals in a controlled environment, underscoring the context-dependent effect of this peptide extensively reported in the human literature (Macdonald and Macdonald, 2010). While OT may have a role in addressing acute alcohol withdrawal and social stress in marijuana dependent individuals, its effects in long-term abstinent cocaine dependent individuals in a controlled environment appear to shift salience toward drug and monetary reward and decrease performance overall on social cognition tasks.
Insel (2003) proposes that drugs of abuse act on neural circuitry underlying motivated behaviors such as maternal and pair-bonding that are themselves mediated by OTand dopamine within mesolimbic reward pathways. Correspondingly, in animal models of social bonding (i.e., prairie voles), drugs of abuse have been shown to reduce affiliative behavior, via altered dopamine signaling in the ventral tegmental area and nucleus accumbens (Young et al., 2011). In theory, administration of OT in this context should augment dopaminergic signaling in mesocorticolimbic pathways in response to drug-related stimuli (Volkow et al., 2006; Wong et al., 2006) and, as a result, shift motivated behaviors toward those that are characteristic of the addicted phenotype: that is, increased desire for drug and monetary reward, with decreasing motivation for social affiliation. Consistent with this hypothesis, the results reported here indicate a significant effect of OT on increasing the desire for drug and monetary reward as well as decreasing measures of social cognition. On social cognition tasks, therefore, (with the exception of the RMET, which is a social perception accuracy task and has no reward contingency), the effect of OT in our study was to shift reward decisions to favor the self rather than others.
In this study, OT was administered to abstinent cocaine dependent subjects who were diverted from incarceration to inpatient treatment for drug dependence. Their last use of cocaine (28 months on average, Table 1) immediately preceded their entry into prison. Jail-based diversion programs attempt to treat a particularly recalcitrant population, beset by multiple socioeconomic deficits (Sirotich, 2009). Relapse during diversion programs results in significant, far-reaching consequences, including return to jail (Sirotich, 2009). For these patients, the combination of loss of freedom, multiple social ills, and the threat of re-incarceration might approximate social defeat. While OT enhanced, albeit modestly, the baseline desire to use, which is consistent with the model proposed by Insel (2003), it augmented cue-induced excitement but not desire to use. In the context of court-ordered treatment, in which drug use is very likely to result in immediate re-incarceration, the cue-induced enhancement of excitability without enhanced desire to use may reflect the social threat that drug use carries for these individuals. The increase in excitement under OT in response to drug-related stimuli, therefore, may be similar to the heightened vigilance to threat that has been reported in other human studies with OT (Grillon et al., 2013;Striepens et al., 2012) and consistent with the potentiation of fear after social defeat seen in the mouse model (Guzman et al., 2013).
The correlations between trait anger/anxiety and cue reactivity outcome measures at baseline and in response to drug cues, showed that the positive significant correlation between state anger and desire to use and other cue reactivity measures under PL was absent under OT. Thus, OT mitigated the effect of state anger on several cue reactivity measures. Drug users have a significantly higher state as well as trait anger (Aharonovich et al., 2001) concurrent with less control over their anger (De Moja and Spielberger, 1997). Further, as state anger is known to play a role in drug taking behavior and relapse (Fox et al., 2008), this result is particularly intriguing. State anger is also highly correlated with subjective perceptions of emotional entrapment (Allan and Gilbert, 2002) which contributes to social defeat. OT enhanced excitability to drug cues, which may have represented heightened social threat in this population, while simultaneously interfering with the relationship between state anger and various measures of cue reactivity. The observed effect of OT on these parameters is especially noteworthy given the long abstinence of the participants in this study. The correlation between state anger and cue-reactivity may represent a long-term neuroadaptation due to chronic drug use. These results raise the question of whether OT is impacting this neuroadaptation as has been demonstrated in preclinical studies (Sarnyai and Kovacs, 1994) and suggested in recent human study (Pedersen et al., 2013). If so, OT would provide an important potential treatment for addiction.
While OT treatment has been proposed to remediate deficits in endogenous OT seen in addiction to various substances (McGregor and Bowen, 2012;McGregor et al., 2008), or to promote social bonding that has been shown to decrease the rewarding effects of psychostimulants (Liu et al., 2011), this pilot study highlights the complexity of OT’s actions (Bartz et al., 2011) in this population of cocaine dependent patients in a controlled environment. Its effect to disrupt the relationship between anger and cue reactivity may be useful therapeutically. For example, as OT can be administered intranasally, it could be used in advance ‘as needed’, prior to situations carrying high risk for anger-related relapse by interrupting the driving force of state anger on drug taking behavior.
Nevertheless, it should be emphasized that these results are preliminary. The specific population and the controlled environment where the study was conducted limit extrapolation to the broader outpatient context where exposure to drug cues and availability is more prominent. Baseline measures of desire to use as well as cue-induced craving were at low levels; and the magnitude of drug effect on these parameters was small, albeit statistically significant, necessitating further study with, perhaps, more robust craving paradigms. Further, the sample size is small and results for cue responsivity would not survive Bonferroni correction. While the analysis for a drug effect on MR/SC measures was conducted in a single AVOVA, resulting in greater confidence in the statistical result, combining measures into single MR and SC scales, while having face validity, has not been reported to date in the literature. Further studies should be done to examine the effect of OT on individual measures of SC and MR. Finally, some subjects were on concomitant medications that could possibly interact with the action of OT and with task performance. Certainly, further research in humans is needed to explore the effect of OT in addiction and the mechanisms underlying OT’s effect on neurocircuitry related to reward and social salience in drug addiction.
While this study reports results from a single administration of OT, it raises caution with respect to OT as a stand-alone therapeutic tool in drug dependence, as it may trigger desire to use and may not increase intended social affiliation to non-drug related stimuli. However its potential ability to disrupt the relationship between state anger and cue reactivity may prove useful therapeutically. If considered as a therapy for addiction (McGregor and Bowen, 2012), careful consideration should be given to the context of its administration, keeping in mind the complexity of its actions.
Acknowledgments
Role of funding source
This study was supported by the NIDA-IRP and NIDA Residential Research Support Services Contract HHSN271200599091CADB. The NIDA-IRP had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We wish to thank Ms Loretta Spurgeon and Ms Kim Modo for their assistance with data collection. We also wish to thank Dr. Jordan Grafman for providing the charitable donation task.
Footnotes
Contributors
MRL, EAS, BJS designed the study and wrote the protocol. MRL and MG conducted the research. MRL, JS and BKC analyzed the data. MRL wrote the first draft of the manuscript. All authors contributed to and have approved the manuscript.
Disclosures: This study was supported by the NIDA-IRP and NIDA Residential Research Support Services Contract HHSN271200599091CADB.
Conflict of interest
Dr. Kelly receives grant support from Bristol Meyers Squibb. No other authors have any conflicts of interest to report.
References
- Aharonovich E, Nguyen HT, Nunes EV. Anger and depressive states among treatment-seeking drug abusers: testing the psychopharmacological specificity hypothesis. Am J Addict. 2001;10:327–334. [PubMed] [Google Scholar]
- Allan S, Gilbert P. Anger and anger expression in relation to perceptions of social rank, entrapment and depressive symptoms. Personal Individ Differ. 2002;32:551–565. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. Proc Natl Acad Sci USA. 2010;107:21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Snifffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. J Psychopharmacol. 2013;27:231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- De Moja CA, Spielberger CD. Anger and drug addiction. Psychol Rep. 1997;81:152–154. doi: 10.2466/pr0.1997.81.1.152. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Flores PJ. Addiction as an attachment disorder: implications for group therapy. Int J Group Psychother. 2001;51:63–81. doi: 10.1521/ijgp.51.1.63.49730. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Szabo G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci USA. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci. 2011;31:7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM, Brune M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38:2831–2843. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Relapse prevention: Theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. pp. 3–67. [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neuro-transmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30:1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19:85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sirotich F. The criminal justice outcomes of jail diversion programs for persons with mental illness: a review of the evidence. J Am Acad Psychiatry Law. 2009;37:461–472. [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory, Form Y. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Scheele D, Kendrick KM, Becker B, Schafer L, Schwalba K, Reul J, Maier W, Hurlemann R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA. 2012;109:18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfish S, Massey R, Krone A. Anxiety and anger among abusers of different substances. Drug Alcohol Depend. 1990;25:253–256. doi: 10.1016/0376-8716(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Wong DF, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Yang JY, Qi J, Han WY, Wang F, Wu CF. Inhibitory role of oxytocin in psychostimulant-induced psychological dependence and its effects on dopaminergic and glutaminergic transmission. Acta Pharmacol Sin. 2010;31:1071–1074. doi: 10.1038/aps.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, Wang Z. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Res. 2011;1367:213–222. doi: 10.1016/j.brainres.2010.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]