Significance
We examine brain dynamics during a common social experience—social exclusion—to determine whether cohesive networks in the brain support navigation of the social world and contribute to the shape of friendship networks. Specifically, exclusion is associated with increased cohesion within brain networks that support understanding what other people think and feel. Furthermore, using social network analysis, we find that variability in brain dynamics is associated with the shape of participants’ friendship networks. Bringing together findings related to brain network dynamics and social network dynamics illuminates ways that psychological processes may shape and be shaped by social environments.
Keywords: social exclusion, mentalizing, fMRI, functional connectivity, social networks
Abstract
Social ties are crucial for humans. Disruption of ties through social exclusion has a marked effect on our thoughts and feelings; however, such effects can be tempered by broader social network resources. Here, we use fMRI data acquired from 80 male adolescents to investigate how social exclusion modulates functional connectivity within and across brain networks involved in social pain and understanding the mental states of others (i.e., mentalizing). Furthermore, using objectively logged friendship network data, we examine how individual variability in brain reactivity to social exclusion relates to the density of participants’ friendship networks, an important aspect of social network structure. We find increased connectivity within a set of regions previously identified as a mentalizing system during exclusion relative to inclusion. These results are consistent across the regions of interest as well as a whole-brain analysis. Next, examining how social network characteristics are associated with task-based connectivity dynamics, we find that participants who showed greater changes in connectivity within the mentalizing system when socially excluded by peers had less dense friendship networks. This work provides insight to understand how distributed brain systems respond to social and emotional challenges and how such brain dynamics might vary based on broader social network characteristics.
Humans are fundamentally motivated to connect with others, spending considerable time and energy investing in social relationships. A lack of social connection resulting from social isolation has a significant negative impact on health and well-being (1, 2), with risks comparable to those associated with lifelong smoking (3). In fact, the acute disruption of ties through social exclusion has a marked effect on our thoughts and feelings, and hence brain responses (4). This neural responsiveness to exclusion is thought to help maintain social bonds (5). However, people vary in how strongly they respond to social challenges such as exclusion (6). One important factor that can moderate stress responses and that may contribute to individual differences in reactivity to social challenges such as exclusion is social network resources (7–9). In particular, individual differences in how people react to being excluded may influence the types of social networks they build and thus the resources that limit the risks related to exclusion; likewise, different social environments may influence how a given individual approaches and reacts to stressful events (10). Therefore, an individual’s network structure represents a potential moderator for the cognitive processing of social exclusion.
Previous neuroimaging studies have investigated the neural substrates of social exclusion by using experimental designs in which participants are socially isolated through rejection by others; one frequently used task is Cyberball, a virtual ball-tossing game where the experimental participant is excluded from the game and receives no ball throws from other players (11, 12). Research on social exclusion has identified the role of two proposed networks: (i) a social pain system associated with distress during exclusion (4, 5), characterized by enhanced activations in the anterior cingulate cortex (ACC) and the anterior insula (aINS); and (ii) a mentalizing system with consistent activity in the dorsal and ventral medial prefrontal cortex (mPFC), precuneus, and bilateral temporo–parietal junction (TPJ) (13, 14). Although described rhetorically as two cohesive networks, limited research has examined functional interactions, or network dynamics, among the regions during social tasks. A small number of studies have focused on a single hotspot of univariate activation effects in the dorsal ACC (dACC) and have assessed its connectivity during social exclusion (15–17); however, no research has examined broader network dynamics during social exclusion. Furthermore, no research has examined how social environments might moderate brain network dynamics in the face of social interaction. This gap limits our ability to draw conclusions about how brain network and social network dynamics might underlie human processing of social interactions.
To this end, we first capitalized on recent advances in network neuroscience to study the functional connectivity relationships among multiple brain regions (18, 19) during social exclusion and inclusion. Although initially focused on the resting state, these novel techniques have recently been extended to assess the dynamics of connectivity patterns during task performance (20–27). Taking a dynamic network neuroscience perspective, we set out to examine the social pain and mentalizing networks holistically during social exclusion as well as broader network dynamics across the whole brain as a function of social exclusion and inclusion.
Second, we examine how network connectivity during social exclusion relates to an individual’s social network structure (28, 29). Sociologists have long shown that social network variables can characterize the social structure in which people are embedded and that social network structure can explain important outcomes ranging from measures of happiness to susceptibility to disease (30–32); likewise, individual differences in personality can shape the structure of social networks (33–36). Building from recent advances in computational social science (37), social network analysis can objectively characterize social network structures related to brain dynamics (28). Specifically, we focus on the density of an individual’s ego-network: A dense network indicates a participant whose friends are also friends with one another, whereas a sparse network indicates a participant whose friends do not know each other. Perhaps most significantly, dense ego-networks are more close-knit (38, 39) and are less likely to include diverse communities. Thus, the friends of the ego are more likely to know, interact, and share with each other (38, 39). On one hand, this density may make the consequences of being excluded more dire, but such close-knit groups also confer social support that may buffer an individual’s response to exclusion (40, 43).
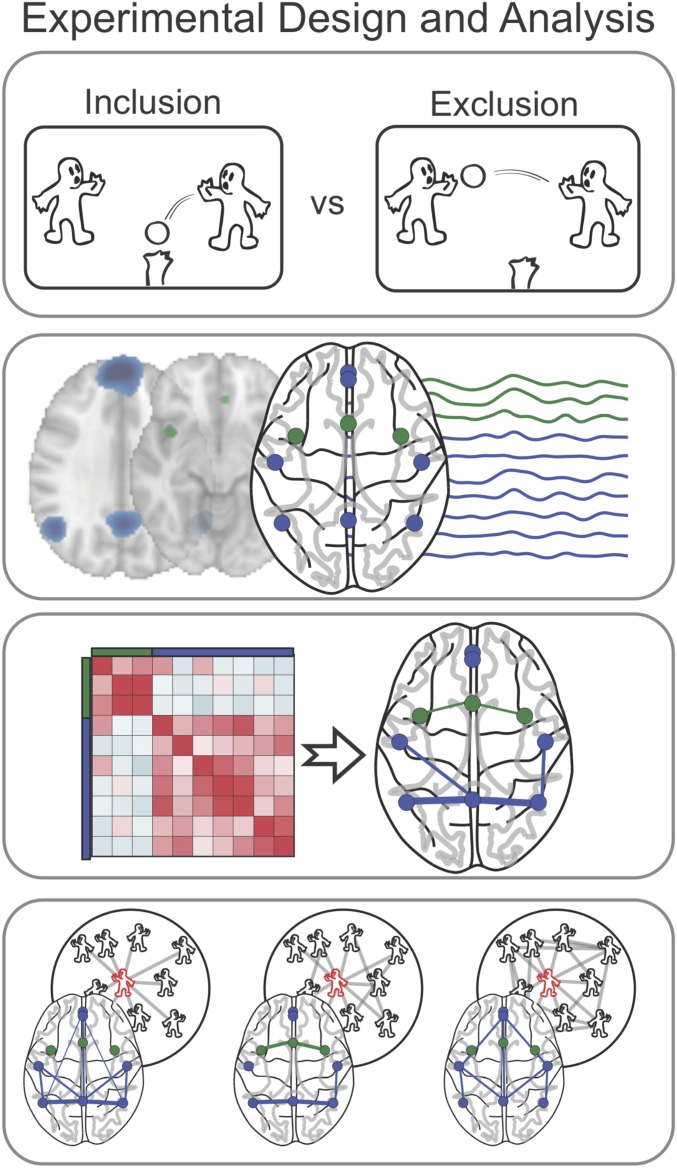
Our analysis focuses on a sample of 80 adolescent males because social ties become especially important during adolescence (42, 43). For each participant, we derived ego-network density using objective logs from the Facebook API. Then, while undergoing fMRI, participants completed Cyberball, a virtual ball-tossing game used experimentally to study the effects of social exclusion (11, 12). An overview of the analysis is shown in Fig. 1. We tested the prediction that connectivity during exclusion would increase, relative to inclusion, in regions of the social pain and mentalizing networks derived from meta-analyses of previous research in Neurosynth (36). We also explored whether connectivity between these two networks would increase during exclusion relative to inclusion. To confirm the effects within these theoretically relevant networks, we also conducted a whole-brain analysis based on a large-scale cortical parcellation comprising 264 regions, including regions that overlap the social pain and mentalizing systems (44). In both analyses, we find that social exclusion is associated with increased connectivity within the regions of the mentalizing network. Furthermore, we tested the prediction that brain dynamics reflecting sensitivity to exclusion will be associated with individual differences in social network structure. Specifically, we test competing hypotheses that increased changes in brain dynamics within the social pain and mentalizing systems might be associated with (i) denser friendship networks, given greater consequences of exclusion in a dense network, or (ii) less dense friendship networks, because those with less dense networks may have less deep social support to buffer the effects of exclusion. Here, we find support for the latter hypothesis, that participants who show greater connectivity changes in the mentalizing system during social exclusion have less dense friendship networks.
Fig. 1.
Overview of task and analysis schema. While undergoing fMRI scanning, participants played Cyberball, a virtual game during which they were socially excluded. Using a priori theorized regions from the social pain and mentalizing networks derived from two meta-analyses, we extracted the nodal time series to construct and compare brain network connectivity during social exclusion and inclusion. Finally, we investigated the relationship between each individual’s ego-network density and their brain connectivity during social exclusion. Adapted from ref. 64.
Results
Stronger Connectivity in the Mentalizing Network During Social Exclusion than Inclusion.
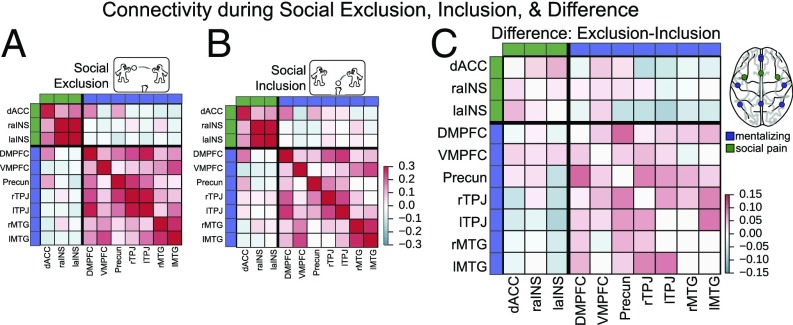
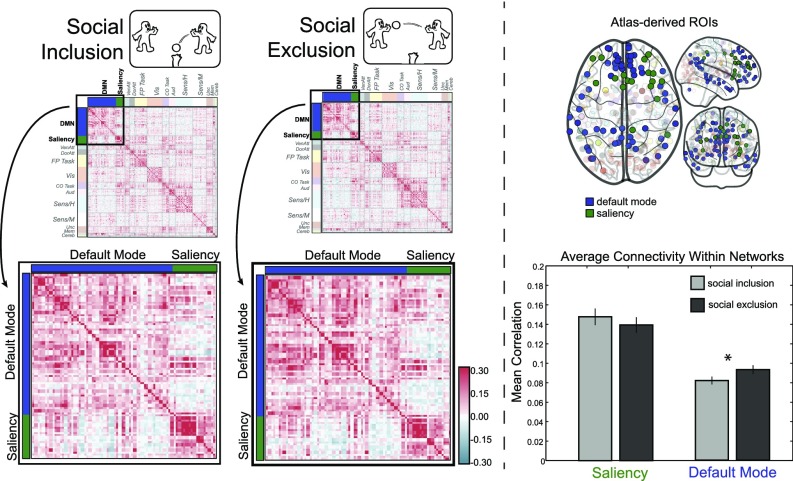
We first tested the prediction that functional connectivity would increase in the social pain and mentalizing systems during exclusion relative to inclusion. We also explored whether connectivity between the two networks changed during exclusion relative to inclusion. To do so, we examined the functional connectivity between regional time series during social exclusion and social inclusion within a priori networks for social pain processing and mentalizing derived from meta-analyses of each of these constructs (Fig. 1; also see SI Materials and Methods). We computed connectivity matrices as the Pearson correlation between the time series of every pair of nodes in the two networks. The resulting connectivity matrices for each individual then were group-averaged for display in Fig. 2. As can be seen in Fig. 2, nodes exhibited high connectivity within both the social pain and mentalizing networks and only weak connectivity across networks during both inclusion and exclusion; this result supports previous research that has postulated that these regions form two cohesive, segregated networks or modules (45).
Fig. 2.
Functional connectivity during social exclusion (A), social inclusion (B), and the difference between exclusion and inclusion (C). For each participant, a functional connectivity matrix was derived as the Pearson correlation coefficient between the time series of every pair of nodes for social exclusion and inclusion, respectively. Group-average functional connectivity matrices then were computed by averaging all participants’ connectivity matrices for each task (after Fisher z-transformation). To isolate the effect of social exclusion, we subtracted the inclusion connectivity matrix from the exclusion connectivity matrix.
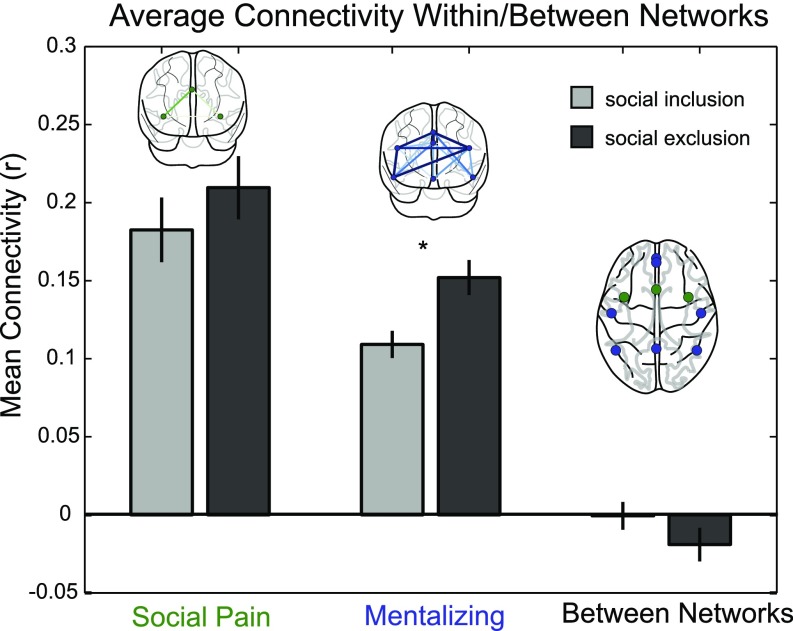
Using this functional subdivision of the nodes, we computed the within-system connectivity as the mean strength of the functional interactions within each system and the between-system connectivity as the mean of interactions between nodes from different systems (46). We then compared the resulting within- and between-system connectivity values for social inclusion and exclusion with a paired t test. As shown in Fig. 3, connectivity significantly increased on average across participants during social exclusion within the mentalizing network, t(79) = 3.67, P < 0.001, but not within the social pain network or between the social pain and mentalizing networks [t(79) = 1.23 and t(79) = −1.37, both not significant]. We next asked whether the strength of individual edges, i.e., the connectivity between two nodes, was modulated by exclusion. Similarly, we found that social exclusion was associated with stronger connectivity between edges comprising the mentalizing network (Fig. S1). Thus, when people were socially excluded, on average, we observed greater regional connectivity between regions of the mentalizing network but not between regions of the social pain network or between regions of the two networks.
Fig. 3.
Social exclusion was associated with increased connectivity within the mentalizing network. The strength of recruitment of each network was captured by computing the within-system connectivity as the mean strength of the functional interactions within the social pain (green) and mentalizing (blue) networks and the between-system connectivity as the mean of interactions between nodes from the social pain and mentalizing networks (blue nodes to green nodes). Connectivity graphs above each bar illustrate the relative difference in connectivity between social exclusion and inclusion within the respective network. The edge width corresponds to differences in connectivity magnitudes between 0 and 0.1. The asterisk indicates significant differences between social inclusion and exclusion in the mentalizing network, t(79) = 3.67, P < 0.001.
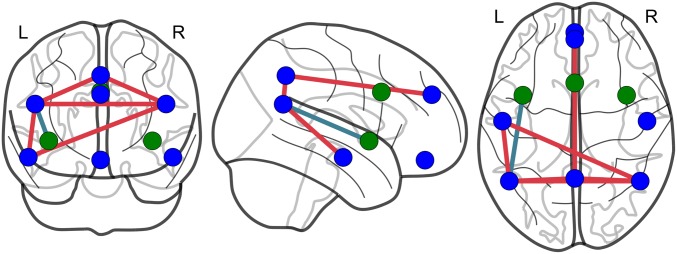
Fig. S1.
Social exclusion is associated with increased connectivity in the mentalizing network. Node colors indicate the network/community membership of each node (blue = mentalizing/default mode network, green = social pain/saliency network); edge colors indicate the direction of the difference in connectivity between social exclusion vs. inclusion (i.e., red edges = increased connectivity during exclusion; blue edges = decreased connectivity; these results are thresholded at P < 0.05, uncorrected, for exploratory purposes). The following edges are significant: dmPFC–precuneus: t(79) = 3.042, P = 0.003; precuneus–rTPJ: t(79) = 2.569; P = 0.012; precuneus–lTPJ: t(79) = 2.487; P = 0.015; rTPJ-lMTG: t(79) = 2.277, P = 0.025; lTPJ-rTPJ: t(79) = 2.323, P = 0.023; lTPJ-lMTG: t(79) = 2.764. P = 0.007; laINS-lTPJ: t(79) = −2.304, P = 0.024.
To complement our connectivity analysis within a priori hypothesized networks, we ran secondary analyses that applied the same analysis pipeline using a whole-brain parcellation both to examine the robustness of our results and to identify effects in regions outside our hypothesized networks of interest. This analysis included 264 brain regions that were previously assigned to 13 large-scale functional brain systems (44); as before, we computed both within-network connectivity for each of the 13 systems and between-network connectivity for all pairs of the networks. Two of these systems have substantial overlap with our a priori networks of interest: The default mode network is a more distributed variant of the mentalizing network, and the saliency network is a more distributed variant of the social pain network. This whole-brain analysis also confirmed increased connectivity in the default mode system during social exclusion [t(79) = 2.58, P = 0.011], as shown in Fig. 4. There was no effect within the salience system, which overlaps with the social pain network (t = −0.86, P = 0.39) (Fig. 4); however, we did observe higher connectivity within the cingulo-opercular system (t = 2.08, P = 0.041), which overlaps with components of the social pain system. The uncorrected results for all other systems in the Power parcellation were as follows: ventral attention, t(79) = 0.54, P = 0.592; dorsal attention, t(79) = −1.51, P = 0.136; fronto-parietal, t(79) = 0.99, P = 0.325; visual, t(79) = 2.31, P = 0.023; auditory, t(79) = 2.84, P = 0.006; somatosensory/-motor, t(79) = 1.19; P = 0.238; subcortical, t(79) = −1.47, P = 0.146; memory, t(79) = 1.78, P = 0.078; cerebellum, t(79) = 0.63, P = 0.529; uncertain, t(79) = -1.23, P = 0.222.
Fig. 4.
Results from a whole-brain analysis of 264 nodes (44) assigned to 13 systems converged with earlier findings: We observed increased connectivity within the default mode system during social exclusion. For the saliency system, which overlaps with the social pain network, there was no change in connectivity during exclusion.
Individual Differences in Ego-Network Density Are Linked to Brain Connectivity Effects.
Our first set of analyses identified a relationship between connectivity in the mentalizing network and social exclusion across participants in our sample on average, but we hypothesized that the strength of this relationship would be related to individual differences in participants’ social network characteristics. In particular, recent studies suggest a relationship between the mentalizing network and the social landscape that people navigate on a daily basis (47, 48); further, psychological factors can shape the structure of social networks (33–36). Consequently, we characterized each individual’s ego-network density from objectively logged Facebook data obtained with participants’ consent from the Facebook API. Participants have a denser ego-network when their friends are also friends with one another and have a sparser network when the participant’s friends are not friends with each other. Especially for adolescent samples, Facebook friendships provide an accurate proxy for offline friendships (49); such social network structures, in turn, can provide a broader window into stable personality differences that are related to important social and emotional outcomes (30).
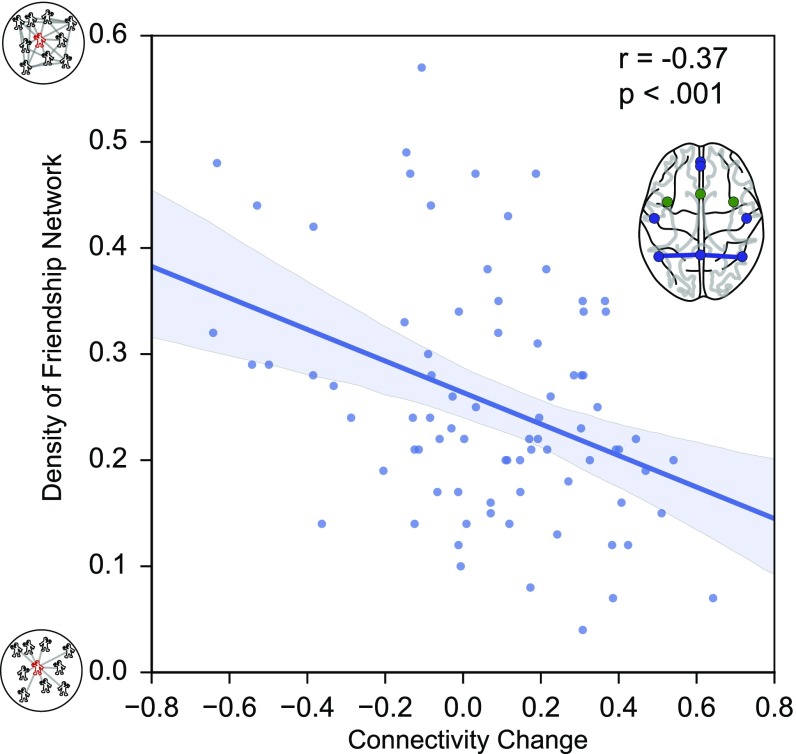
To determine the relationships between the participants’ ego-network data and their brain dynamics during social exclusion and inclusion, we regressed the density of participants’ ego networks onto brain connectivity during social exclusion, controlling for connectivity during social inclusion. Note that we also estimated the correlation coefficient between ego network density and the differences in connectivity during exclusion and inclusion, which yielded the same results. We first entered as connectivity measures the mean strength across nodes from the mentalizing and social pain systems, respectively, but found no significant effects. Next we entered the connectivity of individual edges, i.e., the correlations between the time courses from each pair of nodes. After correcting for multiple comparisons using the false-discovery rate (FDR, q = 0.05), we found that connectivity between the left and right TPJ, two key nodes of the mentalizing network, was negatively related to ego-network density (Fig. 5). In other words, individuals who exhibit a stronger TPJ coupling during exclusion tend to have a less dense friendship network. We did not observe effects between any pairs of nodes from the social pain network, either corrected or uncorrected. Together, these results suggest that brain dynamics during key social experiences such as exclusion may contribute to the type of network structure people occupy. Likewise, the amount of interconnections among friends of the excluded participant’s social network may also influence the impact of exclusion on brain connectivity.
Fig. 5.
Scatterplot illustrating the significant relationship between the functional connectivity between the lTPJ and rTPJ and the density of a participant’s ego-friendship network. Participants who show stronger regional coupling during exclusion have sparsely connected networks in which fewer of their friends are also friends with each other.
SI Materials and Methods
Definition of Regions.
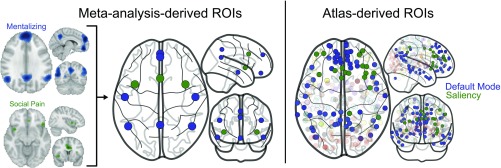
To define the nodes for our analysis, we used Neurosynth (36) to perform two automated meta-analyses of the functional neuroimaging literature on mentalizing and social pain, respectively. In particular, for mentalizing, we queried the Neurosynth database (as of January, 2016) for published studies on the topic of “mentali*” (threshold = 0.001). This query resulted in a set of 112 studies with associated MNI coordinates, which we then submitted to a Neurosynth meta-analysis and saved the FDR 0.01-corrected reverse inference (RI) map. We proceeded analogously for studies of social pain (search term “social* & pain*”; 39 studies). Finally, we extracted result clusters from the mentalizing and social pain RI maps using BSPMview. In addition, we consulted two separate researcher-curated meta-analyses of mentalizing (61) and social pain (4), respectively. Overall, there was good agreement between the coordinates obtained via automated meta-analysis, with minor differences for the “social & pain” Neurosynth analysis, which returned additional coordinates not found in researcher-curated analyses. Closer inspection revealed that these likely stem from leakage of studies on physical pain, and thus they were excluded. To select network coordinates, we discarded regions with cluster sizes below 140 mm3 and symmetrized the coordinates of bilateral nodes. For instance, the center coordinates for the insula were 40, 6, −4 and −36, 8, −4, and these were symmetrized to 38, 7, −4, i.e., the midpoint. This procedure provided the following coordinates for the mentalizing network (Fig. S2): DMPFC, (0, 53, 30); vmPFC, (0, 48, −18); precuneus, (0, −54, 44); rTPJ, (48, −56, 23); lTPJ, (−48, −56, 23); rMTG, (53, −12, −16); and lMTG, (−53, −12, −16) and the following coordinates for the social pain network: dACC, (0, 16, 32); r-aINS, (38, 7, −4); and l-aINS, (−38, 7, −4). Results reported are substantively similar with or without inclusion of these nodes: cerebellum, (−24, −50, −45); brainstem1, (−3, −25, −23); brainstem2, (−5, −22, −42); brainstem3, (5, −22, −42); somatosensory1, (62, 28, 42); and somatosensory2, (−60, −22, 34); furthermore, substantively similar results are also obtained with asymmetric node-coordinates.
Fig. S2.
The nodes for social pain and mentalizing networks as derived by synthesizing automated and researcher-curated meta-analyses on mentalizing (58) and social pain (11), respectively. The brain images (Left) show the meta-analytic contrast of the RI maps (pFgA; FDR, q = 0.01; smoothed 8 mm FWHM), which are combined to yield the parcellation shown in the schematic brain figure (Center). The Right pictures the regions used in the whole-brain analysis, with the default mode network colored in blue to reflect its overlap with the mentalizing network and the saliency network colored green to capture its overlap with the social pain network.
Stronger Connectivity in the Mentalizing Network During Social Exclusion: Edge-Level Results.
The results reported in the main paper are based on the connectivity within and between the social pain and mentalizing networks, respectively. To assess results at a finer spatial resolution, we focused on individual edges and asked whether edge strength differed significantly between social exclusion and inclusion. The results of this analysis, shown in Fig. S2, revealed that the edges that exhibited changes in connectivity between exclusion and inclusion mainly connected regions from the mentalizing network but not from the social pain network (Fig. S1, thresholded at P < 0.05, uncorrected, for exploratory purposes).
Assessing the Robustness of Effects Within the Mentalizing Network.
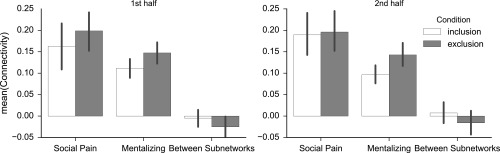
The robustness of the dynamic fluctuations within the mentalizing network was investigated in a control analysis that compared time courses from the first and second half of each block, confirming our main findings that showed increased within-network connectivity was sustained throughout the first and second halves of each block [first half: tMentalizing first half: exclusion vs. inclusion(79) = 2.23; P = 0.029, and second half: tMentalizing second half: exclusion vs. inclusion(79) = 2.57, P = 0.012], and that connectivity did not differ between the first and second halves [tMentalizing, exclusion, first vs. second(79) = 0.24, P = 0.812; tMentalizing, inclusion, first vs. second(79) = 0.94, P = 0.348] (Fig. S3). This analysis suggests not that participants were increasingly mind-wandering or otherwise engaging in default mode activity over time but rather that our analysis likely tracks social cognitive processes relevant to the task.
Fig. S3.
Social exclusion was associated with increased connectivity within the mentalizing network. This figure is based on the analyses illustrated in Fig. 3 in the main text but was computed using data from only the first or second half of each block, respectively.
Discussion
Social relationships are a driving force for human behavior, and previous research on social exclusion has identified robust involvement of two sets of brain regions activated during social pain and mentalizing. We bring a network neuroscience perspective to examine how social experiences may engage and reconfigure these brain networks in support of social cognition and how individual differences in the way people use their brains may correspondingly influence or be influenced by the structure of their social networks.
Specifically, we first tested the prediction that functional connectivity would increase within the social pain and mentalizing networks during social exclusion relative to inclusion. We also explored the relationships between the networks during both exclusion and inclusion. Here, we find support for tight coupling of the two proposed networks (social pain and mentalizing) during social inclusion and exclusion. Next, we find that connectivity within the mentalizing system increases during exclusion relative to inclusion. This result is confirmed using a whole brain analysis that identified significant effects within the so-called default mode network. This atlas-derived default mode network overlaps with an a priori hypothesized mentalizing network.
Previous research has highlighted the role of the social pain system in responding to exclusion (4, 50). Building on this starting point, the few studies examining brain connectivity during social exclusion (15–17, 51) have focused on dACC seed regions as a hotspot of univariate activation effects in the social pain network. Our findings highlight a broader network perspective. Although connectivity is strong within both social pain and mentalizing networks during both social exclusion and inclusion, connectivity increases within the mentalizing system, but not the social pain system, during exclusion relative to inclusion. This first finding highlights the critical value of coordination between regions of the same subnetwork, revealing that within-network instead of between-network interactions were strongly correlated with the social exclusion condition. The second finding complements existing univariate accounts of increased activity within specific nodes of the social pain and mentalizing networks by highlighting a distinction in the temporal dynamics of the social pain and mentalizing processes that was not captured by average univariate activation results. Overall, these findings are in line with the wider literature arguing that connectivity and activity provide complementary information (52, 53) and thus can provide complementary insights about cognitive processes.
Functionally, this stronger coupling among mentalizing regions may support considering the intentions of the individuals who are excluding the participant in the social event or internal ruminations about the relevance of potential ongoing exclusion for one’s broader social relations. In fact, verbal reports from participants who have undergone this type of exclusion show that such thoughts are frequent (11), although not uniformly distributed across people. One benefit of the connectivity methods used in the present paper is that they are, in principle, also sensitive to thought processes that wax and wane multiple times during the exclusion period, such as fluctuations in mentalizing (e.g., about others’ thoughts and motives). Thus, we suggest that increased connectivity in the mentalizing network may tap into such dynamic aspects of the response to social exclusion over a period. One possible function of such a dynamic process may be to make sense of the situation and to reflect more broadly on the meaning of that experience, thereby supporting coping-related functions (54). These data highlight the value of examining functional connectivity in addition to mean activation because connectivity can provide different insight into the dynamics of psychological phenomena such as exclusion; although average activation in both mentalizing and social pain systems are commonly observed in response to exclusion, changes in connectivity were significant in this dataset only within the mentalizing system.
Our social-cognitive interpretation of changing connectivity within the mentalizing system as a means of coping with exclusion is also supported by the fact that this effect covaries with participants’ broader social network structures. Specifically, participants who showed increased connectivity during exclusion relative to inclusion between the left and right TPJ, two regions of the mentalizing system, also had less dense social network structures. One possibility is that outside the safety of dense ego-networks, which tend to be more close-knit (38, 39), individuals use greater mentalizing resources, especially during stressful social interactions such as exclusion. This effect is consistent with neurocognitive models that link intrapersonal effects of social exclusion to the interpersonal contexts surrounding individuals during daily life (53). Social exclusion, by its very nature, does not occur in a vacuum. Rather, preexisting characteristics of one’s social network provide the background or contextual standard from which any new episode of exclusion is understood and compared (55). Within this framework, our measure of social network density can be understood as a social inclination. When excluded, people who interact more with unconnected others may use mentalizing resources differently than those with denser ego-friendship networks. That said, it is also likely that those who use mentalizing resources differently may position themselves differently in their social networks, and that the ways in which individuals use their brains during social experiences shapes their preferences and tendencies to occupy different types of network positions. For example, the extant literature suggests that the lack of diverse communities in denser networks may relate to the degree of mentalizing that individuals engage in during social tasks. Indeed, prior work has shown that network brokerage, which is inversely related to density and associated with bridging diverse communities, can moderate mentalizing activity during social decision making (56). The present findings substantially extend prior research linking personality to the shape of social networks (33–36) by showing that individual differences in brain dynamics may underpin such links. In other words, the relationship between brain network dynamics and social network dynamics is likely bidirectional, and the current findings open avenues to understand both.
Our results add to a growing literature that links individual differences and social network properties, and they also bring neuroscientific methods to bear in characterizing individual differences. For example, past research has linked social personality tendencies, such as extraversion, self-monitoring, and cognitive empathy (related to mentalizing) to personal network structure (35, 57). Our results also complement work across other social species, including research with macaques (58). Researchers have studied the structure and connectivity of brain regions that belong to the default mode network, including the posterior superior temporal sulcus (pSTS), which parallels our TPJ results. These regions are modulated on the basis of social network characteristics, such as network size and position in a social hierarchy (59). Our data complement these findings by showing that properties of an individual’s social networks are associated with intrapersonal reactions to exclusion, which then might reciprocally influence the interpersonal relationships that underlie these social networks. Taken together, these results also highlight the need to consider jointly the structural characteristics of personal networks in combination with cognitive responses to acute episodes of exclusion.
In sum, we find that social exclusion is associated with increased connectivity within the mentalizing system. Furthermore, we explore whether the impact of social exclusion on brain connectivity relates to the structure of participants’ friendship networks, finding that participants with sparser friendship networks show increased connectivity within key brain systems when excluded. These findings demonstrate that networked brain dynamics during social-cognitive tasks can provide relevant information in addition to univariate activity. In particular, our connectivity analysis highlights the fundamental importance of the mentalizing system in responding to socially salient events, and it indicates how a participant’s social network structure may capture critical differences between individuals in how social exclusion influences these key dynamics of the underlying brain networks for social cognition. Together, our connectivity analysis offers insights into the neural and social responses to common social experiences such as exclusion and delineates how distributed brain networks respond to powerful socio-affective challenges that manifest in social networks indexed by online media.
Materials and Methods
Participants.
Eighty neurotypical 16- to 17-y-old adolescent males were recruited through the Michigan state driver registry database as part of a larger study on peer influences on adolescent driving. Participants met standard MRI safety criteria. In accordance with Institutional Review Board approval from the University of Michigan, legal guardians provided written informed consent, and adolescents provided written assent.
Social Exclusion Task.
Participants completed the Cyberball game, which has been validated in a number of behavioral and neuroimaging studies as a reliable way of simulating the experience of social exclusion (5, 60), while undergoing fMRI scanning. The Cyberball game consisted of two 3-min rounds, and the order of rounds was held constant to preserve the psychological experience across participants. In the inclusion round, the participant and two virtual players received the ball equally often, whereas during the exclusion game the participant and virtual players started out playing the ball, but the participant was left out after a few throws, simulating social exclusion. After the scan, participants completed a set of questionnaires.
Social Network Assessment.
In addition to the fMRI tasks, participants also provided information about their social networks. This information was assessed from logged online friendships using the Facebook API (collected in 2011–2013). Following this data acquisition, density was computed for participants’ egocentric networks. In particular, we constructed a friendship network for a given individual (“ego”) and then removed the ego from the network (because all friends are logically connected to it), and computed the density among the remaining nodes (n) of friends, assessing the proportion of existing connections (m) between friends vs. all possible connections [i.e., n × (n − 1)/2 if all the ego’s friends are also friends with each other]. In other words, the density of the ego-network measures the extent to which participants’ Facebook friends are interconnected. After first removing the ego from each individual’s friendship network, density was computed using NetworkX with the formula: d = 2m/(n × (n − 1)), where m denotes the number of edges between persons and n denotes the number of nodes.
fMRI Acquisition and Analysis.
Functional images were recorded using a reverse spiral sequence (repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, 43 axial slices, field of view = 220 mm, slice thickness = 3 mm, voxel size = 3.44 × 3.44 × 3.0 mm). We also acquired in-plane T1-weighted images (43 slices, slice thickness = 3 mm, voxel size = 0.86 × 0.86 × 3.0 mm) and high-resolution T1-weighted images [spoiled gradient recall (SPGR) acquisition, 124 slices, slice thickness = 1.02 × 1.02 × 1.2 mm] for use in coregistration and normalization. Functional data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London). The first four volumes were discarded before analysis. Functional images were despiked using the 3dDespike program as implemented in the AFNI toolbox, corrected for differences in slice time acquisition, and spatially realigned to the first functional image. Functional and structural images were coregistered using a two-stage procedure. First, in-plane T1 images were registered to the mean functional image. Next, high-resolution T1 images were registered to the in-plane image. Structural images then were skull-stripped and normalized to the skull-stripped MNI template provided by FSL (Oxford Centre for functional MRI of the Brain).
Meta-Analytical Definition of Regions.
Nodes for the social pain and mentalizing networks were derived from semiautomated and researcher-curated meta-analysis: We used Neurosynth (36) to perform two automated meta-analyses of the functional neuroimaging literature on “mentalizing” and “social pain,” respectively. In addition, we consulted two separate researcher-curated meta-analyses of mentalizing (61) and social pain (4), respectively (SI Materials and Methods for additional details). This procedure provided the following coordinates for the mentalizing network (Fig. S2): dorsal medial prefrontal cortex (dmPFC) (0, 53, 30); ventromedial PFC (vmPFC) (0, 48, −18); precuneus (0, −54, 44); right TPJ (rTPJ) (48, −56, 23); left TPJ (lTPJ) (−48, −56, 23); right middle temporal gyrus (rMTG) (53, −12, −16); and left MTG (lMTG) (−53, −12, −16) and the following coordinates for the social pain network: dACC (0, 16, 32); right aINS (r-aINS) (38, 7, −4); and left aINS (l-aINS) (−38, 7, −4). In addition to the regions of these two a priori networks, we also examined connectivity changes during social exclusion using a whole-brain parcellation that assigns 264 brain regions to 1 of 13 functional networks (Fig. S2) (44).
Functional Connectivity Analysis.
Analysis of functional connectivity was performed in Python 2.7 using the nilearn package (61). Data were bandpass filtered between 0.06–0.12 Hz (22), detrended, standardized, and extracted from 8-mm-radius spheres around the nodes specified above. Artifacts were reduced using frame censoring and regression. In particular, frames with framewise displacement (FD) >0.5 mm were censored; nuisance regressors included white matter and ventricular signals, the realignment parameters, and high-variance confounds as implemented in the nilearn package. From the extracted time series corresponding to the social inclusion and exclusion runs, we used Pearson’s correlation coefficient to construct task-specific undirected and weighted functional connectivity matrices for each participant. To determine whether results observed could be explained by increased mind-wandering over time in the scanner, an additional set of split-half analyses was also implemented using parallel methods on the first and second half of each block (inclusion and exclusion), respectively (Fig. S3). Analyses were implemented using python packages and in-house functions, and visualizations were created with Nilearn, Matplotlib, and Seaborn (61–63).
Acknowledgments
We thank Nicole Cooper, Qawi Telesford, and Marcelo Mattar for valuable comments and discussions; the University of Michigan Transportation Research Institute for research assistance; the staff of the University of Michigan fMRI Center; and Raymond Bingham, Jean Shope, Marie Claude Ouimet, Anuj Pradhan, Bruce Simons-Morton, Kristin Shumaker, Elizabeth Beard, Jennifer LaRose, Farideh Almani, and Johanna Dolle. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under Award R21HD073549, by NIH New Innovator Award 1DP2DA03515601 (to E.B.F.), by the Army Research Laboratory under Cooperative Agreement Number W911NF-10-2-0022, and the Defense Advanced Research Projects Agency Young Faculty Award YFA-D14AP00048 (to E.B.F.). D.S.B. received support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Army Research Office through Contract W911NF-14-1-0679, National Institute of Mental Health Grant 2-R01-DC-009209-11, National Institute of Child Health and Human Development Grant 1R01HD086888-01, the Office of Naval Research Award CRCNS BCS-1441502, CAREER PHY-1554488, and the National Science Foundation. The views and the conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH and its subdivisions, the Army Research Laboratory, or the US Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616130114/-/DCSupplemental.
References
- 1.Muscatell KA, Eisenberger NI. Neurocognitive and Physiological Mechanisms Linking Stress and Health. Brain Mapping. Elsevier; Amsterdam: 2015. pp. 29–33. [Google Scholar]
- 2.Cacioppo JT, Cacioppo S. Social relationships and health: The toxic effects of perceived social isolation. Soc Personal Psychol Compass. 2014;8:58–72. doi: 10.1111/spc3.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotge J-Y, et al. A meta-analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci. 2015;10:19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 6.Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci USA. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb S. Presidential Address-1976. Social support as a moderator of life stress. Psychosom Med. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cassel J. The contribution of the social environment to host resistance: The Fourth Wade Hampton Frost Lecture. Am J Epidemiol. 1976;104:107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzer R, Leppin A. Social support and health: A meta-analysis. Psychol Health. 1989;3:1–15. [Google Scholar]
- 10.Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 11.Williams KD, Cheung CK, Choi W. Cyberostracism: Effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- 12.Williams KD, Forgas JP, Von Hippel W. The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. Psychology Press; East Sussex, UK: 2013. [Google Scholar]
- 13.Beyer F, Münte TF, Krämer UM. Increased neural reactivity to socio-emotional stimuli links social exclusion and aggression. Biol Psychol. 2014;96:102–110. doi: 10.1016/j.biopsycho.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Soc Cogn Affect Neurosci. 2013;8:151–157. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolling DZ, et al. Development of neural systems for processing social exclusion from childhood to adolescence. Dev Sci. 2011;14:1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puetz VB, et al. Neural response to social rejection in children with early separation experiences. J Am Acad Child Adolesc Psychiatry. 2014;53:1328–1337.e8. doi: 10.1016/j.jaac.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Meyer ML, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc Cogn Affect Neurosci. 2013;8:446–454. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullmore ET, Bassett DS. Brain graphs: Graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DS, et al. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett DS, et al. Task-based core-periphery organization of human brain dynamics. PLOS Comput Biol. 2013;9:e1003171. doi: 10.1371/journal.pcbi.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18:744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun U, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci USA. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doron KW, Bassett DS, Gazzaniga MS. Dynamic network structure of interhemispheric coordination. Proc Natl Acad Sci USA. 2012;109:18661–18668. doi: 10.1073/pnas.1216402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekman M, Derrfuss J, Tittgemeyer M, Fiebach CJ. Predicting errors from reconfiguration patterns in human brain networks. Proc Natl Acad Sci USA. 2012;109:16714–16719. doi: 10.1073/pnas.1207523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantzaris AV, et al. Dynamic network centrality summarizes learning in the human brain. J Complex Netw. 2013;1:83–92. [Google Scholar]
- 27.Mattar MG, Cole MW, Thompson-Schill SL, Bassett DS. A functional cartography of cognitive systems. PLOS Comput Biol. 2015;11:e1004533. doi: 10.1371/journal.pcbi.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell MB, Falk EB. Big data under the microscope and brains in social context integrating methods from computational social science and neuroscience. Ann Am Acad Pol Soc Sci. 2015;659:274–289. [Google Scholar]
- 29.Schilbach L, et al. Toward a second-person neuroscience. Behav Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- 30.Burt RS. Structural Holes: The Social Structure of Competition. Harvard Univ Press; Cambridge, MA: 1992. [Google Scholar]
- 31.Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360–1380. [Google Scholar]
- 32.Smith KP, Christakis NA. Social networks and health. Annu Rev Sociol. 2008;34:405–429. [Google Scholar]
- 33.Andersson MA. dispositional optimism and the emergence of social network diversity. Sociol Q. 2012;53:92–115. [Google Scholar]
- 34.Burt RS, Jannotta JE, Mahoney JT. Personality correlates of structural holes. Soc Networks. 1998;20:63–87. [Google Scholar]
- 35.Kalish Y, Robins G. Psychological predispositions and network structure: The relationship between individual predispositions, structural holes and network closure. Soc Networks. 2006;28:56–84. [Google Scholar]
- 36.Landis B. Personality and social networks in organizations: A review and future directions. J Organ Behav. 2016;37:S107–S121. [Google Scholar]
- 37.Abraham A, Hassanien A-E, Snášel V, editors. Computational Social Network Analysis: Trends, Tools and Research Advances. Springer; London: 2010. [Google Scholar]
- 38.Hurlbert JS, Haines VA, Beggs JJ. Core networks and tie activation: What kinds of routine networks allocate resources in nonroutine situations? Am Sociol Rev. 2000;65:598–618. [Google Scholar]
- 39.Parks MR. The Sage Handbook of Interpersonal Communication. SAGE Publications; Thousand Oaks, CA: 2011. Social networks and the life of relationships; pp. 355–388. [Google Scholar]
- 40.Lin N. Social Capital: A Theory of Social Structure and Action. Cambridge Univ Press; Cambridge, UK: 2002. [Google Scholar]
- 41.Monge PR, Contractor NS. Theories of Communication Networks. Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- 42.Masten CL, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23:162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Gu S, et al. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci USA. 2015;112:13681–13686. doi: 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RIM. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell JL, Lewis PA, Dunbar RIM, García-Fiñana M, Roberts N. Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia. 2010;48:3554–3562. doi: 10.1016/j.neuropsychologia.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Arnaboldi V, Guazzini A, Passarella A. Egocentric online social networks: Analysis of key features and prediction of tie strength in Facebook. Comput Commun. 2013;36:1130–1144. [Google Scholar]
- 50.Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 51.Onoda K, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc Cogn Affect Neurosci. 2010;5:385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Medaglia JD, Lynall M-E, Bassett DS. Cognitive network neuroscience. J Cogn Neurosci. 2015;27:1471–1491. doi: 10.1162/jocn_a_00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doré BP, Ochsner KN. Emotion Regulation. Brain Mapping. Elsevier; Amsterdam: 2015. pp. 53–58. [Google Scholar]
- 55.Kawamoto T, Ura M, Nittono H. Intrapersonal and interpersonal processes of social exclusion. Front Neurosci. 2015;9:62. doi: 10.3389/fnins.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Donnell MB, Bayer J, Cascio CN, Falk EB. Neural bases of recommendations differ according to social network structure. Soc Cogn Affect Neurosci. 2017;12:61–69. doi: 10.1093/scan/nsw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts SGB, Wilson R, Fedurek P, Dunbar RIM. Individual differences and personal social network size and structure. Pers Individ Dif. 2008;44:954–964. [Google Scholar]
- 58.Sallet J, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 59.Mars RB, et al. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams KD, Sommer KL. 1997 Social ostracism by coworkers: Does rejection lead to loafing or compensation? Pers Soc Psychol Rev. Available at: journals.sagepub.com/doi/abs/10.1177/0146167297237003. Accessed April 14, 2017.
- 61.Abraham A, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:14. doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunter JD. 2007. Matplotlib: A 2D graphics environment. Comput Sci Eng 10.1109/MCSE.2007.55.
- 63.Waskom M, et al. 2014. seaborn: v0.5.0 (November 2014) (ZENODO), 10.5281/zenodo.12710.
- 64.Schmälzle R, et al. December 23, 2016. Brain connectivity dynamics during social interaction reflect social network structure. bioRxiv, 10.1101/096420.