Significance
Conjugated polymers (CPs) are some of the most attractive organic semiconductors for flexible optoelectronic device applications. The structural and optoelectronic properties of CPs are dependent on both the conformation of individual polymer chains and interactions between chains. Chemists have sought to control these interactions by creating more planar polymers that can pack tightly together and share strong electronic interactions. Single-molecule spectroscopy (SMS) studies of a fluorinated CP show that backbone fluorination does, in fact, lead to more planar polymer chains and highly ordered aggregates. Interestingly though, the electronic interactions are found to extend along individual chains rather than between chains. The studies demonstrate how SMS can test hypotheses about structure–property relationships in large molecular systems.
Keywords: fluorinated polythiophenes, photophysics, single-molecule spectroscopy, morphology, organic electronics
Abstract
Fluorination represents an important strategy in developing high-performance conjugated polymers for photovoltaic applications. Here, we use regioregular poly(3-ethylhexylthiophene) (P3EHT) and poly(3-ethylhexyl-4-fluorothiophene) (F-P3EHT) as simplified model materials, using single-molecule/aggregate spectroscopy and molecular dynamic simulations, to elucidate the impacts of backbone fluorination on morphology and excitonic coupling on the molecular scale. Despite its high regioregularity, regioregular P3EHT exhibits a rather broad distribution in polymer chain conformation due to the strong steric hindrance of bulky ethylhexyl side chains. This conformational variability results in disordered interchain morphology even between a few chains, prohibiting long-range effective interchain coupling. In stark contrast, the experimental and molecular dynamic calculations reveal that backbone fluorination of F-P3EHT leads to an extended rod-like single-chain conformation and hence highly ordered interchain packing in aggregates. Surprisingly, the ordered and close interchain packing in F-P3EHT does not lead to strong excitonic coupling between the chains but rather to dominant intrachain excitonic coupling that greatly reduces the molecular energetic heterogeneity.
Morphology and excitonic coupling are among the most important factors dictating the functions and performance of conjugated polymers (CPs) in a variety of optoelectronic applications (1–4). To tune and optimize the morphological and optoelectronic properties of CPs, chemical structure modification of the backbone and side chains is one of the main and most effective approaches (5–7). It has been revealed that the position, size, and length of side chains dramatically affect the morphology and optoelectronic properties of CPs. In the efforts to develop high-performance photovoltaic CPs, the introduction of fluorine atoms onto aromatic comonomers, i.e., the fluorination of polymer backbone, offers a very appealing strategy to tune the electronic properties, morphology, and photochemical stability (6–8). It is proposed that the inherent electron-withdrawing nature of fluorine atoms lowers the highest energy occupied molecular orbital energy levels, thereby increasing the open-circuit voltage in photovoltaics. In addition, the strong electronegativity might induce F–S and F–H interactions and potentially modifies the molecular conformation and intermolecular organization (6). Furthermore, it has been shown that fluorination often leads to enhanced thermal and oxidative stability (9–11). Given a combination of these desirable properties, fluorine-containing CPs have led to most of the best-performing polymer-based solar cells to date (12–15).
For typical CPs, the primarily created exciton is Coulombically bound upon photoexcitation mainly due to the low dielectric constant and strong electron–phonon interaction in polymers. The interchain and intrachain morphology not only determines the ultrafast exciton delocalization and relaxation pathways but also affects the longer timescale behavior including exciton migration, recombination, dissociation, and charge transfer (1, 16–18). Therefore, in an endeavor to design high-performance CPs, an investigation into how the backbone fluorination affects polymer morphology and exciton properties is warranted (6). Compared with structurally complicated fluorine-containing donor–acceptor polymers, fluorinated poly(3-alkylthiophenes) (P3AT) represent a simplified and prototypical system to interrogate the effects of fluorination. Recently, we have reported the manipulation of the P3AT backbone by incorporating fluorine in the vacant 4-position to make poly(3-alkyl-4-fluoro)-thiophenes (7). It was revealed that backbone fluorination appeared to promote aggregation or short-range ordering in thin films but frustrate long-range ordering (crystallization). Despite the reduced long-range crystallinity, thin-film transistors of F-P3EHT exhibited better electrical performance than their nonfluorinated analogs. Nevertheless, fundamental and critical knowledge about the influence of fluorination on single-chain conformation, intermolecular morphology, and excitonic coupling on a molecular basis is still lacking. Single-molecule/aggregate spectroscopy in conjunction with nanoscale aggregates fabricated via a solvent-vapor-annealing (SVA) technique has proven to be an unprecedented and elegant approach in elucidating complex relations between morphology and photophysics in CPs (2, 19), while circumventing ensemble averaging effects due to bulk measurements and morphological heterogeneity in bulk samples (2, 19, 20).
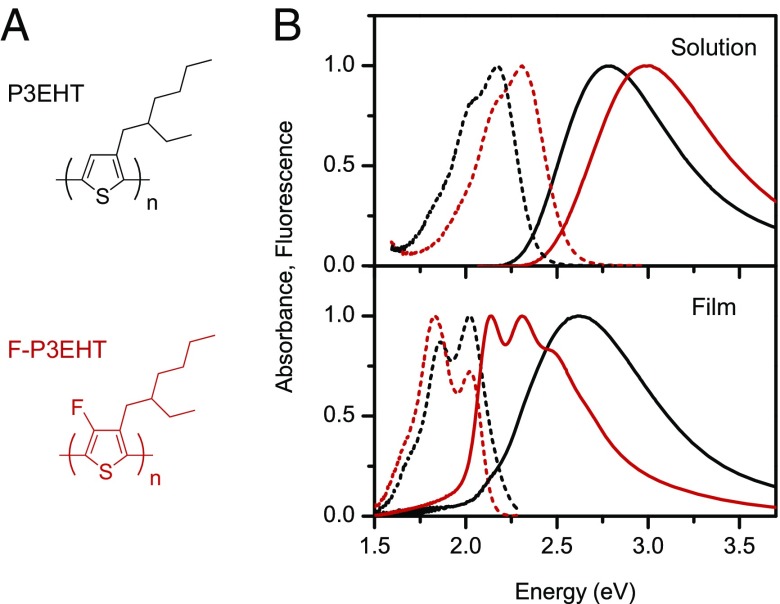
In the present contribution, we investigate the impacts of fluorination of the polythiophene backbone on nanoscale morphology and excitonic coupling, using simple model polythiophenes, namely, poly(3-ethylhexylthiophene) (P3EHT) and poly(3-ethylhexyl-4-fluorothiophenes) (F-P3EHT) (Fig. 1A) (SI Materials and Methods for details). To avoid the complexity of intramolecular folding-induced interchain interactions, we use polymers with a low number average molecular mass of 13 kDa, for which there is no detectable folding of the polyalkylthiophene chain according to our previous studies (2, 21, 22). Fluorescence excitation polarization experiments and molecular dynamics (MD) simulations reveal that backbone fluorination dramatically changes the polymer chain conformation from disordered coil-like for P3EHT into extended rod-like for F-P3EHT. Our photon correlation data, fluorescence transients, and spectral data obtained for SVA-assembled nanoscale aggregates reveal that the modified single-chain conformation due to fluorination surprisingly limits efficient interchain coupling. However, the high order in the molecular aggregates of the F-P3EHT leads to a narrowing of the distribution of polymer chain energies such that the collection of chains shows less heterogeneity than the individuals.
Fig. 1.
(A) Chemical structures of P3EHT and F-P3EHT. (B) Absorption and fluorescence spectra for solutions (Top) and films (Bottom) of P3EHT (13 kDa, black) and F-P3EHT (13 kDa, red). The solid and dashed curves are for absorption and fluorescence spectra, respectively.
Results and Discussion
Absorption and Emission of Bulk Solution and Film.
The absorption and emission spectra of chloroform solutions and films of P3EHT and F-P3EHT are shown in Fig. 1B. In solution, the main absorption peak of F-P3EHT (3.0 eV) is blue-shifted by about 0.20 eV with respect to that of P3EHT (2.8 eV). Similarly, the photoluminescence spectrum of F-P3EHT is blue-shifted by 0.15 eV compared with P3EHT. The spectral blue shift of F-P3EHT relative to P3EHT could be attributed to a combination of a more twisted backbone conformation as a result of the bulkier fluorine in F-P3EHT (as we will show later in MD Simulations) and/or the electron withdrawing of F atom (7). Going from solution to film, P3EHT exhibits a red shift (0.17 eV) in the absorption. Furthermore, the absence of clear vibronic structures in the low-energy range of the film spectrum (23) indicates unfavorable interchain interactions in solid-state P3EHT, despite its high head-to-tail (HT) regioregularity of 97%. This observation implies a destructive effect of the bulky ethylhexyl side chains on interchain interaction in P3EHT.
Despite the blue-shifted absorption spectrum of F-P3EHT in solution relative to P3EHT, F-P3EHT exhibits a large red shift from solution to film. In addition, distinct vibronic structures with a strong 0–0 transition appear in the F-P3EHT film spectrum. These stark changes in F-P3EHT suggest that there is a dramatic variation in the F-P3EHT chain conformation from solution to solid state, presumably from a random coil to an extended chain conformation. This conformational variation could be due to a combined effect of planarization of individual chains upon going from solution to solid state (due to a small rotational barrier that will be discussed in MD Simulations) and interchain packing interaction. The fluorescence spectrum of F-P3EHT film exhibits a Stokes shift of ∼0.12 eV, which is smaller than ∼0.20 eV of typical P3HT films (H-aggregate) but larger than ∼0.06 eV of P3HT J-aggregate nanofibers (24, 25). To provide more in-depth understanding of the morphological and photophysical properties while avoiding the heterogeneity of bulk samples, we will focus on single chains and controllably self-assembled molecular aggregates in what follows.
Morphology of Single Chains and Aggregates.
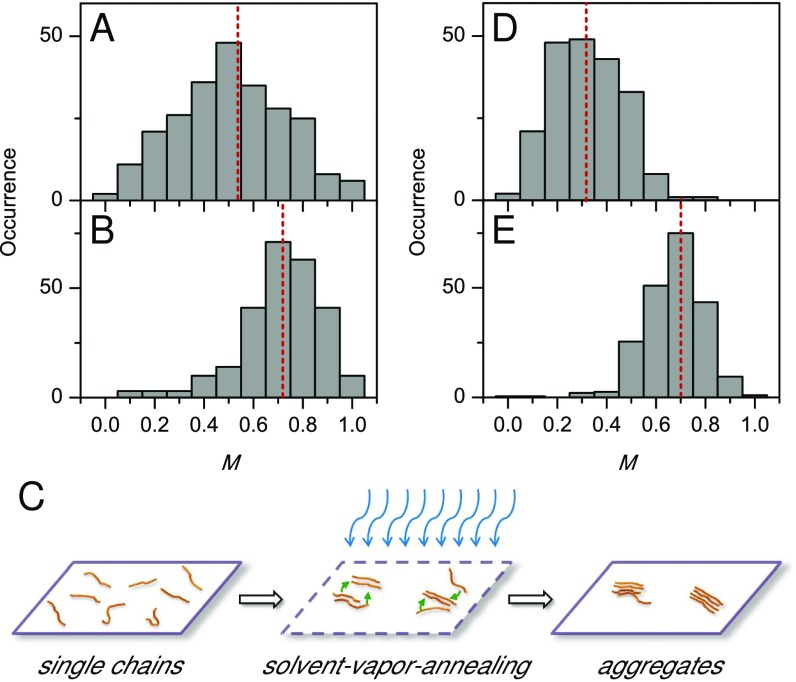
To examine how fluorination affects the morphology of single-polymer chains and aggregates, fluorescence excitation polarization analysis was performed (SI Materials and Methods for details). In these experiments, the fluorescence intensity traces were modulated with a rotating linearly polarized excitation light and then fitted with , where is the excitation polarization angle and is the polarization angle at maximum absorption (2, 26, 27). The modulation depth, , represents the anisotropy of the absorption (excitation) tensor projected on the x−y plane of the laboratory frame and is related to the morphological order of individual molecules or aggregates. Fig. 2 A and B presents the distribution histograms of single chains of P3EHT and F-P3EHT, respectively, embedded in poly(methyl methacrylate) (PMMA) matrix. Although P3EHT investigated in the present work has a high HT regioregularity of 97%, the distribution is spread broadly from 0 to 1, meaning single-chain conformations range from highly disordered to highly ordered, with a mean value of 0.53. This result is similar to that observed for regiorandom P3HT with a regioregularity of 60% (2, 28). The highly disordered single-chain conformations of P3EHT are assumed to be due to a twisted backbone as a result of the strong steric hindrance of branched and bulky ethylhexyl side chains, which will be further elucidated by the theoretical simulations shown below. Interestingly, the fluorination of P3EHT thiophene rings significantly enhances the ordering of single chains. As displayed in Fig. 2B, the values for F-P3EHT single chains are much higher than that of P3EHT with a large majority over 0.60 and a mean of 0.72. Apparently, fluorine atom substitution at the vacant 4-position in thiophene rings in F-P3EHT greatly alleviates the disorder caused by the ethylhexyl side chains and renders more extended backbone conformation.
Fig. 2.
Distribution histograms of fluorescence excitation modulation depth, M, for single chains of P3EHT (A) and F-P3EHT (B). (C) Formation of aggregates using the SVA approach. (D and E) M histograms for SVA-assembled aggregates of P3HT and F-P3EHT, respectively.
The effect of backbone fluorination on the nanoscale interchain morphology was then explored by studying small aggregates, which were assembled using the previously reported SVA technique in conjunction with the wide-field imaging (2, 19). Briefly, during SVA a concentrated single-chain sample in PMMA is swollen with a mixed organic solvent vapor of chloroform (good solvent) and acetone (poor solvent) (Fig. 2C). Here, chloroform is capable of dissolving both PMMA and CP chains, whereas acetone is a poor solvent for CPs but still can dissolve PMMA. The diffusion of CP molecules in the swollen and softened PMMA causes the aggregation of CP chains that usually can be described by an Ostwald ripening mechanism. By using mixed solvents of chloroform and acetone with a vapor ratio of 38/62 (volume ratio of 55/45), we constructed P3EHT and F-P3EHT aggregates composed of a few chains. For detailed descriptions about the aggregate fabrication by the SVA, please refer to SI Materials and Methods and our recent work in ref. 2. Fig. 2 D and E demonstrates the distribution histogram of (5 ± 1)-chain P3EHT aggregates and (4 ± 1)-chain F-P3EHT aggregates, respectively. Highly disordered interchain morphology is observed for P3EHT aggregates as evidenced by the dominant low- values below 0.5 with a mean near 0.32 (Fig. 2D). The bulky side chains together with disordered single-chain configuration lead to unfavorable packing, even between a few P3EHT chains. In stark contrast to P3EHT, F-P3EHT aggregates exhibit an enhanced extent of ordering evidenced by a higher mean- value of 0.70 (Fig. 2E). In addition, the distribution is also narrower in comparison with single chains. This indicates that in F-P3EHT aggregates the conformation of the constituting single chains becomes ordered upon packing. The different observations between these two polymers suggest that extended chains of F-P3EHT as a result of F substitution facilitate packing between F-P3EHT chains despite the bulky side chains. The result obtained for the nanoscale aggregates is in agreement with our previous data, which revealed short-range ordering in F-P3EHT film (7).
MD Simulations.
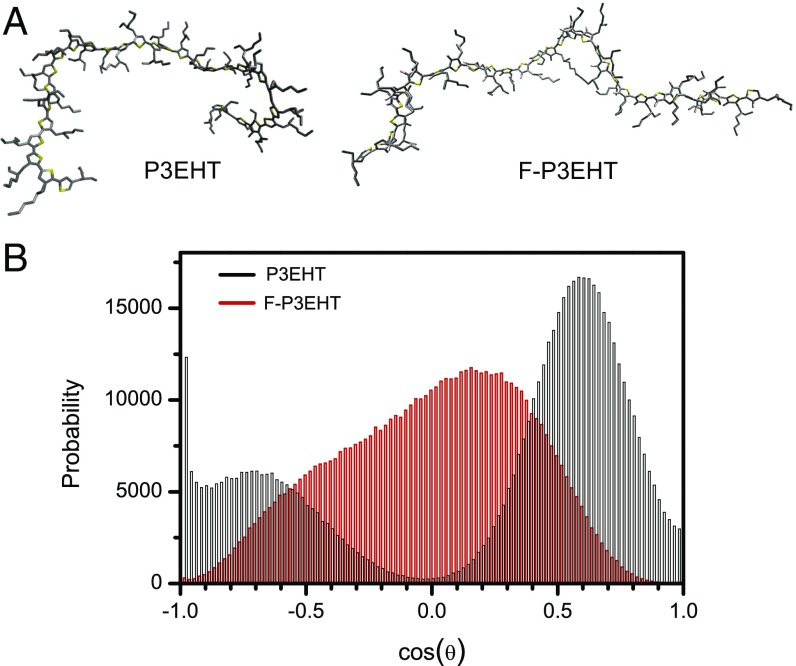
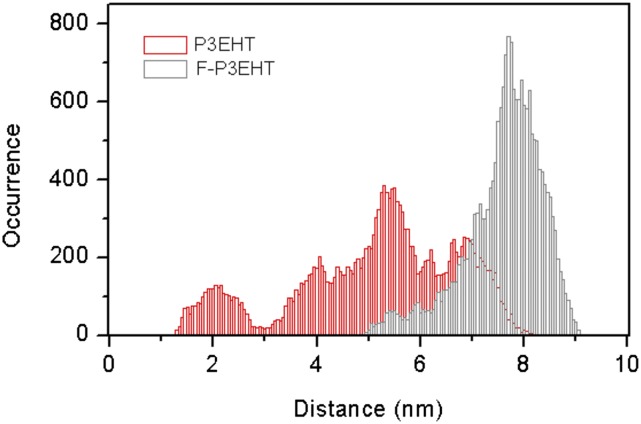
To further elucidate the role of fluorine atom substitution in impacting polymer chain morphology, we performed MD simulations with a classical potential. Please refer to SI Materials and Methods for details. For P3EHT, we use the optimized potentials for liquid simulations-all atom (OPLS-AA) model of DuBay et al. with standard OPLS description of the side chain (29). Modeling of the F-P3EHT required modifying both the partial charges and the dihedral potential due to the inclusion of the fluorine atom. The partial charges were taken from the charges from electrostatic potentials using a grid-based method (CHELPG) calculations on dimers and trimers (Table S1), a common method for fitting partial charges (30). The dihedral potential was fitted using the second-order Møller–Plesset perturbation theory (MP2) potential surface from the previous calculation (Figs. S1 and S2 and Table S2). For the MD simulation, 30-mers of P3EHT and F-P3EHT were simulated in a box of chloroform. Fig. 3A demonstrates the representative simulated structures of the 30-mers of P3EHT and F-P3EHT. By visual inspection, the F-P3EHT adopts more elongated configurations than P3EHT. This is verified by calculation of the end-to-end distance of the polymer during the simulation where the F-P3EHT end-to-end distance is more than 50% larger than for the P3EHT (Fig. S3). The greater elongation observed for the F-P3EHT compared with the P3EHT is also consistent with the modulation depth profiles observed in the single-molecule samples where the F-P3EHT consistently shows more anisotropic conformations as displayed in Fig. 2 A and D. Despite the elongation, the backbone dihedral distribution shown in Fig. 3B reveals that the F-P3EHT is decidedly nonplanar in solution with a broad dihedral angle θ-distribution and a maximum at 75°. In contrast, P3EHT exhibits a slightly skewed conformation as we observed for P3HT (26), although with a moderate preference for cis configurations.
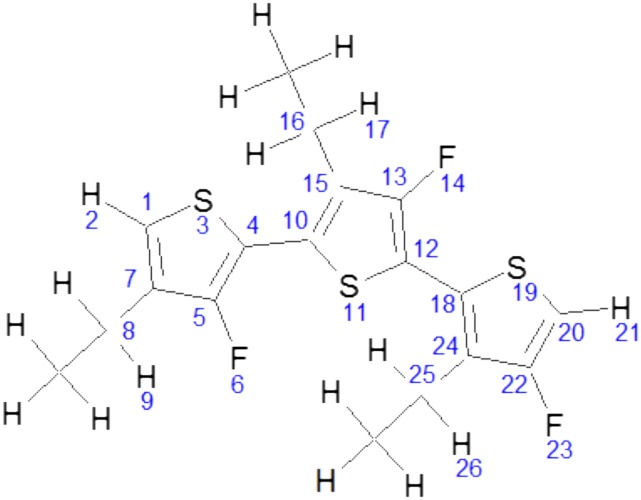
Table S1.
Partial charges of F-P3EHT used in the MD simulations
| Atom | q | Atom | q | Atom | q |
| 1 | −0.275 | 10 | 0.012 | 18 | −0.012 |
| 2 | 0.21 | 11 | −0.052 | 19 | −0.015 |
| 3 | −0.015 | 12 | −0.012 | 20 | −0.275 |
| 4 | −0.012 | 13 | 0.24 | 21 | 0.21 |
| 5 | 0.24 | 14 | −0.2 | 22 | 0.24 |
| 6 | −0.2 | 15 | −0.224 | 23 | −0.2 |
| 7 | −0.11 | 16 | 0.454 | 24 | −0.11 |
| 8 | 0.262 | 17 | −0.109 | 25 | 0.262 |
| 9 | −0.05 | 26 | −0.05 |
Fig. S1.
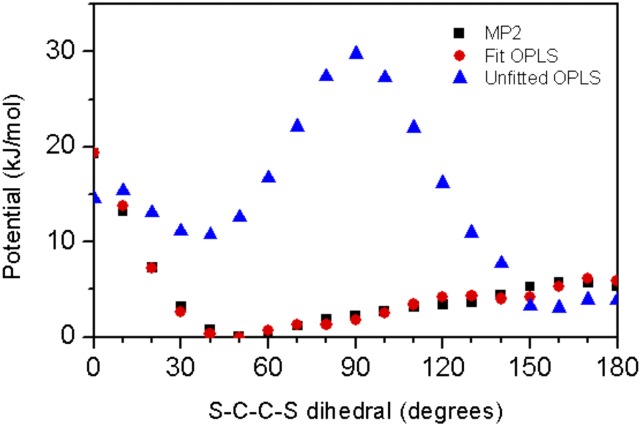
Results of the dihedral potential fit for F-P3EHT dimer structures produced by the relaxed dihedral scan. The unfitted OPLS potential uses the same dihedral potential as the P3EHT model, but with F-P3EHT partial charges. The fit OPLS potential includes dihedral terms to resolve the discrepancy between the unfit potential surface and electronic structure results.
Fig. S2.
Atom labels used for interpreting the partial charges of F-P3EHT listed in Table S1. Unlabeled atoms indicate that partial charges were not modified in the MD simulations.
Table S2.
Dihedral parameters used in the MD simulations for F-P3EHT
| Interring bond | C1 | C2 | C3 | C4 | C5 | C6 |
| S–C–C–S | −1.468 | 6.961 | −9.174 | −15.000 | 4.700 | 0.000 |
| S–C–C–C | −10.474 | 1.448 | 5.274 | −5.919 | −14.677 | 0.000 |
| C–C–C–C | −0.174 | −4.355 | 3.710 | 4.032 | −2.749 | 0.000 |
The three dihedrals listed here are for the interactions which contain two atoms in each of the neighboring rings. The constants listed are in the GROMACS standard of the Ryckaert–Bellemans potential, and are in units of kilojoule per mole.
Fig. 3.
(A) Representative structures of single chains taken from the explicit solvent trajectory. (B) Backbone dihedral distributions from the MD simulations of the 30-mers of P3EHT and F-P3EHT.
Fig. S3.
Distribution of end-to-end length for the 30-mers of P3EHT and F-P3EHT. The distance was calculated using the sulfur atom coordinates from the terminal monomer units on the 30-mer chains.
As shown in Fig. 1B for absorption and emission spectra, F-P3EHT is blue-shifted in solution but red-shifted in film relative to P3EHT. From the above simulation we think that F-P3EHT is less planar along the backbone than P3EHT in solution; this observation is consistent with the blue-shifted electronic transitions. In neat films, the spectrum can red-shift as a combined effect of the relatively flat dihedral potential surface of individual chains and the interchain packing facilitated by the more elongated initial structure of F-P3EHT. This is supported by the Raman data for films in our previous work (7). Additionally, F-P3EHT has a more ordered modulation depth profile than P3EHT (Fig. 2) for both single chains and aggregates while having blue- and red-shifted absorption spectra, respectively.
Excitonic Coupling.
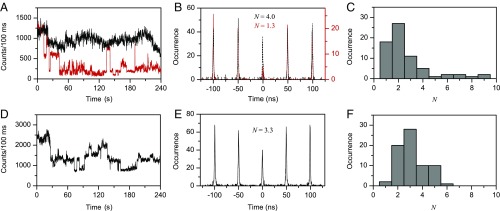
Interchain excitonic coupling was examined by performing fluorescence intensity time trace and photon correlation measurements on individual aggregates constructed with the SVA technique. Please refer to SI Materials and Methods for technique details. Note that the fluorescence transients were taken in the presence of a random generation of photochemical quenchers at high laser excitation power density, while photon correlation data were simultaneously collected until detectable photodegradation occurred. For P3EHT aggregates composed of 5 ± 1 chains, ∼65% of the aggregates exhibit gradual or approximately stepwise (type I) photodegradation behavior, whereas ∼35% show random and large intensity fluctuation, i.e., photoblinking or photobleaching (type II). Fig. 4A presents two exemplary fluorescence transients, shown as black and red curves, respectively, for these two types of aggregates. Corresponding photon correlation histograms taken for the same aggregates with a pulsed 473-nm laser at a repetition rate of 20 MHz are displayed in Fig. 4B. The number of emitters, N, in individual aggregates can be estimated by comparing the integrated area of the central peak at time 0 to the average area of the side peaks. It should be noted that to correctly estimate N the photon correlation analyses were performed before photodegradation. If N is significantly smaller than (close to) the number of chains estimated from SVA experiment, then we can deduce that there is efficient (no or limited) interchain coupling. For the type I aggregate with the transient shown in Fig. 4A, N is calculated to be ∼4.0 (Fig. 4B). Generally, P3EHT aggregates with type I degradation behavior have a broad distribution of N from 2 to 9, but with a dominant population around 2, which is smaller than the mean number of chains in the aggregates (Fig. 4C). This observation suggests a wide variety of interchain morphology and interaction for these types of aggregates, ranging from aggregates with several domains in which few chains are locally coupled, to those with individual component chains acting as discrete emitters. For the aggregates with large intensity fluctuation (type II, red trace in Fig. 4A), the central peak at correlation time 0 is greatly depressed relative to the side peaks, characteristic of photon antibunching as displayed in Fig. 4B (red). This type of aggregate usually contains only one or two emitters according to photon correlation measurements. We think that in these type II aggregates there exists a high ordering of interchain morphology either throughout the whole aggregates or within very few local domains. This ordered morphological characteristic facilitates effective interchain excitonic coupling or energy transfer––hence the observation of blinking behavior and photon antibunching as a signature of a single emitter in some of the type II aggregates.
Fig. 4.
Representative fluorescence intensity transients (A), photon correlation functions (B), and distribution histogram of number of emitters N (C), for P3EHT aggregates. (D–F) Corresponding data for F-P3EHT aggregates. For fluorescence trajectories in A and D, the background counts rate is ∼50 counts per 100 ms. The inset values of N in B and E are for respective photon correlation data shown in the panel.
Given that the F-P3EHT aggregates have a fairly high interchain ordering (Fig. 2E) and close interchain stacking distance of ∼3.8 Å (7, 31), one would anticipate an effective interchain excitonic coupling signified by fluorescence blinking and photon antibunching. Contrary to this expectation, typical (4 ± 1)-chain F-P3EHT aggregates exhibit a stepwise degradation. As shown for the aggregate in Fig. 4D, approximately four steps of degradation are observed and the N estimated from the photon correlation measurement for the same aggregate is 3.3 (Fig. 4E). For ∼70 aggregates examined, a mean N value of 3.5 is obtained as displayed in Fig. 4F. These results unambiguously suggest the absence of efficient excitonic coupling between F-P3EHT chains regardless of the high ordering and close packing between polymer chains. In conjugated polymers, excitonic coupling between adjacent chromophoric segments can occur not only along single-polymer chains but also between closely packed polymer chains. Prior studies on the excitonic coupling in CPs have revealed that there exists a competition between the interchain and intrachain coupling (2, 32, 33). With extended chain conformation, the intrachain coupling is improved; this weakens the interchain coupling because the interaction between the extended chains scales inversely with conjugation length (34). We think that the limited interchain excitonic coupling in F-P3EHT aggregates is primarily caused by the strong and dominant intrachain coupling due to its relatively extended backbone when the chains are packed. Our results show that although the fluorination of P3EHT backbone extends the backbone in the solid state and promotes interchain morphological order, the resultant structural features significantly restrain the excitonic coupling between chains. The observation made here is of importance in the rational design of potential fluorinated polymers for specific optoelectronic applications. For instance, the backbone fluorination on one hand improves the backbone and interchain morphology and therefore enhances the charge transport (7, 33). On the other hand, the resultant extended chain conformation turns out to be detrimental to the interchain excitonic coupling, which would therefore limit the 3D exciton delocalization and diffusion demanded for photovoltaic applications.
Fluorescence Spectra.
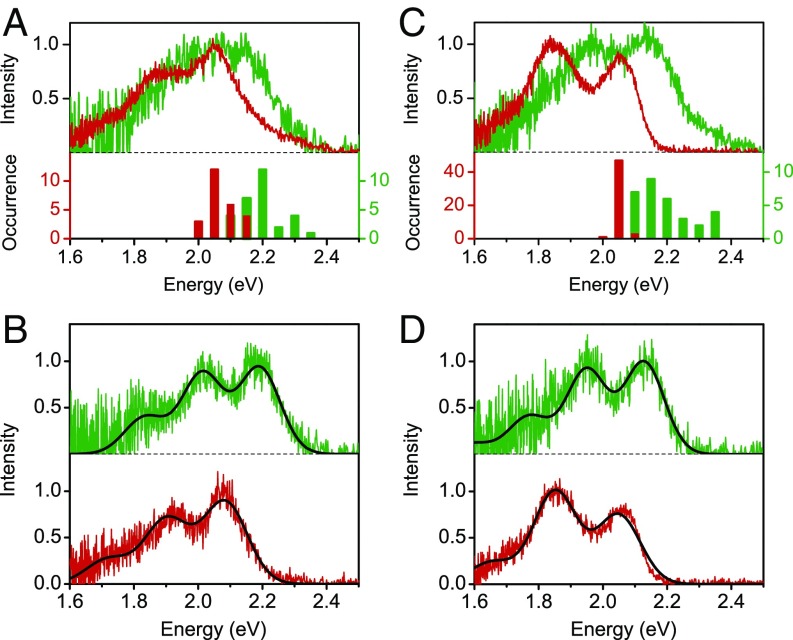
Next, we interrogated fluorescence emission spectral features to provide more detailed understanding about the consequence of fluorine substitution on the photophysics of CPs. Fig. 5A shows the ensemble spectra and the 0–0 transition energy () distributions of single chains and aggregates of P3EHT. Compared with the spectrum of single chains, the spectrum of aggregates is red-shifted, probably due to a slightly increased conjugation length (i.e., planarity) of the chains upon chain packing in aggregates. The broad distribution for both single chains and aggregates of P3EHT is indicative of their energetic inhomogeneity, consistent with their broad morphological variations. As exhibited in Fig. 5B, the spectra of single chains and aggregates of P3EHT can be satisfactorily fit with a single Franck–Condon progression (25, 35). Although two types of fluorescence transient behavior have been detected for P3EHT aggregates (Fig. 4A), we did not find two types of aggregate emission spectra. This is probably due to similar electronic structure of P3EHT chains in the aggregates and the weak excitonic coupling between polymer chains.
Fig. 5.
Ensemble fluorescence emission spectra of single chains (green) and aggregates (red) for P3EHT (A) and F-P3EHT (C). The histograms at the bottom of A and C present the 0–0 transition energy, E0, distribution for single chains (green) and aggregates (red). B and D exhibit a typical spectrum for single chain (green) and aggregate (red) of P3EHT and F-P3EHT, respectively. Corresponding fits (black) of the Franck–Condon model or the H-aggregate model are overlaid on the spectra in B and D.
With respect to F-P3EHT, from single chains to aggregates, a red shift of 0.1 eV in emission with a slight variation in vibronic structures is observed. Despite the ordered single-chain conformation, the F-P3EHT single chains are energetically heterogeneous. As displayed in Fig. 5C, single F-P3EHT chains exhibit a broad distribution in values of , from 2.1 to 2.4 eV, analogous to morphologically disordered P3EHT chains. This observation is presumably due to a variety of chromophore sizes as a result of the relatively small interring dihedral angle in F-P3EHT as we discussed above. Most notably, however, the distribution of F-P3EHT aggregates is much narrower than that of single chains. One might think that this could result from energy funneling to low-energy sites in aggregates. However, this seems unlikely because photon antibunching, or at least a smaller number of emitters than composing chains, should be expected in this case, but these were not observed in the photon correlation measurement (Fig. 4 E and F). Instead, we believe that the narrower distribution of implies that when packed together in the aggregates, individual chains act as discrete emitters that are all nearly identical in energy. Whereas often we assume that interchain interaction in the solid state leads to an array of interactions and broad distribution in electronic energies, in the case of the F-P3EHT aggregates the interactions between the chains significantly reduce the heterogeneity by increasing the intrachain coupling along each chain.
Although the emission spectra of F-P3EHT single chains can be fit with a single Franck–Condon progression (Fig. 5D), such a fit does not work well for the spectra of F-P3EHT aggregates and film. The dampened 0–0 emission in the aggregate and film spectra signifies the formation of H aggregation. The “H-like” emission of F-P3EHT aggregates and film seems contradictory to the “J-like” absorption of film (Fig. 1B). However, such an asymmetry actually has been demonstrated both theoretically and experimentally. First, according to Spano’s model, the 0–0/0–1 ratio in absorption is related to the exciton bandwidth, whereas the 0–0/0–1 ratio in emission is related to the exciton coherence (that is, it is more sensitive to the subtle competition between the intrachain and interchain coupling) (32). Second, compared with the absorption, the emission undergoes fast dynamic processes. Paquin et al. (36) and Parkinson et al. (37) both have revealed a dynamic evolution from an initial vibrationally hot excited state (low symmetry) to a geometrically relaxed state (high symmetry) (i.e., dynamic torsional planarization of the backbones) for P3HT, which results in a dynamic and strong decrease of the relative 0–0 intensity within ∼10 ps. With time-resolved absorption measurements, Ade and co-workers have reported the interchain coupling involves a dynamic process evolving from J-like to H-like within a few hundreds of picoseconds for P3HT in polyethylene oxide matrix* (38).
In the H-aggregate model (25, 36), the 0–0 transition from the first excited state to the ground state is symmetry forbidden and the emission can be described by a modified Franck–Condon progression:
where is the scaling factor that quantifies the amplitude of the 0–0 transition, is the refraction index of the surrounding environment, is the vibration level, is the Gaussian line-width function that represents the inhomogeneously broadened spectral line of the vibronic replica, and represent the energy of the 0–0 transition and carbon–carbon stretching vibration (∼0.18 eV), respectively, and denotes the Huang–Rhys factor (which represents the coupling strength between the electronic transition and a phonon mode). Fig. 5D displays a typical spectrum of a single F-P3EHT aggregate fit with the H-aggregate model. The obtained Huang–Rhys factor for aggregates is 0.7 ± 0.2, which is consistent with 0.7 obtained for the F-P3EHT film and is much smaller than the value of 1.3 ± 0.2 obtained for F-P3EHT single chains. This suggests an increase in the intrachain coupling and that the HJ-aggregate model, in which the intrachain and the interchain coupling are treated equally, is more accurate in describing the F-P3EHT (36). There are several key findings that support this view. First, as shown in Fig. 1B, the 0–0 band in the F-P3EHT film absorption spectrum is much stronger than that in typical P3HT films (25, 36), implying a stronger intrachain coupling in F-P3EHT that is consistent with the assertion of the extended F-P3EHT polymer chains. Second, the I0–0/I0–1 ratio in F-P3EHT film emission is about 0.75 according to Fig. 1, which is ∼2–3× higher than what is typically measured in H-like P3HT films (25, 36). In addition, the Stokes shift of F-P3EHT lies in between that of the H-like P3HT film/aggregates and the much more highly ordered J-like P3HT nanofibers (24). These two observations suggest weaker interchain coupling in F-P3EHT than in the H-like P3HT films, which is in alignment with the limited exciton coupling in F-P3EHT deduced from the photon correlation measurement. Collectively, we think the spectral data of F-P3EHT aggregates and film can be reasonably described by the HJ-aggregate model and support the conclusion of a more extended F-P3EHT backbone even with the H-like emission.
Conclusion
The influence of backbone fluorination in conjugated polymers on morphology and excitonic coupling at the molecular and nanoscale levels were studied with two prototypical polythiophenes, P3EHT and F-P3EHT. Due to the presence of bulky ethylhexyl side chains in P3EHT, there is a broad variety of morphology for both single chains and nanoscale aggregates. Because of the morphological variability in P3EHT aggregates, the excitonic coupling between polymer chains is widely distributed but generally not efficient, i.e., from localized coupling between only a few chains to strongly limited. Fluorescence excitation polarization experiments in combination with MD revealed that backbone fluorination greatly extends the single-chain conformation of P3EHT. Consequently, the fluorinated single chains can assemble to form aggregates with a high extent of interchain ordering. Despite the ordered and close interchain packing in F-P3EHT, the interchain excitonic coupling is surprisingly inhibited. This is attributed to the dominant and competitive coupling along F-P3EHT chains due to fluorine-induced extended backbone. Furthermore, the highly ordered packing in F-P3EHT surprisingly leads to a reduction in the energetic heterogeneity of the individual chains as the intrachain coupling is increased. This provides the unusual result that the ensemble of chains in the aggregate has a narrower distribution of energies than the corresponding distribution of individual polymer chains. Our study pinpoints the importance of thorough and systematic efforts to understand the role that structure and morphology play in determining how inter- and intrachain excitonic coupling affect the electronic properties of conjugated polymers for optoelectronic applications.
Materials and Methods
The synthesis of P3EHT and F-P3EHT was reported in our previous work (7). The crude polymers were further fractionated to obtain a molecular mass of 13 kDa and dispersity of 1.1. Single-molecule samples were spin cast from diluted chloroform solutions containing 2 wt % PMMA on clean coverslips to form PMMA films with conjugated polymer chains embedded inside. The aggregates were prepared via the SVA technique with a vapor ratio of 38/62 of solvent mixture of chloroform and acetone that was detailed previously (2). Single-molecule/aggregate spectroscopy was performed on a typical wide-field and laser scanning confocal microscope (2). In order to do MD simulations, we first performed electronic structure oligomer optimizations and classical force-field parameter derivation. MD simulations included a 30-mer of F-P3EHT or P3EHT in explicit chloroform solvent. All simulations were calculated with Gromacs v4.6.7 (39) using the OPLS-AA force field (40). More details of all materials, methods, and procedures can be found in SI Materials and Methods.
SI Materials and Methods
P3EHT and Fluorinated Polymer F-P3EHT.
The synthesis of P3EHT and F-P3EHT was reported in our previous work (7). The crude polymers after synthesis were further fractionated using gel-permeation chromatography (Viscotek, GPCmax VE-2001) to obtain a number average molecular mass of 13 kDa and dispersity (Ð) of 1.1. This molecular weight was chosen to avoid possible intramolecular folding-induced interchain interaction. PMMA (number average molecular mass = 45 kDa, Ð = 2.2) was purchased from Sigma-Aldrich. Dry solvents including toluene, acetone, chloroform, and chlorobenzene were purchased from Acros Organics. Glass coverslips were cleaned in an acid piranha solution (1:3 vol/vol hydrogen peroxide and sulphuric acid) and thoroughly rinsed with nanopure water before use.
Single Molecule and Aggregate Samples Preparation.
Single-molecule (SM) samples and concentrated precursor samples used for the preparation of aggregates via the SVA method were spin cast in a N2-filled glove box from chloroform solutions containing 2 wt % PMMA on clean coverslips to form 200-nm-thick PMMA films with conjugated polymer chains embedded inside. For SM samples, in the field of view of ∼40 µm in diameter under wide-field microscope the number of CP molecules is typically ∼40 to avoid possible agglomeration. The number of chains (∼430 in the field of view) in the precursor concentrated SM samples used for aggregate preparation was calculated using the dilution factor based on the number of chains observed in more dilute SM samples. The polythiophene aggregates were then prepared using the SVA technique with a vapor ratio of 38/62 of solvent mixture of chloroform and acetone (volume ratio of 55/45) that was detailed previously (2). The number of chains in polymer aggregates (i.e., the size of aggregates) was estimated based on the change in the number of emitting spots before and after SVA. An intensity threshold above the intensity of single chains was applied to the SVA-treated samples to measure the number of aggregates.
Single-Molecule/Aggregate Spectroscopy.
The wide-field optical microscope uses an inverted microscope (Zeiss, Axiovert 200) with a 1.25-N.A. objective lens (Zeiss, Achrostigmat, 100×, oil immersion) A collimated laser beam at 457 nm (DPSS laser, MBL-F-457–10mW, Changchun New Industries Optoelectronics Tech. Co., Ltd.) with a power density of ∼1.5 W/cm2 was used to excite the molecules in an area with a diameter of ∼40 μm. For the fluorescence excitation polarization experiment, a rotating linearly polarized excitation light was obtained via a combination of an electrooptical modulator (Fastpulse Technology, model 3079–4) and a quarter-wave plate. The fluorescence signal was filtered with a dichroic mirror and 473-nm edge filter (Chroma) and then detected with an electron multiplying charge-coupled device (EMCCD) detector (Andor, model iXon+ DU-897E) under a wide-field detection scheme. The fluorescence spectra were taken under sample-scanning confocal mode using the same microscope and objective lens as in the wide-field microscopy mode. The emission spectra were taken at excitation power of ∼30 W/cm2. The emitted light was filtered with a 473-nm blocking edge long-pass filter (Semrock) and delivered through a spectrograph (Princeton Instruments Acton SP-150) that is coupled to a liquid N2-cooled CCD camera (Princeton Instruments). The spectrum of SM or aggregate was obtained by averaging over four consecutive spectra collected with an integration time of 20 s each.
A setup similar to a Hanbury Brown-Twiss type of setup was used to perform the photon correlation experiments with the optical microscope under confocal mode. The excitation source was changed to a 20-MHz, 473-nm pulsed laser (BDL-473-SMC, Becker & Hickl GmbH) and a 1.4-N.A. objective lens (Zeiss, Plan-Apochromat, 100×, oil immersion) was used to improve the collection efficiency. The fluorescence emission, cleaned with a 514-nm long-pass edge filter, was split and detected on two single-photon avalanche photodiodes (APDs) (PerkinElmer, SPCM-AQR-15). The signals from the APDs were fed into a time-correlated single-photon-counting SPC-830 card (Becker & Hickl) with one APD delayed by longer cable to place the coincidence point in the center of the recorded time interval. To estimate the number of emitters in the aggregates or molecules, the discrete photon correlation peaks were integrated and the number N was calculated by , where and represent the area of the central peak and the averaged area of the side peaks, respectively. The photon correlation analyses were only performed before photodegradation to estimate the number of emitters correctly.
The fluorescence transients were collected with the same APDs and recorded with a separate photon counter (PMS-400, Becker & Hickl). These measurements were conducted under N2, with a high laser power density (∼100 W/cm2) to induce photodegradation behavior due to generation of photochemical quenchers. It should be noted that fluorescence degradation events were intentionally initiated under high laser power excitation, whereas under normal imaging power excitation (∼30 W/cm2) the majority of aggregates are stable, without fast photodegradation.
Theoretical Calculations.
T1: Electronic structure oligomer optimizations.
To gain a basic understanding of the behavior of F-P3EHT and gauge the feasibility of using existing classical potentials to model a chain, we calculated relaxed structures for oligomers consisting of 2-5 monomer units. We examined methyl- and ethyl-substituted fluorothiophene, and used both DFT and post-Hartree–Fock methods in B3LYP and MP2 model chemistries, respectively. In agreement with the results of Fei et al. (7), methyl-substituted oligomers of length greater than or equal to 4 monomer units adopt a planar conformation in the methyl-substituted B3LYP/6–31+G(d) calculations. Interestingly, ethyl-substituted oligomers adopt decidedly nonplanar confirmations, with dihedral angles in excess of 30°. Additionally, MP2 calculations adopt similarly nonplanar structures for all combinations of methyl- and ethyl substitution and oligomer length from 2 to 5 monomer units. This apparent dichotomy is the result of a local minimum for the trans planar structures in F-P3EHT oligomers. This is much more pronounced in methyl- than ethyl-substituted oligomers, and in B3LYP versus MP2 model chemistries.
T2: Classical force-field parameter derivation.
The results from the electronic structure relaxations of the fluorinated oligomers indicated that modifications to existing polythiophene potentials were required for MD simulation. The two areas which we sought to replace existing potentials were the partial charge distribution and dihedral parameters. The remaining parameters were taken from that derived by DuBay et al. (29) with P3EHT parameters being used unmodified.
Partial charges were taken as a composite of the charges from electrostatic potentials using a grid-based method (CHELPG) calculations on relaxed dimer and trimer fluoropolymer structures (30). The aliphatic side chain was reduced to an ethyl group for ease of computation. For the trimer and dimer, calculations were performed using the Becke-3-Lee-Yang-Parr density functional theory with the 6-311G(d, p) basis set [B3LYP/6-311G(d,p)]. For the dimer, additional calculations were performed with the second-order Møller–Plesset perturbation theory (MP2) using the 6-311G (d, p) basis set and the B3LYP with aug-cc-pvtz basis set, which collectively did not demonstrate large variations in partial charge estimates. We required that each monomer unit be net neutral, and that standard optimized potentials for liquid simulations (OPLS) charges were to be used for all aliphatic carbons and hydrogens save for the first carbon/hydrogen unit off of the thiophene ring.
Using the new partial charges, modified dihedral parameters were determined by fitting a relaxed dihedral scan. The scan itself was performed by increasing the S–C–C–S dihedral angle in an ethyl-substituted fluorothiophene dimer using the resolution of the identity (RI) approximation MP2 (RI-MP2)/aug-cc-pvdz. The dihedral was varied from 0 to 180° in increments of 10°. The three unique dihedral parameters which determine backbone twisting (S–C–C–S, S–C–C–C, and C–C–C–C) were fitted using a genetic algorithm. The size of the parameter space was limited to prohibit parameters from drifting into unphysical regions. The success of the fit can be seen in Fig. S1, and information regarding the values used for molecular dynamics simulations are found in Fig. S2 and Tables S1 and S2.
T3: Classical simulation protocol.
A Gromacs/4.6.7 simulation (39) of a 30-mer of F-P3EHT or P3EHT in explicit OPLS-AA (40) chloroform solvent was prepared as follows. An outstretched initial configuration of the polymer was solvated with chloroform and energy minimized with 10,000 steps of steepest descent and a time-step of 2 fs. A conjugant gradient minimization followed, with convergence achieved in roughly 1,200 steps for both polymers. The box size was equilibrated with a 1-ns isothermal–isobaric (NPT) simulation, and the output was then scaled to the equilibrium value as determined by the fluctuations in the second half of the NPT trajectory. A 1-ns trajectory in the canonical (NVT) ensemble was then performed as a final preparatory step, before a production run of 20 ns. Configurations were saved every picosecond from the final trajectory, and used for postprocessing.
Acknowledgments
This work was supported by National Science Foundation Grant CHE-1310222 (to D.A.V.B.), National Science Foundation Grant CHE-1362381(to P.J.R.), Robert A. Welch Foundation Grant F-0019 (to P.J.R.), and a doctoral training grant through the Engineering and Physical Sciences Research Council (to M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Gautam B, et al., APS March Meeting 2014, March 3–7, 2014, Denver, CO, abstr BAPS.2014.MAR.T20.2.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620722114/-/DCSupplemental.
References
- 1.Hwang I, Scholes GD. Electronic energy transfer and quantum-coherence in pi-conjugated polymers. Chem Mater. 2011;23:610–620. [Google Scholar]
- 2.Hu Z, et al. Excitonic energy migration in conjugated polymers: The critical role of interchain morphology. J Am Chem Soc. 2014;136:16023–16031. doi: 10.1021/ja508112k. [DOI] [PubMed] [Google Scholar]
- 3.Noriega R, Salleo A, Spakowitz AJ. Chain conformations dictate multiscale charge transport phenomena in disordered semiconducting polymers. Proc Natl Acad Sci USA. 2013;110:16315–16320. doi: 10.1073/pnas.1307158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiessen A, et al. Unraveling the chromophoric disorder of poly(3-hexylthiophene) Proc Natl Acad Sci USA. 2013;110:E3550–E3556. doi: 10.1073/pnas.1307760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perepichka IF, Perepichka DF, Meng H, Wudl F. Light-emitting polythiophenes. Adv Mater. 2005;17:2281–2305. [Google Scholar]
- 6.Jackson NE, et al. Controlling conformations of conjugated polymers and small molecules: The role of nonbonding interactions. J Am Chem Soc. 2013;135:10475–10483. doi: 10.1021/ja403667s. [DOI] [PubMed] [Google Scholar]
- 7.Fei Z, et al. Influence of backbone fluorination in regioregular poly(3-alkyl-4-fluoro)thiophenes. J Am Chem Soc. 2015;137:6866–6879. doi: 10.1021/jacs.5b02785. [DOI] [PubMed] [Google Scholar]
- 8.Son HJ, et al. Synthesis of fluorinated polythienothiophene-co-benzodithiophenes and effect of fluorination on the photovoltaic properties. J Am Chem Soc. 2011;133:1885–1894. doi: 10.1021/ja108601g. [DOI] [PubMed] [Google Scholar]
- 9.Takeda Y, Andrew TL, Lobez JM, Mork AJ, Swager TM. An air-stable low-bandgap n-type organic polymer semiconductor exhibiting selective solubility in perfluorinated solvents. Angew Chem Int Ed Engl. 2012;51:9042–9046. doi: 10.1002/anie.201204066. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Deng Z, Li C, Chen L, Chen Y. Structure evolution of fluorinated conjugated polymers based on benzodithiophene and benzothiadiazole for photovoltaics. J Phys Chem C. 2015;119:8038–8045. [Google Scholar]
- 11.Boufflet P, et al. Using molecular design to increase hole transport: backbone fluorination in the benchmark material poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]-thiophene (pBTTT) Adv Funct Mater. 2015;25:7038–7048. [Google Scholar]
- 12.Baran D, et al. Reducing the efficiency-stability-cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat Mater. 2017;16:363–369. doi: 10.1038/nmat4797. [DOI] [PubMed] [Google Scholar]
- 13.Bin H, et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat Commun. 2016;7:13651. doi: 10.1038/ncomms13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharber MC. On the efficiency limit of conjugated polymer:Fullerene-based bulk heterojunction solar cells. Adv Mater. 2016;28:1994–2001. doi: 10.1002/adma.201504914. [DOI] [PubMed] [Google Scholar]
- 15.Chen J-D, et al. Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv Mater. 2015;27:1035–1041. doi: 10.1002/adma.201404535. [DOI] [PubMed] [Google Scholar]
- 16.Cornil J, dos Santos DA, Crispin X, Silbey R, Bredas JL. Influence of interchain interactions on the absorption and luminescence of conjugated oligomers and polymers: A quantum-chemical characterization. J Am Chem Soc. 1998;120:1289–1299. [Google Scholar]
- 17.Chang MH, Frampton MJ, Anderson HL, Herz LM. Intermolecular interaction effects on the ultrafast depolarization of the optical emission from conjugated polymers. Phys Rev Lett. 2007;98:027402. doi: 10.1103/PhysRevLett.98.027402. [DOI] [PubMed] [Google Scholar]
- 18.Spano FC, Silva C. H- and J-aggregate behavior in polymeric semiconductors. Annu Rev Phys Chem. 2014;65:477–500. doi: 10.1146/annurev-physchem-040513-103639. [DOI] [PubMed] [Google Scholar]
- 19.Vogelsang J, Adachi T, Brazard J, Vanden Bout DA, Barbara PF. Self-assembly of highly ordered conjugated polymer aggregates with long-range energy transfer. Nat Mater. 2011;10:942–946. doi: 10.1038/nmat3127. [DOI] [PubMed] [Google Scholar]
- 20.Vogelsang J, Lupton JM. Solvent vapor annealing of single conjugated polymer chains: Building organic optoelectronic materials from the bottom up. J Phys Chem Lett. 2012;3:1503–1513. doi: 10.1021/jz300294m. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Liu J, Simón-Bower L, Zhai L, Gesquiere AJ. Influence of backbone rigidness on single chain conformation of thiophene-based conjugated polymers. J Phys Chem B. 2013;117:4461–4467. doi: 10.1021/jp308497k. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, et al. Insight into how molecular structures of thiophene-based conjugated polymers affect crystallization behaviors. Polymer (Guildf) 2011;52:2302–2309. [Google Scholar]
- 23.Hu Z, et al. An insight into non-emissive excited states in conjugated polymers. Nat Commun. 2015;6:8246. doi: 10.1038/ncomms9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niles ET, et al. J-aggregate behavior in poly-3-hexylthiophene nanofibers. J Phys Chem Lett. 2012;3:259–263. [Google Scholar]
- 25.Clark J, Silva C, Friend RH, Spano FC. Role of intermolecular coupling in the photophysics of disordered organic semiconductors: Aggregate emission in regioregular polythiophene. Phys Rev Lett. 2007;98:206406. doi: 10.1103/PhysRevLett.98.206406. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, et al. Effect of the side-chain-distribution density on the single-conjugated-polymer-chain conformation. ChemPhysChem. 2013;14:4143–4148. doi: 10.1002/cphc.201300751. [DOI] [PubMed] [Google Scholar]
- 27.Adachi T, et al. Highly ordered single conjugated polymer chain rod morphologies. J Phys Chem C. 2010;114:20896. [Google Scholar]
- 28.Adachi T, et al. Regioregularity and single polythiophene chain conformation. J Phys Chem Lett. 2011;2:1400–1404. [Google Scholar]
- 29.DuBay KH, et al. Accurate force field development for modeling conjugated polymers. J Chem Theory Comput. 2012;8:4556–4569. doi: 10.1021/ct300175w. [DOI] [PubMed] [Google Scholar]
- 30.Breneman CM, Wiberg KB. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem. 1990;11:361–373. [Google Scholar]
- 31.Himmelberger S, et al. Role of side-chain branching on thin-film structure and electronic properties of polythiophenes. Adv Funct Mater. 2015;25:2616–2624. [Google Scholar]
- 32.Yamagata H, Spano FC. Interplay between intrachain and interchain interactions in semiconducting polymer assemblies: The HJ-aggregate model. J Chem Phys. 2012;136:184901. doi: 10.1063/1.4705272. [DOI] [PubMed] [Google Scholar]
- 33.Gierschner J, et al. Excitonic versus electronic couplings in molecular assemblies: The importance of non-nearest neighbor interactions. J Chem Phys. 2009;130:044105. doi: 10.1063/1.3065267. [DOI] [PubMed] [Google Scholar]
- 34.Barford W. Exciton transfer integrals between polymer chains. J Chem Phys. 2007;126:134905. doi: 10.1063/1.2714516. [DOI] [PubMed] [Google Scholar]
- 35.Ho PKH, Kim JS, Tessler N, Friend RH. Photoluminescence of poly(p-phenylenevinylene)-silica nanocomposites: Evidence for dual emission by Franck-Condon analysis. J Chem Phys. 2001;115:2709–2720. [Google Scholar]
- 36.Paquin F, et al. Two-dimensional spatial coherence of excitons in semicrystalline polymeric semiconductors: Effect of molecular weight. Phys Rev B. 2013;88:155202. [Google Scholar]
- 37.Parkinson P, Mueller C, Stingelin N, Johnston MB, Herz LM. Role of ultrafast torsional relaxation in the emission from polythiophene aggregates. J Phys Chem Lett. 2010;1:2788–2792. [Google Scholar]
- 38.Hellmann C, et al. Controlling the interaction of light with polymer semiconductors. Adv Mater. 2013;25:4906–4911. doi: 10.1002/adma.201300881. [DOI] [PubMed] [Google Scholar]
- 39.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen WL, Maxwell DS, Tirado-Rives J. Development and testing of the OPLS All-Atom Force Field on conformational energetics and properties of organic liquids. J Am Chem Soc. 1996;118:11225–11236. [Google Scholar]