Abstract
Objective
Thoracoabdominal aortic aneurysm (TAAA) repair remains a significant challenge with considerable perioperative morbidity and mortality. A hybrid approach visceral debranching with endovascular aneurysm exclusion has been used to treat high-risk patients and therefore to allow repair in more patients. Limited data exist regarding long-term outcomes with this procedure as well as comparison to conventional open repair. This study describes our institutional algorithmic approach to TAAA repair using both open and hybrid techniques.
Methods
Hybrid and open TAAA repairs performed between July 2005 and August 2015 were identified from a prospectively maintained institutional aortic surgery database. Perioperative morbidity and mortality, freedom from reintervention, and long-term and aorta-specific survival were calculated and compared between the two groups.
Results
During the study period, 165 consecutive TAAA repairs were performed, including 84 open repairs and 81 hybrid repairs. Patients in the hybrid repair group were significantly older, were more frequently female, and had a generally greater comorbid disease burden, including significantly more chronic kidney disease. Despite the older and sicker cohort, there was no difference in in-hospital mortality between the two groups (9.9% hybrid vs 7.1% open; P = .59). Major morbidity rates differed by procedure, with patients undergoing open repair having a significantly higher rate of postoperative stroke (9.5% open vs 0% hybrid; P = .017), whereas patients undergoing hybrid repair had a higher rate of new permanent dialysis (14.8% hybrid vs 3.6% open; P = .043). There was no difference between groups in the rate of postoperative permanent paraplegia/paresis (8.3% open vs 7.4% hybrid; P = .294). There was a significantly increased rate of reintervention in the hybrid repair group (12.3% hybrid vs 1.2% open, P = .004), with all hybrid reinterventions performed because of endoleak. One-year survival was similar between groups at 69% in hybrid repairs vs 77% in open repairs. Long-term survival was worse in the hybrid group (5-year survival, 32% hybrid vs 56% open), although late survival appeared to be influenced mainly by comorbid disease burden, given the similar long-term aorta-specific survival between groups.
Conclusions
Use of an algorithmic approach whereby higher risk patients with TAAA are treated by a hybrid approach and lower risk patients with conventional open repair yields satisfactory short- and long-term outcomes. The availability of multiple options for TAAA repair within a single center likely allows repair in more patients with consequent decrease in the risk of aorta-related death, at the expense of increased reinterventions for endoleak.
The management of thoracoabdominal aortic aneurysms (TAAAs) remains a formidable surgical challenge, which is compounded by the fact that the majority of patients presenting with this disease are elderly with multiple comorbidities. Conventional open TAAA repair (OTAAAR) remains associated with substantial perioperative morbidity and mortality, despite improvements in perioperative management and surgical technique.1–4 Although feasible in the elderly, OTAAAR has been associated with worse perioperative outcomes in this population, including prolonged length of hospital and intensive care unit stay, increased mortality, and the need for additional procedures.5
The elderly and multiply comorbid are, however, the fastest growing segment of the population, with recent estimates predicting the total number of individuals older than 65 years to increase by 50% during the next 15 years.6 In concert, thoracic aneurysmal disease is increasing in incidence and remains one of the top 15 causes for mortality in this population.7 Furthermore, owing to the dismal natural history of TAAA8 and despite its hazards, surgical treatment remains favored for this group. As such, the majority of patients being treated for TAAA in the coming years will be high risk, making the decision of whether to offer repair difficult.
Given the risks of conventional open repair in the elderly and multiply comorbid, hybrid TAAA repair (HTAAAR) techniques have been developed that use extra-anatomic visceral bypass (“debranching”) with endovascular aneurysm exclusion.9,10 These techniques, which carry the potential of lower perioperative risk,11 may allow repair in higher risk patients.9,10 However, the published literature to date on HTAAAR is limited regarding long-term outcomes. Furthermore, large studies comparing hybrid with open techniques for TAAA repair in concurrently treated cohorts are lacking, and there are no consensus guidelines for operative decision-making in the high-risk patient.12–16 Given these limitations of the current literature, the aims of this study were to present long-term outcomes with hybrid repair as well as to describe comparative outcomes in a concurrently treated cohort undergoing OTAAAR with guided by an institutional algorithmic approach to TAAA repair.17,18
METHODS
Patient population and data collection
This retrospective study was approved by the Institutional Review Board of Duke University Medical Center, and the need for individual patient consent was waived. Hybrid and open TAAA repairs performed between July 2005 and August 2015 were identified from a prospectively maintained, institutional aortic surgery database. Operative techniques used for HTAAAR have been described previously9,10,17–19 and were performed jointly by a team including cardiothoracic and vascular surgeons. Operative techniques for OTAAAR involved the use of a “clamp and sew” technique for extent IV repairs, whereas extent I–III repairs were performed using cardiopulmonary bypass support with deep hypothermia as described by Kouchoukos.20 Intercostal vessels were reimplanted when feasible in extent I–III repairs, and neurophysiologic intraoperative monitoring with somatosensory and motor evoked potentials was routine with both hybrid and open repairs.21,22
Indications for surgery were classified as degenerative aneurysm, chronic dissection, or other (eg, pseudoaneurysm after prior OTAAAR). Patient selection criteria for hybrid vs open repair were as presented previously,9,17,18 with hybrid approaches generally favored in patients with advanced age (≥65 years), frailty, significant cardiac pulmonary disease, renal insufficiency, prior left-sided chest surgery, and prior open abdominal or descending/TAAA repair (Fig 1). However, these criteria were only relative factors in the surgical planning process and did not serve as absolute indications for or contraindications to a specific repair strategy. Follow-up protocols for patients undergoing HTAAAR were as previously described,23 with computed tomography angiography at 1 month, 6 months, and 12 months postoperatively and annually thereafter. OTAAAR patients likewise underwent postoperative surveillance with cross-sectional imaging 3 to 6 months after surgery and annually thereafter. Comorbid conditions were defined by Society of Thoracic Surgeons definitions (www.sts.org).
Fig 1.
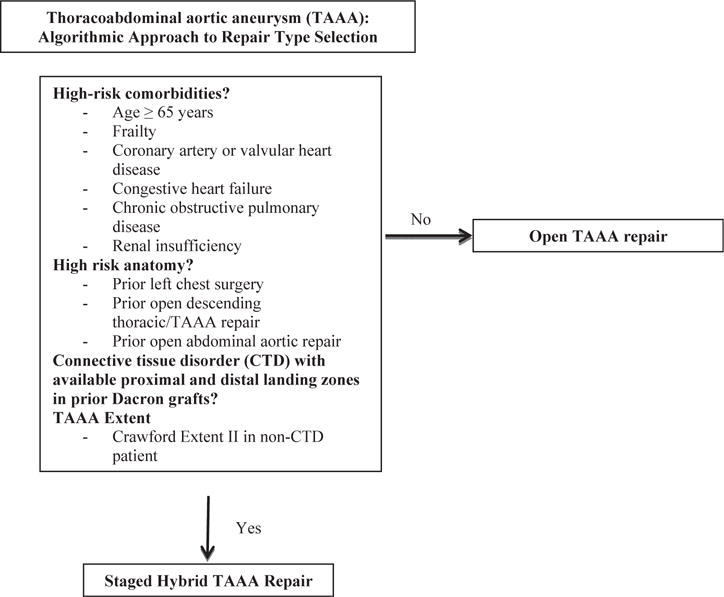
Institutional algorithm for hybrid thoracoabdominal aortic aneurysm (TAAA) repair. These criteria are relative factors in the decision-making process and are not absolute indications or contraindications. The decision for open vs hybrid TAAA repair should be made by a joint vascular and cardiac surgical team with expertise in both techniques. Institutional results with each approach should further influence the decision-making process.
Statistical analyses
Aortic reinterventions, whether through open or endovascular means, for aortic disease within the segment of aorta originally treated at the index HTAAAR or OTAAAR were reviewed and compared between groups. Procedures for metachronously developing aortic disease in a different segment of the thoracic or abdominal aorta distant to the aortic segment originally treated with HTAAAR or OTAAAR were not counted as reinterventions. Freedom from reintervention and survival were assessed at 1-, 3-, and 5-year time points using individual chart review and dedicated follow-up. Categorical variables were compared between groups using χ2 and Fisher exact tests, and continuous variables were compared using Wilcoxon rank sum tests. Freedom from reintervention and overall and aorta-specific survival were estimated using the Kaplan-Meier method. Statistical analyses and calculations were conducted using R software, version 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographic and procedural characteristics
During the study period, 165 consecutive TAAA repair procedures were performed by two principal co-surgeons (R.L.M. and G.C.H.) for repair of thoracoabdominal aortic disease, including 81 HTAAARs and 84 OTAAARs. This represents approximately 10% (n = 165 of 1703) of the total thoracic aortic procedural volume performed at our institution during this period. Patient demographics are presented in Table I. The median patient age for the entire cohort was 69 years, with a significantly younger age in the OTAAAR group (66 vs 72 years; P < .001). In addition, a higher overall percentage of patients undergoing OTAAAR were of male gender (67.9% vs 50.6%; P = .03). Much of the comorbidity profile for the OTAAAR and HTAAAR groups was similar, although a significantly higher proportion of patients in the HTAAAR group had baseline renal insufficiency (37.0% vs 17.9%; P = .008), whereas a significantly higher proportion of patients undergoing OTAAAR had a prior diagnosis of aortic dissection (36.9% vs 17.3%; P = .005). Unsurprisingly, a significantly higher percentage of patients undergoing OTAAAR had a connective tissue disorder (14.5% vs 3.7%; P = .03); at our institution, hybrid procedures are avoided in these patients unless proximal and distal landing zones lie within prior Dacron grafts,24 as was the case for all three patients with connective tissue disease treated with HTAAAR.
Table I.
Demographics
| Variablea | Total (N = 165) | Open (n = 84) | Hybrid (n = 81) | P value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | 69 (61–74) | 66 (53–72) | 72 (66–76) | <.001 |
| Male gender | 98 (59.3) | 57 (67.9) | 41 (50.6) | .03 |
| White race | 116 (70.3) | 59 (70.2) | 57 (70.4) | .99 |
| Body mass index, kg/m2 | 25.7 (22.5–29.1) | 26.4 (23.0–30.4) | 25.1 (22.1–28.8) | .14 |
| Patient comorbidities | ||||
| Hypertension | 154 (93.3) | 79 (94.0) | 75 (92.6) | .76 |
| Hyperlipidemia | 111 (67.2) | 58 (69.0) | 53 (65.4) | .74 |
| Active or recent smoker | 118 (71.5) | 62 (73.8) | 56 (69.1) | .61 |
| Ejection fraction, % | 55 (55, 55) | 55 (54, 55) | 55 (55, 55) | .63 |
| Diabetes mellitus | 15 (9) | 10 (11.9) | 5 (6.2) | .28 |
| Coronary artery disease | 58 (35.1) | 30 (35.7) | 28 (34.6) | .99 |
| Congestive heart failure | 8 (4.8) | 7 (8.3) | 1 (1.2) | .253 |
| History of stroke | 29 (17.5) | 13 (15.5) | 16 (19.8) | .54 |
| Peripheral vascular disease | 59 (35.7) | 26 (31.0) | 33 (40.7) | .2 |
| COPD | 65 (39.3) | 31 (36.9) | 34 (42.0) | .53 |
| Renal insufficiency, baseline creatinine >1.5 mg/dL | 45 (27.2) | 15 (17.9) | 30 (37.0) | .008 |
| Connective tissue disease | 15 (9) | 12 (14.5) | 3 (3.7) | .03 |
| Median baseline serum creatinine, mg/dL | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) | 1.2 (0.9–1.5) | .44 |
| Prior aortic surgery | 89 (53.9) | 41 (48.8) | 48 (59.3) | .21 |
| Previous aortic dissection | 45 (27.2) | 31 (36.9) | 14 (17.3) | .005 |
COPD, Chronic obstructive pulmonary disease.
Data are presented as median (interquartile range) for continuous variables and as a number (%) for categorical variables.
Procedural characteristics are presented in Table II and were similar between groups, with no significant differences in maximum aortic diameter, procedure status, or American Society of Anesthesiologists physical status scores. There was noted to be a significant difference (P < .0001) in the anatomic considerations (Crawford classification) for TAAA repair, with the overwhelming majority of HTAAAR being performed for extents II and III TAAA (42.0% and 45.7%, respectively), whereas OTAAAR was performed in a somewhat more evenly dispersed anatomic distribution. All patients with Crawford extent IV classification TAAA underwent OTAAAR consistent with our institutional preference for open treatment using a clamp and sew technique for this specific TAAA (Fig 1). Cardiopulmonary bypass (CPB) was used in 70.2% of open repairs (median CPB time, 173 minutes), whereas no HTAAARs used CPB support (P < .0001).
Table II.
Operative characteristics by procedure
| Variablea | Total (N = 165) | Open (n = 84) | Hybrid (n = 81) | P value |
|---|---|---|---|---|
| Maximum aortic diameter, cm | 6.4 (5.8–7.1) | 6.3 (5.7–7.0) | 6.4 (5.8–7.4) | .21 |
| Indication | .008 | |||
| Atherosclerotic aneurysm | 115 (69.7) | 50 (59.5) | 65 (80.2) | |
| Chronic dissection | 42 (25.5) | 30 (35.7) | 12 (14.8) | |
| Other | 8 (4.8) | 4 (4.8) | 4 (4.9) | |
| ASA score | .52 | |||
| ASA 3 | 108 | 57 (67.9) | 51 (63.0) | |
| ASA 4 | 57 | 27 (32.1) | 30 (37.0) | |
| Procedure status | .921 | |||
| Elective | 136 | 70 | 66 | |
| Urgent or emergency | 29 | 14 | 15 | |
| Crawford classification | <.001 | |||
| Extent I | 27 (16.4) | 20 (23.8) | 7 (8.6) | |
| Extent II | 45 (27.3) | 11 (13.1) | 34 (42.0) | |
| Extent III | 61 (37.0) | 24 (28.6) | 37 (45.7) | |
| Extent IV | 29 (17.8) | 29 (34.5) | 0 (0) | |
| Staged procedures | 45 (27.3) | NA | 45 (55.6) | NA |
| Intraoperative lumbar drain | 106 (64.2) | 52 (61.9) | 54 (66.7) | .532 |
| Performed on CPB | 59 (35.7) | 59 (70.2) | 0 | <.0001 |
| CPB time, median minutes | 173 (130–219.5) | 173 (130–219.5) | NA | NA |
ASA, American Society of Anesthesiologists; CPB, cardiopulmonary bypass; NA, not applicable.
Data are presented as median (interquartile range) for continuous variables and as a number (%) for categorical variables.
Regarding HTAAAR procedural characteristics (Table III), the initial 34 consecutive procedures were performed as a single-stage procedure with visceral debranching followed immediately by endovascular exclusion of the TAAA, with the end of this contiguous run of “simultaneous” HTAAAR corresponding to the beginning of the “staged” HTAAAR era in January 2010 and coincident with our prior findings of the advantageous nature of the staged approach.17,18 Only 2 additional simultaneous HTAAARs have been performed in the 5 years since (none since September 2011), for a total of 36 single-staged HTAAARs, representing 44.4% of all HTAAARs. The remaining 45 HTAAARs (55.6%) have been planned as staged procedures, with (97.7%) performed during the same hospitalization. A median of 6 days passed between visceral debranching and endovascular exclusion (range, 1–70 days). In two patients, TAAA exclusion was incomplete (2.5%) owing to inability to perform endovascular aneurysmal exclusion secondary to death (both due to mesenteric ischemia) after first-stage debranching, for a technical success rate of 97.5%.
Table III.
Operative characteristics of hybrid thoracoabdominal aortic aneurysm repair (HTAAAR)
| Variable | Hybrid (n = 81) |
|---|---|
| Staged HTAAAR procedure | 45 (55.6) |
| Performed during same hospitalization | 42 (97.7) |
| Duration between abdominal debranching and TEVAR, days, median | 6 (4–7) |
| Aneurysm exclusion incomplete | 2 (2.5) |
| Total bypasses performed | 296 |
| No. of vessels bypassed, median | 4 (3–4) |
| No. of patients undergoing bifurcated abdominal endografting | 13 (16.5) |
| LSCA coverage (partial or complete) | 14 (17.3) |
| No. with LSCA coverage undergoing revascularization | 9 (64.3) |
| Total endovascular stents used | 234 |
| No. of stents per patient, median | 3 |
| IVUS used | 13 (16.0) |
IVUS, Intravascular ultrasound; LSCA, left subclavian artery; TEVAR, thoracic endovascular aortic repair.
Data are presented as number (%) unless otherwise indicated.
A total of 296 visceral bypasses were performed in the HTAAAR group, with a median of 4 bypasses per patient. A total of 17.3% of patients (n = 14) had partial or complete coverage of the left subclavian artery, of which 9 of 14 (64.3%) underwent revascularization, all through left common carotid to left subclavian artery bypass. Intravascular ultrasound was used in 16.0% of cases, which roughly corresponds to the percentage of HTAAAR cases done for the indication of chronic dissection. Thoracic endovascular stent grafts used included Gore TAG and C-TAG (W. L. Gore & Associates, Flagstaff, Ariz), Cook Zenith TX2 (Cook Medical Inc, Bloomington, Ind), and Medtronic Valiant and Talent (Medtronic Vascular, Santa Rosa, Calif) devices. Bifurcated abdominal endovascular stent grafts used in the 13 patients (16.5%) requiring distal seal in the iliac arteries or iliac limbs of an existing infrarenal abdominal bifurcated Dacron graft included Endologix AFX (Endologix, Irvine, Calif), Excluder (Gore), Endurant and Endurant II (Medtronic), and Zenith (Cook).
Thirty-day/in-hospital outcomes
Perioperative outcomes are presented in Table IV. Despite the age and comorbidity difference in the respective cohorts, there was no difference in 30-day or in-hospital death between the HTAAAR and OTAAAR groups, with an in-hospital death rate of 9.9% in the hybrid group vs 7.1% in the open group (P = .59) and a 30-day death rate of 12.3% in the hybrid group vs 8.3% in the open group (P = .45). Major perioperative morbidity rates were found to differ between the two groups, however, with a significantly higher rate of postoperative stroke in the OTAAAR cohort (9.5% OTAAAR vs 0% HTAAAR; P = .007). The HTAAAR cohort was found to have a significantly higher rate of new permanent dialysis (14.8% HTAAAR vs 3.6% OTAAAR; P = .01) and acute kidney injury (43.2% HTAAAR vs 26.2% OTAAAR; P = .02). In addition, postoperative length of stay was found to be significantly longer in the HTAAAR group (12 vs 8 days; P = .002), although much of this can be attributed to the trend toward performing staged hybrid repair during a single hospitalization. Postoperative spinal cord ischemia (SCI) rates did not differ by operative strategy (7.4% HTAAAR vs 8.3% OTAAAR; P = .99). In the HTAAAR series, 50% (n = 3/6) of all permanent SCI events occurred in extent III aneurysms, with two cases in extent II patients (33.3%; n = 2/6) and one case in a patient with extent I (16.7%) TAAA. This contrasts to the OTAAAR group, in which 71.4% (n = 5/7) of patients with SCI had extent I TAAA, with the remaining two being extent II (28.6%). The incidence of SCI was equivocal between simultaneous and staged HTAAAR.
Table IV.
Thirty-day/in-hospital outcomes
| Variablea |
Total (N = 165) |
Open (n = 84) |
Hybrid (n = 81) |
P value |
|---|---|---|---|---|
| Perioperative (30-day) death | 17 (10.3) | 7 (8.3) | 10 (12.3) | .45 |
| In-hospital death | 14 (8.4) | 6 (7.1) | 8 (9.9) | .59 |
| Stroke | 8 (4.8) | 8 (9.5) | 0 | .007 |
| Permanent paraparesis or paraplegia | 13 (7.8) | 7 (8.3) | 6 (7.4) | .99 |
| New dialysis requirement | 15 (9) | 3 (3.6) | 12 (14.8) | .01 |
| Acute kidney injuryb | 57 (34.5) | 22 (26.2) | 35 (43.2) | .02 |
| Tracheostomy | 9 (5.4) | 6 (7.1) | 3 (3.7) | .5 |
| Prolonged ventilation (>24 hours) | 27 (16.3) | 15 (17.9) | 12 (14.8) | .68 |
| Length of stay, days | 10 (7–15) | 8 (6–13) | 12 (8–16) | .002 |
| Reoperation for bleeding | 7 (4.2) | 4 (4.8) | 3 (3.7) | .99 |
Data are presented as median (interquartile range) for continuous variables and as a number (%) for categorical variables.
Defined by new postoperative dialysis requirement, creatinine increase to >2.0 mg/dL, or increase in postoperative creatinine to ≥2× baseline.
Follow-up, outcomes, and reinterventions
During a median follow-up of 16.7 months (12.8 months for OTAAAR vs 20.8 months for HTAAAR; P = .03), there was a significantly higher rate of reintervention in the HTAAAR group at 12.3% (n = 10 patients) vs 1.2% (n = 1) in the OTAAAR group (P = .004). Freedom from reintervention is demonstrated in Fig 2 and was significantly lower in those undergoing HTAAAR (P = .01). The median time to reintervention was 15 months in the hybrid group. All reinterventions (n = 13 total in 10 patients) in the HTAAAR group were due to endoleak (Table V), for an endoleak rate of 12.3%. The single reintervention in the OTAAAR group was a late (7 months) left renal graft stenosis requiring endovascular stenting. Endoleak types were type Ib, 15.4% (n = 2); type II, 23.1% (n = 3); type III, 53.8% (n = 7); and type V, 7.7% (n = 1). Of the HTAAAR patients requiring reintervention for endoleak, the overwhelming anatomic classification was extent II TAAA (n = 8/10; 80%), with the majority of patients reintervened on (n = 6/10; 60%) having undergone HTAAAR with a TAG endovascular device. However, two of the seven total observed type III endoleaks (in five patients) were in those who had had the Gore TAG/C-TAG device implanted at the time of HTAAAR, with the remainder of type III endoleaks (n = 5/7) observed in three patients having had either a Cook Zenith TX2 or Medtronic Valiant device. In one of the two patients with type III endoleak originally treated with a Gore TAG device, the type III endoleak was thought to result from an initial reintervention in which a Palmaz stent (Cordis Corp, Miami Lakes, Fla) was placed for partial endograft collapse in the very early postoperative period, and the Palmaz stent eroded through the TAG device, causing late type III endoleak.
Fig 2.
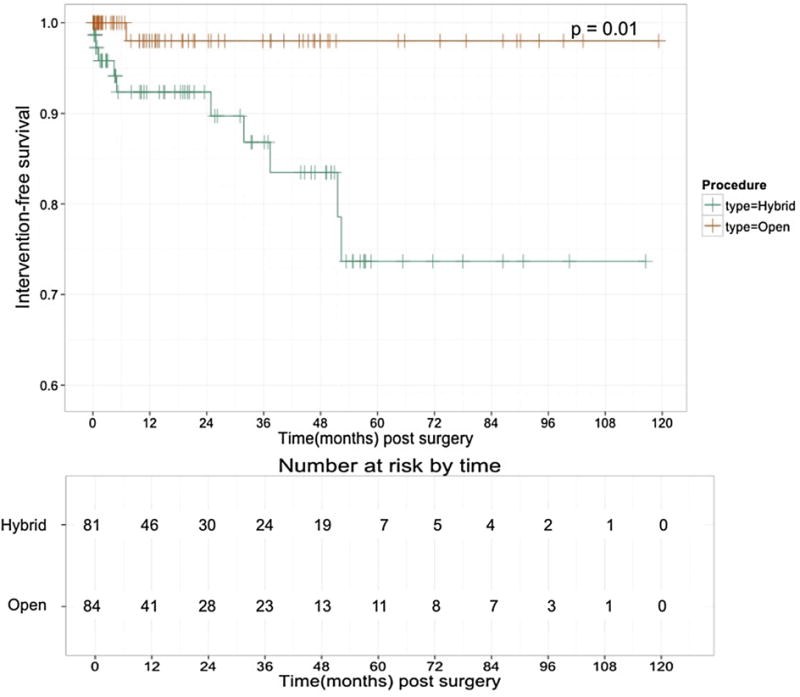
Kaplan-Meier freedom from reintervention after hybrid vs open thoracoabdominal aortic aneurysm (TAAA) repair. Freedom from reintervention was significantly greater after open repair (P = .01).
Table V.
Reinterventions in the hybrid thoracoabdominal aortic aneurysm repair (HTAAAR) group
| Patient | Crawford classification | Original endovascular device | No. of stents at initial repair | Endoleak type | Reintervention performed | Time to reintervention, days |
|---|---|---|---|---|---|---|
| 1 | Extent II | Zenith TX2 | 5 | Type III | TEVAR/proximal and distal extension prostheses | 746 |
| 1 | Extent II | Zenith TX2 | 5 | Type III | TEVAR/distal extension prosthesis | 907 |
| 2 | Extent II | Zenith TX2 | 6 | Type III | TEVAR | 1571 |
| 3 | Extent II | Medtronic Valiant | 4 | Type III | TEVAR/proximal extension prosthesis | 15 |
| 3 | Extent II | Medtronic Valiant | 4 | Type III | TEVAR/EVAR | 1008 |
| 4 | Extent II | Gore TAG | 2 | Type II | Coil embolization of collateral vessel | 1121 |
| 5 | Extent III | Gore TAG | 2 | Type Ib | EVAR | 1548 |
| 6 | Extent II | Gore TAG | 6 | Type Ib | Distal extension prosthesis | 34 |
| 7 | Extent II | Gore TAG | 4 | Type II | Coil embolizationa | 127 |
| 8 | Extent II | Zenith TX2 | 2 | Type V | TEVAR | 583 |
| 9 | Extent I | Gore TAG | 3 | Type III | TEVAR/proximal extension prosthesis | 147 |
| 10 | Extent II | Gore TAG | 5 | Type II | TEVAR/LSCA plug occlusion | 3 |
| 10 | Extent II | Gore TAG | 5 | Type III | TEVAR | 617 |
EVAR, Endovascular aneurysm repair; LSCA, left subclavian artery; TEVAR, thoracic endovascular aortic repair.
Performed at an outside facility.
Kaplan-Meier estimates of unadjusted overall survival are demonstrated in Fig 3. Overall unadjusted survival for the HTAAAR group at 1 year was 69%, which was comparable to that of the OTAAAR cohort (77%). Midterm survival was also comparable between the two groups, with a 3-year unadjusted survival of 61% and 49% in the OTAAAR and HTAAAR groups, respectively. Longer term survival differed significantly between groups, however, with the HTAAAR group having a 5-year unadjusted overall survival of approximately 32%, whereas the OTAAAR group had an unadjusted overall 5-year survival of 56%. Aorta-specific survival (Fig 4) was not significantly different between the two groups during the study period and is estimated at 81.8% in the HTAAAR group and 91.7% in the OTAAAR group at 5 years (P = .23).
Fig 3.
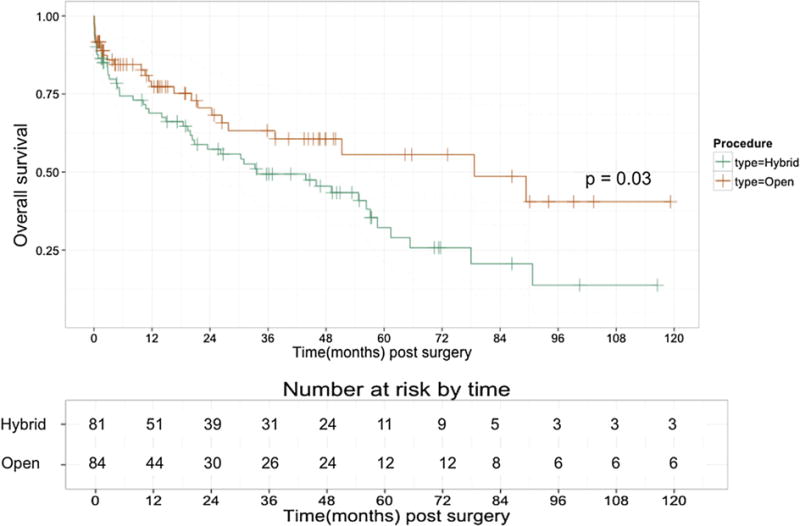
Kaplan-Meier unadjusted overall survival by procedure type. Unadjusted long-term survival was significantly lower (P = .03) after hybrid vs open thoracoabdominal aortic aneurysm (TAAA) repair.
Fig 4.
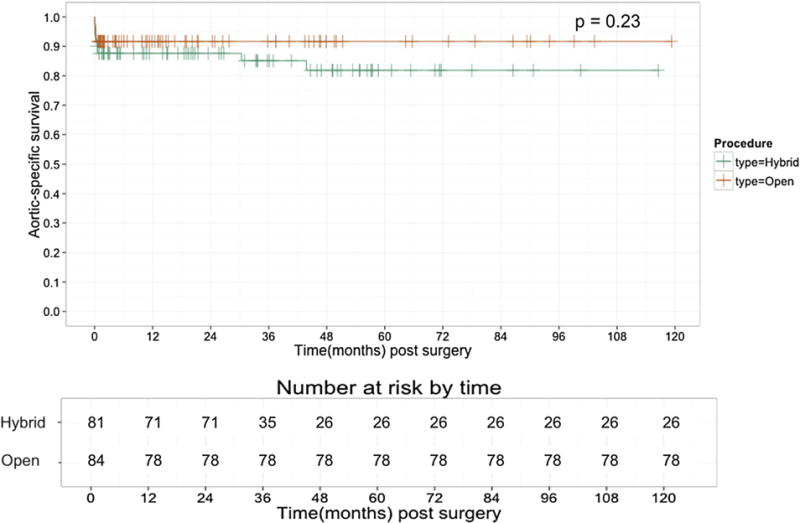
Aorta-specific survival by procedure type. On long-term follow-up, there was no difference in aorta-specific survival between hybrid and open thoracoabdominal aortic aneurysm (TAAA) repairs (P = .23).
Institutional use of open and hybrid TAAA repair
Institutional use of hybrid and open approaches to TAAA repair is presented in Fig 5. Overall institutional TAAA volume reached a peak from the years 2009 to 2012, an era in which the hybrid approach was favored. During this time frame, 83 total TAAA repairs were performed (50.3% of the current study population), with the hybrid approach favored 62.7% of the time (n = 52 HTAAARs). In the years following (2013-present),17,18 48 total TAAA repairs have been performed (29.1% of the study population), with a trend toward OTAAAR (70.8%; n = 34) during this time frame.
Fig 5.
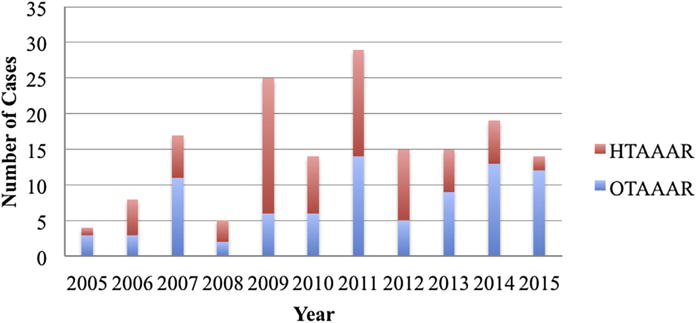
Institutional procedural volumes by year for open thoracoabdominal aortic aneurysm repair (OTAAAR) and hybrid thoracoabdominal aortic aneurysm repair (HTAAAR) demonstrating a shift from a hybrid-predominant to an open-predominant strategy in the latter years of the series.
DISCUSSION
The management of TAAA remains complex, with an aging population whose increased incidence of thoracic aortic disease and susceptibility to death from this disease further confounds delineation of optimal treatment strategies.7,25 Available strategies include open conventional repair of TAAA, which has been demonstrated to be a durable option1,26,27 but represents a significant perioperative morbidity and mortality risk that may preclude certain. Total endovascular repair using fenestrated or branched endografts,28 although a promising option, is not widely available. Commercially manufactured devices are not currently approved by the Food and Drug Administration and may require a degree of endovascular expertise precluding widespread adoption. Hybrid visceral debranching with subsequent endovascular aneurysmal exclusion strategies, which lessen the physiologic challenge typical of OTAAAR, may therefore provide a currently available preferential option in the high-risk patient. Unfortunately, there are limitations in the reported literature comparing open and hybrid TAAA repairs during the same era,14,15 and hence guidelines for favoring one approach over another are lacking. The results of this study would suggest that HTAAAR is an overall safe and effective treatment strategy for patients thought to be less suitable for open repair, with comparable rates of 30-day and in-hospital mortality, SCI, tracheostomy, and prolonged ventilation as well as a decreased rate of stroke compared with the OTAAAR cohort. The comparable perioperative mortality outcomes do come at the expense of increased rates of reintervention and new permanent dialysis, along with a longer length of hospital stay in the HTAAAR group. Given the higher average age and comorbid disease profile of the hybrid repair group, long-term survival was, not surprisingly, worse in this cohort, although a similar aorta-specific survival indicates that these older and sicker patients succumb to causes of death. The perioperative survival of the hybrid repair group in the current study is similar and in many cases superior to that reported by others, which has ranged from 2.3% to 34.2%.14,15,29–37
A continued unique disadvantage of the HTAAAR technique is the susceptibility of this repair to endoleak. This study reports a reintervention rate of 12.3% for endoleak, which compares favorably with the pooled endoleak rate of 21.1% to 22.7% as reported in recent meta-analyses by Moulakakis et al, with the bulk of these previously reported endoleaks being either type I or type II.12,13 Interestingly, the majority of endoleaks observed in the current series were type III endoleaks (53.8%; n = 7/13), which is somewhat discordant with prior HTAAAR literature. In addition, all but one of the type III endoleaks occurred in patients with extent II TAAA, which is the subgroup of TAAA with the most extensive aneurysmal disease and also 80% of our total study endoleak cohort. It is postulated that the incidence of type III endoleak is high in extent II TAAA because of long-segment aortic pavement as well as remodeling of the aorta over time, thus creating a scenario in which the graft material may become fatigued and have a tendency to migrate, thus disrupting seal zones.38,39 In a study by Parmer et al,39 it was demonstrated that the length of aorta treated by stent grafting was predictive for subsequent endoleak. Thus, judicious postoperative surveillance of patients with extent II HTAAAR, in particular, should be maintained, given their apparently increased risk for endoleak, especially as they compose a significant proportion of HTAAAR patients in this and other series.
The majority of type III endoleaks (n = 5/7; 71%) were in patients who had deployment of a Dacron-lined endograft (Valiant, Zenith TX2), which has been thought to be at increased risk for graft fatigue owing to poor fixation, with subsequent reliance of the graft on its inherent mechanical strength.40 The Cook Zenith TX2 proximal component device may present a unique risk for type III endoleak when it is employed in HTAAAR as the proximal barbs of this device, although postulated to be protective against stent graft migration,41,42 may cause graft injury when it is implanted inside another graft.
Given the frequent need for extensive thoracoabdominal aortic pavement with HTAAAR, the concern for SCI complications is raised. The current results demonstrating no difference in the rate of SCI between OTAAAR (8.3%) and HTAAAR (7.4%) compare with those reported in prior direct comparisons of open and hybrid repairs (open, 3.9%-15.1%; hybrid, 3.4%-4.3%),14,15 with a pooled rate of 7.5% of SCI in recent hybrid series.13
This study demonstrates generally comparable in-hospital mortality for OTAAAR and HTAAAR. The in-hospital mortality outcomes for open repair (7.1%) reported herein are similar to those reported by Wong et al in a report of contemporary outcomes with OTAAAR (7.9%) at a high-volume center,1 and the hybrid repair outcomes, although slightly higher, should be interpreted in the setting of a more elderly and comorbid (baseline kidney disease, prior stroke) cohort, factors that led to such patients being selected for hybrid over open repair. It is in this setting that HTAAAR, even in the modern era of advanced anesthesia and end-organ protection adjuncts, remains a valuable repair alternative. Owing to the complexity of intervention for TAAA, patients are best served with an individualized approach to repair strategy, as demonstrated herein. High-volume institutions experienced with thoracic aortic repair will continue to have superior outcomes43 and provide optimal treatment through determining the patient’s suitability and offering all options.
This study has several limitations. First, it is limited by the potential bias inherent in any single-institution, retrospective analysis. Furthermore, the small sample size, differences in age and comorbidity profiles of the patients, and relatively low event rates precluded propensity-matched analysis, and such an analysis has yet to be reported in the literature owing to these differences. Although the open repair group can be thought of as a control population, because of the lack of prospective design, comparisons are limited.
CONCLUSIONS
Hybrid repair of TAAAs is a generally safe approach for the treatment of TAAA in patients who are at high risk for conventional open repair. The comparable in-hospital mortality rates and similar aorta-specific long-term survival after hybrid vs open repair support that both approaches are effective in mitigating death from aortic disease, albeit at the cost of lower freedom from reintervention in the hybrid repair group. The approach presented herein demonstrates that a patient-specific, algorithmic approach whereby higher risk patients with TAAA are treated using a “hybrid-first” strategy and lower risk patients with conventional open repair yields satisfactory short- and long-term outcomes. Caution must be exercised in treating patients with the hybrid approach, as endoleak remains a risk with the long-segment aortic pavement required in extent II TAAA and Dacron-lined devices in particular.
DISCUSSION
Dr Adam W. Beck (Gainesville, Fla): I would like to thank the program committee for their invitation to review this manuscript and thank the authors for getting the manuscript to me in a timely manner. I congratulate the authors on a nice study and impressive results treating a highly complex patient population.
In this study, the cardiovascular team at Duke reports their series of thoracoabdominal aneurysms treated over a 10-year period from 2005 to 2015 using conventional open repair with or without cardiopulmonary bypass vs hybrid endovascular repair with visceral debranching.
My overall sense of the paper is the authors intend to demonstrate that by using these two methodologies selectively, they have had acceptable morbidity and mortality for both groups.
I have one comment and three questions for you.
Comment: Due to the sobering results of hybrid repair, at the University of Florida we have largely abandoned hybrid repair in our practice in favor of total endovascular repair vs conventional open repair. It’s interesting to me that you have continued to do these procedures in light of many reports in the literature with similar concerning results to our own. I applaud your results, but I would encourage you to evaluate your results in comparison to those performing total endovascular repair.
Realizing that your institution may not have branched/fenestrated repair availability for thoracoabdominal aneurysm repair, you certainly have expertise close by. Where do you see branched/fenestrated repair fitting into your algorithm? Do you refer any patients out of your institution?
The algorithm for thoracoabdominal repair includes frailty as an indication for hybrid repair. I tend to use that term when I choose not to intervene on patients who I feel are too high risk. How frail is frail in your study? Do you have any objective measure that you use in your clinical practice?
Looking at your overall volumes and the number of patients treated with hybrid repair, there seems to be a period of great enthusiasm for hybrid repair followed by a progressive drop in hybrid volume. Why is that? Are you losing interest in hybrid repair because of sobering results? Have referral patterns changed? Or have you elected to just treat the patients with a conventional open repair rather than hybrid repair?
Thank you again for the opportunity to review this manuscript.
Dr Ehsan Benrashid: Thank you for your questions and comments. One, we do not refer anyone out. We have a pretty robust fenestrated endovascular aneurysm repair program with Dr McCann, who is the senior vascular surgeon on this study, so we do not elect to do it that way. Regarding our volumes, and I will link to the previous volumes question, it is a moving target type thing. We do real-time-based outcomes analyses of our data and procedures as performed by Drs Hughes and McCann, and with our prospectively maintained and easily retrospectively reviewed aortic surgery database, we have the opportunity to change the way we manage things in real time. Additionally, our referral patterns have changed a little bit in talking to Dr Hughes and Dr McCann. We now have one of the larger connective tissue disease cohorts on the East Coast, and in those patients, as mentioned, we avoid hybrid repair because of disease progression. Additionally, during the era between 2009 and 2012, the enthusiasm for hybrid thoracoabdominal aortic aneurysm repair was such that maybe some patients offered hybrid repair that were “super high risk” lived several years longer and probably would not have otherwise because of rupture risk. In regard to the frailty comment, we have previously published results looking at frail patients at Duke undergoing proximal aortic surgery, although that is a little bit of a different animal. People tend to use objective measures such as albumin levels, low body mass index, age older than 80 years, prior strokes, anemia, and some radiographic features to objectively determine frailty. However, our sense is that really you need to look at someone in clinic and see what frailty is to you and whether or not they would be suitable to undergo any type of repair. So, it is a subjective measure more than an objective measure.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: RM, GH
Analysis and interpretation: EB, HW, NA, JK, RM, GH
Data collection: EB, HW, NA, JK
Writing the article: EB, RM, GH
Critical revision of the article: EB, HW, NA, JK, RM, GH
Final approval of the article: EB, HW, NA, JK, RM, GH
Statistical analysis: EB, HW
Obtained funding: Not applicable
Overall responsibility: GH
Author conflict of interest: G.C.H. has received a consulting fee from and is on the speakers bureau for W. L. Gore & Associates. He is on the speakers bureau for Vascutek, Inc.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented as an oral presentation during Scientific Session III, Thoracic Aorta, of the 2016 Annual Meeting of the Southern Association for Vascular Surgery, Cancun, Mexico, January 20–23, 2016.
References
- 1.Wong DR, Parenti JL, Green SY, Chowdhary V, Liao JM, Zarda S, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg. 2011;212:569–79. doi: 10.1016/j.jamcollsurg.2010.12.041. discussion: 579–81. [DOI] [PubMed] [Google Scholar]
- 2.Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–9. doi: 10.1067/mva.2002.122024. [DOI] [PubMed] [Google Scholar]
- 3.Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:953–60. doi: 10.1016/j.jtcvs.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Lemaire SA, Jones MM, Conklin LD, Carter SA, Criddell MD, Wang XL, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:11–9. doi: 10.1016/j.jvs.2008.08.048. discussion: 19. [DOI] [PubMed] [Google Scholar]
- 5.Aftab M, Songdechakraiwut T, Green SY, Zarda S, Price MD, Nalty CC, et al. Contemporary outcomes of open thoracoabdominal aortic aneurysm repair in octogenarians. J Thorac Cardiovasc Surg. 2015;149(Suppl):S134–41. doi: 10.1016/j.jtcvs.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Perler BA. Charles Dickens, Coach K, Pogo, and the recent history and future of vascular surgery. J Vasc Surg. 2010;51:1286–92. doi: 10.1016/j.jvs.2010.02.262. [DOI] [PubMed] [Google Scholar]
- 7.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841–57. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 8.Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3:578–82. doi: 10.1067/mva.1986.avs0030578. [DOI] [PubMed] [Google Scholar]
- 9.Hughes GC, McCann RL. Hybrid thoracoabdominal aortic aneurysm repair: concomitant visceral revascularization and endovascular aneurysm exclusion. Semin Thorac Cardiovasc Surg. 2009;21:355–62. doi: 10.1053/j.semtcvs.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008;136:21–8. 28.e1–6. doi: 10.1016/j.jtcvs.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 11.Hughes GC, Sulzer CF, McCann RL, Swaminathan M. Endovascular approaches to complex thoracic aortic disease. Semin Cardiothorac Vasc Anesth. 2008;12:298–319. doi: 10.1177/1089253208328667. [DOI] [PubMed] [Google Scholar]
- 12.Moulakakis KG, Mylonas SN, Antonopoulos CN, Liapis CD. Combined open and endovascular treatment of thoracoabdominal aortic pathologies: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2012;1:267–76. doi: 10.3978/j.issn.2225-319X.2012.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moulakakis KG, Mylonas SN, Avgerinos ED, Kakisis JD, Brunkwall J, Liapis CD. Hybrid open endovascular technique for aortic thoracoabdominal pathologies. Circulation. 2011;124:2670–80. doi: 10.1161/CIRCULATIONAHA.111.041582. [DOI] [PubMed] [Google Scholar]
- 14.Patel HJ, Upchurch GR, Jr, Eliason JL, Criado E, Rectenwald J, Williams DM, et al. Hybrid debranching with endovascular repair for thoracoabdominal aneurysms: a comparison with open repair. Ann Thorac Surg. 2010;89:1475–81. doi: 10.1016/j.athoracsur.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 15.Patel R, Conrad MF, Paruchuri V, Kwolek CJ, Chung TK, Cambria RP. Thoracoabdominal aneurysm repair: hybrid versus open repair. J Vasc Surg. 2009;50:15–22. doi: 10.1016/j.jvs.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118:808–17. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, Andersen ND, Hanna JM, McCann RL. Thoracoabdominal aortic aneurysm: hybrid repair outcomes. Ann Cardiothorac Surg. 2012;1:311–9. doi: 10.3978/j.issn.2225-319X.2012.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes GC, Barfield ME, Shah AA, Williams JB, Kuchibhatla M, Hanna JM, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg. 2012;56:621–9. doi: 10.1016/j.jvs.2011.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TC, Andersen ND, Williams JB, Bhattacharya SD, McCann RL, Hughes GC. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg. 2011;92:97–102. doi: 10.1016/j.athoracsur.2011.03.089. discussion: 102–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouchoukos NT. Thoracoabdominal aortic aneurysm repair using hypothermic cardiopulmonary bypass and circulatory arrest. Ann Cardiothorac Surg. 2012;1:409–11. doi: 10.3978/j.issn.2225-319X.2012.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husain AM, Rendahl R, Hughes GC. Aortic surgery. In: Husain AM, editor. A practical approach to neurophysiologic intraoperative montoring. 2nd. New York: Demos Medical Publishing, LLC; 2015. pp. 227–57. [Google Scholar]
- 22.Husain AM, Swaminathan M, McCann RL, Hughes GC. Neurophysiologic intraoperative monitoring during endovascular stent graft repair of the descending thoracic aorta. J Clin Neurophysiol. 2007;24:328–35. doi: 10.1097/WNP.0b013e31811ebf6e. [DOI] [PubMed] [Google Scholar]
- 23.Hughes GC, Daneshmand MA, Swaminathan M, Nienaber JJ, Bush EL, Husain AH, et al. “Real world” thoracic endografting: results with the Gore TAG device 2 years after U.S. FDA approval. Ann Thorac Surg. 2008;86:1530–7. doi: 10.1016/j.athoracsur.2008.07.089. discussion: 1537–8. [DOI] [PubMed] [Google Scholar]
- 24.Ganapathi AM, Andersen ND, Hanna JM, Gaca JG, McCann RL, Hughes GC. Comparison of attachment site endoleak rates in Dacron versus native aorta landing zones after thoracic endovascular aortic repair. J Vasc Surg. 2014;59:921–9. doi: 10.1016/j.jvs.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 25.Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N Engl J Med. 1997;336:1876–88. doi: 10.1056/NEJM199706263362606. [DOI] [PubMed] [Google Scholar]
- 26.Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:S862–4. doi: 10.1016/j.athoracsur.2006.10.088. discussion: S890–2. [DOI] [PubMed] [Google Scholar]
- 27.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–68. discussion: 368–70. [PubMed] [Google Scholar]
- 28.Clough RE, Modarai B, Bell RE, Salter R, Sabharwal T, Taylor PR, et al. Total endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2012;43:262–7. doi: 10.1016/j.ejvs.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Lin PH, Kougias P, Bechara CF, Weakley SM, Bakaeen FG, Lemaire SA, et al. Clinical outcome of staged versus combined treatment approach of hybrid repair of thoracoabdominal aortic aneurysm with visceral vessel debranching and aortic endograft exclusion. Perspect Vasc Surg Endovasc Ther. 2012;24:5–13. doi: 10.1177/1531003511432768. [DOI] [PubMed] [Google Scholar]
- 30.Tshomba Y, Melissano G, Logaldo D, Rinaldi E, Bertoglio L, Civilini E, et al. Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms. Ann Cardiothorac Surg. 2012;1:293–303. doi: 10.3978/j.issn.2225-319X.2012.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bockler D, Kotelis D, Geisbusch P, Hyhlik-Durr A, Klemm K, von Tengg-Kobligk H, et al. Hybrid procedures for thoracoabdominal aortic aneurysms and chronic aortic dissectionsda single center experience in 28 patients. J Vasc Surg. 2008;47:724–32. doi: 10.1016/j.jvs.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Lee WA, Brown MP, Martin TD, Seeger JM, Huber TS. Early results after staged hybrid repair of thoracoabdominal aortic aneurysms. J Am Coll Surg. 2007;205:420–31. doi: 10.1016/j.jamcollsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Chiesa R, Tshomba Y, Melissano G, Marone EM, Bertoglio L, Setacci F, et al. Hybrid approach to thoracoabdominal aortic aneurysms in patients with prior aortic surgery. J Vasc Surg. 2007;45:1128–35. doi: 10.1016/j.jvs.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Drinkwater SL, Bockler D, Eckstein H, Cheshire NJ, Kotelis D, Wolf O, et al. The visceral hybrid repair of thoraco-abdominal aortic aneurysmsda collaborative approach. Eur J Vasc Endovasc Surg. 2009;38:578–85. doi: 10.1016/j.ejvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Kuratani T, Kato M, Shirakawa Y, Shimamura K, Sawa Y. Long-term results of hybrid endovascular repair for thoraco-abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2010;38:299–304. doi: 10.1016/j.ejcts.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Black SA, Wolfe JH, Clark M, Hamady M, Cheshire NJ, Jenkins MP. Complex thoracoabdominal aortic aneurysms: endovascular exclusion with visceral revascularization. J Vasc Surg. 2006;43:1081–9. doi: 10.1016/j.jvs.2005.12.071. discussion: 1089. [DOI] [PubMed] [Google Scholar]
- 37.Rosset E, Ben Ahmed S, Galvaing G, Favre JP, Sessa C, Lermusiaux P, et al. Editor’s choicedhybrid treatment of thoracic, thoracoabdominal, and abdominal aortic aneurysms: a multicenter retrospective study. Eur J Vasc Endovasc Surg. 2014;47:470–8. doi: 10.1016/j.ejvs.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Mohan IV, Harris PL, Van Marrewijk CJ, Laheij RJ, How TV. Factors and forces influencing stent-graft migration after endovascular aortic aneurysm repair. J Endovasc Ther. 2002;9:748–55. doi: 10.1177/152660280200900606. [DOI] [PubMed] [Google Scholar]
- 39.Parmer SS, Carpenter JP, Stavropoulos SW, Fairman RM, Pochettino A, Woo EY, et al. Endoleaks after endovascular repair of thoracic aortic aneurysms. J Vasc Surg. 2006;44:447–52. doi: 10.1016/j.jvs.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Malina M, Brunkwall J, Ivancev K, Jonsson J, Malina J, Lindblad B. Endovascular healing is inadequate for fixation of Dacron stent-grafts in human aortoiliac vessels. Eur J Vasc Endovasc Surg. 2000;19:5–11. doi: 10.1053/ejvs.1999.0867. [DOI] [PubMed] [Google Scholar]
- 41.Chuter TA. Durability of endovascular infrarenal aneurysm repair: when does late failure occur and why? Semin Vasc Surg. 2009;22:102–10. doi: 10.1053/j.semvascsurg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Tonnessen BH, Sternbergh WC, 3rd, Money SR. Mid- and long-term device migration after endovascular abdominal aortic aneurysm repair: a comparison of AneuRx and Zenith endografts. J Vasc Surg. 2005;42:392–400. doi: 10.1016/j.jvs.2005.05.040. discussion: 400–1. [DOI] [PubMed] [Google Scholar]
- 43.Cowan JA, Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR., Jr Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37:1169–74. doi: 10.1016/s0741-5214(03)00085-5. [DOI] [PubMed] [Google Scholar]


