Abstract
The persistence of high morbidity and mortality from systemic fungal infections despite the availability of novel antifungals points to the need for effective treatment strategies. Treatment of invasive fungal infections is often hampered by drug toxicity, tolerability, and specificity issues, and added complications often arise due to the lack of diagnostic tests and to treatment complexities. Combination therapy has been suggested as a possible approach to improve treatment outcome. In this article, we undertake a historical review of studies of combination therapy and also focus on recent studies involving newly approved antifungal agents. The limitations surrounding antifungal combinations include nonuniform interpretation criteria, inability to predict the likelihood of clinical success, strain variability, and variations in pharmacodynamic/pharmacokinetic properties of antifungals used in combination. The issue of antagonism between polyenes and azoles is beginning to be addressed, but data regarding other drug combinations are not adequate for us to draw definite conclusions. However, recent data have identified potentially useful combinations. Standardization of assay methods and adoption of common interpretive criteria are essential to avoid discrepancies between different in vitro studies. Larger clinical trials are needed to assess whether combination therapy improves survival and treatment outcome in the most seriously debilitated patients afflicted with life-threatening fungal infections.
INTRODUCTION
High morbidity and mortality persist for systemic fungal infections due to pathogenic yeast (47, 55, 91, 191) and molds (42, 119, 169). The Food and Drug Administration has approved several antifungal agents belonging to different chemical classes (polyenes, pyrimidines, azoles, and echinocandins) as therapeutic options for fungal infections (reviewed in references 50 and 79). However, treatment is often complicated by high toxicity, low tolerability, or narrow spectrum of activity. These difficulties have driven recent efforts to determine the efficacy of combination therapy in the treatment and management of invasive infections. The most common rationales behind the studies focused on combination therapy are based on (i) mechanisms of action, combining agents with complementary targets within the fungal cells (polyenes plus azoles or echinocandins, antifungals plus immune factors, etc.), (ii) spectrum of action (combining agents potent against different organisms), and (iii) stability and pharamacokinetic/pharmacodynamic characteristics. Of these three main rationales, most combination therapy studies are based on the rationale of combining agents that have complementary mechanisms of action (Fig. 1). Potential benefits of using combination therapy include broad spectrum of efficacy, greater potency than either of the drugs used in monotherapy, improved safety and tolerability, and reduction in the number of resistant organisms (127).
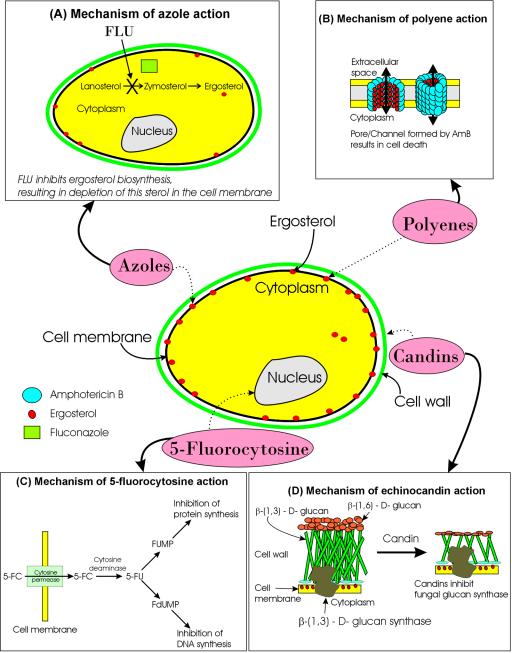
FIG. 1.
Schematic description of sites of action of different antifungal agents. Candins cause disruption of cell wall, allowing other antifungals (polyenes, azoles, and 5-FC) to enter. Azoles and polyenes can inhibit or bind to ergosterol, leading to cell lysis and allowing 5FC to enter the cell and inhibit nucleic acid synthesis. Dashed arrows indicate the site of action for each antifungal class. The mechanisms of action for each class of antifungal agent are depicted in panels A through D.
Antifungal combination therapy was recognized as an important area nearly a quarter of a century ago by Bennett et al. (19), who compared amphotericin B (AmB) alone and in combination with 5-fluorocytosine (5FC) in the treatment of cryptococcal meningitis. However, adoption of this approach for the treatment of invasive fungal infections has been slow, limited to AmB plus 5FC or 5FC plus fluconazole (FLU), and fraught with controversy regarding the use of a polyene combined with an azole. With the approval of the third-generation azole voriconazole (VORI) and the candins (e.g., caspofungin [CAS]), there is rekindled interest in antifungal combination therapy, especially since these agents have different mechanisms of action. Unlike antibacterial and antiviral agents, studies of combinations of antifungal agents are in the early stages of investigation and, consequently, are highly dynamic.
Interactions between different drugs are described variously as synergistic, indifferent, additive, or antagonistic. Assessments of in vitro drug interactions are usually based on the “no interaction” theory, which assumes that drugs in combination do not interact with each other. When the observed effect of the drug combination is more than that predicted from the “no interaction” theory, synergy is claimed. On the other hand, antagonism is claimed when the observed effect is less than that predicted (88). Although several categories including “additive,” “subadditive,” and “indifferent” have been used to describe intermediate drug-drug interactions, the emerging consensus has been to group all of them under the “no interaction” category (163).
Inherent problems associated with susceptibility testing of single antifungal agents are also relevant to testing antifungal combinations. Evaluation of drug-drug interactions against filamentous fungi is further problematic for a number of reasons: (i) in spite of concerted efforts which resulted in a published reference method for the evaluation of susceptibility of conidia-forming filamentous fungi, in vitro and in vivo correlation are not yet well-defined (68, 90, 176, 180, 235); (ii) susceptibility testing of the candins is known to be influenced by different factors (48, 155, 175), and, despite recent attempts, a standard method for this class has not been developed so far; (iii) spectrophotometric reading, which is successfully used to measure the drug MICs for yeast, is less useful for filamentous fungi; and (iv) response to treatment (damage, recovery, or viability) varies differentially for apical and subapical hyphal components (155).
In this article, we present an overview of methods commonly applied to assess the effects of combination therapy, historical and current uses of combination therapy, and potential limitations of antifungal combination therapy. This review demonstrates the increased interest in antifungal combination and clearly illustrates the dynamic nature of this field, with all its complexities.
METHODS TO DETERMINE THE IN VITRO EFFICACY OF ANTIFUNGAL AGENTS IN COMBINATION
The methods for determining antifungal susceptibilities have been extensively reviewed, especially for single antifungal agents (189), and recommendations for determination of the MIC of single antifungals for yeasts and molds are currently available (65, 66, 158, 159, 189). The MIC of an antifungal agents is defined as the minimum concentration of the drug resulting in 80% (or 50%, in some cases) inhibition of fungal growth relative to the control (with no drug exposure). Antifungal susceptibility testing of individual agents is based on the following principles: (i) MIC is not a physical or chemical measure, (ii) host factors are often more important than susceptibility test results in determining clinical outcome, (iii) susceptibility of a microorganism in vitro does not predict successful therapy, and (iv) resistance in vitro should often predict therapeutic failure (186, 187).
In vitro susceptibility assays are highly dependent on the fungal species under investigation and on the testing conditions of exposure time, incubation temperature, media, and other method-specific factors. Many in vitro and in vivo modifications have been devised to better approximate human disseminated disease (130) and to examine the influence of immunosuppression (149) or neutropenia (106) on treatment success. Another complexity is derived from the fact that the activity of antifungals in combination is dependent not only on the drug-drug concentration but also the absolute ratio of the drugs (74, 76, 78). The majority of published results from studies using combination treatments tend to focus on two-drug combinations, although this limit is likely to be revised as methodological and analytical techniques evolve that will allow evaluation of three or more drugs in combination. Additionally, these methods are based on static drug concentrations, and interaction is determined at a single time point; therefore, direct correlation between in vitro and in vivo interactions is not always possible. These principles are also applicable to susceptibility testing of antifungals in combination, resulting in considerably more complex problems. The MICs of different drugs in combination can be determined using the checkerboard method, time-kill method, or Epsilometer test (E-test) (105, 123, 173, 174, 187). In this section, we briefly present an overview of the application of these methods to determine the interactions between antifungal agents used in combination.
Checkerboard Method
The checkerboard method involves the determination of percent growth inhibition of fungal cells in the presence of different combinations of drugs. Percent growth inhibition is calculated relative to growth in control wells which contain only cells and no drug. The specific merits and limitations of checkerboard testing have been described and summarized in detail by others (11, 15, 105, 163, 171). Briefly, the checkerboard method is relatively simple to perform and the results are easily interpreted, making them useful for extensive screening. However, this method also has some important limitations. (i) The checkerboard method appears to be less useful for detecting changes in antimicrobial tolerance over time and may fail to detect changes in susceptibility end points that permit interpretations of synergy or antagonism (105, 124, 178, 189). (ii) Results are relative and not actual measurements of the efficacy of drug combinations. (iii) Combinations with AmB are frequently complicated by MIC clustering, which precludes the discrimination of small differences in susceptibility over time (14, 188, 189). (iv) An assumption in most checkerboard titration systems is that all drugs in combination in the system possess identical, generally linear dose-response curves (105, 108, 123, 125) and a comparable time-course of activity. This assumption may be especially problematic for polyene-azole combinations, since the in vitro activity of azoles against Candida and Cryptococcus species is initiated considerably more slowly than that of the polyenes (109, 110), preexposure to FLU affects the in vitro interaction between AmB and FLU (61, 173), and the rapid onset of AmB activity may result in failure to detect synergic or antagonistic antifungal interactions. Finally, data from the checkerboard method sometimes results in interpretations contradictory to those obtained from other methods like the time-kill or E-test methods (123). In general, checkerboard testing is easy to carry out and interpret but does not provide details about the pharmacodynamic characteristics of antifungal combinations. The checkerboard-based determination of MICs of antifungal agents in combination is often followed by further analysis employing the nonparametric fractional inhibitory concentration index (FICI) (20-22), or the fully parametric response surface model (RSM) proposed by Greco et al. (88, 89). These methods are outlined in the next sections.
Fractional inhibitory concentration index.
Most studies investigating the in vitro efficacy of antifungal agents in combination interpret results in terms of the FICI, which is defined by the following equation:
 |
where MICA and MICB are the MICs of drugs A and B, respectively. Over the years, several investigators have categorized interactions between antifungal agents by using the FICI in different terms, leading to sometimes confusing nomenclature and interpretations of results. Recently, for the sake of uniformity in interpretation, Odds (163) proposed that an FICI of <0.5 should be considered synergy, a FICI of >4 should be considered antagonism, and a FICI of 0.5 to 4 should be considered no interaction. The interpretation of these criteria has also been discussed in detail in a recent review (103). We feel that additivity or indifference implies that the drugs in combination do not have a detrimental effect on the response, even though they are not synergistic. In agreement with the proposed system of interpretation of FICI values, we suggest that all future studies should follow this system while interpreting FICI data.
Ease of use, simplicity, and feasibility of performance make FICI the method of choice for analyses of drug-drug interactions in most clinical laboratories (57). However, this method also has some significant disadvantages: (i) it is dependent on dilution-based determination of MICs and hence may lead to interexperimental errors; (ii) it does not differentiate between the possibility that at some concentrations in the checkerboard there may be synergy while at other dilutions there may be indifference or antagonism; (iii) for some antifungal combinations, the choice of MIC end point is not clear, leading to difficulties in calculating the FICI; (iv) it is not amenable to statistical analyses; and (v) the definition of FICI varies greatly between different studies (Table 1) (146-148, 223). Attempts to overcome the difficulty of statistical analyses of the FICI include determining the median and range from several replicates of MIC determinations (31), concordant FICI values from several replicates (147), and establishment of consensus guidelines regarding interpretation of the FICI values (163).
TABLE 1.
Inconsistent interpretations of FICI values in different studies investigating efficacies of antifungal combinations against fungal organismsa
| Interaction | FICI value for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Candida
|
Cryptococcus
|
Aspergillus
|
Scedosporium
|
|||||||
| Ref 141 | Ref 98 | Ref 223 | Ref 223 | Ref 160 | Ref 214 | Ref 31 | Ref 2 | Ref 148 | Ref 147 | |
| Synergism | <1.0 | <0.5 | <1.0 | <1.0 | <1.0 | <1.0 | ≤0.5 | ≤0.5 | ≤0.5 | <1.0 |
| Additivity | <1.0 | 1.0 | 1.0 | 1.0 | 1.0 | >0.5-1.0 | >0.5-1.0 | >0.5-4.0 | 1.0 | |
| Indifference | 1.0-2.0 | >1.0-2.0 | >1.0-4.0 | |||||||
| Antagonism | >1.0 | >4.0 | >1.0 | >1.0 | >2.0 | >2.0 | >2.0 | >4.0 | >4.0 | >1.0 |
Ref, reference.
Response surface-modeling method.
An alternative method for assessment of drug-drug interactions is the RSM method described by Greco et al. (88), which is based on the calculation of the interaction coefficient alpha (ICα) and its associated 95% confidence interval (CI). These parameters are calculated using the following equation:
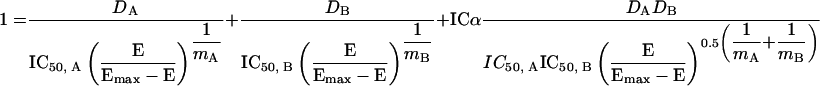 |
where DA and DB represent the MICs of drugs A and B, respectively, ICα is the interaction coefficient, IC50 represents the drug concentrations resulting in 50% inhibition, mA and mB are the slope parameters for drugs A and B, E is the measured response (optical density [OD]) and Emax is the control response. Interactions with an ICα of >0 indicate synergy, while an ICα of <0 indicates antagonism. Additivity is claimed when ICα equals zero. ICα is considered statistically significant if the associated CI does not overlap zero. Although the RSM approach is increasingly being advocated by different studies to be more robust than the FICI method (146-148, 223), this model is also not without some major drawbacks: (i) the model involves complicated mathematical data-fitting and modeling steps; (ii) although specialized software for these analyses have been reported, they are not commonly accessible to clinical laboratories; (iii) since the model relies on regression analysis based on data fitting, results generated are dependent on factors such as initial parameters, ways of calculating sum of squares, variance, and weighting parameters; and (iv) it may give erroneous results (e.g., very high interaction parameters) when IC50 is not known, i.e., no combination results in 50% inhibition (147).
The RSM method has been used in a number of in vitro studies in an attempt to better characterize antifungal drug-drug interactions as a function of specific drugs, by taking into account the absolute and relative concentrations of drugs in combination (76). RSM methods are especially useful for investigations of double and triple drug combinations over a very wide range of doses. Drug concentration and interaction data must be analyzed mathematically to generate descriptions of fungal growth response over a range of drug concentrations and ratios; the results can be visualized using three-dimensional contour or surface response plots.
Although RSM has a place when an in-depth analysis of drug-drug interactions is required, the testing will likely be undertaken by specialized laboratories having the appropriate expertise. Some investigators have suggested using a combination of the FICI and RSM approach and have shown that results obtained from the two correlate well (2, 222, 223). However, the inherent complexity of the RSM method makes it less attractive as the primary method to evaluate the interactions between antifungal agents in combination, and the FICI method has remained the method of choice for most studies.
Time-Kill Method
Time-kill methods are capable of detecting differences in the rate and extent of antifungal activity over time and are better suited for assessing changes in the antifungal activity of AmB (26, 105, 108, 123, 173). In this method, a standardized cell suspension (usually 5 × 105 cells/ml) is exposed to different concentrations of drug combinations for different time intervals (179, 219, 226). After a specified treatment time, cells are retrieved, plated onto agar medium, and incubated to allow growth. The CFU for each incubation time point per milliliter is determined, and plotted as a function of time, resulting in “time-kill” curves for each drug combination tested. Time-kill methods have been commonly used for testing bactericidal activity of antimicrobial agents (5, 9, 25, 92, 102, 131), and recent studies have focused on using this method to determine the efficacy of antifungal agents also, both singly and in combination (27, 60, 62, 63, 105, 107, 108, 123, 126, 129). For antifungal interactions tested by time-kill methods (at 24 to 48 h), the following criteria are commonly followed: (i) synergy is defined as a ≥2 log10 decrease in CFU per milliliter compared to the most active constituent, (ii) antagonism is defined as a ≥2 log10 increase in CFU per milliliter compare to the least active agent, (iii) additivity is defined as a <2 but >1 log10 decrease in CFU per milliliter compared to the most active agent, and (iv) indifference is defined as a <2 but >1 log10 increase in CFU per milliliter compared to the least active agent (123, 126, 127), (Fig. 2; Table 2).
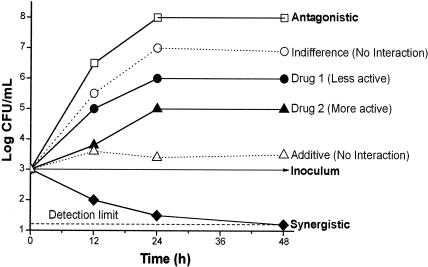
FIG. 2.
Schematic representation of the criteria used to interpret results from time-kill studies of drug-drug interactions. The following criteria are commonly followed: synergy, a ≥2-log10 decrease in CFU/ml compared to the most active constituent; antagonism, a ≥2-log10 increase in CFU/ml compared to the least active agent; additivity, a <2- but >1-log10 decrease in CFU/ml compared to the most active agent; and indifference, a <2- but >1-log10 increase in CFU/ml compared to the least active agent.
TABLE 2.
Criteria used for in vitro evaluation of drug interactions
| Method | Drug interaction
|
Reference(s) | ||
|---|---|---|---|---|
| Synergy | Antagonism | No interaction | ||
| FICI | FICI < 0.5 | FICI > 4 | FICI = 0.5-4 | 163 |
| RSM | ICα > 0 | ICα < 0 | ICα = 0 (additivity) | 88 |
| Time-kill | ≥2 log10 decrease in CFU/ml compared to the most active constituent | ≥2 log10 increase in CFU/ml compared to the least active agent | Additivity: <2 but >1 log10 decrease in CFU/ml compared to the most active agent; indifference: <2 but >1 log10 increase in CFU/ml compared to the least active agent | 123, 126, 127 |
| E-Test | Decrease of ≥3 dilutions of the resultant MIC | Increase of ≥3 dilutions of the MIC | Additivity: decrease of ≥2 but <3 dilutions of the MIC; indifference: decrease of <2 dilutions of the MIC | 36, 123, 202, 203 |
Although the time-kill curve assay provides growth kinetic information over time and give a more detailed picture of the effect of drug combinations on cell viability, this method also has its drawbacks. In the time-kill method, the dependence on calculation of CFU dictates that the cells being evaluated be grown as suspension of single cells, which works fine for bacteria (which grow in suspension as single cells). However, many fungi (e.g., Candida albicans) can grow as both single cells and filaments that tend to coaggregate, making it difficult to assess inhibition. This characteristic of fungi necessitates careful selection of media (e.g., yeast nitrogen base) and growth conditions that do not encourage filamentation. Likewise, in molds, filamentation compromises our ability to count CFU. Time-kill studies are laborious to perform, but they measure the effects of the antifungal interaction on the rate and extent of fungal killing, thus providing some pharmacodynamic information regarding the drug combination tested.
Epsilometer Strip Test (E-test)
The epsilometer test (E-test; AB Biodisk, Solna, Sweden) is also used to determine the in vitro efficacy of antifungal agents (36, 202, 203). MICs are determined from the point of intersection of a growth inhibition zone by using a calibrated strip impregnated with a gradient of antimicrobial concentration and placed on an agar plate lawned with the microbial isolate under test. This method has been adapted to a number of antifungal agents. Several studies have demonstrated good correlation between E-test and broth macro- and microdilution testing methods for singly tested antifungal agents (32, 52, 67, 143). Recent studies have also investigated the feasibility of using the E-test method to determine the activity of combinations of antifungal agents (111, 174, 230). Based on the manufacturer's (AB Biodisk) interpretations, the following interactions between antifungal agents using the E-test method have been proposed: (i) synergy is defined as a decrease of ≥3 dilutions in the resultant MIC, (ii) additivity is defined as a decrease of ≥2 but <3 dilutions, (iii) indifference is defined as a decrease of <2 dilutions, and (iv) antagonism is defined as an increase of ≥3 dilutions for the antifungal combination. In a recent study, Lewis et al. (123) compared the ability of the checkerboard, time-kill, and E-test methods to evaluate antifungal interactions against Candida species, and demonstrated a good agreement between the checkerboard and E-test methods as well as a between the time-kill and E-test methods.
Although simple to perform, the E-test method has drawbacks: (i) it has not undergone extensive testing using different organisms, with at least one report suggesting a species-dependent variation in MIC determination for Candida (203); (ii) it has not been tested against different antifungal agents, (iii) the effect of different growth media needs to be ascertained (RPMI-based agars generally appearing most useful) (177); (iv) growth of the fungal lawn tends to be nonuniform in some cases; and (v) the presence of a feathered or trailing growth edge can make the MIC determination confusing.
In spite of its limitations, checkerboard-based methodols remain the most popular approach for evaluating antifungal drug-drug interactions, evident in the fact that 75% of the drug interaction papers published from 1998 to 2002 in the Journal of Antimicrobial Chemotherapy used the checkerboard method in their analyses (163). Criteria commonly used to interpret in vitro methods to evaluate antifungal combinations are summarized in Table 2.
METHODS TO DETERMINE THE IN VIVO EFFICACY OF ANTIFUNGAL AGENTS IN COMBINATION
In vivo assessment of combination therapy is based on animal studies followed by clinical trials, case series, or anecdotal reports. Most animal studies evaluate the efficacy of combination therapy based on tissue fungal burden of target organs (liver, kidneys, brain, etc.), tissue histopathology (sterilization of tissue), and/or survival studies. Several animal models have been developed, and involve mice (97, 98, 130, 154, 156, 211), rats (140), or rabbits (172). Factors that need to be considered while performing in vivo studies include variable drug absorption, distribution, and metabolism among animal species. Clinical trials evaluating the interactions between different antifungal agents have been very limited. This is not a surprise due to the costs involved and the fact that many companies that market these antifungal agents often do not encourage the idea of combining drugs. Clinical trials of combination therapy have been performed with nonneutropenic patients, and recent trials determined the efficacy of FLU plus AmB combination therapy compared to monotherapy with AmB (185). This clinical trial showed no detectable antagonism when using the FLU plus AmB combination and was instrumental in demonstrating that in vitro antagonism seen in combination therapy may not always be present in the clinical setting (see below).
COMBINATION THERAPY IN PRACTICE GUIDELINES FOR TREATMENT OF FUNGAL INFECTIONS
The Practice Guidelines for the Treatment of Fungal Infections also suggest the use of specific drug combinations in certain situations (207). These guidelines addressed each pathogen separately and provided a guide for the use of single and combination therapy. The following sections provide a brief review of these guidelines, focusing on the recommendations to use combination therapy to treat fungal infections.
Dual Combinations in the Practice Guidelines for Candidiasis
Dual combinations of intravenous AmB or oral FLU in combination with 5FC are specifically mentioned in the Practice Guidelines for candidemia, candidal endocarditis, pericarditis, suppurative phlebitis, candidal meningitis, and endophthalmitis (168, 190). Guidelines for treatment of invasive candidiasis, based on data from clinical trials performed for acute hematogenous candidiasis and case series or anecdotal reports for other forms of invasive candidiasis, have suggested combinations of 5FC, AmB, or FLU as treatment options. In general, 5FC is added in the setting of more severe infection or refractory infection and occasionally is used as prophylaxis in neutropenic patients with well-defined high risk based on their immune status, such as those receiving chemotherapy for leukemia or bone marrow transplant (BMT) recipients (190). The latest version of these guidelines suggest AmB (0.7 mg/kg/day) combined with FLU (800 mg/day), followed by maintenance therapy with FLU (800 mg/day), as alternative therapy for nonneutropenic patients with candidemia. All intravascular catheters should be removed, and the duration of therapy should be 14 days after the last positive culture and resolution of signs and symptoms. For endocarditis patients, a combination of liposomal formulations of AmB (LF-AmB, 3.0 to 6.0 mg/kg/day) and 5FC (25-37.5 mg/kg orally [p.o.] four times a day [qid]) is suggested as primary therapy (for a duration of at least 6 weeks after valve replacement). The guideline to use different antifungal combinations to treat Candida infections at different sits is based primarily on evidence from studies reporting that activity of antifungal combinations is influenced by the site of infection.
Abel-Horn et al. (1) demonstrated site-dependent variation in activity of the same antifungal combination. These investigators performed a prospective, randomized study to compare FLU monotherapy with the combination of AmB plus 5FC for treatment of 72 nonneutropenic intensive-care patients with systemic candidiasis. One arm (n = 36) received FLU 400 mg on day 1 followed by 200 mg/day for 14 days, while the other arm (n = 36) received AmB (1.0 to 1.5 mg/kg of body weight every other day) and 5FC (2.5 g three times a day) for 14 days. For pneumonia and sepsis, treatment success was comparable between the two groups: 18 of 28 FLU patients and 17 of 27 combination patients (P > 0.05, not significant). For peritonitis, however, the combination was more effective than FLU monotherapy with respect to both cure rate (55 and 25%, respectively) and pathogen eradication (86 and 50%, respectively). Such site-specific differences in the activity of antifungal combinations may be due to differences in tissue distribution of antifungal agents and to different effects of the in situ environments on the pharmacodynamic and pharmacokinetic characteristics of the drugs.
An open, prospective randomized trial compared combination therapy with AmB plus 5FC to FLU monotherapy in 40 surgical patients with deep-seated candidal infection (113). Most infections were due to gastrointestinal perforations; C. albicans was the most frequent isolate. Patients received either AmB (0.5 mg/kg of body weight) in combination with 5FC 2.5 g (three times a day; n = 20) or monotherapy with FLU (400 mg on the first day followed by 300 mg daily; n = 20). Although there was no difference between regimens with respect to cure rates, patients receiving combination therapy showed earlier elimination of fungal pathogens than did patients receiving monotherapy with FLU (median elimination time, 8.5 days in the FLU arm and 5.5 days in the AmB-plus-5FC group). Additional studies in support of AmB-plus-5FC and FLU-plus-5FC combinations include those performed by Levine et al. (122), Smego et al. (206), and Marr et al. (142), indicating the clinical usefulness of these combinations.
Dual Combinations in the Practice Guidelines for Cryptococcosis
Combination treatment for cryptococcal diseases has progressed to the point where it is universally recommended as necessary for successful clinical treatment. Dual combinations of intravenous AmB or oral FLU in combination with 5FC are included in the Practice Guidelines for therapy of Cryptococcal Disease to treat central nervous system (CNS) infections in both human immunodeficiency virus (HIV)-infected and non-HIV-infected patients (195). 5FC, a small water-soluble molecule, penetrates the cerebrospinal, vitreous, and peritoneal fluids to predictable levels, comprising the basis for most of its concurrent use with either AmB or FLU (39).
Among non-HIV patients with cryptococcal meningitis, combination therapy with AmB plus 5FC for 4 to 6 weeks is effective (19, 51). However, adverse yet non-life-threatening reactions to 5FC were reported in 32% of the patients (11 of 34) (19). Due to the toxicity issues associated with this regimen, a frequently used alternative treatment for cryptococcal meningitis in immunocompetent patients has been induction with AmB (0.5 to 1 mg/kg/day) plus 5FC (100 mg/kg/day) for 2 weeks, followed by consolidation therapy with FLU (400 mg/day) for an additional 8 to 10 weeks (167). Lower doses of FLU (200 mg/day) for longer periods (6 to 12 weeks) have also been suggested as maintenance therapy (195). For immunocompromised patients with low tolerance for 5FC, a similar therapy of induction, consolidation, and suppression has been suggested: AmB (0.7 to 1 mg/kg/day) for ≥2 weeks followed by FLU (400 to 800 mg/day) for 8 to 10 weeks followed by 6 to 12 months of lower-dose FLU (200 mg/day) (166, 195).
For patients with severe form of AIDS-related cryptococcal pneumonia, a combination of FLU (400 mg/day) plus 5FC (100 to 150 mg/day) for 10 weeks is suggested (166, 195). However, toxicity issues have been reported for this regimen (195). For HIV-associated cryptococcal meningitis, the primary treatment suggested is induction with AmB (0.7 to 1 mg/kg/day) plus 5FC (100 mg/kg/day) for 2 weeks followed by FLU (400 mg/day) for a minimum of 10 weeks (184, 227). If the severity of disease abates, the FLU dose can be subsequently reduced (200 mg/day) but should be continued for life. An alternative regimen is AmB (0.7 to 1 mg/kg/day) plus 5FC (100 mg/kg/day) for 6 to 10 weeks followed by FLU as a maintenance therapy (117, 118, 196, 236). Larsen et al. (117) showed that combination therapy with FLU (400 to 800 mg/day) plus 5FC (100 to 150 mg/kg/day for 6 weeks) is an effective regimen for cryptococcal meningitis in AIDS patients but reported toxicity in 28% of these patients (117). In a separate randomized trial, Mayanja-Kizaa et al. (145) determined the efficacy of combination therapy with low-dose FLU (200 mg/day for 2 months) + short-term 5FC (150 mg/kg/day for the first 2 weeks) followed by FLU maintenance therapy (200 mg three times per week for 4 months) against cryptococcal meningitis. These investigators showed that this combination therapy prevented death within 2 weeks and significantly increased the survival rate, with no serious adverse reactions (145).
Dual Combinations in the Practice Guidelines for Aspergillosis and Coccidioidomycosis
Clinical studies have not shown conclusive evidence in support of use of AmB combined with 5FC for aspergillosis. The guidelines suggested the use of AmB combined with rifampin or itraconazole (ITRA) for CNS infection and renal or prostatic disease due to Aspergillus (215). In support of these recommendations, two studies were cited. In the first study, in vitro susceptibility testing using a macrodilution broth method (>100 isolates) showed that for AmB plus rifampin (36 of 39 isolates (92%) showed synergy and for AmB plus 5FC only 6 of 26 (23%) of the isolates showed antagonism whereas 6 (23%) showed synergy and 13 (50%) showed no interaction. In five AmB-plus-ITRA combination studies, synergy was seen in two while the rest gave results indicating that the combination was noninteractive (44). However, in a separate study, the combination of ITRA plus AmB was shown to be antagonistic both in vitro and in a murine model of aspergillosis (199). These studies involved sequential addition of ITRA followed by AmB and thus tended to support the “depletion theory” of antagonism (see below). When tested in vitro against six isolates of Aspergillus fumigatus after exposure to subfungicidal concentrations of ITRA, AmB lost its activity. Similarly, prior treatment of mice with ITRA abolished the protective effect of AmB, even when ITRA treatment was stopped before AmB therapy was started (199). A recent review of 6,281 clinical cases (from 1966 to 2001) of invasive aspergillosis showed that simultaneous combination and sequential antifungal therapy led to improvement in 63 and 68% of the cases, respectively (211).
The only other organism for which the guidelines mention combination therapy is coccidiodomycoses (71). AmB is often selected for treatment of patients with respiratory failure due to Coccidioides immitis or rapidly progressive coccidioidal infections. With other more chronic manifestations of coccidioidomycosis, additional treatment with FLU, ITRA, or ketoconazole (KETO) is common. The duration of therapy often ranges from many months to years; for some patients, chronic suppressive therapy is needed to prevent relapses (71). In cases of chronic fibrocavity pneumonia caused by C. immitis, the Practice Guidelines suggest an initial treatment with an azole (usually FLU) followed by higher doses of the azole. If the infection persists, adding AmB therapy is suggested. The guidelines also suggest that severe cases of coccidiodal meningitis and hydrocephalus be treated with a combination of AmB and azoles. These guidelines did not address combination therapy with the new agents due to the limited data available at the time these guidelines were being written. Studies focusing on these agents are described below.
ANTIFUNGAL COMBINATIONS AGAINST CANDIDA SPECIES
Established Antifungal Agents in Combination
Amphotericin B plus fluconazole against candidiasis. (i) In vitro studies.
Wide variations have been reported for the AmB-plus-FLU combination against Candida species; in certain cases this combination was additive, while in other cases no interaction was seen (Table 3). In an early study, three-dimensional contour mapping (surface response plots) was used to evaluate in vitro activity of two- and three-drug combinations of AmB, FLU, and 5FC against C. albicans (76). The two-drug combinations were additive (AmB plus FLU) or indifferent (FLU plus 5FC, AmB plus 5FC) against C. albicans, while the three-drug combinations (AmB plus FLU plus 5FC) were additive against C. albicans. Interestingly, no antagonism was observed in AmB-plus FLU combinations in vitro (76). Lewis et al. (129) used an in vitro model of bloodstream infection that simulates human serum pharmacokinetic parameters for these antifungals to evaluate the pharmacodynamic activities of FLU and AmB alone and in combination against C. albicans. These investigators showed that the simultaneous administration of FLU plus AmB resulted in no interaction, while sequential addition of FLU followed by AmB resulted in substantial antagonism (129).
TABLE 3.
Efficacy of drug combinations against Candida species
| Study | Combination | Regimen effect | Reference(s) |
|---|---|---|---|
| In vitro | FLU + AmB | No effect or antagonistic | 135 |
| FLU + AmB | Antagonistic | 220 | |
| FLU + AmB | Better than alone | 76 | |
| 5FC + FLU | Synergistic (63%), additive (6%), indifferent (24%), antagonistic (none) | 160 | |
| 5FC + FLU | Antagonistic | 223 | |
| 5FC + FLU | Additive | 82 | |
| 5FC + AmB | Indifferent (100%) | 105 | |
| 5FC + AmB (low) | Additive | 76 | |
| 5FC + AmB (high) | Antagonistic | 76 | |
| VORI + 5FC | C. albicans, synergistic or additive (50%); C. glabrata, indifferent (50%) or antagonistic (50%); C. tropicalis, synergistic or additive | M. A. Ghannoum and N. Isham, Abstr. 29th Annu. Meet. Eur. Group Blood Marrow. Transplant. abstr. P671, 2003a | |
| AmB + 5FC | C. albicans, synergistic or additive (50%); C. glabrata, additive (60%); C. krusei, additive (70%) | M. A. Ghannoum and N. Isham, Abstr. 29th Annu. Meet. Eur. Group Blood Marrow Transplant. abstr. P671, 2003a | |
| VORI + AmB | Additive (17%), indifferent (83%) | M. A. Ghannoum and N. Isham, Abstr. 29th Annu. Meet. Eur. Group Blood and Marrow Transplant. abstr. P671, 2003a; M. A. Ghannoum, N. Isham, M. A. Hossain, and D. J. Sheehan, Abstr. Trends Invasive Fungal Infect., 2001a | |
| VORI + FLU | Additive (42%), indifferent (58%) | M. A. Ghannoum, N. Isham, M. A. Hossain, and D. J. Sheehan, Abstr. Trends Invasive Fungal Infect. 2001a | |
| VORI + MICAF | Synergistic (17%), additive (17%), indifferent (58%) | M. A. Ghannoum, N. Isham, M. A. Hossain, and D. J. Sheehan, Abstr. Trends Invasive Fungal Infect., 2001a | |
| CAS + AmB | Additive | 98 | |
| In vivo | AmB + FLU | No antagonism | 220 |
| FLU + AmB | Antagonistic against FLU-susceptible or mid-resistant strains, indifferent against FLU-resistant strains | 134 | |
| FLU + AmB | Survival, significantly increased; CFUs, increased clearance | 198 | |
| FLU than AmB + FLU (sequential) | Antagonistic | 135 | |
| FLU + 5FC | No effect | 86 | |
| FLU + 5FC | Better CFU clearance | 7, 136 | |
| CAS + AmB | Survival, increased, but not significant; CFUs, significant reduction in kidneys but not in brain | 98 | |
| Clinicalb | FLU + AmB | No antagonism (clinical trial) | 185 |
| FLU + 5FC | Resolution of candidiasis | 201 | |
| FLU + 5FC | Successful resolution of candidal sepsis and meningitis in a very-low-birth-weight infant | 142 | |
| FLU + 5FC | Cleared C. krusei and C. glabrata infections | 82 | |
| TERB + FLU | Resolved infection | 75 |
Cited in the text.
Case reports.
In a study demonstrating the importance of order of addition of drugs on antifungal interaction, Ernst et al. (61) showed that preexposure of C. albicans cells to FLU for ≥8 h resulted in drastic inhibition of AmB activity. However, removal of FLU from the culture medium reversed the AmB inhibition within a very short period (<6 h). Similar studies were performed recently by Louie et al. (135), who used in vitro time-kill studies to show that preincubation of C. albicans with FLU for 18 h prior to the addition of AmB resulted in transient AmB antagonism. The duration of this induced AmB resistance was directly related to the duration of FLU preincubation. These in vitro findings were also reflected in an in vivo model used by these investigators (described below) (135).
In a separate study, Lewis et al. (123) used checkerboard dilution, E-test, and time-kill methods to determine the activities of FLU plus AmB, FLU plus 5FC, and AmB plus 5FC against six Candida isolates (three C. albicans isolates and one isolate each of C. glabrata, C. krusei, and C. tropicalis). The checkerboard method showed that all three combinations were indifferent or synergistic against all three C. albicans isolates, while the E-test showed that AmB plus FLU was antagonistic for two of the three C. albicans isolates and indifferent for the third isolate. Similar differences in interpretation between checkerboard and E-test methods were observed for exposure of C. glabrata to the three combinations, which were synergistic by checkerboard but indifferent by E-test. In general, there was better agreement between results from the E-test and time-kill methods.
These wide variations in results can often be traced to different types of strains, drug concentrations, method of evaluation, and the criteria of interpretation, and they underscore the necessity of a uniform set of guidelines (similar to the NCCLs guidelines for in vitro susceptibility testing of antifungals tested singly) for testing of antifungal combinations before results from these studies can be widely correlated.
(ii) In vivo studies.
The first study to examine whether antagonism in vitro was pertinent to experimental candidiasis in vivo compared AmB plus FLU in three murine models of invasive candidiasis: acute and subacute infections in immunocompetent mice, and subacute infection in immunosuppressed mice (220). This study showed that the order of administration of FLU with respect to AmB, using low-dose and high-dose regimens for each drug, was not a factor in rate of animal survival. In acute infection in immunocompetent mice, the combination was not antagonistic. On day 9 postinfection, the survival of mice treated with the antifungal combination was significantly increased compared to those treated with FLU alone (P < 0.01). In contrast, survival when the combination was used was as effective as that when AmB was used alone (75 and 90%, respectively; P > 0.1). For treatment of subacute infection in immunocompetent mice, the results were similar: no antagonism was noted, and survival rates were comparable (P > 0.1) for groups of mice receiving the combination or AmB alone. For treatment of less acute infection in immunocompromised mice, the combination was more effective on day 30 than was FLU alone (100 and 10% survival, respectively; P < 0.01), and was as effective as AmB alone (100 and 70% survival, respectively; P > 0.1).
In a separate in vivo study, Sanati et al. (198) demonstrated no antagonism between AmB and FLU in combination therapy in two candidiasis models: a neutropenic-mouse model of hematogenously disseminated candidiasis and a rabbit model of infective endocarditis. In the former model, the combination was responsible for significantly increased survival and reduced fungal densities in both the kidneys and the brain; in the latter model, significant reductions of fungal densities in cardiac vegetations were observed. Louie et al. (134) further evaluated the efficacy of FLU plus AmB in a murine model of systemic candidiasis for one FLU-susceptible strain (MIC, 0.5 μg/ml), two strains with intermediate FLU resistance (FLU-midresistant strains) (MIC, 64 and 128 μg/ml), and one highly FLU-resistant strain (MIC, 512 μg/ml) of C. albicans. Comparison parameters were survival and fungal densities in the kidneys of infected mice. For the FLU-susceptible and FLU-midresistant strains, the FLU-plus-AmB combination was antagonistic, as shown by both quantitative culture results and survival. Interestingly, for the highly FLU-resistant strain (which is where combination therapy is most likely to be used), no antagonism was observed (134). These results need to be verified by further studies. Although the breakpoints defining FLU susceptibility and resistance are arbitrary and do not follow the NCCLS breakpoints, these studies indicates that antagonism between AmB and FLU in animal models of candidiasis may also be influenced by the azole resistance phenotype of C. albicans.
(iii) Clinical studies.
A crucial question was whether the pattern seen in animal models will also be present in the clinical setting. A recent clinical trial attempted to address this issue, in nonneutropenic patient populations (185). These authors compared high-dose FLU monotherapy and high-dose FLU in combination with conventional AmB in a prospective clinical study of nonneutropenic patients with candidemia. Patients with positive blood culture for Candida spp. other than C. krusei and with fever or hypotension within 4 days or signs of local candidal infection within 2 days were randomized to receive either FLU (800 mg/day plus placebo), or FLU plus AmB (0.7 mg/kg/day). In both regimens, placebo or AmB was given only for the first 3 to 8 days while FLU was given for 14 days after symptom resolution. Analysis of 211 patients (FLU, n = 104; AmB plus FLU, n = 107) defined clinical success as blood culture clearance and symptom resolution. Infections identified at baseline were due to C. albicans (60%), C. glabrata (15%), C. parapsilosis (12%), C. tropicalis (9%), or other species (4%). Patient groups were comparable at baseline, except that those receiving FLU monotherapy had higher mean (± standard error) acute physiology and chronic health evaluation II (APACHE II) scores (16.9 ± 0.6 versus 15.1 ± 0.7; P = 0.046). Both overall success rates and blood clearance rates were statistically higher for the combination. Success rates were 68% for the combination and 56% for FLU monotherapy (P = 0.045); blood clearance failure rates were 17 and 7% for FLU monotherapy and the combination, respectively (P = 0.02). Additionally, the success rate by species was the same between regimens. Mortality rates at 30 and 90 days were not significantly different between groups. Results were also comparable between groups for treatment failures secondary to both renal toxicity and hepatotoxicity; patients receiving combination treatment had expectedly higher elevations in creatinine levels (16%) compared with those receiving monotherapy (6%; P = 0.021). The odds of treatment failure were decreased by having an infection attributable to C. parapsilosis (odds ratio [OR] = 0.208; P = 0.018) or by receiving combination treatment (OR = 0.681, 0.22); the odds of treatment failure were increased for patients with higher APACHE II scores (OR = 1.090 per point; P = 0.0006) or a requirement for total parenteral nutrition (TPN) (OR = 3.00; P = 0.0008). Treatment response was independent of prestudy antifungal therapy. The authors concluded that combination treatment with FLU plus AmB was not antagonistic compared with FLU monotherapy. Although the analysis was qualified by differences in baseline APACHE II scores, combination treatment overall trended toward better success and more rapid blood culture clearance, similar overall mortality for both regimens, but more episodes of renal toxicity for the combination (185). This is a seminal study because it adds significant input to the antagonism debate, at least in this patient population.
Amphotericin B plus 5-fluorocytosine: in vitro studies.
Keele et al. (105) reported that no antagonistic or additive effects were observed in vitro for the combination of AmB and 5FC against C. albicans and C. neoformans. Using time-kill analysis, three isolates each of C. albicans and Cryptococcus neoformans were tested with three monotherapy and two combination drug regimens of 5FC and AmB. Monotherapy regimens included 5FC (50 μg/ml), low-dose AmB (0.125 μg/ml), and high dose AmB (2.4 μg/ml). Combinations used a fixed dose of 5FC with either low-dose AmB (5FC at 50 μg/ml plus AmB at 0.125 μg/ml) or high-dose AmB (5FC at 50 μg/ml plus AmB at 2.4 μg/ml). None of the interactions were antagonistic. There were no differences between combination regimens with respect to either 5FC preexposure or timing, i.e., staggered versus simultaneous administration. In both the low-dose and high-dose combination regimens, drug-drug interactions were indifferent. Furthermore, regardless of the AmB concentration, no antagonism or additive effects were observed. The authors stated the importance of their finding of a lack of any antagonistic interaction between the two drugs in combination, reflecting its potential for improved clinical treatment of certain fungal infections based on the different mechanisms of action, distinct toxicity profiles, and complementary pharmacokinetic profiles (105).
Amphotericin B plus 5-fluorocytosine plus fluconazole: in vitro studies.
Using a microdilution plate technique, Ghannoum et al. (76) studied one-, two-, and three-drug regimens of AmB, FLU, and 5FC against three isolates each of C. albicans and C. neoformans. Results of two-drug combinations against both C. albicans and C. neoformans showed that inhibition by AmB plus FLU was greater than inhibition by either drug alone. At low concentrations of AmB, addition of 5FC enhanced the growth-inhibitory effect against C. albicans, but antagonism was noted at higher concentrations of AmB. Data for the three-drug pairs (AmB plus FLU, AmB plus 5FC, and FLU plus 5FC) were presented as contour plots, which showed distinct upward or downward contour plots for C. neoformans and C. albicans (Fig. 3). Results of the three-drug combinations for C. neoformans showed inhibition with AmB plus different concentrations of FLU plus a single fixed dose of 5FC. In the presence of 5FC, the combined effects of AmB and FLU on the growth of C. neoformans remained indifferent; when the AmB concentration was greater than approximately 1 to 1.2 μg/ml, addition of 5FC had no further effect on growth. These investigators suggested that the effects of a drug combination on in vitro fungal growth depends on the ratios and concentrations of the drugs used, as well as the fungal strains tested, apart from other differences related to variations in study design, pathogens, drug conditions, and regimens.
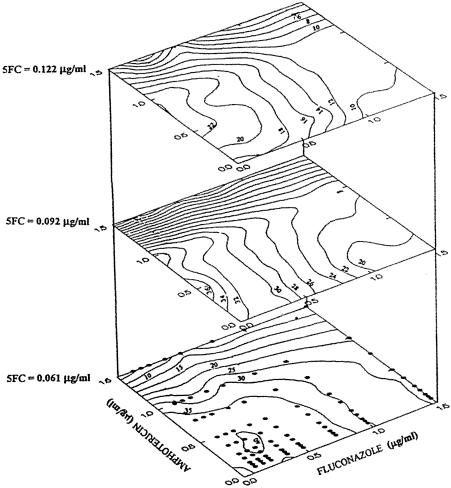
FIG. 3.
Stacked contour plots showing the combined effects of AmB, FLU, and 5-FC on the growth of C. neoformans. Susceptibilities of two- and three-drug combinations were determined using a microdilution method. The range of concentrations of drugs used for the combinations was based on the individual MICs and included concentrations both above and below the MICs. Analysis of antifungal drug concentrations and interactions affecting fungal growth was performed using multifactorial design models. Eighty data points were generated describing growth responses to a wide range of antifungal concentrations and ratios. The data were used to generate contour plots and surface response plots and to develop mathematical equations with coefficients providing the best empirical fit of the experimental data. The numerical values on the contours indicate the percentages of maximal fungal growth in the presence of the various combined antifungal concentrations. The points on the lower contour plane indicate the concentrations of AmB and FLU used at each of the three levels of 5-FC shown to develop the contour plots. Contour plots with generally upward concave form suggest favorable interactions, while those with concave downward form suggest less favorable interactions. The equation describing the best fit of the growth data was as follows: growth = 101 − 27(FLU) − 33(AmB) − 173(5FC) + 7(FLU)(AmB) + 19(FLU)(5FC) + 36(AmB)(5FC) − 7(AmB)(FLU)(5FC) + 2(FLU)2 + 78(5FC)2. These data demonstrate that at AmB concentrations of >1.0 to 1.2 μg/ml, addition of Flu had no further effect on growth. Reprinted from reference 76 with permission.
Amphotericin B plus fluconazole in sequential combinations against candidiasis.
The theoretical concept that using polyenes and azoles in combination may be antagonistic prompted the question whether using these two drug classes sequentially may circumvent the potential antagonism. Consequently, Vazquez et al. (229) investigated the interaction between AmB and FLU against Candida cells and demonstrated that preexposure of C. albicans to the azole leads to transient protection against subsequent exposure to AmB (229). In a subsequent study of FLU and AmB interactions, the order of drug initiation in this combination was examined in a two-part study using four Candida isolates (135). The in vivo implications of in vitro antagonism were examined in the first part by using the time-kill method and macrobroth dilutions (incubation for up to 48 h) and FLU preincubation (up to 40 hs). Assessments included log CFUs per milliliter and the effect of FLU preincubation on AmB (0.5 and 1.5 μg/ml). Results confirmed a transient, induced resistance to AmB as a result of FLU preincubation, with the increased duration of resistance being related to the increased time of FLU preincubation. For yeasts sequentially incubated with FLU followed by AmB plus FLU, fungistatic growth kinetics were similar to those of fungi exposed to FLU alone, with antagonism persisting for a minimum of 24 h. When the drugs were given concomitantly, the activity was similar to that of AmB alone at concentrations of ≥1 μg/ml. The second part of the study used a rabbit model of endocarditis and pyelonephritis. Groups of rabbits received eight different drug regimens, including monotherapy (untreated controls, FLU, and AmB), a simultaneous regimen (FLU plus AmB), and four sequential regimens (AmB followed by FLU, FLU followed by AmB, FLU followed by AmB plus FLU, AmB followed by AmB plus FLU). In vivo doses of FLU were based on the dose that resulted in the area under the concentration-time curve (AUC) for rabbit serum that mimicked the steady-state AUC measured in humans receiving FLU at 800 mg/day. The dose of AmB was based on the dose resulting in serum trough and AUC values similar in humans given 1 mg/kg of body weight/day. Endpoint parameters used to assess efficacy included concentrations of FLU and AmB in serum, tissue fungal burden for kidney and cardiac vegetations, and the effect of FLU given for 1 or 5 days prior to changing regimens to AmB plus FLU to AmB. Results showed that simultaneous (AmB plus FLU) and sequential (FLU followed by AmB plus FLU) regimen is were slower in clearing fungi from tissue then was AmB monotherapy or the sequential regimen initiated with AmB (AmB followed by AmB plus FLU). FLU preexposure (FLU for 1 day followed by AmB) delayed the clearance of fungi from cardiac vegetations and kidneys with respect to AmB monotherapy. Longer FLU preexposure (FLU for 5 days followed by AmB) required more time for AmB to effect clearance. The authors concluded that there were no negative consequences of switching from AmB to FLU during the treatment of deep-seated Candida infections, since this is the usual order of events in the clinic, with AmB empirical therapy switched to FLU once the infection is determined to be FLU susceptible. These investigators showed no therapeutic advantage in using FLU and AmB in sequential protocols (AmB followed by AmB plus FLU or FLU followed by AmB plus FLU) or simultaneously. Several other studies have demonstrated that both the order and sequence of drug administration play critical roles in combination therapy, and favor the use of either sequential approach, starting with AmB, or both drugs given concomitantly (61, 135, 138).
Fluconazole plus 5-fluorocytosine against candidiasis.
Since FLU and 5FC act on the fungal cell by completely different routes, it is expected that a combination of these two drugs will not be antagonistic and should be synergistic or noninteractive. The efficacy of this combination has been tested both in vitro and in vivo, as described below.
(i) In vitro studies.
Only a limited number of studies have been performed with the FLU-plus-5FC combination against Candida infections. In a recent study, Te Dorsthorst et al. (223) evaluated the in vitro efficacy of FLU plus 5FC against 27 Candida species including C. albicans (n = 9), C. glabrata (n = 9), and C. krusei (n = 9). These investigators defined drug interactions as synergy if the FICI was <1, additive if the FICI was 1, and antagonistic if the FICI was >1. Synergism and antagonism were observed for five and four C. albicans isolates, respectively. For C. krusei, synergy was observed for only one isolate, and antagonism was observed for eight isolates. Notably, this combination was antagonistic for all the C. glabrata isolates tested (223).
(ii) In vivo studies.
In vivo studies with 5FC-plus-FLU combinations have shown both synergistic and antagonistic interactions. Graybill et al. (86) evaluated the in vivo efficacy of combining 5FC with azoles in a murine model of disseminated C. tropicalis infection. Survival and tissue fungal burden of the spleen and kidneys were used to evaluate the efficacy of antifungal therapy. These studies showed that combining 5FC with FLU did not increase efficacy against C. tropicalis infection (86). In a separate study, Atkinson et al. (7) established an immunosuppressed-mouse model of C. glabrata infection to evaluate the efficacy of combinations of AmB, 5FC, and FLU treatments in vivo. Treatment with FLU, 5FC, AmB, or a combination was begun 1 day after infection. Kidneys and spleen CFU counts following 5 days of treatment revealed that the FLU-plus-5FC combination was superior to these agents alone in reducing the tissue fungal burden in the kidneys for one isolate of C. glabrata. High doses of FLU alone produced modest reductions in kidneys counts but did not reduce spleen tissue fungal burden. Moreover, there was a poor correlation between in vitro MICs and in vivo results (7). In a subsequent study, the efficacies of FLU plus 5FC and FLU plus AmB were evaluated in a rabbit model of C. albicans endocarditis, endophthalmitis, and pyelonephritis (136). In all the treatment groups except 5FC monotherapy, 93% of animals appeared well and survived until they were sacrificed. On day 5, the relative decreases in CFU per gram in the vitreous humor were greater in groups receiving FLU alone and in combination with 5FC or AmB than in groups receiving AmB or 5FC monotherapies (P < 0.005). However, the results were similar thereafter. In the choroid retina. 5FC was the least active drug. However, there were no differences in choroidal fungal densities between the other treatment groups. Both 5FC plus FLU and 5FC plus AmB demonstrated enhanced killing in cardiac vegetations compared with monotherapies (P < 0.03). In contrast, AmB plus FLU demonstrated antagonism in the treatment of C. albicans endocarditis and in reduction of kidney CFU (136).
(iii) Case reports.
Although studies using animal models suggested mixed interactions for 5FC-plus-FLU combinations, some clinical case reports have indicated successful treatment with this regimen. Scheven et al. (201) reported successful treatment of C. albicans sepsis with FLU-plus-5FC combination therapy. Recently, Girmenia et al. (82) reported two cases of a 46-year-old patient (with C. krusei infection) and a 10-year-old child (with C. glabrata infection) who were successfully treated with FLU-plus-5FC regimens. In vitro susceptibility testing of both strains showed resistance to FLU (MIC, >64 μg/ml). Combination therapy included FLU (600 mg/day) plus 5FC (4 g three times a day [tid], 150 mg/kg/day) for C. krusei; and a regimen of FLU (300 mg/day) plus 5FC (2 g/tid, 150 mg/kg/day) for C. glabrata infection. Combination therapy resulted in clearing of symptoms within 1 to 2 weeks. Subsequent in vitro susceptibility testing using FICI and time-kill methods revealed no antagonistic interactions (FICI = 1.0019) against both isolates.
Therefore, the in vitro and animal model results for the FLU-plus-5FC combination are not accurately predictive for clinical application; this may be due to differences in drug availability and tissue distribution of the drug in the in vivo setting or to differences in strains used in the in vitro animal experiments and clinical isolates. Although these studies suggest that combining FLU and 5FC may have utility in the treatment of azole-resistant Candida, further evaluation is necessary.
Newer Antifungals in Combination
Recently, two new antifungal agents, VORI and CAS, were approved for the treatment of fungal infections. In this section, we review the papers and abstracts that investigate the use of these agents in combination therapy.
Voriconazole combinations. (i) In vitro studies.
Checkerboard methods and visual and colorimetric end points were used to assess drug-drug interactions of 5FC combined in vitro with either AmB or VORI against C. albicans, C. glabrata, C. krusei, and C. tropicalis isolates (M. A. Ghannoum and N. Isham, Abstr. 29th Annu. Meet. Eur. Group Blood Marrow Transplant. abstr. P671, 2003). Interactions of 5FC with either VORI or AmB were synergistic or additive against 50% of C. albicans isolates tested. Interactions against C. glabrata isolates were additive for 5FC plus AmB (60%), with no antagonism noted; however, VORI-plus-5FC interactions were indifferent (50%) or antagonistic (50%). Additionally, 5FC-plus-AmB interactions were additive against 70% of the C. krusei isolates, which are innately resistant to FLU. Finally, VORI-plus-5FC exhibited synergistic or additive interactions against 40% of C. tropicalis. In another study, Ghannoum et al. (M. A. Hossain, M. A. Ghannoum, and D. J. Sheehan, Abstr. Trends Invasive Fungal Infect., abstr. P-59, 2001) determined the efficacy of VORI in combination with conventional AmB, liposomal AmB (LAmB), 5FC, FLU, micafungin (FK 463, MICAF), or CAS against isolates of Candida and Cryptococcus. No antagonism was observed for any combination evaluated. Across all combinations of VORI and another agent, 74% of the interactions were indifferent while 26% were synergistic or additive. Combinations of VORI and MICAF were synergistic against C. albicans and non-albicans species.
A separate study by Ghannoum et al. (M. A. Ghannoum, N. Isham, M. A. Hossain, and D. J. Sheehan, Abstr. Int. Immunocompromised Host Soc. Conf. abstr. 65, 2002) supported the results of an earlier trial using the same methods and definitions against nine Candida isolates. All the isolates were susceptible to VORI plus AmB and to CAS plus MICAF. Overall, VORI combinations were 33% additive, 4% synergistic, and 63% indifferent, with no observed antagonism. VORI-plus-AmB combinations were 17% additive and 83% indifferent. VORI-plus-FLU combinations were 42% additive and 58% indifferent. VORI-plus-MICAF combinations were 17% synergistic, 17% additive, and 58% indifferent. As before, the interactions were strain specific and correlations with clinical outcome were not determined. These studies emphasize the notion that VORI is unlikely to be antagonistic when combined with other antifungal agents.
Ernst et al. (63) used broth microdilution and E-test methods, and time-kill studies to determine the antifungal activities of AmB, FLU, 5FC, and VORI alone and in combination against 11 isolates of C. lusitaniae. AmB demonstrated fungicidal activity against most isolates tested, whereas FLU, VORI, and 5FC demonstrated primarily fungistatic activities. For AmB-resistant isolates (MIC, ≥3μg/ml), a trend toward shorter times taken to reach the fungicidal end point was observed in cells exposed to AmB plus 5FC, compared to those exposed to AmB alone (9.3 and 14.5 h, respectively; P = 0.068). These isolates (3 of 4) also showed a 0.5-log-greater reduction in fungal growth in the presence of AmB plus 5FC, indicating a greater extent of activity by this combination (63). Other combinations did not produce improvement in activity compared to single agents (63).
(ii) In vivo studies.
Recently, VORI has been studied in combination with 5-FC or AmB against systemic candidiasis in immunocompetent guinea pigs (C. A. Hitchcock, R. J. Andrews, B. G. H. H. Lewis, G. W. Pye, G. P. Oliver, and P. F. Troke, Int. J. Antimicrob. Agents 19(SI):S74, 2002; 4th Eur. Congr. Chemother. Infect. 2002), and the results are reviewed below.
(iii) Combination with 5-fluorocytosine or amphotericin B against systemic candidiasis in immunocompetent guinea pigs.
When VORI (0.1, 1, and 5 mg/kg p.o. twice daily [bid] for 5 days) was combined with 5-FC (5 mg/kg p.o. bid for 5 days), there was no evidence of antagonism in terms of survivors or reductions in kidney fungal loads. At 0.1 and 1 mg of VORI per kg, addition of 5-FC caused significantly greater decreases in fungal loads compared with the same VORI doses alone (P < 0.01). When combined with AmB (1 mg/kg intraperitoneally [i.p.]) VORI (0.1 and 1 mg/kg p.o. bid for 5 days) showed no antagonism and was significantly more effective in reducing fungal loads than was the corresponding VORI monotherapy (P < 0.001). The mean fungal load in animals treated with a combination of the highest dose of VORI (5 mg/kg p.o. bid for 5 days) and AmB (1 mg/kg i.p.) was significantly higher (P < 0.05) than that in animals receiving VORI monotherapy. Parallel groups of animals dosed with FLU (0.1, 1, and 5 mg/kg p.o. bid for 5 days) were included for comparison with VORI in these studies. As expected, when combined with AmB, FLU demonstrated a similar trend to that observed for VORI. Thus, addition of AmB (1 mg/kg i.p. once a day for 5 days) significantly (P < 0.01) increased the reduction in kidney fungal load at the lowest doses of FLU (0.1 and 1 mg/kg p.o. bid for 5 days). At the highest dose of FLU (5 mg/kg p.o. bid for 5 days), there was no significant difference (P < 0.01) in fungal load between the monotherapy and combination therapy groups. In general, combinations with VORI, when tested against Candida, did not display any antagonism. Clinical data are needed to determine the validity of these interpretations.
Caspofungin combinations.
The candins, which inhibit cell wall synthesis through disruption of 1,3-β-d-glucan synthesis and possibly 1,6-β-d-glucan synthesis (45, 85, 121, 165), may enhance the activity of either the azoles or AmB by increasing the rate or degree of their access to the cell membrane (Fig. 1 and 4). Hossain et al. (98) recently evaluated in vitro and in vivo efficacies of CAS plus AmB against an azole-resistant strain of C. albicans isolated from a patient who failed to respond to FLU therapy. In vitro antifungal susceptibility testing was performed based on the M27-A method and drug interactions assessed using the checkerboard technique. Interactions were defined as synergy (FICI < 0.5), positive (FICI < 1), negative (FICI > 1), or antagonism (FICI > 4.0). These investigators showed that combination of CAS and AmB resulted in a two- and fourfold reduction in MIC of AmB and CAS, respectively, and exhibited a positive in vitro interactive effect (FICI = 0.75). Furthermore, in vivo studies showed that the combination of CAS (0.002 mg/kg) plus AmB (0.016 mg/kg) significantly prolonged animal survival compared with untreated controls (P = 0.006). The proportions of mice treated with CAS plus AmB survived longer (72%) than those treated with the single drugs alone (50% for AmB, 22% for CAS), although this difference was not statistically significant (P = 0.36) compared to the effect of AmB alone. The CAS-plus-AmB combination was the only treatment that resulted in significant reduction in kidney CFU counts (P = 0.05). Compared to untreated controls, CFU counts in the brains of infected mice were significantly reduced when the animals were treated with CAS plus AmB (P = 0.005) or CAS alone (P = 0.05). However, no significant difference was observed between animals treated with CAS alone and CAS plus AmB (P = 0.094). Interestingly, no antagonistic interactions were observed between the two agents, which tended to result in favorable interactions in vivo and in vitro (98). Graybill et al. (87) recently investigated the in vivo efficacy of the CAS-plus-FLU combination in a murine model of candidiasis. FLU was administered at doses ranging from 0.06 to 5 mg/kg, while CAS doses ranged from 0.0005 to 5 mg/kg/day. Kidney tissue fungal burden was used as a measure of efficacy. These studies showed that none of the CAS-plus-FLU combinations led to any benefit over using the individual drugs alone.
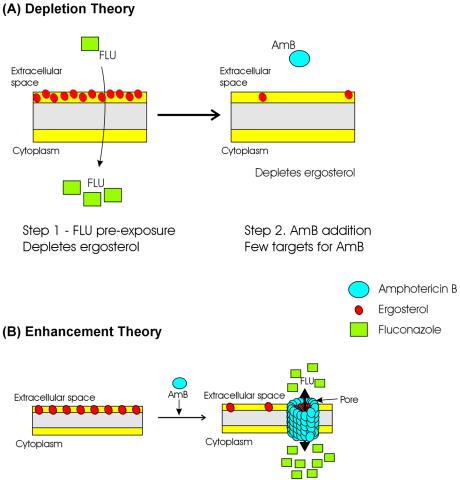
FIG. 4.
Schematic representation of depletion and enhancement theories of interactions between polyenes and azoles. (A) Depletion. Preexposure to azoles depletes the fungal cells of ergosterol, leading to fewer targets for the polyene, resulting in antagonism. (B) Enhancement. Exposure to AmB leads to the formation of pores that facilitate greater access of azoles to the intracellular space, inhibiting the enzymes involved in ergosterol biosynthesis, resulting in increased efficacy of the drug combination.
Although studies evaluating CAS in combination are limited, available data show a promise for using CAS combined with AmB. However, confirmation of these data clinically is warranted.
Fluconazole plus terbinafine combination against candidiasis: case report.
Although terbinafine (TERB) is approved for the treatment of superficial fungal infections including onychomycosis and tinea pedis, this drug attracted great interest in being used in combination with other antifungal agents to treat invasive mycoses. This interest stems from the fact that TERB inhibits fungal growth by blocking squalene epoxidase, an enzyme catalyzing the initial steps in ergosterol biosynthesis. Ghannoum and Elewski (75) described a 39-year-old woman who presented with white patches on her buccal mucosa, tongue, and palate with a bright erythematous erosive base. A fungal culture revealed C. albicans. The patient, who had been taking FLU for over 2 years previously (100 mg/day for 6 months, then 400 mg once a week) for a diagnosis of onychomycosis, failed to respond to the initially prescribed FLU therapy (200 mg/day for 2 weeks). In vitro testing of the culture from the patient showed elevated FLU, ITRA, and TERB MICs (32, 0.5, and 64 μg/ml, respectively). Administration of TERB (250 mg/day) combined with FLU (200 mg/day) for 2 weeks resulted in clearing of clinical symptoms, and the patient was successfully asymptomatic for more than 12 months posttreatment (75). Recent studies have also supported the observations that FLU-plus-TERB combinations are effective against Candida infections (D. Marriott, T. Pfeiffer, D. Ellis, and J. Harkness, Abstr. Trends Fungal Infect., vol. 5, abstr. P6.17, 1999). This combination is very interesting and should be pursued further.
ANTIFUNGAL COMBINATIONS AGAINST CRYPTOCOCCUS SPECIES
Established Antifungal Agents in Combination
Fluconazole plus 5-fluorocytosine against cryptococcosis. (i) In vitro studies.
Nguyen et al. (160) tested FLU plus 5FC (0.125 to 128 μg/ml range for each) against 50 clinical strains of C. neoformans. Combination of FLU with 5FC resulted in significant reductions in the geometric mean FLU MIC (from 5 to 1 μg/ml; P = 0.001) and of the 5FC MIC (from 12 to 0.1 μg/ml; P = 0.0001). Synergy (FICI < 1.0) was observed in 31 of 50 cases (62%), while antagonism (FICI > 2.0) was not observed. For cases in which synergy was achieved, the median reduction in MICs were fourfold (range, 2- to 16-fold) for FLU and fourfold (range, 2- to >1,000-fold) for 5FC. Addition of FLU greatly affected the in vitro inhibitory action of 5FC; the 5FC MICs for Cryptococcus isolates were markedly decreased to concentrations which were severalfold lower than the achievable 5FC concentration in cerebrospinal fluid. However, if the initial FLU MIC for the isolate was ≥8 μg/ml, addition of 5FC did not greatly enhance the in vitro activity of FLU (160). A drug interaction study of the FLU-plus-5FC combination against 35 yeast isolates (including Candida and Cryptococcus spp.) using the FICI and RSM methods demonstrated that by both methods, the combination was antagonistic against most Candida species and synergistic for some (223). However, for Cryptococcus species, the interaction for both combinations was highly dependent on the tested isolate and the method used (223) (Table 4).
TABLE 4.
Efficacy of drug combinations against Cryptococcus species
| Study | Combination | Regimen effect | Reference |
|---|---|---|---|
| In vitro | AmB + FLU | Synergistic (7%), additive (67%), indifferent (26%) | 12 |
| AmB + ITRA | Synergistic (7%), additive (73%), indifferent (20%) | 12 | |
| AmB + POSA | Synergistic (33%), additive (53%), indifferent (14%) | 12 | |
| 5FC + ITRA | Synergistic (63%), additive (31%), indifferent (6%) | 11 | |
| FC + POSA | Synergistic (33%), additive (67%) | 13 | |
| CAS + AmB | Synergistic (100%) | 70 | |
| CAS + FLU | Synergistic (22%), additive (50%), indifferent (28%) | 70 | |
| In vivo | VORI + 5-FC, VORI + AmB | Some antagonism at high doses of VORI (5-10 mg/kg p.o. bid for 10 days) | C. Hitchcock, R. J. Andrews, B. G. H. Lewis, G. W. Pye, G. P. Oliver, and P. F. Troke, Abstr. Int. J. Antimicrob. Agents 19(S1):S74, 2002; 4th Eur. Congr. Chemother. Infect. 2002a |
Cited in the text.
(ii) In vivo and clinical studies.
Nguyen et al. (161) evaluated the efficacy of the FLU-plus-5FC combination as therapy for cryptococcosis in a murine model of meningitis. Three strains of C. neoformans for which the range of FLU MICs was wide—2 μg/ml (susceptible strain), 8 μg/ml (moderately susceptible strain), and 32 μg/ml (resistant strain)—were used to challenge the mice and establish infection. At 1 day postinfection, the mice were randomized into eight treatment groups: placebo; 5FC (40 mg/kg of body weight/day); FLU at 3 mg/kg/day (low dosage), 10 mg/kg/day (moderate dosage), or 20 mg/kg/day (high dosage); and combined 5FC and FLU at low, moderate, or high doses of FLU. These studies showed that (i) MICs for the isolates correlated with the in vivo efficacy of FLU as assessed by the reduction in cryptococcal brain burden, (ii) a dose-response curve can be created for these studies (a higher dose of FLU was significantly more efficacious than a lower dose [P < 0.001]), and (iii) the combination of FLU plus 5FC was superior to therapy with either agent alone (P < 0.01) (161). Similar synergistic effects of FLU-plus-5FC combination therapy were demonstrated in vivo by Larsen et al. (116).
In a separate study using a murine model of cryptococcal meningitis and a study design based on dose response surface modeling, Ding et al. (49) attempted to identify the regions of various dose combinations of 5FC and FLU with the highest fungicidal activity and to determine the effect of a delay in the start of treatment. The range of doses used included 5FC at 0 to 140 mg/kg/day, FLU at 0 to 40 mg/kg/day (0 to 50 mg/kg/day for the day 7 group), and FLU plus 5FC in combination, with approximately four or five mice per group. Infection severity was varied by using a delay in treatment onset (3, 5, or 7 days postinoculation). Analysis of outcomes (duration and percent survival per group, weight change, and tissue fungal burden) relied on a Loess regression model (35) to characterize the effect of severity of cryptococcal meningitis. The combination had the most potent antifungal effects, but the range of effective dose combinations was progressively reduced as the severity of meningitis increased. In addition, the magnitude of the effect as measured by the fungal tissue burden (CFU per gram of brain tissue) was also reduced with increased severity of meningitis. Higher doses of FLU were required to achieve equivalent levels of activity with increased severity of the disease. This study suggested that combining higher doses of FLU with lower doses of 5FC could improve treatment for patients—especially AIDS patients—with cryptococcal meningitis and result in lower toxicity.
The efficacy of the FLU-plus-5FC regimen in the clinical setting was demonstrated by Cook et al. (37), who reported successful treatment of thoracic cryptococcal osteomyelitis with the combination of FLU plus 5FC. These studies supported the earlier clinical trial performed by Larsen et al. (117) showing a higher rate of clinical success with the FLU-plus-5FC combination compared to monotherapies.
Amphotericin B plus fluconazole against cryptococcosis
(i) In vitro studies.
Ghannoum et al. (76) evaluated the activity of two- and three-drug combinations of AmB, FLU, and 5FC against C. neoformans, using the RSM method. Contour plot analyses revealed that none of the three combinations exhibited antagonism but were indifferent in their activity against C. neoformans. Even the triple combination (AmB plus FLU plus 5FC) was not antagonistic (76). In a separate study, Barchiesi et al. (12) investigated the in vitro activity of AmB combined with FLU, ITRA, and posaconazole (POSA; previously known as SCH 56592) against 15 clinical isolates of C. neoformans by using the checkerboard method. The response for 7% of the isolates was synergistic for both the FLU-plus-AmB and ITRA-plus-AmB combinations. AmB plus POSA was synergistic (FICI < 0.5) for 33% of the C. neoformans isolates studied. Additivity (FICI, >0.50 to 1.0) was observed for 67, 73, and 53% of the isolates for the FLU-plus-AmB, ITRA-plus-AmB, and POSA-plus-AmB combinations, respectively. Indifference (FICI, >1.0 to ≤2.0) was observed for 26, 20, and 14% of the isolates for the FLU-plus-AmB, ITRA-plus-AmB, and POSA-plus-AmB combinations, respectively. Antagonism (FIC, >2.0) was not observed.
Overall these studies showed that in vitro, the AmB-plus-azole combination is synergistic or shows no interaction but is not antagonistic against C. neoformans.
(ii) In vivo studies.
Diamond et al. (46) examined double and triple combinations of AmB colloidal dispersion (ABCD) combined with 5FC with or without FLU over a wide range of doses for treatment of murine cryptococcal meningitis. Regimens included ABCD monotherapy (0 to 12.5 mg/kg of body weight intravenously [i.v.] for 3 days/week), FLU (0 to 50 mg/kg/day), ABCD plus 5FC (0 to 110 mg/kg/day), and all three drugs in combination. The dual 5FC-plus-FLU regimens tested low to moderate dose ranges (5FC and FLU at 10 to 45 mg/kg/day) and high dose ranges (5FC at >60 mg/kg/day plus FLU at >40 mg/kg/day). Results were partially analyzed using RSM for each of three end points (survival, weight change relative to baseline, and fungal burden in brain tissue) in an attempt to identify the ranges of effective dose combinations, as opposed to conventional analytical approaches that characterize the effects of specific doses. The addition of FLU to ABCD was required to achieve a maximum antifungal effect (P < 0.00001) and prevent weight loss (P < 0.00001). No mortalities were observed at FLU doses of ≥20 mg/kg/day (P < 0.00001). The only region of dose combinations for which the 99% CIs indicated <100 CFU/g of brain tissue was determined for the triple combination of ABCD (5.0 to 7.5 mg/kg) plus 5FC (20 to 60 mg/kg/day) plus (FLU 30 to 40 mg/kg/day). The triple combination ABCD plus 5FC plus FLU, within these defined ranges for each drug, was needed to achieve greatest antifungal activity. The authors showed that the most promising therapeutic effects were defined by higher doses of FLU combined with low to moderate doses of 5FC plus ABCD, rather than the combination of all three drugs at their respective maximum tolerated doses (46).
Larsen et al. (115) evaluated the antifungal activities of AmB (0.3 to 1.3 mg/kg of body weight/day), FLU (10 to 40 mg/kg/day), and 5FC (20 to 105 mg/kg/day), alone and in combination, in a murine model of cryptococcal meningitis. Activity was determined in terms of brain CFU count. The association between the response and the dose combination was evaluated by the RSM method. Administration of AmB alone led to 95% survival, regardless of the FLU or 5FC dose used, while the AmB-plus-FLU combination (with or without 5FC) gave the highest activity. Therefore, AmB plus FLU may be a viable alternative to the 5FC regimen for the treatment of cryptococcal meningitis.
Barchiesi et al. (12) described the effects of the AmB-plus-FLU combination in vivo by using an experimental model of systemic cryptococcosis in BALB/c mice. A clinical isolate of C. neoformans (strain 2337, serotype D), against which AmB plus FLU in vitro had an additive response, was injected i.v. into mice, and the tissue fungal burden and survival of mice were onitored. These investigators showed that AmB plus FLU (0.5 and 10 mg/kg/day, respectively) was more efficacious than FLU therapy (P < 0.0001) in survival studies but less efficacious than AmB monotherapy (P > 0.05). In the same investigation, tissue fungal burdens were analyzed with two combinations involving low (3 mg/kg/day) or high (10 mg/kg/day) FLU dose combined with AmB (0.5 mg/kg/day). The data showed that both combinations were more effective than monotherapies with either AmB or FLU in clearing all organs studied (lungs, kidneys, brain, liver, and spleen). In the same study, the effect of sequential therapy (FLU followed by AmB) was determined, and it was shown that sequential therapy was better than both monotherapies in reducing fungal burdens in lung, brain and spleen. Efficacy of sequential therapy was better than only one of the single drugs in the liver (better than AmB but not FLU) and kidneys (better than FLU but not AmB) (12). Notably, no antagonism was observed in either in vitro or in vivo effects of the AmB-plus-FLU combination against cryptococcosis. Thus, the simultaneous and sequential addition of AmB and triazoles interact differently with C. neoformans cells, suggesting that FLU preexposure leads to some degree of adaptation that protects the cells against the action of AmB (12). These studies also lend support to the “depletion” and “enhancement” theories for explaining the interactions between AmB and FLU (see below).
Newer Antifungals in Combination
Triazoles in combination. (i) In vitro studies.
The efficacy of combinations of the new triazoles against C. neoformans was investigated in separate studies (12, 13). These studies also attempted to determine any possible correlation between the in vitro and in vivo efficacies of these combinations. Drug interactions for AmB plus FLU, AmB plus ITRA, and AmB plus POSA were determined in vitro by using the checkerboard titration microdilution-based NCCLS method. When tested in vitro, all three combinations with AmB (AmB plus FLU, AmB plus ITRA, and AmB plus POSA) resulted in significant reductions in the geometric mean MICs of individual drugs (12). Thus, for the AmB-plus-FLU combination, the MIC AmB was reduced from 0.73 to 0.07 μg/ml (P = 0.0001) and that of FLU was reduced from 4.1 to 1.8 μg/ml (P = 0.029). For the AmB-plus-ITRA combination, the MIC of AmB was reduced from 0.83 to 0.10 μg/ml (P = 0.0001), while the ITRA MIC was reduced from 0.41 to 0.17 μg/ml (P = 0.009). Similarly, for the AmB-plus-POSA combination, the MIC of AmB was reduced from 0.57 to 0.15 μg/ml (P = 0.0001) while the POSA MIC was reduced from 0.45 to 0.08 μg/ml (P = 0.0001). Synergy was observed in ≥7% of the isolates tested for each combination, while no interaction was observed in ≥20% of the isolates tested for each combination. Importantly, antagonism was not detected for any of the combinations studied (12). In vitro studies evaluating the efficacy of 5FC plus POSA against C. neoformans showed that this combination led to significant reduction in the MICs of both 5FC (from 1.26 to 0.39 μg/ml; P = 0.0001) and POSA (0.13 to 0.02 μg/ml; P = 0.0001) (13). In a separate study, the effect of 5FC plus ITRA against 16 strains of C. neoformans was studied (11). Combination therapy revealed different results for the various strains, including synergy in 63% and no interaction in 37%, while antagonism was not observed in any of the interactions (11). Taken together, these studies clearly demonstrated the strain-dependent in vitro efficacy of combinations of AmB, triazoles, and 5FC against C. neoformans.
(ii) In vivo studies.
Several studies are described in the literature describing the efficacy of the 5FC-plus-triazole combination against cryptococcosis in vivo. In one the early studies, Polak (181) used a murine model of cryptococcosis and showed that the 5FC-plus-FLU combination is indifferent against C. neoformans. In a separate study using a hamster model of cryptococcosis, Iovanitti et al. (100) showed that the combination of 5FC+ITRA was less effective than monotherapy with individual drugs (100). Van Cutsem et al. (225) used a guinea pig model of cryptococcosis and showed that the combinations of 5FC plus ITRA or AmB plus ITRA were more efficacious than monotherapy. More recently, Barchiesi et al. (13) determined the effectiveness of 5FC plus POSA using a murine model of cryptococcosis and showed that combination therapy led to a significant reduction in the number of CFU compared to that observed with single drugs alone (P = 0.0001). Results obtained from both systemic cryptococcosis and cryptococcal meningitis indicated that combination therapy was more effective than either monotherapy (P < 0.05) (13). In another study, combinations of VORI plus 5FC (11%) and VORI plus CAS (33%) were additive against all Cryptococcus isolates tested, while VORI-plus-FLU, VORI-plus-AmB, and VORI-plus-MICAF combinations were indifferent (M. A. Hossain, M. A. Ghannoum, and D. J. Sheehan, Abstr. Trends Invasive Fungal Infect., abstr. P-59, 2001). When evaluated in immunocompetent guinea pig models of intracranial or pulmonary cryptococcosis (C. neoformans), VORI (5 mg/kg bid for 9 days) demonstrated efficacy comparable with the same dose of FLU on the basis of reductions in brain and lung fungal loads, respectively (C. Hitchcock, R. J. Andrews, B. G. H. Lewis, G. W. Pye, G. P. Oliver, and P. F. Troke, Program Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2740, 1995). The activity of VORI in combination with 5-FC or AmB was also investigated in the intracranial cryptococcosis infection model in immunocompetent guinea pigs. In common with systemic candidiasis in guinea pigs, the lower doses of VORI (0.1 and 1 mg/kg p.o. bid for 9 days) were not antagonized when combined with either 5-FC (5 mg/kg p.o. bid for 9 days) or AmB (2.5 mg/kg i.p. on alternate days for 9 days). By contrast, at the highest doses of VORI (5 to 10 mg/kg p.o. bid for 10 days), both 5-FC and AmB combinations showed some antagonism, but they were still effective in significantly reducing brain fungal loads compared with vehicle-treated control animals (Hitchcock et al., Int. J. Antimicrob. Agents 19(S1):S74, 2002).
Echinocandins in combination.
Echincandins represent a novel class of antifungals, targeted against the fungal cell wall biosynthesis, and combination of these drugs with other antifungal classes held the promise of being efficacious against fungi, without being antagonistic. Candins of interest include CAS, anidulafungin (ANIDU, VER-002; formerly LY 303366), and MICAF. CAS when used singly has no activity in vitro against C. neoformans (16). However, Franzot and Casadevall (70) showed that a combination of CAS with AmB or FLU resulted in enhanced efficacy against C. neoformans. These investigators performed in vitro combination testing based on the checkerboard method with 18 clinical and environmental C. neoformans strains. The combination of AmB (0.03 to 1.0 μg/ml) and CAS (8 to 16 μg/ml) led to a median eightfold reduction in the MICs of both drugs. The CAS-plus-AmB combinations exhibited 100% synergy against all the strains tested. In comparison, combining CAS with FLU (0.24 to 4 μg/ml) resulted in a twofold reduction in the MICs of each drug and in synergistic (22%), additive (50%), or indifferent (28%) interactions. No antagonism in the CAS-plus-FLU combination was observed. This is not surprising since, unlike polyenes and azoles, which have the same fungal cellular target, candins and azoles act at completely different sites, and no antagonism is expected.
For the FLU (1 μg/ml)-plus-CAS combination, significantly reduced fungal growth was observed only when the CAS concentration was 16 μg/ml, while no effect was detected in presence of 4 or 8 μg of CAS per ml. In the same study, fungal cell damage in the presence of different combinations of CAS with AmB or FLU was determined using a metabolic dye-based assay. These studies showed that combination of CAS (8 μg/ml) with increasing concentrations of AmB (0.03 to 1 μg/ml) resulted in significantly greater fungal damage compared to each drug used alone. Similarly, while CAS (16 μg/ml) alone induced 35% fungal damage, a combination of this echinocandin with FLU (0.25 to 4 μg/ml) increased fungal damage to the 56 to 65% range (70). Notably, and in contrast to CAS plus AmB, the effect of combining CAS with FLU was independent of the azole concentration (70). Overall, these investigators used the FICI method to show that the in vitro anticryptococcal activity was less pronounced for CAS plus FLU (22% synergism) compared to CAS plus AmB (100%). However, CFU studies and fungal damage {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide [XTT]} assays revealed that addition of CAS enhances the activities of both AmB and FLU against C. neoformans in vitro. Although the activity of both AmB and FLU is dependent on their efficient diffusion across the cell membrane (79), the exact mechanisms of action of CAS plus AmB and CAS plus FLU have not been elucidated. Franzot and Casadevall (70) proposed that CAS can enhance the activities of AmB and FLU by inhibiting cell wall synthesis, resulting in increased access of these drugs to the cell membrane and leading to enhanced efficacy of the combinations. One caveat in these studies is the high concentrations of CAS used (8 to 16 μg/ml) used. Interestingly, pharmacokinetic studies have demonstrated that administration of a 70-mg loading dose on day 1, followed by 50 mg of CAS daily, maintains mean plasma CAS concentrations at ∼1 μg/ml (218). Therefore the combination studies with CAS described above were based on very high levels of CAS, which renders the clinical relevance of these findings questionable.
In a separate study, Roling et al. (193) used a time-kill method to assess the efficacies of a low-dose CAS-plus-FLU-combination (2 and 20 μg/ml, respectively) and a very-low-dose combination of CAS and FLU (0.007 and 0.5 μg/ml, respectively) against two C. neoformans isolates. Mean CFU data (log10 CFU per milliliters) were plotted at different time points of fungal growth, and a ≥3 log10 (99.9%) reduction in CFUs from the starting inoculum was defined as fungicidal. Fungistatic activity was defined as <99.9% reduction in growth. Interactions in both these combinations resulted in indifference, with no antagonistic effects.
Taken together, these studies demonstrated that the efficacy of CAS plus FLU against Cryptococcus is influenced by the drug doses, with higher doses resulting in more synergistic interactions. Differences between the studies may also be attributed to (i) the use of different number of strains, i.e., 2 versus 18; (ii) different methods of testing, i.e., time-kill versus checkerboard; (iii) strain differences; and (iv) drug concentrations used. Since the concentration range used by Roling et al. (193) was lower than that used earlier by Franzot and Casadevall (70), the anticryptococcal activity of the CAS-plus-FLU combination appears to be concentration dependent and is enhanced in the presence of ≥8 μg of CAS per ml. Despite some differences, both studies reported no antagonism in any of the CAS-plus-FLU or CAS-plus-AmB combinations tested.
ANTIFUNGAL COMBINATIONS AGAINST ASPERGILLUS AND FUSARIUM SPECIES
Although the vast majority of the published literature is focused on combination therapy of candidiasis and cryptococcosis, more recent studies have investigated this approach for managing the difficult-to-treat mold infections (Table 5) (for a review, see reference 224 and Reference Guide for Difficult-to-Treat Fungal Infections, J. Chemother. 15(Suppl. 2), 2004, M. A. Ghannoum [guest editor]). This is not surprising, since the treatment of invasive mold infections, especially CNS aspergillosis and fusariosis, is plagued by high failure rates, necessitating the exploration of alternative means for therapy. Several studies, including clinical trials, have been performed to determine the efficacy of combination therapy against aspergillosis. These studies are summarized below.
TABLE 5.
Efficacy of drug combinations against Aspergillus species
| Study | Combination | Regimen effect | Reference |
|---|---|---|---|
| In vitro | VORI + CAS | Antagonistic (66%) | E. Dannaoui, O. Lortholary, and F. Dromer, Abstr. 13th Eur. Congr. Clin. Microbiol. Infect. Dis. abstr. P1493, 2003a |
| VORI + 5FC | Indifferent (42%), antagonistic (58%) | ||
| VORI + CAS | Synergistic (87.5%), additive (4.2%), subadditive (indifferent, 8.3%) | 170 | |
| VORI + CAS | Indifferent | 139 | |
| VORI + 5FC | Synergistic or additive (40%), indifferent (60%), antagonistic (none) | M. A. Ghannoum and N. Isham, Abstr. 29th Annu. Meet. Eur. Group Blood Marrow Transplant. abstr. P671, 2003a | |
| 5FC + FLU | Varied by strain and method (FICI or RSM) | 223 | |
| 5FC + CAS | Synergistic (92%) | E. Dannaoui, O. Lortholary, and F. Dromer, Abstr. 13th Eur. Cong. Clin. Microbiol. Infect. Dis., abstr. P1493, 2003a | |
| CAS + AmB | Additive (66%) | ||
| CAS + AmB | Synergistic (14%), additive (50%), indifferent (36%) | 6 | |
| ITRA + TERB | Synergistic | 222 | |
| ITRA + AmB | Antagonistic | 222, 223 | |
| 5FC + AmB | Synergistic | 223 | |
| ITRA + 5FC | Antagonistic | 223 | |
| TERB + AmB | Antagonistic | 222 | |
| TERB + AmB | Synergistic or additive | 194 | |
| TERB + ITRA | Synergistic | 194 | |
| TERB + VORI | Synergistic | 194 | |
| TERB + ITRA | Synergistic or additive | 151 | |
| TERB + FLU | Synergistic or additive | 151 | |
| TERB + AmB | Indifferent or antagonistic | 151 | |
| TERB + 5FC | Indifferent or antagonistic | 151 | |
| In vivo | VORI + CAS | Mortality, none; survival, complete; CFU/g, significantly less that monotherapy | 106 |
| MICF + RAVU | Significantly reduced | 172 | |
| ITRA + AmB | Antagonistic | 130 | |
| Clinical studyb | FLU + 5FC | Successful treatment of cryptococcal osteomyelitis | 37 |
| ITRA + FLU | Effective | 144 | |
| ITRA + LAmB | Effective | 144 | |
| ITRA + AmB | Cure in 82% of patients compared to 50% in monotherapy group | 183 | |
| CAS + AmB | Favorable response in 60-75% of patients | 4 | |
| CAS + LAmB | Good response to invasive aspergillosis in 2-yr-old patient with acute lymphoblastic leukemia | 56 | |
| VORI + CAS | Invasive aspergillosis resolved | T. Gentina, S. do Botton, S. Alfandri, J. Delomez, S. Jaillard, L. Leroy, C. J. Marquette, G. Beaucaire, F. Bauters, and P. Fenaux, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-860, 2002a | |
| LAmB + CAS | Invasive aspergillosis resolved | T. Gentina, S. do Botton, S. Alfandri, J. Delomez, S. Jaillard, L. Leroy, C. J. Marquette, G. Beaucaire, F. Bauters, and P. Fenaux, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-860, 2002a | |
| VORI + AmB | Favorable response in 37% of patients | A. Thiébaut, D. Antal, M. C. Breyesse, and C. Pivot, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-859, 2002a | |
| CAS + AmB | |||
| MICAF + AmB | 84 patients successfully treated | V. Ratanatharathorn, P. Flynn, J. A. van Burik, P. McSweeney, D. Niederwieser, and D. Kontoyiannis, Abstr. 17th Annu. Sci. Meet. Am. Soc. Hypertension, abstr. 2472, 2002a | |
| MICAF + AmB + triazole | |||
| MICAF + azole | Effective against aspergillosis; no adverse effects | A. J. Ullman, J. A. van Burik, P. McSweeney, V. Ratanatharathorn, J. Raymond, V. L. de Morais, J. McGurik, W. Lau, D. Facklam, S. Koblinger, M. Reusch, K. Marr, T. F. Patterson, and D. W. Denning, Abstr. 13th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. O-400, 2003a |
Cited in the text.
Case report, clinical trials, retrospective study, etc.
Established Antifungals in Combination
Amphotericin B, triazoles, and 5-fluorocytosine against Aspergillus and Fusarium: in vitro and in vivo studies.
Te Dorsthorst et al. (223) employed both FICI and RSM methods to determine in vitro interactions of AmB-plus-ITRA, AmB-plus-5FC, and ITRA-plus-5FC combinations against isolates of A. fumigatus, A. flavus, and A. terreus. As expected, these investigators observed higher MICs of 5FC (median MIC, 128 μg/ml) than either AmB (MIC, 0.50 μg/ml) or ITRA (MIC, 0.25 μg/ml) for all 20 isolates. Interactions (FICI) tended to vary by species and isolates for combinations of AmB plus ITRA and ITRA plus 5FC, with antagonism noted for all three species tested for both combinations (median FICI, 2.5 and 2.062, respectively). For AmB plus 5FC, synergy was observed against A. fumigatus isolates (FICI = 0.75) while antagonism was noted against A. flavus and A. terreus isolates (FICI = 2.5). Investigators were unable to determine FICIs for ITRA plus 5FC against ITRA-resistant A. fumigatus isolates. A similar species and strain variation was noted when the authors used ICα to define drug-drug interactions. For combinations of AmB plus ITRA and ITRA plus 5FC, they again found antagonism (median ICα = −0.04 and −0.05, respectively). Furthermore, for the combination of AmB plus ITRA, results were antagonistic for ITRA-sensitive isolates of A. fumigatus and A. flavus (ICα = −0.05) but synergistic for isolates of A. terreus (ICα = 0.08). No reliable ICα values were found for ITRA-resistant A. fumigatus isolates with the combination AmB plus ITRA. For AmB plus 5FC, they observed synergy for all Aspergillus isolates tested (ICα = 0.65). Although there were occasional discrepancies among the results between the two models, both indicated that the combination of AmB plus 5FC was the most potent combination against the tested Aspergillus spp. in vitro.
In a separate study, Kontoyiannis et al. (111) used the E-test method to evaluate the in vitro efficacy of ITRA (0.002 to 32 μg/ml) combined with AmB (0.002 to 32 μg/ml), added sequentially or concomitantly, against 12 A. fumigatus isolates. Both sequential and concomitant treatments resulted in antagonism, but the antagonism in sequential addition was greater than that in simultaneous addition.
Lewis et al. (130) used an established murine model of invasive pulmonary aspergillosis to evaluate the efficacy of several AmB doses (0.25, 0.5, 1.0, and 3.0 mg/kg) given alone or following preexposure to ITRA. The end points used to examine the efficacy of antifungal therapy included lung tissue fungal burden; mortality at 96 h, and histopathology of representative lung sections. At AmB doses of >0.5 mg/kg/day, fewer ITRA-preexposed mice versus non-ITRA-preexposed mice were alive at 96 h (0 to 20% and 60%, respectively). At all time points, the fungal lung burden was consistently and significantly higher in animals preexposed to ITRA, as measured by the CFU counts (P = 0.001) and the chitin assay (P = 0.03). Higher doses of AmB did not overcome this antagonism. ITRA preexposure was associated with poorer mycological efficacy and survival in mice treated subsequently with AmB for invasive pulmonary aspergillosis (130). However, in a recent study, Najvar et al. (154) investigated the interlaboratory variations in determining the in vivo efficacy of the AmB-plus-POSA combination in a murine model of A. flavus infection and reported consistent results from both sites. These investigators found that no antagonism existed between AmB and POSA in vivo, even when the experiments were designed to maximize the likelihood of antagonism. Thus, in vivo antagonism observed between AmB and some triazoles may be dependent on the actual azole being used (ITRA versus POSA).
One possible reason for the different interactions between AmB and triazoles in vivo may be related to variations in the pharmacodynamic and pharmacokinetic properties (drug absorption, distribution, and metabolism) of different triazoles.
Amphotericin B plus itraconazole against aspergillosis: clinical studies.
Popp et al. (183) examined the role of ITRA in the adjunctive treatment of invasive aspergillosis in a small, retrospective review conducted with patients having definite or probable aspergillosis (1995 to 1997) who were treated with conventional AmB alone or in combination with ITRA. Of 21 patients, 10 received AmB and 11 received the combination. The two groups were comparable at baseline, including similar mean APACHE II scores, and both groups received similar doses and duration of AmB. A higher proportion of patients who received combination therapy (9 of 11; 82%) were cured or showed improvement compared with those who received AmB monotherapy (5 of 10; 50%). In this clinical setting, ITRA and AmB in combination were not antagonistic. Results of a subsequent, large retrospective chart review (n = 595) of patients with aspergillosis who received either ITRA alone or in combination with AmB were considered inconclusive (169). The most severely immunosuppressed patients received AmB alone (n = 187); patients who were less severely immunosuppressed received either ITRA alone (58 patients) or AmB followed by ITRA (93 patients). Mortality rates for patients receiving AmB alone (65%) were much higher than that for patients receiving ITRA alone (26%) or AmB followed by ITRA (36%). These differential results were attributed to selection bias with respect to the treatment of patients included in the chart review and to the conclusion that immunosuppression (rather than drug regimen) was the single most important factor determining outcome (127).
Fluconazole plus itraconazole compared with amphotericin B against aspergillosis.
The rationale of using two azoles to treat fungal infections is based on filling in therapeutic gaps of the respective spectrum of activity for each, in that FLU is active against yeast while ITRA has a broader spectrum (incidentally, this is an example of the second rationale for using combination therapy [see above]). Mattiuzi et al. (144) recently published the results of a randomized clinical trial comparing prophylactic regimens of intravenous monotherapy with LAmB to the combination of oral FLU plus oral ITRA in patients undergoing induction chemotherapy for either acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS). oses used were FLU (200 mg every 12 h [q12]) + ITRA (200 mg q12h; n = 67) and LAmB (3.0 mg/kg i.v. three times weekly; n = 70). Clinical outcomes included time to fungal infection, tissue and blood cultures, signs and symptoms, occurrence of either fever of unknown origin or pneumonia of unknown etiology, and survival. The two groups were comparable at baseline, except for LAmB patients, who were more likely to be younger (P = 0.006) and to have had a history of an unfavorable previous malignancy (P = 0.032). However, response rates were comparable between the two regimens (LAmB, 60%; FLU plus ITRA, 56%), as was survival (10 deaths for patients receiving LAmB; 8 deaths for patients receiving FLU plus ITRA in combination). Two patients in the FLU-plus-ITRA group had infections due to Aspergillus; the authors proposed that these infections may have been due to inadequate ITRA absorption and recommended that higher doses of ITRA be considered for this patient group. They concluded that LAmB monotherapy and combination FLU plus ITRA showed comparable efficacy as prophylaxis against fungal infections in patients with AML or MDS undergoing chemotherapy and that the combination of FLU and ITRA was well tolerated, but that LAmB may be better for some patients, i.e., those who either cannot take oral medications or who may have absorption problems (144).
Newer Antifungals in Combination against Aspergillosis
Although VORI was introduced recently, a significant amount of data is already available for single and combination studies with this antifungal agent. Increasing interest has been focused recently on combining VORI and the candins with polyenes. In contrast, studies with CAS in combination are limited.
Voriconazole plus echinocandins and other antifungals. (i) In vitro studies.
The in vitro activity of two-drug combinations of VORI, 5FC, CAS, and AmB against clinical isolates of Aspergillus spp., including A. fumigatus and A. terreus, was recently investigated using a checkerboard modification of the NCCLS M-38P microdilution broth technique (E. Dannaoui, O. Lortholary, and F. Dromer, Abstr. 13th Eur. Congr. Clin. Microbiol. Infect. Dis. abstr. P1493, 2003). Combinations of CAS plus 5FC and VORI plus 5FC were tested against 14 isolates (A. fumigatus, n = 12; A. terreus, n = 2), while combinations of AmB plus CAS and VORI plus CAS were tested against 35 isolates (A. fumigatus, n = 30; A. terreus; n = 5). MICs were defined as the lowest concentrations showing 50% inhibition (CAS, 5FC, and VORI) or 100% inhibition (AmB) at 48 h. Combinations were generally additive (FICI, >0.5 to ≤1) against A. fumigatus for AmB plus CAS (66%) and VORI plus CAS (68%). Marked synergy (FICI, ≤0.5) was observed for 5FC plus CAS (92%). Combinations of VORI plus 5FC were either indifferent (FICI, > 1 to ≤4; 42%) or antagonistic (FICI, >4; 58%).
Dannaoui et al. (41) performed in vitro evaluation of two- and three-drug combinations of CAS, AmB, VORI, and 5FC against A. fumigatus and A. terreus. Combinations of CAS with either AmB or VORI were additive for all the isolates, and antagonism was not observed (the highest FICI values ranged from 1.00 to 2.5). The CAS-plus-5FC combination was synergistic for 7 of 12 A. fumigatus isolates (58%) and additive for 5 isolates (42%); antagonism was not observed (FICI, 0.62 to 1.0). In contrast, VORI plus 5FC was antagonistic against most (93%) of the isolates. The triple combination of CAS plus 5FC plus AmB was mostly synergistic (FICI, 0.04 to 0.41), while CAS plus 5FC plus VORI was synergistic for 8 of 12 isolates (67%) and additive for 4 isolates (33%). For both the triple combinations, complex interactions were obtained for some isolates, with synergy or antagonism noted for some concentrations of CAS and VORI.
Ghannoum et al. (M. A. Ghannoum, N. Isham, and D. J. Sheehan, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. abstr. M-855, 2002) investigated the efficacy of combining VORI with AmB, Abelcet (AB), FLU, MICAF, ravuconazole (RAVU), or CAS against different filamentous fungi by using the checkerboard technique. Filamentous fungi tested included three strains each of A. fumigatus, A. terreus, Pseudallescheria boydii, Scedosporium prolificans, and Acremonium sp. Isolates were chosen to include strains with high FLU, MICAF, and CAS MICs. All isolates were susceptible to VORI and RAVU, except S. prolificans (mean MICs, 28.24 and 4.0 μg/ml, respectively). All isolates were resistant to FLU, MICAF, and CAS. Susceptibility to AmB and AB varied. VORI-plus-CAS combinations were 33.3% additive and 66.6% indifferent, while VORI-plus-AmB combinations were 20% additive and 80% indifferent. Both VORI-plus-MICAF and VORI-plus-RAVU combinations were 13% additive and 87% indifferent. VORI-plus-FLU and VORI-plus-AB combinations were 100% indifferent. Overall, 14.5% of all VORI combinations against these filamentous isolates were additive while 85.5% were indifferent. These results indicated enhanced efficacy of several VORI drug combinations, but correlation with clinical outcome remains to be determined.
Using NCCLS broth microdilution methods to determine MICs, Perea et al. (170) studied interactions between VORI and CAS in 48 Aspergillus isolates from patients with invasive aspergillosis. Interactions were defined for synergy (FICI, <1), additivity (FICI, 1.0), subadditivity, indifference (FICI, 2), and antagonism (FICI, >2). This is the only study where the terms “subadditivity” (FICI, 1.0 to 2.0), “marked synergy” (FICI, ≤0.5) and “weak synergy” (FICI, 0.50 to 1.0) were used to describe drug interactions. These investigators reported that drug interactions were synergistic (87.5%), additive (4.2%), or subadditive (8.3%), while no antagonism was observed (170). In a separate study, Manavathu et al. (139) used an in vitro method to evaluate the efficacies of CAS plus VORI, CAS plus ITRA, CAS plus POSA, and CAS plus RAVU against A. fumigatus. These investigators showed that while CAS plus VORI or CAS plus RAVU resulted in no interaction (FICI, 0.61 and 1.61, respectively), CAS plus ITRA and CAS plus POSA exhibited synergism (FICI, 0.49 and 0.32, respectively) (139).
Arikan et al. (6) evaluated the in vitro interactions for CAS and AmB alone and in combination against Aspergillus (14 isolates) and Fusarium spp (6 isolates). In addition to the MICs, these investigators determined the minimum effective concentrations, defined as lowest concentration of the drug that results in the formation of aberrantly growing, unusual hyphal tips (114). Using checkerboard microdilution methods, susceptibilities and interactions were determined for isolates of Aspergillus and Fusarium, with 24-h and 48-h interpretations for MICs MECs, and FICI end points. Combination of CAS plus AmB resulted in a reduction of the CAS MIC by three- to ninefold, while that of AmB was reduced only slightly (one- to twofold) (6). For Aspergillus, drug interactions at 24 h were synergistic for 14% (2 of 14), additive for 50% (7 of 14), indifferent for 36% (5 of 14) of the isolates. The corresponding interactions for Fusarium isolates at 24 h were 50% synergistic (3 of 6), 17% additive (1 of 6), and 33% indifferent (2 of 6). Notably, no antagonism was observed (6). Results at 48 h were qualitatively similar to that obtained at 24 h. Similar to the study with C. neoformans performed previously (70), Arikan et al. (6) also hypothesized that the enhanced efficacy of the CAS-plus-AmB combination against Aspergillus may be due to CAS-mediated depletion of fungal cell wall, leading to greater penetration of the cell membrane by AmB (Fig. 1 and 4) (6).
(ii) In vivo studies.
Kirkpatrick et al. (106) used an immunosuppressed transiently neutropenic guinea pig model of invasive aspergillosis to evaluate the efficacy of VORI alone and in combination with CAS. Immunosuppression and transient neutropenia in the model were produced by triamcinolone (20 mg/kg of body weight/day subcutaneously [s.c.] for 4 days prior to challenge) and cyclophosphamide (150 mg/kg i.p. given 1 day prior to challenge), respectively. Seven drug regimens with 12 animals per group were used: untreated controls; four monotherapy regimens (CAS, i.p. 1 mg/kg/day and 2.5 mg/kg/day; VORI, i.p. 5 mg/kg/day; AmB, i.p. 1.25 mg/kg/day) and two combination regimens (VORI [5 mg/kg/day] plus low-dose CAS [1 mg/kg/day] or high-dose CAS [2.5 mg/kg/day]). Efficacy end points included mortality, survival time, and fungal burden (CFU in the liver, lungs, kidneys, and brain). Mortality on day 6 post-challenge was lower for all regimens than for controls (P < 0.0025). Furthermore, no mortality was observed for VORI monotherapy or either of the VORI-plus-CAS combinations. Semiquantitative cultures of liver, lung, kidney, and brain tissue revealed that CAS (1 mg/kg/day) reduced the Aspergillus fungal burden by 10- to 50-fold in brain, liver, and kidney tissue compared to that in untreated controls. In contrast, a higher dosage of CAS (2.5 mg/kg/day) resulted in only a 10-fold reduction in fungal burden in the kidneys. AmB, VORI, and the VORI-plus-CAS combinations also significantly reduced CFU in liver, brain and kidney tissues compared to those in untreated control tissues (P < 0.0025). Lung fungal burdens were only slightly reduced by CAS alone, but treatment with VORI-plus-CAS combinations resulted in significantly reduced CFU in the lung tissues (106). Organ cultures from guinea pigs killed 96 h after completion of therapy revealed that the VORI-plus-CAS combinations were significantly more effective than any of the other monotherapies in sterilizing the liver, lung, brain, and kidney tissues (P < 0.0025). Overall, about 25% of the animals receiving combination therapy with VORI plus CAS (1.5 mg/kg/day) had any organs positive for Aspergillus by culture. In contrast, about 92 to 100% of the animals receiving therapy with single drugs alone had any organs positive for Aspergillus culture (106). These studies supported previous in vitro investigations and suggest that the combination of CAS plus VORI may be an alternative to using either drug alone, especially in difficult-to-treat patients.
In the systemic-aspergillosis models (immune normal and neutropenic animals), the efficacy of VORI was not antagonised in combination either with 5-FC or AmB (Hitchcock et al., Int. J. Antimicrob. Agents 19(S1):S75, 2002). In animals receiving combinations of VORI (0.1 to 5 mg/kg p.o. bid for 5 days) and 5-FC (5 mg/kg p.o. bid for 5 days), no significant interaction between the antifungal agents was observed at any dose compared with the VORI monotherapy group, in terms of either reduction of fungal burden or cure rates. In analogous studies where 5-FC was replaced with AmB, treatment with VORI (1 mg/kg p.o. for 5 days) combined with AmB (2.5 mg/kg i.p. once daily for 5 days) was significantly more effective than the corresponding VORI monotherapy in reducing the fungal burden of the livers (P < 0.001), although cure rates between these two treatment groups were similar. At higher doses of VORI (5 and 10 mg/kg p.o.), addition of AmB had no significant effect on efficacy.
In common with neutropenic guinea pigs, the corresponding studies in immunocompetent animals showed that the efficacy of VORI was not antagonised by 5-FC or AmB; similarly, at lower concentrations of VORI, significant improvements in reducing fungal burden were achieved by the addition of AmB, whereas 5-FC showed no interaction at any of the doses compared with VORI monotherapy.
In a recent study, Sivak et al. (205) assessed the antifungal activity and renal and hepatic toxicity of AmB lipid complex (ABLC; Abelcet) following coadministration of CAS to rats infected with A. fumigatus. An inoculum of 1.3 × 107 to 2.3 × 107 CFU of A. fumigatus was injected via the jugular vein, followed by a single i.v. dose of AmB (1 mg/kg), ABLC (1 or 5 mg of AmpB/kg), or an equivalent volume of normal saline (vehicle control) once daily for 4 days. Rats were further randomized into groups to receive 3 mg of CAS per kg or physiologic saline i.v. once daily for 4 days. Antifungal activity was assessed from tissue fungal burden of brain, lung, heart, liver, spleen, and kidney sections. Renal and hepatic toxicity was assessed from serum creatinine and aspartate aminotransferase levels. Combinations of CAS (3 mg/kg) plus ABLC (1 or 5 mg/kg) significantly decreased the total number of A. fumigatus CFU found in all organs analyzed compared to the number after treatment with CAS alone and nontreated controls. However, the combination of CAS and ABLC was not more efficient than ABLC alone in reducing the tissue fungal burden. These findings suggest that combination of CAS plus ABLC is not antagonistic in vivo, although ABLC alone (5 mg/kg once daily for 4 days) appears to be the best therapeutic choice in this animal model (205).
(iii) Clinical studies.
In a recently reported clinical study involving six leukemia patients with invasive aspergillosis (IA) refractory to AmB, Gentina et al. (T. Gentina, S. do Botton, S. Alfandri, J. Delomez, S. Jaillard, L. Leroy, C. J. Marquette, G. Beaucaire, F. Bauters, and P. Fenaux, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-860, 2002) evaluated antifungal therapies combining VORI (200 mg bid) or LAmB (5 mg/kg/day) with CAS (70 mg on day 1, followed by 50 mg/day). Combination therapies were started 8 days after the initial IA diagnosis. The duration of neutropenia after initiation of combination therapy ranged 4 to 25 days. All patients had pulmonary IA, including one with disseminated IA. In all patients, sequential computed tomograms demonstrated improvement, with a rapid reduction of the size of the lesions. Improvement allowed administration of consolidation chemotherapy in three patients without recurrence of IA. During antifungal therapy, three patients died; none of those deaths were related to IA. No toxicity of antifungal therapy was observed. These results suggest that combination antifungal therapy of IA with VORI, CAS, and LAmB is a useful salvage therapy for IA refractory to AmB.
In a separate study, Thiébault et al. (A. Thiébaut, D. Antal, M. C. Breyesse, and C. Pivot, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-859, 2002) evaluated VORI-plus-AmB or CAS-plus-AmB combinations in six patients with refractory aspergillosis. These investigators showed that overall a 37% favorable response was detected. Furthermore, Ratanatharathorn et al. (V. Ratanatharathorn, P. Flynn, J. A. van Burik, P. McSweeney, D. Niederwieser, and D. Kontoyiannis, Abstr. 17th Annu. Sci. Meet. Am. Soc. Hypertension, abstr. 2472, 2002) reported significant success in treating 84 refractory aspergillosis patients with either a double (MICAF plus AmB) or a triple (MICAF plus AmB plus azole) combination of antifungals. These data were subsequently supported by Ullman et al. (A. J. Ullman, J. A. van Burik, P. McSweeney, V. Ratanatharathorn, J. Raymond, V. L. de Morais, J. McGurik, W. Lau, D. Facklam, S. Koblinger, M. Reusch, K. Marr, T. F. Patterson, and D. W. Denning, Abstr. 13th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. O-400, 2003), who performed an open phase II clinical trial of 283 patients with refractory IA and administered MICAF alone or in combination with azoles. These investigators reported that MICAF alone and in combination were both effective against aspergillosis, and no adverse effects were noted. These studies indicated that an echinocandin-plus-azole combination may represent a new strategy for treatment of pulmonary IA.
Recently, Durand-Joly et al. (54) reported the efficacy of VORI plus ABLC in the treatment of disseminated Fusarium oxysporum infection with skin localization in a woman with a relapse of B-acute leukemia during induction chemotherapy. The infection was refractory to ABLC alone (5 mg/kg of body weight/day) but responded successfully when VORI (loading dose, 6 mg/kg/day, followed by 4 mg/kg/day i.v. every 12 h) was added. No relapse was observed during a follow-up of 9 months.
Aliff et al. (4) reported clinical results for a retrospective chart review of CAS and AmB or LAmB in combination for refractory aspergillosis pneumonia in 30 patients with acute leukemia. Infections were determined as proven (6 patients), probable (4 patients), or possible (20 patients). The antifungal response was based on clinical and radiographic evidence; the response was further graded as favorable (complete or partial resolution of all radiographic evidence of fungal infection accompanied by definitive improvement in associated clinical signs and symptoms) or unfavorable (all other responses). The impact of neutrophil recovery as a potential confounding factor in the determination of AmB resistance was determined by the incidence and timing of neutropenia. At the time CAS treatment was initiated, 27 of 30 patients (90%) were receiving LAmB. All patients had progression of their fungal disease while they were receiving AmB or LAmB; 9 of 30 patients (30%) had fungal disease resistant to ITRA, of whom 4 had persistent fungal disease despite the combination of AmB and ITRA. The Median duration of ITRA monotherapy was 12 days (range, 4 to 65 days), while the median dose of LAmB monotherapy was 7.8 mg/kg (range, 4.2 to 66.1 mg/kg). The median duration of combination therapy was 24 days (range, 3 to 74 days). A favorable response was reported for 18 patients (60%; 95% CI, 42 to 78%), of whom 6 exhibited complete resolution of clinical signs and complete or near complete resolution of radiographic evidence of fungal pneumonia. Twenty patients with acute leukemia received combination for fungal pneumonias arising during chemotherapy; 15 of the patients (75%) had a favorable response independent of their response to leukemia treatment. Survival to discharge was significantly better (P < 0.001) for patients having a favorable response. Mild to moderate nephrotoxicity was reported for half of the patients, who subsequently received LAmB (4). These investigators showed that combination therapy had an overall favorable activity against Aspergillus in the patient groups studied and suggested that the CAS-plus-AmB combination is a safe and feasible option for high-risk patients with hematologic disorders and presumed AmB-resistant fungal infections (4). In a separate study, Elanjikal et al. (56) reported that in a 24-month-old girl with acute lymphoblastic leukemia and IA, combination therapy with CAS plus LAmB achieved a good response. These investigators suggested that combination therapy could be a useful treatment option in children with invasive fungal disease. In another study, Castagnola et al. (28) reported successful use of CAS combined with liposomal AmB or VORI as rescue therapy in two cases of documented and one case of possible invasive fungal infection in children with acute leukemia or undergoing allogeneic BMT (28).
A combination antifungal therapy including CAS could represent an effective therapy for invasive mycoses refractory to single-agent antifungal therapy. In view of these encouraging studies, large-scale randomized clinical trials need to be performed to assess whether an echinocandins-plus-AmB/LAmB combination is more efficacious than monotherapies.
Terbinafine plus triazoles and other antifungals against Aspergillus.
Te Dorsthorst et al. (222) reported that TERB plus ITRA was a potent combination showing synergy against ITRA-resistant and ITRA-sensitive strains of A. fumigatus. These investigators applied RSM to evaluate the interactions between AmB, ITRA, and TERB against 10 ITRA-susceptible and 5 ITRA-resistant clinical strains of A. fumigatus, using a modified checkerboard microdilution method that employs a foramzan dye (222). Their data revealed that the ITRA-plus-TERB combination was synergistic for both IT-S and IT-R strains while the AmB-plus-TERB and AmB-plus-ITRA combinations were antagonistic in vitro (222).
Other studies have supported the observation that ITRA-resistant fungi can be treated with TERB-plus-azole combinations. TERB in combination with either ITRA, FLU, AmB, or 5FC was tested against isolates of A. fumigatus (three isolates, one of which was ITRA-resistant) and A. flavus, A. niger, and A. terreus (two isolates each) using a broth microdilution-based method (151). MICs, fractional inhibitory concentration (FICs), and fractional fungicidal concentration (FFCs) were the determined endpoints. In vitro interactions of TERB in combination with ITRA or FLU against Aspergillus spp were favorable, while combinations of TERB plus AmB or 5FC were less effective. ITRA-plus-TERB was synergistic or additive against all strains tested (FICI, 0.15 to 1.0). FLU-plus-TERB was synergistic against A. fumigatus, A. terreus, and A. flavus (FICI, 0.3 to 0.5) and indifferent against A. niger (FICI, 2). TERB-plus-AmB interactions were primarily indifferent or antagonistic (FICI, 1.0 to 4.02), as were interactions for TERB-plus-5FC (FICI, 0.63 to 8.5). In this study, FFCs were generally in aggreement with FICIs (151).
Ryder and Leitner (194) tested the in vitro activity of TERB alone and in combination with other antifungal agents against isolates of A. fumigatus, A. flavus, and A. niger. Testing was performed by a modified NCCLS macrodilution broth assay, and interactions were examined using a checkerboard design. These investigators demonstrated that TERB was highly active (MIC, 0.01 to 2 μg/ml) and fungicidal (MFC, 0.02 to 4 μg/ml) against Aspergillus isolates; AmB was also highly active and fungicidal (MIC, 1 μg/ml; MFC, 1 to 4 μg/ml) (194). The triazoles ITRA and VORI were highly active but showed variable degrees of fungicidal activity against the different strains, with VORI having the more potent cidal activity. As expected, FLU had no significant activity (MIC, >128 μg/ml). Drug combinations (TERB combined with AmB, ITRA, or VORI) were tested against A. fumigatus and A. niger strains. The TERB-plus-AmB combination showed synergism or no interaction, depending on the isolate. Combinations of TERB-plus-ITRA or VORI-plus-TERB displayed potent synergistic interactions and fungicidal activity against all isolates. In general, TERB combined with a triazole appears to be more efficacious than combined with a polyene. Although many in vitro studies suggest synergy between TERB and other agents, in vivo confirmation is warranted.
ANTIFUNGAL COMBINATIONS AGAINST SCEDOSPORIUM SPECIES
In immunocompromised patients, Scedosporium prolificans can cause pulmonary or disseminated infection similar to aspergillosis or fusariosis (146, 233). S. prolificans infections are difficult to treat due to this organism's inherently poor response against available antifungals (133, 146, 182, 209). In vitro interactions between TERB, VORI, miconazole (MCZ), and ITRA were recently evaluated against five clinical isolates of S. prolificans by using a microdilution checkerboard technique (147). Antifungal effects of the drugs alone and in combination were based on determination of fungal biomass and metabolic activity at 48 and 72 h, using a spectrophotometric method and two colorimetric methods to generate cutoffs for MIC-1 (75% growth inhibition) and MIC-2 (50% inhibition). Interactions were analyzed using parametric, nonparametric, and semiparametric approaches. The investigators found statistically significant synergy between each of three azoles and TERB in all cases, but with different degrees of reduction of the geometric means of the MICs in combination for TERB (27- to 64-fold) and the azoles (16- to 90-fold). Corresponding FICIs ranged from <1 to 0.02. The strongest synergy among the azoles was found with the TERB-plus-VORI and TERB-plus-MCZ combinations, and synergistic effects on both fungal growth and metabolic activity were more potent at 72 hs. These studies demonstrated the in vitro efficacy of combining TERB with VORI or MCZ against S. prolificans (147) (Table 6).
TABLE 6.
Efficacy of drug combinations against uncommon molds
| Study | Organism | Combination | Regimen effect | Reference(s) |
|---|---|---|---|---|
| In vitro | S. prolificans | VORI + CAS | Synergistic | 212 |
| VORI + TERB | Synergistic | 147, 148 | ||
| TERB + ITRA | Synergistic | 147, 148 | ||
| TERB + MCZ | Synergistic | 147, 148 | ||
| P. boydii | AmB + FLU | Additive or synergistic | 234 | |
| AmB + ITRA | Additive or synergistic | 234 | ||
| AmB + MCZ | Additive or synergistic | 234 | ||
| Histoplasma | FLU + AmB | Synergistic (60%), additive (39%), indifferent (10%) | 120 | |
| Zygomycetes | AmB + RIF | Synergistic (69%), additive (31%) | 40 | |
| AmB + 5FC | Additive (100%) | 40 | ||
| AmB + TERB | Synergistic (20%), additive (80%) | 40 | ||
| VORI + TERB | Synergistic (44%), additive (56%) | 40 | ||
| In vivo | Histoplasma | FLU + AmB | No antagonism; CFUs, high (no clearance in lung) | 96, 120 |
| ITRA + AmB | No antagonism; CFUs, 0 (completely sterilized lung) | 120 | ||
| Clinical studya | S. prolificans | VORI + CAS | Synergistic | 212 |
| VORI + TERB | Cure of orthopedic infection | 84 | ||
| VORI + TERB | Control of disseminated infection | 99 | ||
| P. lilacinus | ITRA + CAS | Complete resolution | 197 | |
| Fonsecaea pedrosoib | ITRA + TERB | Synergistic | 93 |
Case report, clinical trials, etc.
Chromoblastomycosis.
Recently, Steinbach et al. (212) described a case of S. prolificans-associated osteomyelitis in a 5-year-old immunocompetent child. The patient was given empirical FLU monotherapy 4 weeks into the infection, followed by intravenous ITRA to expand mold coverage. The fungal isolate was identified as S. prolificans, and the therapy was changed to VORI (4 mg/kg/day) for 2 months, followed by a higher dose of VORI (6 mg/kg/day). Continued failure to resolve the infection prompted the addition of CAS to the treatment regimen at an initial load of 1 mg/kg followed by maintenance at 0.75 mg/kg/day. Surgical debridement was performed twice during systemic combination therapy (6-week course) and led to successful resolution of the infection. Subsequent in vitro antifungal susceptibility testing of the isolated S. prolificans strain revealed that the VORI MIC was 8 μg/ml while the CAS MIC was >4 μg/ml. Microdilution checkerboard-based determination of drug interactions revealed that the combination of VORI-plus-CAS resulted in synergistic interaction (FICI = 0.25) (212). In a recent case report, Gosbell et al. (84) reported the use of VORI-plus-TERB to successfully cure an orthopedic infection due to S. prolificans. Howden et al. (99) recently reported successful treatment of disseminated S. prolificans infection in a BMT patient by using a VORI-plus-TERB combination with aggressive surgical debridement. These studies suggest that difficult-to-treat fungal infections can benefit from combination treatment strategies employing VORI in combination with TERB or an echinocandins.
P. boydii, the sexual teleomorph of S. apiospermum, is resistant to AmB (233). Walsh et al. (234) determined the MICs, minimum lethal concentrations (MLCs; lowest concentrations of antifungals resulting in growth of three or fewer colonies), and FICIs of AmB alone and in combination with either ITRA, FLU, or MCZ against 22 clinical isolates of P. boydii. Except for MCZ, there was a broad range of antifungal activity, indicated by high and low MICs of the individual agents. Mean MICs ± standard error were as follows: AmB (1.1 ± 0.15 μg/ml; range, 0.25 to 2.0 μg/ml), ITRA (0.45 ± 0.20 μg/ml; range, 0.03 to 4.0 μg/ml), FLU (18 ± 2.2 μg/ml; range, 2.0 to 32 μg/ml), and MCZ (0.36 ± 0.04 μg/ml; range, 0.125 to 0.50 μg/ml). However, the mean MLCs of all four agents were 5 to 33 times higher than the corresponding MICs (AmB, 12 ± 3.0 μg/ml; ITRA, 15 ± 2.8 μg/ml; FLU, 87 ± 11 μg/ml; MCZ, 6.9 ± 1.8 μg/ml). Drug interactions (FICIs) were defined for synergy (≤0.05), additivity (>.05 but ≤1), indifference (>1 but ≤4), and antagonism (>4). The majority of AmB-azole interactions (16 of 22; 67%) were additive or synergistic; antagonism was not observed. The mean MIC of AmB was reduced in the presence of ITRA (P = 0.042), FLU (P = 0.11), or MCZ (P = 0.026). The investigators noted a strain-dependent response to AmB: 7 of the 22 isolates (32%) were resistant to AmB at concentrations ≥2.0 μg/ml, but 8 isolates (36%) were sensitive to this antifungal at concentrations ≤0.05 μg/ml. However, the overall disparity between the mean MICs and MLCs for the single agents, and the observed strain-dependent activity, led the authors to conclude that fungicidal effects were not likely to be attained at safely achievable concentrations in serum, limiting their use as individual agents in immunocompromised patients. The authors also concluded that their results were compatible with a mechanism of increased AmB-mediated permeability of the fungal cell membrane, permitting higher intracytoplasmic azole concentrations at the C-14-demethylase-cytochrome P-450 binding site, resulting in further inhibition of ergosterol synthesis in fungal cell membranes (Fig. 4). Clearly, more in vitro and in vivo studies are needed to investigate different combinations to identify appropriate candidates to treat infections caused by Scedosporum (particularly S. prolificans). However, based on the limited data, combinations employing VORI and TERB or an echinocandin may be promising.
ANTIFUNGAL COMBINATIONS AGAINST OTHER FUNGI
The efficacy of antifungal combinations against other fungi, such as Histoplasma, Paecilomyces, and zygomycetes isolates, have not been studied in much detail, and only a handful of studies are described in the literature (Table 6). A brief description of these studies is given.
Lemonte et al. (120) compared the in vitro efficacy of triazoles (ITRA or FLU) plus-AmB against 10 clinical isolates of Histoplasma and showed that drug interactions were synergistic for 60% (6 of 10), additive for 30% (3 of 10), and indifferent for 10% (1 of 10) of the isolates. No antagonism was observed for any of the isolates. In an animal model of histoplasmosis based on lung and spleen CFU determinations, the FLU-plus-AmB combination was shown to be antagonistic. However, these investigators suggested using brain tissue burden as an indicator of efficacy for more accurate analyses of these combinations. Haynes et al. (96) used a murine model of CNS histoplasmosis and brain tissue burden as an indicator of efficacy to demonstrate antagonism between FLU and AmB. Although FLU penetrates into the brain tissue, it was less effective as a single agent than AmB in its ability to clear the brain fungal burden (96). Addition of ITRA to AmB therapy neither improved nor hindered fungal clearance. Although limited; these studies suggest that AmB-plus-FLU combination therapy may not be effective for the treatment of CNS histoplasmosis, while the AmB-plus-ITRA combination is not antagonistic (96, 120).
Paecilomyces lilacinus is an emerging opportunistic pathogen in humans and can cause extensive infection in immunocompromised patients, especially infections of the skin, manifesting as erythematous macules, vesicles, pustules, and lesions (33, 101). Treatment in most cases is extremely difficult due to variable resistance of P. lilacinus to TERB, AmB, 5FC, and most azoles (33, 43, 64, 101). The susceptibility of P. lilacinus to ITRA and CAS is uncertain (33, 43), although single reports have described successful treatment with ITRA (94) or TERB (33). Recently, a case of rapidly progressive cutaneous P. lilacinus infection that responded to CAS-plus-ITRA combination antifungal therapy was reported (197). Initial treatment consisted of oral ITRA (600 mg qid for 3 days) followed by maintenance on 400 mg qid. Lack of response to ITRA for 7 days (demonstrated by the development of new skin lesions) prompted the administration of CAS at 70 mg on the first day and then 50 mg qid. thereafter. Complete resolution of the P. lilacinus infection was observed after 4 weeks of combination therapy (197). Treatment with combination therapy was continued for approximately 3 months, with no significant side effects.
In vitro evaluation of AmB-plus-TERB and ITRA-plus-TERB combinations against 17 clinical isolates of Zygomycota by using a checkerboard technique revealed that the ITRA-plus-TERB combination was synergistic effect against most of the strains. The AmB-plus-TERB combination was indifferent against Rhizopus oryzae and additive for the other species tested (83). In a separate study, Dannaoui et al. (40) reported the in vitro susceptibilities of 35 zygomycetes isolates (Rhizopus, n = 15; Absidia, n = 10; and other zygomycetes, n = 10) to four dual combinations of antimicrobial agents: AmB-plus-rifampin, AmB-plus-5FC, or AmB-plus-TERB, and VORI-plus-TERB (40). TERB was used at 0.25 μg/ml for the combinations, a level similar to that seen in serum (40). These investigators noted no antagonism for any of the four combination regimens. Drug interactions for AmB-plus-rifampin were either synergistic (69%) or additive (31%); those for AmB-plus-5FC were completely additive (100%); those for AmB-plus-TERB were synergistic (20%) or additive (80%); and those for VORI-plus-TERB were synergistic (44%) or additive (56%). As can be seen, VORI-plus-TERB showed the highest synergy among antifungal combinations, suggesting this combination as a possible alternative therapeutic option. Overall, these studies suggest that antifungal combinations can, in some cases, be useful therapies for treating infections due to uncommon fungi. However, more detailed in vitro and in vivo studies and more clinical experience are warranted in this area.
MISCELLANEOUS COMBINATIONS
In addition to antifungal-antifungal combinations, studies evaluating the efficacy of antifungals combined with antibacterial, anticancer, or immunomodulator agents have been undertaken. Since these studies are exploratory and limited in number, they are described briefly, and interested readers are encouraged to consult the original articles.
Combinations of Antifungal, Antibacterial, and Anticancer Agents
Although some studies have investigated the efficacies of antifungal, antibacterial and anticancer agents in combination, more studies need to be performed before a general conclusion may be reached regarding their effectiveness in the clinical setting. Here we present a brief summary of some of these studies.
The antifungal activities of the antibiotics ciprofloxacin and trovafloxacin, alone and in combination with AmB or FLU, were investigated in vivo against C. albicans in a murine model of hematogenously disseminated candidiasis, and in vitro against several molds with trovafloxacin (221). The two fluoroquinolones did not augment the in vitro activity of either AmB or FLU. However, in vivo, the combination of both ciprofloxacin and trovafloxacin with FLU was more effective than FLU alone in prolonging survival. The aromatic diamidine pentamidine (PN) displays multiples effects against protozoa, bacteria and fungi (17, 53, 58, 132, 137, 217a, 237). Among the fungi, its effect has been mostly studied against Pneumocystis carinii (95, 228), C. neoformans (10), C. albicans (150, 162), and A. fumigatus (3). The in vitro effect of PN-plus-ITRA and PN-plus-KETO combinations on 11 C. albicans strains (including one azole-resistant isolate) has also been studied (217a). These studies showed that the KETO-plus-PN combination had no significant effect on drug susceptibility of most strains tested while ITRA-plus-PN was fungicidal for eight strains in either combinations (217a). In another study, Afeltra et al. (J. Afeltra, E. Dannaoui, J. F. G. M. Meis, and P. E. Verweij, Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. abstr. M-849, 2002) investigated the effect of AmB combined with co-trimoxazole (SXT) against 60 clinical isolates of Aspergillus species grown in RPMI 1640 or AM3 media. These investigators demonstrated that in RPMI, AmB-plus-SXT was antagonistic for 45 of 60 isolates (75%), while in AM3 the combination was antagonistic against 29 of 60 (48%), and synergistic against 13 of 60 (21.6%). Although these results need to be confirmed in vivo, available data suggest that AmB-SXT is antagonistic against Aspergillus species (Afeltra et al., 42nd ICAAC, abstr. M-849, 2002). In a recent study, the in vitro efficacy of AmB and PN, alone or in combination, against 30 clinical isolates of S. prolificans was determined by using the FICI and RSM methods (2). These investigators showed that the combination of AmB-plus-PN exhibited synergy against 28 of 30 isolates (93.3%) and aditivity for 2 of 30 isolates (6.7%), while the RSM method predicted 100% synergy (2). Although these combinations showed promise during in vitro and in vivo studies, their clinical utility can be determined only after further studies.
The effect of combining antifungal agents with anticancer drugs such as tamoxifen or 5-fluorouracil against fungal pathogens has been studied in vitro (8, 18). Ghannoum et al. (78) evaluated the efficacies of combinations of two, three, and four drugs, including antineoplastic drugs, antifungal drugs, and combinations of both. Their data allowed predictions of the effects of combinations that provide maximum effectiveness in growth inhibition with minimum levels of the test drugs (78). In a separate study, Ghannoum et al. (77) investigated the effects of combinations of antifungal (AmB, 5FC, or MCZ) with antineoplastic (methotrexate, cyclophosphamide, or 5-fluorouracil) drugs on inhibition of the growth of yeasts. It was shown that interactive effects among antineoplastic and antifungal drugs may be very large and that drug combinations which were effective at low levels in inhibiting one test yeast were also generally effective against other species. However, the levels of susceptibilities and, to a lesser extent, the best ratios of drugs in the test combinations varied with species (77). The levels of drugs required for inhibition in combination drug treatments are critically dependent on the ratios as well as the absolute concentrations of drugs tested (74). The clinical relevance of these combinations remains undetermined.
Antifungals Combined with Immunomodulators
Since fungal infections occur mainly in immunosuppressed patients, it is reasoned that adding an immunomodulator or stimulator to an antifungal agent may improve the chance of a successful outcome. Consequently, researchers have sought to determine the effects of adding immune factors (e.g., granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor) or effector cells (e.g., primarily macrophages, polymorphonuclear neutrophils [PMNs], and monocytes) to antifungal drug regimens in an attempt to manipulate both innate and adaptive host defenses. Effective antifungal agents may act in collaboration with host effector cells at various intracellular and extracellular locations, or during different postinfection intervals corresponding to different phases and mechanisms of host defense response (29, 30, 213). Overall, various antifungals combined with immunomodulators against candidiasis have been shown to be generally more effective than monotherapy (30, 38, 112, 149, 232). Adjunctive immunotherapy using antibody-based therapies has been investigated for C. neoformans and A. fumigatus infections and generally shows enhanced activity with combination therapies (34, 69, 152, 153, 157, 192, 231). Different studies in this field have been summarized in previous reviews (213, 216, 217).
Some case reports have been described supporting the notion that immunomodulators can influence the efficacy of antifungal agents (29, 59). These studies suggested that immunomodulators may be acting via neutrophils (Th1 response) or monocytes (inducing tumor necrosis factor and macrophage inflammatory protein 1α). In separate studies, VORI, POSA, and ITRA enhanced the antifungal functions of human PMNs against hyphae of S. prolificans and S. apiospermium (81). Similarly, AmB lipid complex plus PMN displayed a significant additive effect against both Scedosporium species (22% for S. prolificans and 81% for S. apiospermum; P < 0.04) (80). Efficacies of LAmB plus granulocyte colony-stimulating factor have also been demonstrated in vivo by using an immunosuppressed murine model of disseminated Scedosporium infection (164). Recently, Steinbach et al. (210) used disk diffusion, microdilution checkerboard, and gross and microscopic morphological analyses to demonstrate that combination of the immunosuppressants cyclosporine or tacrolimus (FK506) with CAS exhibit a positive interaction against A. fumigatus.
Taken together, these studies show that combining an antifungal agent with concomitant improvement of host immune response through the use of an immunostimulator is a promising area that needs to be investigated through experimental animal systems and clinical trials. A clear demonstration of the clinical relevance of this approach is the decrease in the incidence of esophageal candidiasis in the HIV/AIDS setting, resulting from host immune reconstitution brought about by the use of highly active antiretroviral therapy (73). Although combining an antifungal with another therapeutic class has shown promise, more studies are needed to determine whether these combinations have widespread clinical relevance.
GENERALIZATIONS REGARDING ANTIFUNGAL COMBINATIONS
Based on the reviewed studies, the following generalizations can be drawn regarding antagonistic interactions, interpretation criteria of the FICI method, commonly used combinations, and in vitro-in vivo correlation of data.
Antagonistic Interactions
A primary concern, especially in the clinic, has been whether any antagonism exists between antifungals used in combination. Debate over combining a polyene with an azole is particularly relevant and has attracted considerable attention, with controversial and hotly debated data. This controversy stems from the fact that polyenes and azoles act on the same biochemical targets, namely, fungal membrane sterols. While polyenes bind to membrane sterols, the azoles inhibit the biosynthesis of these sterols.
Opponents of polyene-azole combinations have cited the antagonism observed in some cases as evidence of the “depletion theory.” According to this theory, preexposure of the fungus to azoles depletes ergosterol, which is the prime target for AmB action. Consequently, added AmB will not have the cellular target necessary for its activity, resulting in antagonistic interaction (105, 128, 130, 200). The “enhancement theory” (in which the efficacy of AmB is augmented by the addition of azoles) is based on the hypothesis that AmB, by binding to fungal membrane sterols and creating a pore, provides greater access to azoles into the cytoplasm, leading to increased inhibition of ergosterol synthesis (70, 147, 148, 223, 234).
Proponents of the use of polyene-azole combinations have argued that the lack of antagonism is due to subtle differences in the molecular mechanisms among the azoles (76, 79, 204); there is also good evidence that the action of AmB involves, in addition to its physicochemical interactions with sterols, other mechanisms including induction of cation permeability; interactions with membrane phospholipids, especially saturated fatty acids, which act as an important parameter for lipid peroxidation (23, 24, 72, 104); and the fact that azole treatment does not completely eliminate membrane sterols, so that the residual amount of ergosterol in the cell membrane may be sufficient for AmB to bind and inhibit fungal growth (72).
Interestingly, recent combination studies with the newer antifungal agents which have been developed (VORI and CAS) or are under development (POSA, RAVU, MICAF, and ANIDU) show no antagonistic interactions. More prominent among the studies demonstrating no antagonism between polyenes and azoles has been the recent clinical trial by Rex et al. (185), which compared AmB monotherapy with AmB-plus-FLU combination therapy. These investigators showed that the AmB-plus-FLU combination was not antagonistic in the treatment of candidemia in nonneutropenic patients. This is a landmark study, since it is the only randomized clinical trial that provides an insight into the lack of antagonism between AmB and FLU, tilting the scale in favor of no antagonistic interactions.
Interpretation of FICI Values
One of the main difficulties in our attempt to correlate different studies was related to the widely varying criteria used to interpret FICI results for drug-drug interactions (Table 1). Using a single system of interpretation will no doubt be of tremendous benefit to our ability to reduce the confusion that exists in the current literature. We recommend that the recently introduced interpretive criteria (synergy, FICI < 0.5; antagonism, FICI > 4.0; no interaction, FICI = 0.5 to 4.0 [Table 2] [163]) should be followed in future studies of antifungal combinations. This will facilitate a uniform interpretation of data and permit more relevant comparisons between different studies.
Potentially Useful Combinations
In vitro, in vivo, and clinical data have identified potentially useful combinations. The most commonly used combination of antifungal agents is that of 5FC with AmB or FLU, both against cryptococcosis. Recently approved drugs such as VORI and CAS have also been used in combination. Combinations of the new agents that may have clinical utility for treating Candida infections include VORI-plus-MICAF, 5FC-plus-CAS, CAS-plus-AmB, or FLU-plus-TERB, while combinations that showed potential promise for treating filamentous fungi (particularly Aspergillus) include VORI-plus-CAS, VORI-plus-LAmB, VORI-plus-TERB, and 5FC-plus-CAS. One caveat is that since antifungal drug-drug interactions are strain specific, it is not possible to select one combination to suit all members of a given species. This necessitates testing of the optimal combination for each particular clinical strain. Obviously, this adds enormous burden in terms of time and expense and therefore should not be conducted as a matter of routine. Therefore, in the clinical setting, antifungal combination therapy should be undertaken only in patients unresponsive to monotherapy and for whom the MICs of individual antifungals are elevated.
CONCLUSIONS
The recent increase in the number of publications and presentations at scientific and medical meetings, as well as the willingness of physicians to treat patients with more than one antifungal agent, clearly demonstrates that antifungal combination therapy is becoming a reality in the fields of infectious diseases and medical mycology. The discrepancies noted between different studies in vitro call for method standardization and adoption of a common interpretive criterion. Recent clinical data go a long way to addressing the controversy regarding antagonism between azoles and polyenes. Moreover, data generated using the newly approved antifungal agents VORI and CAS combined with other agents demonstrate that antagonism is highly unlikely. The goal of future studies should be to determine whether combination therapy with these new, promising agents improves survival and treatment outcome in the most seriously debilitated patients who are afflicted with life-threatening fungal infections.
REFERENCES
- 1.Abele-Horn, M., A. Kopp, U. Sternberg, A. Ohly, A. Dauber, W. Russwurm, W. Buchinger, O. Nagengast, and P. Emmerling. 1996. A randomized study comparing fluconazole with amphotericin B/5-flucytosine for the treatment of systemic Candida infections in intensive care patients. Infection 24:426-432. [DOI] [PubMed] [Google Scholar]
- 2.Afeltra, J., E. Dannaoui, J. F. Meis, J. L. Rodriguez-Tudela, and P. E. Verweij. 2002. In vitro synergistic interaction between amphotericin B and pentamidine against Scedosporium prolificans. Antimicrob. Agents Chemother. 46:3323-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afeltra, J., J. F. Meis, R. G. Vitale, J. W. Mouton, and P. E. Verweij. 2002. In vitro activities of pentamidine, pyrimethamine, trimethoprim, and sulfonamides against Aspergillus species. Antimicrob. Agents Chemother. 46:2029-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliff, T. B., P. G. Maslak, J. G. Jurcic, M. L. Heaney, K. N. Cathcart, K. A. Sepkowitz, and M. A. Weiss. 2003. Refractory Aspergillus pneumonia in patients with acute leukemia: successful therapy with combination caspofungin and liposomal amphotericin. Cancer 97:1025-1032. [DOI] [PubMed] [Google Scholar]
- 5.Alou, L., F. Cafini, D. Sevillano, I. Unzueta, and J. Prieto. 2004. In vitro activity of mupirocin and amoxicillin-clavulanate alone and in combination against staphylococci including those resistant to methicillin. Int. J. Antimicrob. Agents 23:513-516. [DOI] [PubMed] [Google Scholar]
- 6.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson, B. A., C. Bouthet, R. Bocanegra, A. Correa, M. F. Luther, and J. R. Graybill. 1995. Comparison of fluconazole, amphotericin B and flucytosine in treatment of a murine model of disseminated infection with Candida glabrata in immunocompromised mice. J. Antimicrob. Chemother. 35:631-640. [DOI] [PubMed] [Google Scholar]
- 8.Aziz, N. H., S. E. Farag, L. A. Mousa, and M. A. Abo-Zaid. 1998. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 93:43-54. [PubMed] [Google Scholar]
- 9.Babalola, C. P., K. B. Patel, C. H. Nightingale, and D. P. Nicolau. 2004. Synergistic activity of vancomycin and teicoplanin alone and in combination with streptomycin against Enterococcus faecalis strains with various vancomycin susceptibilities. Int. J. Antimicrob. Agents 23:343-348. [DOI] [PubMed] [Google Scholar]
- 10.Barchiesi, F., M. Del Poeta, V. Morbiducci, F. Ancarani, and G. Scalise. 1994. Effect of pentamidine on the growth of Cryptococcus neoformans. J. Antimicrob. Chemother. 33:1229-1232. [DOI] [PubMed] [Google Scholar]
- 11.Barchiesi, F., D. Gallo, F. Caselli, L. F. Di Francesco, D. Arzeni, A. Giacometti, and G. Scalise. 1999. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J. Antimicrob. Chemother. 44:65-70. [DOI] [PubMed] [Google Scholar]
- 12.Barchiesi, F., A. M. Schimizzi, F. Caselli, A. Novelli, S. Fallani, D. Giannini, D. Arzeni, S. Di Cesare, L. F. Di Francesco, M. Fortuna, A. Giacometti, F. Carle, T. Mazzei, and G. Scalise. 2000. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barchiesi, F., A. M. Schimizzi, L. K. Najvar, R. Bocanegra, F. Caselli, S. Di Cesare, D. Giannini, L. F. Di Francesco, A. Giacometti, F. Carle, G. Scalise, and J. R. Graybill. 2001. Interactions of posaconazole and flucytosine against Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry, A. L., M. A. Pfaller, S. D. Brown, A. Espinel-Ingroff, M. A. Ghannoum, C. Knapp, R. P. Rennie, J. H. Rex, and M. G. Rinaldi. 2000. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 38:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basselin, M., and M. Robert-Gero. 1998. Alterations in membrane fluidity, lipid metabolism, mitochondrial activity, and lipophosphoglycan expression in pentamidine-resistant Leishmania. Parasitol. Res. 84:78-83. [DOI] [PubMed] [Google Scholar]
- 18.Beggs, W. H. 1994. Comparative activities of miconazole and the anticancer drug tamoxifen against Candida albicans. J. Antimicrob. Chemother. 34:186-187. [DOI] [PubMed] [Google Scholar]
- 19.Bennett, J. E., W. E. Dismukes, R. J. Duma, G. Medoff, M. A. Sande, H. Gallis, J. Leonard, B. T. Fields, M. Bradshaw, H. Haywood, Z. A. McGee, T. R. Cate, C. G. Cobbs, J. F. Warner, and D. W. Alling. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N. Engl. J. Med. 19:126-131. [DOI] [PubMed] [Google Scholar]
- 20.Berenbaum, M. C. 1985. The expected effect of a combination of agents: the general solution. J. Theor. Biol. 114:413-431. [DOI] [PubMed] [Google Scholar]
- 21.Berenbaum, M. C. 1977. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin. Exp. Immunol. 28:1-18. [PMC free article] [PubMed] [Google Scholar]
- 22.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 23.Bolard, J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257-304. [DOI] [PubMed] [Google Scholar]
- 24.Bolard, J., and J. Milhaud. 1996. Interaction of the anti-Candida amphotericin B (and other polyene antibiotics) with lipids, p. 253-274. In R. Prasad and M. A. Ghannoum (eds.), Lipids of pathogenic fungi. CRC Press, Inc., Boca Raton, Fla.
- 25.Burgess, D. S., R. G. Hall, and T. C. Hardin. 2003. In vitro evaluation of the activity of two doses of Levofloxacin alone and in combination with other agents against Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 46:131-137. [DOI] [PubMed] [Google Scholar]
- 26.Burgess, D. S., and R. W. Hastings. 2000. A comparison of dynamic characteristics of fluconazole, itraconazole, and amphotericin B against Cryptococcus neoformans using time-kill methodology. Diagn. Microbiol. Infect. Dis. 38:87-93. [DOI] [PubMed] [Google Scholar]
- 27.Burgess, D. S., R. W. Hastings, K. K. Summers, T. C. Hardin, and M. G. Rinaldi. 2000. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 36:13-18. [DOI] [PubMed] [Google Scholar]
- 28.Castagnola, E., M. Machetti, B. Cappelli, A. C. Molinari, G. Morreale, P. Dodero, P. Toma, and M. Faraci. 2004. Caspofungin associated with liposomal amphotericin B or voriconazole for treatment of refractory fungal pneumonia in children with acute leukaemia or undergoing allogeneic bone marrow transplant. Clin. Microbiol. Infect. 10:255-257. [DOI] [PubMed] [Google Scholar]
- 29.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2001. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 39:99-103. [DOI] [PubMed] [Google Scholar]
- 30.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2002. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Med. Mycol. 40:21-26. [DOI] [PubMed] [Google Scholar]
- 31.Chiou, C. C., N. Mavrogiorgos, E. Tillem, R. Hector, and T. J. Walsh. 2001. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45:3310-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chryssanthou, E., and M. Cuenca-Estrella. 2002. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J. Clin Microbiol. 40:3841-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark, N. M. 1999. Paecilomyces lilacinus infection in a heart transplant recipient and successful treatment with terbinafine. Clin. Infect. Dis. 28:1169-1170. [DOI] [PubMed] [Google Scholar]
- 34.Clemons, K. V., and D. A. Stevens. 2001. Overview of host defense mechanisms in systemic mycoses and the basis for immunotherapy. Semin. Respir. Infect. 16:60-66. [DOI] [PubMed] [Google Scholar]
- 35.Cleveland, W. S., E. Grosse, and W. M. Shyu. 1991. Local regression models, p. 309-376. In J. M. Chambers and T. Hastie (ed.), Statistical models. Chapman and Hall, New York, N.Y.
- 36.Colombo, A. L., F. Barchiesi, D. A. McGough, A. W. Fothergill, and M. G. Rinaldi. 1995. Evaluation of the E test system versus a microtitre broth method for antifungal susceptibility testing of yeasts against fluconazole and itraconazole. J. Antimicrob. Chemother. 36:93-100. [DOI] [PubMed] [Google Scholar]
- 37.Cook, P. P. 2001. Successful treatment of cryptococcal osteomyelitis and paraspinous abscess with fluconazole and flucytosine. South. Med. J. 94:936-938. [PubMed] [Google Scholar]
- 38.Coste, A., M. D. Linas, S. Cassaing, J. Bernad, S. Chalmeton, J. P. Seguela, and B. Pipy. 2002. A sub-inhibitory concentration of amphotericin B enhances candidastatic activity of interferon-gamma- and interleukin-13-treated murine peritoneal macrophages. J. Antimicrob. Chemother. 49:731-740. [DOI] [PubMed] [Google Scholar]
- 39.Daneshmend, T. K., and D. W. Warnock. 1983. Clinical pharmacokinetics of systemic antifungal drugs. Clin. Pharmacokinet. 8:17-42. [DOI] [PubMed] [Google Scholar]
- 40.Dannaoui, E., J. Afeltra, J. F. Meis, and P. E. Verweij. 2002. In vitro susceptibilities of zygomycetes to combinations of antimicrobial agents. Antimicrob. Agents Chemother. 46:2708-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dannaoui, E., O. Lortholary, and F. Dromer. 2004. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 48:970-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasbach, E. J., G. M. Davies, and S. M. Teutsch. 2000. Burden of aspergillosis-related hospitalizations in the United States. Clin. Infect. Dis. 31:1524-1528. [DOI] [PubMed] [Google Scholar]
- 43.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denning, D. W., L. H. Hanson, A. M. Perlman, and D. A. Stevens. 1992. In vitro susceptibility and synergy studies of Aspergillus species to conventional and new agents. Diagn. Microbiol. Infect. Dis. 15:21-34. [DOI] [PubMed] [Google Scholar]
- 45.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 46.Diamond, D. M., M. Bauer, B. E. Daniel, M. A. Leal, D. Johnson, B. K. Williams, A. M. Thomas, J. C. Ding, L. Najvar, J. R. Graybill, and R. A. Larsen. 1998. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob. Agents Chemother. 42:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding, J. C., M. Bauer, D. M. Diamond, M. A. Leal, D. Johnson, B. K. Williams, A. M. Thomas, L. Najvar, J. R. Graybill, and R. A. Larsen. 1997. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob. Agents Chemother. 41:1589-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dismukes, W. E. 2000. Introduction to antifungal drugs. Clin. Infect. Dis. 30:653-657. [DOI] [PubMed] [Google Scholar]
- 51.Dismukes, W. E., G. Cloud, H. A. Gallis, T. M. Kerkering, G. Medoff, P. C. Craven, L. G. Kaplowitz, J. F. Fisher, C. R. Gregg, C. A. Bowles, et al. 1987. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N. Engl. J. Med. 317:334-341. [DOI] [PubMed] [Google Scholar]
- 52.Dogruman, A. F., E. Aktas, A. Ayyildiz, N. Yigit, and E. Tuncel. 2003. Determination of antifungal susceptibilities of Candida species blood culture isolates by using the macrodilution method and E-test. J. Chemother. 15:515-516. [DOI] [PubMed] [Google Scholar]
- 53.Donkor, I. O., and A. M. Clark. 1999. In vitro antimicrobial activity of aromatic diamidines and diimidazolines related to pentamidine. Eur. J. Med. Chem. 34:639-643. [DOI] [PubMed] [Google Scholar]
- 54.Durand-Joly, I., S. Alfandari, Z. Benchikh, M. Rodrigue, A. Espinel-Ingroff, B. Catteau, C. Cordevant, D. Camus, E. Dei-Cas, F. Bauters, L. Delhaes, and S. De Botton. 2003. Successful outcome of disseminated Fusarium infection with skin localization treated with voriconazole and amphotericin B-lipid complex in a patient with acute leukemia. J. Clin. Microbiol. 41:4898-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 56.Elanjikal, Z., J. Sorensen, H. Schmidt, W. Dupuis, K. Tintelnot, G. Jautzke, T. Klingebiel, and T. Lehrnbecher. 2003. Combination therapy with caspofungin and liposomal amphotericin B for invasive aspergillosis. Pediatr. Infect. Dis. J. 22:653-656. [DOI] [PubMed] [Google Scholar]
- 57.Eliopoulos, G. M., and R. C. Moellering, Jr. 1991. Antimicrobial combinations, p. 432-492. In U. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, Md.
- 58.Ellenberger, T. E., and S. M. Beverley. 1989. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J. Biol. Chem. 264:15094-15103. [PubMed] [Google Scholar]
- 59.Ellis, M., R. Watson, A. McNabb, M. L. Lukic, and M. Nork. 2002. Massive intracerebral aspergillosis responding to combination high dose liposomal amphotericin B and cytokine therapy without surgery. J. Med. Microbiol. 51:70-75. [DOI] [PubMed] [Google Scholar]
- 60.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 61.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 1998. In vitro interaction of fluconazole and amphotericin B administered sequentially against Candida albicans: effect of concentration and exposure time. Diagn. Microbiol. Infect. Dis. 32:205-210. [DOI] [PubMed] [Google Scholar]
- 62.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ernst, E. J., K. Yodoi, E. E. Roling, and M. E. Klepser. 2002. Rates and extents of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 46:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J . Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espinel-Ingroff, A., and A. Rezusta. 2002. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 40:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espinel-Ingroff, A., D. W. Warnock, J. A. Vazquez, and B. A. Arthington-Skaggs. 2000. In vitro antifungal susceptibility methods and clinical implications of antifungal resistance. Med. Mycol. 38:293-304. [PubMed] [Google Scholar]
- 69.Feldmesser, M., J. Mukherjee, and A. Casadevall:1996. Combination of 5-flucytosine and capsule-binding monoclonal antibody in the treatment of murine Cryptococcus neoformans infections and in vitro. J. Antimicrob. Chemother. 37:617-622. [DOI] [PubMed] [Google Scholar]
- 70.Franzot, S. P., and A. Casadevall. 1997. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 41:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galgiani, J. N., N. M. Ampel, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2000. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin. Infect. Dis. 30:658-661. [DOI] [PubMed] [Google Scholar]
- 72.Ghannoum, M. A. 1997. Susceptibility testing of fungi and correlation with clinical outcome. J. Chemother. 9(Suppl. 1):19-24. [PubMed] [Google Scholar]
- 73.Ghannoum, M. A. 2001. Candida: a causative agent of an emerging infection. J. Investig. Dermatol. 6:188-196. [DOI] [PubMed] [Google Scholar]
- 74.Ghannoum, M. A., K. H. Abu-Elteen, M. S. Motawy, M. A. Abu-Hatab, A. S. Ibrahim, and R. S. Criddle. 1990. Combinations of antifungal and antineoplastic drugs with interactive effects on inhibition of yeast growth. Chemotherapy 36:308-320. [DOI] [PubMed] [Google Scholar]
- 75.Ghannoum, M. A., and B. Elewski. 1999. Successful treatment of fluconazole-resistant oropharyngeal candidiasis by a combination of fluconazole and terbinafine. Clin. Diagn. Lab. Immunol. 6:921-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghannoum, M. A., Y. Fu, A. S. Ibrahim, L. A. Mortara, M. C. Shafiq, J. E. J. Edwards, and R. S. Criddle. 1995. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob. Agents Chemother. 39:2459-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghannoum, M. A., M. S. Motawy, M. A. Abu Hatab, K. H. Abu Elteen, and R. S. Criddle. 1989. Interactive effects of antifungal and antineoplastic agents on yeasts commonly prevalent in cancer patients. Antimicrob. Agents Chemother. 33:726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghannoum, M. A., M. S. Motawy, M. A. Abu Hatab, A. S. Ibrahim, and R. S. Criddle. 1989. Multifactorial analysis of effects of interactions among antifungal and antineoplastic drugs on inhibition of Candida albicans growth. Antimicrob. Agents Chemother. 33:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gil-Lamaignere, C., E. Roilides, A. Maloukou, I. Georgopoulou, G. Petrikkos, and T. J. Walsh. 2002. Amphotericin B lipid complex exerts additive antifungal activity in combination with polymorphonuclear leucocytes against Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 50:1027-1030. [DOI] [PubMed] [Google Scholar]
- 81.Gil-Lamaignere, C., E. Roilides, J. Mosquera, A. Maloukou, and T. J. Walsh. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Girmenia, C., M. Venditti, and P. Martino. 2003. Fluconazole in combination with flucytosine in the treatment of fluconazole-resistant Candida infections. Diagn. Microbiol. Infect. Dis. 46:227-231. [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Lopez, A., M. Cuenca-Estrella, E. Mellado, and J. L. Rodriguez-Tudela. 2003. In vitro evaluation of combination of terbinafine with itraconazole or amphotericin B against Zygomycota. Diagn. Microbiol. Infect. Dis. 45:199-202. [DOI] [PubMed] [Google Scholar]
- 84.Gosbell, I. B., V. Toumasatos, J. Yong, R. S. Kuo, D. H. Ellis, and R. C. Perrie. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233-236. [DOI] [PubMed] [Google Scholar]
- 85.Graybill, J. R. 2001. The echinocandins, first novel class of antifungals in two decades: will they live up to their promise? Int. J. Clin. Pract. 55:633-638. [PubMed] [Google Scholar]
- 86.Graybill, J. R., L. K. Najvar, J. D. Holmberg, and M. F. Luther. 1995. Fluconazole, D0870, and flucytosine treatment of disseminated Candida tropicalis infections in mice. Antimicrob. Agents Chemother. 39:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graybill, J. R., R. Bocanegra, L. K. Najvar, S. Hernandez, and R. A. Larsen. 2003. Addition of caspofungin to fluconazole does not improve outcome in murine candidiasis. Antimicrob. Agents Chemother. 47:2373-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 89.Greco, W. R., H. S. Park, and Y. M. Rustum. 1990. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-β-d-arabinofuranosylcytosine. Cancer Res. 50:5318-5327. [PubMed] [Google Scholar]
- 90.Groll, A. H., and T. J. Walsh. 2002. Antifungal chemotherapy: advances and perspectives. Swiss Med. Wkly. 132:303-311. [DOI] [PubMed] [Google Scholar]
- 91.Gudlaugsson, O., S. Gillespie, K. Lee, B. J. Vande, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 92.Gunderson, B. W., K. H. Ibrahim, L. B. Hovde, T. L. Fromm, M. D. Reed, and J. C. Rotschafer. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 47:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta, A. K., P. R. Taborda, and A. D. Sanzovo. 2002. Alternate week and combination itraconazole and terbinafine therapy for chromoblastomycosis caused by Fonsecaea pedrosoi in Brazil. Med. Mycol. 40:529-534. [DOI] [PubMed] [Google Scholar]
- 94.Gutierrez-Rodero, F., M. Moragon, V. Ortiz, de la Tabla, M. J. Mayol, and C. Martin. 1999. Cutaneous hyalohyphomycosis caused by Paecilomyces lilacinus in an immunocompetent host successfully treated with itraconazole: case report and review. Eur. J. Clin. Microbiol. Infect. Dis. 18:814-818. [DOI] [PubMed] [Google Scholar]
- 95.Hatfield, C., A. Kasarskis, and C. Staben. 1991. Pentamidine sensitivity and resistance in Saccharomyces cerevisiae as a model for pentamidine effects on Pneumocystis carinii. J. Protozool. 38:70S-71S. [PubMed] [Google Scholar]
- 96.Haynes, R. R., P. A. Connolly, M. M. Durkin, A. M. LeMonte, M. L. Smedema, E. Brizendine, and L. J. Wheat. 2002. Antifungal therapy for central nervous system histoplasmosis, using a newly developed intracranial model of infection. J. Infect. Dis. 185:1830-1832. [DOI] [PubMed] [Google Scholar]
- 97.Hossain, M. A., P. K. Mukherjee, G. Reyes, L. Long, and M. A. Ghannoum. 2002. Effects of fluconazole singly and in combination with 5-fluorocytosine or amphotericin B in the treatment of cryptococcal meningoencephalitis in an intracranial murine model. J. Chemother. 14:351-360. [DOI] [PubMed] [Google Scholar]
- 98.Hossain, M. A., G. Reyes, L. Long, P. K. Mukherjee, and M. A. Ghannoum. 2003. Efficacy of caspofungin combined with amphotericin B against azole-resistant Candida albicans. J. Antimicrob. Chemother. 51:1427-1429. [DOI] [PubMed] [Google Scholar]
- 99.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 100.Iovannitti, C., R. Negroni, J. Bava, J. Finquelievich, and M. Kral. 1995. Itraconazole and flucytosine + itraconazole combination in the treatment of experimental cryptococcosis in hamsters. Mycoses 38:449-452. [DOI] [PubMed] [Google Scholar]
- 101.Itin, P. H., R. Frei, S. Lautenschlager, S. A. Buechner, C. Surber, A. Gratwohl, and A. F. Widmer. 1998. Cutaneous manifestations of Paecilomyces lilacinus infection induced by a contaminated skin lotion in patients who are severely immunosuppressed. J. Am. Acad. Dermatol. 39:401-409. [DOI] [PubMed] [Google Scholar]
- 102.Jacqueline, C., J. Caillon, M. Le, V, A. F. Miegeville, P. Y. Donnio, D. Bugnon, and G. Potel. 2003. In vitro activity of linezolid alone and in combination with gentamicin, vancomycin or rifampicin against methicillin-resistant Staphylococcus aureus by time-kill curve methods. J. Antimicrob. Chemother. 51:857-864. [DOI] [PubMed] [Google Scholar]
- 103.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joly, V., J. Bolard, L. Saint-Julien, C. Carbon, and P. Yeni. 1992. Influence of phospholipid/amphotericin B ratio and phospholipid type on in vitro renal cell toxicities and fungicidal activities of lipid-associated amphotericin B formulations. Antimicrob. Agents Chemother. 36:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keele, D. J., V. C. DeLallo, R. E. Lewis, E. J. Ernst, and M. E. Klepser. 2001. Evaluation of amphotericin B and flucytosine in combination against Candida albicans and Cryptococcus neoformans using time-kill methodology. Diagn. Microbiol. Infect. Dis. 41:121-126. [DOI] [PubMed] [Google Scholar]
- 106.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klepser, M. E., E. J. Ernst, R. E. Lewis, M. E. Ernst, and M. A. Pfaller. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 42:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klepser, M. E., D. Malone, R. E. Lewis, E. J. Ernst, and M. A. Pfaller. 2000. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob. Agents Chemother. 44:1917-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klepser, M. E., E. J. Wolfe, and M. A. Pfaller. 1998. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J. Antimicrob. Chemother. 41:397-401. [DOI] [PubMed] [Google Scholar]
- 111.Kontoyiannis, D. P., R. E. Lewis, N. Sagar, G. May, R. A. Prince, and K. V. Rolston. 2000. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: an E-test-based strategy. Antimicrob. Agents Chemother. 44:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhara, T., K. Uchida, and H. Yamaguchi. 2000. Therapeutic efficacy of human macrophage colony-stimulating factor, used alone and in combination with antifungal agents, in mice with systemic Candida albicans infection. Antimicrob. Agents Chemother. 44:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kujath, P., K. Lerch, P. Kochendorfer, and C. Boos. 1993. Comparative study of the efficacy of fluconazole versus amphotericin B/flucytosine in surgical patients with systemic mycoses. Infection 21:376-382. [DOI] [PubMed] [Google Scholar]
- 114.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsen, R. A., M. Bauer, A. M. Thomas, and J. R. Graybill. 2004. Amphotericin B and fluconazole, a potent combination therapy for cryptococcal meningitis. Antimicrob. Agents Chemother. 48:985-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Larsen, R. A., M. Bauer, J. M. Weiner, D. M. Diamond, M. E. Leal, J. C. Ding, M. G. Rinaldi, and J. R. Graybill. 1996. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob. Agents Chemother. 40:2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Larsen, R. A., S. A. Bozzette, B. E. Jones, D. Haghighat, M. A. Leal, D. Forthal, M. Bauer, J. G. Tilles, J. A. McCutchan, and J. M. Leedom. 1994. Fluconazole combined with flucytosine for treatment of cryptococcal meningitis in patients with AIDS. Clin. Infect. Dis. 19:741-745. [DOI] [PubMed] [Google Scholar]
- 118.Larsen, R. A., M. A. Leal, and L. S. Chan. 1990. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann. Intern. Med. 113:183-187. [DOI] [PubMed] [Google Scholar]
- 119.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LeMonte, A. M., K. E. Washum, M. L. Smedema, C. Schnizlein-Bick, S. M. Kohler, and L. J. Wheat. 2000. Amphotericin B combined with itraconazole or fluconazole for treatment of histoplasmosis. J. Infect. Dis. 182:545-550. [DOI] [PubMed] [Google Scholar]
- 121.Letscher-Bru, V., and R. Herbrecht. 2003. Caspofungin: the first representative of a new antifungal class. J. Antimicrob. Chemother. 51:513-521. [DOI] [PubMed] [Google Scholar]
- 122.Levine, J., D. B. Bernard, B. A. Idelson, H. Farnham, C. Saunders, and A. M. Sugar. 1989. Fungal peritonitis complicating continuous ambulatory peritoneal dialysis: successful treatment with fluconazole, a new orally active antifungal agent. Am. J. Med. 86:825-827. [DOI] [PubMed] [Google Scholar]
- 123.Lewis, R. E., D. J. Diekema, S. A. Messer, M. A. Pfaller, and M. E. Klepser. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345-351. [DOI] [PubMed] [Google Scholar]
- 124.Lewis, R. E., and M. E. Klepser. 1999. The changing face of nosocomial candidemia: epidemiology, resistance, and drug therapy. Am. J. Health Syst. Pharm. 56:525-533. [PubMed] [Google Scholar]
- 125.Lewis, R. E., M. E. Klepser, and M. A. Pfaller. 1998. Update on clinical antifungal susceptibility testing for Candida species. Pharmacotherapy 18:509-515. [PubMed] [Google Scholar]
- 126.Lewis, R. E., M. E. Klepser, and M. A. Pfaller. 2000. In vitro pharmacodynamic characteristics of flucytosine determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 36:101-105. [DOI] [PubMed] [Google Scholar]
- 127.Lewis, R. E., and D. P. Kontoyiannis. 2001. Rationale for combination antifungal therapy. Pharmacotherapy 21:149S-164S. [DOI] [PubMed] [Google Scholar]
- 128.Lewis, R. E., D. P. Kontoyiannis, R. O. Darouiche, I. I. Raad, and R. A. Prince. 2002. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream Infection. Antimicrob. Agents Chemother. 46:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lewis, R. E., B. C. Lund, M. E. Klepser, E. J. Ernst, and M. A. Pfaller. 1998. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 42:1382-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lewis, R. E., R. A. Prince, J. Chi, and D. P. Kontoyiannis. 2002. Itraconazole preexposure attenuates the efficacy of subsequent amphotericin B therapy in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li, R. C. 2000. New pharmacodynamic parameters for antimicrobial agents. Int. J. Antimicrob. Agents 13:229-235. [DOI] [PubMed] [Google Scholar]
- 132.Libman, M. D., M. A. Miller, and G. K. Richards. 1990. Antistaphylococcal activity of pentamidine. Antimicrob. Agents Chemother. 34:1795-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lopez, L., L. Gaztelurrutia, M. Cuenca-Estrella, A. Monzon, J. Barron, J. L. Hernandez, and R. Perez. 2001. Infection and colonization by Scedosporium prolificans. Enferm. Infecc. Microbiol. Clin. 19:308-313. [DOI] [PubMed] [Google Scholar]
- 134.Louie, A., P. Banerjee, G. L. Drusano, M. Shayegani, and M. H. Miller. 1999. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 43:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Louie, A., P. Kaw, P. Banerjee, W. Liu, G. Chen, and M. H. Miller. 2001. Impact of the order of initiation of fluconazole and amphotericin B in sequential or combination therapy on killing of Candida albicans in vitro and in a rabbit model of endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 45:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Louie, A., W. Liu, D. A. Miller, A. C. Sucke, Q. F. Liu, G. L. Drusano, M. Mayers, and M. H. Miller. 1999. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob. Agents Chemother. 43:2831-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ludewig, G. and C. Staben. 1994. Sensitivity of taxonomically diverse fungi to pentamidine isethionate. J. Eukaryot. Microbiol. 41:103S. [PubMed] [Google Scholar]
- 138.Maesaki, S., S. Kawamura, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 1999. Effect of sequential combination of amphotericin B and azole antifungal agents against Aspergillus fumigatus. J. Infect. Chemother. 5:125-129. [DOI] [PubMed] [Google Scholar]
- 139.Manavathu, E. K., G. J. Alangaden, and P. H. Chandrasekar. 2003. Differential activity of triazoles in two-drug combinations with the echinocandin caspofungin against Aspergillus fumigatus. J. Antimicrob. Chemother. 51:1423-1425. [DOI] [PubMed] [Google Scholar]
- 140.Marchetti, O., P. Moreillon, J. M. Entenza, J. Vouillamoz, M. P. Glauser, J. Bille, and D. Sanglard. 2003. Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob. Agents Chemother. 47:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marr, B., S. Gross, C. Cunningham, and L. Weiner. 1994. Candidal sepsis and meningitis in a very-low-birth-weight infant successfully treated with fluconazole and flucytosine. Clin. Infect. Dis. 19:795-796. [DOI] [PubMed] [Google Scholar]
- 143.Matar, M. J., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2003. Correlation between E-test, disk diffusion, and microdilution methods for antifungal susceptibility testing of fluconazole and voriconazole. Antimicrob. Agents Chemother. 47:1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattiuzzi, G. N., E. Estey, I. Raad, F. Giles, J.Cortes, Y. Shen, D. Kontoyiannis, C. Koller, M. Munsell, M. Beran, and H. Kantarjian. 2003. Liposomal amphotericin B versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer 97:450-456. [DOI] [PubMed] [Google Scholar]
- 145.Mayanja-Kizza, H., K. Oishi, S. Mitarai, H. Yamashita, K. Nalongo, K. Watanabe, T. Izumi, J. Ococi, K. Augustine, R. Mugerwa, T. Nagatake, and K. Matsumoto. 1998. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin. Infect. Dis. 26:1362-1366. [DOI] [PubMed] [Google Scholar]
- 146.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. G. M. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mencacci, A., E. Cenci, A. Bacci, F. Bistoni, and L. Romani. 2000. Host immune reactivity determines the efficacy of combination immunotherapy and antifungal chemotherapy in candidiasis. J. Infect. Dis. 181:686-694. [DOI] [PubMed] [Google Scholar]
- 150.Miletti, K. E., and M. J. Leibowitz 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 44:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mosquera, J., A. Sharp, C. B. Moore, P. A. Warn, and D. W. Denning. 2002. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J. Antimicrob. Chemother. 50:189-194. [DOI] [PubMed] [Google Scholar]
- 152.Mukherjee, J., M. Feldmesser, M. D. Scharff, and A. Casadevall. 1995. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob. Agents Chemother. 39:1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1994. Cryptococcus neoformans infection can elicit protective antibodies in mice. Can. J. Microbiol. 40:888-892. [DOI] [PubMed] [Google Scholar]
- 154.Najvar, L. K., A. Cacciapuoti, S. Hernandez, J. Halpern, R. Bocanegra, M. Gurnani, F. Menzel, D. Loebenberg, and J. R. Graybill. 2004. Activity of posaconazole combined with amphotericin B against Aspergillus flavus infection in mice: comparative studies in two laboratories. Antimicrob. Agents Chemother. 48:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakai, T., J. Uno, F. Ikeda, S. Tawara, K. Nishimura, and M. Miyaji. 2003. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakajima, R., A. Kitamura, K. Someya, M. Tanaka, and K. Sato. 1995. In vitro and in vivo antifungal activities of DU-6859a, a fluoroquinolone, in combination with amphotericin B and fluconazole against pathogenic fungi. Antimicrob. Agents Chemother. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nassar, F., E. Brummer, and D. A. Stevens. 1995. Different components in human serum inhibit multiplication of Cryptococcus neoformans and enhance fluconazole activity. Antimicrob. Agents Chemother. 39:2490-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.NCCLS. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standard M38-P. NCCLS, Wayne, Pa.
- 159.NCCLS. 2003. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed. NCCLS document M27-A2. NCCLS, Wayne, Pa.
- 160.Nguyen, M. H., F. Barchiesi, D. A. McGough, V. L. Yu, and M. G. Rinaldi. 1995. In vitro evaluation of combination of fluconazole and flucytosine against Cryptococcus neoformans var. neoformans. Antimicrob. Agents Chemother. 39:1691-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nguyen, M. H., L. K. Najvar, C. Y. Yu, and J. R. Graybill. 1997. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob. Agents Chemother. 41:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nolan, A., P. J. Lamey, T. W. MacFarlane, T. C. Aitchison, J. Shaw, and J. Y. Sirel. 1994. The effect of nebulised pentamidine on the concentration of intra-oral Candida albicans in HIV-infected patients. J. Med. Microbiol. 41:95-97. [DOI] [PubMed] [Google Scholar]
- 163.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 164.Ortoneda, M., J. Capilla, I. Pujol, F. J. Pastor, E. Mayayo, J. Fernandez-Ballart, and J. Guarro. 2002. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 49:525-529. [DOI] [PubMed] [Google Scholar]
- 165.Pacetti, S. A., and S. P. Gelone. 2003. Caspofungin acetate for treatment of invasive fungal infections. Ann. Pharmacother. 37:90-98. [DOI] [PubMed] [Google Scholar]
- 166.Pappas, P. G. 2001. Therapy of cryptococcal meningitis in non-HIV-infected patients. Curr. Infect. Dis. Rep. 3:365-370. [DOI] [PubMed] [Google Scholar]
- 167.Pappas, P. G., J. R. Perfect, G. A. Cloud, R. A. Larsen, G. A. Pankey, D. J. Lancaster, H. Henderson, C. A. Kauffman, D. W. Haas, M. Saccente, R. J. Hamill, M. S. Holloway, R. M. Warren, and W. E. Dismukes. 2001. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 33:690-699. [DOI] [PubMed] [Google Scholar]
- 168.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 169.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, J. R. Graybill, and the I3 Aspergillus Study Group. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 170.Perea, S., G. Gonzalez, A. W. Fothergill, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 46:3039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Perea, S., G. Gonzalez, A. W. Fothergill, D. A. Sutton, and M. G. Rinaldi. 2002. In vitro activities of terbinafine in combination with fluconazole, itraconazole, voriconazole, and posaconazole against clinical isolates of Candida glabrata with decreased susceptibility to azoles. J. Clin. Microbiol. 40:1831-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 173.Petrou, M. A., and T. R. Rogers. 1991. Interactions in vitro between polyenes and imidazoles against yeasts. J. Antimicrob. Chemother. 27:491-506. [DOI] [PubMed] [Google Scholar]
- 174.Petrou, M. A., and D. C. Shanson. 2000. Susceptibility of Cryptococcus neoformans by the NCCLS microdilution and Etest methods using five defined media. J. Antimicrob. Chemother. 46:815-818. [DOI] [PubMed] [Google Scholar]
- 175.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, and D. J. Diekema. 2003. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference M38-A microdilution methods for determining posaconazole MICs. Diagn. Microbiol. Infect. Dis. 45:241-244. [DOI] [PubMed] [Google Scholar]
- 177.Pfaller, M. A., S. A. Messer, K. Mills, and A. Bolmstrom. 2000. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J. Clin. Microbiol. 38:3359-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pfaller, M. A., J. H. Rex, and M. G. Rinaldi. 1997. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin. Infect. Dis. 24:776-784. [DOI] [PubMed] [Google Scholar]
- 179.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pfaller, M. A., and W. L. Yu. 2001. Antifungal susceptibility testing. New technology and clinical applications. Infect. Dis. Clin. North Am. 15:1227-1261. [DOI] [PubMed] [Google Scholar]
- 181.Polak, A. 1987. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy 33:381-395. [DOI] [PubMed] [Google Scholar]
- 182.Ponton, J., R. Ruchel, K. V. Clemons, D. C. Coleman, R. Grillot, J. Guarro, D. Aldebert, P. Ambroise-Thomas, J. Cano, A. J. Carrillo-Munoz, J. Gene, C. Pinel, D. A. Stevens, and D. J. Sullivan. 2000. Emerging pathogens. Med. Mycol. 38:225-236. [DOI] [PubMed] [Google Scholar]
- 183.Popp, A. I., M. H. White, T. Quadri, L. Walshe, and D. Armstrong. 1999. Amphotericin B with and without itraconazole for invasive aspergillosis: a three-year retrospective study. Int. J. Infect. Dis. 3:157-160. [DOI] [PubMed] [Google Scholar]
- 184.Powderly, W. G. 1993. Cryptococcal meningitis and AIDS. Clin. Infect. Dis. 17:837-842. [DOI] [PubMed] [Google Scholar]
- 185.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, and J. Booth. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 186.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 187.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 188.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 191.Ribaud, P., C. Chastang, J. P. Latge, L. Baffroy-Lafitte, N. Parquet, A. Devergie, H. Esperou, F. Selimi, V. Rocha, H. Esperou, F. Selimi, V. Rocha, F. Derouin, G. Socie, and E. Gluckman. 1999. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin. Infect. Dis. 28:322-330. [DOI] [PubMed] [Google Scholar]
- 192.Roilides, E., C. A. Lyman, J. Filioti, O. Akpogheneta, T. Sein, C. G. Lamaignere, R. Petraitiene, and T. J. Walsh. 2002. Amphotericin B formulations exert additive antifungal activity in combination with pulmonary alveolar macrophages and polymorphonuclear leukocytes against Aspergillus fumigatus. Antimicrob. Agents Chemother. 46:1974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roling, E. E., M. E. Klepser, A. Wasson, R. E. Lewis, E. J. Ernst, and M. A. Pfaller. 2002. Antifungal activities of fluconazole, caspofungin (MK0991), and anidulafungin (LY 303366) alone and in combination against Candida spp. and Crytococcus neoformans via time-kill methods. Diagn. Microbiol. Infect. Dis. 43:13-17. [DOI] [PubMed] [Google Scholar]
- 194.Ryder, N. S., and I. Leitner. 2001. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med. Mycol. 39:91-95. [DOI] [PubMed] [Google Scholar]
- 195.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 196.Saag, M. S., W. G. Powderly, G. A. Cloud, P. Robinson, M. H. Grieco, P. K. Sharkey, S. E. Thompson, A. M. Sugar, C. U. Tuazon, J. F. Fisher, The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. 1992. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N. Engl. J. Med. 326:83-89. [DOI] [PubMed] [Google Scholar]
- 197.Safdar, A. 2002. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin. Infect. Dis. 34:1415-1417. [DOI] [PubMed] [Google Scholar]
- 198.Sanati, H., C. F. Ramos, A. S. Bayer, and M. A. Ghannoum. 1997. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob. Agents Chemother. 41:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schaffner, A., and A. Bohler. 1993. Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses 36:421-424. [DOI] [PubMed] [Google Scholar]
- 200.Schaffner, A., and P. G. Frick. 1985. The effect of ketoconazole on amphotericin B in a model of disseminated aspergillosis. J. Infect. Dis. 151:902-910. [DOI] [PubMed] [Google Scholar]
- 201.Scheven, M., K. Junemann, H. Schramm, and W. Huhn. 1992. Successful treatment of a Candida albicans sepsis with a combination of flucytosine and fluconazole. Mycoses 35:315-316. [DOI] [PubMed] [Google Scholar]
- 202.Schmalreck, A. F., I. Kottmann, A. Reiser, U. Ruffer, E. Scharr, and E. Vanca. 1995. An evaluation of seven methods of testing in vitro susceptibility of clinical yeast isolates to fluconazole. Mycoses 38:359-368. [DOI] [PubMed] [Google Scholar]
- 203.Sewell, D. L., M. A. Pfaller, and A. L. Barry. 1994. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J. Clin. Microbiol. 32:2099-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sivak, O., K. Bartlett, V. Risovic, E. Choo, F. Marra, D. S. Batty, Jr., and K. M. Wasan. 2004. Assessing the antifungal activity and toxicity profile of amphotericin B lipid complex (ABLC; Abelcet(R)) in combination with caspofungin in experimental systemic aspergillosis. J. Pharm. Sci. 93:1382-1389. [DOI] [PubMed] [Google Scholar]
- 206.Smego, R. A., J. R. Perfect, and D. T. Durack. 1984. Combined therapy with amphotericin B and 5-fluorocytosine for Candida meningitis. Rev. Infect. Dis. 6:791-801. [DOI] [PubMed] [Google Scholar]
- 207.Sobel, J. D. for the Mycoses Study Group. 2000. Practice guidelines for the treatment of fungal infections. Clin. Infect. Dis. 30:652. [DOI] [PubMed] [Google Scholar]
- 208.Reference deleted.
- 209.Steinbach, W. J., and J. R. Perfect. 2003. Scedosporium species infections and treatments. J. Chemother. 15(Suppl. 2):16-27. [DOI] [PubMed] [Google Scholar]
- 210.Steinbach, W. J., W. A. Schell, J. R. Blankenship, C. Onyewu, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Steinbach, W. J., D. A. Stevens, and D. W. Denning. 2003. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin. Infect. Dis. 37(Suppl. 3):S188-S224. [DOI] [PubMed] [Google Scholar]
- 212.Steinbach, W. J., W. A. Schell, J. L. Miller, and J. R. Perfect. 2003. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 41:3981-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stevens, D. A. 1998. Combination immunotherapy and antifungal chemotherapy. Clin. Infect. Dis. 26:1266-1269. [DOI] [PubMed] [Google Scholar]
- 214.Stevens, D. A. 2000. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother. 44:2547-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 216.Stevens, D. A., B. J. Kullberg, E. Brummer, A. Casadevall, M. G. Netea, and A. M. Sugar. 2000. Combined treatment: antifungal drugs with antibodies, cytokines or drugs. Med. Mycol. 38(Suppl. 1):305-315. [PubMed] [Google Scholar]
- 217.Stevens, D. A., T. J. Walsh, F. Bistoni, E. Cenci, K. V. Clemons, G. Del Sero, C. Fe d'Ostiani, B. J. Kullberg, A. Mencacci, E. Roilides, and L. Romani. 1998. Cytokines and mycoses. Med. Mycol. 36(Suppl. 1):174-182. [PubMed] [Google Scholar]
- 217a.St. Germain, G. 1990. Effects of pentamidine alone and in combination with ketoconazole or itraconazole on the growth of Candida albicans. Antimicrob. Agents Chemother. 34:2304-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stratton, C. W. 1987. The role of the microbiology laboratory in the treatment of infective endocarditis. J. Antimicrob. Chemother. 20(Suppl. A):41-49. [DOI] [PubMed] [Google Scholar]
- 220.Sugar, A. M., C. A. Hitchcock, P. F. Troke, and M. Picard. 1995. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob. Agents Chemother. 39:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sugar, A. M., X. P. Liu, and R. J. Chen. 1997. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob. Agents Chemother. 41:2518-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Te Dorsthorst, D. T., P. E. Verweij, J. F. Meis, N. C. Punt, and J. W. Mouton. 2002. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against itraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 46:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Te Dorsthorst, D. T. A., P. E. Verweij, J. Meletiadis, M. Bergervoet, N. C. Punt, J. F. G. M. Meis, and J. W. Mouton. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Torres, H. A., I. I. Raad, and D. P. Kontoyiannis. 2003. Infections caused by Fusarium species. J. Chemother. 15(Suppl. 2):28-35. [DOI] [PubMed] [Google Scholar]
- 225.Van Cutsem, J. 1993. Therapy of experimental meningeal and disseminated cryptococcosis. Mycoses 36:357-367. [DOI] [PubMed] [Google Scholar]
- 226.Van der Auwera, P., J. P. Thys, F. Meunier-Carpentier, and J. Klastersky. 1984. The combination of oxacillin with rifampicin in staphylococcal infections: a review of laboratory and clinical studies of the Institut Jules Bordet. J. Antimicrob. Chemother. 13(Suppl. C):31-42. [DOI] [PubMed] [Google Scholar]
- 227.van der Horst, C. M., M. S. Saag, G. A. Cloud, R. J. Hamill, J. R. Graybill, J. D. Sobel, P. C. Johnson, C. U. Tuazon, T. Kerkering, B. L. Moskovitz, W. G. Powderly, W. E. Dismukes, National Institute of Allergy and Infectious Diseases Mycoses Study Group, and AIDS Clinical Trials Group. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 337:15-21. [DOI] [PubMed] [Google Scholar]
- 228.van Dross, R. T., and M. M. Sanders. 2002. Molecular characterization of recombinant Pneumocystis carinii topoisomerase I: differential interactions with human topoisomerase I poisons and pentamidine. Antimicrob. Agents Chemother. 46:2145-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vazquez, J. A., M. T. Arganoza, J. K. Vaishampayan, and R. A. Akins. 1996. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob. Agents Chemother. 40:2511-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Viviani, M. A., M. C. Esposto, M. Cogliati, and A. M. Tortorano. 2003. Flucytosine and cryptococcosis: which in vitro test is the best predictor of outcome? J. Chemother. 15:124-128. [DOI] [PubMed] [Google Scholar]
- 231.Vora, S., S. Chauhan, E. Brummer, and D. A. Stevens. 1998. Activity of voriconazole combined with neutrophils or monocytes against Aspergillus fumigatus: effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 42:2299-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vora, S., N. Purimetla, E. Brummer, and D. A. Stevens. 1998. Activity of voriconazole, a new triazole, combined with neutrophils or monocytes against Candida albicans: effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 42:907-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transplant. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 234.Walsh, T. J., J. Peter, D. A. McGough, A. W. Fothergill, M. G. Rinaldi, and P. A. Pizzo. 1995. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob. Agents Chemother. 39:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Warnock, D. W., B. A. Arthington-Skaggs, and R. K. Li. 1999. Antifungal drug susceptibility testing and resistance in Aspergillus. Drug Resist. Updat. 2:326-334. [DOI] [PubMed] [Google Scholar]
- 236.White, M., C. Cirrincione, A. Blevins, and D. Armstrong. 1992. Cryptococcal meningitis: outcome in patients with AIDS and patients with neoplastic disease. J. Infect. Dis. 165:960-963. [DOI] [PubMed] [Google Scholar]
- 237.Zierdt, C. H. 1991. Blastocystis hominis—past and future. Clin. Microbiol. Rev. 4:61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]