Abstract
β adrenoceptor (βAR) signaling is finely regulated to mediate the sympathetic nervous system control of cardiovascular function. In neonatal cardiac myocytes, β1AR activates the conventional Gs/cAMP pathway, whereas β2AR sequentially activates both the Gs and Gi pathways to regulate the myocyte contraction rate. Here, we show that phosphodiesterase 4D (PDE4D) selectively impacts signaling by β2AR in neonatal cardiac myocytes, while having little or no effect on β1AR signaling. Although β2AR activation leads to an increase in cAMP production, the cAMP generated does not have access to the protein kinase A-dependent signaling pathways by which the β1AR regulates the contraction rate. However, this restricted access is lost in the presence of PDE4 inhibitors or after ablation of PDE4D. These results not only suggest that PDE4D is an integral component of the β2AR signaling complex, but also underscore the critical role of subcellular cAMP regulation in the complex control of receptor signaling. They also illustrate a mechanism for fine-tuned βAR subtype signaling specificity and intensity in the cardiac system.
Keywords: cAMP, heart, knockout
The β adrenoceptors (βARs) play important roles in the regulation of cardiovascular function by the sympathetic nervous system. βARs belong to the family of heptahelical receptors that couple to G proteins to activate several effectors. Cardiomyocytes express all three subtypes of βARs: stimulation of β1AR and β2AR leads to an increase in heart rate and contractility, whereas stimulation of β3AR may have a negative inotropic effect, but the role of this subtype has not been fully defined (1). In most instances, β1ARs and β2ARs are coupled to Gs, leading to stimulation of adenylyl cyclase, an elevation of cAMP levels, and protein kinase A (PKA) activation. Once activated, PKA phosphorylates several proteins important for muscle contractility such as the L-type calcium channel and phospholamban, thereby mediating the catecholamine effect on cardiac performance (1). Experiments performed in mouse neonatal cardiomyocytes suggest that the catecholamine-dependent increase of cardiac contraction rate is regulated predominantly by the activation of the cAMP pathways (2).
Despite the similarities between the cAMP signals elicited by the β1ARs and β2ARs when expressed in fibroblasts (3), β1AR is significantly more efficient than β2AR at increasing cardiac contractility, suggesting that a more complex level of regulation takes place in cardiomyocytes than in fibroblasts. An explanation for the different effects of the two receptor subtypes may be that β1AR activates the classic Gs/cAMP pathway, whereas β2AR sequentially activates both the Gs and Gi pathways in cardiomyocytes (2). In myocytes from β1AR knockout (KO) mice, β2AR activation leads to Gs coupling with an initial increase in the rate of contraction followed by a switch to Gi coupling and a sustained decrease in the rate of contraction (2). This peculiar behavior of β2AR can be explained in part by its subcellular localization in a caveolin-enriched domain of the plasma membrane and subsequent internalization upon agonist stimulation, likely allowing its selective interaction with Gi protein (4-6).
Subtype-specific regulation of βAR signaling also may exist at the level of cAMP degradation. The cAMP signal is terminated via hydrolysis of the second messenger to 5′ AMP. This inactivation is catalyzed by a large multigenic superfamily of enzymes. Of the 11 families of phosphodiesterases (PDEs) thus far identified, members of at least five families are expressed in heart: the Ca2+/calmodulin-dependent PDE1 family; the PDE2 family comprising dual-specificity PDEs stimulated by cGMP; the cGMP-inhibited cAMP PDE, PDE3 family; the cAMP-specific PDE4 that is sensitive to rolipram inhibition (7); and the cGMP-specific PDE5 (8). The isoforms belonging to the PDE3 and PDE4 families appear to play a critical role in the modulation and compartmentation of the cAMP signal in heart of different species (9, 10). In particular, the PDE4D3 isoform has been shown to physically interact with the muscle-selective PKA-anchoring protein and the PKA holoenzyme in rat heart (11). Furthermore, it has been shown that PDE4Ds interact with a large coil-coiled protein, myomegalin, and that both proteins are localized to the Z-band and the T-tubules in close proximity of myofibrils in cardiac and skeletal tissue (12). It has been reported recently that during β2AR desensitization the cytoplasmic adaptor β-arrestin recruits the cAMP-specific PDE4D isoforms to the plasma membrane (13), where they may regulate receptor coupling to G proteins (14). According to this model, the binding of catecholamine to β2AR could induce an increase of cAMP degradation in a localized subcellular compartment near the receptor. This hypothesis would be in agreement with data obtained from real-time imaging of cAMP signaling in rat neonatal cardiac myocytes where the β-adrenergic stimulation gives rise to a transient cAMP accumulation that appeared in specific subcellular domains under the tight control of PDE activity (15).
To examine the contribution of PDE4 isoforms to β1AR- and β2AR-specific signaling in the heart, we have taken advantage of an in vitro beating assay performed on cultured neonatal cardiomyocytes (2). Using cardiomyocytes from WT mice, as well as from mice having disruptions in the genes encoding different βARs or the genes encoding the PDE4 isoforms, it has been possible to study the integrated response to βAR and PDE4 modulation in beating cardiomyocytes and to identify the subtype-specific regulation of β2AR signaling by PDE4.
Methods
Measurement of Myocyte Contraction Rate. Spontaneously beating neonatal cardiac myocytes were prepared from hearts of new-born mouse pups (WT, β1AR-KO, β2AR-KO, PDE4A-KO, PDE4B-KO, and PDE4D-KO) as described (2). The generation of PDE4A-KO, PDE4B-KO, and PDE4D-KO mice has been described (16, 17). After preplating, the myocyte-enriched cells were plated in 35-mm dishes for contraction rate studies and 12-well plates for measuring cAMP or PDE activity. Myocyte cultures were maintained in DMEM containing 10% Nu serum, 10% bovine fetal serum, and 1× Gentamycin. The culture media were changed every 24 h. Measurement of spontaneous contraction rate was carried out as described (5). Stimulation on βARs on mouse neonatal cardiac myocytes leads to a robust increase in contraction rate with an average increase from ≈220 beats per min to ≈270 beats per min. In time-course experiments, statistical significance between groups was analyzed with two-way ANOVA with prism software (GraphPad, San Diego).
Drug Treatment. Neonatal myocytes were treated with the following inhibitors: rolipram (10 μM, gift of Schering A.G., Berlin) or RS25344 (0.2 μM, gift of Roche Biosciences) as PDE4 inhibitors, Cilostamide (10 μM, gift of Otsuka Pharmaceuticals, Rockville, MD) as a PDE3 inhibitor, or 3-isobutyl-1-methylxanthine (100 μM, Sigma) as a nonselective PDE inhibitor (18, 19). These agents were added together with isoproterenol (10 μM; Sigma) and incubated at 37°C. For some contraction assays, pertussis toxin (PTX, 0.75 μg/ml; Sigma) or the myristoylated PKA inhibitor (PKI) amide 14-22 (20 μM; EMD/Calbiochem) was used with rolipram. PTX and PKI treatments were carried out as described (2).
Measurement of cAMP Accumulation and PDE Activity. To measure intracellular cAMP, myocytes were cultured in 12-well plates (2.5 × 105 cells per well). Cells were rinsed three times with 1× PBS before feeding with 1× DMEM with 0.5% fatty acid-free BSA for 1 h. Cells then were stimulated with isoproterenol (10 μM) at 37°C for different times. In some wells, 10 μM rolipram was added with isoproterenol. The assay was terminated by the aspiration of the incubation buffer and the addition of 0.4 ml of 100% ice-cold ethanol with 0.1% trichloroacetic acid to each well. The cell lysates then were collected. Aliquots were dried in a spin vacuum, and cAMP in the residue was determined by using a RIA (2).
The PDE activity of myocytes was determined as described (20). Briefly, samples were assayed in a reaction mixture of 200 μl containing 40 mM Tris·HCl (pH 8.0), 10 mM MgCl2, 5 mM β-mercaptoethanol, 1 μM cAMP, 0.75 mg/ml BSA, and 0.1 μCi of [3H]cAMP for 10 min at 33°C. The reaction was terminated by adding 200 μl of 10 mM EDTA in 40 mM Tris·HCl (pH 8.0) followed by heat inactivation in a boiling water bath for 1 min. The PDE reaction product, 5′ cAMP, then was hydrolyzed by incubation of the assay mixture with 50 μg of Crotalus atrox snake venom for 20 min at 33°C. The resulting adenosine was separated by anion exchange chromatography using 1 ml of AG1-X8 resin and counted by scintillation counting.
Results
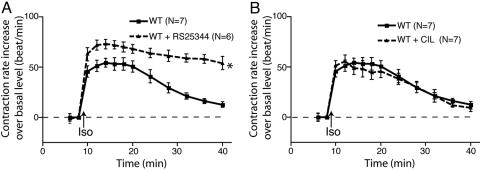
PDE4 Is Involved in βAR-Mediated Contraction Rate Response in Neonatal Cardiac Myocytes. Activation of βARs expressed on mouse neonatal cardiac myocytes leads to a robust increase in contraction rate. In WT myocytes, the increase in contraction rate that follows isoproterenol stimulation was further enhanced by the PDE4-specific inhibitor RS25344 (Fig. 1A). Moreover, the time-dependent return to the basal contraction rate was markedly reduced when PDE4s were inhibited with RS25344. A similar effect on the pattern on β-adrenergic-regulated contraction rate was observed in myocytes treated with rolipram, another PDE4-specific inhibitor, or with the nonselective PDE inhibitor 3-isobutyl-1-methylxanthine (Fig. 5, which is published as supporting information on the PNAS web site). In contrast, the isoproterenol-stimulated contraction rate was not affected when WT myocytes were treated with the PDE3-specific inhibitor cilostamide (Fig. 1B). These data provide an indication that PDE4s play an important role in the control of β-adrenergic-regulated contraction rate in this in vitro cardiac model.
Fig. 1.
Inhibition of PDE4 affects βAR regulation of the contraction rate in cardiac myocytes. (A) Inhibiting PDE4 with RS25344 enhanced the increase in the contraction rate after stimulation of βAR in WT myocytes. (B) Inhibiting PDE3 with cilostamide did not change the response in the contraction rate after stimulation of βAR in WT myocytes. The data represent the mean ± SE of experiments from at least three different myocyte preparations. *, P < 0.05; time course significantly different by two-way ANOVA.
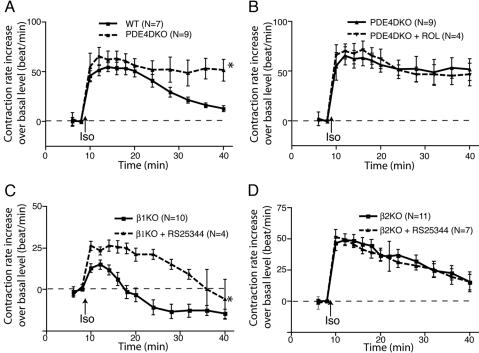
PDE4D has been implicated in βAR signaling in HEK293 cells and rat myocytes (13, 14). In agreement with the effect of the acute inhibition of PDE4s with RS25344, myocytes lacking PDE4D (PDE4D-KO) responded to isoproterenol with an altered pattern of contraction when compared with the WT control. An increase in the maximum contraction rate was followed by a sustained rate of contraction for up to 30 min (Fig. 2A). Inhibiting the remaining PDE4 activity in the PDE4D-KO myocytes with rolipram did not have further effects on the response (Fig. 2B), suggesting that PDE4D is the major PDE regulating the βAR signaling that controls contraction rate. In the same vein, myocytes lacking PDE4A (PDE4A-KO) or PDE4B (PDE4B-KO) displayed an isoproterenol-stimulated contraction rate similar to that of WT controls; inhibition of the residual PDE4 activity in PDE4A-KO or PDE4B-KO myocytes with rolipram led to further enhancement of the contraction rate (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 2.
PDE4D is selectively involved in β2AR signaling for contraction rate response in cardiac myocytes. (A) PDE4D-KO myocytes display a higher contraction rate response to isoproterenol stimulation than do WT myocytes. (B) Inhibition of the residual PDE4 activity in PDE4D-KO myocytes with rolipram does not alter the contraction rate response to isoproterenol stimulation. (C) Inhibition of PDE4 activity enhances the contraction rate response to isoproterenol stimulation in β1AR-KO myocytes. (D) Inhibition of PDE4 activity with rolipram does not alter the contraction rate response to isoproterenol stimulation in β2AR-KO myocytes. The data represent the mean ± SE of experiments from at least three different myocyte preparations. *, P < 0.05; time course found to be significantly different by two-way ANOVA.
β2AR Signaling Is Regulated by PDE4D Activity. β1AR and β2AR activation have distinct effects on myocyte contraction rate (2). We therefore analyzed the role of PDE4D in βAR subtype-mediated signaling in cardiac myocytes isolated from β1AR-KO or β2AR-KO mice. In β1AR-KO myocytes, β2AR-mediated maximum response was greatly enhanced by PDE4-specific inhibitors, rolipram and RS25344, as well as by the nonselective PDE inhibitor 3-isobutyl-1-methylxanthine (Fig. 2C and Fig. 7, which is published as supporting information on the PNAS web site). However, none of these drugs had a significant effect on the contraction rate response mediated by isoproterenol stimulation of β1AR in β2AR-KO myocytes (Figs. 2D and 7).
To rule out the possibility that the observed effects on β2AR signaling by PDE4 inhibitors were caused by altered PDE4 expression in β1AR-KO myocytes, PDE activity was measured on β1AR-KO, β2AR-KO, or WT myocytes. The total PDE activity measured in cultured WT myocytes was ≈58.75 ± 3.2 pmol/mg of protein per min (Table 1). Approximately 63% of PDE activity was rolipram-sensitive, indicating the prevalence of PDE4, whereas 28% of PDE activity was inhibited by cilostamide, a PDE3-selective inhibitor. There was no appreciable difference in PDE activities expressed in myocytes from WT, β1AR-KO, or β2AR-KO mice (Table 1).
Table 1. PDE activity in myocytes from different KO strains.
| Activity | WT, pmol/min × mg | β1AR-KO, pmol/min × mg | β2AR-KO, pmol/min × g |
|---|---|---|---|
| Total PDE activity | 58.75 ± 3.2 | 61.77 ± 10.26 | 62.31 ± 4.89 |
| Rolipram-sensitive | 37.05 ± 3.48 | 39.47 ± 5.95 | 37.13 ± 4.56 |
| PDE4 | (62.78%) | (64.13%) | (60.57%) |
| Cilostamide-sensitive | 16.59 ± 3.21 | 15.93 ± 1.62 | 15.77 ± 2.72 |
| PDE3 | (27.95%) | (26.4%) | (24.97%) |
Total myocyte PDE activity from cell lysates and rolipram-sensitive and cilostamide-sensitive activities were measured as detailed in Methods. No differences were observed in PDE activities from different KO mouse strains.
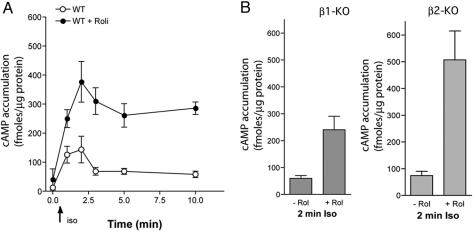
Inhibition of PDE4 Enhances cAMP Accumulation by both β1AR and β2 AR Activation in Cardiac Myocytes. Upon agonist activation, β1AR and β2AR can couple to Gs protein, which leads to activation of adenylyl cyclase and an increase in intracellular cAMP. cAMP accumulation also is controlled by PDE activity. To test the role of PDE4 in βAR-mediated cAMP accumulation in cardiac myocytes, we measured cellular cAMP accumulation in myocytes isolated from WT, β1AR-KO, and β2AR-KO mice in the absence or presence of rolipram. WT myocytes displayed a transient cellular cAMP accumulation that peaked at 2 min after the addition of isoproterenol (Fig. 3A). Inhibiting PDE4 with rolipram greatly enhanced cAMP accumulation, and cAMP levels remained constant for 10 min of stimulation (Fig. 3A). Stimulation of the βAR subtypes in β1AR-KO or β2AR-KO myocytes led to an intracellular cAMP accumulation similar to that observed in WT myocytes, and inhibition of PDE4 with rolipram further enhanced both β1AR- and β2AR-mediated cAMP accumulation (Fig. 3B). The increase in cAMP in β2AR-KO myocytes induced by isoproterenol in the presence of rolipram remained elevated for at least 10 min (Fig. 8, which is published as supporting information on the PNAS web site). Conversely, incubation of β1AR-KO myocytes with isoproterenol in the presence of rolipram produced an enhanced but transient cAMP accumulation (Fig. 8), in good agreement with the contraction rate response measured under the same experimental conditions (Fig. 4B).
Fig. 3.
Inhibition of PDE4 enhances the global cAMP level in response to isoproterenol in myocytes from different KO strains. (A) Stimulation of βAR induces a transient cAMP accumulation that peaks at 2 min of isoproterenol treatment in WT myocytes. Inhibition of PDE4 activity significantly enhanced cAMP accumulation and attenuated the degradation of cAMP. (B) The peak cAMP accumulation induced by either β1AR (Left) or β2AR (Right) activation at 2 min of isoproterenol stimulation is further enhanced by the PDE4 inhibitor rolipram. Each point is the mean + SEM of at least three distinct experiments.
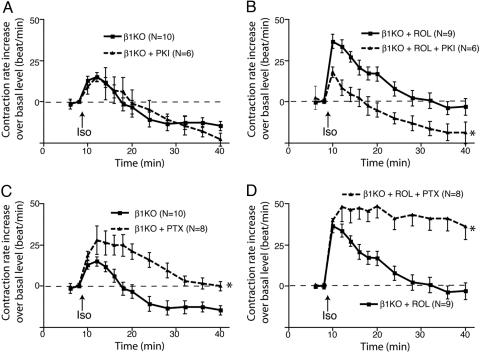
Fig. 4.
Inhibition of PDE4 evokes an increase in the PKA-dependent contraction rate in β2AR-stimulated cardiac myocytes. (A) Effect of PKI on the contraction rate of myocytes from β1AR-KO mice. (B) The effect of PKI on the contraction rate of myocytes from β1AR-KO mice after inhibiting PDE4 with rolipram. (C) Effect of the Gi inhibitor PTX on the contraction rate of myocytes from β1AR-KO mice. (D) Effect of the Gi inhibitor PTX on the contraction rate of myocytes from β1AR-KO mice after inhibiting PDE4 with rolipram. The data represent the mean ± SE of experiments from at least three different myocyte preparations. *, P < 0.05; time course found to be significantly different by two-way ANOVA.
Inhibiting PDE4 Leads to PKA-Dependent Signaling by the β2AR. Our previous report (2) suggested that PKA does not play a role in β2AR/Gs coupling or the β2AR-regulated contraction rate in neonatal cardiac myocytes. Thus, in β1AR-KO myocytes, pretreatment with PKI did not significantly affect the contraction rate increase by isoproterenol stimulation (Fig. 4A and ref. 2). However, PKI completely reversed the changes in the contraction rate response observed upon PDE4 inhibition (Fig. 4B). This finding indicates that inhibition of PDE4 evokes an aberrant PKA-dependent component in β2AR signaling. Recent studies suggest a role of PDE4D in β2AR coupling to the Gi pathway in HEK293 cells and rat neonatal myocytes. We therefore tested the effect of PDE4D inhibition by rolipram on β2AR signaling through Gi in β1AR-KO myocytes. In control cells, pretreatment with PTX, a selective Gi inactivator, significantly enhanced the contraction rate increase by isoproterenol stimulation (Fig. 4C). In comparison, in β1AR-KO myocytes treated with rolipram, inhibition of Gi with PTX led to a further enhancement of contraction rate in response to isoproterenol stimulation, demonstrating that the β2AR coupling to Gi was not inhibited (Fig. 4D). Of interest, isoproterenol stimulation of β2ARs in β1AR-KO myocytes treated with both PTX and rolipram produces a response similar in magnitude and duration to that observed upon stimulation of β1ARs in β2AR-KO myocytes (compare Figs. 2D and 4D). When cAMP was measured in β1AR-KO myocytes treated with PTX and rolipram, the isoproterenol-stimulated cAMP accumulation was significantly higher than that observed in the cells treated with rolipram alone (Fig. 8).
Discussion
Although activated by the same ligands, β1 and β2 ARs play distinct roles in adjusting the cardiac responses to sympathetic activation. To fulfill these subtype-specific functions, receptor signaling is fine-tuned by a complex array of control mechanisms (21). The findings presented here demonstrate that PDE4D selectively regulates signaling by the β2AR in neonatal cardiac myocytes, while having little or no effect on β1AR signaling. These data underscore the critical role of subcellular cAMP regulation in the complex control of receptor signaling.
Our studies were performed in neonatal cardiac myocytes, which beat spontaneously in culture. This is an excellent model system for studying β1AR and β2AR subtype-specific signaling in the context of a differentiated cell. We have reported previously (2) that β1ARs and β2ARs regulate the rate of spontaneous myocyte contraction by different mechanisms. β1AR stimulation leads to a robust PKA-dependent increase in contraction rate. In contrast, stimulation of cardiac β2AR leads to a PKA-independent increase followed by a Gi-mediated decrease in contraction rate. This differential signaling of β1ARs and β2ARs in cardiac myocytes led several investigators to propose the existence of subtype-specific signaling domains that consist of receptors, specific G proteins, effectors, regulatory proteins, and scaffold proteins that assemble the signaling and regulatory molecules in large signaling complexes (9). These macromolecular complexes function in association with the cytoskeleton and within discrete domains of membrane specialization (9, 22, 23). The present results further support the existence of such signaling domains and indicate an important role for PDE4 in defining these domains. Although β2AR activation leads to an increase in cAMP production, the cAMP generated does not have access to the PKA-dependent signaling pathways by which the β1AR regulates contraction rate. Rather, β2AR activation may lead to an increase in contraction rate via activation of a cAMP-sensitive, nonselective ion channel or through a direct interaction of the receptor with L-type Ca channels (24, 25). This restricted access is lost in the presence of PDE4D inhibitors or when PDE4D is genetically inactivated. Indeed, in the absence of PDE4D, β2AR signaling becomes sensitive to PKI inhibition. The increase in contraction rate response observed in β1AR-KO myocytes treated with rolipram may be explained by diffusion of cAMP from the β2AR signaling compartment into the β1AR signaling compartment or other compartments containing the appropriate PKA-sensitive signaling molecules. These results strongly suggest that PDE4D is an integral component of the β2AR signaling complex, and that its activity is required to define the β2AR signaling properties. Given the number of different G protein-coupled receptors expressed in cardiac myocytes and the array of signaling pathways they activate, it is likely that other cardiac-expressed PDEs form complexes with receptors other than the βARs. Therefore, physically discrete pools of cAMP may exist throughout the entire surface of these cells. Recent studies have provided evidence that may explain the difference in the efficacy of PDE4 inhibitors on β1AR and β2AR subtype signaling in cardiac myocytes. In HEK293 cells overexpressing human β2AR, activation of the receptor with isoproterenol results in recruitment of PDE4D isoenzymes to the plasma membrane. This recruitment is not observed in cells lacking β-arrestin, a protein already implicated in receptor desensitization (13). A β-arrestin-PDE4D complex is detectable in the cytosol of the cell before stimulation; the activation-dependent association of the β-arrestin-PDE4D complex with the receptors localizes the PDE4D activity to the same microenvironment as the activated adenylyl cyclase. In contrast to β2AR, activated β1AR has a relatively low binding affinity for arrestin (26). Thus, the β-arrestin-PDE4D complex may not be recruited efficiently during β1AR stimulation, a property that would explain the different sensitivity of β1AR and β2AR activation to PDE4 inhibition. Also consistent with this view is the finding that β2AR stimulation of contraction is very transient, whereas β1AR stimulation dissipates slowly. A difference in PDE4/β-arrestin recruitment may explain the differences in duration of responses after β1AR and β2AR occupancy.
The above scenario, however, is not entirely sufficient to explain all of the properties of the two β-adrenergic signaling compartments. Whereas β2AR signaling is clearly affected by PDE4 activity in cardiac myocytes from β1AR-KO mice, the β1AR-regulated contraction rate in β2AR-KO mice is not affected by inhibition of PDE4 activity (Fig. 2). This observation is somewhat surprising given the fact that β1AR-induced cAMP accumulation is increased after PDE4 inhibition. There are several possible explanations for the lack of a rolipram response in contraction rate in β2AR-KO myocytes despite a robust effect on cAMP accumulation. (i) β1AR coupling to contraction rate may become rapidly saturated by small local cAMP changes so that no further increase in rate is observed when high and diffuse cAMP levels accumulate in the β1AR signaling compartment after PDE4 inhibition with rolipram. This scenario underscores the fact that measurements of global cAMP levels in a cell do not predict the effect on myocyte contraction rate. (ii) There is possibly no diffusion of cAMP from the β1AR signaling compartment to the β2AR signaling compartment, even in the presence of rolipram, i.e., these two compartments may be physically sequestered (27, 28). Thus, the rolipram-sensitive signaling observed in both WT and β1AR-KO myocytes may be caused entirely by the extra cAMP generated in the β2AR compartment and acting on signaling molecules within that or adjacent compartments. (iii) β2AR coupling to the myocyte contraction rate may be completely cAMP-independent, with the stimulatory phase being mediated by direct receptor or G protein coupling to an ion channel, a possibility suggested by data in neurons where β2AR directly couples to L-type Ca2+ channels (25). Therefore, diffusion of cAMP from the β1AR into the β2AR compartment as a result of PDE4D inhibition would have no effect. (iv) The signaling molecules that couple β2AR activation to an increase in contraction rate are absent in the β2AR KO myocytes. This absence may be expected if the β2AR forms the nodes for its signaling complex. Thus, in β2AR-KO myocytes treated with rolipram, cAMP diffuses throughout the cell, but there are no β2AR-associated signaling molecules upon which to act. Although more work is required to distinguish among the possibilities, the data strongly support the view that PDE4D controls a functionally and perhaps physically discrete pool of cAMP, possibly by acting as a barrier to diffusion of cAMP out of the β2AR signaling compartment.
A complex array of PDE forms are expressed in heart, even though marked species differences have been reported (29-31). On the basis of their biochemical characterization and pharmacological profiling, the cGMP-inhibited PDE3s are thought to play a major role in the control of myocardial contractility, particularly in view of the marked inotropic effects of the PDE3 inhibitors amrinone and milrinone (32). PDE1, PDE2, and PDE5 also have been detected in heart extract (29, 33). Our data indicate that PDE4D is the primary PDE associated with β-adrenergic regulation of the mouse myocyte contraction rate. This conclusion is based on the fact that PDE4D ablation produces effects similar to inhibition of all PDE4s with rolipram or RS25344. Furthermore, inhibition of all PDEs with 3-isobutyl-1-methylxanthine does not produce additional effects. This finding of a specialized function of PDE4D is reminiscent of data suggesting PDE4D localization in the Z band where β-adrenergic receptors and L-type channels are localized. Interaction of PDE4D with A kinase anchoring proteins or myomegalin may serve to anchor this PDE in regions crucial for excitation contraction coupling (12, 23, 34). Underscoring the physiological relevance of our findings, ablation of PDE4D in mice has an impact on cardiac function. Aging PDE4D homozygous null develop a dilated cardiomyopathy and cardiac failure, associated with increased phosphorylation of the ryanodine receptors (unpublished data).∥ Our data suggest that a macromolecular complex involving PDE4D in close proximity of the β2AR must be present in these cardiomyocytes.
In summary, our findings provide insight into the physiological role of β1ARs and β2ARs and the domains in which they function. Our results also provide evidence that the function of PDEs is highly specialized, such that only one of the several PDEs expressed in cardiomyocytes impacts the β-adrenergic-mediated contraction rate. It is likely that other receptors and other PDEs are functionally interacting in these cells to control different components of the contractile machinery. Thus, the emerging picture is that subsets of cAMP-related components are functionally coupled to create microdomains of signaling. Understanding the properties of these domains will be critical for new pharmacological strategies aimed at improving the performance of the failing heart. The possibility also should be considered that β1AR and/or β2AR microdomains are functioning in cells other than cardiac myocytes, and that PDE4D plays a role in establishing these compartments. In the same vein, it is possible that some of the phenotypes observed upon PDE4D ablation (35) are caused by the disruption of β1AR and/or β2AR compartments.
Supplementary Material
Acknowledgments
We thank L. Lan for her technical support in the PDE assays. F.N. was supported by a scholarship from the Fulbright Foundation. This work was supported by National Institutes of Health Grants 1R01 HL71078-01 (to B.K.) and 1R01-HD20788-18 (to M.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AR, adrenoceptor; PDE, phosphodiesterase; PKA, protein kinase A; PKI, PKA inhibitor; KO, knockout; PTX, pertussis toxin.
Footnotes
Lehnart, S. E., Wehrens, X. H. T., Reiken, S. R., Jin, S. L. C., Vest, J. A., Conti, M. & Marks, A. R., Annual American Heart Association Scientific Sessions, Nov. 7-11, 2004, New Orleans (abstr.).
References
- 1.Lohse, M. J., Engelhardt, S. & Eschenhagen, T. (2003) Circ. Res. 93, 896-906. [DOI] [PubMed] [Google Scholar]
- 2.Devic, E., Xiang, Y., Gould, D. & Kobilka, B. (2001) Mol. Pharmacol. 60, 577-583. [PubMed] [Google Scholar]
- 3.Green, S. A., Holt, B. D. & Liggett, S. B. (1992) Mol. Pharmacol. 41, 889-893. [PubMed] [Google Scholar]
- 4.Ostrom, R. S., Gregorian, C., Drenan, R. M., Xiang, Y., Regan, J. W. & Insel, P. A. (2001) J. Biol. Chem. 276, 42063-42069. [DOI] [PubMed] [Google Scholar]
- 5.Xiang, Y., Rybin, V. O., Steinberg, S. F. & Kobilka, B. (2002) J. Biol. Chem. 277, 34280-34286. [DOI] [PubMed] [Google Scholar]
- 6.Rybin, V. O., Xu, X., Lisanti, M. P. & Steinberg, S. F. (2000) J. Biol. Chem. 275, 41447-41457. [DOI] [PubMed] [Google Scholar]
- 7.Verde, I., Vandecasteele, G., Lezoualc'h, F. & Fischmeister, R. (1999) Br. J. Pharmacol. 127, 65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti, M., Nemoz, G., Sette, C. & Vicini, E. (1995) Endocr. Rev. 16, 370-389. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg, S. F. & Brunton, L. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 751-773. [DOI] [PubMed] [Google Scholar]
- 10.Jurevicius, J. & Fischmeister, R. (1996) Proc. Natl. Acad. Sci. USA 93, 295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodge, K. L., Khouangsathiene, S., Kapiloff, M. S., Mouton, R., Hill, E. V., Houslay, M. D., Langeberg, L. K. & Scott, J. D. (2001) EMBO J. 20, 1921-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verde, I., Pahlke, G., Salanova, M., Zhang, G., Wang, S., Coletti, D., Onuffer, J., Jin, S. L. & Conti, M. (2001) J. Biol. Chem. 276, 11189-11198. [DOI] [PubMed] [Google Scholar]
- 13.Perry, S. J., Baillie, G. S., Kohout, T. A., McPhee, I., Magiera, M. M., Ang, K. L., Miller, W. E., McLean, A. J., Conti, M., Houslay, M. D. & Lefkowitz, R. J. (2002) Science 298, 834-836. [DOI] [PubMed] [Google Scholar]
- 14.Baillie, G. S., Sood, A., McPhee, I., Gall, I., Perry, S. J., Lefkowitz, R. J. & Houslay, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 940-945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zaccolo, M. & Pozzan, T. (2002) Science 295, 1711-1715. [DOI] [PubMed] [Google Scholar]
- 16.Jin, S. L., Richard, F. J., Kuo, W. P., D'Ercole, A. J. & Conti, M. (1999) Proc. Natl. Acad. Sci. USA 96, 11998-12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin, S. L. & Conti, M. (2002) Proc. Natl. Acad. Sci. USA 99, 7628-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sette, C. & Conti, M. (1996) J. Biol. Chem. 271, 16526-16534. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez, R., Sette, C., Yang, D., Eglen, R. M., Wilhelm, R., Shelton, E. R. & Conti, M. (1995) Mol. Pharmacol. 48, 616-622. [PubMed] [Google Scholar]
- 20.Thompson, W. J. & Appleman, M. M. (1971) Biochemistry 10, 311-316. [PubMed] [Google Scholar]
- 21.Brunton, L. L. (2003) Sci. STKE 2003, PE44. [DOI] [PubMed] [Google Scholar]
- 22.Cong, M., Perry, S. J., Lin, F. T., Fraser, I. D., Hu, L. A., Chen, W., Pitcher, J. A., Scott, J. D. & Lefkowitz, R. J. (2001) J. Biol. Chem. 276, 15192-15199. [DOI] [PubMed] [Google Scholar]
- 23.Fraser, I. D., Cong, M., Kim, J., Rollins, E. N., Daaka, Y., Lefkowitz, R. J. & Scott, J. D. (2000) Curr. Biol. 10, 409-412. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig, A., Zong, X., Jeglitsch, M., Hofmann, F. & Biel, M. (1998) Nature 393, 587-591. [DOI] [PubMed] [Google Scholar]
- 25.Davare, M. A., Avdonin, V., Hall, D. D., Peden, E. M., Burette, A., Weinberg, R. J., Horne, M. C., Hoshi, T. & Hell, J. W. (2001) Science 293, 98-101. [DOI] [PubMed] [Google Scholar]
- 26.Shiina, T., Kawasaki, A., Nagao, T. & Kurose, H. (2000) J. Biol. Chem. 275, 29082-29090. [DOI] [PubMed] [Google Scholar]
- 27.Rich, T. C., Fagan, K. A., Nakata, H., Schaack, J., Cooper, D. M. & Karpen, J. W. (2000) J. Gen. Physiol. 116, 147-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich, T. C., Fagan, K. A., Tse, T. E., Schaack, J., Cooper, D. M. & Karpen, J. W. (2001) Proc. Natl. Acad. Sci. USA 98, 13049-13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weishaar, R. E., Kobylarz-Singer, D. C. & Kaplan, H. R. (1987) J. Mol. Cell. Cardiol. 19, 1025-1036. [DOI] [PubMed] [Google Scholar]
- 30.Movsesian, M. A., Smith, C. J., Krall, J., Bristow, M. R. & Manganiello, V. C. (1991) J. Clin. Invest. 88, 15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauffman, R. F., Crowe, V. G., Utterback, B. G. & Robertson, D. W. (1986) Mol. Pharmacol. 30, 609-616. [PubMed] [Google Scholar]
- 32.Stoclet, J. C., Keravis, T., Komas, N. & Lugnier, C. (1995) Exp. Opin. Invest. Drugs 4, 1081-1199. [Google Scholar]
- 33.Kostic, M. M., Erdogan, S., Rena, G., Borchert, G., Hoch, B., Bartel, S., Scotland, G., Huston, E., Houslay, M. D. & Krause, E. G. (1997) J. Mol. Cell. Cardiol. 29, 3135-3146. [DOI] [PubMed] [Google Scholar]
- 34.Kapiloff, M. S. (2002) Mol. Pharmacol. 62, 193-199. [DOI] [PubMed] [Google Scholar]
- 35.Conti, M., Richter, W., Mehats, C., Livera, G., Park, J. Y. & Jin, C. (2003) J. Biol. Chem. 278, 5493-5496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.