Abstract
Background
Sleep problems are common among university students. Poor sleep is associated with impaired daily functioning, increased risk of psychiatric symptoms, and somatic complaints such as pain. Previous results suggest that poor sleep exacerbates pain, which in turn negatively affects sleep. The purpose of the present study was to determine prevalence rates, comorbidity, and role of depression as a factor of moderating the relationship between sleep and physical complaints in German university students.
Samples and methods
In total, 2443 German university students (65% women) completed a web survey. Self-report measures included the Pittsburg Sleep Quality Index, three modules of the Patient Health Questionnaire, and a questionnaire on the functional somatic syndromes (FSSs).
Results
More than one-third (36.9%) reported poor sleep as assessed by the Pittsburgh Sleep Quality Index. Somatoform syndrome was identified in 23.5%, and the prevalence of any FSS was 12.8%. Self-reported sleep quality, sleep onset latency, sleep disturbances, use of sleep medications, and daytime dysfunctioning were significant predictors of somatoform syndrome, whereas sleep efficiency and sleep duration influenced somatic complaints indirectly. Moderate correlations were found between stress, anxiety, somatoform syndrome, depression, and overall sleep quality. The effect of somatic complaints on sleep quality was associated with the severity of depression. Anxiety shows direct effects on somatization and depression but only indirect associations with sleep quality.
Keywords: sleep quality, pain, depression, anxiety, subjective measures
Introduction
Prevalence of somatic complaints in university students
Physical complaints are highly prevalent in university students.1 Somatoform syndromes with a rate of 9.1% are among the most frequently observed syndromes in a student sample when screening for various psychological symptoms according to Diagnostic and Statistical Manual of Mental Disorders-fourth edition (DSM-IV) in self-report questionnaires.2 In another study, 9.5% of students were found to have at least one functional somatic syndrome (FSS), with a female preponderance.3 The prevalence rates range from 0.1% for globus pharyngis and chronic low back pain (CLBP) up to 1.9% for functional dyspepsia (FD) in the student sample. Students with at least one FSS were more frequently affected by mental disorders, such as somatoform syndrome, major depressive disorder, or anxiety disorder, than non-affected. These findings are particularly relevant, since they indicate that FSS was found in approximately one of ten students.3 Furthermore, somatoform syndrome was associated with functional impairments.2
Prevalence of sleep problems in university students
In addition to somatic complaints, sleep problems impact the physical and mental health of the students and their daytime functioning. Sleep patterns change considerably from secondary school to university because of alterations in external time triggers, such as class schedules and lifestyle preferences.4 Academic demands, part-time jobs, friends, family, relationships, lectures, and free-time activities may contribute to a delay of sleep. These lifestyle habits and especially the postponement of sleep are suspected to induce irregular sleep patterns.5 In the American college students, 27% were at risk for at least one sleep disorder,6 whereas in general population, 20%–30% of young adults reported insomnia symptoms.6–8 Prevalence rates for sleep difficulties in students range from 5% to 73%,5,9–14 showing that students are particularly affected by sleep problems.5,13,14 This broad range of prevalence rates is based on a diversity of criteria. Particularly short sleep duration is a risk factor for poor perceived health and persistent psychological distress.15,16 Difficulties falling asleep and maintaining sleep are common complaints among university students.13,17
The link between sleep and somatic complaints
Asai et al evaluated the association between somatic complaints and sleep. In this study, participants with somatic complaints reported significantly more sleep problems as difficulties falling asleep and perceived poor sleep quality.18 In a longitudinal study, insomnia symptoms were associated with somatic complaints 1 year later.8 In university students, very late bedtime was predictive for somatoform disorder and lower academic motivation when controlling for sleep quality.19 Furthermore, patients with irritable bowel syndrome (IBS) often report significantly increased sleep problems, such as poorer sleep efficiency, daytime dysfunction, and prolonged sleep onset latency than healthy controls.20 Gulewitsch et al found in a German student sample that 16.7% of the 434 students with IBS had trouble falling asleep (n=2399).1 Patients with severe and moderate symptoms of FD had more sleep-related difficulties compared to a healthy control group.21
To better understand the pain–sleep relationship, it is necessary to distinguish between different pain syndromes. Headaches during the night or early in the morning are often related to sleep disturbances.22 Sweileh et al found sleep deprivation and stress to trigger headaches.23 In addition, in a study by Cho et al, poor sleep quality, insomnia, and daytime sleepiness were significantly more frequent in those reporting headaches, compared to headache-free individuals.24
Taking into account results from pain research, Finan et al reviewed 17 longitudinal studies investigating the association between sleep and pain in different samples (eg, adolescents, general population, older adults, and patients with chronic pain).25 Studies assessing the unidirectional effect of sleep on future pain indicate that sleep impairments increase the risk and worsen the long-term prognosis for pain. Pain leads to poor sleep quality with prolonged latency falling asleep. Since the pain perception is more intense at nighttime, the sleep disturbing effect accumulates, which in turn leads to prolonged sleep onset latency.26 Overall, pain leads to a general physiological and psychological activation; therefore, pain can be the cause of disturbed sleep.27,28 This results in the reduction of sleep duration and efficiency and in restless sleep because of frequent changes in sleep stage.23,29 Findings from studies investigating the bidirectional effects of sleep and pain provide evidence that sleep impairments are a stronger predictor of pain than vice versa. Nevertheless, the direction of causality between sleep impairment and pain has not been conclusively answered. The predominant view that sleep and pain are reciprocally related is supported by large, longitudinal cohort studies with subjective assessments of sleep and pain. Finan et al showed that sleep impairment predicted new incidents and exacerbations of pain.25 Equally, Brand et al found a bidirectional relation between sleep and pain among Swiss university students.30 They also stated that other cognitive-emotional processes such as depressive symptoms should be considered while investigating the pain–sleep relationship.
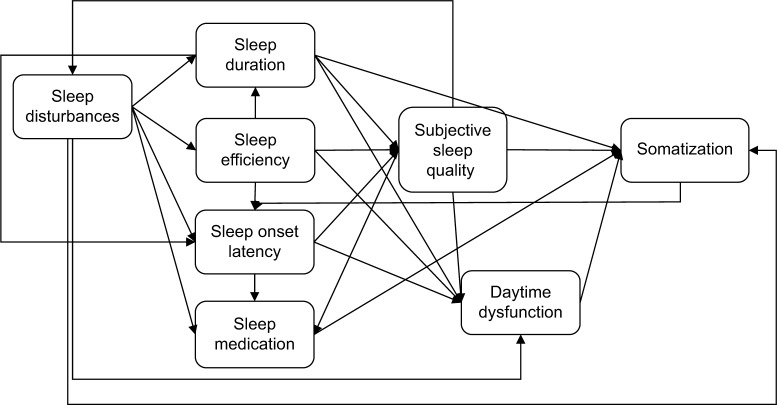
Several studies have shown disconcerting findings with strong associations between non-restorative sleep, somatic pain, depression, and anxiety separately. Sleep quality, depression, and anxiety seem to be strongly associated with one another and so are somatic syndrome, depression, and anxiety.31,32 Based on these previous research results, this study developed a theoretical model including all relevant factors influencing somatic complaints in students (Figure 1).
Figure 1.
Model of sleep and somatization.
Objectives
This study aimed to investigate the relationship between complaints and sleep quality in university students. It was hypothesized that 1) somatic complaints are positively correlated with subjectively poor sleep quality, difficulties falling asleep, and less habitual sleep efficiency. It was also assumed that somatic complaints are negatively associated with total sleep duration and that sleep disturbances and daytime dysfunctioning are predicted by somatic complaints. The model on sleep and somatization as shown in Figure 1 was tested. Furthermore, it was hypothesized that 2) the association between somatic complaints and poor sleep quality are negatively associated with depression. Lastly, it was assumed that 3) sleep quality is predicted by a combination of anxiety, depression, and somatization.
Methods
Measurements
Questionnaire on Functional Somatic Syndromes (Fragebogen zur Erfassung funktioneller somatoformer Syndrome [FFSS])
Somatic syndromes were assessed by using the German questionnaire on Functional Somatic Syndromes (FFSS).33 The first part refers to the symptom-related screening process on the basis of diagnostic criteria of six selected functional clinical pictures: chronic pelvic pain (CPP), tension-type headache (TTH), globus (hystericus), IBS, FD, and CLBP. According to current frequency of occurrence, the symptoms are rated on a 4-point Likert scale from minimum “never” to maximum “almost always”. In a second step, the duration of the symptoms is assessed. FSS was only identified in the absence of a physician diagnosis accounting for the reported symptoms. The results were interpreted using the standard criteria and algorithms of the FFSS. Fischer et al reported good psychometric properties, regarding internal consistency (Cronbach’s a=0.94) and retest reliability (r=0.80–0.94).3 In the present sample, there was high internal consistency (Cronbach’s a=0.91).
Pittsburgh Sleep Quality Index (PSQI)
A validated German version of the PSQI34 (German version35) was used to assess a wide variety of factors related to sleep quality. The PSQI is a 19-item self-rating questionnaire, which differentiates between “good”- and “poor”-quality sleepers. It yields a global score between 0 and 21 by evaluating seven areas retrospectively: 1) subjective sleep quality, 2) sleep onset latency, 3) sleep duration, 4) habitual sleep efficiency, 5) sleep disturbances, 6) use of sleep medications, and 7) daytime dysfunctions. Answers should indicate the most accurate reply for the majority of days and nights over the past 4 weeks. Scoring is based on a Likert scale from minimum score 0 (better) to maximum score 3 (worse). The resulting global sleep quality scores are continuous with high scores (>5) indicating poor sleep quality. The Cronbach’s a in this study equals 0.76. This is in line with numerous studies using the PSQI, demonstrating its high validity and high reliability (Cronbach’s a from 0.70 to 0.78).34,35
Patient Health Questionnaire – German Version (PHQ-D)
The PHQ-D36 (German version37) was used to assess the severity of somatization (PHQ-15), depressive symptoms (PHQ-9), generalized anxiety disorder (GAD-7), panic (15-item panic module), alcohol abuse and stress (10-item stress module). All the modules refer to the occurrence of symptoms over the past 2–4 weeks and are rated on a 4-point Likert scale. Mean scores are calculated for all subscales, and cutoffs for mild, moderate, and severe symptomatology are reported for PHQ-15, PHQ-9, GAD-7, and the stress module. In the present study, the internal consistency is satisfying for all modules (a=0.67–0.89); for alcohol syndrome, the internal consistency is a=0.43, whereas previous studies showed Cronbach’s a from 0.78 to 0.89.36,37
Sample
University students were invited through email circulators to fill out the online survey. Prior to the survey, all students were informed about the purpose and nature of the study. The students had a chance to enter into a raffle at the end of the survey to win one of five rewards (50€ Amazon gift vouchers) as an incentive for participation. By clicking the confirmation button, every student agreed to participate voluntarily. Furthermore, prior to the beginning, they had been informed about the duration (~20 min) and that they can cancel the survey without disadvantages at any time according to the Declaration of Helsinki. The study was approved by Bielefeld University ethics committee. All data were collected anonymously.
Participants
Out of 2676 participants, 2443 students (91.29%) completed the study and were included in the analyses. Among these students, 1587 (65.0%) were female. Their age ranged from 17 to 68 years (mean [M] =24 years, standard deviation [SD] =3.8). Of the total sample, 111 (1.2%) were aged >30 years. The duration of studies was counted in semesters (2 semesters =1 year). The duration of the studies ranged from 1 to 29 semesters (M =5.8 semesters, SD =4.06).
In total, 2443 students were enrolled in Christian-Albrechts-University Kiel and six students from other universities filled out the questionnaires. Table 1 provides an overview of faculties of the entire university and the study sample.
Table 1.
Faculties of the CAU in percentage of the entire university and in the present sample
| Faculty | Percentage of the entire university | Total number (percentage) in the sample | |
|---|---|---|---|
| 1 | Faculty of Theology | 1.08 | 13 (0.5) |
| 2 | Faculty of Law | 8.01 | 134 (5.5) |
| 3 | Faculty of Mathematics and Natural Sciences | 20.73 | 471 (19.3) |
| 4 | Faculty of Agricultural and Nutritional Sciences | 8.63 | 251 (10.3) |
| 5 | Faculty of Medicine | 8.89 | 162 (6.6) |
| 6 | Faculty of Business, Economics and Social Sciences | 8.69 | 237 (9.7) |
| 7 | Faculty of Arts and Humanities | 34.96 | 812 (33.2) |
| 8 | Faculty of Engineering | 8.98 | 136 (5.6) |
| No answer | 227 (9.3) | ||
Abbreviation: CAU, Christian-Albrechts-University Kiel.
Data analysis
As part of the classification and diagnostics of clinical pictures by the FFSS, 36 participants with relevant medical differential diagnosis explaining the established symptomatology were not further rated as functional clinical picture.33 A total of 18 students affected with CLBP, nine with FD, eight students with IBS, and 1 student with globus indicated a relevant medical diagnosis.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) Statistics version 21.0 (IBM, Chicago, IL, USA) and IBM SPSS Amos version 22.0. Relationships between variables were assessed by Pearson product moment correlation coefficients, linear regressions, and path models computed using Amos. Independent sample t-tests were conducted for comparisons between men and women, as well as between cases with any FSS and non-FSS cases. In view of the large sample size, the significance level was set at a=0.01 one-tailed for analysis.38 For fitting the path models, recommendations for a good fit are reported and followed (Table 5).
Table 5.
Model fit of saturated and revised model of sleep and somatization
| Fit indices | Saturated model | Revised model | Recommendations for a good fit |
|---|---|---|---|
| χ2/df | 17.900 | 2.489 | <5 |
| TLI | 0.833 | 0.986 | >0.95 |
| CFI | 0.951 | 0.995 | >0.95 |
| RMSEA | 0.084 | 0.025 | <0.05 |
Note: Sex and age were included as control variables influencing somatization, sleep efficiency, sleep disturbances, and sleep duration. Bold font indicates a good fit.
Abbreviation: df, degrees of freedom; TLI, Tucker-Lewis-Index; CFI, comparative fit index; RMSEA, root mean square error of approximation.
Results
Prevalence rates of FSSs
A total of 369 (15.1%) students reported the occurrence of any FSS. At least one FSS was reported by 312 (12.8%) students, two FSS were found in 53 students (2.2%), and three FSS in four students (0.3%). Independent sample t-test revealed that within this group, women (M =0.18, SD =0.39) were significantly (p<0.01) more likely to report any FSS than men (M =0.09, SD =0.29) (t (2441) =6.239).
Because of the report about relevant medical differential diagnosis explaining the established symptomatology, 36 participants were not further rated as showing a FSS (Table 2, based on the classification and diagnostics of FSS).
Table 2.
Prevalence rates of FSS measured by the FFSS
| FSS | Total (n=2443) | Men (n=856) | Women (n=1587) | p-Value |
|---|---|---|---|---|
| Tension-type headache | 105 (4.3%) | 18 (2.1%) | 87 (5.5%) | 0.000 |
| Globus | 27 (1.1%) | 7 (0.8%) | 20 (1.3%) | 0.318 |
| Irritable bowel syndrome | 160 (6.5%) | 33 (3.9%) | 127 (8.0%) | 0.000 |
| Functional dyspepsia | 114 (4.7%) | 20 (2.3%) | 94 (5.9%) | 0.000 |
| Chronic pelvic pain | 2 (0.1%) | 0 | 2 (0.1%) | – |
| Chronic low back pain | 24 (1.0%) | 9 (1.1%) | 15 (0.9%) | 0.803 |
Note: Bold font indicates p<0.01.
Abbreviations: FSS, functional somatic syndrome; FFSS, Fragebogen zur Erfassung funktioneller somatoformer Syndrome.
Prevalence of sleep disturbances as measured by the PSQI
Over one-third (36.9%, n=889) of the university students had PSQI scores >5, indicating poor sleep quality. The average bedtime was 12.20 a.m. (SD =105 min) with a sleep onset latency of 34.37 min (SD =34.49 min). On average, participants reported to wake up at 8:00 a.m. (SD =85 min). The mean total sleep time was 7.26 h (SD =1.26 h) per night. Prolonged sleep onset latency (>30 min according to DSM-fifth edition (DSM-5) criteria) for three or more times a week was reported by 21.0% of the students (n=513). Frequent nocturnal awakenings and early awakenings three or more times a week were reported by 14.2% (n=347) of the students. The vast majority of participants (83.6%, n=2043) declared that they did not have any trouble sleeping because of pain over the last month.
Prevalence rates of mental disorders measured by the PHQ-D
Students reported a somatoform syndrome with a prevalence of 23.5% (n=574) and alcohol syndrome with a prevalence rate 21.4% (n=523) as the most common syndromes according to the PHQ-D, followed by the major depressive syndrome with 10.4% (n=255) of the 2443 students as indicated in Table 3. Approximately 9.4% (n=232) were above cutoff for somato-form syndrome and reported major depression at the same time. Women suffered significantly more often from most of these syndromes (as seen in Table 3). Equally, with regard to stress, female students scored higher than men, whereas male students scored significantly higher in alcohol syndrome than female students. No sex differences were found for other symptoms.
Table 3.
Prevalence rates of mental disorders measured by the PHQ
| Mental disorders | Total (n=2443) | Men (n=856) | Women (n=1587) | p-Value |
|---|---|---|---|---|
| Somatoform syndrome | 574 (23.5%) | 101 (11.8%) | 473 (29.8%) | 0.000 |
| Major depressive syndrome | 255 (10.4%) | 74 (8.6%) | 181 (11.4%) | 0.033 |
| Other depressive syndromes | 103 (4.2%) | 35 (4.1%) | 68 (4.3%) | 0.818 |
| Generalized anxiety disorder | 113 (4.6%) | 27 (3.2%) | 86 (5.4%) | 0.011 |
| Panic syndrome | 87 (3.6%) | 24 (2.8%) | 63 (4.0%) | 0.138 |
| Stress | 116 (4.7%) | 23 (2.7%) | 93 (5.9%) | 0.000 |
| Alcohol syndrome | 523 (21.4%) | 271 (31.7%) | 252 (15.9%) | 0.000 |
Note: Bold font indicates p<0.01.
Abbreviation: PHQ, Patient Health Questionnaire – German Version.
Correlations between somatic complaints according to PHQ-D and self-reported sleep quality
Pearson product moment correlation coefficients were computed to assess the relationships between the existence of any FSS, the total PSQI score, and the PHQ-D subscales. Presence of FSS correlated significantly with somatoform syndrome (r=0.208), stress (r=0.142), anxiety (r=0.127), and depression (r=0.150) (p<0.01). Somatoform syndrome according to PHQ-D and FSS correlated weakly but significantly. No significant correlation between the occurrence of any FSS and panic syndrome or alcohol abuse has been found. Every diagnostic module of the PHQ-D correlated significantly (p<0.01) with the total PSQI score. Moderate correlations were found for stress (r=0.454), anxiety (r=0.496), somatoform syndrome (r=0.432), depression (r=0.588), and total PSQI score. Also Pearson product moment correlation coefficients were computed to assess the relationship between the total PSQI score, the PHQ-15 score, and the seven FSSs as measured by the FSS (Table 4).
Table 4.
Pearson product moment coefficients
| FSS | Pearson product moment correlation (r)
|
||
|---|---|---|---|
| PSQI total score | FSS existing | PHQ-15 | |
| Chronic pelvic pain | 0.036 | −0.012 | 0.022 |
| Tension-type headache | 0.014 | 0.502** | 0.076** |
| Globus | 0.043 | 0.251** | 0.074** |
| Irritable bowel syndrome | 0.099** | 0.623** | 0.222** |
| Functional dyspepsia | 0.036 | 0.525** | 0.118** |
| Chronic low back pain | 0.045 | 0.236** | 0.114** |
| FSS existing | 0.097** | 1 | 0.242** |
Note:
p<0.001, r>0.10 = weak, r>0.30 = moderate, r>0.50 = strong. Bold font indicates p<0.01.
Abbreviations: FSS, functional somatic syndrome; PSQI, Pittsburgh Sleep Quality Index; PHQ, Patient Health Questionnaire – German Version.
Path models
After testing the assumptions for multiple regression models, linear regression models were conducted to predict somatic complaints, since no assumption was violated. No outliers or influential cases were found. As expected, in a multiple regression of FSS and sleep quality, the R2 was very weak (R2=0.01); hence, no variance in the outcome was accounted for by the FSS.
Table 5 shows the model fit of sleep and somatization model (variables assessed with PSQI and PHQ-15). As the model fit was not satisfying, revisions were made and the revised path model fitted again revealed a good fit (Table 5).
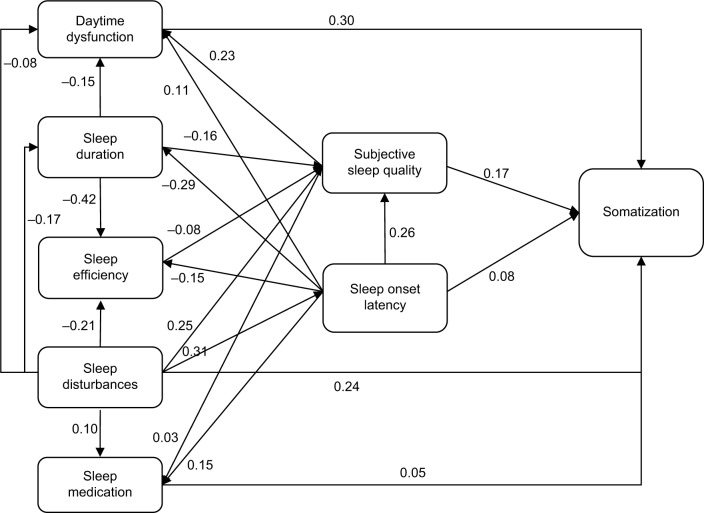
Figure 2 shows the path model. Somatization is influenced by subjective sleep quality, daytime dysfunction, sleep onset latency, and number of sleep disturbances. There is an association of sleep medication and somatization, whereas total sleep duration and sleep efficiency indirectly influence somatization through subjective sleep quality. PSQI-sub-scales interact as shown in Figure 2.
Figure 2.
Model of subjective sleep and somatization.
Note: Sex and age were included as control variables influencing somatization, sleep efficiency, sleep disturbances, and sleep duration. Path coefficients display standardized regression weights.
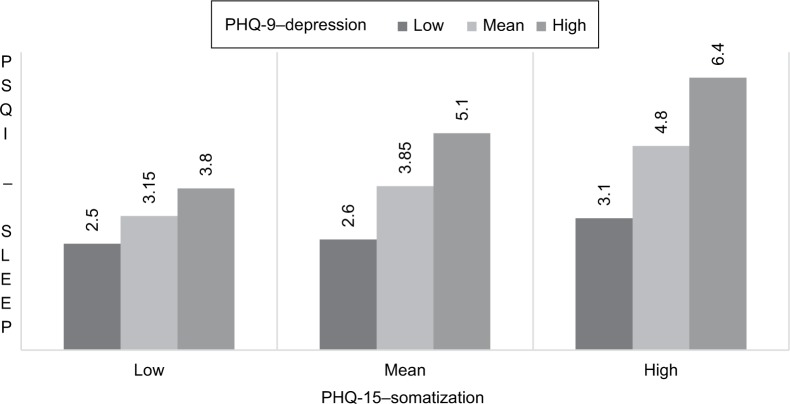
A multiple regression model was implemented to test whether the association between somatic complaints (PHQ-15 – somatoform syndrome) and poor sleep quality depends on depression (PHQ-9 – depression) (hypothesis 2). Results indicated that both higher scores in depression and higher scores in somatization were associated with poor sleep quality. Somatic complaints were more strongly related to poor sleep quality for high levels of depression (b =2.18, standard error [SE] B =0.167, t=13.16, p<0.01) than for mean level (b =1.50, SE B =0.125, t=11.98, p<0.01) or lower levels (b=0.825, SE B =0.125, t=6.61, p<0.01) of depression. The effect of somatization on sleep quality depended on the severity of depression.
Sleep quality scores (PSQI – sleep quality) are shown for low, mean, and high levels of somatization (PHQ-15 – somatization) at the low, mean, and high levels of depression (PHQ-9 – depression) in Figure 3.
Figure 3.
Sleep problems (PSQI), somatization (PHQ-15) in low, mean, and high depression (PHQ-9).
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; PHQ, Patient Health Questionnaire – German Version.
Hypothesis 3 tests whether anxiety (measured by GAD-7 – anxiety) and somatoform syndrome (measured by PHQ-15 – somatization) were predictors of poor sleep quality (measured by PSQI – sleep quality) (Figure 4). A path model including depression was tested, showing a sufficient fit (Table 6).
Figure 4.
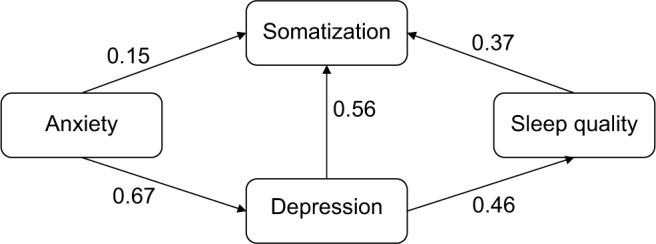
Model of somatization, sleep quality, depression, and anxiety.
Note: Path coefficients display standardized regression weights.
Table 6.
Fit indices of the model on sleep quality, somatization, depression, and anxiety
| Saturated model | Recommendations for a good fit | |
|---|---|---|
| χ2/df | 5.988 | <5 |
| TLI | 0.991 | >0.95 |
| CFI | 0.998 | >0.95 |
| RMSEA | 0.046 | <0.05 |
Note: Good fit according to recommendations is indicated in bold letters.
Abbreviation: df, degrees of freedom; TLI, Tucker-Lewis-Index; CFI, comparative fit index; RMSEA, root mean square error of approximation.
The results suggest a genuine positive relationship between depression and good/poor sleep quality and somatoform syndrome. Anxiety directly influences somatization and depression, whereas sleep quality is indirectly affected through depression severity. It can be concluded that the occurrence of anxiety, depression, and poor sleep quality is a risk for somatoform syndrome.
Discussion
Prevalence of somatic complaints in university students
The prevalence rate of one FSS was found to be nearly one in ten students (12.8%), and the most frequently reported FSSs were IBS, FD, and TTH with prevalence rates of 6.5%, 4.7%, and 4.3%, respectively. These prevalence rates are higher than the prevalence rates observed by Fischer et al in a Swiss student sample (n=3054), with prevalence rates of 0.9% for TTH, 1.3% for IBS, and 1.9% for FD.3 For IBS, in German student sample, Gulewitsch et al found a relatively high prevalence rate of 18.1%.1 A possible explanation for these inconsistent findings is different criteria for diagnoses. Fischer et al assessed very strict “red flags” for functional somatic symptoms, which might lead to an underestimation of prevalence rates.1,3 More information and objective data are required because of the remarkable high prevalence rate for IBS in different populations as mentioned earlier.
The somatoform syndrome was found in 574 students, that is, 23.5% of the total student sample. In comparison with other German student samples, this prevalence rate is fairly high, since Seliger and Brähler39 and Bailer et al2 ascertained (equally assessed by the PHQ-15) only a prevalence rate of 13.4% and 9.1%, respectively. Somatoform syndrome is often seen as an equivalent to the diagnosis of FSS.3 However, in this study, somatoform syndrome and FSS correlated weakly (r=0.21, p<0.01), and equally only 44.4% of the students having any FSS fulfilled the criteria for somatoform syndrome. These findings underline the need of differentiation between somatoform syndrome and FSS. Somatoform syndrome is a very unspecific diagnosis. FSS as a more descriptive diagnosis can help in the processes of diagnostics and therapy.40,41
In the present study, in comparison with students without any FSS, students with FSS showed a significantly higher proportion of somatoform syndrome, generalized anxiety disorders, major depressive syndrome, stress, and poor sleep quality. These wide range of other impairments were also found by Fischer et al.3
With regard to sex, differences between the prevalence rates of somatoform syndrome, stress, and alcohol abuse as measured by the PHQ-D were found. This is consistent with the findings from Bailer et al, indicating equally a female preponderance in somatoform syndrome (females: 14.2%, males: 2.8%), whereas reporting a significantly higher alcohol consumption for males (males: 44.0%, females: 19.0%).2 However, no significant difference in the frequency of depressive symptoms among female and male students was found. Likewise, six other studies could not find any statistically significant sex differences.42–47 For stress, Faber et al also found a higher impairment in women.48
Prevalence of sleep problems in university students
Overall, the results show that poor sleep quality and somatoform syndrome are highly prevalent in university students. In this study, nearly one-third (36.9%) of the students reported poor sleep quality in line with most studies of university students worldwide, reporting prevalence rates of 25%–27%.6,49 Solely, 13.5% students endorsed their overall sleep quality as very good, whereas 27.1% indicated their sleep quality as fairly bad and 4.6% as very bad. The average sleep time of 7.26 h is shorter compared to another German student sample, as Schlarb et al found an average total sleep time of 7.6 h, but is in accordance with Ahrberg et al13,50 observing total sleep time in their German student sample in the “pre-exam” time. In average, the whole sample had a sleep onset latency of 34.37 min. Based on DSM-5 criteria, >30 min sleep latency may be clinical cutoff for insomnia. The lack of sleep due to insomnia symptoms was profound as students reported much daytime sleepiness, the use of hypnotic drugs and a connection between sleep deprivation and sleeping to learning and working problems.9,51,52,53 In addition, poor nighttime sleep is associated with daytime sleepiness.17 Also, global sleep quality was found to be a moderator of alcohol consumption in students.54 Similarly, Nyer et al found in a college student sample that sleep disturbances were associated with depression and anxiety.14 A complex pathway is reported regarding a bidirectional relationship of depression, anxiety, and sleep problems.31 Furthermore, several studies have shown strong associations between non-restorative sleep, somatic pain, depression, and anxiety.2,9,14,53
The link between sleep, pain, depression, and anxiety
The main purpose of this study was to assess the relationship between somatic complaints and self-reported sleep quality. The path model on subjective sleep and somatization revealed that somatic complaints are positively associated with subjectively poor sleep quality, difficulties falling asleep, daytime impairments, sleep disturbances, and sleep medication, whereas less habitual sleep efficiency and lower sleep duration only indirectly influence somatization (Figure 2). This shows the importance of subjective parameters of sleep rather than more objective ones for somatization. Surprisingly, the existence of any FSS correlated with the total PSQI score only very weakly. It is important to note that path models are able to reveal associations but not causality. However, students having any FSS were significantly more likely to report poor sleep quality than students without any FSS.
Compared to the present study, Elsenbruch et al showed that PSQI subcomponents habitual sleep efficiency, daytime dysfunction, and sleep onset latency were significantly higher in individuals with IBS than in healthy controls.20 Nevertheless, subjective sleep quality was not significantly associated with IBS.20 In an experimental design, Affleck et al studied 50 patients with fibromyalgia and revealed that pain intensity of the previous day influences quality of sleep during the following night, which in turn determines the intensity of pain the next day.55 Interestingly, in the present study, only IBS and reporting the existence of any FSS correlated significantly with poor sleep quality, whereas reporting a single FSS did not. Longer sleep onset latency was found to be a predictor for IBS in a sample of university students, which is also shown by the path model on somatization.1 Since this study investigated only whether the presence of any FSS is associated with the total PSQI score, future research is required with more in-depth analysis investigating the association between FSS and the components of the PSQI more thoroughly. Somatic complaints were not negatively associated with the total sleep duration, similarly previous research indicated that quality and non-quantity of sleep are related to overall well-being.5,17
The association between somatic complaints and sleep quality was significantly stronger for students with high levels of depression, indicating this group of participants being more vulnerable. Beta weights indicate a higher decrease (×2.18) of sleep quality along with more somatic complaints in higher depressed participants.
The path model on sleep quality, somatization, depression, and anxiety shows associations between all variables. Sleep quality, somatization, and depression are directly connected. However, anxiety impacts sleep quality only indirectly via influencing somatization and depression. The model indicated that overall poor subjective sleep quality is a predictor of somatoform syndrome. Nevertheless, it seems that several variables interact. Findings from Brand et al are congruent with this assumption. They ascertained a bidirectional relationship between sleep and pain in a student sample.30 Poor sleep may influence the elaboration of pain only indirectly through depressive symptoms, low quality of life, and other factors. Somatic complaints and depressive symptoms on the other hand are often correlated strongly. In longitudinal studies, the association remained bidirectional, likewise in the current sample, comorbidity occurs often and 9.4% were above cuttoff for depression and somatoform disorder.56 Studies showed that comorbidities are risk factors, in particular somatoform syndrome, depression, and anxiety independently predicted dysfunctional illness behavior (eg, reconfirmation of diagnosis).57 This study shows that self-reported depression, somatization, and poor sleep quality are positively related. Thereof, negative emotion occurs to play a decisive part. Consistently, in this study, the regression model of depression had a sufficient fit in the relationship between poor sleep quality and somatic complaints. The model of anxiety on the association between sleep quality, somatic complaints, and depression suggests a genuine positive relationship between anxiety and good/poor sleep quality moderated by depression. These findings suggest a significant role of somatic complaints in student’s mental health and several other factors as depression, anxiety, and sleep need to be taken into account.
Limitations
Several limitations need to be considered while interpreting the results. First, as mentioned earlier, since most findings are correlational, causal conclusions cannot be made. In order to evaluate the causal relationships, longitudinal data should be assessed. Second, data confirmation of the diagnosis through a laboratory assessment or physical examination would be helpful to ensure diagnosis. Future research could use more detailed subjective instruments like sleep logs and objective data (eg, actigraphy). In addition, analyses more thoroughly across different faculties and degrees might be interesting to see whether distinct schedules and demands influence the association between sleep, somatic complaints, depression, and anxiety. However, others found no significant differences between faculties.2,58
Conclusion
As this study illustrated, poor sleep quality and somatic complaints are linked. This can become a vicious circle, which students are unaware of and unable to alter. In addition, the association shows a high comorbidity with stress and anxiety and is moreover moderated by depression. Therefore, university students need to know how to avoid sleep problems and somatic complaints that can easily occur as a result of their lifestyle. Universities should provide prevention and early intervention programs especially addressing students field of learning and living (shared flats, exam schedule, etc). It might be helpful if university information centers would encourage students to follow, for example, sleep hygiene practices and structured daily routines. In addition, a specialized prevention and intervention program for students should be developed as this group has special learning and living conditions.
Acknowledgments
The authors would like to thank Barbara Herbert for her help in the study. They further acknowledge the support for the article processing charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gulewitsch MD, Enck P, Hautzinger M, Schlarb AA. Irritable bowel syndrome symptoms among German students: prevalence, characteristics, and associations to somatic complaints, sleep, quality of life, and childhood abdominal pain. Eur J Gastroenterol Hepatol. 2011;23(4):311–316. doi: 10.1097/MEG.0b013e3283457b1e. [DOI] [PubMed] [Google Scholar]
- 2.Bailer J, Schwarz D, Witthoft M, Stubinger C, Rist F. Prevalence of mental disorders among college students at a German university. Psychother Psychosom Med Psychol. 2008;58(11):423–429. doi: 10.1055/s-2007-986293. [DOI] [PubMed] [Google Scholar]
- 3.Fischer S, Gaab J, Ehlert U, Nater UM. Prevalence, overlap, and predictors of functional somatic syndromes in a student sample. Int J Behav Med. 2013;20(2):184–193. doi: 10.1007/s12529-012-9266-x. [DOI] [PubMed] [Google Scholar]
- 4.Urner M, Tornic J, Bloch KE. Sleep patterns in high school and university students: a longitudinal study. Chronobiol Int. 2009;26(6):1222–1234. doi: 10.3109/07420520903244600. [DOI] [PubMed] [Google Scholar]
- 5.Buboltz WC, Brown F, Soper B. Sleep habits and patterns of college students: a preliminary study. J Am Coll Health. 2001;50(3):131–135. doi: 10.1080/07448480109596017. [DOI] [PubMed] [Google Scholar]
- 6.Gaultney JF. The prevalence of sleep disorders in college students: impact on academic performance. J Am Coll Health. 2010;59(2):91–97. doi: 10.1080/07448481.2010.483708. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alapin I, Fichten CS, Libman E, Creti L, Bailes S, Wright J. How is good and poor sleep in older adults and college students related to daytime sleepiness, fatigue, and ability to concentrate? J Psychosom Res. 2000;49(5):381–390. doi: 10.1016/s0022-3999(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 10.Moo-Estrella J, Pérez-Benítez H, Solís-Rodríguez F, Arankowsky-Sandoval G. Evaluation of depressive symptoms and sleep alterations in college students. Arch Med Res. 2005;36(4):393–398. doi: 10.1016/j.arcmed.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Nadorff MR, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicidal ideation in a college student sample. Sleep. 2011;34(1):93–98. doi: 10.1093/sleep/34.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweileh WM, Ali IA, Sawalha AF, Abu-Taha AS, Zyoud SH, Al-Jabi SW. Sleep habits and sleep problems among Palestinian students. Child Adolesc Psychiatry Ment Health. 2011;5(1):25. doi: 10.1186/1753-2000-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlarb AA, Kulessa D, Gulewitsch MD. Sleep characteristics, sleep problems, and associations of self-efficacy among German university students. Nat Sci Sleep. 2012;4:1–7. doi: 10.2147/NSS.S27971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyer M, Farabaugh A, Fehling K, et al. Relationship between sleep disturbance and depression, anxiety, and functioning in college students. Depress Anxiety. 2013;30(9):873–880. doi: 10.1002/da.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaranayake CB, Arroll B, Fernando AT3. Sleep disorders, depression, anxiety and satisfaction with life among young adults: a survey of university students in Auckland, New Zealand. N Z Med J. 2014;127(1399):13–22. [PubMed] [Google Scholar]
- 16.Glozier N, Martiniuk A, Patton G, et al. Short sleep duration in prevalent and persistent psychological distress in young adults: the DRIVE study. Sleep. 2010;33(9):1139–1145. doi: 10.1093/sleep/33.9.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res. 1997;42(6):583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 18.Asai T, Kameita Y, Uchiyama M, et al. Epidemiological study of the relationship between sleep disturbances and somatic and psychological complaints among the Japanese general population. Sleep Biol Rhythms. 2006;4(1):55–62. [Google Scholar]
- 19.Nagane M, Suge R, Watanabe S-I. Time or retiring and sleep quality may be predictors of academic performance and psychosomatic disorder in university students. Biol Rhythm Res. 2015;47(2):329–337. [Google Scholar]
- 20.Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. Am J Gastroenterol. 1999;94(9):2447–2452. doi: 10.1111/j.1572-0241.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- 21.Lacy BE, Everhart K, Crowell MD. Functional dyspepsia is associated with sleep disorders. Clin Gastroenterol Hepatol. 2011;9(5):410–414. doi: 10.1016/j.cgh.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Parrino L, Zucconi M, Terzano MG. Sleep and Pain. Seattle: International Association for the Study of Pain Press; 2007. Sleep Fragmentation and Arousal in the Pain Patient; pp. 213–234. [Google Scholar]
- 23.Sweileh WM, Sawalha AF, Zyoud SH, Al-Jabi SW, Shamseh FFB, Khalaf HS. Epidemiological, clinical and pharmacological aspects of headache in a university undergraduate population in Palestine. Cephalalgia. 2010;30(4):439–446. doi: 10.1111/j.1468-2982.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 24.Cho S, Yun C, Park S, Chu M. Relationships between sleep and headache in non-clinical population. Sleep Med. 2013;14:e98. [Google Scholar]
- 25.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zulley J. Schlaf und Schmerz [Sleep and Pain] Schlafmedizin. 2013;2:5–7. German. [Google Scholar]
- 27.Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91(1):36–41. doi: 10.1016/j.biopsycho.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25(1):106–116. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 29.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23(1):1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 30.Brand S, Gerber M, Pühse U, Holsboer-Trachsler E. The relation between sleep and pain among a non-clinical sample of young adults. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):543–551. doi: 10.1007/s00406-010-0113-2. [DOI] [PubMed] [Google Scholar]
- 31.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression. Psychosom Med. 2003;65(4):528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 33.Nater UM, Fischer S, Latanzio S, Ruoss D, Gaab J. FFSS – Fragebogen zur Erfassung funktioneller somatischer Syndrome. Verhaltenstherapie. 2011;21(4):263–265. [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 37.Löwe B, Spitzer RL, Zipfel S, Herzog W. Gesundheitsfragebogen für Patienten (PHQ D). Komplettversion und Kurzform. Testmappe mit Manual, Fragebögen, Schablonen. Karlsruhe: Pfizer; 2002. [Google Scholar]
- 38.Bortz J, Schuster C. Statistik für Human- und Sozialwissenschaftler [Statistics for human and social science] Berlin, Heidelberg: Springer Berlin Heidelberg; 2010. German. [Google Scholar]
- 39.Seliger K, Brähler E. Psychische Gesundheit von Studierenden der Medizin [Mental health in medical students] Psychotherapeut. 2007;52(4):280–286. German. [Google Scholar]
- 40.Kapfhammer HP. Somatoforme Störungen [Somatoforme syndromes] Der Nervenarzt. 2001;72(7):487–500. doi: 10.1007/s001150170072. [DOI] [PubMed] [Google Scholar]
- 41.Häuser W, Lempa M. Funktionelle somatische Schmerzsyndrome [Functional somatic pain syndromes] [Accessed February 16, 2017];Schmerz. 2004 18(2):97. doi: 10.1007/s00482-003-0281-3. Available from: http://link.springer.com/content/pdf/10.1007%2Fs00482-003-0281-3.pdf. [DOI] [PubMed] [Google Scholar]
- 42.Arslan G, Ayranci U, Unsal A, Arslantas D. Prevalence of depression, its correlates among students, and its effect on health-related quality of life in a Turkish university. Upsala J Med Sci. 2009;114(3):170–177. doi: 10.1080/03009730903174339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg D, Gollust SE, Golberstein E, Hefner JL. Prevalence and correlates of depression, anxiety, and suicidality among university students. Am J Orthopsychiatry. 2007;77(4):534–542. doi: 10.1037/0002-9432.77.4.534. [DOI] [PubMed] [Google Scholar]
- 44.El-Gendawy S, Hadhood M, Shams R, Khair A. Epidemiological aspects of depression among Assiut University students. Assiut Med J. 2005;2:81–89. [Google Scholar]
- 45.Kaya M, Genc M, Kaya B, Pehlivan E. Prevalence of depressive symptoms, ways of coping, and related factors among medical school and health services higher education students. Turk Psikiyatri Dergisi. 2007;18(2):137–146. [PubMed] [Google Scholar]
- 46.Tjia J, Givens JL, Shea JA. Factors associated with undertreatment of medical student depression. J Am Coll Health. 2005;53(5):219–224. doi: 10.3200/JACH.53.5.219-224. [DOI] [PubMed] [Google Scholar]
- 47.Zong J-G, Cao X-Y, Cao Y, et al. Coping flexibility in college students with depressive symptoms. Health Qual Life Outcomes. 2010;8:66. doi: 10.1186/1477-7525-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faber J, Schlarb A. The relation of sleep, distress, and coping strategies - what male and female students can learn from each other? Health. 2016;8(13):1356–1367. [Google Scholar]
- 49.Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1549–1556. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Ahrberg K, Dresler M, Niedermaier S, Steiger A, Genzel L. The interaction between sleep quality and academic performance. J Psychiatr Res. 2012;46(12):1618–1622. doi: 10.1016/j.jpsychires.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Schleider K, Güntert M. Merkmale und Bedingungen studienbezogener Lern-und Arbeitsstörungen–eine Bestandsaufnahme [Characteristics and conditions of learning and working circumstances while studying] Beiträge zur Hochschulforschung. 2009;31:8–27. German. [Google Scholar]
- 52.Clegg-Kraynok MM, McBean AL, Montgomery-Downs HE. Sleep quality and characteristics of college students who use prescription psychostimulants nonmedically. Sleep Med. 2011;12(6):598–602. doi: 10.1016/j.sleep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 53.American College Health Association-National College Health Assessment Spring 2008 Reference Group Data Report (abridged): the American College Health Association. J Am Coll Health. 2009;57(5):477–488. doi: 10.3200/JACH.57.5.477-488. [DOI] [PubMed] [Google Scholar]
- 54.Kenney SR, LaBrie JW, Hummer JF, Pham AT. Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addict Behav. 2012;37(4):507–512. doi: 10.1016/j.addbeh.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68(2–3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 56.Lieb R, Zimmermann P, Friis RH, Höfler M, Tholen S, Wittchen H-U. The natural course of DSM-IV somatoform disorders and syndromes among adolescents and young adults: a prospective-longitudinal community study. Eur Psychiatry. 2002;17(6):321–331. doi: 10.1016/s0924-9338(02)00686-7. [DOI] [PubMed] [Google Scholar]
- 57.Rief W, Martin A, Klaiberg A, Brahler E. Specific effects of depression, panic, and somatic symptoms on illness behavior. Psychosom Med. 2005;67(4):596–601. doi: 10.1097/01.psy.0000171158.59706.e7. [DOI] [PubMed] [Google Scholar]
- 58.Friedrich A, Claßen M, Schlarb AA. Sag mir, was Du studierst, und ich sag Dir, wie Du schläfst [Tell me what you study and I’ll tell you how you sleep] Somnologie. 2016;20(4):281–287. German. [Google Scholar]