Abstract
Objectives
Preeclampsia is one of the main contributers to maternal and fetal morbidity and mortality during pregnancy. A history of preeclampsia puts mother and offspring at an increased cardiovascular risk in later life. We hypothesized that at the time of birth functional impairments of fetal endothelial cells can be detected in pregnancies complicated by preeclampsia and that a therapeutic intervention using 1,25 (OH)2 vitamin D3 can reverse the adverse effects of preeclampsia on cell function.
Methods
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords obtained from preeclamptic (N = 12) and uncomplicated pregnancies (N = 13, control). Placental villous tissue fragments from uncomplicated term pregnancies were incubated in explant culture for 48 h at 2% (hypoxia), 8% or 21% O2. Explant conditioned media (CM) was collected and pooled according to oxygen level. We compared the ability of preeclampsia vs. control HUVEC to migrate, proliferate, and form tubule-like networks in a Matrigel assay, in the presence/absence of CM and 1,25(OH)2 vitamin D3.
Results
HUVEC from preeclamptic pregnancies showed reduced migration (P = 0.04) and tubule formation (P = 0.04), but no change in proliferation (P = 0.16) compared to healthy pregnancies. Placental villous explant CM derived from 2% O2 incubations significantly reduced HUVEC migration, when compared to non-CM (P = 0.04). Vitamin D3 improved HUVEC function in neither of the groups. There was no significant difference in VEGF gene expression between healthy and preeclamptic pregnancies and no effect of Vitamin D3 on VEGF expression.
Conclusions
Reduced functional abilities of fetal endothelial cells from preeclamptic pregnancies suggests that disease pathways, possibly originating from the dysfunctional placenta, negatively impact fetal endothelium. The neutral effect of 1,25(OH)2 vitamin D3 contrasts with previous findings that vitamin D rescues the poor migration, proliferation and tubule formation exhibited by cord blood fetal endothelial progenitor cells from preeclamptic pregnancies. Further investigations to distinguish pathways by which offspring exposed to preeclampsia are at risk for cardiovascular disease are needed.
Introduction
Long-term follow-up studies find that offspring from mothers with preeclampsia exhibit alterations of the cardiovascular system that predispose them to a higher risk for adverse cardiovascular events. Exposure to a preeclamptic in utero environment increases the systolic and diastolic blood pressure and body mass index (BMI) already in children and young adults [1, 2]. In their teen years, male and female offspring of mothers with preeclampsia display marked vascular dysfunction as evidenced by a higher pulmonary artery pressure, reduced flow-mediated dilation, and increased carotid intima media thickness, the latter a subclinical marker of atherosclerosis [2, 3]. Preeclampsia is associated with an increased risk of stroke [4, 5] and cardiovascular dysfunction [6, 7] in the adult offspring.
It is hypothesized that the associations between preeclampsia and later cardiovascular function relate to the impaired placental development with placental insufficiency and restricted oxygen supply in preeclampsia. The release of pathogenic factors, e.g. antiangiogenic factors and reactive oxygen species, is thought to trigger a systemic oxidative and inflammatory reaction during the pregnancy [8]. However, the impact of such factors on fetal programming remains unclear.
We have shown previously that fetal endothelial colony-forming cells (ECFC), a subgroup of endothelial progenitor cells (EPC) are reduced in cord blood of preeclamptic pregnancies and exhibit reduced migratory, proliferative and angiogenic capabilities in vitro [9, 10]. These differences might represent a first sign of impairment of the neonatal cardiovascular system at the time of delivery. In addition to maternal pregnancy outcome history these findings might help to identify infants at risk for cardiovascular dysfunction already at the time of birth.
Vitamin D is known for its important role in calcium homeostasis and bone metabolism, but it also influences the cardiovascular system through unclear mechanisms [11]. A deficiency of vitamin D is associated with increased cardiovascular disease and all-cause mortality, various cardiovascular risk factors and coronary heart disease [11]. Preeclampsia is characterized by changes in vitamin D and calcium metabolism compared to normal pregnancies [12]. Several observational studies have demonstrated a significant relationship between maternal vitamin D deficiency and the incidence of preeclampsia [13, 14]. Moreover, vitamin D supplementation studies showed protective effects on preeclampsia incidence [14, 15]. Not least we recently showed a significant promotion of in vitro angiogenesis by 1,25 (OH)2 vitamin D3 in fetal ECFC derived from preeclamptic pregnancies, suggesting a regulatory role of vitamin D for ECFC function [9].
As the isolation and characterization of ECFC is time and resource intense, and because adverse effects of a preeclamptic intrauterine environment may not be limited to progenitor endothelial populations, we tested whether preeclampsia impairs the angiogenic properties also of human umbilical vein endothelial cells (HUVEC) and if functional characteristics can be improved by vitamin D. HUVEC are predominantly mature endothelial cells, that are used frequently to study the regulation of endothelial cell function, blood vessel repair, the development of atherosclerotic plaques and angiogenesis [16–18]. As with cord blood endothelial progenitor cells, these fetal endothelial cells may be impacted by pathogenic factors released by the placenta.
Materials and methods
Patients
The Ethical Committee at Hannover Medical School approved the study (1507 / 2012). Informed written consent was obtained from each patient. HUVEC derived from umbilical veins of 13 healthy women with uncomplicated, normotensive pregnancies (controls) and 12 women with preeclampsia, at 36.1 +/- 3.9 (control) and 34.0 +/- 4.3 (PE) weeks of gestation, delivered by vaginal or Cesarean section, were used. Four out of 12 preeclamptic women developed early-onset (29.4 +/-2.9 weeks of gestation) and eight late-onset (36.3 +/-2.1 weeks of gestation) preeclampsia. All women had singleton pregnancies. Preeclampsia was defined as gestational hypertension and proteinuria beginning after 20 weeks of pregnancy with resolution of clinical symptoms postpartum [19]. Gestational hypertension was defined as persistent, new onset hypertension (absolute blood pressure ≥140 mmHg systolic and/or ≥90 mmHg diastolic) appearing after 20 weeks of gestation. Proteinuria was defined as ≥300 mg per 24-h urine collection, ≥2+ protein on voided urine sample, ≥1+ protein on catheterized urine specimen, or a protein-creatinine ratio of ≥0.3. Women were classified as having an uncomplicated pregnancy if they were without proteinuria and normotensive throughout gestation and if they delivered healthy babies. All study subjects were non-smokers by self-report, and were without clinical history of preexisting renal, vascular, or metabolic disease. Pre-pregnancy weight, self-reported at enrollment, and measured height were used to calculate pre- pregnancy BMI. Maternal race was by self- report at enrollment. Gestational age-specific birth weight percentiles, adjusted for infant sex and race, were based upon data from Hannover Medical Center (Hannover, Germany).
Vitamin D3 measurement in patient serum and umbilical cord blood
25(OH) Vitamin D3 concentrations in the individual maternal serum prior delivery and cord blood serum samples after delivery were determined in the clinical laboratory at Hannover Medical School using a LIAISON 25(OH) Vitamin D3 TOTAL Assay (DiaSorin Inc., USA), as per the manufacturers recommendations.
HUVEC isolation, culture and phenotyping
HUVEC were isolated from umbilical cord according to the protocol of Jaffe et al. with some modificatios [20]. Briefly, umbilical cords were collected immediately after delivery and stored at 4°C until the cells were isolated between one and four days after delivery under sterile conditions. After incubation of the umbilical cord with collagenase enriched cord buffer for 25 min at 37°C the detached endothelial cells were washed in 10 ml growth medium containing 2.44% (v/v) supplements (human recombinant epidermal growth factor, fibroblast growth factor, VEGF, ascorbic acid, hydrocortisone, recombinant insulin-like growth factor in supplier-recommended concentrations, Lonza, Walkersville, MD, USA), 8% fetal calf serum (FCS, Biochrom, Berlin, Germany) and 1.2% penicillin/ streptomycin (Biochrom, Berlin, Germany) and transferred to a T75 cell culture flask. The next day the medium was refreshed. The HUVEC were cultured at 37°C and 5% CO2 in T75 cell culture flasks until they were grown to confluence. After trypsinization they were used for phenotyping or frozen in freezing medium containing 92% FCS and 8% DMSO (Sigma Aldrich, Steinheim, Germany) and stored in liquid nitrogen until they were used for experiments. All experiments were performed with HUVEC in passage 2 to 6. For each functional experiment, HUVEC from a preeclamptic and a corresponding control patient were run in tandem. Using flow cytometry the typical endothelial cell phenotype (CD31+, CD90-) was confirmed. During the cell culture assays HUVEC were incubated with 0 nM (vehicle) or 10 nM of 1,25 (OH)2 vitamin D3 (Sigma Aldrich, St. Louis, MO). The concentration of 1,25 (OH)2 vitamin D3 was intended to approximate physiological levels in pregnancy [14] and was previously found to rescue ECFC functions in vitro [9, 10, 21].
Culture of placental villous explants and preparation of conditioned medium
Placental villous explant preparation and culture was carried out according to published protocols with some modifications [22]. Briefly, placentas from eigth uncomplicated, healthy pregnancies delivered by vaginal or Cesarean section were obtained within 10 min of delivery. After removal of the decidua, biopsies were excised from the maternal side of the placenta. The tissue was immediately transported to the laboratory in ice-cold phosphate buffered saline (PBS) containing 2% penicillin/streptomycin. After rinsing the placental pieces in PBS to wash out blood, large vessels and decidua were removed by blunt dissection. Placental villous explants (1–2 mg each in size) were dissected and used for experiments under different oxygen conditions. An average of 50 mg of finely dissected villous tissue was placed into each well of a 12-well plate containing 1.5 ml of Medium 199 (Sigma-Aldrich, St- Louis, MO, USA) supplemented with 2% FCS and 1% penicillin/streptomycin. The plates were incubated under controlled oxygen conditions (2% O2, 8% O2 and 21% O2) in three separate incubator chambers (Xvivo, Biospherix Inc., USA) at 37°C, 5% CO2 for 48 h. The conditioned medium (CM) was centrifuged (3,200 rpm, 4°C, 5 min). The cell-free supernatants were pooled according to the oxygen conditions and stored in aliquots at -80°C. As control, M199 medium supplemented with 2% FBS, 1% penicillin/streptomycin (non-conditioned medium, NCM) was employed in the same ratio as CM.
In vitro angiogenesis assay
We used an in vitro angiogenesis assay (endothelial tubule formation in Matrigel) as a test of HUVEC function. In a 96-well plate 8,000 cells per well were seeded in 150 ul treatment medium with 30 ul growth factor reduced Matrigel (BD Biosciences, Bedford, MA). HUVEC from preeclampsia and a corresponding control patient were run in tandem. The experiments were performed in the presence and absence of 10 nM 1,25(OH)2 vitamin D3. After six hours of incubation at 37°C each well was photographed with a LEICA DMI 6000 B microscope. The formed tubules were quantified using Image-J software (National Institutes of Health). All experiments were performed in triplicate wells from which values were averaged (N = number of experiments).
Cell migration assay
To analyze HUVEC migratory ability, 10,000 to 50,000 HUVEC were seeded in each well of a 12-well-plate with growth medium containing 2.44% supplements, 8% FCS and 1.2% penicillin/ streptomycin. After reaching confluence the HUVEC monolayer was scratched using a sterile P200 pipette tip to produce a lane free of cells as described before [23]. HUVEC from preeclampsia and a corresponding control patient were run in tandem. The experiments were performed in the presence and absence of 10 nM 1,25(OH)2 vitamin D3. Additionally, CM from three different oxygen levels or NCM were added at 50% v/v concentration. The experiments were performed in the presence or absence of 10 nM 1,25(OH)2 vitamin D3. Light microscopic images were obtained immediately after the scratch (T0) and at the end of the experiment after 18h (T18). Migration into the scratch wound was analysed using Image J software and calculated as percentage of wound closure (percentage of original area at T0 that became occupied by cells by migration into the wound area at T18). All experiments were done in quadruplicate wells from which values were averaged.
Proliferation assay
To determine the proliferative capacity of HUVEC derived from uncomplicated and preeclamptic pregnancies in the presence or absence of 1,25(OH)2 vitamin D3, 10,000 cells were seeded per well of 24-well culture plates in EGM supplemented with 8% (v/v) FCS and 1% penicillin/streptomycin. Medium was changed the next day and cells were incubated with 0 nM (vehicle) or 10 nM of 1,25 (OH)2 vitamin D3. After 24 h, 48 h and 72 h of treatment, the cell number was counted in a Neubauer chamber with 1:2 trypan-blue dilution. Population doubling time was calculated as following: log2/ (logNt–logNo), t = time period (h), Nt = number of cells at time t, No = initial cell number.
RT-PCR for quantification of VEGF gene expression
The RNA isolation of HUVEC was performed according to the protocol of Chomczynski et al. [24] which was slightly modified. The concentration of RNA of each sample was determined spectrophotometrically (BIO photometer, Eppendorf, Germany) at 260/280 nm and aliquots (2 mg) of this RNA were fractionated on agarose/ethidium bromide gels to check RNA integrity.
For the cDNA synthesis 2 ug of RNA was diluted with diethylpyrocarbonate (DEPC) water to a volume of 8 ul and denatured at 68°C for 10 minutes in a thermocycler (PTC 200, Biozym Scientific GmbH, Germany). Then 12 ul of High Capacity cDNA Reverse transcription (RT) master mix were added. This is made up of RT buffer, RT Random primer, deoxyribonucleoside triphosphate (dNTP) mix (100 mM), MultiScribeTMReverse transcriptase (Applied Biosystems, USA) and DEPC. The generated cDNA was initially diluted 1: 2 with DEPC water. In each case, 1.5 ul diluted cDNA and 10.5 ul master mix were pipetted into the appropriate strip tubes (0.1 ml). For each treatment, medium triplets were created and three RT-PCR runs were done for each patient. For normalization, 18S rRNA served as housekeeping gene. Primer sequences used were as follows: VEGF-A forward primer 5-CTGGAGTGTGTGCCCACTGA-3, VEGF-A reverse primer 5-TCCTATGTGCTGGCCTTGGT-3, 18S rRNA forward primer 5-GAGCGAAAGCATTTGCCAAG-3 239, 18S rRNA reverse primer 5—GGCATCGTTTATGGTCGGAA-3). Ct values were automatically generated and relative quantification of gene expression was calculated by standard ΔCt method using the expression of 18S rRNA as reference. Relative expression levels of HUVEC from uncomplicated pregnancies and pregnancies complicated by preeclampsia with and without vitamin D treatment were finally compared.
Statistical analysis
Demographic data are expressed as means and standard deviation. Experimental data are presented as means and standard error. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with ANOVA, Kruskal-Wallis, unpaired t-test, Mann-Whitney or Wilcoxon-signed rank test, as appropriate. Data were analyzed with Prism 4 software package (GraphPad Software Inc., La Jolla, CA). Where specified, to account for interassay variation, data are given as fold change in functional variables relative to untreated HUVEC from uncomplicated pregnancies or relative to untreated HUVEC from preeclamptic pregnancies.
Results
Patient demographics
The clinical and demographic data for the pregnant women who provided umbilical cord for the analysis of HUVEC function are given in Table 1. Maternal age, maternal pre-pregnancy BMI, race and parity, and baby sex were not statistically different between the preeclampsia and control groups. The percentage of women who delivered by Cesarean section versus vaginal delivery did not differ by outcome group. By definition, women with preeclampsia had higher systolic and diastolic blood pressures at delivery compared to the uncomplicated study group. Patients were matched by gestational age for the cell culture experiments.
Table 1. Clinical and demographic data of patients who provided maternal blood samples (data are mean +/- SD).
| Uncomplicated pregnancy (n = 13) |
Preeclampsia (n = 12) |
P value |
|
|---|---|---|---|
| Maternal age | 32.2 +/-5.2 | 31.1 +/-4.1 | 0.55 |
| Gestational age at delivery (wks) | 36.1 +/-3.9 | 34.0 +/-4.3 | 0.23 |
| Multiparous- n (%) | 5 (38%) | 3 (25%) | 0.70 |
| Maternal pre-pregnancy BMI (kg/m2) | 24.9 +/-5.3 | 26.6 +/-6.3 | 0.51 |
| Gestational SBP, pre-delivery (mm Hg) | 117 +/-13.0 | 170 +/-19.6 | <0.0001 |
| Gestational SBP before 20 week gestation (mm Hg) | 119 +/-9.2 | 127 +/-14.1 | 0.054 |
| Gestational DBP, pre-delivery (mm Hg) | 67.1 +/-7.9 | 99.6 +/-15.6 | <0.0001 |
| Gestational DBP before 20 week gestation (mm Hg) | 72.9 +/-8.9 | 80.6 +/-14.0 | 0.12 |
| Birth weight (g) | 2637 +/- 1026 | 1907 +/- 857 | 0.08 |
| Birth weight percentile | 34.8 +/- 26.2 | 20.9 +/- 25.6 | 0.08 |
| Birth weight percentile <10th- n (%) | 3 (23%) | 6 (50%) | 0.48 |
| Caesarean delivery- n (%) | 9 (69%) | 9 (75%) | 1.0 |
| Maternal Race, Black- n (%) | 0 (0%) | 0 (0%) | 1.0 |
| Baby sex, male- n (%) | 9 (69%) | 4 (33%) | 0.49 |
Vitamin D3 concentrations in maternal serum and umbilical cord blood
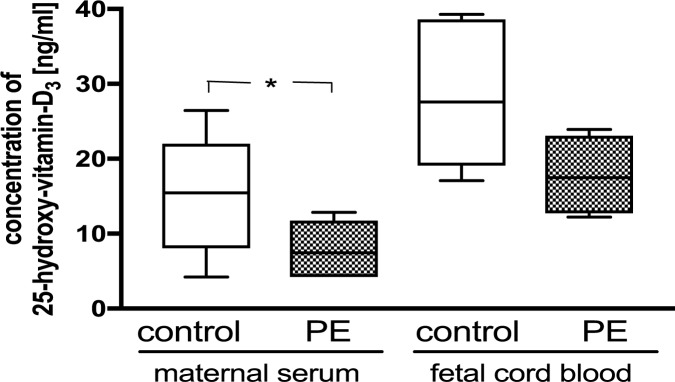
Women with preeclampsia had significantly lower 25(OH) vitamin D3 levels compared to women with healthy pregnancies (Control: 15.5 +/- 2.4 ng/ml; PE: 7.5 +/- 1.5 ng/ml; P = 0.04), (Fig 1). The 25(OH) vitamin D3 levels in umbilical cord blood of preeclamptic pregnancies were marginally lower compared to cord blood of control patients (Control: 27.6 +/- 2.3 ng/ml; PE: 17.5 +/- 2.7 ng/ml; P = 0.06). Umbilical cord blood concentrations of 25(OH) vitamin D3 were significantly higher than in maternal sera (Control: maternal vs. umbilical cord blood: P = 0.005; preeclampsia: maternal vs. umbilical cord blood: P = 0.009). Of all patients, 70% of the control group and 100% of the preeclamptic group were vitamin D deficient when defined as 25(OH) vitamin D3 concentrations ≤ 20 ng/ml.
Fig 1. 25(OH) vitamin D concentrations (ng/ml) in the individual maternal serum samples prior delivery and umbilical cord blood after delivery.
*P < 0.05 vs. control. The ends of the whiskers represent the maximum and minimum measured values. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with unpaired t-test.
In vitro angiogenesis
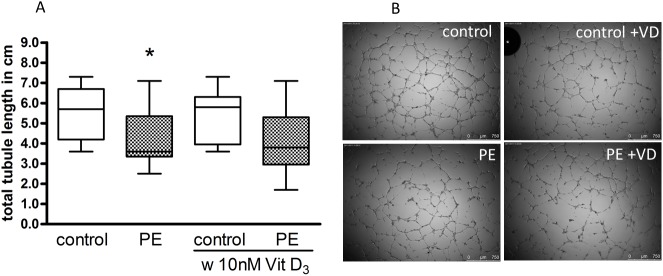
A Matrigel angiogenesis model was used to assess the capacity of HUVEC to differentiate into tubule-like structures (Fig 2). Tubule assemblage by preeclampsia-derived HUVEC was markedly impaired (Control: 5.5 +/- 0.4 cm; PE: 4.4 +/- 0.4 cm; 72% of control, P = 0.04). The supplementation with 10 nM 1,25(OH)2 vitamin D3 did not impact the tubule formation of HUVEC from uncomplicated pregnancies (Control: 5.2 +/- 0.4 cm; 96% of control, P = 0.54) or preeclamptic pregnancies (PE: 4.1 +/- 0.5 cm, 94% of control, P = 0.89).
Fig 2. Building of tubule-like structures by uncomplicated pregnancy (control) and preeclampsia (PE) HUVEC, and the effect of 1,25(OH)2 vitamin D3, on capillary-tube formation by HUVEC in a Matrigel assay.
(A) Capillary-tube formation (average total tubule length per microscopic field) was analyzed after 6 h by visual microscopy at 2.5 magnification. Data are expressed as total tubule length in cm. *P < 0.05 vs. control. The ends of the whiskers represent the maximum and minimum measured values. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with Mann-Whitney test. (B) Representative photomicrographs of HUVEC after incubation in Matrigel. Scale bar represents 750 μm.
Migration
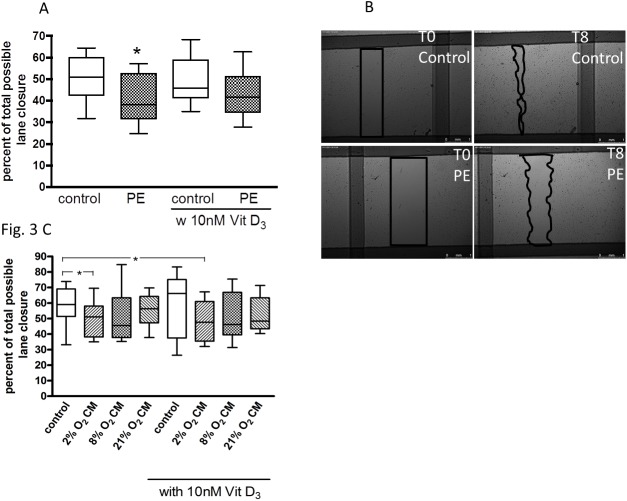
With scratch wound area filling expressed as percent of total possible lane closure (100%), the migration of HUVEC from preeclamptic pregnancies (41% +/- 3.2%) was significantly impaired, compared to control (50% +/- 2.9%; P = 0.04) (Fig 3A and 3B). After treatment of HUVEC from healthy and preeclamptic pregnancies with 1,25(OH)2 vitamin D3 the two groups were no longer significantly different (P = 0.22). However, 1,25(OH)2 vitamin D3 did not significantly improve HUVEC migration in the control group (49% +/- 2.9%, 92% of control, P = 0.81) or the preeclamptic pregnancy outcome group (44% +/-3.1%, 98% of control, P = 0.53), suggesting at best a marginal impact of vitamin D.
Fig 3. Effect of preeclampsia (PE), preeclampsia-like conditions and 1,25(OH)2 vitamin D3 on HUVEC migration.
(A) HUVEC of uncomplicated (control) and PE pregnancies were cultured in endothelial basal medium (EBM) and treated with or without 10 nM 1,25(OH)2 vitamin D3. The migration of PE HUVEC into the scratch wound assessed after incubation for 18 h was decreased compared to control. (B) "scratch wound" at 0 h and 8 h incubation is shown. Scale bar represents 750 μm. (C) Effect of villous explant conditioned medium (2% O2, 8% O2, 21% O2 CM) and 1,25(OH)2 vitamin D3 on HUVEC migration with a reduction at 2% O2. *P < 0.05 vs. untreated control. The ends of the whiskers represent the maximum and minimum measured values. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with unpaired t-test (A) or Wilcoxon-signed rank test (C).
Clinical and demographic data for the uncomplicated pregnant women who provided placental samples for villous explant culture are given in Table 2. Treatment with placental villous explant CM derived from 2% O2 incubations led to a reduced HUVEC migration (49% +/- 3.5%, 85% of NCM; P = 0.04), when compared to NCM (59% +/- 3.7%). Compared to NCM, explant CM from 8% O2 impaired HUVEC migration, but this effect was not statistically significant (50% +/- 5.2%, 87% of NCM; P = 0.38), (Fig 3C). Explant CM from 21% O2 did not affect HUVEC migration (55% +/- 3.1%, 99% of NCM; P = 0.46). Compared to absence of vitamin D, 1,25(OH)2 vitamin D3 did not improve endothelial cell migration in any CM group.
Table 2. Clinical and demographic data for the uncomplicated pregnant women who provided placental samples for villous explant culture (data are mean +/2 SD).
| (n = 8) |
|
|---|---|
| Maternal age | 33.3 +/-4.9 |
| Gestational age at delivery (wks) | 38.2 +/-2.4 |
| Multiparous- n (%) | 4 (50%) |
| Maternal pre-pregnancy BMI (kg/m2) | 29.2 +/-9.3 |
| Gestational SBP, pre-delivery (mm Hg) | 119.8 +/-11.5 |
| Gestational SBP before 20 week gestation (mm Hg) | 75.0 +/-7.6 |
| Birth weight (g) | 3626 +/-831 |
| Birth weight percentile | 72.3 +/-30.8 |
| Birth weight percentile <10th- n (%) | 1 (12.5%) |
| Caesarean delivery- n (%) | 6 (75%) |
| Maternal Race, Black–n (%) | 0 (0%) |
| Baby gender, male- n (%) | 4 (50%) |
Proliferation
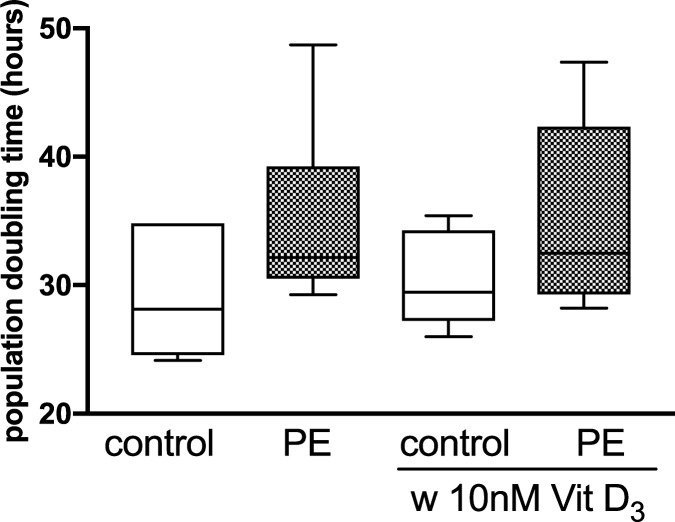
The population doubling time (proliferation) of HUVEC isolated from preeclamptic pregnancies was only marginally imparied (34.8 h) compared to uncomplicated pregnancy (29.7 h) during 72 h of culture (P = 0.16) (Fig 4). Concentrations of 10 nM 1,25(OH)2 vitamin D3 did not change the doubling time of uncomplicated or preeclamptic pregnancies.
Fig 4. Effect of pregnancy outcome and 1,25(OH)2 vitamin D3 on HUVEC population doubling time.
HUVEC of uncomplicated (control) and preeclamptic (PE) pregnancies were incubated in the presence and absence of 1,25(OH)2 vitamin D3 (10 nM). Cell numbers were counted and population doubling time calculated after 72 h. The ends of the whiskers represent the maximum and minimum measured values. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with Mann-Whitney test.
RT-PCR for quantification of the VEGF gene expression
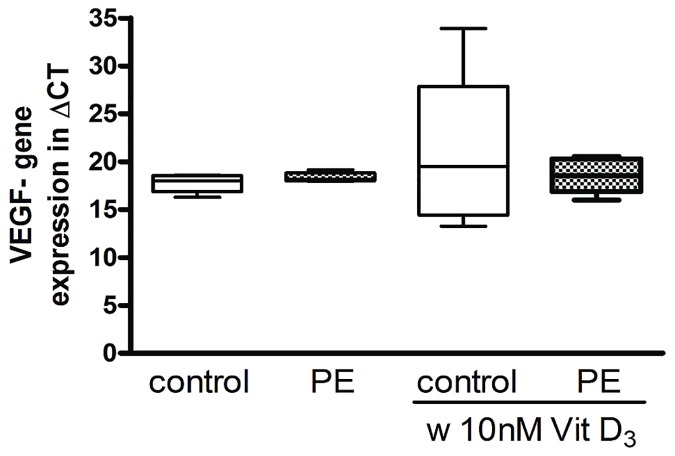
There was no significant difference in the VEGF gene expression of HUVEC from healthy pregnancies compared to HUVEC from peeclamptic pregnancies (P = 0.39) (Fig 5). The treatment with 10nM 1,25(OH)2 vitamin D3 did not change the VEGF gene expression neither in the control group (P = 0.37) nor in the preeclamptic group (P = 0.75).
Fig 5. VEGF gene expression of HUVEC from uncomplicated (control) and preeclamptic (PE) pregnancies in the presence and absence of 1,25(OH)2 vitamin D3 (10 nM) in ug/ml.
For each treatment medium triplets were created and three RT-PCR runs were performed for each patient. The ends of the whiskers represent the maximum and minimum measured values. Distribution was examined using Kolmogorov- Smirnov test. Continuous data were compared with Mann-Whitney test.
Discussion
Adverse changes of the cardiovascular system in offspring of women with preeclampsia have been described already in childhood and in young adulthood. However, at the time of birth when most babies appear to be healthy and classical risk factors for cardiovascular disease (e.g. high BMI, smoking, hypertension) are not present, merely the maternal history and the pregnancy outcome are used to predict an infant’s risk for chronic disease. Therefore, we thought to use a fetal endothelial cell model (HUVEC), to examine if the effect of preeclampsia on endothelial function can be detected already this early in life and if a therapeutic intervention using 1,25(OH)2 vitamin D3 can improve cell characteristics.
In line with our hypothesis we showed a negative impact of preeclampsia on functional characteristics, e.g. tubule formation and migration, of endothelial cells derived from the umbilical vein. A similar effect on migration was observed after treatment of HUVEC with CM derived from placental explant cultures at 2% O2 compared to NCM. To our knowledge we are the first to who show a neutral effect of 1,25(OH)2 vitamin D3, which is in contrast to previous studies, in which the impaired migration, proliferation and tubule formation of fetal, cord blood-derived endothelial progenitor cells from preeclamptic pregnancies was improved [9, 10, 21].
Placental vasculogenesis and angiogenesis are important for a healthy pregnancy. In preeclampsia various angiogenic abnormalities in the feto-placental unit and alterations of circulating angiogenic factors have been described [25, 26]. Our finding here of a reduced average tubule length under standard culturing conditions (21% O2) in an in vitro angiogenesis model in preeclampsia is consistent with a functional study that described inherent differences of the angiogenic capacity in HUVEC from women with the disease [27]. The migration of endothelial cells marks an important contribution to angiogenesis. Therefore, impaired migration and tubule formation as observed in HUVEC of preeclamptic pregnancies in this study may be germane to angiogenic abnormalities observed in preeclampsia. Although the generation times of HUVEC from preeclamptic pregnancies were 17% higher they did not statistically differ from the generation times of HUVEC from healthy patients.
Incompletely remodeled spiral arteries and the associated dysfunctional blood flow are central features in the pathophysiology of preeclampsia [28, 29]. Fluctuating oxygen concentrations lead to oxidative stress and the release of inflammatory cytokines, antiangiogenic factors and reactive oxygen species, that target endothelial cells causing generalized maternal endothelial dysfunction [30–35]. To simulate these non-physiologic conditions in vitro and to assess the effect of aberrant oxygen conditions on endothelial cell migration we used 25% v/v of placental explant CM obtained from incubations at 2% O2 (to mimic placental hypoxia in the second half of pregnancy), 8% O2 (to approximate placental normoxia in the second half of pregnancy) and 21% O2 (standard culture conditions). To the best of our knowledge this is the first study to show that hypoxic placental CM induced a reduction in HUVEC migration. Previously, Moyes et al. described a significant reduction of tubule formation of HUVEC in vitro incubated at 0.5% O2. [27]. Our findings are in line with a report of decrease in endothelial cell ATP levels, mitochondrial dehydrogenase activity, and mitochondrial membrane potential and increased endothelial cell death with medium conditioned by hypoxic explants [34]. However, direct exposure to hypoxia of HUVEC [36], human breast cancer cells [37] or murine stem cells [38] stimulated cell migration. These results are in contrast to our findings but could be explained by different effects of direct hypoxia that stimulates angiogenesis compared to hypoxic CM that we deployed. Hypoxic CM contains potentially pathogenic factors, e.g. sFlt-1 and inflammatory cytokines [39] implicated in the endothelial cell dysfunction of preeclampsia. Moreover, chronic hypoxia as employed in some of the studies might not exactly model the effects of fluctuations (hypoxia-reoxygenation) thought to frequently occur with preeclampsia [40].
Vitamin D deficiency is associated with adverse cardiovascular outcomes and an increased risk for the development of preeclampsia (14,15,17,18,19). Compared with uncomplicated pregnancies, there is a reduced maternal, placental and fetal vitamin D pool [41]. Although in this in vitro study the overall patient numbers were low (N = 12 and 13) we confirmed significantly lower maternal serum concentrations of 25(OH) vitamin D in preeclamptic participants. Interestingly, although not statistically different fetal cord blood concentrations in preeclampsia were 63% lower compared to healthy pregnancies. This in accordance with previous findings [10]. Four out of 12 preeclamptic cases were early-onset preeclampsia < 34 weeks gestation. While early cases are often clinically more severe and one could expect a different experimental response, we performed a subanalysis of our data. Here, we didn’t see a clear pattern of a stronger impairment of HUVEC functional characteristics in early-onset cases compared to late-onset preeclampsia.
In contrast to our hypothesis we did not confirm a significant effect of 1,25 (OH)2 vitamin D3 on endothelial cell function. Neither HUVEC of healthy nor HUVEC from preeclamptic pregnancies changed their angiogenic, migratory or proliferative behaviour when incubated with 10 nM 1,25 (OH)2 vitamin D3. Previous studies on the effect of 1,25 (OH)2 vitamin D3 yielded controversial results. While a negative effect of 1,25 (OH)2 vitamin D3 on endothelial cells isolated from the bovine aorta and a decrease of migration of endothelial cells from the pulmonary artery [42] have been found [43], an increased migration of vascular smooth muscle cells (VSMC) [44] and no effect on the migration of retinal endothelial cells [45] have been described. Our previous work showed a significant inhibitory effect of preeclampsia, preeclamptic serum or hypoxic CM on fetal ECFC migration, proliferation and tubule formation [9, 10, 21]. In these studies, 1,25 (OH)2 vitamin D3 rescued fetal ECFC function under the described conditions [9, 10, 21]. ECFC are a subset of endothelial progenitor cells and critical to blood vessel formation and repair [46]. ECFC dysfunction may represent an early risk factor or risk marker for cardiovascular disease [47]. ECFCs reportedly differ from HUVECs or human umbilical artery endothelial cells in the expression of differentiation-related surface markers (such as CD44) or activities such as proliferation or telomerase activities [48]. However, the reason for the distinct proangiogenic response to vitamin D by fetal ECFC is presently unclear. Apparently, ECFC may be more plastic and adaptable and thus capable of responding compared to the more mature and differentiated HUVEC. In addition, angiogenesis could be regulated by different vitamin D receptor cascades, which vary depending on differentiation, localization or species of the cells. Our data in fetal endothelial cell models suggest that the vitamin D effect is mediated via the fetal progenitor endothelial cells and not via mature fetal endothelial cells.
The effect of preeclampsia on VEGF gene expression in HUVEC has not been studied yet. In this study, the VEGF gene expression of HUVEC from preeclampsia was not different from the gene expression of healthy pregnancies. However, an increase of the VEGF receptor expression in preeclamptic placentas [33], an increase of VEGF gene expression in HUVEC under hypoxic conditions [36, 49], and a decrease of free VEGF in fetal hematopoietic EPCs from preeclamptic women [50] was described previously. In contrast to our assumption 1,25 (OH)2 vitamin D3 did not affect VEGF gene expression in HUVEC from either healthy nor preeclamptic pregnancies. Cardus et al. showed a stimulating effect of 1,25 (OH)2 vitamin D3 on VEGF gene expression in VSMC and others confirmed this finding in embryo fibroblasts [51], osteoblasts [52] and fetal ECFC [21].
At the time of birth the umbilical cord and cord blood provide easy accessible tissue and cells. ECFC and HUVEC of the developing fetus are exposed to an adverse metabolic milieu during preeclamptic pregnancies. They might serve as a source for the development of predictive models for later life disease risk. However, at this point it is unclear, if the observed impairments of cell function and metabolism persist throughout child- and adulthood or if they are a temporary effect of the acute disease. Therefore, long-term follow-up studies need to assess the feasibility of using these vascular cells for risk prediction and if an association of functional observations with other clinical measures of endothelial dysfunction exists. Since cardiovascular events remain the main cause of death in the developed world the identification of offspring at risk for cardiovascular alterations as early in life as possible is urgently needed and will allow for closer observation and risk-reducing interventions already in early childhood.
Acknowledgments
We gratefully acknowledge the assistance of Prof. Ralf Hass and co-workers (Hannover, Germany) with the flow cytometry and thank the staff of Labor and Delivery of Hannover Medical School for support in obtaining blood and placental samples. We thank Prof. Carl A. Hubel (Magee-Womens Research Institute and Foundation, Pittsburgh, USA) for scientific discussions and assistance with the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
The study was funded by a grant from the Deutsche Forschungsgemeinschaft (VE490/7-1) to Frauke von Versen-Höynck. http://www.dfg.de/en/.
References
- 1.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61. doi: 10.1542/peds.2011-3093 [DOI] [PubMed] [Google Scholar]
- 2.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56(1):159–65. Epub 2010/05/19. doi: 10.1161/HYPERTENSIONAHA.110.150235 [DOI] [PubMed] [Google Scholar]
- 3.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122(5):488–94. Epub 2010/07/21. doi: 10.1161/CIRCULATIONAHA.110.941203 [DOI] [PubMed] [Google Scholar]
- 4.Kajantie E, Phillips DI, Andersson S, Barker DJ, Dunkel L, Forsen T, et al. Size at birth, gestational age and cortisol secretion in adult life: foetal programming of both hyper- and hypocortisolism? Clin Endocrinol (Oxf). 2002;57(5):635–41. Epub 2002/10/23. [DOI] [PubMed] [Google Scholar]
- 5.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–80. Epub 2009/03/07. doi: 10.1161/STROKEAHA.108.538025 [DOI] [PubMed] [Google Scholar]
- 6.Lewandowski AJ, Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum Dev. 2014;90(11):725–9. Epub 2014/09/12. doi: 10.1016/j.earlhumdev.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 7.Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127(2):197–206. Epub 2012/12/12. doi: 10.1161/CIRCULATIONAHA.112.126920 [DOI] [PubMed] [Google Scholar]
- 8.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30 Suppl A:S38–42. Epub 2009/01/14. [DOI] [PubMed] [Google Scholar]
- 9.von Versen-Höynck F, Brodowski L, Dechend R, Myerski AC, Hubel CA. Vitamin D antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function. PLoS One. 2014;9(6):e98990 PubMed Central PMCID: PMC4044051. doi: 10.1371/journal.pone.0098990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodowski L, Burlakov J, Myerski AC, von Kaisenberg CS, Grundmann M, Hubel CA, et al. Vitamin D prevents endothelial progenitor cell dysfunction induced by sera from women with preeclampsia or conditioned media from hypoxic placenta. PLoS One. 2014;9(6):e98527 PubMed Central PMCID: PMCPMC4041729. doi: 10.1371/journal.pone.0098527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–56. Epub 2008/12/06. doi: 10.1016/j.jacc.2008.08.050 [DOI] [PubMed] [Google Scholar]
- 12.August P, Marcaccio B, Gertner JM, Druzin ML, Resnick LM, Laragh JH. Abnormal 1,25-dihydroxyvitamin D metabolism in preeclampsia. Am J Obstet Gynecol. 1992;166(4):1295–9. Epub 1992/04/01. [DOI] [PubMed] [Google Scholar]
- 13.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720–6. doi: 10.1097/EDE.0b013e3181a70f08 [DOI] [PubMed] [Google Scholar]
- 14.Halhali A, Villa AR, Madrazo E, Soria MC, Mercado E, Diaz L, et al. Longitudinal changes in maternal serum 1,25-dihydroxyvitamin D and insulin like growth factor I levels in pregnant women who developed preeclampsia: comparison with normotensive pregnant women. J Steroid Biochem Mol Biol. 2004;89-90(1–5):553–6. Epub 2004/07/01. doi: 10.1016/j.jsbmb.2004.03.069 [DOI] [PubMed] [Google Scholar]
- 15.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr. 1990;64(3):599–609. Epub 1990/11/01. [DOI] [PubMed] [Google Scholar]
- 16.Pan C, Xing JH, Zhang C, Zhang YM, Zhang LT, Wei SJ, et al. Aldehyde dehydrogenase 2 inhibits inflammatory response and regulates atherosclerotic plaque. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munarin F, Coulombe KL. A novel 3-dimensional approach for cardiac regeneration. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2015;2015:1741–4. PubMed Central PMCID: PMC4710856. [DOI] [PMC free article] [PubMed]
- 18.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvascular research. 2003;66(2):102–12. [DOI] [PubMed] [Google Scholar]
- 19.Group NHBPEPW. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American journal of obstetrics and gynecology. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 20.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. The Journal of clinical investigation. 1973;52(11):2745–56. PubMed Central PMCID: PMC302542. doi: 10.1172/JCI107470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303(9):C954–62. PubMed Central PMCID: PMC3492823. doi: 10.1152/ajpcell.00030.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Versen-Höynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta. 2009;30(5):434–42. Epub 2009/03/24. PubMed Central PMCID: PMC2674514. doi: 10.1016/j.placenta.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bainbridge SA, Roberts JM, von Versen-Höynck F, Koch J, Edmunds L, Hubel CA. Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers. Am J Physiol Cell Physiol. 2009;297(2):C440–50. Epub 2009/06/19. PubMed Central PMCID: PMC2724097. doi: 10.1152/ajpcell.00593.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nature protocols. 2006;1(2):581–5. doi: 10.1038/nprot.2006.83 [DOI] [PubMed] [Google Scholar]
- 25.Roberts JM. Preeclampsia: what we know and what we do not know. Seminars in perinatology. 2000;24(1):24–8. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven't we cured the disease? J Reprod Immunol. 2013;99(1–2):1–9. doi: 10.1016/j.jri.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyes AJ, Maldonado-Perez D, Gray GA, Denison FC. Enhanced angiogenic capacity of human umbilical vein endothelial cells from women with preeclampsia. Reprod Sci. 2011;18(4):374–82. Epub 2010/10/22. doi: 10.1177/1933719110385131 [DOI] [PubMed] [Google Scholar]
- 28.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–82. Epub 2009/04/21. PubMed Central PMCID: PMCPMC2697319. doi: 10.1016/j.placenta.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613–21; discussion 23–6. Epub 1997/11/19. [DOI] [PubMed] [Google Scholar]
- 30.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111(5):649–58. PubMed Central PMCID: PMC151901. doi: 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kingdom JC, Kaufmann P. Oxygen and placental vascular development. Advances in experimental medicine and biology. 1999;474:259–75. [DOI] [PubMed] [Google Scholar]
- 32.Trollmann R, Amann K, Schoof E, Beinder E, Wenzel D, Rascher W, et al. Hypoxia activates the human placental vascular endothelial growth factor system in vitro and in vivo: up-regulation of vascular endothelial growth factor in clinically relevant hypoxic ischemia in birth asphyxia. American journal of obstetrics and gynecology. 2003;188(2):517–23. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–91. Epub 2004/10/09. doi: 10.1161/01.RES.0000147365.86159.f5 [DOI] [PubMed] [Google Scholar]
- 34.Robinson NJ, Wareing M, Hudson NK, Blankley RT, Baker PN, Aplin JD, et al. Oxygen and the liberation of placental factors responsible for vascular compromise. Laboratory investigation; a journal of technical methods and pathology. 2008;88(3):293–305. doi: 10.1038/labinvest.3700746 [DOI] [PubMed] [Google Scholar]
- 35.Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Human pathology. 2002;33(11):1069–77. doi: 10.1053/hupa.2002.129420 [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, et al. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. Journal of cellular and molecular medicine. 2012;16(8):1840–55. PubMed Central PMCID: PMC3822696. doi: 10.1111/j.1582-4934.2011.01479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Indelicato M, Pucci B, Schito L, Reali V, Aventaggiato M, Mazzarino MC, et al. Role of hypoxia and autophagy in MDA-MB-231 invasiveness. Journal of cellular physiology. 2010;223(2):359–68. doi: 10.1002/jcp.22041 [DOI] [PubMed] [Google Scholar]
- 38.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem cells. 2003;21(3):337–47. doi: 10.1634/stemcells.21-3-337 [DOI] [PubMed] [Google Scholar]
- 39.Dunn WB, Brown M, Worton SA, Crocker IP, Broadhurst D, Horgan R, et al. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta. 2009;30(11):974–80. doi: 10.1016/j.placenta.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 40.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215(1):27–35. PubMed Central PMCID: PMC2714636. doi: 10.1111/j.1469-7580.2008.00978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halhali A, Diaz L, Barrera D, Avila E, Larrea F. Placental calcitriol synthesis and IGF-I levels in normal and preeclamptic pregnancies. J Steroid Biochem Mol Biol. 2013. Epub 2014/01/01. [DOI] [PubMed] [Google Scholar]
- 42.Hisa T, Taniguchi S, Tsuruta D, Hirachi Y, Ishizuka S, Takigawa M. Vitamin D inhibits endothelial cell migration. Archives of dermatological research. 1996;288(5–6):262–3. [DOI] [PubMed] [Google Scholar]
- 43.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circulation research. 2000;87(3):214–20. [DOI] [PubMed] [Google Scholar]
- 44.Rebsamen MC, Sun J, Norman AW, Liao JK. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circulation research. 2002;91(1):17–24. [DOI] [PubMed] [Google Scholar]
- 45.Albert DM, Scheef EA, Wang S, Mehraein F, Darjatmoko SR, Sorenson CM, et al. Calcitriol is a potent inhibitor of retinal neovascularization. Investigative ophthalmology & visual science. 2007;48(5):2327–34. [DOI] [PubMed] [Google Scholar]
- 46.Sipos PI, Crocker IP, Hubel CA, Baker PN. Endothelial progenitor cells: their potential in the placental vasculature and related complications. Placenta. 2010;31(1):1–10. doi: 10.1016/j.placenta.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 47.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England journal of medicine. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 48.Egorova AD, DeRuiter MC, de Boer HC, van de Pas S, Gittenberger-de Groot AC, van Zonneveld AJ, et al. Endothelial colony-forming cells show a mature transcriptional response to shear stress. In Vitro Cell Dev Biol Anim. 2012;48(1):21–9. doi: 10.1007/s11626-011-9470-z [DOI] [PubMed] [Google Scholar]
- 49.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145(11):4838–45. Epub 2004/07/31. doi: 10.1210/en.2004-0533 [DOI] [PubMed] [Google Scholar]
- 50.Hwang HS, Maeng YS, Park YW, Koos BJ, Kwon YG, Kim YH. Increased senescence and reduced functional ability of fetal endothelial progenitor cells in pregnancies complicated by preeclampsia without intrauterine growth restriction. Am J Obstet Gynecol. 2008;199(3):259 e1–7. Epub 2008/09/06. [DOI] [PubMed] [Google Scholar]
- 51.Levine MJ, Teegarden D. 1alpha,25-dihydroxycholecalciferol increases the expression of vascular endothelial growth factor in C3H10T1/2 mouse embryo fibroblasts. The Journal of nutrition. 2004;134(9):2244–50. [DOI] [PubMed] [Google Scholar]
- 52.Schlaeppi JM, Gutzwiller S, Finkenzeller G, Fournier B. 1,25-Dihydroxyvitamin D3 induces the expression of vascular endothelial growth factor in osteoblastic cells. Endocrine research. 1997;23(3):213–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.