Abstract
The development of Dictyostelium discoideum is a model for tissue size regulation, as these cells form groups of ≈2 × 104 cells. The group size is regulated in part by a negative feedback pathway mediated by a secreted multipolypeptide complex called counting factor (CF). CF signal transduction involves decreasing intracellular CF glucose levels. A component of CF, countin, has the bioactivity of the entire CF complex, and an 8-min exposure of cells to recombinant countin decreases intracellular glucose levels. To understand how CF regulates intracellular glucose, we examined the effect of CF on enzymes involved in glucose metabolism. Exposure of cells to CF has little effect on amylase or glycogen phosphorylase, enzymes involved in glucose production from glycogen. Glucokinase activity (the first specific step of glycolysis) is inhibited by high levels of CF but is not affected by an 8-min exposure to countin. The second enzyme specific for glycolysis, phosphofructokinase, is not regulated by CF. There are two corresponding enzymes in the gluconeogenesis pathway, fructose-1,6-bisphosphatase and glucose-6-phosphatase. The first is not regulated by CF or countin, whereas glucose-6-phosphatase is regulated by both CF and an 8-min exposure to countin. The countin-induced changes in the Km and Vmax of glucose-6-phosphatase cause a decrease in glucose production that can account for the countin-induced decrease in intracellular glucose levels. It thus appears that part of the CF signal transduction pathway involves inhibiting the activity of glucose-6-phosphatase, decreasing intracellular glucose levels and affecting the levels of other metabolites, to regulate group size.
Much remains to be understood about how an organism can regulate the size of its components during development. Dictyostelium discoideum is an excellent system in which to study size regulation, since it forms relatively evenly sized groups of about 2 × 104 cells. Dictyostelium cells grow on soil surfaces as individual amoeba, feeding on bacteria when the food source is ample (for reviews, see references 32 and 42). Upon starvation, cells signal each other that they are starving by secreting a cell density-sensing factor called conditioned-medium factor (27, 43, 59). As more and more cells secrete this factor, the concentration of the factor rises, and when the concentration goes above a threshold, cells start aggregation with relayed pulses of cyclic AMP (cAMP) as a chemoattractant (8, 48). The cAMP pulses also regulate the expression of many genes that are specific to early development (for review, see reference 54). The aggregates of cells develop into fruiting bodies, each of which is a spore mass held atop a column of stalk cells ≈1 to 2 mm high.
The main purpose of forming a fruiting body is to disperse spores. If a spore mass is too big, it will slide down the stalk or the stalk will not support the weight and the structure will collapse. Even if the stalk can support a large spore mass, it is conceivable that a large spore mass might not desiccate quickly, and the resulting clumpy spores might not disperse well. If a fruiting body is too small, the spore mass will be too close to the ground for optimal spore dispersal. Therefore, it is of utmost importance for a field of starving Dictyostelium cells to form an optimal number of optimally tall fruiting bodies (for review, see references 13 and 22). Dictyostelium cells use several mechanisms to form aggregates of ≈2 × 104 cells (26, 38, 51). One of them involves a secreted factor that can sense how many cells are in a group.
We previously identified a 450-kDa protein complex which is involved in group size determination in Dictyostelium discoideum (9, 11). This protein complex, called counting factor (CF), is secreted in moderate amounts by wild-type cells. Calculations of CF diffusion suggested that the accumulated CF concentration would allow cells to sense how many cells are in the cells' vicinity (11). Computer simulations predicted that CF could regulate group size by decreasing cell-cell adhesion and increasing motility, thereby breaking up large aggregation streams flowing into an aggregation center, and we found that CF has these predicted effects on adhesion and motility (50, 52). Disruption of the smlA gene causes the oversecretion of CF, resulting in the formation of small fruiting bodies. In smlA mutant cells, the aggregation streams are severely fragmented, forming large numbers of small fruiting bodies. When a component of CF is disrupted, cells secrete essentially undetectable levels of CF activity (10-12). In these cells, the aggregation streams seldom break, forming small numbers of large fruiting bodies, which tend to collapse.
Garrod and Ashworth showed that wild-type cells grown with a high concentration of glucose (86 mM) formed larger fruiting bodies than control cells (21). We found that glucose appears to be a part of the CF signal transduction pathway (28). Transformants lacking bioactive CF and wild-type cells with extracellular CF depleted by antibodies have high intracellular glucose levels, while transformants oversecreting CF have low intracellular glucose levels. A component of CF, countin, affects group size in a manner similar to CF, and an 8-min exposure of cells to recombinant countin (the shortest time we could add countin and then collect cells by centrifugation) decreases intracellular glucose levels. The addition of 1 mM glucose to starving cells increases the levels of intracellular glucose. The addition of glucose to starving cells does not change CF secretion, and a significant amount of glucose is not secreted into conditioned medium. Adding 1 mM glucose to starving cells negates the effect of high levels of extracellular CF on group size and mimics the effect of depleting CF on intracellular glucose levels, cell-cell adhesion, cAMP pulse size, actin polymerization, myosin assembly, and motility (28). Addition of phosphodiesterase or pulsing cells with cAMP did not have any effect on intracellular glucose levels. With computer simulations to model stream breakup, we observed that although changing adhesion alone or motility alone by the amounts observed when 1 mM glucose was added to cells only slightly increased group size, changing both adhesion and motility by these amounts increased group size by roughly the amount observed when 1 mM glucose was added to cells. How intracellular glucose levels are regulated by CF is unknown.
Glucose is metabolized in glycolysis to yield pyruvate and lactate. These compounds can be further metabolized in the tricarboxylic acid cycle (40). Glycolysis is an almost universal central pathway of glucose catabolism where the largest flux of carbon occurs in most cell types. The rate of glycolysis is tightly regulated by allosteric regulation of two glycolytic enzymes, phosphofructokinase and pyruvate kinase. In addition to glycolysis, excess glucose can be polymerized into glycogen. The synthesis of glycogen is catalyzed by a series of enzyme reactions, and the activity of one of the enzymes, glycogen synthase, is regulated by phosphorylation of two serine hydroxyl groups (40). In cells, glucose is synthesized by the breakdown of glycogen or by gluconeogenesis. Glycogen phosphorylase and amylase break down glycogen to form glucose. Like glycogen synthase, the activity of glycogen phosphorylase is regulated by phosphorylation of a serine residue (40). Glycogen synthase and glycogen phosphorylase are reciprocally regulated, so that when one is activated, the other is deactivated. During gluconeogenesis, glucose is synthesized from pyruvate. Although many of the reactions of gluconeogenesis use the same enzymes used in glycolysis, three enzymes, glucose-6-phophatase, fructose-1,6-bisphosphatase, and phosphoenolpyruvate carboxykinase, catalyze irreversible reactions and are thus specific for gluconeogenesis.
In higher eukaryotes, blood glucose levels are regulated by secreted signals such as insulin, epinephrine, and glucagon (40). When blood glucose levels increase, the secretion of insulin from pancreatic β cells is increased. When blood glucose levels are higher than necessary, as indicated to cells by high levels of insulin, glucose is converted to glycogen inside cells such as muscle and liver. Insulin activates glycogen synthase and inactivates glycogen phosphorylase. Insulin also activates phosphofructokinase and pyruvate dehydrogenase, stimulating the breakdown of glucose by glycolysis. Epinephrine activates glycogen phosphorylase and inactivates glycogen synthase, increasing blood glucose levels. In addition, epinephrine stimulates glycolysis in muscle by raising the concentration of fructose-2,6-bisphosphate, an allosteric activator of phosphofructokinase. Epinephrine also stimulates the secretion of glucagon, which signals low blood glucose. Glucagon stimulates the breakdown of glycogen by activating glycogen phosphorylase and deactivating glycogen synthase. At the same time, glucagon also inhibits glucose breakdown by deactivating phosphofructokinase and pyruvate kinase and increases glucose synthesis by activating fructose-1,6-bisphosphatase in the liver.
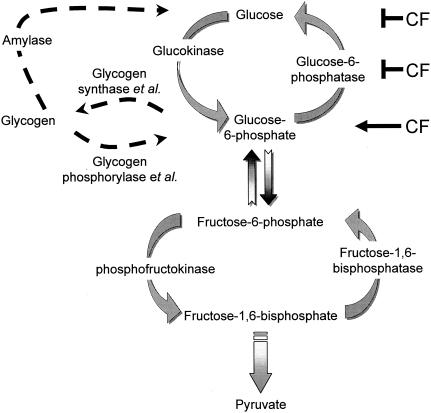
To determine how CF decreases glucose levels inside cells, we measured the activities of enzymes that are involved in glycogen breakdown, the levels of glycolytic/gluconeogenic intermediates, and the activities of enzymes that are involved in glycolysis and gluconeogenesis in smlA mutant, wild-type, and countin mutant cells (see Fig. 7). In this report, we provide evidence that CF decreases intracellular glucose levels at least in part by decreasing the activity of the gluconeogenic enzyme glucose-6-phosphatase.
FIG. 7.
Summary of glucose regulation. The major enzymes that are involved in the regulation of glucose levels are shown. Amylase, glycogen phosphorylase, and glycogen synthase catalyze part of the reactions that are shown in the drawing and are therefore noted as enzyme et al. The results suggest that glucose-6-phosphatase is inhibited by CF to decrease glucose levels and increase glucose-6-phosphate levels in cells.
MATERIALS AND METHODS
Cell culture and glucose assay.
Dictyostelium discoideum Ax4 wild-type, smlA mutant (HDB7YA), countin mutant (HDB2B/4), ugpB mutant (HJBDH1UGP1) (7), and parental cell line DH1 were grown in HL5 medium (with maltose as the carbohydrate source) and starved in PBM buffer (20 mM KH2PO4, 10 μM CaCl2, 1 mM MgCl2, pH 6.1) at 5 × 106 cells/ml as previously described (9, 11, 28). At 0, 2, 4, and 6 h of development, cells were collected as previously described (28). The pellets were lysed by freezing in dry ice-ethanol and thawed on ice.
Recombinant countin was prepared by the method of Gao et al. (20), and when indicated, cells were starved in PBM containing 200 ng of recombinant countin per ml . For rapid exposure of cells to recombinant countin (20), cells were starved in PBM in shaking cultures for 6 h and then treated with recombinant countin for 1 min. Where indicated, cells were collected by centrifugation and resuspended in PBM before the recombinant countin was added. The cells were collected by centrifugation at 500 × g for 2 min, and the pellets were resuspended in ≈600 μl of supernatant (to keep the cells exposed to the CF they were secreting or the exogenous recombinant countin), transferred to Eppendorf tubes, and collected by centrifugation at 10,000 × g for 15 s. The pellets were quick frozen in dry ice-ethanol. It took approximately 8 min from the exposure to recombinant countin to freezing. Glucose assays were performed by the method of Jang et al. (50).
Determination of glycogen.
Glycogen assays were performed by the method of Lo et al. (41); 2 ml of cells starving in shaking culture (107 cells) were collected and lysed by freeze lysis; 100 μl of PBM was added to the lysed cells, and 50 μl of lysates was mixed with 500 μl of 30% KOH saturated with anhydrous Na2SO4 and heated to 100°C in a boiling water bath for 30 min. Samples were cooled on ice for 5 min, mixed with 600 μl of 95% ethanol, and kept on ice for 30 min. The samples were then centrifuged at 900 × g for 30 min at 4°C, and the supernatants were carefully aspirated. The glycogen precipitates were then dissolved in 5 ml of distilled water; 500 μl of this solution was transferred to glass tubes and then mixed with 500 μl of 5% phenol (LC18195-1, Labchem Inc., Pittsburgh, Pa.); 2.5 ml of 98% H2SO4 was added to the tube within 20 s. The tubes were allowed to stand for 10 min at room temperature and then shaken and placed in a 30°C water bath for 30 min. The absorbance was measured at 490 nm, with oyster glycogen (Sigma, St. Louis, Mo.) as a standard. At the same time, 2 μl of cell lysate was used for a protein assay (Bio-Rad, Hercules, Calif.), with bovine serum albumin as a standard.
Amylase and glycogen phosphorylase assays.
Amylase and glycogen phosphorylase assays were performed by the method of Jones and Wright (29); 50 ml of cells starving in shaking culture were collected and lysed by freeze lysis; 1.4 ml of PB (3.2 mM Na2HPO4, 7.0 mM KH2PO4, pH 6.5) was added to the pellets, and the lysate was clarified by centrifugation at 19,000 × g for 2 min; 100 μl of clarified cell lysate was used per enzyme reaction. At the same time, 2 μl of lysate was used for protein determination. The Vmax and Km values for amylase and all other enzymes whose activity was measured as a function of the substrate concentration were calculated with nonlinear regressions to fit the activity to the Michaelis-Menten equation with the Prism software package (GraphPad Software, San Diego, Calif.).
Glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-bisphosphate assay.
The glucose-6-phosphate and fructose-6-phosphate assays were performed by the method of Land and Michal (39), and fructose-1,6-bisphosphate assays were performed by the method of Michal and Beutler (44). Cells were starved in PBM buffer at 5 × 106 cells/ml as previously described. At 6 h of development, 200 ml of cells was collected by centrifugation at 500 × g for 2 min, and the supernatant was carefully aspirated. This step was repeated to remove any traces of liquid in the sample. The weight of the cell pellet was then determined. The pellets were lysed by freezing in a dry ice-ethanol bath and thawing on ice for 10 min; 5 ml of 0.6 N perchloric acid was added to the pellets, and the mixture was quickly vortexed. Cell lysates were clarified by centrifugation at 3,000 × g for 10 min at 4°C. After removing the supernatant, 2 ml of 0.3 N perchloric acid was added to the sediment, and this was clarified by centrifugation at 3,000 × g for 10 min. The supernatants from both steps were mixed, and the pH was adjusted to 3.5 with 5 M K2CO3. The final volume of the sample was brought to 15 ml with H2O, and the sample was kept on ice for 15 min. This material was designated sample A.
For the glucose-6-phosphate and fructose-6-phosphate assays, 1 ml of sample A was mixed with 1 ml of 1 M triethanolamine (pH 7.6), 40 μl of 10-mg/ml NADP, and 20 μl of 0.5 M MgCl2. The reaction mixture was then brought to 22°C. The absorbance at 340 nm was taken at 0, 3, and 6 min; 10 μl of glucose-6-phosphate dehydrogenase (1 U/μl; Sigma) was added to the reaction mixture, and the absorbance at 340 nm was measured at 16, 19, and 21 min. Then, 4 μl of phosphoglucoisomerase (1 U/μl; Sigma) was added to the reaction mixture, and the absorbance at 340 nm was measured at 31, 34, and 37 min. All times used the beginning of the 22°C incubation as 0 min. For the fructose-1,6-bisphosphatase assay, 1 ml of sample A was mixed with 1 ml of 1 M triethanolamine (pH 7.6) and 100 μl of 5 mM NADH, and the reaction mixture was then brought to 22°C. The absorbance at 340 nm was taken at 0, 3, and 6 min; 50 μl of glycerol-3-phosphate dehydrogenase (40 U/ml; Sigma) was added to the reaction mixture, and at 21 min, 50 μl of triosephosphate isomerase (250 U/ml; Sigma) was added to the reaction mixture. The absorbance at 340 nm was measured at 26, 28, and 30 min. Then, 50 μl of aldolase (13.5 U/ml; Sigma) was added to the reaction mixture, and the absorbance at 340 nm was measured at 39, 42, and 45 min.
Glucokinase and glucose-6-phosphatase assays.
Cell lysates were prepared as described for amylase assays; 100 μl of clarified lysate was used for both assays, and 2 μl of the supernatant was used for a Bio-Rad protein assay. Glucokinase assays were performed by the method of Baumann (5) with the exception that the assay buffer contained 40 mM triethanolamine-HCl, pH 7.5, 0.2 mM EDTA, 1.5 mM MgCl2, 1 mM dithiothreitol, 0.3 mM NADP, 2 mM ATP, and 1.2 U of glucose-6-phosphate dehydrogenase per ml. Glucose-6-phosphatase assays were performed by the method of Alegre et al. (1). Glucose-6-phosphatase activity is associated with microsomes, which fractionate into the clarified lysate prepared as described (53).
Phosphofructokinase and fructose-1,6-bisphophatase assays.
Phosphofructokinase activity was measured by the method of Donnicke et al. (16), and fructose-1,6-bisphophatase activity was measured by the method of Baumann and Wright (6) with a minor modification. Cell lysates were prepared as described for amylase assays; 100 μl of clarified lysate was used for both the phosphofructokinase and fructose-1,6-bisphosphatase assays, and 2 μl of the supernatant was used for a Bio-Rad protein assay. In the fructose 1,6-bisphosphatase assay, the clarified lysates were mixed with 0.9 ml of assay buffer (40 mM triethanolamine-HCl, pH 7.5, 1.5 mM MgCl2, 1 mM dithiothreitol, 0.3 mM NADP, 1.2 U of glucose-6-phosphate dehydrogenase per ml, 1 U of phosphoglucoisomerase per ml, 0.2 mM EDTA) containing different concentrations of fructose-1,6-bisphosphate. Nonlinear regression curve fits to a Hill equation and F tests to determine if there was cooperative binding were done with the Prism software package.
RESULTS
Both glucose and glycogen levels are decreased in smlA mutant cells.
We previously found that intracellular glucose levels are increased in countin mutant cells and are decreased in smlA mutant cells and in wild-type cells treated with recombinant countin (28). To determine how intracellular glucose levels are regulated in Dictyostelium cells, we first measured the levels of glycogen. In cells, excess glucose is stored as glycogen, and thus glycogen serves as a reservoir of glucose (40). If smlA mutant cells have decreased intracellular glucose levels due to an increased conversion of glucose to glycogen, we would expect to see increased glycogen levels in smlA mutant cells.
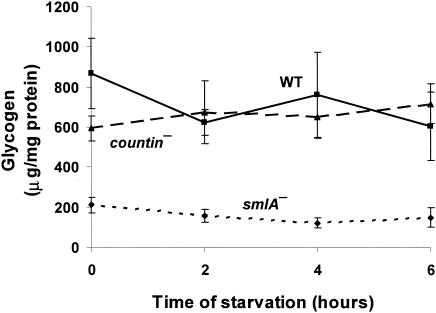
smlA mutant, wild-type, and countin mutant cells were starved in shaking culture, and levels of glycogen were measured in these cells. During both vegetative growth and development in submerged shaking culture, wild-type and countin mutant cells had very similar amounts of glycogen, while smlA mutant cells had lower levels of glycogen (Fig. 1). This suggests that there is not an inverse correlation between glucose levels and glycogen levels. Previously observed glycogen concentrations were ≈200 to 600 μg of glycogen/mg of protein depending on the growth conditions (3, 57), which is quite similar to what we observed.
FIG. 1.
Differences in glycogen levels in cell lines with different extracellular CF activity. smlA mutant, wild-type (WT), and countin mutant cells were starved by shaking in PBM and collected at the times indicated. The levels of glycogen were measured in the three cell lines. Paired t tests indicated that the levels of glycogen in smlA mutant were significantly different from that of the wild type at 0, 2, 4, and 6 h of development, with P < 0.05. Values are means ± standard error of the mean from four independent assays.
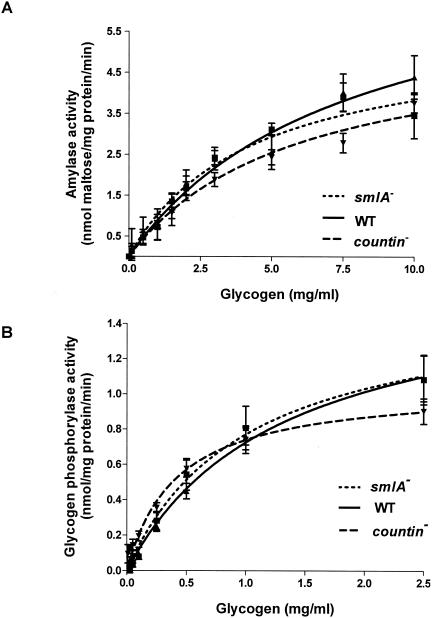
To determine whether the different levels of intracellular glucose in smlA mutant, wild-type, and countin mutant cells are due to the differences in the breakdown of glycogen, we measured the activities of the two enzymes that break down glycogen to glucose. The first enzyme, amylase, breaks down glycogen to glucose. The activities of amylase were essentially indistinguishable in smlA mutant, wild-type, and countin mutant cells at 0, 2, 4, and 6 h of development, suggesting that amylase activities are not affected in cell lines with different extracellular CF activity and that amylase is not responsible for the differences in intracellular glucose levels in smlA mutant, wild-type, and countin mutant cells during the first 6 h of development (data not shown and Fig. 2A).
FIG. 2.
Activities of amylase and glycogen phosphorylase in cell lines with different extracellular CF activity. countin mutant, Ax4 (WT), and smlA mutant cells were starved by shaking in PBM and harvested at 6 h of development, and the activities of amylase and glycogen phosphorylase were measured. (A) Maltose was used to prepare a calibration curve for reducing sugar produced by amylase. Values are means ± standard error of the mean from six independent assays. The lines show Michaelis-Menten equations fit to the data with nonlinear regression. The Vmax of wild-type cells was 7.7 nmol/mg of protein/min, and the Km was 7.7 mg/ml. (B) The activity of glycogen phosphorylase was measured by coupling the reaction to NADH production. Values are means ± standard error of the mean from five independent assays. The lines show Michaelis-Menten equations fit to the data with nonlinear regression. The Vmax of wild-type cells was 1.7 nmol/mg of protein/min, and the Km was 1.3 mg/ml.
The activity of the other enzyme known to hydrolyze glycogen, glycogen phosphorylase, was also measured in smlA mutant, wild-type, and countin mutant cells. Glycogen phosphorylase breaks down glycogen to glucose-1-phosphate, which is converted to glucose-6-phosphate by phosphoglucomutase, and glucose-6-phosphate in turn can be converted to glucose by glucose-6-phosphatase. During vegetative growth, the three cell lines had indistinguishable activities of glycogen phosphorylase (data not shown). The activity of glycogen phosphorylase at 6 h of development was not statistically different between smlA mutant, wild-type and countin mutant cells (Fig. 2B). This suggested that glycogen phosphorylase is not significantly regulated by CF. The activity of glycogen phosphorylase was lower than that of amylase, in agreement with the previous observation that amylase is the predominant glycogen-degrading enzyme up to aggregation (29). We found that high concentrations of glycogen cause turbidity in the assay buffer and interfere with the spectroscopy, so we were unable to measure activities of amylase and glycogen phosphorylase at the physiological levels of glycogen.
In addition, we indirectly looked at the effect of altered glycogen synthesis on the levels of glucose by measuring intracellular glucose levels in a mutant that has disrupted glycogen synthesis. UgpB is one of two uridine diphosphoglucose pyrophosphorylase enzymes in Dictyostelium discoideum (7). These enzymes catalyze the formation of uridine diphosphoglucose from glucose-1-phosphate and UDP. Uridine diphosphoglucose can then be incorporated into glycogen by glycogen synthase (40).
ugpB mutant cells have a reduced ability to synthesize glycogen during development and therefore have almost no glycogen when starved (7). However, the development of ugpB mutant cells was essentially indistinguishable from that of the parent strain during the first 16 h of development, during which period cells initiate development and form aggregates. At 0, 2, 4, and 6 h of development, the intracellular glucose levels of ugpB mutant cells were not significantly different from that of the parental strain (for instance, at 6 h, the intracellular glucose concentrations were 6.7 ± 0.8 nmol/mg of protein for wild-type DH1 and 9.2 ± 2.7 nmol/mg of protein for the ugpB mutant; means ± standard error of the mean, n = 3), indicating that even though ugpB mutant cells have low levels of glycogen during that time, disruption of ugpB does not affect group size and does not have a statistically significant effect on intracellular glucose levels. The values that we obtained were similar to the previously observed intracellular glucose levels in wild-type cells (23, 28, 58). Together, the data suggest that CF does not regulate intracellular glucose levels by altering the activities of two enzymes that are known to break down glycogen and that changing glycogen levels does not affect group size or intracellular glucose levels.
Levels of metabolites are affected in cell lines with different extracellular CF activity.
Two other pathways that can affect glucose levels are glycolysis and gluconeogenesis. To begin to assess whether CF affects one or both of these pathways, we measured levels of three of the glycolytic metabolites, glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-bisphosphate, in three cell lines that accumulate different levels of extracellular CF activity. At 6 h of development, smlA mutant cells had the highest levels of glucose-6-phosphate and fructose-6-phosphate but the lowest levels of fructose-1,6-bisphosphate (Table 1). Making the assumption that the density of packed cells is ≈1 g/ml and that ≈70% of the volume of the packed cells is cytosol, 6-h-starved wild-type cells would then contain ≈114 μM glucose-6-phosphate, ≈43 μM fructose-6-phosphate, and ≈914 μM fructose-1,6-bisphosphate.
TABLE 1.
Levels of glycolytic intermediatesa
| Enzyme | Mean concn (μmol/g of packed cells) ± SEM
|
||
|---|---|---|---|
| smlA mutant | Wild type | countin mutant | |
| Glucose-6-phosphate | 0.13 ± 0.02 | 0.080 ± 0.008 | 0.050 ± 0.006 |
| Fructose-6-phosphate | 0.052 ± 0.004 | 0.030 ± 0.003 | 0.023 ± 0.002 |
| Fructose-1,6-bisphosphate | 0.47 ± 0.04 | 0.63 ± 0.05 | 0.66 ± 0.07 |
Cells were starved by shaking in PBM for 6 h, and the levels of glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-bisphosphate were measured. T-tests indicated that the levels of glucose-6-phosphate in mutant cells were significantly different from those in wild-type cells, with P < 0.02. In addition, the levels of fructose-6-phosphate and fructose-1,6-bisphosphate in smlA cells were significantly different from those in wild-type cells with P < 0.005 and P < 0.03, respectively. Values are means from eight (glucose-6-phosphate and fructose-6-phosphate) or nine (fructose-1,6-bisphosphate) independent assays.
The concentration of glucose-6-phosphate was similar to the previously observed concentration (15). However, we observed concentrations of fructose-6-phosphate and fructose-1,6-bisphosphate that were much higher than the concentrations reported by Cleland (15). With Cleland's data converted to our units, Cleland (15) found that the concentration of fructose-1,6-bisphosphate was 0.018 μmol/g of packed cells, which is about ≈35 times lower than what we obtained. In addition, Cleland (15) reported that fructose-6-phosphate was undetectable.
There are several differences that might explain these disparities. First, Cleland (15) used cells that were grown to stationary phase on bacteria lawns. Axenically grown cells and bacterially grown cells are known to have very different levels of carbohydrates, and cells in stationary phase have quite different characteristics compared to cells that are in mid-log phase (for review, see reference 42). In addition, Cleland used 20% KOH to neutralize the acid-hydrolyzed sample and brought the pH up to 7.0 to 7.5 for the extraction of intermediates. Fructose-6-phosphate and fructose-1,6-bisphospate are known to rapidly dephosphorylate when titrated with a strong base (personal communication, Sigma technical support). These may explain the low yield of fructose-6-phosphate and fructose-1,6-bisphosphate in her system. Together, our data suggest that CF increases levels of glucose-6-phosphate and fructose-6-phosphate and that high concentrations of CF decreases levels of fructose-1,6-bisphosphate.
Short exposure to countin does not affect two key glycolytic enzymes.
Since CF appears to regulate intracellular glucose levels, we measured the activities of some of the enzymes involved in glycolysis and gluconeogenesis in smlA mutant, wild-type, and countin mutant cells. We examined hexokinase and phosphofructokinase, enzymes that are specific to glycolysis, and the activities of their gluconeogenic counterparts, glucose-6-phosphatase and fructose-1,6-bisphosphatase. Other enzymes that catalyze reversible reactions were not examined in this study.
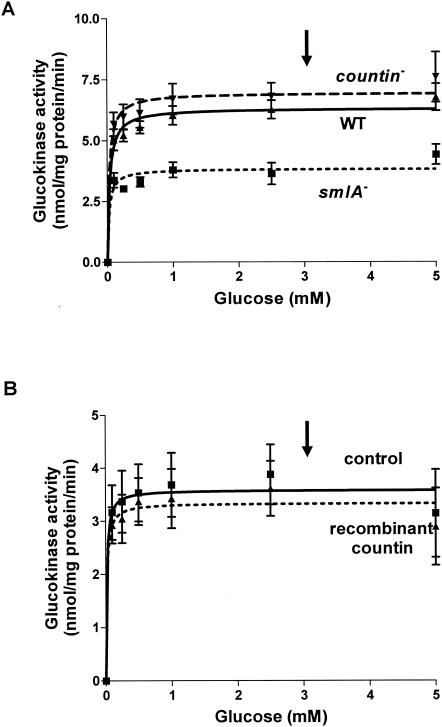
Hexokinase catalyzes the phosphorylation of glucose and other hexoses, which is the first step of glycolysis. However, the hexokinase in Dictyostelium discoideum has a high substrate specificity for glucose and cannot catalyze the phosphorylation of mannose, fructose, or 2-deoxyglucose and thus is called glucokinase (5). To determine whether glucokinase activity is affected in cells with different levels of extracellular CF activity, we measured glucokinase activities in smlA mutant, wild-type, and countin mutant cells. At 6 h of development, glucokinase activities were indistinguishable in wild-type and countin mutant cells but lower in smlA mutant cells, suggesting that this enzyme may be regulated by CF (Fig. 3A). When wild-type cells were harvested and washed before being assayed for glucokinase activity, the apparent Vmax of the enzyme decreased (Fig. 3B). However, a short exposure of wild-type cells to recombinant countin did not significantly change the activity of glucokinase (Fig. 3B). The total amount of time that cells were exposed to recombinant countin was the same as when we previously measured a significant change in intracellular glucose levels in response to a short exposure of cells to recombinant countin (28). The arrows in Fig. 3A and B indicate the physiological level of glucose in wild-type cells (28).
FIG. 3.
Activity of glucokinase in cell lines with different extracellular CF activity. (A) smlA mutant, wild-type (WT), and countin mutant cells were starved by shaking in PBM and collected 6 h later. The activity of glucokinase was measured by varying the concentration of glucose in the assay buffer and by coupling the reaction to the formation of NADPH. Values are means ± standard error of the mean from five independent assays. The Vmax of wild-type cells was 6.3 nmol/mg of protein/min, and the Km was 0.03 mM. (B) Wild-type cells were starved by shaking in PBM and harvested at 6 h of starvation. Cells were treated with 200 ng of bovine serum albumin per ml (control) or recombinant countin for 1 min and then collected. The activity of glucokinase was measured as in panel A. Values are means ± standard error of the mean from five independent assays. The lines show Michaelis-Menten equations fit to the data with nonlinear regression. The arrows indicate the physiological level of glucose (≈3 mM).
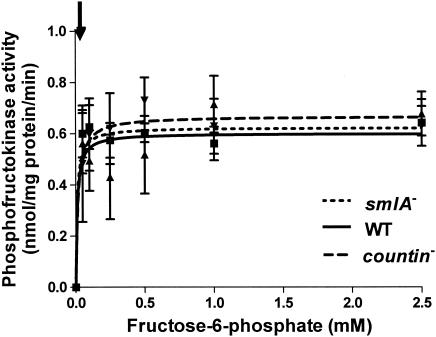
Phosphofructokinase catalyzes the phosphorylation of fructose-6-phosphate and has several regulatory sites where allosteric inhibitors (ATP and citrate) or activators (fructose-2,6-bisphosphate, ADP, and AMP) bind to affect the activity of the enzyme (40). However, unlike in other systems, phosphofructokinase in Dictyostelium discoideum is not affected by fructose-2,6-bisphosphate (2). To determine whether different levels of extracellular CF activity affect phosphofructokinase activities, smlA mutant, wild-type, and countin mutant cells were starved in shaking culture, and the activities of the enzyme were measured. At 6 h of development, phosphofructokinase activities were indistinguishable in the three cell lines, suggesting that CF does not affect this enzyme (Fig. 4). The arrow in Fig. 4 indicates the physiological level of fructose-6-phosphate in wild-type cells (≈43 μM).
FIG. 4.
Activity of phosphofructokinase in cell lines with different extracellular CF activity. smlA mutant, wild-type (WT), and countin mutant cells were starved by shaking in PBM and collected 6 h later. The activity of phosphofructokinase was measured by varying the concentration of fructose-6-phosphate in the assay buffer and by coupling the reaction to the consumption of NADPH. Values are means ± standard error of the mean from five independent assays. The lines show Michaelis-Menten equations fit to the data with nonlinear regression. The Vmax of wild-type cells was 0.6 nmol/mg of protein/min, and the Km was 0.01 mM. The arrow indicates the physiological level of fructose-6-phosphate (≈43 μM).
Key gluconeogenesis enzyme is regulated in cells with high levels of extracellular CF activity.
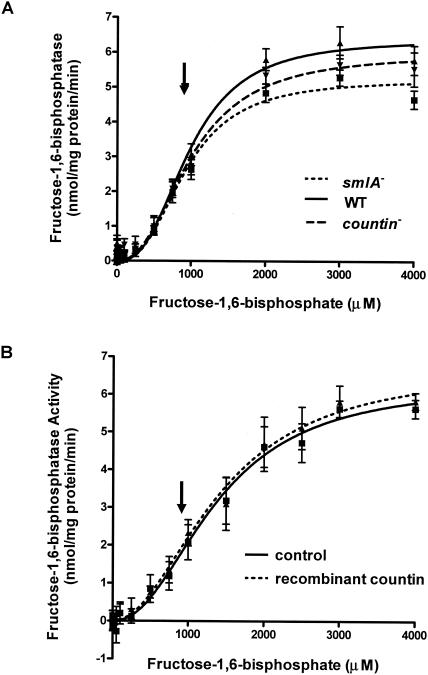
To determine if the activity of the gluconeogenic counterpart of phosphofructokinase is altered in cells with different levels of extracellular CF activity, fructose-1,6-bisphosphatase activities were measured in 6-h-starved smlA mutant, wild-type (Ax4), and countin mutant cells (Fig. 5A). This enzyme converts fructose-1,6-bisphosphate to fructose-6-phosphate. The activities of fructose-1,6-bisphosphatase in lysates of the three cell lines had sigmoidal saturation curves, where the activity increased and reached a plateau at ≈2,000 μM fructose-1,6-bisphosphate. The physiological concentration of fructose-1,6-bisphosphate in wild-type cells is indicated by the arrow.
FIG. 5.
Activity of fructose-1,6-bisphosphatase in cell lines with different extracellular CF activity. (A) smlA mutant, wild-type (WT), and countin mutant cells were starved by shaking in PBM and collected 6 h later. The activities of fructose-1,6-bisphosphatase were measured by varying the concentration of fructose-1,6-bisphosphate in the assay buffer and by coupling the reaction to the formation of NADPH. Values are means ± standard error of the mean from five independent assays. The lines show Hill equations fit to the data with nonlinear regression. For wild-type cells, the Vmax was 6.4 nmol/mg of protein/min, the Km was 980 μM, and the Hill coefficient was 2.7. (B) Wild-type cells were starved by shaking in PBM and harvested at 6 h of starvation. Cells were treated with 200 ng of bovine serum albumin per ml (control) or recombinant countin for 1 min and then collected. The activity of fructose-1,6-bisphosphatase was measured as in panel A. Values are means ± standard error of the mean from five independent assays. The lines show Hill equations fit to the data with nonlinear regression. The arrows indicate the physiological level of fructose-1,6-bisphosphate (≈914 μM).
The activity curves of fructose-1,6-bisphosphatase in the three cell lines had sigmoidal shapes instead of the hyperbolic curves observed for other enzymes. A change in the substrate saturation curve from hyperbolic to sigmoidal was previously observed when fructose-1,6-bisphosphatase was inhibited by low concentrations of fructose-2,6-bisphosphate (2). An F test indicated that a Hill equation with cooperative binding (A = Vmax × SN/[KmN + SN], where A is the enzyme activity, S is the substrate concentration, and N is the Hill coefficient) (45) fit the data better than a standard Michaelis-Menten equation, with P < 0.0001. This statistic takes into account the fact that the cooperative binding model has more degrees of freedom (http://www.graphpad.com/curvefit/2_models_1_dataset.htm).The Hill coefficient was ≈2.6 in smlA mutant and wild-type cells and ≈2.4 in countin mutant cells, indicating cooperative binding on the enzyme. There did not appear to be significant differences in the activity of fructose-1,6-bisphosphatase at the physiological substrate level between the three cell lines.
To determine whether recombinant countin can affect the activity of fructose-1,6-bisphosphatase by a fast signal transduction pathway, wild-type cells were exposed to recombinant countin for a short period by the method of Jang et al. (28), and the activity of the enzyme was measured (Fig. 5B). The activity curve for cells treated with recombinant countin was fit better with a Hill equation (P < 0.001; F test), giving a Hill coefficient of 2.1 to 2.2. Exposure of cells to recombinant countin had no significant effect on the activity of fructose-1,6-bisphosphatase.
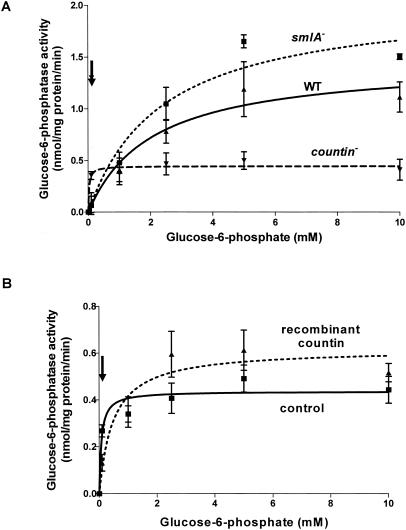
Since the activity of glucokinase was altered in smlA mutant cells, we measured the activities of its gluconeogenic counterpart (glucose-6-phosphatase) in smlA mutant, wild-type, and countin mutant cells (Fig. 6A). The glucose-6-phosphatase activities of smlA mutant and wild-type cells increased slowly and reached a plateau at ≈5 mM glucose-6-phosphate. countin mutant cells reached a plateau at ≈0.1 mM glucose-6-phosphate. Even though smlA mutant cells had higher activities at substrate concentrations above 2.5 mM, countin mutant cells had the highest enzyme activities at the physiological concentration of glucose-6-phosphate (≈114 μM for wild-type cells). The Km and Vmax of the enzyme calculated from nonlinear regression curve fitting are shown in Table 2. The Km of glucose-6-phosphatase in lysates of countin mutant cells was ≈89-fold less than that of the smlA mutant, suggesting that glucose-6-phosphatase in the countin mutant is regulated in such a way that it has a higher affinity for the substrate. In addition, exposure of wild-type cells to recombinant countin for a short period by the method of Jang et al. (28) increased the Km and Vmax of glucose-6-phosphatase, suggesting that the enzyme is regulated by a fast signal transduction pathway (Fig. 6B and Table 3).
FIG. 6.
smlA mutant cells and wild-type (WT) cells given an 8-min treatment with recombinant countin have altered glucose-6-phosphatase activity. (A) smlA mutant, Ax4 (WT), and countin mutant cells were starved by shaking in PBM and harvested at 6 h of starvation. The activity of glucose-6-phosphatase was measured by varying the concentration of glucose-6-phosphate in the assay buffer and by coupling the reaction to the formation of NADH. At 0.1 mM glucose-6-phosphate, t tests indicated that the activities of glucose-6-phosphatase in the lysate of countin mutant cells were significantly different from that of the wild-type and smlA mutant, with P < 0.03 and P < 0.01, respectively. Values are means ± standard error of the mean from three independent assays. (B) Wild-type cells were starved by shaking in PBM and collected after 6 h. Cells were washed, resuspended, and treated with 200 ng of bovine serum albumin per ml (control) or recombinant countin for 1 min and then collected. The activity of glucose-6-phosphatase was measured as in panel A. At 0.1 and 2.5 mM glucose-6-phosphate, paired t tests indicated that the activities of glucose-6-phosphatase in the lysates of cells treated with recombinant countin were significantly different from that of the control, with P < 0.05 and P < 0.03, respectively. Values are means ± standard error of the mean from eight independent assays. The lines show Michaelis-Menten equations fit to the data with nonlinear regression. The arrows indicate the physiological level of glucose-6-phosphate (≈114 μM).
TABLE 2.
Vmax and Km of glucose-6-phosphatasea
| Cells | Vmax (nmol/mg of protein/mm) | Km (mM) |
|---|---|---|
| smlA mutant | 2.1 | 2.4 |
| Wild-type Ax4 | 1.5 | 2.1 |
| countin mutant | 0.45 | 0.027 |
Differences in the Vmax and Km of glucose-6-phosphatase in cell lines with different extracellular CF activity were determined. Cells were starved by shaking in PBM for 6 h, and the activity of glucose-6-phosphatase was measured (Fig. 6A). The Vmax and Km values were calculated by nonlinear regressions to fit the activity (as a function of the substrate concentration) to the Michaelis-Menten equation.
TABLE 3.
Vmax and Km of glucose-6-phosphate after exposure to countina
| Cells | Vmax (nmol/mg of protein/mm) | Km (mM) |
|---|---|---|
| Countin treated | 0.62 | 0.48 |
| Control | 0.44 | 0.073 |
The Vmax and Km of glucose-6-phosphatase in washed wild type cells treated with recombinant countin were determined. Wild-type cells were starved by shaking in PBM and harvested at 6 h of starvation. Cells were washed in PBM, resuspended, and treated with bovine serum albumin (control) or recombinant countin for 1 min and collected, and the activity of glucose-6-phosphatase was measured (Fig. 6B). Because of the time required to collect cells by centrifugation, the total exposure time to recombinant countin was ∼8 mins. The Vmax and Km values were calculated as in Table 2.
DISCUSSION
CF does not seem to regulate intracellular glucose by regulating glycogen breakdown.
Exposure of cells to CF decreases the levels of intracellular glucose in cells, which in turn regulate cell-cell adhesion and motility to regulate group size in Dictyostelium discoideum (28). We found that at physiological substrate levels, the activity of a key enzyme that is specific to gluconeogenesis, glucose-6-phosphatase, was increased in countin mutant cells (which secrete low levels of CF activity). The activity of the enzyme was decreased in wild-type cells treated with recombinant countin. The inhibition of the activity of glucose-6-phosphatase by CF may thus help explain how CF decreases intracellular glucose levels. Some of the enzymes, key products, and intermediates involved in regulating intracellular glucose levels are shown in Fig. 7.
Since, as for most biochemical assays, we measured enzyme activities in vitro by diluting cell lysates, it is possible that some of the natural regulation, such as allosteric inhibition, may have been lost. In addition, labile covalent modifications might be lost during the lysis and assays. Therefore, as for most biochemical assays, what we observed in vitro may be different from what actually takes place in vivo. However, the fact that we were able to observe an effect of recombinant countin on the activity of glucose-6-phosphatase suggests that at least some of the natural regulation mechanisms are not completely lost in our assays.
The amounts of glycogen stored in cells were significantly different between smlA mutant and wild-type cells, which suggests that high levels of CF decrease glycogen synthesis. However, there were no significant differences in glycogen levels between wild-type and countin mutant cells, and it is unclear whether the apparent effect of high levels of CF on glycogen levels is due to the effect of CF on the levels of starting material (glucose) or to the inhibition of enzymes involved in glycogen synthesis. Glycogen synthase uses uridine diphosphoglucose as its substrate for glycogen synthesis. A ugpB mutant lacking an enzyme to produce uridine diphosphoglucose thus has very little glycogen (7). The ugpB mutant cells still had normal levels of intracellular glucose and normal group size, indicating that the amount of glycogen does not play a significant role in the ability of CF to regulate intracellular glucose levels or group size.
If CF regulates group size by regulating glycogen synthesis, one might expect that decreasing glycogen synthesis would affect group size. Since the ugpB mutant cells had different levels of glycogen synthesis but not group size, it is likely that CF does not decrease group size by regulating glycogen synthesis. In addition, CF had no significant effects on the activities of amylase or glycogen phosphorylase. Thus, it appears that although high levels of CF decreases glycogen levels, CF does not regulate group size by regulating glycogen levels, glycogen synthesis, or glycogen breakdown.
CF regulates glucokinase.
The observation that the activity of glucokinase was not affected by a short exposure to recombinant countin while the smlA mutant had lower glucokinase activity compared to wild-type and countin mutant cells suggests either that the whole CF complex itself may decrease the activity of glucokinase but not recombinant countin or that CF regulates glucokinase through a slow signal transduction pathway. The observation that the activity of phosphofructokinase was not different in smlA mutant, wild-type, and countin mutant cells suggests that this enzyme may not play a role in the decrease in intracellular glucose levels by CF. Together, the data suggest that the enzymes specific to glycolysis (glucokinase and phosphofructokinase) do not make significant contributions to the decrease in intracellular glucose levels observed when cells are treated with recombinant countin for a short period.
CF appears to regulate intracellular glucose by regulating gluconeogenesis enzymes.
Treating wild-type cells with recombinant countin for a short period of time increased the Vmax and Km of glucose-6-phosphatase, thereby decreasing the activity of the enzyme at the physiological concentration of glucose-6-phosphate, suggesting that recombinant countin regulates this enzyme. The results suggest that CF causes glucose-6-phosphatase to have a decrease in its affinity for glucose-6-phosphate. When wild-type cells were harvested, washed, and then treated with bovine serum albumin for a short period of time, the Vmax and Km of glucose-6-phosphatase were different from the values seen in wild-type cells but close to the values seen in countin mutant cells. The untreated wild-type cells were harvested and frozen without being washed, and so were harvested while being exposed to the CF that they had been secreting. The bovine serum albumin-treated cells had the CF that they were secreting removed by the wash step, so this suggests that the activity of glucose-6-phosphatase in the bovine serum albumin-treated cells was similar to that of countin mutant cells due to the sudden decrease in the concentration of CF. This then suggests that removing CF and adding countin both cause rapid changes in the activity of glucose-6-phosphatase.
We also found that different strains of wild-type cells (Ax4 and Ax2) having different group sizes had different activities of glucose-6-phosphatase; at the physiological glucose-6-phosphate level of ≈114 μM, strains that formed large groups had high glucose-6-phosphatase activities, and strains that formed small groups had low glucose-6-phosphatase activities (data not shown). The variations in group sizes of different clones from the same wild-type strain also correlated with glucose-6-phosphatase activities at the physiological glucose-6-phosphate concentration (data not shown). Although we do not know at this point whether the differences in group sizes in the wild-type strains and clones is the result of differences in glucose-6-phosphatases or vice versa, this suggests that there is a close relationship between group size and glucose-6-phosphatase activity.
We previously observed that treatment of wild-type cells for a short time with recombinant countin decreased the intracellular glucose levels (28). Even though the actual treatment of cells with recombinant countin was for 1 min, it took about ≈8 min to harvest and freeze the cells. The difference in intracellular glucose levels between control cells and cells treated with recombinant countin was 0.83 ± 0.16 nmol/mg of protein. Since CF inhibits the activity of glucose-6-phosphatase, this could potentially explain why CF causes intracellular glucose levels to decrease. To determine if this could account for the differences in the intracellular glucose levels, we calculated the amount of glucose generated by glucose-6-phosphatase with the observed glucose-6-phosphate concentration of ≈114 μM and the predicted activity at this substrate concentration from our observed values of the Km and Vmax of glucose-6-phosphatase.
From the Michaelis-Menten equation A = Vmax × S/(Km + S), where A is the enzyme activity and S is the substrate concentration, the activities of glucose-6-phosphatase would be 0.27 nmol/mg of protein/min in the lysates of control cells and 0.12 nmol/mg of protein/min in the lysates of cells treated with recombinant countin for 1 min. Assuming that recombinant countin was active for 8 min, the total differences in the amount of glucose generated by glucose-6-phosphatase between control cells and cells treated with recombinant countin would thus be roughly (0.23 − 0.12 nmol/mg of protein/min) × 8 min = 1.2 nmol/mg of protein. This is roughly comparable to the observed change. Our working hypothesis is thus that recombinant countin, and CF itself, decreases intracellular glucose levels by decreasing the activity of glucose-6-phosphatase.
Glucose-6-phosphatase is an important enzyme in gluconeogenesis, which hydrolyzes glucose-6-phosphate to glucose and Pi. It is made of multiple transmembrane components, with the active site in the lumen of the endoplasmic reticulum (for review, see references 14, 18, 19, 46, and 47). Some of the components transport glucose-6-phosphate from the cytosol into the endoplasmic reticulum, and others transport glucose out. The multicomponent nature of the glucose-6-phosphatase system provides several sites for inhibition. In rat liver, epinephrine stimulates glucose-6-phosphatase, suggesting that in higher eukaryotes as well as in Dictyostelium discoideum extracellular factors can regulate this enzyme (4).
CF may inhibit glucose cycling.
In cells, glucose can be phosphorylated to glucose-6-phosphate by glucokinase, and glucose-6-phosphate can also be dephosphorylated to glucose by glucose-6-phosphatase. This suggests that glucose could be phosphorylated to glucose-6-phosphate, which could then be dephosphorylated to glucose with the resulting consumption of ATP. This cycle is called a futile cycle, and such cycles occur in the liver (30, 31). It is possible that a different compartmentalization of glucokinase and glucose-6-phosphatase allows cells to regulate a futile cycle. Increased glucose cycling between glucose and glucose-6-phosphate is characteristic of the insulin resistance and hyperglycemia seen with type II diabetes (17), although it is unclear whether glucose cycling contributes to the hyperglycemia in diabetic animals or not, and the results vary in different types of diabetes (24, 25, 36, 55).
Glucose-6-phosphatase activity and glucose cycling are increased in the liver and hypothalamus of obese hyperglycemic (ob/ob) mice (33-37). Weber et al. (56) suggested that the role of glucose cycling is to maintain a constant glucose concentration. Hunger is a consequence of a decrease in the blood glucose concentration and is related to changes in glucose uptake in cells in the hypothalamus (49), which is known as the center of satiety and feeding. Khan et al. (37) suggested that increased glucose cycling in the hypothalamus of obese hyperglycemic (ob/ob) mice decreases glucose utilization in the hypothalamus, which results in increased appetite, leading to obesity in ob/ob mice. The activities of the two enzymes involved in glucose cycling, glucokinase and glucose-6-phosphatase, were decreased in smlA mutant cells. This suggests that high levels of CF decrease glucose cycling in Dictyostelium cells.
Our data suggest that exposure of cells to CF decreases the activity of glucose-6-phosphatase and thus lowers intracellular glucose levels. We do not know whether the actual signal that regulates adhesion and motility and thus group size is glucose per se, glucose-6-phosphate, fructose-6-phosphate, fructose-1,6-bisphosphate, or some other metabolite. Our working hypothesis is that, via a signal transduction pathway, CF decreases the activity of glucose-6-phosphatase, resulting in changes in the amount of one or more signal molecules, which in turn regulate adhesion and motility and thus regulate group size during Dictyostelium development.
Acknowledgments
We thank R. Diane Hatton for assistance with preparation of the manuscript and Fred Rudolph and Steve Olson for suggestions and guidance.
R.H.G. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grant C-1555 from the Robert A. Welch Foundation.
Footnotes
This paper is dedicated to the memory of Fred Rudolph.
REFERENCES
- 1.Alegre, M., C. J. Ciudad, C. Fillat, and J. J. Guinovart. 1988. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 173:185-189. [DOI] [PubMed] [Google Scholar]
- 2.Aragon, J. J., V. Sanchez, and L. Boto. 1986. Fructose-2,6-bisphosphatase in Dictyostelium discoideum—independence of cAMP production and inhibition of fructose-1,6-bisphosphatase. Eur. J. Biochem. 161:757-761. [DOI] [PubMed] [Google Scholar]
- 3.Ashworth, J. M., and D. J. Watts. 1970. Metabolism of the cellular slime mold Dictyostelium discoideum grown in axenic culture. Biochem. J. 119:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bady, I., C. Zitoun, L. Guignot, and G. Mithieux. 2002. Activation of liver G-6-pase in response to insulin-induced hypoglycemia or epinephrine infusion in the rat. Am. J. Physiol. Endocrinol. Metab. 282:E905-E910. [DOI] [PubMed] [Google Scholar]
- 5.Baumann, P. 1969. Glucokinase of Dictyostelium discoideum. Biochemistry 8:5011-5015. [DOI] [PubMed] [Google Scholar]
- 6.Baumann, P., and B. E. Wright. 1969. The fructose 1,6-diphosphatase of Dictyostelium discoideum. Biochemistry 8:1655-1659. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, J. D., B. C. Moon, F. Harrow, D. Ratner, R. H. Gomer, R. P. Dottin, and D. T. Brazill. 2002. A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J. Biol. Chem. 277:32430-32437. [DOI] [PubMed] [Google Scholar]
- 8.Brazill, D. T., D. F. Lindsey, J. D. Bishop, and R. H. Gomer. 1998. Cell density sensing mediated by a G protein-coupled receptor activating phospholipase C. J. Biol. Chem. 273:8161-8168. [DOI] [PubMed] [Google Scholar]
- 9.Brock, D. A., F. Buczynski, T. P. Spann, S. A. Wood, J. Cardelli, and R. H. Gomer. 1996. A Dictyostelium mutant with defective aggregate size determination. Development 122:2569-2578. [DOI] [PubMed] [Google Scholar]
- 10.Brock, D. A., K. Ehrenman, R. Ammann, Y. Tang, and R. H. Gomer. 2003. Two components of a secreted cell number-counting factor bind to cells and have opposing effects on cAMP signal transduction in Dictyostelium. J. Biol. Chem. 278:52262-52272. [DOI] [PubMed] [Google Scholar]
- 11.Brock, D. A., and R. H. Gomer. 1999. A cell-counting factor regulating structure size in Dictyostelium. Genes Dev. 13:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brock, D. A., R. D. Hatton, D.-V. Giurgiutiu, B. Scott, R. Ammann, and R. H. Gomer. 2002. The different components of a multisubunit cell number-counting factor have both unique and overlapping functions. Development 129:3657-3668. [DOI] [PubMed] [Google Scholar]
- 13.Brown, J. M., and R. A. Firtel. 2000. Just the right size: Cell counting in Dictyostelium. Trends Genet. 16:191-193. [DOI] [PubMed] [Google Scholar]
- 14.Burchell, A., B. B. Allan, and R. Hume. 1994. Glucose-6-phosphatase proteins of the endoplasmic reticulum. Mol. Membr. Biol. 11:217-227. [DOI] [PubMed] [Google Scholar]
- 15.Cleland, S. V. 1969. Gluconeogenesis and glycolysis in Dictyostelium discoideum. Ph.D. thesis. Northwestern University, Evanston, Ill.
- 16.Donnicke, M., H. W. Hofer, and D. Pette. 1972. Influence of enzyme concentration on the reaction of rabbit muscle phosphofructokinase with antibodies. FEBS Lett. 20:187-190. [DOI] [PubMed] [Google Scholar]
- 17.Efendic, S., A. Wajngot, and M. Vranic. 1985. Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes. Proc. Natl. Acad. Sci. USA 82:2965-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, J. D., and R. C. Nordlie. 2002. The biochemistry and molecular biology of the glucose-6-phosphatase system. Exp. Biol. Med. (Maywood) 227:601-608. [DOI] [PubMed] [Google Scholar]
- 19.Foster, J. D., B. A. Pederson, and R. C. Nordlie. 1997. Glucose-6-phosphatase structure, regulation, and function: an update. Proc. Soc. Exp. Biol. Med. 215:314-332. [DOI] [PubMed] [Google Scholar]
- 20.Gao, T., K. Ehrenman, L. Tang, M. Leippe, D. A. Brock, and R. H. Gomer. 2002. Cells respond to and bind countin, a component of a multisubunit cell number counting factor. J. Biol. Chem. 277:32596-32605. [DOI] [PubMed] [Google Scholar]
- 21.Garrod, D. R., and J. M. Ashworth. 1972. Effect of growth conditions on development of the cellular slime mould Dictyostelium discoideum. J. Embryol. Exp. Morph. 28:463-479. [PubMed] [Google Scholar]
- 22.Gomer, R. H. 2001. Not being the wrong size. Nat. Rev. Mol. Cell. Biol. 2:48-54. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson, G. L., and B. E. Wright. 1972. Analysis of approaches used in studying differentiation of the cellular slime mold. Crit. Rev. Microbiol. 1:453-478. [Google Scholar]
- 24.Henly, D. C., M. C. Chauvet, and J. W. Phillips. 1999. Hepatic glucose cycling does not contribute to the development of hyperglycemia in Zucker diabetic fatty rats. Diabetes 48:342-346. [DOI] [PubMed] [Google Scholar]
- 25.Henly, D. C., J. W. Phillips, and M. N. Berry. 1996. Suppression of glycolysis is associated with an increase in glucose cycling in hepatocytes from diabetic rats. J. Biol. Chem. 271:11268-11271. [DOI] [PubMed] [Google Scholar]
- 26.Hohl, H. R., and K. B. Raper. 1964. Control of sorocarp size in the cellular slime mold Dictyostelium discoideum. Dev. Biol. 9:137-153. [Google Scholar]
- 27.Jain, R., I. S. Yuen, C. R. Taphouse, and R. H. Gomer. 1992. A density-sensing factor controls development in Dictyostelium. Genes Dev. 6:390-400. [DOI] [PubMed] [Google Scholar]
- 28.Jang, W., B. Chiem, and R. H. Gomer. 2002. A secreted cell-number counting factor represses intracellular glucose levels to regulate group size in Dictyostelium. J. Biol. Chem. 277:31972-31979. [DOI] [PubMed] [Google Scholar]
- 29.Jones, T. H. D., and B. E. Wright. 1970. Partial purification and characterization of glycogen phosphorylase from Dictyostelium discoideum. J. Bacteriol. 104:754-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz, E. R. 1978. Cellular slime mold genetics. Bioscience 28:692-697. [PubMed] [Google Scholar]
- 31.Katz, J., and R. Rognstad. 1976. Futile cycles in the metabolism of glucose. Curr. Top. Cell Regul. 10:237-289. [DOI] [PubMed] [Google Scholar]
- 32.Kessin, R. H. 2001. Dictyostelium evolution, cell biology, and the development of multicellularity. Cambridge University Press, New York, N.Y.
- 33.Khan, A., V. Chandramouli, C. G. Ostenson, B. Ahren, W. C. Schumann, H. Low, B. R. Landau, and S. Efendic. 1989. Evidence for the presence of glucose cycling in pancreatic islets of the ob/ob mouse. J. Biol. Chem. 264:9732-9733. [PubMed] [Google Scholar]
- 34.Khan, A., V. Chandramouli, C. G. Ostenson, P. O. Berggren, H. Low, B. R. Landau, and S. Efendic. 1990. Glucose cycling is markedly enhanced in pancreatic islets of obese hyperglycemic mice. Endocrinology 126:2413-2416. [DOI] [PubMed] [Google Scholar]
- 35.Khan, A., V. Chandramouli, C. G. Ostenson, H. Low, B. R. Landau, and S. Efendic. 1990. Glucose cycling in islets from healthy and diabetic rats. Diabetes 39:456-459. [DOI] [PubMed] [Google Scholar]
- 36.Khan, A., C. Hong-Lie, and B. R. Landau. 1995. Glucose-6-phosphatase activity in islets from ob/ob and lean mice and the effect of dexamethasone. Endocrinology 136:1934-1938. [DOI] [PubMed] [Google Scholar]
- 37.Khan, A., L. Zong-Chao, S. Efendic, and B. R. Landau. 1998. Glucose-6-phosphatase activity in the hypothalamus of the ob/ob mouse. Metabolism 47:627-629. [DOI] [PubMed] [Google Scholar]
- 38.Kopachik, W. J. 1982. Size regulation in Dictyostelium. J. Embryol. Exp. Morphol. 68:23-35. [PubMed] [Google Scholar]
- 39.Lang, G., and G. Michal. 1974. d-Glucose-6-phosphate and d-fructose-6-phosphate, p. 1238-1242. In H. V. Bergmeyer (ed.), Methods of enzymatic analysis, vol. 3. Academic Press, New York, N.Y.
- 40.Lehninger, A. L., D. L. Nelson, and M. M. Cox. 1993. Principles of biochemistry, 2nd ed. Worth Publishing, New York, N.Y.
- 41.Lo, L., J. C. Russell, and A. W. Taylor. 1970. Determination of glycogen in small tissue samples. J. Appl. Physiol. 28:234-236. [DOI] [PubMed] [Google Scholar]
- 42.Loomis, W. F. 1975. Dictyostelium discoideum: a developmental system. Academic Press, New York, N.Y.
- 43.Mehdy, M. C., and R. A. Firtel. 1985. A secreted factor and cyclic AMP jointly regulate cell-type-specific gene expression in Dictyostelium discoideum. Mol. Cell. Biol. 5:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michal, G., and H.-O. Beutler. 1974. d-Fructose-1,6-diphosphate, dihydroxyavetone phosphate and d-glyceraldehyde-3-phosphate, p. 1314-1319. In H. V. Bergemeyer (ed.), Methods of enzymatic analysis, vol. 3. Academic Press, New York, N.Y.
- 45.Neet, K. 1996. Cooperativity in enzyme functions: equilibrium and kinetic aspects. .In K. Neet (ed.), Contemporary enzyme kinetics and mechanisms. Academic Press, San Diego, Calif.
- 46.Nordlie, R. C., A. M. Bode, and J. D. Foster. 1993. Recent advances in hepatic glucose 6-phosphatase regulation and function. Proc. Soc. Exp. Biol. Med. 203:274-285. [DOI] [PubMed] [Google Scholar]
- 47.Nordlie, R. C., J. D. Foster, and A. J. Lange. 1999. Regulation of glucose production by the liver. Annu. Rev. Nutr. 19:379-406. [DOI] [PubMed] [Google Scholar]
- 48.Parent, C. A., and P. N. Devreotes. 1999. A cell's sense of direction. Science 284:765-770. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds, R. W., and J. Kimm. 1965. Effect of glucose on food intake in hypothalamic hyperphagic rats. J. Comp. Physiol. Psychol. 60:438-440. [DOI] [PubMed] [Google Scholar]
- 50.Roisin-Bouffay, C., W. Jang, and R. H. Gomer. 2000. A precise group size in Dictyostelium is generated by a cell-counting factor modulating cell-cell adhesion. Mol. Cell 6:953-959. [PubMed] [Google Scholar]
- 51.Shaffer, B. M. 1957. Variability of behavior of aggregating cellular slime moulds. Q. J. Microbiol. Sci. 98:393-405. [Google Scholar]
- 52.Tang, L., T. Gao, C. McCollum, W. Jang, M. G. Vickers, R. Ammann, and R. H. Gomer. 2002. A cell number-counting factor regulates the cytoskeleton and cell motility in Dictyostelium. Proc. Natl. Acad. Sci. USA 99:1371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Werve, G., A. Lange, C. Newgard, M. C. Mechin, Y. Li, and A. Berteloot. 2000. New lessons in the regulation of glucose metabolism taught by the glucose 6-phosphatase system. Eur. J. Biochem. 267:1533-1549. [DOI] [PubMed] [Google Scholar]
- 54.Verkerke-van Wijk, I., and P. Schaap. 1997. cAMP, a signal for survival, p. 145-162. In Y. Maeda, K. Inouye, and I. Takeuchi (ed.), Dictyostelium—a model system for cell and developmental biology. Universal Academy Press, Tokyo, Japan.
- 55.Wajngot, A., A. Khan, A. Giacca, M. Vranic, and S. Efendic. 1990. Dexamethasone increases glucose cycling, but not glucose production, in healthy subjects. Am. J. Physiol. 259:E626-E632. [DOI] [PubMed] [Google Scholar]
- 56.Weber, J. M., S. Klein, and R. R. Wolfe. 1990. Role of the glucose cycle in control of net glucose flux in exercising humans. J. Appl. Physiol. 68:1815-1819. [DOI] [PubMed] [Google Scholar]
- 57.Weeks, G., and J. M. Ashworth. 1972. Glycogen synthetase and the control of glycogen synthesis in the cellular slime mould Dictyostelium discoideum during the growth (myxamoebal) phase. Biochem. J. 126:617-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright, B. E., and G. L. Gustafson. 1972. Expansion of the kinetic model of differentiation in Dictyostelium discoideum. J. Biol. Chem. 247:7875-7884. [PubMed] [Google Scholar]
- 59.Yuen, I. S., R. Jain, J. D. Bishop, D. F. Lindsey, W. J. Deery, P. J. M. Van Haastert, and R. H. Gomer. 1995. A density-sensing factor regulates signal transduction in Dictyostelium. J. Cell Biol. 129:1251-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]