Significance
Parasitic plants are pests of many plants, including major crop species. An important step toward creating resistance to parasitic plants is gaining a better understanding of how these pathogens control the physiology and development of their hosts. We combined genetic, cell-biological, and biochemical methods to identify the plant hormone cytokinin as a mobile signal between the hemiparasitic plant Phtheirospermum japonicum and the host Arabidopsis thaliana. Transport of parasite-derived cytokinins induced morphological changes in host roots, revealing insights into how parasitic plants manipulate host development and laying the foundation for future explorations for bioactive molecule transfer from parasitic plants to hosts.
Keywords: cytokinin, transport, hypertrophy, parasitism, Arabidopsis
Abstract
Parasitic plants share a common anatomical feature, the haustorium. Haustoria enable both infection and nutrient transfer, which often leads to growth penalties for host plants and yield reduction in crop species. Haustoria also reciprocally transfer substances, such as RNA and proteins, from parasite to host, but the biological relevance for such movement remains unknown. Here, we studied such interspecies transport by using the hemiparasitic plant Phtheirospermum japonicum during infection of Arabidopsis thaliana. Tracer experiments revealed a rapid and efficient transfer of carboxyfluorescein diacetate (CFDA) from host to parasite upon formation of vascular connections. In addition, Phtheirospermum induced hypertrophy in host roots at the site of infection, a form of enhanced secondary growth that is commonly observed during various parasitic plant–host interactions. The plant hormone cytokinin is important for secondary growth, and we observed increases in cytokinin and its response during infection in both host and parasite. Phtheirospermum-induced host hypertrophy required cytokinin signaling genes (AHK3,4) but not cytokinin biosynthesis genes (IPT1,3,5,7) in the host. Furthermore, expression of a cytokinin-degrading enzyme in Phtheirospermum prevented host hypertrophy. Wild-type hosts with hypertrophy were smaller than ahk3,4 mutant hosts resistant to hypertrophy, suggesting hypertrophy improves the efficiency of parasitism. Taken together, these results demonstrate that the interspecies movement of a parasite-derived hormone modified both host root morphology and fitness. Several microbial and animal plant pathogens use cytokinins during infections, highlighting the central role of this growth hormone during the establishment of plant diseases and revealing a common strategy for parasite infections of plants.
Parasitic plants are widespread agricultural pests and account for ∼1% of known flowering plants species (1). Parasitism ranges from holoparasites, which depend entirely on nutrient supply from host plants, to hemiparasites, which obtain nutrients via their own photosynthesis and from their hosts (1). Many hemiparasites do not depend on parasitism but often parasitize when conditions are suitable. These hemiparasitic plants include parasitic plants such as the commonly studied Orobanchaceae species Rhinanthus minor, Triphysaria versicolor, and Phtheirospermum japonicum. Both hemiparasites and holoparasites form specialized organs called haustoria that undergo a developmental transition from proto-haustoria to mature haustoria during the penetration and infection of host tissues to acquire nutrients and water (2). Some parasitic plants such as Striga or Rhinanthus form vascular connections exclusively to host xylem via xylem bridges (xylem-feeding), whereas haustoria of other plants such as Cuscuta or Orobanche also form symplastic phloem-to-phloem connections to host plants (phloem-feeding) (1). In addition to water and nutrients, other small substances are transferred across haustoria, including RNAs and proteins (3–5), but the biological relevance for this movement is not clear. Beyond parasitic plants, various plant-pathogenic microbes, insects, and nematodes produce compounds that move into the host and contribute to their virulence, including the plant hormone cytokinin (6–8).
Cytokinins participate in many physiological and developmental plant processes such as cell division, growth, vascular development, senescence, photosynthesis, and nutrient allocation (9). Cytokinins are isoprenoid substituted adenines and in plants, isoprenoid transfer by isopentenyltransferases (IPTs) is the rate-limiting and crucial step for producing various types of cytokinins including cis-zeatin (cZ), N6-(∆2-isopentenyl)-adenine (iP), trans-zeatin (tZ), and dihydrozeatin (DZ) (10). tZ is the most abundant and the most potent cytokinin in Arabidopsis (9). Cytokinins are further metabolized and inactivated through conjugation to sugars or through cleavage by cytokinin oxidases (CKXs) (11). Cytokinins act at the site of biosynthesis and are also mobile within the plant vascular system (12, 13). Grafting experiments with Arabidopsis ipt1,3,5,7 mutants demonstrated root-to-shoot movement of tZ-type cytokinins and an opposing shoot-to-root movement of iP-type cytokinins (13), consistent with the idea that tZ-type cytokinins predominate in the xylem, whereas iP-types predominate in the phloem (13). ARABIDOPSIS HISTIDINE KINASE 2, 3 and 4 (AHK2, AHK3, AHK4) receptors directly bind cytokinins and trigger downstream responses (14), including the transcriptional induction of A-type ARABIDOPSIS RESPONSE REGULATOR (ARR) genes such as ARR5 (15). Although Arabidopsis cytokinin receptors act largely redundantly, combinations of ahk double and triple mutants show various cytokinin-deficient phenotypes (14).
Increased cytokinin levels were previously reported during infection by parasitic plants such as Cuscuta spp., mistletoes, Santalum album, and several Orobanchaceae species, but the source of the cytokinins and the biological relevance were unknown (16, 17). Here, we demonstrate the parasitic plant Phtheirospermum increases its cytokinin levels upon infection, and these cytokinin species move into the host Arabidopsis. We further demonstrate these cytokinin species are bioactive in Arabidopsis roots and induce changes in gene response, cell division, and cell differentiation that leads to modifications in host root morphology and impacts host fitness.
Results
Phtheirospermum Parasitizes Arabidopsis.
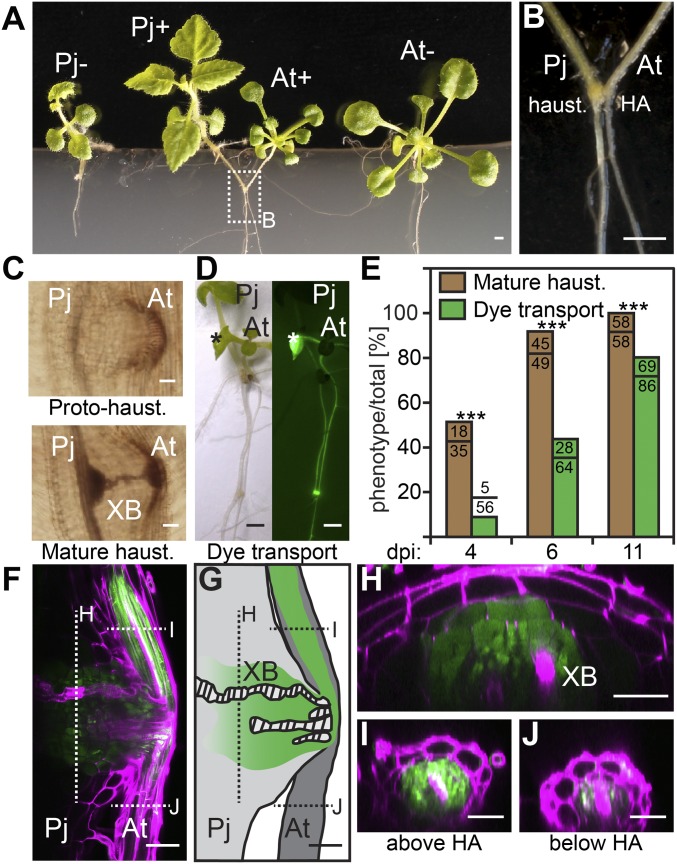
Phtheirospermum infects a variety of plant species including rice, maize, and Arabidopsis (18, 19). We sought to identify conditions that promote Arabidopsis infection because growth of Phtheirospermum does not absolutely depend on parasitism. As previously described, haustorium development occurs when Phtheirospermum comes in contact with its host on water-agar with no additional nutrients (18, 19). We used a similar water-only setup, but substituted Whatman filter paper and nylon membrane for agar to anchor the plants, similar to an experimental setup used for Arabidopsis grafting (20). This low nutrient environment allowed efficient infection, consistent with observations that low levels of nitrogen are beneficial for infection by other parasitic plants such as Striga hermonthica (21). Under these conditions, Phtheirospermum formed haustoria on Arabidopsis roots within 3 d (Fig. 1 A and B). At 7 d postinfection (dpi), infected and noninfected controls were moved to 1/2 strength Murashige and Skoog (MS) growth medium to assess the long-term effects of Phtheirospermum parasitism on Arabidopsis in the absence of low nutrient stress. Infected and noninfected plants were of similar size at 7 dpi, but infected Arabidopsis developed poorly in contrast to uninfected controls and showed a clear reduction in size at 30 dpi (Fig. 1A and Movie S1). Meanwhile, Phtheirospermum-infecting Arabidopsis grew larger than Phtheirospermum growing solitarily (Fig. 1A and Movie S1). Safranin-O staining of haustoria confirmed the formation of xylem bridges from Phtheirospermum to Arabidopsis during transition from protohaustoria to mature haustoria, typically 3–4 dpi, consistent with normal haustorial development (Fig. 1 C and E) (18).
Fig. 1.
Phtheirospermum parasitizes Arabidopsis. (A) Phtheirospermum growing alone (Pj-) or infecting (Pj+) Arabidopsis (At+) show increased Phtheirospermum size and decreased Arabidopsis size compared with uninfected controls (At-) at 30 dpi. (B) Detail image of Phtheirospermum-infecting Arabidopsis with Phtheirospermum haustorium (haust.) attachment site (HA). (C) Images of Safranin-O stained proto- and mature haustoria show differences in xylem bridge (XB) formation. (D) CFDA transport ability of 11 dpi haustoria were assayed 90 min after application of CFDA onto host leaves (asterisk). (E) Ratios of mature haustoria (brown bar) and haustoria with CFDA transport ability (green bar) (n = 35–86) were quantified for the indicated time points (Fisher Exact Test, P < 0.001). (F) A fluorescent image of a single optical plane of the haustorium 90 min after CFDA application onto a host leaf. CFDA fluorescence is green, and cell walls were stained with propidium iodide in magenta. (G) Schematic representation of F with indicated optical section of the haustorium (H), the host root above (I) or below (J) the HA. (Scale bars: A, B, and D, 1 mm; C, F, and G, 50 µm; H and I, 25 µm.)
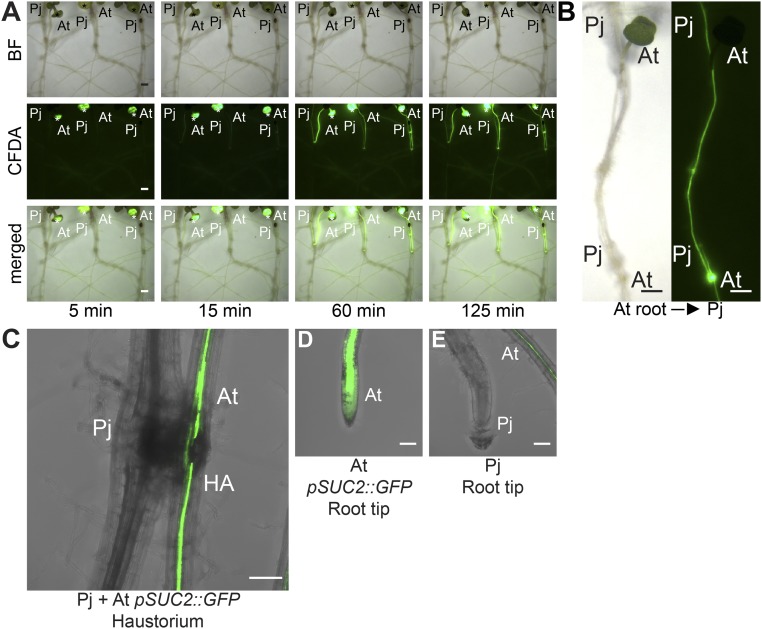
To characterize the functionality of nascent haustoria, we monitored haustorial transport activity by using the vascular transport dye carboxyfluorescein diacetate (CFDA) (22). After CFDA application to host roots or shoots, fluorescent signals rapidly propagated from host vasculature into Phtheirospermum haustoria and shoots, indicating efficient transport from host to parasite as early as 15 min after CFDA application (Fig. 1D, Fig. S1 A and B, and Movie S2). To investigate the role of xylem bridges for CFDA transport, we compared the ratio of CFDA uptake to the ratio of mature haustoria with xylem bridges at different time points (Fig. 1E). More than 50% of Phtheirospermum developed haustoria with at least one xylem bridge at 4 dpi, whereas less than 10% of Phtheirospermum transported CFDA at this time point (Fig. 1E). The percentage of Phtheirospermum transporting CFDA gradually increased at 6 and 11 dpi when the majority of haustoria reached maturity (Fig. 1E). A strong fluorescent signal was detected in mature haustorial tissues surrounding the xylem bridges within the Phtheirospermum haustorium (Fig. 1 F and H). The fluorescence signal decreased gradually toward the proximal haustorial tissue (Fig. 1 F–H). At the same time, fluorescence in host roots above the haustorium attachment site was stronger than below, indicating preferential CFDA uptake from the host vasculature via the haustorium (Fig. 1 I and J). To investigate the possibility of transport via phloem connections, we infected Phtheirospermum to Arabidopsis pSUC2::GFP plants that express mobile GFP in the phloem (23). GFP was not detected in Phtheirospermum haustoria or root tips, whereas GFP was efficiently unloaded in root tips of infected Arabidopsis pSUC2::GFP plants (Fig. S1 C–E). Furthermore, no gradient in fluorescence intensities along haustoria was detected for phloem-mobile GFP in infected Arabidopsis pSUC2::GFP roots (Fig. S1C).
Fig. S1.
Different transport routes between Phtheirospermum and host plants. (A) Leaves of three Arabidopsis (At) plants each infected with one Phtheirospermum (Pj) plant at 11 dpi were labeled with CFDA and CFDA movement to Pj was monitored over time (same experiment as shown in Movie S2). (B) Images show CFDA transport from infected At roots to Pj shoots 90 min after CFDA was applied to At roots tips. (C) Arabidopsis pSUC2::GFP (At) expressing plants were infected with Phtheirospermum (Pj) and confocal stacks were taken of the haustorium attachment site (HA) at 11 dpi or root tips of At (D) and Phtheirospermum (E) to show GFP unloading in sink tissue. (Scale bars: A and B, 1 mm; C–E, 100 µm.)
Phtheirospermum Induces Hypertrophy and Cytokinin Responses in Host Plants.
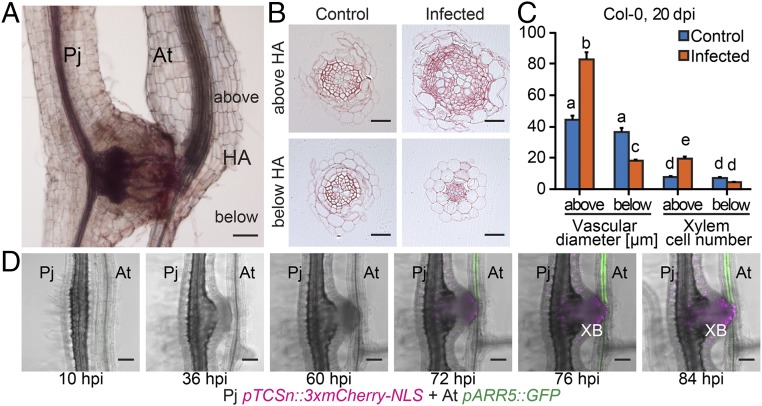
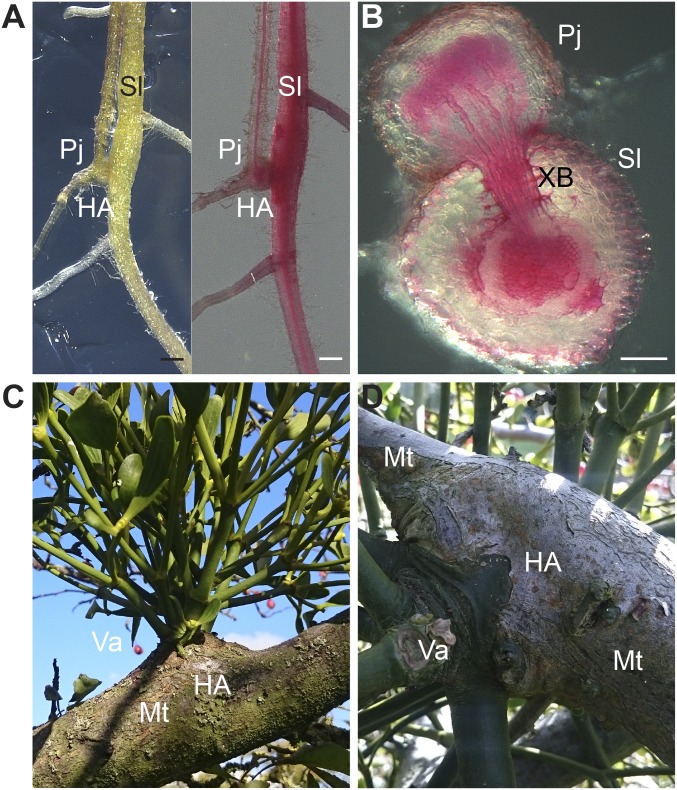
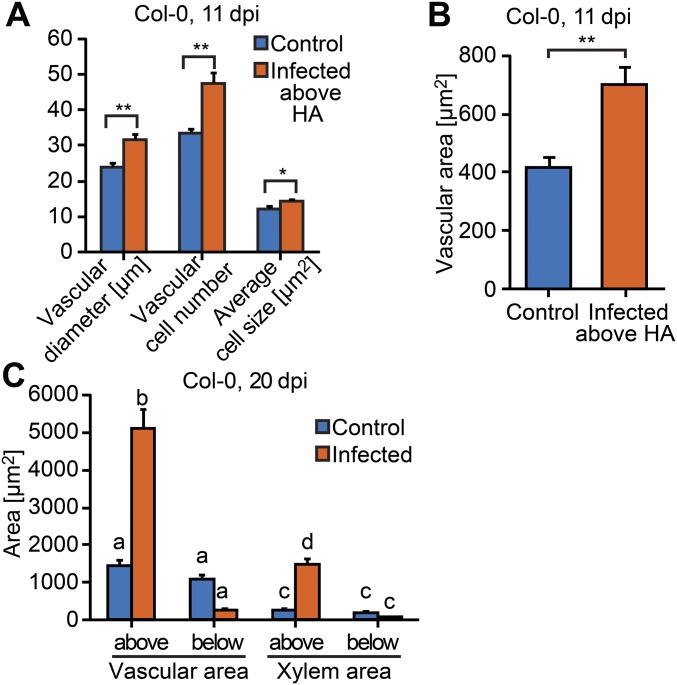
After Phtheirospermum established xylem connections to Arabidopsis, we observed a swelling of Arabidopsis tissue above haustorium attachment sites (Figs. 1B and 2A), a phenomenon referred to as hypertrophy (1). Hypertrophy resulted from an increase in vascular cell size (hypertrophy) and cell number (hyperplasia) that enlarged the vascular and xylem area in host tissue above haustoria attachment sites compared with host tissue below the haustoria or uninfected controls (Fig. 2 B and C). Hypertrophy was not specific to Arabidopsis because a similar effect also occurred in tomato (Solanum lycopersicum) infected by Phtheirospermum (Fig. S2 A and B). Increases in vascular size were already detected above the haustoria at 11 dpi (Fig. S3 A and B), and by 20 dpi, quantifications of root sections prepared above and below the haustorium confirmed a further increase in vascular and xylem diameter and cell number above haustorium attachment sites, whereas no significant differences in uninfected plants were detected (Fig. 2C). The vascular and xylem areas above haustorium attachment sites were ∼4 times greater than in uninfected plants (Fig. S3C), which was substantial considering that infected Arabidopsis showed an overall growth reduction compared with uninfected plants of the same age (Movie S1). The alteration in morphology suggested that Phtheirospermum locally induced secondary growth in the host root, a phenomenon that resembles other cases of host hypertrophy caused by parasitic plants such as mistletoes (Viscum album) infecting crabapple trees (Malus toringoides) (Fig. S2 C and D).
Fig. 2.
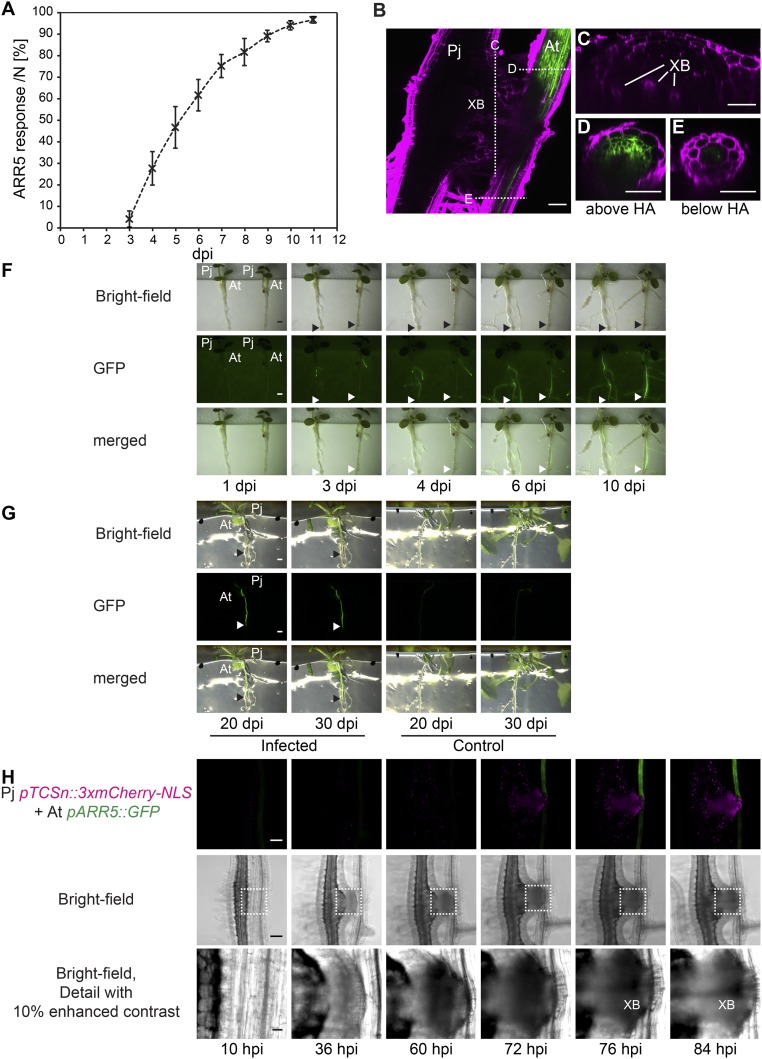
Phtheirospermum induces hypertrophy in Arabidopsis. (A) Safranin-O stained Phtheirospermum (Pj) infected Arabidopsis (At) Col-0 root with haustorium attachment site (HA) at 20 dpi. (B) Ruthenium red-stained transverse sections were taken 8 mm (1–3 mm above the HA) and 13 mm below the hypocotyl-root junction (2–4 mm below the HA) of control and infected Col-0 plants at 20 dpi to quantify vascular diameter and xylem cell number of control (blue) and infected Col-0 plants (orange) in C (mean ± SE, n = 6–20, ANOVA, P < 0.01). (D) Confocal images were taken between 10 and 84 hpi of Pj hairy roots expressing pTCSn::3xmCherry-NLS (magenta) and host roots (At pARR5::GFP, green) before and after xylem bridge (XB) formation. (Scale bars: A and D, 100 µm; B, 25 µm.)
Fig. S2.
Hypertrophy induced by parasitic plants. (A) Tomato plants, Solanum lycopersicum (Sl), were infected with Phtheirospermum (Pj) and surveyed for morphological changes at haustorium attachment sites (HA). Pictures show an unstained (Left) and Safranin-O stained (Right) infected root at 30 dpi. (B) Safranin-O–stained sections of Phtheirospermum haustoria on tomato root with xylem bridges (XB) at 30 dpi. (C and D) Hypertrophy on crabapple trees, Malus toringoides (Mt), induced by European mistletoe, Viscum album (Va). Photographs were taken at Cambridge University Botanic Garden, Cambridge, United Kingdom (October 22, 2016). (Scale bars: A, 0.5 mm; B, 100 µm.)
Fig. S3.
Characterization of Phtheirospermum-induced hypertrophy. Root transverse sections of Arabidopsis Col-0 control (blue) and Phtheirospermum (Pj) infected (orange) plants were used to determine changes in vascular diameter, vascular cell number, and average vascular cell size (A) and vascular area at 11 dpi (n = 28–31, **P < 0.01, *P < 0.05; Student’s t test) (B). (C) Quantification of vascular area and xylem area at 20 dpi (n = 6–31, ANOVA, P < 0.01). Bars represent mean ± SE.
Root secondary growth depends on and is promoted by the plant hormone cytokinin (13). To test whether infection altered cytokinin signaling, we monitored cytokinin response in Arabidopsis by using the transcriptional reporter line pARR5::GFP (24). Infected plants showed a substantial increase in GFP fluorescence at 3–4 dpi and, by 6 dpi, most host plants showed high GFP fluorescence (Fig. S4A). The increase in GFP expression was restricted to tissue above haustoria attachment sites (Fig. S4B). Spatial localization of fluorescence at 11 dpi revealed pARR5::GFP expression overlapped with host tissues undergoing hypertrophic growth (Fig. S4 B–E). Temporally, increased cytokinin response occurred during hypertrophic growth because pARR5::GFP expression remained high even after 20 dpi (Fig. S4 F and G). These data were consistent with an increase in cytokinin response occurring in tissues undergoing increased cell division and xylem differentiation.
Fig. S4.
Phtheirospermum induces pARR5::GFP response in Arabidopsis. (A) The ratio of Arabidopsis pARR5::GFP plants showing GFP expression above haustoria (ARR5 response) was quantified between 3 and 11 dpi in four independent experiments with each containing between 34 and 46 infected plants (mean ± SD). (B) Z projection of 17 images separated by 2 µm of a two-photon microscope scanned Phtheirospermum (Pj) haustorium on Arabidopsis pARR5::GFP (At) at 11 dpi with indicated optical cross-section through the haustorium (C), the Arabidopsis root above (D) and below (E) the haustorium. Propidium iodide-stained cell walls are shown in magenta, GFP in green, XB (xylem bridge). Images of At pARR5::GFP plants infected with Pj were taken at indicated time points during cultivation in infection media (1–10 dpi) (F) and on growth media (1/2 MS agar, 20 dpi and 30 dpi) (G). Arrows indicate haustorium attachment sites. (H) Fluorescent and bright-field images as shown in Fig. 2D of Pj hairy roots expressing pTCSn::3xmCherry-NLS (magenta) and infecting host roots (At pARR5::GFP, green) with detailed images of the parasite–host interface as indicated by dotted lines in the bright-field panel. (Scale bars: B–E, 50 µm; F and G, 1 mm; H, 100 µm.)
To address the source of this response, we generated transgenic Phtheirospermum hairy roots containing the cytokinin responsive promotor sequence of the Two Component signaling Sensor new (pTCSn) fused to a triple mCherry-NLS (pTCSn::3xmCherry-NLS) (25). We then followed cytokinin responses simultaneously in Phtheirospermum expressing pTCSn::3xmCherry-NLS infecting Arabidopsis expressing pARR5::GFP. No cytokinin responses in host and parasite were detected during penetration of host tissue (10–60 h postinfection, hpi), but during the transition from proto- to mature haustoria (72–84 hpi) a parallel increase of cytokinin response in parasite and host preceded the formation of the first xylem bridge (84 hpi) (Fig. 2D and Fig. S4H). Hairy roots of Phtheirospermum resembled haustoria of nontransgenic Phtheirospermum roots in development and transcriptional induction of pARR5::GFP in host plants (Fig. 1E and Fig. S4).
Phtheirospermum-Induced Host Hypertrophy Depends on Host Cytokinin Signaling but Not on Host Cytokinin Biosynthesis Genes.
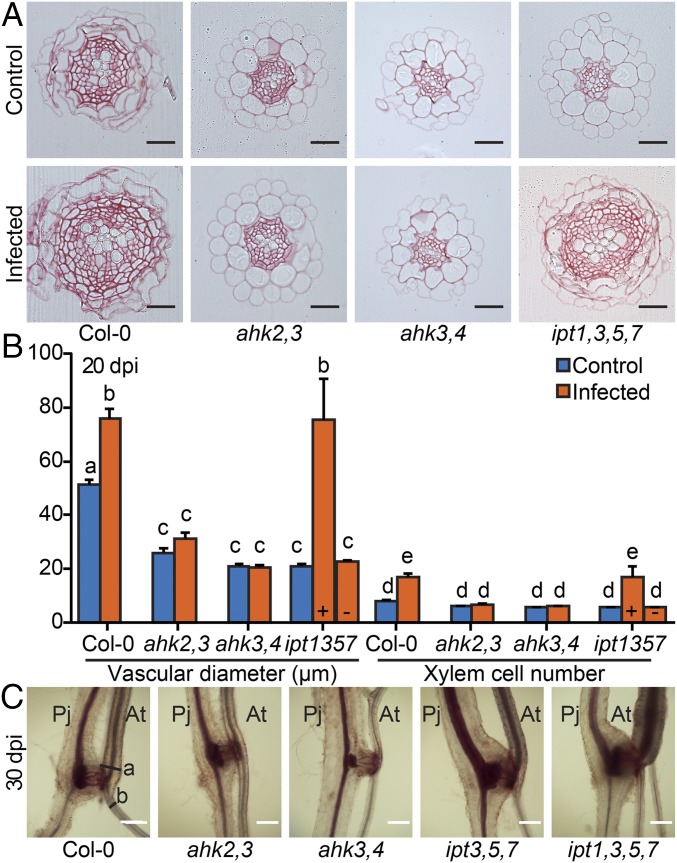
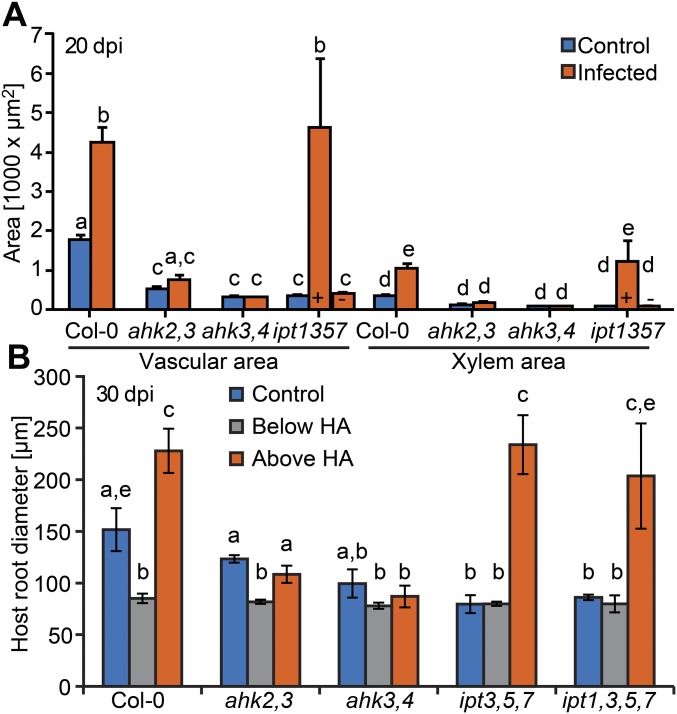
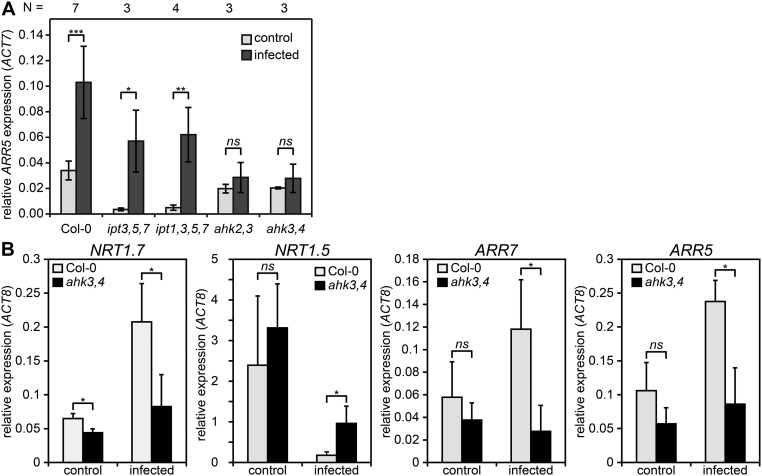
The spatial and temporal overlap between host cytokinin responses and hypertrophy suggested that they might be directly linked. To test this possibility, we infected various Arabidopsis cytokinin signaling and biosynthesis mutants. Infected Col-0 wild type showed more secondary growth including increased vascular diameter and xylem number at 20 dpi (Fig. 3 A and B and Fig. S5A) and developed roots with twofold larger diameters above haustoria compared with diameters below haustoria at 30 dpi (Fig. 3C and Fig. S5B). Roots of infected Col-0 were even larger than uninfected roots of the same age, despite their reduced shoot growth (Fig. 1A and Fig. S5B). Hypertrophy was blocked in the cytokinin signaling mutants ahk2,3 and ahk3,4, but cytokinin biosynthesis mutants ipt3,5,7 and ipt1,3,5,7 had hypertrophy levels similar to Col-0, thus partially rescuing the mutant root phenotype (Fig. 3 and Fig. S5). Consistent with these observations, transverse sections above the haustoria at 20 dpi showed an increase in vascular diameter, vascular area, xylem cell number, and xylem area for Col-0 and ipt1,3,5,7, but not for ahk3,4 and only marginally for ahk2,3 (Fig. 3 A and B and Fig. S5A). Hypertrophy in ipt1,3,5,7 was more variable at 20 dpi with some plants showing no macroscopic signs of hypertrophy. Increased cytokinin response occurred throughout the plant because endogenous ARR5 transcripts levels were also up-regulated at 11 dpi in shoots of infected Col-0, ipt3,5,7, and ipt1,3,5,7, but were not up-regulated in infected ahk2,3 and ahk3,4 (Fig. S6A). Likewise, genes downstream of cytokinin signaling including NRT1.7 and NRT1.5 showed substantial changes upon infection in Col-0 that were not observed in ahk3,4 at 11 dpi (Fig. S6B) (26).
Fig. 3.
Phtheirospermum-induced hypertrophy depends on host cytokinin signaling. (A) Ruthenium red-stained transverse sections of Arabidopsis (At) roots were used to quantify (B) control (blue) and Phtheirospermum (Pj) infected (orange) vasculature diameters and xylem cell numbers above haustorium attachment sites (HA) at 20 dpi. “+” and “–” indicate ipt1,3,5,7 sub-populations with (+) and (-) without hypertrophy (n = 6–33, mean values ± SE, ANOVA, P < 0.01). (C) Safranin-O staining of infected Arabidopsis roots at 30 dpi (a, above; b, below HA). (Scale bars: A, 25 µm, C, 200 µm.)
Fig. S5.
Characterization of Phtheirospermum-induced hypertrophy in different Arabidopsis genotypes. (A) Root transverse sections above haustoria corresponding to 8 mm below the hypocotyl-root junction of uninfected Arabidopsis Col-0 (control, blue) and Phtheirospermum (Pj) infected (orange) plants were used to determine changes in vascular and xylem area at 20 dpi (+ with hypertrophy, - without hypertrophy, n = 6–33, mean ± SE, ANOVA, P < 0.01). (B) Safranin-O staining of 30 dpi Phtheirospermum (Pj) infections of At roots were used to measure host root diameters above (orange) or below (gray) haustoria and of control plants (blue) (mean ± SD of three independent experiments; ANOVA, P < 0.05).
Fig. S6.
Hypertrophy is linked to transcriptional cytokinin responses during Phtheirospermum infection in different host genotypes. (A) ARR5 expression relative to Arabidopsis ACT7 in shoots of host plants was quantified by qRT-PCR at 11 dpi of n = 3–7 independent experiments. (B) qRT-PCR expression analysis of host genes regulated by cytokinin relative to ACT8 in Col-0 (gray) and ahk3,4 (black) root segments above haustorium attachment sites at 11 dpi and control experiments (n = 3). All graphs show mean expression ± SD with ***P < 0.001; **P < 0.01; *P < 0.05, P > 0.05 was considered not significant (ns) by using a Student’s t test.
Phtheirospermum Elevates Host Cytokinin Levels Independently of Host Cytokinin Biosynthesis Genes.
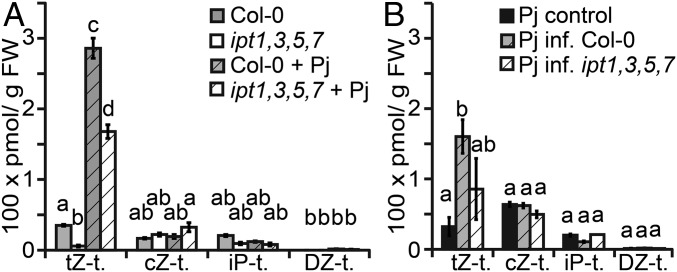
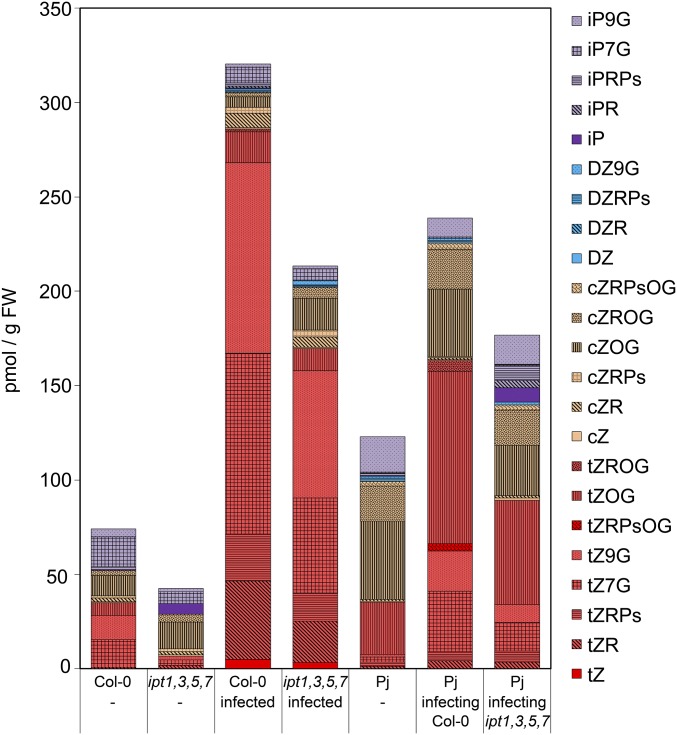
Our data demonstrated that an induction of host cytokinin responses was independent of several host IPT cytokinin biosynthesis genes, but it remained unknown whether increased cytokinin response resulted from an increase of cytokinin levels in host plants during infection. To address this question, we quantified cytokinin species in host roots (including hypocotyls) and parasite roots above haustoria at 11 dpi in Col-0 and ipt1,3,5,7 and compared the resulting cytokinin profiles to those of uninfected controls. Uninfected ipt1,3,5,7 were greatly depleted in tZ-type cytokinins compared with uninfected Col-0, consistent with previous findings (13) (Fig. 4A, Fig. S7, and Dataset S1). Upon infection, both infected Col-0 and infected ipt1,3,5,7 showed significant increases of tZ-type cytokinins. Compared with uninfected plants, tZ-type cytokinins increased 8-fold in Col-0 and 29-fold in ipt1,3,5,7 (Fig. 4A). Detailed profiling of cytokinins showed an increase of all detected species of tZ-type cytokinins including bioactive tZ and xylem mobile precursor tZ-riboside (tZR, Fig. S7 and Dataset S1). tZ-type cytokinins also increased in Phtheirospermum upon infection, however, the fold change was lower than compared with tZ-type cytokinin accumulation in host plants (Fig. 4B). No significant changes during infection were detected for cZ-, iP-, or DZ-type of cytokinins in either host or parasite (Fig. 4).
Fig. 4.
Cytokinin accumulation in the host is independent of host cytokinin biosynthesis. (A) Different species of cytokinin were quantified by UPLC-tandem mass spectrometry and categorized to different types (t.) from tissue of uninfected (open) and infected (hatched) Col-0 (gray) or ipt1,3,5,7 (white) above haustoria at 11 dpi. (B) Cytokinin quantifications in solitary grown Phtheirospermum (Pj) (black) or Pj infecting Col-0 (gray) or ipt1,3,5,7 (hatched). Bars show mean values ± SE (n = 4, ANOVA, P < 0.05).
Fig. S7.
Cytokinin profiles in different hosts and Phtheirospermum. Cytokinin profiles were determined by UPLC-tandem mass spectrometry of control plants (-) or infected plants on Phtheirospermum, Col-0 or ipt1,3,5,7 mutants. Bars show average values of four independent experiments at 11 dpi. iP, N6-(∆2-isopentenyl)adenine; iPR, iP riboside; iPRPs, iPR 5′-phosphates; iP7G, iP-7-N-glucoside; iP9G, iP-9-N-glucoside; DZ, dihydrozeatin; DZR, DZ riboside; DZRPs, DZR 5′-phosphates; DZ9G, dihydrozeatin-9-N-glucoside; cZ, cis-zeatin; cZR, cZ riboside; cZRPs, cZR 5′-phosphates; cZOG, cZ-O-glucoside; cZROG, cZR-O-glucoside; cZRPsOG, cZRPs-O-glucoside; tZ, trans-zeatin; tZR, tZ riboside; tZRPs, tZR 5′-phosphates; tZ7G, tZ-7-N-glucoside; tZ9G, tZ-9-N-glucoside; tZOG, tZ-O-glucoside; tZROG, tZR-O-glucoside; tZRPsOG, tZRPs-O-glucoside.
Host Hypertrophy Inducing Cytokinins Are Derived from Phtheirospermum.
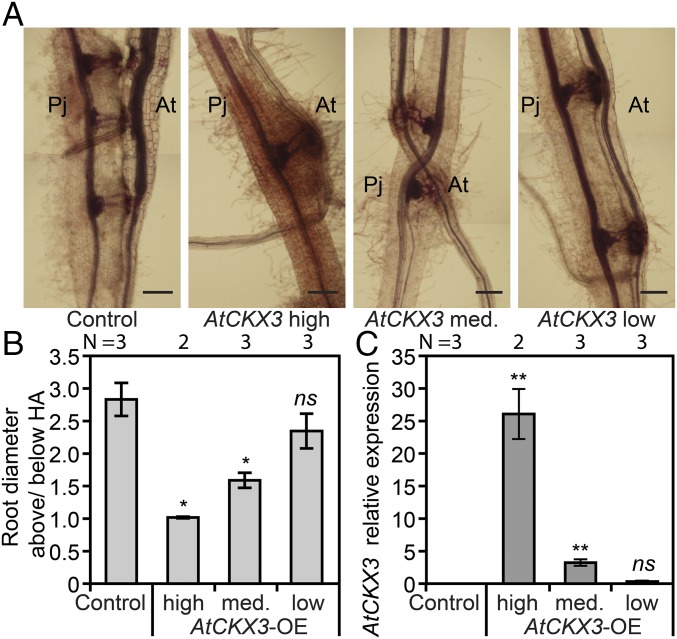
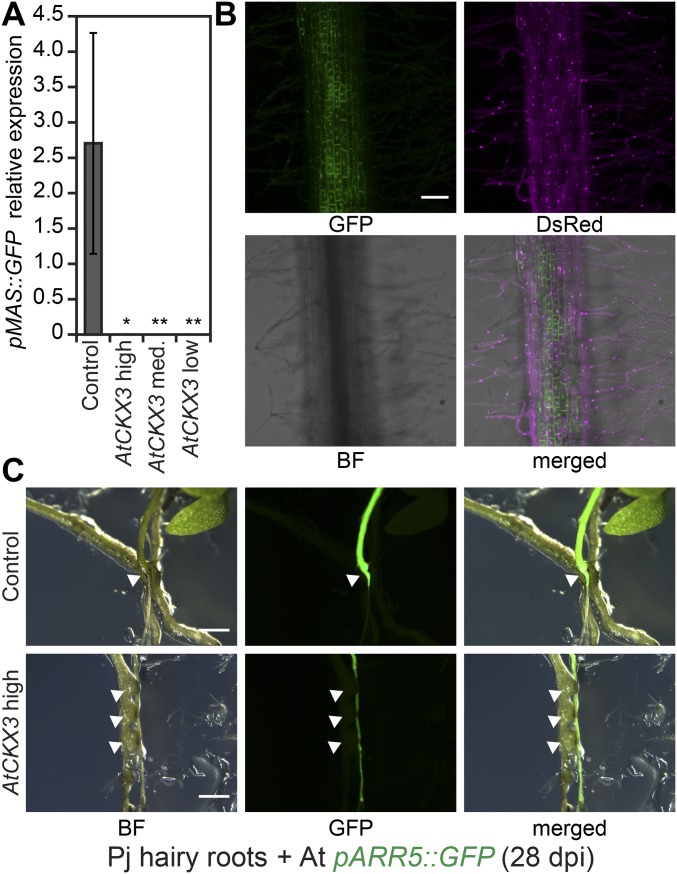
The presence of hypertrophy (Fig. 3) and the increase of tZ-type cytokinins in infected ipt1,3,5,7 similar to levels found in Col-0 (Fig. 4A) suggested that host tZ-type cytokinins were derived from Phtheirospermum. To address this possibility, we expressed an Arabidopsis cytokinin-degrading enzyme, AtCKX3, in hairy roots of Phtheirospermum (Fig. 5). Overexpression of AtCKX3 reduces cytokinin levels in a distant relative of Phtheirospermum, Nicotiana tabacum (27), and caused various cytokinin-deficient phenotypes in Arabidopsis and N. tabacum (11, 28). Thus, we reasoned that overexpressing AtCKX3 in Phtheirospermum would also reduce parasite cytokinin levels. We quantified host hypertrophy during infection of Phtheirospermum hairy roots expressing either high, medium, or low levels of pMAS::AtCKX3 and of control hairy roots expressing pMAS::GFP (Fig. 3 and Fig. S8 A and B). Whereas Phtheirospermum roots expressing pMAS::GFP induced similar levels of host hypertrophy compared with nontransgenic roots at 28 dpi (Figs. 3C and 5 A and B and Fig. S5B), Arabidopsis hypertrophy decreased with increasing AtCKX3 expression in Phtheirospermum (Fig. 5). No hypertrophy was observed for Phtheirospermum hairy roots with the highest AtCKX3 expression. Hairy roots expressing pMAS::AtCKX3 had similar morphology and ability to develop haustoria compared with hairy roots transformed with pMAS::GFP; however, hairy roots expressing high levels of pMAS::AtCKX3 induced lower levels of pARR5::GFP expression in the host compared with pMAS::GFP controls (Fig. 5A and Fig. S8C).
Fig. 5.
Host hypertrophy depends on Phtheirospermum-derived cytokinins. (A) Manually reassembled microscopy images show transgenic Phtheirospermum (Pj) roots expressing pMAS::GFP (Control) and high, medium (med.), and low levels of pMAS::AtCKX3. These transformed hairy roots infected Arabidopsis (At) plants and were stained at 28 dpi with Safranin-O. (B) The amount of hypertrophy in hairy roots was quantified by measuring root diameters above haustoria relative to the root diameter below haustoria (n = 2–3). (C) Relative AtCKX3 transgene expression in Phtheirospermum hairy roots was determined by real-time quantitative PCR (RT-qPCR) using the expression of PjUBC2 as reference. (B and C) Bars represent mean ± SE (*P < 0.05, **P < 0.01 Student’s t test). (Scale bars: 100 µm.)
Fig. S8.
Characterization of transgenic Phtheirospermum roots. (A) Average pMAS::GFP transgene expression in individual Phtheirospermum hairy root was quantified by RT-qPCR using expression of Phtheirospermum UBC2 as reference (mean ± SE, difference to control in Student t test, *P < 0.05, **P < 0.01). (B) Confocal images (Z projection) of a Phtheirospermum hairy root overexpressing pMAS::GFP (green) and the transformation marker p35S::DsRed (magenta). (C) pARR5::GFP expression of infected Arabidopsis pARR5::GFP plants at 28 dpi show differences in GFP accumulation in host plants above haustoria (white triangle) compared with host plants infected with control hairy roots (Upper) or hairy roots showing high AtCKX3 expression (Lower). Note that pMAS::GFP expression in control hairy roots was not detected at this exposure, but validated by RT-qPCR (A). BF, bright field. (Scale bars: B, 100 µm; C, 1 mm.)
Hypertrophy Correlates with Reduced Host Biomass and Increased Haustoria Density.
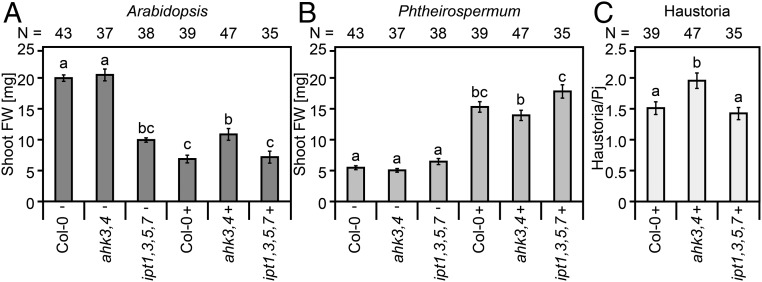
Because Phtheirospermum infection caused Arabidopsis to retard growth and reduce biomass (Fig. 1A and Movie S1), we tested whether hypertrophy contributed to this phenomenon by infecting Col-0 and ipt1,3,5,7 that showed hypertrophy along with ahk3,4 that is hypertrophy resistant (Fig. 3). At 30 dpi, infection reduced Col-0 shoot weight by 66% compared with uninfected plants, whereas infected plants reduced ahk3,4 shoot weights by only 48% compared with uninfected plants, resulting in significantly heavier plants (Fig. 6A). Shoot weights of infected ipt1,3,5,7 showed an even lower reduction of 28% compared with uninfected controls (Fig. 6A), likely due to the smaller shoot size of uninfected ipt1,3,5,7 or possibly because parasite-derived cytokinins partially rescued the ipt1,3,5,7 phenotype. Conversely, Phtheirospermum shoot weights increased ∼2.8 fold at 30 dpi when infecting any of the three tested genotypes. Significant Phtheirospermum weight differences were only observed when it infected ipt1,3,5,7 compared with slightly lower weights when it infected ahk3,4 (Fig. 6B). However, Phtheirospermum produced more haustoria on ahk3,4 than on either Col-0 or ipt1,3,5,7 (Fig. 6C).
Fig. 6.
Hypertrophy is linked to host biomass reduction in host plants. (A and B) Shoot fresh weights (FW) of different Arabidopsis genotypes and Phtheirospermum were assessed from three independent experiments of plants growing next to Phtheirospermum (-) and of plants infected with Phtheirospermum (+) at 30 dpi (n = 35–47). (C) Average number of haustoria per Phtheirospermum (Pj) on corresponding genotypes at 30 dpi. Bars represent mean values ± SE (ANOVA P < 0.05).
Discussion
Combining the two model species Arabidopsis and Phtheirospermum created an experimental framework to study parasitic plants. Infection of Arabidopsis by Phtheirospermum was thereby truly parasitic because it caused growth benefits for the parasite and growth penalties for the host (Figs. 1 and 6). Consistent with a redistribution of nutrients, the infection modified the source-sink movement of molecules including CFDA dye that was efficiently transported from the Arabidopsis shoot to Phtheirospermum (Fig. 1D), but inefficiently transported to the Arabidopsis root below the haustorium attachment site (Fig. 1F). Transport of CFDA occurred after differentiation of the xylem bridge, suggesting CFDA may be transported via the xylem. Similar transport routes between parasitic plants and host plants were proposed for xylem-feeding parasites such as Striga (29). Consistent with Phtheirospermum acting as a xylem-feeding parasite, we did not observe uptake of phloem-mobile GFP from host to parasite. This absence of uptake contrasts with haustoria of the phloem-feeding parasite Orobanche aegyptiaca, which forms phloem-to-phloem connections and uptakes GFP during parasitism of tomato plants expressing pSUC2::GFP (3). However, we cannot completely exclude the possibility of phloem or symplastic connections because CFDA moves in both the xylem and phloem (20), and its small size could allow it to move more easily through plasmodesmata than the larger GFP protein.
Successful parasites uptake water and nutrients from their hosts, but less is known about the role of substance transport from parasite to host. Here, we demonstrate that upon infection, Phtheirospermum increases cytokinin levels and transports these across the haustorium to the Arabidopsis host. These results are analogous to the phloem-mediated, bidirectional exchange of proteins, RNAs, and viruses across haustoria (1, 5). However, the biological relevance of RNA and protein movement is unclear and might result from bulk flow transport of RNAs and proteins normally found in the parasite phloem or host phloem (5). Conversely, the increase of cytokinin levels in the parasite upon infection suggests an active process related to parasitism, and indeed, we observed morphological changes including hypertrophic root growth in the host that depended on the host cytokinin-signaling pathway. Thus, these data describe molecular movement from parasitic plant to host that has a clear physiological and developmental effect.
Cytokinin responses in both parasite and host were detected several hours after successful penetration of host tissue, but also several hours before xylem bridges were fully formed in haustoria (Fig. 2D). This early detection of host cytokinin responses triggered by proto-haustoria rather than mature haustoria suggests a transport mechanism that is initially independent of xylem bridges and, thus, uncoupled from bulk nutrient influxes from host to parasite. Different cytokinin species move in the phloem compared with the xylem, in particular, tZ-type species are typically found in the xylem (12). We detected mainly transport of tZ-type species, consistent with the idea that Phtheirospermum is a xylem feeder, and at least some of the tZ-type species detected could be moving through the xylem bridge after haustoria maturation. Notably, Arabidopsis cytokinin receptor mutants formed normal haustoria (Fig. 3), whereas Phtheirospermum hairy roots overexpressing AtCKX3 could also form haustoria (Fig. 5), suggesting that cytokinins play a role in plant parasitism after haustorium formation.
Parasitic plants actively manipulate host physiology (1), and we propose that one mechanism for this manipulation is by actively transporting cytokinins to the host. One visible physiological change that occurs during parasitism is hypertrophic root or stem growth that occurs in a variety of hosts infected by Alectra vogelii, mistletoes, or Cuscuta japonica (Fig. S2 C and D) (1, 16). Phtheirospermum induced such symptoms on Arabidopsis and tomato suggesting that cytokinin transport could be a widely conserved mechanism used by parasitic plants. It is likely that other parasitic plants in addition to Phtheirospermum produce cytokinins at the haustorial infection site. A gene homologous to IPT1 was transcriptionally induced in haustoria of sandalwood (Santalum album) (30), and cytokinin responsive genes showed elevated levels in rice infected with Striga hermonthica (31). We propose that hypertrophy makes the parasite a more efficient sink to uptake nutrients from the host because host biomass partially depended on hypertrophy (Fig. 6A). This increase in host vascular size could lead to increased sink strength at haustorium attachment sites, which could improve nutrient withdrawal by parasitic plants. Indeed, Phtheirospermum produced additional haustoria on hypertrophy-deficient ahk3,4, and this increase might have improved sink strength by partially compensating for the absence of hypertrophy (Fig. 6C). However, we did not observe substantial differences in Phtheirospermum weights upon infecting hypertrophic or nonhypertrophic Arabidopsis genotypes (Fig. 6B). It remains to be shown whether this effect on biomass is conserved or stronger in different parasitic plant–host systems and whether such sink-source relationships become more important in different environmental conditions.
Cytokinin production appears to be a widespread strategy used by a variety of different plant pathogens. Well-known plant pathogens that induce cytokinin production include the crown gall-inducing bacterium Agrobacterium tumefaciens (6), the fungal rice blast pathogen Magnaporthe oryzae (7), and phytopathogenic nematodes such as Heterodera schachtii (8). Interspecies cytokinin transport thus may be a widely used mechanism for infectivity, and in parasitic plants, we suggest a scenario whereby multiple transport routes at the haustorium contribute to the bidirectional transfer of molecules that affects both host and parasite physiology.
Materials and Methods
Plant Material.
Phtheirospermum and its transformation was described in ref. 19. Arabidopsis ahk2-2,3–3; ahk3-3,4 (cre1-12) (32); ipt3,5,7 and ipt1,3,5,7 (10), Col-0 pSUC2::GFP (23), and Ws pARR5::GFP (24) marker lines were described previously. Detailed protocol descriptions are available as SI Materials and Methods and Dataset S2.
Cytokinin Quantification.
Extraction and quantification of cytokinins from 11 dpi hypocotyl and root segments above the first haustorium of Phtheirospermum and Arabidopsis and corresponding tissues of uninfected plants were performed with ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (AQUITY UPLC System/XEVO-TQS; Waters) with an ODS column (AQUITY UPLC BEH C18, 1.7 µm, 2.1 × 100 mm, Waters) as described (33).
SI Materials and Methods
Growth Conditions and Infection Assays.
Phtheirospermum and Solanum lycopersicum M82 seeds were sterilized for 5 min with 6% sodium hypochlorite, washed with H2O, and then air dried on filter paper. Phtheirospermum seeds were then transferred to 1/2 MS (Nippon Seiyaku), 1% Bacto Agar (Difco). After 2 d of stratification, plates were arranged vertically in a Biotron (NK System) growth chamber with a 8-h light/16-h dark cycle, 22 °C, and approximately 60 μmol⋅m−2⋅s−1 for 7 d. Sterilized Arabidopsis seeds were germinated under the same condition on full strength MS. For infection, Phtheirospermum and Arabidopsis roots were aligned next to each other on two layers of wet filter paper and one layer of a Hybond N+ membrane (Ge Healthcare) as described for Arabidopsis micrografts (20). Infections exceeding 11 dpi were moved at 7 dpi onto 1/2 MS agar plates. Plate position was randomized every 2–3 d. S. lycopersicum seeds were transferred after sterilization to 1/2 MS, 1% bacto agar plates, and grown for 13 d under long day conditions (16-h light/8-h dark cycle, 22 °C). For infections, Phtheirospermum and S. lycopersicum were aligned next to each other on water-1% bacto agar plates and sampled at 30 dpi.
Safranin-O-Staining.
Dissected root parts were fixed in ethanol-acetic acid, stained with Safranin-O solution (0.1%), cleared with chloral hydrate for 2–3 d and then observed with an Olympus BX51 microscope as described (18).
CFDA Transport Assays.
5-(and-6)-Carboxyfluorescein diacetate (CFDA, Biotium Inc.) was dissolved in DMSO and added to a final concentration of 0.66 µg/µL in 1% Gamborg’s B5 Agar (Nippon Seiyaku), mixed and then drop inoculated on host root tips or locally wounded host leaves. Fluorescence was monitored with a stereo microscope (Leica M165 FC and M205 FA).
Two-Photon Microscopy.
To observe the cell shape of Phtheirospermum haustorium, Phtheirospermum roots were stained with 10 μg/mL propidium iodide (PI) for 10 min. Fluorescent images were obtained with an upright two-photon microscope (FVMPE-RS; Olympus) equipped with dual femtosecond pulse lasers (InSight DeepSee Dual-OL; Spectra-Physics) and a 25× water immersion objective lens (XLPLN25XWMP2, N.A. = 1.05, WD = 2.00 mm; Olympus). The excitation wavelength for GFP was 860 nm, CFDA was 880 nm, and PI was 1040 nm, respectively.
Histology.
Root samples from Arabidopsis and Phtheirospermum were fixed by using a solution containing 1% glutaraldehyde, 4% formaldehyde, 0.05 M sodium phosphate. After ethanol dehydration, the samples were infiltrated and then embedded with Leica basic resin by using homemade chambers. Five-micrometer-thin transverse sections were cut at various positions of the roots and stained with ruthenium red. Pictures were taken with a Zeiss Axioimager.M2.
Phtheirospermum Hairy Root Transformation.
Six-day-old Phtheirospermum seedlings grown on Gamborg’s B5 media (0.8% agar) supplemented with 1% sucrose were cocultivated with Agrobacterium rhizogenes AR1193 for 2 d at 22 °C in dark after sonication and vacuum infiltration of a bacterial suspension as described in ref. 19. Phtheirospermum with hairy roots were then transferred to water agar plates (0.8%) for additional 2 d, before infection with 6-d-old Arabidopsis plants. Infected plants were then either analyzed by confocal microscopy (Leica, TCS SP5 II) or after 1 wk transferred onto 1/2 MS agar plates.
Constructs Design and Cloning.
Binary vectors pAGM4723-pMAS::GFP:tMas + p35S::DsRED:t35S and pAGM4723-pMAS::AtCKX3:tMas + p35S::DsRED:t35S for Phtheirospermum hairy root transformation were assembled from preexisting modules of the Golden Gate library (34). AtCKX3 (AT5G56970) was first cloned from Col-0 cDNA into pGEM-T (Promega) and then into a level-0 universal vector with parallel silent site-directed mutagenesis of intrinsic BpiI sites. The pTCSn promoter module was amplified from the pTCSn::GFP plasmid (25). The 3xmCherry-NLS module was cloned as described (18). All constructs were sequenced before A. rhizogenes transformation. Primers sequences are listed in Dataset S2.
RT-qPCR.
RNA was isolated from Phtheirospermum hairy roots tissues by using RNeasy Plant Mini Kit (Qiagen), followed by cDNA synthesis using SuperScript III (Invitrogen) and quantified by qPCR (Thunderbird SYBR Green and ROX, Toyobo) using a Stratagene mx3000p real time thermal cycler. PjUBC2 primers are based on published Phtheirospermum transcriptome data (35). Primers sequences are given in Dataset S2.
Data Analyses and Statistics.
One-way ANOVA and Tukey post hoc were calculated by using the AOV and TukeyHSD function in R. Fisher exact and Student’s t test were done in Microsoft Excel 2010. We used CLC Main Workbench (Version 6.9, Qiagen) for vector design and sequence analyses.
Supplementary Material
Acknowledgments
We thank Ruth Stadler for providing pSUC2::GFP seeds, Mikiko Kojima and Yumiko Takebayshi for cytokinin quantification, Simon Saucet for technical help, and Nicola Patron for sharing the Modular Cloning Toolkit. This work is partially supported by Ministry of Education, Culture, Sports, Science and Technology KAKENHI Grants 24228008 and 15H05959 (to K.S.), 25114521, 25711019, and 25128716 (to S.Y.), and 15H05962 (to S.M.); Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship (to T.S.); JSPS Research Fellowship for Young Scientist (to T.W.); the RIKEN Special Postdoctoral Researchers Program (to T.S.); and Gatsby Charitable Trust Grants GAT3272/C and GAT3273-PR1 (to C.W.M).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.J.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619078114/-/DCSupplemental.
References
- 1.Heide-Jørgensen HS, Heide-Jorgensen H. Parasitic Flowering Plants. Brill Academic Publishers; Leiden, The Netherlands: 2008. [Google Scholar]
- 2.Yoshida S, Cui S, Ichihashi Y, Shirasu K. The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol. 2016;67:643–667. doi: 10.1146/annurev-arplant-043015-111702. [DOI] [PubMed] [Google Scholar]
- 3.Aly R, et al. Movement of protein and macromolecules between host plants and the parasitic weed Phelipanche aegyptiaca Pers. Plant Cell Rep. 2011;30:2233–2241. doi: 10.1007/s00299-011-1128-5. [DOI] [PubMed] [Google Scholar]
- 4.Birschwilks M, Sauer N, Scheel D, Neumann S. Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec. Planta. 2007;226:1231–1241. doi: 10.1007/s00425-007-0571-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim G, LeBlanc ML, Wafula EK, dePamphilis CW, Westwood JH. Plant science. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science. 2014;345:808–811. doi: 10.1126/science.1253122. [DOI] [PubMed] [Google Scholar]
- 6.Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon MP. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA. 1984;81:5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanclud E, et al. Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence Emilie. PLoS Pathog. 2016;12:e1005457. doi: 10.1371/journal.ppat.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddique S, et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc Natl Acad Sci USA. 2015;112:12669–12674. doi: 10.1073/pnas.1503657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieber JJ, Schaller GE. Cytokinins. Arabidopsis Book. 2014;12:e0168. doi: 10.1199/tab.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyawaki K, et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner T, et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose N, et al. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto-Kitano M, et al. Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA. 2008;105:20027–20031. doi: 10.1073/pnas.0805619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolz A, et al. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 2011;67:157–168. doi: 10.1111/j.1365-313X.2011.04584.x. [DOI] [PubMed] [Google Scholar]
- 16.Furuhashi T, et al. Morphological and plant hormonal changes during parasitization by Cuscuta japonica on Momordica charantia. J Plant Interact. 2013;9:220–232. [Google Scholar]
- 17.Zhang X, et al. Endogenous hormone levels and anatomical characters of haustoria in Santalum album L. seedlings before and after attachment to the host. J Plant Physiol. 2012;169:859–866. doi: 10.1016/j.jplph.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Cui S, et al. Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant Phtheirospermum japonicum. Plant Physiol. 2016;170:1492–1503. doi: 10.1104/pp.15.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida JK, Yoshida S, Ito M, Namba S, Shirasu K. Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS One. 2011;6:e25802. doi: 10.1371/journal.pone.0025802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnyk CW, Schuster C, Leyser O, Meyerowitz EM. A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr Biol. 2015;25:1306–1318. doi: 10.1016/j.cub.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cechin I, Press MC. Nitrogen relations of the sorghum-Striga hermonthica host-parasite association: Germination, attachment and early growth. New Phytol. 1993;124:681–687. doi: 10.1111/j.1469-8137.1993.tb03858.x. [DOI] [PubMed] [Google Scholar]
- 22.Oparka KJ, Duckett CM, Prior OAM, Fisher DB. Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 1994;6:759–766. [Google Scholar]
- 23.Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 1999;11:309–322. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai O, et al. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol. 2005;15:1566–1571. doi: 10.1016/j.cub.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 25.Zürcher E, et al. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 2013;161:1066–1075. doi: 10.1104/pp.112.211763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiba T, Kudo T, Kojima M, Sakakibara H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J Exp Bot. 2011;62:1399–1409. doi: 10.1093/jxb/erq410. [DOI] [PubMed] [Google Scholar]
- 27.Polanská L, et al. Altered cytokinin metabolism affects cytokinin, auxin, and abscisic acid contents in leaves and chloroplasts, and chloroplast ultrastructure in transgenic tobacco. J Exp Bot. 2007;58:637–649. doi: 10.1093/jxb/erl235. [DOI] [PubMed] [Google Scholar]
- 28.Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pageau K, Simier P, Le Bizec B, Robins RJ, Fer A. Characterization of nitrogen relationships between Sorghum bicolor and the root-hemiparasitic angiosperm Striga hermonthica (Del.) Benth. using K15 NO3 as isotopic tracer. J Exp Bot. 2003;54:789–799. doi: 10.1093/jxb/erg081. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, et al. RNA-Seq analysis identifies key genes associated with haustorial development in the root hemiparasite Santalum album. Front Plant Sci. 2015;6:661. doi: 10.3389/fpls.2015.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swarbrick PJ, et al. Global patterns of gene expression in rice cultivars undergoing a susceptible or resistant interaction with the parasitic plant Striga hermonthica. New Phytol. 2008;179:515–529. doi: 10.1111/j.1469-8137.2008.02484.x. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima M, et al. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: An application for hormone profiling in Oryza sativa. Plant Cell Physiol. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engler C, et al. A golden gate modular cloning toolbox for plants. ACS Synth Biol. 2014;3:839–843. doi: 10.1021/sb4001504. [DOI] [PubMed] [Google Scholar]
- 35.Ishida JK, et al. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell. 2016;28:1795–1814. doi: 10.1105/tpc.16.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.