Abstract
Human herpesvirus 6 (HHV-6) is a betaherpesvirus that is closely related to human cytomegalovirus. It was discovered in 1986, and HHV-6 literature has expanded considerably in the past 10 years. We here present an up-to-date and complete overview of the recent developments concerning HHV-6 biological features, clinical associations, and therapeutic approaches. HHV-6 gene expression regulation and gene products have been systematically characterized, and the multiple interactions between HHV-6 and the host immune system have been explored. Moreover, the discovery of the cellular receptor for HHV-6, CD46, has shed a new light on HHV-6 cell tropism. Furthermore, the in vitro interactions between HHV-6 and other viruses, particularly human immunodeficiency virus, and their relevance for the in vivo situation are discussed, as well as the transactivating capacities of several HHV-6 proteins. The insight into the clinical spectrum of HHV-6 is still evolving and, apart from being recognized as a major pathogen in transplant recipients (as exemplified by the rising number of prospective clinical studies), its role in central nervous system disease has become increasingly apparent. Finally, we present an overview of therapeutic options for HHV-6 therapy (including modes of action and resistance mechanisms).
INTRODUCTION
THE HUMAN HERPESVIRUSES
To date, the human Herpesviridae family counts eight members. Basedon genetic analysis of the conserved structural protein gH, they are divided into three subfamilies, designated alpha, beta, and gamma. Their main shared characteristic is their ability to remain in a latent or persistent state in their host for life and to reactivate during periods of relative immunosuppression. Apart from herpes simplex virus 2 (HSV-2) and human herpesvirus 8, their prevalence in the adult population is high, although geographic variations exist. The Alphaherpesvirinae HSV-1 and -2 were the first to be discovered; they are neurotropic and cause fever blisters and genital sores, respectively, and occasionally encephalitis. The varicella-zoster virus belongs to this subfamily as well. It is the only herpesvirus for which a vaccine (Varivax, Varilrix) is available, which is routinely administered in the United States and has recently been recommended for implementation in Europe. Primary varicella-zoster virus infection causes chickenpox (varicella), usually in children. Reactivations are common in immunosuppressed and elderly persons and result in shingles (zoster), a self-resolving skin disease that may be complicated by postherpetic neuralgia in a subset of patients. The Gammaherpesvirinae Epstein-Barr virus (EBV; the cause of the majority of mononucleosis cases) and human herpesvirus 8 (HHV-8; which has etiologically been linked to Kaposi's sarcoma and other malignancies) are lymphotropic and are the only human herpesviruses with an established oncogenic potential. The Betaherpesvirinae human cytomegalovirus (HCMV), HHV-6, and HHV-7 have a less-confined cell tropism, and their clinical spectra are still being defined. HCMV congenital infection is a major cause of hearing loss and mental retardation. Moreover, reactivation of the virus is an important concern in immunocompromised patients, such as transplant recipients and AIDS patients (although less so in the latter group since the introduction of highly active antiretrovirus therapy), causing retinitis, pneumonitis, and generalized infection. Chronic HCMV infection has recently been associated with atherosclerosis and restenosis (372). HHV-7 has not definitively been linked to any disease yet, but it is responsible for a subset of exanthema subitum (ES) (see below) and other exanthema cases. In transplant recipients, HHV-7 reactivation would facilitate development of HCMV disease. HHV-6 exists as two closely related variants, HHV-6A and HHV-6B. HHV-6A has not been etiologically linked to any disease; HHV-6B is the causative agent of ES, a childhood disease characterized by high fever and a mild skin rash, occasionally complicated by seizures or encephalitis. Like HCMV and HHV-7, it is increasingly being recognized as a pathogen in immunocompromised hosts.
BIOLOGY OF HHV-6
Ultrastructure
All herpesviruses consist of three main structural elements: (i) a nucleocapsid with icosahedral symmetry and a diameter of 90 to 110 nm and which contains the viral DNA genome; (ii) an envelope, in which the viral glycoproteins are embedded; and (iii) the tegument, which consists of a protein mixture that occupies the space between nucleocapsid and envelope. Mature virions are approximately 200 nm in diameter (37, 442).
Genome
Structure.
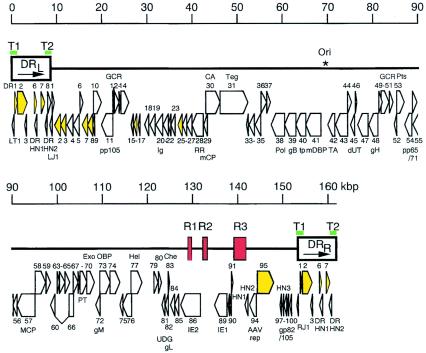
The HHV-6 genome is a linear, double-stranded DNA molecule, 160 to 162 kb in size, and consists of a 143- to 145-kb unique (U) region, flanked by terminal direct repeats (DR) of 8 to 9 kb and interrupted by three intermediate repeats, designated R1, R2, and R3, in the immediate-early A (IE-A) region (Fig. 1). The DRs contain the cleavage-packaging motifs, pac-1 and pac-2, and reiterations of the hexanucleotide (GGGTTA)n (403), which is a characteristic sequence of the telomeres of vertebrate chromosomes (283). These reiterations have been hypothesized to play a role in DNA replication and in maintenance of the viral genome in latently infected cells (142). The genomic organization of the unique region shows similarities to that of the unique long (UL) region of HCMV and is colinear to that of HHV-7. The HHV-6B genome contains 119 open reading frames (ORFs) (encoded by 97 genes), 9 of which are absent in HHV-6A (109). On the other hand, nine ORFs in HHV-6A described by Gompels et al. (143) do not have counterparts in the HHV-6B genome as a result of either the lack of an initiator codon, truncation, or frameshift mutations. The genes in the unique region are termed U1 to U100 and ORFs within the direct repeats are designated DR1 to DR7, whereas the 9 ORFs unique to HHV-6B are abbreviated as B1 to B9.
FIG. 1.
Schematic representation of the HHV-6B genomic organization. The terminal direct repeats (DRL and DRR) are boxed, and the intermediate repeats (R1, R2, and R3) are shown as red boxes. Telomeric sequences (T1 and T2) are denoted as green bars. The origin of lytic replication (Ori) is indicated by an asterisk. Protein coding regions are represented by open arrows; arrows colored yellow denote genes belonging to the HCMV US22 gene family. GCR, G-protein-coupled receptor; Ig, immunoglobulin superfamily; RR, ribonucleotide reductase; mCP, minor capsid protein; CA, capsid assembly protein; Teg, large tegument protein; Pol, DNA polymerase; tp, transport protein; mDBP, major single-stranded DNA binding protein; TA, conserved herpesvirus transactivator; dUT, dUTPase; Pts, protease/assembly protein; MCP, major capsid protein; PT, phosphotransferase; Exo, exonuclease; OBP, origin binding protein; Hel, helicase; UDG, uracil-DNA glycosylase; Che, chemokine; AAV/rep, adeno-associated virus-2 replication protein homolog. Reproduced from Isegawa et al. (183) with permission of the publisher.
Genes coding for structural virion components or enzymes required for nucleotide metabolism and DNA replication, and that are conserved among all herpesviruses, cluster into seven gene blocks. An additional cluster of genes, the so-called US22 gene family, is conserved among β-herpesviruses only. Their function is not fully understood, although several members have transactivating potential (201, 304, 375). Several genes (such as U83 and U94) (404, 463) are unique to HHV-6; their function will be explained later.
Interstrain variation.
The overall nucleotide sequence identity between HHV-6A and -B variants is 90%. It decreases from 95 to 98% in the central, conserved regions of the genome towards the genome ends, with the divergence in the IE-1 region (genes U86 to U95, except U94) reaching 31% at the nucleotide level (109, 183). HHV-6A and HHV-6B have characteristic restriction endonuclease profiles which allow clinical isolates to be classified unambiguously as A or B variants (1, 24). As mentioned above, the highest degree of sequence divergence is found in the IE-1 region (68, 437). Its splicing patterns and temporal transcription regulation differ between A and B variants (109), suggesting that this region may be responsible for certain biological differences between the two variants. Another determinant of the phenotypic difference between A and B variants is the U97-U100 region, encoding the virion envelope glycoprotein complex gp82-gp105. An exon of this gene, designated HN3, is found only in HHV-6B (183). Extensive splicing of its mRNA transcripts results in different proteins containing variant-specific neutralizing epitopes (325, 326). As the 80-kDa U100 gene product, designated gQ, is involved in CD46 receptor binding and subsequent membrane fusion (296), divergence in this region might contribute to differences in cell tropism between A and B variants. Other regions of divergence are LT1, DR2, DR7, DR8, LJ1, RJ1, U1, and U47 (183). Except for the protein encoded by DR7 (pDR7), whose function will be discussed in detail below, the biological functions of the other encoded proteins are not yet known. It is plausible, however, that high divergence in amino acid sequence explains biological and pathogenic differences between variants A and B.
Intrastrain variation.
As for the difference between A and B variants, the degree of heterogeneity between HHV-6 isolates within the same variant depends on which gene or region is being analyzed. The highest degree of intrastrain variation for HHV-6B maps to the left end of both DR regions (131, 143, 239, 272, 324, 403). Although the IE-A region shows a high level of sequence variation between A and B variants, it is highly conserved among HHV-6B isolates, whether obtained from clinical samples or after passage in vitro. Remarkably, the HHV-6B IE-A sequences were observed to further segregate into two distinct subgroups, represented by the laboratory strains Z29 and HST (374).
Replication Cycle
Both the A and B variants of HHV-6 enter the cell through interaction with CD46 (352), which is present on the membrane of all nucleated cells and is physiologically involved in complement regulation (241) (Fig. 2). This glycoprotein also serves as the receptor for vaccine strains of measles virus (111, 302), although the interactions with measles virus and HHV-6 occur via different domains of CD46 (149). It was shown for HHV-6A that the gH-gL-gQ complex (the glycoproteins H, L, and Q being encoded by the HHV-6 genes U48, U82, and U100, respectively) serves as the viral ligand for human CD46 (296). gH is the factor responsible for binding at the short consensus repeat domains SCR2 and SCR3 of CD46 (351). The HHV-6 gB (encoded by U39) is involved in the fusion process as well, through an as-yet-undefined mechanism (390). After binding to its receptor and gH-gL-gQ-mediated fusion of the viral envelope to the cell membrane, the incoming nucleocapsid is transported through the cytoplasm (most likely by association with the microtubule network) to nuclear pore complexes, where the viral DNA genome is released into the nucleoplasm. The cytosolic transport mechanisms have not been investigated in detail for HHV-6, but they probably show strong similarity to those described for HSV-1 (260) and HCMV (314). The virus then uses the cellular transcription and translation machinery to produce three kinetic classes of viral proteins (IE, early [E], and late [L]). HHV-6 IE proteins are synthesized within a few hours after infection and regulate the expression of other genes. The expression of E genes is dependent on IE protein synthesis and yields proteins that are mainly involved in DNA metabolism and replication. Dependency on viral DNA polymerase activity temporally discerns E and L proteins, although exceptions (so called leaky transcripts) exist (318). Late proteins are often glycosylated and serve as components of the mature virus particles, but also the chemokine-encoding U83 gene is expressed at late time points (463).
FIG. 2.
Schematic representation of the HHV-6 lytic replication cycle. The successive events of entry, replication, maturation, and egress are described in detail in “Replication cycle” in the text.
Herpesvirus DNA replication requires seven virally encoded factors. First, the origin binding protein (encoded by the HHV-6 U73 gene), which is absent in HCMV and in gammaherpesvirinae, binds to the origin of lytic replication (ori-lyt) and denatures a portion of the circular viral DNA genome (100, 179, 180). This gap is maintained by the helicase/primase complex (consisting of the U43, U74, and U77 gene products), which also provides RNA primers for the lagging-strand DNA synthesis (303). The single-stranded DNA in the “replication bubble” is stabilized by the major DNA binding protein (encoded by U41) until second-strand synthesis is catalyzed by the DNA polymerase (pU38) (399), driven by a processivity factor (pU27) (238). The four proteins encoded by the U79 and U80 genes of HHV-6 are suspected of being involved in DNA replication as well, although their role is not yet understood (396). As the new strand grows, the circular replication structure is nicked to form a rolling circle intermediate. Long concatameric strands of progeny DNA are encapsidated by the interaction of cleavage and packaging proteins with specific packaging (pac) signals at the end of the viral genomes (97). It is of interest that ori-lyt and pac sequences are different for the A and B variants of HHV-6 (414). The mature capsids bud out of the nucleus (thereby temporarily acquiring an intermediate membrane devoid of glycoproteins) into the cytoplasm, where they acquire a tegument and a secondary spiked viral envelope at the Golgi complex or at annulate lamellae, where viral glycoproteins accumulate (50). These are sequentially glycosylated in transport vesicles prior to release of the mature virus particle into the extracellular space by exocytosis. The HHV-6 maturation pathway is different from that of the other herpesviruses in that no viral glycoproteins are detectable in the cell membrane of infected cells (73, 411). The total time from infection to release of new virions (i.e., one replication cycle) takes approximately 72 h (38).
Cell Tropism
Although the initial designation of HHV-6 was human B-lymphotropic virus (350), both HHV-6A and HHV-6B replicate most efficiently in vitro in CD4+ T lymphocytes (388). All HHV-6 isolates infect activated peripheral or cord blood mononuclear cells (PBMCs or CBMCs, respectively), but their ability to infect particular T-cell lines is determining for their classification into the variant types (1). Grivel et al. (152) reported that CD4+ T lymphocytes expressing a nonnaive phenotype were preferentially infected by both variants, while CD8+ T cells were efficiently infected only with HHV-6A. Cord blood cells offer the advantage of being free of latent HHV-6, which is frequent in adult PBMCs. Nevertheless, HHV-6 is able to infect a wide variety of cell types, both in vitro and in vivo, which is consistent with the ubiquitous nature of its receptor, CD46. Still, the efficiency of lytic replication must rely on as-yet-unknown cell type-related factors.
Besides T lymphocytes, fibroblasts (246), natural killer cells (257), continuous liver cells (57), epithelial cells (65), endothelial cells (55), fetal astrocytes (160), oligodendrocytes and microglia (9) have been successfully infected with HHV-6 in vitro. The host tissue range of HHV-6 in vivo is even broader. It includes brain tissue (60, 110, 248), liver tissue (155, 186, 319), tonsillar tissue (347), salivary glands (130), and endothelium (55). Bone marrow progenitors were found to harbor latent HHV-6, which may be transmitted longitudinally to differentiated blood cells of different lineages (251). This is confirmed by the demonstration of HHV-6 in monocytes/macrophages (47, 215, 216) and dendritic cells (198).
HHV-6 has a restricted range of susceptible species, and a rodent model for HHV-6 infection does not exist. Nevertheless, antibodies to HHV-6 or a closely related cross-reacting simian virus have been demonstrated in monkeys (163), simian HHV-6 homologs have been identified (227), and experimental infections with HHV-6 in different monkey species and macaque tissue in vitro have been successful (436).
Latency
Like the other human herpesviruses, HHV-6 is capable of persisting in the host after primary infection. It can be inferred from experimental data that this persistence involves both a true latent state (with no production of infectious virus) and a low-level chronic replication, each occurring at different anatomic sites. Whereas salivary glands and brain tissue are suspected of harboring persistent HHV-6 infection (60, 110, 130, 194), candidate sites for latency are monocytes (215) and early bone marrow progenitor cells (251). Low levels of HHV-6 DNA are also found in PBMCs from healthy individuals; the U94 gene product was shown to enable the establishment and/or maintenance of latent infection in these cells (346). U94 is expressed at low levels during lytic replication but is a major transcript during latent infection (334, 346). Kondo et al. identified a set of latency-associated transcripts (LTs) from the HHV-6 IE-A region (220). They contained ORFs that were common to the productive-phase transcripts IE1 and IE2 (see below). However, different transcription start sites were used for LTs, and the structures of their 5′ noncoding regions differed from those of the productive-phase transcripts. Abundant expression of these HHV-6 LTs was observed just prior to the onset of viral reactivation, which was assessed by the presence of IE1 and IE2. Interestingly, this was observed both in patients, after the onset of immunosuppressive therapy, and in vitro, after phorbol stimulation of cultures harboring latent HHV-6 (219). Thus, cellular stimuli must be responsible for the expansion of LTs, which may induce the start of IE1 and IE2 productive-phase transcription, which in turn may enable viral reactivation from latency. The regulation of LT expression and the proteins encoded by them were found to share similarity to what has been described for HCMV (217).
HHV-6 can be reactivated from latency by superinfection with HHV-7, presumably through transacting mechanisms (see below) (203). More evidence for the latency-establishing potential of HHV-6 comes from reports on the integration of the HHV-6 genome into the host cell DNA, both in vivo and in vitro, at the ends of chromosomes 1, 17, and 22 (88, 89, 250). These findings are supported by the presence of human telomeric-like repeat sequences at the HHV-6 genome termini (142), since the integration site in chromosome 17 was shown to be within the telomeric sequences (297). Furthermore, HHV-6 pU94 may function in a similar way as the adeno-associated virus (AAV) rep gene products, which mediate site-specific DNA integration in human cells (383).
Regulation of HHV-6 Gene Expression
Transcription of IE genes occurs within minutes after virus entry into the cell, is independent of de novo protein synthesis, and often relies on virion-associated proteins (such as the tegument proteins) for proper expression. Little attention has been paid in the literature to the regulatory mechanisms for the expression of HHV-6 IE genes, although the intermediate repeat sequence R3 has been predicted to modulate the transcription of IE-A genes (Fig. 3), as it contains multiple putative binding sites for cellular transcription factors (such as PEA3, NF-κB, and AP-2) and is located upstream of the IE-A region (271, 405). Takemoto et al. (391) showed that the R3 region of HHV-6B strongly enhances the promoter activity of U95 through the interaction with NF-κB. The expression of HHV-6A U95 may be regulated similarly, although the repetitive organization of the R3 sequence diverges between both variants, the A variant R3 counting more NF-κB binding sites. Therefore, whereas HHV-6A R3 may be involved in the transcriptional regulation of the entire IE-A region, this is very unlikely for HHV-6B (391). It has been shown that, depending on the stage of infection, transcription of IE-A genes can be initiated from multiple promoters, resulting in multiple transcripts with different sizes and kinetics (147, 355).
FIG. 3.
Schematic diagram of the HHV-6A IE-A region. The region encodes two proteins (termed IE1 and IE2), which correspond to the open reading frames U90-89 and U90-86/87, respectively. Reproduced from reference 321 with permission of the publisher.
Although efforts have been made to temporally map HHV-6 transcripts (287, 318), the mechanisms at the basis of transcriptional regulation have only been investigated in detail for a few early or late HHV-6 genes. Among these are the U38 DNA pol gene (4), the U27-encoded p41 processivity factor (401), and the U83 chemokine-encoding gene (131). As for the IE genes (129, 220), differential splicing and the use of multiple transcription sites, as well as posttranscriptional modifications, contribute to the complex temporal expression patterns of early and late HHV-6 proteins (396). These, moreover, may be dependent on the cell line or methodology used for virus propagation and may differ among HHV-6 variants. This was confirmed by Øster and Hollsberg, who by using sensitive real-time PCR were able to detect many transcripts—even those encoding late proteins—as early as 1 h postinfection (318).
Transactivation by HHV-6 Proteins
Transactivating proteins by definition regulate some step in the expression of their target genes by binding to cis-acting elements (e.g., a promoter sequence). The HHV-6 genome encodes a number of transactivating proteins; most of them are expressed at immediate-early time points, which is not surprising given the fact that IE viral proteins control the temporal cascade of viral gene expression. HSV-1, EBV, and HCMV encode proteins capable of transactivating in vitro the gene expression of human immunodeficiency virus type 1 (HIV-1), directed by the long terminal repeat (LTR) (8, 90, 204). As HHV-6 and HIV-1 target the same cell type (CD4+ T lymphocytes) and have been found to coinfect the same cells (253), this interaction may have biological relevance. The transactivating potential of HHV-6 infection on the HIV-1 LTR was the first to be reported (121); since then, these and other transactivating functions have been assigned to an increasing number of individual HHV-6 genes, including the following.
(i) pDR7.
The protein encoded by the HHV-6A DR7 gene (expressed from 18 h postinfection) has been shown to transform NIH 3T3 fibroblast cells, to transactivate the HIV-1 LTR promoter, and to increase HIV-1 replication (201, 400). Its oncogenic potential relates to its capacity for binding and inactivating the tumor suppressor protein p53 (200).
(ii) pU3.
The U3 gene is a positional homolog of HCMV UL24 and a member of the US22 family, which contains multiple genes whose products function as gene regulators. Likewise, the U3-encoded protein was found to transactivate the HIV-1 LTR promoter in monkey kidney CV-1 cells (295).
(iii) IE-B proteins (U16 to U19).
The U16 to U19 genes are members of the HCMV US22 gene family and share similarities with the IE HCMV UL36 to UL38 genes, which encode transactivators that up-regulate viral and cellular transcription (76). Temporal regulation and splicing patterns of the U16/U17 genes are complex. The immediate-early expressed spliced gene products of U16/17 and the IE U18- and U19-encoded proteins have all been shown to independently transactivate the HIV-1 LTR promoter in vitro (129, 136, 304).
(iv) U27.
The DNA polymerase processivity factor, encoded by the HHV-6 U27 gene, was shown to transactivate the HIV-1 LTR in CV-1 cells. The presence of NF-κB binding sites was mandatory for the response to pU27 (461).
(v) IE-A proteins.
The HHV-6 IE-A region is a positional homolog of the HCMV major IE locus; both regions express extensively spliced transcripts and show CpG suppression. However, they exhibit very limited similarity on the nucleotide sequence level. The HHV-6 IE-A locus encodes two proteins, IE1 and IE2, corresponding to the open reading frames U89 and U86-87, respectively (Fig. 3). Both are expressed from spliced mRNAs, and each contains an exon derived from the U90 gene (308). Although both IE1 and IE2 proteins are detectable at immediate-early time points, data on the temporal regulation of expression of U90/U86-87 transcripts seem conflicting (148, 318, 321, 355). There is a consensus, however, that IE1 is expressed prior to IE2 and that IE2, in contrast to IE1, continues to increase over time (148). IE1 of HHV-6A was shown capable of transactivating heterologous promoters, suggesting a putative role for IE1 in transcriptional regulation (127, 271). Compared to the A variant of IE1, the HHV-6B IE1 protein was found to exhibit much lower transactivating potential on the HIV-1 LTR (147). IE2 was shown to bind to a DNA fragment containing the putative IE-A promoter; whether it is capable of shutting off promoter activity, like its HCMV homolog IE86, has yet to be demonstrated (321). IE2 activates multiple promoters that have no regulatory element in common, such as the complex HIV-1 LTR promoter, or simple promoters containing no or only one response element (NF-κB, CRE, or NFAT) (148). This suggests that IE2 may play an important role in controlling the expression of both cellular and viral genes, as illustrated by its abilities to transactivate the CD4 promoter (127) and to enhance cyclooxygenase-2 expression (193). U95 is a member of the HCMV US22 gene family and is located upstream of R3. It is a positional homolog of the murine CMV IE2 gene (which is known to exhibit transactivating potential) and has been shown to be expressed as an IE protein (391); whether pU95 acts as a transactivator has not been determined yet.
(vi) U94-encoded AAV-2 rep homolog.
U94 is an analog of the human AAV rep gene that among herpesviruses is unique for HHV-6 (404). HHV-6 pU94 acts as a transactivator by binding to a transcription factor, human TATA binding protein (294). Also, pU94 by itself possesses single-stranded DNA binding capacity, which is enhanced by cellular nuclear factors (102). pU94-encoded protein was found to activate the HIV-1 LTR promoter in fibroblast cells (406) and is capable of suppressing transformation by the human H-ras oncogenic protein (16).
Also worth mentioning here are the products of the HHV-6 genes U42, a transactivator conserved among herpesviruses (430), U51, which specifically down-regulates transcription of the gene coding for RANTES (for regulated upon activation, normal T-cell expressed and secreted) (286), and U54, a homolog of the HCMV tegument protein pUL82 (243).
Molecular Interactions between HHV-6 and Other Viruses
HIV-1.
HHV-6 has been proposed as a cofactor in AIDS (254). To substantiate this claim, the following arguments were raised, which are based on in vitro data rather than on clinical observations (see “HIV patients” below). (i) HHV-6, like HIV-1, has a tropism for CD4+ T cells (258), and lytic HHV-6 infection may contribute to the decline of this cell population in HIV-infected individuals. (ii) As described above, several HHV-6 proteins are capable of transactivating the HIV-1 LTR promoter, thereby stimulating HIV-1 expression (121, 277). Moreover, HHV-6 proteins and the HIV-1 transactivating protein TAT were shown to interact synergistically in this respect (105, 134). The reciprocal effects of TAT on HHV-6 are less clear, as it has been shown to both inhibit HHV-6 replication in the Jurkat T-cell line (105) and to enhance HHV-6 titers and protein synthesis in cord blood lymphocytes and continuous CD4+ JJHAN T cells (359). (iii) HHV-6 induces CD4 expression, thus rendering nonpermissive cells susceptible to HIV-1 infection (127, 252, 255, 257). (iv) HHV-6 influences the expression of various immunological mediators, such as tumor necrosis factor alpha (TNF-α) (124), which activate HIV-1 expression (116). Productive coinfections with HHV-6 and HIV-1 have been reported both in vitro and in vivo (21, 123, 139, 253, 332). However, conflicting reports exist regarding the effect of HHV-6 on HIV-1 replication, with some studies showing activation (81, 121, 167, 253) and others showing repression of HIV replication (39, 53, 235). In an in vivo model using SCID mice engrafted with human liver or thymus tissue, both viruses did not seem to affect each other's cytopathicity (139). As mentioned below in “HIV patients,” the clinical evidence for a role of HHV-6 in HIV disease is inconclusive.
EBV.
Given their prevalence in a broad array of human tissues, particularly of lymphoid origin, it may not come as a surprise that HHV-6 and EBV are frequently detected simultaneously (34, 415). HHV-6 infection was shown to activate EBV replication from latency by a transacting mechanism targeting a cyclic AMP response element within the EBV Zebra promoter (126, 128), to increase the expression of the EBV early genes BZLF1 and BMRF1 through transactivation of their promoters (82), and to enhance EBV's transforming capacity (84). In return, the presence of the EBV genome rendered B cells more susceptible to HHV-6 infection (82). The relevance of these in vitro data is unclear, since clinical data have shown that, contrary to EBV, HHV-6 has no important role in common lymphoproliferative disorders (see “Neoplasia,” below).
AAV-2.
During its evolution, HHV-6 has acquired the AAV-2 rep gene that, apart from its essential role in AAV-2 DNA replication, has transactivating potential and inhibitory effects on cell transformation (404). Although both genes evolved differently and their products have different transacting activities, HHV-6 pU94 was found able to complement replication of a rep-deficient AAV-2 genome (406).
HPV.
HHV-6 was shown to infect human cervical epithelial cultures harboring latent human papillomavirus (HPV), thereby enhancing HPV mRNA expression. Moreover, the proteins encoded by the HHV-6 U16 gene (previously designated pZVB-70) and the genomic clone pZVH-14 (encompassing U30) transactivated HPV-transforming genes. However, as outlined in “Neoplasia” below, clinical investigations argue against an association between HHV-6 infection and HPV-induced neoplasia (59, 66, 342, 413).
Molecular Interactions between HHV-6 and the Immune System
HHV-6-encoded chemokine and chemokine receptors.
During their evolution, many viruses have developed molecular piracy and mimicry mechanisms so that acquired host genes within virus genomes produce proteins capable of interfering with the normal host defense response (10). The protein encoded by the HHV-6 U83 gene, after being identified as a functional chemokine (463), was recently found to be a highly selective and effective CCR2 agonist (259). pU83, produced by HHV-6-infected cells, attracts CCR2-expressing cells (such as monocytes/macrophages), in which the virus may establish a productive or latent infection, thus facilitating virus spread. In contrast to the U83 gene which, among herpesviruses, is unique to HHV-6, U12 and U51 have counterparts in the other betaherpesviruses and encode G-coupled receptors similar to chemokine receptors. pU12 is a functional β-chemokine receptor, related to CCR-1, -3, and -5 and expressed at the late stage of HHV-6 infection in CBMCs and monocytes/macrophages. pU12 is activated by the β-chemokines RANTES, macrophage inflammatory protein 1α and 1β, and monocyte chemoattractant protein 1, but not by the α-chemokine interleukin-8 (IL-8) (184, 216). U51 is expressed at early times postinfection. When expressed in epithelial cells, pU51 was shown to specifically bind RANTES, an event associated with morphological alterations and transcriptional down-regulation of RANTES, which are both proposed to play a role in the systemic spread of virus from infected epithelia (286). Remarkably, pU51 accumulated in the endoplasmic reticulum of transfected human epithelial kidney and osteosarcoma cell lines, whereas in transfected and HHV-6-infected T cells it was found integrated in the cell membrane, indicating that a T-lymphocytic function is required for expression of pU51 at the plasma membrane (282). Although this factor has not yet been elucidated, it can be speculated that this cell type-dependent expression profile may involve selective recruitment of circulating inflammatory cells to enhance infection.
Modulation of the host's immune response by HHV-6.
Modulation of expression of the host's immune factors is yet another mechanism of evading the immune response or creating an environment in which the virus can survive (161, 419). This is particularly important for herpesviruses, which persist in their host throughout life. Mutual development of adaptive immune responses by the host, as reviewed for HCMV by Basta and Bennink (30), has not been investigated for HHV-6. For the purpose of immune modulation, HHV-6 exploits its transactivating capacities as well as other mechanisms that are not related to transcription.
(i) IFNs.
Interferons (IFNs) are part of the cellular innate immune response to viruses in general and are therefore likely to influence the course of HHV-6 infection. HHV-6 infection of mononuclear cells resulted in an increased production of IFN-α (as has been shown for other herpesviruses [299]), even when UV-irradiated (but not heat-inactivated) virus was used. IFN-α in turn was shown to suppress HHV-6 replication (207, 212). In contrast, IFN-γ release was inhibited by HHV-6 in PBMCs (19), whereas in continuous T cells IFN-γ levels were unaltered after HHV-6 infection (275).
(ii) TNF-α and ILs.
HHV-6-induced up-regulation of TNF-α and IL-1β in PBMCs was found to depend solely on virus entry-related events, since viral de novo protein synthesis was not required (124, 145). Similarly, the transcriptional down-regulation of IL-2 required no active viral replication (125). TNF-α release was also found to be up-regulated in HHV-6-infected differentiated U937 monocytoma cells (18). With an immunomicroarray, Mayne et al. (275) observed that both A and B variants of HHV-6 increased expression in the Sup-T1 T-cell line for the genes for IL-18, the IL-2 receptor, members of the TNF-α superfamily receptors, mitogen-activated protein kinase, and Janus kinase signaling proteins. Furthermore, the CCR7 receptor was up-regulated when CD4+ T cells were infected with HHV-6 (158). Previous studies in infected PBMCs suggested that HHV-6 induces the T-helper cell profile to shift from Th1 to Th2 by up-regulating IL-10 and down-regulating IL-12 in infected PBMCs (19). However, Mayne et al. (275) reported that type 2 cytokines, including IL-10, its receptor, and IL-14, were down-regulated by HHV-6 infection in continuous T cells. Conflicting evidence concerning IL-10 and IL-12 expression also originated from experiments using monocytes/macrophages. According to Li et al. (236), both cytokines were induced in HHV-6-infected monocytes and continuous THP-1 monocytic cells, although simultaneous stimulation with IFN-γ increased IL-12 and decreased IL-10 production. In a more recent study by Smith et al. (365), however, IL-12 production in stimulated macrophages was found to be profoundly impaired upon HHV-6 infection, the IL-12 levels being affected at the posttranscriptional level, independently of viral replication. The effect of HHV-6 on IL-18 expression is yet another issue on which conflicting data have been reported (17, 275). Obviously, the regulation of cytokine expression is a tightly controlled process that may be altered according to the microenvironment and differentiation status of the cell. Thus, especially when studying the interactions between viruses and cytokine expression, one must realize that the experimental design may bias the observations. Furthermore, HHV-6 has been reported to induce IL-6 both in vitro (275, 446) and in vivo (138) and to up-regulate the production of monocyte chemoattractant protein 1 and IL-8 (55, 178).
HHV-6A was observed to down-regulate the de novo synthesis of human leukocyte antigen (HLA) class I molecules in mature dendritic cells, while HLA class II expression was unaffected (164). In contrast, HHV-6 induced HLA class I and II molecules at the cell surface of immature dendritic cells; however, in these cells, antigen capture and/or processing was impaired after HHV-6 infection (198). Finally, an interesting feature of HHV-6 infection is that, during the course of infection, it down-regulates cell surface expression of its own receptor, CD46, in infected as well as in uninfected cells, both in vitro and in an ex vivo virus propagation system (152, 352). In light of the speculations on a role of HHV-6 in AIDS progression (254), its ability to increase CD4 protein levels in continuous T cells (275), hematopoietic progenitor cell lines (418), CD4− γδ T cells (255), and lymphoid tissue ex vivo (152) is an important feature. Transactivation of the CD4 promoter by the IE1 and IE2 proteins of HHV-6 was found to underlie this event (127). HHV-6 infection, through down-regulation of the HIV-1 receptor CXCR4, may render CD4+ T lymphocytes resistant to HIV-1 infection in vitro (157, 439). However, expression levels of neither CXCR4 nor CCR5 were affected by HHV-6 in an ex vivo propagation system (151). Moreover, HHV-6 replication enhances the production of the CCR5 ligand RANTES (54, 151, 152) and thus provides a mechanism for the selective blockade of CCR5-tropic HIV-1, as proven by the suppression of CCR5-tropic but not CXCR4-tropic HIV-1 replication by HHV-6 (151).
(iii) T-cell activation.
IL-2-induced activation of primary T lymphocytes is an absolute requirement for efficient HHV-6 replication, but at higher concentrations (>10 U/ml), IL-2 strongly inhibits the virus-induced cytopathic effect (132, 339). The requirement for T-cell activation as a stimulus for HHV-6 was also illustrated by pretreatment of PBMCs with anti-CD3 (206). CD3 is transcriptionally down-regulated by newly formed HHV-6 proteins, resulting in reduced surface expression of the CD3/T-cell receptor complex (152, 256). Although T-cell activation may be a prerequisite for HHV-6 replication, the T-cell proliferative response to mitogen or antigen presentation was seen to be severely impaired after HHV-6 infection (125, 166), indicating that HHV-6 infection induces a state of immune suppression.
Natural killer (NK) cells are important in the host's early immune response to tumor cells or viruses, as they can kill virus-infected cells in an HLA-independent manner. NK cells were found to have enhanced killing potential towards HHV-6-infected cells through a mechanism mediated by IL-15, which triggers the synthesis of IFN-γ from both CD4+ and NK cells, thereby modulating the cytotoxic potential of NK cells (146).
HHV-6 and Apoptosis
CD4+ continuous JJHAN T cells, infected with the A or B variant of HHV-6, displayed increased surface expression of TNF receptor 1, resulting in apoptosis. The degree of apoptosis was augmented by TNF-α and anti-Fas antibody. Remarkably, apoptotic cells in infected cultures harbored little or no virus, and apoptosis could be induced by virus-free culture supernatants, indicating that indirect mechanisms may be at the basis of these observations (181). Although they found evidence of apoptotic CD4+ cells in HHV-6-infected CBMC cultures, Ichimi et al. (174) demonstrated that the apoptotic marker 7A6 antigen and HHV-6 antigen colocalized to the same cells and that neither Fas nor TNF-α was involved in the induction of apoptosis by HHV-6. It is unclear whether the different experimental set-ups (continuous versus primary CD4+ T lymphocytes) can explain these conflicting data. The ability of HHV-6 to induce apoptosis was further illustrated in vivo in CD4+ lymphocytes from patients, isolated during acute HHV-6 infection (440), and in lymphocytes and macrophages from patients with Kikuchi-Fujimoto's disease (225). Kirn et al. (209) demonstrated elevated levels of p53 and p21waf in HHV-6-infected CBMCs. We also reported an increasing fraction of apoptotic CBMCs after HHV-6 infection, probably mediated by a strong and early up-regulation of p53. The effect was more pronounced after infection with variant A of HHV-6 and was found to be independent of p21 expression (92).
CLINICAL FEATURES OF HHV-6
Epidemiology
The reported seroprevalence of HHV-6, especially in the earlier reports, differs according to a number of variables, such as differences in serologic assay sensitivity and interpretation, cross-reaction of certain antibodies with HHV-6 and HHV-7 antigens in immunofluorescence assays, and geographic variations. Nevertheless, the seropositivity in the adult population is now estimated to be >95% for either or both HHV-6 variants in developed countries, the antibody titers showing a decline with advancing age (27, 175, 322, 354). HHV-6 is widespread throughout the world, with geographic differences in HHV-6 prevalence varying between 70 and 100% (36, 69, 240, 306, 409, 432); given the above-mentioned limitations, these differences may be insignificant. A serologic test discriminating between HHV-6 variants is not available. Two studies in 24 and 36 patients in which variant typing was performed using a lymphoproliferative response assay indicated that all patients who responded to HHV-6A antigen also responded to HHV-6B antigen and that variant B was more seroprevalent than variant A (58 versus 25%) (422, 423).
HHV-6 is generally acquired between 6 and 15 months of age (120, 316), and the incubation period is 1 to 2 weeks. Primary HHV-6 infection accounts for 20% of all cases of acute fever in children between 6 and 12 months old (154) and is almost exclusively caused by HHV-6B, not HHV-6A (101, 356). It is currently not known at what age seroconversion to the A variant, which is frequently recovered from adults, takes place, but it is thought to occur after acquisition of the B variant and without manifest clinical symptoms. Interestingly, several studies report the isolation of more than one HHV-6 strain (from both variants) in individual patients, both adults and children (80, 226, 417, 454), indicating that reinfection with HHV-6 may occur.
Transmission
The frequent detection of HHV-6 DNA in saliva and salivary gland tissue (77, 104, 379, 460) suggests that salivary glands are a potential site for HHV-6 persistence and that saliva is a vehicle for transmission of the virus, either from mother to child or between children (300, 395, 417). Remarkably, all HHV-6 isolates from saliva were B variants (77). The detection of HHV-6 DNA in cord blood specimens of healthy newborns in the absence of serum immunoglobulin M (IgM) and in fetuses following spontaneous abortion supports the possibility of intrauterine transmission (3, 25). The incidence of vertical HHV-6 transmission is about 1 to 2% of all births and is not related to intrauterine HCMV transmission (41, 85). One case report describes severe neurological complications after intrauterine HHV-6 infection (228). Although HHV-6B DNA sequences were found in the genital tract of 20% of pregnant women, perinatal transmission seems unlikely (265, 315). Fecal-oral spread, a common transmission route among young children, has not been documented for HHV-6, although stool specimens were found positive for HHV-6 DNA (377, 379). Thus, the most probable route for HHV-6 transmission (at least for the B variant) is through saliva.
Clinical Manifestations of HHV-6 Infection
Primary infection.
Primary infection with HHV-6 causes acute febrile illness, usually in children between 6 months and 1 year old (22). Fever is high (≥40°C) and lasts for three (sometimes up to seven) days, after which it suddenly declines. A subset of patients subsequently develops a mild skin rash, mainly on the trunk, neck, and face, that is diagnosed as ES (roseola infantum, sixth disease); for unknown reasons, a larger portion of patients presents with clinically defined ES in Japan than in other countries (22, 154, 328). The association between ES and primary HHV-6B infection (demonstrated by seroconversion and virus isolation) was discovered by Yamanishi et al. (438). Since then, HHV-6B has been identified as the causative agent of illnesses similar to ES in young children (101, 154, 328, 331).
The most common complications are malaise, inflamed tympanic membranes, and gastrointestinal and respiratory tract symptoms (154). Febrile seizures were reported in approximately 10% of children with primary HHV-6 infection, representing 10 to 20% of febrile seizure cases in children under 2 years old (22, 29, 154, 218, 426). More severe central nervous system (CNS) complications, such as meningoencephalitis and encephalopathy, have been reported but are rare (185, 196, 202, 278, 378, 445). One case report describing an infant with afebrile convulsions due to primary infection with HHV-6B demonstrated that HHV-6 infection can have a direct effect on the brain, causing convulsions in the absence of fever (459). HHV-6 DNA has been detected in cerebrospinal fluid (CSF) of children with convulsions during primary infection or reactivation (218, 378). Thus, neurological complications are likely to result from direct infection of the CNS by HHV-6. It must be noted that the above-mentioned reports overestimate the proportion of children suffering complications of primary HHV-6 infection, as they are limited to those who required urgent medical care. Most HHV-6 infections, although characterized by high fever, have a benign and self-resolving course. Nonetheless, case reports highlight the versatile pathogenicity of HHV-6, with cases of fulminant hepatitis (23, 186, 280), liver dysfunction (385, 386, 392), thrombocytopenia (159, 210, 349, 448, 453), and hemophagocytic syndrome (327, 380, 387).
Given the high seroprevalence in the population, primary HHV-6 infection in adults is very rare; it is not clear whether disease manifestations are similar to those observed in children. For a few cases, a more severe outcome, such as infectious mononucleosis-like syndrome (6, 140, 182, 305, 376, 382) or fulminant hepatitis (367), has been reported in immunocompetent adults, although some early reports did not differentiate between primary infection and reactivation. In immunocompromised patients, the consequences of primary HHV-6 infection may be fatal. A number of case reports have described a primary infection in transplant recipients that was introduced by an HHV-6-positive graft (79, 229, 344, 427).
CNS disease.
The high fever, characteristic of HHV-6 primary infection, is often accompanied by seizures, and HHV-6 reactivation in immunocompromised patients is associated with severe encephalitis and/or encephalopathy. There have been case reports on immunocompetent and immunocompromised adults with severe CNS disease due to HHV-6 active infection, which was characterized by a fulminant multifocal demyelinating disease (32, 115, 262, 313, 329, 410). The neuroinvasiveness of the virus is proven by the fact that HHV-6 DNA is frequently detected in specimens from divergent regions of the brain (incidence rate of individuals with positive HHV-6 PCR on brain tissue, 32 to 85%) (60, 61, 83, 110, 249). Both A and B variants of HHV-6 have been found in a proportion reflecting their seroprevalence (the B variant being approximately three times more frequent than the A variant). Some patients harbor both variants, though at different locations within the brain (60). However, in a large prospective study including 2,716 children with acute ES, either HHV-6A or HHV-6B DNA, but not both, was detected in the CSF during the active phase (153). In patients with dual infection, only HHV-6A persisted in CSF, which may suggest that HHV-6A has greater neurotropism. Altogether, these studies prove that the brain is an important site for active and latent HHV-6 infection.
HHV-6 has been proposed as a cofactor in the etiology of progressive multifocal leukoencephalopathy (PML), a demyelinating disease of the CNS occurring in individuals with impaired cell-mediated immunity. PML is caused by the JC polyoma virus (JCV), but recent evidence suggests that HHV-6 and JCV cooperate as etiologic agents in PML. In these studies, active HHV-6 infection was demonstrated by immunocytochemistry within the demyelinating lesions of PML (and not in control tissues), and HHV-6 and JCV antigens colocalized to the same affected oligodendrocytes (86, 290).
A similar line of evidence was followed by Challoner et al. (58) to propose an association of HHV-6 with the etiology or pathogenesis of multiple sclerosis (MS), the most common human demyelinating disease. In this study, HHV-6 was identified as a commensal of the brain, yet HHV-6 antigen was only found in oligodendrocytes in MS lesions (plaques) and not in unaffected brain tissue of MS patients and non-MS individuals (58). Oligodendrocytes, the myelin-producing cells, play a primordial role in the pathogenesis of MS, a disease primarily characterized by an immune-mediated focal breakdown of myelin sheaths around neuronal axons, which results in impaired conduction of nerve impulses. Secondary to myelin destruction, T-cell and macrophage infiltration, astrocytic scar formation and, at a later stage, axonal damage are hallmarks of MS lesions (312).
Challoner's findings provoked substantial controversy, and a whole literature of conflicting data and comments has arisen, as critically reviewed by Moore and Wolfson (293). A first point of criticism is related to the viral parameter and the assay chosen to demonstrate HHV-6. Detection of anti-HHV-6 antibodies (IgG or IgM) in serum or CSF may not discriminate between active and latent (or chronic persistent) infection. Likewise, PCR or quantitative PCR detection of HHV-6 DNA in PBMCs may have little significance, while the same assay performed on serum or CSF should be indicative of an active HHV-6 infection. Other valuable techniques include virus isolation and culture from CSF or a lymphoproliferative assay to measure the reactivity of the patient's lymphocytes against HHV-6 antigens. Although immunohistochemical detection of HHV-6 antigen in brain specimens appears to be most conclusive, the scarcity of this material (in particular for specimens obtained at the onset of MS disease) limits the number of patients that can be studied. Any assay should include positive control samples (such as serum or CSF obtained from children with ES) and allow discrimination between HHV-6A and HHV-6B. A second consideration is related to the heterogeneity of MS disease. HHV-6 activity may depend on whether the patient is in an early or late stage of MS disease, in relapse or remission, or receiving immunosuppressive therapy such as corticosteroids. Nearly all published studies are case reports or retrospective analyses with ill-defined criteria for selection of MS patients. Finally, an increased presence of active HHV-6 in MS brains does not necessarily imply a causative role for the virus, since HHV-6 may merely benefit from the immunological dysfunction in MS. For all these reasons, the data gathered so far (and briefly summarized below) urge for a multicentered prospective study on a large number of MS patients in different disease stages, in which HHV-6 is being determined by a standardized assay, with negative and positive controls, and performed in a blinded fashion. Until then, the hypothesis on the association between HHV-6 and MS remains fascinating but unproven.
To establish a role of HHV-6 in the pathogenesis of MS, several groups have focused on patient material obtained at the onset of MS disease. Knox et al. (211) observed that HHV-6 viremia (measured by virus culture assay) was associated with early stages of MS disease. Using immunohistochemical analysis, HHV-6 antigen was found to be present in MS plaques but not in healthy tissue (218), consistent with the data from Challoner et al. (58). Biopsy specimens representing the earliest-manifesting MS lesions were studied by Goodman et al. (144). HHV-6 DNA was found at much higher frequency in MS patients than in controls, especially in oligodendrocytes and microglia, but HHV-6 antigen was only detected in reactive astrocytes and microglia in the absence of HHV-6 DNA. One reason for this discrepancy may be the different sensitivity of both assays. Alternatively, the detection of HHV-6 antigen in lesional astrocytes and microglia may reflect their phagocytic action towards HHV-6-infected dying oligodendrocytes, which is no longer present when MS disease has progressed to a chronic stage (144).
Detection of HHV-6 DNA in PBMCs requires a high PCR sensitivity that may not always be achieved. Although some studies claim this parameter to be increased in MS patients, the relevance of these data is limited, as they may reflect latent HHV-6 (12, 13, 270, 276, 345). Active HHV-6 infection, demonstrated by the presence of HHV-6 DNA in serum and/or CSF or by virus isolation, has frequently been observed at a higher incidence in MS patients than in controls (7, 63, 211, 397, 429), although other reports have presented negative results (11, 141, 237, 270, 288). The same is true for reports on an altered humoral or tissue-specific immune response in MS patients (expressed as serum IgM or CSF IgG levels or lymphoproliferative responses towards HHV-6 antigens) (63, 74, 119, 368, 370, 397). In one study, higher prevalence and higher antibody titers towards HHV-6 pU94 were found and it was concluded that MS patients may undergo a different exposure to HHV-6 than controls (56). Several authors who found HHV-6A more prevalent than HHV-6B have pointed at the importance of subtyping HHV-6-positive cases (7, 13, 208, 370). A correlation between the geographic distribution of MS and its possible causative agent(s), an evident requirement for etiological linkage, is missing for HHV-6B. The geographic distribution of HHV-6A, however, has not been studied. Subtyping of HHV-6 variants in the context of MS should therefore be considered. Finally, some studies have shown a correlation between active HHV-6 infection and clinical exacerbations (33, 63), whereas others have not (13). As already explained, this HHV-6 reactivation might be interpreted as a bystander effect from the immune dysfunction at the time of an MS exacerbation.
Several intriguing observations made in the past 3 years may provide an explanation on how HHV-6 could be associated (whether or not etiologically) with MS. (i) Elevated levels in serum and CSF of soluble CD46 have been described in several autoimmune disorders. In MS patients, these CD46 levels were found to be correlated with the presence of HHV-6 DNA in serum (369). This may point at an active HHV-6 infection and an increased vulnerability to complement lysis. (ii) HHV-6 is known to encode a highly selective and potent CCR-2 agonist, pU83 (259), which may attract CCR-2-expressing cells, such as macrophages, to the site of infection. Interestingly, a CCR2 gene polymorphism was recently related to the development of MS (and its animal model, experimental autoimmune encephalomyelitis) (190, 289). (iii) MS is considered an autoimmune disorder, although an autoantigen has not been definitively identified. Residues 4 to 10 of HHV-6 pU24 are identical to residues 96 to 102 of myelin basic protein, a candidate autoantigen in MS. Importantly, both T-cell and antibody responses to this peptide sequence were found elevated in MS patients (398). (iv) Soluble factors induced by HHV-6 infection were found to enhance necrosis of oligodendrocyte precursor cells and oligodendroglioma M03.13 cells. This effect is stronger for HHV-6A than for HHV-6B (221). More recently, in vitro infection of glial precursor cells by HHV-6 was found to impair cell replication and increase the expression of oligodendrocyte markers, suggesting that HHV-6 infection of the CNS may influence the neural repair mechanisms (103).
A definite answer on the role of HHV-6 in MS may be expected from clinical trials with antiviral therapy. The efficacy of IFN-β1a, which is routinely used in MS patients, may relate to its antiviral properties. A small-scale study by Hong et al. (165) indicated decreased levels of markers of active HHV-6 infection after IFN-β1a treatment. In one randomized placebo-controlled double-blind study, acyclovir was shown to reduce the exacerbation rate in relapsing-remitting MS patients yet to have no effect on the overall neurological deterioration (261). In another study, the oral prodrug valacyclovir effectuated a significant reduction in new lesion formation in patients with high disease activity (31). The acyclovir and valacyclovir doses used were nonimmunosuppressive and sufficiently high for suppression of most herpesvirus infections, including HHV-6. However, the antiviral activity may have been limited by inadequate levels of acyclovir in CSF (about fivefold lower than in serum). The major shortcoming of both studies was a lack of viral end points. Further studies on antiherpesvirus therapy in this subpopulation of MS patients are warranted, and the use of more active compounds (especially with respect to HHV-6) would provide more convincing data. Unfortunately, no such compounds with a long-term safety profile as favorable as that of acyclovir are currently available.
Neoplasia.
Two human herpesviruses, EBV and HHV-8, have been identified as oncogenic agents. As the first HHV-6 strains were isolated from patients with lymphoproliferative disorders (350), there has been continuous interest to explore its possible role in malignancy, especially in the context of immunosuppression. This interest was further fostered by the discovery of HHV-6 genes that encode transactivating proteins, one of which was shown to possess transforming capacity (pDR7; see “Transactivation by HHV-6 proteins”) (200). On the other hand, pU94 can suppress Ras-induced transformation and might counter tumorigenesis (16). Thus, multiple regulatory and cell-interacting mechanisms play a role during HHV-6 infection, and no transforming events were ever detected after HHV-6 infection in vitro. Second, although HHV-6 has tentatively been linked to certain proliferative disorders, no evidence could be provided for its oncogenic properties in vivo. Indeed, the ubiquitous nature of the pathogen and its ability to remain in a latent state and to integrate into the host cell chromosomal DNA (87, 250) complicate the interpretation of PCR analysis on tumor tissues. Moreover, the altered immune status in these patients, induced by disease or medication, may induce some degree of virus reactivation, with HHV-6 being an opportunistic rather than a causal pathogen.
A few studies have focused on HHV-6 antigen expression in lymphoid lesional tissues, representative of several lymphoproliferative disorders, that tested positive for HHV-6 DNA (247, 264, 341, 415). Their conclusions, mainly based on the absence of HHV-6 proteins from Reed-Sternberg cells, argue against a major pathogenic role of the virus in human lymphomas such as Hodgkin's and non-Hodgkin's lymphoma. A possible involvement of HHV-6 in Rosai-Dorfman disease (a rare, benign, pediatric lymphadenopathy) has been suggested (234, 247). Evidence has also been found for involvement of HHV-6 in S100-positive T-cell chronic lymphoproliferative disease, a rare but aggressive hematologic disorder (43).
The detection of HHV-6 in cervical epithelium (66, 435) and its transactivating properties towards HPV (65) have prompted the investigation of its role in the pathogenesis of cervical carcinoma (106). In a large prospective study, cervical tissue samples from 388 women were PCR analyzed for HHV-6 DNA, and the association between herpesvirus infection and the degree of cervical lesion was determined with respect to HPV status. It was concluded that HHV-6 (as well as HCMV and HHV-7) are bystanders rather than cofactors in the oncogenesis of cervical cancer (59). Other groups have confirmed that a causative role of HHV-6 in cervical cancer is unlikely (342, 413). A role for HHV-6 in the pathogenesis of oral carcinoma has been tentatively suggested, based upon the frequent detection of HHV-6 antigen in malignant and nonmalignant oral lesions (434), but this has not been further investigated.
Reactivation or reinfection in immunocompromised hosts.
HHV-6 infection is frequently detected in immunosuppressed patients. Most of these HHV-6 infections are due to reactivation of HHV-6B (114, 423), and the incidence peaks at 2 to 4 weeks posttransplantation (447). The incidence varies between 48% (range, 28 to 75%) for bone marrow transplant (BMT) recipients and 32% (range, 0 to 82%) for solid organ transplant (SOT) recipients (Tables 1 and 2). Similarly to what was outlined in “CNS disease,” this considerable variation in laboratory data may reflect patient heterogeneity, but it may also result from different methodologies with different assay sensitivities and varying size and nature of clinical samples. Again, the lack of a standardized method for the demonstration of active HHV-6 infection hampers the comparison of data. Most reports on severe complications of HHV-6 infection are from case studies or retrospective analyses. Although these individual cases are strongly suggestive of the clinical relevance of HHV-6 infection posttransplantation, the described medical cases are often complex and a careful interpretation of disease associations is warranted. Nevertheless, the numerous prospective studies realized during the past 5 years (Tables 1 and 2) have confirmed that HHV-6 reactivation is associated with some disease in a subset of transplant recipients, and the main clinical observations (encephalitis, fever, and rash) are consistent with early case reports.
TABLE 1.
Prospective studies on HHV-6 reactivation after BMT
| Detection methoda | Sampleb | No. of patients | Incidence of active HHV-6 infection (%) | Observed diseased | HHV-6 variant | Reference |
|---|---|---|---|---|---|---|
| PCR | PBMC, BALF | 41 | 46 | Vascular endothelial damage, GVHD | Not determined | 389 |
| PCR | PBMC, plasma | 92 | 42.5 | Myelosuppression, fever, delayed platelet and neutrophil engraftment | Not determined | 177 |
| PCR | PBL | 22 | 60 | Delayed platelet engraftment | B | 266 |
| PCR | PBL, plasma | 61 | 28 | Fever, engraftment failure | B | 62 |
| PCR | PBMC | 37 | Not given | Delayed platelet and granulocyte engraftment | B | 421 |
| PCR | PBL, oral lavage fluid, urine | 57 | 60 | Acute GVHD | 90% B, 10% A | 428 |
| PCR, IHC | PBL, skin | 57 | Not given | GVHD | Not determined | 15 |
| qPCR | PBL, CSF | 74 | 78 | Delayed platelet engraftment | Not determined | 245 |
| qPCR | PBMC | 20c | Not given | Rash and fever (2/20) | Not determined | 79 |
| VI | PBMC | 82 | 38 | Rash | B | 447 |
| VI | PBMC | 22 | Not given | Skin rash | Not determined | 450 |
| VI | PBMC | 26 | 46 | None | B | 197 |
| VI | PBMC and/or bone marrow | 25 | 48 | Skin rash | Not determined | 456 |
RT-PCR, reverse transcriptase PCR; qPCR, quantitative (real-time) PCR; VI, virus isolation; IHC, immunohistochemistry.
PBL, peripheral blood lymphocytes; BALF, bronchoalveolar lavage fluid.
Patients in this study were all HCMV seronegative.
GVHD, graft-versus-host disease.
TABLE 2.
Prospective studies on HHV-6 reactivation after SOT
| Transplant type | Detection methodb | Samplec | No. of patients | Incidence of active HHV-6 infection (%) | Observed diseasee | HHV-6 variant | Reference |
|---|---|---|---|---|---|---|---|
| Heart | PCR | PBMC | 21 | 0 | No disease | 298 | |
| Kidney | PCR | PBMC | 107 | Not givend | No disease | Not determined | 320 |
| Kidney | qPCR | PBMC | 52 | 23 | No disease | Not determined | 205 |
| Kidney | VI, serology | PBMC | 65 | 55 | None | Not determined | 455 |
| Kidney and/or liver | VI, serology | PBMC | 32 | 31 | None (unless concomitant HCMV infection) | Not determined | 162 |
| Kidney and/or pancreas | PCR, serology | Urine, serum | 30 | 50 | Fever | Not determined | 335 |
| Liver | IHC | Liver tissue, PBMC | 32 | Not given | Acute liver failure | Not determined | 155 |
| Liver | IHC | PBMC | 34 | 29 | HCMV disease | Not determined | 232 |
| Liver | qPCR | PBMC | 200 | 28 | Opportunistic infections, HCMV disease, acute graft rejection | Not determined | 171 |
| Liver | qPCR, serology | PBMC | 33 | 9 | HCMV disease | Not determined | 281 |
| Liver | qPCR | PBMC | 88 | 54 | HCMV disease | Not determined | 173 |
| Liver | PCR, VI, serology | Plasma | 47 | 49 | Fever | B | 452 |
| Liver | VI | PBMC | 80 | 39 | CNS disease, fungal infections | Not determined | 340 |
| Liver | Serology, IHC | PBMC | 51 | 22 | Graft dysfunction | B | 233 |
| Liver | qPCR | PBMC | 60 | 32 | Graft rejection | Not determined | 150 |
| Liver | Serology | Serum | 247 | 24 | HCMV disease | Not determined | 108 |
| Liver | PCR | PBMC | 46 | Not given | None | 10% A, 90% B | 357 |
| Livera | qPCR | PBMC | 66 | 54 | Increased severity of HCV-related fibrosis or hepatitis | Not determined | 172 |
| Livera | VI | PBMC | 51 | 41 | Increased severity of HCV-related fibrosis | Not determined | 364 |
| Liver heart/lung | VI, PCR | PBMC, BALF | 30 | 27 | Higher mortality rate, fungal infections | B | 192 |
Study population restricted to hepatitis C virus-positive patients undergoing liver transplantation.
qPCR, quantitative PCR; VI, virus isolation; IHC, immunohistochemistry.
BALF, bronchoalveolar lavage fluid.
Unclear whether PCR was able to discriminate active from latent infection.
HCV, hepatitis C virus.
Given the pharmacologically induced state of immunosuppression in transplant recipients, HHV-6 is likely to reactivate from latency and act as an opportunistic pathogen. On the other hand, as HHV-6 is harbored by many tissues such as mononuclear cells, bone marrow, lung, and liver, there is also the possibility of graft-induced (re-)infection with HHV-6, the virus being transferred from donor to recipient (see “Primary infection”). Of special interest is the observation that the rate of HHV-6 reactivation is higher among transplant recipients receiving anti-CD3 (antithymocyte globulin), which is used for the treatment of rejection (98, 457).
As already mentioned, standard PCR on tissue samples (including PBMCs), the most widely used method for HHV-6 detection until a few years ago, cannot discriminate between latent and active HHV-6 infection. Virus isolation and cocultivation with PBMCs or CBMCs, although highly indicative of active infection, is less sensitive and is a difficult and time-consuming procedure. PCR on small amounts of PBMCs has been suggested to discern active from latent infection (150), as well as serum-plasma PCR (170, 317) and reverse transcription-PCR (311, 416, 444), which allow detection of circulating viral genomes and viral mRNAs, respectively. More recently, however, quantitative PCR analysis, which allows fast, sensitive, and absolute quantitation of viral load, has become the method of choice (20, 135, 176, 394). No specific clinical syndrome has been linked to active HHV-6 infection in immunosuppressed patients. In some studies, HHV-6 infection was found to be asymptomatic; other studies documented various pathologies (see below).
(i) Bone marrow and stem cell transplant recipients.
Case reports have associated active HHV-6 infection after bone marrow or stem cell transplantation with a variety of complications. The most frequently reported clinical disease is encephalitis or encephalopathy (35, 115, 263, 333, 338, 407, 424, 441), followed by pneumonitis (46, 78, 309, 333, 394) and delayed engraftment (266, 333, 343), whereas only a few reports mention fever and skin rash as a result of HHV-6 infection (333, 353, 394). HHV-6 has been suspected of causing vascular endothelial damage in BMT recipients, a process that may lead to thrombotic microangiopathy, which is a major complication of BMT (273, 389). Lastly, HHV-6 infection has been associated with bone marrow suppression (52, 113), an observation that is supported by in vitro data on the suppressive effects of HHV-6 on proliferation of stromal cells of granulocyte/macrophage, erythroid, or megakaryocyte lineages (48, 187-189, 212). A recent retrospective study by Boutolleau et al. (42) in 78 stem cell transplant recipients found a correlation between HHV-6 viral load in PBMCs and delayed neutrophil engraftment, severe graft-versus-host disease, and classical HHV-6 manifestations, such as fever and rash. The incidence of HHV-6 viremia appears to be higher in allogeneic than in autologous BMT recipients. Furthermore, pretransplantation conditions that were found to predispose to developing HHV-6 viremia posttransplantation were leukemia or lymphoma and low HHV-6 antibody titers (447). One study (performed in 25 BMT recipients) reported a higher incidence for HHV-6A DNA in plasma (24 of 25) than in the corresponding peripheral blood leukocyte (PBL) samples (1 of 25), whereas HHV-6B was detected at similar rates in PBLs (22 of 25) and in plasma (21 of 25); 80% of patients had both variants circulating in plasma throughout the transplantation period (310). These findings, if confirmed by other studies, would imply that in these patients, HHV-6A regularly reactivates from tissues other than PBLs. The outcome of several prospective studies on HHV-6 infection in BMT recipients is summarized in Table 1.
(ii) SOT recipients.
As mentioned above, the frequency of active HHV-6 infection after SOT is generally lower than that after BMT. This may relate to the patient population, which is generally older for SOT than for BMT; the majority of SOT recipients are adults. Clinical manifestations of HHV-6 reactivation or reinfection in SOT recipients are similar to those described in BMT patients. These include fever and/or rash as the most frequently seen disease manifestation of active HHV-6 infection (150, 363, 384, 449, 451), followed by encephalitis or encephalopathy (291, 323, 363, 384), hepatitis (150, 384, 427), graft dysfunction or rejection (2, 15, 168, 231), bone marrow suppression (362, 363), and pneumonitis (362). Table 2 provides a summary of prospective studies in SOT patients.
A fatal case of primary HHV-6A infection was reported in a seronegative kidney recipient with acute hemophagocytic syndrome with hepatitis and encephalopathy (344). In a recent retrospective study in pediatric liver transplant recipients, primary HHV-6 infection was found to be associated with graft rejection (122). A second retrospective study by DesJardin et al. found a high degree of HHV-6 reactivation, which predisposed to the development of HCMV disease (98, 99). Singh et al. observed a prolonged and specific suppression of anti-HHV-6 T-helper cell response after liver transplantation, which may represent a predisposing factor for the development of HHV-6 infection (361). Also, HHV-6 was shown retrospectively to increase lymphocyte infiltration in liver allografts, thereby impairing liver function (230). The outcome of several prospective studies is summarized in Table 2. Most interesting (although not confirmed in all studies) is the observation that in SOT patients, HHV-6 (alone or along with HHV-7), either through direct virus interactions or through modulation of the host's immune status, or both, positively influences the HCMV reactivation rate and alters the course of HCMV disease (62, 98, 99, 108, 171-173, 335). In addition, a temporal pattern of reactivation of the three betaherpesviruses was found to underlie clinical symptoms, with HHV-6 being reactivated first (median time after SOT, 20 days), followed by HHV-7 (26 days) and HCMV (36 days) (150). Furthermore, concomitant HCMV infection was shown to aggravate clinical symptoms of HHV-6 infection (162). Lastly, the immunomodulating properties of HHV-6 may also trigger reactivation of unrelated opportunistic events, such as fungal infections (107, 192, 340).
HIV patients.
Although HHV-6 active infection is frequently detected in HIV-infected individuals, it is unclear whether HHV-6 merely acts as an opportunistic pathogen or may accelerate HIV disease progression. Several mechanisms for a molecular interaction between HHV-6 and HIV-1 (see “Molecular interactions between HHV-6 and other viruses”), both negatively and positively affecting HIV-1 replication, have been demonstrated in vitro, and it is unclear which would prevail in the in vivo situation. PCR analysis of 141 autopsy specimens obtained from 11 AIDS patients demonstrated that the organ presence of HHV-6 was significantly associated with HIV-1 proviral load (118). In a similar study detecting HHV-6A antigen in lymph node specimens from HIV-infected individuals, active HHV-6 infection was demonstrated in all samples tested (51). These histological data may suggest a role for HHV-6 as an opportunistic agent in AIDS, and active HHV-6 infection in HIV-seropositive patients has indeed been associated with encephalitis (213, 214) and pneumonitis (307), although not consistently (40). There have been investigations on a possible etiological link between HHV-6 and AIDS-associated retinitis, which showed that HHV-6 can superinfect HCMV-infected retinae from AIDS patients (123), although HIV-positive retinae harboring actively replicating HHV-6 in the absence of HCMV infection have been described as well (332). However, most case reports indicate that, unlike in transplant recipients, HHV-6 infection causes no specific clinical syndrome or major complications in HIV-seropositive patients. The issue on whether HHV-6 may alter the course of HIV disease was addressed in an early clinical investigation (373). HHV-6 antibody titers were not different between HIV-seropositive and HIV-seronegative adults and appeared not to be associated with HIV progression (373). Likewise, two studies in HIV-infected infants found no evidence that primary HHV-6 infection may alter the progression of AIDS (307, 358). In another study, 41 HIV-positive infants were carefully monitored during the first year of life. Lower CD4+ cell numbers resulted in lower rates of primary HHV-6 infection, and those children acquiring HHV-6 showed a more rapid progression towards AIDS (222).
ANTIVIRAL THERAPY FOR HHV-6
As outlined above, HHV-6 infections in immunocompetent children are self-limiting and do not require treatment. In immunocompromised individuals, however, reactivation of latent virus may cause life-threatening complications. As an effective vaccine is not available, safe and reliable drugs for the treatment of HHV-6 infections are needed. No controlled trials of antiviral therapy against HHV-6 have been conducted, and no compounds have been formally approved for the treatment of HHV-6 infections. Therefore, the drugs clinically used against HHV-6 are the same as those used in HCMV therapy or prophylaxis and consist of the nucleoside analogs ganciclovir (and valganciclovir) and, to a lesser extent, acyclovir (and valaciclovir), the nucleotide analogue cidofovir, and the pyrophosphate analogue foscarnet (Fig. 4). Most of these classical antiherpesvirus compounds have important drawbacks; therefore, new strategies for the treatment of infections with HHV-6 (or, rather, herpesviruses in general) are currently being explored (reviewed in reference 75).
FIG. 4.
Chemical structures of clinically used anti-HHV-6 compounds.
Ganciclovir and Acyclovir
Mechanism of action.
The acyclic nucleoside analogs ganciclovir and acyclovir target the viral DNA polymerase, and their triphosphate metabolites inhibit enzyme function by competition with the natural substrate (dGTP for ganciclovir and acyclovir), by incorporation in the growing DNA chain (effecting chain termination in case of absence of a hydroxyl group in the acyclic chain), and by dead-end complex formation (336) (Fig. 5). Their selectivity relies on a greater affinity of their triphosphates for viral DNA polymerases compared to cellular DNA polymerases and, most importantly, selective phosphorylation (activation) by a viral kinase. Unlike alpha- and gammaherpesviruses, betaherpesviruses do not express a thymidine kinase but encode a phosphotransferase which is capable of converting nucleoside analogs to their monophosphate. The two subsequent phosphorylations to the active triphosphate form are carried out by the cellular enzymes deoxyguanylate (dGMP) kinase and nucleoside diphosphate (NDP) kinase (133, 274). In the case of HCMV, the conversion of ganciclovir to its monophosphate is catalyzed by the HCMV pUL97 phosphotransferase (242, 381). HCMV pUL97 also phosphorylates acyclovir, although less efficiently than observed for ganciclovir (393, 462). The homologous kinase encoded by the U69 gene of HHV-6 (pU69) was shown to phosphorylate ganciclovir, since recombination with pU69 conferred ganciclovir sensitivity to baculoviruses (14). In human cells infected with recombinant vaccinia viruses expressing pU69 or pUL97, the efficiency for ganciclovir phosphorylation by HHV-6 pU69 was found to be about 10-fold less compared to its HCMV counterpart (93). The reason for this is presently unclear. The HCMV pUL97 and HHV-6 pU69 phosphotransferases are closely related and share homology with cellular kinases and bacterial phosphotransferases (64). Similarly to pUL97, pU69 phosphotransferase is expressed as a nuclear protein (93, 285). Both are able to autophosphorylate (mainly on serine residues) (284) or to use exogenous substrates such as histones, although optimal reaction conditions are different for HHV-6 pU69 and HCMV pUL97 (14, 26). The role of HHV-6 pU69 in the virus replication cycle remains to be investigated. HCMV pUL97 plays an essential role in HCMV replication (330) and has been proposed to be involved in viral DNA synthesis (by binding to the HCMV DNA polymerase processivity factor) (224, 269), in nuclear egress and capsid assembly (223, 431), and in regulation of HCMV gene expression (26).
FIG. 5.
Metabolic activation of acyclic nucleoside analogs (exemplified by ganciclovir) and acyclic nucleoside phosphonates (represented by cidofovir). Abbreviations are explained in the text in “Ganciclovir and acyclovir” and “Cidofovir.”
In vitro activity against HHV-6.
Evaluation of the anti-HHV-6 activity of nucleoside analogs is somewhat hampered by their relatively high cytotoxicity in the fast-growing T-cell lines that are routinely used for the propagation of this virus. Much better selectivity indices are obtained in CBMCs (95). The in vitro activity of ganciclovir against HHV-6 is superior to that of acyclovir and penciclovir, irrespective of the propagation system (5, 49, 267, 443). Among the newer nucleoside analogs with good and selective activity against HHV-6 are the cyclopropyl derivative A-5021 [(1′S,2′R)-9-[[1′,2′-bis(hydroxymethyl)-cycloprop-1′-yl]methyl]guanine] and the N7-substituted derivative S2242 [2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine] (95). Both A and B variants of HHV-6 have proven to be equally sensitive to all nucleoside compounds evaluated to date.
In vivo activity against HHV-6.
As mentioned above, no controlled trials have been conducted on HHV-6 therapy. Several case reports support the clinical effectiveness of ganciclovir (Cymevene) against HHV-6 disease in BMT or SOT recipients, manifesting, in the majority of cases, with HHV-6 encephalitis (195, 292, 323, 338, 441), although some cases of fulminant HHV-6 infection showed no response (344, 407). Also, prophylactic therapy with ganciclovir, but not acyclovir (Zovirax), proved effective in preventing HHV-6 reactivation in BMT or stem cell transplant recipients (333, 408, 424). A drawback for ganciclovir is its low oral bioavailability, making intravenous administration necessary for most indications. Recently, the valyl ester valganciclovir (Valcyte) has become available as an oral prodrug, offering a ganciclovir exposure comparable to the intravenous formulation (45). It is currently indicated for the prevention of HCMV disease in certain subsets of transplant recipients. Another limitation to the use of ganciclovir is that it may cause important dose-dependent side effects, mainly neutropenia (which may be irreversible) and thrombocytopenia (279). In order to prevent these side effects of ganciclovir treatment, patients at risk of acquiring herpesviral opportunistic infections generally receive low-dose ganciclovir prophylaxis, although this may facilitate resistance development (112). Preemptive therapy (i.e., therapy initiated after systemic detection of the virus, but prior to disease manifestation) has been suggested as an alternative to minimize drug exposure but is still an issue of controversy (117, 156, 360).
Foscarnet
Foscarnet (phosphonoformic acid; Foscavir) is a pyrophosphate analog (Fig. 4) with broad-spectrum activity against all herpesviruses. It targets the viral DNA polymerase without requiring activation by cellular or viral enzymes, by reversible binding close to the pyrophosphate binding site of the enzyme. In this manner, cleavage of pyrophosphate from deoxynucleoside triphosphates is prevented, resulting in an inhibition of pyrophosphate release or chain termination (420). The higher affinity for viral than cellular DNA polymerases determines its selectivity. Foscarnet has excellent in vitro activity against HHV-6 and exhibits low cytotoxicity, even in continuous cell lines (49, 95, 267, 337). Intravenous foscarnet is indicated for the treatment of HCMV retinitis in AIDS patients and, in transplant recipients, it is reserved for individuals unresponsive to ganciclovir. Reversible dose-dependent nephrotoxicity is frequently associated with the use of foscarnet and is the main reason why (val)ganciclovir is preferred, especially in prophylaxis. Most case studies have shown successful use of foscarnet in the treatment of HHV-6 encephalitis in transplant recipients (35, 458); others have had a disappointing result (344, 407). No comparative and prospective data are available to conclude whether ganciclovir or foscarnet should be preferred for HHV-6 therapy.
Cidofovir
Cidofovir (Vistide) is an acyclic nucleoside phosphonate analog of (deoxy)CMP (Fig. 4). Cidofovir exhibits activity against a broad spectrum of DNA viruses (including herpes-, papilloma-, polyoma-, adeno-, and poxviruses) and has been approved for the treatment of HCMV retinitis in AIDS patients (94, 301). Unlike ganciclovir and acyclovir, cidofovir does not require metabolization by a viral enzyme (Fig. 5). Due to the presence of the phosphonate moiety, two subsequent phosphorylations by cellular enzymes (pyrimidine nucleoside monophosphate kinase and NDP kinase, pyruvate kinase or creatine kinase, respectively) are sufficient for cidofovir to reach its metabolically active state (70). Cidofovir diphosphate was shown to act as a competitive inhibitor of dCTP and an alternate substrate for the HCMV DNA polymerase. Its incorporation does not directly result in chain termination (433). Like foscarnet, cidofovir diphosphate owes its selectivity to the higher affinity for viral DNA polymerases compared to cellular DNA polymerases (301). Cidofovir displays strong activity against HHV-6 in vitro (337) and, along with foscarnet, it is the most active compound available for the treatment of HHV-6 infections, with a very favorable selectivity index in CBMCs (91, 443). However, due to the considerable risk of developing nephrotoxicity, the clinical use of cidofovir for controlling systemic HCMV infections is restricted to second-line therapy of ganciclovir- or foscarnet-resistant virus strains (244). Clinical reports on cidofovir for the treatment of HHV-6 infection are limited to a recent case study in an immunocompetent patient with HHV-6 encephalomyelitis (96). In this study, ganciclovir displayed superior efficacy and safety compared to cidofovir in suppressing HHV-6 replication and resolving neurological symptoms.
New antiherpesvirus compounds
The antiherpesvirus drugs described so far all target the viral DNA polymerase, and in the case of ganciclovir and acyclovir, a viral thymidine or protein kinase is required for their activation. In patients kept under a long-term low-dose regimen (as in prophylaxis), resistant viruses with reduced sensitivity to the drug (by amino acid substitutions in the target enzyme) may benefit and colonize the host. The mechanisms of HHV-6 resistance will be discussed below. In the cases where these mutations give rise to cross-resistance, the number of alternative therapeutic options may be very limited. Therefore, new antiherpesvirus compounds are currently being developed that act during distinct stages of herpesvirus replication (reviewed in reference 75). For the benzimidazole derivative maribavir, which is currently being evaluated for HCMV therapy (425) and is supposed to act by inhibition of HCMV pUL97 phosphotransferase, the lack of in vitro activity against HHV-6 excludes its use in HHV-6 therapy. Likewise, the chemically related compound BDCRB, targeted at the HCMV terminase, has no activity against HHV-6 (412). Another new class of compounds, the 4-oxo-dihydroxyquinolines, act as nonnucleoside inhibitors of herpesvirus DNA polymerases, with potent and broad-spectrum antiviral activity and very low cytotoxicity (44). They are potent inhibitors of six of the eight human herpesviruses, including HCMV, but are not active against HHV-6 and HHV-7 due to a single amino acid change in the conserved domain III (see Fig. 7, below) of their DNA polymerases (402).
FIG. 7.
Schematic representation of HCMV (A) and HHV-6 (B) DNA polymerases. Conserved regions are boxed. Functional domains are indicated by arrows. Bars indicate codons mapped to resistance to ganciclovir (GCV), cidofovir (CDV), and foscarnet (PFA). Regions showing variation in drug-sensitive isolates (Var) are indicated by shaded boxes. APB, accessory protein binding.
HHV-6 resistance
As mentioned above, no formally approved treatment for HHV-6 infections is available. In many cases, patients under immunosuppressive therapy already receive acyclovir or ganciclovir prophylaxis to prevent HSV and HCMV reactivations, respectively. For ganciclovir, this prophylaxis seems effective in preventing HHV-6 reactivation as well (333, 408, 424). Clinical HHV-6 resistance to antivirals has therefore not been documented to date. However, when considering dynamics and mechanisms of resistance development, some important features of HCMV resistance may apply to HHV-6 as well.
First, the incidence of HCMV resistance in ganciclovir-naive patients is low (below 3%). Emergence of resistant strains is facilitated (i) when virus is actively replicating, (ii) after prolonged drug exposure, and (iii) when suboptimal doses are being used. Suboptimal dosage is often related to maintenance therapy with oral ganciclovir, which is known to have an oral bioavailability of only 3 to 7% (371). It may also be a consequence of insufficient drug delivery at privileged sites of HCMV replication (112). The first two criteria for resistance development are met in AIDS patients with HCMV retinitis or CNS disease, who receive anti-HCMV therapy after onset of clinical symptoms and require life-long maintenance treatment. The incidence of HCMV resistance in AIDS patients was shown to increase to approximately 30% after 9 months of treatment with ganciclovir, foscarnet, or cidofovir (112). High titers of replicating HCMV are also present in HCMV-seronegative transplant recipients from HCMV-seropositive donors. In these patients, the incidence of HCMV resistance is markedly higher and correlates to treatment duration, reaching 5 to 10% after 3 months of ganciclovir treatment, both after preemptive therapy and when receiving prophylactic oral ganciclovir (137).
Second, ganciclovir resistance relies on a dual mechanism. Low-level ganciclovir-resistant HCMV mutants isolated after short-term ganciclovir treatment mostly carry mutations in the UL97 gene (encoding the HCMV pUL97 phosphotransferase), whereas high-level resistance requires long-term therapy and exhibits mutations in both the UL97 and DNA pol genes (137, 191, 366) (Fig. 6 and Fig. 7). Most of the reported UL97 mutations are comprised within a region close to the substrate recognition site (codons 590 to 607). For the DNA pol mutants, nearly all mutations lie within eight conserved domains (Fig. 7). Mutations outside these domains (at the DNA pol termini) are likely the result of interstrain variation (67). Depending on their location, mutations may decrease the affinity of DNA pol for the inhibitor, decrease the incorporation of inhibitors into the elongating DNA chain, or enhance the DNA pol proofreading (3′-5′ exonuclease) activity (71, 72, 199). Certain mutations in the conserved region III (which is, together with domains I and II, involved in substrate recognition [169]) are associated with a multidrug resistance phenotype (Fig. 7).
FIG. 6.
ClustalW alignment of the HHV-6 pU69 and HCMV pUL97 protein sequences. The overall identity between HHV-6 GS and Z29 pU69 proteins is 94%. Identical residues are indicated by an asterisk. The colons and periods represent strong and weak functional similarities, respectively. Published mutations conferring ganciclovir phenotypic resistance (74, 277, 356) are boxed. Conserved domains are shaded. Amino acid residues that are highly conserved in protein kinases are highlighted within conserved domains. The GXGXXG motif thus indicated in domains I and II is the putative nucleotide binding site. The (A)ACR(AL) motif at amino acid positions 590 to 595 in pUL97 is essential for ganciclovir phosphorylation (381) and is among protein kinases unique to HHV-6 and HCMV.
Thus far, only one report has been published on the resistance of HHV-6 to antiviral compounds. Notably, an HHV-6 mutant virus obtained after serial passage in vitro under increasing ganciclovir pressure was found to be cross-resistant to ganciclovir and cidofovir, exhibiting a 24- and 52-fold decreased sensitivity, respectively (268). Two mutations were identified that yielded amino acid substitutions in the pU69 phosphotransferase (M318V, analogous to the M460V/I/L substitution in HCMV pUL97) (28) and in the DNA pol (A961V). Interestingly, the M318V mutant was also detected by PCR in HHV-6-infected PBMCs from an AIDS patient who had received long-term ganciclovir therapy. Recently, Safronetz et al. (348) evaluated the impact of a series of HHV-6 U69 mutations introduced at positions corresponding to ganciclovir resistance-conferring codons in HCMV UL97. Substitutions in positions 318, 448, and 463 (corresponding to UL97 codons 460, 592, and 607, respectively) were shown to severely impair ganciclovir phosphorylation by pU69 expressed in a baculovirus system. The impact of the A961V substitution in the HHV-6 DNA polymerase, reported by Manichanh et al. (268), is not fully understood.
REFERENCES
- 1.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 2.Acott, P. D., S. H. Lee, H. Bitter-Suermann, J. G. Lawen, and J. F. Crocker. 1996. Infection concomitant with pediatric renal allograft rejection. Transplantation 62:689-691. [DOI] [PubMed] [Google Scholar]
- 3.Adams, O., C. Krempe, G. Kogler, P. Wernet, and A. Scheid. 1998. Congenital infections with human herpesvirus 6. J. Infect. Dis. 178:544-546. [DOI] [PubMed] [Google Scholar]
- 4.Agulnick, A. D., J. R. Thompson, and R. P. Ricciardi. 1994. An ATF/CREB site is the major regulatory element in the human herpesvirus 6 DNA polymerase promoter. J. Virol. 68:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agut, H., J. T. Aubin, and J. M. Huraux. 1991. Homogeneous susceptibility of distinct human herpesvirus 6 strains to antivirals in vitro. J. Infect. Dis. 163:1382-1383. [DOI] [PubMed] [Google Scholar]
- 6.Akashi, K., Y. Eizuru, Y. Sumiyoshi, T. Minematsu, S. Hara, M. Harada, M. Kikuchi, Y. Niho, and Y. Minamishima. 1993. Brief report: severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N. Engl. J. Med. 329:168-171. [DOI] [PubMed] [Google Scholar]
- 7.Akhyani, N., R. Berti, M. B. Brennan, S. S. Soldan, J. M. Eaton, H. F. McFarland, and S. Jacobson. 2000. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J. Infect. Dis. 182:1321-1325. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht, M. A., N. A. DeLuca, R. A. Byrn, P. A. Schaffer, and S. M. Hammer. 1989. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J. Virol. 63:1861-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albright, A. V., E. Lavi, J. B. Black, S. Goldberg, M. J. O'Connor, and F. Gonzalez-Scarano. 1998. The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: oligodendrocytes and microglia. J. Neurovirol. 4:486-494. [DOI] [PubMed] [Google Scholar]
- 10.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 11.Al Shammari, S., R. F. Nelson, and A. Voevodin. 2003. HHV-6 DNAaemia in patients with multiple sclerosis in Kuwait. Acta Neurol. Scand. 107:122-124. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Lafuente, R., C. Martin-Estefania, V. de las Heras, C. Castrillo, I. Cour, J. J. Picazo, E. Varela de Seijas, and R. Arroyo. 2002. Prevalence of herpesvirus DNA in MS patients and healthy blood donors. Acta Neurol. Scand. 105:95-99. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Lafuente, R., C. Martin-Estefania, V. de las Heras, C. Castrillo, J. J. Picazo, E. Varela de Seijas, and R. A. Gonzalez. 2002. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch. Neurol. 59:929-933. [DOI] [PubMed] [Google Scholar]
- 14.Ansari, A., and V. C. Emery. 1999. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J. Virol. 73:3284-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appleton, A. L., L. Sviland, J. S. Peiris, C. E. Taylor, J. Wilkes, M. A. Green, A. D. Pearson, P. J. Kelly, A. J. Malcolm, S. J. Proctor, et al. 1995. Human herpesvirus-6 infection in marrow graft recipients: role in pathogenesis of graft-versus-host disease. Bone Marrow Transplant. 16:777-782. [PubMed] [Google Scholar]
- 16.Araujo, J. C., J. Doniger, F. Kashanchi, P. L. Hermonat, J. Thompson, and L. J. Rosenthal. 1995. Human herpesvirus 6A ts suppresses both transformation by H-ras and transcription by the H-ras and human immunodeficiency virus type 1 promoters. J. Virol. 69:4933-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arena, A., D. Iannello, D. Gazzara, A. Speranza, L. Bonina, and P. Mastroeni. 2001. Role of interleukin-18 in peripheral blood mononuclear cells infected with human herpes virus type 6. Intervirology 44:250-254. [DOI] [PubMed] [Google Scholar]
- 18.Arena, A., M. C. Liberto, A. B. Capozza, and A. Foca. 1997. Productive HHV-6 infection in differentiated U937 cells: role of TNF alpha in regulation of HHV-6. New Microbiol. 20:13-20. [PubMed] [Google Scholar]
- 19.Arena, A., M. C. Liberto, D. Iannello, A. B. Capozza, and A. Foca. 1999. Altered cytokine production after human herpes virus type 6 infection. New Microbiol. 22:293-300. [PubMed] [Google Scholar]
- 20.Aritaki, K., J. H. Ohyashiki, A. Suzuki, T. Ojima, K. Abe, N. Shimizu, K. Yamamoto, K. Ohyashiki, and A. Hoshika. 2001. A rapid monitoring system of human herpesviruses reactivation by LightCycler in stem cell transplantation. Bone Marrow Transplant. 28:975-980. [DOI] [PubMed] [Google Scholar]
- 21.Asada, H., V. Klaus-Kovtun, H. Golding, S. I. Katz, and A. Blauvelt. 1999. Human herpesvirus 6 infects dendritic cells and suppresses human immunodeficiency virus type 1 replication in coinfected cultures. J. Virol. 73:4019-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano, Y., T. Yoshikawa, S. Suga, I. Kobayashi, T. Nakashima, T. Yazaki, Y. Kajita, and T. Ozaki. 1994. Clinical features of infants with primary human herpesvirus 6 infection (exanthem subitum, roseola infantum). Pediatrics 93:104-108. [PubMed] [Google Scholar]
- 23.Asano, Y., T. Yoshikawa, S. Suga, T. Yazaki, K. Kondo, and K. Yamanishi. 1990. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet 335:862-863. [DOI] [PubMed] [Google Scholar]
- 24.Aubin, J. T., H. Collandre, D. Candotti, D. Ingrand, C. Rouzioux, M. Burgard, S. Richard, J. M. Huraux, and H. Agut. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubin, J. T., L. Poirel, H. Agut, J. M. Huraux, C. Bignozzi, Y. Brossard, N. Mulliez, J. Roume, F. Lecuru, and R. Taurelle. 1992. Intrauterine transmission of human herpesvirus 6. Lancet 340:482-483. [DOI] [PubMed] [Google Scholar]
- 26.Baek, M. C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 27.Baillargeon, J., J. Piper, and C. T. Leach. 2000. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J. Clin. Virol. 16:149-157. [DOI] [PubMed] [Google Scholar]
- 28.Baldanti, F., D. Michel, L. Simoncini, M. Heuschmid, A. Zimmermann, R. Minisini, P. Schaarschmidt, T. Schmid, G. Gerna, and T. Mertens. 2002. Mutations in the UL97 ORF of ganciclovir-resistant clinical cytomegalovirus isolates differentially affect GCV phosphorylation as determined in a recombinant vaccinia virus system. Antivir. Res. 54:59-67. [DOI] [PubMed] [Google Scholar]
- 29.Barone, S. R., M. H. Kaplan, and L. R. Krilov. 1995. Human herpesvirus-6 infection in children with first febrile seizures. J. Pediatr. 127:95-97. [DOI] [PubMed] [Google Scholar]
- 30.Basta, S., and J. R. Bennink. 2003. A survival game of hide and seek: cytomegaloviruses and MHC class I antigen presentation pathways. Viral Immunol. 16:231-242. [DOI] [PubMed] [Google Scholar]
- 31.Bech, E., J. Lycke, P. Gadeberg, H. J. Hansen, C. Malmestrom, O. Andersen, T. Christensen, S. Ekholm, S. Haahr, P. Hollsberg, T. Bergstrom, B. Svennerholm, and J. Jakobsen. 2002. A randomized, double-blind, placebo-controlled MRI study of anti-herpes virus therapy in MS. Neurology 58:31-36. [DOI] [PubMed]
- 32.Beovic, B., N. Pecaric-Meglic, J. Marin, J. Bedernjak, I. Muzlovic, and M. Cizman. 2001. Fatal human herpesvirus 6-associated multifocal meningoencephalitis in an adult female patient. Scand. J. Infect. Dis. 33:942-944. [DOI] [PubMed] [Google Scholar]
- 33.Berti, R., M. B. Brennan, S. S. Soldan, J. M. Ohayon, L. Casareto, H. F. McFarland, and S. Jacobson. 2002. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J. Neurovirol. 8:250-256. [DOI] [PubMed] [Google Scholar]
- 34.Bertram, G., N. Dreiner, G. R. Krueger, A. Ramon, D. V. Ablashi, S. Z. Salahuddin, and N. Balachandram. 1991. Frequent double infection with Epstein-Barr virus and human herpesvirus-6 in patients with acute infectious mononucleosis. In Vivo 5:271-279. [PubMed] [Google Scholar]
- 35.Bethge, W., R. Beck, G. Jahn, P. Mundinger, L. Kanz, and H. Einsele. 1999. Successful treatment of human herpesvirus-6 encephalitis after bone marrow transplantation. Bone Marrow Transplant. 24:1245-1248. [DOI] [PubMed] [Google Scholar]
- 36.Bhattarakosol, P., C. Pancharoen, J. Mekmullica, and P. Bhattarakosol. 2001. Seroprevalence of anti-human herpes virus-6 IgG antibody in children of Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 32:143-147. [PubMed] [Google Scholar]
- 37.Biberfeld, P., B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1987. Ultrastructural characterization of a new human B lymphotropic DNA virus (human herpesvirus 6) isolated from patients with lymphoproliferative disease. J. Natl. Cancer Inst. 79:933-941. [PubMed] [Google Scholar]
- 38.Black, J. B., K. C. Sanderlin, C. S. Goldsmith, H. E. Gary, C. Lopez, and P. E. Pellett. 1989. Growth properties of human herpesvirus-6 strain Z29. J. Virol. Methods 26:133-145. [DOI] [PubMed] [Google Scholar]
- 39.Bonura, F., A. M. Perna, F. Vitale, M. R. Villafrate, E. Viviano, R. Guttadauro, G. Mazzola, and N. Romano. 1999. Inhibition of human immunodeficiency virus 1 (HIV-1) by variant B of human herpesvirus 6 (HHV-6). New Microbiol. 22:161-171. [PubMed] [Google Scholar]
- 40.Bossolasco, S., R. Marenzi, H. Dahl, L. Vago, M. R. Terreni, F. Broccolo, A. Lazzarin, A. Linde, and P. Cinque. 1999. Human herpesvirus 6 in cerebrospinal fluid of patients infected with HIV: frequency and clinical significance. J. Neurol. Neurosurg. Psychiatry 67:789-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutolleau, D., D. Cointe, A. Gautheret-Dejean, M. Mace, H. Agut, L. Grangeot-Keros, and D. Ingrand. 2003. No evidence for a major risk of roseolovirus vertical transmission during pregnancy. Clin. Infect. Dis. 36:1634-1635. [DOI] [PubMed] [Google Scholar]
- 42.Boutolleau, D., C. Fernandez, E. Andre, B. M. Imbert-Marcille, N. Milpied, H. Agut, and A. Gautheret-Dejean. 2003. Human herpesvirus (HHV)-6 and HHV-7: two closely related viruses with different infection profiles in stem cell transplantation recipients. J. Infect. Dis. 187:179-186. [DOI] [PubMed] [Google Scholar]
- 43.Braun, D. K., P. E. Pellett, and C. A. Hanson. 1995. Presence and expression of human herpesvirus 6 in peripheral blood mononuclear cells of S100-positive, T cell chronic lymphoproliferative disease. J. Infect. Dis. 171:1351-1355. [DOI] [PubMed] [Google Scholar]
- 44.Brideau, R. J., M. L. Knechtel, A. Huang, V. A. Vaillancourt, E. E. Vera, N. L. Oien, T. A. Hopkins, J. L. Wieber, K. F. Wilkinson, B. D. Rush, F. J. Schwende, and M. W. Wathen. 2002. Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. Antivir. Res. 54:19-28. [DOI] [PubMed] [Google Scholar]
- 45.Brown, F., L. Banken, K. Saywell, and I. Arum. 1999. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin. Pharmacokinet. 37:167-176. [DOI] [PubMed] [Google Scholar]
- 46.Buchbinder, S., A. H. Elmaagacli, U. W. Schaefer, and M. Roggendorf. 2000. Human herpesvirus 6 is an important pathogen in infectious lung disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 26:639-644. [DOI] [PubMed] [Google Scholar]
- 47.Burd, E. M., and D. R. Carrigan. 1993. Human herpesvirus 6 (HHV-6)-associated dysfunction of blood monocytes. Virus Res. 29:79-90. [DOI] [PubMed] [Google Scholar]
- 48.Burd, E. M., K. K. Knox, and D. R. Carrigan. 1993. Human herpesvirus-6-associated suppression of growth factor-induced macrophage maturation in human bone marrow cultures. Blood 81:1645-1650. [PubMed] [Google Scholar]
- 49.Burns, W. H., and G. R. Sandford. 1990. Susceptibility of human herpesvirus 6 to antivirals in vitro. J. Infect. Dis. 162:634-637. [DOI] [PubMed] [Google Scholar]
- 50.Cardinali, G., M. Gentile, M. Cirone, C. Zompetta, L. Frati, A. Faggioni, and M. R. Torrisi. 1998. Viral glycoproteins accumulate in newly formed annulate lamellae following infection of lymphoid cells by human herpesvirus 6. J. Virol. 72:9738-9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrigan, D. R., D. Harrington, and K. K. Knox. 1996. Variant A human herpesvirus six as a cofactor in the pathogenesis of AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovir. 13:97-98. [DOI] [PubMed] [Google Scholar]
- 52.Carrigan, D. R., and K. K. Knox. 1994. Human herpesvirus 6 (HHV-6) isolation from bone marrow: HHV-6-associated bone marrow suppression in bone marrow transplant patients. Blood 84:3307-3310. [PubMed] [Google Scholar]
- 53.Carrigan, D. R., K. K. Knox, and M. A. Tapper. 1990. Suppression of human immunodeficiency virus type 1 replication by human herpesvirus-6. J. Infect. Dis. 162:844-851. [DOI] [PubMed] [Google Scholar]
- 54.Caruso, A., F. Favilli, A. Rotola, M. Comar, D. Horejsh, G. Alessandri, M. Grassi, D. Di Luca, and S. Fiorentini. 2003. Human herpesvirus-6 modulates RANTES production in primary human endothelial cell cultures. J. Med. Virol. 70:451-458. [DOI] [PubMed] [Google Scholar]
- 55.Caruso, A., A. Rotola, M. Comar, F. Favilli, M. Galvan, M. Tosetti, C. Campello, E. Caselli, G. Alessandri, M. Grassi, E. Garrafa, E. Cassai, and D. Di Luca. 2002. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J. Med. Virol. 67:528-533. [DOI] [PubMed] [Google Scholar]
- 56.Caselli, E., M. Boni, A. Bracci, A. Rotola, C. Cermelli, M. Castellazzi, D. Di Luca, and E. Cassai. 2002. Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J. Clin. Microbiol. 40:4131-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cermelli, C., M. Concari, F. Carubbi, G. Fabio, A. M. Sabbatini, M. Pecorari, P. Pietrosemoli, M. Meacci, E. Guicciardi, N. Carulli, and M. Portolani. 1996. Growth of human herpesvirus 6 in HepG2 cells. Virus Res. 45:75-85. [DOI] [PubMed] [Google Scholar]
- 58.Challoner, P. B., K. T. Smith, J. D. Parker, D. L. MacLeod, S. N. Coulter, T. M. Rose, E. R. Schultz, J. L. Bennett, R. L. Garber, and M. Chang. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 92:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan, P. K., M. Y. Chan, W. W. Li, D. P. Chan, J. L. Cheung, and A. F. Cheng. 2001. Association of human beta-herpesviruses with the development of cervical cancer: bystanders or cofactors. J. Clin. Pathol. 54:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan, P. K., H. K. Ng, M. Hui, and A. F. Cheng. 2001. Prevalence and distribution of human herpesvirus 6 variants A and B in adult human brain. J. Med. Virol. 64:42-46. [DOI] [PubMed] [Google Scholar]
- 61.Chan, P. K., H. K. Ng, M. Hui, M. Ip, J. L. Cheung, and A. F. Cheng. 1999. Presence of human herpesviruses 6, 7, and 8 DNA sequences in normal brain tissue. J. Med. Virol. 59:491-495. [DOI] [PubMed] [Google Scholar]
- 62.Chan, P. K., J. S. Peiris, K. Y. Yuen, R. H. Liang, Y. L. Lau, F. E. Chen, S. K. Lo, C. Y. Cheung, T. K. Chan, and M. H. Ng. 1997. Human herpesvirus-6 and human herpesvirus-7 infections in bone marrow transplant recipients. J. Med. Virol. 53:295-305. [DOI] [PubMed] [Google Scholar]
- 63.Chapenko, S., A. Millers, Z. Nora, I. Logina, R. Kukaine, and M. Murovska. 2003. Correlation between HHV-6 reactivation and multiple sclerosis disease activity. J. Med. Virol. 69:111-117. [DOI] [PubMed] [Google Scholar]
- 64.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 65.Chen, M., N. Popescu, C. Woodworth, Z. Berneman, M. Corbellino, P. Lusso, D. V. Ablashi, and J. A. DiPaolo. 1994. Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. J. Virol. 68:1173-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen, M., H. Wang, C. D. Woodworth, P. Lusso, Z. Berneman, D. Kingma, G. Delgado, and J. A. DiPaolo. 1994. Detection of human herpesvirus 6 and human papillomavirus 16 in cervical carcinoma. Am. J. Pathol. 145:1509-1516. [PMC free article] [PubMed] [Google Scholar]
- 67.Chou, S., N. S. Lurain, A. Weinberg, G. Y. Cai, P. L. Sharma, C. S. Crumpacker, and Adult AIDS Clinical Trials Group-CMV Laboratories. 1999. Interstrain variation in the human cytomegalovirus DNA polymerase sequence and its effect on genotypic diagnosis of antiviral drug resistance. Antimicrob. Agents Chemother. 43:1500-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chou, S., and G. I. Marousek. 1994. Analysis of interstrain variation in a putative immediate-early region of human herpesvirus 6 DNA and definition of variant-specific sequences. Virology 198:370-376. [DOI] [PubMed] [Google Scholar]
- 69.Chua, K. B., N. S. Khairullah, and P. S. Hooi. 1996. Seroepidemiology of human herpesvirus 6 in a population seen in the University Hospital, Kuala Lumpur, Malaysia. Southeast Asian J. Trop. Med. Public Health 27:91-95. [PubMed] [Google Scholar]
- 70.Cihlar, T., and M. S. Chen. 1996. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 50:1502-1510. [PubMed] [Google Scholar]
- 71.Cihlar, T., M. D. Fuller, and J. M. Cherrington. 1998. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J. Virol. 72:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cihlar, T., M. D. Fuller, A. S. Mulato, and J. M. Cherrington. 1998. A point mutation in the human cytomegalovirus DNA polymerase gene selected in vitro by cidofovir confers a slow replication phenotype in cell culture. Virology 248:382-393. [DOI] [PubMed] [Google Scholar]
- 73.Cirone, M., G. Campadelli-Fiume, L. Foa-Tomasi, M. R. Torrisi, and A. Faggioni. 1994. Human herpesvirus 6 envelope glycoproteins B and H-L complex are undetectable on the plasma membrane of infected lymphocytes. AIDS Res. Hum. Retrovir. 10:175-179. [DOI] [PubMed] [Google Scholar]
- 74.Cirone, M., L. Cuomo, C. Zompetta, S. Ruggieri, L. Frati, A. Faggioni, and G. Ragona. 2002. Human herpesvirus 6 and multiple sclerosis: a study of T cell cross-reactivity to viral and myelin basic protein antigens. J. Med. Virol. 68:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coen, D. M., and P. A. Schaffer. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2003. 2:278-288. [DOI] [PubMed] [Google Scholar]
- 76.Colberg-Poley, A. M., L. D. Santomenna, P. P. Harlow, P. A. Benfield, and D. J. Tenney. 1992. Human cytomegalovirus US3 and UL36-38 immediate-early proteins regulate gene expression. J. Virol. 66:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collot, S., B. Petit, D. Bordessoule, S. Alain, M. Touati, F. Denis, and S. Ranger-Rogez. 2002. Real-time PCR for quantification of human herpesvirus 6 DNA from lymph nodes and saliva. J. Clin. Microbiol. 40:2445-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cone, R. W., R. C. Hackman, M. L. Huang, R. A. Bowden, J. D. Meyers, M. Metcalf, J. Zeh, R. Ashley, and L. Corey. 1993. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N. Engl. J. Med. 329:156-161. [DOI] [PubMed] [Google Scholar]
- 79.Cone, R. W., M. L. Huang, L. Corey, J. Zeh, R. Ashley, and R. Bowden. 1999. Human herpesvirus 6 infections after bone marrow transplantation: clinical and virologic manifestations. J. Infect. Dis. 179:311-318. [DOI] [PubMed] [Google Scholar]
- 80.Cone, R. W., M. L. Huang, R. C. Hackman, and L. Corey. 1996. Coinfection with human herpesvirus 6 variants A and B in lung tissue. J. Clin. Microbiol. 34:877-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Csoma, E., A. Bacsi, X. Liu, J. Szabo, P. Ebbesen, Z. Beck, J. Konya, I. Andirko, E. Nagy, and F. D. Toth. 2002. Human herpesvirus 6 variant A infects human term syncytiotrophoblasts in vitro and induces replication of human immunodeficiency virus type 1 in dually infected cells. J. Med. Virol. 67:67-87. [DOI] [PubMed] [Google Scholar]
- 82.Cuomo, L., A. Angeloni, C. Zompetta, M. Cirone, A. Calogero, L. Frati, G. Ragona, and A. Faggioni. 1995. Human herpesvirus 6 variant A, but not variant B, infects EBV-positive B lymphoid cells, activating the latent EBV genome through a BZLF-1-dependent mechanism. AIDS Res. Hum. Retrovir. 11:1241-1245. [DOI] [PubMed] [Google Scholar]
- 83.Cuomo, L., P. Trivedi, M. R. Cardillo, F. M. Gagliardi, A. Vecchione, R. Caruso, A. Calogero, L. Frati, A. Faggioni, and G. Ragona. 2001. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J. Med. Virol. 63:45-51. [PubMed] [Google Scholar]
- 84.Cuomo, L., P. Trivedi, U. de Grazia, A. Calogero, M. D'Onofrio, W. Yang, L. Frati, A. Faggioni, L. Rymo, and G. Ragona. 1998. Upregulation of Epstein-Barr virus-encoded latent membrane protein by human herpesvirus 6 superinfection of EBV-carrying Burkitt lymphoma cells. J. Med. Virol. 55:219-226. [PubMed] [Google Scholar]
- 85.Dahl, H., G. Fjaertoft, T. Norsted, F. Z. Wang, M. Mousavi-Jazi, and A. Linde. 1999. Reactivation of human herpesvirus 6 during pregnancy. J. Infect. Dis. 180:2035-2038. [DOI] [PubMed] [Google Scholar]
- 86.Daibata, M., N. Hatakeyama, M. Kamioka, Y. Nemoto, M. Hiroi, I. Miyoshi, and H. Taguchi. 2001. Detection of human herpesvirus 6 and JC virus in progressive multifocal leukoencephalopathy complicating follicular lymphoma. Am. J. Hematol. 67:200-205. [DOI] [PubMed] [Google Scholar]
- 87.Daibata, M., T. Taguchi, M. Kamioka, I. Kubonishi, H. Taguchi, and I. Miyoshi. 1998. Identification of integrated human herpesvirus 6 DNA in early pre-B cell acute lymphoblastic leukemia. Leukemia 12:1002-1004. [DOI] [PubMed] [Google Scholar]
- 88.Daibata, M., T. Taguchi, Y. Nemoto, H. Taguchi, and I. Miyoshi. 1999. Inheritance of chromosomally integrated human herpesvirus 6 DNA. Blood 94:1545-1549. [PubMed] [Google Scholar]
- 89.Daibata, M., T. Taguchi, H. Taguchi, and I. Miyoshi. 1998. Integration of human herpesvirus 6 in a Burkitt's lymphoma cell line. Br. J. Haematol. 102:1307-1313. [DOI] [PubMed] [Google Scholar]
- 90.Davis, M. G., S. C. Kenney, J. Kamine, J. S. Pagano, and E. S. Huang. 1987. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 84:8642-8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Bolle, L., G. Andrei, R. Snoeck, Y. Zhang, A. van Lommel, M. Otto, A. Bousseau, C. Roy, E. De Clercq, and L. Naesens. 2004. Potent, selective and cell-mediated inhibition of human herpesvirus 6 at an early stage of viral replication by the non-nucleoside compound CMV423. Biochem. Pharmacol. 67:325-336. [DOI] [PubMed] [Google Scholar]
- 92.De Bolle, L., S. Hatse, E. Verbeken, E. De Clercq, and L. Naesens. 2004. Human herpesvirus 6 infection arrests cord blood mononuclear cells in G2 phase of the cell cycle. FEBS Lett. 560:25-29. [DOI] [PubMed] [Google Scholar]
- 93.De Bolle, L., D. Michel, T. Mertens, C. Manichanh, H. Agut, E. De Clercq, and L. Naesens. 2002. Role of the human herpesvirus 6 U69-encoded kinase in the phosphorylation of ganciclovir. Mol. Pharmacol. 62:714-721. [DOI] [PubMed] [Google Scholar]
- 94.De Clercq, E. 2003. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin. Microbiol. Rev. 16:569-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Clercq, E., L. Naesens, L. De Bolle, D. Schols, Y. Zhang, and J. Neyts. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381-395. [DOI] [PubMed] [Google Scholar]
- 96.Denes, E., L. Magy, K. Pradeau, S. Alain, P. Weinbreck, and S. Ranger-Rogez. 2004. Successful treatment of human herpesvirus 6 encephalomyelitis in immunocompetent patient. Emerg. Infect. Dis. 10:729-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng, H., and S. Dewhurst. 1998. Functional identification and analysis of cis-acting sequences which mediate genome cleavage and packaging in human herpesvirus 6. J. Virol. 72:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DesJardin, J. A., E. Cho, S. Supran, L. Gibbons, B. G. Werner, and D. R. Snydman. 2001. Association of human herpesvirus 6 reactivation with severe cytomegalovirus-associated disease in orthotopic liver transplant recipients. Clin. Infect. Dis. 33:1358-1362. [DOI] [PubMed] [Google Scholar]
- 99.DesJardin, J. A., L. Gibbons, E. Cho, S. E. Supran, M. E. Falagas, B. G. Werner, and D. R. Snydman. 1998. Human herpesvirus 6 reactivation is associated with cytomegalovirus infection and syndromes in kidney transplant recipients at risk for primary cytomegalovirus infection. J. Infect. Dis. 178:1783-1786. [DOI] [PubMed] [Google Scholar]
- 100.Dewhurst, S., S. C. Dollard, P. E. Pellett, and T. R. Dambaugh. 1993. Identification of a lytic-phase origin of DNA replication in human herpesvirus 6B strain Z29. J. Virol. 67:7680-7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dewhurst, S., K. McIntyre, K. Schnabel, and C. B. Hall. 1993. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J. Clin. Microbiol. 31:416-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dhepakson, P., Y. Mori, Y. B. Jiang, H. L. Huang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol. 83:847-854. [DOI] [PubMed] [Google Scholar]
- 103.Dietrich, J., B. Blumberg, M. Roshal, J. V. Baker, S. D. Hurley, M. Mayer-Pröschel, and D. J. Mock. 2004. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J. Neurosci. 24:4875-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Luca, D., P. Mirandola, T. Ravaioli, R. Dolcetti, A. Frigatti, P. Bovenzi, L. Sighinolfi, P. Monini, and E. Cassai. 1995. Human herpesviruses 6 and 7 in salivary glands and shedding in saliva of healthy and human immunodeficiency virus positive individuals. J. Med. Virol. 45:462-468. [DOI] [PubMed] [Google Scholar]
- 105.Di Luca, D., P. Secchiero, P. Bovenzi, A. Rotola, A. Caputo, P. Monini, and E. Cassai. 1991. Reciprocal in vitro interactions between human herpesvirus-6 and HIV-1 Tat. AIDS 5:1095-1098. [DOI] [PubMed] [Google Scholar]
- 106.Di Paolo, J. A., N. C. Popescu, D. V. Ablashi, P. Lusso, D. B. Zimonjic, and C. D. Woodworth. 1994. Multistage carcinogenesis utilizing human genital cells and human papillomaviruses. Toxicol. Lett. 72:7-11. [DOI] [PubMed] [Google Scholar]
- 107.Dockrell, D. H., J. C. Mendez, M. Jones, W. S. Harmsen, D. M. Ilstrup, T. F. Smith, R. H. Wiesner, R. A. Krom, and C. V. Paya. 1999. Human herpesvirus 6 seronegativity before transplantation predicts the occurrence of fungal infection in liver transplant recipients. Transplantation 67:399-403. [DOI] [PubMed] [Google Scholar]
- 108.Dockrell, D. H., J. Prada, M. F. Jones, R. Patel, A. D. Badley, W. S. Harmsen, D. M. Ilstrup, R. H. Wiesner, R. A. Krom, T. F. Smith, and C. V. Paya. 1997. Seroconversion to human herpesvirus 6 following liver transplantation is a marker of cytomegalovirus disease. J. Infect. Dis. 176:1135-1140. [DOI] [PubMed] [Google Scholar]
- 109.Dominguez, G., T. R. Dambaugh, F. R. Stamey, S. Dewhurst, N. Inoue, and P. E. Pellett. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 73:8040-8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donati, D., N. Akhyani, A. Fogdell-Hahn, C. Cermelli, R. Cassiani-Ingoni, A. Vortmeyer, J. D. Heiss, P. Cogen, W. D. Gaillard, S. Sato, W. H. Theodore, and S. Jacobson. 2003. Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 61:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 112.Drew, W. L., C. V. Paya, and V. Emery. 2001. Cytomegalovirus (CMV) resistance to antivirals. Am. J. Transplant. 1:307-312. [PubMed] [Google Scholar]
- 113.Drobyski, W. R., W. M. Dunne, E. M. Burd, K. K. Knox, R. C. Ash, M. M. Horowitz, N. Flomenberg, and D. R. Carrigan. 1993. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J. Infect. Dis. 167:735-739. [DOI] [PubMed] [Google Scholar]
- 114.Drobyski, W. R., M. Eberle, D. Majewski, and L. A. Baxter-Lowe. 1993. Prevalence of human herpesvirus 6 variant A and B infections in bone marrow transplant recipients as determined by polymerase chain reaction and sequence-specific oligonucleotide probe hybridization. J. Clin. Microbiol. 31:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drobyski, W. R., K. K. Knox, D. Majewski, and D. R. Carrigan. 1994. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N. Engl. J. Med. 330:1356-1360. [DOI] [PubMed] [Google Scholar]
- 116.Duh, E. J., W. J. Maury, T. M. Folks, A. S. Fauci, and A. B. Rabson. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA 86:5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Emery, V. C. 2001. Prophylaxis for CMV should not now replace pre-emptive therapy in solid organ transplantation. Rev. Med. Virol. 11:83-86. [DOI] [PubMed] [Google Scholar]
- 118.Emery, V. C., M. C. Atkins, E. F. Bowen, D. A. Clark, M. A. Johnson, I. M. Kidd, J. E. McLaughlin, A. N. Phillips, P. M. Strappe, and P. D. Griffiths. 1999. Interactions between beta-herpesviruses and human immunodeficiency virus in vivo: evidence for increased human immunodeficiency viral load in the presence of human herpesvirus 6. J. Med. Virol. 57:278-282. [DOI] [PubMed] [Google Scholar]
- 119.Enbom, M., F. Z. Wang, S. Fredrikson, C. Martin, H. Dahl, and A. Linde. 1999. Similar humoral and cellular immunological reactivities to human herpesvirus 6 in patients with multiple sclerosis and controls. Clin. Diagn. Lab. Immunol. 6:545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Enders, G., M. Biber, G. Meyer, and E. Helftenbein. 1990. Prevalence of antibodies to human herpesvirus 6 in different age groups, in children with exanthema subitum, other acute exanthematous childhood diseases, Kawasaki syndrome, and acute infections with other herpesviruses and HIV. Infection 18:12-15. [DOI] [PubMed] [Google Scholar]
- 121.Ensoli, B., P. Lusso, F. Schachter, S. F. Josephs, J. Rappaport, F. Negro, R. C. Gallo, and F. Wong-Staal. 1989. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 8:3019-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feldstein, A. E., R. R. Razonable, T. G. Boyce, D. K. Freese, M. El Youssef, J. Perrault, C. V. Paya, and M. B. Ishitani. 2003. Prevalence and clinical significance of human herpesviruses 6 and 7 active infection in pediatric liver transplant patients. Pediatr. Transplant. 7:125-129. [DOI] [PubMed] [Google Scholar]
- 123.Fillet, A. M., I. Reux, C. Joberty, J. G. Fournier, J. J. Hauw, P. Le Hoang, F. Bricaire, J. M. Huraux, and H. Agut. 1996. Detection of human herpes virus 6 in AIDS-associated retinitis by means of in situ hybridization, polymerase chain reaction and immunohistochemistry. J. Med. Virol. 49:289-295. [DOI] [PubMed] [Google Scholar]
- 124.Flamand, L., J. Gosselin, M. D'Addario, J. Hiscott, D. V. Ablashi, R. C. Gallo, and J. Menezes. 1991. Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J. Virol. 65:5105-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flamand, L., J. Gosselin, I. Stefanescu, D. Ablashi, and J. Menezes. 1995. Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation. Blood 85:1263-1271. [PubMed] [Google Scholar]
- 126.Flamand, L., and J. Menezes. 1996. Cyclic AMP-responsive element-dependent activation of Epstein-Barr virus zebra promoter by human herpesvirus 6. J. Virol. 70:1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flamand, L., F. Romerio, M. S. Reitz, and R. C. Gallo. 1998. CD4 promoter transactivation by human herpesvirus 6. J. Virol. 72:8797-8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flamand, L., I. Stefanescu, D. V. Ablashi, and J. Menezes. 1993. Activation of the Epstein-Barr virus replicative cycle by human herpesvirus 6. J. Virol. 67:6768-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flebbe-Rehwaldt, L. M., C. Wood, and B. Chandran. 2000. Characterization of transcripts expressed from human herpesvirus 6A strain GS immediate-early region B U16-U17 open reading frames. J. Virol. 74:11040-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fox, J. D., M. Briggs, P. A. Ward, and R. S. Tedder. 1990. Human herpesvirus 6 in salivary glands. Lancet 336:590-593. [DOI] [PubMed] [Google Scholar]
- 131.French, C., P. Menegazzi, L. Nicholson, H. Macaulay, D. DiLuca, and U. A. Gompels. 1999. Novel, nonconsensus cellular splicing regulates expression of a gene encoding a chemokine-like protein that shows high variation and is specific for human herpesvirus 6. Virology 262:139-151. [DOI] [PubMed] [Google Scholar]
- 132.Frenkel, N., E. Roffman, E. C. Schirmer, G. Katsafanas, L. S. Wyatt, and C. H. June. 1990. Cellular and growth-factor requirements for the replication of human herpesvirus 6 in primary lymphocyte cultures. Adv. Exp. Med. Biol. 278:1-8. [DOI] [PubMed] [Google Scholar]
- 133.Gallois-Montbrun, S., B. Schneider, Y. Chen, V. Giacomoni-Fernandes, L. Mulard, S. Morera, J. Janin, D. Deville-Bonne, and M. Veron. 2002. Improving nucleoside diphosphate kinase for antiviral nucleotide analogs activation. J. Biol. Chem. 277:39953-39959. [DOI] [PubMed] [Google Scholar]
- 134.Garzino-Demo, A., M. Chen, P. Lusso, Z. Berneman, and J. A. DiPaolo. 1996. Enhancement of TAT-induced transactivation of the HIV-1 LTR by two genomic fragments of HHV-6. J. Med. Virol. 50:20-24. [DOI] [PubMed] [Google Scholar]
- 135.Gautheret-Dejean, A., C. Manichanh, F. Thien-Ah-Koon, A. M. Fillet, N. Mangeney, M. Vidaud, N. Dhedin, J. P. Vernant, and H. Agut. 2002. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J. Virol. Methods 100:27-35. [DOI] [PubMed] [Google Scholar]
- 136.Geng, Y. Q., B. Chandran, S. F. Josephs, and C. Wood. 1992. Identification and characterization of a human herpesvirus 6 gene segment that trans activates the human immunodeficiency virus type 1 promoter. J. Virol. 66:1564-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilbert, C., J. Bestman-Smith, and G. Boivin. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Update 5:88-114. [DOI] [PubMed] [Google Scholar]
- 138.Go, T., and K. Nakamura. 2002. Frequent seizures with elevated interleukin-6 at the eruptive stage of exanthema subitum. Eur. J. Paediatr. Neurol. 6:221-223. [DOI] [PubMed] [Google Scholar]
- 139.Gobbi, A., C. A. Stoddart, G. Locatelli, F. Santoro, C. Bare, V. Linquist-Stepps, M. E. Moreno, N. W. Abbey, B. G. Herndier, M. S. Malnati, J. M. McCune, and P. Lusso. 2000. Coinfection of SCID-hu Thy/Liv mice with human herpesvirus 6 and human immunodeficiency virus type 1. J. Virol. 74:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goedhard, J. G., J. M. Galama, and J. H. Wagenvoort. 1995. Active human herpesvirus 6 infection in an adolescent male. Clin. Infect. Dis. 20:1070-1071. [DOI] [PubMed] [Google Scholar]
- 141.Goldberg, S. H., A. V. Albright, R. P. Lisak, and F. Gonzalez-Scarano. 1999. Polymerase chain reaction analysis of human herpesvirus-6 sequences in the sera and cerebrospinal fluid of patients with multiple sclerosis. J. Neurovirol. 5:134-139. [DOI] [PubMed] [Google Scholar]
- 142.Gompels, U. A., and H. A. Macaulay. 1995. Characterization of human telomeric repeat sequences from human herpesvirus 6 and relationship to replication. J. Gen. Virol. 76:451-458. [DOI] [PubMed] [Google Scholar]
- 143.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 144.Goodman, A. D., D. J. Mock, J. M. Powers, J. V. Baker, and B. M. Blumberg. 2003. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J. Infect. Dis. 187:1365-1376. [DOI] [PubMed] [Google Scholar]
- 145.Gosselin, J., L. Flamand, M. D'Addario, J. Hiscott, I. Stefanescu, D. V. Ablashi, R. C. Gallo, and J. Menezes. 1992. Modulatory effects of Epstein-Barr, herpes simplex, and human herpes-6 viral infections and coinfections on cytokine synthesis: a comparative study. J. Immunol. 149:181-187. [PubMed] [Google Scholar]
- 146.Gosselin, J., A. TomoIu, R. C. Gallo, and L. Flamand. 1999. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood 94:4210-4219. [PubMed] [Google Scholar]
- 147.Gravel, A., J. Gosselin, and L. Flamand. 2002. Human herpesvirus 6 immediate-early 1 protein is a sumoylated nuclear phosphoprotein colocalizing with promyelocytic leukemia protein-associated nuclear bodies. J. Biol. Chem. 277:19679-19687. [DOI] [PubMed] [Google Scholar]
- 148.Gravel, A., A. TomoIu, N. Cloutier, J. Gosselin, and L. Flamand. 2003. Characterization of the immediate-early 2 protein of human herpesvirus 6, a promiscuous transcriptional activator. Virology 308:340-353. [DOI] [PubMed] [Google Scholar]
- 149.Greenstone, H. L., F. Santoro, P. Lusso, and E. A. Berger. 2002. Human herpesvirus 6 and measles virus employ distinct CD46 domains for receptor function. J. Biol. Chem. 277:39112-39118. [DOI] [PubMed] [Google Scholar]
- 150.Griffiths, P. D., M. Ait-Khaled, C. P. Bearcroft, D. A. Clark, A. Quaglia, S. E. Davies, A. K. Burroughs, K. Rolles, I. M. Kidd, S. N. Knight, S. M. Noibi, A. V. Cope, A. N. Phillips, and V. C. Emery. 1999. Human herpesviruses 6 and 7 as potential pathogens after liver transplant: prospective comparison with the effect of cytomegalovirus. J. Med. Virol. 59:496-501. [DOI] [PubMed] [Google Scholar]
- 151.Grivel, J. C., Y. Ito, G. Faga, F. Santoro, F. Shaheen, M. S. Malnati, W. Fitzgerald, P. Lusso, and L. Margolis. 2001. Suppression of CCR5- but not CXCR4-tropic HIV-1 in lymphoid tissue by human herpesvirus 6. Nat. Med. 7:1232-1235. [DOI] [PubMed] [Google Scholar]
- 152.Grivel, J. C., F. Santoro, S. Chen, G. Faga, M. S. Malnati, Y. Ito, L. Margolis, and P. Lusso. 2003. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 77:8280-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hall, C. B., M. T. Caserta, K. C. Schnabel, C. Long, L. G. Epstein, R. A. Insel, and S. Dewhurst. 1998. Persistence of human herpesvirus 6 according to site and variant: possible greater neurotropism of variant A. Clin. Infect. Dis. 26:132-137. [DOI] [PubMed] [Google Scholar]
- 154.Hall, C. B., C. E. Long, K. C. Schnabel, M. T. Caserta, K. M. McIntyre, M. A. Costanzo, A. Knott, S. Dewhurst, R. A. Insel, and L. G. Epstein. 1994. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N. Engl. J. Med. 331:432-438. [DOI] [PubMed] [Google Scholar]
- 155.Harma, M., K. Hockerstedt, and I. Lautenschlager. 2003. Human herpesvirus-6 and acute liver failure. Transplantation 76:536-539. [DOI] [PubMed] [Google Scholar]
- 156.Hart, G. D., and C. V. Paya. 2001. Prophylaxis for CMV should now replace pre-emptive therapy in solid organ transplantation. Rev. Med. Virol. 11:73-81. [DOI] [PubMed] [Google Scholar]
- 157.Hasegawa, A., M. Yasukawa, I. Sakai, and S. Fujita. 2001. Transcriptional down-regulation of CXC chemokine receptor 4 induced by impaired association of transcription regulator YY1 with c-Myc in human herpesvirus 6-infected cells. J. Immunol. 166:1125-1131. [DOI] [PubMed] [Google Scholar]
- 158.Hasegawa, H., Y. Utsunomiya, M. Yasukawa, K. Yanagisawa, and S. Fujita. 1994. Induction of G protein-coupled peptide receptor EBI 1 by human herpesvirus 6 and 7 infection in CD4+ T cells. J. Virol. 68:5326-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hashimoto, H., H. Maruyama, K. Fujimoto, T. Sakakura, S. Seishu, and N. Okuda. 2002. Hematologic findings associated with thrombocytopenia during the acute phase of exanthem subitum confirmed by primary human herpesvirus-6 infection. J. Pediatr. Hematol. Oncol. 24:211-214. [DOI] [PubMed] [Google Scholar]
- 160.He, J., M. McCarthy, Y. Zhou, B. Chandran, and C. Wood. 1996. Infection of primary human fetal astrocytes by human herpesvirus 6. J. Virol. 70:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus-survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 162.Herbein, G., J. Strasswimmer, M. Altieri, M. L. Woehl-Jaegle, P. Wolf, and G. Obert. 1996. Longitudinal study of human herpesvirus 6 infection in organ transplant recipients. Clin. Infect. Dis. 22:171-173. [DOI] [PubMed] [Google Scholar]
- 163.Higashi, K., H. Asada, T. Kurata, K. Ishikawa, M. Hayami, Y. Spriatna, Sutarman, and K. Yamanishi. 1989. Presence of antibody to human herpesvirus 6 in monkeys. J. Gen. Virol. 70:3171-3176. [DOI] [PubMed] [Google Scholar]
- 164.Hirata, Y., K. Kondo, and K. Yamanishi. 2001. Human herpesvirus 6 downregulates major histocompatibility complex class I in dendritic cells. J. Med. Virol. 65:576-583. [PubMed] [Google Scholar]
- 165.Hong, J., M. V. Tejada-Simon, V. M. Rivera, Y. C. Zang, and J. Z. Zhang. 2002. Anti-viral properties of interferon beta treatment in patients with multiple sclerosis. Mult. Scler. 8:237-242. [DOI] [PubMed] [Google Scholar]
- 166.Horvat, R. T., M. J. Parmely, and B. Chandran. 1993. Human herpesvirus 6 inhibits the proliferative responses of human peripheral blood mononuclear cells. J. Infect. Dis. 167:1274-1280. [DOI] [PubMed] [Google Scholar]
- 167.Horvat, R. T., C. Wood, S. F. Josephs, and N. Balachandran. 1991. Transactivation of the human immunodeficiency virus promoter by human herpesvirus 6 (HHV-6) strains GS and Z-29 in primary human T lymphocytes and identification of transactivating HHV-6 (GS) gene fragments. J. Virol. 65:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoshino, K., T. Nishi, H. Adachi, H. Ito, Y. Fukuda, K. Dohi, and T. Kurata. 1995. Human herpesvirus-6 infection in renal allografts: retrospective immunohistochemical study in Japanese recipients. Transplant. Int. 8:169-173. [DOI] [PubMed] [Google Scholar]
- 169.Huang, L., K. K. Ishii, H. Zuccola, A. M. Gehring, C. B. Hwang, J. Hogle, and D. M. Coen. 1999. The enzymological basis for resistance of herpesvirus DNA polymerase mutants to acyclovir: relationship to the structure of alpha-like DNA polymerases. Proc. Natl. Acad. Sci. USA 96:447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang, L. M., P. F. Kuo, C. Y. Lee, J. Y. Chen, M. Y. Liu, and C. S. Yang. 1992. Detection of human herpesvirus-6 DNA by polymerase chain reaction in serum or plasma. J. Med. Virol. 38:7-10. [DOI] [PubMed] [Google Scholar]
- 171.Humar, A., D. Kumar, A. M. Caliendo, G. Moussa, A. Ashi-Sulaiman, G. Levy, and T. Mazzulli. 2002. Clinical impact of human herpesvirus 6 infection after liver transplantation. Transplantation 73:599-604. [DOI] [PubMed] [Google Scholar]
- 172.Humar, A., D. Kumar, J. Raboud, A. M. Caliendo, G. Moussa, G. Levy, and T. Mazzulli. 2002. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am. J. Transplant. 2:461-466. [DOI] [PubMed] [Google Scholar]
- 173.Humar, A., G. Malkan, G. Moussa, P. Greig, G. Levy, and T. Mazzulli. 2000. Human herpesvirus-6 is associated with cytomegalovirus reactivation in liver transplant recipients. J. Infect. Dis. 181:1450-1453. [DOI] [PubMed] [Google Scholar]
- 174.Ichimi, R., T. Jin-no, and M. Ito. 1999. Induction of apoptosis in cord blood lymphocytes by HHV-6. J. Med. Virol. 58:63-68. [DOI] [PubMed] [Google Scholar]
- 175.Ihira, M., T. Yoshikawa, J. Ishii, M. Nomura, H. Hishida, M. Ohashi, Y. Enomoto, S. Suga, K. Iida, Y. Saito, Y. Nishiyama, and Y. Asano. 2002. Serological examination of human herpesvirus 6 and 7 in patients with coronary artery disease. J. Med. Virol. 67:534-537. [DOI] [PubMed] [Google Scholar]
- 176.Ihira, M., T. Yoshikawa, K. Suzuki, M. Ohashi, S. Suga, K. Horibe, N. Tanaka, H. Kimura, S. Kojima, K. Kato, T. Matsuyama, Y. Nishiyama, and Y. Asano. 2002. Monitoring of active HHV-6 infection in bone marrow transplant recipients by real time PCR; comparison to detection of viral DNA in plasma by qualitative PCR. Microbiol. Immunol. 46:701-705. [DOI] [PubMed] [Google Scholar]
- 177.Imbert-Marcille, B. M., X. W. Tang, D. Lepelletier, B. Besse, P. Moreau, S. Billaudel, and N. Milpied. 2000. Human herpesvirus 6 infection after autologous or allogeneic stem cell transplantation: a single-center prospective longitudinal study of 92 patients. Clin. Infect. Dis. 31:881-886. [DOI] [PubMed] [Google Scholar]
- 178.Inagi, R., R. Guntapong, M. Nakao, Y. Ishino, K. Kawanishi, Y. Isegawa, and K. Yamanishi. 1996. Human herpesvirus 6 induces IL-8 gene expression in human hepatoma cell line, Hep G2. J. Med. Virol. 49:34-40. [DOI] [PubMed] [Google Scholar]
- 179.Inoue, N., T. R. Dambaugh, J. C. Rapp, and P. E. Pellett. 1994. Alphaherpesvirus origin-binding protein homolog encoded by human herpesvirus 6B, a betaherpesvirus, binds to nucleotide sequences that are similar to ori regions of alphaherpesviruses. J. Virol. 68:4126-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Inoue, N., and P. E. Pellett. 1995. Human herpesvirus 6B origin-binding protein: DNA-binding domain and consensus binding sequence. J. Virol. 69:4619-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Inoue, Y., M. Yasukawa, and S. Fujita. 1997. Induction of T-cell apoptosis by human herpesvirus 6. J. Virol. 71:3751-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Irving, W. L., and A. L. Cunningham. 1990. Serological diagnosis of infection with human herpesvirus type 6. Br. Med. J. 300:156-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Isegawa, Y., T. Mukai, K. Nakano, M. Kagawa, J. Chen, Y. Mori, T. Sunagawa, K. Kawanishi, J. Sashihara, A. Hata, P. Zou, H. Kosuge, and K. Yamanishi. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 73:8053-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Isegawa, Y., Z. Ping, K. Nakano, N. Sugimoto, and K. Yamanishi. 1998. Human herpesvirus 6 open reading frame U12 encodes a functional beta-chemokine receptor. J. Virol. 72:6104-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ishiguro, N., S. Yamada, T. Takahashi, Y. Takahashi, T. Togashi, T. Okuno, and K. Yamanishi. 1990. Meningo-encephalitis associated with HHV-6 related exanthem subitum. Acta Paediatr. Scand. 79:987-989. [DOI] [PubMed] [Google Scholar]
- 186.Ishikawa, K., K. Hasegawa, T. Naritomi, N. Kanai, M. Ogawa, Y. Kato, M. Kobayashi, N. Torii, and N. Hayashi. 2002. Prevalence of herpesviridae and hepatitis virus sequences in the livers of patients with fulminant hepatitis of unknown etiology in Japan. J. Gastroenterol. 37:523-530. [DOI] [PubMed] [Google Scholar]
- 187.Isomura, H., M. Yamada, M. Yoshida, H. Tanaka, T. Kitamura, M. Oda, S. Nii, and Y. Seino. 1997. Suppressive effects of human herpesvirus 6 on in vitro colony formation of hematopoietic progenitor cells. J. Med. Virol. 52:406-412. [DOI] [PubMed] [Google Scholar]
- 188.Isomura, H., M. Yoshida, H. Namba, N. Fujiwara, R. Ohuchi, F. Uno, M. Oda, Y. Seino, and M. Yamada. 2000. Suppressive effects of human herpesvirus-6 on thrombopoietin-inducible megakaryocytic colony formation in vitro. J. Gen. Virol. 81:663-673. [DOI] [PubMed] [Google Scholar]
- 189.Isomura, H., M. Yoshida, H. Namba, and M. Yamada. 2003. Interaction of human herpesvirus 6 with human CD34 positive cells. J. Med. Virol. 70:444-450. [DOI] [PubMed] [Google Scholar]
- 190.Izikson, L., R. S. Klein, I. F. Charo, H. L. Weiner, and A. D. Luster. 2000. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192:1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jabs, D. A., B. K. Martin, M. S. Forman, J. P. Dunn, J. L. Davis, D. V. Weinberg, K. K. Biron, and F. Baldanti. 2001. Mutations conferring ganciclovir resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J. Infect. Dis. 183:333-337. [DOI] [PubMed] [Google Scholar]
- 192.Jacobs, F., C. Knoop, F. Brancart, P. Gilot, C. Melot, B. Byl, M. L. Delforge, M. Estenne, and C. Liesnard. 2003. Human herpesvirus-6 infection after lung and heart-lung transplantation: a prospective longitudinal study. Transplantation 75:1996-2001. [DOI] [PubMed] [Google Scholar]
- 193.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 194.Jarrett, R. F., D. A. Clark, S. F. Josephs, and D. E. Onions. 1990. Detection of human herpesvirus-6 DNA in peripheral blood and saliva. J. Med. Virol. 32:73-76. [DOI] [PubMed] [Google Scholar]
- 195.Johnston, R. E., A. M. Geretti, H. G. Prentice, A. D. Clark, A. C. Wheeler, M. Potter, and P. D. Griffiths. 1999. HHV-6-related secondary graft failure following allogeneic bone marrow transplantation. Br. J. Haematol. 105:1041-1043. [DOI] [PubMed] [Google Scholar]
- 196.Jones, C. M., H. G. Dunn, E. E. Thomas, R. W. Cone, and J. M. Weber. 1994. Acute encephalopathy and status epilepticus associated with human herpes virus 6 infection. Dev. Med. Child Neurol. 36:646-650. [DOI] [PubMed] [Google Scholar]
- 197.Kadakia, M. P., W. B. Rybka, J. A. Stewart, J. L. Patton, F. R. Stamey, M. Elsawy, P. E. Pellett, and J. A. Armstrong. 1996. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation. Blood 87:5341-5354. [PubMed] [Google Scholar]
- 198.Kakimoto, M., A. Hasegawa, S. Fujita, and M. Yasukawa. 2002. Phenotypic and functional alterations of dendritic cells induced by human herpesvirus 6 infection. J. Virol. 76:10338-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kariya, M., S. Mori, and Y. Eizuru. 2000. Comparison of human cytomegalovirus DNA polymerase activity for ganciclovir-resistant and -sensitive clinical strains. Antivir. Res. 45:115-122. [DOI] [PubMed] [Google Scholar]
- 200.Kashanchi, F., J. Araujo, J. Doniger, S. Muralidhar, R. Hoch, S. Khleif, E. Mendelson, J. Thompson, N. Azumi, J. N. Brady, M. Luppi, G. Torelli, and L. J. Rosenthal. 1997. Human herpesvirus 6 (HHV-6) ORF-1 transactivating gene exhibits malignant transforming activity and its protein binds to p53. Oncogene 14:359-367. [DOI] [PubMed] [Google Scholar]
- 201.Kashanchi, F., J. Thompson, M. R. Sadaie, J. Doniger, J. Duvall, J. N. Brady, and L. J. Rosenthal. 1994. Transcriptional activation of minimal HIV-1 promoter by ORF-1 protein expressed from the SalI-L fragment of human herpesvirus 6. Virology 201:95-106. [DOI] [PubMed] [Google Scholar]
- 202.Kato, Z., R. Kozawa, T. Teramoto, K. Hashimoto, S. Shinoda, and N. Kondo. 2003. Acute cerebellitis in primary human herpesvirus-6 infection. Eur. J. Pediatr. 162:801-803. [DOI] [PubMed] [Google Scholar]
- 203.Katsafanas, G. C., E. C. Schirmer, L. S. Wyatt, and N. Frenkel. 1996. In vitro activation of human herpesviruses 6 and 7 from latency. Proc. Natl. Acad. Sci. USA 93:9788-9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kenney, S., J. Kamine, D. Markovitz, R. Fenrick, and J. Pagano. 1988. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc. Natl. Acad. Sci. USA 85:1652-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kidd, I. M., D. A. Clark, C. A. Sabin, D. Andrew, A. F. Hassan-Walker, P. Sweny, P. D. Griffiths, and V. C. Emery. 2000. Prospective study of human betaherpesviruses after renal transplantation: association of human herpesvirus 7 and cytomegalovirus co-infection with cytomegalovirus disease and increased rejection. Transplantation 69:2400-2404. [DOI] [PubMed] [Google Scholar]
- 206.Kikuta, H., H. Lu, K. Tomizawa, and S. Matsumoto. 1990. Enhancement of human herpesvirus 6 replication in adult human lymphocytes by monoclonal antibody to CD3. J. Infect. Dis. 161:1085-1087. [DOI] [PubMed] [Google Scholar]
- 207.Kikuta, H., A. Nakane, H. Lu, Y. Taguchi, T. Minagawa, and S. Matsumoto. 1990. Interferon induction by human herpesvirus 6 in human mononuclear cells. J. Infect. Dis. 162:35-38. [DOI] [PubMed] [Google Scholar]
- 208.Kim, J. S., K. S. Lee, J. H. Park, M. Y. Kim, and W. S. Shin. 2000. Detection of human herpesvirus 6 variant A in peripheral blood mononuclear cells from multiple sclerosis patients. Eur. Neurol. 43:170-173. [DOI] [PubMed] [Google Scholar]
- 209.Kirn, E., E. Krueger, S. Boehmer, J. P. Klussmann, and G. R. Krueger. 1997. In vitro cytobiological effects of human herpesviruses 6 and 7: immunohistological monitoring of apoptosis, differentiation and cell proliferation. Anticancer Res. 17:4623-4632. [PubMed] [Google Scholar]
- 210.Kitamura, K., H. Ohta, T. Ihara, H. Kamiya, H. Ochiai, K. Yamanishi, and K. Tanaka. 1994. Idiopathic thrombocytopenic purpura after human herpesvirus 6 infection. Lancet 344:830. [DOI] [PubMed] [Google Scholar]
- 211.Knox, K. K., J. H. Brewer, J. M. Henry, D. J. Harrington, and D. R. Carrigan. 2000. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin. Infect. Dis. 31:894-903. [DOI] [PubMed] [Google Scholar]
- 212.Knox, K. K., and D. R. Carrigan. 1992. In vitro suppression of bone marrow progenitor cell differentiation by human herpesvirus 6 infection. J. Infect. Dis. 165:925-929. [DOI] [PubMed] [Google Scholar]
- 213.Knox, K. K., and D. R. Carrigan. 1995. Active human herpesvirus (HHV-6) infection of the central nervous system in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9:69-73. [PubMed] [Google Scholar]
- 214.Knox, K. K., D. P. Harrington, and D. R. Carrigan. 1995. Fulminant human herpesvirus six encephalitis in a human immunodeficiency virus-infected infant. J. Med. Virol. 45:288-292. [DOI] [PubMed] [Google Scholar]
- 215.Kondo, K., T. Kondo, T. Okuno, M. Takahashi, and K. Yamanishi. 1991. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 72:1401-1408. [DOI] [PubMed] [Google Scholar]
- 216.Kondo, K., T. Kondo, K. Shimada, K. Amo, H. Miyagawa, and K. Yamanishi. 2002. Strong interaction between human herpesvirus 6 and peripheral blood monocytes/macrophages during acute infection. J. Med. Virol. 67:364-369. [DOI] [PubMed] [Google Scholar]
- 217.Kondo, K., and E. S. Mocarski. 1995. Cytomegalovirus latency and latency-specific transcription in hematopoietic progenitors. Scand. J. Infect. Dis. Suppl. 99:63-67. [PubMed] [Google Scholar]
- 218.Kondo, K., H. Nagafuji, A. Hata, C. Tomomori, and K. Yamanishi. 1993. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J. Infect. Dis. 167:1197-1200. [DOI] [PubMed] [Google Scholar]
- 219.Kondo, K., J. Sashihara, K. Shimada, M. Takemoto, K. Amo, H. Miyagawa, and K. Yamanishi. 2003. Recognition of a novel stage of betaherpesvirus latency in human herpesvirus 6. J. Virol. 77:2258-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kondo, K., K. Shimada, J. Sashihara, K. Tanaka-Taya, and K. Yamanishi. 2002. Identification of human herpesvirus 6 latency-associated transcripts. J. Virol. 76:4145-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kong, H., Q. Baerbig, L. Duncan, N. Shepel, and M. Mayne. 2003. Human herpesvirus type 6 indirectly enhances oligodendrocyte cell death. J. Neurovirol. 9:539-550. [DOI] [PubMed] [Google Scholar]
- 222.Kositanont, U., C. Wasi, N. Wanprapar, P. Bowonkiratikachorn, K. Chokephaibulkit, S. Chearskul, K. Chimabutra, R. Sutthent, S. Foongladda, R. Inagi, T. Kurata, and K. Yamanishi. 1999. Primary infection of human herpesvirus 6 in children with vertical infection of human immunodeficiency virus type 1. J. Infect. Dis. 180:50-55. [DOI] [PubMed] [Google Scholar]
- 223.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Krosky, P. M., M. C. Baek, W. J. Jahng, I. Barrera, R. J. Harvey, K. K. Biron, D. M. Coen, and P. B. Sethna. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Krueger, G. R., M. L. Huetter, J. Rojo, M. Romero, and H. Cruz-Ortiz. 2001. Human herpesviruses HHV-4 (EBV) and HHV-6 in Hodgkin's and Kikuchi's diseases and their relation to proliferation and apoptosis. Anticancer Res. 21:2155-2161. [PubMed] [Google Scholar]
- 226.Kusuhara, K., K. Ueda, K. Okada, C. Miyazaki, K. Tokugawa, M. Hirose, K. Takahashi, and K. Yamanishi. 1991. Do second attacks of exanthema subitum result from human herpesvirus 6 reactivation or reinfection? Pediatr. Infect. Dis. J. 10:468-470. [DOI] [PubMed] [Google Scholar]
- 227.Lacoste, V., P. Mauclere, G. Dubreuil, J. Lewis, M. C. Georges-Courbot, J. Rigoulet, T. Petit, and A. Gessain. 2000. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. J. Virol. 74:11993-11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lanari, M., I. Papa, V. Venturi, T. Lazzarotto, G. Faldella, L. Gabrielli, B. Guerra, M. P. Landini, and G. P. Salvioli. 2003. Congenital infection with human herpesvirus 6 variant B associated with neonatal seizures and poor neurological outcome. J. Med. Virol. 70:628-632. [DOI] [PubMed] [Google Scholar]
- 229.Lau, Y. L., M. Peiris, G. C. Chan, A. C. Chan, D. Chiu, and S. Y. Ha. 1998. Primary human herpes virus 6 infection transmitted from donor to recipient through bone marrow infusion. Bone Marrow Transplant. 21:1063-1066. [DOI] [PubMed] [Google Scholar]
- 230.Lautenschlager, I., M. Harma, K. Hockerstedt, K. Linnavuori, R. Loginov, and E. Taskinen. 2002. Human herpesvirus-6 infection is associated with adhesion molecule induction and lymphocyte infiltration in liver allografts. J. Hepatol. 37:648-654. [DOI] [PubMed] [Google Scholar]
- 231.Lautenschlager, I., K. Hockerstedt, K. Linnavuori, and E. Taskinen. 1998. Human herpesvirus-6 infection after liver transplantation. Clin. Infect. Dis. 26:702-707. [DOI] [PubMed] [Google Scholar]
- 232.Lautenschlager, I., M. Lappalainen, K. Linnavuori, J. Suni, and K. Hockerstedt. 2002. CMV infection is usually associated with concurrent HHV-6 and HHV-7 antigenemia in liver transplant patients. J. Clin. Virol. 25(Suppl. 2):S57-S61. [DOI] [PubMed] [Google Scholar]
- 233.Lautenschlager, I., K. Linnavuori, and K. Hockerstedt. 2000. Human herpesvirus-6 antigenemia after liver transplantation. Transplantation 69:2561-2566. [DOI] [PubMed] [Google Scholar]
- 234.Levine, P. H., N. Jahan, P. Murari, M. Manak, and E. S. Jaffe. 1992. Detection of human herpesvirus 6 in tissues involved by sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). J. Infect. Dis. 166:291-295. [DOI] [PubMed] [Google Scholar]
- 235.Levy, J. A., A. Landay, and E. T. Lennette. 1990. Human herpesvirus 6 inhibits human immunodeficiency virus type 1 replication in cell culture. J. Clin. Microbiol. 28:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li, C., J. M. Goodrich, and X. Yang. 1997. Interferon-gamma (IFN-gamma) regulates production of IL-10 and IL-12 in human herpesvirus-6 (HHV-6)-infected monocyte/macrophage lineage. Clin. Exp. Immunol. 109:421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liedtke, W., R. Malessa, P. M. Faustmann, and A. M. Eis-Hubinger. 1995. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J. Neurovirol. 1:253-258. [DOI] [PubMed] [Google Scholar]
- 238.Lin, K., and R. P. Ricciardi. 1998. The 41-kDa protein of human herpesvirus 6 specifically binds to viral DNA polymerase and greatly increases DNA synthesis. Virology 250:210-219. [DOI] [PubMed] [Google Scholar]
- 239.Lindquester, G. J., and P. E. Pellett. 1991. Properties of the human herpesvirus 6 strain Z29 genome: G+C content, length, and presence of variable-length directly repeated terminal sequence elements. Virology 182:102-110. [DOI] [PubMed] [Google Scholar]
- 240.Linhares, M. I., Y. Eizuru, S. Tateno, and Y. Minamishima. 1991. Seroprevalence of human herpesvirus 6 infection in Brazilian and Japanese populations in the north-east of Brazil. Microbiol. Immunol. 35:1023-1027. [DOI] [PubMed] [Google Scholar]
- 241.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 242.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 243.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ljungman, P., G. L. Deliliers, U. Platzbecker, S. Matthes-Martin, A. Bacigalupo, H. Einsele, J. Ullmann, M. Musso, R. Trenschel, P. Ribaud, M. Bornhauser, S. Cesaro, B. Crooks, A. Dekker, N. Gratecos, T. Klingebiel, E. Tagliaferri, A. J. Ullmann, P. Wacker, C. Cordonnier, et al. 2001. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. Blood 97:388-392. [DOI] [PubMed] [Google Scholar]
- 245.Ljungman, P., F. Z. Wang, D. A. Clark, V. C. Emery, M. Remberger, O. Ringden, and A. Linde. 2000. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br. J. Haematol. 111:774-781. [PubMed] [Google Scholar]
- 246.Luka, J., M. Okano, and G. Thiele. 1990. Isolation of human herpesvirus-6 from clinical specimens using human fibroblast cultures. J. Clin. Lab. Anal. 4:483-486. [DOI] [PubMed] [Google Scholar]
- 247.Luppi, M., P. Barozzi, R. Garber, A. Maiorana, G. Bonacorsi, T. Artusi, R. Trovato, R. Marasca, and G. Torelli. 1998. Expression of human herpesvirus-6 antigens in benign and malignant lymphoproliferative diseases. Am. J. Pathol. 153:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Luppi, M., P. Barozzi, A. Maiorana, R. Marasca, and G. Torelli. 1994. Human herpesvirus 6 infection in normal human brain tissue. J. Infect. Dis. 169:943-944. [DOI] [PubMed] [Google Scholar]
- 249.Luppi, M., P. Barozzi, A. Maiorana, R. Marasca, R. Trovato, R. Fano, L. Ceccherini-Nelli, and G. Torelli. 1995. Human herpesvirus-6: a survey of presence and distribution of genomic sequences in normal brain and neuroglial tumors. J. Med. Virol. 47:105-111. [DOI] [PubMed] [Google Scholar]
- 250.Luppi, M., P. Barozzi, R. Marasca, and G. Torelli. 1994. Integration of human herpesvirus-6 (HHV-6) genome in chromosome 17 in two lymphoma patients. Leukemia 8(Suppl. 1):S41-S45. [PubMed] [Google Scholar]
- 251.Luppi, M., P. Barozzi, C. Morris, A. Maiorana, R. Garber, G. Bonacorsi, A. Donelli, R. Marasca, A. Tabilio, and G. Torelli. 1999. Human herpesvirus 6 latently infects early bone marrow progenitors in vivo. J. Virol. 73:754-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lusso, P., A. De Maria, M. Malnati, F. Lori, S. E. DeRocco, M. Baseler, and R. C. Gallo. 1991. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature 349:533-535. [DOI] [PubMed] [Google Scholar]
- 253.Lusso, P., B. Ensoli, P. D. Markham, D. V. Ablashi, S. Z. Salahuddin, E. Tschachler, F. Wong-Staal, and R. C. Gallo. 1989. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature 337:370-373. [DOI] [PubMed] [Google Scholar]
- 254.Lusso, P., and R. C. Gallo. 1995. Human herpesvirus 6 in AIDS. Immunol. Today 16:67-71. [DOI] [PubMed] [Google Scholar]
- 255.Lusso, P., A. Garzino-Demo, R. W. Crowley, and M. S. Malnati. 1995. Infection of gamma/delta T lymphocytes by human herpesvirus 6: transcriptional induction of CD4 and susceptibility to HIV infection. J. Exp. Med. 181:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lusso, P., M. Malnati, A. De Maria, C. Balotta, S. E. DeRocco, P. D. Markham, and R. C. Gallo. 1991. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 147:685-691. [PubMed] [Google Scholar]
- 257.Lusso, P., M. S. Malnati, A. Garzino-Demo, R. W. Crowley, E. O. Long, and R. C. Gallo. 1993. Infection of natural killer cells by human herpesvirus 6. Nature 362:458-462. [DOI] [PubMed] [Google Scholar]
- 258.Lusso, P., P. D. Markham, E. Tschachler, F. di Marzo Veronese, S. Z. Salahuddin, D. V. Ablashi, S. Pahwa, K. Krohn, and R. C. Gallo. 1988. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J. Exp. Med. 167:1659-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Luttichau, H. R., I. Clark-Lewis, P. O. Jensen, C. Moser, J. Gerstoft, and T. W. Schwartz. 2003. A highly selective CCR2 chemokine agonist encoded by human herpesvirus 6. J. Biol. Chem. 278:10928-10933. [DOI] [PubMed] [Google Scholar]
- 260.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 261.Lycke, J., B. Svennerholm, E. Hjelmquist, L. Frisen, G. Badr, M. Andersson, A. Vahlne, and O. Andersen. 1996. Acyclovir treatment of relapsing-remitting multiple sclerosis. A randomized, placebo-controlled, double-blind study. J. Neurol. 243:214-224. [DOI] [PubMed] [Google Scholar]
- 262.Mackenzie, I. R., D. R. Carrigan, and C. A. Wiley. 1995. Chronic myelopathy associated with human herpesvirus-6. Neurology 45:2015-2017. [DOI] [PubMed] [Google Scholar]
- 263.MacLean, H. J., and A. G. Douen. 2002. Severe amnesia associated with human herpesvirus 6 encephalitis after bone marrow transplantation. Transplantation 73:1086-1089. [DOI] [PubMed] [Google Scholar]
- 264.Maeda, A., T. Sata, H. Enzan, K. Tanaka, H. Wakiguchi, T. Kurashige, K. Yamanishi, and T. Kurata. 1993. The evidence of human herpesvirus 6 infection in the lymph nodes of Hodgkin's disease. Virchows Arch. Pathol. Anat. Histopathol. 423:71-75. [DOI] [PubMed] [Google Scholar]
- 265.Maeda, T., T. Okuno, K. Hayashi, M. Nagata, M. Ueda, K. Terashima, T. Kawashima, H. Miyamoto, T. Mori, and Y. Yamada. 1997. Outcomes of infants whose mothers are positive for human herpesvirus-6 DNA within the genital tract in early gestation. Acta Paediatr. Jpn. 39:653-657. [DOI] [PubMed] [Google Scholar]
- 266.Maeda, Y., T. Teshima, M. Yamada, K. Shinagawa, S. Nakao, Y. Ohno, K. Kojima, M. Hara, K. Nagafuji, S. Hayashi, S. Fukuda, H. Sawada, K. Matsue, K. Takenaka, F. Ishimaru, K. Ikeda, K. Niiya, and M. Harada. 1999. Monitoring of human herpesviruses after allogeneic peripheral blood stem cell transplantation and bone marrow transplantation. Br. J. Haematol. 105:295-302. [PubMed] [Google Scholar]
- 267.Manichanh, C., P. Grenot, A. Gautheret-Dejean, P. Debre, J. M. Huraux, and H. Agut. 2000. Susceptibility of human herpesvirus 6 to antiviral compounds by flow cytometry analysis. Cytometry 40:135-140. [DOI] [PubMed] [Google Scholar]
- 268.Manichanh, C., C. Olivier-Aubron, J. P. Lagarde, J. T. Aubin, P. Bossi, A. Gautheret-Dejean, J. M. Huraux, and H. Agut. 2001. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J. Gen. Virol. 82:2767-2776. [DOI] [PubMed] [Google Scholar]
- 269.Marschall, M., M. Freitag, P. Suchy, D. Romaker, R. Kupfer, M. Hanke, and T. Stamminger. 2003. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 311:60-71. [DOI] [PubMed] [Google Scholar]
- 270.Martin, C., M. Enbom, M. Soderstrom, S. Fredrikson, H. Dahl, J. Lycke, T. Bergstrom, and A. Linde. 1997. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol. Scand. 95:280-283. [DOI] [PubMed] [Google Scholar]
- 271.Martin, M. E., J. Nicholas, B. J. Thomson, C. Newman, and R. W. Honess. 1991. Identification of a transactivating function mapping to the putative immediate-early locus of human herpesvirus 6. J. Virol. 65:5381-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Martin, M. E., B. J. Thomson, R. W. Honess, M. A. Craxton, U. A. Gompels, M. Y. Liu, E. Littler, J. R. Arrand, I. Teo, and M. D. Jones. 1991. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J. Gen. Virol. 72:157-168. [DOI] [PubMed] [Google Scholar]
- 273.Matsuda, Y., J. Hara, H. Miyoshi, Y. Osugi, H. Fujisaki, K. Takai, H. Ohta, K. Tanaka-Taya, K. Yamanishi, and S. Okada. 1999. Thrombotic microangiopathy associated with reactivation of human herpesvirus-6 following high-dose chemotherapy with autologous bone marrow transplantation in young children. Bone Marrow Transplant. 24:919-923. [DOI] [PubMed] [Google Scholar]
- 274.Matthews, T., and R. Boehme. 1988. Antiviral activity and mechanism of action of ganciclovir. Rev. Infect. Dis. 10(Suppl. 3):S490-S494. [DOI] [PubMed] [Google Scholar]
- 275.Mayne, M., C. Cheadle, S. S. Soldan, C. Cermelli, Y. Yamano, N. Akhyani, J. E. Nagel, D. D. Taub, K. G. Becker, and S. Jacobson. 2001. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J. Virol. 75:11641-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mayne, M., J. Krishnan, L. Metz, A. Nath, A. Auty, B. M. Sahai, and C. Power. 1998. Infrequent detection of human herpesvirus 6 DNA in peripheral blood mononuclear cells from multiple sclerosis patients. Ann. Neurol. 44:391-394. [DOI] [PubMed] [Google Scholar]
- 277.McCarthy, M., D. Auger, J. He, and C. Wood. 1998. Cytomegalovirus and human herpesvirus-6 trans-activate the HIV-1 long terminal repeat via multiple response regions in human fetal astrocytes. J. Neurovirol. 4:495-511. [DOI] [PubMed] [Google Scholar]
- 278.McCullers, J. A., F. D. Lakeman, and R. J. Whitley. 1995. Human herpesvirus 6 is associated with focal encephalitis. Clin. Infect. Dis. 21:571-576. [DOI] [PubMed] [Google Scholar]
- 279.McGavin, J. K., and K. L. Goa. 2001. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 61:1153-1183. [DOI] [PubMed] [Google Scholar]
- 280.Mendel, I., M. de Matteis, C. Bertin, B. Delaporte, D. Maguer, H. Collandre, and C. Buffet-Janvresse. 1995. Fulminant hepatitis in neonates with human herpesvirus 6 infection. Pediatr. Infect. Dis. J. 14:993-997. [DOI] [PubMed] [Google Scholar]
- 281.Mendez, J. C., D. H. Dockrell, M. J. Espy, T. F. Smith, J. A. Wilson, W. S. Harmsen, D. Ilstrup, and C. V. Paya. 2001. Human beta-herpesvirus interactions in solid organ transplant recipients. J. Infect. Dis. 183:179-184. [DOI] [PubMed] [Google Scholar]
- 282.Menotti, L., P. Mirandola, M. Locati, and G. Campadelli-Fiume. 1999. Trafficking to the plasma membrane of the seven-transmembrane protein encoded by human herpesvirus 6 U51 gene involves a cell-specific function present in T lymphocytes. J. Virol. 73:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Meyne, J., R. L. Ratliff, and R. K. Moyzis. 1989. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 86:7049-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Michel, D., S. Kramer, S. Hohn, P. Schaarschmidt, K. Wunderlich, and T. Mertens. 1999. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J. Virol. 73:8898-8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Milne, R. S., C. Mattick, L. Nicholson, P. Devaraj, A. Alcami, and U. A. Gompels. 2000. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J. Immunol. 164:2396-2404. [DOI] [PubMed] [Google Scholar]
- 287.Mirandola, P., P. Menegazzi, S. Merighi, T. Ravaioli, E. Cassai, and D. Di Luca. 1998. Temporal mapping of transcripts in herpesvirus 6 variants. J. Virol. 72:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mirandola, P., A. Stefan, E. Brambilla, G. Campadelli-Fiume, and L. M. Grimaldi. 1999. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology 53:1367-1368. [DOI] [PubMed] [Google Scholar]
- 289.Miyagishi, R., M. Niino, T. Fukazawa, I. Yabe, S. Kikuchi, and K. Tashiro. 2003. C-C chemokine receptor 2 gene polymorphism in Japanese patients with multiple sclerosis. J. Neuroimmunol. 145:135-138. [DOI] [PubMed] [Google Scholar]
- 290.Mock, D. J., J. M. Powers, A. D. Goodman, S. R. Blumenthal, N. Ergin, J. V. Baker, D. H. Mattson, J. G. Assouline, E. J. Bergey, B. Chen, L. G. Epstein, and B. M. Blumberg. 1999. Association of human herpesvirus 6 with the demyelinative lesions of progressive multifocal leukoencephalopathy. J. Neurovirol. 5:363-373. [DOI] [PubMed] [Google Scholar]
- 291.Montejo, M., F. J. Ramon, M. Testillano, A. Valdivieso, K. Aguirrebengoa, C. Varas, A. Olaizola, and J. O. De Urbina. 2002. Encephalitis caused by human herpesvirus-6 in a liver transplant recipient. Eur. Neurol. 48:234-235. [DOI] [PubMed] [Google Scholar]
- 292.Mookerjee, B. P., and G. Vogelsang. 1997. Human herpesvirus-6 encephalitis after bone marrow transplantation: successful treatment with ganciclovir. Bone Marrow Transplant. 20:905-906. [DOI] [PubMed] [Google Scholar]
- 293.Moore, F. J. A., and C. Wolfson. 2002. Human herpesvirus 6 and multiple sclerosis. Acta Neurol. Scand. 106:63-83. [DOI] [PubMed] [Google Scholar]
- 294.Mori, Y., P. Dhepakson, T. Shimamoto, K. Ueda, Y. Gomi, H. Tani, Y. Matsuura, and K. Yamanishi. 2000. Expression of human herpesvirus 6B rep within infected cells and binding of its gene product to the TATA-binding protein in vitro and in vivo. J. Virol. 74:6096-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mori, Y., H. Yagi, T. Shimamoto, Y. Isegawa, T. Sunagawa, R. Inagi, K. Kondo, Y. Tano, and K. Yamanishi. 1998. Analysis of human herpesvirus 6 U3 gene, which is a positional homolog of human cytomegalovirus UL 24 gene. Virology 249:129-139. [DOI] [PubMed] [Google Scholar]
- 296.Mori, Y., X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Morris, C., M. Luppi, M. McDonald, P. Barozzi, and G. Torelli. 1999. Fine mapping of an apparently targeted latent human herpesvirus type 6 integration site in chromosome band 17p13.3. J. Med. Virol. 58:69-75. [DOI] [PubMed] [Google Scholar]
- 298.Moschettini, D., A. De Milito, M. Catucci, A. Marconi, C. Rinina, M. L. Bianchi-Bandinelli, and P. E. Valensin. 1998. Detection of human herpesviruses 6 and 7 in heart transplant recipients by a multiplex polymerase chain reaction method. Eur. J. Clin. Microbiol. Infect. Dis. 17:117-119. [DOI] [PubMed] [Google Scholar]
- 299.Mossman, K. L. 2002. Activation and inhibition of virus and interferon: the herpesvirus story. Viral Immunol. 15:3-15. [DOI] [PubMed] [Google Scholar]
- 300.Mukai, T., T. Yamamoto, T. Kondo, K. Kondo, T. Okuno, H. Kosuge, and K. Yamanishi. 1994. Molecular epidemiological studies of human herpesvirus 6 in families. J. Med. Virol. 42:224-227. [DOI] [PubMed] [Google Scholar]
- 301.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 302.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nicholas, J. 1994. Nucleotide sequence analysis of a 21-kbp region of the genome of human herpesvirus-6 containing homologues of human cytomegalovirus major immediate-early and replication genes. Virology 204:738-750. [DOI] [PubMed] [Google Scholar]
- 304.Nicholas, J., and M. E. Martin. 1994. Nucleotide sequence analysis of a 38.5-kilobase-pair region of the genome of human herpesvirus 6 encoding human cytomegalovirus immediate-early gene homologs and transactivating functions. J. Virol. 68:597-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Niederman, J. C., C. R. Liu, M. H. Kaplan, and N. A. Brown. 1988. Clinical and serological features of human herpesvirus-6 infection in three adults. Lancet 2:817-819. [DOI] [PubMed] [Google Scholar]
- 306.Nielsen, L., and B. F. Vestergaard. 1996. Competitive ELISA for detection of HHV-6 antibody: seroprevalence in a danish population. J. Virol. Methods 56:221-230. [DOI] [PubMed] [Google Scholar]
- 307.Nigro, G., G. Luzi, A. Krzysztofiak, F. D'Orio, and F. Aiuti. 1995. Detection of IgM antibodies to human herpesvirus 6 in Romanian children with nonprogressive human immunodeficiency virus disease. Pediatr. Infect. Dis. J. 14:891-894. [DOI] [PubMed] [Google Scholar]
- 308.Nikolaou, K., L. Varinou, N. Inoue, and M. Arsenakis. 2003. Identification and characterization of gene products of ORF U90/89 of human herpesvirus 6. Acta Virol. 47:17-26. [PubMed] [Google Scholar]
- 309.Nishimaki, K., S. Okada, K. Miyamura, I. Ohno, Y. Ashino, T. Sugawara, T. Kondo, and T. Hattori. 2003. The possible involvement of human herpesvirus type 6 in obliterative bronchiolitis after bone marrow transplantation. Bone Marrow Transplant. 32:1103-1105. [DOI] [PubMed] [Google Scholar]
- 310.Nitsche, A., C. W. Muller, A. Radonic, O. Landt, H. Ellerbrok, G. Pauli, and W. Siegert. 2001. Human herpesvirus 6A DNA is detected frequently in plasma but rarely in peripheral blood leukocytes of patients after bone marrow transplantation. J. Infect. Dis. 183:130-133. [DOI] [PubMed] [Google Scholar]
- 311.Norton, R. A., M. T. Caserta, C. B. Hall, K. Schnabel, P. Hocknell, and S. Dewhurst. 1999. Detection of human herpesvirus 6 by reverse transcription-PCR. J. Clin. Microbiol. 37:3672-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Noseworthy, J. H., C. Lucchinetti, M. Rodriguez, and B. G. Weinshenker. 2000. Multiple sclerosis. N. Engl. J. Med. 343:938-952. [DOI] [PubMed] [Google Scholar]
- 313.Novoa, L. J., R. M. Nagra, T. Nakawatase, T. Edwards-Lee, W. W. Tourtellotte, and M. E. Cornford. 1997. Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult. J. Med. Virol. 52:301-308. [DOI] [PubMed] [Google Scholar]
- 314.Ogawa-Goto, K., K. Tanaka, W. Gibson, E. Moriishi, Y. Miura, T. Kurata, S. Irie, and T. Sata. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 77:8541-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Okuno, T., H. Oishi, K. Hayashi, M. Nonogaki, K. Tanaka, and K. Yamanishi. 1995. Human herpesviruses 6 and 7 in cervixes of pregnant women. J. Clin. Microbiol. 33:1968-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Okuno, T., K. Takahashi, K. Balachandra, K. Shiraki, K. Yamanishi, M. Takahashi, and K. Baba. 1989. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J. Clin. Microbiol. 27:651-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Osiowy, C., I. Prud'homme, M. Monette, and S. Zou. 1998. Detection of human herpesvirus 6 DNA in serum by a microplate PCR-hybridization assay. J. Clin. Microbiol. 36:68-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Oster, B., and P. Hollsberg. 2002. Viral gene expression patterns in human herpesvirus 6B-infected T cells. J. Virol. 76:7578-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ozaki, Y., H. Tajiri, K. Tanaka-Taya, S. Mushiake, A. Kimoto, K. Yamanishi, and S. Okada. 2001. Frequent detection of the human herpesvirus 6-specific genomes in the livers of children with various liver diseases. J. Clin. Microbiol. 39:2173-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Pacsa, A. S., S. Essa, A. Voevodin, A. el Shazly, H. Kazak, M. R. Nampoory, K. V. Johny, T. Said, and W. Al Nakib. 2003. Correlation between CMV genotypes, multiple infections with herpesviruses (HHV-6, 7) and development of CMV disease in kidney recipients in Kuwait. FEMS Immunol. Med. Microbiol. 35:125-130. [DOI] [PubMed] [Google Scholar]
- 321.Papanikolaou, E., V. Kouvatsis, G. Dimitriadis, N. Inoue, and M. Arsenakis. 2002. Identification and characterization of the gene products of open reading frame U86/87 of human herpesvirus 6. Virus Res. 89:89-101. [DOI] [PubMed] [Google Scholar]
- 322.Parker, C. A., and J. M. Weber. 1993. An enzyme-linked immunosorbent assay for the detection of IgG and IgM antibodies to human herpesvirus type 6. J. Virol. Methods 41:265-275. [DOI] [PubMed] [Google Scholar]
- 323.Paterson, D. L., N. Singh, T. Gayowski, D. R. Carrigan, and I. R. Marino. 1999. Encephalopathy associated with human herpesvirus 6 in a liver transplant recipient. Liver Transplant. Surg. 5:454-455. [DOI] [PubMed] [Google Scholar]
- 324.Pellett, P. E., G. J. Lindquester, P. Feorino, and C. Lopez. 1990. Genomic heterogeneity of human herpesvirus 6 isolates. Adv. Exp. Med. Biol. 278:9-18. [DOI] [PubMed] [Google Scholar]
- 325.Pfeiffer, B., Z. N. Berneman, F. Neipel, C. K. Chang, S. Tirwatnapong, and B. Chandran. 1993. Identification and mapping of the gene encoding the glycoprotein complex gp82-gp105 of human herpesvirus 6 and mapping of the neutralizing epitope recognized by monoclonal antibodies. J. Virol. 67:4611-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pfeiffer, B., B. Thomson, and B. Chandran. 1995. Identification and characterization of a cDNA derived from multiple splicing that encodes envelope glycoprotein gp105 of human herpesvirus 6. J. Virol. 69:3490-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Portolani, M., C. Cermelli, M. Meacci, P. Pietrosemoli, M. Pecorari, A. M. Sabbatini, S. Leoni, A. Guerra, and G. Bonaccorsi. 1997. Primary infection by HHV-6 variant B associated with a fatal case of hemophagocytic syndrome. New Microbiol. 20:7-11. [PubMed] [Google Scholar]
- 328.Portolani, M., C. Cermelli, A. Moroni, M. F. Bertolani, D. Di Luca, E. Cassai, and A. M. Sabbatini. 1993. Human herpesvirus-6 infections in infants admitted to hospital. J. Med. Virol. 39:146-151. [DOI] [PubMed] [Google Scholar]
- 329.Portolani, M., M. Pecorari, M. G. Tamassia, W. Gennari, F. Beretti, and G. Guaraldi. 2001. Case of fatal encephalitis by HHV-6 variant A. J. Med. Virol. 65:133-137. [PubMed] [Google Scholar]
- 330.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pruksananonda, P., C. B. Hall, R. A. Insel, K. McIntyre, P. E. Pellett, C. E. Long, K. C. Schnabel, P. H. Pincus, F. R. Stamey, and T. R. Dambaugh. 1992. Primary human herpesvirus 6 infection in young children. N. Engl. J. Med. 326:1445-1450. [DOI] [PubMed] [Google Scholar]
- 332.Qavi, H. B., M. T. Green, D. E. Lewis, F. B. Hollinger, G. Pearson, and D. V. Ablashi. 1995. HIV-1 and HHV-6 antigens and transcripts in retinas of patients with AIDS in the absence of human cytomegalovirus. Investig. Ophthalmol. Vis. Sci. 36:2040-2047. [PubMed] [Google Scholar]
- 333.Rapaport, D., D. Engelhard, G. Tagger, R. Or, and N. Frenkel. 2002. Antiviral prophylaxis may prevent human herpesvirus-6 reactivation in bone marrow transplant recipients. Transplant. Infect. Dis. 4:10-16. [DOI] [PubMed] [Google Scholar]
- 334.Rapp, J. C., L. T. Krug, N. Inoue, T. R. Dambaugh, and P. E. Pellett. 2000. U94, the human herpesvirus 6 homolog of the parvovirus nonstructural gene, is highly conserved among isolates and is expressed at low mRNA levels as a spliced transcript. Virology 268:504-516. [DOI] [PubMed] [Google Scholar]
- 335.Ratnamohan, V. M., J. Chapman, H. Howse, K. Bovington, P. Robertson, K. Byth, R. Allen, and A. L. Cunningham. 1998. Cytomegalovirus and human herpesvirus 6 both cause viral disease after renal transplantation. Transplantation 66:877-882. [DOI] [PubMed] [Google Scholar]
- 336.Reardon, J. E., and T. Spector. 1989. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 264:7405-7411. [PubMed] [Google Scholar]
- 337.Reymen, D., L. Naesens, J. Balzarini, A. Holy, H. Dvorakova, and E. De Clercq. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antivir. Res. 28:343-357. [DOI] [PubMed] [Google Scholar]
- 338.Rieux, C., A. Gautheret-Dejean, D. Challine-Lehmann, C. Kirch, H. Agut, and J. P. Vernant. 1998. Human herpesvirus-6 meningoencephalitis in a recipient of an unrelated allogeneic bone marrow transplantation. Transplantation 65:1408-1411. [DOI] [PubMed] [Google Scholar]
- 339.Roffman, E., and N. Frenkel. 1990. Interleukin-2 inhibits the replication of human herpesvirus-6 in mature thymocytes. Virology 175:591-594. [DOI] [PubMed] [Google Scholar]
- 340.Rogers, J., S. Rohal, D. R. Carrigan, S. Kusne, K. K. Knox, T. Gayowski, M. M. Wagener, J. J. Fung, and N. Singh. 2000. Human herpesvirus-6 in liver transplant recipients: role in pathogenesis of fungal infections, neurologic complications, and outcome. Transplantation 69:2566-2573. [DOI] [PubMed] [Google Scholar]
- 341.Rojo, J., A. V. Ferrer, U. Klueppelberg, G. R. Krueger, E. Eidt, D. V. Ablashi, J. Luka, and H. Tesch. 1994. Semi-quantitative in situ hybridization and immunohistology for antigen expression of human herpesvirus-6 in various lymphoproliferative diseases. In Vivo 8:517-526. [PubMed] [Google Scholar]
- 342.Romano, N., F. M. Romano, E. Viviano, F. Vitale, M. R. Villafrate, A. M. Perna, F. Bonura, and R. Guttadauro. 1996. Rare association of human herpesvirus 6 DNA with human papillomavirus DNA in cervical smears of women with normal and abnormal cytologies. J. Clin. Microbiol. 34:1589-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rosenfeld, C. S., W. B. Rybka, D. Weinbaum, D. R. Carrigan, K. K. Knox, D. F. Andrews, and R. K. Shadduck. 1995. Late graft failure due to dual bone marrow infection with variants A and B of human herpesvirus-6. Exp. Hematol. 23:626-629. [PubMed] [Google Scholar]
- 344.Rossi, C., M. L. Delforge, F. Jacobs, M. Wissing, O. Pradier, M. Remmelink, B. Byl, J. P. Thys, and C. Liesnard. 2001. Fatal primary infection due to human herpesvirus 6 variant A in a renal transplant recipient. Transplantation 71:288-292. [DOI] [PubMed] [Google Scholar]
- 345.Rotola, A., E. Cassai, M. R. Tola, E. Granieri, and D. Di Luca. 1999. Human herpesvirus 6 is latent in peripheral blood of patients with relapsing-remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 67:529-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rotola, A., T. Ravaioli, A. Gonelli, S. Dewhurst, E. Cassai, and D. Di Luca. 1998. U94 of human herpesvirus 6 is expressed in latently infected peripheral blood mononuclear cells and blocks viral gene expression in transformed lymphocytes in culture. Proc. Natl. Acad. Sci. USA 95:13911-13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Roush, K. S., R. K. Domiati-Saad, L. R. Margraf, K. Krisher, R. H. Scheuermann, B. B. Rogers, and D. B. Dawson. 2001. Prevalence and cellular reservoir of latent human herpesvirus 6 in tonsillar lymphoid tissue. Am. J. Clin. Pathol. 116:648-654. [DOI] [PubMed] [Google Scholar]
- 348.Safronetz, D., M. Petric, R. Tellier, B. Parvez, and G. A. Tipples. 2003. Mapping ganciclovir resistance in the human herpesvirus-6 U69 protein kinase. J. Med. Virol. 71:434-439. [DOI] [PubMed] [Google Scholar]
- 349.Saijo, M., H. Saijo, M. Yamamoto, M. Takimoto, H. Fujiyasu, K. Murono, and K. Fujita. 1995. Thrombocytopenic purpura associated with primary human herpesvirus 6 infection. Pediatr. Infect. Dis. J. 14:405. [DOI] [PubMed] [Google Scholar]
- 350.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, and B. Kramarsky. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 351.Santoro, F., H. L. Greenstone, A. Insinga, M. K. Liszewski, J. P. Atkinson, P. Lusso, and E. A. Berger. 2003. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 278:25964-25969. [DOI] [PubMed] [Google Scholar]
- 352.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 353.Sashihara, J., K. Tanaka-Taya, S. Tanaka, K. Amo, H. Miyagawa, G. Hosoi, T. Taniguchi, T. Fukui, N. Kasuga, T. Aono, M. Sako, J. Hara, K. Yamanishi, and S. Okada. 2002. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood 100:2005-2011. [PubMed] [Google Scholar]
- 354.Saxinger, C., H. Polesky, N. Eby, S. Grufferman, R. Murphy, G. Tegtmeir, V. Parekh, S. Memon, and C. Hung. 1988. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J. Virol. Methods 21:199-208. [DOI] [PubMed] [Google Scholar]
- 355.Schiewe, U., F. Neipel, D. Schreiner, and B. Fleckenstein. 1994. Structure and transcription of an immediate-early region in the human herpesvirus 6 genome. J. Virol. 68:2978-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Schirmer, E. C., L. S. Wyatt, K. Yamanishi, W. J. Rodriguez, and N. Frenkel. 1991. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc. Natl. Acad. Sci. USA 88:5922-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Schmidt, C. A., F. Wilbron, K. Weiss, V. Brinkmann, H. Oettle, R. Lohmann, J. M. Langrehr, P. Neuhaus, and W. Siegert. 1996. A prospective study of human herpesvirus type 6 detected by polymerase chain reaction after liver transplantation. Transplantation 61:662-664. [DOI] [PubMed] [Google Scholar]
- 358.Sever, J. L., T. A. Rakusan, M. Ellaurie, N. Frenkel, L. S. Wyatt, J. M. Campos, R. M. O'Donnell, and M. V. Price. 1995. Coinfection with herpesviruses in young children of HIV-infected women. Pediatr. AIDS HIV Infect. 6:75-82. [PubMed] [Google Scholar]
- 359.Sieczkowski, L., B. Chandran, and C. Wood. 1995. The human immunodeficiency virus tat gene enhances replication of human herpesvirus-6. Virology 211:544-553. [DOI] [PubMed] [Google Scholar]
- 360.Singh, N. 2001. Preemptive therapy versus universal prophylaxis with ganciclovir for cytomegalovirus in solid organ transplant recipients. Clin. Infect. Dis. 32:742-751. [DOI] [PubMed] [Google Scholar]
- 361.Singh, N., C. Bentlejewski, D. R. Carrigan, T. Gayowski, K. K. Knox, and A. Zeevi. 2002. Persistent lack of human herpesvirus-6 specific T-helper cell response in liver transplant recipients. Transplant. Infect. Dis. 4:59-63. [DOI] [PubMed] [Google Scholar]
- 362.Singh, N., D. R. Carrigan, T. Gayowski, and I. R. Marino. 1997. Human herpesvirus-6 infection in liver transplant recipients: documentation of pathogenicity. Transplantation 64:674-678. [DOI] [PubMed] [Google Scholar]
- 363.Singh, N., D. R. Carrigan, T. Gayowski, J. Singh, and I. R. Marino. 1995. Variant B human herpesvirus-6 associated febrile dermatosis with thrombocytopenia and encephalopathy in a liver transplant recipient. Transplantation 60:1355-1357. [PubMed] [Google Scholar]
- 364.Singh, N., S. Husain, D. R. Carrigan, K. K. Knox, K. E. Weck, M. M. Wagener, and T. Gayowski. 2002. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin. Transplant. 16:92-96. [DOI] [PubMed] [Google Scholar]
- 365.Smith, A., F. Santoro, G. Di Lullo, L. Dagna, A. Verani, and P. Lusso. 2003. Selective suppression of IL-12 production by human herpesvirus 6. Blood 102:2877-2884. [DOI] [PubMed] [Google Scholar]
- 366.Smith, I. L., J. M. Cherrington, R. E. Jiles, M. D. Fuller, W. R. Freeman, and S. A. Spector. 1997. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 176:69-77. [DOI] [PubMed] [Google Scholar]
- 367.Sobue, R., H. Miyazaki, M. Okamoto, M. Hirano, T. Yoshikawa, S. Suga, and Y. Asano. 1991. Fulminant hepatitis in primary human herpesvirus-6 infection. N. Engl. J. Med. 324:1290. [DOI] [PubMed] [Google Scholar]
- 368.Soldan, S. S., R. Berti, N. Salem, P. Secchiero, L. Flamand, P. A. Calabresi, M. B. Brennan, H. W. Maloni, H. F. McFarland, H. C. Lin, M. Patnaik, and S. Jacobson. 1997. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 3:1394-1397. [DOI] [PubMed] [Google Scholar]
- 369.Soldan, S. S., A. Fogdell-Hahn, M. B. Brennan, B. B. Mittleman, C. Ballerini, L. Massacesi, T. Seya, H. F. McFarland, and S. Jacobson. 2001. Elevated serum and cerebrospinal fluid levels of soluble human herpesvirus type 6 cellular receptor, membrane cofactor protein, in patients with multiple sclerosis. Ann. Neurol. 50:486-493. [DOI] [PubMed] [Google Scholar]
- 370.Soldan, S. S., T. P. Leist, K. N. Juhng, H. F. McFarland, and S. Jacobson. 2000. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann. Neurol. 47:306-313. [PubMed] [Google Scholar]
- 371.Spector, S. A., D. F. Busch, S. Follansbee, K. Squires, J. P. Lalezari, M. A. Jacobson, J. D. Connor, D. Jung, A. Shadman, B. Mastre W. Buhles, W. L. Drew, et al. 1995. Pharmacokinetic, safety, and antiviral profiles of oral ganciclovir in persons infected with human immunodeficiency virus: a phase I/II study. J. Infect. Dis. 171:1431-1437. [DOI] [PubMed] [Google Scholar]
- 372.Speir, E., Z. X. Yu, and V. J. Ferrans. 1998. Infectious agents in coronary artery disease: viral infection, aspirin, and gene expression in human coronary smooth muscle cells. Rev. Port. Cardiol. 17(Suppl. 2):II.33-II.39. [PubMed] [Google Scholar]
- 373.Spira, T. J., L. H. Bozeman, K. C. Sanderlin, D. T. Warfield, P. M. Feorino, R. C. Holman, J. E. Kaplan, D. B. Fishbein, and C. Lopez. 1990. Lack of correlation between human herpesvirus-6 infection and the course of human immunodeficiency virus infection. J. Infect. Dis. 161:567-570. [DOI] [PubMed] [Google Scholar]
- 374.Stanton, R., G. W. Wilkinson, and J. D. Fox. 2003. Analysis of human herpesvirus-6 IE1 sequence variation in clinical samples. J. Med. Virol. 71:578-584. [DOI] [PubMed] [Google Scholar]
- 375.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J. Virol. 66:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Steeper, T. A., C. A. Horwitz, D. V. Ablashi, S. Z. Salahuddin, C. Saxinger, R. Saltzman, and B. Schwartz. 1990. The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am. J. Clin. Pathol. 93:776-783. [DOI] [PubMed] [Google Scholar]
- 377.Suga, S., T. Yazaki, Y. Kajita, T. Ozaki, and Y. Asano. 1995. Detection of human herpesvirus 6 DNAs in samples from several body sites of patients with exanthem subitum and their mothers by polymerase chain reaction assay. J. Med. Virol. 46:52-55. [DOI] [PubMed] [Google Scholar]
- 378.Suga, S., T. Yoshikawa, Y. Asano, T. Kozawa, T. Nakashima, I. Kobayashi, T. Yazaki, H. Yamamoto, Y. Kajita, and T. Ozaki. 1993. Clinical and virological analyses of 21 infants with exanthem subitum (roseola infantum) and central nervous system complications. Ann. Neurol. 33:597-603. [DOI] [PubMed] [Google Scholar]
- 379.Suga, S., T. Yoshikawa, Y. Kajita, T. Ozaki, and Y. Asano. 1998. Prospective study of persistence and excretion of human herpesvirus-6 in patients with exanthem subitum and their parents. Pediatrics 102:900-904. [DOI] [PubMed] [Google Scholar]
- 380.Sugita, K., H. Kurumada, M. Eguchi, and T. Furukawa. 1995. Human herpesvirus 6 infection associated with hemophagocytic syndrome. Acta Haematol. 93:108-109. [DOI] [PubMed] [Google Scholar]
- 381.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 382.Sumiyoshi, Y., M. Kikuchi, K. Ohshima, M. Takeshita, Y. Eizuru, and Y. Minamishima. 1995. A case of human herpesvirus-6 lymphadenitis with infectious mononucleosis-like syndrome. Pathol. Int. 45:947-951. [DOI] [PubMed] [Google Scholar]
- 383.Surosky, R. T., M. Urabe, S. G. Godwin, S. A. McQuiston, G. J. Kurtzman, K. Ozawa, and G. Natsoulis. 1997. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J. Virol. 71:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sutherland, S., G. Christofinis, J. O'Grady, and R. Williams. 1991. A serological investigation of human herpesvirus 6 infections in liver transplant recipients and the detection of cross-reacting antibodies to cytomegalovirus. J. Med. Virol. 33:172-176. [DOI] [PubMed] [Google Scholar]
- 385.Tajiri, H., O. Nose, K. Baba, and S. Okada. 1990. Human herpesvirus-6 infection with liver injury in neonatal hepatitis. Lancet 335:863. [DOI] [PubMed] [Google Scholar]
- 386.Tajiri, H., K. Tanaka-Taya, Y. Ozaki, S. Okada, S. Mushiake, and K. Yamanishi. 1997. Chronic hepatitis in an infant, in association with human herpesvirus-6 infection. J. Pediatr. 131:473-475. [DOI] [PubMed] [Google Scholar]
- 387.Takagi, M., A. Unno, T. Maruyama, K. Kaneko, and K. Obinata. 1996. Human herpesvirus-6 (HHV-6)-associated hemophagocytic syndrome. Pediatr. Hematol. Oncol. 13:451-456. [DOI] [PubMed] [Google Scholar]
- 388.Takahashi, K., S. Sonoda, K. Higashi, T. Kondo, H. Takahashi, M. Takahashi, and K. Yamanishi. 1989. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 63:3161-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Takatsuka, H., T. Wakae, A. Mori, M. Okada, Y. Fujimori, Y. Takemoto, T. Okamoto, A. Kanamaru, and E. Kakishita. 2003. Endothelial damage caused by cytomegalovirus and human herpesvirus-6. Bone Marrow Transplant. 31:475-479. [DOI] [PubMed] [Google Scholar]
- 390.Takeda, K., T. Okuno, Y. Isegawa, and K. Yamanishi. 1996. Identification of a variant A-specific neutralizing epitope on glycoprotein B (gB) of human herpesvirus-6 (HHV-6). Virology 222:176-183. [DOI] [PubMed] [Google Scholar]
- 391.Takemoto, M., T. Shimamoto, Y. Isegawa, and K. Yamanishi. 2001. The R3 region, one of three major repetitive regions of human herpesvirus 6, is a strong enhancer of immediate-early gene U95. J. Virol. 75:10149-10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Takikawa, T., H. Hayashibara, Y. Harada, and K. Shiraki. 1992. Liver dysfunction, anaemia, and granulocytopenia after exanthema subitum. Lancet 340:1288-1289. [DOI] [PubMed] [Google Scholar]
- 393.Talarico, C. L., T. C. Burnette, W. H. Miller, S. L. Smith, M. G. Davis, S. C. Stanat, T. I. Ng, Z. He, D. M. Coen, B. Roizman, and K. K. Biron. 1999. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob. Agents Chemother. 43:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Tanaka, N., H. Kimura, Y. Hoshino, K. Kato, T. Yoshikawa, Y. Asano, K. Horibe, S. Kojima, and T. Morishima. 2000. Monitoring four herpesviruses in unrelated cord blood transplantation. Bone Marrow Transplant. 26:1193-1197. [DOI] [PubMed] [Google Scholar]
- 395.Tanaka-Taya, K., T. Kondo, T. Mukai, H. Miyoshi, Y. Yamamoto, S. Okada, and K. Yamanishi. 1996. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J. Med. Virol. 48:88-94. [DOI] [PubMed] [Google Scholar]
- 396.Taniguchi, T., T. Shimamoto, Y. Isegawa, K. Kondo, and K. Yamanishi. 2000. Structure of transcripts and proteins encoded by U79-80 of human herpesvirus 6 and its subcellular localization in infected cells. Virology 271:307-320. [DOI] [PubMed] [Google Scholar]
- 397.Tejada-Simon, M. V., Y. C. Zang, J. Hong, V. M. Rivera, J. M. Killian, and J. Z. Zhang. 2002. Detection of viral DNA and immune responses to the human herpesvirus 6 101-kilodalton virion protein in patients with multiple sclerosis and in controls. J. Virol. 76:6147-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Tejada-Simon, M. V., Y. C. Zang, J. Hong, V. M. Rivera, and J. Z. Zhang. 2003. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol. 53:189-197. [DOI] [PubMed] [Google Scholar]
- 399.Teo, I. A., B. E. Griffin, and M. D. Jones. 1991. Characterization of the DNA polymerase gene of human herpesvirus 6. J. Virol. 65:4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Thompson, J., S. Choudhury, F. Kashanchi, J. Doniger, Z. Berneman, N. Frenkel, and L. J. Rosenthal. 1994. A transforming fragment within the direct repeat region of human herpesvirus type 6 that transactivates HIV-1. Oncogene 9:1167-1175. [PubMed] [Google Scholar]
- 401.Thompson, J. R., A. D. Agulnick, and R. P. Ricciardi. 1994. A novel cis element essential for stimulated transcription of the p41 promoter of human herpesvirus 6. J. Virol. 68:4478-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Thomsen, D. R., N. L. Oien, T. A. Hopkins, M. L. Knechtel, R. J. Brideau, M. W. Wathen, and F. L. Homa. 2003. Amino acid changes within conserved region III of the herpes simplex virus and human cytomegalovirus DNA polymerases confer resistance to 4-oxo-dihydroquinolines, a novel class of herpesvirus antiviral agents. J. Virol. 77:1868-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Thomson, B. J., S. Dewhurst, and D. Gray. 1994. Structure and heterogeneity of the a sequences of human herpesvirus 6 strain variants U1102 and Z29 and identification of human telomeric repeat sequences at the genomic termini. J. Virol. 68:3007-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Thomson, B. J., S. Efstathiou, and R. W. Honess. 1991. Acquisition of the human adeno-associated virus type-2 rep gene by human herpesvirus type-6. Nature 351:78-80. [DOI] [PubMed] [Google Scholar]
- 405.Thomson, B. J., and R. W. Honess. 1992. The right end of the unique region of the genome of human herpesvirus 6 U1102 contains a candidate immediate early gene enhancer and a homologue of the human cytomegalovirus US22 gene family. J. Gen. Virol. 73:1649-1660. [DOI] [PubMed] [Google Scholar]
- 406.Thomson, B. J., F. W. Weindler, D. Gray, V. Schwaab, and R. Heilbronn. 1994. Human herpesvirus 6 (HHV-6) is a helper virus for adeno-associated virus type 2 (AAV-2) and the AAV-2 rep gene homologue in HHV-6 can mediate AAV-2 DNA replication and regulate gene expression. Virology 204:304-311. [DOI] [PubMed] [Google Scholar]
- 407.Tiacci, E., M. Luppi, P. Barozzi, G. Gurdo, A. Tabilio, S. Ballanti, G. Torelli, and F. Aversa. 2000. Fatal herpesvirus-6 encephalitis in a recipient of a T-cell-depleted peripheral blood stem cell transplant from a 3-loci mismatched related donor. Haematologica 85:94-97. [PubMed] [Google Scholar]
- 408.Tokimasa, S., J. Hara, Y. Osugi, H. Ohta, Y. Matsuda, H. Fujisaki, A. Sawada, J. Y. Kim, J. Sashihara, K. Amou, H. Miyagawa, K. Tanaka-Taya, K. Yamanishi, and S. Okada. 2002. Ganciclovir is effective for prophylaxis and treatment of human herpesvirus-6 in allogeneic stem cell transplantation. Bone Marrow Transplant. 29:595-598. [DOI] [PubMed] [Google Scholar]
- 409.Tolfvenstam, T., M. Enbom, H. Ghebrekidan, U. Ruden, A. Linde, M. Grandien, and B. Wahren. 2000. Seroprevalence of viral childhood infections in Eritrea. J. Clin. Virol. 16:49-54. [DOI] [PubMed] [Google Scholar]
- 410.Torre, D., F. Speranza, R. Martegani, P. Ferrante, E. Omodeo-Zorini, R. Mancuso, and G. P. Fiori. 1998. Meningoencephalitis caused by human herpesvirus-6 in an immunocompetent adult patient: case report and review of the literature. Infection 26:402-404. [DOI] [PubMed] [Google Scholar]
- 411.Torrisi, M. R., M. Gentile, G. Cardinali, M. Cirone, C. Zompetta, L. V. Lotti, L. Frati, and A. Faggioni. 1999. Intracellular transport and maturation pathway of human herpesvirus 6. Virology 257:460-471. [DOI] [PubMed] [Google Scholar]
- 412.Townsend, L. B., R. V. Devivar, S. R. Turk, M. R. Nassiri, and J. C. Drach. 1995. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-d-ribofuranosyl)benzimidazoles. J. Med. Chem. 38:4098-4105. [DOI] [PubMed] [Google Scholar]
- 413.Tran-Thanh, D., A. Koushik, D. Provencher, P. Drouin, J. Dubuc-Lissoir, P. Gauthier, G. Allaire, J. Kornegay, E. Franco, and F. Coutlee. 2002. Detection of human herpes virus type 6 DNA in precancerous lesions of the uterine cervix. J. Med. Virol. 68:606-610. [DOI] [PubMed] [Google Scholar]
- 414.Turner, S., D. DiLuca, and U. Gompels. 2002. Characterisation of a human herpesvirus 6 variant A “amplicon” and replication modulation by U94-Rep “latency gene.” J. Virol. Methods 105:331-341. [DOI] [PubMed] [Google Scholar]
- 415.Valente, G., P. Secchiero, P. Lusso, M. C. Abete, C. Jemma, G. Reato, S. Kerim, R. C. Gallo, and G. Palestro. 1996. Human herpesvirus 6 and Epstein-Barr virus in Hodgkin's disease: a controlled study by polymerase chain reaction and in situ hybridization. Am. J. Pathol. 149:1501-1510. [PMC free article] [PubMed] [Google Scholar]
- 416.Van den Bosch, G., G. Locatelli, L. Geerts, G. Faga, M. Ieven, H. Goossens, D. Bottiger, B. Oberg, P. Lusso, and Z. N. Berneman. 2001. Development of reverse transcriptase PCR assays for detection of active human herpesvirus 6 infection. J. Clin. Microbiol. 39:2308-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.van Loon, N. M., S. Gummuluru, D. J. Sherwood, R. Marentes, C. B. Hall, and S. Dewhurst. 1995. Direct sequence analysis of human herpesvirus 6 (HHV-6) sequences from infants and comparison of HHV-6 sequences from mother/infant pairs. Clin. Infect. Dis. 21:1017-1019. [DOI] [PubMed] [Google Scholar]
- 418.Vignoli, M., G. Furlini, M. C. Re, E. Ramazzotti, and M. La Placa. 2000. Modulation of CD4, CXCR-4, and CCR-5 makes human hematopoietic progenitor cell lines infected with human herpesvirus-6 susceptible to human immunodeficiency virus type 1. J. Hematother. Stem Cell Res. 9:39-45. [DOI] [PubMed] [Google Scholar]
- 419.Vossen, M. T., E. M. Westerhout, C. Soderberg-Naucler, and E. J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics 54:527-542. [DOI] [PubMed] [Google Scholar]
- 420.Wagstaff, A. J., and H. M. Bryson. 1994. Foscarnet: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs 48:199-226. [DOI] [PubMed] [Google Scholar]
- 421.Wang, F. Z., H. Dahl, A. Linde, M. Brytting, A. Ehrnst, and P. Ljungman. 1996. Lymphotropic herpesviruses in allogeneic bone marrow transplantation. Blood 88:3615-3620. [PubMed] [Google Scholar]
- 422.Wang, F. Z., H. Dahl, P. Ljungman, and A. Linde. 1999. Lymphoproliferative responses to human herpesvirus-6 variant A and variant B in healthy adults. J. Med. Virol. 57:134-139. [PubMed] [Google Scholar]
- 423.Wang, F. Z., A. Linde, H. Dahl, and P. Ljungman. 1999. Human herpesvirus 6 infection inhibits specific lymphocyte proliferation responses and is related to lymphocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 24:1201-1206. [DOI] [PubMed] [Google Scholar]
- 424.Wang, F. Z., A. Linde, H. Hagglund, M. Testa, A. Locasciulli, and P. Ljungman. 1999. Human herpesvirus 6 DNA in cerebrospinal fluid specimens from allogeneic bone marrow transplant patients: does it have clinical significance? Clin. Infect. Dis. 28:562-568. [DOI] [PubMed] [Google Scholar]
- 425.Wang, L. H., R. W. Peck, Y. Yin, J. Allanson, R. Wiggs, and M. B. Wire. 2003. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ward, K. N., and J. J. Gray. 1994. Primary human herpesvirus-6 infection is frequently overlooked as a cause of febrile fits in young children. J. Med. Virol. 42:119-123. [DOI] [PubMed] [Google Scholar]
- 427.Ward, K. N., J. J. Gray, and S. Efstathiou. 1989. Brief report: primary human herpesvirus 6 infection in a patient following liver transplantation from a seropositive donor. J. Med. Virol. 28:69-72. [DOI] [PubMed] [Google Scholar]
- 428.Wilborn, F., V. Brinkmann, C. A. Schmidt, F. Neipel, H. Gelderblom, and W. Siegert. 1994. Herpesvirus type 6 in patients undergoing bone marrow transplantation: serologic features and detection by polymerase chain reaction. Blood 83:3052-3058. [PubMed] [Google Scholar]
- 429.Wilborn, F., C. A. Schmidt, V. Brinkmann, K. Jendroska, H. Oettle, and W. Siegert. 1994. A potential role for human herpesvirus type 6 in nervous system disease. J. Neuroimmunol. 49:213-214. [DOI] [PubMed] [Google Scholar]
- 430.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68:3943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Wu, Z., G. Mu, and L. Wang. 1997. Seroprevalence of human herpesvirus-6 in healthy population in two provinces of north China. Chin. Med. Sci. J. 12:111-114. [PubMed] [Google Scholar]
- 433.Xiong, X., J. L. Smith, C. Kim, E. S. Huang, and M. S. Chen. 1996. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem. Pharmacol. 51:1563-1567. [DOI] [PubMed] [Google Scholar]
- 434.Yadav, M., M. Arivananthan, A. Chandrashekran, B. S. Tan, and B. Y. Hashim. 1997. Human herpesvirus-6 (HHV-6) DNA and virus-encoded antigen in oral lesions. J. Oral Pathol. Med. 26:393-401. [DOI] [PubMed] [Google Scholar]
- 435.Yadav, M., M. Arivananthan, and S. Kumar. 1996. HHV-6 antigen and viral DNA detected in cervical cells from archived tissue using histochemical staining and hybridization. Clin. Diagn. Virol. 7:23-33. [DOI] [PubMed] [Google Scholar]
- 436.Yalcin, S., T. Mukai, K. Kondo, Y. Ami, T. Okawa, A. Kojima, T. Kurata, and K. Yamanishi. 1992. Experimental infection of cynomolgus and African green monkeys with human herpesvirus 6. J. Gen. Virol. 73:1673-1677. [DOI] [PubMed] [Google Scholar]
- 437.Yamamoto, T., T. Mukai, K. Kondo, and K. Yamanishi. 1994. Variation of DNA sequence in immediate-early gene of human herpesvirus 6 and variant identification by PCR. J. Clin. Microbiol. 32:473-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed] [Google Scholar]
- 439.Yasukawa, M., A. Hasegawa, I. Sakai, H. Ohminami, J. Arai, S. Kaneko, Y. Yakushijin, K. Maeyama, H. Nakashima, R. Arakaki, and S. Fujita. 1999. Down-regulation of CXCR4 by human herpesvirus 6 (HHV-6) and HHV-7. J. Immunol. 162:5417-5422. [PubMed] [Google Scholar]
- 440.Yasukawa, M., Y. Inoue, H. Ohminami, K. Terada, and S. Fujita. 1998. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J. Gen. Virol. 79:143-147. [DOI] [PubMed] [Google Scholar]
- 441.Yoshida, H., K. Matsunaga, T. Ueda, M. Yasumi, J. Ishikawa, Y. Tomiyama, and Y. Matsuzawa. 2002. Human herpesvirus 6 meningoencephalitis successfully treated with ganciclovir in a patient who underwent allogeneic bone marrow transplantation from an HLA-identical sibling. Int. J. Hematol. 75:421-425. [DOI] [PubMed] [Google Scholar]
- 442.Yoshida, M., F. Uno, Z. L. Bai, M. Yamada, S. Nii, T. Sata, T. Kurata, K. Yamanishi, and M. Takahashi. 1989. Electron microscopic study of a herpes-type virus isolated from an infant with exanthem subitum. Microbiol. Immunol. 33:147-154. [DOI] [PubMed] [Google Scholar]
- 443.Yoshida, M., M. Yamada, T. Tsukazaki, S. Chatterjee, F. D. Lakeman, S. Nii, and R. J. Whitley. 1998. Comparison of antiviral compounds against human herpesvirus 6 and 7. Antivir. Res. 40:73-84. [DOI] [PubMed] [Google Scholar]
- 444.Yoshikawa, T., S. Akimoto, N. Nishimura, T. Ozaki, M. Ihira, M. Ohashi, M. Morooka, S. Suga, Y. Asano, M. Takemoto, and Y. Nishiyama. 2003. Evaluation of active human herpesvirus 6 infection by reverse transcription-PCR. J. Med. Virol. 70:267-272. [DOI] [PubMed] [Google Scholar]
- 445.Yoshikawa, T., and Y. Asano. 2000. Central nervous system complications in human herpesvirus-6 infection. Brain Dev. 22:307-314. [DOI] [PubMed] [Google Scholar]
- 446.Yoshikawa, T., Y. Asano, S. Akimoto, T. Ozaki, T. Iwasaki, T. Kurata, F. Goshima, and Y. Nishiyama. 2002. Latent infection of human herpesvirus 6 in astrocytoma cell line and alteration of cytokine synthesis. J. Med. Virol. 66:497-505. [PubMed] [Google Scholar]
- 447.Yoshikawa, T., Y. Asano, M. Ihira, K. Suzuki, M. Ohashi, S. Suga, K. Kudo, K. Horibe, S. Kojima, K. Kato, T. Matsuyama, and Y. Nishiyama. 2002. Human herpesvirus 6 viremia in bone marrow transplant recipients: clinical features and risk factors. J. Infect. Dis. 185:847-853. [DOI] [PubMed] [Google Scholar]
- 448.Yoshikawa, T., Y. Asano, I. Kobayashi, T. Nakashima, and T. Yazaki. 1993. Exacerbation of idiopathic thrombocytopenic purpura by primary human herpesvirus 6 infection. Pediatr. Infect. Dis. J. 12:409-410. [DOI] [PubMed] [Google Scholar]
- 449.Yoshikawa, T., M. Ihira, H. Furukawa, S. Suga, K. Asonuma, K. Tanaka, and Y. Asano. 1998. Four cases of human herpesvirus 6 variant B infection after pediatric liver transplantation. Transplantation 65:1266-1269. [DOI] [PubMed] [Google Scholar]
- 450.Yoshikawa, T., M. Ihira, M. Ohashi, S. Suga, Y. Asano, H. Miyazaki, M. Hirano, K. Suzuki, K. Matsunaga, K. Horibe, S. Kojima, K. Kudo, K. Kato, T. Matsuyama, and Y. Nishiyama. 2001. Correlation between HHV-6 infection and skin rash after allogeneic bone marrow transplantation. Bone Marrow Transplant. 28:77-81. [DOI] [PubMed] [Google Scholar]
- 451.Yoshikawa, T., M. Ihira, K. Suzuki, S. Suga, Y. Asano, K. Asonuma, K. Tanaka, and Y. Nishiyama. 2001. Primary human herpesvirus 6 infection in liver transplant recipients. J. Pediatr. 138:921-925. [DOI] [PubMed] [Google Scholar]
- 452.Yoshikawa, T., M. Ihira, K. Suzuki, S. Suga, K. Iida, Y. Saito, K. Asonuma, K. Tanaka, and Y. Asano. 2000. Human herpesvirus 6 infection after living related liver transplantation. J. Med. Virol. 62:52-59. [PubMed] [Google Scholar]
- 453.Yoshikawa, T., M. Morooka, S. Suga, Y. Niinomi, T. Kaneko, K. Shinoda, Y. Muraki, K. Takahashi, N. Sugaya, and Y. Asano. 1998. Five cases of thrombocytopenia induced by primary human herpesvirus 6 infection. Acta Paediatr. Jpn. 40:278-281. [DOI] [PubMed] [Google Scholar]
- 454.Yoshikawa, T., T. Nakashima, Y. Asano, S. Suga, T. Yazaki, S. Kojima, T. Mukai, and K. Yamanishi. 1992. Endonuclease analyses of DNA of human herpesvirus-6 isolated from blood before and after bone marrow transplantation. J. Med. Virol. 37:228-231. [DOI] [PubMed] [Google Scholar]
- 455.Yoshikawa, T., S. Suga, Y. Asano, T. Nakashima, T. Yazaki, Y. Ono, T. Fujita, K. Tsuzuki, S. Sugiyama, and S. Oshima. 1992. A prospective study of human herpesvirus-6 infection in renal transplantation. Transplantation 54:879-883. [DOI] [PubMed] [Google Scholar]
- 456.Yoshikawa, T., S. Suga, Y. Asano, T. Nakashima, T. Yazaki, R. Sobue, M. Hirano, M. Fukuda, S. Kojima, and T. Matsuyama. 1991. Human herpesvirus-6 infection in bone marrow transplantation. Blood 78:1381-1384. [PubMed] [Google Scholar]
- 457.Zerr, D. M., T. A. Gooley, L. Yeung, M. L. Huang, P. Carpenter, J. C. Wade, L. Corey, and C. Anasetti. 2001. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 33:763-771. [DOI] [PubMed] [Google Scholar]
- 458.Zerr, D. M., D. Gupta, M. L. Huang, R. Carter, and L. Corey. 2002. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:309-317. [DOI] [PubMed] [Google Scholar]
- 459.Zerr, D. M., L. C. Yeung, R. M. Obrigewitch, M. L. Huang, L. M. Frenkel, and L. Corey. 2002. Case report: primary human herpesvirus-6 associated with an afebrile seizure in a 3-week-old infant. J. Med. Virol. 66:384-387. [DOI] [PubMed] [Google Scholar]
- 460.Zhao, J., H. Fan, G. Mu, X. Shen, and X. Cheng. 1997. Detection of human herpesvirus 6 (HHV-6) DNA in salivary glands by the polymerase chain reaction. Chin. Med. Sci. J. 12:126-128. [PubMed] [Google Scholar]
- 461.Zhou, Y., C. K. Chang, G. Qian, B. Chandran, and C. Wood. 1994. trans-activation of the HIV promoter by a cDNA and its genomic clones of human herpesvirus-6. Virology 199:311-322. [DOI] [PubMed] [Google Scholar]
- 462.Zimmermann, A., D. Michel, I. Pavic, W. Hampl, A. Luske, J. Neyts, E. De Clercq, and T. Mertens. 1997. Phosphorylation of aciclovir, ganciclovir, penciclovir and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antivir. Res. 36:35-42. [DOI] [PubMed] [Google Scholar]
- 463.Zou, P., Y. Isegawa, K. Nakano, M. Haque, Y. Horiguchi, and K. Yamanishi. 1999. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 73:5926-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]