Significance
Mitigating the spread of invasive species remains difficult—substantial variability in invasion speed is increasingly well-documented, but the sources of this variability are poorly understood. We report a mechanism for invasion speed variability. The combined action of density dependence in demography and dispersal can cause invasions to fluctuate, even in constant environments. Speed fluctuations occur through creation of a pushed invasion wave that moves forward not from small populations at the leading edge but instead, from larger, more established populations that “jump” forward past the previous invasion front. Variability in strength of the push generates fluctuating invasion speeds. Conditions giving rise to fluctuations are widely documented in nature, suggesting that an important source of invasion variability may be overlooked.
Keywords: Allee effects, biological invasion, density-dependent dispersal, integrodifference equations, invasive species
Abstract
Density dependence plays an important role in population regulation and is known to generate temporal fluctuations in population density. However, the ways in which density dependence affects spatial population processes, such as species invasions, are less understood. Although classical ecological theory suggests that invasions should advance at a constant speed, empirical work is illuminating the highly variable nature of biological invasions, which often exhibit nonconstant spreading speeds, even in simple, controlled settings. Here, we explore endogenous density dependence as a mechanism for inducing variability in biological invasions with a set of population models that incorporate density dependence in demographic and dispersal parameters. We show that density dependence in demography at low population densities—i.e., an Allee effect—combined with spatiotemporal variability in population density behind the invasion front can produce fluctuations in spreading speed. The density fluctuations behind the front can arise from either overcompensatory population growth or density-dependent dispersal, both of which are common in nature. Our results show that simple rules can generate complex spread dynamics and highlight a source of variability in biological invasions that may aid in ecological forecasting.
Fluctuations in population size have long fascinated ecologists and fueled a now-classic debate over whether populations are governed by extrinsic environmental factors or intrinsic self-limitation (1). One of the most important advances of 20th century ecology was the discovery that intrinsic density feedbacks can cause population densities to fluctuate, even in constant environments (2–4). This discovery helped resolve the important role of density dependence in population regulation, revealing that strong regulating forces can generate dynamics superficially consistent with no regulation at all. Our understanding of temporal fluctuations in population size stands in sharp contrast with our relatively poor understanding of fluctuations in the spatial dimension of population growth: spread across landscapes.
Understanding the dynamics of population spread takes on urgency in the current era of human-mediated biological invasions and range shifts in response to climate change. The velocity of spread or “invasion speed” is a key summary statistic of an expanding population and an important tool for ecological forecasting (5). Estimates of invasion speed are often derived from regression methods that describe change in spatial extent with respect to time (6–8). Implicit in this approach is the assumption that the true spreading speed is constant and that deviations from it represent “error” in the underlying process or human observation of the process. This assumption is reinforced by long-standing theoretical predictions that, under a wide range of conditions, a population will asymptotically spread with a constant velocity. Invasion at a constant speed can arise from both pulled waves [where the advancing wave moves forward by dispersal and rapid growth of low-density populations far in front of the advancing wave (9–12)] and pushed waves [where the invasion is driven by reproduction and dispersal from high-density populations behind the invasion front (13–15)]. The conventional wisdom of a long-term constant invasion speed is widely applied (16, 17).
In contrast to classic approaches that emphasize a long-term constant speed, there is growing empirical recognition that invasion dynamics can be highly variable and idiosyncratic (18–25). There are several theoretical explanations for fluctuations in invasion speed (which we define here as any persistent temporal variability in spreading speed), including stochasticity in either demography or dispersal (24–28) and temporal or spatial environmental heterogeneity (29–34). Indeed, empirical studies often attribute temporal variation in speed to differences in the environments encountered by the invading population (7, 35). Predator–prey dynamics can also induce fluctuating invasion speeds (30, 36). Notably, Dwyer and Morris (36) showed that density feedbacks can produce fluctuations in spreading speed, but we still have an incomplete understanding of the conditions under which fluctuations in speed arise. Surprisingly, few theoretical studies have since investigated these density feedbacks, especially with respect to their effect on endogenously driven speed fluctuations, despite recent empirical work on invasion variability (16, 20–22).
Here, we develop deterministic, single-species mathematical models of spatial spread to ask under what conditions the invasion speed of an expanding population can fluctuate in a spatially uniform and temporally constant environment. As a starting point, we took inspiration from the relatively complete understanding of fluctuations in population size generated by density dependence in nonspatial models (4). We conjectured that density-dependent feedbacks might similarly generate fluctuating invasion speeds pursuing the suggestion first made in ref. 36. Because spread dynamics are jointly governed by demography (local births and deaths) and dispersal (spatial redistribution), we considered several types of density feedbacks (37), including positive density dependence in population growth (i.e., Allee effects) at the low-density invasion front (38) and density-dependent movement (36, 39).
Our analysis uncovered density-dependent mechanisms that can induce variability in invasion speed, with fluctuations ranging from stable two-point cycles to more complicated aperiodic dynamics. By showing that simple invasion models can generate complex spread dynamics, our results reveal previously undescribed sources of variability in biological invasions and provide a roadmap for empirical studies to detect these processes in nature.
Models and Results
We use integrodifference equations (11) to model population growth and spread. These models describe the change in population density [] from time to time as the result of demography and dispersal. First, individuals at location generate offspring and then die. Second, a fraction of these offspring disperse. The probability that a dispersing individual moves from location to location is given by the dispersal kernel, . The remaining fraction remains at their natal location. Concatenating reproduction and dispersal, we have (40–43)
| [1] |
We will assume that , so that the population has an equilibrium at the carrying capacity , and that the tails of the dispersal kernel are thin (i.e., go to zero at least exponentially fast), so that the probability that an individual disperses an extremely large distance is exceedingly small.
In general, both the dispersing fraction and the dispersal kernel may depend on the population density at the natal location as does the reproduction function . The way that the functions , , and depend on population density determine the dynamics of Eq. 1. In the simplest case, the reproduction function is strictly compensatory: that is, is an increasing but decelerating function of density [ and ]. For strictly compensatory models, the population will spread at a constant asymptotic speed (Fig. 1A) if three conditions hold: small populations grow [], all individuals disperse (), and dispersal distance is independent of population density. Here, the speed is determined by the growth and spread of the low-density populations far ahead of the main invasion front (9); the dynamics at high densities do not matter—the hallmark of a pulled invasion.
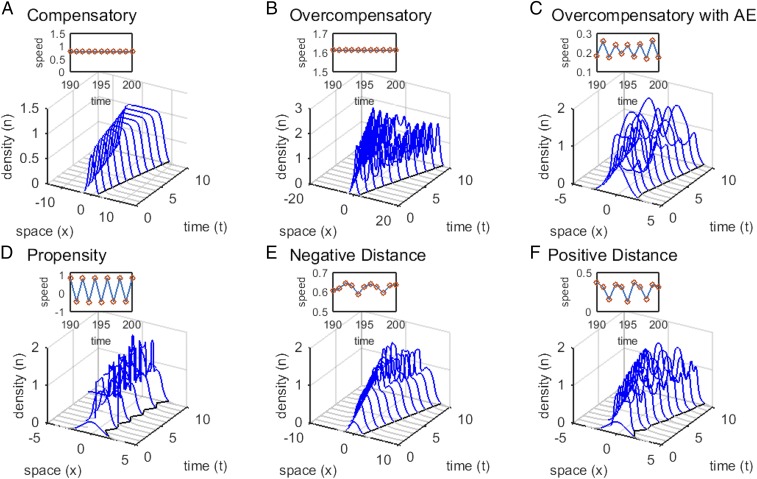
Fig. 1.
Invasion dynamics under different types of density dependence and dispersal. (A) With compensatory growth at high densities, the wave shape and invasion speed are both constant, which is true with and without low-density Allee effects (AE) (overcompensatory model: , , and ) (SI Appendix, Fig. S1A). (B) With overcompensatory population growth and no Allee effect, population density exhibits fluctuations behind the front, but the leading edge progresses at a constant speed (overcompensatory model: , , and ) (SI Appendix, Fig. S1A). (C) However, when overcompensation combines with low-density Allee effects, the invasion speed fluctuates (overcompensatory model: , , and ) (SI Appendix, Fig. S1A). Variability in invasion speed can also occur when Allee effects combine with density dependence in (D) the proportion of dispersing offspring (SI Appendix, Fig. S1 B and C) (propensity model: , , , , , and ) or (E and F) dispersal distance (SI Appendix, Fig. S1 B and D). In the latter model, dispersal distance (E) decreases with population density (distance model: , , , , , and ) or (F) increases with density (distance model: parameters as in E except ). Initial population densities are either (A–C) 2 or (D–F) 0.8 times the standard normal probability density truncated at .
Constant asymptotic invasion speeds are not, however, limited to the simple case just described. In the absence of Allee effects, they can also occur if the reproduction function produces overcompensation—declining offspring production with increasing population density [so that ]. As with classic nonspatial models, overcompensation produces oscillations in population density (2–4), which in turn, cause dynamic changes in the shape of the wave behind the invasion front. Despite these complex fluctuations at high population densities, the invasion speeds of overcompensatory models (without Allee effects) remain constant (Fig. 1B) and are still determined by the dynamics at low densities (44).
Long-standing theory suggests that invaders subject to Allee effects at low population density and compensatory dynamics at larger population density will also eventually spread at a constant speed if their initial population sizes are sufficiently large and the Allee effect is not too strong (13, 14). Allee effects cause invasion waves to be pushed from behind their leading edge (11, 14). When Allee effects are sufficiently strong, the invasion speed no longer depends on the pull of populations at low densities in front of the wave but rather, depends on the strength of the push from the high-density populations behind it. In our models, we show that, when low-density Allee effects combine with spatiotemporal population density fluctuations (created through overcompensation or density-dependent dispersal), the invasion speed may not be constant asymptotically as expected under classic invasion theory but may rather exhibit persistent fluctuations (Fig. 1 C–F).
Allee Effects and Overcompensation.
First, we investigated whether combining an Allee effect with overcompensation at high population density could induce fluctuating invasion speeds when dispersal is density-independent and all offspring disperse (i.e., ). This model (the “overcompensatory model”) (Materials and Methods and SI Appendix, Fig. S1A) has two important parameters: , which affects both the growth rate at low density and the strength of density dependence at carrying capacity, and , the Allee threshold. We assume that, when the population density falls below , no offspring are produced there (a strong Allee effect). If the population density falls below everywhere, the population is doomed to extinction.
Simulations (described in Materials and Methods) revealed that this model generates variable speed invasions (Fig. 1C), but only when the low-density Allee threshold is of intermediate value and high-density overcompensation is strong () (Fig. 2A). For , the local equilibrium density is unstable, leading to sustained fluctuations in local density. Our simulations suggest that is a necessary condition for fluctuating invasion speeds in the overcompensatory model. If the Allee threshold () is too large, the spreading population eventually falls below the threshold everywhere and is extirpated. If is sufficiently small, the invasion proceeds with an apparently constant speed (Fig. 2A).
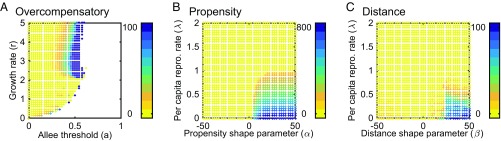
Fig. 2.
Amplitude of fluctuations in invasion speed normalized by the mean speed for populations with Allee effects and (A) overcompensatory growth, (B) density-dependent dispersal propensity, and (C) density-dependent dispersal distance. Darker colors (blue) indicate where fluctuations create large differences from the mean invasion speed. Values of zero (yellow) indicate invasion waves that move at a constant speed. White regions indicate where invasions fail. Parameter values: (A and B) ; (B and C) and ; (B) and ; (C) and . Initial population densities are equivalent to those in Fig. 1.
These fluctuations are induced by the combination of a strong Allee effect, which produces a pushed wave, and strong overcompensation, which produces large spatiotemporal variation in density behind the invasion front and thus, variation in the strength of the push (Fig. 3). When the population density at any location is smaller than the Allee threshold (), such as at the leading edge of the wave, the population vanishes before the next time step. Populations just above become large after reproduction, but as the population size increases beyond , the offspring population size declines as a result of overcompensation (SI Appendix, Fig. S1A). Therefore, when reproduction occurs [transition between and ] (Fig. 3, black vs. blue), populations with the highest density become populations of low density, and populations with density just above become high density. Through time, these transitions creates variability in the size of the push by varying the size of the region contributing to the wave front, leading to fluctuating invasion speeds (Fig. 3D and SI Appendix, Fig. S2 A–F). The speed fluctuations can be periodic or more complex (SI Appendix, Fig. S3). They vary in amplitude by as much as of the mean speed, with some parameter combinations reaching amplitudes of of the mean speed (Fig. 2A).
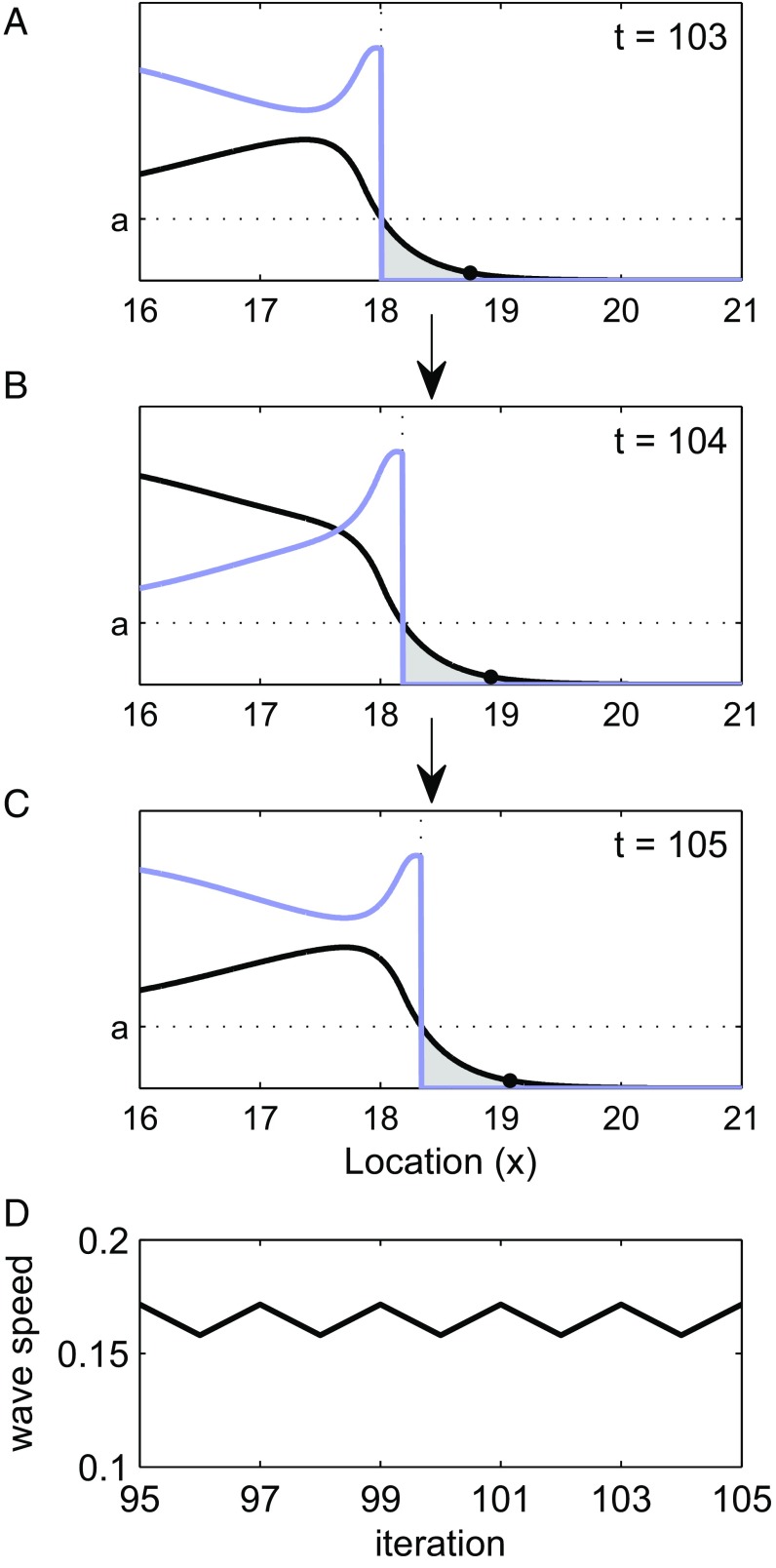
Fig. 3.
Population density before [; black curves] and after [; blue curves] growth (overcompensatory model with Allee effects) (Eq. 3) at (A–C) sequential time steps. Gray regions represent locations that go extinct because of Allee effects [light gray; )], and the solid points show the edges of the wave. (D) The wave speed over time corresponds to A–C. Parameter values include , and .
This mechanism for variable speed invasion does not depend on the discreteness of time. We developed a continuous time version of the overcompensatory model, where we find fluctuating invasion speeds as long as density fluctuations behind the wave front combine with strong low-density Allee effects (SI Appendix, Figs. S4–S6).
Allee Effects and Density-Dependent Dispersal.
Overcompensation is not the only mechanism that can generate the spatiotemporal variability in population density that is necessary to produce fluctuating invasion speeds when combined with Allee effects. Density-dependent dispersal, manifest either as density dependence in the propensity to disperse () or in the shape of the dispersal kernel (), can generate this high-density variability in the pushing force as well. We show this result with two models (the “propensity model” and the “distance model,” respectively) (Materials and Methods), both built on a piecewise linear growth function that is compensatory at high population density (SI Appendix, Fig. S1B). We continue to include low-density Allee effects. When the population size falls below the threshold density , individuals produce offspring at the constant per capita rate . Alternatively, if the population size exceeds , the population goes to carrying capacity.
In the propensity model, population density influences the propensity to disperse . In particular, we assume that the proportion of offspring that disperse is given by a logistic function of local population density [] (Eq. 5) with four parameters: the minimum () and maximum () dispersal proportions; a location parameter , which is the density at which the dispersal propensity is halfway between and ; and a shape parameter . The sign of determines if the proportion dispersing increases () or decreases () with density (SI Appendix, Fig. S1C). The larger the magnitude of , the steeper the density response, which is centered around .
The propensity model can also generate invasions that spread at fluctuating speeds (Fig. 1D and SI Appendix, Fig. S7). We found that these fluctuations persist only when Allee effects are strong (), that dispersal propensity increases with population density (), and that the dispersal response occurs at a population density that is larger than the Allee threshold (). Fluctuations in speed are nearly always periodic (SI Appendix, Figs. S7C and S8 A–D) and of large amplitude, altering the invasion speed by 100–750% relative to the mean speed (Fig. 2B). These large amplitude periodic fluctuations often include positive and negative speeds, meaning that invasions alternate between steps forward and smaller steps backward (Fig. 1D).
As before, spreading speed fluctuations are created through variations in the dispersing population that pushes the invasion forward from behind the front (Fig. 1D). The magnitude of the push depends on the width of the region contributing dispersing individuals and the proximity of this region to the front (SI Appendix, Fig. S2 G–L). When density dependence in dispersal is strong and positive (large ), the population directly adjacent to the front is below the Allee effect threshold () and therefore, decays to zero (SI Appendix, Fig. S2 G and H). Farther behind the front, density is above but below the dispersal midpoint (); thus, this region of the population reproduces but does not disperse (SI Appendix, Fig. S2 H and I). This action results in a large push from behind the wave front that moves the invasion forward at the next time step when the nondispersing population eventually disperses (SI Appendix, Fig. S2 I–K). Subsequently, the region of the nondispersing population is much smaller and farther from the invasion front at the next time step, resulting in a much smaller push (SI Appendix, Fig. S2K).
With the distance model, we explore a second type of density-dependent dispersal, where density alters the dispersal distance. Here, all offspring disperse (), but density alters the variance () of the dispersal kernel (Eq. 6). Four parameters control this dependence: and , which are the lower and upper bounds of the variance; the location parameter , which is the density at which dispersal variance is halfway between and ; and a shape parameter . The dispersal variance increases with population density when is positive and decreases with density when is negative. The larger the absolute value of , the sharper the response (SI Appendix, Fig. S1D).
The distance model also produces the necessary spatiotemporal variability in population density behind the invasion front to induce fluctuating invasion speeds (Fig. 1 E and F and SI Appendix, Fig. S7). As in the propensity model, the invasion speed only fluctuates when Allee effects are strong (). However, unlike the propensity model, we find that persistent fluctuations are possible when density-dependent dispersal is both positive () and negative () (Fig. 2C). The speed fluctuations are more frequently aperiodic (SI Appendix, Fig. S8 E–H) than the two-cycle fluctuations seen in the propensity model, with the largest amplitude when dispersal distance increases with density () (Fig. 2C and SI Appendix, Fig. S7F). In general, fluctuations are larger as both Allee effects and density-dependent dispersal are stronger and alter the invasion speed by 5–100% () and 1–9% () relative to the mean speed (Fig. 2C and SI Appendix, Fig. S7F).
When the dispersal distance exhibits strong positive density dependence (SI Appendix, Fig. S2 M–R), populations at densities above the dispersal threshold disperse long distances, and those below disperse short distances. In this model, each push forward is made up of a combination of both short- and long-distance dispersers. The size of this push changes depending on the proportion of the push made up of each type of disperser, which is temporally variable, creating fluctuating invasion speeds. A similar mechanism operates when (SI Appendix, Fig. S2 S–X); however, instead, high-density populations disperse short distances and vice versa.
Discussion
Our work provides insight into mechanisms behind invasion variability: fluctuations in invasion speed can occur solely because of endogenous density dependence. In the models that we examine, both a strong low-density Allee effect [creating a pushed wave (17, 45)] and large variations in population density behind the invasion front are necessary to create fluctuating invasion speeds. We show that the necessary spatiotemporal variability can be generated via two types of density feedbacks: overcompensatory density dependence or density-dependent dispersal. When combined with Allee effects, either of these factors can cause the strength of the invasion push from high-density populations to vary, leading to varying spreading speeds. The potential for deterministic, density-dependent processes to generate complex fluctuations in local population density is a canonical result of theoretical population biology (1–4) and has proven influential in basic and applied empirical settings (46). By considering the spatial dimension of population growth, which is increasingly relevant in the context of global change, our results flesh out understanding of complex population dynamics arising from endogenous mechanisms. We conjecture that there is some generality to this mechanism, because we also see fluctuating speeds in continuous time (SI Appendix, Fig. S4), although we recognize that fluctuations can occur through other means (25, 30, 36). Our results are potentially consistent with the highly variable spreading speeds seen in empirical invasion studies (20–25).
Processes capable of generating fluctuations in population density that create the variable pushing force behind the invasion vanguard are common in nature. First, many invasive species show the combination of high intrinsic growth rates and conspecific interference at high density that gives rise to overcompensatory population fluctuations (46, 47). Second, density-dependent dispersal as a distinct source of spatiotemporal density fluctuations can arise, even with strictly compensatory density dependence in population growth. We found fluctuating invasion speeds with positive density-dependent dispersal propensity, which is common in organisms with environmentally inducible dispersal polymorphisms, including many insects. For example, wingless aphids (48, 49) and plant hoppers (50) can produce winged morphs when densities become high. When density dependence alters dispersal distance, fluctuations in speed were seen under both positive and negative density dependence. Mobile organisms can increase their dispersal distance with increasing density by altering behavioral responses (39). Alternatively, dispersal distances can decrease with density when crowding decreases reproductive and dispersal ability (39, 51, 52) or in animals (notably small mammals) with strong group behavior (39, 53, 54).
Allee effects, a common density-dependent process (55, 56), influence small populations by decreasing low-density vital rates [e.g., reproduction (40)]. We find in all of our models that Allee effects and the pushed invasions that they generate are a necessary ingredient of fluctuating speeds. Interestingly, this result contrasts with that of Dwyer and Morris (36). Working with a two-species model, they found that fluctuating speeds can occur when predator dispersal distance depends on prey density (a type of density-dependent movement) but without an explicit Allee effect. We conjecture that predator–prey dynamics in their model may, in fact, give rise to an implicit Allee effect, which is known to occur in other predator–prey models (30). Biologically, density-dependent movement can contribute to an Allee effect by reducing mate-finding abilities at low densities, especially when the movement is sex biased (16, 57). In this way, the study by Dwyer and Morris (36), although superficially inconsistent with our study, may nonetheless satisfy the conditions that we identify as necessary for variable invasions.
Thoroughly accounting for the sources of variability in the speed of biological invasions may improve invasion forecasting. Our work suggests that intrinsic density dependence can create complex invasion dynamics, consistent with the highly variable spreading speeds seen in empirical invasion studies (20–25). However, they remain open questions of whether and how often these processes affect the ecological dynamics of spread given the pervasive influences of environmental heterogeneity (29–34) and demographic stochasticity (26–28) and their roles in invasion variability. To begin to answer this question, we suggest coupling models and empirical data, which has proven to be a fruitful approach to understanding the intrinsic mechanisms behind fluctuations in local population density (3, 4). Collecting long-term data can be difficult, but some patterns might be straightforward to identify from existing datasets. In particular, the strong two-cycle speed fluctuations generated when invaders experience both Allee effects and density-dependent dispersal propensity would likely be detectable in data. Few empirical studies have tested for endogenous mechanisms of fluctuating invasion speeds, including studies for which variability in speed was an explicit focus (16, 18–21, 25). Thus, signatures of endogenous variability may be embedded in existing data, and we encourage empiricists to reexamine variable invasion data in the context of these density-dependent mechanisms.
Materials and Methods
The models that we studied are each a special case of Eq. 1. They all use the Laplace dispersal kernel with variance :
| [2] |
Qualitative results are robust to kernel choice (i.e., normal or Cauchy).
Overcompensatory Model.
We combine low-density Allee effects with the possibility of overcompensation at high density (SI Appendix, Fig. S1A):
| [3] |
Dispersal is independent of density in this model [, a constant], and all offspring disperse ().
Propensity Model.
Here, we used a linear constant model for growth:
| [4] |
where (SI Appendix, Fig. S1B). Dispersal propensity depends on the population density [] via a logistic form similar to other models with density-dependent dispersal (SI Appendix, Fig. S1C) (58):
| [5] |
As in the overcompensatory model, the distance moved by dispersing individuals is independent of density [, a constant].
Distance Model.
For this model, we use the reproduction function (Eq. 4) but assume that all offspring disperse () after a dispersal distribution with variance that is a logistic function of parental density [] (SI Appendix, Fig. S1D); that is,
| [6] |
We simulated each model for 200 iterations across a domain of length of 1,200 with spatial nodes. Within each simulation, we defined the location of the invasion front at each time step as the location where the density of the invasion wave first exceeded a density threshold of 0.05. We then used this location to calculate (i) the instantaneous invasion speed (i.e., the distance traveled by the front between consecutive time steps), (ii) the mean invasion speed averaged over the last 50 time steps, and (iii) the amplitude of invasion speed fluctuations (the difference between the maximum and minimum speeds over the last 20 time steps). SI Appendix, Table S1 has a list of parameters and definitions. Code to run these models and recreate all figures is available from the Dryad Digital Repository: dx.doi.org/10.5061/dryad.69sq3.
Supplementary Material
Acknowledgments
We thank J. Pruszenski, E. Strombom, R. Williams, and two anonymous reviewers for comments and support. The initial idea was developed during the 2014 ACKME Nantucket Mathematical Ecology retreat with input from participants and funding from the Woods Hole Oceanographic Institute Sea Grant. The University of Minnesota (UMN) Minnesota Supercomputing Institute provided resources that contributed to the research results reported within this paper (www.msi.umn.edu). The paper was funded in part by the Commonwealth Center for Humanities and Society, University of Louisville. L.L.S. and A.K.S. were supported by startup funds from the UMN (to A.K.S.), B.L. was supported by National Science Foundation (NSF) Grant DMS-1515875, T.E.X.M. was supported by NSF Grant DEB-1501814, and M.G.N. was supported by NSF Grants DEB-1257545 and DEB-1145017.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The code and data to create the figures and run the models have been deposited in the Dryad Digital Repository (dx.doi.org/10.5061/dryad.69sq3).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618744114/-/DCSupplemental.
References
- 1.Kingsland SE. Modeling Nature. Univ of Chicago Press; Chicago: 1995. [Google Scholar]
- 2.May RM. Biological populations with nonoverlapping generations: Stable points, stable cycles, and chaos. Science. 1974;186:645–647. doi: 10.1126/science.186.4164.645. [DOI] [PubMed] [Google Scholar]
- 3.Costantino RF, Desharnais RA, Cushing JM, Dennis B. Chaotic dynamics in an insect population. Science. 1997;275:389–391. doi: 10.1126/science.275.5298.389. [DOI] [PubMed] [Google Scholar]
- 4.Turchin P. Complex Population Dynamics: A Theoretical/Empirical Synthesis. Princeton Univ Press; Princeton: 2003. [Google Scholar]
- 5.Fagan WF, Lewis MA, Neubert MG, van den Driessche P. Invasion theory and biological control. Ecol Lett. 2002;5:148–157. [Google Scholar]
- 6.Miller TEX, Tenhumberg B. Contributions of demography and dispersal parameters to the spatial spread of a stage-structured insect invasion. Ecol Appl. 2010;20:620–633. doi: 10.1890/09-0426.1. [DOI] [PubMed] [Google Scholar]
- 7.Andow DA, Kareiva PM, Levin SA, Okubo A. Spread of Invading Organisms. Landsc Ecol. 1990;4:177–188. [Google Scholar]
- 8.van den Bosch F, Hengeveld R, Metz JAJ. Analysing the velocity of animal range expansion. J Biogeogr. 1992;19:135–150. [Google Scholar]
- 9.Weinberger HF. Long-time beahvior of a class of biological models. SIAM J Math Anal. 1982;13:353–396. [Google Scholar]
- 10.Skellam JG. Random dispersal in theoretical populations. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- 11.Kot M, Lewis MA, van den Driessche P. Dispersal data and the spread of invading organisms. Ecology. 1996;77:2027–2042. [Google Scholar]
- 12.Neubert MG, Caswell H. Demography and dispersal: Calculation and sensitivity analysis of invasion speed for structured populations. Ecology. 2000;81:1613–1628. [Google Scholar]
- 13.Lui R. Existence and stability of travelling wave solutions of a nonlinear integral operator. J Math Biol. 1983;16:199–220. [Google Scholar]
- 14.Wang MH, Kot M, Neubert MG. Integrodifference equations, Allee effects, and invasions. J Math Biol. 2002;44:150–168. doi: 10.1007/s002850100116. [DOI] [PubMed] [Google Scholar]
- 15.van Saarloos W. Front propagation into unstable states. Phys Rep. 2003;386:29–222. [Google Scholar]
- 16.Wagner NK, Ochocki BM, Crawford KM, Compagnoni A, Miller TE. Genetic mixture of multiple source populations accelerates invasive range expansion. J Anim Ecol. 2017;86:21–34. doi: 10.1111/1365-2656.12567. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi SR, Yurtsev EA, Korolev KS, Gore J. Range expansions transition from pulled to pushed waves as growth becomes more cooperative in an experimental microbial population. Proc Natl Acad Sci USA. 2016;113:6922–6927. doi: 10.1073/pnas.1521056113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melbourne BA, Hastings A. Highly variable spread rates in replicated biological invasions: Fundamental limits to predictability. Science. 2009;325:1536–1539. doi: 10.1126/science.1176138. [DOI] [PubMed] [Google Scholar]
- 19.Miller TEX, Inouye BD. Sex and stochasticity affect range expansion of experimental invasions. Ecol Lett. 2013;16:354–361. doi: 10.1111/ele.12049. [DOI] [PubMed] [Google Scholar]
- 20.Ochocki BM, Miller TEX. Rapid evolution of dispersal ability makes biological invasions faster and more variable. Nat Commun. 2017;8:14315. doi: 10.1038/ncomms14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss-Lehman C, Hufbauer RA, Melbourne BA. Rapid trait evolution drives increased speed and variance in experimental range expansions. Nat Commun. 2017;8:14303. doi: 10.1038/ncomms14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JL, Kendall BE, Levine JM. Rapid evolution accelerates plant population spread in fragmented experimental landscapes. Science. 2016;353:482–485. doi: 10.1126/science.aaf6268. [DOI] [PubMed] [Google Scholar]
- 23.Chen H. A spatiotemporal pattern analysis of historical mountain pine beetle outbreaks in British Columbia, Canada. Ecography. 2014;37:344–356. [Google Scholar]
- 24.Walter JA, Johnson DM, Tobin PC, Haynes KJ. Population cycles produce periodic range boundary pulses. Ecography (Cop.) 2015;38:1200–1211. [Google Scholar]
- 25.Johnson DM, Liebhold AM, Tobin PC, Bjørnstad ON. Allee effects and pulsed invasion by the gypsy moth. Nature. 2006;444:361–363. doi: 10.1038/nature05242. [DOI] [PubMed] [Google Scholar]
- 26.Pachepsky E, Levine JM. Density dependence slows invader spread in fragmented landscapes. Am Nat. 2011;177:18–28. doi: 10.1086/657438. [DOI] [PubMed] [Google Scholar]
- 27.Kot M, Medlock J, Reluga T, Walton DB. Stochasticity, invasions, and branching random walks. Theor Popul Biol. 2004;66:175–184. doi: 10.1016/j.tpb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Shen W. Traveling waves in diffusive random media. J Dyn Diff Equ. 2004;16:1011–1060. [Google Scholar]
- 29.Shigesada N, Kawasaki K, Teramoto E. Traveling periodic waves in hetergeneous environments. Theor Popul Biol. 1986;30:143–160. [Google Scholar]
- 30.Neubert MG, Kot M, Lewis MA. Invasion speeds in fluctuating environments. Proc R Soc Lond B Biol Sci. 2000;267:1603–1610. doi: 10.1098/rspb.2000.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger HF. On spreading speeds and traveling waves for growth and migration models in a periodic habitat. J Math Biol. 2002;45:511–548. doi: 10.1007/s00285-002-0169-3. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger HF, Kawasaki K, Shigesada N. Spreading speeds of spatially periodic integro-difference models for populations with nonmonotone recruitment functions. J Math Biol. 2008;57:387–411. doi: 10.1007/s00285-008-0168-0. [DOI] [PubMed] [Google Scholar]
- 33.Caswell H, Neubert MG, Hunter CM. Demography and dispersal: Invasion speeds and sensitivity analysis in periodic and stochastic environments. Theor Ecol. 2011;4:407–421. [Google Scholar]
- 34.Schreiber SJ, Ryan ME. Invasion speeds for structured populations in fluctuating environments. Theor Ecol. 2011;4:423–434. [Google Scholar]
- 35.Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW. Spatial synchrony in forest insect outbreaks: Roles of regional stochasticity and dispersal. Ecology. 2002;83:3120–3129. [Google Scholar]
- 36.Dwyer G, Morris WF. Resource-dependent dispersal and the speed of biological invasions. Am Nat. 2006;167:165–176. doi: 10.1086/498944. [DOI] [PubMed] [Google Scholar]
- 37.Sakai AK, et al. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–332. [Google Scholar]
- 38.Taylor CM, Hastings A. Allee effects in biological invasions. Ecol Lett. 2005;8:895–908. [Google Scholar]
- 39.Matthysen E. Density-dependent dipsersal in birds and mammals. Ecography. 2005;28:403–416. [Google Scholar]
- 40.Veit RR, Lewis MA. Dispersal, population growth, and the Allee Effect: Dynamics of the house finch invasion of eastern North America. Am Nat. 1996;148:255–274. [Google Scholar]
- 41.Volkov D, Lui R. Spreading speed and travelling wave solutions of a partially sedentary population. IMA J Appl Math. 2007;72:801–816. [Google Scholar]
- 42.Lutscher F. Density-dependent dispersal in integrodifference equations. J Math Biol. 2008;56:499–524. doi: 10.1007/s00285-007-0127-1. [DOI] [PubMed] [Google Scholar]
- 43.Lutscher F, Van Minh N. Traveling waves in discrete models of biological populations with sessile stages. Nonlinear Anal Real World Appl. 2013;14:495–506. [Google Scholar]
- 44.Li B, Lewis MA, Weinberger HF. Existence of traveling waves for integral recursions with nonmonotone growth functions. J Math Biol. 2009;58:323–338. doi: 10.1007/s00285-008-0175-1. [DOI] [PubMed] [Google Scholar]
- 45.Mendez V, Llopis I, Campos D, Horsthemke W. Effect of environmental fluctuations on invasion fronts. J Theor Biol. 2011;281:31–38. doi: 10.1016/j.jtbi.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Pardini EA, Drake JM, Chase JM, Knight TM. Complex population dynamics and control of the invasive biennial Alliaria petiolata (garlic mustard) Ecol Appl. 2009;19:387–397. doi: 10.1890/08-0845.1. [DOI] [PubMed] [Google Scholar]
- 47.Zipkin EF, Kraft CE, Cooch EG, Sullivan PJ. When can efforts to control nuisance and invasive species backfire? Ecol Appl. 2009;19:1585–1595. doi: 10.1890/08-1467.1. [DOI] [PubMed] [Google Scholar]
- 48.Harrison RG. Dispersal polymorphisms in insects. Annu Rev Ecol Syst. 1980;11:95–118. [Google Scholar]
- 49.Johnson B. Wing polymorphism in aphids II. Interactions between aphids. Entomol Exp Appl. 1965;8:49–64. [Google Scholar]
- 50.Denno RF, Roderick GK. Density-related dispersal in planthoppers: Effects of interspecific crowding. Ecology. 1992;73:1323–1334. [Google Scholar]
- 51.Marchetto KM, Jongejans E, Shea K, Isard SA. Plant spatial arrangement affects projected invasion speeds of two invasive thistles. Oikos. 2010;119:1462–1468. [Google Scholar]
- 52.Donohue K. Maternal determinants of seed dispersal in Cakile edentula: Fruit, plant, and site traits. Ecology. 1998;79:2771–2788. [Google Scholar]
- 53.Ims RA, Andreassen HP. Density-dependent dispersal and spatial population dynamics. Proc R Soc Lond B Biol Sci. 2005;272:913–918. doi: 10.1098/rspb.2004.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreassen HP, Ims RA. Dispersal in patchy vole populations: Role of patch configuration, density dependence, and demography. Ecology. 2001;82:2911–2926. [Google Scholar]
- 55.Kramer AM, Dennis B, Liebhold AM, Drake JM. The evidence for Allee effects. Pop Ecol. 2009;51:341–354. [Google Scholar]
- 56.Morris DW. Measuring the Allee effect: Positive density dependence in small mammals. Ecology. 2002;83:14–20. [Google Scholar]
- 57.Shaw AK, Kokko H, Neubert MG. Sex differences and Allee effects shape the dynamics of sex-structured invasions. J Anim Ecol. February 20, 2017 doi: 10.1111/1365-2656.12658. [DOI] [PubMed] [Google Scholar]
- 58.Smith MJ, Sherratt JA, Lambin X. The effects of density-dependent dispersal on the spatiotemporal dynamics of cyclic populations. J Theor Biol. 2008;254:264–274. doi: 10.1016/j.jtbi.2008.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.