Significance
Common deletions affecting multiple genes that cause multiple birth defects can be studied by investigating each gene’s independent role in embryonic development. This study shows that a specific gene, CRKL, which lies within the commonly deleted region at chromosome locus 22q11.2, is required for normal overall embryonic growth, and normal development of the kidneys and testes. Deletion of only the Crkl gene in mice is sufficient to cause increased incidence of birth defects commonly seen in humans who possess deletion at 22q11.2. This study shows that CRKL is one of the key genes whose deletion contributes to the urogenital birth defects associated with multiple-gene deletion at chromosome 22q11.2, indicating a new target for gene therapy in affected patients.
Keywords: del22q11.2, haploinsufficient, genitourinary, urogenital, congenital defects
Abstract
The spectrum of congenital anomalies affecting either the upper tract (kidneys and ureters) or lower tract (reproductive organs) of the genitourinary (GU) system are fundamentally linked by the developmental origin of multiple GU tissues, including the kidneys, gonads, and reproductive ductal systems: the intermediate mesoderm. Although ∼31% of DiGeorge/del22q11.2 syndrome patients exhibit GU defects, little focus has been placed on the molecular etiology of GU defects in this syndrome. Among del22q11.2 patients exhibiting GU anomalies, we have mapped the smallest relevant region to only five genes, including CRKL. CRKL encodes a src-homology adaptor protein implicated in mediating tyrosine kinase signaling, and is expressed in the developing GU-tract in mice and humans. Here we show that Crkl mutant embryos exhibit gene dosage-dependent growth restriction, and homozygous mutants exhibit upper GU defects at a microdissection-detectable rate of 23%. RNA-sequencing revealed that 52 genes are differentially regulated in response to uncoupling Crkl from its signaling pathways in the developing kidney, including a fivefold up-regulation of Foxd1, a known regulator of nephron progenitor differentiation. Additionally, Crkl heterozygous adult males exhibit cryptorchidism, lower testis weight, lower sperm count, and subfertility. Together, these data indicate that CRKL is intimately involved in normal development of both the upper and lower GU tracts, and disruption of CRKL contributes to the high incidence of GU defects associated with deletion at 22q11.2.
Commonly referred to as DiGeorge syndrome, 22q11.2 deletion syndrome affects ∼1 in 4,000 births and is associated with a wide range of developmental abnormalities, including craniofacial, heart, and thymus defects, as well as cognitive deficits and developmental delay (1). Among the less penetrant yet equally detrimental congenital anomalies associated with 22q11.2 deletion are defects of the genitourinary (GU) system affecting ∼31% of patients (2). The clear association of 22q11.2 dosage variation with GU anomalies indicates that one or more genes within the commonly deleted region are involved in the normal development of the GU system. Identifying and characterizing these genes provides valuable information not just for diagnosing and treating patients with clinical DiGeorge syndrome, but potentially a wider spectrum of GU patients harboring mutations within a common signaling network.
Both syndromic and isolated congenital anomalies of the GU tract are common. Lower-tract defects occur at frequencies of ∼5.9% of male births for cryptorchidism (3), 1:240 for hypospadias (4) (USA cohort), and a conservative estimate of 1:4,500 for the less-common defect of ambiguous genitalia (5). Upper-tract defects, commonly referred to as congenital anomalies of the kidney and urinary tract (CAKUT), are also common, affecting 0.3–0.6% of births (6). CAKUT refers to a spectrum of renal defects, including agenesis, hypoplasia, and anomalies of the ureter accounting for 20–30% of all structural birth defects (6). Despite congenital GU anomalies of the upper and lower systems being common, requiring extensive surgical interventions and exhibiting high morbidity and mortality rates, the conditions are often socially stigmatized and the molecular basis of many of these conditions remains largely unknown.
CRKL encodes an adaptor protein with SH2 and SH3 homology domains (7) and has been previously linked to a plethora of signaling pathways in a wide variety of normal and diseased tissues, including PI3K signaling (8, 9), BCR/ABL signaling (10), and FGF signaling (11, 12). CRKL lies within the standard deleted region of 22q11.2 patients and was previously linked via murine models to a variety of the congenital malformations associated with the disease, including thymic aplasia, heart defects, and neurocristopathies (13). In 2010, a large-scale array comparative genomic hybridization (aCGH) screen of both syndromic and nonsyndromic patients with GU anomalies further indicated that 22q11.2 copy number variations (CNVs) are associated with GU defects of both the upper and lower tracts (14).
A recent study of 135 murine mutant lines harboring congenital heart disease (CHD) indicated that 29% of CHD mutant lines also harbor CAKUT, indicating mechanistic links between cardiovascular and renal malformations (15). Although not part of the CHD study, Crkl-null mice exhibit cardiac outflow anomalies (16) and are therefore more likely to harbor a cooccurrence of GU defects. Indeed, a podocyte-specific model of Crkl deletion indicates that Crkl and Crk are partially redundant in podocytes and codeletion results in albuminuria and altered podocyte process architecture (17). The goal of this study is to further our understanding of normal and abnormal GU development on a molecular and genetic level, and improve the diagnosis and treatment of both syndromic and nonsyndromic 22q11.2 variation patients by examining the role of CRKL in GU development.
Results
CRKL Lies Within the Minimal Region for 22q11.2 Patients with GU Anomalies and Is Expressed During GU Development.
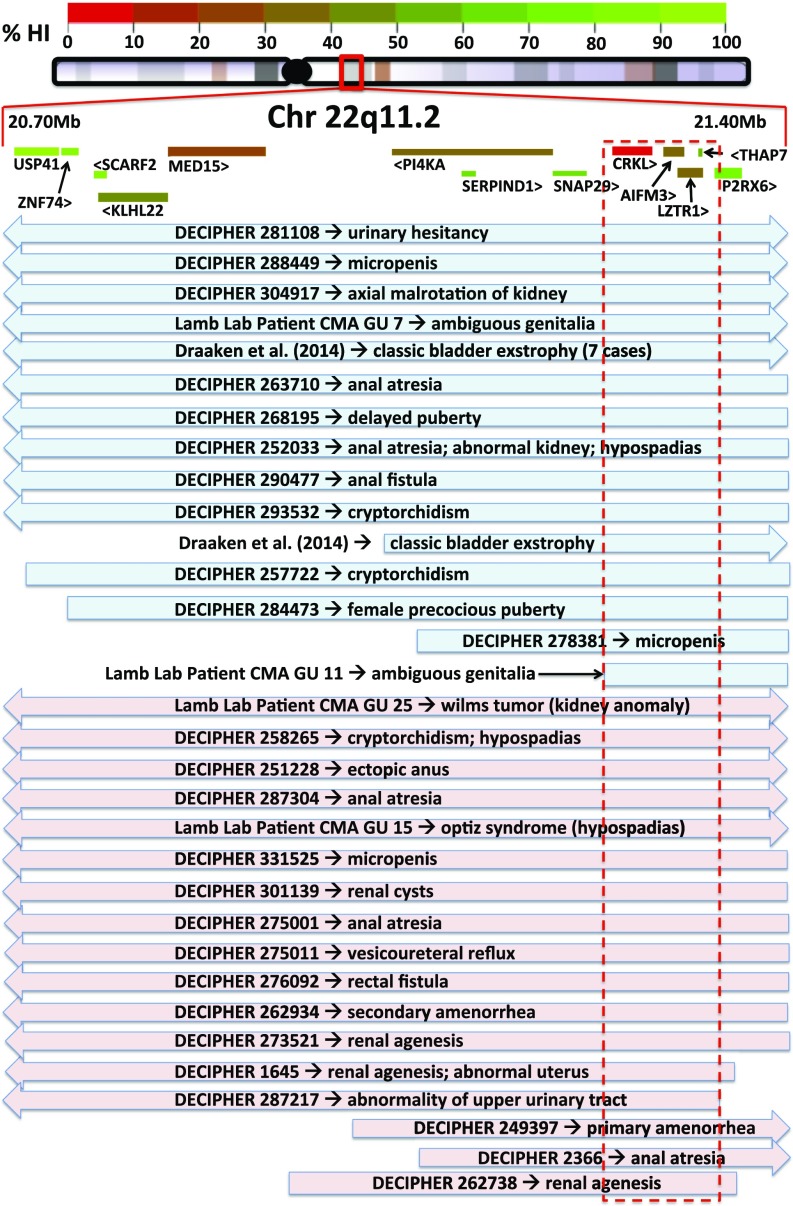
By retrospectively analyzing 22q11.2 patients from the DECIPHER database, previously published literature (18), and patient data acquired from aCGH previously performed by our laboratory (14), we compiled an overlap map of patients with 22q11.2 dosage variation who also exhibit GU phenotypes (Fig. 1). The smallest region covered by all of the mapped GU 22q11.2 CNVs encompasses five protein-coding genes: CRKL, AIFM3, LZTR1, THAP7, and P2RX6. We referred to the ExAC database of 60,706 individual genomes (exac.broadinstitute.org) to determine the likelihood of haploinsufficiency for each gene (probability of loss-of-function intolerance; pLI score). Of the five genes within the minimal region, CRKL harbors the highest pLI score at 0.16. Scores for the other four genes within the minimal region were AIFM3 pLI = 0, LZTR1 pLI = 0, THAP7 pLI = 0.03, and P2RX6 pLI = 0. Additionally, only two truncating mutations of CRKL are reported in the ExAC database, both of which are located at the end of the 303-aa protein sequence: p.Gln297Ter and p.Glu301Ter. The absence of any early truncating mutations in the 60,706 genomes of the ExAC database suggests an unappreciated intolerance to loss-of-function mutations in CRKL.
Fig. 1.
Map of GU abnormal patients with CNVs covering 22q11.2 indicates CRKL as candidate gene. CRKL is within the minimal region of overlap and harbors the highest pLI score (pLI = 0.16). Data are gleaned from DECIPHER database, literature (18), as well as original data from D.J.L.’s laboratory (14). Blue indicates duplication; pink indicates deletion. Arrows indicate CNV expands beyond map. Red dotted box indicates minimal region of maximum CNV overlap.
An aCGH and CNV qPCR screen showed duplications (n = 2) and deletions (n = 2) encompassing CRKL (Fig. 1, “Lamb Lab Patients”) were enriched in a population of GU-abnormal patients and present at 1.4% (n = 277; P = 0.0004) compared with the unselected general population where CRKL-encompassing CNVs are present at 0.088% (6 of 6,813; only duplications found) (19). CNVs in CRKL are also enriched in our cohort of GU-abnormal patients compared with rates in the generally disease-enriched cohort of the ExAC database (19 deletions; 47 duplications; 66 of 60,706; 0.1%; all analyzed ethnic subgroups represented; P = 0.03). Our laboratory’s cohort of GU-abnormal patients is heavily biased toward lower-tract defects, and our findings may underestimate the frequency of CRKL encompassing CNVs in GU-abnormal patients because of underrepresentation of patients with upper-tract phenotypes.
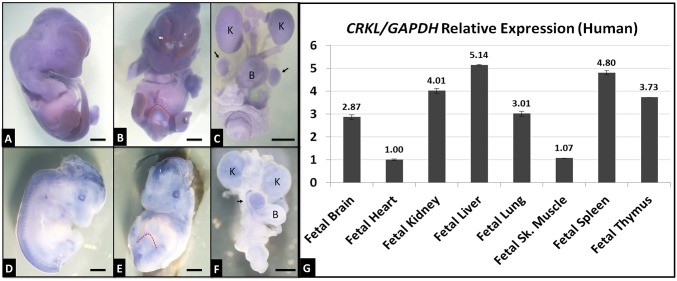
To define Crkl expression in the developing GU system to ask whether these observed CNVs might contribute to GU defects associated with 22q11.2 dosage variation, in situ hybridization was performed on murine tissues (Fig. 2 A–F), as well as a qPCR screen of human fetal cDNAs from various tissues (Fig. 2G). Crkl is expressed throughout the developing mouse at modest levels (Fig. 2A), and at moderate levels in the developing GU tract with emphasis on the E12.5 genital tubercle (Fig. 2B, red dotted outline) and the E16.5 kidneys, bladder, and testis (Fig. 2C). E16.5 Crkl expression patterns are largely corroborated by E15.5 data from the GUDMAP database (www.gudmap.org; entry #10685). Human CRKL is expressed throughout a variety of fetal tissues, including liver, lung, and skeletal muscle, and, importantly for DiGeorge syndrome phenotypes, heart, spleen, thymus, brain, and kidney (Fig. 2G). CRKL is expressed in the human fetal kidney at levels comparable to or higher than human fetal organs affected in Crkl murine deletion models (thymus and heart, respectively).
Fig. 2.
Murine Crkl and human CRKL expression patterns. Crkl antisense (A–C) and sense control (D–F) probes were used to stain E12.5 (A, B, D, and E) whole embryos and E16.5 isolated GU tracts (C and F). Blue areas indicate probe hybridization. Crkl is expressed at moderate levels (higher than sense probe background) throughout the developing embryo, including genital tubercle (red dotted outline), kidneys (K), bladder (B), and testes (black arrows). (Scale bars, 2 mm.) CRKL qPCR was performed on cDNAs (G) from spontaneously aborted human fetuses. Expression levels of tissues shown relative to heart expression. Error bars represent SEM.
Loss of Crkl Adapter Functions Mimics Null-Deletion Models and Indicates Haploinsufficiency via Dose-Dependent Intrauterine Growth Restriction in Mice.
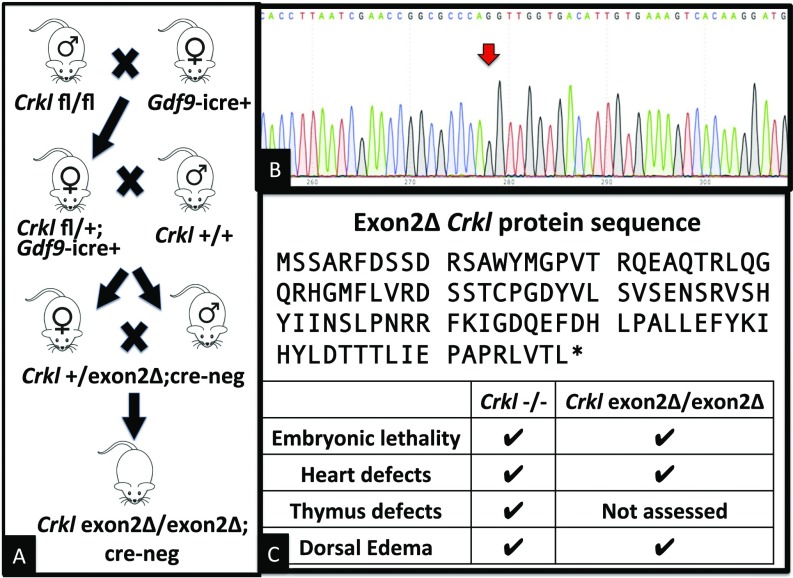
To determine a role that Crkl may play in GU development as implicated above, we used a cre-dependent conditional allele in which exon 2 of murine Crkl is flanked by two loxP sites. The second exon of Crkl encodes the SH3n domain and a short portion of the SH3c domain. The remainder of the SH3c domain is encoded in the third exon, which is transcribed but not translated in cre-recombined tissues because of the introduction of a premature stop codon. Crkl exon2Δ/exon2Δ embryos were produced using a three-generation cross between Crkl exon2 fl/fl and GDF9-icre oocyte-specific cre-recombinase mice (Fig. 3A). Upon generation of full-bodied Crkl exon2Δ/exon2Δ embryos, RNA was isolated from E16.5 kidneys and cDNA was sequenced with Sanger sequencing to validate splicing of Crkl exon 1 to exon 3 (Fig. 3B). Crkl exon2Δ/exon2Δ animals are embryonic-lethal at E18.5 with no recovery of live null animals perinatally suggesting a tight phenocopy of the Crkl−/− embryos developed and assessed previously (Fig. 3C) (11, 13). Upon analysis of mutant embryos it was immediately apparent that both Crkl exon2Δ/exon2Δ as well as Crkl+/exon2Δ embryos exhibit intrauterine growth restriction (Fig. 4 A and B). Because loss of a single copy of wild-type Crkl is sufficient to cause restricted intrauterine growth, Crkl is haploinsufficient for normal embryonic development and is likely to contribute to growth restriction associated with 22q11.2 deletion in humans (20).
Fig. 3.
Generation of Crkl exon2Δ/exon2Δ embryos. (A) A multigenerational cross was used to generate Crkl exon2Δ/exon2Δ embryos. (B) Deletion was validated by Sanger sequencing of cDNA indicating splicing of exon 1 to exon 3 (red arrow). (C) Exon 2-deleted protein sequence showing premature stop, and Inset chart comparing predominant phenotypes of Crkl−/− to Crkl exon2Δ/exon2Δ.
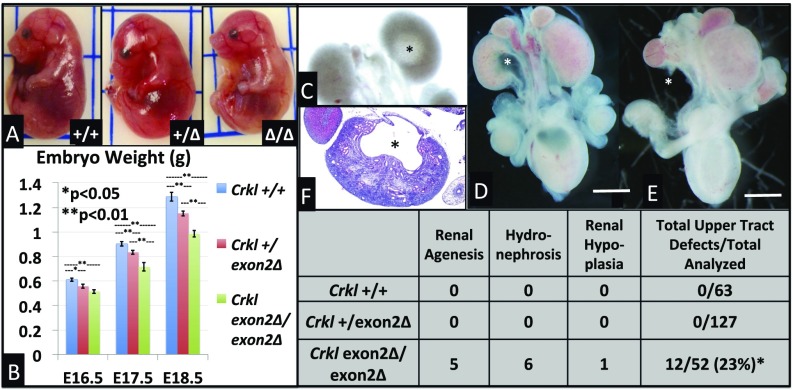
Fig. 4.
Crkl exon 2 deletion results in dose-dependent intrauterine growth restriction and Crkl exon2Δ/exon2Δ embryos exhibit 23% incidence of upper-tract phenotypes. Crkl+/+, Crkl+/exon2Δ, and Crkl exon2Δ/exon2Δ embryos were collected at E16.5 (A), E17.5, and E18.5. Loss of one copy of wild-type Crkl is sufficient to confer intrauterine growth restriction as early as E16.5 (B). n for each group ≥ 7. Error bars represent SEM. GU tracts were microdissected from E16–E18. Upper-tract phenotypes were observed and penetrance documented (Inset chart). Phenotypes included mild (C) to moderate (D and F) hydronephrosis, unilateral renal agenesis (E), and unilateral renal hypoplasia. Asterisks indicate abnormalities. Asterisk in table indicates P < 0.05 by Fisher exact test. (Scale bars, 2 mm.)
Murine Loss of Crkl Exon 2 Results in 23% Incidence of Upper GU-Tract Embryonic Phenotypes.
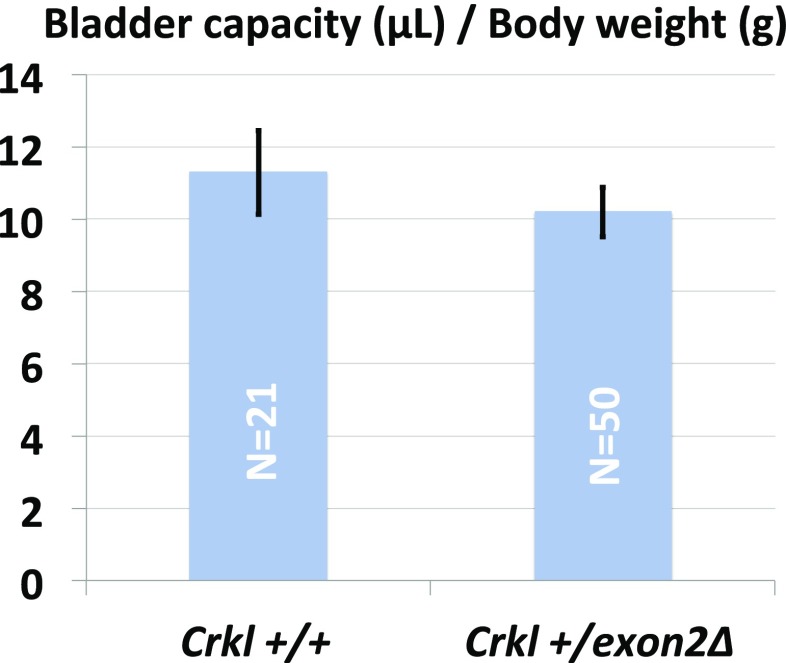
Crkl exon2Δ/exon2Δ embryos and their control littermates were analyzed by microdissection for overt GU structural anomalies. A total of 242 embryos were analyzed and upper tract GU phenotypes including hydronephrosis (Fig. 4 C, D, and F) and unilateral renal agenesis (Fig. 4E) were found in 23% (12 of 52) of Crkl exon2Δ/exon2Δ embryos, and not present in 63 Crkl+/+ and 127 Crkl+/exon2Δ littermates (Fig. 4, Inset chart). This incidence is considered statistically significant by Fisher’s exact test between Crkl exon2Δ/exon2Δ and Crkl+/+, with P = 0.0001. No overt embryonic phenotypes were present in other microdissected GU tissues, including bladder, gonads, reproductive ducts, and genital tubercle. Because embryos were assessed only for overt structural anomalies, the incidence described here likely underrepresents the true incidence of renal phenotypes in Crkl-deficient embryos. Vesicoureteral reflux (VUR) assay on 52 adult male Crkl+/exon2Δ and 57 wild-type controls (Fig. S1) revealed a higher-trending but statistically nonsignificant (P = 0.19 by Fisher exact test) rate of VUR in 32.7% (17 of 52) of Crkl+/exon2Δ males versus 21% (12 of 57) of Crkl+/+ controls. Heterozygous mutants exhibited normal bladder capacity normalized to body weight (Fig. S2) and no anomalies of the bladder. Although embryonic and early postnatal VUR studies in other backgrounds indicate wild-type incidences ranging from 0 to 59% (21–23), and embryonic and early postnatal VUR in C57/BL6 occurs at low rates (24, 25), this is the largest study to date showing background incidence of VUR in C57/BL6 adult age-matched males. One of the 52 Crkl+/exon2Δ mutants assayed for VUR exhibited unilateral cystic kidney and apparent ureteropelvic junction (UPJ) obstruction as assessed by micro-CT scanning (Fig. S3).
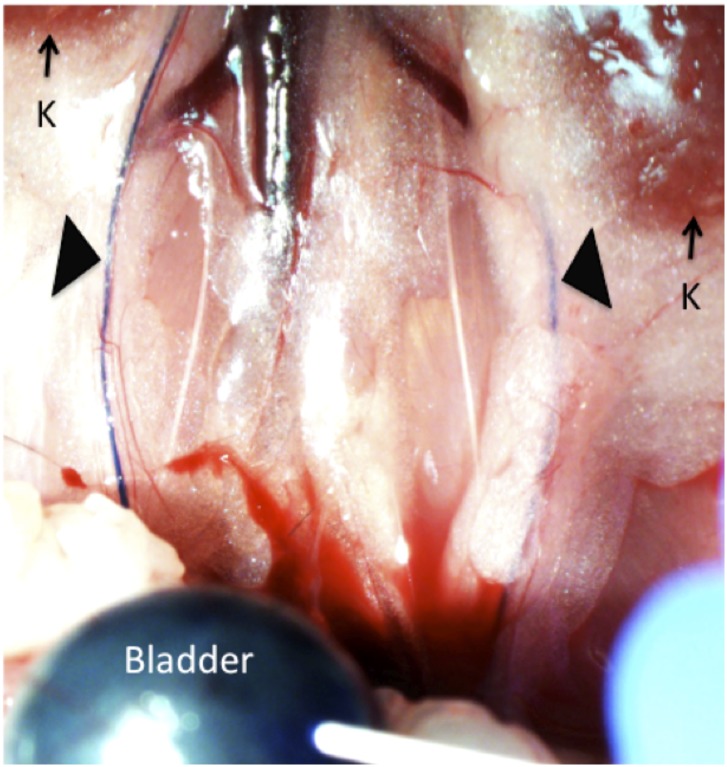
Fig. S1.
Crkl+/exon2Δ age matched males exhibit higher-trending but statistically nonsignificant rates of VUR compared with Crkl+/+ males. Methylene blue dye is injected into the bladders of anesthetized age-matched males until urethral leakage. When VUR is present, blue dye can be seen flowing retrograde from the bladder through the ureters (black arrows) toward the kidneys (K). VUR was observed in 32.7% (17 of 52) of Crkl+/exon2Δ males versus 21% (12 of 57) of Crkl+/+ controls (P = 0.19).
Fig. S2.
Crkl+/exon2Δ age matched males exhibit normal bladder capacity to body weight ratios. After aspiration of any present urine, methylene blue dye is injected into the bladders of anesthetized age-matched males until urethral leakage. Bladder capacity is defined as total injected volume before leakage.
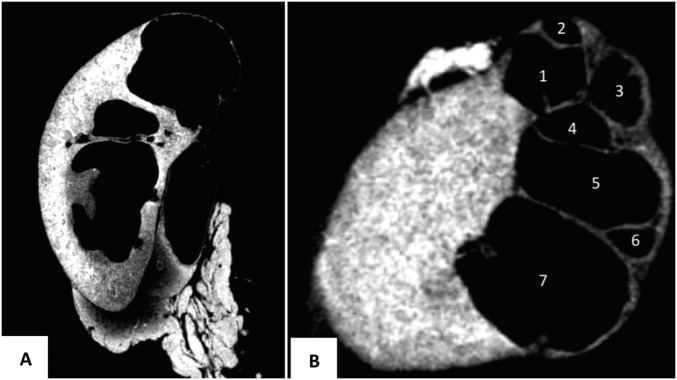
Fig. S3.
Cystic kidney found in 1 of 52 Crkl+/exon2Δ adult males. Micro-CT scan was performed to visualize seven independent cysts resulting from apparent UPJ obstruction. Images were taken from 3D reconstructions showing sagittal (A) and transverse (B) sections of the cystic kidney. (Voxel size, 9.5 μm.)
Crkl Facilitates Renal Signaling Pathways Affecting a Minimum of 52 Transcripts.
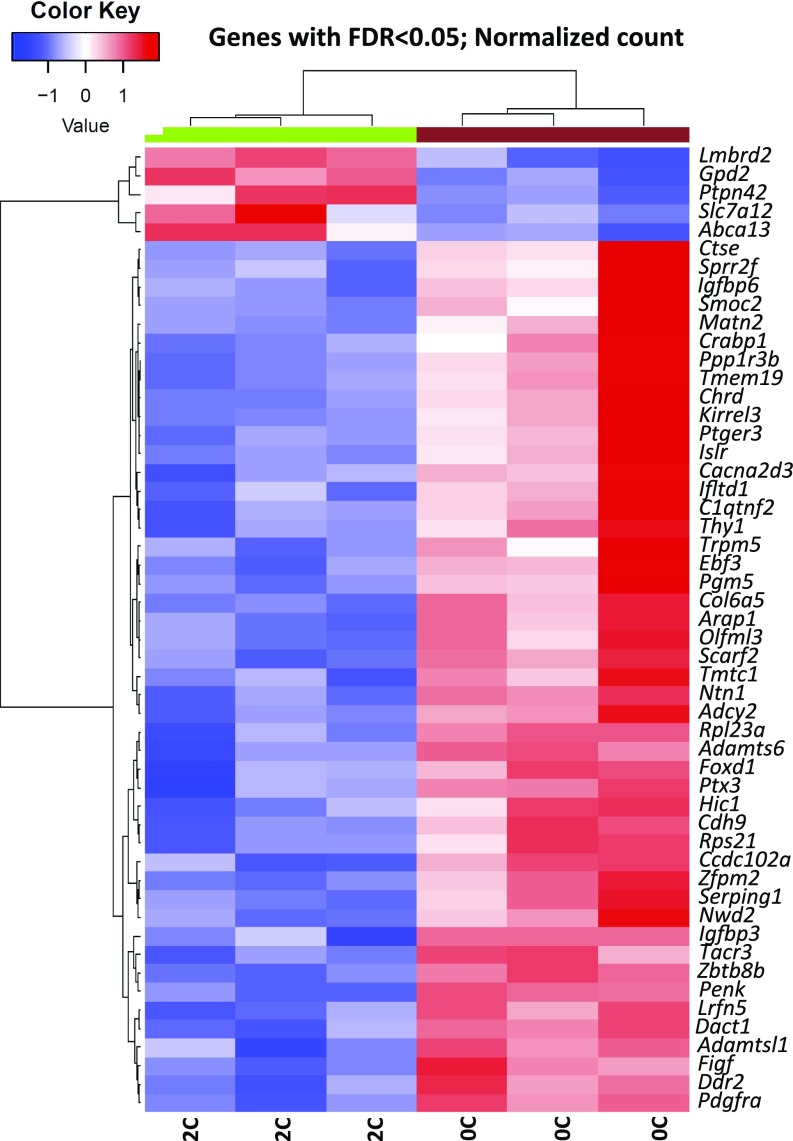
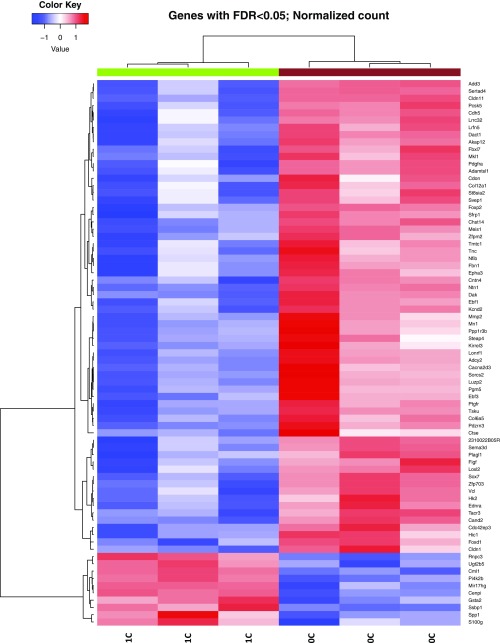
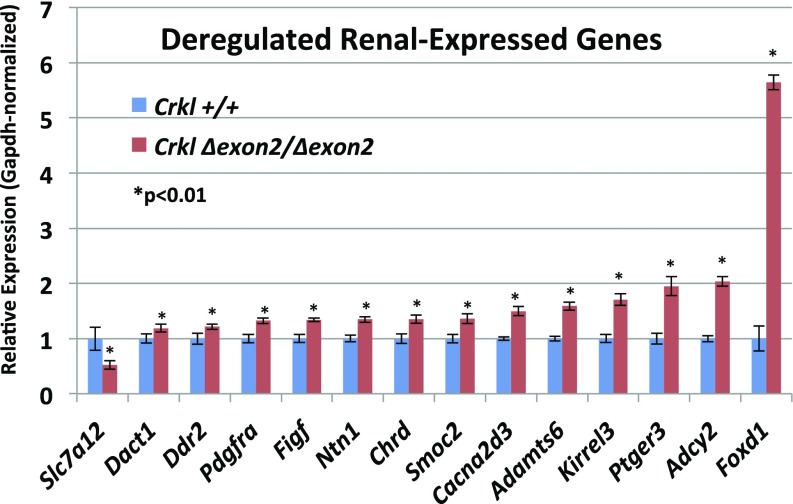
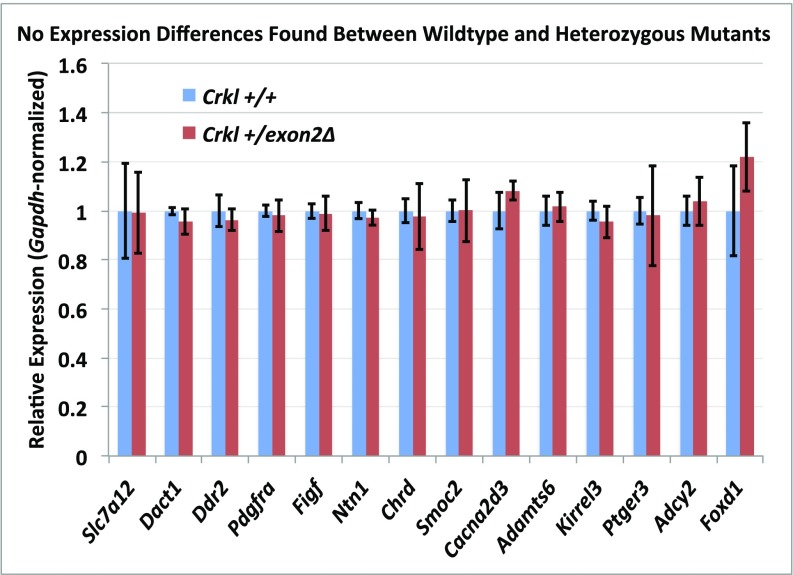
Because Crkl facilitates the transduction of a wide variety of signaling pathways in an even wider variety of cell types, it is imperative to our understanding of Crkl function specific to renal development to attempt to address which pathways are aberrantly affected when Crkl cannot perform its normal adaptor functions within developing kidney tissue. To address this question, mRNA from n ≥ 3 E16.5 kidney pairs of phenotypically normal Crkl exon2Δ/exon2Δ male embryos and Crkl+/+ male control embryos was next-generation sequenced at 80 million reads per sample. Phenotypically normal kidneys were used so as to isolate transcript changes primary to Crkl dosage variation and exclude transcript differences secondary to organ stress. Fifty-two transcripts are differentially expressed when Crkl is uncoupled from its transduction networks in embryonic renal tissue, with a heavy bias toward genes becoming aberrantly up-regulated. This finding suggests that a main function of Crkl in the developing kidney is to aid in maintaining suppression of a wide panel of transcripts (Fig. 5). Because of the evidence for Crkl haploinsufficiency in intrauterine growth, we also sequenced RNA from Crkl+/exon2Δ embryonic kidneys and found that although similar genes are affected in the comparison between Crkl+/exon2Δ kidneys and Crkl exon2Δ/exon2Δ kidneys (Fig. S4), Crkl+/exon2Δ kidney transcripts did not vary from Crkl+/+ (Datasets S1–S4). This result correlates with the finding that although Crkl is haploinsufficient for intrauterine growth, Crkl+/exon2Δ embryos do not exhibit increased incidence of overt upper-tract GU phenotypes. To validate the RNA-seq findings, qPCR was performed on cDNA reverse-transcribed from the RNA samples. Fourteen genes were selected for validation, several of which are involved in renal development (Fig. 6). Of the validated genes, Foxd1 is the most significantly affected and exhibits a 5.5-fold induction when Crkl is uncoupled from its renal signaling networks. The same cohort of genes was tested for differences between wild-type and heterozygous mutant samples, but no differences were found (Fig. S5).
Fig. 5.
Fifty-two genes are differentially expressed in Crkl exon2Δ/exon2Δ E16.5 kidneys compared with Crkl+/+ E16.5 kidneys at a normalized false-discovery rate (FDR) < 0.05. Total RNA collected from E16.5 kidneys was extracted and next-generation sequenced. 2C indicates two copies of wild-type Crkl; 0C indicates zero copies of wild-type Crkl.
Fig. S4.
Differentially expressed genes between Crkl+/exon2Δ and Crkl exon2Δ/exon2Δ E16.5 kidneys. Several genes from this analysis overlap to those found in wild-type versus homozygous mutant analysis. More total genes were found to be differentially expressed in this comparison partially because of lower interreplicate variability. 1C indicates one copy of wild-type Crkl; 0C indicates 0 copies of wild-type Crkl.
Fig. 6.
A subset of genes differentially expressed by RNA-sequencing were chosen to be validated by qPCR. RNAs from the same samples used for sequencing were reverse-transcribed and used to validate differential expression via the higher sensitivity TaqMan qPCR assay. Most of the validated genes showed more robust fold change values by qPCR than indicated by RNA-seq analysis. Error bars represent SEM.
Fig. S5.
A subset of genes differentially expressed between wild-type and homozygous mutants by RNA-sequencing were assayed by qPCR to compare expression between wild-type and heterozygous mutants. RNAs from the same samples used for sequencing were reverse-transcribed and used in TaqMan qPCR to interrogate differential expression. Although all of the genes shown here were differentially expressed between wild-type and homozygous mutants, no differences were apparent when comparing wild-type and heterozygous mutants. Biological triplicates; error bars represent SEM.
Adult Crkl Exon 2 Deletion Heterozygotes Exhibit Lower-Tract GU Defects and Subfertility.
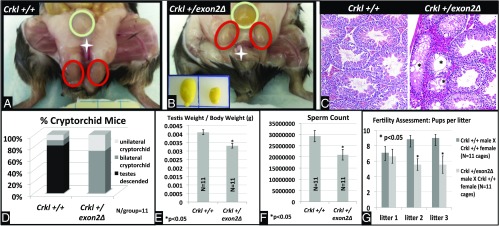
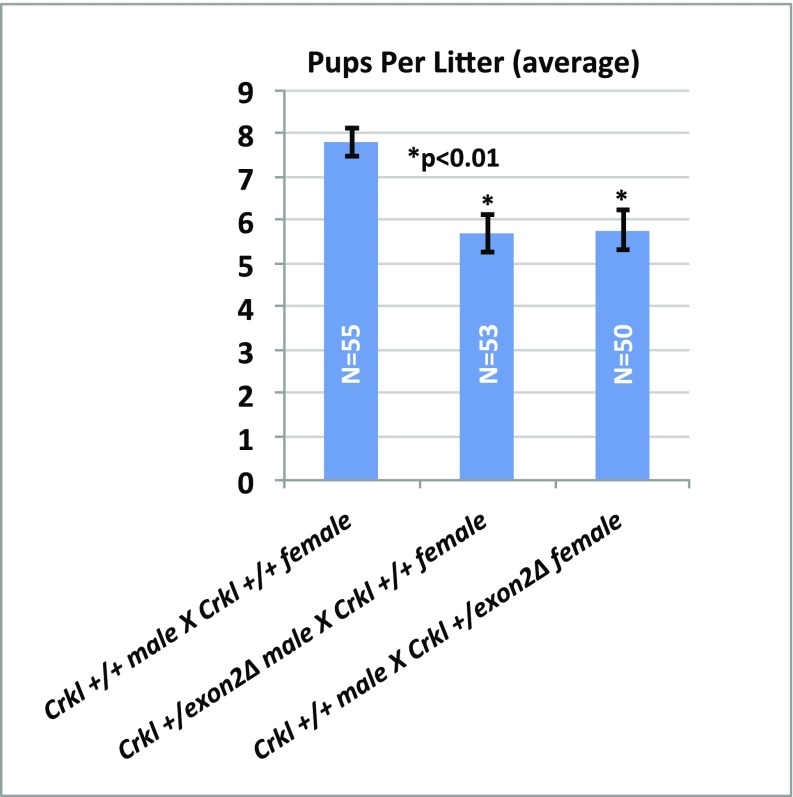
As we were performing our crosses to derive Crkl exon2Δ/exon2Δ embryos and maintain the mutant colony, it became apparent that Crkl+/exon2Δ males frequently exhibited lower-than-average pups per litter when crossed to wild-type females, and often would become infertile earlier than their male wild-type littermates. To quantify this observation, a two-branched approach to analyze anatomical and physiological lower-tract GU phenotypes was used. To address anatomical defects, testicular parameters of males and controls (n = 11 per group) were defined at the age of observed subfertility onset (∼20 wk). To address physiological defects, an age-matched fertility study of n = 11 breeding cages per group across three consecutive litters with breeding starting at 11 wk of age when fertility of mutants was comparable to wild-types was undertaken. Crkl+/exon2Δ males exhibit a high penetrance of cryptorchidism (Fig. 7 A and D), smaller testes and lower testis-to-body-weight ratio (Fig. 7 B and E), lower sperm concentration (Fig. 7F), and abnormal testicular histology (Fig. 7C) compared with Crkl+/+ age-matched controls. Physiologically, Crkl+/exon2Δ males sire ∼30% fewer pups per litter than Crkl+/+ controls after their first litter, which is of normal size (Fig. 7G). All Crkl+/exon2Δ male mice tested produced at least one litter, and two of the experimental breeding cages stopped producing litters at or before their third litter. Approximately even numbers of Crkl+/+ and Crkl+/exon2Δ pups were recovered from experimental cages; this indicates that a potential loss of mutant embryos does not account for the lower pups per litter observed in experimental cages. Additionally, time between litters is unaffected (Fig. S6), indicating that although subfertile by their second litter, Crkl+/exon2Δ males are breeding at rates comparable to wild-type controls. Sperm motility was also unaffected (Fig. S7). Based on these findings, Crkl+/exon2Δ males exhibit age-related subfertility secondary to testicular atresia. Because the histological findings vary from that typically expected for cryptorchid testicular histology (26,27), the histology observed is likely primary to a role of Crkl in adult testicular function rather than secondary to cryptorchidism. These data indicate that Crkl is haploinsufficient for murine male fertility, and CRKL mutations may play a role in age-related subfertility and infertility in humans. Additionally, subfertility was observed in Crkl+/exon2Δ females (Fig. S8). It is important to note that female subfertility is not a result of oocyte malfunction, because females with exon 2 deleted specifically in oocytes (Crkl fl/fl;Gdf9-icre+) produced viable full-bodied Crkl+/exon2Δ offspring when mated to wild-type males.
Fig. 7.
Crkl+/exon2Δ males exhibit cryptorchidism, smaller testes, fewer sperm, and subfertility compared with Crkl+/+ controls. Eighteen- to 20-wk-old males (n = 11 per group) were analyzed for cryptorchidism (A) (testicles circled in red; bladder in green, penis indicated by white star), testicular size (B) (Inset: Left wild-type, Right Crkl+/exon2Δ). H&E staining of testes (C) confirmed abnormal testicular histology in approximately half of the Crkl+/exon2Δ seminiferous tubules with atresia and vacuolization of tubules apparent (*). (Magnification, 10×.) Distribution of cryptorchidism was graphed (D). Testicle weight was graphed as a proportion of body weight (E). Genotype-blinded sperm count was assessed by hemacytometry (F). n = 11 cages per group were mated at 11 wk of age and assessed for fertility across three litters (G). Error bars represent SEM.
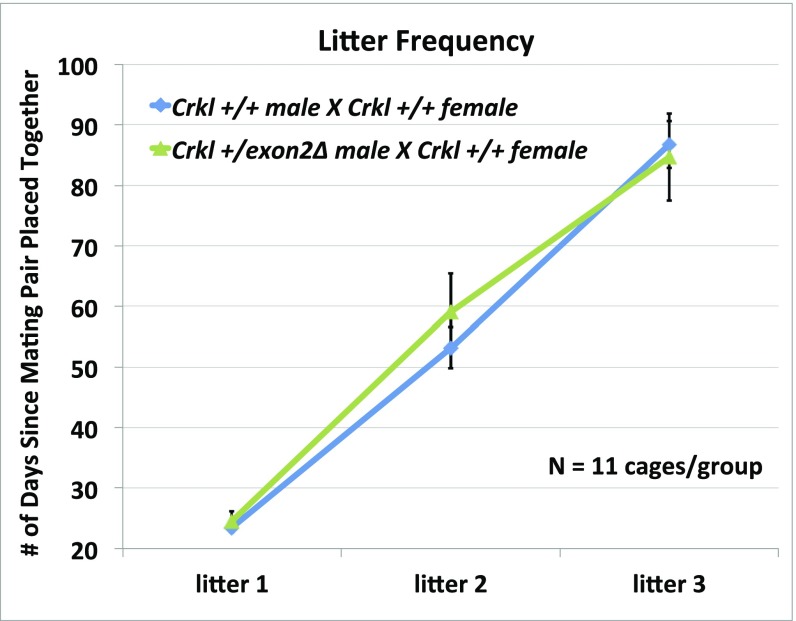
Fig. S6.
Litter frequency is unaffected in Crkl+/exon2Δ adult males. Although they exhibit fewer pups per litter compared with wild-types, Crkl+/exon2Δ adult males mate and produce their first three litters with normal frequency. Error bars represent SEM.
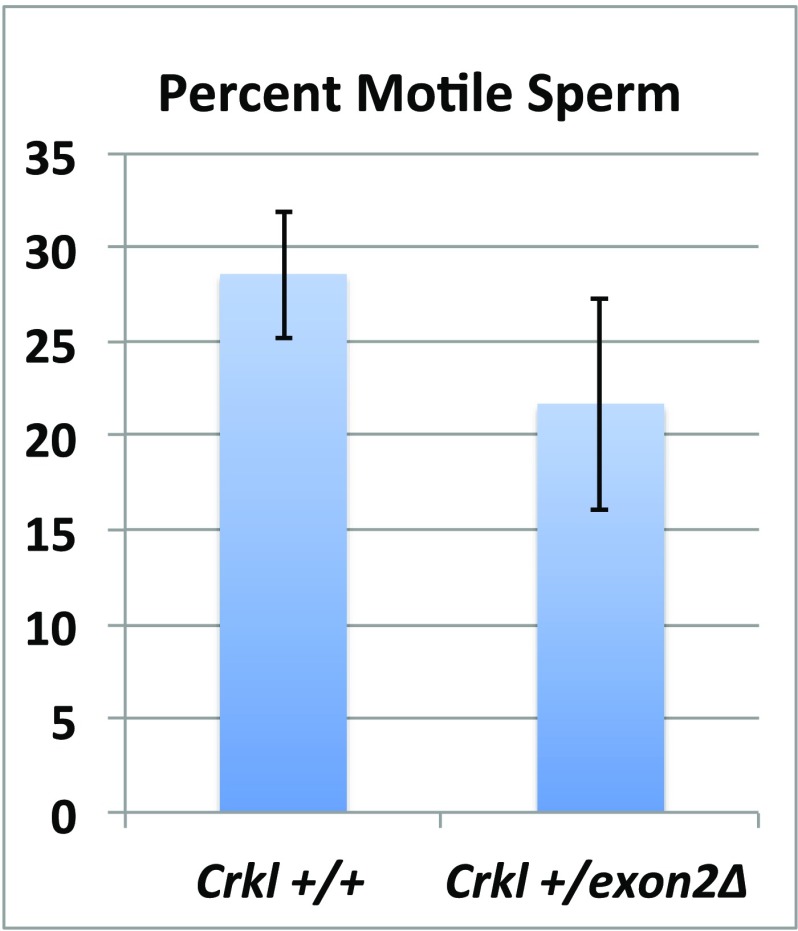
Fig. S7.
Sperm motility is unaffected in Crkl+/exon2Δ adult males. Although they exhibit fewer pups per litter and lower sperm count compared with wild-types, Crkl+/exon2Δ adult males do not exhibit significant motility defects. n = 11 per group. Error bars represent SEM.
Fig. S8.
Average pups per litter is decreased in Crkl heterozygote mutants of both sexes. n ≥ 50 litters per group (5 consecutive litters with n ≥ 11 cages per group) were analyzed for pup number. Heterozygote mutants of both sexes exhibit subfertility. Male subfertility was further assessed; female subfertility is the subject of future studies.
Discussion
The finding that unrelated, seemingly nonsyndromic patients with congenital GU defects exhibit CNVs clustering at 22q11.2 was, perhaps, not surprising given the fact that 22q11.2 dosage variation is a notoriously complex disease with high phenotypic variability and incomplete penetrance across phenotypes. Genetically complex disorders such as these require simplification to be understood. Deletion and overexpression models of individual genes within large copy-variant genomic loci are indispensable tools to unveiling the effect of each gene’s individual impact on a given CNV syndrome. Deletion of murine Crkl by our group (exon 2) and others (11, 13, 17) (null and conditional models) revealed that Crkl plays a pivotal role in the proper development of a wide variety of tissues whose structures and functions are deleteriously affected in 22q11.2 patients. The mouse model described in this paper shows that in addition to neural, heart, and thymic congenital defects, loss of Crkl also contributes to the GU defects characteristic of DiGeorge syndrome, and lends insight to the observation that similar GU anomalies are seen in a subset of nonsyndromic patients with smaller 22q11.2 aberrations.
Although not every phenotypic parameter was compared, the full-body Crkl exon 2 deletion model suggests that loss of exon 2 is sufficient to phenocopy a true null allele. This study indicates that loss of one copy of wild-type Crkl is sufficient to confer intrauterine growth restriction and adult testicular phenotypes, implicating that Crkl is haploinsufficient for a subset of biological structures and functions. This is especially important in the context of 22q11.2 deletions because patients generally still harbor one normal copy of each of the affected genes, rendering them functionally heterozygous. One of the main evolutionary benefits to a biallelic genome is the general unlikelihood that having only one copy of a given gene results in the generation of insufficient amounts of protein to perform that protein’s baseline function. In this sense, the second copy of a gene is conventionally thought to represent a backup of all of the data required for that particular genetic program to function. Although haploinsufficient genes are still considered rare, genes are regularly discovered to be dosage-sensitive, and clinically phenotypic syndromes associated with the monoallelic loss of a subset of genes point us in the direction of genes that are haploinsufficient for the development or function of tissues affected in a particular syndrome. In the case of Crkl, it appears that one wild-type copy is insufficient to sustain normal embryonic growth as well as normal adult testicular histology and function. Together, these findings suggest that patients with DiGeorge syndrome or smaller aberrations to the CRKL gene affecting one or both copies are more likely to exhibit embryonic growth restriction and subfertility in males as adults.
Similar to the high phenotypic variability and incomplete penetrance observed in 22q11.2 deletion patients, our Crkl exon 2 deletion mice exhibited partially penetrant congenital renal and ureteral anomalies, ranging from congenital hydronephrosis to unilateral renal hypoplasia and agenesis. The renal agenesis observed uniformly involved apparently normal ureteric growth (Fig. 4E), implying that the renal aplasia resultant from Crkl exon 2 deletion is secondary to impaired metanephric mesenchyme induction or response rather than aplasia of the ureteric bud. RNA-sequencing of Crkl exon 2-deleted embryonic kidneys revealed deregulation of 52 transcripts compared with wild-type controls, with a heavy bias toward transcript induction. This induction bias suggests that in the normal embryonic kidney, Crkl facilitates the maintenance of repression signaling across a variety of transcripts. One of the most heavily up-regulated renal genes in response to Crkl exon 2 deletion from the panel chosen for validation is Foxd1. Foxd1 is a transcription factor that promotes nephron progenitor differentiation (28). It is unclear whether overexpression of Foxd1 is detrimental to renal development; however, Foxd1 duplication is associated with branchial defects (29), which are also common phenotypes observed in 22q11.2 deletion (30). Another gene up-regulated in renal tissue in response to Crkl exon2 deletion is Adcy2. Adcy2 encodes adenylyl cyclase 2, an enzyme responsible for catalyzing the production of the signaling molecule cAMP in a wide range of tissues (31). A review of the DECIPHER database shows a correlation between CNVs (both deletions and duplications) covering ADCY2 and GU phenotypes, including hydronephrosis and ureteral atresia (patient 259229), VUR (patient 262907), and cryptorchidism (patients 279259 and 284051), and previous work has linked ADCY2 CNVs to abnormalities of the GU tract (14).
From these studies several conclusions can be drawn regarding the role of Crkl in GU development, as well as the role of exon 2 in Crkl function. Because the Crkl exon 2 deletion embryos closely mimic phenotypes observed in Crkl-null models, exon 2 deletion, despite producing a truncated protein from translation of exon 1, is most likely a complete loss-of-function mutation. However, it is yet possible that the truncated protein produced by exclusive deletion of Crkl exon 2 may retain some function, allowing overt renal phenotypes to persist only in homozygous but not heterozygous murine mutants; this could explain the discrepancy between renal phenotypes being present in humans heterozygous for 22q11.2 deletion but absent at the microdissection-level in Crkl+/exon2Δ mice. Another explanation could be that heterozygous phenotypes are less penetrant, less severe, and therefore not easily detectable by embryonic microdissection. Indeed, a recent study indicates that subtler, histologically detectable phenotypes are penetrant at low levels in Crkl+/exon2Δ embryos (32). Because loss of Crkl results in dose-dependent intrauterine growth restriction, and because heterozygous animals exhibit high rates of cryptorchidism and subfertility, it can be concluded that Crkl is a haploinsufficient gene for a subset of developmental processes. Additionally, it can be concluded that loss of both copies of wild-type Crkl confers increased susceptibility to structurally overt upper-tract GU defects. In this same vein, one can hypothesize that loss of a single wild-type copy of CRKL, perhaps by itself but especially in combination with modifying genetic aberrations, may be sufficient to induce CAKUT phenotypes of varying severity in patients with 22q11.2 deletion. Additionally, RNA sequencing identified several candidate pathways by which loss of Crkl may induce renal phenotypes. These candidate pathways will need to be further studied to narrow down the molecular means by which Crkl aberrations affect renal development and function. DiGeorge syndrome is among the many syndromic CNVs that can be screened for embryonically. These technological advances allow researchers to hypothesize that by combining molecular and genetic findings with embryonic screening of at-risk pregnancies, eventually it may be possible to manufacture molecular modifiers to help modulate the effects of syndromic CNVs in utero without undertaking the higher-risk tasks of modifying the embryonic genome or performing infant or in utero surgeries.
Materials and Methods
CNV qPCR of GU-abnormal patients was performed by isolation of genomic DNA from blood followed by CNV qPCR using 20-ng input template in triplicate and TaqMan CNV probe HS02102421, with informed consent from patients and Institutional Review Board approval from the Baylor College of Medicine. C. M. Vezina laboratory protocol (33) was used for in situ hybridization. Takara Clontech Human fetal cDNA panel Cat. 636747 was used with CRKL TaqMan probe HS00178304_m1 to determine human expression. Gdf9-icre females (34) (Jax stock #011062) were bred to Crkl fl/fl to generate Crkl exon2Δ mutants. Mutant cDNA was sequenced for validation. Embryonic GU tracts were microdissected for anatomical assessment. RNA from n ≥ 3 male E16.5 kidneys per genotype was sequenced and reads were mapped, analyzed, and a subset was validated by qPCR. Age-matched males were assessed for testicular parameters and prospective fertility study with endpoint of pups per litter. All mouse work was performed in strict concordance to animal protocols approved by the Institutional Animal Care and Use Committee of the University of Chicago, as well as Baylor College of Medicine. Please refer to SI Materials and Methods files for detailed materials and methods and Fig. S9 for RNA-sequencing samples and read quality.
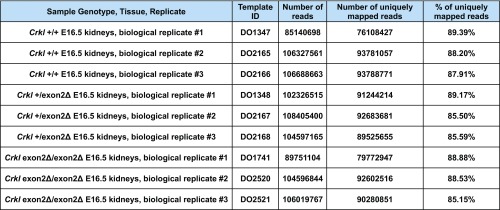
Fig. S9.
RNA-sequencing samples and read quality. Chart indicates raw number of reads per sample, raw number of uniquely mapped reads per sample, and percent of uniquely mapped reads per sample.
SI Materials and Methods
Experimental Design.
This study aimed to map the minimal region of 22q11.2 dosage variation in GU patients and assess the optimal candidate gene within the minimal region (CRKL) for expression and function in GU systems. This was achieved by in situ hybridization and qPCR studies to delineate CRKL/Crkl expression pattern in the GU tract, and by assessment of Crkl mutant embryos. Weights and pictures of littermate embryos were compared, and each embryo was genotype-blind microdissected to reveal overt congenital GU anomalies, which were documented by photograph, histology, and CT scans. The presence of renal phenotypes indicates a need to delineate underlying molecular mechanisms, which was achieved by RNA-sequencing of phenotypically normal embryonic renal tissue from mutants and controls. Additionally, adult male mutants and controls were subjected to a panel of GU phenotype assessments, including dissection, VUR assay, bladder capacity assessment, cryptorchidism assessment, and fertility assessment (sperm motility, sperm count, testis histology, and prospective fertility assay). Genotype-blinded assessments were used wherever possible (during microdissections of embryos, sperm count, and sperm motility measures). Sufficient “n” to establish statistically significant differences were calculated by power analysis after preliminary data were collected.
CNV Overlap Mapping and Identification of Candidate Genes.
The DECIPHER database was queried for copy number gains and losses at the 22q11.2 locus and CNV breakpoints of patients with phenotypes relevant to GU development were mapped. These data were combined with CGH-based microarray analysis data (14) from our own laboratory and all GU-relevant CNVs were mapped to determine the smallest region of maximal CNV overlap indicating the presence of candidate genes. The five genes in the minimal region (CRKL, pLI = 0.16; AIFM3, pLI = 0; LZTR1, pLI = 0; THAP7, pLI = 0.03; and P2RX6, pLI = 0) were then queried in the ExAC database to determine likelihood of haploinsufficiency, indicating CRKL as the gene with the highest likelihood of haploinsufficiency (pLI = 0.16). CNV qPCR of GU-abnormal patients was performed by isolation of genomic DNA from blood (Gentra Puregene Kit, Cat. 158389) followed by CNV qPCR using 20-ng input template in triplicate and TaqMan CNV probe HS02102421 according to the manufacturer’s protocol, with informed consent from patients and Institutional Review Board approval from the Baylor College of Medicine.
In Situ Hybridization.
Primers were designed against Crkl cDNA. T7 promoter sequence was added to the 5′ end of the reverse primer for the antisense probe (antisense forward: GGCCACTGCTCTTGTAACCT; antisense reverse: CGATGTTAATACGACTCA-CTATAGGGACGCTCACAGCAGTTAACCA) and the 5′ end of the forward primer for the sense probe (sense forward: CGATGTTAATACGACTCA-CTATAGGGGGCCACTGCTCTTGTAACCT; sense reverse: ACGCTCACAGCAGTTAACCA). Primers were used to amplify Crkl cDNA from whole embryos. Phusion polymerase PCR was performed and products were purified by gel extraction using Qiagen kit Cat. 28706. RNA probes were in vitro transcribed using Sigma (Roche) DIG RNA Labeling Kit (SP7/T7) Cat. 11175025910. Wild-type ICR embryos were collected at E12.5 for whole-mount embryos and E16.5 for whole-mount isolated GU tracts, fixed in 4% PFA at 4 °C overnight, followed by serial methanol dehydration. Specimens then underwent DIG staining according to C. M. Vezina Laboratory protocol (33) and were imaged using SZX10 Olympus dissection microscope.
Human CRKL Expression Panel.
Human fetal cDNA panel Cat. 636747 was purchased from Takara Clontech consisting of cDNA of spontaneously aborted human fetuses aged 16–40 wk. cDNA was assayed from brain, lung, liver, kidney, heart, spleen, thymus, and skeletal muscle using CRKL TaqMan probe HS00178304_m1 and normalized to GAPDH. The tissue with the lowest CRKL expression (heart) was set to one and all other tissues are represented relative to one.
Derivation of Crkl Exon2Δ/Exon2Δ Embryos.
A cre-dependent conditional allele (Crkl exon2 fl or Crkl f2) was derived from Crkltm1a(EUCOMM)Hmgu after removal of a splice acceptor-IRES-βgal and β-actin-neo cassettes by FLPeR strain [B6;129S4-Gt(ROSA)26Sortm2Dym/J] followed by segregation of the FLPeR. This resultant allele included one FRT and one loxP sites in intron 1, and one loxP site in intron 2 of the Crkl gene, designed to delete exon 2 upon cre-mediated recombination. Crkl exon2 fl/fl males were mated with Gdf9-icre females (34) (Jackson Laboratories stock #011062) to generate oocyte-specific deletion Crkl exon2 fl/+; Gdf9-icre+ females. These females were then bred to C57/BL6 wild-type males to generate Crkl+/exon2Δ progeny, which were then interbred to generate Crkl exon2Δ/exon2Δ animals. Embryonic lethality required our studies to be completed via timed matings and the collection of Crkl exon2Δ/exon2Δ embryos and their littermates at time points E16.5, E17.5, and E18.5. Multiplex genotyping primers are Crkl int1 F2: 5′- TCAGCCAGGAGAGGAGGATCATCAA; Crkl int1 R: 5′-TGGCTCTC-GTCCTGCATAGGTCA; Crkl int2 R1: 5′- GAGGCCAGGACAGCTGGGACTAC with expected band sizes at 484 bp (deleted allele); 358 bp (floxed allele); 194 bp (wild-type allele). Genotyping was performed using Promega GoTaq green Cat. M7123 with 56 °C annealing temperature. Gdf9-icre animals were donated to D.J.L.’s laboratory, courtesy of Austin Cooney, Department of Pediatrics, Dell Pediatric Research Institute, University of Texas at Austin Dell Medical School, Austin, TX. All mouse work was performed in strict concordance to animal protocols approved by the Institutional Animal Care and Use Committee of the University of Chicago as well as that of Baylor College of Medicine. The Crkl conditional mutant strain will be available shortly through The Jackson Laboratory as B6;129S4-Crkl < tm1c(EUCOMM)Hmgu>/ImoJ (Jackson Laboratories stock #30030).
Validation of Crkl Exon 2 Deletion.
Total RNA was collected from E16.5 Crkl exon2Δ/exon2Δ kidneys using a dounce homogenizer and zymo RNA isolation kit Cat. R1055. Next, 500 ng of total RNA was converted to cDNA using TermoFisher High-Capacity Reverse Transcription Kit Cat. 4368814. Primers were designed flanking exon 2 of Crkl cDNA and used to amplify PCR products from cDNA of Crkl exon2Δ/exon2Δ E16.5 kidneys: forward: CCTCCGCCAGGTTTGATTCT; reverse: ACTC-GTTGTCATCGGGGTTC (440-bp product) and sequenced bidirectionally by Genewiz using Sanger Sequencing. Sequences were aligned to RefSeq to verify transcripts are missing exon 2 of Crkl.
Microdissection and Histological Analysis of GU Tracts.
Timed matings between Crkl+/exon2Δ animals were performed and litters were collected at E16.5, E17.5, and E18.5, which included Crkl exon2Δ/exon2Δ embryos. Embryo pictures and weights were documented upon litter collection. GU tracts (kidneys, ureters, bladder, gonads, müllerian and wolffian duct derivatives, and genital tubercle) were collected by microdissection and any observable gross anatomical GU malformations were noted. GU tracts were then fixed in bouins overnight and transferred to 70% ethanol before being oriented in biopsy pads for paraffin embedding. Sections of specimens were taken at 5 μ and stained with H&E for histological analysis.
RNA-Sequencing, Analysis, and Validation.
Total RNA was isolated from n ≥ 3 male wild-type, Crkl+/exon2Δ, and Crkl exon2Δ/exon2Δ E16.5 nonphenotypic kidneys using a dounce homogenizer and zymo RNA isolation kit Cat. R1055. RNA was DNase-treated and next-generation sequenced by BCM GARP RNA-sequencing core at ∼80 million reads per sample. Nonphenotypic kidneys were used to exclude differential expression secondary to organ stress. The Genomic and RNA Profiling Core first conducted sample quality checks using the NanoDrop spectrophotometer and Agilent Bioanalyzer 2100, then used Illumina TruSeq RNA library preparation protocol. A double-stranded DNA library was created using 250 ng of total RNA (measured by picogreen) plus ERCC Spike-in Mixes, preparing the fragments for hybridization onto a flow cell. First, cDNA was created using the fragmented 3′ poly(A) selected portion of total RNA and random primers. During second-strand synthesis, dTTP is replaced with dUTP, which quenches the second strand during amplification, thereby achieving strand specificity. Libraries were created from the cDNA by first blunt ending the fragments, attaching an adenosine to the 3′ end, and finally ligating unique adapters to the ends. The ligated products were then amplified using 15 cycles of PCR. The resulting libraries were quantitated using the NanoDrop spectrophotometer and fragment size assessed with the Agilent Bioanalyzer. A qPCR quantitation was performed on the libraries to determine the concentration of adapter ligated fragments using Applied Biosystems ViiA7 Real-Time PCR System and a KAPA Library Quant Kit. All samples were pooled equimolar, requantitated by qPCR, and reassessed on the Bioanalyzer. Using the pooled concentration from the qPCR assay, the library pool was loaded onto three lanes of a high-output flow cell at a concentration of 30pM and amplified by bridge amplification using the Illumina cBot instrument, v4 chemistry. A paired-end 100 cycle run was used to sequence the flow cell on a HiSEq. 2500 instrument. Differences in number of reads per sample were calculated and used to generate heat maps with FDRs < 0.05. The pair-ended reads were mapped to the mouse genome (UCSC mm10) using STAR (35) with National Center for Biotechnology Information RefSeq genes as the reference. Overall, about 88% of the reads were uniquely mapped to the mouse genome (Fig. S9). To reduce possible PCR biases, read duplicates were removed using Picard tools (broadinstitute.github.io/picard/). HTseq (www-huber.embl.de/users/anders/HTSeq) was used to determine the number of reads falling in the known genes. DESeq2 (36) was used to identify differentially expressed genes between the groups of interest. To perform the differential expression analysis, DESeq2 first normalized the data by calculating a factor for each sample and then scaled the gene-expression value by the factor. After normalization, DESeq2 fitted generalized linear models for the genes and performed tests to identify differentially expressed genes. The FDR of the differentially expressed genes was estimated using Benjamini and Hochberg method. FDR < 0.05 were considered statistically significant. A subset of differentially expressed genes previously indicated in renal development was validated using TaqMan qPCR.
VUR Assessment.
Kidneys and bladder of anesthetized Crkl+/exon2Δ males and wild-type controls aged matched to 10–12 wk were visualized by dissection. A 27-gauge butterfly needle with 5- to 8-inch tubing was used to first aspirate any urine present in the bladder and then inject filtered 2% methylene blue in PBS into the bladder as slowly as possible. Bladder expansion was observed and recorded in units of microliter of dye injected, and bladder capacity was considered reached when urethral leakage was observed. Presence or absence and location of VUR was recorded with the presence indicated by dye fluxing from bladder up ureters to kidney either unilaterally or bilaterally.
Analysis of Testis and Sperm Parameters.
Crkl+/exon2Δ and wild-type control males aged 18–20 wk were analyzed for testis position after being killed, by massaging inguinal canals in a downward direction to avoid false discovery of retractile testis as cryptorchid testis. Mice were then dissected and pictures of testicular position were taken. Position was analyzed relative to the bladder and penis. Testes positioned beside the bladder were considered inguinal cryptorchid; testes positioned beside or directly above the penis were considered prescrotal cryptorchid; testes inside the scrotum were considered to have descended properly. Testes were isolated and weighed; testicular weight was graphed normalized to body weights. Testes were then fixed in bouin’s overnight and moved to 70% ethanol before further dehydration and embedding in paraffin for 5-μm sectioning and H&E analysis. Epididymis were isolated and placed into prewarmed 37 °C HTF media, minced, and allowed to incubate for 30 min at 37 °C before sperm were analyzed by: (i) sperm motility by sperm smear as calculated by percent motile out of total sperm; and (ii) sperm count as calculated by hemacytometer. Sperm assessment was genotype-blinded.
Fertility Assessment.
For fertility assessment, n ≥ 11 cages per group were set up into the following groups with animals aged 11 wk: group 1: wild-type male × wild-type female (control); group 2: Crkl+/exon2Δ male × wild-type female (experimental); group 3: Crkl+/exon2Δ female × wild-type male. Fertility was assessed as pups per litter average, as well as normalized to litter number. Even representation of wild-type and Crkl+/exon2Δ embryos in experimental crosses indicate that Crkl+/exon2Δ embryos are not being lost in utero and are not affecting pups per litter measurements.
Statistical Analysis.
Frequency comparisons (i.e., affected versus unaffected) were assessed by Fisher’s exact test with P < 0.05 considered significant. Professional statistical analyst Q.M. compiled the heat map of RNA-sequencing results with FDR < 0.05. All other statistical comparisons were performed by Student’s t test with P < 0.05 considered significant. Per group “n” values are listed in each figure or figure legend.
Supplementary Material
Acknowledgments
We thank Cenk Cengiz for performing genotype-blinded sperm counts and Marisol O’Neill for performing micro-CT. Portions of this project were made possible by the Baylor College of Medicine Genomic and RNA Profiling (GARP) Core and the UC Transgenics/ES Technology Core. This work was funded by NIH Grants T32DK007763 and R01DK078121.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619523114/-/DCSupplemental.
References
- 1.Oskarsdóttir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: A population-based study in western Sweden. Arch Dis Child. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu HY, et al. Genitourinary malformations in chromosome 22q11.2 deletion. J Urol. 2002;168:2564–2565. doi: 10.1016/S0022-5347(05)64215-2. [DOI] [PubMed] [Google Scholar]
- 3.Acerini CL, Miles HL, Dunger DB, Ong KK, Hughes IA. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch Dis Child. 2009;94:868–872. doi: 10.1136/adc.2008.150219. [DOI] [PubMed] [Google Scholar]
- 4.Springer A, van den Heijkant M, Baumann S. Worldwide prevalence of hypospadias. J Pediatr Urol. 2016;12:152.e1–152.e7. doi: 10.1016/j.jpurol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Hughes IA, Houk C, Ahmed SF, Lee PA. LWPES Consensus Group ESPE Consensus Group Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91:554–563. doi: 10.1136/adc.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaou N, Renkema KY, Bongers EMHF, Giles RH, Knoers NVAM. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol. 2015;11:720–731. doi: 10.1038/nrneph.2015.140. [DOI] [PubMed] [Google Scholar]
- 7.ten Hoeve J, et al. Cellular interactions of CRKL, and SH2-SH3 adaptor protein. Cancer Res. 1994;54:2563–2567. [PubMed] [Google Scholar]
- 8.Segovis CM, et al. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol. 2009;182:6933–6942. doi: 10.4049/jimmunol.0803840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lian X, et al. CrkL regulates SDF-1-induced breast cancer biology through balancing Erk1/2 and PI3K/Akt pathways. Med Oncol. 2015;32:411. doi: 10.1007/s12032-014-0411-z. [DOI] [PubMed] [Google Scholar]
- 10.Senechal K, Halpern J, Sawyers CL. The CRKL adaptor protein transforms fibroblasts and functions in transformation by the BCR-ABL oncogene. J Biol Chem. 1996;271:23255–23261. doi: 10.1074/jbc.271.38.23255. [DOI] [PubMed] [Google Scholar]
- 11.Moon AM, et al. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo J-H, Suenaga A, Hatakeyama M, Taiji M, Imamoto A. Structural and functional basis of a role for CRKL in a fibroblast growth factor 8-induced feed-forward loop. Mol Cell Biol. 2009;29:3076–3087. doi: 10.1128/MCB.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat Genet. 2001;27:293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- 14.Tannour-Louet M, et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS One. 2010;5:e15392. doi: 10.1371/journal.pone.0015392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San Agustin JT, et al. Genetic link between renal birth defects and congenital heart disease. Nat Commun. 2016;7:11103. Erratum in Nat Commun 7:11910. doi: 10.1038/ncomms11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racedo SE, et al. Mouse and human CRKL is dosage sensitive for cardiac outflow tract formation. Am J Hum Genet. 2015;96:235–244. doi: 10.1016/j.ajhg.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George B, et al. Crk1/2 and CrkL form a hetero-oligomer and functionally complement each other during podocyte morphogenesis. Kidney Int. 2014;85:1382–1394. doi: 10.1038/ki.2013.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draaken M, et al. Classic bladder exstrophy: Frequent 22q11.21 duplications and definition of a 414 kb phenocritical region. Birth Defects Res A Clin Mol Teratol. 2014;100:512–517. doi: 10.1002/bdra.23249. [DOI] [PubMed] [Google Scholar]
- 19.Tucker T, et al. Prevalence of selected genomic deletions and duplications in a French-Canadian population-based sample of newborns. Mol Genet Genomic Med. 2013;1:87–97. doi: 10.1002/mgg3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, et al. Subtelomeric rearrangements and 22q11.2 deletion syndrome in anomalous growth-restricted fetuses with normal or balanced G-banded karyotype. Ultrasound Obstet Gynecol. 2006;28:939–943. doi: 10.1002/uog.3884. [DOI] [PubMed] [Google Scholar]
- 21.Boualia SK, et al. Vesicoureteral reflux and other urinary tract malformations in mice compound heterozygous for Pax2 and Emx2. PLoS One. 2011;6:e21529. doi: 10.1371/journal.pone.0021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hains DS, et al. High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J Urol. 2010;183:2077–2084. doi: 10.1016/j.juro.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu OH, Murawski IJ, Myburgh DB, Gupta IR. Overexpression of RET leads to vesicoureteric reflux in mice. Am J Physiol Renal Physiol. 2004;287:F1123–F1130. doi: 10.1152/ajprenal.00444.2003. [DOI] [PubMed] [Google Scholar]
- 24.Bowen SE, Watt CL, Murawski IJ, Gupta IR, Abraham SN. Interplay between vesicoureteric reflux and kidney infection in the development of reflux nephropathy in mice. Dis Model Mech. 2013;6:934–941. doi: 10.1242/dmm.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paredes J, et al. Assessing vesicoureteral reflux in live inbred mice via ultrasound with a microbubble contrast agent. Am J Physiol Renal Physiol. 2011;300:F1262–F1265. doi: 10.1152/ajprenal.00720.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechlin CW, Kogan BA. What lessons can be learned from testicular histology in undescended testes? Transl Androl Urol. 2014;3:365–369. doi: 10.3978/j.issn.2223-4683.2014.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta S, Joshi KR, Sengupta P, Bhattacharya K. Unilateral and bilateral cryptorchidism and its effect on the testicular morphology, histology, accessory sex organs, and sperm count in laboratory mice. J Hum Reprod Sci. 2013;6:106–110. doi: 10.4103/0974-1208.117172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fetting JL, et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balikova I, Devriendt K, Fryns JP, Vermeesch JR. FOXD1 duplication causes branchial defects and interacts with the TFAP2A gene implicated in the branchio-oculo-facial syndrome in causing eye effects in zebrafish. Mol Syndromol. 2010;1:255–261. doi: 10.1159/000327707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- 31.Edelhoff S, Villacres EC, Storm DR, Disteche CM. Mapping of adenylyl cyclase genes type I, II, III, IV, V, and VI in mouse. Mamm Genome. 1995;6:111–113. doi: 10.1007/BF00303253. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Rivera E, et al. Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med. 2017;376:742–754. doi: 10.1056/NEJMoa1609009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abler LL, et al. A high throughput in situ hybridization method to characterize mRNA expression patterns in the fetal mouse lower urogenital tract. J Vis Exp. 2011;54:e2912. doi: 10.3791/2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- 35.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.