Significance
Vimentin is a marker for the epithelial-to-mesenchymal transition, which is thought to lead to cancer metastasis. Without vimentin, planar cells exhibit polarity defects and cannot migrate as single cells. The mechanisms by which vimentin influences cell migration remain mostly unknown, however. Generally, intermediate filaments regulate the mechanical integrity of cells and tissues; thus, we hypothesized that vimentin could be involved in supporting the directionally aligned force transmission required for single-cell migration. We have developed imaging and analysis methods to probe the role of vimentin in mesenchymal cell migration that will have broad utility in intermediate filament research. In summary, our results demonstrate that vimentin governs the alignment of the cell traction forces needed for directed single-cell mesenchymal migration.
Keywords: vimentin, fiber orientation, traction stress, intermediate filaments, mesenchymal migration
Abstract
The intermediate filament vimentin is required for cells to transition from the epithelial state to the mesenchymal state and migrate as single cells; however, little is known about the specific role of vimentin in the regulation of mesenchymal migration. Vimentin is known to have a significantly greater ability to resist stress without breaking in vitro compared with actin or microtubules, and also to increase cell elasticity in vivo. Therefore, we hypothesized that the presence of vimentin could support the anisotropic mechanical strain of single-cell migration. To study this, we fluorescently labeled vimentin with an mEmerald tag using TALEN genome editing. We observed vimentin architecture in migrating human foreskin fibroblasts and found that network organization varied from long, linear bundles, or “fibers,” to shorter fragments with a mesh-like organization. We developed image analysis tools employing steerable filtering and iterative graph matching to characterize the fibers embedded in the surrounding mesh. Vimentin fibers were aligned with fibroblast branching and migration direction. The presence of the vimentin network was correlated with 10-fold slower local actin retrograde flow rates, as well as spatial homogenization of actin-based forces transmitted to the substrate. Vimentin fibers coaligned with and were required for the anisotropic orientation of traction stresses. These results indicate that the vimentin network acts as a load-bearing superstructure capable of integrating and reorienting actin-based forces. We propose that vimentin's role in cell motility is to govern the alignment of traction stresses that permit single-cell migration.
Vimentin is a marker for cancer metastasis and is required for single-cell mesenchymal migration (1). Vimentin is known to interact with both actin and microtubules (MTs) (2, 3), two cytoskeleton polymers that have been well studied in the context of migration. Actin assembly and contraction provide the propulsive and traction forces required for forward translocation of cells (4–6), while at the same time, MTs determine cell orientation (7). Vimentin has been proposed to structurally integrate these networks (8, 9); however, how this occurs in the process of cell migration when the interacting cytoskeletons are under permanent turnover and reorganization is unknown, owing mainly to the lack of appropriate live cell imaging studies and image analysis tools for inferring underlying mechanisms.
Previous work demonstrated that injection of dominant-negative nonpolymeric vimentin into a fibroblast resulted in local lamellipod formation (10), a region of rapid actin flow. Recently, vimentin expression was shown to affect the position of the lamellar arc, and also to be dependent on lamellar actin-binding proteins for distribution (11). Moreover, our recent study in epithelial cells undergoing an epithelial-to-mesenchymal transition (EMT) found that vimentin tightly integrated with MTs in a positive feedback loop, in which MTs support the assembly of a vimentin network, which in turn templates MT growth to confer persistence in cell polarity (12). Taken together, the foregoing studies suggest that vimentin's ability to interact with both MTs and actin could reinforce cell polarity and directed migration.
Similar to other intermediate filaments, vimentin structure is essential for the mechanical integrity of cells and tissues. In vitro rheology measurements have demonstrated that in contrast to MTs and actin, the vimentin network is both easily deformable and able to withstand high strains without breaking (13). Vimentin also increases cytoplasmic elasticity in vivo (14). Interestingly, whereas MTs and actin polymerize unidirectionally, vimentin filaments polymerize both longitudinally and laterally without polarity (15). This capacity for variable spatial regulation of vimentin density suggests that the architecture of the network could be a key determinant of its function.
To test this hypothesis, we sought to monitor the structure of the vimentin network in a native mesenchymal environment. We used transcription activator-like effector nuclease (TALEN)-based genome modification to express fluorescent-labeled vimentin in nonimmortalized human foreskin fibroblasts (hFFs). We found that the vimentin network exhibited both longer continuous regions, resembling fibers, as well as shorter, fragmented, mesh-like regions. We have developed image analysis tools to perform orientation analysis on the fibers, and found that these fibers were aligned with fibroblast branching and migration direction. The vimentin network also was spatially correlated with the slowest actin retrograde flow, and regulated the homogeneity of traction force. Finally, we found that vimentin fibers were required for traction stress orientation. Individual fibers were locally aligned with traction stresses, and vimentin level determined the degree of anisotropy in traction stress orientation. These results indicate that the presence of vimentin governs the orientation of traction forces in mesenchymal cells.
Results
Identification of Vimentin Fibers.
We sought to accurately characterize the structure of the vimentin network in living cells while minimizing as much as possible perturbation owing to the overexpression and tagging of vimentin. We introduced the mEmerald gene at the N terminus of the endogenous vimentin locus in nonimmortalized human foreskin fibroblasts (hFFs) using TALENs (12, 16) (Methods). The fraction of mEmerald-vimentin was 4 ± 4% of the total vimentin in edited cells (Fig. S1A). TALEN-edited hFFs migrated at the same speed as control cells (Fig. S1B), and no differences in morphology or vimentin distribution were detected between wild-type (WT) and TALEN-edited hFFs (Fig. S2).
Fig. S1.
TALEN-edited mEmerald-vimentin expression and cell migration in primary hFFs. (A, Top) Western blot quantification of vimentin protein expression in WT and TALEN- modified cells. Cell lysates from WT and genome-edited hFFs were twofold serially diluted, and six samples from the dilution series for WT and edited lysates were run and blotted on the same membrane. Membranes were probed with anti-vimentin together with loading control antibodies for GAPDH for normalization. Blots were scanned on an infrared imaging system, and band intensities were quantified with Image Studio Light 4.0 software (LI-COR). Scans for mouse anti-Vimentin (clone V9; Sigma-Aldrich) and rabbit anti-GAPDH (rabbit GTX100118; GeneTex) are shown. The expected molecular weight of vimentin is 54 kDa, that of mEmerald-vimentin is 70 kDa, and that of GAPDH is 37 kDa. Intensities for bands corresponding to untagged and fusion proteins and the sum of both are plotted. The band intensities suggest that vimentin protein levels are roughly equal in parental and edited cells, and that approximately 10% of vimentin is tagged with fluorescent protein mEmerald. (A, Bottom) Results were confirmed with a second rabbit antibody against the C-terminal peptide of vimentin (C20; Santa Cruz Biotechnology). The GAPDH (mouse GT239; GeneTex) loading control from the same blot is shown. Compared with the parental cell line, in the edited cells, mean vimentin expression was 105 ± 54% (n = 9 blots, two different antibodies). In the edited hFF cell line, 4 ± 4% vimentin was tagged with mEmerald. This could indicate that the fusion protein was repressed or less stable and subject to proteolytic degradation. The high SDs reflect the variation between blots and between the different antibodies. (B, Left) TALEN-edited cells migrated at the same speed as control lentiviral cells, whereas lentiviral knockdown significantly decreased migration speed (P < 0.01). n = 100 cells for each variable. Cells were plated on glass-bottom MatTek dishes coated with 10 μg/mL fibronectin and imaged concurrently for 8 h in an ambient-controlled chamber. Values are mean from four experiments. (B, Right) Western blot of vimentin (clone V9; Sigma-Aldrich) and rabbit anti-GAPDH (GTX100118; GeneTex) from control and vimentin kd lentivirus-transfected cells. The average knockdown efficiency in two separate experiments was 50%.
Fig. S2.
WT hFFs (A) and TALEN genome-edited hFFs (B) appear similar in morphology and vimentin distribution. Cells were plated on glass coated with fibronectin and allowed to spread for 4 h before fixation and staining with anti-vimentin antibody. (Scale bar: 10 μm.)
We then imaged the mEmerald-vimentin network organization in hFFs at high resolution using spinning disk confocal microscopy (Fig. 1A). We observed that polymer density and organization varied from short, mesh-like randomly oriented fragments to longer, linear bundles (Fig. 1B, left to right). To assess this complex organization, we developed image analysis tools to distinguish the more bundled, fibrous regions from the remaining network mesh. In a first step, we used a previously published algorithm (12) to identify the structure of the network. In brief, from the raw image (Fig. 1C), we thresholded the background and applied a multiscale steerable filter (17) to detect curvilinear features (12) (Methods). The resulting filter response map (Fig. 1D) described the confidence for each pixel in belonging to the vimentin network. We next applied nonmaximum suppression (12, 18) to skeletonize the vimentin network. This was followed by a graph-based connection algorithm that assembled, based on their proximity and end-to-end orientation, curvilinear skeleton segments into a complete network (12), the result of which is a map of vimentin fragments of various lengths (Fig. 1E).
Fig. 1.
Characterization of the vimentin network by computational image processing. (A) Z-stack maximum intensity projection of mEmerald-vimentin distribution within an hFF cell. (B) Vimentin mesh (Left and Middle images) and fibrous architecture (Middle and Right images). All images are z-stack maximum intensity projections of mEmerald-vimentin in three representative cells. (Insets) 2× zoom of the areas of detail. (C) Raw spinning disk confocal image of mEmerald-vimentin in an hFF cell. (D) Multiscale steerable filtering was applied to the image in C to enhance curvilinear features. (E–G) Extraction of vimentin fibers. (E) Nonmaximum suppression was applied to D, followed by iterative graph matching of skeletonized curvilinear features in close proximity and orientation to extract a map of network fragments of various lengths. (F) Fragments from E meeting an experimentally determined length threshold (4 μm) were classified as vimentin fibers (magenta). All high- confidence pixels from D not classified as fibers were classified as mesh (gray). (G) Inset of F showing the raw image (Top) and analyzed pixel map (Bottom) of fibrous and mesh-like vimentin. (H) Orientation of vimentin fibers in the image frame of reference. (Scale bar: 10 μm.)
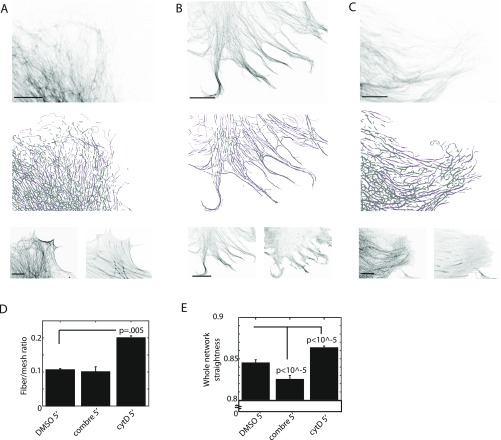
In a second step, we distinguished the longer, bundled fragments from the shorter fragments. To establish a length threshold for vimentin fragments, we sought to subtly perturb the vimentin network organization and classify resistant structures as fibers. No specific reagent against vimentin network assembly has been identified; however, it is well established that vimentin assembly requires intact surrounding actin and MT cytoskeletons (2, 9). Thus, we hypothesized that acute (5 min) disruption of filamentous actin or MTs would affect the finer components of the vimentin network as well (Fig. S3 A–C). Indeed, after actin depolymerization by cytochalasin D, we observed a shift in the vimentin network organization toward a more fibrous state (Fig. S3 A and B). When we applied a length threshold of 4 μm, which matches the length scale of actin stress fibers, the ratio of fiber to remaining network increased twofold for this condition relative to the untreated condition (Fig. S3D), reflecting what we observed. Thus, we applied a 4-μm threshold for fiber identification in all analyses (Fig. 1 F and G, magenta pixels). All high-confidence pixels from the steerable filter map not classified as fibers (i.e., fragments below the fiber length threshold, as well as those so short and with an isotropic orientation that they were eliminated by the nonmaximum suppression and graph-based connection algorithms) were classified as mesh (Fig. 1 F and G, gray pixels). For the pixels belonging to fibers, we also determined the local fiber orientation (12) (Fig. 1H).
Fig. S3.
Vimentin network organization subject to actin and MT depolymerization. (A–C) Cells were subjected to the following treatments at 5 min prior to fixation and staining: DMSO control (A), 1 μM cytochalasin D (B), and 500 nM combretastatin (C). (Top) Raw mEmerald-vimentin in treated hFFs. (Middle) Pixel maps of fibrous vimentin (magenta) overlaid on mesh (gray). (Bottom) Raw MT (Left) and raw actin (Right) images. (Scale bars: 10 μm.) (D and E) Analysis of network architecture for a given treatment. DMSO, n = 17 cells; cytochalasin D, n = 26 cells; combretastatin, n = 9 cells. (D) Percentage of an individual cell covered by fibrous vs. mesh vimentin. (E) Mean straightness of all detected fibers within a single image. Error bars indicate SD.
We tested the ability of our orientation algorithms to measure changes in detected vimentin fibers in response to partial MT depolymerization, which we previously described as disrupting vimentin network polarity (12). Indeed, we found that MT disruption reduced the coalignment between the vimentin fibers within a cell (Fig. S3 A, C, and E), demonstrating that the fibers are not organized randomly.
Vimentin Network Geometry in Migrating Fibroblasts.
We next applied our imaging and analysis tools to measure vimentin network organization in migrating fibroblasts. During random migration, fibroblasts exhibit multiple protrusion branches (Movie S1). Examination of individual branch retraction events showed that the vimentin fibers apparently remained intact, folding inward with slight compaction during cell retraction (Movie S2). Although we could not track individual fibers, we estimated the stability of individual fibers by tracking the fiber/mesh ratio over time. Although the amount of fibers varied among cells (Fig. S4A), the fiber/mesh ratio remained fairly constant for an individual cell over the migration period (Fig. S4 B and C).
Fig. S4.
The fiber/mesh ratio in migrating cells is stable over time. The fiber/mesh pixel ratio was measured over the time course of an entire movie for each cell, giving an estimate of fiber structure stability. (A) The mean fiber ratio for all cells (n = 53) was 0.21 (solid line), with an SD of 0.11 (dotted lines), demonstrating variability within the population. (B) The fiber/mesh ratio was stable within individual cells. Shown is the ratio from a representative migrating cell. (C) The percent change in fiber ratio within a cell was calculated as SD/mean. The median change in fiber ratio within cells was 7.5% (solid line).
We then asked how the fibers were oriented in relation to cell shape and the direction of movement (Fig. 2A). To this end, we first segmented the cell footprint and applied skeletonization to the resulting mask (Methods and Fig. 2B, Left). We then determined the number and orientation of skeletonized branches in a cell within a given frame. We also evaluated displacement of the cell by tracking the position of the center of the mask from frame to frame (Fig. 2B, Right). Vectors connecting the mask centers in consecutive frames defined the instantaneous direction of cell movement (Methods). We found that vimentin fibers aligned with fibroblast branches (Fig. 2C, Left and Movie S3), whereas the mean fiber orientation tended to align with the direction of cell movement (Fig. 2C, Right). These results show that on the time scale of cell migration, the vimentin network is composed of apparently stable fibers embedded in a surrounding mesh, and these fibers align with fibroblast protrusion and the overall direction of cell movement.
Fig. 2.
Vimentin network geometry during mesenchymal migration. (A) Vimentin fiber orientation in migrating hFFs. The arrow points to the same cell over time. Note that the image contrast of this time lapse was adjusted to more clearly visualize peripheral vimentin fibers (Methods). (B) hFFs expressing mEmerald-vimentin were stained with a 642-nm emitting membrane dye to measure cell shape and position. The outline of cells was masked and then skeletonized (Methods). The cell position was determined by the center of the cell mask. The orientation of cell movement was tracked from frame to frame. (C, Left) The angles between the branches of the skeletonized cell shape and vimentin fibers in the same region of the cell were compared for each branch in each frame. Shown are all individual measurements over all time-lapse sequences (8-h time lapse; n = 60 cells). Data are mean values from four separate experiments. The measured distribution deviates from a uniform distribution with P < 10−5 (Kolmogorov–Smirnov test). (C, Right) The mean vimentin fiber orientation angle within a cell was also compared with the orientation of whole cell movement. The measured distribution deviates from a uniform distribution with P < 10−5 (Kolmogorov–Smirnov test). (Scale bar: 10 μm.)
Vimentin Network Position Relative to Actin Flow Speed.
We next sought to test our hypothesis that vimentin architecture could regulate force transmission during single-cell migration. To do this, we first measured interaction of the vimentin network with actin flow, which results from actomyosin contraction as the primary force generator during planar mesenchymal cell migration (19, 20). To observe colocalization between vimentin and actin, we transiently expressed an actin SNAP-tag and labeled it with SNAP-Cell TMR (New England BioLabs) in hFFs expressing mEmerald-vimentin (Fig. 3A). Actin flow speed was measured using particle image velocimetry methods (21, 22). We then compared instantaneous actin flow speeds in regions containing no vimentin with those in regions containing vimentin mesh or fibers (Fig. 3 B–D). Compared with actin flow in regions without vimentin, actin flow speeds were significantly reduced when colocalized with vimentin mesh (Fig. 3D), and even more reduced when colocalized with vimentin fibers (Fig. 3D and Movies S4 and S5). The relationship between actin flow speed and vimentin did not depend on the relative position within the cell; actin flows across the cell periphery and cell interior were fastest in the absence of vimentin and slowest when colocalized with vimentin fibers (Fig. S5A). However, we found no significant correlation between the direction of actin flow and the orientation of vimentin fibers (Fig. S5B).
Fig. 3.
Vimentin network relative to actin flow speed. TALEN-modified hFFs expressing mEmerald-vimentin and transiently expressing actin SNAP-tag were incubated with SNAP-TMR. Both wavelengths were imaged every 5 s. Actin behavior was analyzed using qFSM software (22). (A) SNAP-TMR actin distribution in hFFs. Zoom-in of inset is the raw vimentin image overlaid with the cell outline. (Scale bars: 5 μm.) (B) Detected vimentin fibers overlaid with actin flow vectors (white). (C) Zoom-in of boxed regions in B. Vimentin fibers are shown in red. Each white pixel represents the position of an identified actin speckle. Vector length represents the time interval over which a speckle was tracked. (D) Actin flow speed vs. colocalized vimentin polymer organization (none, mesh, fiber) for n = 7 cells. Shown from left to right: no vimentin, M = 5,046 actin flow tracks; mesh, M = 14,130 flow tracks; and filamentous vimentin, M = 418 flow tracks.
Fig. S5.
Effects of the vimentin network on actin flow speed and orientation. TALEN-modified hFFs expressing mEmerald-vimentin and actin SNAP-tag were incubated with SNAP-TMR to label actin. Both wavelengths were imaged every 5 s. Actin behavior was analyzed using QFSM software as described previously (22). (A) Actin flow speed vs. vimentin intensity throughout the vimentin network. Actin flow data are separated by distance from the center of the vimentin network (left to right, in μm) and by vimentin architecture (top to bottom). (B) The orientation of actin flow vectors was compared with that of colocalized vimentin fibers for a single cell (Left) and for seven cells (Right). The Kolmogorov–Smirnov test indicated no evidence of co-orientation between actin flow vectors and vimentin fibers.
Vimentin Network Effects on Traction Force Magnitude.
Given the spatial correlation between the presence of vimentin and reduced actin flow speed, we hypothesized that the elastic and relatively stable vimentin network could absorb and redistribute forces generated by actomyosin contractility. To assess force transmission in the presence of vimentin, we measured the traction force distribution on 8.5-kPa bead-coated silicone gel substrates (23) in TALEN-modified hFFs expressing either control or vimentin knockdown lentivirus. Traction force distributions appeared to be more homogeneous with control cells than with knockdown cells (Fig. 4 A and B). Measurements of traction force maxima in the cell center indicated that with control cells, traction forces were threefold more homogeneously distributed than with knockdown cells, whereas no difference was observed at the cell periphery, which tends to be a vimentin-depleted region (Fig. 4C). With both control and knockdown cells, vimentin position was anticorrelated with traction force maxima (Fig. 4 D and E). Based on these data, we concluded that the presence and position of vimentin result in significantly more homogeneous force transmission to the substrate.
Fig. 4.
Vimentin network effects on traction forces. (A, Left) Pseudocolor image of traction force exerted by an hFF expressing control lentivirus. (A, Right) Traction force exerted by an hFF expressing shRNA targeting vimentin. Traction force range (minimum to maximum) was adjusted to display the distribution effectively. In the left panel, the range corresponds to 0–0.8 kPa; in the right panel, the range corresponds to 0–1.0 kPa. (Scale bar: 5 μm.) (C, Left) Mean number per cell of traction force peaks in the interior (area >7 μm from the cell edge; Methods) of control and vimentin kd cells. **P = 0.0115, t test. (C, Right) Mean number of traction peaks in the 7-μm-wide peripheral band. Control cells, n = 20; kd cells, n = 19. Error bars represent SEM. (D and E) Zoom-in views of boxes indicated in A, showing vimentin distribution (Left) and traction force distribution (Right). (D) I; (E) II. (F) Interior (Top) and peripheral (Bottom) traction stress vs. colocalized vimentin intensity for representative control (Left) and kd (Right) cells. The counts of traction stresses for given magnitude and vimentin intensity are presented on a pseudocolor scale, with a range of 0–3000.
Vimentin Network Effects on Traction Stress Orientation.
For directed migration, mesenchymal cells must regulate not only the magnitude of transmitted forces, but also their directionality. We found that vimentin fibers aligned along the direction of fibroblast protrusion and migration (Fig. 2), and thus asked whether the vimentin network itself affected the directionality of traction stresses. We first compared vimentin fiber orientation with that of traction stresses (Fig. 5 A and B). In contrast to the absence of alignment between vimentin fibers and actin flow, we found a high degree of alignment between the orientation of cell traction stresses and that of local fibrous vimentin (Fig. 5C). We then checked whether knockdown of vimentin altered local traction stress orientation. To this end, we measured the SD of traction stress orientation within 2.5-μm-radius windows throughout the cell. We found that knockdown cells had nearly randomly oriented traction stresses relative to control cells (Fig. 5D; see Methods for a definition of the alignment index). This result demonstrates that the presence of vimentin is required for traction stress alignment.
Fig. 5.
Effect of vimentin fibers on traction stress orientation. (A, Top) Segmented vimentin fibers color-coded for orientation and traction stress vector orientation for control cell shown in Fig. 4A. (A, Bottom) Zoom-in of box indicated in the top panel. (Scale bars: 5 μm.) (B and C) Illustration (B) and measurement of (C) angular differences between vimentin fibers and colocalized traction force vectors for n = 12 cells. The measured distribution deviates from a uniform distribution with P < 10−5 (Kolmogorov–Smirnov test). (D) Local alignment of traction stress vectors within a circular window of 2.5-μm radius measured in control and vimentin kd cells in regions with and without detectible vimentin. Control cells, n = 12; kd cells, n = 9. (E) Model of the vimentin network as a load-bearing superstructure that redirects actomyosin forces to the peripheral adhesions and aligns the traction force field with the orientation of the vimentin fibers.
Our results show that the vimentin network colocalizes with regions of slowest actin flow, redistributes local actin network-based forces, and is required for traction stress alignment. We conclude that the vimentin network acts as a load-bearing superstructure capable of redistributing and determining the orientation of actomyosoin-generated forces (Fig. 5E). In the absence of vimentin, actomyosin-generated forces are wholly transmitted to the substrate via discrete adhesions throughout the cell–substrate interface; however, in the presence of vimentin fibers, actomyosin-generated forces are partially absorbed by the interacting vimentin superstructure and redirected to adhesions in the peripheral band low in vimentin concentration. As a result, the traction force field is aligned with the colocalized vimentin fibers.
Discussion
EMT and subsequent single-cell migration are marked by the expression of vimentin. The principal manner in which intermediate filaments have been shown to influence cell function is by providing structural integrity to cells; however, how or why vimentin is required for normal mesenchymal migration has remained unclear. Here, we provide evidence that the structural organization of the vimentin network governs the alignment of traction stresses required for single-cell migration.
We found that vimentin fibers co-oriented with fibroblast branching, as well as the overall direction of cell movement (Fig. 2). This is similar to the alignment of MTs with the direction of migration (7), suggesting some convergence of function between these two cytoskeleton components. Recently we showed that vimentin and MT orientations reinforced one another, as well as overall cell polarity, during migration (12). Thus, we hypothesized that because of vimentin's known interaction with both MTs and actin, vimentin could function to integrate the polarizing effect of MTs with actomyosin-based forces that drive planar mesenchymal migration.
Indeed, we measured significantly slower actin flow in the presence of vimentin, particularly vimentin fibers, indicating interaction between these two cytoskeleton systems in fibroblasts. Although it is difficult to determine the cause and effect of protein colocalization, recent work (11) demonstrated that vimentin and actomyosin coregulate their distributions through actin-vimentin linkers, such as plectin (8). Vimentin and actin networks may potentially interact via bulk effects as well; in fact, vimentin has been shown to regulate actin network stiffness by providing steric constraints in vitro (24, 25).
We next investigated whether vimentin, as an elastic network colocalizing with slower actin flow, affected the distribution of forces exerted by the actin cytoskeleton on the substrate. Actin retrograde flow and myosin contractility on the actin cytoskeleton are known to exert stress on the extracellular substrate via discrete adhesions (26). Although it was previously reported that traction stresses in regions of slower actin flow are more homogenously distributed (and independent of adhesion size) (27), why this is so was not clear. We used traction force microscopy to map the points in the cell where intracellularly generated forces are coupled to the extracellular substrate. We found that the highest traction stresses occurred outside the footprint of the vimentin network, whereas forces inside the footprint were spatially more homogeneous. These results lead to the proposal that vimentin serves as a cell-internal load-bearing superstructure that redistributes local actin-generated forces.
One prediction of the vimentin superstructure model is that anisotropic architecture, such as fiber formation, would set the orientation of the traction stresses and through it the direction of cell migration. To test this prediction, we analyzed the spatial correlation of the direction of vimentin fibers and traction stresses and indeed found traction stresses to be aligned with vimentin fibers (Fig. 5). Moreover, the local coalignment of traction stresses was dependent on vimentin expression. Thus, our data and analyses together identify a role for the vimentin network as a secondary superstructure within the cell that reorients actin-based forces and is essential for traction stress alignment. In view of the mutual structural guidance between vimentin and MTs (12), it will be interesting in future studies to examine vimentin’s role as a potential mediator between MT organization and traction force transmission.
Methods
TALEN Assembly.
The N-terminal addition of EGFP or mEmerald to vimentin has been shown to yield functional fusion proteins (10, 28). To stably express mEmerald-vimentin, we used a TALEN-based genome editing approach. The target site selection and the assembly of these TALENs have been described earlier (12, 29).
Cells.
The hFFs were obtained from American Type Culture Collection and grown in high-glucose DMEM containing 25 mM d-glucose, 4 mM l-glutamine, and 110 mg/mL sodium pyruvate (Gibco, Life Technologies) supplemented with 10% FBS (Gibco), 15 mM Hepes (Gibco), and penicillin/streptomycin (Gibco). For Western blot analysis, mouse anti-vimentin (clone V9; Sigma-Aldrich), rabbit anti-vimentin against a C-terminal peptide (C-20; Santa Cruz Biotechnology), and mouse and rabbit anti-GAPDH (GT239 and GTX100118, respectively; GeneTex) antibodies were used. For immunofluorescence, chicken polyclonal to vimentin ab24525 and rabbit monoclonal [EP1332Y] to α-tubulin (Abcam) were used.
Imaging.
All images were collected with a Yokogawa CSU-X1 spinning disk confocal system on a Nikon Ti inverted microscope equipped with a Perfect Focus System for continuous maintenance of focus. Cells were fixed for 15 min at 37 °C with 1% glutaraldehyde and then permeabilized with 0.5% Triton X-100 for 5 min. Actin was labeled with Alexa Fluor 647 phalloidin (Invitrogen). For migration assays, six-well 1.5 MatTek dishes coated with 10 µg/mL fibronectin were used for coimaging of control shRNA and vimentin shRNA hFF lines. Cells were plated at 4 h before imaging and then incubated for 5 min at 37 °C with a CellMask Deep Red plasma membrane stain (Thermo Fisher Scientific) at 1 h before imaging. mEmerald-vimentin was excited with a 488-nm, 100-mW solid-state laser with an ET525/50m emission filter (Chroma Technology). The Deep Red membrane stain was excited with a 642-nm 100-mW laser with an ET700/75m emission filter (Chroma Technology). Images were collected every 5 min, using an exposure time of 50 ms for mEmerald-vimentin and 200 ms for the Deep Red membrane stain. Images were collected with a Nikon Apo 40x 0.95 dry objective lens.
Image Representation.
For fine vimentin structures to be visible in the figure panels, we adjusted the contrast of raw vimentin images using ImageJ software. Raw images first underwent intensity inversion followed by contrast adjustment, such that local structure could be visualized without obscuring any surrounding features in the region shown. For time-lapse images such as those that appear in Fig. 2, the same contrast settings were applied throughout.
shRNA Constructs and Reagents.
Human vimentin expression was inhibited using the target sequence 5′ACGTACGTCAGCAATATGA-3′. The control shRNA expression vector contained a 5′-ATGTACTGCGCGTGGAGA-3′ sequence. The loop sequence between the sense and antisense sequences was 5′-TTCAAGAGA-3′. These sequences were cloned into pSilencer 5.1 H1-retroviral vector (Life Technologies) in accordance with the manufacturer’s instructions.
Traction Force Imaging and Analysis.
Substrates were prepared as described previously (23). Dishes were coated with 10 µg/mL fibronectin and 100 µg/mL 1-ethyl-3-(3-dimethylaminopropyl) carbodimide in PBS for 1 h at 37 °C. Cells were seeded at 4 h before imaging and incubated with Deep Red plasma membrane stain. Red-labeled bead positions were imaged using a 561-nm, 200-mW laser with an ET620/60m emission filter with an exposure time of 300 ms. Beads were imaged before and after cell removal via 15 min of 2.5% Trypsin at 37 °C. Images were collected with a Nikon Plan Apo VC 1.4 NA oil immersion objective lens. Traction force data were analyzed as described previously (30).
We observed a difference in interior traction force distributions between control and vimentin kd cells. To quantify these differences, we segmented islands with high traction by thresholding the image with a combination of Otsu (31) and Rosin (32) thresholding. Specifically, given the threshold values determined by the Otsu algorithm TO (in which the underlying assumption is a bimodal traction distribution) and by the Rosin algorithm TR (in which the underlying assumption is a unimodal traction distribution with high-force outliers), an empirical threshold level reflecting the mixture of these theoretical distributions was computed as 2/3 TO + 1/3 TR. We considered traction islands with an area >0.2 μm2. To quantify the level of spatial traction homogeneity, we counted the number of high-traction islands in either a peripheral band 7 μm from the cell edge or the cell interior.
For traction stress alignment, the traction stress isotropy was defined as the circular SD (S) of traction stress orientation within a window with a radius of 2.5 μm. The local “alignment” of the traction stresses was defined as the complement of the mean value of S for a given window (SD of uniform orientation distribution - |S|). The distribution shown is for all windows in all cells.
Actin Flow Imaging and Analysis.
Cells were transfected with SNAP-tag actin using the Neon Transfection System (Invitrogen) at 24 h before the experiment. At 1.5 h before seeding, cells were incubated with SNAP-tag TMR ligand (New England BioLabs) following the manufacturer's protocol. At 1 h before imaging, DMEM imaging medium containing 10% FBS, 1% oxyfluor, 10 μM dl-lactate, 15 mM Hepes, and no phenol red was added to the cells. A layer of mineral oil was also added to prevent evaporation. Images were collected with a Nikon Plan Apo 60× objective. Actin flow data were analyzed as described previously (21, 22).
Vimentin Network Structure Analysis.
We observed that vimentin polymer density and organization varied from short mesh-like, randomly oriented fragments to longer, linear bundles. To assess this complex organization, we first used a previously published algorithm designed to extract filamentous features (12). In brief, we used a steerable filter (17) to enhance the raw image data curvilinear features and then applied a nonmaximum suppression operator that accounts for the local orientation of a putative curvilinear feature. Combined, these two procedures generated a map of 1-pixel-wide vimentin fragments. We next clustered fragments likely belonging to the same fiber based on co-orientation and spatial proximity using an iterative graph-matching algorithm. The graph matching includes the rescue of low-confidence fragments that are structurally well linked to high-confidence fragments. The output of the reconstruction is a network of vimentin fragments, each presented by an ordered chain of pixels and the local fragment orientation.
In a second step, we applied an experimentally determined length threshold of 4 μm to extract long “fibers” from the fragment map. All high-confidence pixels in the steerable filter response map minus the pixels belonging to long fibers were classified as “mesh.”
Cell-Shape Tracking.
To track cell shape over time, a binary mask of the cell was skeletonized using medial axis transformation as implemented in the MATLAB function bwmorph. Skeleton branches were tracked over time to identify cell protrusions and their directionality. Instantaneous cell speed and directionality were determined as the magnitude and orientation of the displacement of the center of the mass of the binary mask between consecutive frames.
Supplementary Material
Acknowledgments
We thank Jennifer Waters and staff of the Nikon Imaging Center at Harvard Medical School for help with light microscopy; Robert Goldman and Saleem Mahammad (Northwestern University Medical School) for help with constructs; and Jessica Tytell (formerly of the G.D. laboratory), Hunter Elliott (Harvard Image and Data Analysis Core), and Jagesh Shah (Harvard Medical School) for valuable discussions. This study was supported by National Institutes of Health PPG Grant P01 GM096971 (to G.D.) and 2R01 GM039565-28 (to T.J.M.). C.J.B. was supported by fellowships from the Swiss National Science Foundation and the Novartis Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614610114/-/DCSupplemental.
References
- 1.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber F, Boire A, López MP, Koenderink GH. Cytoskeletal crosstalk: When three different personalities team up. Curr Opin Cell Biol. 2015;32:39–47. doi: 10.1016/j.ceb.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Hookway C, et al. Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol Biol Cell. 2015;26:1675–1686. doi: 10.1091/mbc.E14-09-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 5.Vallotton P, Danuser G, Bohnet S, Meister JJ, Verkhovsky AB. Tracking retrograde flow in keratocytes: News from the front. Mol Biol Cell. 2005;16:1223–1231. doi: 10.1091/mbc.E04-07-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- 8.Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helfand BT, et al. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell. 2011;22:1274–1289. doi: 10.1091/mbc.E10-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiu Y, et al. Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Reports. 2015;11:1511–1518. doi: 10.1016/j.celrep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Gan Z, et al. Vimentin enhances directed cell migration by stabilizing microtubule-mediated cell polarity. Cell Systems. 2016;3:252–263. doi: 10.1016/j.cels.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo M, et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013;105:1562–1568. doi: 10.1016/j.bpj.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nöding B, Herrmann H, Köster S. Direct observation of subunit exchange along mature vimentin intermediate filaments. Biophys J. 2014;107:2923–2931. doi: 10.1016/j.bpj.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cermak T, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob M, Unser M. Design of steerable filters for feature detection using canny-like criteria. IEEE Trans Pattern Anal Mach Intell. 2004;26:1007–1019. doi: 10.1109/TPAMI.2004.44. [DOI] [PubMed] [Google Scholar]
- 18.Canny J. A computational approach to edge detection. IEEE Trans Pattern Anal Mach Intell. 1986;8:679–698. [PubMed] [Google Scholar]
- 19.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theriot JA, Mitchison TJ, Tilney LG, Portnoy DA. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 21.Lim J, Danuser G. Live cell imaging of F-actin dynamics via fluorescent speckle microscopy (FSM) J Vis Exp. 2009;30:1325. doi: 10.3791/1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza MC, Besson S, Danuser G. 2012. Quantitative fluorescent speckle microscopy (QFSM) to measure actin dynamics. Curr Protoc Cytom 62:2.18:2.18.1–2.18.26. [DOI] [PMC free article] [PubMed]
- 23.Gutierrez E, et al. High refractive index silicone gels for simultaneous total internal reflection fluorescence and traction force microscopy of adherent cells. PLoS One. 2011;6:e23807. doi: 10.1371/journal.pone.0023807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MH, Morris EJ, Goldman RD, Weitz DA. Emergent properties of composite semiflexible biopolymer networks. BioArchitecture. 2014;4:138–143. doi: 10.4161/19490992.2014.989035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esue O, Carson AA, Tseng Y, Wirtz D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J Biol Chem. 2006;281:30393–30399. doi: 10.1074/jbc.M605452200. [DOI] [PubMed] [Google Scholar]
- 26.Gardel ML, et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon M, Moir RD, Prahlad V, Goldman RD. Motile properties of vimentin intermediate filament networks in living cells. J Cell Biol. 1998;143:147–157. doi: 10.1083/jcb.143.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian ML, et al. Targeting G with TAL effectors: A comparison of activities of TALENs constructed with NN and NK repeat variable di-residues. PLoS One. 2012;7:e45383. doi: 10.1371/journal.pone.0045383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han SJ, Oak Y, Groisman A, Danuser G. Traction microscopy to identify force modulation in subresolution adhesions. Nat Methods. 2015;12:653–656. doi: 10.1038/nmeth.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- 32.Rosin PL. Unimodal thresholding. Pattern Recognit. 2001;34:2083–2096. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.