Significance
One of the posttranscriptional modifications of tRNA, 2-thiouridine (s2U), enhances thermostability. Although extensive studies have been conducted to understand the mechanism behind this modification, many ill-defined points remain, because the S-transfer enzyme 2-thiouridine synthetase TtuA has shown very low activity in previous in vitro experiments. Here we demonstrate that TtuA requires oxygen-labile [4Fe-4S] clusters for its activity. Furthermore, we determine the crystal structure of TtuA in complex with the Fe-S cluster and ATP analog and also with its S-donor protein, 2-thiouridine synthesis sulfur carrier protein (TtuB). The combined actions of TtuA and TtuB using the Fe-S cluster aid the S-transfer mechanism.
Keywords: Fe-S cluster, sulfur transfer, tRNA modification, 2-thiouridine, crystal structure
Abstract
Two-thiouridine (s2U) at position 54 of transfer RNA (tRNA) is a posttranscriptional modification that enables thermophilic bacteria to survive in high-temperature environments. s2U is produced by the combined action of two proteins, 2-thiouridine synthetase TtuA and 2-thiouridine synthesis sulfur carrier protein TtuB, which act as a sulfur (S) transfer enzyme and a ubiquitin-like S donor, respectively. Despite the accumulation of biochemical data in vivo, the enzymatic activity by TtuA/TtuB has rarely been observed in vitro, which has hindered examination of the molecular mechanism of S transfer. Here we demonstrate by spectroscopic, biochemical, and crystal structure analyses that TtuA requires oxygen-labile [4Fe-4S]-type iron (Fe)-S clusters for its enzymatic activity, which explains the previously observed inactivation of this enzyme in vitro. The [4Fe-4S] cluster was coordinated by three highly conserved cysteine residues, and one of the Fe atoms was exposed to the active site. Furthermore, the crystal structure of the TtuA-TtuB complex was determined at a resolution of 2.5 Å, which clearly shows the S transfer of TtuB to tRNA using its C-terminal thiocarboxylate group. The active site of TtuA is connected to the outside by two channels, one occupied by TtuB and the other used for tRNA binding. Based on these observations, we propose a molecular mechanism of S transfer by TtuA using the ubiquitin-like S donor and the [4Fe-4S] cluster.
Thiolation of nucleotides is a posttranscriptional modification of RNA widely conserved in three domains of life. To date, 22 types of thionucleosides have been identified (1), classified into four major groups: 2-thiouridine (s2U), 4-thiouridine (s4U), 2-thiocytidine (s2C), and 2-methylthio-N6-alkyladenosine (2). Most of these modifications are found in transfer RNA (tRNA), where they have various important cellular roles, such as controlling the fidelity of translation (3–5), recognizing aminoacyl-tRNA synthetase (6), sensing UV radiation stress (7), and stabilizing the ternary structure of tRNA for growth at high temperatures (8–10).
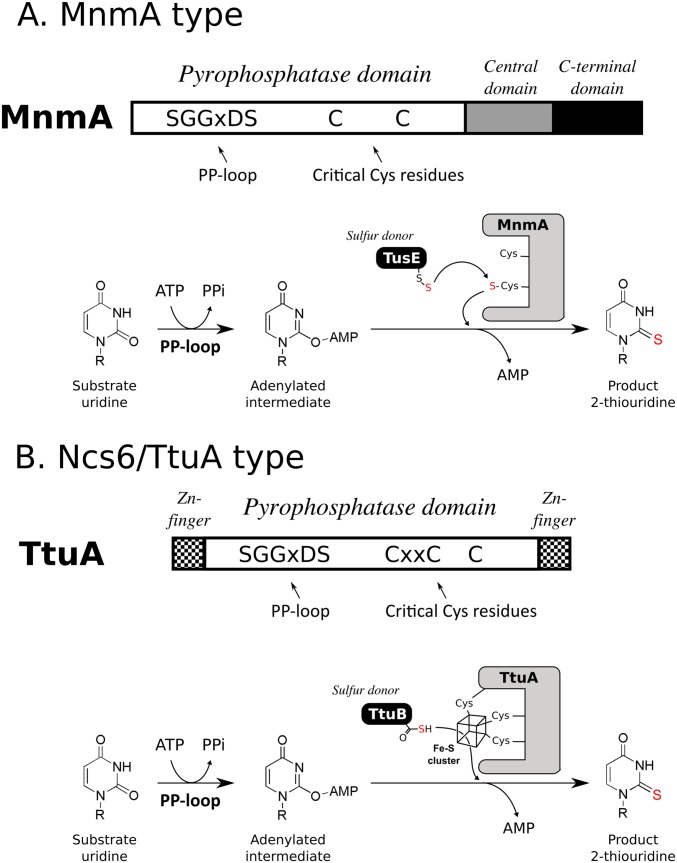
The sulfurtransferases of s2U can be divided into two major groups: tRNA-specific 2-thiouridylase MnmA type and cytoplasmic tRNA 2-thiolation protein Ncs6/2-thiouridine synthetase TtuA type (10). The MnmA type is a well-studied prototype of tRNA thiolation enzymes. Bacterial MnmA is responsible for 5-methylaminomethyl-2-thiouridine at position 34 of several tRNAs (11). The thiolation reaction of this type of enzyme consists of two steps: a first activation step by ATP to form an adenylated intermediate of the target base, and a second nucleophilic substitution step of the sulfur (S) atom for the adenyl group (Fig. S1A) (12). This reaction is conducted by the N-type ATP-pyrophosphatase domain of the enzyme. A PP-loop motif (SGGXDS) located in this domain contributes to the hydrolysis of ATP in the first step. In the second step, a persulfide (R-SSH) S formed on modification enzymes nucleophilically attacks the adenylated intermediate, resulting in formation of the thiocarboxylated base. R-SSH is first generated from cysteine (Cys) by a Cys desulfurase, IscS (13, 14), then transferred directly or via S-carrier proteins to the catalytic Cys residue of MnmA, and finally used as an S donor (Fig. S1A).
Fig. S1.
Schematic representation of the characteristics of MnmA-type (A) and Ncs6/TtyA-type (B) sulfurtransferases. Orientation of the PP-loop and critical Cys residues in the pyrophosphatase domain (Top), as well as the respective sulfur donor and reaction mechanism (Bottom) of MnmA and TtuA, are shown for comparison. The following characteristics of TtuA noted in this study are shown: TtuA has an [4Fe-4S] cluster; this cluster is essential for sulfur transfer activity; and the detailed role of the cluster remains unclear. The transferred sulfur atoms are colored in red.
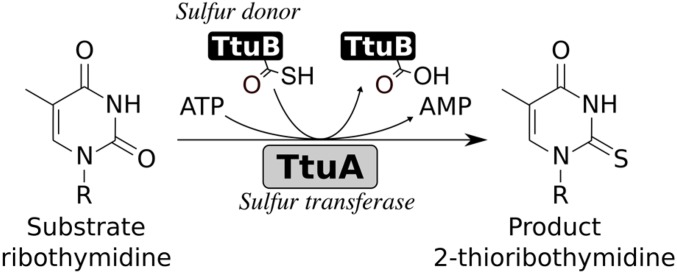
Another prototype of thiolation enzymes, the Ncs6/TtuA type, is found in eukaryotic cytoplasm and thermophilic bacteria. The Ncs6/Ncs2 heterocomplex (15, 16) and TtuA (17) are sulfurtransferases responsible for 5-methoxycarbonylmethyl-2-thiouridine at position 34 of several cytosolic tRNAs (tRNAGlu/Gln/Lys) in eukaryotes and for 5-methyl-2-thiouridine (m5s2U, or s2T) (Fig. 1) at position 54 of almost all tRNA species in Thermus thermophilus, respectively (Fig. 1 and Fig. S1B). These proteins share the same catalytic domain (N-type ATP-pyrophosphatase domain) with MnmA-type enzymes, but have more conserved Cys residues (Figs. S1B and S2). Although a previous structural study has shown that 3 of 10 conserved Cys residues accumulate in the catalytic center, their functional roles remain unclear (18). It should be noted that the full in vitro enzymatic activity could not be confirmed for either Ncs6 or TtuA, and that quite weak activity was detected only after a cell extract was added, which hinders investigation of the molecular basis of S transfer by these types of enzymes. Based on these observations, it has been suggested that additional factor(s) may be required for the enzymatic activity of Ncs6 and TtuA (19, 20). More recently, TtcA, which is a homolog of Ncs6/TtuA but catalyzes s2C synthesis at position 32 (21) of bacterial tRNA, was reported to require an Fe-S cluster for its activity (22). This finding suggests that Ncs6/TtuA also has an Fe-S cluster.
Fig. 1.
Sulfur transfer reaction catalyzed by TtuA and C-terminal thiocarboxylated TtuB.
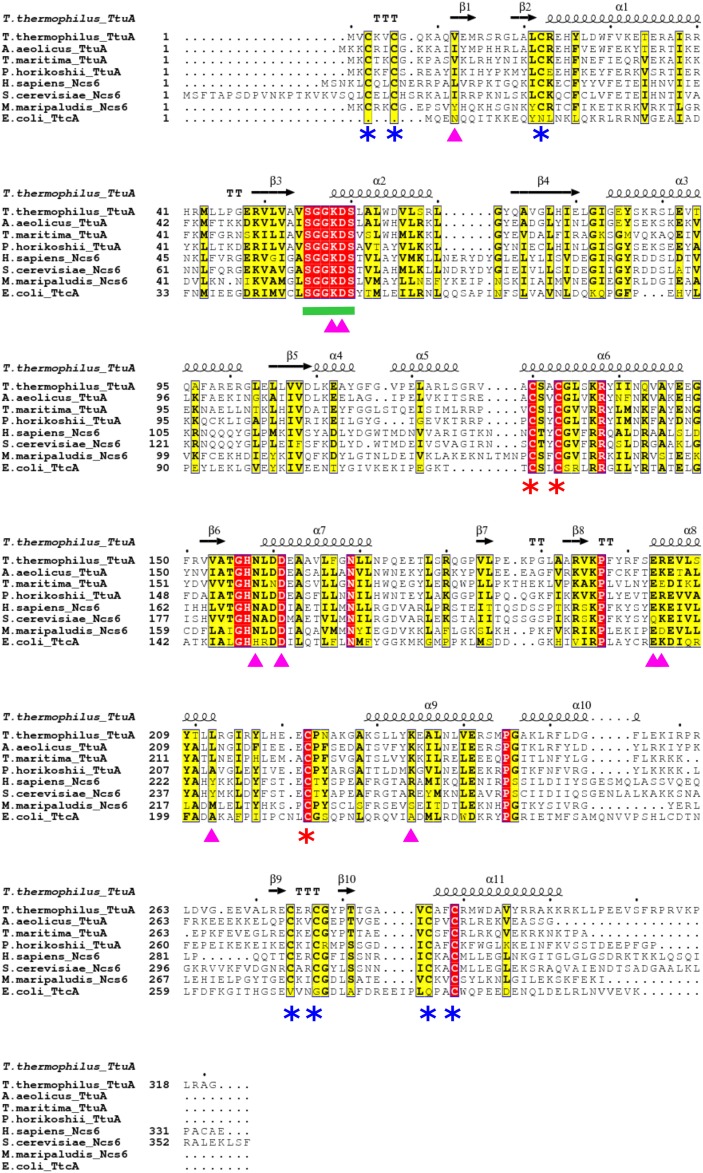
Fig. S2.
Sequence alignment of TtuA and related proteins. The amino acid sequence of TthTtuA is aligned to the sequences of other TtuA/Ncs6 proteins from Aquifex aeolicus, Thermotoga maritima, P. horikoshii, Homo sapiens, Saccharomyces cerevisiae, and M. maripaludis. The amino acid sequence of E. coli TtcA is also aligned. The secondary structure of TthTtuA is shown at the top. The completely conserved residues are highlighted in red, the highly conserved residues are highlighted in yellow, the PP-loop is indicated by the green line, the three Fe-S cluster-binding Cys residues are indicated by red asterisks, and the other Cys residues involved in Zn binding are indicated by blue asterisks.
Distinct from the MnmA-type enzymes, Ncs6/Ncs2 and TtuA have another prominent characteristic on the S donor. The S atom is supplied as a thiocarboxylate moiety (R-COSH) on the C terminus of an ubiquitin-like S-carrier protein, i.e., Urm1 in eukaryotes (15–17, 23–25) and 2-thiouridine synthesis sulfur carrier protein TtuB in thermophiles (17, 20) (Fig. 1 and Fig. S1B). How Urm1/TtuB transfers the activated S atom to Ncs6/TtuA, which is necessary to elucidate the S-transfer mechanism catalyzed by Ncs6/TtuA-type enzymes, remains unclear, however.
In the present study, we characterize the sulfurtransferase TtuA from T. thermophilus from biochemical, spectroscopic, and structural viewpoints. We demonstrate that a [4Fe-4S] type of an Fe-S cluster is essential for the activity of TtuA. Furthermore, we determine the crystal structures of TtuA in complex with the [4Fe-4S] cluster and ATP analog (AMPPNP) and the TtuA-TtuB complex. Based on these results, we reveal a molecular basis of the s2U biosynthesis mechanism involving the Fe-S protein and ubiquitin-like S-donor protein.
Results
TtuA Is an Oxygen-Labile Iron-Sulfur Protein.
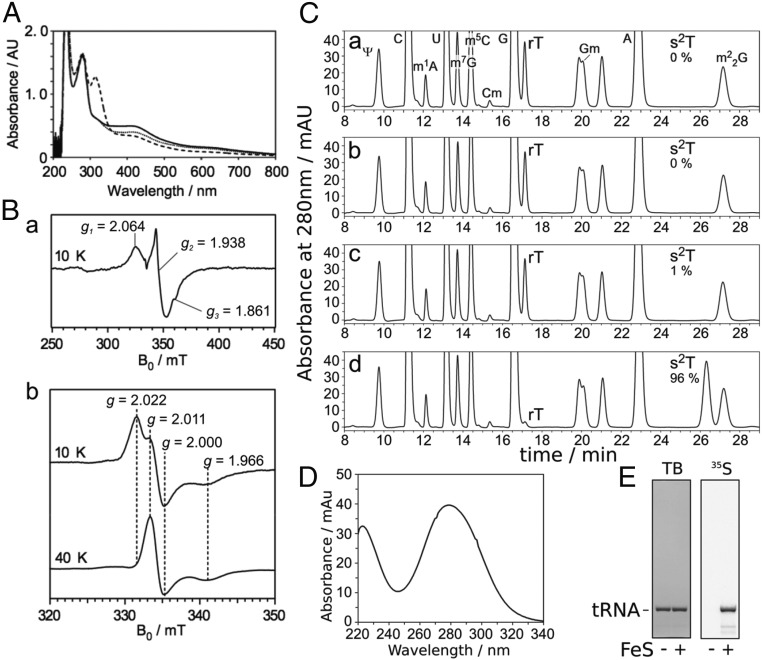
We first investigated T. thermophilus TtuA from a spectroscopic standpoint. TtuA expressed in Escherichia coli and purified under aerobic conditions is almost colorless and exhibits no UV-visible (UV-VIS) spectrum suggestive of an Fe-S cluster (17). Because a component of Fe-S protein is known to be sensitive to oxygen, we attempted anaerobic reconstitution of the TtuA Fe-S cluster. When the aerobically purified TtuA (apo-TtuA) was incubated with Fe and sulfide in anaerobic conditions, a brown solution of the TtuA protein was obtained that exhibited a UV-VIS spectrum with a shoulder at ∼410 nm (Fig. 2A), which is characteristic of Fe-S proteins, namely the [4Fe-4S] form. The Fe content of reconstituted TtuA was determined to be 3.84 ± 0.08 mol per mol of TtuA, which also suggests the presence of a [4Fe-4S]-type Fe-S cluster.
Fig. 2.
The iron-sulfur cluster on TtuA is required for s2T synthesis in vitro. (A) UV-VIS spectrum of TtuA. Reconstituted TtuA (solid line), TtuA treated with dithionite (dashed line), and TtuA treated with ferricyanide (dotted line) are shown. TtuA was diluted to 1.0 mg/mL in buffer A. The peak at around 310 nm in the reduced sample was derived from excess dithionite. (B) EPR spectrum of TtuA treated with dithionite at 10 K (a), and EPR spectra of TtuA treated with ferricyanide at 10 K (b, Upper) and 40 K (b, Lower). Principal g values are shown. (C) Nucleoside analysis of yeast tRNAPhe reacted in vitro. Apo-TtuA was treated with DTT (a), DTT and FeCl3 (b), DTT and Na2S (c), or DTT, FeCl3, and Na2S (d). Using these TtuA samples, tRNA (450 pmol) was reacted at 60 °C for 1 h with 75 pmol TtuA, TtuB, TtuC, TtuD, and SufS in the presence of 0.1 mM Cys and 2.5 mM ATP. The modified nucleosides of reacted RNA were analyzed by HPLC. The amounts of s2T formed are indicated; complete conversion of rT to s2T was set to 100%. (D) UV spectrum of s2T detected at 26.3 min in C, d. (E) Incorporation of 35S-sulfur to s2T from [35S]Cys. Yeast tRNAPhe (450 pmol) was reacted at 60 °C for 1 h with 75 pmol apo- or holo-TtuA, TtuB, TtuC, TtuD, and SufS in the presence of 0.1 mM [35S]Cys and 2.5 mM ATP. The reacted RNA was separated by 10% (wt/vol) denaturing PAGE and then stained with toluidine blue (TB; Left), after which 35S radioactivity was visualized (Right).
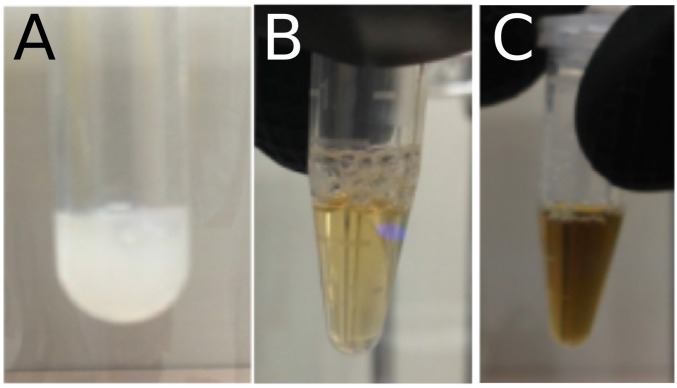
To clarify the properties of the Fe-S center of TtuA in detail, we analyzed reconstituted TtuA by electron paramagnetic resonance (EPR) spectroscopy. Upon reduction by excess dithionite, the absorbance at ∼410 nm was decreased (Fig. 2A). Reduced TtuA exhibited an EPR spectrum with principal g values of 2.06, 1.94, and 1.86 at 10 K (Fig. 2B, a), characteristic of [4Fe-4S]1+ centers in proteins such as aconitase and fumarate and in nitrate reduction transcriptional regulator (FNR) (26, 27). We next analyzed TtuA treated with excess ferricyanide. On oxidation, the absorbance at around 410 nm was diminished (Fig. 2A). Oxidized TtuA exhibited an EPR spectrum with principal g values of 2.02, 2.01, and 2.00 at 10 K (Fig. 2B, b, Upper). At 40 K, the signal with g = 2.01 was the most intense (Fig. 2B, b, Lower). The signals with g = 2.02 and 2.00 obtained at 10 K are characteristic of the [3Fe-4S]1+ center in proteins such as aconitase and RumA (28, 29). The signal with g = 2.01, which can be detected even at high temperatures (40 K), seemed to be an “unidentified radical” species commonly observed when [4Fe-4S] protein is oxidated by ferricyanide (29, 30). Both the [3Fe-4S]1+ center and the radical are thought to be derived from the oxidative degradation of the [4Fe-4S]2+ center (29, 30). These results suggest the existence of a [4Fe-4S]2+ center in reconstituted TtuA. It should be noted that the EPR properties of TtuA are distinct from those of 4-thiouridine synthetase ThiI and O-phospho-l-seryl-tRNA:Cys-tRNA synthase SepCysS, which were recently reported to possess [3Fe-4S]-type clusters (SI Discussion) (31). When reconstituted TtuA was exposed to air, the brown color of the solution quickly disappeared and a white precipitate formed (Fig. S3), demonstrating the oxygen sensitivity of the Fe-S cluster of TtuA. Therefore, we conducted the subsequent biochemical experiments under anaerobic conditions.
Fig. S3.
The state of the TthTtuA sample in purification. (A) A large amount of white precipitation present under aerobic conditions. (B) Light-yellow color of the TthTtuA sample after purification in anaerobic conditions. (C) Dark-yellow color of TthTtuA sample after the Fe-S cluster reconstitution. In both B and C, the concentration is ∼4 mg mL−1.
The Iron-Sulfur Cluster of TtuA Is Required for Sulfurtransferase Activity.
Because TtuA can bind to the oxygen-labile [4Fe-4S]2+ cluster (see above), we investigated the requirement of the Fe-S cluster for the sulfurtransferase activity of TtuA in vitro. Here, under anaerobic conditions, we performed the 2-thiolation reaction of yeast tRNAPhe, which possesses rT54, in the presence of recombinant proteins involved in s2T biosynthesis: TtuA, TtuB, TtuC [an enzyme responsible for generating thiocarboxylated TtuB (20)], TtuD [a homolog of yeast Tum1 (19), an S carrier recently identified to enhance the reaction efficiency (32)], SufS [Cys desulfurase (17)]), 0.1 mM Cys, and 2.5 mM ATP. Several preparations of TtuA samples were used in this experiment (Fig. 2C). Nucleoside analysis of the reacted tRNAs by HPLC indicated that highly efficient s2T formation occurred only after the addition of TtuA treated with FeCl3 and Na2S (Fig. 2C, d). Concomitant with the formation of s2T (retention time, 26.3 min; the UV-VIS spectrum is also shown in Fig. 2D), the amount of substrate rT (retention time, 17.2 min) was reduced. Using 35S-Cys as an S donor, the incorporation of 35S-S in tRNA was readily detected using holo-TtuA (Fig. 2E). Taken together, these data demonstrate that the Fe-S cluster of TtuA is required for its sulfurtransferase activity.
In our previous work, we observed the in vitro formation of s2T in tRNAs under aerobic conditions in quite low yields (∼0.1%) only when the desalted cell extract of T. thermophilus was doped in (20). It is plausible that very small amounts of holo-TtuA in the reaction mixture catalyze the slow 2-thiolation reaction.
Crystal Structures of T. thermophilus TtuA Possessing the Fe-S Cluster and AMPPNP.
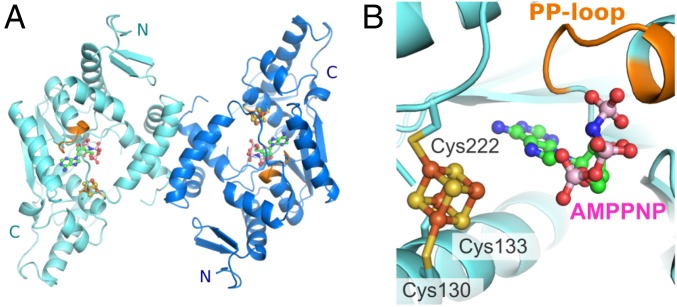
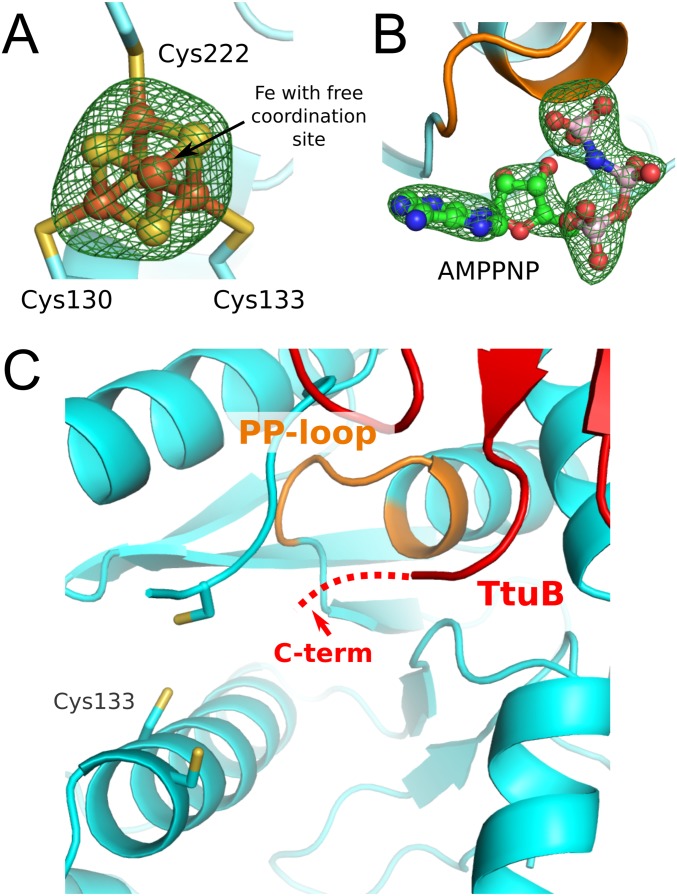
The crystal structures of holo-TtuA from T. thermophilus and its complex with AMPPNP (hereinafter, the TthTtuA-Fe-S-AMPPNP complex) were determined (Fig. 3A). In both crystals, TtuA forms a Z-shaped dimer, as has been observed for apo-TtuA (18). A difference Fourier map of the holo-TtuA crystal showed a clear cube-shaped peak in the vicinity of the three conserved Cys residues (Fig. S4A). Based on this observation, along with the spectroscopic and biochemical studies, we concluded that TthTtuA contains a [4Fe-4S]-type Fe-S cluster. Three of four Fe atoms in the [4Fe-4S] cluster were coordinated by three conserved Cys residues (Cys130, Cys133, and Cys222), and the remaining Fe atom was chelated by three inorganic S atoms of Fe-S without any coordination by main chain or side chain groups of TtuA (Fig. 3B).
Fig. 3.
Structures of TtuA in complex with the 4Fe-4S cluster and AMPPNP. (A) Overall structure of the Z-shaped TtuA dimer. Two protomers are shown in cyan and blue. The PP-loop is colored orange. The bound [4Fe-4S] cluster and AMPPNP are shown as balls and sticks (yellow indicates sulfate; brown, iron; green, carbon; blue, nitrogen; red, oxygen; pink, phosphorous). (B) Close-up view of the catalytic center of TtuA. The Fe-S cluster and AMPPNP are shown as balls and sticks. The PP-loop binding with AMPPNP is colored orange.
Fig. S4.
(A) Difference Fourier map of the bound [4Fe-4S] cluster in the TtuA-4Fe4S structure. The map is contoured at 6.5 σ. (B) Difference Fourier map of the bound AMPPNP in the TtuA-FeS-AMPPNP structure. The map is contoured at 4.5 σ. (C) Close-up view of the catalytic site of the TthTtuA-TtuB (G65C) complex. The Fe-S cluster is absent, and the two terminal residues of TtuB (shown by a red dotted line) are disordered.
In the difference Fourier map of the AMPPNP-soaked crystal, we observed a distinct electron density corresponding to the AMPPNP around the PP-loop motif. The γ-phosphate of AMPPNP was coordinated by the PP-loop (Fig. 3B). A similar AMPPNP-binding mode was observed in Mycobacterium tuberculosis NAD+ synthase (33) [Protein Data Bank (PDB) ID code 3SEQ], a member of the N-type ATP phosphatase group, suggesting that a common ATP recognition manner is adopted in this family. Furthermore, the bound ATP and the PP-loop were located close to the [4Fe-4S] cluster (Fig. S4B), suggesting that this region is a catalytic center of TtuA. The adenine moiety of AMPPNP was bound in a pocket of the catalytic center. No significant conformational change in TtuA was observed on AMPPNP binding.
In Vitro Characterization of Conserved Cys Residues of TtuA.
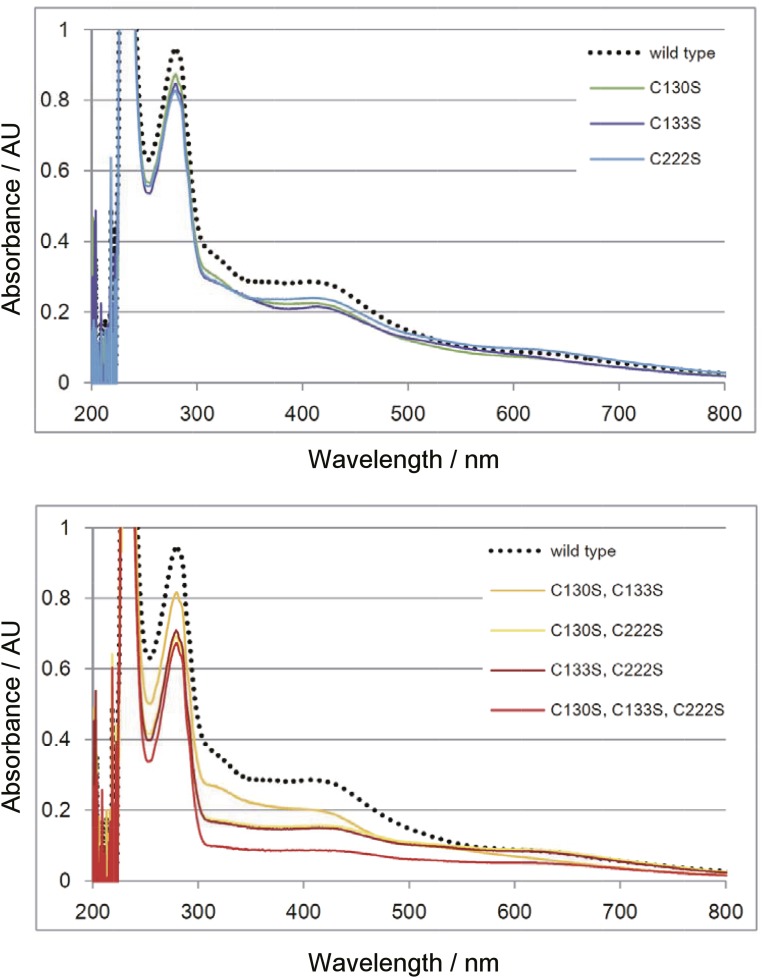
Our structural study revealed that a [4Fe-4S] cluster is coordinated by three conserved Cys residues: Cys130, Cys133, and Cys222 (Fig. 3). To evaluate the importance of these Cys residues, as well as that of the [4Fe-4S] cluster, we reconstituted TtuA with the Fe-S cluster using a series of Ser-substituted mutants of these Cys residues, and then determined their Fe contents and enzymatic activities (Table S1). Fe content was decreased in each of these mutants (Table S1), and the shoulder peaking at ∼410 nm in the UV-VIS spectrum was also diminished (Fig. S5), suggesting a reduced amount of the Fe-S cluster in these mutants. Concomitant with the decrease in quantities of Fe, 2-thiolation activities were also decreased for single mutants or completely abolished for double and triple mutants (Table S1). These data confirm that the Fe-S cluster chelated by the three Cys residues is essential for 2-thiolation activity.
Table S1.
In vitro mutational analysis of TtuA
| Mutations | Amount of Fe,* pmol Fe/pmol TtuA | Enzyme activity* | s2T amounts in vivo,† %‡ | |
| pmol s2T/pmol TtuA/min | %‡ | |||
| WT | 3.84 ± 0.08 | 0.912 ± 0.030 | 100.0 | 100.0 ± 2.35 |
| C130S | 3.27 ± 0.09 | 0.067 ± 0.009 | 7.3 | 9.63 ± 0.31 |
| C133S | 2.82 ± 0.08 | 0.004 ± 0.001 | 0.4 | 0.81 ± 0.12 |
| C222S | 3.07 ± 0.45 | 0.011 ± 0.001 | 1.2 | 4.53 ± 2.56 |
| C130S, C133S | 2.41 ± 0.36 | Not detected | 0 | Not determined |
| C130S, C222S | 1.60 ± 0.11 | Not detected | 0 | Not determined |
| C133S, C222S | 1.64 ± 0.12 | Not detected | 0 | Not determined |
| C130S, C133S, C222S | 1.41 ± 0.14 | Not detected | 0 | Not determined |
Experiments were repeated in triplicate.
The result of in vivo mutational analysis of TtuA (18) are presented.
The values of WT were set to 100.
Fig. S5.
UV-VIS spectrum of TtuA mutants reconstituted with the Fe-S cluster. Reconstituted TtuA samples were diluted to 0.7 mg/mL in buffer A, and the spectrum was measured.
In Vitro Characterization of s2T Synthesis.
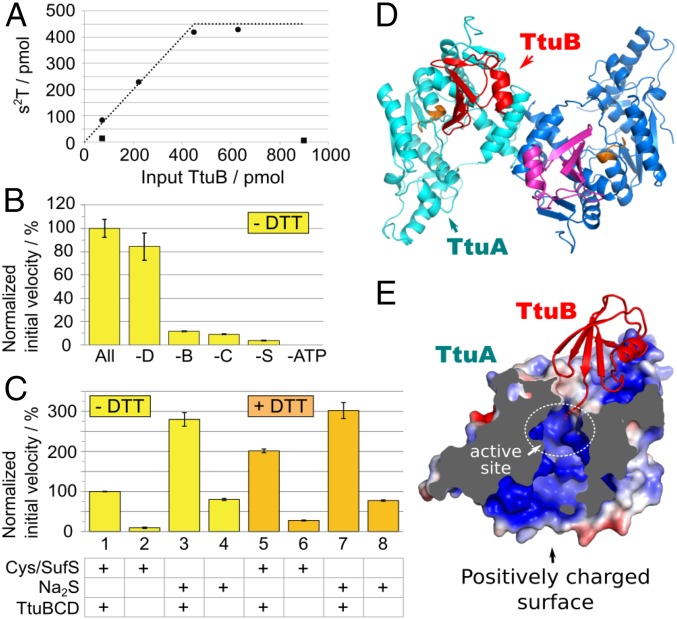
We measured the 2-thiolation reaction of yeast tRNAPhe using various S donors. With use of the thiocarboxylated TtuB (TtuB-COSH), almost all TtuB-COSH added to the reaction mixture was converted to s2T in tRNA. In contrast, almost no s2T was detected when using TtuB-COOH (Fig. 4A). The initial velocity of the 2-thiolation reaction was measured without the addition of DTT (Fig. 4B). The reaction exhibited strong dependency on SufS, TtuB, or TtuC, consistent with in vivo data indicating that ΔttuB and ΔttuC mutants lack s2T (17, 20). When ATP was omitted from the reaction mixture, the 2-thiolation reaction did not occur. We also measured the initial velocity of s2T formation in the presence of 0.1 mM DTT (Fig. 4C). When sulfur was supplied by Cys/SufS, a twofold enhancement of the reaction was detected in the presence of DTT (assays 1 and 5), most likely because bisulfide (S2−) was released from SufS-SSH and both S2− and TtuB-COSH were used by TtuA. Indeed, S2− itself could be engaged by TtuA (assays 4 and 8). Thus, the minimal components for reconstitution of the 2-thiolation reaction in vitro are holo-TtuA, Na2S, and ATP. The reaction kinetics were approximately threefold faster in the assay conditions with 0.1 mM S2− (assay 3) compared with in the conditions with 0.1 mM Cys/SufS (assay 1). The addition of TtuBCD enhanced the reaction by approximately fourfold (assays 3 and 4, or 7 and 8), most likely because TtuB-COSH also may be formed from S2−.
Fig. 4.
(A–C) In vitro characterization of s2T synthesis. The 2-thiolation reaction of yeast tRNAPhe (450 pmol; 7.5 pmol/µL) was examined at 60 °C with various combinations of the recombinant proteins, ATP, and sulfur donors. (A) The 2-thiolation reaction using TtuB-COSH as a sulfur donor. The reaction was performed in the presence of 75 pmol (1.25 pmol/µL) holo-TtuA, 2.5 mM ATP, and various amounts of either TtuB-COSH (closed circle) or TtuB-COOH (closed square) for 1 h. The x- and y-axes represent the amounts of input TtuB and s2T formed, respectively. The dotted line represents the maximum possible values of s2T had all of the sulfur atoms of input TtuB-COSH been incorporated into s2T. The value plateaus at 450 pmol because that was the amount of tRNA present. (B) The reaction was performed in the presence of all protein factors (holo-TtuA, TtuB, TtuC, TtuD, and SufS), 0.1 mM Cys, and 2.5 mM ATP in the absence of DTT (All). −D, −B, −C, −S, and −ATP represent the absence of TtuD, TtuB-COOH, TtuC, SufS, and ATP in each reaction mixture, respectively. Holo-TtuA (9.4–75 pmol; 0.16–1.25 pmol/µL) and other proteins (75 pmol; 1.25 pmol/µL) were included in reaction mixtures. The initial velocity of s2T formation is presented relative to that of the complete mixture (0.349 ± 0.027 pmol s2T/pmol TtuA/min). The experiment was performed in triplicate, and the data are presented with SD values. (C) The initial velocity of s2T formation was measured with 0.1 mM cysteine and 75 pmol (1.25 pmol/µL) SufS (assays 1, 2, 5, and 6), or 0.1 mM Na2S (3, 4, 7, and 8) as sulfur donors. 0.1 mM DTT was included in assays 5–8. Holo-TtuA (9.4–75 pmol; 0.16–1.25 pmol/µL) was included in all of the reaction mixtures. The values are presented relative to assay 1 (0.356 ± 0.002 pmol s2T/pmol TtuA/min). TtuB-COOH, TtuC, and TtuD (75 pmol; 1.25 pmol/µL) were included in assays 1, 3, 5, and 7. The experiment was performed in triplicate, and the data are presented with SD values. (D and E) Crystal structure of TthTtuA-TtuB (G65C) complex. (D) Overall structure of the TtuA-TtuB complex. Two protomers of TtuA dimer are colored in cyan and blue, and two molecules of TtuB are in red and magenta. The PP-loop is shown in orange. (E) Sectional view of the TtuA-TtuB complex. The surface of TtuA is colored according to the electrostatic potential (red, −4 kT/e; blue, +4kT/e) calculated with APBS (48, 49). The position of the catalytic site is indicated by a white dotted circle.
Crystal Structure of the TtuA-TtuB Complex.
In the s2T54 synthesis pathway of T. thermophilus, the S is transferred from the C-terminal thiocarboxylate of TtuB (TtuB-COSH) to the tRNA by the catalytic activity of TtuA. To elucidate the details of S delivery by TtuB-COSH, we determined the crystal structure of the TtuA-TtuB complex in the absence of the Fe-S cluster at a resolution of 2.5 Å, using a TtuB G65C mutant (34). Two copies of the Z-shaped TtuA dimer were present as an asymmetric unit, in which each protomer of TtuA independently captured TtuB (Fig. 4D and Table S2). No significant structural diversity among the four TtuA-TtuB complex copies was observed (rmsd <0.5 Å). On binding with TtuB, the conformation of a loop containing Cys222 of TtuA was fixed, which covers the active site. Consequently, the active site of TtuA connected to the exterior through two tunnels (Fig. 4E). TtuB was bound on the exit of one of the tunnels by its C-terminal loop inserted deep into the tunnel. Although two residues of the C terminus were invisible owing to disorder, the orientation reasonably accounts for the manner of the S transfer of TtuB to TtuA. The absence of the Fe-S cluster is a plausible cause of the disorder of the C terminus of TtuB (Discussion). The other tunnel connects the active site to the positively charged surface, which may allow it to interact with tRNA (18). These structural characteristics strongly suggest that TtuA interacts simultaneously with both tRNA and TtuB during the S-transfer reaction (Discussion).
Table S2.
Data collection and refinement statistics
| Statistic | TtuA-4Fe4S complex | TtuA-FeS-AMPPNP complex | TtuA-TtuB (G65C) complex* |
| Data collection | |||
| Diffraction source | BL32XU, Spring-8 | BL17A, Photon Factory | NW3A, Photon Factory |
| Temperature, K | 100 | 100 | 100 |
| Space group | P6122 | P6122 | P1 |
| Unit-cell parameters, Å | a and b = 93.9; c = 264.5 | a and b = 92.9; c = 266.9 | a = 54.1; b = 93.9; c = 97.5; α = 109.2; β = 104.6; γ = 106.9 |
| Wavelength, Å | 0.98 | 1.35 | 1.28251 |
| Mosaicity, ° | 0.094 | 0.056 | 0.414 |
| Resolution range, Å | 50.0–2.75 (2.91–2.75) | 46.5–2.70 (2.86–2.70) | 47.8–2.50 (2.65–2.50) |
| Total no. of reflections | 195,685 | 274,464 | 207,808 |
| No. of unique reflections | 18,794 | 35,452 | 105,373 |
| Completeness, % | 99.9 (99.9) | 99.8 (98.8) | 94.9 (93.2) |
| Multipicity | 10.4 (10.5) | 7.7 (7.6) | 1.97 (1.87) |
| Average I/σ(I) | 21.6 (4.30) | 18.0 (2.74) | 16.24 (2.79) |
| Rmeas, %† | 8.4 (57.4) | 9.2 (77.3) | 5.5 (39.6) |
| Rsym, %‡ | 7.9 (54.6) | 8.6 (72.1) | 3.9 (28.0) |
| CC1/2, % | 99.9 (92.4) | 99.9 (85.6) | 99.8 (85.8) |
| Overall B factor from Wilson plot, Å2 | 62 | 60 | 51 |
| Data refinement | |||
| Resolution range, Å | 47.96–2.75 (2.90–2.75) | 46.46–2.70 (2.84–2.70) | 47.85–2.50 (2.55–2.50) |
| No. of reflections | 18,794 (2,580) | 19,685 (2,704) | 53,904 (2,561) |
| Rwork, %§ | 21.9 (26.9) | 21.6 (27.0) | 19.6 (25.4) |
| Rfree, %¶ | 25.7 (30.8) | 23.7 (31.0) | 24.7 (32.9) |
| Protomers per asymmetric unit | 1 | 1 | 8‖ |
| No. of atoms | |||
| Protein | 2,328 | 2,397 | 11,741 |
| Ligands | 40 | 77 | 47 |
| Solvent | 25 | 39 | 81 |
| rmsd | |||
| Bond, Å | 0.01 | 0.009 | 0.003 |
| Angle, ° | 1.319 | 0.96 | 0.678 |
| Ramachandran plot | |||
| Favored, % | 97.22 | 97.31 | 98.05 |
| Allowed, % | 2.78 | 2.69 | 1.95 |
| Outliers, % | 0 | 0 | 0 |
| Clashscore | 6.5 | 7.07 | 5.03 |
| PDB ID code | 5B4F | 5B4E | 5GHA |
Values for the outer shell are given in parentheses.
The statistics for the TtuA-TtuB(G65C) complex are from our previous report (34).
Rmeas = Σhkl{N(hkl)/N(hkl) − 1}1/2Σi|Ii(hkl)-I(hkl)|/ΣhklΣiIi(hkl), where Ii(hkl) and N(hkl) are the mean intensity of a set of equivalent reflections and the multiplicity, respectively.
Rsym= ΣhklΣi |Ii(hkl) − I(hkl)|/ΣhklΣiIi(hkl), where Ii(hkl) is the ith observation of reflection hkl and I(hkl) is the weighted average intensity for all observations I of reflection hkl.
Rwork (%) = Σhkl||Fobs(hkl)| − |Fcalc(hkl)||/Σhkl|Fobs(hkl)|.
Rfree (%) = Rwork calculated using 5% of the reflection data chosen randomly and omitted from the refinement.
Four copies of the TtuA-TtuB heterodimer were located in an asymmetric unit.
SI Discussion
Comparison of the Reaction Mechanisms With and Without an Fe-S Cluster.
MnmA, an enzyme responsible for 5-methylaminomethyl-2-thiouridine, is a well-studied enzyme involved in sulfur transfer to tRNA (12). MnmA shares many common features with TtuA, including the substrate (U), the product (s2U), the presence of the catalytic PP-loop domain, and a two-step reaction composed of an initial adenylation reaction and a subsequent substitution reaction (Fig. S1). However, there is a striking difference between TtuA and MnmA: Fe-S cluster dependency. Our present study suggests that the Fe-S cluster of TtuA contributes to the second substitution step. For this reaction, MnmA uses two Cys residues, in which one Cys residue receives an S atom from an S donor protein and then transfers it with the help of another catalytic Cys. Three Cys residues are located on the active site of TtuA, all of which are used for chelating the 4Fe-4S cluster.
Although their evolutionary relevance is unclear (or possibly of no relevance), it is interesting that important Cys residues commonly located in the active site have different roles in the same enzymatic reaction by these two enzymes sharing a similar active site structure. However, if thiolation of TtuA uses the “indirect mechanism,” then the Fe with free a coordination site of the Fe-S cluster might play the same role as the first Cys residue of MnmA in receiving the liberated sulfide.
Thionucleoside Synthetases May Share a Common Fe-S Cluster-Dependent Mechanism.
Methanococcus maripaludis Ncs6 (MmaNcs6), belonging to the same enzyme family as TtuA, reportedly uses R-SSH on the central CXXC motif for its enzymatic reaction (50). Considering the oxygen sensitivity of the Fe-S cluster of TtuA, the R-SSH on the CXXC motif might act as an intermediate for the formation or degradation of an Fe-S cluster; therefore, MmaNcs6 also may be an Fe-S protein, similar to TtuA.
The [4Fe-4S] cluster requirement is shared between different subgroups of S-transfer proteins, i.e., TtuA and TtcA (22). Our crystal structure shows that the characteristic sequence CxxC…C is responsible for Fe-S cluster formation. Notably, it was recently reported that ThiI from M. maripaludis and SepCysS from Methanocaldococcus jannaschii, both of which possess the CxxC…C sequence, also require a [3Fe-4S] type Fe-S cluster for their enzymatic activity (31). Assuming that the [4Fe-4S] cluster in TtuA was used for fixing the orientation of TtuB-COSH in the active site, the presence of the Fe-S cluster to stabilize the S donor is the sole characteristic required, and thus the difference in the type of the cluster (i.e., [4Fe-4S] for TtuA, Ncs6, and TtcA and [3Fe-4S] for ThiI and SepCysS) might not be of importance. Otherwise, engagement of the Fe-S cluster is a general mechanism for sulfur transfer, but the detailed reaction mechanisms remain unclear. The actual mechanism may be dependent on the manner of sulfur supply, for example, thiocarboxylate of the C terminus of ubiquitin-like protein or bare sulfide ion. Although the biological significance of the differences in the type of the Fe-S cluster has not yet been clarified, these results demonstrate a correlation between this sequence motif and presence of the Fe-S cluster. Despite their common enzymatic reactions for the biosynthesis of s4U, ThiI from some organisms, such as Methanobacteriales, Methanococccales, Methanomicrobiales, Methanosarcinales, Methanocellales, and some Thermococcales, possess the CxxC…C motif, whereas others do not (51). These groups may have evolved from or into using the Fe-S cluster for sulfur transfer reaction according to their living environments.
Taking these observations together, we propose that the Fe-S cluster requirement is a common characteristic for some thionucleoside synthetases, but that until now we might have been unaware of its presence owing to the inherent oxygen lability. The Fe-S cluster may be shared more widely than expected in enzyme families, regardless of its enzymatic roles.
Discussion
In Vitro Reconstitution of 2-Thiouridine Synthesis in T. thermophilus.
We were able to characterize s2T54 synthesis in vitro using TtuA reconstituted with the Fe-S cluster under anaerobic conditions (Figs. 2 and 4). Our results indicate that TtuA has a [4Fe-4S] cluster (Fig. 3), and that this cluster is required for in vitro s2T54 synthesis (Fig. 2 C and E).
Distinct from the previously reported in vitro s2T synthesis reaction using doped cell-free extract (20), the present in vitro system used only recombinant proteins, and the efficiency of the reaction was very high, which allowed us to establish the detailed characterization of s2T synthesis in vitro. The S atoms of TtuB-COSH (Fig. 4A) and Cys (Fig. 4B) were effectively incorporated into s2T, and turnover of the S-transfer reaction was observed. Furthermore, holo-TtuA did not form s2T in the absence of the S source (TtuB-COSH or Na2S). These observations demonstrate that the S atom of the [4Fe-4S] cluster is not incorporated into s2T, and thus the Fe-S cluster does not act as S reservoir for the reaction, but must play another important role in the enzymatic reaction.
Structural Implication of the Iron-Sulfur Cluster in TtuA.
Our crystal structure analysis revealed that the [4Fe-4S] cluster was coordinated by three conserved Cys residues: Cys130, Cys133, and Cys222 (Fig. 3). Mutation analysis demonstrated that the substitution of these conserved Cys residues of TtuA caused a significant decrease in enzymatic activity (Table S1). This finding is consistent with the results of a previous mutation analysis in vivo (18); therefore, we concluded that the absence of these residues led to the loss of the Fe-S cluster, which results in the inactivation of TtuA. The [4Fe-4S] cluster reportedly has various roles, such as in stabilization of the protein structure, as an electron donor, for substrate binding, and for enzymatic reactions (35). Most of the Fe-S clusters chelated by the four Cys residues are responsible for structure stabilization and electron transfer. Because Pyrococcus horikoshiii TtuA, which lacks Fe-S clusters, maintained a similar structure as our [4Fe-4S] cluster-bound form even after incubation at 85 °C (rmsd = 0.862 for 217 Cα atoms), its function in TtuA likely extends beyond just structure stabilization. In contrast, the Fe with free coordination sites of Fe-S clusters is often used for substrate binding and in enzymatic reactions. Given that the [4Fe-4S] cluster of TtuA was chelated by three Cys residues, the Fe with a free coordination site of the Fe-S cluster of TtuA may contribute to an enzymatic role.
Structural Insight into the TtuA-TtuB-tRNA Ternary Complex.
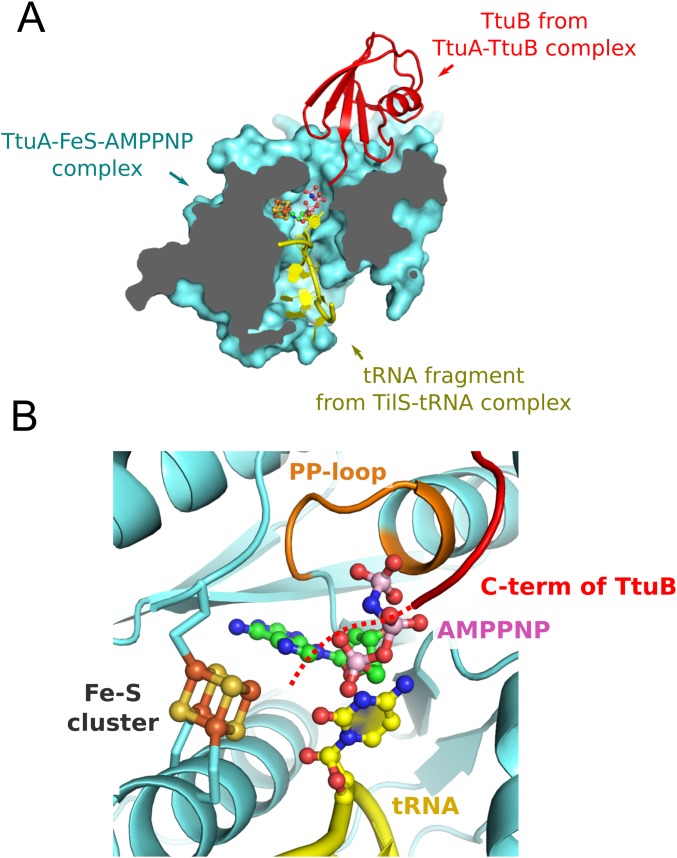
One of the most important benefits of focusing on the structural study of TtuA is that its S donor, TtuB, has been identified. We have elucidated its structural basis in this study. Because TtuA transfers the S atom from the thiocarboxylated C terminus of TtuB to tRNA, TtuA should bind these two macromolecules during the reaction. Our crystal structure of the TtuA-TtuB complex demonstrates that TtuB delivers the S atom by inserting its C terminus into one of the tunnels leading to the active site (Fig. 4E). Furthermore, the structure suggests that the positively charged surface located opposite the TtuB binding site is used for binding with tRNA. This hypothesis is also supported by the superposition of our TtuA structure onto the Geobacillus kaustophilus TilS-tRNA complex (PDB ID code 3A2K). TilS is a tRNA modification enzyme similar to TtuA in terms of function (3) and structure (36, 37); that is, both enzymes adenylate uridine bases using the PP-loop domain. In the superposed model, the substrate tRNA was located within the tunnel connecting the active site and the positive surface (Fig. S6A).
Fig. S6.
The TtuA-TtuB-Fe-S-AMPPNP-tRNA pentamer complex model. The pentamer complex model was prepared by superposing the TtuA-TtuB complex and the TilS-tRNA complex structure (PDB ID code 3A2K) onto the TtuA-Fe-S-AMPPNP complex. For clarity, a part of the tRNA located in the tunnel is shown. Note that the location of the substrate base of TtuA (position 54) is different from that of TilS (position 34). (A) Sectional view of the pentamer complex model (cyan, TtuA; red, TtuB; yellow, tRNA fragment). The [4Fe-4S] cluster and AMPPNP are shown in the same manner as described above. (B) Close-up view of the catalytic center of the model structure. The expected location of the C terminus of TtuB is shown as a red dotted line.
Considering the foregoing observations together, we propose a TtuA-TtuB-tRNA ternary complex structure model in which TtuB and tRNA simultaneously access the active site through two different tunnels (Fig. S6A). This manner of binding allows all of the participants (i.e., the Fe-S cluster, ATP, the thiocarboxylated C terminus of TtuB, and the substrate uridine base) to enter the narrow catalytic site of TtuA. In particular, the Fe with a free coordination site of the Fe-S cluster faces the C terminus of TtuB, ATP, and the uridine base (Fig. S6B), which reasonably suggests an enzymatic role of the Fe-S cluster in s2T generation. Through formation of the TtuA-TtuB-tRNA ternary complex, accurate S transfer between two macromolecules ( i.e., from the C terminus of TtuB to tRNA) could be achieved.
Role of the Iron-Sulfur Cluster of TtuA.
s2T synthesis comprises two reactions, adenylation and thiolation (Fig. S1). An initial adenylation reaction is commonly observed for many other N-type ATP-pyrophosphatases, such as MnmA and TilS. In these enzymes, ATP is bound close to the nucleobase, followed by transfer of the adenyl group from ATP to the nucleobase by the inherent enzymatic activity of the PP-loop domain (38). To our knowledge, no PP-loop domain is reported to catalyze its adenylation reaction in a Fe-S cluster-dependent manner. Furthermore, MnmA does not possess the Fe-S cluster, even though it catalyzes the adenylation of tRNA using the PP-loop domain. Based on the foregoing observations, we can conclude that the Fe-S cluster in TtuA does not contribute to the initial adenylation reaction.
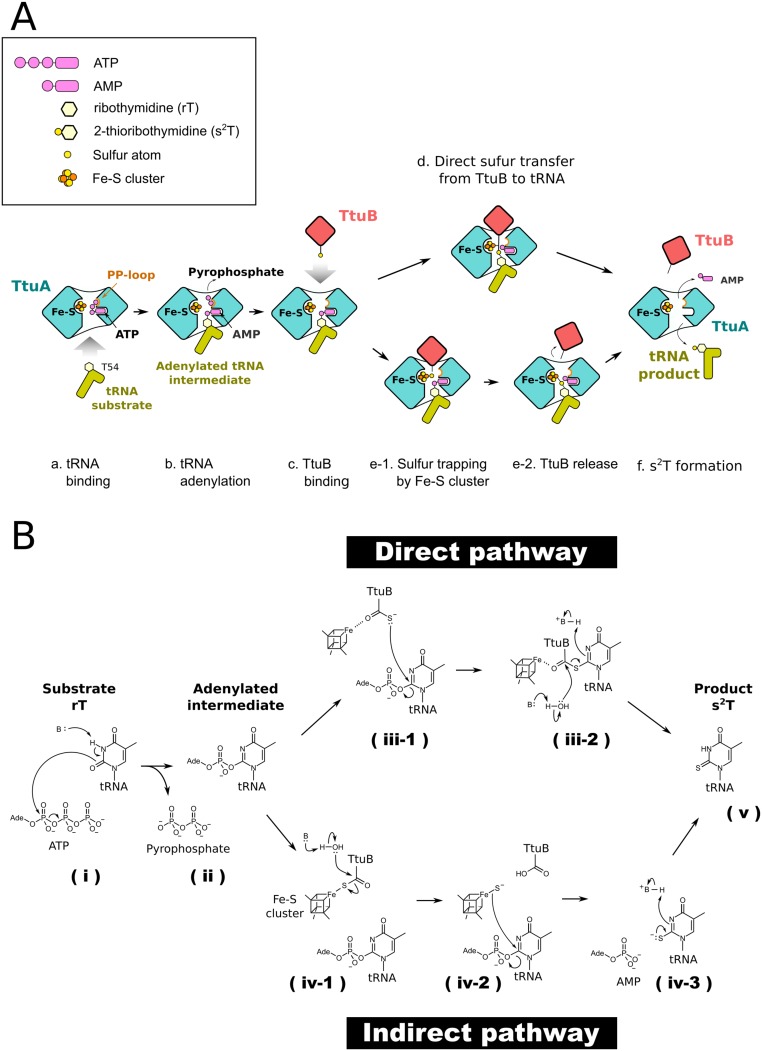
Thiolation makes use of the S atom supplied by the ubiquitin-like protein TtuB. Our in vitro experiments demonstrate the importance of TtuB for the enzymatic activity of TtuA (Fig. 4). Furthermore, a previous report showed that the generation of s2T was completely obstructed in the ttuB gene-deficient strain (17). Such an S-transfer pathway of s2T biosynthesis is reminiscent of thiamin and molybdenum cofactor biosynthesis (39) (40). Ubiquitin-like proteins ThiS and molybdopterin synthase sulfur carrier subunit (MoaD) also serve as S donors in those pathways, in which the S atom of the C-terminal thiocarboxylate directly attacks the substrate nucleophilically. Analogous to these homologous proteins, the S on the C-terminal thiocarboxylate of TtuB nucleophilically attacks the adenylated nucleobase, completing the thiolation reaction (Fig. S7 A, d and B, iii-1 and iii-2). Of note, the C-terminal loop of TtuB appeared quite flexible (Fig. S4C). Although the C terminus Gly (G65) was substituted for Cys so as to mimic the thiocarboxylated state, the two terminal residues of TtuB were completely disordered in all four copies of the determined TtuA-TtuB complex structure. In contrast, the C terminus loops of ThiS and MoaD were rigid in the catalytic sites of their corresponding thiotransfer enzymes, ThiG and MoaE, respectively (PDB ID codes 1TYG and 2QIE). The Fe with a free coordination site at the Fe-S cluster is anticipated to be located close enough to interact with the C terminus of TtuB (Fig. S6). The Fe-S cluster may coordinate and constrain the flexible C terminus of TtuB in an appropriate orientation for the direct attack (Fig. S7 A, d and S7 B, iii-1), which would then enhance the rapidity and fidelity of a series of the S-transfer reactions of TtuA shown in Fig. S7B, iii-1, iii-2, and v), including nucleophilic substitution by an S atom for AMP, an elimination-addition reaction of the thiocarboxylated C terminus of TtuB, double-bond formation between the released S atom and nucleobase, and protonation.
Fig. S7.
Proposed sulfur transfer mechanism by TtuA-TtuB using the iron-sulfur cluster. (A) Schematic representation of the proposed sulfur transfer mechanism. First, substrate tRNA binds to TtuA (a), which is followed by the transfer of the adenyl group of ATP to the substrate base of tRNA; an activated intermediate is then formed (b). Next, the sulfur-carrier protein TtuB inserts the thiocarboxylated C terminus into the active site for supplying the sulfur atom (c). Two possible mechanisms involving the Fe-S cluster have been proposed: the sulfur atom is transferred directly from TtuB to a uridine base, in which the Fe with a free coordination site is used for fixing the position of the C terminus of TtuB (d), or, alternatively, the sulfur atom, once released from TtuB, is captured by the Fe with a free coordination site of the [4Fe-4S] cluster (e-1) and is subsequently transferred to the activated uridine base (e-2). After completion of the thiolation reaction, the sulfur transfer complex is decomposed (f). (B) Deduced chemical reaction of the sulfur transfer. After the binding of substrate rT to TtuA, a basic residue would enhance the nucleophilicity of the O2 of rT by deprotonation of the N3, followed by a nucleophilic attack from O2 to alpha-phosphate of ATP (i), which generates the adenylated intermediate and the pyrophosphate (ii). Two possible mechanisms involving the Fe-S cluster have been proposed (Discussion and A). In the direct pathway, the Fe with a free coordination site is used for fixing the position of the C terminus of TtuB. The anionic S atom attacks position 2 of the rT, which causes the second substitution of the S for the AMP (iii-1). A water molecule deprotonated by a basic residue attacks the carbonyl carbon of TtuB C terminus and causes an elimination-addition reaction. The S atom is released and forms a double bond with the carbon of position 2. Subsequently, the nitrogen of position 3 is protonated by a basic residue (iii-2). In the indirect pathway, a water molecule deprotonated by a basic residue attacks the carbonyl carbon of TtuB C terminus that is coordinated by the cluster, causing an elimination-addition reaction. The released S atom remains on the Fe with a temporary free coordination site (iv-1). Subsequently, the S atom attacks position 2 of the rT, which causes the second substitution of the S for the AMP (iv-2). The S atom deprotonated by a basic residue forms double bond with the carbon of position 2, and the nitrogen of position 3 is protonated by a water molecule (iv-3). The thiolation reaction is completed and generates the product s2T (v).
Another possible scenario is an indirect S-transfer mechanism in which the S atom supplied by TtuB is first extracted from the C-terminal thiocarboxylate and then used as the substrate for TtuA (Fig. S7 A, e-1 and 2 and B, iv-1–iv-3). Our in vitro experiment shows that TtuA can use Na2S as the S source (Fig. 4C), indicating that a bare sulfide ion is sufficient for the reaction in vitro. Furthermore, some organisms possessing TtuA do not have TtuB-like S carriers. These observations suggest that thiocarboxylate is not used in its native form, but rather is used after decomposition to a sulfide ion and carboxylate group. In this mechanism, the Fe-S cluster might receive the S atom liberated from TtuB-COSH on its Fe with a free coordination site owing to the inherent affinity for sulfur, as has been observed for tRNA-2-methylthio-N6-dimethylallyladenosine synthase (MiaB), another sulfur transfer enzyme using Fe with a free coordination site as a receptor of sulfide ions (41).
Materials and Methods
Analysis of tRNA Modification.
Hydrolysates of tRNAs from T. thermophilus and the in vitro assay were prepared and analyzed by HPLC with a photodiode array detector (GL Sciences) as described previously (20). The amount of s2T was quantified using pseudouridine as a standard.
Recombinant Proteins for Biochemical Assays.
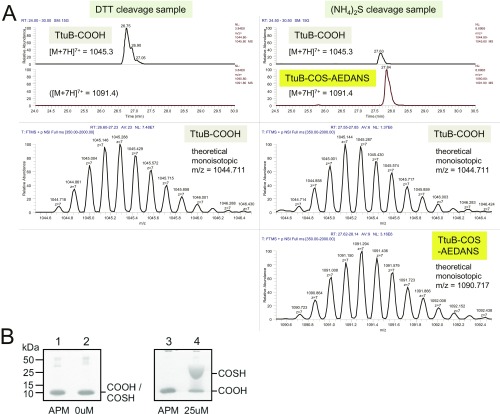
N-terminal His6-tagged T. thermophilus TtuA and TtuD were expressed in E. coli and purified as described in SI Materials and Methods. The N-terminal His6-tagged versions of T. thermophilus TtuB, TtuC, and SufS were purified as described previously (17, 20). Protein concentrations were determined using a Bio-Rad protein assay kit with a BSA standard. The C-terminal carboxylate and thiocarboxylate versions of TtuB were prepared using the intein fusion technique (IMPACT Kit; New England BioLabs) (42). The fusion protein was cleaved in the presence of DTT and (NH4)2S for the preparation of TtuB-COOH and TtuB-COSH, respectively. The production of TtuB-COSH was confirmed by SDS/PAGE with the gels containing [(N-acryloylamino)phenyl]mercuric chloride (APM) (43, 44) and LC/MS (Fig. S8). The APM gel showed that ∼70% of the sample cleaved by sulfide was TtuB-COSH. Detailed procedures for the preparation and confirmation of TtuB-COSH are described in SI Materials and Methods.
Fig. S8.
Confirmation of TtuB-COOH and TtuB-COSH prepared by the intein method. (A) LC/MS analysis. The samples were reacted with I-AEDANS to stabilize the thiocarboxylate and then analyzed by LC/MS. (Left) Data for the DTT cleavage samples. (Right) Data for the (NH4)2S cleavage sample. The extracted ion chromatograms for TtuB-COOH (m/z = 1,045.3, a charge state of 7) and TtuB-COS-AEDANS (m/z = 1,091.4, a charge state of 7) are shown. TtuB-COS-AEDANS was detected only in the (NH4)2S cleavage sample. The scanning spectra of TtuB-COOH and TtuB-COS-AEDANS are shown below the chromatograms. The monoisotopic m/z values are closely matched with the theoretical values. (B) Analysis by SDS/PAGE with gels containing APM. TtuB-COOH (lanes 1 and 3) and TtuB-COSH (lanes 2 and 4) prepared by the intein method were analyzed by SDS/PAGE (Left) and SDS/PAGE containing 25 µM APM (Right).
Reconstitution of the Iron-Sulfur Cluster of TtuA.
Apo-TtuA was purified under aerobic conditions as described in SI Materials and Methods. All manipulations were performed under strictly anaerobic conditions in an anaerobic glove box (Coy Laboratory Equipment) in an atmosphere of 95% N2 and 5% H2. Solutions were made anoxic by bubbling for a minimum of 3 h. TtuA (0.032 mM) was incubated for 3 h at 25 °C in buffer A [50 mM Hepes-KOH pH 7.6, 200 mM (NH4)2SO4, 50 mM NH4OAc, 5 mM MgCl2, 10% (vol/vol) glycerol, and 7 mM β-mercapto-ethanol (β-ME)] with 1.5 mM DTT, 0.3 mM FeCl3, and 0.3 mM Na2S. Excess FeCl3 and Na2S were removed using a NAP 5 gel filtration column (GE Healthcare) with buffer A, after which reconstituted TtuA was concentrated using 10-kDa cutoff Amicon Ultra filters (Merck Millipore). Protein concentrations were determined using a Bio-Rad protein assay kit, and the Fe concentration of protein samples was measured by a colorimetric method with FeSO4 as a standard (BioChain). All samples were stored at 20 °C and kept anoxic in a glove box.
EPR Spectroscopy.
Anaerobically reconstituted TtuA was concentrated to ∼0.9 mM, then reduced or oxidized by 10 eq of sodium dithionite or 5 eq of potassium ferricyanide, respectively. EPR spectra were obtained with a Bruker ELEXSYS E580 spectrometer in continuous-wave mode operating at 9.38 GHz. An Oxford liquid helium flow cryostat was used for cryogenic measurements. The microwave frequency was monitored by a frequency counter (Bruker SuperX-FT bridge), and the magnetic flux density was measured with a Teslameter (Bruker ER 036TM). The microwave power was 1 mW.
In Vitro s2T Formation with Holo-TtuA.
s2T formation on substrate tRNA was assayed in strictly anaerobic conditions in a glove box. The standard assay was performed at 60 °C for the indicated times in 60 µL of buffer H (50 mM Hepes-KOH pH 7.6, 100 mM KCl, 10 mM MgCl2, and 0.1 mM DTT) containing 2.5 mM ATP, 0.1 mM Cys, 20 µM pyridoxal phosphate, 450 pmol yeast tRNAPhe (Sigma-Aldrich), and 75 pmol of each recombinant protein (holo-TtuA, TtuB, TtuC, TtuD, and SufS). After the reactions, RNA was recovered using an acid-guanidinium thiocyanate-phenol-chloroform reagent (Isogen; Wako Chemicals) and precipitated with ethanol. tRNA modifications were analyzed by HPLC and quantified. For the experiment depicted in Fig. 4 B and C, β-ME in the enzyme stock solutions was removed by NAP 5 gel filtration columns (GE Healthcare).
The reaction with 35S-Cys as an S donor was performed using 0.1 mM 35S-Cys (6 µCi). RNA was separated by PAGE on 10% (wt/vol) gels containing 7 M urea, and the gels were stained with 0.025% (wt/vol) toluidine blue. Finally, the gels were dried, exposed on an imaging plate, and analyzed using the BAS 2500 system (Fuji Photo Systems).
Crystal Structure Analysis.
The holo-TtuA used for crystallization was prepared as described in SI Materials and Methods. Crystals of holo-TtuA were obtained from a reservoir solution consisting of 0.1 M sodium acetate (pH 3.8–5.6), and 30–60% (vol/vol) 1,2-propanediol at 25 °C. For the AMPPNP complex, the holo-TtuA crystals were soaked in a crystallization buffer containing 5 mM of AMPPNP for 30 s. Diffraction data for the TtuA-Fe-S-AMPPNP crystal complex were collected using beam line BL17A of the Photon Factory. Data were indexed, integrated, and scaled with XDS (45). The crystal structure was determined by the molecular replacement method with Phase-MR (46) using a polyalanine model of apo-P. horikoshii TtuA (18) as a search probe. Structure refinement was carried out with phenix.refine (47). Finally, the crystallographic Rwork and Rfree factors converged to 21.9% and 25.7%, respectively, for holo-TtuA and to 21.6% and 23.7%, respectively, for the AMPPNP complex. Refinement statistics are summarized in Table S2.
Crystallization, data collection, and initial phase determination of the TtuA-TtuB complex were performed as reported previously (34). Finally, the crystallographic Rwork and Rfree factors converged to 19.6% and 24.7%, respectively.
SI Materials and Methods
Preparation of Recombinant Proteins.
The wild type (WT) and variants of N-terminal His6-tagged TtuA were expressed in E. coli strain Rosetta DE3 (Novagene) (17) and purified in aerobic conditions with a slight modification of buffers. The expression plasmids for variants were constructed by site-directed mutagenesis (QuikChange II Mutagenesis Kit; Stratagene) from the WT plasmids. The sequences of the plasmids were confirmed by sequencing. Cultures were grown to an OD600 of ∼0.6, induced with 0.5 mM isopropyl 1-thio-β-d-galactopyranoside (IPTG), and then cultured for a further 6 h at 37 °C. Cells were sonicated in buffer A [50 mM Hepes-KOH pH 7.6, 200 mM (NH4)2SO4, 50 mM NH4OAc, 5 mM MgCl2, 10% (vol/vol) glycerol, and 7 mM β-ME] containing 0.2 mM phenylmethylsufonyl fluoride (PMSF), and cell debris was removed by centrifugation. The supernatants were heated at 70 °C for 15 min, and insoluble precipitates were removed by a second centrifugation. The cleared lysate was loaded onto an Ni-NTA agarose (Qiagen) column, washed with buffer A containing 1 M NH4Cl and 10 mM imidazole, and eluted with buffer A with 250 mM imidazole. Purified proteins were desalted using a NAP 25 gel filtration column (GE Healthcare) with buffer A. The TtuA samples purified in aerobic conditions were almost colorless (apo-TtuA).
The expression plasmids for TtuD were prepared as follows. The gene encoding TtuD (Ttc0666) of T. thermophilus HB27 was cloned into the NdeI and BamHI sites of pET15b (Novagene), resulting in N-terminal His6-tagged fusion proteins. TtuD was expressed in E. coli strain Rosetta DE3 (Novagene). Cultures were grown to an OD600 of ∼0.6, induced with 0.1 mM IPTG, and cultured for a further 6 h at 37 °C. Cells were sonicated in K100 buffer (50 mM Hepes-KOH pH 7.6, 100 mM KCl, 10 mM MgCl2, 5% (vol/vol) glycerol, and 7 mM β-ME) containing 0.2 mM PMSF, and cell debris was removed by centrifugation. The supernatants were heated at 70 °C for 15 min, and insoluble precipitates were removed by a second centrifugation. The cleared lysate was loaded onto an Ni-NTA agarose (Qiagen) column, washed with K100 buffer containing 1 M NH4Cl and 10 mM imidazole, and eluted with K100 buffer with 250 mM imidazole. Purified proteins were desalted using an NAP 25 gel filtration column (GE Healthcare) with K100 buffer, and all samples were stored at −30 °C.
The C-terminal carboxylate and thiocarboxylate versions of TtuB were prepared using the IMPACT kit (New England BioLabs). The TtuB gene was cloned into the NdeI and KpnI sites of the pTYB1 vector, wherein an intein-binding and a chitin-binding domain were attached at the C terminus of TtuB. Expression vectors were constructed using the following primers: TtuB-INTAIN-F, 5′-agccagctca tatgagggtc gttctgcgcc-3′; TtuB-INATIN-R, 5′-tgcatggtac ccttggcaaa gcaccctccc gagatggcgg agaggac-3′. The sequences of the plasmids were confirmed by sequencing. The fusion protein was expressed in E. coli strain B834 (DE3). Overexpression of the fusion protein was induced with 0.1 M IPTG, and cells were cultured for 20 h at 25 °C. Cells were resuspended in T buffer (20 mM Tris⋅HCl pH 8.5 and 500 mM NaCl) with 0.1% Triton X-100. The cells were disrupted by sonication, and the supernatant was loaded onto a chitin resin (New England BioLabs). After washing with 20 column volumes of T buffer, 3.3 column volumes of T buffer supplemented with 50 mM cleavage reagent were added to the resin, followed by incubation for 20 h at 25 °C. DTT and (NH4)2S were used as cleavage reagents for the preparation of TtuB-COOH and TtuB-COSH, respectively.
After on-column cleavage, the target protein was eluted by T buffer, concentrated using an Amicon Ultra filter (3-kDa cutoff; Merck Milipore), and then loaded on an NAP 25 gel filtration column (GE Healthcare) pre-equilibrated with a buffer containing 14 mM Tris⋅HCl pH 8.5, 350 mM NaCl, and 30% (vol/vol) glycerol. Fractions containing the desired protein were collected and stored at −80 °C. For LC/MS analysis, these proteins were incubated with 0.8 mM 5-({2-[(iodoacetyl)amino]ethyl}amino)naphthalene-1-sulfonic acid (I-AEDANS) for 2 h at 25 °C and then desalted with C18 stage tips (Thermo Fisher Scientific). The samples were separated on a C18 column and analyzed using the positive mode of an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific).
Preparation of Holo-TtuA for Crystallization.
TtuA used for crystallization was expressed by E. coli strain B834 (DE3) with pET26 vector (Novagen) as a C-terminal His6-tagged protein. Cultivation conditions were the same as those for the C-terminal carboxylate and thiocarboxylate versions of TtuB. Expressed TtuA was purified in an anaerobic chamber (Coy Laboratory Products). The buffer used for purification was stirred in anaerobic conditions overnight to remove the dissolved oxygen. Before use, all regents were confirmed to be oxygen-free using an anaerobic indicator (Oxoid Anaerobic Indicator; Thermo Fisher Scientific). Collected cells were resuspended in a purification buffer consisting of 50 mM Hepes-KOH pH 7.6, 200 mM ammonium sulfate, 50 mM ammonium acetate, 5 mM magnesium chloride, 10% (vol/vol) glycerol, and 0.1% Triton X-100. The cells were sonicated for 60 min on ice with an ultrasonic cell disruptor (XL2000; Misonix), followed by heat treatment at 70 °C for 20 min. The cell debris was removed by centrifugation at 7,000 × g for 30 min at 12 °C. The supernatant was filtrated and loaded onto a HisTrap HP column (GE Healthcare) that had been preequilibrated with the purification buffer.
After washing with the purification buffer, the adsorbed protein was eluted with a 0–0.5 M gradient of imidazole in the purification buffer. Fractions containing the desired proteins were further purified using a HiLoad 16/60 Superdex 200 column (GE Healthcare) that had been preequilibrated with the purification buffer. The procedure for reconstituting the Fe-S cluster was as follows. DTT was added to TtuA purified at a final concentration of 5 mM. After 10 min of incubation, a 20-fold molar excess of FeCl3 was added, followed by incubation for another 10 min. Then a 20-fold molar excess of Na2S was added, followed by incubation for 3 h at 4 °C. The precipitation of iron sulfide was removed by filtration, and the excess iron and sulfide ions were removed by a Sephadex NAP-25 desalting column. After concentrating up to 20 mg/mL, holo-TtuA was used for crystallization.
Acknowledgments
We thank Professor Toshiya Senda and Dr. Miki Senda (High-Energy Accelerator Research Organization) for their valuable suggestions regarding the anaerobic experiment. The synchrotron radiation experiments were performed at SPring-8 (proposals 2014B1033, 2015A1114, 2016A2565, and 2016B2565) and the Photon Factory (proposal 2014G080). We thank the beamline staff of SPring-8 and the Photon Factory for their assistance with data collection. This work was supported by the Japan Agency for Medical Research and Development (Platform for Drug Discovery, Informatics, and Structural Life Science 11961); the Japan Science and Technology Agency’s Precursory Research for Embryonic Science and Technology (JPMJPR1517); the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research 15J01961, 24000011, 26291008, 16H00748, 24570173, and 16K07311); and the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.J.B. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5B4E, 5B4F, and 5GHA).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615585114/-/DCSupplemental.
References
- 1.Machnicka MA, et al. MODOMICS: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boschi-Muller S, Motorin Y. Chemistry enters nucleic acids biology: Enzymatic mechanisms of RNA modification. Biochemistry (Mosc) 2013;78:1392–1404. doi: 10.1134/S0006297913130026. [DOI] [PubMed] [Google Scholar]
- 3.Ikeuchi Y, et al. Molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol Cell. 2005;19:235–246. doi: 10.1016/j.molcel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Krüger MK, Pedersen S, Hagervall TG, Sørensen MA. The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J Mol Biol. 1998;284:621–631. doi: 10.1006/jmbi.1998.2196. [DOI] [PubMed] [Google Scholar]
- 5.Yarian C, et al. Accurate translation of the genetic code depends on tRNA modified nucleosides. J Biol Chem. 2002;277:16391–16395. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- 6.Krüger MK, Sørensen MA. Aminoacylation of hypomodified tRNAGlu in vivo. J Mol Biol. 1998;284:609–620. doi: 10.1006/jmbi.1998.2197. [DOI] [PubMed] [Google Scholar]
- 7.Caldeira de Araujo A, Favre A. Induction of size reduction in Escherichia coli by near-ultraviolet light. Eur J Biochem. 1985;146:605–610. doi: 10.1111/j.1432-1033.1985.tb08694.x. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Shinma M, Oshima T, Nishimura S. Heat-induced stability of tRNA from an extreme thermophile, Thermus thermophilus. Biochem Biophys Res Commun. 1976;72:1137–1144. doi: 10.1016/s0006-291x(76)80250-1. [DOI] [PubMed] [Google Scholar]
- 9.Shigi N, et al. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J Biol Chem. 2006;281:2104–2113. doi: 10.1074/jbc.M510771200. [DOI] [PubMed] [Google Scholar]
- 10.Shigi N. Biosynthesis and functions of sulfur modifications in tRNA. Front Genet. 2014;5:67. doi: 10.3389/fgene.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan MA, Cannon JF, Webb FH, Bock RM. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985;161:368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numata T, Ikeuchi Y, Fukai S, Suzuki T, Nureki O. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature. 2006;442:419–424. doi: 10.1038/nature04896. [DOI] [PubMed] [Google Scholar]
- 13.Ikeuchi Y, Shigi N, Kato J, Nishimura A, Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Kambampati R, Lauhon CT. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry. 1999;38:16561–16568. doi: 10.1021/bi991119r. [DOI] [PubMed] [Google Scholar]
- 15.Dewez M, et al. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci USA. 2008;105:5459–5464. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283:27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 17.Shigi N, Sakaguchi Y, Suzuki T, Watanabe K. Identification of two tRNA thiolation genes required for cell growth at extremely high temperatures. J Biol Chem. 2006;281:14296–14306. doi: 10.1074/jbc.M511675200. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H, et al. Crystallographic and mutational studies on the tRNA thiouridine synthetase TtuA. Proteins. 2013;81:1232–1244. doi: 10.1002/prot.24273. [DOI] [PubMed] [Google Scholar]
- 19.Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigi N, Sakaguchi Y, Asai S, Suzuki T, Watanabe K. Common thiolation mechanism in the biosynthesis of tRNA thiouridine and sulphur-containing cofactors. EMBO J. 2008;27:3267–3278. doi: 10.1038/emboj.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäger G, Leipuviene R, Pollard MG, Qian Q, Björk GR. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J Bacteriol. 2004;186:750–757. doi: 10.1128/JB.186.3.750-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvier D, et al. TtcA: A new tRNA-thioltransferase with an Fe-S cluster. Nucleic Acids Res. 2014;42(12):7960–7970. doi: 10.1093/nar/gku508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci USA. 2008;105:18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leidel S, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 25.Nakai Y, Nakai M, Lill R, Suzuki T, Hayashi H. Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway. Mol Cell Biol. 2007;27:2841–2847. doi: 10.1128/MCB.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beinert H, Kennedy MC. 19th Sir Hans Krebs Lecture. Engineering of protein bound iron-sulfur clusters: A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989;186:5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M, et al. ChlR protein of Synechococcus sp. PCC 7002 is a transcription activator that uses an oxygen-sensitive [4Fe-4S] cluster to control genes involved in pigment biosynthesis. J Biol Chem. 2014;289:16624–16639. doi: 10.1074/jbc.M114.561233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy MC, Mende-Mueller L, Blondin GA, Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci USA. 1992;89:11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwalla S, Stroud RM, Gaffney BJ. Redox reactions of the iron-sulfur cluster in a ribosomal RNA methyltransferase, RumA: Optical and EPR studies. J Biol Chem. 2004;279:34123–34129. doi: 10.1074/jbc.M405702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan TV, Stephens PJ, Devlin F, Burgess BK, Stout CD. Selective oxidative destruction of iron-sulfur clusters: Ferricyanide oxidation of Azotobacter vinelandii ferredoxin I. FEBS Lett. 1985;183:206–210. doi: 10.1016/0014-5793(85)80777-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, et al. A [3Fe-4S] cluster is required for tRNA thiolation in archaea and eukaryotes. Proc Natl Acad Sci USA. 2016;113:12703–12708. doi: 10.1073/pnas.1615732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigi N, Asai SI, Watanabe K. Identification of a rhodanese-like protein involved in thiouridine biosynthesis in Thermus thermophilus tRNA. FEBS Lett. 2016;590:4628–4637. doi: 10.1002/1873-3468.12499. [DOI] [PubMed] [Google Scholar]
- 33.Chuenchor W, Doukov TI, Resto M, Chang A, Gerratana B. Regulation of the intersubunit ammonia tunnel in Mycobacterium tuberculosis glutamine-dependent NAD+ synthetase. Biochem J. 2012;443:417–426. doi: 10.1042/BJ20112210. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, et al. Crystallographic study of the 2-thioribothymidine-synthetic complex TtuA-TtuB from Thermus thermophilus. Acta Crystallogr F Struct Biol Commun. 2016;72:777–781. doi: 10.1107/S2053230X16014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 36.Kuratani M, et al. Structural basis of the initial binding of tRNA(Ile) lysidine synthetase TilS with ATP and L-lysine. Structure. 2007;15:1642–1653. doi: 10.1016/j.str.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi K, et al. Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase. Nature. 2009;461:1144–1148. doi: 10.1038/nature08474. [DOI] [PubMed] [Google Scholar]
- 38.Bork P, Koonin EV. A P-loop-like motif in a widespread ATP pyrophosphatase domain: Implications for the evolution of sequence motifs and enzyme activity. Proteins. 1994;20:347–355. doi: 10.1002/prot.340200407. [DOI] [PubMed] [Google Scholar]
- 39.Settembre EC, et al. Thiamin biosynthesis in Bacillus subtilis: Structure of the thiazole synthase/sulfur carrier protein complex. Biochemistry. 2004;43:11647–11657. doi: 10.1021/bi0488911. [DOI] [PubMed] [Google Scholar]
- 40.Daniels JN, Wuebbens MM, Rajagopalan KV, Schindelin H. Crystal structure of a molybdopterin synthase-precursor Z complex: Insight into its sulfur transfer mechanism and its role in molybdenum cofactor deficiency. Biochemistry. 2008;47:615–626. doi: 10.1021/bi701734g. [DOI] [PubMed] [Google Scholar]
- 41.Forouhar F, et al. Two Fe-S clusters catalyze sulfur insertion by radical-SAM methylthiotransferases. Nat Chem Biol. 2013;9:333–338. doi: 10.1038/nchembio.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinsland C, Taylor SV, Kelleher NL, Mclafferty FW, Begley TP. Overexpression of recombinant proteins with a C-terminal thiocarboxylate: Implications for protein semisynthesis and thiamin biosynthesis. Prot Sci. 1998;7(8):1839–1842. doi: 10.1002/pro.5560070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Igloi GL. Interaction of tRNAs and of phosphorothioate-substituted nucleic acids with an organomercurial: Probing the chemical environment of thiolated residues by affinity electrophoresis. Biochemistry. 1988;27:3842–3849. doi: 10.1021/bi00410a048. [DOI] [PubMed] [Google Scholar]
- 44.Van der Veen AG, et al. Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc Natl Acad Sci USA. 2011;108:1763–1770. doi: 10.1073/pnas.1014402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunkóczi G, et al. Phaser.MRage: Automated molecular replacement. Acta Crystallogr D Biol Crystallogr. 2013;69:2276–2286. doi: 10.1107/S0907444913022750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:665–667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolinsky TJ, et al. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007;35:W522-5. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Long F, Wang L, Söll D, Whitman WB. The putative tRNA 2-thiouridine synthetase Ncs6 is an essential sulfur carrier in Methanococcus maripaludis. FEBS Lett. 2014;588:873–877. doi: 10.1016/j.febslet.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, et al. Biosynthesis of 4-thiouridine in tRNA in the methanogenic archaeon Methanococcus maripaludis. J Biol Chem. 2012;287:36683–36692. doi: 10.1074/jbc.M112.405688. [DOI] [PMC free article] [PubMed] [Google Scholar]