Significance
The upper respiratory tract (URT) is the first contact site for inhaled pathogens and intranasal vaccines, and is serviced by a network of lymphoid-tissues, including draining lymph nodes and nasal-associated lymphoid tissues (NALTs). Whether these lymphoid structures have distinct roles in facilitating T-cell immunity to inhaled antigens is unclear. We show, following antigen delivery into the URT, NALTs failed to support naïve T-cell priming; however, they supported the recall expansion of memory T cells. Although antigen delivery to the URT may not induce effective T-cell priming, it would be an effective means of boosting responses in the context of preexisting T-cell immunity. These results have significant implications for intranasal vaccines that deliver antigen to NALTs and aim to elicit protective T-cell immunity.
Keywords: influenza virus, respiratory tract, CD8 T-cell priming, nasal-associated lymphoid tissue
Abstract
The lymphoid tissue that drains the upper respiratory tract represents an important induction site for cytotoxic T lymphocyte (CTL) immunity to airborne pathogens and intranasal vaccines. Here, we investigated the role of the nasal-associated lymphoid tissues (NALTs), which are mucosal-associated lymphoid organs embedded in the submucosa of the nasal passage, in the initial priming and recall expansion of CD8+ T cells following an upper respiratory tract infection with a pathogenic influenza virus and immunization with a live attenuated influenza virus vaccine. Whereas NALTs served as the induction site for the recall expansion of memory CD8+ T cells following influenza virus infection or vaccination, they failed to support activation of naïve CD8+ T cells. Strikingly, NALTs, unlike other lymphoid tissues, were not routinely surveyed during the steady state by circulating T cells. The selective recruitment of memory T cells into these lymphoid structures occurred in response to infection-induced elevation of the chemokine CXCL10, which attracted CXCR3+ memory CD8+ T cells. These results have significant implications for intranasal vaccines, which deliver antigen to mucosal-associated lymphoid tissue and aim to elicit protective CTL-mediated immunity.
The development of a cytotoxic influenza-specific CD8+ T-cell response is beneficial for the rapid recovery from acute influenza virus infection and maintenance of long-term immunity against both homosubtypic and heterosubtypic virus strains (1). The activation of an influenza-specific CD8+ T-cell response requires a coordinated series of events, where antigen-presenting cells (APCs), usually dendritic cells (DCs), present foreign viral antigens sampled from the respiratory mucosae to rare antigen-specific T cells, stimulating their entry into a process of clonal expansion and effector differentiation. This process is facilitated by strategically located lymphoid tissues, which have evolved to augment encounters between naïve lymphocytes and antigen-bearing DCs, and provides a milieu that supports expansion, differentiation, and survival of activated cells (2).
The upper respiratory tract (URT) is one of the first contact sites for inhaled pathogens and is serviced by a network of lymphoid tissues which include encapsulated draining lymph nodes (LNs) and mucosal-associated lymphoid tissue, which are directly embedded in the submucosa of the nasal passage (3). In the human URT, mucosal-associated lymphoid tissue is comprised of the Waldeyer’s ring, which collectively includes the adenoids or nasopharyngeal tonsils, the palatine tonsils, and the bilateral lingual tonsils. The murine equivalent, the nasal-associated lymphoid tissue (NALT), consists of paired lymphoid tissue located at the base of the nasal cavities at the entrance of the nasopharyngeal duct. NALTs in the URT, proposed as the functional equivalent to Peyer’s patches of the gut, are highly organized lymphoid structures covered by ciliated epithelium, which also contains M cells that facilitate antigen transport from the nasal cavity to the underlying lymphoid tissues (4). Whereas the lymphoid tissues of the URT represent an important initiation site for cytotoxic T lymphocyte (CTL) immunity to inhaled antigens, little is known of the mechanisms involved in the induction of this immunity and whether the draining LN and the mucosal-associated lymphoid structures have distinct roles in facilitating these immune responses.
Here, we investigated the priming and recall response of CD8+ T cells in the URT draining lymph nodes and NALTs following both vaccination with live attenuated influenza virus (LAIV) and infection with a pathogenic influenza strain. We showed that, whereas NALTs served as the induction site for the recall expansion of memory CD8+ T cells following influenza virus infection or vaccination of the URT, they failed to support activation of naïve CD8+ T cells, which surprisingly, did not migrate into these structures. The recruitment of T cells into the NALTs was in response to infection-induced elevation of the chemokine CXCL10, which resulted in the migration of CXCR3+ memory CD8+ T cells into these inflamed structures.
Results
Influenza Antigen Is Present Within the NALTs Following Influenza Virus Infection.
NALTs can be identified by microscopy of nasal tissue sections as densely packed clusters of B and T lymphocytes situated on the dorsal surface of the upper palate (Fig. 1 A and B). These lymphoid aggregates are vascularized, harboring high endothelial venules (HEVs) expressing both peripheral node addressin (PNad+) and mucosal addressin cellular adhesion molecule 1 (MadCam-1) (Fig. 1B and Fig. S1), the expression of which facilitates lymphocyte entry. Dendritic cells (CD11c+MHCII+) were enriched beneath the subepithelial dome region (Fig. 1C), the majority of which costained with the marker CD11b (Fig. 1D). NALTs can be teased away from the palate and their cellular composition, which differs markedly from the surrounding nasal tissue, can be assessed by flow cytometry. In naïve mice, B cells (B220+) represent the dominant lymphocyte population within the NALTs (∼80% of total lymphocytes), whereas they are less abundant in the surrounding nasal tissue (∼30% of total lymphocytes) (5) (Fig. 1E). T lymphocytes represent 10% of cells within the NALTs and assessment of the phenotype of these cells revealed that ∼65% of T cells are of memory phenotype (CD44hi). This is in contrast to the T cells present in the cervical lymph nodes, which drain the upper airways, where the T lymphocyte population is predominately naïve phenotype (CD44low) (Fig. 1F).
Fig. 1.
Influenza antigen is present within the NALTs following intranasal influenza infection. (A) Diagram of the nasal tissue highlighting the NALTs, nasal turbinates, nasal septum, and maxillary sinus. (B) Microscopy of NALTs with B220, CD3, peripheral node adressin (PNad), and DAPI staining. (C) Microscopy of NALTs with CD11c, CD8, and MHC II staining. (D) Microscopy of NALTs with CD11c, CD8, CD11b, and MHC II staining. (E) Flow cytometry profiles depicting CD3+ and B220+ expression on cells isolated from the NALTs and nasal tissue from naïve mice. (F) Flow cytometry profiles depicting CD44 and CD62L expression on CD3+ cells isolated from the NALTs and cLNs of naïve mice. (G and H) Flow cytometry analysis of influenza-NP antigen in the NALTs 1–5 d postintranasal infection with influenza virus. (H) Enumeration of NP+ cells in the NALTs. Graph represents the mean ± SEM (n = 6–8 mice per group) (I) Viral titers in the NALTs, nasal tissue, and cLNs of mice 2 d postintranasal infection with influenza virus. Dots represent individual mice, bars represents the mean ± SEM (n = 9 mice per group, one-way ANOVA, Tukey’s multiple comparison).
Fig. S1.
HEV in the NALTs stain positive for PNAd and Madcam-1. (A and B) Microscopy of NALTs with CD3, MHCII, MadCam-1, and PNad staining (B) depicts enlargement of HEV.
To assess the involvement of NALTs in the initiation of a cellular immune response following influenza virus infection, C57BL/6 mice were infected intranasally (i.n.) with influenza virus and we screened the NALTs for the presence of influenza viral antigen. Influenza virus nuclear protein (NP+) was detected within cells in the NALTs from 48 h postinfection (p.i.), peaked on day 3 p.i. and was absent from this site by day 5 (Fig. 1 G and H). We could also recover infectious influenza virus from the NALTs and surrounding nasal tissue within 48 h following influenza virus infection, whereas no virus was recoverable from the cervical lymph nodes (Fig. 1I). The presence of infectious virus within the NALTs will influence the cell types capable of acting as APCs, providing an opportunity for both cross and direct presentation to occur. Overall, the early emergence of influenza antigen-positive cells and the presence of infectious virus in the NALTs is suggestive that these sites may represent active hubs for mucosal immune responses following infection of the upper airways.
NALTs Do Not Support CTL Priming Following URT Infection or Vaccination.
Before determining the involvement of NALTs in CTL-mediated immune responses following URT influenza virus infection, it was first necessary to establish an infection model that would localize the virus to the upper airways. The mouse-adapted A/Puerto Rico/8 (H1N1) (PR8) strain of influenza virus does not disseminate efficiently from the upper (nose) to the lower (lung) respiratory tract (6). Similarly, when a recombinant PR8 virus engineered to express the OVA257–264 epitope within the neuraminidase stalk (PR8-OVA) is administered as an URT infection (10 μL to the nares, no anesthetic), virus replication is largely localized to the upper airways with limited replication in the lung (Fig. 2 A and B). In contrast, following total respiratory tract (TRT) infection (30 μL to anesthetized mice), PR8-OVA replicates in the nose and to high titers in the lung (Fig. 2 A and B).
Fig. 2.
CTL priming occurs in the cervical lymph nodes but not NALTs following influenza virus infection of the URT. Viral titers in (A) nasal tissue and (B) the lungs of mice 3 d after URT or TRT infection with PR8-OVA strain of influenza virus. Symbols represent individual mice; bars represent the mean ± SEM (n = 5 per group; Student’s t test). (C) Absolute number of divided OT-I.CD45.1+ T cells in various tissues from mice injected with 106 CFSE-labeled naïve OT-I.CD45.1 T cells and infected via the URT or TRT with PR8-OVA 3 d earlier. Bars represent the mean ± SEM (n = 6–9 mice per group; two-way ANOVA, Sidak’s multiple comparison test). (D–F) Mice infected via the URT with PR8-LAIV were killed at various times postinfection and the absolute number of CD8+ NP- and PA-tetramer+ cells in the (D) mediastinal LNs (mLNs) (E) cervical LNs (cLNs), and (F) NALTs, was determined. (n = 4–7 mice per group; two-way ANOVA, Sidak’s multiple comparison, black asterisk NP analysis, red asterisk PA analysis).
Using this model, we determined whether NALTs served as an anatomical location for CTL priming following influenza virus infection of the upper airways. Congenically marked (CD45.1) CFSE-labeled OVA-specific naïve OT-I T-cell receptor (TCR) transgenic CD8+ T cells were adoptively transferred into C57BL/6 recipients (CD45.2), which then received an URT infection with a recombinant influenza virus expressing the CD8+ T-cell epitope from the model antigen OVA (PR8-OVA). As a comparison, we also infected a cohort of mice with a TRT infection to determine whether extending the influenza infection along the entire respiratory tract influenced the site for CTL priming. The absolute number of dividing OT-I T cells (CFSElo) in NALTs, cervical LNs (cLNs, draining the URT), mediastinal LNs (mLNs, draining the lower respiratory tract), spleen, nasal tissue, and lung was determined at day 3 p.i. (Fig. 2C). In mice that received an URT infection, the activation of CD8+ T cells was largely confined to the cLNs, as this was where the majority of OT-I cells had undergone division. In contrast, following a TRT infection, the majority of OT-I cells that had undergone division were found in the mLNs, suggesting that the site of T-cell priming differed between the two models of infection. Interestingly, there was no evidence of the infiltration or expansion of naïve OT-I in the NALTs in either infection model, despite these structures clearly harboring cells that stained positive for influenza virus antigen (Fig. 1 G and H).
Determining the anatomical locations of naïve CD8+ T-cell priming following antigen deposition into the URT has important implications for intranasal vaccines that deliver antigen to this region. We next assessed the anatomical location of CTL priming following immunization with the LAIV vaccine, which is engineered to preferentially replicate in the lower temperatures of the upper airways (7–9) and monitored the endogenous CD8+ T-cell response directed against influenza viral proteins. The CD8+ T-cell response following influenza virus infection of C57BL/6 mice is predominately directed against two viral proteins, the nucleoprotein (NP) and acid polymerase (PA) and can be monitored using H-2Db tetramers loaded with the PA224 and NP366 epitopes. Mice were infected via the URT with PR8-LAIV and the absolute number of NP366- and PA224-tetramer positive cells in the NALTs, cLNs, and mLNs was assessed over the acute phase of the infection (Fig. 2 D–F). We first observed significant elevations in NP366- and PA224-tetramer positive CD8+ T cells in the cLNs between days 3 and 5 postvaccination. The earliest influenza-tetramer positive cells observed in the NALTs was between days 5 and 7 postvaccination, which likely reflects that these cells were not activated within these structures but have been recruited to the NALTs after being primed elsewhere. Collectively, these data indicate that NALTs are not an inductive site for CTL responses following URT infection or LAIV vaccination.
NALTs Serve as the Recall Site for Memory CD8+ T-Cell Response Following an URT Infection.
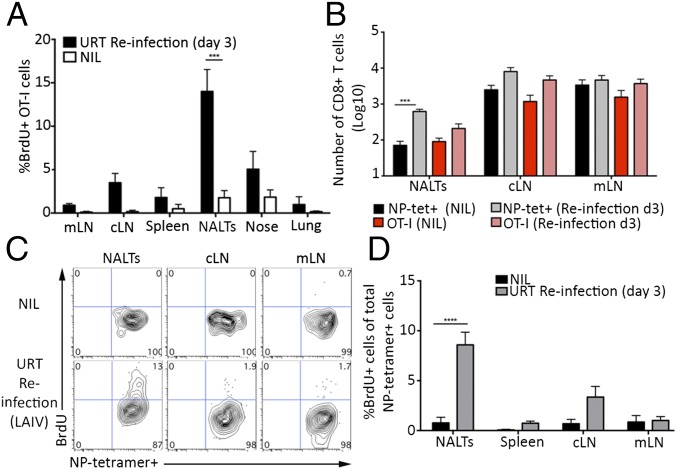
We next assessed whether the NALTs could facilitate the recall expansion of a memory CD8+ T-cell response following a secondary exposure to influenza virus. Mice seeded with naïve OT-I.CD45.1 CD8+ T cells and receiving a TRT infection with X31-OVA were rechallenged 30 d later via an URT infection with a heterologous virus (PR8-OVA) or, alternatively, given PBS as a control (NIL). To determine where the OT-I CD8+ T-cell recall response was occurring, 3-d-postrechallenge mice were administered 5-Bromo-2′deoxyuridine (BrdU), a synthetic nucleoside analog of thymidine, which is incorporated into the DNA of dividing cells, and the presence of BrdU+ OT-I cells in various tissues was assessed 1 h later. Following reinfection with PR8-OVA, BrdU+ OT-I cells were essentially restricted to the nasal tissue, cLNs, and the NALTs (Fig. 3A and Fig. S2). Interestingly, we observed the largest proportion of the BrdU+ OT-I cells in the NALTs, indicating that these structures can support recall expansion of memory CD8+ T cells.
Fig. 3.
NALTs serve as the recall site for memory CD8+ T-cell responses following an URT infection. (A) Mice seeded with 104 naïve OT-I.CD45.1+ T cells and infected with X31-OVA (TRT) were reinfected 30 d later via an URT infection with PR8-OVA or given PBS (NIL). Mice were injected with BrdU on day 3 postreinfection and killed for analysis 1 h later. The proportion of OT-I cells in various tissues that had incorporated BrdU. Bars represent the mean ± SEM (n = 4–8 mice per group; two-way ANOVA, Sidak’s multiple comparison). (B) Mice were injected with 104 naïve OT-I.CD45.1+ T cells before a TRT infection with X31-OVA. At day 30 p.i., mice were either immunized intranasally with PR8-LAIV or given PBS (NIL). Absolute number of CD8+ NP-tetramer+ cells and OT-I.CD45.1+ T cells in the NALTs, cLNs, and mLNs was determined on day 3 postimmunization. Bars represent the mean ± SEM (n = 6–9 mice per group; two-way ANOVA, Sidak’s multiple comparison). (C and D) Mice receiving a TRT infection with X31 30 d earlier were immunized via the URT with PR8-LAIV or given PBS (NIL). Three days later, mice were injected with BrdU and killed for analysis 1 h later. (C) Flow cytomtery analysis of BrdU incorporation in CD8+ NP+ tetramer+ cells in the cLNs, mLNs, and NALTs at day 3 postimmunization. (D) The proportion of NP-tetramer+ CD8+ T cells in the NALTs, cLNs, spleen, and mLNs that had incorporated BrdU. Bars represent the mean ± SEM (n = 4–6 mice per group; two-way ANOVA, Sidak’s multiple comparison).
Fig. S2.
NALTs serve as the recall site for memory CD8 T-cell responses following an URT infection. Mice seeded with 104 naïve CD45.1+ CD8+ OT-I T cells and infected with X31-OVA (TRT) were reinfected 30 d later via an URT infection with PR8-OVA or given PBS (NIL). Mice were injected with BrdU on day 3 postreinfection and killed for analysis 1 h later. Flow cytometry plots of BrdU incorporation in OT-I.CD45-1+ cells from various tissues at day 3 postrechallenge.
We next assessed whether NALTs also served as a site for memory CD8+ T-cell recall expansion following vaccination of immune mice with LAIV. Mice seeded with naïve OT-I.CD45.1 CD8+ T cells were infected via the TRT with X31-OVA and were rested for 30 d, allowing the establishment of memory CD8+ T-cell pool consisting of the transgenic memory OT-I CD8+ T cells as well as an endogenous memory CD8+ T-cell response directed against the influenza viral proteins. On day 30 p.i., mice were vaccinated with PR8-LAIV virus (which lacks the cognate antigen for the OT-I T cells) or alternatively given PBS as a control (NIL) and the absolute number of influenza NP366-tetramer+ cells in the NALTs, cLNs, and mLNs was quantified 3 d later. As an internal control, we quantified the OT-I memory cells in these tissues following vaccination to gauge the level of antigen-independent recruitment of memory CD8+ T cells into the inflamed lymphoid structures that could occur in response to infection-induced inflammation. The number of NP366-tetramer+ cells increased 10-fold in the NALTs in response to vaccination, whereas there was no significant increase in the number of NP366-tetramer+ cells in cLNs and mLNs. The number of OT-I memory cells, which in this experiment represented a nonspecific memory T-cell pool, did not increase in response to vaccination in any site, indicating that the elevation in NP366-tetramer+ cells we observed in the NALTs was an antigen-specific event (Fig. 3B). To determine whether the NP366-tetramer+ cells underwent local expansion in the NALTs following LAIV vaccination, “memory” mice generated as described above (in Fig. 3B) were vaccinated with LAIV and then administered BrdU 3 d later. The presence of BrdU+ NP366-tetramer+ cells in the NALTs, cLNs, and mLNs was assessed 1 h later (Fig. 3 C and D). Following vaccination, the largest proportion of the BrdU+ NP366-tetramer+ cells was found in the NALTs (Fig. 3 C and D). These data indicate that NALTs serve as the initial site for memory CD8+ T-cell recall responses following an URT infection.
NALTs Support the Recruitment and Recall Expansion of Memory CD8+ T Cells but Not Naïve CD8+ T-Cell Priming Following URT Influenza Infection.
To further investigate the disparity in naïve and memory CD8+ T-cell expansion in the NALTs following an URT infection, mice were seeded with equal numbers of cell trace violet-labeled memory OT-I.CD45.1 T cells (generated as described in Materials and Methods) and CFSE-labeled naïve OT-I.CD45.1 T cells. These animals then received an URT infection with PR8-OVA and at day 4 p.i., the location of labeled OT-I.CD45.1 T cells and the extent to which they had divided was determined by flow cytometry. Both naïve and memory OT-I T cells had undergone division in the cLN (as assessed by the dilution of CFSE or CTV, respectively), and consistent with recent reports, naïve CD8+ T cells underwent more divisions compared with their memory counterparts (10, 11). Undivided naïve and memory T cells were evident in the spleen (Fig. 4A). The adoptively transferred memory cells had been recruited and had undergone cellular division in the NALTs, validating that these structures have the capacity to recruit and support expansion of circulating memory T cells following URT influenza virus infection (Fig. 4 A and B). Strikingly, once again there was no evidence of the infiltration or expansion of naïve OT-I T cells in the NALTs (Fig. 4 A and B).
Fig. 4.
NALTs support the recall expansion of memory CD8+ T cells, but not naïve CD8+ T-cell priming, following URT influenza infection. (A) Flow cytometry profiles depicting the proportion of divided naïve and memory OT-I.CD45.1 T cells isolated from the spleen, NALTs, and cLNs of mice injected with 106 naïve (CFSE+) and 106 memory (cell trace violet+) OT-I cells, infected via the URT with PR8-OVA and harvested 4 d later. (B) The proportion of divided cells derived from memory or naïve OT-I populations. Bars represent the mean ± SEM (n = 7–8 mice per group; two-way ANOVA, Sidak’s multiple comparison). (C) Flow cytometry profiles depicting the proportion of divided naïve OT-I.CD45.1+ T cells isolated from the spleen, NALTs, and cLNs of mice injected with 106 naïve (CFSE+) OT-I cells, infected via URT infection with PR8-OVA, and harvested 0, 4, or 6 d later. Data are representative of three experiments. (D) Flow cytometry profiles depicting the proportion of divided naïve and memory OT-I.CD45.1+ T cells isolated from the various tissues of mice injected with 106 naïve (CFSE+) and 106 memory (cell trace violet+) OT-I cells, infected via TRT infection with X31-OVA, or the parental influenza strain X31 or given PBS and harvested 4 d later. Data are representative of three experiments.
To ensure our inability to observe expansion of naïve CD8+ T cells in the NALTs following URT infection was not due to a lag in the infiltration and activation of these cells within this site, we seeded mice with CFSE-labeled naïve OT-I.CD45.1 T cells, infected the URT with PR8-OVA, and checked the cLNs, spleen, and NALTs for the presence of dividing cells 4 and 6 d later (Fig. 4C). Consistent with earlier experiments, on day 4 p.i. we observed proliferating OT-I CD8+ T cells within the cLNs, undivided OT-I cells (CFSEhi) in the spleen, and no detectable OT-I CD8+ T cells in the NALTs. By day 6 p.i., OT-I CD8+ T cells were detectable within the NALTs; however, they were all CFSE−, indicating that these cells had undergone activation and multiple rounds of division elsewhere before infiltrating these structures (Fig. 4C).
We next determined whether recruitment and/or proliferation of memory cells in the NALTs was dependent on antigen or rather driven by the inflammatory milieu. Naïve OT-I T cells (CFSE+) and memory OT-I cells (violet trace+) were transferred into mice that remained uninfected or alternatively received TRT infections with X31-OVA, or the parental X31 virus (which lacks the cognate antigen for the OT-I T cells). Flow cytometric analysis showed that in the absence of infection, undivided memory and naïve OT-I CD8+ T cells were present in mLNs, cLNs, and spleen, but not NALTs (Fig. 4D). Similarly, 4 d after infection with X31, undivided memory and naïve cells were again observed in the mLNs, cLNs, and spleen; however, a population of undivided memory T cells (but not naïve cells) was also evident in the NALTs. As expected, infection with X31-OVA resulted in the proliferation of both memory and naïve OT-I CD8+ T cells in both lymph nodes and spleen and the proliferation of only memory cells in the NALTs (Fig. 4D). Taken together, the data demonstrate that memory, but not naïve, CD8+ T cells are recruited to the NALTs via a mechanism associated with local infection.
We showed that memory CD8+ T cells infiltrated the NALTs in response to an URT infection. In these experiments, the memory pool that we adoptively transferred into mice, was consistently composed of a 1:9 ratio of effector-to-central memory cells (Fig. S3A). To determine whether there was any preferential recruitment of central or effector memory CD8+ T cells into the NALTs, equal numbers of sort-purified CFSE-labeled central (CD44+CD62L+) and violet trace-labeled effector (CD44+CD62L−) OT-I memory cell subsets were injected into mice that were either left untreated (NIL) or were infected with PR8 in the URT to drive recruitment, but not the proliferation, of the transferred cells. We observed similar ratio of central and effector memory OT-I cells in the spleen and a bias of central memory over effector memory CD8+ T cells in both lymph nodes (Fig. S3 B and C). Interestingly, in the NALTs we observed 10-fold more effector memory T cells compared with central memory T cells in response to infection-induced inflammation. This finding is likely reflective of the superior capacity of the effector memory T cells to migrate into sites of inflammation. Notably, in the absence of infection, neither memory CD8+ T-cell subset infiltrated the NALTs (Fig. S3 B and C), demonstrating that these cells are actively recruited to the NALTs after reinfection.
Fig. S3.
Effector memory CD8 T cells preferentially migrate into the inflamed NALTs. (A) Flow cytometry profiles depicting the expression of CD44 and CD62L on naïve and memory OT-I cells. (B and C) Flow cytometry profiles depicting central memory (CD62L+) OT-I.CD45.1+ CD8 T cells (CFSE) and effector memory (CD62L−) OT-I.CD45.1+ CD8 T cells (violet tract) cells isolated from the various tissues of mice injected with 106 of each of these cells, infected via the URT with PR8 and harvested 4 d later. (C) Graph represents the ratio of adoptively transferred effector/central memory OT-I memory CD8 T cells. Bars represent the mean ± SEM (data pooled from two experiments, n = 5).
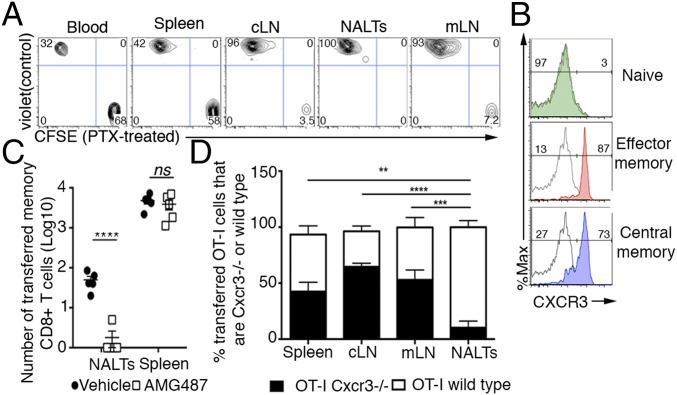
Memory CD8+ T Cells Are Recruited into Inflamed NALTs by CXCR3 Signaling.
To better define the basis for the selective recruitment of memory cells to the NALTs, we assessed the requirement for G-protein signaling in this process, a central feature of chemokine-mediated cellular trafficking. C57BL/6 mice were adoptively transferred with CFSE-labeled memory OT-I cells that had been treated with pertussis toxin (PTX, to block G-protein signaling), along with a violet trace-labeled untreated control population and then infected in URT with PR8, to drive recruitment, but not the proliferation, of the transferred cells. Flow cytometric analysis revealed the presence of both PTX- and control-treated cells in lymph nodes, blood, and spleen, albeit that there were markedly reduced numbers of PTX-treated cells within the draining LNs of infected animals relative to control cells (Fig. 5A). Critically, whereas control-treated cells were evident in the NALTs, treatment with PTX totally abrogated the recruitment of memory OT-I T cells to this site, demonstrating that inhibition of chemokine signaling prevented infiltration of the memory T cells into the inflamed NALTs (Fig. 5A). RT-PCR analyses of chemokine expression in naïve and infected NALTs revealed the up-regulation of transcripts for several chemokines, the most significant change being Cxcl10, which was expressed at 40-fold higher levels following influenza infection (Fig. S4). The receptor for this chemokine, CXCR3 is typically absent in naïve T cells, yet a characteristic feature of both central and effector memory T cells (Fig. 5B), thus providing a plausible explanation for the difference in the recruitment of naïve and memory T cells to infected NALTs.
Fig. 5.
CXCR3 signaling promotes memory CD8+ T-cell infiltration into the inflamed NALTs. (A) Flow cytometry profiles depicting pertussis toxin treated (PTX-CFSE+) or untreated (control violet trace+) memory OT-I.CD45.1+ T cells isolated from various tissues of mice injected with 106 of each of these cells, infected via the URT with PR8 and harvested 4 d later. (B) Expression of CXCR3 on naïve and effector (CD62L−) and central (CD62L+) memory (as generated in Materials and Methods) CD8+ OT-I T cells. Gray line represents unstained control. (C) Absolute number of transferred memory (CD44+) CD8+ T cells in the NALTs and spleen of mice treated with AMG487 (CXCR3 antagonist) or vehicle control at day 4 after URT infection with PR8. Bars represent the mean ± SEM (n = 5 per group; two-way ANOVA, Sidak’s multiple comparison). (D) The proportion of memory OT-I CD8+ T cells that are WT or of Cxcr3−/− origin isolated from the various tissues of mice injected with a mixture of memory OT-I.Cxcr3−/− CD8+ T cells and WT OT-I+CD8+ T cells, (as generated in Materials and Methods) infected via URT infection with PR8 and harvested 4 d later. Bars represent the mean ± SEM (n = 5, two-way ANOVA, Dunnett’s multiple comparison).
Fig. S4.
Elevation of Cxcl10 in the influenza virus-infected NALTs. Shown is the expression of chemokine mRNA transcripts in NALTs isolated from mice 2 d after receiving URT infection with PR8-OVA, relative to NALTs isolated from naïve donors. Data are represented as fold change relative to naïve. Bars represent the mean ± SEM (data were pooled from three independent experiments, n = 3–4 mice pooled per group).
To directly test the involvement of this CXCR3 signaling pathway, mice were seeded with sort-purified memory (CD44+) CD8+ T cells and infected with influenza virus (PR8) in the presence of the CXCR3 inhibitor AMG487. Consistent with a central role for CXCR3 in driving the recruitment of cells to the NALTs, there was a significant decrease in the number of memory T cells infiltrating the NALTs when CXCR3 signaling was blocked (Fig. 5C). To independently validate this mechanism, we then assessed whether memory CD8+ T cells lacking the chemokine receptor CXCR3 could infiltrate inflamed NALTs. In this case, equal numbers of memory OT-I.Cxcr3−/− CD8+ T cells (CFSE labeled) and wild-type memory OT-I cells (violet trace labeled) were adoptively transferred into recipients before URT infection with PR8. Again, in the absence of CXCR3, the recruitment of memory OT-I CD8+ T cells to the NALTs was abrogated, whereas both CXCR3 positive and negative memory OT-I T cells were readily observed in all other sites examined (Fig. 5D). Collectively, these data establish a role for CXCR3 signaling in the emigration of circulating memory CD8+ T cells into the inflamed NALTs.
Discussion
The URT represents an important initiation site of infection for many human respiratory pathogens, including influenza virus, as well as the region where antigen is deposited following intranasal vaccination. The lymphoid organs that have evolved to service the URT facilitate the induction of immune responses to these inhaled antigens. Here, we dissected the contribution of these distinct lymphoid structures in the priming and recall of a CTL response following antigen delivery into the upper airways. Strikingly, we discovered that NALTs served as the induction site for the recall expansion of memory CD8+ T cells following influenza virus infection or vaccination, but failed to support activation of naïve CD8+ T cells, which was an event largely confined to the cLNs.
Surprisingly we found that NALTs, unlike other lymphoid tissues, are not routinely surveyed during the steady state by the circulating T-cell pool. The recruitment of T cells into NALTs occurred in response to local inflammation, which resulted in the migration of memory CD8+ T cells into these structures. In all situations tested, naïve T cells were excluded from these mucosal-associated lymphoid tissues. Why would the circulating T-cell pool have restricted access to this mucosal-associated lymphoid tissue and why are only antigen-experienced cells permitted entry into these structures? Perhaps, an exclusion of naïve T cells from the NALTs serves as a tolerance mechanism, to prevent unwanted activation of a CTL response to the innocuous antigens or symbiotic microbiota that are continually sampled from the mucosal tissue. The M cells that line the epithelium of the NALTs continuously sample an array of antigens from the nasal mucosa, derived from both harmless commensal organisms and environmental irritants as well as harmful inhaled pathogens. Limiting T-cell migration into the NALTs in response to local inflammation provides a mechanism to discriminate between harmful and harmless antigens, thereby facilitating the balance between mucosal immunity and homeostasis (12).
The majority (∼65%) of the T cells within the murine NALTs express a memory phenotype (CD44+), which is also observed in human tonsils (NALT equivalent) where 50–80% of the lymphocytes are CD45RO+ (13). A bias of memory over naïve T cells is also evident in the Peyer’s patches, the mucosal-associated lymphoid tissue that drains the gut (14). The abundance of memory T cells in the NALTs is supported by our observation that only memory CD8+ T cells can infiltrate these structures and do so only in response to local inflammation. Whether these memory T cells present within the NALTs remain resident within these structures or are transiently circulating through this lymphoid tissue and their role in controlling secondary bouts of infection requires further investigation.
Vaccination remains the most cost-effective strategy to protect against annual seasonal influenza. Two types of influenza vaccines are currently available, the trivalent or quadrivalent inactivated influenza vaccine (TIV/QIV), which is given intramuscularly, and the live attenuated influenza vaccine (LAIV), which is administered intranasally. LAIV have been engineered by reverse genetics using the HA and NA genes from circulating viruses and an attenuated, temperature-sensitive, cold-adapted backbone, which prevents replication of the virus at temperatures above 33 °C, thus limiting virus infection to the upper airways (8, 9). Following inhalation of LAIV, the palatine tonsils, the NALT equivalent in humans, is the site where the humoral immune response is initiated (15). Although the vaccine is clearly able to elicit HA-specific neutralizing antibodies, it is still unclear whether protective influenza-specific T cells are generated in humans following LAIV vaccination (16, 17). Here we showed that NALTs can only support memory T-cell expansion and not naïve T-cell priming following antigen delivery into the URT. If human nasal mucosal-associated lymphoid tissues are also bound by these restrictions, then prior immunity to influenza virus and the presence of antigen-experienced T cells may heavily impact on the capacity of the LAIV to prime or boost an influenza-specific CTL pool.
In summary, we demonstrated that following antigen delivery into the URT CD8+ T-cell priming and recall responses are initiated within different lymphoid organs. Specifically, cLNs served as the primary site for naïve CD8+ T-cell activation following the deposition of antigen into the URT, whereas the NALTs represented the initial site for recall expansion of memory CD8+ T cells. These results have important implications for intranasal T cell-based vaccines that deliver antigen-to-mucosal associated lymphoid tissues. The development of better intranasal vaccines hinges on the better understanding of the immune response initiated following antigen delivery into the URT.
Materials and Methods
Mice, Viruses, and Viral Titer Determination.
C57BL/6, OT-I.CD45.1, and OT-I.Cxcr3−/− mice were bred in house and housed in specific pathogen-free (SPF) conditions in the animal facility located at the Doherty Institute of Infection and Immunity, the University of Melbourne, or at the Walter and Eliza Hall Institute. All experiments were done in accordance with the Institutional Animal Care and Use Committee guidelines of the University of Melbourne. X31-OVA or PR8-OVA influenza viruses (18) encode the OVA257–264 epitope within the neuraminidase stalk. Influenza viral titers were determined by plaque assay as described (19). For TRT infection, mice were anesthetized with inhalation anesthetic and infected with 104 pfu of virus in a volume of 30 μL. For URT infection, 10 μL of 104.5 pfu of virus was placed onto the nares of unanesthetized mice. In some experiments, mice received an URT infection with 105 pfu of PR8-LAIV, which was generated as described (7). In some experiments, mice were treated twice daily i.p. with 3 mg/kg of AMG487 or the vehicle control.
In Vitro Activation of OT-I Cells and Generation of Memory OT-I Cells.
OT-I T cells were activated in vitro with 10−6 M SIINFEKL peptide as described (20). Twenty to thirty million effector OT-I cells were injected i.v. into naïve mice that were rested for 10–20 d.
Isolation and Adoptive Transfer of Naïve and Memory T-Cell Isolation.
Naïve OT-I CD8 T cells isolated from naïve OT-I TCR transgenic or memory OT-I isolated from memory mice (generated as described above) were purified via negative selection from all LNs and spleen. Naïve OT-I T-cell preparations were 90–95% pure as determined by flow cytometry. Memory OT-I T cells were further purified by cell sorting CD8+CD44+ cells. A total of 106 purified memory or naïve CD8+ T cells were labeled with either 5 μM CFSE or 5 μM of cell trace violet before i.v. injection into mice. In some experiments, T cells were treated with pertussis toxin (100 μg/mL) for 1 h at 37 °C before transfer.
Flow Cytometry.
Single cell suspensions were prepared from spleens, LNs, and NALTs by mechanical disruption. Lung tissue and nasal tissue were harvested from perfused mice and enzymatically digested for 1 h at 37 °C in collagenase type 3 (3 mg/mL). H2-Db-NP or H2-Db-PA tetramers were made in house. To perform BrdU staining, mice were injected i.p. with 100 mg of BrdU 1 h before harvest. Tissue was processed as described above and samples were stained using a BD BrdU APC staining kit following manufacturer’s instructions.
Real-Time PCR.
RNA extraction, cDNA synthesis, and real-time PCR were performed as previously described (21). Fold change was calculated relative to the geometric mean of three housekeeping genes (RPL13a, HPRT, and IPO8). Primer sequences are as described (22).
Immunofluorescence Microscopy.
Tissue was fixed in 4% paraformaldehyde and then incubated for 48 h at room temperature in 20% EDTA before embedding in OCT. Tissue sections were stained with the specified antibodies: CD3(17A2), B220(RA3-6B2), PNad(MECA-79), MHC-II(M5/114), MadCam-1(MECA-367), CD11b(M1/70), and CD11c(N418).
Statistical Analysis.
Comparison between two study groups was statistically evaluated by unpaired two-tailed t test. Comparison between more than two groups (single factor) was evaluated using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison on log10-transformed values. Two-way ANOVA with Sidak’s multiple comparison on log10-transformed values was used to evaluate more than two groups at different time points. In all tests, statistical significance was quantified as *P < 0.5, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data were pooled from two to three independent experiments.
SI Materials and Methods
Isolation of Nasal Tissue and NALTs.
The nasal tissue, which includes the nasal cavity, nasal turbinates, and NALTs, was obtained by cutting down the vertical plane of the skull and scraping out the tissues and small bones from both sides of the nasal passages. The NALTs were extracted by dissecting away the lower jaw, tongue, and connective tissue to expose the soft palate of the upper jaw. The palate was peeled back from the anterior end, revealing the paired NALT structures at the posterior of the hard palate.
Acknowledgments
We thank Dr. W. Heath (University of Melbourne) for helpful discussions; Dr. S. Turner (Monash University) for the influenza viruses X31-OVA and PR8-OVA; and Mr. J. Smith for technical assistance. We thank Victor C. Huber (University of South Dakota) and Dr. Paul G. Thomas (St. Jude Children's Research Hospital, Memphis) for the reverse genetics-based plasmids (PA, PB1, and PB2) for live attenuated influenza virus. This work was supported by the National Health and Medical Research Council of Australia. K.K. is a National Health and Medical Research Council Senior Research Fellow Level B. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620194114/-/DCSupplemental.
References
- 1.Brown LE, Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol Cell Biol. 2009;87:300–308. doi: 10.1038/icb.2009.16. [DOI] [PubMed] [Google Scholar]
- 2.Goodnow CC. Chance encounters and organized rendezvous. Immunol Rev. 1997;156:5–10. doi: 10.1111/j.1600-065x.1997.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 3.Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: A balancing act with micro-organisms. Mucosal Immunol. 2014;7:455–466. doi: 10.1038/mi.2014.11. [DOI] [PubMed] [Google Scholar]
- 4.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang B, Hyland L, Hou S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J Virol. 2001;75:5416–5420. doi: 10.1128/JVI.75.11.5416-5420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edenborough KM, Gilbertson BP, Brown LE. A mouse model for the study of contact-dependent transmission of influenza A virus and the factors that govern transmissibility. J Virol. 2012;86:12544–12551. doi: 10.1128/JVI.00859-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, et al. Establishment of memory CD8+ T cells with live attenuated influenza virus (LAIV) across different vaccination doses. J Gen Virol. 2016;97:3205–3214. doi: 10.1099/jgv.0.000651. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann E, et al. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J Virol. 2005;79:11014–11021. doi: 10.1128/JVI.79.17.11014-11021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306:18–24. doi: 10.1016/s0042-6822(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter SM, Nunes-Alves C, Booty MG, Way SS, Behar SM. A higher activation threshold of memory CD8+ T cells has a fitness cost that is modified by TCR affinity during tuberculosis. PLoS Pathog. 2016;12:e1005380. doi: 10.1371/journal.ppat.1005380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehlhop-Williams ER, Bevan MJ. Memory CD8+ T cells exhibit increased antigen threshold requirements for recall proliferation. J Exp Med. 2014;211:345–356. doi: 10.1084/jem.20131271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 13.Sada-Ovalle I, et al. Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin Exp Immunol. 2012;168:200–206. doi: 10.1111/j.1365-2249.2012.04573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung EJ, Lee KH, Seong BL. Reverse genetic platform for inactivated and live-attenuated influenza vaccine. Exp Mol Med. 2010;42:116–121. doi: 10.3858/emm.2010.42.2.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohn KG, et al. Live attenuated influenza vaccine in children induces B-cell responses in tonsils. J Infect Dis. 2016;214:722–731. doi: 10.1093/infdis/jiw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XS, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803–811. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 17.Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines (Basel) 2015;3:373–389. doi: 10.3390/vaccines3020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- 19.Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75:615–622. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 20.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infusini G, et al. Respiratory DC use IFITM3 to avoid direct viral infection and safeguard virus-specific CD8+ T cell priming. PLoS One. 2015;10:e0143539. doi: 10.1371/journal.pone.0143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock AT, Smith JM, Carbone FR. Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection. J Exp Med. 2014;211:751–759. doi: 10.1084/jem.20132183. [DOI] [PMC free article] [PubMed] [Google Scholar]