Significance
Aluminum (Al) toxicity is a major constraint for crops grown on acid soils. Various plant species have adopted an Al exclusion/avoidance mechanism and/or internal tolerance mechanisms to ameliorate Al toxicity. In Arabidopsis, the root organic acid exudation-based Al exclusion mechanism has been well characterized; however, no evidence for an internal Al tolerance mechanism has been reported. Here we identify NIP1;2 as an Al-malate transporter involved in Al removal from root cell walls and root-to-shoot Al translocation. We report that the NIP1;2-mediated Al-malate transport is dependent on Al-activated root malate efflux mediated by ALMT1 in Arabidopsis. Taken together, our findings demonstrate the importance of the coordination between Al exclusion and internal Al detoxification mechanisms in Al tolerance in Arabidopsis.
Keywords: aquaporin, nodulin 26-like protein, aluminum tolerance, organic acid exudation, malate
Abstract
Members of the aquaporin (AQP) family have been suggested to transport aluminum (Al) in plants; however, the Al form transported by AQPs and the roles of AQPs in Al tolerance remain elusive. Here we report that NIP1;2, a plasma membrane-localized member of the Arabidopsis nodulin 26-like intrinsic protein (NIP) subfamily of the AQP family, facilitates Al-malate transport from the root cell wall into the root symplasm, with subsequent Al xylem loading and root-to-shoot translocation, which are critical steps in an internal Al tolerance mechanism in Arabidopsis. We found that NIP1;2 transcripts are expressed mainly in the root tips, and that this expression is enhanced by Al but not by other metal stresses. Mutations in NIP1;2 lead to hyperaccumulation of toxic Al3+ in the root cell wall, inhibition of root-to-shoot Al translocation, and a significant reduction in Al tolerance. NIP1;2 facilitates the transport of Al-malate, but not Al3+ ions, in both yeast and Arabidopsis. We demonstrate that the formation of the Al-malate complex in the root tip apoplast is a prerequisite for NIP1;2-mediated Al removal from the root cell wall, and that this requires a functional root malate exudation system mediated by the Al-activated malate transporter, ALMT1. Taken together, these findings reveal a critical linkage between the previously identified Al exclusion mechanism based on root malate release and an internal Al tolerance mechanism identified here through the coordinated function of NIP1;2 and ALMT1, which is required for Al removal from the root cell wall, root-to-shoot Al translocation, and overall Al tolerance in Arabidopsis.
Aluminum (Al) toxicity is a major constraint for crop yields on acid soils worldwide (1–4). To cope with Al stress, plants have adopted several resistance mechanisms, including (i) an Al exclusion mechanism in which plants release organic acids (OAs) from the root apex into the rhizosphere to chelate toxic Al3+ ions and prevent them from entering into root cells, and (ii) an internal Al tolerance mechanism by which Al is sequestered in the vacuole of the root cell and/or translocated to the shoot for sequestration in leaf cell vacuoles (1, 2, 5).
Al exclusion from root tips via Al-activated root OA exudation is the best-characterized mechanism used by many plant species, including wheat (6, 7), sorghum (8), barley (9), Arabidopsis (10, 11), and maize (12). Malate and citrate are the most commonly used OAs, transported by two families of plasma membrane (PM)-localized transporters: the Al-activated malate transporter (ALMT) family of anion channels (13) and the multidrug and toxic compound extrusion (MATE) family of OA/H+ antiporters (8, 9), respectively. In Arabidopsis, ALMT1 is responsible for a larger Al-activated malate release from the root tip (10), whereas MATE facilitates a smaller citrate exudation in root regions behind the root tip (11, 14).
The mechanism underlying internal Al tolerance has been less well characterized. In rice, Al uptake from the root cell wall into the root cytosol is facilitated by a PM-localized Al transporter, Nrat1 (Nramp aluminum transporter 1) (15), which is involved in lowering the concentration of toxic Al3+ in the root cell wall, which could be a key aspect of Al tolerance in rice (15, 16).
Recently, two members of the hydrangea (Hydrangea macrophylla) aquaporin (AQP) family, the H. macrophylla plasma membrane aluminum transporter (HmPALT1) and vacuolar aluminum transporter (HmVALT), have been suggested to facilitate Al transport across the PM into the cytosol and across the vacuolar membrane (VM) into the vacuoles of sepal (flower) cells, respectively (17, 18). The Al form transported by these transporters and the roles of the transporters in Hydrangea Al tolerance remain elusive, however. When heterologously expressed in Arabidopsis, only HmVALT enhances Al tolerance, suggesting that Al may enter the Arabidopsis root cytosol via an unidentified native PM transporter (17). Thus, in the present study, we investigated the role of the Arabidopsis nodulin 26-like intrinsic protein (NIP) subfamily, the closest homologs of HmPALT1, in Al transport (SI Appendix, Fig. S1). We report that NIP1;2 functions as a root PM-localized Al-malate (Al-Mal) transporter involved in a unique Al tolerance mechanism based on lowering root cell wall Al concentrations and facilitating root-to-shoot Al translocation. We demonstrate that these NIP1;2-mediated processes rely on a functional ALMT1-mediated malate release system. Therefore, the coordinated functioning of the external and internal Al detoxification mechanisms plays a critical role in Al tolerance in Arabidopsis.
Results
nip1;2 Mutants Are Sensitive Specifically to Aluminum Stress.
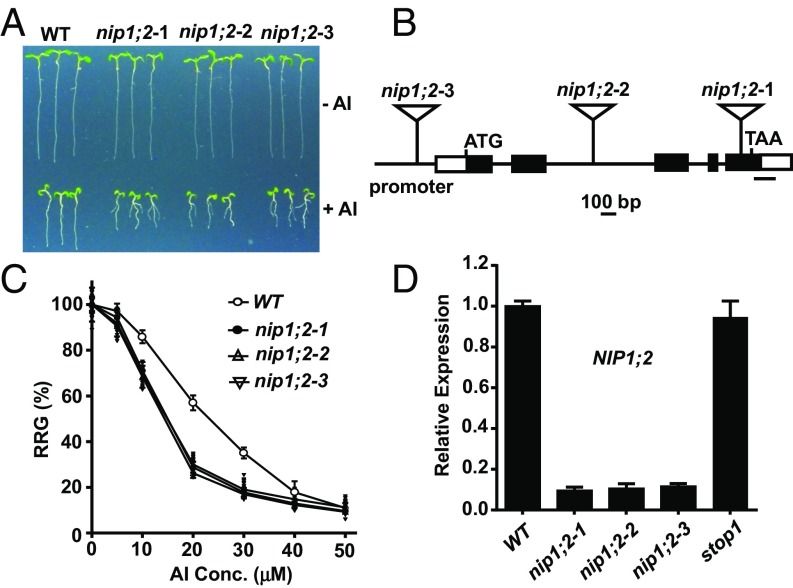
The Arabidopsis NIP subfamily comprises nine members (19). T-DNA insertion lines of the nine NIP members (SI Appendix, Table S1) were acquired from the Arabidopsis Biological Resource Center (ABRC) and tested for Al sensitivity. Three independent NIP1;2 T-DNA lines, nip1;2-1 (SALK_126593), nip1;2-2 (SALK_147353) and nip1;2-3 (SALK_076128), with T-DNA insertions in the exon, intron, and promoter, respectively, showed hypersensitivity to a range of Al concentrations (Fig. 1 A–C), suggesting that NIP1;2 is involved in Al tolerance in Arabidopsis. Compared with nip1;2 mutants, an almt1 T-DNA knockout (KO) mutant (SALK_009629) was more sensitive to Al stress in a range of Al concentrations (0–50 μM) at pH 4.2 (SI Appendix, Fig. S2).
Fig. 1.
NIP1;2 mutations sensitize mutant Arabidopsis plants to Al stress. (A) Al sensitivity of WT and three nip1;2 mutant lines. Seedlings were treated with 0 or 30 μM AlCl3 (pH 4.2) for 7 d. (B) Gene structure of NIP1;2. Box, exon; horizontal line, intron; closed box, coding sequence; triangle, T-DNA insertion; bar underneath the 3′ UTR, qRT-PCR amplification. (C) Relative root growth (RRG%) of WT and nip1;2 mutants under Al stress. (D) qRT-PCR analyses of root NIP1;2 expression in WT as well as nip1;2 and stop1 mutants. Data in C and D are mean ± SD of three biological replicates.
Real-time quantitative RT-PCR (qRT-PCR) analysis indicated that NIP1;2 expression in roots was barely detected in the nip1;2 mutants (Fig. 1D), indicating that they are loss-of-function mutants. In Arabidopsis, STOP1 encodes a master transcription factor that controls the expression of a set of key Al tolerance genes (20), including, but not limited to, ALMT1 (21), MATE (11), and ALS3 (21). NIP1;2 expression was not affected by the loss-of-function stop1 mutation, indicating that STOP1 does not control NIP1;2 gene expression (Fig. 1D). The nip1;2 mutants were hypersensitive specifically to Al stress, but not to other toxic metal ions, including Cd2+, La3+, Zn2+ and Cu2+ (SI Appendix, Fig. S3).
NIP1;2 Expression in Roots Is Rapidly and Specifically Enhanced by Al Stress.
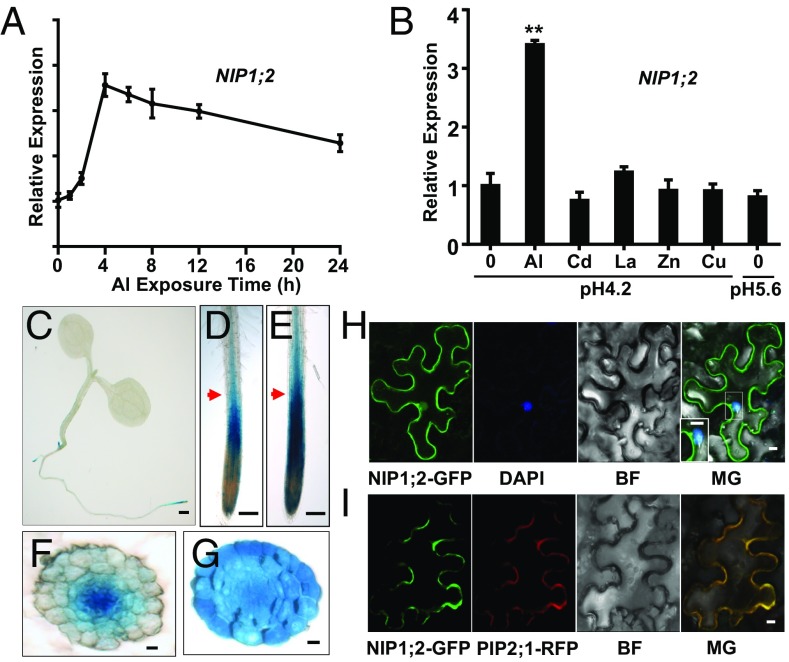
qRT-PCR analyses found NIP1;2 expressed mainly in the root and barely in the shoot (SI Appendix, Fig. S4). Time-course qRT-PCR analyses indicated that NIP1;2 expression was enhanced exponentially within the first 2 h and peaked with a 3.5-fold increase after 4 h of Al treatment (Fig. 2A). In addition, NIP1;2 expression was enhanced specifically by Al3+ but not by other cation metals, including Cd2+, La3+, Zn2+, and Cu2+, or by pH changes (Fig. 2B).
Fig. 2.
NIP1;2 expression patterns. (A) Time course qRT-PCR analysis of NIP1;2 gene expression in WT roots treated with 20 μM AlCl3 at pH 4.2. (B) qRT-PCR analysis of NIP1;2 expression in WT roots in response to different metal ions and pH changes. Here 7-d-old WT plants were treated with 20 μM AlCl3, 10 μM CdCl2, 5 μM LaCl3, 10 μM ZnSO4, or 5 μM CuSO4 at pH 4.2 for 6 h. The −Al experiment was conducted at pH 4.2 or pH 5.6 for 6 h. Asterisks indicate significant differences between 0 and 20 μM Al treatments (**P < 0.01). (C–G) Tissue-specific expression of NIP1;2. GUS activity driven by the NIP1;2 promoter in the whole plant (C) or root tips without (D) or with (E) 50 μM Al treatment for 8 h. Red arrows in D and E point to the positions of the cross-sections for - (F) or + (G) Al treatment, respectively. (Scale bars: 1 mm in C, 150 μm in D and E, and 10 μm in F and G.) (H and I) NIP1;2 is localized to the PM. (H) Confocal laser scanning microscopy of tobacco epidermal cells expressing 35Spro:NIP1;2::GFP. The nucleus (blue) was stained with DAPI. (I) Coexpression of NIP1;2-GFP (green) and the PM marker PIP1;2::RFP (red) in a tobacco epidermal cell. (Scale bar: 10 μm.) BR, brightfield; MG, merged.
Tissue Specificity of NIP1;2 Expression.
Tissue specificity of NIP1;2 expression was examined via NIP1;2 promoter β-glucuronidase (GUS) analysis in transgenic Arabidopsis plants. GUS activity was observed consistently in roots but not in shoots (Fig. 2C). In the primary and lateral roots, GUS activity was localized mainly to the root tip region (Fig. 2C). Under control conditions (−Al), GUS staining was observed between ∼300 μm and ∼1 mm from the root tip (Fig. 2D), a region encompassing the root distal transition zone (RDTZ) and the root elongation zone (REZ), a demonstrated target for Al toxicity (22). GUS staining was also observed in the stele in more mature root regions where root hairs had emerged (Fig. 2D). Al treatment not only significantly enhanced GUS staining intensity in these regions (Fig. 2E), but also caused expanded GUS staining to the nearby tissues/cells that were not stained under the −Al condition (Fig. 2E). For example, in a root cross-section above the RDTZ (indicated by the red arrows in Fig. 2 D and E), GUS staining was observed only in the stele under −Al (Fig. 2F), but was expanded throughout the entire root cross-section under Al treatment (Fig. 2G).
NIP1;2 Is Localized to the Plasma Membrane in Planta.
A NIP1;2-green fluorescence protein (GFP) fusion construct was transiently expressed in epidermal cells of tobacco (Nicotiana benthamiana) leaves driven by a 35S promoter. NIP1;2-GFP fusion protein was localized to the extreme cell periphery of epidermal cells (Fig. 2H) and was colocalized with the red fluorescence protein (RFP)-PIP2;1, a known PM marker (Fig. 2I) (23). The localization of the DAPI-stained nucleus to the cytoplasmic side of the NIP1;2-GFP fluorescence excludes the possibility of a VM localization for NIP1;2 (Fig. 2H). Examination of the root cells of T3 Arabidopsis plants stably transformed with 35S::NIP1;2-GFP also indicated that the NIP1;2-GFP fusion protein was localized to the PM (SI Appendix, Fig. S5). Taken together, these results indicate that NIP1;2 is a PM-localized protein.
NIP1;2 Is Involved in Root Al Uptake in Arabidopsis.
Hematoxylin was used to stain and approximate Al accumulation in the root cell walls of the wild type (WT), nip1;2–1, and nip1;2–2 seedlings (SI Appendix, Fig. S6) (24). After Al treatment, hematoxylin staining was observed mainly in the RDTZ and REZ. Compared with the staining in WT, the staining was much stronger and expanded in the root tip region of the nip1;2 mutant plants (SI Appendix, Fig. S6), suggesting that the nip1;2 mutants hyperaccumulated Al in the root cell walls in the root tip region.
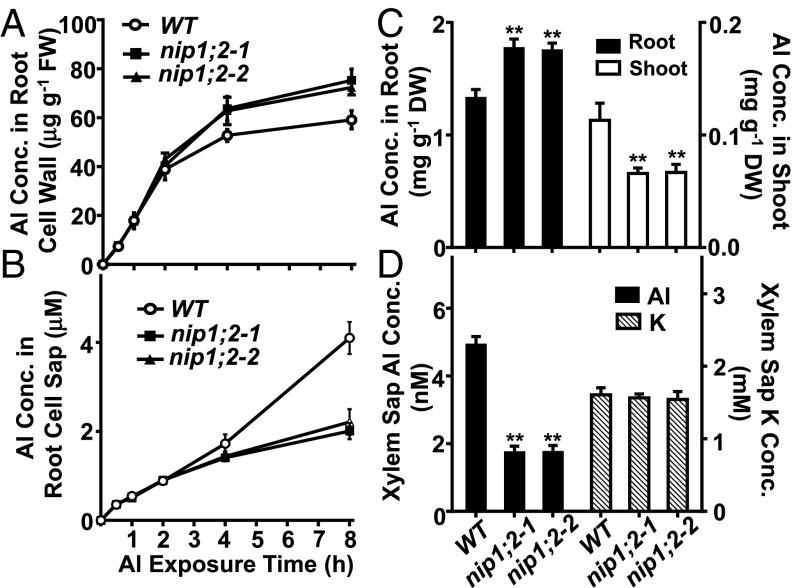
Quantitative measurements of the short-term (0–8 h) root Al uptake by inductively coupled plasma mass spectrometry (ICP-MS) indicated that compared with concentrations in WT, Al concentrations in the root cell wall were significantly higher and Al concentrations in the root cell sap (symplasm) were significantly lower in the nip1;2 mutants after 8 h of Al treatment (Fig. 3 A and B). These results indicate that NIP1;2 is involved in root Al uptake/removal from the root cell wall into the root cytosol.
Fig. 3.
NIP1;2-mediated Al uptake and distribution. (A and B) Al uptake by NIP1;2 in Arabidopsis roots. WT, nip1;2-1, and nip1;2-2 plants (7-d-old) were exposed to 30 μM AlCl3 (pH 4.2) for the indicated times. Al concentrations in the root cell wall (A) and the root cell sap (B) were determined by ICP-MS. Data are mean ± SD of three biological replicates from three magenta boxes. (C) Root and shoot Al concentrations of WT and nip1;2 mutants treated with 30 μM AlCl3 for 8 h. (D) Al and K concentrations in xylem sap. Here 6-wk-old WT and nip1;2 mutant plants were treated with 30 μM AlCl3 (pH 4.2) for 8 h. Xylem sap samples were collected from five plants, and Al or K concentrations were measured using ICP-MS. Data are mean ± SD of three xylem sap samples. **P < 0.01 between WT and individual nip1;2 lines under the indicated treatment conditions. DW, dry weight; FW, fresh weight.
NIP1;2 Is Involved in Root-to-Shoot Al Translocation.
Along with its expression in the root apical region, NIP1;2 was also expressed in the root stele just distal to the root tip (Fig. 2 D and F), suggesting that it also might be involved in long-distance Al transport. Compared with WT, the nip1;2 mutants exhibited significantly higher Al concentrations in the root but lower Al concentrations in the shoot (Fig. 3C). Furthermore, Al concentrations in the root xylem sap were also significantly lower in the nip1;2 mutants, whereas K concentrations in the root xylem sap were comparable in WT and the nip1;2 mutants (Fig. 3D). Compared with WT, in the nip1;2 mutants, Al concentration was 46% lower in the root cell sap (root symplasm; Fig. 3B) and 65% lower in the root xylem sap (Fig. 3D). In the nip1;2 mutants, the larger decrease in Al concentration in root xylem sap compared with that in root symplasm suggests impaired xylem loading of Al. Therefore, NIP1;2 not only may facilitate Al uptake from the root cell wall to the root cytosol, but also may be involved in xylem loading of Al from the xylem parenchyma cells into the xylem vessels in Arabidopsis.
NIP1;2 Facilitates Passive Bidirectional Aluminum Transport in Yeast.
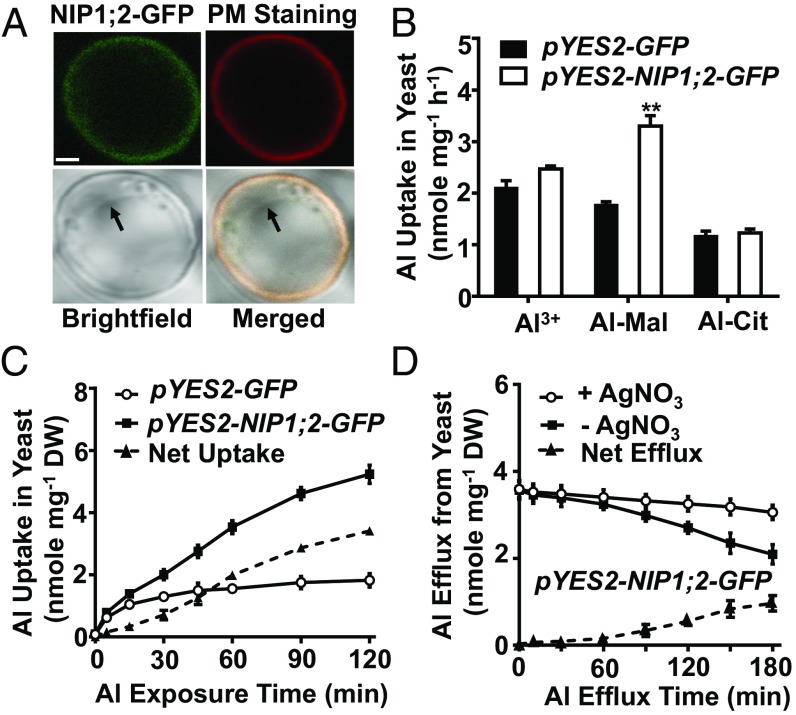
It has been widely accepted that members of the AQP family, including those from the NIP subfamily, transport noncharged molecules (25, 26), although recent studies have suggested that some members of the AQP family could transport charged substrates (27, 28). However, the major Al species at low pH (4.2) is the charged Al3+ ion (29). To identify the Al form transported by NIP1;2, we conducted short-term Al uptake assays with yeast lines expressing the pYES2-GFP (as a control) or the pYES2-NIP1;2-GFP construct with the potential transport substrates Al3+, Al-malate (Al-Mal), or Al-citrate (Al-Cit) at pH 4.2. Microscopic examination of the galactose-induced pYES2-NIP1;2-GFP line indicated that the NIP1;2-GFP fusion protein was localized to the yeast PM (Fig. 4A).
Fig. 4.
NIP1;2 facilitates Al uptake and efflux in yeast. (A) NIP1;2-GFP (green) was colocalized with red CellMask PM Staining (Thermo Fisher Scientific) in a yeast cell carrying pYES2-NIP1;2::GFP. (Scale bar: 1 μm.) Arrows points to the VM. (B) Uptake of Al3+, Al-malate (Al-Mal), and Al-citrate (Al-Cit) by NIP1;2. Yeast lines carrying pYES2-GFP or pYES2-NIP1;2-GFP were treated with AlCl3, Al-Mal, or Al-Cit at pH 4.2 for 1 h. (C) Time-dependent Al-Mal uptake by NIP1;2. Yeast lines were treated with Al-Mal for the indicated times. (D) Time-dependent Al efflux by NIP1;2. Yeast cells carrying pYES2-NIP1;2-GFP were pretreated with Al-Mal for 8 h, then transferred to fresh LPM medium supplemented with 0 or 5 μM AgNO3 at pH 7.0 for the indicated times. Yeast Al concentration was determined by ICP-MS. The net Al uptake (C) and Al efflux (D) at each time point was the difference between two yeast lines (C) or two treatments (D). Data are mean ± SD of three biological replicates from three independent transformation events. **P < 0.01 between two yeast lines under the indicated treatment conditions.
Under galactose induction, no significant differences in Al uptake among these yeast lines were observed when Al3+ or Al-Cit was supplied in the uptake medium (Fig. 4B); however, a significant increase in Al uptake was observed when Al-Mal was supplied (Fig. 4B). When glucose was used in the medium, which is unable to induce the expression of the recombinant proteins, no Al uptake was observed in the yeast line expressing NIP1;2-GFP irrespective of the form of Al provided (SI Appendix, Fig. S7). Short-term Al-Mal uptake assays further confirmed that NIP1;2 transports Al-Mal in a time-dependent (Fig. 4C) and concentration-dependent (SI Appendix, Fig. S8) manner. Other known cytosolic metal ligands, including oxalate, succinate, fumarate, aconite, histidine, glutathione, phytochelatin, and metallothionein, were also tested for NIP1;2-mediated Al-ligand transport in yeast. NIP1;2 transports only Al-Mal in yeast at pH 4.2 or 7.0 (SI Appendix, Fig. S9).
To test the NIP1;2-mediated Al efflux, we pretreated the NIP1;2-GFP-expressing yeast cells with Al-Mal for 8 h (Fig. 4B), then transferred them to fresh LPM medium with or without a supplementation with 5 μM AgNO3, a potent AQP inhibitor (30). Short-term Al efflux from the yeast cells was monitored by ICP-MS. Al efflux from the yeast cells was observed in a time-dependent manner, and was strongly suppressed by AgNO3 at both pH 7.0 (Fig. 4D) and pH 4.2 (SI Appendix, Fig. S10). Taken together, these findings indicate that NIP1;2 facilitates passive bidirectional Al transport in yeast (Fig. 4 C and D).
Positively Charged Al3+ Ions, but Not the Al-Malate Complex, Are Retained in the Root Cell Wall.
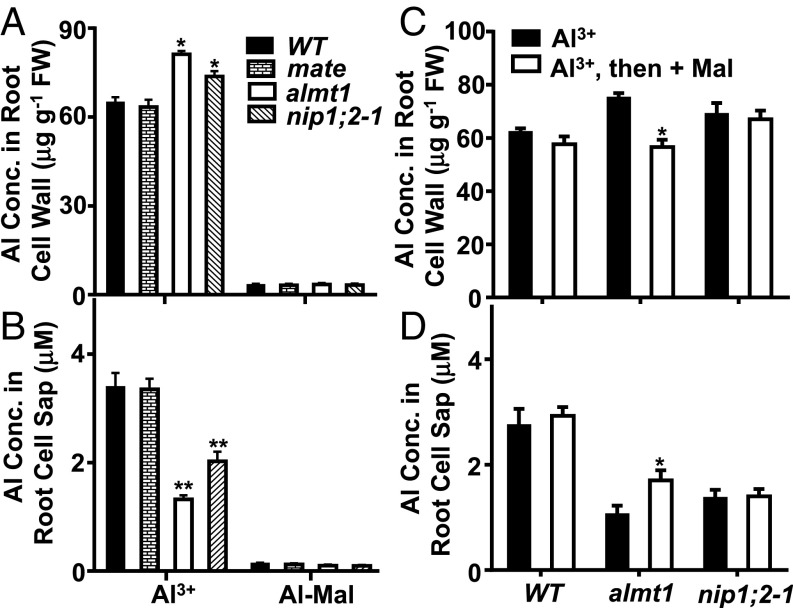
After treatment with 50 μM AlCl3 or 50 μM AlCl3 + 150 μM Malate (Al-Mal) at pH 4.2 for 8 h, Al concentrations in the root cell wall (Fig. 5A) and the root cell sap (Fig. 5B) were measured in WT as well as in mate, nip1;2–1, and almt1 KO mutants using ICP-MS. Little Al was detected in the root cell wall (Fig. 5A) or the root cell sap (Fig. 5B) for all lines treated with Al-Mal; however, large amounts of Al were detected in the root cell wall of all lines exposed to Al3+ (Fig. 5A). The concentrations of Al3+ and Al-Mal remained stable in the treatment solutions during the entire treatment process (SI Appendix, Fig. S11), indicating that Al-Mal in the solution was not precipitated during the treatment procedure. These results indicate that Al3+, but not Al-Mal, could be retained in the root cell walls.
Fig. 5.
NIP1;2-facilitated root Al uptake requires a functional ALMT1-mediated root malate exudation system. (A) Al3+ ions, but not Al-Mal, are retained in the root cell wall. Here 7-d-old seedlings were treated with AlCl3 or Al-Mal (pH 4.2) for 8 h, after which Al concentrations in the root cell wall (A) and root cell sap (B) were measured by ICP-MS. (C and D) Externally supplied malate partially restored NIP1;2-mediated Al uptake in the almt1 KO mutant. Here 7-d-old seedlings were pretreated with AlCl3 (pH 4.2) for 8 h, washed three times with 0.5 mM CaCl2 , and then treated with 200 μM malate (−Al) for 8 h. Al concentrations in the root cell wall (C) and root cell sap (D) were determined by ICP-MS. Data are mean ± SD of three sample replicates from three magenta boxes. In A and B, *P < 0.05, **P < 0.01) between WT and almt1 or nip1;2–1 under the indicated treatment conditions; in C and D, *P < 0.05 between two treatments for almt1.
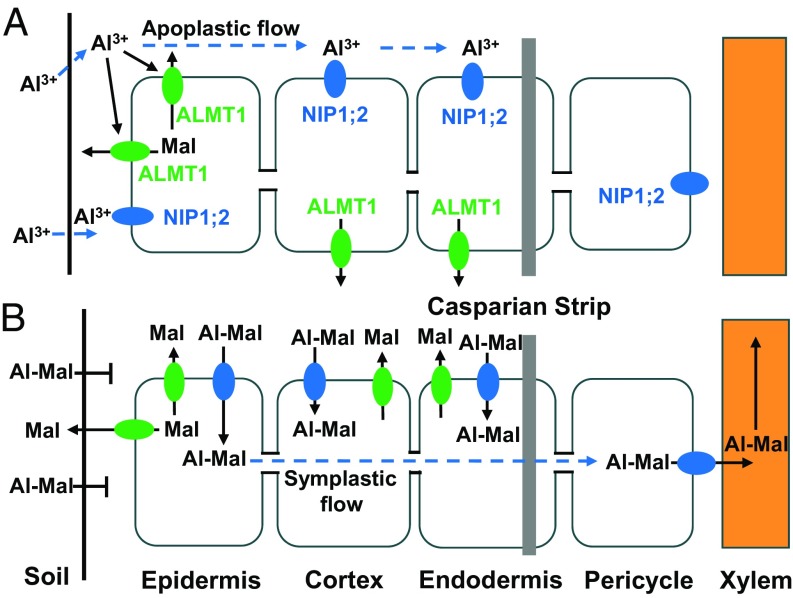
In yeast, NIP1;2 transports Al-Mal, but not Al3+ (Fig. 4B); however, in Arabidopsis, NIP1;2-mediated Al uptake occurred only when WT plants were exposed to Al3+ (Fig. 3 A and B), not when they were exposed to Al-Mal (Fig. 5 A and B). This finding raised a question as to whether NIP1;2 transports Al3+ or Al-Mal in Arabidopsis. We hypothesize that the main reason for this finding is that the PM-localized NIP1;2 can transport Al-Mal only from the root cell wall, whereas the externally supplied Al-Mal cannot be retained in the root cell wall (Fig. 5A). Therefore, we predict that Al3+ retention in the root cell wall is an indispensable first step for the NIP1;2-mediated Al transport in Arabidopsis (Fig. 6). The Al3+ retained in the root cell wall adjacent to the PM activates the ALMT1-mediated malate efflux from the root cytosol into the root cell wall, resulting in formation of Al-Mal in the root cell wall near the NIP1;2 transport protein, which facilitates Al-Mal uptake into the root cytosol (Fig. 6). Therefore, the sequential steps of retention of Al3+ in the root cell wall, Al3+-activated and ALMT1-mediated root malate efflux, and functional NIP1;2-mediated Al-Mal transport could be the key factor in the removal of Al from the root cell wall into the root cytosol (Fig. 6).
Fig. 6.
Schematic diagrams of the coordinated functions of ALMT1 and NIP1;2 in Al tolerance in Arabidopsis. (A) Toxic Al3+ ions that enter the root cell wall activate ALMT1-mediated malate release from the root cells into the root apoplast and rhizosphere, where nontoxic Al-Mal complexes form. (B) Al-Mal in the rhizosphere is unable to enter the root apoplast, whereas the Al-Mal formed within the root cell wall is subjected to NIP1;2-mediated uptake into the root cytosol. Once inside the root cells, Al-Mal moves across the Casparian strip of endodermal cells through symplastic flow into xylem parenchyma cells, where NIP1;2 facilitates xylem loading of Al-Mal into the xylem vessels, followed by translocation of Al-Mal to the shoot. The vertical gray bar on the endodermis represents the Casparian strip.
Functional ALMT1-Mediated Malate Exudation Is Required for Root Al Uptake via NIP1;2.
To test this hypothesis and the possible roles of root malate and citrate exudation in NIP1;2-facilitated Al transport in Arabidopsis, root Al uptake was evaluated for the WT and nip 1;2-1, almt1, and mate KO mutant plants. The nip 1;2-1, almt1, and mate mutant lines lack functional NIP1;2, Al-activated root malate exudation, and Al-activated root citrate exudation, respectively. Under Al treatment, nip1;2-1 and almt1 mutants accumulated significantly higher concentrations of Al in the root cell wall (Fig. 5A) and significantly lower concentrations of Al in the root cell sap (Fig. 5B) compared with WT and the mate mutant, indicating that both nip1;2-1 and almt1 were impaired in Al transport from the root cell wall across the root cell PM.
Given the lack of Al-activated ALMT1-mediated root malate exudation in the almt1 background (10, 11, 14), Al3+ should be a major Al form retained in the root tip cell wall of the almt1 plants treated with AlCl3 at low pH (4.2). Because the almt1 mutant line has a functional NIP1;2, as indicated by its normal expression of intact NIP1;2 cDNAs in the root (SI Appendix, Figs. S12 and S13), the lack of NIP1;2-mediated root Al uptake in the almt1 background (Fig. 5 A and B) suggests that NIP1;2 could not transport the Al3+ ions retained in the root cell walls of the almt1 plants. The mate mutant line accumulated similar levels of Al in the root cell wall and root cell sap as WT (Fig. 5 A and B), further confirming that the MATE-mediated root citrate exudation is not required for Al removal from the root cell wall in Arabidopsis.
To provide direct evidence for Al-Mal as the transport substrate of NIP1;2 in Arabidopsis and to address the role of root malate release in the NIP1;2-mediated root Al uptake, roots of WT, almt1, and nip1;2 plants were pretreated with 50 μM AlCl3 (pH 4.2) for 8 h, which allowed retention of Al3+ in the root cell wall (Fig. 5A). Then the pretreated plants were transferred to fresh hydroponic growth medium (−Al, pH 4.2) supplemented with or without 200 μM malate for another 8 h, which allowed the formation of Al-Mal in the root cell wall (Fig. 5C). Subsequently, Al uptake was measured by ICP-MS. Only the almt1 mutant line exhibited significant differences in Al concentrations in the root cell wall (Fig. 5C) and root cell sap (Fig. 5D) (i.e., evidence for Al uptake) between the treatments with and without malate. The fact that an external supply of malate resumed NIP1;2-facilitated Al uptake in the almt1 mutant pretreated with Al confirmed that the NIP1;2 Al transporter was functional in the almt1 background and Al-Mal is the transport substrate of NIP1;2 in Arabidopsis.
Discussion
In this work, we have demonstrated that NIP1;2 is a bidirectional Al transporter that facilitates the removal of Al from the root cell wall via trans-PM transport into the root cell cytosol and subsequent root-to-shoot Al translocation via Al xylem loading. We also have shown that the Al-malate complex is the transport substrate for NIP1;2, and that the NIP1;2-mediated root Al uptake requires a functional ALMT1-mediated root malate exudation system. Therefore, coordination between the Al exclusion and the internal Al tolerance mechanisms is required to achieve overall Al tolerance in Arabidopsis.
NIP1;2 Is Involved in Aluminum Removal from the Root Tip Cell Wall and Root-to-Shoot Al Translocation.
The cell wall in the root rip region has been recognized as a major target for Al toxicity in plants (22, 31, 32). It has been suggested that the swollen and distorted root tip cells under Al stress are due, at least in part, to disruption of the cell wall structure, integrity, and function (SI Appendix, Fig. S6) (32–34). Therefore, limiting the accumulation of toxic Al3+ in the root tip cell wall could help mitigate Al toxicity in plants. This could be achieved by restricting Al3+ retention in the root cell wall and/or by removing Al from the root cell wall via transport into the root cytosol and subsequent Al sequestration in root cell vacuoles and/or Al translocation from the root to presumably less Al-sensitive shoot tissues. The formation of Al-OA complexes in the rhizosphere is the most effective way to prevent the toxic Al3+ ions from entering the root cell wall (Fig. 5A) (35).
The mechanisms underlying Al uptake from the root cell wall and subsequent sequestration into root and shoot vacuoles are more poorly understood in plants. We have shown that NIP1;2 is a PM-localized bidirectional Al transporter that facilitates Al removal from the root cell wall and Al translocation from the root to the less Al-sensitive shoots (Figs. 3 and 4). A nonfunctional NIP1;2 resulted in impaired root Al uptake, reduced root-to-shoot Al translocation, and hypersensitivity to Al stress, indicating that NIP1;2 plays an important role in Al tolerance in Arabidopsis (Figs. 1 and 3). We also note that nip1;2 mutant plants still accumulate significant amounts of Al in the xylem sap (Fig. 3D) and root symplasm (Fig. 5 B and D), suggesting the possible existence of other root Al transport systems.
NIP1;2 Transports Aluminum-Malate Complex.
It is widely accepted that members of the AQP family, including the NIP subfamily, transport noncharged molecules (25, 26). However, at low pH (4.2), the major Al species is the charged Al3+ ion (29). A set of possible transport substrates, including Al3+, Al-Mal, and Al-Cit, were tested for NIP1;2-mediated Al transport in yeast (Fig. 4B and SI Appendix, Fig. S9). Only the provision of Al-Mal resulted in NIP1;2-mediated Al uptake at pH 4.2 or 7 (SI Appendix, Fig. S9), identifying Al-Mal as the transport substrate for NIP1;2 in yeast.
In Arabidopsis, Al stress induces a rapid and large ALMT1-mediated malate exudation from the root tip (10, 11). The loss-of-function mutant almt1 lacks the Al-activated root malate exudation (10, 11), and no Al-Mal is formed in the root tip cell walls thus under Al3+ stress. Because the almt1 mutant contains a functional NIP1;2 transporter, it is an excellent system for testing whether NIP1;2 transports Al-Mal in Arabidopsis. Under Al3+ treatment, like the nip1;2 mutant line, the almt1 mutant had greatly reduced root Al uptake (Fig. 5 A and B), indicating that NIP1;2 could not transport Al3+ in the almt1 background. However, an external supply of malate, which allowed the formation of Al-Mal in the root cell wall of the almt1 plants pretreated with Al3+, resumed a degree of NIP1;2-mediated Al transport activity in the root tips of the almt1 plants (Fig. 5 C and D), confirming Al-Mal as the transport substrate for NIP1;2 in Arabidopsis. Because nip1;2 mutants are loss-of-function mutants (Fig. 1), externally supplied malate could not restore NIP1;2-mediated root Al uptake in these mutants (Fig. 5 C and D). The reason for no effect of externally supplied malate on Al uptake in WT is that in WT, Al3+ treatment triggers ALMT1-mediated root malate exudation (10, 11), which allows for the formation of the Al-Mal transport substrate in the root cell wall. Thus, under Al3+ stress, malate was not a limiting factor for NIP1;2-mediated Al-Mal transport in WT. The fact that WT and mate had lower levels of Al in the root cell wall and higher levels of Al in the root cell sap compared with nip1;2 and almt1 mutants (Fig. 5 A and B) indicates that ALMT1-dependent and NIP1;2-mediated Al transport from the root cell wall into the cytosol is functional in the WT and mate backgrounds.
Passive Bidirectional Aluminum Transport by NIP1;2.
The processes of root Al uptake and root-to-shoot Al translocation require coordinated transport systems that facilitate Al influx from the root cell wall into the symplasm of root cells, Al efflux across the PM of xylem parenchyma cells into the xylem vessels, and subsequent Al translocation from the root to the shoot through transpirationally driven xylem flow (Fig. 6). We have demonstrated that NIP1;2 is involved in a passive bidirectional Al transport in yeast (Fig. 4 C and D). The fact that the loss-of-function nip1;2 mutants exhibited reduced Al accumulation in the root symplasm (i.e., an Al influx activity; Fig. 3B) and reduced xylem loading of Al (i.e., an Al efflux activity; Fig. 3D) suggests that NIP1;2 also could be involved in bidirectional Al-Mal transport for root Al-Mal uptake and root Al-Mal xylem loading in Arabidopsis. Although ligand exchange reactions could occur after Al-Mal is transported into the root cytosol, among the possible cytosolic Al ligands tested, Al-Mal was the sole transport substrate for NIP1;2 at pH 4.2 and 7.0 (SI Appendix, Fig. S9), suggesting that Al-Mal is likely to be the transport substrate for NIP1;2 during root Al-Mal xylem uploading. Members of the NIP subfamily have been reported to function as passive, bidirectional transporters for metalloid transport. For instance, NIP3;1, NIP5;1, and NIP6;1 are responsible for bidirectional As(III) transport across the PM (36, 37). Therefore, NIPs might be functionally conserved as passive bidirectional transporters for different substrates.
Coordinated Functioning Between External and Internal Aluminum Detoxification Mechanisms.
The external and internal Al detoxification mechanisms are responsible for the total Al resistance; however, how these two mechanisms interact and communicate in plants remains unknown. In this report, we demonstrate that Al-activated, ALMT1-mediated root malate exudation plays a dual role in both the Al exclusion and internal Al tolerance mechanisms. At the rhizosphere, the released malate chelates the toxic Al3+ ions, forming an Al-Mal complex, thereby preventing Al from entering and/or being retained in the root cell walls (i.e., root Al exclusion; Figs. 5A and 6). In the root tip cell wall, the released malate chelates the Al retained in the cell wall to form Al-Mal, which is the transport substrate for NIP1;2 (internal Al detoxification; Figs. 5 and 6). Therefore, we have discovered a coordinated operation between Al exclusion and Al internal tolerance mechanisms linked by ALMT1-mediated root malate exudation and NIP1;2-mediated Al uptake from the root cell wall (Figs. 3, 5, and 6).
Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis T-DNA insertion lines were acquired from the ABRC. Detailed information on these lines and their growth conditions is provided in SI Appendix, Materials and Methods.
GUS Staining Assays.
A 1.89-kb PCR-amplified NIP1;2 promoter was cloned into the pCAMBIA1305.2 vector, and the resulting pCAMBIA1305.2-NIP1;2promoter::β- glucuronidase (GUS) construct was stably transformed into WT (Col-0) through Agrobacterium tumefaciens (strain GV3101)-mediated transformation. Detailed information about the plasmid construction and the procedures for transgenic plant selection and GUS staining is provided in SI Appendix, Materials and Methods.
RT-PCR and qRT-PCR.
Total RNA isolations were conducted using the RNeasy Mini Kit (Qiagen). Additional experimental details are provided in SI Appendix, Materials and Methods.
Yeast Al Uptake and Efflux Analyses.
Yeast line generation, yeast growth conditions, and yeast Al uptake assays are described in detail in SI Appendix, Materials and Methods.
Total Root and Shoot Ion Content Measurement.
Al and other ions were measured in the roots and the shoots of 7-d-old seedlings by ICP-MS analyses. The procedures are described in detail in SI Appendix, Materials and Methods.
Determinations of Al Contents in Cell Sap and Cell Wall Samples.
Details on plant treatment, cell sap and cell wall sample preparation, and Al measurement are provided in SI Appendix, Materials and Methods.
Xylem Sap Sample Collection and Al Content Measurement.
Xylem sap was collected from 6-wk-old plants. Al contents were measured by ICP-MS. Plant treatment, xylem sap sample collection, and Al measurement are described in detail in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Kudla for providing the pGPTV-II-GFP vector, the ABRC for providing the T-DNA insertion lines, M. Srivastava for help with image analysis, and J. Shaff for help with the GEOCHEM-EZ analysis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618557114/-/DCSupplemental.
References
- 1.Kochian LV, Piñeros MA, Liu J, Magalhaes JV. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:571–598. doi: 10.1146/annurev-arplant-043014-114822. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Piñeros MA, Kochian LV. The role of aluminum sensing and signaling in plant aluminum resistance. J Integr Plant Biol. 2014;56:221–230. doi: 10.1111/jipb.12162. [DOI] [PubMed] [Google Scholar]
- 3.Von Uexküll H, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. [Google Scholar]
- 4.Wood S, Sebastian K, Scherr S. Pilot Analysis of Global Ecosystems: Agroecosystems. World Resources Institute; Washington, DC: 2000. [Google Scholar]
- 5.Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007;264:225–252. doi: 10.1016/S0074-7696(07)64005-4. [DOI] [PubMed] [Google Scholar]
- 6.Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan PR, Delhaize E, Randall PJ. Characterisation of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- 8.Magalhaes JV, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa J, et al. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- 10.Hoekenga OA, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 12.Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- 13.Sasaki T, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, et al. A promoter-swap strategy between the AtALMT and AtMATE genes increased Arabidopsis aluminum resistance and improved carbon-use efficiency for aluminum resistance. Plant J. 2012;71:327–337. doi: 10.1111/j.1365-313X.2012.04994.x. [DOI] [PubMed] [Google Scholar]
- 15.Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA. 2010;107:18381–18385. doi: 10.1073/pnas.1004949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J-Y, et al. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc Natl Acad Sci USA. 2014;111:6503–6508. doi: 10.1073/pnas.1318975111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negishi T, et al. Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS One. 2012;7:e43189. doi: 10.1371/journal.pone.0043189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negishi T, Oshima K, Hattori M, Yoshida K. Plasma membrane-localized Al-transporter from blue hydrangea sepals is a member of the anion permease family. Genes Cells. 2013;18:341–352. doi: 10.1111/gtc.12041. [DOI] [PubMed] [Google Scholar]
- 19.Johanson U, et al. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iuchi S, et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA. 2007;104:9900–9905. doi: 10.1073/pnas.0700117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawaki Y, et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150:281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z-B, et al. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell. 2014;26:2889–2904. doi: 10.1105/tpc.114.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson BK, Cai X, Nebenführ A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007;51:1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x. [DOI] [PubMed] [Google Scholar]
- 24.Polle E, Konzak C, Kattrick J. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci. 1978;18:823–827. [Google Scholar]
- 25.Maurel C, Verdoucq L, Luu D-T, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 26.Forrest KL, Bhave M. Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Funct Integr Genomics. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- 27.Rambow J, Wu B, Rönfeldt D, Beitz E. Aquaporins with anion/monocarboxylate permeability: Mechanisms, relevance for pathogen-host interactions. Front Pharmacol. 2014;5:199. doi: 10.3389/fphar.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrt CS, et al. 2016. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca(2+) and pH. Plant Cell Environ, 10.1111/pce.12832.
- 29.Smits MM, Hoffland E. Possible role of ectomycorrhizal fungi in cycling of aluminium in podzols. Soil Biol Biochem. 2009;41:491–497. [Google Scholar]
- 30.Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- 31.Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–163. [Google Scholar]
- 32.Sivaguru M, Liu J, Kochian LV. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013;76:297–307. doi: 10.1111/tpj.12290. [DOI] [PubMed] [Google Scholar]
- 33.Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann Bot (Lond) 2010;106:185–197. doi: 10.1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma JF, Shen R, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 2004;45:583–589. doi: 10.1093/pcp/pch060. [DOI] [PubMed] [Google Scholar]
- 35.Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- 36.Bienert GP, et al. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, et al. Arabidopsis NIP3;1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol Plant. 2015;8:722–733. doi: 10.1016/j.molp.2015.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.