Significance
This work presents a technique for dynamic nuclear polarization (DNP)-enhanced magic-angle spinning (MAS) solid-state NMR studies of complex proteins and biological assemblies. The sequential side-chain correlation approach streamlines the site-specific assignment of NMR peaks in multidimensional spectra, a critical step in determining structural information such as distances. When combined with DNP enhancement, fast MAS, and nonuniform sampling, this technique allows for faster data acquisition than previously possible. Applied to the intact Pf1 bacteriophage, sequential side-chain correlation spectra have enabled a virtually complete assignment using DNP data alone. These assignments shed insight into the chemical shift and linewidth changes associated with cryogenic temperatures. Our data point to hydration as a key variable influencing these parameters.
Keywords: Pf1 bacteriophage, DNP, SSNMR
Abstract
An experimental strategy has been developed to increase the efficiency of dynamic nuclear polarization (DNP) in solid-state NMR studies. The method makes assignments simpler, faster, and more reliable via sequential correlations of both side-chain and Cα resonances. The approach is particularly suited to complex biomolecules and systems with significant chemical-shift degeneracy. It was designed to overcome the spectral congestion and line broadening that occur due to sample freezing at the cryogenic temperatures required for DNP. Nonuniform sampling (NUS) is incorporated to achieve time-efficient collection of multidimensional data. Additionally, fast (25 kHz) magic-angle spinning (MAS) provides optimal sensitivity and resolution. Data collected in <1 wk produced a virtually complete de novo assignment of the coat protein of Pf1 virus. The peak positions and linewidths for samples near 100 K are perturbed relative to those near 273 K. These temperature-induced perturbations are strongly correlated with hydration surfaces.
By providing potential enhancements of up to 300-fold (1, 2), and observed enhancements of 250-fold for biological samples (3), dynamic nuclear polarization (DNP) is revolutionizing solid-state NMR (SSNMR) spectroscopy for many applications (4). DNP spectrometers have eliminated the notion of being “signal-limited” in most practical cases and, together with nonuniform sampling (NUS) techniques (5–7), make it possible to collect 3D and four-dimensional (4D) NMR spectra of biological macromolecules in 12–48 h. For practical purposes, n-dimensional spectra enable correlations between n interacting nuclei. DNP studies of small molecules (8, 9), a variety of biological systems (refs. 3 and 10–16; reviewed in refs. 17 and 18), inorganic clusters (19–21), and material surfaces (20, 22–25) have yielded insights that would have been otherwise impossible.
Despite its successes, DNP has been limited by reduced resolution at the cryogenic temperatures required. Linewidths are typically increased by at least twofold, and peak positions may change. Partial protein assignments have been made (10, 15, 26, 27). In most studies, however, assignments are carried over to the analysis of DNP data. Such approaches, although user-friendly, are problematic if one is interested in resonances that are inaccessible at or around room temperature or those that are sensitive to changes in conformation or solvation at cryogenic temperatures, as is common for 15N chemical shifts (28, 29).
A common paradigm for assignment is the N–Cα–CX (NCACX)-N–C′–Cα (NCOCA) “backbone walk” (26, 30, 31)—first demonstrated on bovine pancreatic trypsin inhibitor (32), ubiquitin (33), and α-spectrin SH3 domain (34) —which allows correlations between neighboring residues based on their amidic 15N chemical shifts (see Fig. 2A for graphical depiction). However, this NCACX-NCOCA backbone walk paradigm does not generally succeed for proteins under DNP conditions. At 100 K, intrinsic protein linewidths are too broad to avoid massive spectral overlap in 2D and 3D 15N–(13C)–13C spectra. For C′ and N, the resonances key to making sequential interresidue assignments, limited chemical-shift dispersion means that the spectral congestion under DNP conditions impedes backbone assignments.
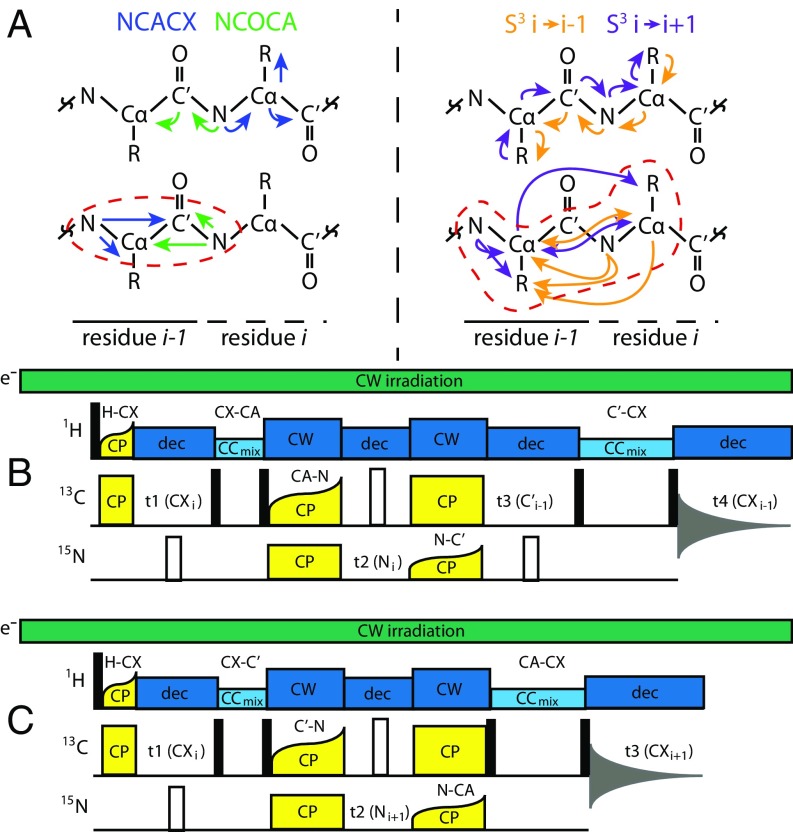
Fig. 2.
(A) Contrast of the polarization transfer pathways (Upper) and key chemical-shift correlations (Lower) for NCACX/NCOCA and S3 assignment strategies, as depicted on a protein backbone. Regions of overlap between the forward and backward correlation experiments for each assignment strategy are shown in dashed outlines. The S3 approach covers the full transfer pathway of both NCACX and NCOCA experiments in a single experiment and provides a much larger overlap region, enabling multiple chemical-shift correlations between neighboring residues (e.g., N–Cα–N and direct Cα–Cα) for maximum robustness and ease of assignment. (B and C) Schematics of the pulse sequences for backward [B; 4D CX(Cα)NC′CX i→i − 1] and forward [C; 3D CX(C′)N(Cα)CX i→i + 1] S3 correlation experiments. In both experiments, a short (60–80 us) H–C tangential CP was used to maximize side-chain 13C polarization, before a short 13C–3C homonuclear recoupling period to maximize transfer to Cα or C′. Ten milliseconds of DARR mixing was typically used for Cα, and 20 ms for C′ (empirically optimized). To transfer polarization to the previous or subsequent residue, 13C–15N SPECIFIC-CP (Cα–N in the backward case, and C′ –N in the forward case), a 15N chemical shift encoding period, followed by 15N–13C SPECIFIC-CP (N–C′ in the backward case, N–Cα in the forward case) was used. After optional 13C chemical-shift encoding on Cα/C′, a second 13C–13C homonuclear recoupling element transferred polarization to the side chain, which was detected directly. This transfer required longer mixing times to achieve several-bond transfers; 50 ms of DARR mixing was used for short-range correlations and 250 ms for long-range correlations. Spinal-64 decoupling (dec) was used during evolution periods; continuous wave (CW) decoupling was applied during cross-polarization.
We show here that sequential assignments at 100 K with DNP enhancement are fully tractable by means of a method we term sequential side-chain–side-chain (S3) correlation spectroscopy. S3 experiments are conceptually similar to the 4D Cα–N–C′–Cα (CANCOCA) experiment, pioneered by Rienstra and coworkers to assign regions with overlapping Cα chemical shifts (35), which has enabled the assignment of complex proteins and assemblies by conventional SSNMR (36–39). Such experiments remain highly challenging near room temperature; data-acquisition times are often on the order of weeks to months, making the experiments very demanding of sample and instrument stability. DNP at cryogenic temperatures, conversely, affords the key benefit of enormously enhanced starting signals, enabling long-range polarization transfers with high sensitivity. In this context, DNP is able to deliver on its promise of acquiring high-quality data in vastly less time.
The S3 method helps to reduce spectral overlap and to compensate for broader linewidths without reducing signal intensity. The method also significantly reduces acquisition time. It is used here to collect and fully assign spectra for the major coat protein of Pf1 in the frozen state near 100 K, without reference to data for unfrozen samples near 273 K. Pf1 is an extensively studied filamentous phage (40–52). It has been used to demonstrate promising enhancements in whole-virus samples by DNP, including assignment of Pf1 DNA resonances (51, 53). Comparison of chemical-shift datasets of Pf1 near 100 K (DNP-enhanced) and 273 K (non-DNP) provides insight into the causes of chemical-shift perturbations (CSPs). The S3 method eliminates the need to use inappropriate near-room-temperature chemical-shift tables for assignment of DNP data.
Results
Information Richness and Rationale for S3 Spectroscopy.
Promising DNP signal enhancements for Pf1 spectra at 600-MHz field have been obtained: ∼35-fold in a 3.2-mm sapphire rotor and 60-fold in a 1.9 mm rotor. However, linewidths of DNP-enhanced spectra taken near 100 K were considerably broadened relative to spectra taken at 273 K (Fig. 1). The broadening for 13C lines ranged from 0.5 to 1.3 ppm (parts per million); for 15N, it was from 2.0 to >4.0 ppm. This broadening caused nuclei such as N or C′ that have small isotropic chemical-shift dispersion to lose value for assignment. In unfavorable cases, a single broad DNP resonance could extend over virtually the entire chemical-shift dispersion of a given residue type (SI Appendix, Fig. S2). Resonances from Cα, the other backbone atom, which have dispersions of several parts per million within a residue type and >10 ppm between residue types, provided sufficient chemical-shift discrimination, but could remain crowded. Amino acid side chains, however, provided maximal chemical-shift dispersion and excellent discrimination between amino acid types. The ratio of dispersion to linewidth serves as a measure of information value; assigning resonances with DNP enhancement should therefore emphasize information-rich Cα and side-chain resonances. Dimensions such as N or C′ have less discrimination value. However, because directing polarization transfers through the N afforded directionality, high efficiency, and simplified phase cycling, the amidic nitrogen dimension was often included in our experiments, whereas carbonyl was omitted.
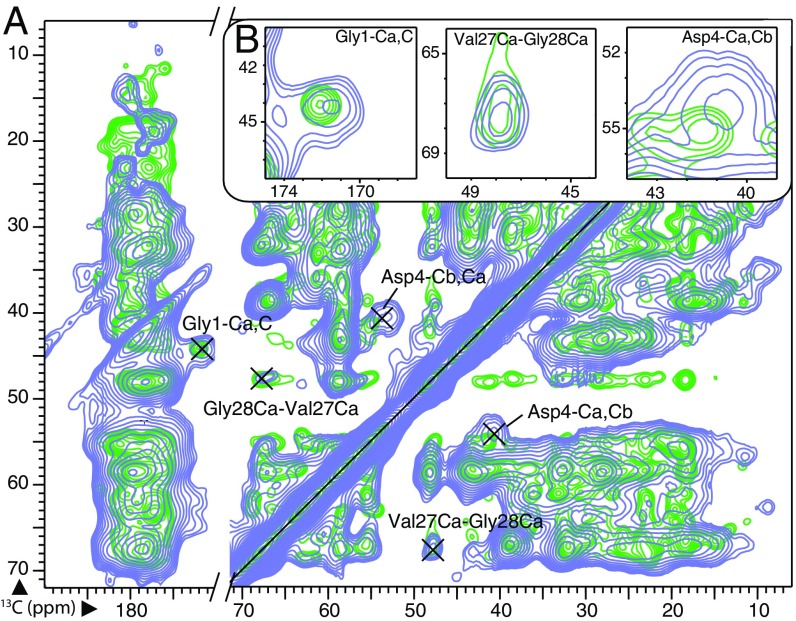
Fig. 1.
(A) The 2D 13C–13C DARR spectra (100 ms mixing time) of Pf1 illustrating linewidth differences between DNP conditions at 106 K (purple) and the same sample at 273 K (green). (B) Expansions around several assigned peaks with unique chemical shifts are shown. Although some well-resolved peaks at 273 K remained well resolved at 106 K, peaks in more crowded regions can become unrecognizable. Even for well-resolved peaks, linewidths broadened approximately twofold, from <100 Hz (0.5 ppm) to >200 Hz (1.3 ppm), making assignments more challenging. See SI Appendix for additional experimental details, including demonstration of 35-fold enhancement in 3.2-mm rotor and 60-fold enhancement in 1.9-mm rotor (SI Appendix, Fig. S1).
Moreover, linewidths were primarily broadened by static inhomogeneity, making absolute peak positions less certain in DNP spectra. In Pf1 datasets at 100 K, absolute 13C chemical shifts for multiple peaks assigned to the same atom ranged over 0.3 ppm, which is double the range for spectra at 273 K. Thus, identification of an amino acid based on a single chemical shift became significantly error-prone. Double-chemical-shift correlations for each residue for confident assignment could be achieved by walking between neighboring residues both forward and backward (Fig. 2A).
To this end, we present a modular pulse sequence that can encode 3D, 4D, or five-dimensional (5D) datasets (Fig. 2 B and C). The S3 pulse sequence exploits the chemical-shift dispersion of the side chains, as correlated through their Cα resonances. In brief, side-chain polarization was transferred first to the corresponding backbone Cα or C′ by 13C–13C recoupling, then through the amide nitrogen and to the preceding or following amino acid via a double-spectrally induced filtering in combination with cross-polarization (SPECIFIC-CP) (54) step (13C→15N, then 15N→13C), and, finally, out to the side chain of the second amino acid by a longer 13C–13C recoupling element. A full 5D S3 experiment correlated CXi–Cαi–Ni–C′i-1–CXi-1 in the “backward,” or i→i − 1, direction, and CXi–C′i –Ni+1–Cαi+1–CXi+1 in the “forward,” or i→i + 1, direction, thus combining information-rich side-chain chemical shifts with directional selectivity to provide a robust and reliable assignment tool. Although the pulse sequence incurred significant signal losses due to the number of transfers involved, a much larger starting signal (relative to non-DNP SSNMR) allowed sufficient signal to noise at detection. Interestingly, we found the efficiencies of the two N↔C transfers to be nonmultiplicative, presumably due to the selection of homogeneous volume by the first transfer. We found that the overall efficiency of a triple cross-polarization (CP) transfer under DNP was quite favorable, at 35% and 21% for S3 i→i − 1 and S3 i→i + 1, respectively. This efficiency allowed S3 spectra to retain adequate signal to noise while maintaining transfer selectivity. S3 data could be collected in 1–2 d for 4D experiments and in ∼1 wk for 5D experiments. The 3D spectra presented here were collected in <1 d, despite substantial signal averaging.
DNP-Based de Novo Assignment of Pf1 Coat Protein.
We demonstrated the robustness of our S3 assignment protocol by using DNP data, including 3D NCACX, NCOCA, and 3D S3 i→i + 1 and S3 i→i − 1 spectra, to assign de novo the Pf1 major coat protein. We assigned 205 13C resonances and 51 15N resonances, corresponding to 100% and 94% completeness, respectively (SI Appendix, Fig. S3). Most of the S3 experiments shown here were performed on sparsely 13C-labeled samples to minimize 13C linewidths by eliminating 13C–13C nearest-neighbor couplings. However, it is important to note that these experiments also worked well for uniformly labeled samples. As Fig. 3 demonstrates, uniform labeling enabled longer-range transfers. A 250-ms dipolar-assisted rotational resonance (DARR) (55) 13C–13C transfer step allowed correlations to distant side-chain sites and, in some cases, to residues beyond the nearest neighbor. For challenging regions with high levels of residue and chemical-shift redundancy, the availability of both short- and long-range S3 data greatly improved the efficiency of assignment (Fig. 4). Additionally, by using faster magic-angle spinning (MAS; up to 25 kHz) in 1.9-mm rotors, we reduced 13C peak widths to 1.0 ppm and 15N widths to 3.5 ppm. The resulting sharper peaks enabled high-resolution and high signal-to-noise spectra despite the presence of multiple internuclear transfers and long mixing times. Furthermore, faster MAS rates allowed spinning sidebands to be placed further away from peaks of interest, preventing problems of overlap. The primary drawback of faster MAS—namely, smaller sample volume as a result of using smaller diameter rotors—was more than offset by the superior DNP enhancement factors afforded (SI Appendix, Fig. S1).
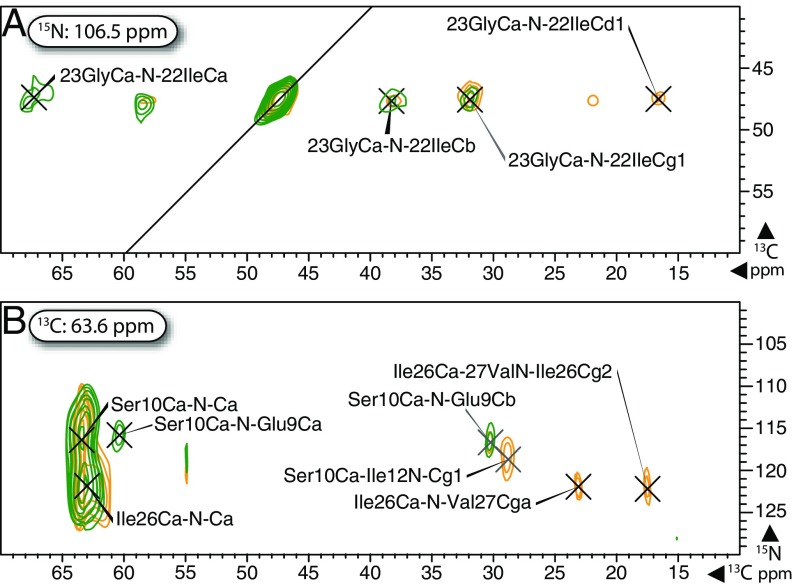
Fig. 3.
Long-range S3 spectra of Pf1 enabled assignment of distant side-chain sites, whereas comparison with short-range S3 helped to identify nearest-neighbor backbone correlations, as shown in 13C–13C planes (A) and 13C–15N planes (B) at the indicated 15N and 13C indirect dimension frequency, respectively, from short-range (green) and long-range (orange) CX(Cα)N(C′)CX i→i − 1 S3 spectra. DARR transfers of 10 and 50 ms were used for the short-range experiment; 50- and 250-ms DARR were used for long-range S3. 13C and 15N linewidths were as low as 1.0 and 3.5 ppm, respectively. See SI Appendix for additional experimental details.
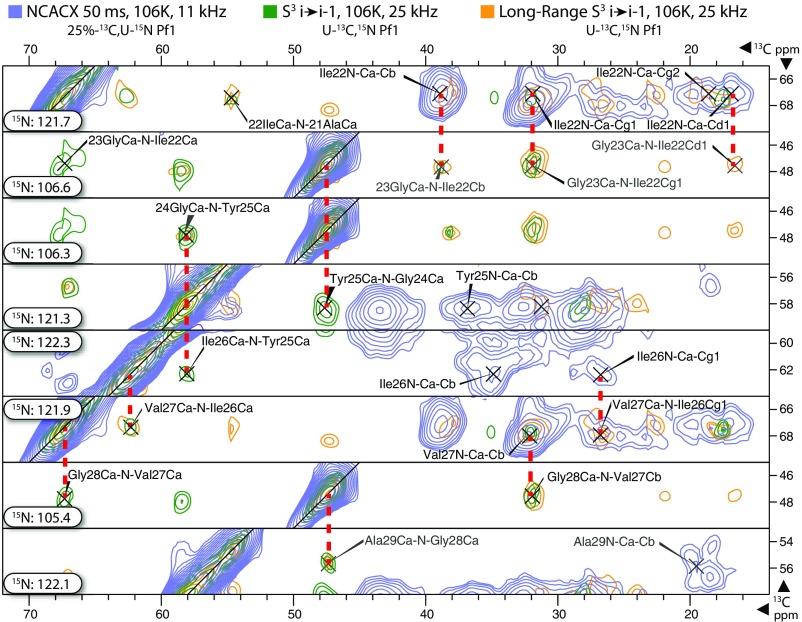
Fig. 4.
Strip plot of assignments for residues 21–29 of the Pf1 coat protein, showing representative backbone and side-chain interresidue “walks.” Data from 50-ms NCACX (blue), “short-range” S3 i→i − 1 10,50 ms (green), and “long-range” S3 i→i − 1 50, 250 ms (orange) are overlaid at the indicated 15N frequency in each slice. Other spectra, including NCOCA and S3 i→i + 1, were used to confirm and extend these assignments. See SI Appendix for more experimental details.
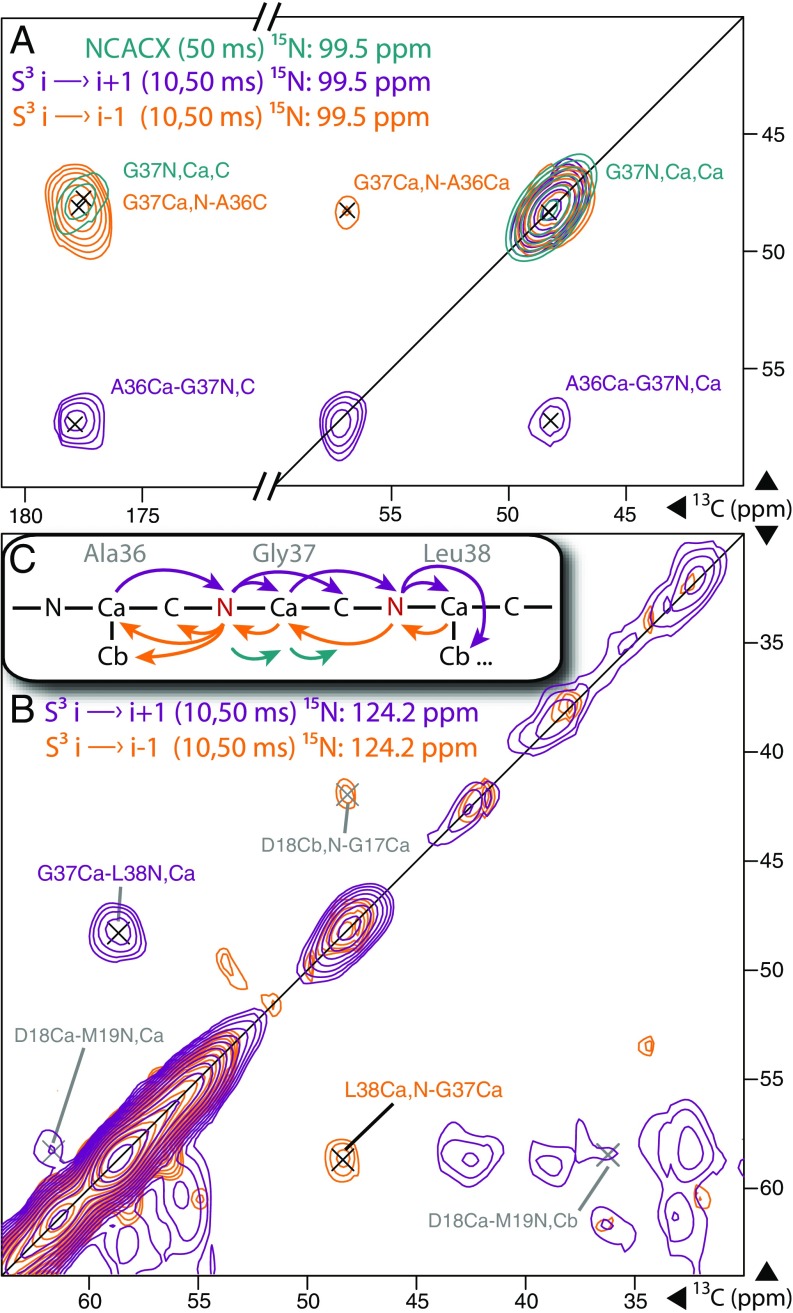
In assigning the highly repetitive, highly helical Pf1 coat protein, NCACX/NCOCA correlations were only useful for small stretches (SI Appendix, Fig. S4). These correlations relied on a single 15N chemical shift to correlate the intraresidue NCACX resonances to the interresidue NCOCA resonances, limiting sequential assignment to the i→i − 1 direction. In regions lacking notable 15N outliers like glycine (the Pf1 coat protein contains no proline or histidine residues), congestion became insurmountable, and NCOCA correlations were largely useless for interresidue walks because both N and C′ resonances were overlapping. In contrast, the S3 technique provided a direct interresidue Cα–Cα correlation. The superior resolution of 13C alongside the larger chemical-shift dispersion of Cα/CX sites allowed for straightforward and bidirectional assignment of the protein backbone via S3 i→i − 1 and i→i + 1. Additionally, S3 provided both intraresidue and interresidue correlations in the same experiment without increased congestion. In the present work, NCACX/NCOCA spectra were used alongside better-resolved S3 data in a confirmatory fashion, where possible.
NCACX/NCOCA spectroscopy relies on the directional selectivity of the N→C transfer, as provided by SPECIFIC-CP (54). The S3 scheme improves on this directional selectivity by using two SPECIFIC-CP N↔C transfers; ideally, each of these transfers passes <10% of the undesired transfer pathway (e.g., N→C′ would be undesired in a selective N→Cα transfer). Because only a single transfer pathway is preferentially followed, cross-peaks in 13C–13C planes show up on one side of the diagonal, as opposed to the diagonally symmetric pattern normally observed in 13C–13C correlations, helping to avoid spectral congestion. Reversing the direction of transfer along the protein backbone causes cross-peaks to appear on the opposite side of the diagonal; the S3 i→i − 1 and i→i + 1 spectra thus complement each other for assignment purposes and, when overlaid, provide chemical-shift redundancy, as shown in Fig. 5. In comparing cross-peak patterns between S3 i→i − 1 and i→i + 1 spectra, we found S3 directional selectivity to be nearly perfect in isolated 15N slices. Importantly, the S3 i→i − 1 experiment showed very few intraresidue 13C–13C cross-peaks. The vast majority of the detected cross-peaks followed the intended i→i − 1 transfer pathway. For S3 i→i + 1 experiments, some intraresidue diagonal intensity was observed due to the lower selectivity of N→Cα transfers (∼25% leakage to C′) relative to N→C′ transfers (∼10% leakage to Cα) as optimized (SI Appendix, Fig. S5). However, the S3 i→i − 1 transfer scheme did effectively suppress most intraresidue correlations, as demonstrated by comparison with an S3 experiment intentionally made nonselective by using a Cα→N→Cα transfer. When overlaid with canonical S3 spectra (SI Appendix, Fig. S6), the nonselective variant had many intraresidue cross-peaks absent in the S3 i→i − 1 experiment.
Fig. 5.
Sequential assignment of A36–L38 shown in 13C–13C slices of 3D NCACX (green), 3D S3 i→i + 1 (purple), and i→i − 1 (orange) at the indicated 15N chemical shifts. Notably, the two S3 spectra show very different patterns. (A) In S3 i→i − 1, G37-A36 cross-peaks appeared at the glycine frequency in the indirect dimension (y axis) and were “read out” at alanine frequencies in the direct dimension (x axis), whereas in S3 i→i + 1, these frequencies were reversed. No S3 i→i − 1 intensity was detected at the glycine frequency, and no S3 i→i + 1 intensity was observed on the alanine slice, except for a diagonal peak representing G37Cα–N–Cα. (B) A similar pattern was observed for G37–L38 interresidue cross-peaks. (C) With the exception of some diagonal intensity, the observed cross-peak patterns were exactly as expected from the transfer diagram, demonstrating the excellent selectivity of S3 experiments. See SI Appendix for additional experimental details.
Although ambiguity arises in many forms throughout the process of assignment, the S3 approach was designed to avoid several common sources. It is clear from a metaanalysis of SSNMR chemical shifts by Fritzsching et al. (56) that C′–Cα correlations are rarely distinctive enough to identify a particular amino acid in congested spectra. Cα–Cβ cross-peaks, conversely, tend to occupy unique fingerprint regions. By relying heavily on the latter, analysis of S3 spectra avoids deconvolution of myriad overlaid C′ –Cα peaks in poorly resolved 15N planes, eliminating a major potential source of error. Also avoided is the need to align two separate spectra to correlate residues, as in the case of NCACX/NCOCA. Spectral alignment is prone to error under DNP conditions, where wide nitrogen resonances can span multiple planes of an n-dimensional spectrum. An example of such difficulties is illustrated in SI Appendix, Fig. S4. Pair ambiguity (e.g., ability to confidently assign peaks of interest to an isoleucine-glycine pair, but inability to specify which isoleucine-glycine pair in the protein sequence) was also largely avoided because S3 data permit forward and backward backbone walks (example shown in Fig. 5). By avoiding ambiguous assignments, the S3 approach not only cut down on assignment error, but also saved analysis time. Compared with the multiple weeks typical of a full de novo protein assignment by SSNMR, all assignments presented here were performed in ∼60 person-hours.
Chemical-Shift Perturbation and Line Broadening Correlated with Hydration.
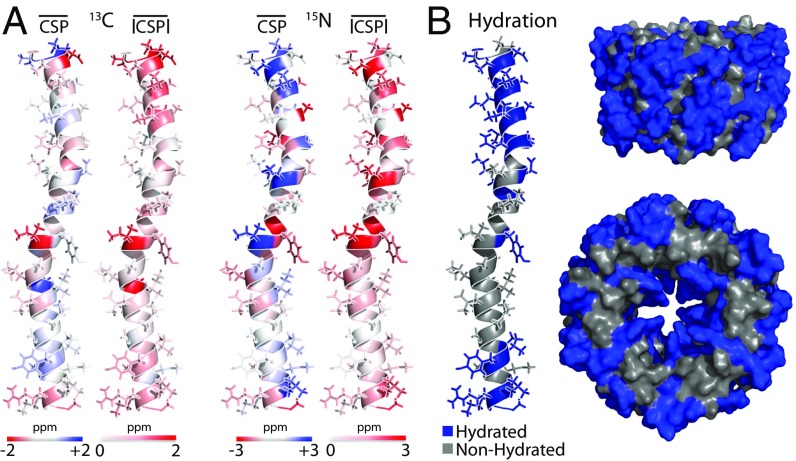
To date, several DNP studies have reported significant CSPs near 100 K (27, 57). Having assigned the coat protein of Pf1 both with and without DNP enhancement independently, we were able to examine the magnitude and sequence/structural distribution of CSPs. Large experimental CSPs (Fig. 6A and SI Appendix, Fig. S7) were clustered into three major regions: the outward-facing N-terminal domain (residues 1–20), the kink region (residues 24–26), and the inward-facing C terminus (residues 40–46), which is the DNA-interaction domain. The spatial distribution of CSPs thus correlated extremely well with the hydration map of the major coat protein (52), derived at 273 K from long-range 1H–13C and 1H–15N NMR contacts (Fig. 6B). The high degree of overlap between hydrated residues and residues with significant CSPs implies that the majority of CSPs under DNP conditions are linked to solvation effects, consistent with earlier results from amyloid-forming oligopeptides (27) and amyloid fibrils (58).
Fig. 6.
(A) Mappings of average 13C and 15N CSP for each residue of the Pf1 coat protein. Signed CSPs and absolute value CSPs (|CSP|) were averaged over all atoms of each residue; CSPs were computed as σ106 K – σ273 K in all cases. The shade and intensity of color correspond to perturbation levels, as indicated. A plot of residue average CSPs by residue number is shown in SI Appendix, Fig. S7. Large CSPs are clustered in three main regions: the outward-facing N terminus, a central kink region, and the inward-facing C terminus, which is the DNA interaction domain. (B) An independent map of where water molecules contact the protein at 278 K shows very similar patterns (52) on a single copy of the coat protein as well as on sections of the Pf1 assembly looking perpendicular to and down the central symmetry axis. The high degree of correlation suggests that solvent exposure represents a significant driving force of chemical shift changes in moving from conditions near 273 K to DNP conditions near 100 K.
A similar analysis to assess trends in 13C and 15N linewidths near 100 K revealed differences between hydrated and nonhydrated residues. Broader linewidths were found predominantly in the hydrated N-terminal region and at the C terminus, with a smaller increase in linewidth observed in the kink region near residue 25 (SI Appendix, Fig. S8). Residues that were not hydrated at 273 K did not exhibit as much line broadening at cryogenic temperatures as residues that were hydrated.
CSPs and linewidth both report on the immediate chemical environment of a residue; linewidth especially can serve as a strong marker of conformational inhomogeneity. As a result, the striking positional similarities between CSPs, linewidths, and solvent-exposed surfaces point to solvation and inhomogeneity being closely linked at cryogenic temperatures. Our data suggest that, when their closest layer of hydration water becomes frozen or glassy at cryogenic temperatures, previously hydrated biomolecular sites are likely to undergo structural rearrangements as a result of coupling to the glassy solvent, causing both broader linewidths and possible changes in chemical shift. Unlike at higher temperatures (59), these effects are not ameliorated by the addition of even high molar ratios of cryoprotectants, such as glycerol, to the aqueous solvent mixture. Sites that are not normally solvent-exposed, however, are not necessarily subject to the same constraints and can maintain favorable linewidths and relatively low CSPs, even at cryogenic temperatures down to 100 K, and potentially lower.
Conclusions
We used S3 correlation experiments to acquire and assign de novo the highly repetitive and almost fully helical coat protein of the Pf1 bacteriophage under DNP conditions. The backbone assignments were complete for all 46 residues, and the overall assignment was 95% complete. Furthermore, we found that S3 experiments allow accurate assignments to be carried out efficiently, with features such as chemical-shift redundancy across the diagonal between S3 i→i − 1 and S3 i→i + 1 spectra. Fast-spinning DNP at 25 kHz gave further improvements in signal to noise and enabled long-range side-chain–side-chain polarization transfers. Together, DNP NCACX and S3 (3D) experiments can be acquired in under 2 d by using NUS, allowing efficient use of DNP instrumentation. With advances in both the speed and ease of assignment, DNP has the potential to provide a wealth of structural information on a timescale not previously possible. In our hands, S3 experiments have been used to aid in the assignment of several biological systems of interest, including viral capsids, amyloid fibrils, and membrane proteins.
Comparison of DNP data collected near 100 K and near 273 K revealed that 13C and 15N CSPs cluster into distinct regions of the protein. Observed DNP 13C and 15N linewidths adhered to a similar pattern, with larger linewidths correlated to larger CSPs. Regions with significant perturbations and increased linewidths were highly correlated with a previously determined hydration map, suggesting that CSPs and linewidths under DNP conditions are both closely related to solvation phenomena, but do not necessarily require exposure to bulk solvent. These observations can be used to predict which regions of a biomolecule are likely to experience significant CSPs at cryogenic temperatures and provide clues toward further DNP sample optimization.
Materials and Methods
Samples of Pf1 bacteriophage were prepared by using published protocols (45, 52). DNP requirements, of high MAS speeds and cryogenic conditions, did not affect phage infectivity on host Pseudomonas aeruginosa, strain K. For U-15N, 25%–13C Pf1, 25% 13C-glucose with random isotopic distribution was used during the growth stage to minimize the effect of 13C–13C J-couplings on linewidth, while retaining sufficient 13C–13C pairs to record 13C homonuclear correlations. For U-15N,13C Pf1, 99% 13C6-glucose was used.
DNP samples were prepared by mixing 2% (wt/vol) PEG 8000 precipitates of Pf1 (from 100 mM Tris buffer at pH 8.4 and 50 mM MgCl2) in D2O with a 60% glycerol-d8/40% D2O solution containing 20 mM AMUPol to achieve a final solvent mixture of 30% glycerol-d8/70% D2O with 10 mM AMUPol. Because the virions were fully protonated, sufficient protons were present in the sample to make the addition of H2O unnecessary. No differences in linewidth, polarization buildup time, or enhancement were observed between 30% and 60% glycerol solvent mixtures; 30% was used to maximize sample concentration in the rotor.
DNP-SSNMR spectroscopy was carried out at the New York Structural Biology Center on a 14.1-T Bruker AVANCE-III 600/89 spectrometer (600-MHz 1H field) equipped with a 395-GHz gyrotron, low-temperature MAS cabinet, and a three-channel 1H–13C–15N (HCN) 3.2-mm DNP probe. A MAS frequency of 11 kHz was used, with variable-temperature (VT)/bearing/drive temperatures all within the range of 98 to 106 K. Maximum field strengths used were 120 kHz 1H, 62.5 kHz 13C, and 50 kHz 15N; high-power proton decoupling was performed at 115 kHz. Where indicated, Pf1 samples were instead packed in a 1.9-mm zirconia DNP rotor, and spectroscopy was performed by using a Bruker 1.9-mm three-channel HCN probe on the same spectrometer, allowing MAS rates up to 25 kHz, with VT/bearing/drive temperatures in the range of 98 to 108 K. Although faster MAS is theoretically possible with 1.9-mm rotors, the density of cold nitrogen gas, coupled with flow limitations, precluded regular spinning >25 kHz near 100 K. Maximum power levels of 150 kHz 1H, 62.5 kHz 13C, and 62.5 kHz 15N were used, with proton decoupling calibrated at 120 kHz.
The 3D spectra were acquired at a level of 25% NUS, by using 50% sampling in each indirect dimension. NUS tables were generated in Bruker Topspin (Version 3.1) by using a double-exponential biasing scheme based on the experimental T2*. NUS spectra were reconstructed by using the MDD protocol in Topspin (Version 3.1) and qMDD (Version 2.2) (60). No significant differences were observed in processed spectra between NUS and uniformly sampled NCACX/NCOCA spectra.
Additional details regarding optimization of NMR parameters, experimental setup, and assignment protocol are presented in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Michael J. Goger of the New York Structural Biology Center (NYSBC) for help with instrumentation; and Dr. Tatyana Polenova and Chris Suiter for many helpful discussions of nonuniform sampling techniques and their application to biological SSNMR. This work was supported by National Science Foundation Grant MCB 1412253 (to A.E.M.), as well as by National Institutes of Health Grant NIH R01 GM 88724 (to A.E.M.). R.R. was supported by the National Institutes of Health Training Program in Molecular Biophysics T32GM008281. A.E.M. is a member of the NYSBC. The data collected at NYSBC was made possible by a grant from Empire State Division of Science Technology and Innovation and Office of Research Infrastructure Programs/NIH facility improvement Grant CO6RR015495. The 600 MHz DNP/NMR spectrometer was purchased with funds from NIH Grant S10RR029249.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701484114/-/DCSupplemental.
References
- 1.Ni QZ, et al. High frequency dynamic nuclear polarization. Acc Chem Res. 2013;46:1933–1941. doi: 10.1021/ar300348n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song C, Hu KN, Joo CG, Swager TM, Griffin RG. TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J Am Chem Soc. 2006;128:11385–11390. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 3.Wenk P, et al. Dynamic nuclear polarization of nucleic acid with endogenously bound manganese. J Biomol NMR. 2015;63:97–109. doi: 10.1007/s10858-015-9972-1. [DOI] [PubMed] [Google Scholar]
- 4.Smith AN, Long JR. Dynamic nuclear polarization as an enabling technology for solid state nuclear magnetic resonance spectroscopy. Anal Chem. 2016;88:122–132. doi: 10.1021/acs.analchem.5b04376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyberts SG, Arthanari H, Wagner G. Applications of non-uniform sampling and processing. In: Billeter M, Orekhov V, editors. Novel Sampling Approaches in Higher Dimensional NMR. Springer; Heidelberg: 2012. pp. 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoch JC, Maciejewski MW, Mobli M, Schuyler AD, Stern AS. Nonuniform sampling and maximum entropy reconstruction in multidimensional NMR. Acc Chem Res. 2014;47:708–717. doi: 10.1021/ar400244v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer MR, Wenrich BR, Stahlfeld P, Rovnyak D. Performance tuning non-uniform sampling for sensitivity enhancement of signal-limited biological NMR. J Biomol NMR. 2014;58:303–314. doi: 10.1007/s10858-014-9823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossini AJ, et al. Dynamic nuclear polarization enhanced NMR spectroscopy for pharmaceutical formulations. J Am Chem Soc. 2014;136:2324–2334. doi: 10.1021/ja4092038. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig C, et al. Application of ex situ dynamic nuclear polarization in studying small molecules. Phys Chem Chem Phys. 2010;12:5868–5871. doi: 10.1039/c002700f. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. Functional and shunt states of bacteriorhodopsin resolved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc Natl Acad Sci USA. 2009;106:9244–9249. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG. DNP enhanced frequency-selective TEDOR experiments in bacteriorhodopsin. J Magn Reson. 2010;202:9–13. doi: 10.1016/j.jmr.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CY, Han S. Dynamic nuclear polarization methods in solids and solutions to explore membrane proteins and membrane systems. Annu Rev Phys Chem. 2013;64:507–532. doi: 10.1146/annurev-physchem-040412-110028. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, et al. Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc Natl Acad Sci USA. 2013;110:16444–16449. doi: 10.1073/pnas.1316290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlova A, et al. Site-specific dynamic nuclear polarization of hydration water as a generally applicable approach to monitor protein aggregation. Phys Chem Chem Phys. 2009;11:6833–6839. doi: 10.1039/b906101k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayro MJ, et al. Intermolecular structure determination of amyloid fibrils with magic-angle spinning and dynamic nuclear polarization NMR. J Am Chem Soc. 2011;133:13967–13974. doi: 10.1021/ja203756x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto K, Caporini MA, Im SC, Waskell L, Ramamoorthy A. Cellular solid-state NMR investigation of a membrane protein using dynamic nuclear polarization. Biochim Biophys Acta. 2015;1848:342–349. doi: 10.1016/j.bbamem.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes AB, et al. High-field dynamic nuclear polarization for solid and solution biological NMR. Appl Magn Reson. 2008;34:237–263. doi: 10.1007/s00723-008-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maly T, et al. Dynamic nuclear polarization at high magnetic fields. J Chem Phys. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunther WR, Michaelis VK, Caporini MA, Griffin RG, Román-Leshkov Y. Dynamic nuclear polarization NMR enables the analysis of Sn-Beta zeolite prepared with natural abundance 119Sn precursors. J Am Chem Soc. 2014;136:6219–6222. doi: 10.1021/ja502113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitzthum V, et al. Dynamic nuclear polarization of quadrupolar nuclei using cross polarization from protons: Surface-enhanced aluminium-27 NMR. Chem Commun (Camb) 2012;48:1988–1990. doi: 10.1039/c2cc15905h. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, et al. Enhanced solid-state NMR correlation spectroscopy of quadrupolar nuclei using dynamic nuclear polarization. J Am Chem Soc. 2012;134:18491–18494. doi: 10.1021/ja307755t. [DOI] [PubMed] [Google Scholar]
- 22.Rossini AJ, et al. Dynamic nuclear polarization surface enhanced NMR spectroscopy. Acc Chem Res. 2013;46:1942–1951. doi: 10.1021/ar300322x. [DOI] [PubMed] [Google Scholar]
- 23.Zagdoun A, et al. A slowly relaxing rigid biradical for efficient dynamic nuclear polarization surface-enhanced NMR spectroscopy: Expeditious characterization of functional group manipulation in hybrid materials. J Am Chem Soc. 2012;134:2284–2291. doi: 10.1021/ja210177v. [DOI] [PubMed] [Google Scholar]
- 24.Zagdoun A, et al. Improved dynamic nuclear polarization surface-enhanced NMR spectroscopy through controlled incorporation of deuterated functional groups. Angew Chem Int Ed Engl. 2013;52:1222–1225. doi: 10.1002/anie.201208699. [DOI] [PubMed] [Google Scholar]
- 25.Zagdoun A, et al. Non-aqueous solvents for DNP surface enhanced NMR spectroscopy. Chem Commun (Camb) 2012;48:654–656. doi: 10.1039/c1cc15242d. [DOI] [PubMed] [Google Scholar]
- 26.Fricke P, Demers JP, Becker S, Lange A. Studies on the MxiH protein in T3SS needles using DNP-enhanced ssNMR spectroscopy. ChemPhysChem. 2014;15:57–60. doi: 10.1002/cphc.201300994. [DOI] [PubMed] [Google Scholar]
- 27.Debelouchina GT, et al. Dynamic nuclear polarization-enhanced solid-state NMR spectroscopy of GNNQQNY nanocrystals and amyloid fibrils. Phys Chem Chem Phys. 2010;12:5911–5919. doi: 10.1039/c003661g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le H, Oldfield E. Correlation between 15N NMR chemical shifts in proteins and secondary structure. J Biomol NMR. 1994;4:341–348. doi: 10.1007/BF00179345. [DOI] [PubMed] [Google Scholar]
- 29.Le HB, Oldfield E. Ab initio studies of amide-N-15 chemical shifts in dipeptides: Applications to protein NMR spectroscopy. J Phys Chem. 1996;100:16423–16428. [Google Scholar]
- 30.McDermott A. Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- 31.Franks WT, et al. Magic-angle spinning solid-state NMR spectroscopy of the beta1 immunoglobulin binding domain of protein G (GB1): 15N and 13C chemical shift assignments and conformational analysis. J Am Chem Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]
- 32.McDermott A, et al. Partial NMR assignments for uniformly (13C, 15N)-enriched BPTI in the solid state. J Biomol NMR. 2000;16:209–219. doi: 10.1023/a:1008391625633. [DOI] [PubMed] [Google Scholar]
- 33.Igumenova TI, Wand AJ, McDermott AE. Assignment of the backbone resonances for microcrystalline ubiquitin. J Am Chem Soc. 2004;126:5323–5331. doi: 10.1021/ja030546w. [DOI] [PubMed] [Google Scholar]
- 34.Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H. Backbone and side-chain 13C and 15N signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 Tesla. ChemBioChem. 2001;2:272–281. doi: 10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Franks WT, Kloepper KD, Wylie BJ, Rienstra CM. Four-dimensional heteronuclear correlation experiments for chemical shift assignment of solid proteins. J Biomol NMR. 2007;39:107–131. doi: 10.1007/s10858-007-9179-1. [DOI] [PubMed] [Google Scholar]
- 36.Gopinath T, Veglia G. Multiple acquisition of magic angle spinning solid-state NMR experiments using one receiver: Application to microcrystalline and membrane protein preparations. J Magn Reson. 2015;253:143–153. doi: 10.1016/j.jmr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wylie BJ, Bhate MP, McDermott AE. Transmembrane allosteric coupling of the gates in a potassium channel. Proc Natl Acad Sci USA. 2014;111:185–190. doi: 10.1073/pnas.1319577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang M, Comellas G, Rienstra CM. Advanced solid-state NMR approaches for structure determination of membrane proteins and amyloid fibrils. Acc Chem Res. 2013;46:2080–2088. doi: 10.1021/ar4000168. [DOI] [PubMed] [Google Scholar]
- 39.Comellas G, Rienstra CM. Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys. 2013;42:515–536. doi: 10.1146/annurev-biophys-083012-130356. [DOI] [PubMed] [Google Scholar]
- 40.Makowski L, Caspar DLD, Marvin DA. Filamentous bacteriophage Pf1 structure determined at 7A resolution by refinement of models for the alpha-helical subunit. J Mol Biol. 1980;140:149–181. doi: 10.1016/0022-2836(80)90101-1. [DOI] [PubMed] [Google Scholar]
- 41.Cross TA, Tsang P, Opella SJ. Comparison of protein and deoxyribonucleic acid backbone structures in fd and Pf1 bacteriophages. Biochemistry. 1983;22:721–726. doi: 10.1021/bi00273a002. [DOI] [PubMed] [Google Scholar]
- 42.Day LA, Marzec CJ, Reisberg SA, Casadevall A. DNA packing in filamentous bacteriophages. Annu Rev Biophys Biophys Chem. 1988;17:509–539. doi: 10.1146/annurev.bb.17.060188.002453. [DOI] [PubMed] [Google Scholar]
- 43.Liu DJ, Day LA. Pf1 virus structure: Helical coat protein and DNA with paraxial phosphates. Science. 1994;265:671–674. doi: 10.1126/science.8036516. [DOI] [PubMed] [Google Scholar]
- 44.Thiriot DS, Nevzorov AA, Zagyanskiy L, Wu CH, Opella SJ. Structure of the coat protein in Pf1 bacteriophage determined by solid-state NMR spectroscopy. J Mol Biol. 2004;341:869–879. doi: 10.1016/j.jmb.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 45.Goldbourt A, Gross BJ, Day LA, McDermott AE. Filamentous phage studied by magic-angle spinning NMR: Resonance assignment and secondary structure of the coat protein in Pf1. J Am Chem Soc. 2007;129:2338–2344. doi: 10.1021/ja066928u. [DOI] [PubMed] [Google Scholar]
- 46.Tomar S, Green MM, Day LA. DNA-protein interactions as the source of large-length-scale chirality evident in the liquid crystal behavior of filamentous bacteriophages. J Am Chem Soc. 2007;129:3367–3375. doi: 10.1021/ja068498d. [DOI] [PubMed] [Google Scholar]
- 47.Lorieau JL, Day LA, McDermott AE. Conformational dynamics of an intact virus: Order parameters for the coat protein of Pf1 bacteriophage. Proc Natl Acad Sci USA. 2008;105:10366–10371. doi: 10.1073/pnas.0800405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldbourt A, Day LA, McDermott AE. Intersubunit hydrophobic interactions in Pf1 filamentous phage. J Biol Chem. 2010;285:37051–37059. doi: 10.1074/jbc.M110.119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuboi M, Tsunoda M, Overman SA, Benevides JM, Thomas GJ., Jr A structural model for the single-stranded DNA genome of filamentous bacteriophage Pf1. Biochemistry. 2010;49:1737–1743. doi: 10.1021/bi901323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straus SK, Scott WRP, Schwieters CD, Marvin DA. Consensus structure of Pf1 filamentous bacteriophage from X-ray fibre diffraction and solid-state NMR. Eur Biophys J. 2011;40:221–234. doi: 10.1007/s00249-010-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergeyev IV, Day LA, Goldbourt A, McDermott AE. Chemical shifts for the unusual DNA structure in Pf1 bacteriophage from dynamic-nuclear-polarization-enhanced solid-state NMR spectroscopy. J Am Chem Soc. 2011;133:20208–20217. doi: 10.1021/ja2043062. [DOI] [PubMed] [Google Scholar]
- 52.Sergeyev IV, Bahri S, Day LA, McDermott AE. Pf1 bacteriophage hydration by magic angle spinning solid-state NMR. J Chem Phys. 2014;141:D533. doi: 10.1063/1.4903230. [DOI] [PubMed] [Google Scholar]
- 53.Bajaj VS, et al. Dynamic nuclear polarization at 9T using a novel 250GHz gyrotron microwave source. J Magn Reson. 2003;160:85–90. doi: 10.1016/s1090-7807(02)00192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baldus M, Petkova AT, Herzfeld J, Griffin RG. Cross polarization in the tilted frame: Assignment and spectral simplification in heteronuclear spin systems. Mol Phys. 1998;95:1197–1207. [Google Scholar]
- 55.Takegoshi K, Nakamura S, Terao T. 13C-1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett. 2001;(344):631–637. [Google Scholar]
- 56.Fritzsching KJ, Yang Y, Schmidt-Rohr K, Hong M. Practical use of chemical shift databases for protein solid-state NMR: 2D chemical shift maps and amino-acid assignment with secondary-structure information. J Biomol NMR. 2013;56:155–167. doi: 10.1007/s10858-013-9732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mak-Jurkauskas ML, et al. Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR. Proc Natl Acad Sci USA. 2008;105:883–888. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bauer T, et al. Line-broadening in low-temperature solid-state NMR spectra of fibrils. J Biomol NMR. 2017;67:51–61. doi: 10.1007/s10858-016-0083-4. [DOI] [PubMed] [Google Scholar]
- 59.Siemer AB, Huang KY, McDermott AE. Protein linewidth and solvent dynamics in frozen solution NMR. PLoS One. 2012;7:e47242. doi: 10.1371/journal.pone.0047242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orekhov VY, Ibraghimov I, Billeter M. Optimizing resolution in multidimensional NMR by three-way decomposition. J Biomol NMR. 2003;27:165–173. doi: 10.1023/a:1024944720653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.