Significance
Inflammatory responses are often characterized by elevated levels of cytokines, but the complex interplay among peptides and cytokines is not often considered. Here, we report that the cytokine IL-33, administered in combination with the proinflammatory peptide substance P (SP), causes a marked increase in tumor necrosis factor synthesis and secretion from cultured human mast cells. These responses are mediated via the activation of the SP receptor NK-1 and the IL-33 receptor ST2 and can be inhibited by the natural flavonoid methoxyluteolin. Our findings reveal interactions that increase the understanding of inflammation and offer new directions for the development of antiinflammatory drugs.
Keywords: mast cells, substance P, interleukin-33, tumor necrosis factor, inflammation
Abstract
The peptide substance P (SP) and the cytokine tumor necrosis factor (TNF) have been implicated in inflammatory processes. Mast cells are recognized as important in inflammatory responses. Here, we report that IL-33 (30 ng/mL), a member of the IL-1 family of cytokines, administered in combination with SP (1 µM), markedly increase (by 1,000-fold) TNF gene expression in cultured human LAD2 and primary mast cells derived from umbilical cord blood. SP (0.01–1 μM) and IL-33 (1–100 ng/mL) in combination also greatly stimulate TNF secretion (by 4,500-fold). Pretreatment of LAD2 cells with two different neurokinin-1 (NK-1) receptor antagonists and siRNA inhibits TNF secretion by 50% (P < 0.001) when stimulated by SP and IL-33. Pretreatment of LAD2 cells with a neutralizing antibody for IL-33 receptor, ST2, inhibits TNF secretion by 50% (P < 0.001), and ST2 siRNA decreases TNF secretion by 30% (P < 0.05), when stimulated by SP and IL-33. Surprisingly, NK-1 antagonists also inhibit 50% of TNF secretion (P < 0.001) when stimulated only by IL-33, and ST2 receptor reduction also decreases SP-stimulated TNF secretion by 30% (P < 0.05), suggesting an interaction between NK-1 and ST2 receptors. Moreover, IL-33 increases NK-1 gene and surface protein expression, as well as IKβ-α phosphorylation. Pretreatment of LAD2 cells with 5,7,3′,4′-tetramethoxyflavone (methoxyluteolin) (1–100 μM) inhibits (P < 0.001) TNF gene expression (98%) and secretion (64%) at 50 µM and phosphorylation of p-IKB-α at 1 μM when stimulated by SP and IL-33. These findings identify a unique amplification process of TNF synthesis and secretion via the interaction of NK-1 and ST2 receptors inhibitable by methoxyluteolin.
Substance P (SP), a peptide originally isolated from the rat brain and characterized by Leeman and Chang (1), has been implicated in inflammatory processes (2–7). SP also has been shown to stimulate mast cells to secrete histamine (8) and tumor necrosis factor (TNF) (9–11). Mast cells are hemopoietically derived tissue immune cells involved in allergic diseases (12), innate and acquired immunity (13), autoimmunity (14), and inflammatory responses through the release of proinflammatory mediators. In addition to histamine and TNF, these mediators include IL-1β, IL-6, IL-8, and vascular endothelial growth factor (VEGF) (15, 16). We have previously reported that SP and IL-33 in combination increase vascular permeability of the skin and VEGF release from cultured human mast cells (16). In fact, murine mast cells derived from bone marrow secrete hemokinin-1, which is structurally related to SP and augments IgE-stimulated mast cells in an autocrine fashion (17).
IL-33 belongs to the IL-1 family of cytokines and plays a crucial role in regulation of the innate and adaptive immune systems (18, 19), as well as in a number of autoimmune, allergic, and inflammatory diseases (20, 21). IL-33 promotes mast cell proliferation and release of proinflammatory mediators (22, 23), and also augments the effects of IgE and nerve growth factor on HMC-1 human leukemic mast cells (24). It is interesting that serine proteases (chymase and tryptase) secreted from mast cells generate a shorter, mature, and more active form of IL-33 (25). IL-33 also has been reported to enhance allergic responses (26) and allergic bronchoconstriction via activation of mast cells in mice (27). IL-33 is expressed in the epidermis (28) and in the human keratinocytes (29). Moreover, IL-33 has been implicated in the pathogenesis of psoriasis via keratinocyte and mast cell activation (30), and has been reported to be elevated in the serum of patients with generalized psoriasis and correlated with high serum TNF levels (31). In addition to the newly synthesized TNF secretion reported here, mast cells are the only immune cells that also store and rapidly secrete preformed TNF (32–36). Given the foregoing findings, we decided to investigate whether the interactions between SP and IL-33 affect human mast cell secretion of TNF.
We previously reported that 5,7,3′,4′-tetramethoxyflavone (methoxyluteolin), in which four hydroxyl groups are replaced by methyl groups, is a more potent mast cell inhibitor than 5,7,3′,4′-tetrahydroxyflavonol (luteolin) (37). Here we report that IL-33 administered in combination with SP potently enhances TNF synthesis and secretion in cultured human mast cells. These effects are mediated via interaction of NK-1 and ST2 receptors and are inhibited by methoxyluteolin. These findings provide insights to the understanding and treatment of inflammatory diseases.
Results
Selection of the Optimal Doses to Study TNF Secretion Stimulated by SP and IL-33 When Administered in Combination.
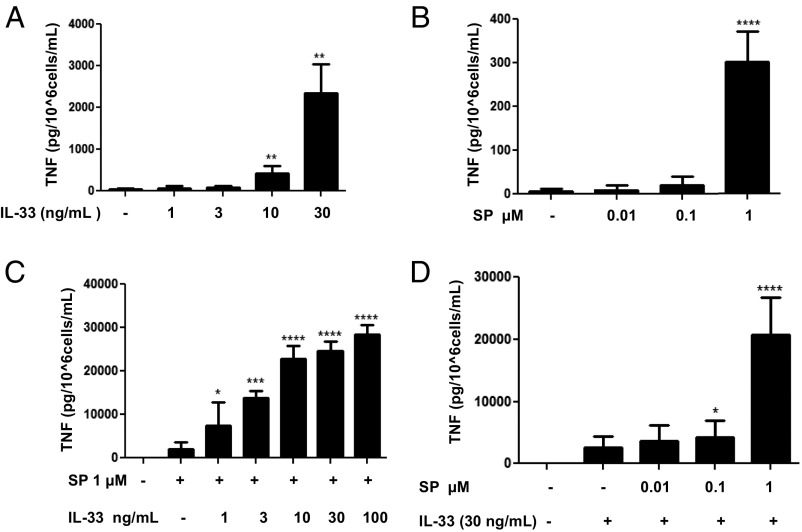
Stimulation of LAD2 cells by IL-33 alone (1–100 ng/mL) resulted in a maximum secretion of ∼2,500 pg/mL TNF at 30 ng/mL (P < 0.01) (Fig. 1A), and stimulation by SP alone (0.01–1 µM) resulted in a maximum secretion of ∼400 pg/mL TNF at 1 µM (P < 0.001) (Fig. 1B). The combination of IL-33 (30 ng/mL) and SP (1 μM) produced a robust augmentation of TNF secretion at ∼25,000 pg/mL (P < 0.001) (Fig. 1 C and D); therefore, we used this combination for further experiments.
Fig. 1.
Selection of the optimal doses to study TNF secretion stimulated by SP and IL-33 when administered in combination. LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates and stimulated with IL-33 (1–30 ng/mL) alone (A), with SP (0.1–1 µM) alone (B), or with SP (0.01–1 μΜ), IL-33 (1-100 ng/mL), or their combination as shown (C and D) for 24 h. Supernatant fluids were collected at the end of the incubation period and assayed by TNF ELISA. n = 3.*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
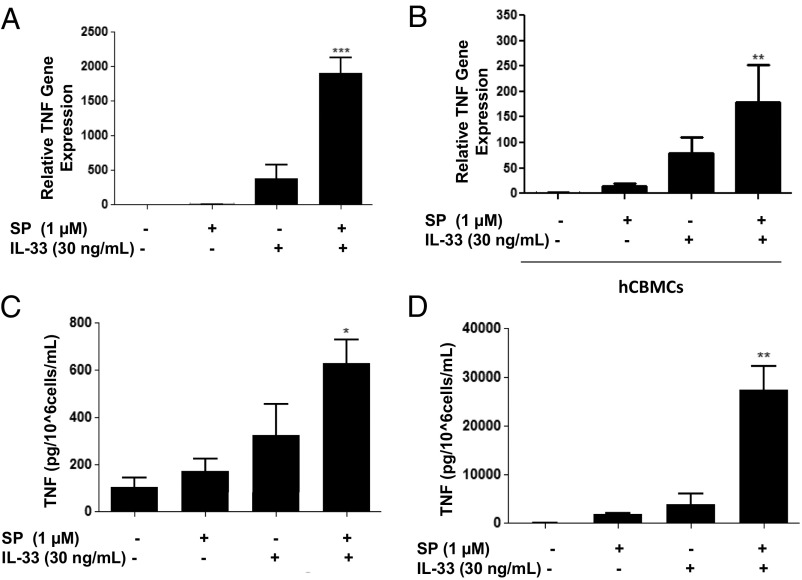
The combination of IL-33 (30 ng/mL) and SP (1 μM) significantly (P < 0.001) increased TNF mRNA gene expression by 1,000-fold (Fig. 2A) in LAD2 cells and by 100-fold (P < 0.001) in human umbilical cord blood-derived mast cells (hCBMCs) (Fig. 1B). IL-33 and SP also significantly increased TNF cellular protein by 600-fold (P < 0.05) and secretion by 4,500-fold (P < 0.01) in LAD2 cells (Fig. 2 C and D).
Fig. 2.
SP and IL-33 markedly enhance TNF gene expression and secretion in human mast cells. (A and B) LAD2 cells (1 × 106 cells per well) (A) and hCBMCs (0.3 × 106 cell per well) (B) were seeded in 12-well culture plates and stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 6 h. TNF mRNA expression levels were measured by qRT-PCR and normalized to human GAPDH endogenous control. (C and D) LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates and stimulated with SP (1 μΜ), IL-33 (30 ng/mL) or their combination as shown for 24 h. Control cells were treated with culture media only. Supernatant fluids (D) and cell lysates (C) were collected at the end of the incubation period and assayed by ELISA. n = 3. *P < 0.05; **P < 0.01.
NK-1 Receptor Antagonists L-733,060 and CP-96345 Inhibit TNF Secretion Stimulated by SP and IL-33 Administered in Combination.
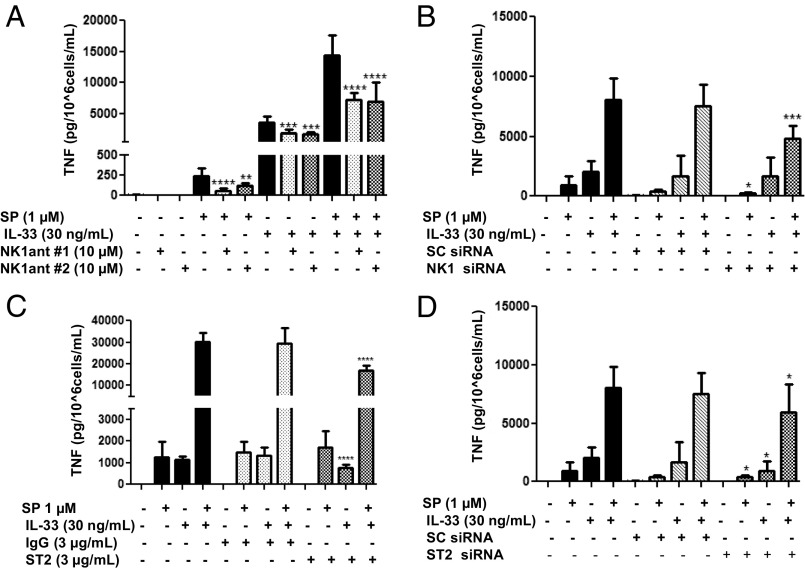
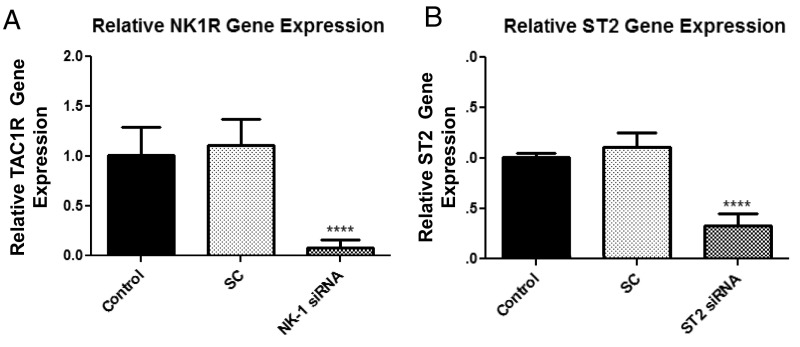
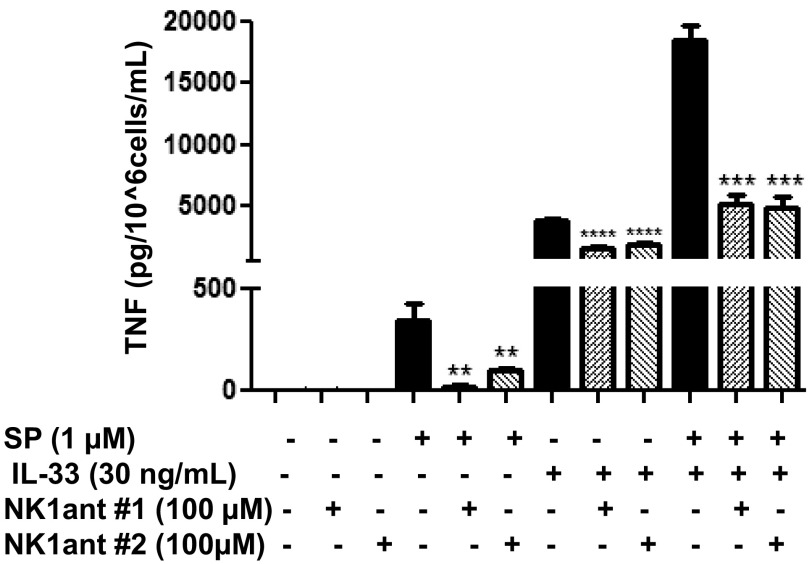
We next investigated whether the enhancing effect of IL-33 and SP is mediated via the neurokinin 1 receptor (NK-1). LAD2 cells were preincubated with the NK-1 antagonist L-733,060 (10 μM) or with CP-96345 (10 µM) for 30 min and then stimulated with SP (1 μM) alone, IL-33 (30 ng/mL) alone, or the combination for 24 h. Preincubation with either antagonist significantly (P < 0.0001) inhibited TNF release by ∼50% when IL-33 and SP were administered in combination (Fig. 3A). These results were confirmed when we transiently decreased NK-1 receptor expression by 92% using NK-1 siRNA (50 µM) (Fig. S1A). NK-1 receptor knockdown significantly (P < 0.001) decreased TNF release by 50% when stimulated by IL-33 and SP in combination (Fig. 3B).
Fig. 3.
NK-1 receptor antagonists and ST2 neutralizing antibody inhibit TNF secretion. (A) LAD2 cells were pretreated with NK-1R antagonists L-733,060 (10 μM) and CP-96345 (10 µM) for 30 min and then stimulated with SP (1 μΜ), IL-33 (30 ng/mL) or their combination for 24 h. (C) LAD2 cells were preincubated with ST2 neutralizing antibody (3 ng/mL) or IgG control (3 ng/mL) for 2 h, then stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. (B and D) LAD2 cells were pretreated with NK-1 receptor siRNA (50 µM) (B), ST2 receptor siRNA (D), or scrambled siRNA (SC) (50 µM) (B and D) for 72–96 h and then stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Collected supernatant fluids were assayed by TNF ELISA. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. S1.
NK-1 and ST2 transient gene knockdown. LAD2 cells were treated with NK-1, ST2, or scramble (SC) siRNA (50 µM) for 72–96 h. Expression levels of NK-1 (A) and ST2 mRNA (B) were measured by qRT-PCR and normalized to human GAPDH endogenous control. n = 3. ****P < 0.0001.
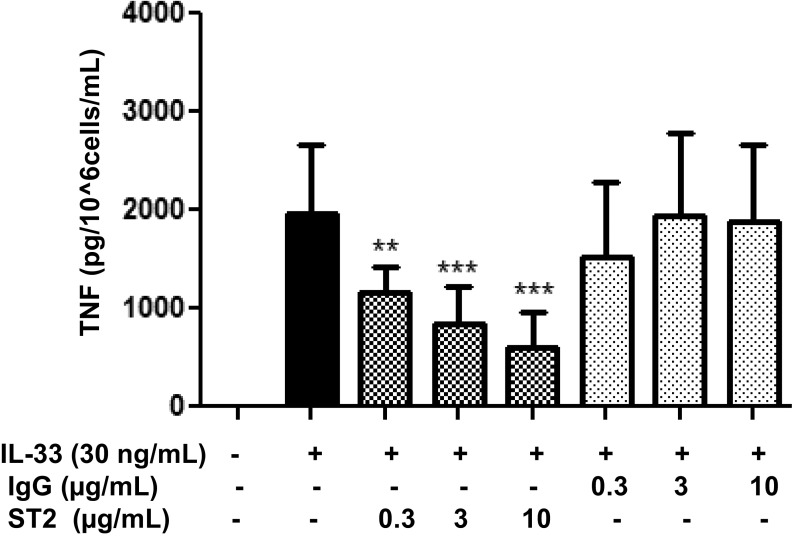
ST2 Receptor Neutralizing Antibody Inhibits TNF Secretion Stimulated by the Combination of SP and IL-33.
Then we investigated whether blockade of the IL-33 receptor ST2 diminishes the enhancing effect of IL-33 and SP. Owing to the absence of ST2 receptor antagonists, LAD2 cells were pretreated with a ST2 receptor neutralizing antibody and a nonspecific IgG control over various concentrations (Fig. S2). LAD2 cells were preincubated with the ST2 neutralizing antibody (3 µg/mL) or IgG control (3 µg/mL) for 2 h and then stimulated with SP (1 μM) alone, IL-33 (30 ng/mL) alone, or the combination for 24 h. Preincubation with the ST2 neutralizing antibody significantly (P < 0.0001) inhibited TNF release by 50% when IL-33 and SP were administered in combination and by 34% when IL-33 was administered alone (Fig. 3C). In addition, a transient 70% reduction in ST2 receptor gene expression using siRNA (Fig. S1B) significantly (P < 0.05) decreased TNF release by ∼30% when IL-33 and SP were administered in combination (Fig. 3D).
Fig. S2.
Dose-dependent blockade of ST2 receptor. LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates, preincubated with ST2 neutralizing antibody (0.3–10 µg/mL) or IgG control (0.3–10 µg/mL) for 2 h, and then stimulated with IL-33 (30 ng/mL) for 24 h. Supernatant fluids were collected at the end of the incubation period and assayed by TNF ELISA. n = 3. **P < 0.01; ***P < 0.001.
NK-1 and ST2 Receptor–Receptor Interactions.
LAD2 cells were preincubated with NK-1 antagonists L-733,060 (10 μM) or CP-96345 (10 µM) for 30 min and then stimulated with IL-33 (30 ng/mL) alone. Interestingly, we observed that both NK-1 antagonists also significantly (P < 0.001) inhibited the effect of IL-33 alone by ∼50% (Fig. 3A), suggesting an interaction between NK-1 and ST2 receptors. Moreover, ST2 receptor knockdown significantly (P < 0.05) decreased SP-stimulated TNF release by 30% (Fig. 3D), again suggesting that decreased ST2 expression results in decreased NK-1 activation.
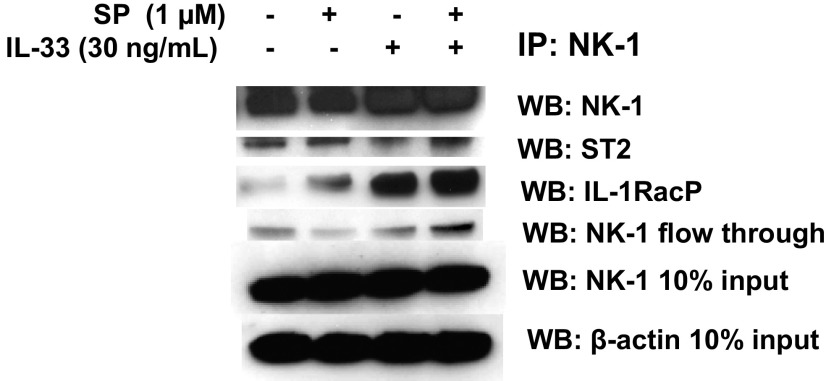
To further explore the interactions between NK-1 and ST2 receptors, we coimmunoprecipitated LAD2 cells using an antibody against the NK-1 receptor and assayed for a ST2 and IL-33 coreceptor, IL-1RacP. We found that even in unstimulated LAD2 cells, NK-1 coimmunoprecipitated with ST2 and IL-1RacP (Fig. S3), suggesting a complex formation. Interestingly, IL-1RacP protein expression was markedly increased when stimulated by the combination of SP and IL-33.
Fig. S3.
NK-1 receptor coimmunoprecipitates with ST2 and IL-1RacP. LAD2 cells (5 × 106 cells) were stimulated with the combination of SP and IL-33. Cell lysates were collected after 30 min and immunoprecipitated for an antibody against NK-1 receptor. IP samples as well as 10% loading control samples were assayed for protein levels of NK-1, ST2, IL-1RacP, and β-actin by Western blot analysis. n = 3.
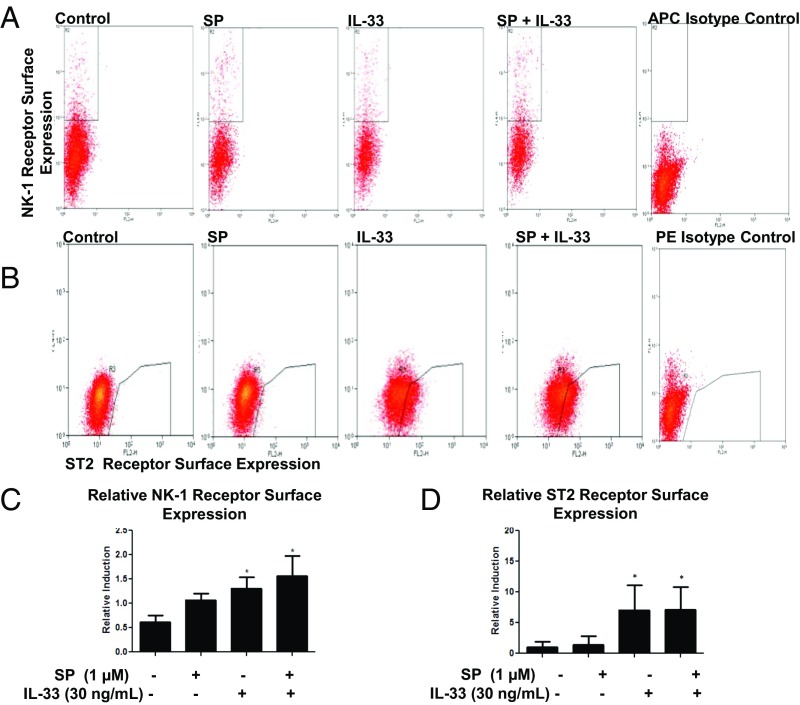
IL-33 and SP Administered Separately or in Combination Increase Their Receptor Expression.
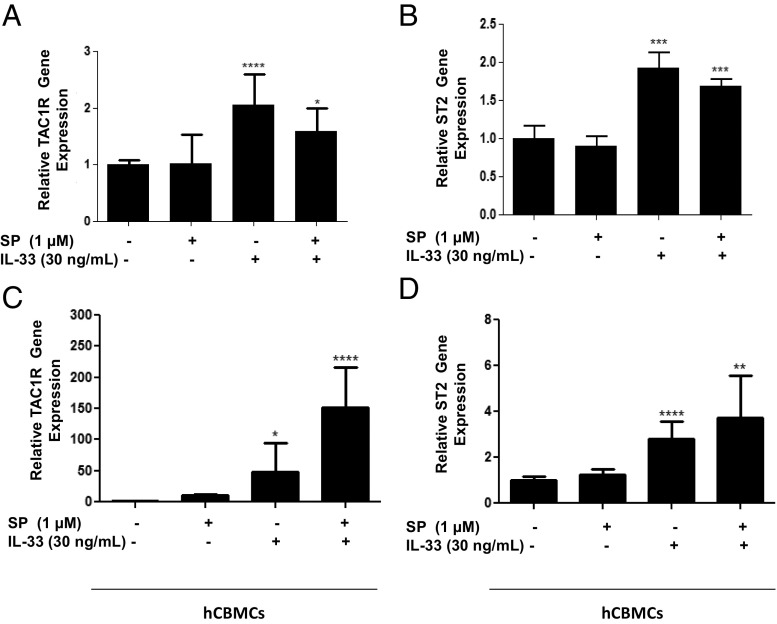
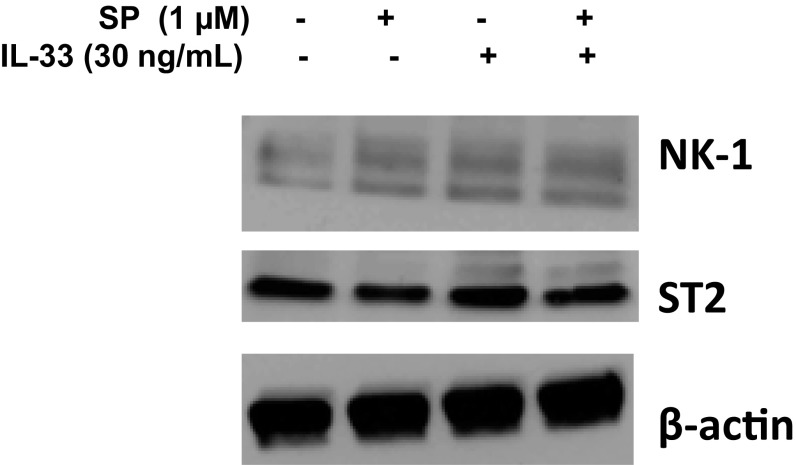
We also investigated the effects of SP alone, IL-33 alone, and the combination on NK-1 and ST2 receptor gene and protein expression. Stimulation with SP (1 μM), IL-33 (30 ng/mL), or their combination for 24 h significantly increased NK-1 (P < 0.05) and ST2 (P < 0.05) receptor gene expression by twofold (Fig. 4 A and B) in LAD2 cells and by 150-fold for NK-1 (P < 0.0001) and by threefold for ST2 (P < 0.01) in hCBMCs (Fig. 3 B and C). Measurement of total protein expression by Western blot analysis showed that IL-33 (30 ng/mL) in combination with SP (1 µM) increased the protein expression of both ST2 and NK-1 receptors (Fig. S4). In addition, fluorescence-activated cell sorting (FACS) analysis data show that IL-33 (30 ng/mL) increased its own ST2 receptor surface expression by sevenfold and NK-1 surface expression by 1.4-fold (Fig. 5 C and D). In contrast, SP (1 μM) increased its own NK-1 receptor surface expression by 1.1-fold, but had no effect on ST2 receptor surface expression. The combination of IL-33 (30 ng/mL) and SP (1 μM) increased NK-1 receptor protein surface expression by 1.5-fold (P < 0.05) (Fig. 5C).
Fig. 4.
Effect of SP and IL-33 on NK-1 and ST2 gene expression. LAD2 cells (1 × 106 cells per well) (A and B) and hCBMCs (0.3 × 106 cell per well) (C and D) were seeded in 12-well culture plates and stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. NK-1 and ST2mRNA expression levels were measured by qRT-PCR and normalized to human GAPDH endogenous control. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. S4.
IL-33 increases NK-1 total protein. LAD2 cells (1 × 106 cells) were stimulated with the combination of SP (1 µM) and IL-33 (30 ng/mL). Cell lysates were collected after 24 h, and protein levels of NK-1 and ST2 were measured by Western blot analysis. β-actin served as a loading control. n = 3.
Fig. 5.
IL-33 increases NK-1 and ST2 expression. (A and B) LAD2 cells (1 × 106 cells per well) were seeded in 12-well culture plates and stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Collected cells were probed for allophylocyanin (APC)-conjugated NK-1 receptor (A) and phycoerythrin (PE)-conjugated ST2 receptor (B). Data are from a representative experiment. (C and D) Relative NK-1 (C) and ST2 (D) expression on cell the surface compared with control. n = 3. *P < 0.05.
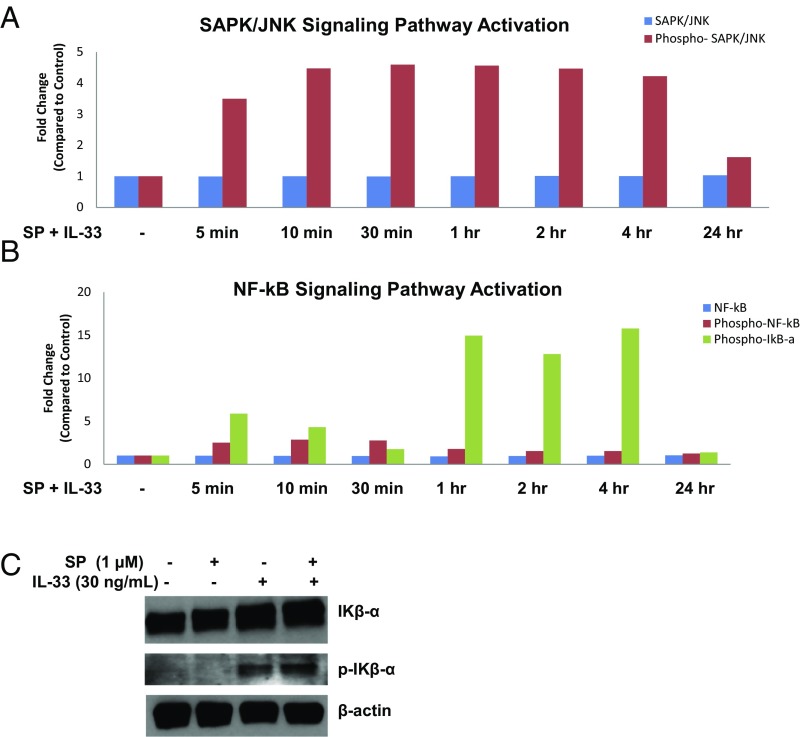
NF-κB and SAPK/JNK Signaling Pathways Are Involved in TNF Gene Expression and Secretion.
We next investigated the signaling pathways involved in the effect of SP and IL-33 administered in combination on TNF gene expression and secretion. PathScan ELISA performed on seven different kinases (ERK1/2, p38 MAPK, MEK1/2, Iκβ-α, NF-κB, SAPK/JNK, and STAT3) showed activation of the NF-κB and SAPK/JNK pathways. We observed a differential time course of the signaling pathway activation over 24 h with combined SP and IL-33 stimulation. The SAPK/JNK pathway was activated as early as 5 min after stimulation and was maintained over the next 4 h (Fig. 6B). In contrast, activation of NF-κB, as shown by phosphorylation of Iκβ-α, was observed starting at 1 h after stimulation (Fig. 6A) and maintained for the next 4 h.
Fig. 6.
NF-κB and SAPK/JNK signaling pathways are involved in TNF gene expression and secretion. (A and B) LAD2 cells (2 × 106 cells) were stimulated with SP (1 μΜ) and IL-33 (30 ng/mL) for 5 min, 10 min, 30 min, 1 h, 2 h, 4 h, and 24 h. Phosphorylation of SAPK/JNK (Th183/Tyr185) (A), NF-κB p65 (Ser536), and IΚβ-α (Ser32) (B) was detected using the PathScan Inflammation Mutli-Target Sandwich ELISA Kit. Whole-cell lysates were assayed at a protein concentration of 1 mg/mL. (C) LAD2 MCs were stimulated with SP (1 μM), IL-33 (30 ng/mL), and their combination. Cell lysates were collected after 1 h, and protein levels of IΚβ-α and phospho-IΚβ-α were measured by Western blot analysis. β-actin served as a loading control. n = 3.
We then stimulated LAD2 cells with SP (1 μM), IL-33 (30 ng/mL), or their combination for 1 h to determine which component of the combination triggers phosphorylation of Iκβ-α. Our Western blot assay revealed that IL-33 is responsible for the phosphorylation of Iκβ-α, and that the combination of SP and IL-33 increases it even further (Fig. 6C).
Methoxyluteolin Inhibits TNF Gene Expression and Secretion.
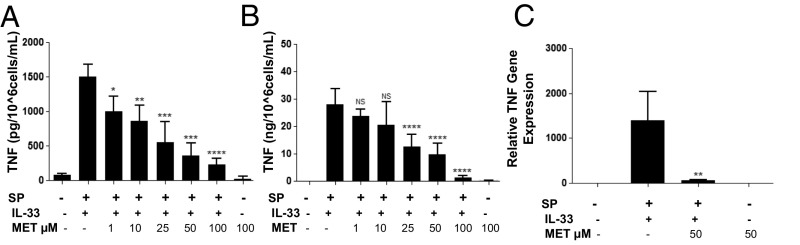
LAD2 cells were preincubated for 2 h with luteolin or methoxyluteolin (1–100 μM) and then stimulated with the combination of SP (1 μM) and IL-33 (30 ng/mL) for 24 h (Fig. S5). TNF cellular protein was significantly (P < 0.05) inhibited by 45% at the lowest flavonoid concentrations (1 μM), and TNF secretion was significantly (P < 0.0001) inhibited by 50% at concentrations ≥25 μM (Fig. 7 A and B). SP and IL-33–induced TNF mRNA gene expression was significantly (P < 0.001) inhibited by 98% at 50 μM (Fig. 7C). Methoxyluteolin was more potent than luteolin in inhibiting TNF secretion throughout the experiment.
Fig. S5.
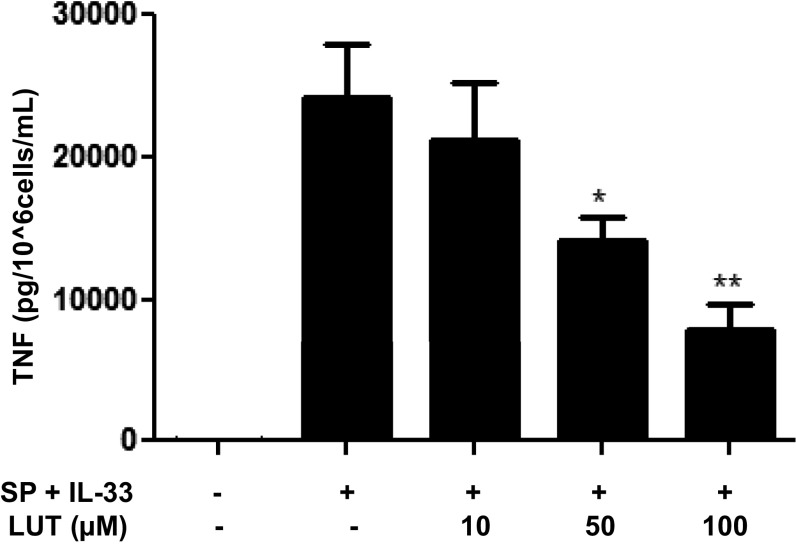
Luteolin inhibits TNF secretion. LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates and preincubated with luteolin (Lut) (10–100 μM), then stimulated with the combination of SP (1 μΜ) and IL-33 (30 ng/mL) for 24 h. Control cells were treated with 0.1% DMSO, the highest concentration corresponding to that of 100 μΜ Lut. Supernatant fluids were assayed for TNF by ELISA. n = 3. *P < 0.05; **P < 0.01, Student’s t test.
Fig. 7.
Methoxyluteolin inhibits TNF gene expression and secretion. (A and B) LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates and preincubated with methoxyluteolin (MET; 1–100 μM), then stimulated with the combination of SP (1 μΜ) and IL-33 (30 ng/mL) for 24 h. Control cells were treated with 0.1% DMSO, the highest concentration corresponding to that of 100 μΜ methoxyluteolin. Collected supernatant fluids (A) and cell lysates (B) were assayed by TNF ELISA. (C) LAD2 cells (1 × 106 cells per well) were seeded in 12-well culture plates and preincubated with methoxyluteolin (50 μM), then stimulated with the combination of SP (1 μΜ) and IL-33 (30 ng/mL) for 6 h. TNF mRNA expression levels were measured by qRT-PCR and normalized to human GAPDH endogenous control. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
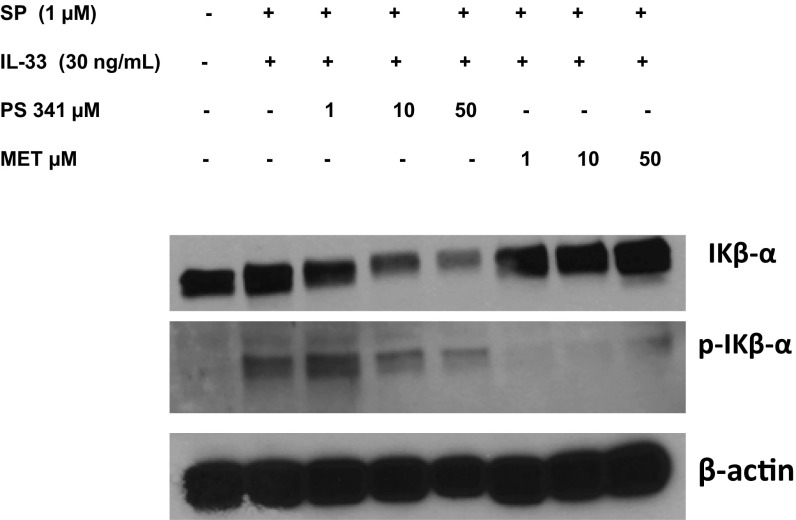
We also investigated the effect of methoxyluteolin on Iκβ-α phosphorylation. LAD2 mast cells were preincubated with the proteasome inhibitor PS 341 (1, 10, and 50 μM) as a positive control or methoxyluteolin (1, 10, and 50 μM) and then stimulated with the combination of IL-33 and SP. Cell lysates were collected after 1 h, and protein levels of IKβ-α and phospho-Ikβ-α were assayed by Western blot analysis. Preincubation with methoxyluteolin (1–50 μM) markedly inhibited the Iκβ-α phosphorylation induced by the combination of IL-33 (30 ng/mL) and SP (1 μM) in LAD2 cells (Fig. 8).
Fig. 8.
Methoxyluteolin inhibits IKβ-α phosphorylation. LAD2 cells (1 × 106 cells) were preincubated with proteasome inhibitor PS 341 (1, 10, and 50 μM) or methoxyluteolin (1, 10, and 50 μM) and then stimulated with the combination of SP and IL-33. Cell lysates were collected after 1 h, and protein levels of IKβ-α and phospho-IΚβ-α were measured by Western blot analysis. β-actin served as a loading control. n = 3.
Discussion
The results presented here demonstrate complex interactions between the peptide SP and the cytokine IL-33 administered in combination that lead to a significant increase in TNF synthesis and secretion in cultured human mast cells. These responses depend on the activation of both NK-1, the receptor for the peptide SP, and ST2, the receptor for the cytokine IL-33.
An important finding is that interference with the NK-1 receptor function results in a decrease in ST2 receptor activation, and that a decrease in ST2 receptor expression results in a decrease in NK-1 receptor signaling. Treatment with 100-fold more of the NK-1 antagonists did not completely inhibit the robust TNF secretion, suggesting that another mechanism is contributing to this effect (Fig. S6). NK-1 inhibition was previously reported to only partially inhibit SP stimulation of skin-derived human mast cells (38), supporting the idea that another pathway might be participating in this response. Furthermore, the NK-1 antagonists inhibit TNF secretion in mast cells stimulated by IL-33 alone, whereas the ST2 siRNA also inhibits SP-stimulated TNF, indicating possible receptor–receptor interaction between NK-1 and ST2. A precedent for this receptor–receptor interaction is a report of cross-activation of c-kit, the receptor for stem cell factor (SCF), via complexing with ST2 and its coreceptor, the IL-1R accessory protein (IL-RAcP), in murine mast cells (39). An analogous structural association could occur among NK-1, ST2, and IL-RAcP via contact; therefore, we coimmunoprecipitated these proteins. The results of these experiments show that coimmunoprecipitation using an antibody specific to NK-1 recovered both ST2 and IL-1RacP. The expression of IL-1RacP was markedly increased after stimulation with the combination of SP and IL-33, suggesting that this protein participates in complexing NK-1 and ST2 receptors. Because coimmunoprecipitation is performed after cell lysis, the association of these proteins cannot be confirmed through this methodology alone. The results merit further investigation to assess the association among NK-1, ST2, and IL-1RacP via techniques that do not require cell lysis.
Fig. S6.
NK-1 receptor antagonists at 100 µM do not completely inhibit TNF secretion. LAD2 cells were pretreated with NK-1R antagonists L-733,060 (100 μM) and CP-96345 (100 µM) for 30 min and then stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Collected supernatant fluids were assayed by TNF ELISA. n = 3. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Mast cells are now recognized as critical in inflammatory processes (40) and are implicated in inflammatory diseases including rheumatoid arthritis (41), inflammatory bowel disease (42), psoriasis (43), multiple sclerosis (44), mastocytosis (45, 46), and fibromyalgia syndrome (47). Independent reports have shown that mast cells, SP, IL-33, and TNF contribute to the inflammatory processes in these diseases (31, 47–51); for example, increased SP plasma levels (0.31 µg/L) that correlate with mast cell load have been reported in patients with mastocytosis (52). In addition, treatment with the TNF inhibitor etanercept has been shown to reduce serum SP levels in patients with rheumatoid arthritis (53). Our findings provide insight into how SP, IL-33, TNF, and mast cells may interact in these inflammatory diseases. The combined enhancing effect of SP and IL-33 on robust secretion of TNF from human mast cells could be a major contributor to the inflammatory processes occurring in the aforementioned pathological conditions.
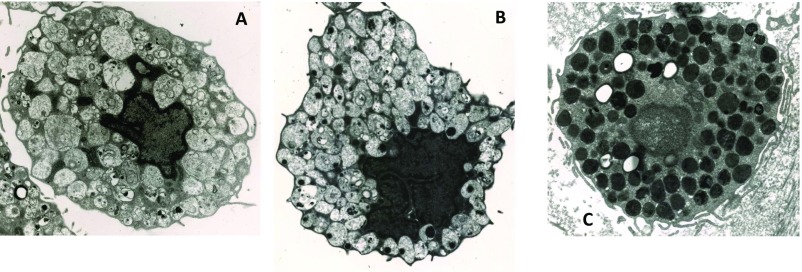
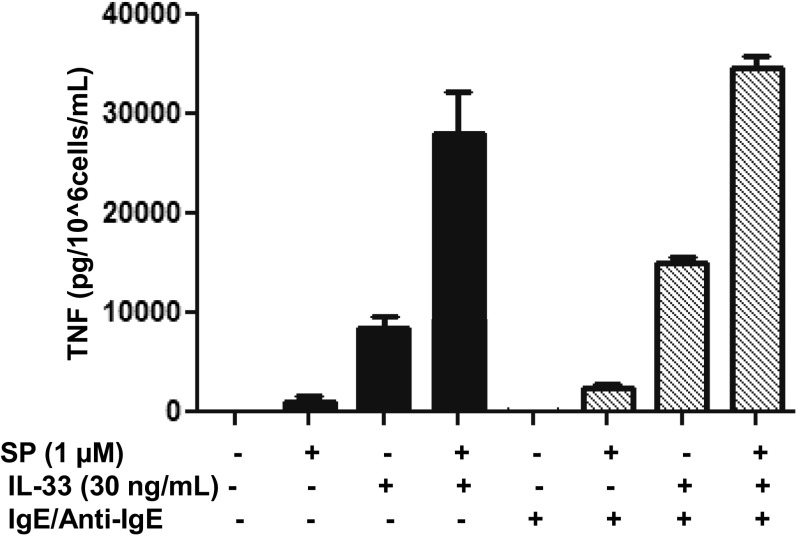
A major challenge in the studies of mast cell pathophysiology is the source of primary mast cells. Ordinarily, mature human mast cells can come from clean margins of breast mastectomies, extracted tissue from fat reduction, or circumcisions. However, these cells vary enormously in their phenotypic characteristics owing to the age of the donor, site of skin used, degree of inflammation, or the presence of adipocytokines. The primary mast cells that we used were isolated from umbilical cord blood collected from healthy donors after uncomplicated deliveries, which have been well characterized (54). At 12 wk, these cells express the major granule mediator tryptase (Fig. S7A) and the surface receptor for SCF, c-kit (Fig. S7B). The drawback of these hCBMC cells is that their secretory granules do not appear to have the morphology of mature adult human mast cells (Fig. S8). SP had previously been shown to stimulate small amounts of TNF from rat peritoneal mast cells (9, 55) and cultured human skin mast cells (10, 11), but at much higher SP concentrations (20–100 µM). One major difference between those studies may be the fact that human skin mast cells that responded to SP were derived from adult skin (10), whereas those cells that did not respond to SP were purified from circumcisions (38). In addition, it has been reported that SP stimulates TNF release from LAD2 cells, but not from purified human skin mast cells (38). In fact, there is great variability in the allergic response to IgE among mast cells derived from circumcisions (25, 56). Moreover, it is important to note that unlike previous reports showing that IL-33 augmented allergic IgE-stimulated histamine release, in the present study it did not augment inflammatory IgE-stimulated TNF release in human cultured mast cells (Fig. S9).
Fig. S7.
hCBMCs contain tryptase and express surface c-kit receptor. Photomicrographs of hCBMCs stained by immunohistochemistry at 12 wk for tryptase content (A) and c-kit receptor surface expression (B). (Magnification: 1,000×.)
Fig. S8.
Lack of secretory granule content maturity in hCBMCs. Transmission electron micrographs of individual human mast cells: (A) hCBMCs, 9-wk culture; (B) hCBMCs, 15-wk culture; (C) normal human detrusor muscle mast cell. (Magnification: 10,000×.) Note the differences in quality, content, and texture of the secretory granules.
Fig. S9.
IgE/anti-IgE does not increase SP and IL-33 stimulated secretion of TNF. LAD2 cells (1 × 105 cells per well) were seeded in 96-well culture plates and preincubated with human IgE (1 µg/mL) or medium overnight, and the next day stimulated with anti-IgE (10 ng/mL) for 2 h and/or SP (1 µM) and IL-33 (30 ng/mL) for 24 h. Supernatant fluids were collected at the end of the incubation period and assayed by TNF ELISA. n = 3.
Our present findings provide an enhanced understanding of the physiological and pathological interactions among peptides, cytokines, and mast cells, which may lead to the discovery of novel pharmacologic approaches to the development of antiinflammatory therapies. We used a naturally occurring molecule, methoxyluteolin, to explore its inhibitory effects on mast cells. Methoxyluteolin inhibits the enhancing effect of SP and IL-33 on TNF gene expression and secretion from human mast cells, indicating that this flavonoid must be acting at some step of the signaling pathway following receptor activation. We have shown that methoxyluteolin is a more potent inhibitor of human mast cells (57) and human keratinocytes (37) compared with luteolin, and that therapeutic doses can be achieved in vivo (Table S1). Flavonoids (58), particularly luteolin (59) and methoxyluteolin (60), are considered safe. The time course of the key signaling steps of stimulation with the combination of IL-33 and SP led to JNK kinase activation within the first 5 min, whereas IKβ-α became active within the first hour, and both remained active over the next 4 h of stimulation. SP/NK-1 receptor signaling has been shown to activate JNK kinase (16), whereas IL-33/ST2 receptor activation has been shown to signal via NK-κB (61). One of the ways in which methoxyluteolin may inhibit TNF gene expression and secretion is through the inhibition of IKβ-α phosphorylation. SP was previously reported to stimulate NF-κB in murine umbilical cord blood mast cells (55) at 10–100 µM and in hCBMCs at 1 µM (57). On phosphorylation, IKβ-α releases NF-κB, which translocates from the cytoplasm into the nucleus and regulates gene the expression of various inflammatory mediators, including TNF (62). IL-33 could contribute to TNF gene expression and secretion stimulated by the combination of IL-33 and SP via translocation into the nucleus, where it could possibly activate some transcription factors, particularly AP-1 (63), an affect that may be inhibited by methoxyluteolin. We previously showed that both luteolin and methoxyluteolin inhibit NF-κB and also decrease NF-кB p65 DNA-binding activity in the nuclear extract of human mast cells (64). Both flavonoids also decrease mRNA expression of two genes encoding different subunits in the NF-κB protein complex, NFKB1 (encoding NF-κB p50 subunit) and RELA (encoding NF-κB p65 subunit) (64).
Table S1.
Therapeutic dose of methoxyluteolin can be achieved in vivo
| Flavonoid | Molecular weight, g/mol | Concentration, µM | 1 L = 1 kg | Average body weight, kg | Flavonoid absorption, % | Capsule weight, mg | Capsules per day |
| Methoxyluteolin | 342.3 | 25 | 8.55 mg/kg | 80 | 50 | 150 | 8–9 |
| Luteolin | 338.3 | 25 | 8.45 mg/kg | 80 | 50 | 150 | 8–9 |
At 25 μM, methoxyluteolin and luteolin significantly inhibit TNF secretion from human cultured mast cells. Assuming that 1 L = 1 kg, the dose of methoxyluteolin then equals MW (342.343 g/mol) × molarity (25 μM) = 342.343 g/mol × 25 μM = 8.55 mg/L= 8.55 mg/kg. If we further assume that adults weigh on average ∼80 kg and maximal absorption of the flavonoids is 50%, we should be administering 8.55 × 80 × 2 = 1,368 mg/day. The structural analog of methoxyluteolin, luteolin is available in 150 mg soft gel capsules (www.algonot.com); thus, we assume that methoxyluteolin can be administrated the same way. Therefore, methoxyluteolin could be taken as 8–9 capsules per day.
Our present results have important clinical implications for the understanding of the complex interplay among SP, IL-33, and mast cells in inflammatory processes. The impressive enhancing effect of IL-33 and SP in combination on TNF gene expression and secretion in human mast cells may be a key step in the pathogenesis of inflammatory diseases. The potent inhibitory effect of methoxyluteolin suggests that it could be developed as systemic or local antiinflammatory treatment. The magnitude of TNF synthesis and secretion, due to interactions between SP and IL-33, suggests new therapeutic approaches through the use of SP and IL-33 receptor antagonists, as well as methoxyluteolin.
Methods
Culture of Human Mast Cells.
LAD2 mast cells derived from a human mast cell leukemia (65) were cultured in StemPro-34 medium (Invitrogen) supplemented with 100 U/mL penicillin/streptomycin and 100 ng/mL recombinant human SCF (rhSCF; StemGen). Cells were maintained at 37 °C and a 95% O2/5% CO2 atmosphere in a humidified incubator. Cell viability was measured by trypan blue exclusion at all SP and IL-33 concentrations tested. The umbilical cord blood was obtained under Tufts University IRB approval No. 12152, which waived the requirement for written informed consent as per Federal regulations for unidentified discarded biologic material.
For culture of primary hCBMCs, human umbilical cord blood was obtained after normal deliveries in accordance with established institutional guidelines (66, 67). Mononuclear cells were isolated by layering heparin-treated cord blood onto Lymphocyte Separation Medium (MP Biomedicals). CD34+ progenitor cells were isolated by means of positive selection of AC133 (CD133+/CD34+) cells using magnetic cell sorting (CD133 Microbead Kit; Miltenyi Biotech). For the first 6 wk, CD34+ progenitor cells were cultured in Iscove’s modified Dulbecco’s medium (Life Technologies) supplemented with 0.1% BSA, 1% insulin-transferrin-selenium, 50 ng/mL IL-6, 0.1% b-mercaptoethanol, 1% penicillin/streptomycin, and 100 ng/mL rhSCF. After 6 wk, the cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 10% FBS, 50 ng/mL IL-6, 0.1% b-mercaptoethanol, 1% penicillin/streptomycin, and 100 ng/mL rhSCF. hCBMCs cultured for at least 12 wk were used for experiments. Cell viability was determined by means of trypan blue (0.4%) exclusion.
Mast Cell Treatments.
LAD2 cells and/or hCBMCs were stimulated with various concentration of SP (0.01–1 µM; Sigma-Aldrich) and IL-33 (1–30 ng/mL; R&D Systems) alone or in combination. In some experiments, LAD2 cells were stimulated with human IgE (1 µg/mL; Millipore) overnight and then triggered with anti-IgE (10 ng/mL; Life Technologies). In some experiments, LAD2 cells were pretreated with the NK-1 antagonists L-733,060 (10 μM; Sigma-Aldrich) and CP-96345 (10 µM; Tocris Biosciences), an ST2 neutralizing antibody (0.3–10 μg/mL; R&D Systems) or a nonspecific IgG antibody (0.3–10 μg/mL; R&D Systems), the proteasome inhibitor PS 341 (1–50 µM; Tocris Biosciences), and methoxyluteolin (1–100 µM; Hangzhou Skyherb Technologies Co., Ltd., Zhejiang, China). Silencer Select siRNA targeting either NK-1 or ST2 receptors, as well as control scramble siRNA (10–100 nM; Life Technologies), were used in Lipofectamine RNAiMAX and OPTI-MEM medium (Life Technologies) to treat LAD2 cells for 72–96 h, to inhibit gene expression of respective receptors. Additional details are provided in SI Methods.
SI Methods
TNF Assays.
LAD2 cells (1 × 105 cells/well) were treated with various concentrations of SP (0.01–1 µM) and IL-33 (1–30 ng/mL) alone or in combination for 24 h. Control cells were treated with the same volume of culture media alone. Supernatants were collected and assayed using the TNF DuoSet ELISA Kit (R&D Systems).
RNA Isolation and qRT-PCR.
LAD2 cells (1 × 106 cells) were stimulated for 6 h with SP (1 μΜ), IL-33 (30 ng/mL), or their combination. Total mRNA was extracted with the RNeasy Mini Kit (Qiagen) in accordance with the manufacturer’s instructions. The iScript cDNA Synthesis Kit (BioRad) was used for reverse-transcription of each mRNA sample. qRT-PCR was performed using TaqMan gene expression assays for TNF, NK-1 receptor, and ST2 receptor (Applied Biosystems). Samples were run at 45 cycles using an Applied Biosystems 7300 real-time PCR system. Relative mRNA levels were determined from standard curves run with each experiment. mRNA gene expressions were normalized to GAPDH endogenous control (Applied Biosystems).
FACS.
LAD2 cells (1 × 106 cells) were treated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h, centrifuged at 500 × g for 5 min, and then washed three times in PBS. Cells were treated with 1 μg of mouse IgG1/human IgG for 15 min at room temperature before staining to block nonspecific binding. The APC-conjugated human NK-1 receptor antibody and PE-conjugated human ST2 receptor antibody (R&D Systems) were added to stimulated cells for 45 min at 2–8 °C. Following incubation, unreacted antibodies were removed by washing the cells three times with PBS. Finally, cells were resuspended in 750 μL of PBS for flow cytometry analysis. Cell surface expressions of NK-1 and ST2 receptors were determined with a FACSCalibur flow cytometer (BD Biosciences).
Multiple Kinase Phosphorylation Assay.
LAD2 cells (2 × 106 cells) were stimulated with SP (1 μΜ) and IL-33 (30 ng/mL) in a time-dependent manner for 5 min, 10 min, 30 min, 1 h, 2 h, 4 h, and 24 h. Phosphorylation of SAPK/JNK (Th183/Tyr185), NF-κB p65 (Ser536), and Iκβ-α (Ser32) was detected with the PathScan Inflammation Multi-Target Sandwich ELISA Kit (7276; Cell Signaling Technology) in accordance with the manufacturer’s instructions. Whole cell lysates were assayed at a protein concentration of 1 mg/mL. Absorbance was read at 450 nm using a LabSystems Multiskan RC microplate reader (Thermo Fisher Scientific). Relative phospho-SAPK/JNK, phospho-NF-κB p65, and phospho-Iκβ-α levels were normalized to control cells.
Immunoprecipitation and Western Blot Assays.
LAD2 cells (1 × 106) were preincubated with the proteasome inhibitor PS 341 (1–50 µM) or methoxyluteolin (1–50 µM) for 2 h and then stimulated with SP (1 μM), IL-33 (30 ng/mL), and/or their combination for 1 h. The reaction was stopped by the addition of ice-cold PBS. Cells were washed once with PBS and then lysed using protein lysis radioimmunoprecipitation buffer (Sigma-Aldrich) in the presence of protease inhibitor mixtures. For immunoprecipitation (IP), LAD2 cells (5 × 106) were treated with SP (1 μM), IL-33 (30 ng/mL), and/or their combination for 30 min, washed once with PBS, and then lysed using Cell Lysis Buffer (Cell Signaling Technology) supplemented with PMSF and EDTA (Cell Signaling Technology). Total protein concentration was determined by bicinchoninic acid assay (BCA) (Thermo Fisher Scientific) using BSA as a standard. For IP, 200 μg of protein was incubated with rabbit anti-human NK-1 primary antibody (Abcam) at a 1:50 ratio overnight at 4 °C and then incubated with 30 µL of 50% Protein A Bead Slurry (Cell Signaling Technology) for 2 h, washed with Cell Lysis Buffer, and boiled for 5 min in 2× SDS. The total cellular proteins (20-μg aliquots) and IP samples were separated using 4–20% Mini Protean TGX Gels (Bio-Rad) under SDS denaturing conditions and then electrotransferred onto PVDF membranes (Bio-Rad). Blocking was carried out with 5% BSA in Tris-buffered saline containing 0.05% Tween-20. The membranes were probed with the following primary antibodies at a dilution: Iκβ-α (1:1,000; Cell Signaling Technology), phospho-Iκβ-α (1:1,000; Cell Signaling Technology), IL-1RacP (1:1,000; R&D Systems), ST2 (1:250; Abcam), and NK-1 (1:10,000 Abcam). β-actin served as a loading control. For detection, the membranes were incubated with the appropriate secondary HRP-conjugated antibody (1:1,000; Cell Signaling Technology), and the blots were visualized with enhanced chemiluminescence SuperSignal West Pico Substrate (Thermo Fisher Scientific).
Immunocytochemistry for Tryptase and c-Kit.
The purity of hCBMCs was determined by immunostaining for mast cell-specific tryptase. Cytospin smears of hCBMCs were prepared using a Cytospin 3 centrifuge (Shandon). After fixation with Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid) for 3 min, the smears were stained for mast cell tryptase and c-kit receptor by the alkaline phosphatase–anti-alkaline phosphatase (APAAP) technique using the Dako APAAP Kit system, as reported previously (66, 67). In brief, the slides were incubated overnight at 4 °C with mouse anti-human tryptase and c-kit monoclonal antibodies (Chemicon) used at 1 mg/mL in Tris⋅HCl-PBS (pH 7.6) plus 10% FBS. The slides were brought to room temperature, then incubated with rabbit anti-serum (Ig fraction) to mouse Ig for 30 min, followed by incubation with the APAAP immune complex for another 30 min. Between each incubation, the slides were rinsed in Tris-buffered saline (pH 7.6) for 10 min. The reaction was finally developed with substrate solution (naphthol AS-MX phosphate, fast red, and levamisole) for 20 min and then rinsed briefly in a water bath. Negative controls were performed either by omitting the primary antibody or by using an isotype-matched mouse IgG1 antibody instead of the primary antibody.
Statistics.
All experiments were performed in triplicate and repeated at least three times (n = 3). Data are presented as mean ± SD. Results were analyzed using the unpaired two-tailed Student’s t test. The significance of comparisons between conditions is denoted by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Acknowledgments
We thank Drs. A. S. Kirshenbaum and D. Metcalfe (National Institutes of Health) for the LAD2 human mast cells, Swedish Orphan Biovitrum for their generous gift of rhSCF, and Dr. D. Kempuraj for the immunohistochemistry staining experiments. This work was supported in part by a Pfizer ASPIRE Rheumatology and Dermatology Award, a translational grant from the Psoriasis Foundation, and the Michael and Margaret Johnson Family Fund (T.C.T.).
Footnotes
Conflict of interest statement: T.C.T. and Cem Akin were coauthors on a 2015 publication. This publication was a review article and did not involve any research collaboration.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524845114/-/DCSupplemental.
References
- 1.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 2.Saraceno R, Kleyn CE, Terenghi G, Griffiths CE. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876–882. doi: 10.1111/j.1365-2133.2006.07518.x. [DOI] [PubMed] [Google Scholar]
- 3.Douglas SD, Leeman SE. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 5.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard AC, et al. The TNF-family cytokine TL1A: From lymphocyte costimulator to disease co-conspirator. J Leukoc Biol. 2015;98:333–45. doi: 10.1189/jlb.3RI0315-095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz M, Coveñas R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 2014;46:1727–1750. doi: 10.1007/s00726-014-1736-9. [DOI] [PubMed] [Google Scholar]
- 8.Fewtrell CMS, et al. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-α gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 10.Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. Human skin mast cells produce TNF-alpha by substance P. Int Arch Allergy Immunol. 1998;117:48–51. doi: 10.1159/000053571. [DOI] [PubMed] [Google Scholar]
- 11.Okabe T, Hide M, Koro O, Yamamoto S. Substance P induces tumor necrosis factor-alpha release from human skin via mitogen-activated protein kinase. Eur J Pharmacol. 2000;398:309–315. doi: 10.1016/s0014-2999(00)00304-6. [DOI] [PubMed] [Google Scholar]
- 12.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 13.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 14.Rottem M, Mekori YA. Mast cells and autoimmunity. Autoimmun Rev. 2005;4:21–27. doi: 10.1016/j.autrev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Theoharides TC, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumpter TL, et al. Autocrine hemokinin-1 functions as an endogenous adjuvant for IgE-mediated mast cell inflammatory responses. J Allergy Clin Immunol. 2015;135:1019–30.e8. doi: 10.1016/j.jaci.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Setting the cytokine trap for autoimmunity. Nat Med. 2003;9:20–22. doi: 10.1038/nm0103-20. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. Targeting IL-33 in autoimmunity and inflammation. J Pharmacol Exp Ther. 2015;354:24–31. doi: 10.1124/jpet.114.222505. [DOI] [PubMed] [Google Scholar]
- 21.Saluja R, et al. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–85. doi: 10.1016/j.molimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Saluja R, Hawro T, Eberle J, Church MK, Maurer M. Interleukin-33 promotes the proliferation of mouse mast cells through ST2/MyD88 and p38 MAPK-dependent and Kit-independent pathways. J Biol Regul Homeost Agents. 2014;28:575–585. [PubMed] [Google Scholar]
- 23.Moulin D, et al. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Silver MR, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm Res. 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 25.Babina M, Guhl S, Artuc M, Trivedi NN, Zuberbier T. Phenotypic variability in human skin mast cells. Exp Dermatol. 2016;25:434–439. doi: 10.1111/exd.12924. [DOI] [PubMed] [Google Scholar]
- 26.Louten J, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 27.Sjöberg LC, Gregory JA, Dahlén SE, Nilsson GP, Adner M. Interleukin-33 exacerbates allergic bronchoconstriction in the mice via activation of mast cells. Allergy. 2015;70:514–521. doi: 10.1111/all.12590. [DOI] [PubMed] [Google Scholar]
- 28.Meephansan J, et al. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J Dermatol Sci. 2013;71:107–114. doi: 10.1016/j.jdermsci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J Invest Dermatol. 2012;132:2593–2600. doi: 10.1038/jid.2012.185. [DOI] [PubMed] [Google Scholar]
- 30.Balato A, et al. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol. 2012;21:892–894. doi: 10.1111/exd.12027. [DOI] [PubMed] [Google Scholar]
- 31.Mitsui A, et al. Serum IL-33 levels are increased in patients with psoriasis 1. Clin Exp Dermatol. 2015;41:183–9. doi: 10.1111/ced.12670. [DOI] [PubMed] [Google Scholar]
- 32.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 33.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI: A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B, et al. Mitochondria distinguish granule-stored from de novo synthesized tumor necrosis factor secretion in human mast cells. Int Arch Allergy Immunol. 2012;159:23–32. doi: 10.1159/000335178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakae S, et al. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempuraj D, et al. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng Z, Patel AB, Vasiadi M, Therianou A, Theoharides TC. Luteolin inhibits human keratinocyte activation and decreases NF-κB induction that is increased in psoriatic skin. PLoS One. 2014;9:e90739. doi: 10.1371/journal.pone.0090739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunol. 2005;163:92–101. doi: 10.1016/j.jneuroim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Drube S, et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 2010;115:3899–3906. doi: 10.1182/blood-2009-10-247411. [DOI] [PubMed] [Google Scholar]
- 40.Theoharides TC, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kritas SK, et al. Mast cell involvement in rheumatoid arthritis. J Biol Regul Homeost Agents. 2013;27:655–660. [PubMed] [Google Scholar]
- 42.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: Implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Kritas SK, et al. Impact of mast cells on the skin. Int J Immunopathol Pharmacol. 2013;26:855–859. doi: 10.1177/039463201302600403. [DOI] [PubMed] [Google Scholar]
- 44.Costanza M, Colombo MP, Pedotti R. Mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci. 2012;13:15107–15125. doi: 10.3390/ijms131115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am. 2006;26:465–485. doi: 10.1016/j.iac.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Castells M, Austen KF. Mastocytosis: Mediator-related signs and symptoms. Int Arch Allergy Immunol. 2002;127:147–152. doi: 10.1159/000048188. [DOI] [PubMed] [Google Scholar]
- 47.Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC. Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther. 2016;356:664–672. doi: 10.1124/jpet.115.230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 49.Singh UP, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. doi: 10.1016/j.cyto.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Victor FC, Gottlieb AB, Menter A. Changing paradigms in dermatology: Tumor necrosis factor alpha (TNF-alpha) blockade in psoriasis and psoriatic arthritis. Clin Dermatol. 2003;21:392–397. doi: 10.1016/j.clindermatol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Probert L. TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Maintz L, et al. Neuropeptide blood levels correlate with mast cell load in patients with mastocytosis. Allergy. 2011;66:862–869. doi: 10.1111/j.1398-9995.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 53.Origuchi T, et al. Reduction in serum levels of substance P in patients with rheumatoid arthritis by etanercept, a tumor necrosis factor inhibitor. Mod Rheumatol. 2011;21:244–250. doi: 10.1007/s10165-010-0384-5. [DOI] [PubMed] [Google Scholar]
- 54.Kourelis T, Manola A, Theoharides TC. Umbilical cord-derived mast cells as models for the study of inflammatory diseases. In: Cetrulo CL, Cetrulo KJ, Cetrulo CLJ, editors. Pregnancy and Placental Stem Cells. Wiley-Blackwell; Hoboken, NJ: 2010. pp. 103–145. [Google Scholar]
- 55.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta. 2003;1643:75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Lefrançais E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weng Z, Patel AB, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2015;135:1044–52.e5. doi: 10.1016/j.jaci.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harwood M, et al. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 59.Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. 2013;35:592–602. doi: 10.1016/j.clinthera.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Theoharides TC. Tolerability and benefit of a tetramethoxyluteolin-containing skin lotion. Int J Immunopathol Pharmacol. 2017 doi: 10.1177/0394632017707610. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milovanovic M, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 62.Müller-Ladner U, Gay RE, Gay S. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep. 2002;4:201–207. doi: 10.1007/s11926-002-0066-1. [DOI] [PubMed] [Google Scholar]
- 63.Kakkar R, Lee RT. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 65.Kirshenbaum AS, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia: Activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 66.Kempuraj D, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 67.Kempuraj D, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]