Significance
Infection with Leishmania (Viannia) parasites can have different manifestations, ranging from localized cutaneous to disseminated and mucocutaneous leishmaniasis, that are prone to relapse after the healing. We previously described the association of the endosymbiont Leishmania RNA virus 1 (LRV1) with increased disease severity. Here, we showed that coinfection with the lymphocytic choriomeningitis virus (LCMV) or Toscana virus exacerbated the outcome of Leishmania guyanensis-induced murine leishmaniasis, favoring parasite persistence and dissemination resulting in metastasis. Both endogenous and exogenous coinfections were dependent upon type I interferon responses. Strikingly, LCMV coinfection after the healing of leishmaniasis induced disease reactivation, overriding the protective adaptive immune response. Thus, viral infections may be a significant risk factor contributing to the pathological spectrum of human leishmaniasis.
Keywords: Leishmania RNA virus 1, Totiviridae, arboviruses, trypanosomatid protozoan parasite, Leishmania subgenus Viannia
Abstract
The presence of the endogenous Leishmania RNA virus 1 (LRV1) replicating stably within some parasite species has been associated with the development of more severe forms of leishmaniasis and relapses after drug treatment in humans. Here, we show that the disease-exacerbatory role of LRV1 relies on type I IFN (type I IFNs) production by macrophages and signaling in vivo. Moreover, infecting mice with the LRV1-cured Leishmania guyanensis (LgyLRV1−) strain of parasites followed by type I IFN treatment increased lesion size and parasite burden, quantitatively reproducing the LRV1-bearing (LgyLRV1+) infection phenotype. This finding suggested the possibility that exogenous viral infections could likewise increase pathogenicity, which was tested by coinfecting mice with L. guyanensis and lymphocytic choriomeningitis virus (LCMV), or the sand fly-transmitted arbovirus Toscana virus (TOSV). The type I IFN antiviral response increased the pathology of L. guyanensis infection, accompanied by down-regulation of the IFN-γ receptor normally required for antileishmanial control. Further, LCMV coinfection of IFN-γ–deficient mice promoted parasite dissemination to secondary sites, reproducing the LgyLRV1+ metastatic phenotype. Remarkably, LCMV coinfection of mice that had healed from L. guyanensis infection induced reactivation of disease pathology, overriding the protective adaptive immune response. Our findings establish that type I IFN-dependent responses, arising from endogenous viral elements (dsRNA/LRV1), or exogenous coinfection with IFN-inducing viruses, are able to synergize with New World Leishmania parasites in both primary and relapse infections. Thus, viral infections likely represent a significant risk factor along with parasite and host factors, thereby contributing to the pathological spectrum of human leishmaniasis.
Protozoan parasites of the genus Leishmania are transmitted as unicellular promastigote forms by sand flies to their mammalian host (humans and dogs). In the skin, Leishmania parasites are phagocytized by tissue resident macrophages, where they survive intracellularly and proliferate as amastigotes. Infection with Leishmania parasites may lead to the development of leishmaniasis, affecting over 12 million people worldwide (1, 2). Leishmaniasis may have different outcomes, ranging from localized cutaneous leishmaniasis to visceral leishmaniasis (1, 2). Infection with Leishmania guyanensis (Lgy) or Leishmania braziliensis (Lbr) principally leads to simple cutaneous lesions; however, up to 10% of patients develop disseminated or mucocutaneous leishmaniasis (DCL or MCL). These latter, more severe, forms of the disease are characterized by the dissemination of the parasites from the primary infection site. Another complication of Lgy or Lbr infection can be relapse, which may occur months to years after the healing of the primary lesion, or after a first-line drug treatment (3, 4). Recently, we correlated the development of these more severe forms of leishmaniasis with the presence of Leishmania RNA virus (LRV1) within different species of Leishmania (3–6). Discovered in the 1980s (7, 8), and since then for a long time neglected, Leishmaniavirus is a genus of double-stranded RNA viruses belonging to the Totiviridae family. Like most other viruses in this family, LRV1 is neither shed nor infectious, and thus can be seen as a persistent, endogenous viral element (9). Two species of LRV have been identified. The LRV1 species is principally found in South America within Lgy and Lbr (10, 11), and the LRV2 species is found within Leishmania major and Leishmania aethiopica in the Old World (6, 12). The increasing reports of LRVs in different Leishmania species could imply a wider role in determining the fate of infection in humans. However, in some instances, metastasis and relapse after drug treatment also occur in the absence of LRV1 (13). The basis for these discrepancies is of considerable interest; the hypothesis put forward includes the significance of the presence of other parasite species, microbial or host factors that are known to play an important role in the development of MCL (14–16).
The disease-exacerbatory role of LRV1 relies principally on its modulation of the innate immune system via its dsRNA genome (5). We recently showed that LRV1-dependent IL-17 promotes the dissemination of the parasite and the consequent formation of metastatic lesions (17). Moreover, we demonstrated that LRV1 increases the life span of Lgy-infected macrophages through a Toll-like receptor 3 (TLR3) and Akt-dependent pathway (18). Further, LRV-containing parasites promote TLR3-dependent secretion of proinflammatory cytokines and chemokines, including IFN-β (5). Interestingly, ablation of LRV1 using the parasite RNAi machinery, or by treatment with compounds selectively inhibiting LRV1, completely abrogated the production of proinflammatory cytokines by infected macrophages (19, 20).
The induction of TLR3-dependent IFN-β production following infection with LRV1+ parasites suggested a potential role in the pathway leading to elevated pathogenicity. Type I interferons (type I IFNs) are mainly known for their antiviral activity, and IFN therapy is currently used to treat several viral infections, including hepatitis B and C and herpes virus (21–23). The role of type I IFNs in bacterial and parasitic infection is less clear, as they are known to protect mice from Plasmodium falciparum infection, but on the other hand, can promote infection pathology with Listeria monocytogenes, Toxoplasma, and Trypanosoma (24–27). During parasite infection, type I IFNs show more variable effects, being either protective or detrimental for the host, depending on the dose, timing of administration, and the parasite species (28, 29). For L. major, type I IFNs have been associated with control (30–32), whereas for other species, especially the New World species L. amazonensis and L. braziliensis, IFN-β has been associated with promotion of parasite survival and/or disease (33–35).
The potential significance of IFN signaling during infections with Leishmania parasites bearing endogenous dsRNA viruses raises the intriguing possibility that coinfections with exogenous viruses inducing type I IFNs could also worsen the disease outcome. Such coinfections could occur at the site of infection by sand flies carrying both Leishmania parasites and Phleboviruses [e.g., Toscana virus (TOSV)], or by another virus inducing systemic production of type I IFNs. Thus far, little is known about coinfection with Leishmania and viruses, with the exception of HIV and the phenotypic change due to its impairment of the adaptive immune response (16, 36).
In this study, we investigated the disease-exacerbatory role of viral-induced type I IFNs in Lgy infection, not only with LRV1-bearing Lgy (LgyLRV1+), but also by coinfecting mice with LRV1-cured Lgy (LgyLRV1−) and lymphocytic choriomeningitis virus (LCMV), or TOSV.
Results
Type I IFNs Exacerbate LgyLRV1+ Infection.
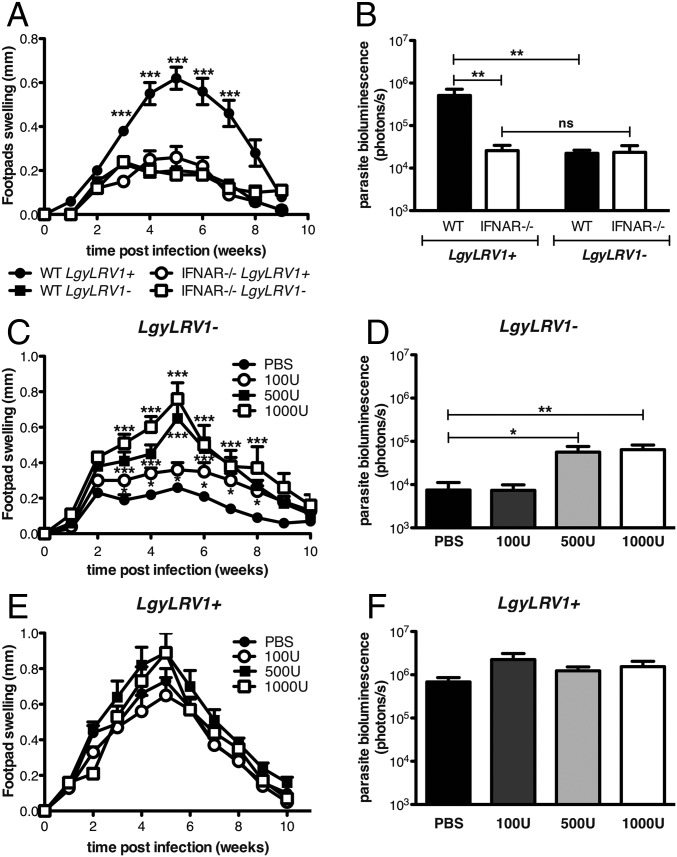
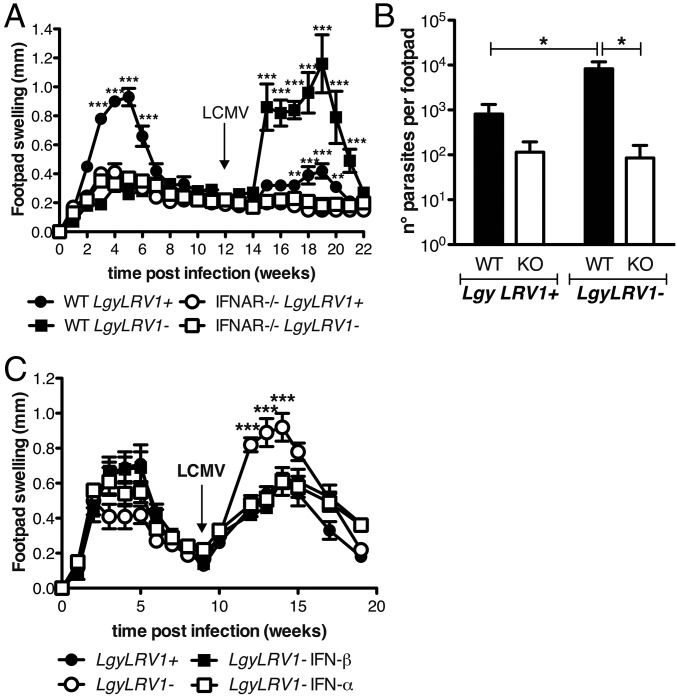
We first analyzed whether type I IFNs could modulate the pathogenicity of Lgy. C57BL/6 wild-type (WT) or type I IFN receptor deficient (ifnar−/−) mice were infected with LgyLRV1− or LgyLRV1+ parasites. Two weeks postinfection, WT mice began to develop lesions that grew until reaching a maximal size at week 5, which then healed 4 wk later, with LgyLRV1+ infection inducing significantly larger lesions compared with LgyLRV1−-infected mice (Fig. 1A). In contrast, LgyLRV1+-infected ifnar−/− mice developed significantly smaller lesions, similar to those developed by ifnar−/− and WT mice infected with LgyLRV1− (Fig. 1A). Parasite numbers were also significantly increased in WT mice infected with LgyLRV1+ compared with the LgyLRV1−-infected counterpart (Fig. 1B). Again, no difference was observed between ifnar−/− mice, independent of the presence of LRV1 in the infecting parasite, as the parasite load was similar to LgyLRV1−-infected WT mice. Thus, the deleterious effect of type I IFNs includes worsening of lesion pathology and increased parasite numbers.
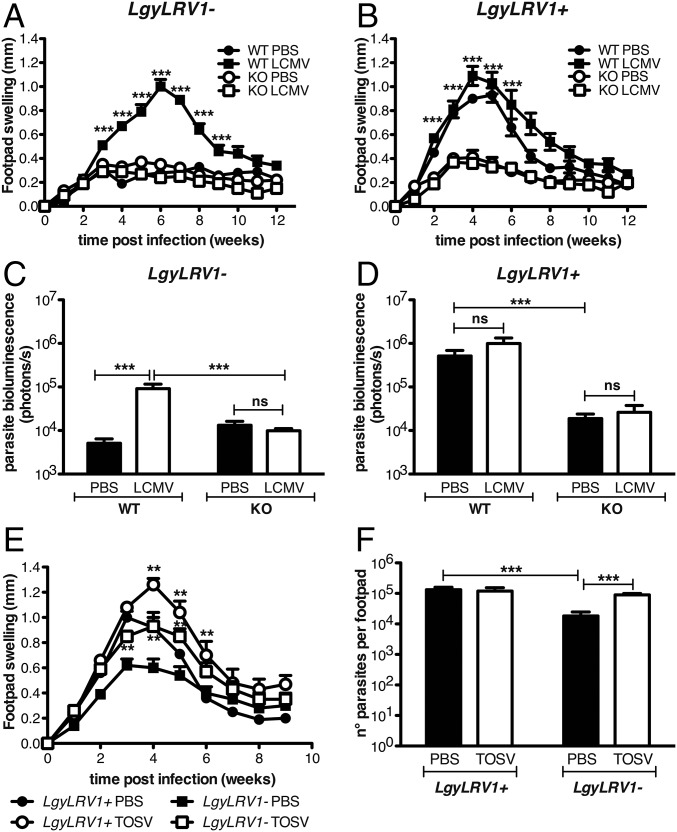
Fig. 1.
Type I IFNs increased the severity of Lgy infection. (A and B) WT or ifnar−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. (C–F) At 6, 24, and 48 h postinfection (p.i.), WT mice were injected with the indicated amount of IFN-β into the footpad. (A, C, and E) Footpad thickness was measured weekly. (B, D, and F) Parasite burden was quantified 5 wk p.i. in vivo by measuring parasite bioluminescence. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA (A, C, and E), two-way ANOVA (B), or Student’s t test (D and F). *P < 0.05, **P < 0.01, ***P < 0.001; ns, nonsignificant.
To further confirm the deleterious role of type I IFNs, WT mice were injected with recombinant IFN-β at early time points post-Lgy infection, to mimic the antiviral response induced by the endogenous dsRNA LRV1 virus (5). In LgyLRV1−-infected mice, IFN-β showed a dose-dependent effect in increasing the lesion size and the parasite load at the peak of infection. In fact, injection of 100 units of IFN-β showed a moderate increase in lesion size and no effect on parasite load, whereas injection of 500 or 1,000 units caused greater increase of both lesion size and parasite burden, quantitatively reproducing the phenotype of LgyLRV1+ infection (Fig. 1 C and D). In contrast, little effect from IFN-β treatment was seen in LgyLRV1+-infected mice, (Fig. 1 E and F). Similar results were obtained with IFN-α treatment (Fig. S1).
Fig. S1.
IFN-α injection increased the size of the lesions of LRV1–Lgy-infected mice. WT mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. At 6, 24, and 48 h p.i., mice were injected with increasing doses of IFN-α s.c. into the footpad. (A and C) Footpad thickness was measured weekly. (B and D) Parasite burden was quantified 4 wk p.i. in vivo by measuring parasite bioluminescence. Results of one representative of two independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA (A and C) or Student’s t test (B and D). ***P < 0.0001; ns, nonsignificant.
Viral Coinfection Increases the Severity of Lgy Leishmaniasis.
The data above, in combination with previous findings (5), suggest that the endogenous dsRNA virus LRV1 acts to promote Lgy virulence through TLR3 and type I IFN signaling. This evidence alluded to the possibility that other agents triggering type I IFN responses might act similarly to promote Leishmania virulence, such as coinfections with other viruses. To test this possibility, we coinfected mice with LgyLRV1− parasites and LCMV Armstrong, an arenavirus, which induces a potent type I IFN response (37). Two different sites of injection were used: i.p. injection for LCMV and s.c. in the footpad for Lgy.
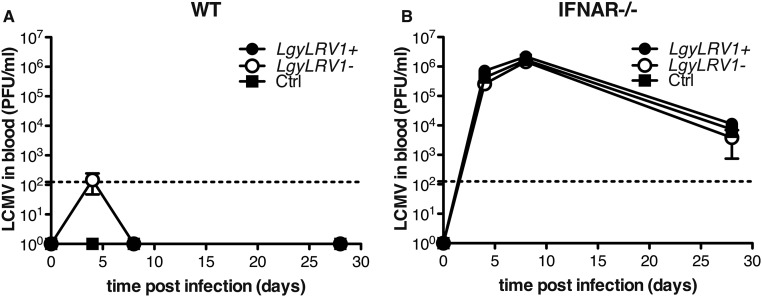
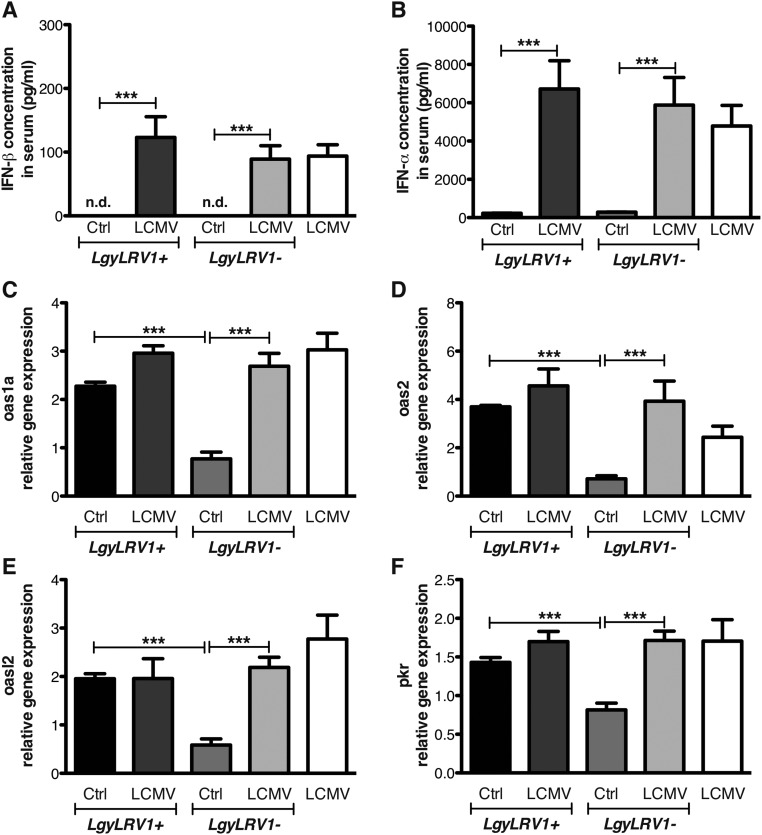
Infection with LCMV Armstrong was shown to be cleared in 8 d by C57BL/6 WT mice with a robust T-cell response (38). Viral titration in mouse serum, following i.p. inoculation, showed only transient and very low viremia (39). As previously reported (40), the presence of Leishmania parasites did not increase LCMV infection (Fig. S2). Similarly, the concentration of type I IFNs in the serum after 24 h of LCMV infection was comparable between mice infected or not with Lgy (Fig. S3). These data demonstrated that the development of the LCMV infection was not affected by the presence of Lgy parasites. Interestingly, type I IFNs were not detected in mouse blood following single infection with LgyLRV1+, suggesting that in this particular case the type I IFN response is only local (Fig. S3).
Fig. S2.
Leishmania coinfection did not affect LCMV clearance. (A) WT or (B) ifnar−/− mice were infected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. At the same time, where indicated, mice were injected in the hind footpad with 3 × 106 stationary phase Lgy promastigotes. LCMV titer in the blood was measured at different times p.i. by plaque assay. Results of one experiment were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA.
Fig. S3.
LRV1 and LCMV coinfection induced a similar IFN response in vivo. WT mice were infected in the hind footpad with 3 × 106 stationary phase Lgy promastigotes, or PBS as control. At the same time, where indicated, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. (A) IFN-β and (B) IFN-α concentration in serum was measured by ELISA 24 h p.i. (C–F) Popliteal LNs were recovered 24 h p.i. and used for RT-qPCR analysis. Results of one representative of two independent experiments were expressed as mean ± SEM (n = 5) of protein concentration (A and B) or as mean ± SEM (n = 5) of transcript increase relative to LgyLRV1-infected mice, and normalized to housekeeping gene l32 (C–F). Statistical significance was assessed by Student’s t test. ***P < 0.001. n.d., nondetectable.
We subsequently investigated whether LCMV-induced type I IFNs stimulated gene expression at the site of Leishmania infection. We observed that genes known to be stimulated by type I IFNs (oas1a, oas2, oasl2, or pkr) were significantly up-regulated in mice infected with LgyLRV1+ or with LgyLRV1− coinfected with LCMV, compared with LgyLRV1−-infected mice (Fig. S3).
We then tested the effect of LCMV coinfection on the progression of leishmaniasis. LCMV injection in LgyLRV1−-infected mice significantly worsened the outcome of leishmaniasis, increasing both pathology and parasite burden, which were very similar to the phenotype induced by LgyLRV1+ infection (Fig. 2 A and C). The LCMV aggravation of Lgy-induced leishmaniasis completely relied on type I IFNs, as no difference was observed when LCMV was injected into LgyLRV1−-infected ifnar−/− mice, neither with regard to footpad swelling nor parasite burden. ifnar−/− mice failed to rapidly clear the virus due to the absence of a proper type I IFN response (Fig. S2) (41). Moreover, this effect could again be related to the type I IFN level, because, as already shown in the type I IFNs injection experiment, no significant effect was observed when LCMV was injected in LgyLRV1+-infected mice (Fig. 2 B and D).
Fig. 2.
Viral coinfection increased the severity of leishmaniasis through type I IFNs. (A–F) WT or ifnar−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. (A–D) Leishmania-infected mice were injected simultaneously with 2 × 105 pfu of LCMV Armstrong or the same volume of PBS as vehicle control intraperitoneally. (E and F) Alternatively, Leishmania-infected mice were simultaneously inoculated with 5 × 105 pfu of TOSV s.c. into the footpad. (A, B, and E) Footpad thickness was measured weekly. (C, D, and F) Parasite burden was measured 5 wk p.i. by measuring parasite bioluminescence or by RT-qPCR. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA (A, B, and E) or two-way ANOVA (C, D, and F). **P < 0.01, ***P < 0.001; ns, nonsignificant.
We furthermore asked whether another virus could similarly affect the course of leishmaniasis. We focused on TOSV, a phlebovirus transmitted to humans by the same insect vector as Leishmania parasites (42). Mimicking the likely biological route of concomitant infection, TOSV was inoculated together with Lgy parasites s.c. into the footpad. As observed with LCMV, LgyLRV1−/TOSV coinfection highly increased footpad swelling and parasite burden at the peak of infection (Fig. 2 E and F). ifnar−/− mice were susceptible to TOSV infection, with over 50% of mortality after 2 wk of infection, precluding further tests on the type I IFN dependency in this coinfection model.
Viral Coinfection Modulates Macrophage Responsiveness to IFN-γ Through Type I IFNs.
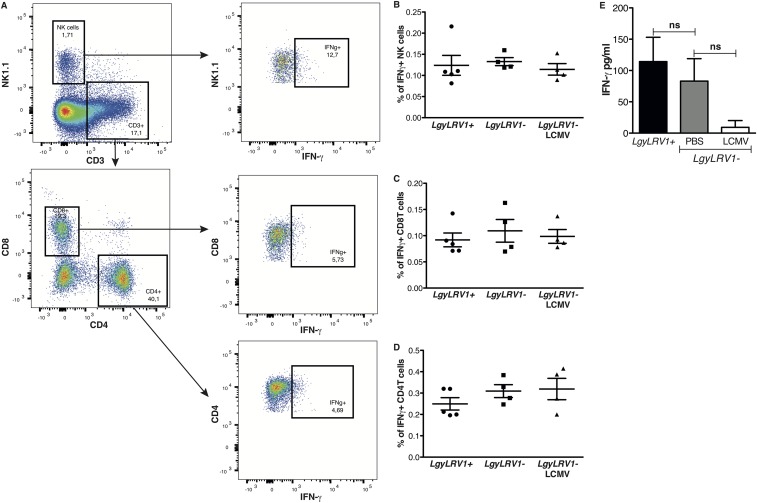
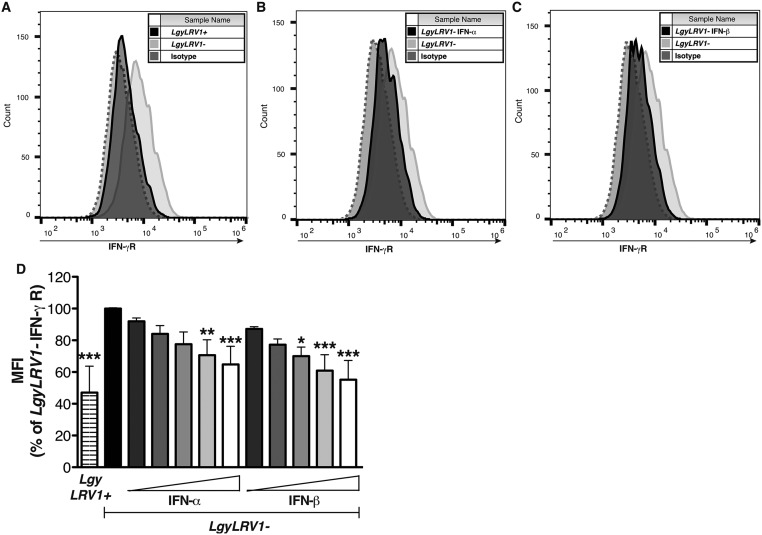
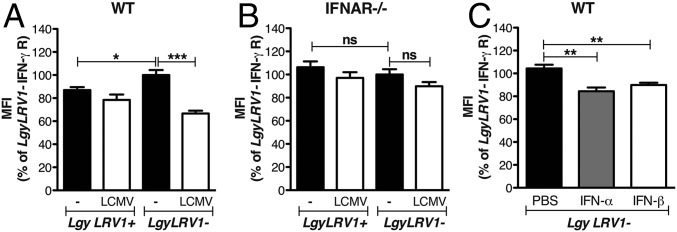
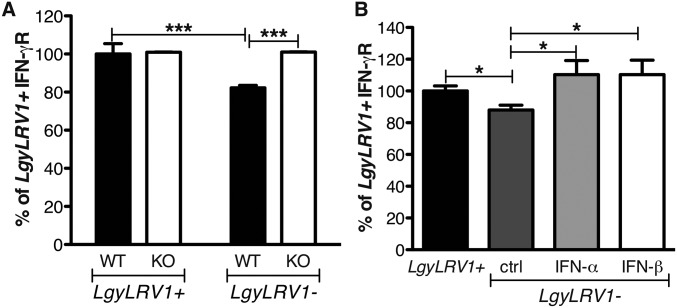
The data above suggested that coinfection with exogenous RNA viruses resulted in worsening of leishmaniasis, quantitatively similar to that seen by parasites bearing endogenous LRV1. We thus focused on defining potential mechanisms responsible for these exacerbated phenotypes. The immune system efficiently clears intracellular Leishmania through the production of IFN-γ (43), and type I IFN responses are known to down-modulate IFN-γ responses (24). The early IFN-γ response is fundamental for the determination of the outcome of Leishmania infection (44), consistent with our finding showing that injection of type I IFNs in the first hours of infection is crucial for the development of disease (Fig. 1 C and D). As LRV1 or LCMV coinfection did not modulate early IFN-γ production (Fig. S4), we wondered whether IFN-γ signaling was modulated during coinfections. To this end, we first measured IFN-γ receptor (R) surface expression on bone marrow-derived macrophages. Infection with LgyLRV1+ parasites induced a significant down-regulation of IFN-γR compared with LgyLRV1−, in a type I IFN-dependent manner (Fig. S5). In this in vivo model of coinfection with LCMV and Leishmania, the pathogens were inoculated at two different sites, lowering the chances for the same cell to be infected by both pathogens and arguing for a systemic production of type I IFNs. Thus, in in vitro experiments, we replaced LCMV coinfection by treating the cells with increasing doses of recombinant IFN-α or IFN-β subsequent to LgyLRV1− infection. Both type I IFNs induced IFN-γR down-regulation in a dose-dependent manner, with IFN-β showing somewhat higher potency (somewhat greater than twofold; Fig. S5D). Subsequently, we confirmed these results during in vivo infection. Flow cytometric analysis 48 h postinfection showed that macrophages from LgyLRV1+-infected or LgyLRV1−/LCMV-infected mice, expressed significantly lower levels of surface IFN-γR, compared with infections by LgyLRV1− alone (Fig. 3A). No significant difference was observed in ifnar−/− mice (Fig. 3B); however, the down-regulation of IFN-γR expression was observed when mice were infected with LgyLRV1− and injected with IFN-α or IFN-β (Fig. 3C). This finding confirmed that the effect depended on type I IFNs.
Fig. S4.
LRV1 or LCMV coinfection did not modulate early IFN-γ production in vivo. WT mice were infected in the hind footpad with 3 × 106 stationary phase Lgy promastigotes. At the same time, where indicated, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong, or PBS as control. Popliteal LN cells were recovered 48 h p.i. (A) Flow cytometry dot plots showing intracellular IFN-γ expression in different cell populations. (B–D) Frequency of IFN-γ–expressing NK, CD8T, and CD4T cells, respectively. Results of one experiment were expressed as mean ± SEM (n ≥ 4). (E) IFN-γ secretion by lymph node cells was measured by ELISA. Results of a pool of two independent experiments were expressed as mean ± SEM (n = 8). Statistical significance was assessed by Student’s t test, results were nonsignificant (ns).
Fig. S5.
LgyLRV1+ infection or type I IFN treatment down-regulated IFN-γR expression on macrophages. WT BMDMs were infected with Lgy parasites. Six hours p.i., LgyLRV1−-infected macrophages were treated with 1,000 units/mL of IFN-α or IFN-β. Forty-eight hours p.i., IFN-γR expression was measured by flow cytometry. Results of one representative of three independent experiments showed IFN-γR surface expression on LgyLRV1+ infected (A), IFN-α (B), or IFN-β (C) treated macrophages compared with LgyLRV1− infection. (D) WT BMDMs were infected with Lgy parasites. Six hours p.i., LgyLRV1−-infected macrophages were treated with increasing doses of IFN-α or IFN-β (50, 100, 250, 500 or 1,000 units/mL). Forty-eight hours p.i., IFN-γR expression was measured by flow cytometry. Results of a pool of two independent experiments were expressed as mean ± SEM of IFN-γR MFI. Statistical significance was assessed by Student's t test (D). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 3.
Lgy–LCMV coinfection modulated IFN-γR expression on macrophages through type I IFNs. (A) WT or (B) ifnar−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. At the same time, where indicated, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. (C) Alternatively, at 6 h p.i., LgyLRV1−-infected WT mice were injected with 1,000 units of IFN-α or IFN-β s.c. into the footpad. Forty-eight hours p.i., popliteal lymph node (LN) cells were recovered and IFN-γ receptor expression at the surface of macrophages was measured by flow cytometry. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by two-way ANOVA (A and B) or Student’s t test (C). *P < 0.05, **P < 0.01, ***P < 0.001.
LCMV–Lgy Coinfection Accelerates Parasite Dissemination.
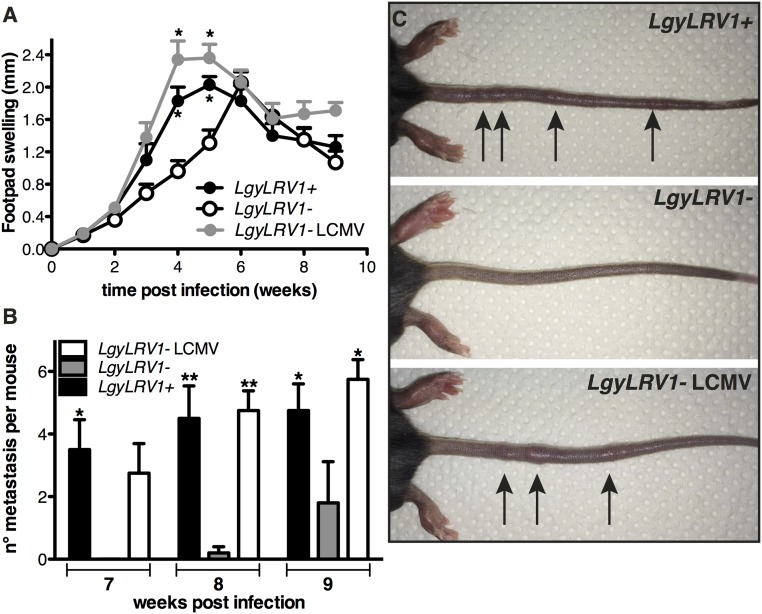
The importance of IFN-γ was further demonstrated as a critical component of our murine model for metastatic leishmaniasis, where IFN-γ−/− mice failed to control Lgy infection, causing multiple metastatic lesions, usually located on the tail (17). In this model, metastases were accelerated in the presence of the endogenous LRV1 within Lgy parasites. Interestingly, IFN-γ−/− mice coinfected with LgyLRV1−/LCMV developed metastasis earlier than those infected with LgyLRV1− alone, thus reproducing the phenotype of LgyLRV1+ parasites. Therefore, exogenous viral coinfection was equally capable of synergizing parasite virulence and metastasis (Fig. 4 A–C).
Fig. 4.
Lgy–LCMV coinfection promoted disease dissemination. (A–C) IFN-γ−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. At the same time, where indicated, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. (A) Footpad thickness was measured weekly. (B) Number of metastatic lesions per mouse at week 7, 8, and 9 p.i. (C) Photographic image of representative mice showing metastatic lesions on the tail at week 8 postinfection. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA (A) or two-way ANOVA (B). *P < 0.05, **P < 0.01.
LCMV Infection Induces Reactivation of Leishmaniasis.
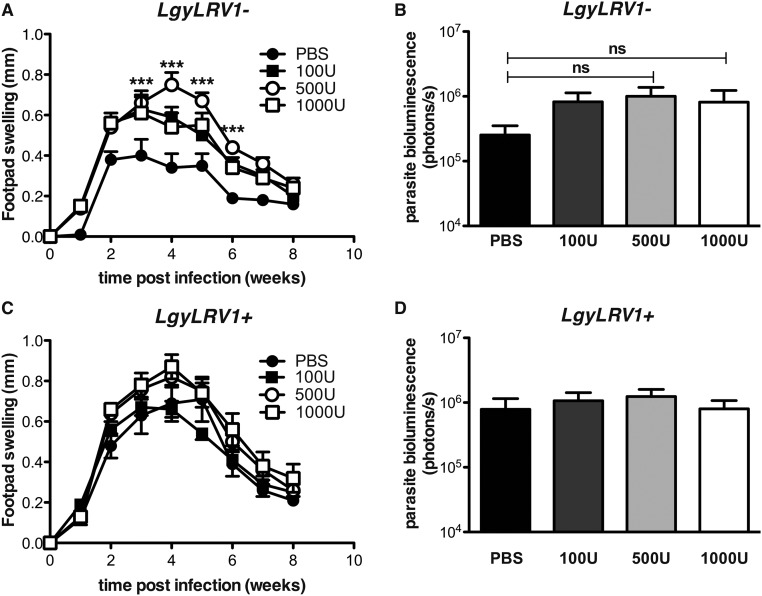
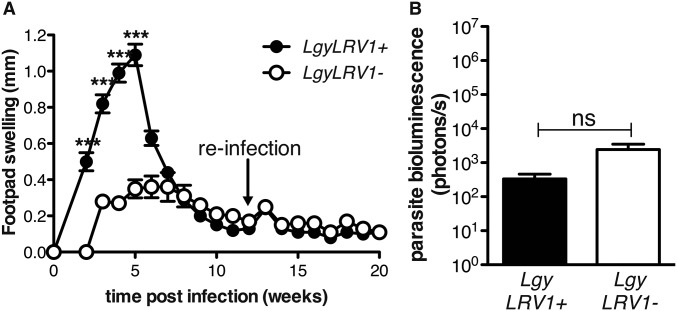
We then asked whether viral infection could lead to reactivation of leishmaniasis following healing, a pathological situation often observed in humans (45). LCMV injection subsequent to lesion healing induced reactivation of the disease, with a reappearance of both lesion pathology and increased parasite numbers, overriding the protective adaptive immune response essential in C57BL/6 mice reinfected with Lgy (Fig. S6). Once reactivated, pathology and parasite burden progressed very similarly to that seen in concomitant LgyLRV1−/LCMV infections, with the lesion pathology and size peaking around 5 wk post-LCMV injection and healing a few weeks later (Fig. 5A). Further, at the peak of LCMV infection, we found higher parasite burden, a sign of reactivation of parasite proliferation (Fig. 5B). The mechanism of relapse relied on type I IFNs, because no footpad swelling or increase in parasite load was observed in ifnar−/− mice infected with either LgyLRV1− or LgyLRV1+ (Fig. 5 A and B). As seen with simultaneous infections, the subsequent delayed posthealing LCMV injection in LgyLRV1−-infected mice induced down-regulation of IFN-γR surface expression on macrophages in a type I IFN-dependent manner (Fig. S7A).
Fig. S6.
C57BL/6 WT mice are protected against Lgy reinfection. WT mice were infected in the hind footpad with 3 × 106 stationary phase Lgy promastigotes. Twelve weeks p.i. mice were reinfected with Lgy promastigotes. (A) Footpad thickness was measured weekly. (B) Parasite burden was quantified 15 wk p.i. in vivo by measuring parasite bioluminescence. Results of one representative of two independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated-measure ANOVA (A), or Student’s t test (B); ***P < 0.001; ns, nonsignificant.
Fig. 5.
LCMV late coinfection induced the relapse of leishmaniasis. (A and B) WT or ifnar−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. (C) At 6, 24, and 48 h p.i., LgyLRV1−-infected WT mice were injected with 1,000 units of IFN-α or IFN-β. Following healing, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. (A and C) Footpad thickness was measured weekly. (B) Parasite burden was measured at week 17 p.i. by RT-qPCR, measuring kmp11 gene expression. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by repeated measure ANOVA (A and C) or Student's t test (B). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S7.
Early type I IFNs inhibited IFN-γR down-regulation following LCMV coinfection. (A) WT or ifnar−/− mice were infected in the hind footpads with 3 × 106 stationary phase Lgy promastigotes. (B) At 6, 24, and 48 h p.i., LgyLRV1−-infected WT mice were injected with 1,000 units of IFN-α or IFN-β. Following healing, mice were injected intraperitoneally with 2 × 105 pfu of LCMV Armstrong. (A and B) Forty-eight hours after LCMV infection, popliteal LN cells were recovered and IFN-γ receptor expression on macrophages was measured by FACS. Results of one representative of two independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by two-way ANOVA (A) or Student’s t test (B). *P < 0.05, ***P < 0.001.
Interestingly, LgyLRV1+-infected WT mice developed only small lesions with low parasite number compared with their LgyLRV1− counterparts, thus reversing the phenotype observed in simple Lgy infection. Indeed, when LgyLRV1−-infected mice were injected with type I IFNs during the first 2 d of infection, they developed less severe relapses when subsequently coinfected with LCMV (Fig. 5C). This result suggests that early type I IFN production could significantly prevent the reactivation of the disease in mice previously infected with LgyLRV1+, thus explaining the difference between the LgyLRV1+ and LgyLRV1− relapsing phenotype. Accordingly, the down-regulation of IFN-γR was not observed in LgyLRV1+-infected, nor in LgyLRV1−/ type I IFN-injected mice (Fig. S7B), suggesting that early type I IFN signaling may inhibit the effect of a subsequent LCMV coinfection.
Discussion
In this study we confirmed in an in vivo murine model of leishmaniasis that the detrimental role of LRV1 borne by Lgy was due to the antiviral response triggered by viral dsRNA recognition, which culminated in type I IFN production. Further, we demonstrated that injection of recombinant type I IFNs during the initial days of infection was sufficient to worsen the outcome of leishmaniasis. Despite the fact that type I IFNs are mainly known for their antiviral role, increasing evidence attests to the involvement of type I IFNs in bacterial and parasitic infection (24–27, 29). Our findings are consistent with earlier reports suggestive of a disease-exacerbatory role for type I interferons in New World Leishmania species (33–35).
Here, we showed that LgyLRV1+ infection, or LgyLRV1− plus LCMV coinfection, induced the down-regulation of IFN-γR on macrophages, in a type I IFN-dependent manner. In this model, both type I IFNs demonstrated activity, with IFN-β showing greater potency in vitro. The importance of IFN-γ production during the first days of infection with Leishmania parasites has been widely described (44). In our model of coinfection, we did not observe differences in early IFN-γ production, nevertheless the lower expression of the IFN-γ receptor likely acts to promote the development of disease. Recently we have shown that infection with LgyLRV1+ highly increased macrophage survival in vitro (18). This result, combined with the down-regulation of IFN-γR, suggests that the presence of viral coinfection increased the persistence of Leishmania parasites. Whereas the down-regulation of IFN-γR was quantitatively modest, this was also observed in studies of the exacerbatory role of type I interferons in Listeria infections (24). Our studies, however, do not rule out other mechanisms of disease exacerbation induced by type I IFNs.
Significantly, exogenous viral coinfection with LCMV or TOSV worsened the outcome of murine leishmaniasis caused by LgyLRV1−, reproducing the phenotype of LgyLRV1+-infected mice (Fig. 2). It was recently reported that mice infected with LCMV present increased lesions when subsequently infected with L. major (46). This phenotype was due to an increased inflammatory response induced by memory T cells not accompanied by an increased parasite burden. Similar results were observed when mice were first infected with L. major and only 2 wk later coinfected with LCMV (40). In this latter case, a transiently decreased anti-Leishmania immune response was observed 1 wk following LCMV coinfection. In our experiments, mice were infected at the same time with parasites and LCMV, leading not only to increased footpad lesions, but also to higher parasite burden, compared with infection with parasites alone. This finding suggests that there was a decrease of macrophage antiparasitic activity, consistent with the lower IFN-γR expression on macrophages; this observation, however, did not exclude other possible systemic effects of LCMV. Interestingly, LCMV coinfection was able to promote the metastatic phenotype in LgyLRV1−-infected IFN-γ–deficient mice (Fig. 4). Further, we showed that disease exacerbation of LRV1 and LCMV coinfection depended completely on type I IFNs (Fig. 2 A and B).
These findings may explain why the tight correlation between disease severity and metastasis with LgyLRV1+ in animal models (5) may be more variable in humans (13, 17, 47, 48). Potentially, coinfection with viruses or other pathogens inducing a sufficient amount of type I IFNs could increase the severity of Lgy infection, contributing to the development of more severe disease manifestations. Certainly, many viral diseases are found in Leishmania endemic regions, which could contribute to increased disease severity (49). A number of arboviruses are transmitted by the sand fly vectors also transmitting Leishmania, including the Massilia and TOSV phleboviruses (50, 51). Coinfection was reported in dogs, whereas seropositivity to TOSV was associated with Leishmania infantum in humans (52, 53); however, the clinical relevance of the coinfection is unknown. As our results suggest that events early in infection were crucial to determine the fate of the disease (Fig. 1 C and D), having the virus transmitted simultaneously with Leishmania (Viannia) could magnify the impact of coinfection. However, i.p. LCMV infections which induce a systemic type I IFN response strongly exacerbated disease, suggesting that coinfections do not require the same entry site.
Finally, we showed that LCMV infection following the resolution of the primary lesion induced relapses of leishmaniasis, overriding the memory immune response of the host. Surprisingly, relapses were more severe in LgyLRV1−-infected mice compared with LgyLRV1+. This result correlated with a type I IFN-dependent down-regulation of IFN-γR observed in LgyLRV1−-infected mice compared with LgyLRV1+, confirming the role of IFN-γR in determining the outcome of the disease. Identification of the mechanism(s) underlying this difference will require future investigation. In humans, relapses are observed following infection with different species of Leishmania and can have varying outcomes, ranging from a simple cutaneous lesion, to DCL or MCL in the case of Lgy or Lbr infection, or postkala-azar dermal leishmaniasis, in the case of Leishmania donovani infection (3, 54, 55). Here, we suggest that viral coinfection and prior exposure to type I IFNs not only could be a risk factor for relapses of leishmaniasis, but could be the trigger of parasite reactivation.
In total, our findings establish a major role for simultaneous or subsequent viral infection in determining the severity of Leishmania (Viannia) infection in animal models and that viral coinfections could contribute toward metastasis and relapse in human patients suffering from leishmaniasis.
Materials and Methods
For additional information please refer to SI Text.
Mice.
C57BL/6 WT mice were purchased from Harlan Laboratories; ifnar−/− mice were obtained from M. Aguet, Swiss Institute of Experimental Cancer Research, Epalinges, Switzerland; IFN-γ−/− mice were purchased from The Jackson Laboratory. Experimentation procedures were undertaken with strict adherence to ethical guidelines set out by the Swiss Federal Veterinary Office and under inspection by the Department of Security and Environment of the State of Vaud, Switzerland.
Parasites and Viruses.
Matched LgyLRV1+ and LgyLRV1− parasites expressing luciferase obtained following limited treatment with anti-LRV1 inhibitors were used in all studies (20). In vivo parasites were quantified by luciferase bioluminescence imaging as described previously (17).
LCMV and TOSV were provided by D.Z. and M.G.C., respectively.
Macrophages.
Bone marrow-derived macrophages were generated as described previously (18).
SI Text
LgyLRV1+ Infection Induced Type I IFN Expression in Vivo.
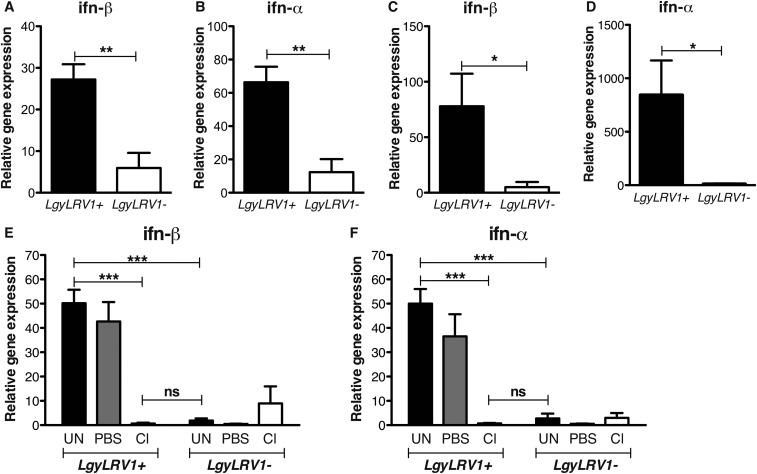
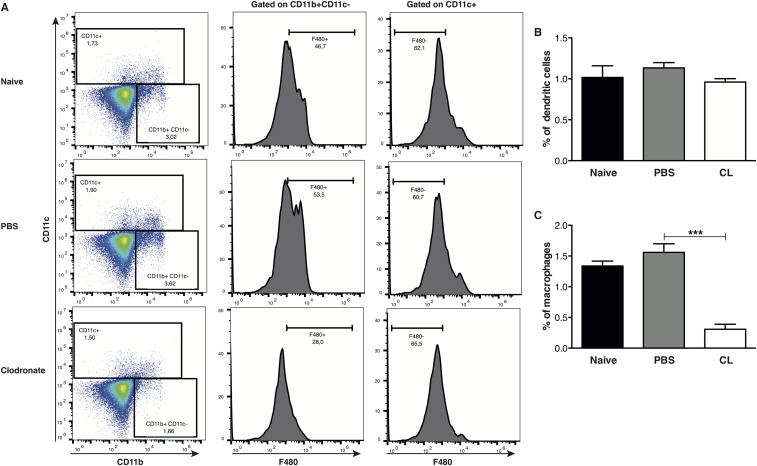
We previously demonstrated that macrophages produce IFN-β upon infection with LgyLRV1+ parasites in vitro (5). To verify whether similar production was occurring in vivo, C57BL/6 WT mice were inoculated with LgyLRV1+ or LgyLRV1− parasites in the hind footpad. At 6 or 12 h postinfection, mice were killed, and the footpads and draining lymph nodes (LNs) were recovered and used for RT-qPCR analysis. ifn-β transcription was up-regulated at early time points post-LgyLRV1+ infection in both footpads and draining lymph nodes (Fig. S8). The increase of transcription was found to be only transient, as 48 h postinfection, type I IFNs were not detectable either in the lymph node, or in the footpad. Similar results were obtained for ifn-α (Fig. S8). Further, in vivo macrophage depletion using clodronate-containing liposomes completely abrogated the induction of ifn-α and ifn-β, demonstrating that macrophages are responsible for type I IFN production in LgyLRV1+ infection (Figs. S8 and S9).
Fig. S8.
LgyLRV1+ parasite infection induced type I IFN up-regulation in vivo. WT mice were infected in the hind footpads with 3 × 106 Lgy stationary-phase promastigotes. ifn-β and ifn-α transcript levels were quantified by RT-qPCR at 6 or 12 h p.i. in footpads (A and B) or draining LNs (C and D). (E and F) Three weeks before infection, mice were injected s.c. into the ankle with 50 μL of PBS- or clodronate-containing liposomes. ifn-β and ifn-α transcript levels were quantified by RT-qPCR in draining LNs 12 h p.i. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5) transcript increase relative to a naive mouse and normalized to housekeeping gene l32. Statistical significance was assessed by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001; ns, nonsignificant.
Fig. S9.
Clodronate depletion of macrophages in vivo. WT mice were injected s.c. in the ankle with 50 μL of PBS- or clodronate-containing liposomes. Three weeks posttreatment, mice were infected in the hind footpads with 3 × 106 Lgy stationary-phase promastigotes. LNs cells were recovered 12 h p.i. (A) FACS plots showed the frequency of macrophages and dendritic cells in LNs. (B and C) Graphs showed the percentage of dendritic cells and macrophages in total LNs cells. Results of one representative of three independent experiments were expressed as mean ± SEM (n = 5). Statistical significance was assessed by Student’s t test. ***P < 0.001.
SI Materials and Methods
Mice.
Four- to 5-wk-old C57BL/6 mice were purchased from Harlan Laboratories, type I IFN receptor-deficient (ifnar−/−) mice were obtained from M. Aguet, Swiss Institute of Experimental Cancer Research (Epalinges, Switzerland), IFN-γ−/− mice were purchased from The Jackson Laboratory. Mice were kept and bred at the animal facility of the Center of Immunity and Immunology, Lausanne (Switzerland) in a pathogen-free environment. All animal protocols were approved by the Swiss Federal Veterinary Office (SFVO), under the authorization numbers 2113.1, 2113.2a, and 2113.2b. Animal experimentation procedures were undertaken with strict adherence to ethical guidelines set out by the SFVO and under inspection by the Department of Security and Environment of the State of Vaud, Switzerland.
Parasites.
LRV1-bearing and LRV1-cured L. guyanensis parasites (LgyLRV+ and LgyLRV−, respectively) were used in all studies. These parasites were obtained following brief drug treatment of the LRV1+ strain of L. guyanensis M4147 containing a firefly luciferase (ffLUC) gene integrated stably into the small subunit gene of the ribosomal RNA locus (LgM4147/SSU:IR2SAT-LUCb LRV1+) as described previously (20).
Parasites were cultured at 26 °C in Schneider’s medium (Sigma-Aldrich) supplemented with 10% FCS, 1% penicillin/streptomycin (P/S), 1% Hepes (Sigma-Aldrich), 0.6 μg/mL of Biopterin (Sigma-Aldrich) and 5 μg/mL of Haemin (Sigma-Aldrich).
Viruses.
Lymphocytic choriomeningitis virus (LCMV) 53b Armstrong was provided by Dietmar Zehn, Technical University of Munich, Germany. The LCMV 53b Armstrong strain was propagated in baby hamster kidney cells and titrated on Vero African green monkey kidney cells according to an established protocol (56). Frozen stocks were diluted in PBS; 2 × 105 plaque-forming units (pfu) of LCMV Armstrong were injected intraperitoneally. Blood samples from LCMV-infected mice were “shock frozen” to release the virus. Diluted samples were used for infection of Vero cells, and viral titers were determined by the LCMV focus-forming assay.
Toscana virus strain 1812 (TOSV) was provided by M.G.C. TOSV was propagated and titrated on Vero cells according to an established protocol (57). The total of 5 × 105 pfu of TOSV in 50 μL of PBS was inoculated s.c. into the footpad.
Mice Infection.
Stationary phase parasites were injected into the hind footpads of mice at a concentration of 3 × 106 parasites per footpad in 50 μL of PBS. Footpad thickness was measured weekly postinfection (p.i.) using a Vernier caliper. Where required, mice were injected in the footpad at 6, 24, and 48 h p.i. with the indicated amount of murine recombinant IFN-β (CellScience), or IFN-α (CellScience) in 50 μL of PBS, or with the same volume of PBS as vehicle control.
Macrophage Extraction and Culture.
To obtain bone marrow-derived macrophages (BMDMs), bone marrow was extracted from tibias and femurs of C57BL/6 or ifnar−/− mice. Macrophages were cultured at 37 °C and 5% CO2 in 10 mL of Dulbecco’s modified Eagle medium (DMEM, Gibco) supplemented with 10% FCS, 1% P/S, 1% Hepes (Sigma-Aldrich) and 50 ng/mL murine recombinant M-CSF (Immunotools). After 3 d, 5 mL of fresh DMEM was added to the culture and 3 d later, BMDMs were used for stimulation assays.
IFN-γ Receptor Measurement.
A total of 5 × 105 BMDMs in 200 μL of DMEM supplemented with 10% FCS, 1% P/S, and 1% Hepes (DMEM complete medium) were seeded in a 48-well plate and incubated at 37 °C and 5% CO2 O/N. BMDMs were subsequently infected with L. guyanensis parasites (Lgy) [multiplicity of infection (moi) 3:1] in 200 μL of DMEM complete medium. At 6 h p.i., BMDMs were treated with 50, 100, 250, 500, or 1,000 units/mL of murine recombinant IFN-α or IFN-β (CellScience). Forty-eight hours p.i., BMDMs were detached with PBS/5 mM EDTA and IFN-γR surface expression was measured by FACS.
To measure IFN-γR expression in vivo, mice were infected with L. guyanensis parasites and coinfected with LCMV or treated with IFN-α or IFN-β as described above. Mice were killed at 48 h p.i., and popliteal lymph node cells were recovered. IFN-γR expression on macrophages was measured by flow cytometry using the following antibodies: IFN-γR1-PE (clone 2E2, dilution 1:100), CD11b-FITC (clone M1/70, 1:500), CD11c-PECy7 (clone N418, 1:500), and F4/80-APC (clone BM8, 1:300) (eBioscience). Flow cytometry was performed with a BD Accuri C6 cytometer and results were analyzed using FlowJo Software (Tree Star).
IFN-γ Measurement in Vivo.
WT mice were infected with L. guyanensis parasites and coinjected with LCMV or PBS as control as described above. Mice were killed at 48 h p.i.; popliteal lymph node cells were subsequently recovered; and intracellular expression of IFN-γ within NK, CD4T, and CD8T cells was measured by flow cytometry, using the following antibodies: CD3-APC-Cy7 (clone 17A2, dilution 1:1,000), CD4-Pacific blue (clone GK1.5, 1:1,000), CD8-PerCP (clone 53–6.7, 1:1,000) (BioLegend), NK1.1-FITC (clone PK136, 1:500), IFN-γ-PE (clone XMG1.2, 1:500) (eBioscience). Flow cytometry was performed with a BD LSR-II flow cytometer (BD Biosciences) and results were analyzed using FlowJo Software (Tree Star). Alternatively, popliteal lymph node cells were cultured for 72 h in complete DMEM. After 72 h, cells were pelleted and supernatant was recovered. IFN-γ protein concentration was measured by ELISA using an IFN-γ ELISA kit (eBioscience) following manufacturer’s instructions.
Type I IFN and IFN-Stimulated Gene Measurement.
Transcript levels of ifn-α and ifn-β were measured in vivo in footpads and draining LNs, at 6 and 12 h p.i., respectively. Alternatively, transcript levels of IFN-stimulated genes (ISGs) were measured in vivo in draining LNs at 24 h p.i. To allow RNA extraction, LNs were stored in RNAlater RNA stabilization solution (Qiagen), whereas footpads were frozen and then homogenized in TRIzol (Life Technologies). Total RNA was extracted from LNs and footpads using the ZR-96 RNA Clean and Concentrator (Zymo Research), and cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen). The cDNA obtained was purified with the ZR-96 DNA Clean and Concentrator 5 (Zymo Research). Real-time quantitative PCR (qRT-PCR) was performed using the LightCycler 480 (Roche Applied Science) to measure SYBR green (LightCycler 480 SYBR Green I Master, Roche) incorporation. The following primers were used: ifn-α: 5′-GGACTTTGGATTCCCGCAGGAGAAG and 5′-GCTGCATCAGACAGCCTTGCAGGTC; ifn-β: 5′-AACCTCACCTACAGGGC, and 5′-CATTCTGGAGCATCTCTTGG; l32: 5′-AAGCGAAACTGGCGGAAAC and 5′-TAACCGATGTTGGGCATCAG; oas1a: 5′-CCCATCTGCATCAGGAGGTG and 5′-GGATCAGGCTTGCTGGAAGT; oas2: 5′-TGAAGAAGCGAAGGAGTGGC and 5′-GGGTCTGCATTACTGGCACTT; oasl2: 5′-CCGCGACATCTGCATCTACT and 5′-CGGTCTCCCTTCAGCTGTTT; pkr: 5′-CAGAAACTTTGGCCACTGGGA and 5′-CCGTGCATCTGGCGGTATT. The results were analyzed using the threshold cycle (CT) method (2-ΔΔCt) for relative quantification of gene expression and normalized to l32. The fold induction was calculated according to the relative expression in naive mice.
Type I IFN protein levels were measured 24 h p.i. in mice serum, using the Verikine mouse IFN-α ELISA kit and Verikine-HS Mouse IFN-β Serum ELISA kit (PBL Assay Science), following manufacturer’s instructions.
Parasite Quantification.
In vivo parasites were quantified at the peak of infection in mouse footpads by injecting 15 mg/kg of luciferin (Invivo Imaging) intraperitoneally and measuring the luminescence produced in the footpads with a Xenogen IVIS Lumina II. The following parameters were used: exposure 10 min, binning medium, F/stop 1.2. Alternatively, the Brucker Extreme II machine was used with the following parameters: exposure 5 min, binning 4, F/stop 1.2.
Macrophage Depletion.
To deplete macrophages from the draining lymph nodes, WT mice were injected s.c. into the ankle with 50 μL of PBS- or clodronate-containing liposomes (ClodronateLiposomes.org). Three weeks posttreatment, mice were infected with 3 × 106 stationary phase L. guyanensis parasites in each footpad. Mice were killed 12 h p.i., draining LNs were taken and macrophage presence was measured by FACS using the Accuri cytometer C6 and data analyzed with FlowJo (version 10.0.7). The following antibodies were used: CD169-PE (clone 3D6.112), CD11b-FITC (clone M1/70), and CD11c-PEcy7 (clone N418) (eBioscience).
Statistical Analysis.
Data obtained from Xenogen and FACS were analyzed using either two-way ANOVA or Student’s t test. Data obtained from qRT-PCR and ELISA were analyzed using a Student’s t test. Data obtained from footpad swelling measurements were analyzed using a repeated measure ANOVA test. When the curves were nonparallel (i.e., the treatment–time interaction was statistically significant), statistical significance of difference between treatments at different time points was assessed using Bonferroni’s multiple test correction.
Statistical significance was set at a P value lower than 0.05. All data are represented with the mean ± the SEM. All analyses were performed using GraphPad Prism software.
Acknowledgments
We thank S. Masina for critical reading of the manuscript and J. Boon for comments on an early draft of the manuscript. This work was funded by the Swiss National Fund for Research (Grants 310030-153204 and IZRJZ3_164176), the Institute for Arthritis Research, the Pierre Mercier Foundation, and the NIH (Grants R56AI099364 and R01AI029646).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621447114/-/DCSupplemental.
References
- 1.Alvar J, et al. WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye P, Scott P. Leishmaniasis: Complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 3.Bourreau E, et al. Presence of Leishmania RNA virus 1 in Leishmania guyanensis increases the risk of first-line treatment failure and symptomatic relapse. J Infect Dis. 2016;213:105–111. doi: 10.1093/infdis/jiv355. [DOI] [PubMed] [Google Scholar]
- 4.Adaui V, et al. Association of the endobiont double-stranded RNA virus LRV1 with treatment failure for human leishmaniasis caused by Leishmania braziliensis in Peru and Bolivia. J Infect Dis. 2015;213:112–121. doi: 10.1093/infdis/jiv354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ives A, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331:775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangger H, et al. Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PLoS Negl Trop Dis. 2014;8:e2836. doi: 10.1371/journal.pntd.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarr PI, et al. LR1: A candidate RNA virus of Leishmania. Proc Natl Acad Sci USA. 1988;85:9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart KD, Weeks R, Guilbride L, Myler PJ. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci USA. 1992;89:8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley MA, Drexler S, Ronet C, Beverley SM, Fasel N. The immunological, environmental, and phylogenetic perpetrators of metastatic leishmaniasis. Trends Parasitol. 2014;30:412–422. doi: 10.1016/j.pt.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salinas G, Zamora M, Stuart K, Saravia N. Leishmania RNA viruses in Leishmania of the Viannia subgenus. Am J Trop Med Hyg. 1996;54:425–429. doi: 10.4269/ajtmh.1996.54.425. [DOI] [PubMed] [Google Scholar]
- 11.Saiz M, et al. Short report: Detection of Leishmaniavirus in human biopsy samples of leishmaniasis from Peru. Am J Trop Med Hyg. 1998;58:192–194. doi: 10.4269/ajtmh.1998.58.192. [DOI] [PubMed] [Google Scholar]
- 12.Scheffter SM, Ro YT, Chung IK, Patterson JL. The complete sequence of Leishmania RNA virus LRV2-1, a virus of an Old World parasite strain. Virology. 1995;212:84–90. doi: 10.1006/viro.1995.1456. [DOI] [PubMed] [Google Scholar]
- 13.Pereira LdeO, et al. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infection in Brazil. Mem Inst Oswaldo Cruz. 2013;108:665–667. doi: 10.1590/0074-0276108052013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellucci LC, et al. Host genetic factors in American cutaneous leishmaniasis: A critical appraisal of studies conducted in an endemic area of Brazil. Mem Inst Oswaldo Cruz. 2014;109:279–288. doi: 10.1590/0074-0276140028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schriefer A, Wilson ME, Carvalho EM. Recent developments leading toward a paradigm switch in the diagnostic and therapeutic approach to human leishmaniasis. Curr Opin Infect Dis. 2008;21:483–488. doi: 10.1097/QCO.0b013e32830d0ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmentier L, et al. Severe cutaneous leishmaniasis in a human immunodeficiency virus patient coinfected with Leishmania braziliensis and its endosymbiotic virus. Am J Trop Med Hyg. 2016;94:840–843. doi: 10.4269/ajtmh.15-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley MA, et al. Leishmaniavirus-dependent metastatic leishmaniasis is prevented by blocking IL-17A. PLoS Pathog. 2016;12:e1005852. doi: 10.1371/journal.ppat.1005852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eren RO, et al. Mammalian innate immune response to a leishmania-resident RNA virus increases macrophage survival to promote parasite persistence. Cell Host Microbe. 2016;20:318–328. doi: 10.1016/j.chom.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brettmann EA, et al. Tilting the balance between RNA interference and replication eradicates Leishmania RNA virus 1 and mitigates the inflammatory response. Proc Natl Acad Sci USA. 2016;113:11998–12005. doi: 10.1073/pnas.1615085113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhlmann FM, et al. Antiviral screening identifies adenosine analogs targeting the endogenous dsRNA Leishmania RNA virus 1 (LRV1) pathogenicity factor. Proc Natl Acad Sci USA. 2017;114:E811–E819. doi: 10.1073/pnas.1619114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooksley WG. The role of interferon therapy in hepatitis B. MedGenMed. 2004;6:16. [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd J, Waugh N, Hewitson P. Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: A rapid and systematic review. Health Technol Assess. 2000;4:1–67. [PubMed] [Google Scholar]
- 23.Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2010;(12):CD002898. doi: 10.1002/14651858.CD002898.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayamajhi M, Humann J, Penheiter K, Andreasen K, Lenz LL. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SH. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Reports. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Bacar A, et al. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 2004;26:315–318. doi: 10.1111/j.0141-9838.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 27.Chessler AD, Caradonna KL, Da’dara A, Burleigh BA. Type I interferons increase host susceptibility to Trypanosoma cruzi infection. Infect Immun. 2011;79:2112–2119. doi: 10.1128/IAI.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stifter SA, Feng CG. Interfering with immunity: Detrimental role of type I IFNs during infection. J Immunol. 2015;194:2455–2465. doi: 10.4049/jimmunol.1402794. [DOI] [PubMed] [Google Scholar]
- 29.Beiting DP. Protozoan parasites and type I interferons: A cold case reopened. Trends Parasitol. 2014;30:491–498. doi: 10.1016/j.pt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Mattner J, et al. Protection against progressive leishmaniasis by IFN-beta. J Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 31.Diefenbach A, et al. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 32.Favila MA, et al. Human dendritic cells exhibit a pronounced type I IFN signature following Leishmania major infection that is required for IL-12 induction. J Immunol. 2014;192:5863–5872. doi: 10.4049/jimmunol.1203230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khouri R, et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: Evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 34.Pereira RM, et al. Novel role for the double-stranded RNA-activated protein kinase PKR: Modulation of macrophage infection by the protozoan parasite Leishmania. FASEB J. 2010;24:617–626. doi: 10.1096/fj.09-140053. [DOI] [PubMed] [Google Scholar]
- 35.Xin L, et al. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol. 2010;184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Griensven J, Zijlstra EE, Hailu A. Visceral leishmaniasis and HIV coinfection: Time for concerted action. PLoS Negl Trop Dis. 2014;8:e3023. doi: 10.1371/journal.pntd.0003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Herrath M, Whitton JL. Animal models using lymphocytic choriomeningitis virus. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1910s36. [DOI] [PubMed] [Google Scholar]
- 39.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crosby EJ, Clark M, Novais FO, Wherry EJ, Scott P. Lymphocytic choriomeningitis virus expands a population of NKG2D+CD8+ T cells that exacerbates disease in mice coinfected with Leishmania major. J Immunol. 2015;195:3301–3310. doi: 10.4049/jimmunol.1500855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 42.Cusi MG, Savellini GG, Zanelli G. Toscana virus epidemiology: From Italy to beyond. Open Virol J. 2010;4:22–28. doi: 10.2174/1874357901004010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decker T, Stockinger S, Karaghiosoff M, Müller M, Kovarik P. IFNs and STATs in innate immunity to microorganisms. J Clin Invest. 2002;109:1271–1277. doi: 10.1172/JCI15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 45.Netto EM, et al. Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with glucantime. Trans R Soc Trop Med Hyg. 1990;84:367–370. doi: 10.1016/0035-9203(90)90321-5. [DOI] [PubMed] [Google Scholar]
- 46.Crosby EJ, Goldschmidt MH, Wherry EJ, Scott P. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS Pathog. 2014;10:e1003970. doi: 10.1371/journal.ppat.1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantanhêde LM, et al. Further evidence of an association between the presence of Leishmania RNA virus 1 and the mucosal manifestations in tegumentary leishmaniasis patients. PLoS Negl Trop Dis. 2015;9:e0004079. doi: 10.1371/journal.pntd.0004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adaui V, et al. Association of the endobiont double-stranded RNA virus LRV1 with treatment failure for human leishmaniasis caused by Leishmania braziliensis in Peru and Bolivia. J Infect Dis. 2016;213:112–121. doi: 10.1093/infdis/jiv354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai TF, Halstead SB. Tropical viral infections. Curr Opin Infect Dis. 1998;11:547–553. doi: 10.1097/00001432-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Es-Sette N, et al. Phlebotomus sergenti a common vector of Leishmania tropica and Toscana virus in Morocco. J Vector Borne Dis. 2014;51:86–90. [PubMed] [Google Scholar]
- 51.Faucher B, et al. Presence of sandflies infected with Leishmania infantum and Massilia virus in the Marseille urban area. Clin Microbiol Infect. 2014;20:O340–O343. doi: 10.1111/1469-0691.12404. [DOI] [PubMed] [Google Scholar]
- 52.Dincer E, Gargari S, Ozkul A, Ergunay K. Potential animal reservoirs of Toscana virus and coinfections with Leishmania infantum in Turkey. Am J Trop Med Hyg. 2015;92:690–697. doi: 10.4269/ajtmh.14-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bichaud L, et al. Epidemiologic relationship between Toscana virus infection and Leishmania infantum due to common exposure to Phlebotomus perniciosus sandfly vector. PLoS Negl Trop Dis. 2011;5:e1328. doi: 10.1371/journal.pntd.0001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Passos VM, et al. American cutaneous leishmaniasis: Use of a skin test as a predictor of relapse after treatment. Bull World Health Organ. 2000;78:968–974. [PMC free article] [PubMed] [Google Scholar]
- 55.Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 56.Battegay M, et al. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 57.Gori Savellini G, et al. Toscana virus induces interferon although its NSs protein reveals antagonistic activity. J Gen Virol. 2011;92:71–79. doi: 10.1099/vir.0.025999-0. [DOI] [PubMed] [Google Scholar]