Highlight
The waxy phenotype of barley CDC_Alamo is associated with a reduction of catalytic activity and deficient targeting of GBSS1a.
Keywords: Amylose, GBSS, starch biosynthesis, starch functionality, subcellular targeting, waxy.
Abstract
Amylose synthesis is strictly associated with activity of granule-bound starch synthase (GBSS) enzymes. Among several crops there are cultivars containing starch types with either little or no amylose known as near-waxy or waxy. This (near) amylose-free phenotype is associated with a single locus (waxy) which has been mapped to GBSS-type genes in different crops. Most waxy varieties are a result of either low or no expression of a GBSS gene. However, there are some waxy cultivars where the GBSS enzymes are expressed normally. For these types, single nucleotide polymorphisms have been hypothesized to represent amino-acid substitutions leading to loss of catalytic activity. We here confirm that the HvGBSSIa enzyme from one such waxy barley variety, CDC_Alamo, has a 90% reduction in catalytic activity. We also engineered plants with expression of transgenic C-terminal green fluorescent protein-tagged HvGBSSIa of both the non-waxy type and of the CDC_Alamo type to monitor their subcellular localization patterns in grain endosperm. HvGBSSIa from non-waxy cultivars was found to localize in discrete concentric spheres strictly within starch granules. In contrast, HvGBSSIa from waxy CDC_Alamo showed deficient starch targeting mostly into unknown subcellular bodies of 0.5–3 µm in size, indicating that the waxy phenotype of CDC_Alamo is associated with deficient targeting of HvGBSSIa into starch granules.
Introduction
Starch is the main polysaccharide in which carbon and energy are stored in higher plants and is the principal dietary source of energy for humans (Zeeman et al., 2010; Tetlow, 2011). Cereal starch accounts for over 90% of the world market for starch (Tetlow, 2011) and is used in several industrial processes including papermaking and first generation bioethanol. Starch is an insoluble, semi-crystalline glucan composed of two polymers of glucose—amylose and amylopectin—that are linked together by α-1,4-D-glycosidic linkages with branch points formed by α-1,6-D-glycosidic linkages. The highly branched amylopectin is the major component of starch and has an estimated molecular mass of 107–109 Da whereas the essentially linear amylose is smaller than amylopectin (105–106 Da) (Buléon et al., 1998). The ratio between amylose and amylopectin in plant starches varies depending on the botanical source, but typically 15–30% of the starch granule is composed of amylose (Tetlow, 2011). This ratio seems to have been optimized in nature for optimal starch granule robustness, digestibility and carbohydrate remobilization as demonstrated for barley seedling establishment (Shaik et al., 2014). Waxy starches are characterized by either being amylose-free (waxy) or having low amylose content (near-waxy) (<5%) (Domon et al., 2002). The texture of cooked starches is influenced by the content of amylose, and the clear starch pastes with good freeze–thaw stability and little tendency to retrograde that are formed by waxy starches are of high value in many processed foods (Denyer et al., 2001). Also, the cloning and characterization of starch modifying enzymes is increasingly important for industrial and agro-biotechnological modification of starches and other carbohydrates (Hebelstrup et al., 2015).
Starch synthesis in cereals involves five classes of enzymes: ADP-glucose pyrophosphorylase (AGPase), starch synthases (ADP-α-D-glucose:[1→4]-α-D-glucan 4-α-D-glucosyltransferase; EC 2.4.1.21, GT5), starch phosphorylase, and starch branching and debranching enzymes (Blennow et al., 2013). Five classes of starch synthases have been identified in barley: granule-bound starch synthase (GBSSI) and starch synthases SSI, SSII, SSIII, and SSIV). All five classes catalyse the transfer of a glucosyl unit from ADP-glucose to the non-reducing end of an existing α-glucan chain (Zeeman et al., 2010). Pioneering work in the 1960s suggested that amylose synthesis is strictly associated with activity of granule-bound starch synthase(s) (reviewed by Nelson and Pan, 1995). Mutations in GBSSIa genes have later been verified in many waxy mutants of cereal crops, where in particular waxy corn (Wessler and Varagona, 1985; Nelson and Pan, 1995) and glutinous rice (Wang et al., 1995; Olsen and Purugganan, 2002) are the major waxy crops produced. Of the two GBSS genes in barley, only HvGBSSIa is expressed in the endosperm (Radchuk et al., 2009), whereas HvGBSSIb is expressed in the pericarp and the embryo (Sreenivasulu et al., 2006). GBSS-type enzymes represent the majority of the protein fraction bound within storage starch granules (Wang et al., 2013), but they do not contain known types of starch binding domains. HvGBSSIa is targeted to amyloplasts by the recognition of an N-terminal transit peptide, which is cleaved off during translocation across plastid membranes. We have previously shown that the first 69 N-terminal amino acids of HvGBSSIa corresponds to such a transit peptide, which can effectively target transgenic enhanced green fluorescent protein (eGFP) to the amyloplast of barley endosperm cells (Hebelstrup et al., 2010). In Arabidopsis, targeting of GBSS to transient leaf starch granules has been shown to be dependent on interaction with PTST (protein targeting to starch), which is a 26 kDa protein containing a glucan-binding family 48 carbohydrate-binding module (CBM48) (Seung et al., 2015). In barley, storage starch accumulates in the starchy endosperm starting around 6 days after pollination and continues throughout the grain-filling period (Radchuk et al., 2009). During grain development the amylose content increases from around 20% to around 30% in the mature grain (Radchuk et al., 2009). The loss of activity from GBBSIa in barley was linked to the waxy phenotype by Hylton et al. (1996) and several waxy barley cultivars have since then been identified and characterized (Domon et al., 2002; Patron et al., 2002; Ma et al., 2010; Asare et al., 2012). Most waxy barley mutants are a result of either low or no expression of the GBSSIa gene caused by deletions or non-silent single nucleotide polymorphisms (SNPs) (Domon et al., 2002; Patron et al., 2002). However, there are some waxy cultivars with no detectable amylose wherein the full GBSSIa gene is expressed at a normal level, and therefore non-silent SNPs in the GBSSIa gene are interpreted to result in catalytic inactivity without affecting enzyme concentration (Asare et al., 2012). However, the enzymes of these presumed loss-of-function alleles were never cloned and characterized in vitro. CDC Alamo is an example of such a waxy barley cultivar, where HvGBSSIa is expressed at a normal level (Patron et al., 2002; Asare et al., 2012). Here we describe what effect the SNPs identified in the HvGBSSIa gene of the waxy barley cultivar CDC Alamo have on the catalytic properties of HvGBSSIa and its subcellular localization. We found that HvGBSSIa from non-waxy cultivars are effectively targeted into concentric spheres within developing starch granules. In contrast, HvGBSSIa from the waxy cultivar CDC Alamo has a 90% loss of catalytic activity, and it is not correctly localized in starch granules. Instead it is localized mostly into unknown subcellular bodies with a size of 0.5–3 µm.
Material and methods
Constructs for expression of barley granule-bound starch synthases Ia
Barley granule-bound starch synthase (HvGBSSIa, GenBank accession: AAM74048) was codon optimized for expression in E. coli and synthesized by DNA2.0 (www.DNA20.com). It was synthesized without the initial 69 amino acid residues, i.e. plastid transit peptide as predicted using TargetP (http://www.cbs.dtu.dk/services/TargetP/). The HvGBSSIa gene contained additional NdeI and HindIII restriction sites at the 5′ and 3′ end, respectively, to facilitate re-cloning into the pET28a expression vector, which contains a 6xHis tag. For expression in barley plants we used the construct pUCEUbi:HvGBSSIa-GFP:NOS, which we have described previously (Carciofi et al., 2012). In brief, this construct was made by cloning the original wild-type full length HvGBSSIa cDNA sequence (including the transit peptide) to generate the plasmid pJ241-HvGBSSIa-eGFP (full length HvGBSSIa with a C-terminally linked green fluorescent protein (GFP) tag). Full length HvGBSSIa-eGFP was then recloned into the pUCE plant transformation vector Ubi:USER:NOS (Hebelstrup et al., 2010).
Site-directed mutagenesis
Mutants for kinetic and in planta studies were constructed by using the QuickChange site-directed mutagenesis kit (Agilent Technologies) with pET28a-HvGBSSIa and pJ241-HvGBSSIa-eGFP as templates (respectively for expression either in E. coli or in barley), and primers listed in Supplementary Table S1 at JXB online. Sequence-confirmed mutants of HvGBSSIa-eGFP were digested with PacI (Fermentas, FastDigest) and recloned into the pUCE vector Ubi:USER:NOS (PacI digested and treated with calf intestine alkaline phosphatase) as described previously (Carciofi et al., 2012).
Expression and purification of barley starch synthases
The expression vectors of HvGBSSIa or mutants were transformed into E. coli Tuner(DE3) cells, which were grown in 1 liter LB medium containing 50 µg ml–1 kanamycin to OD ~0.6, cooled on ice for 10 min, induced with 50 µM isopropyl β-D-1-thiogalactopyranoside and incubated overnight at 16 °C. The cell pellet was resuspended in 10 ml buffer A (20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 40 mM imidazole, 1 M UREA, 10% (v/v) glycerol) per gram pellet together with two protease inhibitor tablets (Roche 11 873580001) and two to three drops of antifoam (Sigma-Aldrich). The cell suspension was lysed using a continuous cell disruptor (1.35 kbar, Constant Systems Ltd). DNAseI (2 µg ml–1, bovine pancreas, grade II, Roche 101041559001) and MgCl2 (final concentration 10 mM) were added to the cell lysate and incubated on ice for 20 min before centrifugation (Beckman, JA-20, 48 400 g, 30 min, 4 °C). The filtered supernatant was loaded on a 6 ml HisTrap column (GE Healthcare), equilibrated with buffer A and eluted using a gradient from 0% to 45% buffer B (as buffer A but with 500 mM imidazole). The eluted fractions were mixed with DTT and EDTA, pH 8.0 (final concentration 10 mM of both) and analysed using SDS-PAGE. Fractions containing HvGBSS were pooled, concentrated using a Vivaspin20 centrifugal concentrator (30 kDa molecular mass cut-off) and loaded on a Superdex75 gel-filtration column (GE Healthcare), equilibrated with buffer C (20 mM Tris–HCl, pH 8.0, 150 mM NaCl, 10% (v/v) glycerol, 1 mM DTT, 1 mM EDTA). Fractions were analysed using SDS-PAGE and those containing HvGBSSIa were pooled. The protein concentration was estimated using the Bradford method with bovine immunoglobulin as a reference, divided into 100 µl aliquots, flash-frozen in liquid nitrogen, and stored at –20 °C.
Coupled glycosyltransferase assay
ADP-glucose, di-ammonium salt was prepared using chemo-enzymatic synthesis as described previously (Cuesta-Seijo et al., 2013). Initial rates were determined by coupling the release of ADP to NADH oxidation via pyruvate kinase and lactate dehydrogenase in a protocol adapted from (Gosselin et al., 1994). Assays were performed in a final volume of 100 µl with the following final concentrations: 50 mM Bicine, pH 8.5, 25 mM potassium acetate, 0.1% (w/v) bovine serum albumin, 2 mM MgCl2, 10 mM DTT, 0.375 mM NADH, 0.7 mM phosphorenolpyruvate tricyclohexylammonium salt, 6 U ml–1 pyruvate kinase, and 30 U ml–1 lactate dehydrogenase (both Sigma-Aldrich, rabbit muscle type II) with 30–800 nM enzyme at 37 °C; 10 mM maltotriose (Sigma-Aldrich M8378) or 1 mg ml–1 of glycogen (Sigma-Aldrich G8876, rabbit liver type III) was used as acceptor. Reactions were initiated by addition of 1 mM ADP-glucose. Enzyme concentrations for activity assays were estimated by the method of Bradford with bovine immunoglobulin as a reference. NADH oxidation was monitored by the decrease in absorbance at 340 nm. For single substrate kinetics, the fixed substrate concentration used was >5 times the Km value. kcat and Km values were calculated by fitting the Michaelis–Menten equation to the initial rates (GraphPad Prism version 4.03, GraphPad Software, San Diego, CA, USA).
Plant transformation
Transgenic callus cultures were induced from immature embryos of H. vulgare var. Golden Promise, by Agrobacterium tumefaciens-mediated transformation as described previously (Carciofi et al., 2011, 2012). Three plant expression vectors were used: pUCEUbi:GBSSIaCDC_Alamo-eGFP for expression of GBSSIa from CDC Alamo, which contains the three amino acid substitutions D219V, M490V, and I491V, was included in this study, to be compared with the wild-type (pUCEUbi:GBSSIaWT-eGFP) and a vector control (pUCEUbi:eGFP) (Carciofi et al., 2012). The pUCEUbi plant expression vectors contain the hygromycin phosphotransferase selection marker gene (hpt), and the common maize ubiquitin promoter for constitutive expression of the transgene (Hebelstrup et al., 2010). Transgenic barley plants were regenerated from callus in selective medium as described in Carciofi et al. (2011). The T1 grains from these plants were used for microscopy.
Western blotting
Developing endosperm was ground in buffer (10 mM Tris–HCl, pH 8.0) in an automatic tissuelyser (FastPrep™ FP120, Thermo Savant) for 20 s. The samples were spun twice at 200 g for 2 min. The supernatant was collected, and it was checked with a microscope that it did not contain any starch granules. The pellet, which consisted of starch granules, was washed 10 times with buffer. Protein concentrations were determined with Bradford reagent (Bio-Rad no. 500-0006) using BSA as standard. The samples were mixed with a 6× SDS-loading buffer to reach the following final concentrations: 50 mM Tris–HCl, pH 6.8, 10% glycerol, 1% SDS, 3% β-mercaptoethanol. The samples were boiled for 10 min and the proteins were separated by SDS-PAGE using NuPAGE Novex Bis-Tris Mini Gels (4–12%, Invitrogen) and Bio-Rad Miniprotean II Multiscreen Apparatus according to the manufacturer’s instructions (Bio-Rad bulletin 1721). Proteins were transferred to polyvinylidene fluoride membranes. Rabbit antiserum raised against HvGBSSIa (Carlsberg Laboratory, Copenhagen, Denmark) was used to detect HvGBSSIa at a dilution of (1:1000). Purified antibodies from rabbit serum raised against eGFP (Sigma-Aldrich, G1544) at a dilution of (1:1000) was used to detect eGFP-tagged HvGBSSIa. The secondary antibody (1:5000) was goat anti-rabbit purified IgG coupled with alkaline phosphatase (Sigma-Aldrich, A3687).
Crossing and genotyping
Transgenic plants with overexpression of the GBSSIa variants were grown in a controlled growth chamber (12–14 °C) under a 16 h day (550 μmol m–2 s–1)/8 h dark cycle, and with a relative humidity of 90–95%. Spikes with non-mature green anthers were identified and emasculated 2 days before trans-pollination with mature anthers from the three varieties: CDC Alamo, CDC Candle and SB94912 of H. vulgare. F1 grains were harvested at maturity and a new generation was propagated to obtain mature F2 grains. Embryos were removed from the F2 grains for genomic DNA purification using the FastDNA® Kit (MP Biomedicals, LLC, OH, USA). The F2 grains were genotyped with respect to the GBSSIa gene by PCR as described by (Asare et al., 2012). In brief, the primers Hor_wx_1F (5′-CAAAAAGCGAAGAGGAAGGA-3′) and Hor_wx_1R (5′-AGAATCGAACCAACCGAGTG-3′) were used to identify the GBSSIa genotypes of CDC Candle and SB94912, and the primers Hor_wx_5F (5′-TTTTGCTAGGTGGCCTTCTG-3′) and Hor_wx_5R (5′-TCCGATCACTCAATCATCCA-3′) combined with digestion of the PCR product with DrdI were used to identify the CDC Alamo GBSSIa genotype (Asare et al., 2012).
Epifluorescence and confocal laser scanning microscopy
Endosperm cells and starch granules from T1 and F2 grains were analysed by fluorescence, Normarski/differential interference contrast (DIC) and transmission light microscopy on a Zeiss Axioplan 2 epifluorescence microscope equipped with an AxioCam MRc5. The filter B1001 (supplied by the manufacturer) was used for detection of eGFP fluorescence and the filter B1000 was used for detection of endosperm cell wall autofluorescence. For transmission light microscopy, starch granules were stained by Lugol’s iodine stain solution (250 mg I2, 2.5 g KI, 125 mL ddH2O) (Carciofi et al., 2012) to identify the waxy phenotype. Granular localization of GFP-tagged GBSSIa and mutants was also determined using a Leica TCS SP5-X MP UV confocal microscope equipped with filters for eGFP or chlorophyll detection. Endosperm was isolated from developing grains harvested 30 days after pollination and from dry mature grains or mature grains imbibed in sterile ddH2O for 2 h. Relative fluorescence from starch granules was determined by using the software supplied by the manufacturer. No fluorescence was detected in starch granules from non-transformed barley grains or in grains from vector control (pUCEUbi:eGFP) transformants.
Amylose determination and qPCR
The amylose content of barley grains was determined using iodine colorimetry as described previously (Wickramasinghe et al., 2009) and expression levels of HvGBSSIa mRNA was determined by qPCR as described previously (Carciofi et al., 2012).
Results
Enzymatic activity of HvGBSSIa from CDC Alamo
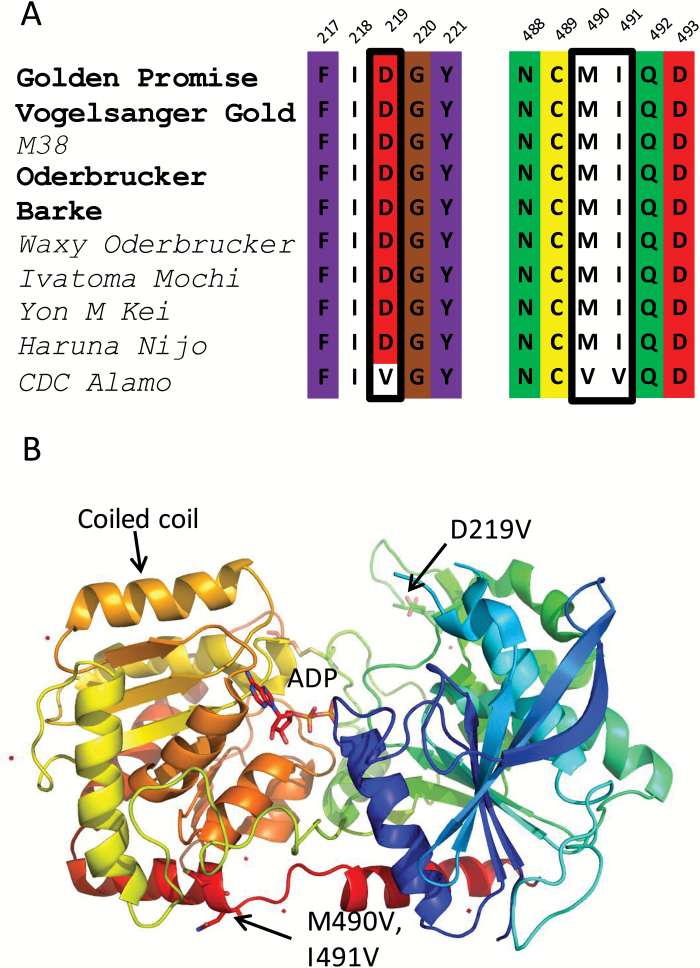
A single SNP which causes an Asp219→Val219 (D219V) mutation in the HvGBSSIa allele of CDC Alamo (HvGBSSIaCDC_Alamo) has previously been reported (Asare et al., 2012). However, aligning the cDNA sequence of the HvGBSSIaCDC_Alamo allele with a number of other HvGBSSIa alleles shows that there are also two other SNPs in HvGBSSIaCDC_Alamo, which deviate from the consensus sequence of HvGBSSIa (Supplementary Table S2). They cause a Met490→Val490 (M490V) mutation and an Ile491→Val491 (I491V) mutation, respectively (Fig. 1A). The structure of GBSSIa from rice has been determined (Momma and Fujimoto, 2012). We mapped Asp219, M490, and I491 to this structure (Fig. 1B). However, none of these amino acids is located in the presumed catalytic site or in the transit peptide. To test if they are important for the efficiency of GBSSIa starch synthesis, we cloned and successfully expressed six different versions of HvGBSSIa in Escherichia coli: (i) a wild-type consensus (HvGBSSIaWT) cloned from the non-waxy barley line Vogelsanger; (ii) The CDC Alamo variant (HvGBSSIaCDC_Alamo) containing all three amino acid substitutions D219V/M490V/I491V; and four different combinations of these, namely (iii) HvGBSSIaM490V, (iv) HvGBSSIa I491V, (v) HvGBSSIa M490V/I491V, and (vi) HvGBSSIa D219V. High enzyme yields in E. coli (>50 mg l–1) were achieved for all six variants. To test the efficiency of each variant, substrate kinetics were determined for all six with either rabbit liver glycogen or ADP-Glc as varying substrate. All five tested variants (HvGBSSIaD219V, HvGBSSIaM490V, HvGBSSIaI491V, HvGBSSIaM490V/I491V, and HvGBSSIaCDC_Alamo) showed activity in vitro, and hence none of the amino acid substitutions resulted in a full loss of catalytic activity (Table 1). The strongest reduction in catalytic activity was observed for HvGBSSIaCDC_Alamo, which showed a 90% reduction of kcat compared with HvGBSSIaWT.
Fig. 1.
(A) Three unique amino acid substitutions were identified in HvGBSSIaCDC_Alamo (D219V, M490V, and I491V) as compared with a number of other waxy and non-waxy (in bold) barley varieties. (B) Mapping of these three amino acid substitutions on the structure of the catalytic domain of rice GBSSIa (Momma and Fujimoto, 2012; PDB entry 4VUF). D219V is located on the N-terminal Rossman fold while M490V and I491V are on the C-terminal Rossman fold. None of the three amino acids are positioned in the catalytic site in which ADP is bound, or involved in any other putative substrate, or protein binding sites.
Table 1.
Glycosyltransferase activity of various HvGBSSIa variants in vitro
| Enzymec | ADP-Glca | Glycogenb | ||||
|---|---|---|---|---|---|---|
| k cat | K m | k cat/Km | k cat | K m | k cat/Km | |
| (min–1) | (µM) | (min–1 µM–1) | (min–1) | (µg ml–1) | (min–1 µg–1 ml) | |
| HvGBSSIaWT | 5.8 ± 0.2 | 160 ± 15 | 3.65 × 10–2 (100%) | 6.3 ± 0.2 | 72 ± 6 | 8.69 × 10–2 (100%) |
| HvGBSSIaD219V | 2.76 ± 0.06 | 127 ± 9 | 2.17 × 10–2 (59%) | 3.2 ± 0.2 | 84 ± 16 | 3.81 × 10–2 (44%) |
| HvGBSSIaM490V | 21.2 ± 0.6 | 71 ± 7 | 2.99 × 10–1 (818%) | 19.4 ± 0.8 | 159 ± 20 | 1.22 × 10–1 (140%) |
| HvGBSSIaI491V | 6.7 ± 0.3 | 114 ± 15 | 5.88 × 10–2 (161%) | 6.5 ± 0.3 | 108 ± 15 | 6.02 × 10–2 (69%) |
| HvGBSSIaM490V/I491V | 0.94 ± 0.04 | 56 ± 8 | 1.70 × 10–2 (47%) | 1.3 ± 0.2 | 175 ± 77 | 7.43 × 10–3 (9%) |
| HvGBSSIaCDC Alamo | 0.56 ± 0.03 | 51 ± 10 | 1.09 × 10–2 (30%) | 0.7 ± 0.1 | 139 ± 62 | 5.30 × 10–3 (6%) |
a ADP-Glc as the varying substrate; the concentration of rabbit liver glycogen was constant at 1 mg ml–1.
b Rabbit liver glycogen as the varying substrate; the concentration of ADP-Glc was constant at 1 mM.
c Wild-type kcat activities correspond to 0.102–0.095 µmol min–1 mg–1.
Starch targeting patterns of HvGBSSIa
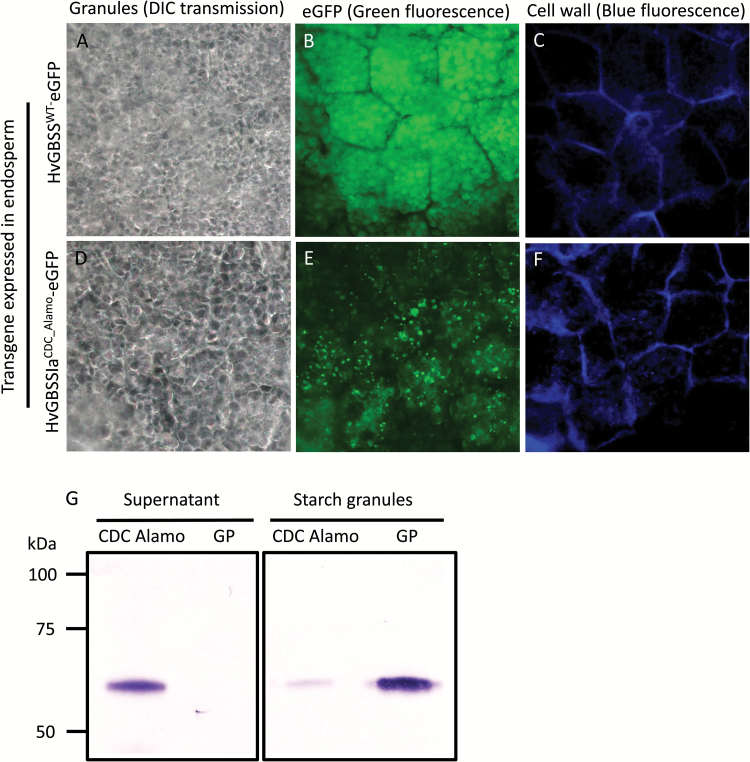
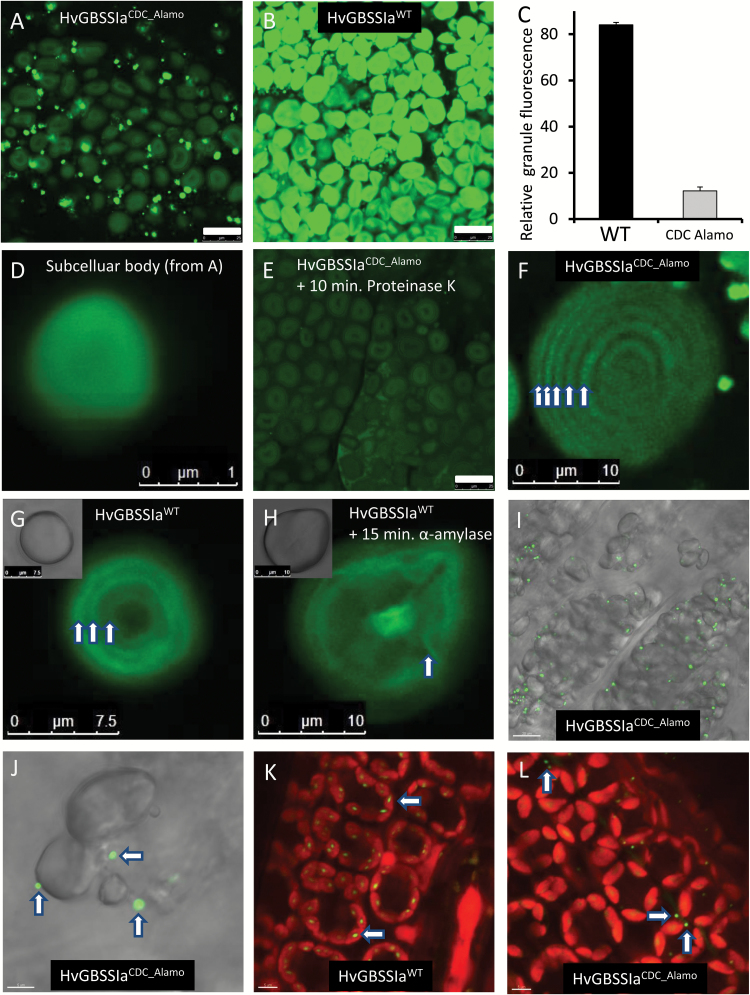
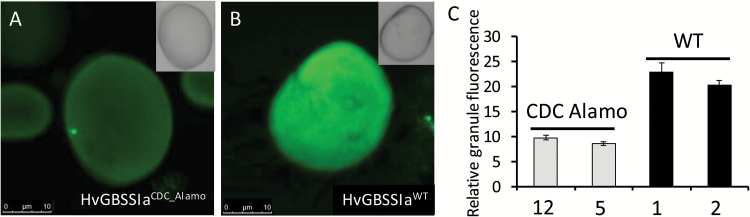
HvGBSSIaCDC_Alamo had a strong reduction in catalytic activity, but was not fully inactive. To examine the subcellular localization patterns of GBSSIa, we generated transgenic barley plants (var: Golden Promise, GP) from calli transformed with constructs for overexpression of either HvGBSSIaCDC_Alamo or HvGBSSIaWT as fusion proteins tagged with eGFP. GBSS-type enzymes are strictly associated with starch granules. In our previous work, we found that HvGBSSIaWT-eGFP fusion protein expressed in callus cells was strictly localized to starch granules (Carciofi et al., 2012), whereas such visual localization of GBSS within storage starch granules from tubers or grains has not previously been reported. Barley grain starch has a bimodal granule morphology consisting of large disc-shaped granules of 15–50 µm (A-type) and small granules of 2–3 µm (B-type) (Jane et al., 1994; Carciofi et al., 2012). Barley callus cell starch granules are much smaller (<3 µm) than the large A-type granules from grains (Carciofi et al., 2012). We therefore studied the cellular localization pattern of HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP in endosperm cells in grains from two independent transgenic barley lines for each of the HvGBSSIa varieties. Starch granules were detected with transmitted light using DIC settings. HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP were detected as green epifluorescence. Cell walls were detected as blue fluorescence. In endosperm cells of developing grains (30 days after pollination (DAP)), HvGBSSIaWT-eGFP was strictly associated with the starch granules (Fig. 2A–C), whereas HvGBSSIaCDC_Alamo-eGFP showed a deficient starch targeting pattern with localization into smaller subcellular bodies rather than into starch granules (Fig. 2D–F). Western blotting with antiserum against HvGBSSI on either the protein fraction of the supernatant or on starch granules purified from developing endosperm showed bands with similar size in the supernatant and starch granules from CDC Candle or starch granules of GP. These bands correspond well to the expected size (59 kDa) of HvGBSSIa without a transit peptide. While GBSSIa was strictly associated with starch granules in GP, GBSSIa in CDC Alamo was detected mainly in the supernatant, with only a smaller quantity in the starch granules (Fig. 2G). Western blotting with eGFP antibody similarly confirmed that transgenic HvGBSSIaWT-eGFP was located in purified starch granules, whereas HvGBSSIaCDC_Alamo-eGFP was mostly associated with the supernatant, rather than starch granules (Supplementary Fig. S1). The localizations, as detected by the eGFP signal of HvGBSSIaWT-eGFP and HvGBSSIaCDC_Alamo-eGFP into starch granules and the smaller subcellular bodies, were further investigated by confocal laser scanning microscopy (Fig. 3). This showed that for both HvGBSSIaWT-eGFP and HvGBSSIaCDC_Alamo-eGFP fluorescence was detected in both large (A-type) and small (B-type) starch granules. However, HvGBSSIaWT-eGFP (Fig. 3A) was more strongly associated with starch granules than HvGBSSIaCDC_Alamo-eGFP (Fig. 3B) when comparing relative difference in granule fluorescence (Fig. 3C). The subcellular bodies associated with HvGBSSIaCDC_Alamo-eGFP were mostly disc shaped with less fluorescence in the center and a diameter of 0.5–3 µm (Fig. 3D). They disappeared completely after a 10 min treatment with proteinase K, leaving both large (A-type) and small (B-type) starch granules intact (Fig. 3E). This suggests that they may be protein bodies. Interestingly, both HvGBSSIa-eGFP types were not uniformly distributed within the starch granules but were concentrated in internal concentric spheres (Fig. 3F–G). These spheres became blurred after a 15 min treatment with α-amylase (Fig. 3H; only HvGBSSIaWT is shown, although HvGBSSIaCDC_Alamo showed a similar blurring pattern). We recorded both transmitted light (with DIC) and eGFP to simultaneously visualize endosperm subcellular structures and HvGBSSIaCDC_Alamo-eGFP subcellular bodies in thin (50 µm) sections of developing endosperm (Fig. 3I, J). While some of the subcellular bodies seemed to be located away from starch granules (Fig. 3J), we were unable to determine if the subcellular bodies were located inside or outside of the amyloplast membrane. However, in leaves where HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP were also expressed, we observed that HvGBSSIaWT-eGFP was localized inside chloroplasts (Fig. 3K), whereas HvGBSSIaCDC_Alamo-eGFP was mostly located outside of chloroplasts (Fig. 3L). In dry, post-harvest mature grains there was only a slight tendency for distribution into concentric spheres in both HvGBSSIaWT-eGFP and HvGBSSIaCDC_Alamo-eGFP starch granules (Fig. 4A, B) and the difference in relative levels of fluorescence between HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP starch granules was smaller (Fig. 4C) than during endosperm development (Fig. 3C).
Fig. 2.
(A–F) Subcellular localization of HvGBSSIaWT-eGFP (A–C) and HvGBSSIaCDC_Alamo-eGFP (D–F) in 100 µm-thick sections of developing endosperm (30 DAP) of grains from transgenic barley plants. Starch granules were detected with transmitted light using differential interference contrast (DIC) settings (A, D); HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP were detected as green epifluorescence (B, E); cell walls were detected as blue epifluorescence (C, F). HvGBSSIaWT-eGFP is strictly targeted to starch granules (B), whereas HvGBSSIaCDC_Alamo-eGFP is mostly targeted into unknown subcellular bodies (E). (G) Western blotting with GBSSI antiserum confirms that HvGBSSIa is strictly targeted to starch granules in the endosperm of Golden Promise, whereas it is mostly found in the supernatant of the endosperm cell fraction of CDC Alamo. All lanes are from the same blot.
Fig. 3.
Confocal laser scanning microscopy of starch granules from the endosperm of developing grains at the late grain filling stage (30 DAP) of transgenic barley plants expressing HvGBSSIaWT-eGFP (B, G, H, K) or HvGBSSIaCDC_Alamo-eGFP (A, E, F). (A), (B), (E) and (F) are recorded with same sensitivity, whereas (D), (G) and (H) were recorded with lower sensitivity to avoid oversaturation. (C) The relative fluorescence intensities were much stronger in HvGBSSIaWT-eGFP starch granules than in HvGBSSIaCDC_Alamo-eGFP starch granules (n=10). Bars indicate standard errors. (D) Magnified subcellular body from HvGBSSIaCDC_Alamo-eGFP endosperm cells from panel A. (E) The subcellular bodies in panel (A) disappeared after treatment with proteinase K (1 mg ml–1 in H2O) for 10 min. (G, H) Both HvGBSSIaWT-eGFP and HvGBSSIaCDC_Alamo-eGFP are localized into concentric spheres (indicates by arrows) within starch granules. (H) These concentric spheres became blurred (indicated by the arrow) upon treatment with α-amylase. (I, J) Transmission light was used to visualize subcellular structures in thin sections of endosperm cells simultaneously with localization of HvGBSSIaCDC_Alamo-eGFP recorded as overlaid eGFP fluorescence. (K, L) HvGBSSIaWT-eGFP (green; K) was located in granules inside chloroplasts (red fluorescence) in leaves whereas HvGBSSIaCDC_Alamo-eGFP (L) was mostly localized in subcellular bodies (green, indicated by arrows) outside of the red chloroplasts. The scale bars in (A, B, E) are 25 µm; the scale bar in (I) is 20 µm; the scale bars in (F, H) are 10 µm; the scale bar in (G) is 7.5 µm; the scale bars in (J, K, L) are 5 µm; and the scale bar in (D) is 1 µm.
Fig. 4.
(A, B) Confocal laser scanning microscopy of starch granules from mature dry grains of transgenic barley plants expressing HvGBSSIaCDC_Alamo-eGFP (A) and HvGBSSIaWT-eGFP (B). (C) Relative fluorescence intensities in HvGBSSIaWT-eGFP and HvGBSSIaCDC_Alamo-eGFP starch granules (n=10) from two different lines each: HvGBSSIaCDC_Alamo-eGFP (lines 12 and 5) and HvGBSSIaWT-eGFP (lines 1 and 2). Bars indicate standard errors.
Deficient starch targeting of HvGBSSIaCDC_Alamo-eGFP is associated with the waxy phenotype
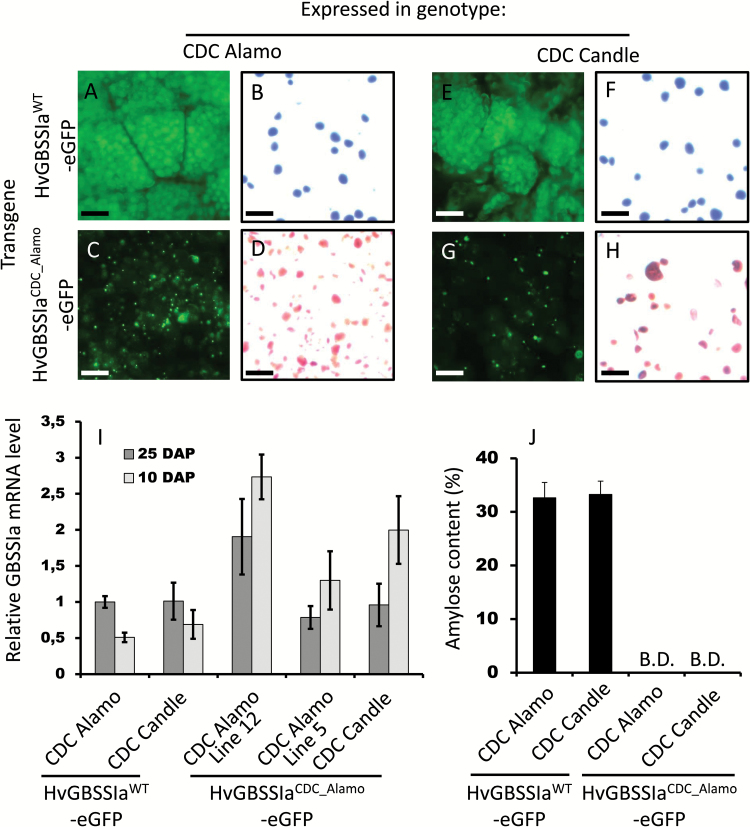
Golden Promise is a non-waxy variety with fairly high amylose content, and overexpression of either HvGBSSIaWT-eGFP or HvGBSSIaCDC_Alamo-eGFP in Golden Promise did not change the amylose content, suggesting that GBSSIa enzyme activity may already be saturated in this variety (not shown). Therefore, to further demonstrate the link between the deficient GBSSIaCDC_Alamo-eGFP starch targeting pattern with the waxy phenotype of CDC Alamo, we crossed the transgenic barley plants expressing either GBSSIaCDC_Alamo-e GFP or HvGBSSIaWT-eGFP with either CDC Alamo or either of the near-waxy barley varieties CDC Candle or SB94912 (♀ Golden Promise_Ubi:GBSSIa(WT or CDC_Alamo)-eGFP × ♂ CDC Alamo or CDC Candle or SB94912). Both of the HvGBSSIaCDC_Candle and HvGBSSIaSB94912 alleles contain a deletion in the promoter and have low expression levels of HvGBSSIa enzyme imparting a near-waxy starch phenotype (Asare et al., 2012). The near-waxy starch phenotype can be identified by a screen with iodine staining of endosperm flour, which will reveal near-waxy starch granules staining with a red color, in contrast to non-waxy starch granules, which are blue. Parents and F2 grains of the crosses were genotyped with respect to the endogenous HvGBSSIa gene as described in ‘Materials and methods’ to identify F2 grains that are homozygous for one of the endogenous waxy alleles HvGBSSIaCDC_Alamo, HvGBSSIaCDC_Candle or HvGBSSIaSB94912. Expression of HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP in the endosperm cells of F1 and segregating F2 grains was detectable with fluorescence microscopy as described above for T1 grains, and also showed localization patterns identical to those observed in T1 grains: HvGBSSIaWT-eGFP was strictly associated with starch granules (Fig. 5A, E), whereas HvGBSSIaCDC_Alamo-eGFP showed a deficient starch targeting pattern with localization into subcellular bodies rather than into starch granules (Fig. 5C, G). Furthermore, there was a difference between HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP in their ability to rescue the waxy phenotype of CDC Alamo, CDC Candle or SB94912: transgenic expression of HvGBSSIaWT-eGFP rescued the three waxy varieties by making non-waxy blue-staining granules (Fig. 5B, F) with an amylose content above 30% in mature grains (Fig. 5J), whereas the three waxy lines with over-expression of HvGBSSIaCDC_Alamo-eGFP retained red-staining waxy starch granules with an amylose content below detection level (Fig. 5J). The effect was the same in the three different cultivars, showing that the deficient starch targeting pattern of HvGBSSIaCDC_Alamo-eGFP as well as the inability of HvGBSSIaCDC_Alamo-eGFP to rescue the waxy phenotype is independent of both the endogenous HvGBSSIa locus and the genetic background in the three different waxy varieties used in this study. The results for SB94912 were identical to those of CDC Candle. So for simplicity, only CDC Candle is shown in Fig. 5. To ensure that the observed deficiencies of HvGBSSIaCDC_Alamo-eGFP was not due to a difference in expression efficiency between the HvGBSSIaCDC_Alamo-eGFP and HvGBSSIaWT-eGFP transgene, mRNA expression levels of total HvGBSSIa were measured by qPCR, which demonstrated that expression of HvGBSSIaCDC_Alamo-eGFP was not significantly lower than expression of HvGBSSIaWT-eGFP. Two independent lines of HvGBSSIaCDC_Alamo-eGFP expression were used (lines 5 and 12) and both failed to complement waxy in any of the three cultivars.
Fig. 5.
(A–H) eGFP fluorescence from endosperm cells (A, C, E, G) and iodine-stained starch granules (B, D, F, H) from developing grains (30 DAP) of plants with transgenic expression of HvGBSSIaWT-eGFP (A, B, E, F) or HvGBSSIaCDC_Alamo-eGFP (C, D, G, H) in the three waxy cultivars CDC Alamo (A–D), CDC Candle (E–H) and SB94912. The results for SB94912 were identical to those of CDC Candle, so for simplicity, only CDC Candle is shown. The scale bars in (A–H) are 50 µm. (I) Relative gene expression levels of HvGBSSIa determined by qPCR in developing grains (10 and 25 DAP) from plants with overexpression of either HvGBSSIaWT-eGFP or HvGBSSIaCDC_Alamo-eGFP (two different lines: 12 and 5) in the cultivars CDC Alamo and CDC Candle. (J) Amylose content in starch isolated from dry mature grains of plants expressing either HvGBSSIaWT-eGFP or HvGBSSIaCDC_Alamo-eGFP in CDC Alamo or CDC Candle.
Discussion
The amylose-free phenotype of barley cv. CDC Alamo has been presumed to be the result of a complete elimination of HvGBSSIa activity caused by a single SNP alteration from adenine to thymine, which replaces the aspartic acid with the hydrophobic amino acid valine at position 219 (Patron et al., 2002; Asare et al., 2012). This mutation was never tested in vitro and only measurement of starch synthase activity associated with granules in developing endosperm of barley cv. CDC Alamo suggested that most or all of GBSSIa protein was inactive (Patron et al., 2002). In addition, a re-examination of the gene entry of barley cv. CDC Alamo (AF486519) revealed two extra SNPs that also resulted in two mutations, M490V and I491V (Fig. 1A).
Examination of Asp219, Met490, and Ile491 in a modeled structure based on the published structure of rice GBSSIa, which shares very close homology with barley HvGBSSIa (Momma and Fujimoto, 2012), did not provide an immediate explanation as to why HvGBSS in CDC Alamo should be inactive (Fig. 1B). GBSSIa belongs to the GT5 family of glycosyltransferases in the CAZy database (Coutinho et al., 2003), which adopts the characteristic GT-B fold (double Rossmann fold). The interface between the two Rossmann folds forms the catalytic site wherein an ADP molecule was identified in the rice GBSSIa structure (Momma and Fujimoto, 2012). Asp219 is neither positioned in this catalytic site nor involved in any putative substrate binding, but is located in the 380s loop of starch synthases; a region that is highly variable among all starch synthase gene classes (Leterrier et al., 2008). However, Asp219 itself is conserved among different plant GBSS genes (Leterrier et al., 2008), indicating that it may have some importance for protein structure and function. Asp219 (HvGBSS numbering) is also in close proximity with the basic Arg196 and Lys229 residues, which could potentially be disturbed by the Asp219→Val substitution in HvGBSSaICDC_Alamo. Met490 and Ile491 are both located on the second to last α-helix in the structure (the last one of the C-terminal Rossman fold). Met490 is buried in the structure of rice GBSSIa where Ile491 (Asn in Rice GBSS) is surface exposed (Momma and Fujimoto, 2012). Met490 is conserved among cereal starch synthase amino acid sequences from all five starch synthase gene classes (Leterrier et al., 2008).
The three identified substitutions D219V, M490V, and I491V were selected as targets for a mutational study of HvGBSSIa to explain the amylose-free phenotype of CDC Alamo. The specific activity of HvGBSSIaWT correlated well with previous studies of purified maize GBSS (Macdonald and Preiss, 1983, 1985). HvGBSSIaD219V showed only a small reduction in activity (44–59%) compared with wild-type (HvGBSSIaWT), while HvGBSSIaM490V/I491V (9–47%) and HvGBSSIaCDC_Alamo (6–30%) activities were much lower. The catalytic properties of HvGBSSIaI491V were essentially unchanged compared with wild-type; however, HvGBSSIaM490V differed significantly from the wild-type by having at least three-fold higher kcat. The Km value towards ADP-glucose HvGBSSIa was in the same range as those previously determined for maize GBSS (Macdonald and Preiss, 1985), but notably lower than that of potato (Edwards et al., 1999) and Chlamydomonas reinhardtii GBSS (Delrue et al., 1992) (10- and 23-fold lower, respectively). In summary, the combination of the three SNPs in the HvGBSSIa gene of CDC Alamo seems to lead to strong reduction in catalytic activity of the enzyme.
Our results indicate that the waxy phenotype is also associated with deficient targeting of HvGBSSIa into starch granules in CDC Alamo. We used eGFP tagging to demonstrate that a consensus HvGBSSIa from non-waxy cultivars is targeted to starch granules, where it is localized into concentric spheres. This is well in line with the general consensus that GBSS-type enzymes are bound tightly to starch granules within the internal granular matrix (Tatge et al., 1999; Wang et al., 2013). Concentric spheres of similar morphology were observed in wheat, maize, and potato starch in a confocal laser scanning microscopy study of starch treated with a protein-specific dye (3-(4-carboxybenzoyl) quinoline-2-carboxaldehyde) (Han and Hamaker, 2002). In line with our observations, these spheres are likely to be GBSSIa, as the concentric spheres were not observed in waxy maize and amylose-free potato starch, which correlated with the lack of expression of GBSSIa (Han and Hamaker, 2002). In contrast, the targeting of HvGBSSIa is deficient in CDC Alamo. Western blotting with HvGBSSIa antiserum showed that HvGBSSIaCDC_Alamo fails to be fully targeted to starch granules in CDC Alamo, where a major fraction of HvGBSSIaCDC_Alamo is found in the soluble fraction of developing endosperms (Fig. 2G). Transgenic expression of eGFP-tagged HvGBSSIaWT or HvGBSSIaCDC_Alamo confirmed that HvGBSSIaCDC_Alamo is unable to target correctly to starch granules and to synthesize amylose in vivo (Fig. 5). HvGBSSIa is generally targeted to amyloplasts by a transit peptide that is cleaved when the protein is transported through the amyloplast membrane. Our previous work has demonstrated that a transit peptide, with an identical 69 amino-acid sequence to that of HvGBSSIaWT and HvGBSSIaCDC_Alamo (which have identitical gene sequences for the transit peptide), effectively transports transgenic eGFP to the amyloplast of developing barley endosperm cells (Hebelstrup et al., 2010). This suggests that the incorrect targeting of HvGBSSIaCDC_Alamo is not due to a deficient transit peptide. This is supported by the western blot (Fig. 2G), which shows that the soluble fraction of HvGBSSIaCDC_Alamo in CDC Alamo has an identical size to that of the HvGBSSIaCDC_Alamo that is correctly targeted to starch granules. This implies that the transit peptide must have been correctly removed from the soluble fraction of HvGBSSIaCDC_Alamo.
GBSSI seems to be the predominant protein in storage starch granules (Wang et al., 2013). In contrast, soluble starch synthases and starch branching enzymes form complexes (Tetlow et al., 2008; Liu et al., 2012). However, GBSS-type enzymes do not contain any identified starch binding modules, nor do they interact in the mentioned complexes with the soluble starch synthases or starch branching enzymes containing starch binding modules. In Arabidopsis leaves, amylose synthesis is dependent on a GBSS type that is targeted to transient starch granules by interaction with the protein PTST (protein targeting to starch), which contains a carbohydrate binding module, and loss-of-function ptst mutant Arabidopsis plants synthesize a waxy-like type of transient starch in their leaves (Seung et al., 2015). A PTST gene homolog exists in the barley genome, and therefore the same mechanism may be necessary for correct targeting of HvGBSSIa to storage starch granules and synthesis of amylose in barley grain endosperm. This also demonstrates that mutations in other genes than GBSS can contribute to the waxy phenotype. However, in CDC Alamo, the three identified mutations in HvGBSSIa seem to be the single cause of deficient targeting to starch granules and the waxy phenotype, because HvGBSSIaCDC_Alamo was also incorrectly targeted and unable to rescue the waxy phenotype when expressed in the cultivars CDC Candle and SB94912 (Fig. 5), which are waxy due to a deletion in the gene promoter and subsequently lack HvGBSSIa enzyme (Asare et al., 2012). Similarly, the waxy phenotype could be rescued in CDC Alamo by expression of functional HvGBSSIaWT resulting in >30% amylose content. Given that binding of GBSS with PTST-type proteins is necessary for correct targeting of GBSS and synthesis of amylose (Seung et al., 2015), it is possible that one or more of the three amino acid substitution (D219V, M490V, and I491V) in HvGBSSIaCDC_Alamo in barley prevents binding to a putative PTST protein, whereby HvGBSSIaCDC_Alamo is incorrectly targeted. However, the binding of GBSS to PTST in Arabidopsis was mediated through a coiled-coil domain identified on the distal helix (Fig. 5B), which is not in the structural area of the three amino acid substitutions of HvGBSSIaCDC_Alamo.
We cannot exclude the possibility that the deficient targeting of HvGBSSIa in CDC Alamo is caused indirectly by the partial loss of catalytic activity. It can also be speculated that HvGBSSIa is a less processive variant, which causes it to fail to remain bound to (or within) the starch granules after correct targeting. The cellular structure of the subcellular bodies where HvGBSSIaCDC_Alamo-eGFP is located is not clear (Figs 2, 3 and 5). Their sensitivity to proteinase K indicates that they are protein-rich bodies. In leaves, at least some of them are located outside of chloroplasts (Fig. 3L). In endosperm cells, it was not clear if they are located outside or on the inside of the amyloplast membrane. However, as discussed above, western blotting suggests that their transit peptide has been correctly removed, indicating that they have been translocated across a plastid membrane. It cannot be excluded that they represent more than one type of cellular structure. They do not seem to be breakdown products of HvGBSSIa, since the western blot showed a single intact protein in the soluble fraction of CDC Alamo with a size similar to that of the HvGBSSIa in the starch granules in Golden Promise (Fig. 2G). Similar results were obtained for a transgene system where HvGBSSIaCDC_Alamo-eGFP expressed in endosperm located mostly to the supernatant with a similar size to that of the correctly targeted HvGBSSIaWT-eGFP and neither showed signs of breakdown products (Supplementary Fig. S1). In summary, our work suggests that the waxy phenotype of CDC Alamo is associated with a combination of a strong reduction of catalytic activity of HvGBSSIa, as well as a deficiency of either targeting or retaining HvGBSSIa within the matrix of starch granules.
Supplementary Material
Acknowledgements
We are grateful to Charlotte Riis for assistance with plant crossing. This work was funded in part by the Danish Council for Independent Research (Technology and Production Sciences, grant no. 1335-00203) and in part by the Carlsberg Foundation.
Glossary
Abbreviations:
- DIC
differential interference contrast
- eGFP
enhanced green fluorescent protein
- GBSS
granule-bound starch synthase
- GP
Golden Promise
- SNP
single nucleotide polymorphism
- SS
starch synthase.
References
- Asare EK, Båga M, Rossnagel BG, Chibbar RN. 2012. Polymorphism in the barley granule bound starch synthase 1 (gbss1) gene associated with grain starch variant amylose concentration. Journal of Agricultural and Food Chemistry 60, 10082–10092. [DOI] [PubMed] [Google Scholar]
- Blennow A, Jensen SL, Shaik SS, Skryhan K, Carciofi M, Holm PB, Hebelstrup KH, Tanackovic V. 2013. Future cereal starch bioengineering: cereal ancestors encounter gene technology and designer enzymes. Cereal Chemistry 90, 274–287. [Google Scholar]
- Buléon A, Colonna P, Planchot V, Ball S. 1998. Starch granules: structure and biosynthesis. International Journal of Biological Macromolecules 23, 85–112. [DOI] [PubMed] [Google Scholar]
- Carciofi M, Blennow A, Nielsen MM, Holm PB, Hebelstrup KH. 2012. Barley callus: a model system for bioengineering of starch in cereals. Plant Methods 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carciofi M, Shaif SS, Jensen SL, Blennow A, Svensson JT, Vincze E, Hebelstrup KH. 2011. Hyperphosphorylation of cereal starch. Journal of Cereal Science 54, 339–346. [Google Scholar]
- Coutinho PM, Deleury E, Davies GJ, Henrissat B. 2003. An evolving hierarchical family classification for glycosyltransferases. Journal of Molecular Biology 328, 307–317. [DOI] [PubMed] [Google Scholar]
- Cuesta-Seijo JA, Nielsen MM, Marri L, Tanaka H, Beeren SR, Palcic MM. 2013. Structure of starch synthase I from barley: insight into regulatory mechanisms of starch synthase activity. Acta Crystallographica. Section D, Biological Crystallography 69, 1013–1025. [DOI] [PubMed] [Google Scholar]
- Delrue B, Fontaine T, Routier F, Decq A, Wieruszeski JM, Van Den Koornhuyse N, Maddelein ML, Fournet B, Ball S. 1992. Waxy Chlamydomonas reinhardtii: monocellular algal mutants defective in amylose biosynthesis and granule-bound starch synthase activity accumulate a structurally modified amylopectin. Journal of Bacteriology 174, 3612–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer KAY, Johnson P, Zeeman S, Smith AM. 2001. The control of amylose synthesis. Journal of Plant Physiology 158, 479–487. [Google Scholar]
- Domon E, Saito A, Takeda K. 2002. Comparison of the waxy locus sequence from a non-waxy strain and two waxy mutants of spontaneous and artificial origins in barley. Genes & Genetic Systems 77, 351–359. [DOI] [PubMed] [Google Scholar]
- Edwards A, Borthakur A, Bornemann S, Venail J, Denyer K, Waite D, Fulton D, Smith A, Martin C. 1999. Specificity of starch synthase isoforms from potato. European Journal of Biochemistry 266, 724–736. [DOI] [PubMed] [Google Scholar]
- Gosselin S, Alhussaini M, Streiff MB, Takabayashi K, Palcic MM. 1994. A continuous spectrophotometric assay for glycosyltransferases. Analytical Biochemistry 220, 92–97. [DOI] [PubMed] [Google Scholar]
- Han XZ, Hamaker BR. 2002. Location of starch granule-associated proteins revealed by confocal laser scanning microscopy. Journal of Cereal Science 35, 109–116. [Google Scholar]
- Hebelstrup KH, Christiansen MW, Carciofi M, Tauris B, Brinch-Pedersen H, Holm PB. 2010. UCE: A uracil excision (USER)-based toolbox for transformation of cereals. Plant Methods 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebelstrup KH, Sagnelli D, Blennow A. 2015. The future of starch bioengineering: GM microorganisms or GM plants? Frontiers in Plant Science 6, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton CM, Denyer K, Keeling PL, Chang MT, Smith AM. 1996. The effect of waxy mutations on the granule-bound starch synthases of barley and maize endosperms. Planta 198, 230–237. [Google Scholar]
- Jane JL, Kasemsuwan T, Leas S, Zobel H, Robyt JF. 1994. Anthology of starch granule morphology by scanning electron-microscopy. Starch – Stärke 46, 121–129. [Google Scholar]
- Leterrier M, Holappa LD, Broglie KE, Beckles DM. 2008. Cloning, characterisation and comparative analysis of a starch synthase IV gene in wheat: functional and evolutionary implications. BMC Plant Biology 8, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Romanova N, Lee EA, Ahmed R, Evans M, Gilbert EP, Morell MK, Emes MJ, Tetlow IJ. 2012. Glucan affinity of starch synthase IIa determines binding of starch synthase I and starch-branching enzyme IIb to starch granules. The Biochemical Journal 448, 373–387. [DOI] [PubMed] [Google Scholar]
- Ma JA, Jiang QT, Wei YM, Andre L, Lu ZX, Chen GY, Liu YX, Zheng YL. 2010. Molecular characterization and comparative analysis of two waxy alleles in barley. Genes & Genomics 32, 513–520. [Google Scholar]
- Macdonald FD, Preiss J. 1983. Solubilization of the starch-granule-bound starch synthase of normal maize kernels. Plant Physiology 73, 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald FD, Preiss J. 1985. Partial purification and characterization of granule-bound starch synthases from normal and waxy maize. Plant Physiology 78, 849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momma M, Fujimoto Z. 2012. Interdomain disulfide bridge in the rice granule bound starch synthase I catalytic domain as elucidated by X-ray structure analysis. Bioscience, Biotechnology, and Biochemistry 76, 1591–1595. [DOI] [PubMed] [Google Scholar]
- Nelson O, Pan D. 1995. Starch synthesis in maize endosperms. Annual Review of Plant Physiology and Plant Molecular Biology 46, 475–496. [Google Scholar]
- Olsen KM, Purugganan MD. 2002. Molecular evidence on the origin and evolution of glutinous rice. Genetics 162, 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron NJ, Smith AM, Fahy BF, Hylton CM, Naldrett MJ, Rossnagel BG, Denyer K. 2002. The altered pattern of amylose accumulation in the endosperm of low-amylose barley cultivars is attributable to a single mutant allele of granule-bound starch synthase I with a deletion in the 5′-non-coding region. Plant Physiology 130, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radchuk VV, Borisjuk L, Sreenivasulu N, Merx K, Mock HP, Rolletschek H, Wobus U, Weschke W. 2009. Spatiotemporal profiling of starch biosynthesis and degradation in the developing barley grain. Plant Physiology 150, 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung D, Soyk S, Coiro M, Maier BA, Eicke S, Zeeman SC. 2015. Protein targeting to starch is required for localising granule-bound starch synthase to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biology 13, e1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik SS, Carciofi M, Martens HJ, Hebelstrup KH, Blennow A. 2014. Starch bioengineering affects cereal grain germination and seedling establishment. Journal of Experimental Botany 65, 2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasulu N, Radchuk V, Strickert M, Miersch O, Weschke W, Wobus U. 2006. Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds. The Plant Journal 47, 310–327. [DOI] [PubMed] [Google Scholar]
- Tatge H, Marshall J, Martin C, Edwards EA, Smith AM. 1999. Evidence that amylose synthesis occurs within the matrix of the starch granule in potato tubers. Plant, Cell and Environment 22, 543–550. [Google Scholar]
- Tetlow IJ. 2011. Starch biosynthesis in developing seeds. Seed Science Research 21, 5–32. [Google Scholar]
- Tetlow IJ, Beisel KG, Cameron S, Makhmoudova A, Liu F, Bresolin NS, Wait R, Morell MK, Emes MJ. 2008. Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiology 146, 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Hassani ME, Crossett B, Copeland L. 2013. Extraction and identification of internal granule proteins from waxy wheat starch. Starch – Stärke 65, 186–190. [Google Scholar]
- Wang ZY, Zheng FQ, Shen GZ, Gao JP, Snustad DP, Li MG, Zhang JL, Hong MM. 1995. The amylose content in rice endosperm is related to the posttranscriptional regulation of the waxy gene. The Plant Journal 7, 613–622. [DOI] [PubMed] [Google Scholar]
- Wessler SR, Varagona MJ. 1985. Molecular-basis of mutations at the waxy locus of maize: correlation with the fine-structure genetic-map. Proceedings of the National Academy of Sciences of the United States of America 82, 4177–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe HAM, Blennow A, Noda T. 2009. Physico-chemical and degradative properties of in-planta re-structured potato starch. Carbohydrate Polymers 77, 118–124. [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. 2010. Starch: its metabolism, evolution, and biotechnological modification in plants. Annual Review of Plant Biology 61, 209–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.