Abstract
Identification of gram-negative bacilli, both enteric and nonenteric, by conventional methods is not realistic for clinical microbiology laboratories performing routine cultures in today's world. The use of commercial kits, either manual or automated, to identify these organisms is a common practice. The advent of rapid or “spot” testing has eliminated the need for some commonly isolated organisms to be identified with the systems approach. Commercially available systems provide more in-depth identification to the species level as well as detect new and unusual strains. The answers obtained from these systems may not always be correct and must be interpreted with caution. The patient demographics, laboratory workload and work flow, and technologist's skill levels should dictate the system of choice. Cost considerations introduce another variable into the equation affecting choice. Each system has its own strengths and weaknesses, and each laboratory must decide on the level of sophistication that fulfills its particular needs.
INTRODUCTION
The accurate identification of Enterobacteriaceae and other glucose-fermenting and nonfermenting gram-negative bacilli has been the subject of many hundreds of publications over the years. With the introduction of each new or upgraded commercial product or system, the question once again becomes “Is newer better?” That is followed almost immediately by a plethora of evaluations by scientists hoping to contribute their data for consideration by others in the decision-making process.
Of more recent concern is the identification of possible agents of bioterrorism (BT) (65, 84). Bacillus anthracis, Yersinia pestis, and Franciscella tularensis are categorized as biothreat level A organisms whose identification is imperative. While there is relatively little information as to the accuracy of such identifications by commercial methods, such an identification remains the first indication a lab might have that their unknown isolate could be one of these organisms, and it should not be disregarded as incorrect without further investigation.
This review provides a comprehensive list of all commercial products, both manual and automated, currently available for the identification of both Enterobacteriaceae and other glucose-fermenting and nonfermenting gram-negative bacilli. The review begins with some historical perspective on how the industry has progressed over the last 30-plus years. Also included for each product is information on the component substrates, packaging and storage temperatures, suspension and incubation, additional reagents and tests that might be required, and quality control. A discussion of the current database contents completes the technical information and leads to relevant literature citations. Each section also includes the appropriate website for additional information or company contacts.
HISTORICAL PERSPECTIVE
Even before the turn of the 20th century, efforts were being made to find biochemical substrates that would help differentiate among the species contained within the two major groups of gram-negative organisms, widely known as the enterics and the nonfermenters. Both groups are common causes of bacterial infections in humans and occasionally cause widespread outbreaks of epidemiologic importance (36).
In 1898, Voges and Proskauer first observed that an eosin color was released upon the addition of caustic potash to certain bacterial suspensions but not others (92). In 1911, Russell described a double-sugar tube medium that would allow for separation of typhoid, paratyphoid, and dysentery organisms (77). Simmons (82) demonstrated that citrate, when used as a sole source of carbon, could differentiate among genera and species as described earlier by Koser (50) but that it worked even better when agar and bromthymol blue were added to the medium. Levine et al. (57) reported that the detection of H2S production could be improved by using a medium that did not contain lead acetate as described by Kligler (49). In 1946, Christensen introduced a medium that would detect the presence of the enzyme urease (15). In 1955, Møller detailed the pH shift of bromcresol purple that he observed while demonstrating the decarboxylation of several amino acids, namely, lysine, arginine, ornithine, and glutamic acid (63).
However, even as biochemical tests were being developed to differentiate among bacterial genera and species, other efforts were being made to decrease the amount of time that was required not only to obtain a positive test result but also to generate a correct identification. In 1948, Arnold and Weaver described a microtechnique to detect indole production in bacteria in as little as 6 min (range, up to 2 h) by using a heavy inoculum of organism and 1-ml quantities of medium (3). In 1949, Soto described a process to test carbohydrate fermentation by using paper disks with the carbohydrate and bromcresol purple incorporated into them (86). This effectively decreased the amount of tube medium that needed to be kept on hand and allowed results to be obtained within 8 h. In 1962, LeMinor and Hamida demonstrated that the test results for the enzyme β-galactosidase (σ-nitrophenyl-β-d-galactopyranoside) could reliably be read at the end of only 1 h of incubation (56). By 1963, Vracko and Sherris had adapted the concept of using paper disks and strips to develop “spot” tests, beginning with the test for indole production (93). They obtained excellent correlation when they compared their results to the results from conventional Kovacs' tube tests.
In 1964, the General Diagnostics Division of Warner-Chilcott Laboratories introduced the PathoTec reagent-impregnated paper strips, which were used to test for some of the specific enzymes produced by clinically significant bacteria. These included lysine and ornithine decarboxylase, esculin hydrolysis, urease, and indole production (61) and phenylalanine deaminase (33). With this successful commercial modification of tube-based technology, the door was opened to a plethora of systems, both manual and automated, that would accurately identify bacterial species.
CONCEPTS OF TECHNOLOGY
All commercial identification systems are based on one of five different technologies or a combination thereof. These include pH-based reactions that require from 15 to 24 h of incubation, enzyme-based reactions that require 2 to 4 h, utilization of carbon sources, visual detection of bacterial growth, or detection of volatile or nonvolatile fatty acids via gas chromatography (72).
In pH-based reactions, a positive test is indicated by a change in the color of one or more dyes. When a carbohydrate is utilized, the pH becomes acidic; when protein is utilized or there is release of a nitrogen-containing compound, the pH becomes alkaline. These reactions are influenced by the inoculum size, incubation time, and temperature of the reaction.
In 1980, Bascomb and Spencer described several rapid automated methods for measuring the enzyme activity of bacterial suspensions that could provide results within 6 h (7). Color changes in the enzyme-based system were due to the hydrolysis of a colorless complex by an appropriate enzyme with the resulting release of a chromogen or fluorogen. Because the incubation times needed for assay of enzymatic activities were shorter than those required for pH-based media, chance contamination was not a critical factor.
In the third type of reaction, utilization of carbon sources, there is a transfer of electrons from an organic product to the dye tetrazolium violet, which is incorporated within each test well. That transfer causes a colorimetric change in the dye, signaling the increased cellular respiration that occurs during the oxidation process. These reactions may occur in as little as 4 h.
The fourth method is a simple visual detection of growth of the test organism (increased turbidity) in the presence of a substrate. Results are determined by comparing a control well to the test well and may utilize a Wickerham card to read turbidity. This type of reaction may be difficult to read and always involves a minimum of overnight incubation.
The last technology, which is not commonly used, is more complex. It involves detecting the end products of cellular fatty acid metabolism. The end products are displayed on chromatographic tracings that are compared to a library of known patterns.
CHALLENGES INVOLVING TAXONOMY AND DATABASE MATRICES
As can be seen from Table 1, changes in the taxonomy of the Enterobacteriaceae have been almost logarithmic over the past 64 years. Changes within the non-glucose-fermenting or oxidase-positive glucose-fermenting gram-negative bacilli have been even more plentiful. Even as these attempts to classify bacteria are taking place, they present problems not only for the clinical microbiologist who must keep pace with them but also for the manufacturers whose databases must incorporate them. Taxonomy has always been a “hot topic” as there are definite camps of “splitters” and “lumpers.” The splitters would like to have all organisms completely characterized and identified to species level, corresponding to their genetic phylogeny. The lumpers, on the other hand, would prefer to group similar organisms into fewer genera or species groups, particularly if there is no difference in the clinical management of the disease. In 1983, Brenner wrote of the impact of modern taxonomy on the clinical laboratory, saying that while changes are constant, it is imperative that microbiologists stay current with those changes (12). However, cost is also a consideration that must be taken into account when a laboratory is trying to decide how completely they will identify an organism. It may be that an antimicrobial susceptibility test along with a “presumptive” identification using minimal biochemical tests will suffice, while the final identification is completed at a reference laboratory.
TABLE 1.
Increase in taxa of Enterobacteriaceae over 64 years
| Yr | Reference | No. of:
|
|
|---|---|---|---|
| Genera | Species | ||
| 1939 | Bergey’s Manual of Determinative Bacteriology (9) | 9 | NAa |
| 1952 | Kauffmann and Edwards (43) | 8 | 15 |
| 1962 | Edwards and Ewing (24) | 10 | 24 |
| 1974 | Ewing (28) | 13 | 30 |
| 1986 | Bailey and Scott’s Diagnostic Microbiology, 7th ed. (30) | 24 | 74 |
| 1995 | Centers for Disease Control and Prevention, unpublished data | 28 | 114 |
| 2003 | Manual of Clinical Microbiology, 8th ed. (29) | 31 | 130 |
NA, not available (species not given in listings).
Once the testing of a given organism is completed, the individual test results are compared to the database matrix of the respective product. These matrices are constructed by using thousands of previously characterized strains of bacteria, which are then tested in the new test system to determine the substrate profile that would be given to the isolate using the new system. These newly generated profiles are then stored in the computer and form the new database for the new test system. Tests are chosen based on their discriminatory ability to differentiate among numerous taxa. Bayes' theorem is one of the statistical methods used by manufacturers to arrive at a certain taxon based on the biochemical reaction profile produced by the unknown clinical isolate (96). Bayes' theorem considers two important issues to reach an accurate conclusion: (i) P(ti/R) is the probability that an organism exhibiting test pattern R belongs to taxon ti, and (ii) P(R/ti) is the probability that members of taxon ti will exhibit test pattern R. Prior to testing, we make an assumption that an unknown isolate has an equal chance of being any taxon and that each test used to identify the isolate is independent of all other tests. In this case, Bayes's theorem can be written as P(ti/R) = [P(R/ti)]/[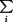 P(R/ti). By observing reference identification charts generated from conventional biochemical tests, we know the expected pattern of the population of taxon ti (e.g., Escherichia coli is indole positive, citrate negative, etc.). R in the formula is the test pattern composed of R1, R2,…, Rn, where R1 is the result for test 1, R2 is the result for test 2, etc., for a given taxon. The percentages (likelihoods that ti will exhibit R1, etc.) are incorporated into Bayes' theorem to arrive at an accurate taxon (72). Bascomb et al. (6), Friedman et al. (31), and Lapage et al. (52) were very instrumental in adapting these principles to identification of bacteria by using computer software. For more detailed information, the reader is referred to those publications.
P(R/ti). By observing reference identification charts generated from conventional biochemical tests, we know the expected pattern of the population of taxon ti (e.g., Escherichia coli is indole positive, citrate negative, etc.). R in the formula is the test pattern composed of R1, R2,…, Rn, where R1 is the result for test 1, R2 is the result for test 2, etc., for a given taxon. The percentages (likelihoods that ti will exhibit R1, etc.) are incorporated into Bayes' theorem to arrive at an accurate taxon (72). Bascomb et al. (6), Friedman et al. (31), and Lapage et al. (52) were very instrumental in adapting these principles to identification of bacteria by using computer software. For more detailed information, the reader is referred to those publications.
MANUAL IDENTIFICATION SYSTEMS
Many of the manual systems that will be discussed here are no longer in demand by the market and thus have not been the subject of recent evaluations. This paper will present more recent data, keeping in mind that one or more revisions of the identification software may have occurred. The studies cited are those which compared product identifications to identifications obtained by using conventional biochemicals. If there is an exception, it is noted. The reader is referred to Table 2 for many of the details relevant to each product or system.
TABLE 2.
Features of manual identification products
| Name of product (catalog no.) | Manufacturer | No. of tests on product | Organisms in databasea
|
No. of products per package | Storage temp (°C) | Incubation time (h), incubation temp (°C) | No. of additional reagents | No. of organisms for quality control | List cost per testb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacteriaceae | Vibrionaceae and Aeromonadaceae | Glucose nonfermenters | |||||||||
| API 20E (20100) | bioMérieux | 20c | × | × | × | 25 | 4 | 18-24, 35 | 6 | 5 | $6.56 |
| API 20NE (20050) | bioMérieux | 20 | × | × | 25 | 4 | 24, 30 | 3 | 4 | $5.18 | |
| API RapiD 20E (20700) | bioMérieux | 20 | × | × | × | 25 | 4 | 4, 35 | 2 | 3 | $5.96 |
| Crystal E/NF (24500) | BDd | 30 | × | × | × | 20 | 2-25 | 18-20, 35 | 2 | 6 | $5.91 |
| Enterotube II (211832) | BD | 12 | × | 25 | 4 | 18-24, 35 | 2 | 4 | $9.89 | ||
| EPS (V1107) | bioMérieux | 10 | × | 20 | 4 | 4-6, Online | 1 | 4 | $4.85 | ||
| GN2 Microplate (1101) | Biolog | 95 | × | × | × | 1 | 4 | 4-6, 16-24 (see text) | 0 | 0 | $7.40 |
| ID Tri-Panel | BD | 30 | × | × | × | −20 to −70 | 18-24 | 6 | 5 | $8.94 | |
| ID 32E | bioMérieux | 32 | × | × | × | 25 | 2-8 | 24, 37 | 1 | 5 | €68 |
| Microbact | Oxoid | 24 | × | × | × | 40, 60, 80, or 120 | 4 | 18-24, 35 | 4 | 5 | $3.97 |
| Micro-ID (38145) | Remel | 15 | × | 10 | 4 | 4, 35 | 2 | 4 | $6.94 | ||
| Oxi-Ferm II (212116) | BD | 12 | × | × | 25 | 2-8 | 48, 35 | 1 | 4 | $9.88 | |
| RapID NF Plus (8311005) | Remel | 17 | × | × | 20 | 4 | 4, 35 | 3 | 4 | $4.65 | |
| RapID onE (8311006) | Remel | 19 | × | × | 20 | 4 | 4, 35 | 1 | 4 | $5.05 | |
| RapID SS/u (8311004) | Remel | 12 | × | 20 | 4 | 2, 35 | 3 | 5 | $2.73 | ||
| r/b Enteric Differential (73-100-02e) | Remel | 15 | × | Various | 2-8 | 18-24, 35 | 1 | 7 | $3.22f | ||
| UID/UID-3 (V1106/V1102) | bioMérieux | 9 | × | × | 20 | 4 | 1-13, online | 0 | 5 | $4.60/$2.01 | |
| Uni-N/F-Tek (7310050g) | Remel | 5 or 13h | × | 20 tubes, 10 plates | 4 | 18-48, 35 or 42 | 1 | 4 | $10.38 | ||
×, included in database.
List cost as of 5 March 2004.
An additional six tests are necessary for glucose nonfermenters.
BD, Becton Dickinson.
There are different catalog numbers for each component of the r/b system.
$3.22 per tube for rb1, rb2, or Cit/Rham expander.
There are different catalog numbers for each component of the Uni-N/F-Tek.
The number of tests depends on whether the plate or just the screen tube is inoculated.
API 20E
In 1971, Washington et al. published the first evaluation of the API 20E, originally owned by the Analytab Products Division of American Home Products, which has been owned since 1986 by bioMérieux, Inc. (Durham, N.C.) (95). An impermeable plastic backing supports 20 cupules that contain pH-based substrates that have not changed since the product was originally designed in 1970.
The database has expanded from 87 taxa in 1977 to 102 taxa in 2003 and includes Y. pestis (Table 3). The current database is version 4.0. The website http://biomerieux-usa.com/support/techlibrary/api/index.asp provides ordering information as well as package inserts.
TABLE 3.
Potential agents of bioterrorism included in product databases
| System | Organisms in databasea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biothreat level A
|
Biothreat level B
|
||||||||
| Bacillus anthracis | Yersinia pestis | Francisella tularensis | Brucella spp. | Burkholderia mallei | Salmonella spp. | Shigella dysenteriae | Escherichia coli O157:H7 | Vibrio cholerae | |
| Manual systems | |||||||||
| API 20E | × | × | × | × | × | × | |||
| API 20NE | × | ||||||||
| RapiD 20E | × | × | × | × | |||||
| Crystal E/NF | × | × | × | × | |||||
| Enterotube II | × | × | × | ||||||
| EPS | × | × | |||||||
| GN2 Microplate | × | × | × | × | × | × | × | × | × |
| ID 32E | × | × | × | × | |||||
| ID Tri-Panel | × | × | × | × | |||||
| Micro ID | × | × | × | ||||||
| RapiD NF Plus | × | × | |||||||
| RapID onE | × | × | |||||||
| r/b Enteric Differential | × | × | |||||||
| Automated systems | |||||||||
| Phoenix NID | × | × | × | ||||||
| Vitek GNI+ | × | × | × | × | × | ||||
| Vitek 2 ID-GNB | × | × | × | × | × | × | × | × | |
| MicroScan Neg ID type 2 | × | × | × | × | × | ||||
| MicroScan Rapid Neg ID type 3 | × | × | × | × | × | ||||
| MIDI | × | × | × | × | × | × | × | × | |
| ARIS2X GNID | × | × | × | × | |||||
The databases of Oxiferm, RapID SS/u, UID, and Uni-N/F Tek include none of these organisms. Biothreat levels are from reference 83. ×, included in database.
In the evaluation by Washington et al., approximately 93.0% of the 129 Enterobacteriaceae and five Aeromonas strains were correctly identified to species level (95). Smith et al. in 1972 showed an overall accuracy rate of 96.4% in testing 366 enteric strains (83). Over the next 20 years, the API 20E strip was compared to many other identification systems and, because of its large acceptance by the clinical microbiology laboratory market, became somewhat of a “gold standard” among commercial systems.
Because of the continued expansion of the database while the original substrate pattern in the strip was maintained, O'Hara et al. in 1992 reevaluated the strip for its accuracy in the identification of Enterobacteriaceae (70). For those organisms routinely isolated in clinical laboratories (e.g., E. coli, Klebsiella pneumoniae, and Proteus mirabilis), the strip accurately identified 87.7% at 24 h and 96.3% at 48 h. For organisms less routinely isolated, e.g., Providencia stuartii or Escherichia vulneris, the API strip identified only 78.7% at 24 h.
There have been several studies of accuracy aimed at individual genera. In 1987, Archer et al. reported accuracies of 66 and 51% in the identification of Yersinia spp. when incubation was at 28 and 37°C, respectively (2). They also reported greater accuracy in identification of Yersinia enterocolitica biogroups 1 and 2 as opposed to biogroups 3 and 4 (97 to 100% as opposed to 27 to 47% at either temperature). This report indicated that many of the misidentifications were due to the inability of organisms to ferment melibiose and rhamnose at 37°C. Sharmer et al. reported accuracies of 97% for all biogroups of Y. enterocolitica when incubated at 28°C and of 90% for Yersinia spp. overall (81). They also reported problems with the fermentation of melibiose and rhamnose, as well as inositol. Wilmoth et al. tested 12 human strains of Y. pestis and reported only 58.3% accuracy (98). If those same code numbers were used with the current database, the percent correct would remain at 58.3%, but the other five strains would have Y. pestis as the first choice at a probability of approximately 80%. In 1998, Neubauer et al. compared the accuracy of four systems for their ability to identify Yersinia spp. (66). Of 118 isolates tested, 93 (78.8%) were correctly identified with the API 20E strip. Lowe et al. reported an accuracy of 99% for identification of Burkholderia pseudomallei (60). O'Hara et al. tested eight species of Vibrio and reported that Vibrio alginolyticus, Vibrio parahaemolyticus, and Photobacterium damselae exceeded 90% accuracy compared to conventional biochemicals (71). Identifications of Vibrio cholerae were only 50% accurate, while 9 of 10 identifications each of Vibrio fluvialis and Vibrio hollisae were correct, but all at the “good likelihood, low selectivity” level of probability.
API 20NE
Constructed along the same lines as the API 20E, the API 20NE (bioMérieux) has 20 cupules that contain 8 conventional substrates and 12 assimilation tests. Suspensions are prepared in 0.85% NaCl for inoculation into the 8 conventional substrates and in AUX medium for inoculation into the 12 assimilation cupules. The database contains 32 genera and 64 species of nonfastidious gram-negative rods not belonging to the Enterobacteriaceae. The seven-digit profile number is converted to an identification by using the APILAB software, version 3.3.3. The API 20NE website is the same as cited above for the API 20E.
Most of the evaluations of this product have been performed on a single genus or a single species. Lampe and van der Reijden, however, tested 198 isolates and compared their identifications to those obtained with conventional biochemicals (51). These strains included species of Pseudomonas, Acinetobacter, Achromobacter, Bordetella, Flavobacterium (Chryseobacterium), Alcaligenes, and Moraxella. They reported 92% overall agreement, with only the less common species of Pseudomonas being less than 93% accurate.
van Pelt et al. compared the identifications of 114 strains of Burkholderia spp. to identifications obtained with a combination of commercial assays and PCR-restriction fragment length polymorphism (91). Only 74.6% of the API 20NE identifications were correct; none of the Burkholderia gladioli strains were identified correctly.
In 1996, Bernards et al. tested 130 strains belonging to 18 genomic species of Acinetobacter whose identifications had been confirmed by DNA hybridization (10). Although their results were based on version 5.1 of the APILAB software, the article included profile numbers. When those numbers were put into the current software (version 6.1), many of the misidentifications were no longer in error. As the database now includes two more genomic species than it did in the previous version, one would be led to believe that the 87% accuracy might be somewhat higher. The authors concluded at that time that the discriminative power of the test in the API 20NE was insufficient for correct identification of all Acinetobacter genomic species.
Lowe et al. reported that 98% of 103 clinical isolates of B. pseudomallei were correctly identified by the API 20NE (60). These results parallel those of Dance et al., who reported an accuracy of 97.5% for 400 strains (18). Even with the updated software, the accuracy reported by Dance et al. exceeds 90%. Inglis et al. expressed concern that the API 20NE was overcalling B. pseudomallei and that some of the isolates were actually Chromobacterium violaceum (39). As their report included no raw profile numbers, it was not possible to see whether the current database had resolved the problem.
Two reports in the literature cite the misidentification of Brucella melitensis by the API 20NE. In one report, the organism was responsible for a laboratory-acquired infection and was identified as Moraxella phenylpyruvica (8); in the other report, it was identified as Ochrobactrum anthropi (26). When the profile numbers were entered into the current APILAB software, the answers remained incorrect. Both articles emphasize the need for caution in the interpretation of answers when the clinical diagnosis might lead one to suspect brucellosis.
Pacova and Dlouhy reported 97.1% accuracy when identifying 35 strains of Pseudomonas stutzeri (73), and Barr et al. reported 72.9% accuracy with 140 isolates of Pseudomonas aeruginosa (4). Clarridge and Zighelboim-Daum in 1985 reported the isolation of an “unidentified” organism from a patient who had suffered a hand wound while handling catfish (16). Although the database at that time would not identify the isolate of V. damselae, entry of the profile number into the current database would yield the correct answer.
Teng et al. published a case report of a patient with persistent bacteremia caused by an unusual clone of Burkholderia cepacia (90). While the identifications were initially correct on the 20NE, a very unusual antibiogram prompted the laboratory to positively confirm the identification by using cellular fatty acid analysis and 16S rRNA gene technology.
API RapiD 20E
Originally marketed in 1982 as the Rapid E system (DMS Laboratories, Flemington, N.J.) but owned since 1986 by API and subsequently by bioMérieux Inc., the API RapiD 20E system is designed to identify Enterobacteriaceae in 4 h. Similar to the API 20E in its test configuration, this system has 20 microtubes that contain substrates for the demonstration of enzymatic activity or fermentation of carbohydrates. The seven-digit profile number that is compiled from the test reactions is entered into the APILAB software. The current version of the RapiD 20E software is 3.0. The database contains 26 genera and 65 species. Identifications are also available by using the Analytical Profile Index. Package inserts and ordering information are available at http://biomerieux-usa.com/support/techlibrary/api/index.asp.
Most of the published evaluations of this product were completed prior to 1986, when API bought the product. The database has since been updated on multiple occasions, but data from these publications are likely to be outdated and are not reviewed here.
Crystal E/NF
Introduced in 1993 by Becton Dickinson (Sparks, Md.), the BBL Crystal Enteric/Nonfermenter (E/NF) ID kit is for the identification of Enterobacteriaceae and more commonly isolated glucose-fermenting and nonfermenting gram-negative bacilli. The plastic panels include 30 tests for the fermentation, oxidation, degradation, or hydrolysis of various substrates. Once the panel is inoculated and snapped together with its lid, it becomes a completely sealed system posing little risk of exposure to the technologist. The current software version is 4.0 and contains 38 genera and 104 species. The category of miscellaneous gram-negative bacilli includes an additional 20 taxa that may require up to 17 additional off-line tests for completion of an identification via an algorithm. V. cholerae is the only BT-related organism included in the database (Table 3).
There are four comprehensive published evaluations of this product, although all four utilized previous editions of the software. An evaluation of 131 isolates encompassing nine species of Vibrio showed an overall accuracy of 80.9% (71). Among the API 20E, Crystal E/NF, MicroScan Neg ID type 2, and Rapid Neg ID type 3 panels and Vitek GNI+ and Vitek ID-GNB, only the Crystal was able to identify accurately more than 90% of V. cholerae isolates (n = 30) in comparison to conventional biochemicals. Correct identifications of P. damselae, V. hollisae, and V. vulnificus also exceeded 90% accuracy. A study by Soler et al. included 52 clinical isolates and 22 reference strains of Aeromonas species (85). Of the 74 isolates, however, only 48 of the reference identifications were contained in the E/NF database; 18 (37.5%) were correctly identified. In 1996, Wilmoth et al. tested 12 human isolates of Y. pestis and reported 91.7% accuracy (98). Because the authors reported profile code numbers in the publication, we were able to subsequently determine that the percent accuracy has not changed.
EPS
Marketed by bioMérieux, the Enteric Pathogen Screen (EPS) is to be used in conjunction with the Vitek Legacy instrument as a screen for isolates of the common oxidase-negative enteric pathogens, which include Edwardsiella tarda, Salmonella spp., Shigella sonnei and other Shigella spp., and Y. enterocolitica. The card is designed to be presumptive only, and any identifications must be confirmed with a GNI+ card and serology where appropriate. A report of “negative for Salmonella, Shigella, and Yersinia” may also be given.
The cards are self-contained, and each card contains 10 substrates. Incubations are carried out in the instrument, and reports are generated automatically at the end of the cycle.
The most recent evaluation of this product, in 1993, reported a sensitivity of 99.5% in the screening for possible enteric pathogens (38).
GN2 MicroPlate
The GN2 MicroPlate was designed in 1989 by Biolog, Inc. (Hayward, Calif.) for use with any one of three Microbial Identification/Characterization systems: the OmniLog ID, a fully automated system; the MicroLog MicroStation, a semiautomated system; and the MicroLog 1 and 2, which are manual-read systems. This product is based on the exchange of electrons produced during an organism's respiration, leading to a subsequent tetrazolium-based color change. Each of the 96 wells of the microtiter-style plate contains tetrazolium dye, which changes from colorless to purple as the actively growing cells oxidize the carbon source.
The organisms to be tested are grown on Biolog Universal Growth agar containing 5% sheep blood, after which suspensions are made in a proprietary GN inoculating fluid. The inoculated plates are incubated at either 30 or 35°C (depending on the suspected organism) for 4 to 6 h or for 16 to 24 h. The current GN database is release 6.01 and contains identification patterns for 526 species or taxa that encompass not only Enterobacteriaceae but many other gram-negative nonfermenters and fastidious organisms. There is also a Dangerous Pathogens database that supplements the GN database and includes B. anthracis, B. melitensis, Y. pestis, F. tularensis, Burkholderia mallei, and B. pseudomallei.
While there have been multiple abstracts at scientific meetings on the ability of the GN2 plate to identify gram-negative bacilli, there have been only isolated reports in the clinical literature; most of these studies used prior versions of the software and therefore are not included in this review.
One presentation (J. C. David, W. L. Thomas, R. J. Burgess, and T. L. Hadfield, Abstr. 101st Meet. Am. Soc. Microbiol., abstr. C-335, p. 229, 2001) compared the MicroLog ML3 system to the Vitek 32 for the identification of select biological warfare agents. The gram-positive and gram-negative microplates were able to identify eight of the nine agents tested on the first attempt.
Additional detailed information is available at the company website www.biolog.com.
ID Tri-Panel
The ID Tri-Panel was introduced by the PASCO Laboratories division of Difco Laboratories in 1984. Upon the sale of Difco Laboratories in 1997, the panel became the property of Becton Dickinson.
The panel will accommodate the testing of three isolates at one time or can be used as part of a combination MIC-ID configuration. It contains 30 colorimetric-based substrates. A profile number is generated, and the answer is obtained from either an Electro-Code computer program or the data management system.
The current database contains 31 genera and 118 species, including Brucella spp. (Table 3). Several taxonomic changes have been made since the last update.
The most recent evaluation was in 1994 by Edinger et al., who reported that 86% of 127 non-glucose-fermenting isolates were correctly identified (23). A total of 91% (93 of 102 isolates) of the Pseudomonas-Xanthomonas group and the Acinetobacter group were correctly identified to species level.
ID 32E
The ID 32E (bioMérieux, Marcy l'Etoile, France) is not currently for sale to the clinical market in the United States but is widely used in Europe. It is an upgraded version of the API 20E and contains 32 substrates in a plastic-strip configuration similar to the API. While there is an automated ATB reader, it is possible to read the strip visually.
A numerical profile is generated and entered into the APILAB PLUS software for an identification or for a list of the 17 additional tests that might be necessary for the completion of an identification. The current database is version 3.3.3, which contains 40 genera and 103 species.
Comprehensive evaluations of this product used previous versions of the software. Leclerq et al. reported on the ability of the system to discriminate between isolates of E. coli O157:H7 and non-O157. Even though the O157 strains showed atypical biochemical reactions, the identifications were correct at the species level. There were no unique biochemical profile numbers for the O157 strains, but the numbers were distinct from those of other serotypes (54).
The Rapid ID 32E is a 4-h configuration of this product, but it is also unavailable to the U.S. clinical market.
Microbact
The newest product to be released in the United States is manufactured by Oxoid, Ltd. (Basingstoke, England) and is used for the identification of Enterobacteriaceae and miscellaneous gram-negative bacilli. Identification is based on pH changes in various substrates and substrate utilization tests. The product consists of two separate substrate strips labeled 12A and 12B. Each strip contains 12 different biochemicals. The 12A strip is used alone for identification of oxidase-negative, nitrate-positive glucose fermenters and is used in conjunction with the 12B strip to identify oxidase-positive, nitrate-negative glucose nonfermenters. There are also two configurations of the same 24 substrates in a solid microplate format that will identify the same taxa of organisms. Two unusual items relative to this product are (i) a precaution notice that once the foil package of strips is opened, the remaining strips must be used within 7 days, and (ii) a precaution that colonies grown on selective media may have to be grown in peptone water for 4 h before being suspended in saline for use in the strip.
The reactions are converted into an octal code and then entered into the Microbact computerized identification package, which provides the identification. The database of the 12A strip contains 14 genera and 34 species. In the “Limitations” portion of the package insert, there is the notation that the use of the 12A strip alone necessitates that Klebsiella spp., Enterobacter spp., or Serratia spp. be reported as “Klebsiella/Enterobacter/Serratia group,” since there are insufficient data to provide accurate species identification within the group. It is recommended that the 12B strip always be included in the setup. Additional testing may also be required for Yersinia spp., which do include Y. pestis (Table 3). When the 12B strip is included in the initial processing, the list of taxa increases to 29 genera, 109 species, 7 Centers for Disease Control and Prevention (CDC) enteric groups of oxidase-negative organisms, and 12 genera and 31 species of oxidase-positive organisms. Because the product is new, there are no current evaluations in the literature.
RapID NF Plus
The RapID NF Plus was originally designed in 1989 and marketed by Innovative Diagnostic Systems (IDS) (Norcross, Ga.) but is now marketed by Remel (Lexena, Kans.). It employs both conventional and chromogenic substrates for the identification of non-glucose-fermenting, gram-negative bacilli and selected glucose-fermenting bacilli not belonging to the family Enterobacteriaceae. The plastic panel has molded into it 10 reaction cavities that contain 17 substrates. The addition of reagents allows seven of the cavities to be bifunctional, containing two separate tests in the same cavity. The reactions are recorded and the resulting microcode referenced in the RapID NF Plus Code Compendium or the Electronic RapID Compendium (ERIC) for an identification. The current database is dated 30 April 2003 and contains 31 genera, 68 species, and two CDC groups.
One of the most recent evaluations is that of 345 strains by Kitch et al., who reported an accuracy of 90.1% and an error rate of 3.8% at the end of the initial incubation period (47). Another evaluation, in 1996 by Kiska et al., compared the results of four commercial identification systems for 150 gram-negative bacilli isolated from cystic fibrosis patients, including 58 strains of B. cepacia (46). The RapID NF Plus system correctly identified 80% of all isolates and 81% of the B. cepacia strains. Oliver reported that a blood isolate of O. anthropi was misidentified as Shewanella putrefaciens. His report underscored the difficulty that is sometimes encountered in the identification of nonfermentative organisms, since they may have clinical significance and unusual susceptibility patterns (J. Oliver, Letter, J. Clin. Microbiol. 41:4486, 2003).
RapID onE
Like the RapID NF Plus, the RapID onE was designed originally by IDS and is now marketed by Remel. It employs conventional and chromogenic substrates for the identification of Enterobacteriaceae and other clinically relevant oxidase-negative, gram-negative bacilli from human sources. The same plastic panel with 18 reaction cavities will give 19 test results, as one cavity is bifunctional after the addition of a single reagent.
As with the RapID NF Plus, the reactions are recorded and the resulting microcode is referenced in the RapID NF Plus Code Compendium or the ERIC for an identification. The current database is dated 30 April 2003 and contains 28 genera, 60 species, and several biogroups within species.
Two studies in 1994 reported accuracy rates exceeding 91%. Kitch et al. evaluated 364 strains of Enterobacteriaceae and 15 strains of oxidase-negative, gram-negative nonfermenters and found an accuracy rate of 97.6% without additional tests (48). Lee et al. (55) studied 125 strains of Enterobacteriaceae and Acinetobacter calcoaceticus. They reported accuracy rates of 92.9% with fresh clinical isolates and 90.2% with frozen stock isolates.
RapID SS/u
A third product in the RapID line, designed by IDS in 1986 and now also marketed by Remel, is the RapID SS/u, a combination of conventional and chromogenic substrates for the identification of organisms isolated from urine. This plastic panel has 10 reaction cavities, with one being bifunctional after the addition of spot indole reagent.
The current database is dated 29 April 2003 and contains nine gram-negative and two gram-positive genera as well as two taxa of yeasts. Only Morganella morganii, E. coli, Candida albicans, and Candida glabrata are separated into species. Once the reactions are recorded, the resulting microcode is entered in the RapID SS/u Differential Chart, the RapID SS/u Code Compendium, or the ERIC for an identification.
An evaluation by Halstead et al. reported that 95.9% of 170 isolates were identified correctly in 2 h (34). A subsequent evaluation by DeGirolami et al. reported an accuracy of 86.5% for 185 isolates (20). Although the database has been updated recently, its contents do not appear to have been changed since these two studies were completed.
UID/UID-3
The Urine Identification screen card (UID/UID-3) was designed by bioMérieux Inc. in the late 1970s for the detection, enumeration, and selective identification of some Enterobacteriaceae, P. aeruginosa, group D enterococci, Staphylococcus spp., and yeasts. The Enterobacteriaceae include E. coli, Proteus spp., Klebsiella and Enterobacter spp., and Citrobacter freundii.
There are 10 wells in the UID card, 9 of which contain substrates and metabolic inhibitors whose reactions are specific for a given genus. The UID-3 card, which contains an identical set of biochemicals, can identify three isolates on each card.
Reconstitution of the dried substrates is with urine diluted in 0.45% sterile saline. Incubation is online in any Vitek instrument other than a Vitek 2. A colony count (CFU per milliliter) will also be given if the positive control well indicates a higher count than the selective wells.
Huber reported that 90.1% of 1,634 specimens were both correctly enumerated and identified within 9 h with the UID-3 card (37). Dalton et al. studied the use of the UID-3 as a screening test for bacteriuria and reported a sensitivity and a specificity of 93 and 55%, respectively, when the colony counts were ≥105 CFU/ml (17).
Uni-N/F-Tek
The Uni-N/F-Tek method is commonly referred to as the N/F system and also incorporates the N/F Screen 42P and N/F Screen GNF. The Uni-N/F-Tek was originally designed and marketed by Diagnostic Research, Inc., a division of Corning Medical Products, Inc. (Roslyn, N.Y.), was sold to Flow Diagnostics, and is now marketed by Remel. The system is used for the identification of nonenteric aerobic gram-negative bacilli.
If the unknown organism is oxidase positive, the 42P and GNF tubes are inoculated and incubated. Depending on the reactions from these two tubes, a Uni-N/F-Tek plate may be inoculated. For oxidase-negative organisms, the r/b Enteric Differential system (see below) is inoculated first. If there is growth but no color changes, the Uni-N/F-Tek plate is inoculated. The Uni-N/F-Tek is a plastic circular plate divided into 11 independently sealed wells and a central well. Each of the 12 wells has a different conventional medium, with the central one being bifunctional, for a total of 13 test results.
Bacterial colonies are used to inoculate the 42P and GNF tubes; the inoculum for the Uni-N/F-Tek plate is a suspension of the organism in sterile distilled water. The current database is dated 25 September 2000 and contains 19 genera, 43 species, and 1 CDC group. There do not appear to have been any evaluations of this product since 1979.
The following four products are still available for purchase from their manufacturers even though their databases are at least 10 years old. With the current trend toward improvements in global medicine, there are specific instances where they would serve as a simple solution. They are detailed here, but without reference to previous evaluations.
Enterotube II
The Enterotube II, first designed and marketed by Roche Diagnostics in 1969, was eventually sold to Becton Dickinson and is used for the identification of Enterobacteriaceae and four commonly isolated oxidase-negative nonfermenters. The self-contained, compartmented plastic tube contains 12 conventional media and an inoculating wire. When the wire is pulled the length of the tube, it simultaneously inoculates all of the media from a single bacterial colony. A wax overlay in the glucose compartment allows for the determination of gas production from that particular carbohydrate. A five-digit profile number is generated, and the Computer Coding Identification System is consulted for the identification. The current version of the Computer Coding Identification System code book is dated August 1993, and the database includes 22 genera, 79 species, and 6 CDC enteric groups (three of which have been named since 1993). Two of the organisms in the database have also been renamed since this code book was released.
Micro-ID
The Micro-ID kit was designed for the identification of Enterobacteriaceae by the General Diagnostics Division of Warner-Lambert Pharmaceutical Company; it was released in approximately 1978. The product was subsequently owned by Organon Teknika and is now owned by Remel.
The Micro-ID is a self-contained plastic unit containing 15 reagent-impregnated disks that detect the presence of specific enzymes and/or metabolic end products produced by the microorganism. A five-digit, octal number is composed from the 15 reactions, and the MICRO-ID Identification Manual is consulted for the identification. Package inserts are available at the company website http://www.remel.com/products/clinical. The database is dated January 1981, which is also the last time that the product was evaluated. There have been no revisions of the database since then.
This is one of the few manual products on the market for which there is a procedure for the presumptive identification of organisms taken directly from blood cultures without routine subculture (21).
Oxi/Ferm II
The Oxi/Ferm II was introduced in approximately 1976 by Roche Diagnostics but was eventually sold to Becton Dickinson. Similar in design to the Enterotube II, the Oxi/Ferm was intended to identify oxidative-fermentative, aerobic, gram-negative rods other than Enterobacteriaceae and is a self-contained, compartmented plastic tube that also contains an inoculating wire. An initial oxidase test is performed to ensure that the Oxi/Ferm tube rather than the Enterotube II should be used. Direct inoculation of the media is accomplished by withdrawing the wire through all of the compartments.
The 15 reactions are converted to a five-digit code that is then located in the Oxi/Ferm Biocode Manual. The current version of the manual is dated June 1993 and contains 14 genera and 40 species. Because of the rapidly changing taxonomic status of many of these species, approximately 25% of the organism names in the database have been changed since this version was released.
r/b Enteric Differential System
The two-tube r/b Enteric Differential System, whose name was taken from the initials of its coinventors, William Rollender and Orville Beckford, was originally manufactured by Diagnostic Research, Inc., was sold to Corning Diagnostics, and is now marketed by Remel. The two tubes, designated r/b1 and r/b2, are the components of the r/b system, along with an auxiliary tube, the Cit/Rham Expander. If the isolate is suspected of being Enterobacter aerogenes, Hafnia alvei, Serratia marcescens, Serratia liquefaciens, or a non-H2S-producing Salmonella strain, a Soranase tube is also added to the set.
The current database is dated October 1990 and includes 13 genera and 37 species. An organism can be identified by using the chart in the package insert or by generating a biogram code number and using the computer code book.
AUTOMATED IDENTIFICATION SYSTEMS
With the advancement of automated testing in chemistry and hematology laboratories in the mid-1970s, it was only logical that some degree of automation in microbiology would eventually follow. In 1976, Williams commented at the Symposium on Rapid Methods and Automation in Microbiology that “… medical bacteriologists have tended to impose their own circadian rhythm on their bacterial cultures and to work in units of 18 to 24 h” (97), and in 1979, Randall observed, “Indeed, this commitment to the traditional ‘overnight’ incubation period has further delayed the automation period” (76).
In the early days of the space race, McDonnell Douglas Astronautics Co. Bioscience Laboratory introduced the concept of detecting, enumerating, and identifying microorganisms in a spacecraft environment. From that, in 1973, the AutoMicrobic System (AMS) (McDonnell Douglas Corp., St. Louis, Mo.) was born. It incorporated a disposable miniaturized plastic specimen-handling system, solid-state optics for microbial detection, and a minicomputer for control and processing and today is recognized as the first generation of the Vitek instruments (1). Results obtained in an elapsed time of only 13 h demonstrated detection and identification at levels of 92% or higher positive correlation when levels of the organism were ≥7 × 104 CFU. Within 10 years, Vitek's competitors included the MS-2 (Abbott Diagnostics, Inc., Chicago, Ill.), the Autobac IDX (Pfizer Inc., Groton, Conn. and General Diagnostics, Morris Plains, N.J.), and the AutoScan-3 (MicroScan Corp., Hillsdale, N.J.). Both the MS-2 and the Autobac were originally introduced for urine screening but were eventually used for bacterial identification and antimicrobial susceptibility testing. Other products, such as the BBL Sceptor (Becton Dickinson) and the Quantum (Abbott Diagnostics) made brief appearances but were short-lived in this very competitive marketplace. Technology had enabled valid results to be obtained in as little as 4 h. Microbiology was definitely on the fast track to rapid testing and shorter turnaround times.
Table 4 presents the most important features of each of the seven automated instruments currently available.
TABLE 4.
Features of automated identification products
| Name of product (catalog no.) | No. of tests on product | No. of products per package | Storage temp (°C) | Reagents required | Incubation time (h) | Additional tests required | No. of organisms for quality control | Cost of instrumenta | Cost per testa | Identification-susceptibility combination panels |
|---|---|---|---|---|---|---|---|---|---|---|
| BD Phoenix NID (448007) | 45 | 25 | RTb | No | 2-12 | Spot tests only | 2 | $95,000 | $7.40 | Yes |
| Vitek GNI+ (V1311) | 28 | 20 | 4 | No | 2-12 | 70 | 8 | $137,850 | $5.35 | No |
| Vitek 2 ID-GNB (21312) | 41 | 20 | 4 | No | 3 | 5 | 9 | $159,000 | $7.15 | No |
| MicroScan Neg ID type 2 (B1017-27) | 32 | 20 | 2-30 | 6 | 16-20 | Yes | 4 | $153,038 | $12.79c | Yes |
| MicroScan Rapid Neg ID type 3 (B1017-110) | 36 | 20 | 2-8 | 1 | 2 h 20 min | Yes | 4 | $153,038 | $14.39d | Yes |
| MIDI | NAe | NA | RT | No | NA | No | 0 | $60,888 | $1.50 | NA |
| Trek Sensititre GNID (GNID) | 32 | 10 | RT | No | 5-24 | 4 | 6 | $72,380 | $9.41 | No |
List cost as of 4 March 2004.
RT, room temperature.
Does not include the minute amounts of six reagents that are needed.
Does not include the minute amounts of rapid indole reagent that is needed.
NA, not applicable because the system is chromatographic in nature.
BD Phoenix 100
The BD Phoenix 100 is the newest instrument in the automated identification market, having been introduced in 2003. Designed and marketed by Becton Dickinson, its goal is the rapid identification of gram-negative and gram-positive bacteria of human origin. The Phoenix 100 instrument is capable of processing 99 panels at one time; one panel holder is reserved for the internal thermometer. Once the panels are inoculated and loaded into the instrument, all operations are totally automated and results print when each panel is completed.
NID
The NID panel is for gram-negative identifications and has 45 wells containing dried biochemical substrates and 2 fluorescent control wells. There are 16 enzymatic substrates, 23 carbon source substrates, and 5 utilization and growth inhibition tests. The panels are available as an identification panel only or as part of a combination identification-antimicrobial susceptibility test panel. The current database is version 4.05 and contains 60 genera, 155 species, and 5 CDC enteric groups.
Endimiani et al. tested 136 nonfermenting gram-negative bacilli and reported 95.6% agreement between the Phoenix 100 and the ATB/ID32GN (bioMérieux, Marcy l’Etoile, France) (27). Brisse et al. tested 134 isolates of the B. cepacia complex that had been identified by using four different molecular methods and reported an accuracy rate of 50% (13). Stefaniuk et al. reported an accuracy rate of 92.5% compared to the API 20E in the testing of 120 strains representing only eight of the most commonly encountered species of Enterobacteriaceae (87a). The same study showed an accuracy of 96.3% when the Phoenix 100 was compared to the API 20NE in the identification of 54 strains of P. aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. When Donay et al. used the same two reference systems for comparison to the Phoenix, 130 strains of Enterobacteriaceae and 57 strains of nonenteric organisms showed accuracy rates of 94.6 and 89.4%, respectively (20a). O'Hara found an agreement of 90.4% with conventional biochemicals for 500 strains of Enterobacteriaceae (unpublished data). Colodner et al. reported 98.0% accuracy when identifying 51 strains of Vibrio vulnificus biotype 3 (R. Colodner, L. Lerner, J. Kopelowitz, I. Meir, Z. Lazarovich, Y. Keness, and R. Raz, Abstr. 12th Eur. Cong. Clin. Microbiol. Infect. Dis., abstr. P637, 2002).
bioMérieux Vitek
The AMS instrument began as part of McDonnell Douglas Corporation's program to enumerate and identify organisms found in space. Shortly thereafter, Vitek Systems, Inc., became its own entity, and in 1980 the AMS was reconfigured to identify Enterobacteriaceae from any clinical specimen by using an Enterobacteriaceae biochemical card (EBC). An early evaluation by Isenberg et al. reported an accuracy of 97.8% for 1,020 isolates compared to conventional biochemicals. Turnaround times averaged 8 h (40). The AMS became commonly known as the Vitek. By 1982, a new EBC+ card could also identify several nonenteric organisms and reduced turnaround times to 4 h in many cases (5). In 1988, Vitek Systems, Inc., was purchased by bioMérieux, Inc., and by 1989, the gram-negative identification (GNI) card was introduced. This was followed in 1996 by the GNI+ card, which increased the number of organism profiles in the database, improving the accuracy of identification while shortening the time to reporting from 5.7 to 4.1 h (11). In 1997, bioMérieux introduced the next generation of the instrument, the Vitek 2, and its associated ID-GNB card. The year 2004 has seen the advent of the new colorimetric card for the Vitek 2, the 2GN.
The original Vitek (i.e., Vitek Legacy) can process 32 or 120 cards at a time; up to four instruments may be linked to one system. The list price of the instrument in Table 4 is for a 100-card capacity unit and includes the filler-sealer module and the computer. The 32-card instrument incorporates a filler, making that module unnecessary.
The Vitek 2 can process 60 or 120 cards at one time. The list price of the 120-card instrument in shown in Table 4. The Vitek 2 is a self-contained instrument that incorporates both the filler and the sealer, making these two external modules unnecessary.
GNI+.
The 30-well GNI+ card is used for the identification of Enterobacteriaceae, Vibrionaceae, and a selected group of non-glucose-fermenting gram-negative bacilli. The current version of the software is 7.01 and contains 48 genera and 112 species, including Y. pestis, B. mallei, and B. pseudomallei (Table 3).
There have been no general evaluations of the GNI+ since 1998, and there have been two software upgrades since that time. Lowe et al. studied the identification of B. pseudomallei and reported 99% accuracy for 100 clinical isolates compared to the API 20NE (60). O'Hara et al. reported an accuracy of 73.5% for six species of Vibrio compared to conventional biochemicals (71). Only V. alginolyticus and V. parahaemolyticus were identified at >90% accuracy. Park et al. reported that an isolate of Aeromonas hydrophila was misidentified as V. alginolyticus (74).
The Vitek was the first automated instrument that allowed the direct inoculation of positive blood cultures into the identification cards (64). The most recent study of this technique reported that the GNI+ card correctly identified 75% of 169 isolates within 6 h when inoculated directly with a suspension of organisms from a positive blood culture bottle (35).
ID-GNB.
The 64-well ID-GNB card is used in the Vitek 2 for the identification of clinically significant fermenting and nonfermenting gram-negative bacilli. It contains 41 biochemical tests that include both conventional substrates and preformed enzymes, which is 13 substrates more than in the GNI+ card. Once the card and suspension tube are loaded into the Vitek 2, all manipulations are automatic and incubation is online. The database is version R04.00 (7 June 2004). It contains 48 genera and 99 species. It is capable of identifying Brucella spp., B. pseudomallei, and Y. pestis (Table 3).
There have been several recent evaluations of this product (32, 41, 58, 69, 80). Accuracies of identifications ranged from 84.7% (32) when compared to conventional biochemicals to 95.0% (58) when compared to the API 20E. Joyanes et al. specifically addressed the identification of P. aeruginosa (146 strains characterized with the API 20NE and some conventional phenotypic tests), A. baumannii (25 strains characterized with conventional biochemicals), and S. maltophilia (27 strains characterized with API 20NE) and reported accuracies of 60.3, 68.0, and 100%, respectively (42). Lowe et al. noted that only 19 of 100 B. pseudomallei strains were identified correctly (60). O'Hara et al. reported that only 77.7% of eight species of Vibrio that are included in the database were identified accurately, although strains of P. damselae, V. fluvialis, V. mimicus, and V. parahaemolyticus were correctly identified ≥90.0% of the time (71).
Another important aspect of the Vitek 2 and the ID-GNB is the accuracy of identification when the inoculum is taken directly from a positive blood culture bottle without first being subcultured overnight. Bruins et al. reported that 93% of 344 Enterobacteriaceae and P. aeruginosa results were correct using an inoculum taken directly from a positive Bactec 9240 blood culture bottle compared to the identification results obtained after overnight subcultures were also processed in ID-GNB cards (14). In a similar study, Ling et al. reported that 82.2% (97 of 118 strains) of enterics and nonfermenters were correctly identified when the inoculum was taken directly from a positive BacT/Alert blood culture bottle and inoculated into the ID-GNB card (58). These identifications were compared to those obtained from overnight subcultures that were used with the API 20E, API 20NE, or other standard biochemical tests. Of the 21 unidentified strains, 13 were nonfermenters.
2GN.
The newly released 64-well 2GN card to be used on the Vitek 2 contains substrates for 47 tests instead of the 41 on the original ID-GNB card. These 47 tests are a combination of 26 tests from the ID-GNB card and 21 new tests and are entirely colorimetric in nature. All Vitek 2 instruments will be retrofitted with new optics to read this new card, at which point the production of the ID-GNB card will be discontinued. The current database is version 4.01 and contains 55 genera and 130 species of Enterobacteriaceae and aerobic, nonenteric, gram-negative bacilli. The database includes B. mallei, E. coli O157, F. tularensis, Salmonella spp., V. cholerae, and Y. pestis (Table 3).
A recent evaluation by Funke and Funke-Kissling, testing 655 isolates from 54 taxa, demonstrated an accuracy of 97.3% at the end of the initial incubation period (31a). There were no instances of a “no identification” call and only 0.6% misidentifications when compared to reference identifications from a combination of conventional methods, the ID32GN, and the API 20NE. At 7 h, 91.6% of all identifications were complete; at 8 h, 95.0% were complete. By 10 h, all identifications were complete.
A study by Bassel et al. that tested 447 fresh clinical isolates had an accuracy rate of 96.0% compared to ID-GNB results, while another study by Renaud tested 416 isolates for an accuracy rate of 97.6% compared to identifications from the ID32GN, API 20E, and API 20NE (A. Bassel, K. Kuhne, B. Celliere, J-S. Bonin, B. Blanc, M. Desmonceaux, R. Fillet, D. Monget, W. M. Dunne, and D. Pincus, Abstr. 104th Annu. Meet. Am. Soc. Microbiol., abstr. C-179, 2004; F. N. R. Renaud, S. Tigaud, C. Fuhrmann, B. Gravagna, and J. Freney, Abstr. 104th Annu. Meet. Am. Soc. Microbiol., abstr. C-180, 2004). Both studies had error rates of less than 1.0%.
A study that focused only on the identification of potential agents of bioterrorism, specifically, Brucella spp., B. mallei, B. pseudomallei, F. tularensis subsp. holartica, Y. pestis, and B. anthracis, was reported by Garin-Bastuji et al. (B. Garin-Bastuji, S. Chatellier, J. Vaissaire, D. Albert, C. LeDoujet, C. Mendy, M. Thiébaud, B. Blanc, C. Celliere, and G. Bossy, Abstr. 104th Annu. Meet. Am. Soc. Microbiol., abstr. C-177, 2004). Of the 92 strains from both human and animal origins, 98% were correctly identified. Only one strain, a strain of B. mallei, was misidentified.
Dade Behring MicroScan
In 1981, American MicroScan (then located in Hillsdale, N.J.) introduced the autoSCAN-3, a semiautomated instrument that utilized microdilution trays containing frozen conventional substrates for identification of bacterial isolates. An early evaluation, incorporating both Enterobacteriaceae and nonfermenters, by Ellner and Myers in 1981 reported an agreement of 95.0% between visually read and automated identifications, thus ensuring that machines were capable of accurate interpretations of the reactions in each well (25). The company then introduced the autoSCAN-4 in 1983, which brought with it improved dry panels that did not require refrigeration and included an updated database. Baxter Healthcare Corporation and subsequently Dade Behring have owned the company since its move to West Sacramento, Calif. In 1986, the autoSCAN-WalkAway came into the marketplace. This instrument is a combination incubator-reader that monitors the growth in bacterial identification panels in a completely “hands-off” method. This instrument has become known as the WalkAway. One of MicroScan's goals was to shorten the turnaround times for test results by using fluorogenic substrates in the panels. These “rapid” panels were first marketed in 1989.
The WalkAway SI, which is the present configuration of the system, can process 96 panels at one time. The data management system, called LabPro, runs on an adjacent computer.
Neg ID type 2.
The Neg ID type 2 panel was introduced in 1988 for the identification to species level of aerobic and facultatively anaerobic gram-negative bacilli and was designed to be read either manually or on the WalkAway instrument. The clear plastic 96-well tray contains 26 conventional substrates and 6 antimicrobials for inhibition of growth, all in dried form, and requires overnight incubation.
If the autoSCAN-4 is being used, the user must read the panel manually and convert the 34 reactions to a profile number. The identification is then obtained from a printed code book. The LabPro software (current version 1.51) will automatically identify a panel and organism that is processed in the WalkAway. The database contains 48 genera and 123 species.
This panel has been tested with selected groups of organisms. Saiman et al. reported that only 57% of nonmucoid and 40% of mucoid strains of P. aeruginosa isolated from cystic fibrosis patients could be identified by using this panel and the AutoSCAN-4 instrument (79).
Although the LabPro software was updated in 2004, the database for the Neg ID type 2 panel did not change. The most recent comprehensive evaluation of this panel with the WalkAway was by Sung et al. (88). In that study, 71.4% of 301 non-glucose-fermenting isolates were correctly identified at a probability level of ≥85% at the end of the initial incubation period compared to conventional tube biochemicals. Another 24.6% were correctly identified but at a low level of probability.
van Pelt et al. reported that of 70 isolates of B. cepacia, Ralstonia pickettii, S. maltophilia, and P. aeruginosa from cystic fibrosis patients tested on the WalkAway, only 68% of the B. cepacia strains were identified correctly, although the other identifications were reported accurately (91). O'Hara et al. tested 122 strains of eight species of Vibrio and reported an accuracy rate of 63.1% when 0.85% saline was added to the inoculum (71). Without the extra NaCl, only 51.6% were identified correctly. All strains of P. damselae were correctly identified.
Rapid Neg ID type 3.
The Rapid Neg ID type 3 panel was introduced in approximately 1998 as an update of the Neg ID type 2 panel. The Rapid Neg ID type 3 replaced 10 of the substrates on the Neg ID type 2 panel with newer ones and eliminated the need for the mineral oil overlay on the decarboxylase test. It also increased the shelf life from 6 months to 1 year when stored at 2 to 8°C. The rapid panel utilizes 36 substrates that work by one of the following mechanisms: hydrolysis of fluorogenic substrates, pH changes following substrate utilization, production of specific metabolic by-products, or evaluation of the rate of production of specific metabolic by-products after 2.5 h of incubation (67). These panels can be processed only on a WalkAway instrument, as their opaque color prevents a visual read of the wells. The bacterial suspensions must be made from 18 to 24 h colonies grown on MacConkey agar plates with lactose and crystal violet.
The current database is LabPro 1.51, which contains 44 genera and 125 species of both Enterobacteriaceae and oxidase-positive glucose-fermenting and nonfermenting gram-negative bacilli. The database includes Y. pestis, V. cholerae, and E. coli O157:H7 (Table 3).
O'Hara and Miller tested 511 organisms and reported an accuracy of 88.5% for Enterobacteriaceae and 78.8% for 170 nonenteric glucose-fermenting and nonfermenting gram-negative bacilli at the end of the initial incubation period (67, 68). O'Hara et al. reported 100% accuracy for V. alginolyticus, V. furnissii, V. hollisae, V. mimicus, and P. damselae compared to conventional biochemicals (71).
There have been several reports indicating the usefulness and accuracy of direct bacterial identification with inocula from positive blood culture bottles. A study by Waites et al. indicated 99% concordance between gram-negative identifications when blood was concentrated and the bacterial pellet was used to directly inoculate the panels and identifications resulting from standard biochemical methods (94). Although the panels and software have been updated since that study, it is reasonable to suspect that the same level of accuracy would be achieved with the SI instrument and the Rapid Neg ID type 3 panels.
Sherlock Microbial Identification System
The Sherlock Microbial Identification System, commonly known as the MIDI, was brought to the market in 1985 by Midi, Inc., Newark, Del. (www.midi-inc.com). The fully automated system combines fatty acid analysis with computerized high-resolution gas chromatography. The system analyzes fatty acids of 9 to 20 carbons in length and automatically identifies and quantitates them. The fatty acid profiles are then compared to a library of more than 100,000 entries, and a bacterial identification is rendered.
Isolates for identification are grown on Trypticase soy base, and while the list of consumables needed for sample preparation and chromatographic analysis is quite lengthy, they are all stored at room temperature. The initial investment for the instrument itself is comparable to those for other identification systems on the market.
The current clinical database, CLIN50, contains 63 genera and 164 species. There is also a bioterrorism library (BTR20) that contains 7 genera and 21 species, including B. anthracis, B. melitensis, B. mallei, B. pseudomallei, F. tularensis, and Y. pestis.
Recent publications of clinical interest include a study of 72 unusual isolates by Tang et al. that demonstrated an agreement with conventional biochemicals to the species level of 52.0% for 25 fermenters and 77.5% for 47 nonfermenters, for an overall agreement of 67.7% (89). The assignment of CDC weak oxidizer group 2 to the new genus Pandoraea, as published by Daneshvar et al., utilized this technology along with DNA relatedness (19). Khashe and Janda reported Shewanella alga to be the predominant Shewanella species associated with clinical specimens rather than S. putrefaciens (44). Several recent publications have addressed the identification of agents of bioterrorism (45, 53). Srinivasan et al. reported a case of laboratory-acquired glanders in a microbiologist in which the causative agent, B. mallei, was identified by using the MIDI instrument (87). The preliminary identification obtained by using an unidentified “automated bacterial identification system” had indicated the organism to be either Pseudomonas fluorescens or Pseudomonas putida.
Trek Diagnostic Systems
Trek Diagnostic Systems, located in Cleveland, Ohio, was founded in 1999 after having acquired the Sensititre line of products from AccuMed International, Inc. It currently has two instrument configurations on the market, one of which is the Sensititre AutoReader, a microprocessor-controlled, fully automatic fluorometer-reader that transfers test results to the associated data management system for processing, interpretation, and report generation. The second configuration, the Sensititre ARIS2X, is a fully automatic, bench top incubating and reading system. The ARIS2X utilizes internal bar codes to identify panels and assign appropriate incubation times. At the end of the assigned time, the machine automatically transports the panels to the fluorometric AutoReader for measurements. While the AutoReader can process an unlimited number of panels at one time because of its off-line incubation requirement, the ARIS2X has a 64-plate capacity. Both of the instruments use the same GNID plate for testing gram-negative organisms.
Sensititre GNID.
The GNID plate is a fluorometric reagent-based plate that contains both biochemical and enzyme tests. The 32 substrates, repeated three times across a 96-well plate, are grouped into one of four categories: sugar fermentation, fluorogenic substrate, carbon source utilization, and enzymatic. Because each 96-well plate contains three complete sets of biochemicals, three separate organisms may be tested simultaneously.
The current database of February 2004 contains 55 genera and 128 species in the clinical taxon list and contains Y. pestis and V. cholerae. To date, there have been no independent evaluations of the GNID plate.
TAXONOMIC CHANGES AND DATABASE UPDATES
As discussed earlier, taxonomic changes are constant. The current edition of Bergey's Manual of Systematic Bacteriology was published in 1984. The new edition, expected during 2005, will contain many hundreds of newly described organisms within the prokaryotes and many changes in the names of previously described species. These continuous changes, many of which have been made possible by advances in molecular technology, will take time to integrate into the laboratory's information system, making it a challenge for the clinical microbiologist to become familiar with the new names and the biochemical characteristics of the organisms. The manufacturers of diagnostic instruments will have to weigh the importance of providing accurate and current identifications versus the cost of an updated database. Just as important is the fact that adding more taxa to a database does not necessarily improve the accuracy of the system, particularly if the given product is not redesigned to accommodate the biochemical characteristics of the newly characterized organisms. A case in point is the API 20E database, which in 1977 contained 87 taxa, in 1992 contained 110 taxa, and in 2002 contained 102 taxa, with no redesign of the product itself. The accuracy of identifications, compared to those from conventional methods, was 91.1% in 1977 (78), but in 1992, with the revised database, the accuracy had declined to 78.7% at 24 h (70). It is imperative that clinical microbiologists not rely solely on an answer obtained from a commercial identification kit or system. This information should be evaluated together with colonial morphology, the results of antimicrobial susceptibility tests, and the specimen source information to arrive at a definitive identification.
Because the identification of BT agents has become a high priority, Table 3 has been included here as a quick reference to the inclusion of these agents in product databases.
IS THIS EVALUATION VALID?
In 1998, Congress passed legislation that relieved manufacturers of bacterial identification systems from the requirement to provide the Food and Drug Administration with data previously submitted in a 510K application (75). Prior to that time, such products were cleared by the Food and Drug Administration for diagnostic use based on those data submissions, as remains the case today with antimicrobial susceptibility products. Now, clinical microbiologists must rely on evaluations of new or upgraded products that have been performed in large hospitals or by reference laboratories. Miller, in 1991 (62), discussed the intricacies of the evaluation process, while Edberg and Konowe, in 1982, provided guidelines for conducting a valid instrument evaluation (22). While both of these publications are now over 10 years old, the concepts that they present are still very valid. As noted in the 8th edition of the Manual of Clinical Microbiology, each diagnostic laboratory must decide what levels of discrimination and accuracy to accept from their identification systems (72). A large laboratory with more resources might be more willing to accept a lower percent accuracy, knowing that they would have the resources of employees, time, and money to further investigate or confirm an identification. A smaller lab, conversely, might set their limits higher before they sent the isolate on to a reference lab for identification or confirmation. In compliance with the Clinical Laboratory Improvement Amendment of 1988, verification of a new method must be completed before the old method is replaced (72). What is acceptable in terms of accuracy for one laboratory may not be acceptable for another based on their patient population and specimen sources.
CONCLUSION
The choice of a method for the identification of bacteria involves many variables. This article has presented a comprehensive review of the choices available for bacterial identification products along with pertinent information on each one that should aid laboratories in making such decisions. The choice of an identification method depends on a variety of very important factors, some of which a laboratorian may have little control over. The capital resources, workforce technical acumen, physical laboratory size, patient population, and laboratory throughput are the primary driving forces that lead to a final decision. Since the goal of the microbiology laboratory is to provide results that are accurate and clinically relevant, it stands to reason that selecting the identification method contributes to the accuracy component of this paradigm, with the caveat that good microbiologists will use interpretive judgment when accepting a result from any automated instrument. Blind acceptance of species identification without skilled review of potential inaccuracies will eventually lead to misdiagnosis and inappropriate therapy—laboratory errors that we cannot afford to make. Therefore, one must not simply conclude that purchasing an automated identification instrument helps justify employing fewer or less skilled microbiology specialists for any clinical laboratory. Utilizing the information presented in this article, however, should assist management and technologists in the decision process constantly used to optimize our role in infectious disease diagnostics.
Acknowledgments
I sincerely appreciate the assistance given by the following individuals in updating much of the product information presented here: Paul Campognone (Becton Dickinson), Dave Pincus (bioMérieux), Linda Dominy (MicroScan), Craig Kunitsky (Midi), Sonia Guindon (Oxoid), Donna Price (Remel), and Jenny Lorbach (Trek). I also thank Karla Tomfohrde for her recollections of the initial days of commercial identifications.
REFERENCES
- 1.Aldridge, C., P. W. Jones, S. Gibson, J. Lanham, M. Meyer, R. Vannest, and R. Charles. 1977. Automated microbiological detection/identification system. J. Clin. Microbiol. 6:406-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, J. R., R. F. Schell, D. R. Pennell, and P. D. Wick. 1987. Identification of Yersinia spp. with the API 20E system. J. Clin. Microbiol. 25:2398-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, W. M., Jr., and R. H. Weaver. 1948. Quick microtechniques for the identification of cultures. I. Indole production. J. Lab. Clin. Med. 33:1334-1337. [PubMed] [Google Scholar]
- 4.Barr, J. G., A. M. Emmerson, G. M. Hogg, and E. T. M. Smyth. 1989. API-20NE and Sensititre autoidentification systems for identifying Pseudomonas spp. J. Clin. Pathol. 42:1113-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry, A. L., and R. E. Badal. 1982. Reliability of early identifications obtained with Enterobacteriaceae-Plus biochemical cards in the AutoMicrobic system. J. Clin. Microbiol. 16:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bascomb, S., S. P. LaPage, M. A. Curtis, and W. R. Willcox. 1973. Identification of bacteria by computer: identification of reference strains. J. Gen. Microbiol. 77:291-315. [DOI] [PubMed] [Google Scholar]
- 7.Bascomb, S., and R. C. Spencer. 1980. Automated methods for identification of bacteria from clinical specimens. J. Clin. Pathol. 33:36-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batchelor, B. I., R. J. Brindle, G. F. Gilks, and J. B. Selkon. 1992. Biochemical mis-identification of Brucella melitensis and subsequent laboratory-acquired infections. J. Hosp. Infect. 22:159-162. [DOI] [PubMed] [Google Scholar]
- 9.Bergey, D. H., R. S. Breed, E. G. D. Murray, and A. P. Hutchens. 1939. Bergey's manual of determinative bacteriology, p. 75-76. Biotech Publications, Geneva, N.Y.
- 10.Bernards, A. T., J. van der Toorn, C. P. A. van Boven, and L. Dijkshoorn. 1996. Evaluation of the ability of a commercial system to identify Acinetobacter genomic species. Eur. J. Clin. Microbiol. Infect. Dis. 15:303-308. [DOI] [PubMed] [Google Scholar]
- 11.Bourbeau, P. P., and B. J. Heiter. 1998. Comparison of Vitek GNI and GNI+ cards for identification of gram-negative bacteria. J. Clin. Microbiol. 36:2775-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner, D. J. 1983. Impact of modern taxonomy on clinical microbiology. ASM News 49:58-63. [Google Scholar]
- 13.Brisse, S., S. Stefani, J. Verhoef, A. Van Belkum, P. Vandamme, and W. Goessens. 2002. Comparative evaluation of the BD Phoenix and Vitek 2 automated instruments for identification of isolates of the Burkholderia cepacia complex. J. Clin. Microbiol. 40:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruins, M. J., P. Bloembergen, G. J. H. M. Ruijs, and M. J. H. M. Wolfhagen. 2004. Identification and susceptibility testing of Enterobacteriaceae and Pseudomonas aeruginosa by direct inoculation from positive BACTEC blood culture bottles into Vitek 2. J. Clin. Microbiol. 42:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen, W. B. 1946. Urea decomposition as a means of differentiating Proteus and paracolon cultures from each other and from Salmonella and Shigella types. J. Bacteriol. 52:461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarridge, J. E., and S. Zighelboim-Daum. 1985. Isolation and characterization of two hemolytic phenotypes of Vibrio damselae associated with a fatal wound infection. J. Clin. Microbiol. 21:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton, M. T., S. Comeau, B. Rainnie, K. Lambert, and K. R. Forward. 1993. A comparison of the API Uriscreen with the Vitek Urine Identification-3 and the leukocyte esterase or nitrite strip as a screening test for bacteriuria. Diagn. Microbiol. Infect. Dis. 16:93-97. [DOI] [PubMed] [Google Scholar]
- 18.Dance, D. A. B., V. Withiekanun, P. Naigowit, and N. J. White. 1989. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API 20NE. J. Clin. Pathol. 42:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneshvar, M. I., D. G. Hollis, A. G. Steigerwalt, A. M. Whitney, L. Spangler, M. P. Douglas, J. G. Jordan, J. P. MacGregor, B. C. Hill, F. C. Tenover, D. J. Brenner, and R. S. Weyant. 2001. Assignment of CDC weak oxidizer group 2 (WO-2) to the genus Pandoraea and characterization of three new Pandoraea genomospecies. J. Clin. Microbiol. 39:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGirolami, P. C., J. S. Poliferno, L. S. Mills, and K. A. Eichelberger. 1988. Evaluation of the IDS RapID SS/u system for rapid identification of urinary microorganisms. Am. J. Clin. Pathol. 89:791-793. [DOI] [PubMed] [Google Scholar]
- 20a.Donay, J.-L., D. Mathieu, P. Fernandes, C. Prégermain, P. Bruel, A. Wargnier, I. Casin, F. X. Weill, P. H. Lagrange, and J. L. Herrmann. 2004. Evaluation of the automated Phoenix system for potential routine use in the clinical microbiology laboratory. J. Clin. Microbiol. 42:1542-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edberg, S. C., D. Clare, M. H. Moore, and J. M. Singer. 1979. Rapid identification of Enterobacteriaceae from blood cultures with the Micro-ID system. J. Clin. Microbiol. 10:693-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edberg, S. C., and L. S. Konowe. 1982. A systematic means to conduct a microbiology evaluation. In V. Lorian (ed.), Significance of medical microbiology in the care of patients, 2nd ed. Williams & Wilkins, Baltimore, Md.
- 23.Edinger, R., R. R. Courtney, and G. E. Hollick. 1994. Evaluation of the Pasco gram-negative nonfermenter identification system. Diagn. Microbiol. Infect. Dis. 19:61-63. [DOI] [PubMed] [Google Scholar]
- 24.Edwards, P. R., and W. H. Ewing. 1962. Identification of Enterobacteriaceae. Burgess Publishing Co., Minneapolis, Minn.
- 25.Ellner, P. D., and D. A. Myers. 1981. Preliminary evaluation of the autoSCAN-3, an instrument for automated reading and interpretation of microdilution trays: identification of aerobic gram-negative bacilli. J. Clin. Microbiol. 14:326-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsaghir, A. A. F., and E. A. James. 2003. Misidentification of Brucella melitensis as Ochrobactrum anthropi by API 20NE. J. Med. Microbiol. 52:441-442. [DOI] [PubMed] [Google Scholar]
- 27.Endimiani, A., F. Luzzaro, A. Tamborini, G. Lombardi, V. Elia, R. Belloni, and A. Toniolo. 2002. Identification and antimicrobial susceptibility testing of clinical isolates of nonfermenting gram-negative bacteria by the Phoenix automated microbiology system. Microbiologica 25:323-329. [PubMed] [Google Scholar]
- 28.Ewing, W. H. 1974. Differentiation of Enterobacteriaceae by biochemical reactions, p. 3-5. Centers for Disease Control, Atlanta, Ga.
- 29.Farmer, J. J., III. 2003. Enterobacteriaceae: introduction and identification, p. 636-653. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 30.Finegold, S. M., and E. J. Baron. 1986. Bailey and Scott's diagnostic microbiology, 7th ed. The C. V. Mosby Co., St. Louis, Mo.
- 31.Friedman, R. B., D. Bruce, J. MacLowry, and V. Brenner. 1973. Computer-assisted identification of bacteria. Am. J. Clin. Pathol. 60:395-403. [DOI] [PubMed] [Google Scholar]
- 31a.Funke, G., and P. Funke-Kissling. 2004. Evaluation of the new Vitek 2GN card for identification of clinically relevant gram-negative rods. J. Clin. Microbiol. 42:4067-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funke, G., D. Monnet, C. deBernardis, A. von Graevenitz, and J. Freney. 1998. Evaluation of the Vitek 2 system for rapid identification of medically relevant gram-negative rods. J. Clin. Microbiol. 36:1948-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandelman, A. L., and P. H. Mann. 1965. An evaluation of reagent-impregnated paper strips for use in the process of identifying certain species of clinically important bacteria. Curr. Ther. Res. 7:130-138. [PubMed] [Google Scholar]
- 34.Halstead, D. C., M. R. Hoffert, and G. C. Colasante. 1987. Premarket evaluation of IDS RapID SS/u system for identification of urine isolates. J. Clin. Microbiol. 25:42-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen, D. S., A. G. Jensen, N. Nørskov-Lauritsen, R. Skov, and B. Bruun. 2002. Direct identification and susceptibility testing of enteric bacilli from positive blood cultures using Vitek (GNI+/GNS-GA). Clin. Microbiol. Infect. 8:38-44. [DOI] [PubMed] [Google Scholar]
- 36.Hardy, A. V., R. P. Mason, and G. A. Martin. 1952. The dysenteries in the armed forces. Am. J. Trop. Med. Hyg. 1:171-175. [DOI] [PubMed] [Google Scholar]
- 37.Huber, T. W. 1985. The Automicrobic system for detection of bacteriuria: efficacy of revised urine identification cards. Am. J. Clin. Pathol. 84:637-642. [DOI] [PubMed] [Google Scholar]
- 38.Imperatrice, C. A., and I. Nachamkin. 1993. Evaluation of the Vitek EPS enteric pathogen screen card for detecting Salmonella, Shigella, and Yersinia spp. J. Clin. Microbiol. 31:433-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inglis, T. J. J., D. Chiang, G. S. H. Lee, and L. Chor-Kiang. 1998. Potential misidentification of Burkholderia pseudomallei by API 20NE. Pathology 30:62-64. [DOI] [PubMed] [Google Scholar]
- 40.Isenberg, H. D., T. L. Gavan, P. B. Smith, A. Sonnenwirth, W. Taylor, W. J. Martin, D. Rhoden, and A. Balows. 1980. Collaborative investigation of the AutoMicrobic system Enterobacteriaceae biochemical card. J. Clin. Microbiol. 11:694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jossart, M.-F., and R. J. Courcol. 1999. Evaluation of an automated system for identification of Enterobacteriaceae and nonfermenting bacilli. Eur. J. Clin. Microbiol. Infect. Dis. 18:902-907. [DOI] [PubMed] [Google Scholar]
- 42.Joyanes, P., M. D. C. Conejo, L. Martinez-Martinez, and E. J. Perea. 2001. Evaluation of the Vitek 2 system for the identification and susceptibility testing of three species of nonfermenting gram-negative rods frequently isolated from clinical samples. J. Clin. Microbiol. 39:3247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kauffmann, F., and P. R. Edwards. 1952. Classification and nomenclature of Enterobacteriaceae. Int. Bull. Bacteriol. Nomen. Tax. 2:2-8. [Google Scholar]
- 44.Khashe, S., and J. M. Janda. 1998. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J. Clin. Microbiol. 36:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim, W. Y., T. W. Song, M. O. Song, J. Y. Nam, C. M. Park, K. J. Kim, S. I. Chung, and C. S. Choi. 2001. Analysis of cellular fatty acid methyl esters (FAMEs) for the identification of Bacillus anthracis. J. Kor. Soc. Microbiol. 35:31-40. [Google Scholar]
- 46.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitch, T. T., M. R. Jacobs, and P. C. Appelbaum. 1992. Evaluation of the 4-hour RapID NF Plus method for identification of 345 gram-negative nonfermentative rods. J. Clin. Microbiol. 30:1267-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitch, T. T., M. R. Jacobs, and P. C. Appelbaum. 1994. Evaluation of RapID onE system for identification of 379 strains in the family Enterobacteriaceae and oxidase-negative, gram-negative nonfermenters. J. Clin. Microbiol. 32:931-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kligler, I. J. 1918. Modifications of culture media used in the isolation and differentiation of typhoid, dysentery, and allied bacilli. J. Exp. Med. 28:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koser, S. A. 1924. Correlation of citrate utilization by members of the colon-Aerogenes group with other differential characteristics and with habitat. J. Bacteriol. 9:59-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lampe, A. S., and T. J. K. van der Reijden. 1984. Evaluation of commercial test systems for the identification of nonfermenters. Eur. J. Clin. Microbiol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 52.LaPage, S. P., S. Bascomb, W. R. Willcox, and M. A. Curtis. 1973. Identification of bacteria by computer: general aspects and perspectives. J. Gen. Microbiol. 77:273-290. [DOI] [PubMed] [Google Scholar]
- 53.Leclercq, A., A. Guiyoule, M. El Lioui, E. Carniel, and J. Decallonne. 2000. High homogeneity of the Yersinia pestis fatty acid composition. J. Clin. Microbiol. 38:1545-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leclerq, A., B. Lambert, D. Picard, and J. Mahillon. 2001. Particular biochemical profiles for enterohemorrhagic Escherichia coli O157:H7 isolates on the ID32E system. J. Clin. Microbiol. 2001:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee, J. H., M. Z. Huang, J. L. Wu, and W. C. Tsai. 1994. Assessment of a new four-hour diagnostic kit-RapID onE system for the identification of enteric bacteria. Chinese J. Microbiol. Immunol. 27:133-139. [PubMed] [Google Scholar]
- 56.LeMinor, L., and F. B. Hamida. 1962. Avantages de la recherche de la β-galactosidase sur celle de la fermentation du lactose en milieu complexe dans le diagnostic bactériologique, en particulier des Enterobacteriaceae. Ann. Inst. Pasteur 102:267-277. [Google Scholar]
- 57.Levine, M., S. S. Epstein, and R. H. Vaughn. 1934. Differential reactions in the colon group of bacteria. Am. J. Public Health 24:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ling, T. K. W., P. C. Tam, Z. K. Liu, and A. F. B. Cheng. 2001. Evaluation of Vitek 2 rapid identification and susceptibility testing system against gram-negative clinical isolates. J. Clin. Microbiol. 39:2964-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu, T. K. W., Z. K. Liu, and A. F. B. Cheng. 2003. Evaluation of the Vitek 2 system for rapid direct identification and susceptibility testing of gram-negative bacilli from positive blood cultures. J. Clin. Microbiol. 41:4705-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lowe, P., C. Engler, and R. Norton. 2002. Comparison of automated and nonautomated systems for identification of Burkholderia pseudomallei. J. Clin. Microbiol. 40:4625-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mann, P. H., and A. L. Gandelman. 1965. An evaluation of reagent-impregnated paper strips for use in the process of identifying certain species of clinically important bacteria. II. Testing for the production of indole. Curr. Ther. Res. 7:510-512. [PubMed] [Google Scholar]
- 62.Miller, J. M. 1991. Evaluating biochemical identification systems. J. Clin. Microbiol. 29:1559-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Møller, V. 1955. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol. Microbiol. Scand. 36:158-172. [DOI] [PubMed] [Google Scholar]
- 64.Moore, D. F., S. S. Hamada, E. Marso, and W. J. Martin. 1981. Rapid identification and antimicrobial susceptibility testing of gram-negative bacilli from blood cultures by the AutoMicrobic system. J. Clin. Microbiol. 13:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morse, S. A. 2001. Bioterrorism: laboratory security. Lab. Med. 32:303-306. [Google Scholar]
- 66.Neubauer, H., T. Sauer, H. Becker, S. Aleksic, and H. Meyer. 1998. Comparison of systems for identification and differentiation of species within the genus Yersinia. J. Clin. Microbiol. 36:3366-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Hara, C. M., and J. M. Miller. 2000. Evaluation of the MicroScan Rapid Neg ID3 panel for identification of Enterobacteriaceae and some common gram-negative nonfermenters. J. Clin. Microbiol. 38:3577-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Hara, C. M., and J. M. Miller. 2002. Ability of the MicroScan Rapid Gram-Negative ID Type 3 panel to identify nonenteric glucose-fermenting and nonfermenting gram-negative bacilli. J. Clin. Microbiol. 40:3750-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Hara, C. M., and J. M. Miller. 2003. Evaluation of the Vitek 2 ID-GNB assay for identification of members of the family Enterobacteriaceae and other nonenteric gram-negative bacilli and comparison with the Vitek GNI+ card. J. Clin. Microbiol. 41:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Hara, C. M., D. L. Rhoden, and J. M. Miller. 1992. Reevaluation of the API 20E identification system versus conventional biochemicals for identification of members of the family Enterobacteriaceae: a new look at an old product. J. Clin. Microbiol. 30:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Hara, C. M., E. G. Sowers, C. A. Bopp, S. B. Duda, and N. A. Strockbine. 2003. Accuracy of six commercially available systems for identification of members of the family Vibrionaceae. J. Clin. Microbiol. 41:5654-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Hara, C. M., M. P. Weinstein, and J. M. Miller. 2003. Manual and automated systems for detection and identification of microorganisms, p. 185-207. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 73.Pacova, Z., and M. Dlouhy. 1998. Identification of Pseudomonas stutzeri with the NEFERM test, NEFERM test 24 and API 20NE commercial test kits. Epidemiol. Mikrobiol. Immunol. (Czech) 47:47-51. [PubMed] [Google Scholar]
- 74.Park, T. S., S. H. Oh, E. Y. Lee, T. K. Lee, K. H. Park, M. J. Figueras, and C. L. Chang. 2003. Misidentification of Aeromonas veronii biovar sobria as Vibrio alginolyticus by the Vitek system. Lett. Appl. Microbiol. 37:349-353. [DOI] [PubMed] [Google Scholar]
- 75.Pugliese, G., and M. S. Favero (ed.). 1998. CDC to take over FDA's evaluation of bacterial identification methods. Infect. Cont. Hosp. Epidemiol. 19:929. [Google Scholar]
- 76.Randall, E. L. 1979. The future of automation in microbiology. Clin. Microbiol. Newsl. 1:5-6. [Google Scholar]
- 77.Russell, F. F. 1911. The isolation of typhoid bacilli from urine and feces with the description of a new double sugar tube medium. J. Med. Res. 25:217-229. [PMC free article] [PubMed] [Google Scholar]
- 78.Rutherford, I., V. Moody, T. L. Gavan, L. W. Ayers, and D. L. Taylor. 1977. Comparative study of three methods of identification of Enterobacteriaceae. J. Clin. Microbiol. 5:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saiman, L., J. L. Burns, D. Larone, Y. Chen, E. Garber, and S. Whittier. 2003. Evaluation of MicroScan Autoscan for identification of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 41:492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanders, C. C., M. Peyret, E. S. Moland, S. J. Cavalieri, C. Shubert, K. S. Thomson, J.-M. Boeufgras, and W. E. Sanders, Jr. 2001. Potential impact of the Vitek 2 system and the advanced expert system on the clinical laboratory of a university-based hospital. J. Clin. Microbiol. 39:2379-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharmer, N. K., P. W. Doyle, S. A. Gerbasi, and J. H. Jessop. 1990. Identification of Yersinia by the API 20E. J. Clin. Microbiol. 28:1443-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmons, J. S. 1926. A culture medium for differentiating organisms of typhoid-colon aerogenes groups and for isolation of certain fungi. J. Infect. Dis. 39:209-217. [Google Scholar]
- 83.Smith, P. B., K. M. Tomfohrde, D. L. Rhoden, and A. Balows. 1972. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl. Microbiol. 24:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snyder, J. W. 2003. Role of the hospital-based microbiology laboratory in preparation for and response to a bioterrorism event. J. Clin. Microbiol. 41:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soler, L., F. Marco, J. Vila, M. R. Chacón, J. Guarro, and M. J. Figueras. 2003. Evaluation of two miniaturized systems, MicroScan W/A and BBL Crystal E/NF, for identification of clinical isolates of Aeromonas spp. J. Clin. Microbiol. 41:5732-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soto, O. B. 1949. Fermentation reactions with dried paper discs containing carbohydrate and indicator. Puerto Rico J. Public Health. 25:96-100. [PubMed] [Google Scholar]
- 87.Srinivasan, A., C. N. Kraus, D. DeShazer, P. M. Becker, J. D. Dick, L. Spacek, J. G. Bartlett, W. R. Byrne, and D. L. Thomas. 2001. Glanders in a military research microbiologist. N. Engl. J. Med. 345:287-289. [DOI] [PubMed] [Google Scholar]
- 87a.Stefaniuk, E., A. Baraniak, M. Gniadkowski, and W. Hryniewicz. 2003. Evaluation of the BD Phoenix automated identification and susceptibility testing system in clinical microbiology laboratory practice. Eur. J. Clin. Microbiol. Infect. Dis. 22:479-485. [DOI] [PubMed] [Google Scholar]
- 88.Sung, L. L., D. I. Yang, C. C. Hung, and H. T. Ho. 2000. Evaluation of autoSCAN-W/A and the Vitek GNI+ AutoMicrobic system for identification of non-glucose-fermenting gram-negative bacilli. J. Clin. Microbiol. 38:1127-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang, Y.-W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teng, L.-J., P.-R. Hsueh, H.-J. Pan, S.-W. Ho, and K.-T Luh. 2001. Persistent bacteraemia caused by a single clone of Burkholderia cepacia with unusual phenotype. J. Infect. 42:202-205. [DOI] [PubMed] [Google Scholar]
- 91.van Pelt, C., C. M. Verduin, W. H. F. Goessens, M. C. Vos, B. Tümmler, C. Segonds, F. Reubsaet, H. Verbrugh, and A. van Belkum. 1999. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J. Clin. Microbiol. 37:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voges, O., and B. Proskauer. 1898. Beitrag zur Ernahrungs physiologie und sur differential diagnose der Backerien der hamorrhagiahen septicamie. Zeit. Hyg. 28:20-37. [Google Scholar]
- 93.Vracko, R., and J. C. Sherris. 1963. Indole-spot test in bacteriology. Am. J. Clin. Pathol. 39:429-432. [PubMed] [Google Scholar]
- 94.Waites, K. B., E. S. Brookings, S. A. Moser, and B. L. Zimmer. 1998. Direct bacterial identification from positive BacT/Alert blood cultures using MicroScan overnight and rapid panels. Diagn. Microbiol. Infect. Dis. 32:21-26. [DOI] [PubMed] [Google Scholar]
- 95.Washington, J. A., II, P. K. W. Yu, and W. J. Martin. 1971. Evaluation of accuracy of multitest micromethod system for identification of Enterobacteriaceae. Appl. Microbiol. 22:267-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Willcox, W. R., S. P. LaPage, S. Bascomb, and M. A. Curtis. 1973. Identification of bacteria by computer: theory and programming. J. Gen. Microbiol. 77:317-330. [DOI] [PubMed] [Google Scholar]
- 97.Williams, R. E. O. 1977. Introduction, p. 1. In H. H. Johnston and S. W. B. Newsom (ed.), Second International Symposium on Rapid Methods and Automation in Microbiology. Research Studies Press, Inc., Forest Grove, Oreg.
- 98.Wilmoth, B. A., M. C. Chu, and T. J. Quan. 1996. Identification of Yersinia pestis by BBL Crystal Enteric/Nonfermenter identification system. J. Clin. Microbiol. 34:2829-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]


