Abstract
An important theme that emerges from all early historical accounts is that in addition to the decreased virulence of Treponema pallidum, the incidence of secondary syphilis has decreased drastically over the past three centuries. Even in the early 20th century, most syphilologists were of the opinion that the disease had undergone changes in its manifestations and that they were dealing with an attenuated form of the spirochete. Such opinions were based primarily on the observations that violent cutaneous reactions and fatalities associated with the secondary stage had become extremely rare. The rate of primary and secondary syphilis in the United States increased in 2002 for the second consecutive year. After a decade-long decline that led to an all-time low in 2000, the recent trend is attributable, to a large extent, by a increase in reported syphilis cases among men, particularly homosexual and bisexual men having sex with men. The present review addresses the clinical and diagnostic criteria for the recognition of secondary syphilis, the clinical course and manifestations of the disease if allowed to proceed past the primary stage of disease in untreated individuals, and the treatment for this stage of the disease.
INTRODUCTION
Historical Perspectives Regarding Secondary Syphilis
Among etiologic agents and their respective infectious disease entities, Treponema pallidum and syphilis unquestionably have one of the richest recorded histories known to humankind. Although biblical and ancient Chinese writings are consistent with descriptions of late syphilis, the relationship between the cutaneous eruptions of secondary syphilis and the primary lesions or genital sores does not seem to have been recognized until late in the 15th century (40, 66). The disease, first termed morbus gallicus (Gallic [or French] disease) and later named lues venerea (the venereal pest) prior to being called syphilis, swiftly swept across Europe in the 15th and 16th centuries as if it alone were one of the Horsemen of the Apocalypse. The origin of that epidemic, which coincided with the return of Columbus' sailors to Barcelona from the New World in 1493, remains shrouded in uncertainty and controversy. Whereas many historians have speculated that the disease was transmitted from natives in the West Indies and carried back to the nonimmune populations of Europe, thus favoring the “New World origin of syphilis” (39, 66, 68), there is convincing evidence that the disease arose via mutational events from a spirochetal disease similar to yaws that had existed for centuries in central Africa (43). While attempts to resolve the issue concerning the introduction of syphilis into Europe are beyond the scope of this review, there is little doubt concerning the fierceness of its first strike on the European continent.
The incidence of syphilis across all strata of society during the first 50 years of its reign in Europe was tremendous, and the lesions, described by Fracastoro (29) and later by the layman Ulrich von Hutten, were characteristically much more severe than those described today (20, 32, 43, 72). Syphilis even caused substantial mortality, with a death rate approaching 20 to 40%.
The first book dealing with syphilis, In Pustulis Malas Morbum quem Malum de Francia vulgus appellat, que sunt de genere Formicarum (The Disease of Bad Pustules, also Popularly Called the French Malady, which is Formicarum), was published by Konrad Schelling in 1495 to 1496 (40). Other early written accounts in the period 1496 to 1497 include De Pustulis et Morbo qui Vulgo Mal de Franzos appellatur (Of Pustules and Other Disease which is Commonly Called the French Malady) by Hans Widmann and De Pustulis quae Asphati nominatur (Of Pustules which are called Asphati) by Juan de Fogueda. It is worthy of special note that, in Tractatus de Pestilentia Scorra (Tractate on the Scorra Pestilence, 1498), Joseph Grunpecks accurately described the lesions of primary and secondary syphilis and their incubation periods. Three decades later, the name of the disease, syphilis, was coined by the Italian physician Girolamo Fracastoro in his tale of the shepherd Syphilus, who contracted the disease after insulting the pagan Sun god. Another three centuries, however, would pass before the term would gain both medical and general acceptance. In 1564, Gabriele Falloppio recharacterized the primary chancre in his classic work De Morbo Gallico (The French Disease) (40, 66).
Two centuries later, in 1738, Jean Astruc compiled perhaps the most comprehensive history of syphilis and venereal disease in De Morbis Venereais (On Venereal Disease). In the late 18th century, the English physician John Hunter (1728 to 1793) was regarded as a great authority on this subject. He accepted the prevailing notion that the gonorrheal discharge and syphilitic ulcer were caused by the same venereal infection. To test his theory, he undertook a brave but foolish experiment, inoculating himself with a gonorrheal discharge from a patient who, in retrospect, also was infected with syphilis. Hunter contracted both syphilis and gonorrhea, thus serving as a living example of his misguided theory that the two were manifestations of a single venereal disease.
Writing in Philadelphia in 1823, Samuel Hahnemann, the famous German homeopathic physician, disagreed with Hunter. He realized that the natures of the two venereal “miasms” were different, and he called the gonorrheal one “sycosis” after the Greek word for “fig.” Nevertheless, the unitary theory of veneral disease held its ground without further challenge until 1837, when the eminent French venereologist Phillipe Ricord showed the specificity of the two diseases through a better controlled series of experimental inoculations from syphilitic chancres. Another French physician, Jean-Louis Marc Alibert, is credited with introducing the term “dermatoses syphilids” for cutaneous lesions (42). A few years later, Laurent Théodore Biett, a leading clinical teacher, devised the classification of syphilitic eruptions which is still the basis of all current classifications. Ricord, however, was the first to clearly differentiate primary, secondary, and tertiary syphilis, the three stages of infection, and to describe the characteristic skin lesions of each stage. Other invaluable contributions were made throughout the late 19th century, such the description of the pathology of syphilis by Rudolf Virchow in 1859, in his Ueber die Natur der Constitutionellsyphilitischen Affectionen (On the Nature of Generalized Syphilitic Infection). Virchow, a leading advocate of the modern germ theory, described and attempted to explain clinical variations in latent and active periods of disease. He is also credited with having discovered the systemic nature of syphilitic infection and its transmissibility by blood exposure. In 1879, at the beginning of the golden age of bacteriology, Albert Neisser provided bacteriologic confirmation of the separate nature of gonorrhea; Neisseria gonorrhoeae was subsequently named for him. Jean Alfred Fournier, one of the greatest syphilologists of the 19th century, studied the relationship of syphilis to social problems. His La Syphilis Hereditaire Tardive (Late Appearing Congenital Syphilis) in 1886 includes a discussion of syphilis as a cause of degenerative diseases (40, 42).
An important theme that emerges from all these early historical accounts is the spontaneous diminution in the virulence of syphilitic infection to the level that we recognize today. In the early 20th century, most syphilologists were of the opinion that the disease had undergone changes in its manifestations and that they were dealing with an attenuated form of the spirochete (42). Such opinions were based primarily on the observations that violent cutaneous reactions and fatalities associated with the secondary stage had become extremely rare by the early 1900s. Explanations other than a decrease in virulence are certainly possible. Among these, many of the early historical accounts of the preceding centuries may have been inaccurate. Many of the patients with secondary syphilis may have actually been coinfected with other microbial agents, making diagnosis extremely difficult in the absence of both a known etiology and confirmatory laboratory tests. It seems quite plausible that coinfections with smallpox and other chronic infectious diseases such as hepatitis and tuberculosis may have adversely impacted the presentation, morbidity, and mortality attributed to secondary syphilis in those early accounts. Irrespective of whether one questions the decreased virulence of the organism in the first three centuries following its advent, T. pallidum does not appear to have continued its journey toward avirulence over the past 100 years. There is no scientific evidence or proof that the Nichols strain or street strains are less virulent today than they were at the turn of the 20th century.
Status of Secondary Syphilis in the 20th Century
In addition to the decreased virulence of the organism, the incidence of syphilis has decreased drastically over the past three centuries, with the major changes occurring with the dawning of the antibiotic era. At the turn of the 20th century, the prevalence of syphilis was a matter of considerable discussion and uncertainty. Although some estimates were as high as 10% (42), it is likely that the incidence was about 2 to 5% of the population when both sexes and all races were taken into consideration. Such an incidence would be consistent with the rate estimated by Fournier and others in the late 1800s. Hazen's analysis in 1928 estimated that there were about 8 × 106 syphilitics in the United States, and Parran estimated that there were approximately 681,000 new cases each year in the United States (42, 65).
It is perhaps unfortunate that as a consequence of our fast-paced technoelectronically based lives, detailed accounts of the cause of syphilis seem relatively unimportant and, in the realm of things, are often relegated to pages of forgotten history. The stories that are told in those accounts not only represent more than four centuries of medical thought but also illustrate how close many of the distinguished bacteriologists of the late 1800s and early 1900s, (Metchnikoff, Roux, Neisser, Bordet, and Gengou) came to discovering the true cause of syphilis (20, 32, 45, 72). In 1905, Schaudinn and Hoffmann successfully identified Spirochaeta pallida as the etiologic agent of syphilis (74), which in turn led to over 700 publications in the next 2 years confirming their findings. Despite numerous attempts, fulfillment of Koch's postulates, except for cultivation of the organism on artificial medium, was delayed until it was achieved by Noguchi 1911 (63).
The turn of the 20th century also witnessed new classifications of the cutaneous lesions of secondary syphilis based on factors such as the time of appearance and the clinical manifestations of the lesions. Fournier, based on Alibert's earlier classification, distinguished between lesions of the skin proper and those of mucous membranes. Mucous membrane lesions included those involving the nostrils, lips, mouth, tongue, palate, tonsils, and throat. Those early classifications led Gerorge Henry Fox in 1913 to suggest to the American Dermatological Association (that the lesions of secondary syphilis be divided into two groups: the early and late secondary syphilids (40, 42). Official approval of that classification scheme by the American Dermatological Association followed a year later. The term “syphilid,” or welt, although used throughout the first half of the century, never really gained wide acceptance by physicians and epidemiologists and is only infrequently used today to denote a syphilitic lesion. Various types of early and late secondary lesions in accord with that classification are presented in Tables 1 and 2. The major problem with this approach is that there is no sharp demarcation between the stages of syphilis and that the division of the disease into stages is a means of simplifying clinical presentation.
TABLE 1.
Characteristics of early secondary lesions
| Type of lesion | Characteristic appearance | Distribution on the body | Differential diagnosis |
|---|---|---|---|
| Macular | A pale pink, flat, and somewhat elliptical spot usually 4 to 8 mm in diameter. These may be violaceous in darker-skinned individuals. The central area is always more highly colored, whereas the periphery tends to blend into the surrounding skin. The reddish hue, due to localized hyperemia, resolves under pressure. Though probably the most common type of cutaneous lesion, these are often overlooked or misdiagnosed. | These lesions never appear on the face, in contrast to all other types of syphilids. | Erythema muliforme, seborrheic dermatitis, pityriasis rosea, tinea versicolor, tinea corporis, leprosy, measles, German measles, typhoid fever (Rose spots), toxic dermatitis, and dermatitis medicamentosa (drug rashes). |
| Small papular | Small, distinct, localized elevations of skin which are readily palpated. Solid, rounded, resistant to touch. Varies in color depending on skin pigmentation. May assume a pustular character with no exudate. In most areas such as the genital areas, the groin, and the axilla, they may appear in the form of flat condylomas. | Most common; favor the trunk. May appear on any part of the body, including flexor surfaces of the limbs, forehead, and temples. These lesions often border the scalp in the form of the so-called crown of Venus. | Erythema multiforme, psoriasis, pityriasis, keratosis, variola, lichen planus, papulonerotic tuberculid, urticaria pigmentosa, leprosy, mycosis fungoides, and adenoma sebaceum. |
| Follicular or pseudovesicular | Round or pointed papules which develop around the orifices of hair follicles and sweat glands. Vary in size from pinpoint to pinhead. Vary in color depending on skin pigmentation. Pigmentary deposits are frequent. Scaling often appears at the apex of the lesion. May also be involuted. Frequently larger in the genital and anal regions. Itching may be frequent due to sweat decomposition. | May appear on any part of the body. These lesions tend to group and are generally most abundant on the back, upper trunk, and arms. They are also frequently found on the thighs and face. | Lichen planus, lichen scrofulosorum, lichen nitidus, tineal dermatitis, seborrheic dermatitis, pityriasis rubra pilaris, keratosis pilaris, and lichen spinulosus. |
| Lichenoid | Flattened and angulated lesions resembling lichen planus. | May appear on any part of the body. These lesions also are generally most abundant on the back, upper trunk, and arms. | Lichen planus. |
| Vesicular | Pointed, very small, and ruptured only with difficulty. Reddish, raised base without an inflammatory areola. Often found in combination with small papular lesions. May constitute a transient intermediate type of lesion. Existence is questionable, as the vesicles are of short duration and often present for only a few hours. | May appear on any part of the body. | Erythema multiforme, psoriasis, pityriasis, keratosis, variola, lichen planus, papulonerotic tuberculid, urticaria pigmentosa, leprosy, mycosis fungoides, and adenoma sebaceum. |
| Psoriasiform | These lesions resemble those of psoriasis in color distribution and scaling. They differ from psoriasis in that the lesions never bleed when the scale is removed. | Found predominantly on the palms of the hands and soles of the feet. May also occur on the face, elbows, and knees. The scalp is exempt. | Psoriasis. |
| Corymbiform | Appearance of a nipple with a well-marked areola. These tend to be very unusual in that they are large lesions or plaques surrounded by a number of smaller lesions. | May appear on any part of the body. | No other dermatologic disease causes this type of lesion. |
TABLE 2.
Characteristics of late secondary lesions
| Type of lesion | Characteristic appearance | Distribution on the body | Differential diagnosis |
|---|---|---|---|
| Large Papular | Much like the small papular variety except that they then to be flattened, although distinctly elevated and less round. Marked tendency toward grouping and plaque formation. Seldom crowded together; often sparsely scattered. May coalesce to form lesions suggestive of tuberculids. | May appear on any part of the body. Predilection for the forehead, face, back of the neck, limb bends, and inner thighs. | Psoriasis, pityriasis, keratosis, variola, pityriasis lichenoides chronica, lichen planus, papulonecrotic tuberculid, urticaria pigmentosa, nodular leprosy, mycosis fungoides, erythema multiforme, and adenoma sebaceum. |
| Annular | Papular syphilids which have a circinate configuration. One form appears to develop from a single papule, spreading peripherally and forming a ring or taking the form of gyrate patches. A number of papules may unite to form a hollow or solid ring, giving rise to the second type. Usually annular are sparsely distributed. | May appear on any part of the body. Predilection for the mucocutaneous junction of the nasal alae and the oral comissures. Usually not on the limbs. Unusual in Caucasians. | Tinea corporis, seborrheic dermatitis (annular type), impetigo contagiosa, pityriasis rosea, erythema multiforme, granuloma annulare, chronic urticaria, and psoriasis. |
| Condylomata lata | These begin as ordinary papules which become flattened, macerated, and covered with a thick, tenancious, mucoid exudate. Like annular syphilids, they appear in two forms; one is a flat, moist papule varying in size but excoriated in the center, and the elevated (verrucous) or cauliflower type is large, not excoriated, and grayish in appearance and has a vile odor. | Most frequently occur around the rectum, on the scrotum and vulva, as well as in the groin. | Verruca vulgaris, lymphogranuloma inguinale, granuloma inguinale, intertriginous (mycotic) dermatitis, lichen planus, pyodermia, aphthae, pemphigus, epithelioma, and tuberculosis. |
| Pustular | The typical pustule is indolent, originating as a vesicle. Initially the lesion resembles a papule covered with scales. It then becomes flaccid and ruptures. If a scab forms, beneath it is a punched-out ulcer surrounded by a slight inflammatory arcola, usually with a livid or bluish tingue. These contain very little pus and tend to group into gyrate configurations. If no scab forms, the unencrusted lesions simply appear as punched-out ulcerations. | May appear on any part of the body. Predilection for the face, especially the nose, the flanks and thighs, the palms of the hands, and the soles of the feet. | Variola, pyodermia, acne serofulosorum and cachecticorum, ecthyma, acne necrotica, papulonecrotic tuberculids, acne varioliformis, psoriasis, tineal (corporis) dermatitis, dermatitis medicamentosa, impetigo contagiosa, and acne vulgaris. |
| Rupial | Really large pustules which have piled-up crusts. These are typically an encrusted, impetiginous eruption without an inflammatory areola. | May appear on any part of the body. | Variola, pyodermia, acne scrofulosorum and cachecticorum, ecthyma, acne necrotica, papulonecrotic tuberculids, acne varioliformis, psoriasis, tineal (corporis) dermatitis, dermatitis medicamentosa, impetigo contagiosa, and acne vulgaris. |
| Frambesiform | A hypertrophic type of papular lesion characterized by raspberrylike growths of various shapes and sizes. These lesions are moist, violaceous, and softly verrucous with a high serous content and an offensive odor. | May appear on any part of the body. Predilection for the face and scalp, especially the mouth or nose. Also found in the axilla or the anal and genital regions. | Fungating condylomas. |
| Pigmentary | These lesions vary in size and are not raised above the skin's surface. They may be hypopigmented (depigmented) or hyperpigmented. | May appear on any part of the body. Predilection for the arms and trunk. | Vitiligo, dermatitis medicamentosa, tinea versicolor, xeroderma pigmentosum, erythema aboigne, factitial dermatitis, scleroderma, leprosy, chloasma, residual inflammatory dermatitis, and pigmented scars. |
We have already alluded to the diminishing virulence of syphilis in the late 19th and early 20th centuries. A second factor that, in the 20th century, has had a major impact on the evolution of syphilitic infection has been treatment. Through trial and error, Ehrlich discovered that arsphenamine was effective in treating syphilis (66). Treatment was long, arduous, and fraught with complications but was largely successful; for example, 6 months of daily injections might be utilized to treat primary infection. Thus, the discovery of penicillin and its application to treating syphilis represented a major advance. In fact, the widespread, and often indiscriminate, use of this miraculous new antibiotic in the late 1940s and early 1950s had a remarkable effect on the prevalence of active syphilitic infection in all stages (19).
One of the great ironies of medical science is that the availability of remarkably effective treatment for syphilis did not succeed in eliminating it, supporting the theory that eradication of disease is more depending on education and prevention than on therapy. In the last decade of the 20th century, the United States essentially stood alone as the only industrialized country that had failed to control the disease. In the late 1980s and early 1990s, the rate of incidence of syphilis reached its highest level in 40 years (8, 70). In 1989, 18.4 in 100,000 Americans were treated for syphilis, up from the 13.7 cases per 100,000 treated in 1981. The 1989 rate was higher than at any time since 1949. By the end of 1990, the total number of reported cases had peaked at slightly over 134,000, resulting in a rate of 200 per 100,000 (69). The number of new cases has declined in the past few years. One recent report has even suggested that the decline in syphilis in men in the 1990s may be attributed to AIDS mortality (16). Roughly 16,500 and 11,400 cases of primary and secondary syphilis, respectively, were reported for 1995 and 1996 (8). The rate of decline in reported primary and secondary syphilis from 1990 to 1997 was 84% (9). Despite that decline, the number of reported cases of primary and secondary syphilis increased slightly in 2001.
We have known for the last 60 years that, in the United States, syphilis was largely an affliction of minority groups in the population. Data from the Selective Service Boards during World War II indicate that significantly higher rates existed in African-Americans: 25% versus 1.7% in Caucasians (80). Although the overall rate of infection has declined substantially, an even greater racial differential persists today. The rate for African-Americans in the last decade was nearly six times the rate of Caucasians, with a rate of 46.2 per 100,000 Americans (9). Syphilis remains a major sexually transmitted disease (STD) health problem, particularly in Southern states, as well as in developing countries. The association with other STDs such as human immunodeficiency virus (HIV) infection increases the prevalence and complexity of the problem (9). In fact, since syphilis enhances HIV transmission, prevention of syphilis is considered very important for controlling HIV infection.
RECOGNITION AND TREATMENT OF SECONDARY SYPHILIS
General Considerations
To fully appreciate the differentiation of this chronic disease into early and late infection, one must begin with the primary or initial lesion. At 14 to 21 days after inoculation of Treponema pallidum into a dermal site, a red, painless papule 0.5 to 2 cm in diameter appears at the site of inoculation. Within a few days the papule ulcerates, producing the typical chancre of primary syphilis, an ulcerated area sometimes covered by a slight yellowish or grayish exudate and surrounded by a slightly indurated margin. Remarkably, considering their location, syphilitic chancres are painless. They are generally round, although they may be elongated, following tissue lines. Modest enlargement of inguinal lymph nodes, frequently bilaterally, is observed in the majority of patients who have genital lesions. Although solitary lesions were once said to be characteristic, multiple lesions frequently occur (14, 42).
Because of their venereal origin, primary syphilitic chancres occur most frequently in the genital, perineal, anal, or oral area; however, any part of the body may be affected. Most chancres are found on the penis of men and on the labia, fourchette, or cervix of women. Chancres in the anus or rectum are particularly common in men who have sex with other men (MSM). When these lesions cause pain on defecation or rectal bleeding, they can be confused with hemorrhoids or even a neoplasm (23), but they often go unnoticed, as do those on the labia and cervix. In most instances, active syphilis in women and in MSM is usually not diagnosed until the secondary stage.
If untreated, syphilitic chancres heal spontaneously within 3 to 8 weeks and disappear. A local immune reaction, rather than a generalized one, accounts for the healing, because secondary lesions regularly appear during or after the regression of the primary one(s). Differentiating a syphilitic chancre from chancroid, the soft chancre caused by infection with Haemophilus ducreyi, may be impossible on clinical grounds, although a great degree of tenderness, a purulent exudate over the lesion, or striking inguinal lymphadenopathy with thin and shiny overlying skin is suggestive of chancroid. Simple trauma, especially to the penis, or a fixed drug eruption may cause lesions resembling chancre. Lesions of herpes simplex virus infection usually are not difficult to differentiate from syphilitic chancres, but coinfection by both organisms may occur (42, 62). The clinical criteria for the recognition of secondary syphilis and the clinical manifestations of the disease are presented in Tables 3 and 4, respectively.
TABLE 3.
Clinical criteria for recognition of secondary syphilis
| Generalized eruptions, especially if indolent, associated with generalized lymphadenopathy and otherwise vague signs of disease |
| Eruptions that are universal, with the exception of macular rashes, and symmetrically distributed, almost always involving the face and forehead (individual lesions tend to be indurated; the color may vary considerably, most often presenting as a subdued red rather than a bright red lesion) |
| Macular eruptions highly associated with papules on the genitalia or within the oral cavity |
| Papular lesions on the palms of the hands or the soles of the feet and, in the absence of dermatitis, elsewhere on the body and involvement of the genitalia |
| Generalized macular or papular lesions that persist for more than 1 week and are associated with a sore throat |
| Generalized pustular or follicular lesions in the absence of oral and genitalia involvment |
| Vesicular lesions, which, although uncommon, are not rare in darker-skinned subjects |
| Secondary syphilitic lesions, which tend to disappear without leaving permanent scars (depigmentary changes, although infrequent, tend to be permanent, whereas hyperpigmentation changes are not) |
| Generalized lymphadenopathy, uniformly associated with secondary syphilis |
TABLE 4.
Clinical manifestations of secondary syphilisa
| Rash |
| Condyloma latum in intertriginous areas |
| Lymphadenopathy |
| Hepatitis (subclinical) |
| Systemic: fever, malaise, weight loss |
| Neurologic: headache, meningismus, meningitis, cranial nerve disorders (optic neuritis, deafness, otitis), cerebrovascular accident |
| Periostitis |
| Uvieitis, iritis |
| Glomerulonephritis |
| Arthritis |
| Alopecia |
Early neurosyphilis, as seen in HIV-infected persons, is specifically not included (see the text).
Clinical Course
In untreated individuals, treponemes proliferate in the chancre and are carried via lymphatics to the bloodstream, from which they disseminate throughout the body (42, 62). The time at which the secondary lesions make their appearance basically depends on two factors: the virulence of the treponeme and the systemic response of the host. Pathologically the cutaneous lesions of secondary syphilis can be regarded as local reactions induced in highly susceptible tissue by metastatic accumulations of treponemes. As such, the blood vessels and perivascular lymphatics play an important role in their epidermal and subepidermal development. Our inability to readily propagate this organism in vitro has made it difficult to examine components that contribute to tissue damage, much less define which ones may be causative versus associated. Despite numerous studies aimed at identifying and characterizing treponemal polypeptides, particularly membrane-associated proteins (reviewed in reference 64), the roles of adhesins, secreted enzymes, and other factors in the pathogenesis of T. pallidum remain unclear.
The generalized lymphadenopathy that results from the passage of treponemes through satellite glands or buboes of the primary chancre into the lymphatic channels sets the stage for subsequent events in untreated individuals. Contrasted with the regional involvement seen in primary syphilis, early secondary syphilis involves all lymphatic structures. The lymphadenopathy is typically without any relationship to cutaneously involved areas. Lymphatic involvement usually runs an indolent course similar to that seen with the primary or bubo stage. There is a tendency for the nodes to become firm and painless for weeks or months prior to spontaneous resolution.
Neurologic manifestations that appear in early syphilis have received increased attention, especially because of the relationship with concurrent HIV infection, which is discussed in detail later in this review. Before the occurrence of HIV infection, abnormalities in the cerebrospinal fluid (CSF)—increased white blood cell counts, elevated protein level, a positive Venereal Disease Research Laboratory (VDRL) test, or the presence of viable T. pallidum organisms—were detected in up to 40% of patients with secondary syphilis in the absence of neurologic abnormalities (2). However, no more than 1 to 2% of patients with secondary syphilis were found to have symptoms or signs of central nervous system involvement, including meningismus, meningitis, headaches, and mental changes; cranial nerve abnormalities such as ocular palsy, deafness, or nystagmus, and internuclear ophthalmoplegia; cerebrovascular accidents; or signs of spinal cord or nerve root involvement such as tingling, weakness, and hyporeflexia. These findings are sometimes called early neurosyphilis. Before the penicillin era, they were included under the term “neurorecurrence” because they nearly always occurred in subjects who had received inadequate therapy for syphilis (61). There may be some degree of overlap between these neurologic manifestations of early secondary syphilis and some of the classic manifestations of late or tertiary neurosyphilis. It is important to note, however, that at this stage the symptoms are reversible with treatment. Progression to late neurosyphilis was uncommon, even in the prepenicillin era, if treatment was continued until the CSF returned to normal. As discussed later and reviewed elsewhere (61), both the frequency and the severity of neurologic involvement in secondary syphilis are greatly increased in persons with HIV infection.
Lesions of secondary syphilis result from the hematogenous dissemination of treponemes from syphilitic chancres. Thus, the term “disseminated syphilis” may be more appropriate (62). Typically, more than 3 weeks elapses between the deposition of T. pallidum in the dermis and emergence of disseminated lesions. This delay in onset relative to primary infection and the failure of the involved sites to develop into lesions that resemble primary chancres reflect a degree of humoral and/or cellular immunity capable of modifying the evolution of infection. Secondary-stage lesions generally appear 4 to 10 weeks after the initial appearance of primary lesions; however, in some patients who present with disseminated lesions there is an overlap, and a careful examination may disclose a primary chancre. Malignant syphilis (lues maligna), wherein disseminated lesions resemble primary chancres, is rare, despite several recent case reports in the literature (7, 21, 37, 52, 59, 71, 73, 78, 81). Host factors, other than often an association with HIV infections, that contribute to or allow this unusual manifestation of syphilis have not been identified. The tendency for the skin to be the most extensively involved of all organs may reflect the slightly lower temperature; rabbits that have been experimentally infected develop lesions only at sites where their fur has been shaved.
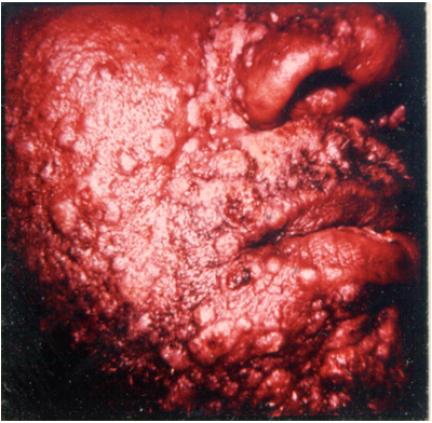
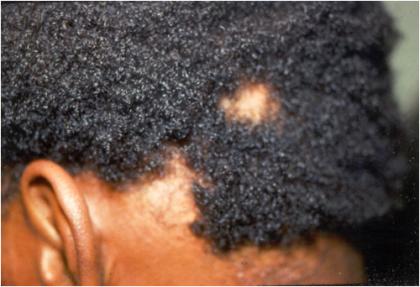
The initial finding in disseminated syphilis is an evanescent macular rash that is often overlooked. The macular lesions of early secondary syphilis differ from all other secondary lesions in several aspects (Table 5). Were it not for the fact that macular lesions are often overlooked or misdiagnosed, there is little question that they are probably the most common of all secondary lesions. A few days later, a symmetric papular eruption appears, involving the entire trunk and the extremities (Fig. 1), including the palms of the hands (Fig. 2) and the soles of the feet. They are generally scaly, although they may be smooth, follicular, or rarely, pustular. Vesicles usually do not occur, although vesiculopustular lesions are seen on rare occasions and are common on the palms or soles. Mucosal lesions are also quite common and characteristic of secondary syphilis. An example of malignant syphilis (lues maligna) is shown in Fig. 3. Alopecia also occurs in untreated cases, reflecting involvement of hair follicles (Fig. 4).
TABLE 5.
Key differences between macular lesions and all other forms of secondary lesions
| Macular lesions never appear on the face but predominate on the body and limbs |
| Macular lesions are transitory, generally lasting for only 2 or 3 days, as opposed to all other secondary lesions, which may last for several weeks |
| Treponemes are not present in macular lesions by dark-field examination |
| In the preantibiotic era, cutaneous Jarisch-Herxheimer reactions never occurred following arsenical injections |
FIG. 1.
Acutely inflamed, disseminated lesions in a patient with secondary syphilis. Photograph courtesy of the late John Knox. Reprinted from reference (33a) with permission of the publisher.
FIG. 2.
Pustular lesions on the palms of a patient with secondary syphilis. (Copyright John Knox.)
FIG. 3.
Malignant lues. (Copyright John Knox.)
FIG. 4.
Alopecia in a patient with secondary syphilis. (Copyright John Knox.)
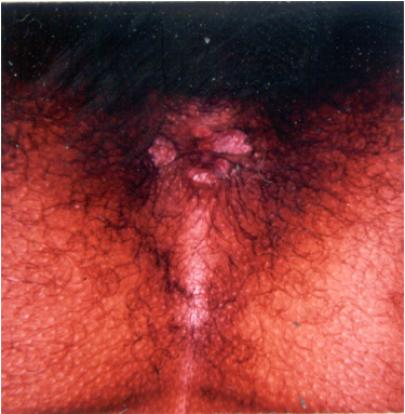
Condyloma latum refers to one or more large, raised, white or gray lesions found in warm, moist areas. These lesions, originally described as a manifestation of well-established secondary syphilis, reflect a local breakdown of secondary lesions with extension of infection in areas of tissue trauma, most frequently involving the axilla and groin (76). More common, at present, are condylomatous lesions that appear in the perineum or around the anus before or soon after the generalized lesions (Fig. 5). In this situation, treponemes have spread from a primary lesion and proliferated in a favorable environment. Such lesions represent an intermediate stage of infection because, strictly speaking, they do not reflect dissemination.
FIG. 5.
Condylomata in the perineal area of a patient with secondary syphilis. (Copyright John Knox.)
Secondary syphilis is typically a systemic disease, with the patient often presenting with a variety of symptoms, such as malaise, sore throat, headache, weight loss, low-grade fever, pruritus, and muscle aches, in addition to the dermatologic manifestations. Lymph node enlargement is present in the great majority of patients. Subclinical hepatitis can be detected in approximately 10% of all patients by appropriate laboratory studies and is supported by histologic findings (49). Symptomatic hepatitis is occasionally seen; however, one cannot be certain that this manifestation does not reflect concurrent viral hepatitis. Iritis, anterior uveitis, arthritis, and glomerulonephritis or nephrotic syndrome also occur in secondary syphilis. Circulating immune complexes that contain treponemal antigen and human fibronectin, together with antibody and complement, are present in this stage of infection, and their deposition in relevant organs is thought to play a role in the pathogenesis of these syndromes (3, 25, 48, 53).
Diagnosis
Virtually all patients who have secondary syphilis have antibody to cardiolipin, detectable in tests such as the Rapid Plasma Reagin (RPR) or VDRL tests (4). The RPR test is quantitated, and in the secondary stage it is usually reactive at a titer of ≥1:32. Antibody to treponemal outer membrane proteins, as measured in tests such as the MHA-TP (microhemagglutinating antibody to Treponema pallidum) test, is also uniformly positive. Although it is possible to detect treponemes in secondary syphilitic lesions, very few medical personnel are trained to do so, and the diagnosis remains clinical (based on the appearance) with serologic support. One can envision a scenario in which a misleading clinical diagnosis of a skin rash (e.g., infectious mononucleosis or chicken pox) can be associated with a so-called false-positive RPR test (one in which the positive reaction results from some disease other than syphilis); the erroneous diagnosis might thought to be supported by a positive microhemagglutination-T. pallidum (MHA-TP) test that has persisted from a remote bout of syphilis. Abnormal results of serologic testing (unusually high, unusually low, and fluctuating titers) have been observed among HIV-infected patients. For such patients, the use of other tests (e.g., biopsy and direct microscopy) should be considered. Serologic tests in general, however, appear to be accurate and reliable for the diagnosis of syphilis and for evaluation of treatment response for the vast majority of HIV-infected patients.
The natural history of untreated secondary syphilis is marked by spontaneous resolution after 3 to 12 weeks, leaving the patient free of lesions and of symptoms. If treatment has not been given, this naturally attained asymptomatic state is called latency. Latent syphilis is defined as the period after infection with T. pallidum in which patients are seroreactive but show no other evidence of disease.
Pathologic Changes
Irrespective of how the lesions of the second stage of syphilis are classified, it is important to remember that they represent different stages of a single process and are not independent lesions or mutually exclusive of one another. At one end of the spectrum, the changes in the macular lesions are initially slight, while the changes in the papular lesions are more well developed. At the opposite end of the spectrum are changes seen in the pustular and ulcerative lesions, which tend to be overdeveloped. At the far extreme are eruptions involving specialized pathological changes in the epithelium such as the condylomas and rupial and frambesiform lesions.
In general, the histopathologic pictures of all lesions, irrespective of classification, are essentially identical (42, 62). The cellular infiltrate consists primarily of lymphocytes, plasma cells, macrophages, some polymorphonuclear leukocytes, epithelioid cells, and occasional giant cells. The areas involved are the cutis, the lower portion of the rete malpighii, and in certain instances the cuticular appendages. The vessels around the lesions typically show inflammatory proliferative changes and cellular infiltrates in their walls, along with various degrees of dilatation.
The treponemal burden in the various lesions, with the exception of the macular lesions, constitutes additional proof that all lesions represent progressive stages of the same lesion.
Early Latency, Relapses, or Recurrent Secondary Eruptions
In the preantibiotic era, 25% of patients whose infection had become latent had a recrudescence of active, secondary syphilis within 12 months (17). According to the early literature, papular and pustular lesions exhibited a tendency to recur in groups forming definite ring patterns. The reappearance of lesions was indicative of an exacerbation of spirochetemia resulting from insufficient or improper treatment. Because the majority of the relapses occurred within 1 year of the onset of latency, this period was called early latency. Relapses after this time were rare, and so after 1 year without recurrence of disease and before the onset of tertiary syphilis untreated persons were said to have entered the late latent period. Patients with late latent syphilis are refractory to reinfection with T. pallidum (56). Although infection stimulates vigorous humoral and cellular immune responses that contribute to lowering the infectious burden in the tissues, there is little evidence in support of solid or protective immunity. Having syphilis once does not protect a person from getting it again. Following successful treatment, individuals are still susceptible to reinfection. Today, if one sees a recurrence of secondary-stage disease, it is more likely to be indicative of reinfection rather than of inadequate antibiotic therapy.
Manifestations in HIV-Infected Individuals
Somewhat surprisingly, there is little to suggest that the dermatologic manifestations of secondary syphilis are more striking in HIV-infected persons, nor has an increase in syphilitic hepatitis, arthritis, or osteitis been specifically documented. This may be due, in part, to the fact that the lesions of secondary syphilis are immunologically mediated, owing to deposition of circulating immune complexes (48). In contrast, concurrent HIV infection appears to have a profound impact on neurologic involvement in syphilis (28, 41, 50, 61). In the AIDS era, neurosyphilis has been seen often and in HIV-infected persons who are relatively young. Many cases have been described in the context of therapeutic failure with conventional doses of penicillin (see “Treatment” below); their importance in terms of our understanding the pathogenesis of syphilis in these subjects is just as great. Numerous individual case reports have documented the rapid progression of early syphilis to neurosyphilis, manifested in meningitis, optic neuritis, deafness, or the symptoms of nervous system disorders (35, 36, 38, 46, 60, 61, 84, 85). Cranial nerve defects appear with or without meningitis, and vasculitis with appropriate radiographic documentation has also been described, causing cerebrovascular accidents. Currently, from the standpoint of clinical diagnosis, it is important to realize that cases of early neurosyphilis, which were nearly unheard of before 1980, have become commonplace. Patients with this kind of disease may have AIDS or be at an earlier stage of infection with HIV. In many cases, the concurrent HIV infection was documented for the first time when neurologic complications of syphilis were recognized.
Diagnostic Criteria for Clinical Recognition
Secondary syphilis is clinically the least difficult to diagnose, with the possible exception of the macular rashes, which may be transient and disappear in the absence of specific therapy (42, 62). The macular rash of secondary syphilis may mimic a variety of dermatological diseases as well as several systemic conditions with cutaneous manifestations. Additionally, since the rashes may vary with age and race and the duration of the infection, they can be readily overlooked. The other disseminated lesions of secondary syphilis are certainly easy to recognize, although they may be confused with a variety of conditions, including, but by no means limited to, eczema, psoriasis, drug eruption, and pityriasis rosea. In general, physicians should be suspicious of any symmetrical, generalized eruption and should promptly consider an appropriate serologic test to help in the diagnosis. As stated above, the clinical criteria for recognition of secondary syphilis which basically have not changed over the last 60 years are listed in Table 5.
Laboratory Confirmation
As noted above, dark-field microscopic examination is actually the only test that specifically establishes the diagnosis of syphilis. Using this technique, a well-trained technician is likely to make the correct diagnosis in 100% of cases of untreated primary syphilitic chancre. Collectively, the treponemal and nontreponemal serologic laboratory tests that are used diagnostically are referred to as serologic tests for syphilis (4). The two nontreponemal tests, the RPR and VDRL tests (described above), measure antibody to anticardiolipin. These tests, developed as economical tests for mass screening, are not specific for T. pallidum. Therefore, a variety of acute and chronic conditions may result in biological false-positive reactions. Despite that fact, both tests are reactive in nearly all patients with secondary and latent syphilis (>98%). In contrast, the nontreponemal tests are less sensitive in patients with primary syphilis (4); titers are generally ≤1:8 and are reactive in only 80 to 86% of patients at the time they seek medical attention for the primary stage of the disease (4). Tests that measure antibody to surface proteins of T. pallidum by a hemagglutination assay (such as the T. pallidum hemagglutination assay or MHA-TP) also are highly specific (>98%) and sensitive in patients with secondary syphilis. Despite the increased specificities of these tests in patients with primary infections, they are less sensitive, yielding positive reactive in about 90 to 95% of such cases. In patients with primary syphilis, a negative result does not exclude the diagnosis. Nor does a positive MHA-TP establish the diagnosis, because once this antibody test becomes positive, it tends to remain so for life, a feature that largely limits its usefulness to excluding a diagnosis of syphilis if it is negative. Official recommendations are to repeat the RPR test if it does not return to normal within a year, and to re-treat the patient. The true meaning of such an occurrence is unclear, and persisting RPR reactivity after primary syphilis probably reflects reinfection or primary infection in someone who had already developed early latent syphilis.
Similar to the situation with primary syphilis, dark-field microscopic examination is actually the only test that specifically establishes the diagnosis of secondary syphilis. With the exception of macular lesions, all cutaneous and mucous membrane lesions of secondary syphilis contain treponemes. Thus, material for dark-field examination secured from an eroded moist lesion will uniformly yield positive results. Nevertheless, many laboratories do not have a working dark-field microscope, and secondary lesions are not easy to study by this technique. Therefore, the diagnosis of secondary syphilis remains largely clinical with support by serologic methods. In secondary syphilis, the RPR and VDRL tests are uniformly positive, nearly always at a high dilution (at least 1:32). Thus, a negative or nonreactive RPR or VDRL result in a patient with a disseminated rash that is thought to be syphilitic actually excludes the diagnosis. Most laboratories do not perform serum dilutions unless the physician indicates that the diagnosis of syphilis is suspected clinically; in this situation, the prozone phenomenon, in which a high concentration of antibody is present but is not detected in undiluted serum; may obscure a positive reading. HIV-infected subjects may have positive reactions with unusually high titers. Isolated case reports of secondary syphilis with a negative VDRL result in an HIV-infected patient (38) represent the rare exception that proves the rule.
To summarize, even though the MHA-TP test is highly sensitive and specific for T. pallidum infection, clinically it is not useful for establishing a diagnosis of syphilis in most situations because a positive result may reflect an earlier infection. The principal usefulness of the MHA-TP test is to obtain a negative result, which excludes any diagnosis of syphilis other than early primary disease. Serologic tests for syphilis, on the other hand, are neither specific nor highly sensitive, although positivity at high titer (≥1:16) generally signifies active infection with T. pallidum. Detecting treponemes by dark-field examination remains the surest way in which the laboratory can support a clinical diagnosis of syphilis. As a good general principle, consideration of one venereal disease should lead to consideration of another; for example, any person with syphilis should be studied for antibody to HIV, hepatitis B virus, and others, and vice versa.
Treatment
Treatment schedules for syphilis are given in standard textbooks as well as in brochures available from the Centers for Disease Control and Prevention and from state and city departments of public health. Treatment of secondary syphilis with 2.4 × 106 units of benzathine penicillin seems to cure the vast majority of patients, even though some authorities have recorded a serologic failure rate as high as 25% (53). Fiumara has claimed that two such treatments 7 days apart causes the VDRL result to return to negative in every instance, but he tended to exclude patients whose test remained positive, regarding them as having been reinfected (27). It seems quite clear that a small proportion of patients who are cured of their secondary syphilis retain low-grade VDRL reactivity throughout life (the so-called serofast state) (75). Although conventional wisdom recommends repeated treatment, it is unclear what the persistence actually means from biological point of view. These treatments probably do not produce a bacteriologic cure; treponemes persist in lymph nodes and the central nervous systems of treated patients and experimental animals (18, 82), a fact that becomes important in considering treatment of HIV-infected subjects.
If T. pallidum travels to seemingly privileged areas, such as the lymph nodes and central nervous system, where it escapes the normally lethal effects of penicillin, what might be expected in the presence of severe immunosuppression? Although one early report (30) suggested that HIV-infected patients with early syphilis respond slowly to 2.4 × 106 units of benzathine penicillin, two subsequent studies (35, 44) have shown that the clinical response of early syphilis to therapy is not altered by HIV infection, although serologic responses may be delayed (70). What is impressive is the number of cases in which treatment with benzathine penicillin is followed by rapid progression to neurosyphilis (1, 5, 47, 61), a clinical finding that has been supported by documentation of persisting treponemes in the CSF of HIV-infected patients (54, 55). It is not clear, however, that larger doses or repeated injections eliminate the problem of relapse to early neurosyphilis. Thus, treatment of secondary infection without neurologic involvement in HIV-infected patients should be with 2.4 × 106 units of benzathine penicillin; specifically, there is no benefit to adding oral amoxicillin and probenecid for 10 days (57).
If a macrolide (erythromycin or azithromycin) or tetracycline (doxycycline) is used because of a history of penicillin allergy, both the physician and the patient should be fully aware of the need for close monitoring and the concern about failure to cure neurologic disease, since penetration of the blood-brain barrier is poor and efficacy in preventing neurosyphilis is not established even in non-HIV-infected persons. For syphilis with neurologic (including ophthalmologic or otologic) involvement, 10 days of intravenous penicillin at 2.4 × 106 units per day is recommended. Alternative therapy, including ceftriaxone at 1 g per day, is equally effective and can be administered far more easily. Both of these regimens are associated with rates of failure plus relapse that approach 30% (22, 34). It is entirely possible that daily procaine penicillin for 10 to 14 days would be as effective. Clearly, with any treatment regimen, careful attention needs to be given both to the clinical response and to the subsequent serum VDRL reaction.
Before AIDS was recognized, isolated case reports showed that 2.4 × 106 units of benzathine penicillin may arrest all but the neurologic manifestations of secondary syphilis (79). This finding is consistent with the minuscule levels of penicillin that are present in the central nervous system after benzathine penicillin therapy (31). The rarity of neurosyphilis before 1980 argues that, in practice, recommended doses of penicillin were remarkably successful in curing syphilis, and it seems highly likely, in retrospect, that the relapses occurred in persons who had unrecognized HIV infection, especially when the actual case reports are read closely.
An unusual feature of the treatment of syphilis is the Jarisch-Herxheimer reaction. Although an adverse reaction to treatment has been postulated for a number of chronic infections, in no disease is this as well documented as it is in syphilis. In a prospective study, we (83) demonstrated that 79% of patients who were treated with penicillin for secondary syphilis developed malaise, fever (as high as 104°F in one case), and/or increased prominence of the lesions. The mechanism of this reaction remains uncertain, but it seems most likely that release of endotoxin or endotoxin-like constituents from treponemes is responsible.
SECONDARY SYPHILIS IN THE 21ST CENTURY
Syphilis, and especially its transmission, continues to be an important, complex, and perplexing public health problem, which in turn is only one facet of a larger problem involving all STDs. In the absence of a specific vaccine, federal, state, and local efforts are focused on the identification and targeting of high-risk groups, offering expanded screening and treatment programs for both those infected and their sex partners and instituting effective behavioral intervention and surveillance programs. Such is the case with the National Syphilis Elimination Plan initiated by the Centers for Disease Control and Prevention in 1999 with target efforts focused on syphilis in the southern United States and among minority populations (13, 77). The national goals of that plan are to reduce the annual number of primary and secondary syphilis cases to <1,000 cases (rate, 0.4 per 100,000 population) and to increase the number of syphilis-free counties to 90% by 2005. Syphilis elimination as defined in that report is the absence of sustained transmission (i.e., no transmission after 90 days of the report of an imported index case).
Despite continuing efforts such as those to curtail the transmission of syphilis and the other STDs, programs are often impeded by societal and human factors, which in turn make for a very convoluted problem. Clear associations between drug use and STD transmission exist (8, 9, 24, 58), especially among prostitutes and persons who exchange sexual favors for drugs. Even if the level of education is high enough to know about prevention of infection, concerns may be overridden by desperation and the lack of resources. Drug-addicted individuals in need of money to support their habits and homeless young people without education, job training, or means of support often turn to prostitution. These individuals often forego safe-sex demands, particularly condom use, as a result of both gender power imbalances and their economic dependence on prostitution (26). A strong distrust of both police and public health authorities may further complicate the situation and adversely affect screening and intervention programs. Treatment all too frequently represents a last resort, even if it is readily available.
Today, more than at any other time in our history, we are a highly mobile, global society, unrestricted by the borders of past eras. Thus, it may take only one “patient zero” (using terminology from world experience with HIV infection) today to set in motion an epidemic halfway round the world tomorrow. This translates into a need for continued vigilance aimed at detecting imported cases before they result in new outbreaks, a need that is underscored by the close association between codissemination of HIV infection and syphilis (9).
In the last 3 years, the rate of primary and secondary syphilis among men has continued to increase, especially among MSM. In fact, outbreaks among MSM in large U.S. cities are well documented (6, 10, 11, 12, 51, 67). That population also has a high rate of HIV coinfection, in addition to often practicing unsafe sex with increased risks. To single out the year 2002, it was estimated that >40% of cases reported occurred among MSM. A substantial number of cases of syphilis in MSM appear to involve meeting anonymous partners in venues such as bathhouses and meeting people in person who were first contacted in Internet chat rooms. These facts, as the Centers for Disease Control and Prevention acknowledges, not only represents a major challenge for the National Syphilis Elimination Plan but also warrant developing and assessing new strategic approaches for locating and treating sex partners. The declining infection rates among non-Hispanic African Americans and the increasing rate of infection among non-Hispanic Caucasians in the United States has decreased the disparity in rates of infection between the two populations. Furthermore, the decline among non-Hispanic African Americans has occurred predominantly in women whereas the increase among non-Hispanic Caucasians has occurred exclusively in men. Geographically, the disease continues to plague large U.S. cities, reflecting the fact that it is concentrated in urban areas. As is also acknowledged by CDC, reported findings with respect to demographics are subject to limitations. Chief among those limitations is the fact that the quality of surveillance data varies at both the local and state levels. Additionally, national reporting is incomplete since case reports are dependent on individuals having known sex partners and their willingness to identify their partners. The latter is borne out in a recent study which has suggested that anonymity may actually decrease the number of cases detectable by contact tracing (33).
Logically, preventive health measures should always begin with education. Like other STDs, syphilis has been and continues to be a disease of younger, sexually active individuals. The youth of our nation, besides being one of our richest natural resources, are also often disenfranchised and vulnerable. Teenagers continue to be at high risk for acquiring and transmitting STDs. In 1990 it was estimated that the rate of infection of teenagers with an STD was once every 12.6 s. Patterns of change over the last decade are not clearly evident. Today, two-thirds of all STDs occur in people younger than 25 years, one in four new STD cases occur in adolescents, and approximately half of all sexually active teenagers will contract a STD by age 25 years. Currently, education about HIV and other STDs is mandated in 38 states and the District of Columbia, whereas only 22 states and the District of Columbia require a broader sexuality education curriculum. Today there are 12 million children in the United States living below the poverty line, many of them the homeless “street kids” who, far too often, turn to prostitution following a history of childhood abuse or neglect. Adolescent pregnancy and birth rates in the United States, although having decreased over the last 10 years to a record low of 45.9 per 1,000 in 2001, remain by far the highest among industrialized nations. Many of our states continue to have deplorable teenage pregnancy rates; approximately 106 teenage girls in the United States become pregnant each year. Their babies are often of low birth weight and experience disproportionately high infant mortality rates in addition to the likelihood of being poor. All these factors, along with inaccessibility to health care in the late 1980s, clearly contributed to the surge in the numbers of new cases of congenital syphilis. As far as education goes, our youth need and deserve comprehensive sex education. Irrespective of how ugly the truth may be, denial will only result in long-term negative consequences.
History has taught us some rather important lessons regarding the transmission of syphilis and elimination of this disease. We can only optimistically hope that achieving the goals set forth in the National Syphilis Elimination Plan will be successful despite existing challenges posed by both teenagers and MSM. However, similar, albeit erroneous, predictions were made regarding the elimination of tuberculosis in the early 1990s. Reemerging infectious diseases and the development of antibiotic-resistant strains are now a matter of record. Thus far, we have been spared the latter with regard to T. pallidum. Realistically, worldwide intervention, surveillance, and prevention programs cannot be expected to eliminate the disease completely, even if such programs were feasible. If we lower our resolve and adopt an attitude that an understanding of the immunology of disease and a vaccine are relatively unimportant, the consequences could be drastic.
Acknowledgments
This work was supported by Merit funds from the Department of Veterans Affairs.
REFERENCES
- 1.Bayne, L. L., J. W. Schmidley, and D. S. Goodin. 1986. Acute syphilitic meningitis. Its occurrence after clinical and serologic cure of secondary syphilis with penicillin G. Arch. Neurol. 43:137-138. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, T. J., E. V. Price, and J. C. Cutler. 1952. Spinal fluid examinations among patients with primary or secondary syphilis. Am. J. Syph. 36:309-•••. [PubMed] [Google Scholar]
- 3.Baughn, R. E., M. C. McNeeley, J. L. Jorizzo, and D. M. Musher. 1986. Characterization of the antigenic determinants and host components in immune complexes from patients with secondary syphilis. J. Immunol. 136:1406-1414. [PubMed] [Google Scholar]
- 4.Baughn, R. E., and R. F. Schell. 2001. Immune responses to Treponema pallidum and Borrelia burgdorefi, p. 27.1-27.7. In R. R. Rich (ed.), Principles and practice of clinical immunology, 2nd ed. Mosby, St. Louis, Mo.
- 5.Berry, C. D., T. M. Hooton, A. C. Collier, and S. A. Lukehart. 1987. Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N. Engl. J. Med. 6:1587-1589. [DOI] [PubMed] [Google Scholar]
- 6.Bronzan, R., L. Echavarria, J. Hermida, M. Trepka, T. Burns, and K. Fox. 2002. Syphilis among men who have sex with men (MSM) in Miami-Dade County, Florida. Program Abstr. 2002 Nat. STD Prev. Conf., abstr. P135.
- 7.Carnauba, D., Jr., A. Bittencourt, and C. Brites. 2003. Atypical presentation of syphilis in an HTLV-1 infected patient. Braz. J. Infect. Dis. 7:273-277. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1995. Morb. Mortal. Wkly. Rep. 44:75. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1998. Primary and secondary syphilis—United States, 1997. Mob. Mortal. Wkly. Rep. 47:493-497. [PubMed]
- 10.Centers for Disease Control and Prevention. 1999. Resurgent bacterial sexually transmitted disease among men who have sex with men—King County, Washington, 1997-1999. Morb. Mortal. Wkly. Rep. 48:773-777. [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention. 2001. Outbreak of syphilis among men who have sex with men—Southern California, 2000. Morb. Mortal. Wkly. Rep. 50:117-120. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2002. Primary and secondary syphilis among men who have sex with men—New York City, 2001. Morb. Mortal. Wkly. Rep. 51:853-856. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1999. The national plan to eliminate syphilis from the United States. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for HIV, STD, and TB Prevention, Atlanta, Ga.
- 14.Chapel, T. A. 1978. The variability of syphilitic chancres. Sex. Transm. Dis. 5:68-70. [DOI] [PubMed] [Google Scholar]
- 15.Chapel, T. A. 1980. The signs and symptoms of secondary syphilis. Sex. Transm. Dis. 7:161-164. [DOI] [PubMed] [Google Scholar]
- 16.Chesson, H. W., T. S. Dee, and S. O. Aral. 2003. AIDS mortality may have contributed to the decline in syphilis in the United States in the 1990s. Sex. Transm. Dis. 30:419-424. [DOI] [PubMed] [Google Scholar]
- 17.Clark, E. G., and N. Danbolt. 1964. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med. Clin. North Am. 48:613-621. [Google Scholar]
- 18.Collart, P., L.-J. Borel, and P. Durel. 1964. Significance of spiral organisms found, after treatment, in late human and experimental syphilis. Br. J. Vener. Dis. 40:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danehower, W. F. 1964. Penicillin fallout and infectious syphilis. Med. Clin. North Am. 48:747-753. [Google Scholar]
- 20.Dennis, C. C. 1962. A history of syphilis. Charles C Thomas, Springfield, Ill.
- 21.Don, P. C., R. Rubinstein, and S. Christie. 1995. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int. J. Dermatol. 34:403-407. [DOI] [PubMed] [Google Scholar]
- 22.Dowell, M. E., P. G. Ross, D. M. Musher, T. R. Cate, and R. E. Baughn. 1992. Response of latent syphilis or neurosyphilis to ceftriaxone in persons infected with human immunodeficiency virus. Am. J. Med. 93:481-488. [DOI] [PubMed] [Google Scholar]
- 23.Drusin, L. M., C. Singer, A. J. Valenti, and D. Armstrong. 1977. Infectious syphilis mimicking neoplastic disease. Arch. Intern. Med. 137:156-160. [PubMed] [Google Scholar]
- 24.Edlin, B. R., K. L. Irwin, D. D. Ludwig, et al. 1992. High-risk sex behavior among young street-recruited crack cocaine smokers in three American cities: an interim report. J. Psychoactive Drugs 23:363-371. [DOI] [PubMed] [Google Scholar]
- 25.Engel, S., and W. Diezel. 1980. Persistent serum immune complexes in syphilis. Br. J. Vener. Dis. 56:221-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falck, R. S., J. Wang, R. G. Carlson, and H. A. Siegal. 1997. Factors influencing condom use among heterosexual users of infection drugs and crack cocaine. Sex. Transm. Dis. 24:204-210. [DOI] [PubMed] [Google Scholar]
- 27.Fiumara, N. J. 1980. Treatment of primary and secondary syphilis. Serological response. JAMA 243:2500-2502. [PubMed] [Google Scholar]
- 28.Folk, J. C., T. A. Weingeist, J. J. Corbett, L. A. Lobes, and R. C. Watzke. 1983. Syphilitic neuroretinitis. Am. J. Ophthalmol. 95:480-486. [DOI] [PubMed] [Google Scholar]
- 29.Fracastoro, H. 1930. De contagione et contagiosis morbis et eorum curatione, libri III. Translated by W. C. Wright. G. P. Putnam's Sons, New York, NY.
- 30.Frederick, W. R., R. Delapenha, S. Barnes, et al. 1988. Secondary syphilis and HIV infection. Program. Abstr. 28th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1175, p. 320.
- 31.Goh, B. T., G. W. Smith, L. Samarasinghe, V. Singh, and K. S. Lim. 1984. Penicillin concentrations in serum and cerebrospinal fluid after intramuscular injection of aqueous procaine penicillin 0.6 MU with and without probenecid. Br. J. Vener. Dis. 60:371-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman, H. 1943. Notable contributions to the knowledge of syphilis. Froben Press, New York, N.Y.
- 33.Gorbach, P. M., S. O. Aral, C. Celum, et al. 2000. To notify or not to notify: STD patients' perspectives of partner notification in Seattle. Sex. Transm. Dis. 27:193-200. [DOI] [PubMed] [Google Scholar]
- 33a.Gorbach, S. L., J. G. Bartlett, and N. R. Blacklow (ed.). 2003. Infectious diseases, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 34.Gordon, S. M., M. E. Eaton, R. George, et al. 1994. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N. Engl. J. Med. 331:1469-1473. [DOI] [PubMed] [Google Scholar]
- 35.Gourevitch, M. N., P. A. Selwyn, K. Davenny, et al. 1993. Effects of HIV infection on the serologic manifestations and response to treatment of syphilis in intravenous drug users. Ann. Intern. Med. 118:350-355. [DOI] [PubMed] [Google Scholar]
- 36.Harris, R. L., P. A. Rutecki, D. T. Donovan, M.-W. Bradshaw, and T. W. Williams, Jr. 1985. Fever, headache and hearing loss in a young homosexual man. Hosp. Pract. 20:167-170. [DOI] [PubMed] [Google Scholar]
- 37.Held, J. L., M. Ross, V. Beltrani, Jr., S. R. Kohn, and M. E. Grossman. 1990. Noduloulcerative or “malignant” syphilis in an otherwise healthy woman: report and review of a dramatic dermatosis. Cutis 45:119-122. [PubMed] [Google Scholar]
- 38.Hicks, C. B., P. M. Benson, G. P. Lupton, and E. C. Tramont. 1987. Seronegative secondary syphilis in a patient infected with the human immunodeficiency virus (HIV) with Kaposi sarcoma. Ann. Intern. Med. 107:492-495. [DOI] [PubMed] [Google Scholar]
- 39.Hoff, H., and J. A. Shaby. 1940. Nervous manifestations of bejel. Trans. Ro. Soc. Trop. Med. Hyg. 33:549-551. [Google Scholar]
- 40.Holcombe, R. C. 1938. The holy wood and the Haitian myth of the origin of syphilis, p. 12-19. In Syphilis. Publication 6. American Association for the Advancement of Science, Washington, D.C.
- 41.Holtom, P. D., R. A. Larsen, M. E. Leal, and J. M. Leedom. 1992. Prevalence of neurosyphilis in human immunodeficiency virus-infected patients with latent syphilis. Am. J. Med. 93:9-12. [DOI] [PubMed] [Google Scholar]
- 42.Howles, J. K. 1943. A synopsis of clinical syphilis, p. 59-98 and p. 466-499. The C. V. Mosby Co., St. Louis, Mo.
- 43.Hudson, E. H. 1956. Treponematosis. Current Lit. Vener. Dis., Spec. Issue, p. 56.
- 44.Hutchinson, C. M., E. W. Hook III, and M. Shepherd. 1994. Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann. Intern. Med. 121:94-100. [DOI] [PubMed] [Google Scholar]
- 45.Ingraham, N. R., Jr. 1938. Spirochaeta pallida and the etiology of syphilis, p. 40-46. In Syphilis. Publication 6. American Association for the Advancement of Science, Washington, D.C.
- 46.Johns, D. R., N. I. Tierney, and D. Felsenstein. 1987. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N. Engl. J. Med. 316:1569-1572. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen, J., G. Tikjob, and K. Weisman. 1986. Neurosyphilis after treatment of latent syphilis with benzathine penicillin. Genitourin. Med. 62:129-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorizzo, J. L., M. C. McNeely, R. E. Baughn, A. R. Solomon, T. Cavalls, and E. B. Smith. 1986. Role of circulating immune complexes in human secondary syphilis. J. Infect. Dis. 153:1014-1022. [DOI] [PubMed] [Google Scholar]
- 49.Jozsa, L., M. Timmer, T. Somogyi, and J. Feher. 1977. Hepatitis syphilitica: a clinico-pathological study of 25 cases. Acta Hepatogastroenterol. 24:344-347. [PubMed] [Google Scholar]
- 50.Katz, D. A., J. A. Berger, and R. C. Duncan. 1993. Neurosyphilis: a comparative study of the effects of infection with human immunodeficiency virus. Arch. Neurol. 50:243-249. [DOI] [PubMed] [Google Scholar]
- 51.Katz, M. H., S. K. Schwarcz, T. A. Kellogg, et al. 2002. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am. J. Public Health 92:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar, B., and S. Muralidhar. 1998. Malignant syphilis: a review. AIDS Patient Care STDs 12:921-925. [DOI] [PubMed] [Google Scholar]
- 53.Leslie, N. 1964. Treatment of early infectious syphilis with benzathine penicillin G, p. 12-19. In Proc. World Forum Syphilis Other Treponematoses. USPHS publication 997. U.S. Public Health Service, Washington, D.C.
- 54.Lukehart, S. A., E. W. Hook III, S. A. Baker-Zander, A. C. Collier, C. W. Critchlow, and H. H. Handsfield. 1988. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann. Intern. Med. 109:855-862. [DOI] [PubMed] [Google Scholar]
- 55.Lukehart, S. A., E. W. Hook III, S. A. Baker-Zander, A. C. Collier, C. W. Critchlow, and H. H. Handsfield. 1988. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann. Intern. Med. 109:855-862. [DOI] [PubMed] [Google Scholar]
- 56.Magnuson, H. J., E. W. Thomas, S. Olansky, et al. 1956. Inoculation syphilis in human volunteers. Medicine (Baltimore) 35:33-82. [DOI] [PubMed] [Google Scholar]
- 57.Marra, C. M., P. Boutin, J. C. McArthur, et al. 2000. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin. Infect. Dis. 30:540-544. [DOI] [PubMed] [Google Scholar]
- 58.McCusker, J., A. M. Stoddard, J. G. Zapka, and M. Zorn. 1993. Use of condoms by heterosexually active drug abusers before and after AIDS education. Sex. Transm. Dis. 20:81-88. [DOI] [PubMed] [Google Scholar]
- 59.Misery, L., V. Besnard, R. Badel, E. Marneffe-Delalande, M. Faure, and J. Thiovolet. 1996. Malignant syphilis in human immunodeficiency virus infection. Ann. Dermatol. Venereol. 123:732-734. [PubMed] [Google Scholar]
- 60.Morgello, S., and H. Laufer. 1989. Quaternary neurosyphilis. N. Engl. J. Med. 319:1549. [DOI] [PubMed] [Google Scholar]
- 61.Musher, D. M., R. J. Hamill, and R. E. Baughn. 1990. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann. Intern. Med. 113:872-881. [DOI] [PubMed] [Google Scholar]
- 62.Musher, D. M., and J. M. Knox. 1983. Syphilis and yaws, p. 101-120. In R. E. Schell and D. M. Musher (ed.), Pathogenesis and Immunology of treponemal infections. Marcel Dekker, Inc., New York, N.Y.
- 63.Noguchi, H. 1911. A cutaneous reaction in syphilis. J. Exp. Med. 14:557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norris, S. J., and the T. pallidum Polypeptide Research Group. 1993. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional; and immunological roles. Microbiol. Rev. 57:750-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parran, T. 1938. Syphilis: a public health problem, p. 187-193. In Syphilis. Publication 6. American Association for the Advancement of Science, Washington, D.C.
- 66.Pusey, W. A. 1933. The history and epidemiology of syphilis. Charles C Thomas, Springfield, Ill.
- 67.Rietmeijer, C. A., J. L. Patnaik, F. N. Judson, and J. M. Douglas. 2003. Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex. Transm. Dis. 30:562-567. [DOI] [PubMed] [Google Scholar]
- 68.Riverius, L. 1946. Of causes of French pox. Urol. Cutan. Rev. 50:510-515. [PubMed] [Google Scholar]
- 69.Rolfs, R. T. 1993. Surveillance for primary and secondary syphilis—United States, 1991. Morb. Mortal. Wkly. Rep. 42:13-19. [PubMed] [Google Scholar]
- 70.Rolfs, R. T., R. Joesoef, E. F. Hendershot, et al. 1997. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. N. Engl. J. Med. 337:307-314. [DOI] [PubMed] [Google Scholar]
- 71.Romero-Jimenez, M. J., L. I. Suarez, P. J. M. Fajardo, and F. B. Baron. 2003. Malignant syphilis in patient with human immunodeficiency virus (HIV); case report and literature review. An. Med. Intern. 20:373-376. [PubMed] [Google Scholar]
- 72.Rosebury, T. 1971. Microbes and morals: the strange story of venereal disease. Viking Press, New York, N.Y.
- 73.Sapra, S., and L. Weatherhead. 1989. Extensive nodular secondary syphilis. Arch. Dermatol. 125:1666-1669. [PubMed] [Google Scholar]
- 74.Schaudinn, F., and E. Hoffmann. 1905. Bericht uber das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen. Arb. Kaiserl. Gesurndheisamte 22:527-532. [Google Scholar]
- 75.Schroeter, A. L., J. B. Lucas, E. V. Price, and V. H. Falcone. 1972. Treatment of early syphilis and reactivity of serological tests. JAMA 221:471-496. [PubMed] [Google Scholar]
- 76.Shrivastav, S. N., and C. Singh. 1977. Extensive condyloma lata. Br. J. Vener. Dis. 53:23-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.St. Louis, M. E., T. A. Farley, and S. O. Aral. 1996. Untangling the persistence of syphilis in the south. Sex. Transm. Dis. 23:1-4. [DOI] [PubMed] [Google Scholar]
- 78.Tosca A., P. G. Stavropouloos, E. Hatziolou, A. Arvanitis, N. Stavrianeas, M. Hatzivassiliou, and J. D. Stratigos. 1990. Malignant syphilis HIV-infected patients. Int. J. Dermatol. 29:575-578. [DOI] [PubMed] [Google Scholar]
- 79.Tramont, E. C. 1976. Persistence of Treponema pallidum following penicilling G therapy. A report of two cases. JAMA >236>:2206-2207. [PubMed] [Google Scholar]
- 80.Vonderlehr, R. A. 1942. Impact of war on venereal-disease problems. N. Engl. J. Med. 227:203-205. [Google Scholar]
- 81.Witkowski, J. A., and L. C. Parish. 2002. The great imitator: malignant syphilis with hepatitis. Clin. Dermatol. 20:156-163. [DOI] [PubMed] [Google Scholar]
- 82.Yobs, A. R., J. W. Clark, Jr., S. E. Mothershed, J. C. Bullard, and C. W. Artley. 1968. Further observations on the persistence of Treponema pallidum after treatment in rabbits and humans. Br. J. Vener. Dis. 44:116-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young, E. J., N. M. Weingarten, R. E. Baughn, and W. C. Duncan. 1982. Studies on the pathogenesis of the Jarisch-Herxheimer reaction: development of an animal model and evidence against a role for classical endotoxin. J. Infect. Dis. 146:606-615. [DOI] [PubMed] [Google Scholar]
- 84.Zaidman, G. W. 1986. Neurosyphilis and retrobulbar neuritis in a patient with AIDS. Ann. Ophthalmol. 18:260-261. [PubMed] [Google Scholar]
- 85.Zambrano, W., G. M. Perez, and J. L. Smith. 1987. Acute syphilitic blindness in AIDS. J. Clin. Neurol. Ophthalmol. 7:1-5. [DOI] [PubMed] [Google Scholar]