Highlight
Pi deficiency suppressed the transcription factor gene StMYB44, encoding the protein that interacts with Arabidopsis WRKY6 and StWRKY6 and negatively affects Pi distribution by suppressing the expression of PHOSPHATE1 in potato.
Keywords: Gene expression, phosphate deficiency, PHOSPHATE1, potato, protein–protein interaction, RNA-Seq, StMYB44
Abstract
Phosphorus is an important macronutrient for plant growth, but often deficient in soil. To understand the molecular basis of the complex responses of potato (Solanum tuberosum L.) to phosphate (Pi) deficiency stress, the RNA-Seq approach was taken to identify genes responding to Pi starvation in potato roots. A total of 359 differentially expressed genes were identified, among which the Solanum tuberosum transcription factor gene MYB44 (StMYB44) was found to be down-regulated by Pi starvation. StMYB44 was ubiquitously expressed in potato tissues and organs, and StMYB44 protein was exclusively localized in the nucleus. Overexpression of StMYB44 in potato resulted in lower accumulation of Pi in shoots. Transcriptomic analysis indicated that the abundance of S. tuberosum PHOSPHATE1 (StPHO1), a Pi transport-related gene, was reduced in StMYB44 overexpression lines. In contrast, knock-out of StMYB44 by a CRISPR/Cas9 system failed to increase transcription of StPHO1. Moreover, StMYB44 was found to interact in the nucleus with AtWRKY6, a known Arabidopsis transcription factor directly regulating PHO1 expression, and StWRKY6, indicating that StMYB44 could be a member of the regulatory complex controlling transcription of StPHO1. Taken together, our study demonstrates that StMYB44 negatively regulates Pi transport in potato by suppressing StPHO1 expression.
Introduction
Inorganic phosphates (Pi) are taken up by plants to meet the phosphorus (P) requirements for a variety of structural and physiological functions. Inadequate supply of P in soil negatively affects plant development and growth (Raghothama, 1999). Reshaping root architecture and development is one of the mechanisms to increase Pi uptake, mobilization, and utilization upon Pi deficiency (Devaiah et al., 2007b). Changing expression of Pi-responsive genes, and altering metabolic and developmental processes are molecular adaptations in this regard (Wu et al., 2003; Thibaud et al., 2010; Secco et al., 2013; Puga et al., 2014). Systematic transcriptional regulation of Pi-responsive genes is believed to be the major regulatory step in maintaining Pi homeostasis (Hammond et al., 2004). A number of transcription factors mediating plant responses to Pi starvation have been identified in Arabidopsis and rice, including MYB transcription factors, PHR1/OsPHR2, PSR1, AtMYB2, MYB62, and OsMYB2P-1 (Wykoff et al., 1999; Rubio et al., 2001; Zhou et al., 2008; Devaiah et al., 2009; Dai et al., 2012; Baek et al., 2013), WRKY transcription factors AtWRKY6, AtWRKY42, AtWRKY45, and AtWRKY75 (Devaiah et al., 2007a; Chen et al., 2009; H. Wang et al., 2014; Su et al., 2015), basic helix–loop–helix transcription factors OsPTF1 and bHLH32 (Yi et al., 2005; Chen et al., 2007, zinc-finger transcription factor ZAT6 (Devaiah et al., 2007b), and APETALA2/ETHYLENE RESPONSE FACTOR, AtREF070 (Ramaiah et al., 2014). Each transcription factor specifically activates or suppresses a single or multiple Pi-related genes in response to Pi starvation (Chen et al., 2009; H. Wang et al., 2014; Su et al., 2015). Nuclear proteins SPX1 and SPX2 carry an SPX domain, which exists in Pi sensors and other Pi starvation signaling proteins in yeast and plants. These proteins are found to inhibit the activity of PHR1 and OsPHR2 transcription factors by protein–protein interactions in response to Pi availability in Arabidopsis and rice (Puga et al., 2014; Z. Wang et al., 2014). It demonstrates the vital role of transcription factors in Pi signaling pathways by linking Pi perception and gene expression. Hence, identification of additional transcription factors will further broaden our understanding about the signaling process in plant responses to Pi deficiency.
Among various MYB families, R2R3-type transcription factors are the largest MYB family in plants (Stracke et al., 2001). Based on amino acid sequence similarities, 126 Arabidopsis R2R3-type MYB transcription factors are categorized into 22 subgroups, and the last subgroup of MYB transcription factors mainly mediates hormone signaling and abiotic stress responses (Jung et al., 2008). One of its members, AtMYB77, mediates auxin signaling by interacting with auxin response factors and regulating expression of auxin-inducible genes to control lateral root growth and development (Shin et al., 2007). Another member of this subgroup, AtMYB44, positively regulates drought tolerance by enhancing stomatal closure (Jung et al., 2008). In addition, AtMYB44 has also been shown to induce expression of ETHYLENE INSENSITIVE2 (EIN2), a central component in the ethylene signaling pathway (Liu et al., 2011). Interaction of MYBR1/AtMYB44 with ABA receptor PYR1-LIKE8 (PYL8) mediates leaf senescence and responds to stress and wounding (Jaradat et al., 2013), implying that members of this subgroup are involved in diverse physiological processes in plants.
Potato (Solanum tuberosum L.), the fourth largest food crop in the world, faces an array of abiotic stresses including drought, cold, and mineral deficiency (Leone et al., 1999). Unlike Arabidopsis and rice, little is known about the mechanisms to maintain mineral homeostasis in potato since relatively few genes involved in regulation of mineral uptake and distribution have been identified in this species.
The present study was designed to carry out RNA-Seq-based identification of genes, particularly those encoding transcription factors, whose expression is affected in potato roots by Pi starvation. The current study is to explore how StMYB44 (previously named tuber-specific and sucrose-inducible element-binding factor), one of the transcription factors identified, is involved in regulation of Pi uptake and distribution in potato plant.
Materials and methods
Plant materials and growth conditions
Tetraploid potato (Solanum tuberosum L.), Désirée, plants were grown in a greenhouse under a 14 h light/10 h dark regime at 25 °C. Arabidopsis thaliana (ecotype Columbia) were grown in a growth chamber under a 14 h light/10 h dark cycle at 23 °C. Hoagland solution was used in hydroponic growth of potato plants, and was changed every other day. The Pi starvation was initiated by withdrawing Pi from the Hoagland solution when the potato plants were 1 month old. Roots were collected 5 d after the treatment and stored at –80 °C before RNA extraction.
Plasmid construction, and transformation of Arabidopsis and potato
The coding region of StMYB44 without the stop codon was amplified by PCR and cloned into the pAVA393 vector (von Arnim et al., 1998) to make the StMYB44:GFP fusion gene, which was then subcloned behind a double Cauliflower mosaic virus (CaMV) 35S promoter in the binary vector pCAMBIA1300S (Zhou et al., 2015). The complete vector was verified by sequencing and transformed into Agrobacterium tumefaciens GV3101 by electroporation. Arabidopsis transformation was performed by the floral-dip method (Clough and Bent, 1998).
For the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeat/Cas9) vector, the sequence GAAGATGATACTATCATCAGG of the StMYB44 gene was used as the target sequence. Two primers were synthesized and annealed to form the dsDNA and cloned between two BsaI sites of the pKSE401 vector by Golden Gate cloning (Xing et al., 2014). The complete vector was verified by sequencing.
The 1.5 kb StMYB44 promoter upstream of the translation start codon was inserted between HindIII and BamHI sites of pBI101.2, and then transformed into A. tumefaciens GV3101.
The complete vectors were introduced into potato by Agrobacterium-mediated transformation as previously described (Chronis et al., 2013).
Protein structure analysis and phylogenetic tree analysis
Predicted StMYB44 and homologs from Arabidopsis, tomato, tobacco, and cotton were aligned by using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was built with the Molecular Evolutionary Genetics Analysis (MEGA) software. Bootstrap analysis of the phylogenetic tree was performed using 100 replicates.
RNA extraction, library construction, RNA-Seq, and quantitative RT-PCR
Total RNA was extracted from roots of potato plants by using an E.Z.N.A.® Total RNA Kit I (Omega Bio-tek, Norcross, GA, USA). A 5 μg aliquot of total RNA was used for library preparation as previously described (Zhong et al., 2011). Sequencing was conducted on an Illumina HiSeq2500 at the Genomics Resources Core Facility of Weill Cornell Medical College.
Total RNA samples were treated with RQ1 DNase (Promega, Madison, WI, USA) for 30 min to remove genomic DNA, and then converted into cDNA using iScript™ Reverse Transcription Supermix (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR (qRT-PCR) was conducted in a CFX Connect Real-Time System with iTaq Universal SYBR Green Supermix (Bio-Rad). The thermal cycle involves 95 °C for 3 min, and 40 cycles of 95 °C 15 s and 60 °C for 60 s, followed by melt curve analysis to verify the specificity of amplification. The ΔΔCt method was used to calculate RT-PCR results with the potato Actin gene as an internal control.
RNA-Seq data processing and analysis
Libraries were sequenced on a HiSeq2500 (Illumina) using 101 base, single-end sequencing, and the quality of RNA-Seq data was determined by using FASTQC (v 0.10.1) (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were mapped to the reference S. tuberosum Group Phureja DM1-3 genome assembly PGSC v4.03 pseudomolecules (http://solanaceae.plantbiology.msu.edu/pgsc_download.shtml) using TopHat2 (Kim et al., 2013), allowing up to two mismatches. Differentially expressed genes were identified using cuffdiff following normalization of transcript count information to RPKM (reads per kilobase of exon model per million mapped reads) (Mortazavi et al., 2008). Genes with a P-value <0.05 were considered to be differentially expressed.
GUS staining
β-Glucuronidase (GUS) activity was assayed as previously described (Jefferson et al., 1987) in transgenic potato seedlings, leaves, flowers, and tubers expressing the ProMYB44:GUS chimeric gene using two independent transgenic lines for analysis.
To compare expression of the StMYB44 promoter upon Pi starvation, transgenic seedlings were transferred onto fresh medium with Pi (Hoagland solution) or medium without Pi (Hoagland solution without Pi) and grown for 5 d. GUS activity in the seedlings was examined as above.
Subcellular localization of StMYB44
Agrobacterium cells containing 35S:StMYB44-GFP and 35S:GFP plasmids, respectively, were infiltrated into 4-week-old Nicotiana benthamiana leaves. Three days after infiltration, the leaves were detached and green fluorescent protein (GFP) signals were examined under a Leica TCS-SP5 confocal microscope (Leica Microsystems Exton, PA, USA) with excitation wavelength at 488 nm and emission wavelength at 500–520 nm.
Six-day-old transgenic Arabidopsis seedlings expressing the 35S:StMYB44-GFP and 35S:GFP transgenes were used to study subcellular localization. Nuclei of root cells were stained with DAPI solution at 10 μg ml–1 (w/v) for 10 min, and then washed three times with water. Transgenic Arabidopsis seedlings expressing 35S:GFP were used as the control. GFP and DAPI signals were examined using a Leica TCS-SP5 confocal microscope with excitation wavelengths 488 nm for GFP and 405 nm for DAPI (Zhou et al., 2011).
Pi content determination
Pi content was determined as previously described (Jain et al., 2007) by grinding 6–20 mg of fresh shoot or root samples to a fine power in liquid nitrogen. The ground samples were suspended in 500 µl of 1% glacial acetic acid and immediately frozen in liquid nitrogen again and thawed. After centrifugation at 13 000 rpm for 1 min, 50 µl of supernatant were used in a phosphomolybdate colorimetric assay (Ames, 1966). To make Pi contents comparable, seedlings of wild-type and individual transgenic potato lines were grown in the same Magenta box containing 4.3 g l–1 Murashige and Skoog (MS) salt, 0.17 g l–1 NaH2PO4·H2O, 0.1 g l–1 inositol, 0.4 mg l–1 thiamine HCl, 30 g l–1 sucrose, and 1.8 g l–1 gelrite. Two weeks after subculture, shoot and root samples were collected for Pi content determination.
Protein–protein interaction by BiFC
The coding sequences of StMYB44, AtWRKY6(At1g62300), and StWRKY6 (NM_001318697, initially named StWRKY31, but it is more similar to AtWRKY6), a homolog of WKRY6 from potato, without stop codons were amplified by PCR and cloned into the KpnI and XmaI sites of the bimolecular fluorescence complementation (BiFC) vectors pSPYCE and pSPYNE, respectively (Waadt et al., 2008). After confirmation by sequencing, the vectors were transferred into A. tumefaciens GV3101 and agroinfiltrated into 4-week-old N. benthamiana leaves. Three days after infiltration, the leaf discs were detached and examined by confocal microscopy for the yellow fluorescent protein (YFP) signal with excitation wavelength at 488 nm and emission filter at 520 nm.
Results
Identification of Pi starvation-responsive genes in potato roots by RNA-Seq
To investigate the regulatory mechanism of potato in response to Pi deficiency, differentially expressed genes in roots under Pi-sufficient (Hoagland solution with 0.5 mM KH2PO4) and Pi-deficient (Hoagland solution without Pi) conditions were examined by RNA-Seq. A previous study on rice subjected to Pi starvation elucidated a 2- to 3-fold change in Pi content in shoots and roots, but substantial numbers of differentially expressed genes were not observed until 3–7 d (Secco et al., 2013). Therefore, to obtain a relatively comprehensive list of genes involved in the responses triggered by Pi deficiency, plant materials examined in this study were collected 5 d after Pi withdrawal.
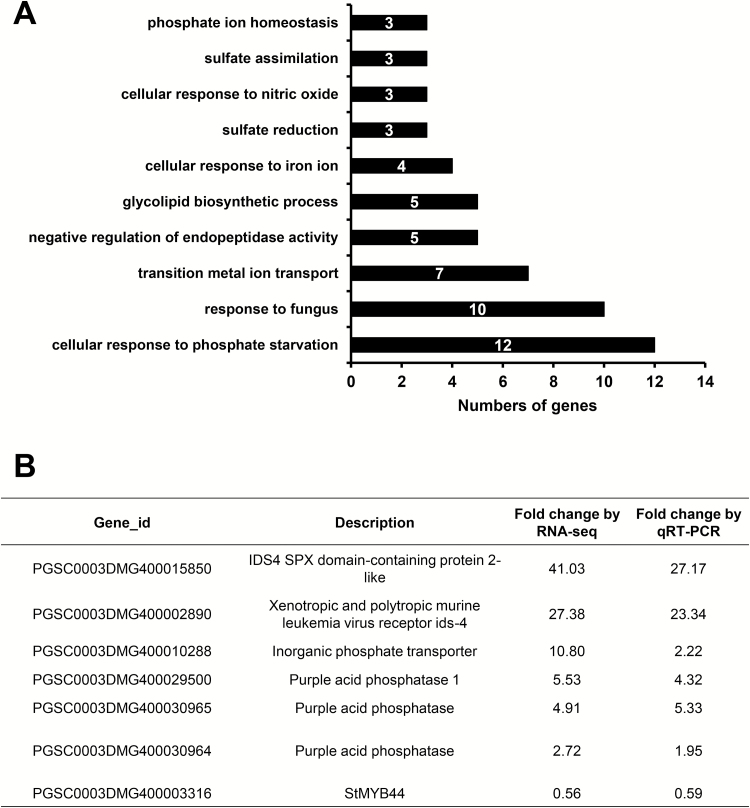
A total of 31.5 million reads were sequenced from six libraries generated from three biological repeats of Pi-deficient and sufficient samples. Statistical analysis indicated the differential expression of 359 genes upon Pi limitation, of which 221 genes were expressed at a minimum 1.6-fold higher level (see Supplementary Table S1 at JXB online). The rest of the genes were found to be reduced >1.6-fold upon Pi starvation as compared with control (Supplementary Table S2). Functional categorization of these genes revealed their involvement in diverse biological processes including cellular response to phosphate starvation and phosphate ion homeostasis (Fig. 1A). Further analysis indicated that several genes, including those encoding Inorganic Phosphate Transporter, four purple acid phosphatases, and three SPX domain-containing proteins were strongly up-regulated after Pi starvation treatment. In contrast, PHOSPHATE2 (PHO2), a gene encoding a ubiquitin-conjugating E2 enzyme mediating the degradation of Phosphate Transporter 1 (PHT1) and PHOSPHATE1 (PHO1), was observed to be dramatically suppressed in potato roots (Supplementary Table S2). These results indicated that a 5 d Pi starvation treatment had successfully triggered comprehensive molecular responses in potato.
Fig. 1.
Identification of phosphate starvation-responsive genes in potato roots using RNA-Seq. (A) Functional categories of genes differentially expressed between Pi-sufficient and Pi-deficient potato roots. (B) Verification of gene expression by qRT-PCR. qRT-PCR was carried out with two biological repeats and three technical trials.
The replacement of phospholipids in membranes with glycolipids and sulfolipids is one of the typical responses of plants to Pi starvation (Härtel et al., 2000). In this study, five genes, namely those encoding two glycosyltransferases, 1,2-diacylglycerol 3-beta-galactosyltransferase, digalactosyldiacylglycerol synthase 2 (DGD2), and riboflavin kinase/FMN adenylyltransferase, involved in the glycolipid biosynthetic process were identified. The Sulfate Transporter 3.4-encoding gene was observed to have greater abundance (17.5-fold increase) upon Pi starvation (Supplementary Table S1), suggesting an increase in S uptake or transport to meet the demand for the elevated biosynthesis of sulfolipids (Misson et al., 2005).
Previous studies have indicated that starch accumulates in response to Pi deprivation (Calderon-Vazquez et al., 2008; Hammond and White, 2008). The abundances of transcripts of starch synthase VI and two phosphofructokinase genes, involved in starch synthesis, were observed to be ~3-fold higher in Pi-depleted potato roots (Supplementary Table S1). The increased expression of these genes was also reported in Pi-deficient potato leaves (Hammond et al., 2011).
The expression of several members of gene families involved in secondary metabolism and stress responses was altered by Pi starvation, including those encoding cytochrome P450s (eight genes), peroxidases (10 genes), and nodulins (five genes) (Supplementary Tables S1, S2), consistent with previous observations in maize, Arabidopsis, and rice (Misson et al., 2005; Calderon-Vazquez et al., 2008; Secco et al., 2013).
Verification of gene expression by quantitative RT-PCR
qRT-PCR was used to verify the expression of several genes potentially involved in Pi uptake and signaling, including those encoding IDS4 SPX Domain-containing Protein 2-Like, Xenotropic and Polytropic Murine Leukemia Virus Receptor IDS-4, Inorganic Phosphate Transporter, Purple Acid Phosphatase 1, and two purple acid phosphatases. Altered expression of these selected genes was consistent with that from the RNA-Seq approach although the scale of the fold changes differed between two approaches (Fig. 1B).
Among the Pi starvation-responsive genes, a number of targets, including seven up-regulated and nine down-regulated transcription factors, with potential signaling functions in response to Pi starvation were identified (Supplementary Table S3). StMYB44 (PGSC0003DMG400003316), a potato homolog to AtMYB44 and a member of the important MYB family subgroup 22, was down-regulated in roots by Pi starvation, as shown by both RNA-Seq and qRT-PCR (Fig. 1B). This gene was selected for a more comprehensive analysis of its involvement in regulation of Pi starvation responses.
Isolation and structure analysis of potato StMYB44
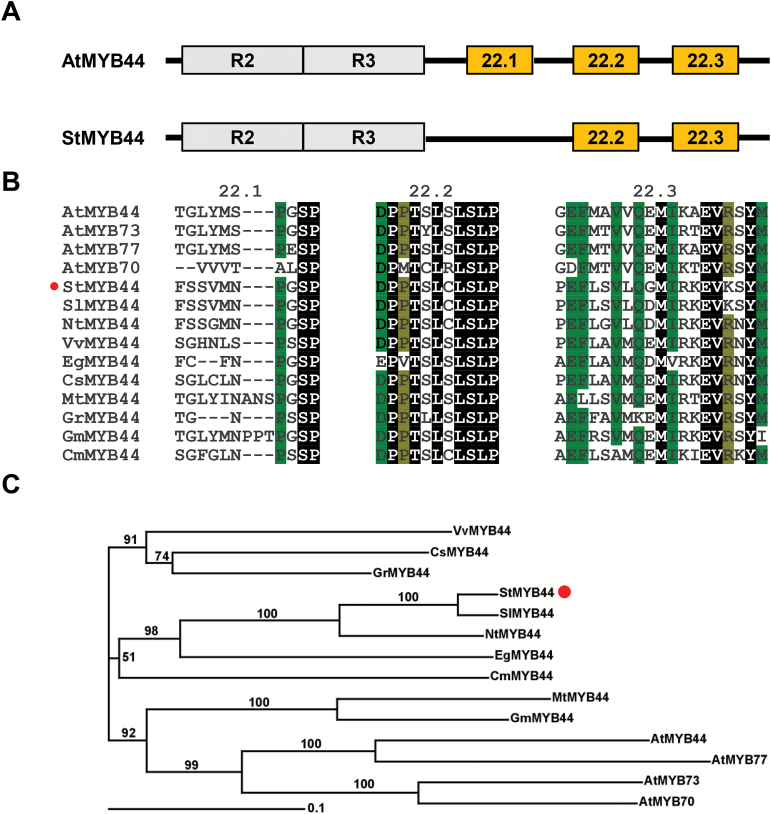
The ORF of StMYB44 was isolated from potato cultivar Désirée by PCR. Sequencing analysis showed that the 963 bp long ORF encoded a protein of 320 amino acid residues with a predicted molecular mass of 35.02 kDa and an isoelectric point of 9.24 by using Compute pI/Mw software online (http://web.expasy.org/compute_pi/). The deduced protein shared 49% sequence identity with MYB44 in Arabidopsis. AtMYB44 belongs to R2R3-MYB subgroup 22 carrying R2 and R3 MYB repeat domains (Fig. 2A; Supplementary Fig. S1). Most members of this group in Arabidopsis contained the conserved motifs 22.1 (TGLYMSPxSP) and 22.3 (GxFMxVVQEMIxxEVRSYM) (Stracke et al., 2001). Further analysis indicated that another conserved motif, 22.2, (D/EPP/MTxLSLP) is present between motifs 22.1 and 22.3 among the members of this group in Arabidopsis. StMYB44 carried the 22.2 and 22.3 motifs but lacked the 22.1 motif (Fig. 2A, B), indicating that it could have different physiological roles from its homologs in Arabidopsis. In addition, phylogenetic analysis showed that StMYB44 and its orthologs from tomato and tobacco form one clade with high bootstrap numbers (Fig. 2C), indicating that the divergence of StMYB44 occurred after the split of Solanaceae and Brassicaceae.
Fig. 2.
Isolation and analysis of StMYB44. (A) Schematic structures of StMYB44 and AtMYB44. (B) Conserved domains in MYB44 proteins. Alignment was conducted using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). (C) Phylogenetic tree analysis of StMYB44 and homologs from other plant species. The GenBank accession numbers for the amino acid sequences are XP_006367421 for Solanum tuberosum MYB44, XP_004238123 for Solanum lycopersicum MYB44, NP_001311792 for Nicotiana tabacum MYB44, AT5G67300 for Arabidopsis thaliana MYB44, AT4G37260 for Arabidopsis thaliana MYB73, AT3G50060 for Arabidopsis thaliana MYB77, AT2G23290 for Arabidopsis thaliana MYB70, XP_002285015 for Vitis vinifera MYB44, XP_012851720 for Erythranth eguttata MYB44, NP_001275798 for Citrus sinensis MYB44, XP_003611666 for Medicago truncatula MYB44, XP_012451049 for Gossypium raimondii MYB44, NP_001238087 for Glycine max MYB44 (previously named MYB50), and NP_001315374 for Cucumis melo MYB44.
Expression and subcellular localization of StMYB44
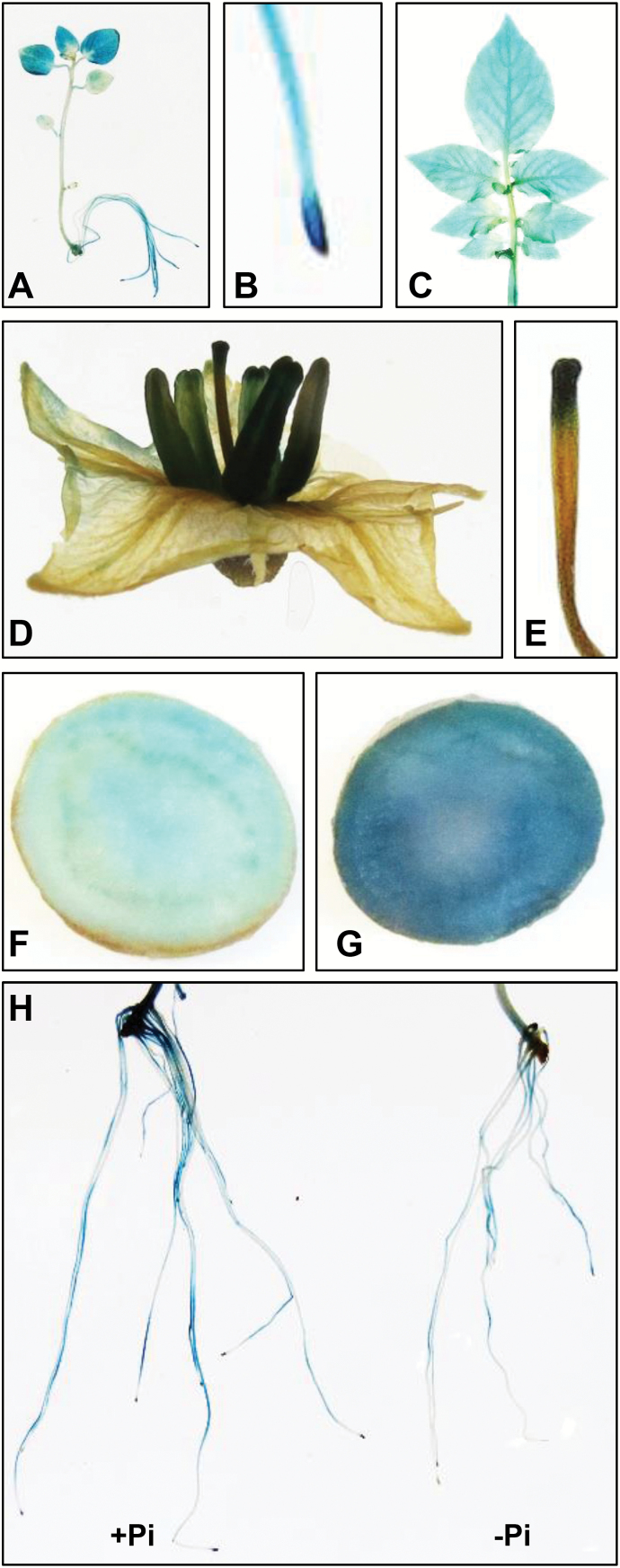
To examine the tissue-specific expression patterns of StMYB44, a 1.5 kb fragment upstream of the start codon was fused to the GUS reporter gene, and transformed into potato. GUS assay showed StMYB44 expression in almost all potato tissues, including young seedlings, roots, mature leaves, flowers, and tubers, although the expression in young leaves, root tips, stigma, and anthers was stronger than that in other tissues (Fig. 3A–G). Examination of GUS activity in ProStMYB44:GUS seedlings grown in either Pi-sufficient or deficient conditions showed reduced staining in roots responding to Pi deficiency, further confirming the results of RNA-Seq and qRT-PCR (Fig. 3H).
Fig. 3.
Tissue-specific expression pattern of StMYB44 in Désirée. GUS staining of transgenic potato carrying the ProStMYB44:GUS transgene. Tissues or organs at different stages included 2-week-old seedling (A), root tip from young seedling (B), mature leaf (C), flower (D), pistil (E), tuber stained for 3 h (F), and tuber stained for 6 h (G). (H) GUS activity in ProStMYB44:GUS transgenic seedlings grown under Pi-sufficient and Pi deficient conditions.
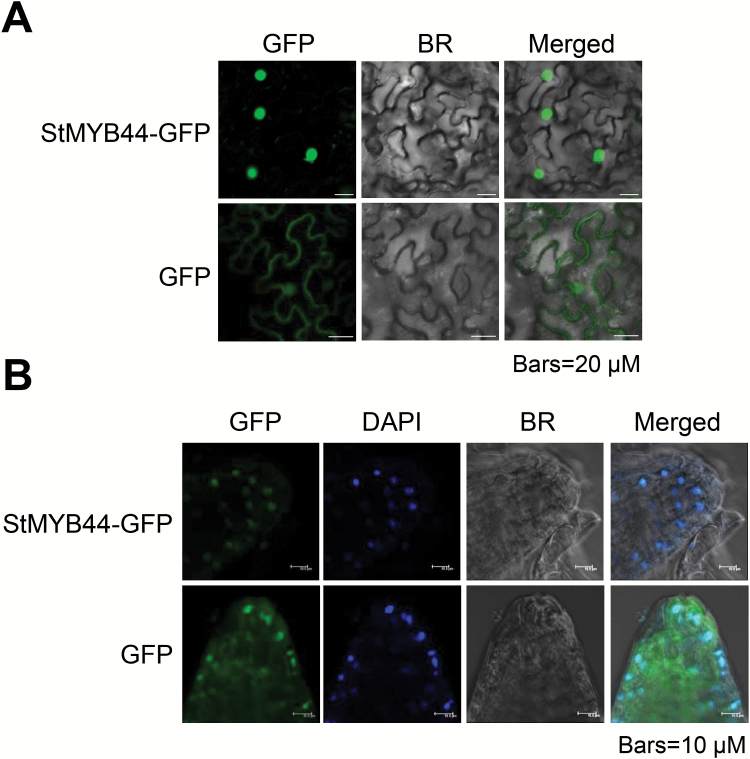
To determine the subcellular localization of StMYB44, a StMYB44:GFP fusion protein was expressed in tobacco leaves by agroinfiltration. Confocal microscopic analysis showed the exclusive accumulation of StMYB44:GFP in the nucleus, whereas only GFP protein driven by the same CaMV 35S promoter was found in the cytosol and nucleus (Fig. 4A).
Fig. 4.
Subcellular localization of StMYB44. (A) Subcellular localization of StMYB44 in tobacco leaves. Agrobacterium carrying the 35S:StMYB44:GFP and 35S:GFP genes was infiltrated into N. benthamiana leaves. Images were taken 2 d after agroinfiltration by confocal microscopy. Scale bars=20 µm. (B) Subcellular localization of StMYB44 in Arabidopsis roots. Roots from 6-day-old transgenic Arabidopsis expressing 35S:StMYB44:GFP and 35S:GFP were stained with DAPI for 10 min. GFP and DAPI fluorescence signals were observed by confocal microscopy. Scale bars=10 µm.
In addition, roots of 6-day-old seedlings of two stable transgenic Arabidopsis lines expressing 35S:StMYB44:GFP were stained with DAPI, a reagent specifically staining the nucleus. The overlap of the GFP and DAPI signals verified the nuclear localization of the StMYB44 protein, consistent with its function and the transient localization studied in tobacco leaf. As a control, GFP was detected in both the cytosol and nucleus (Fig. 4B).
Overexpression of StMYB44 results in low Pi accumulation in potato shoots
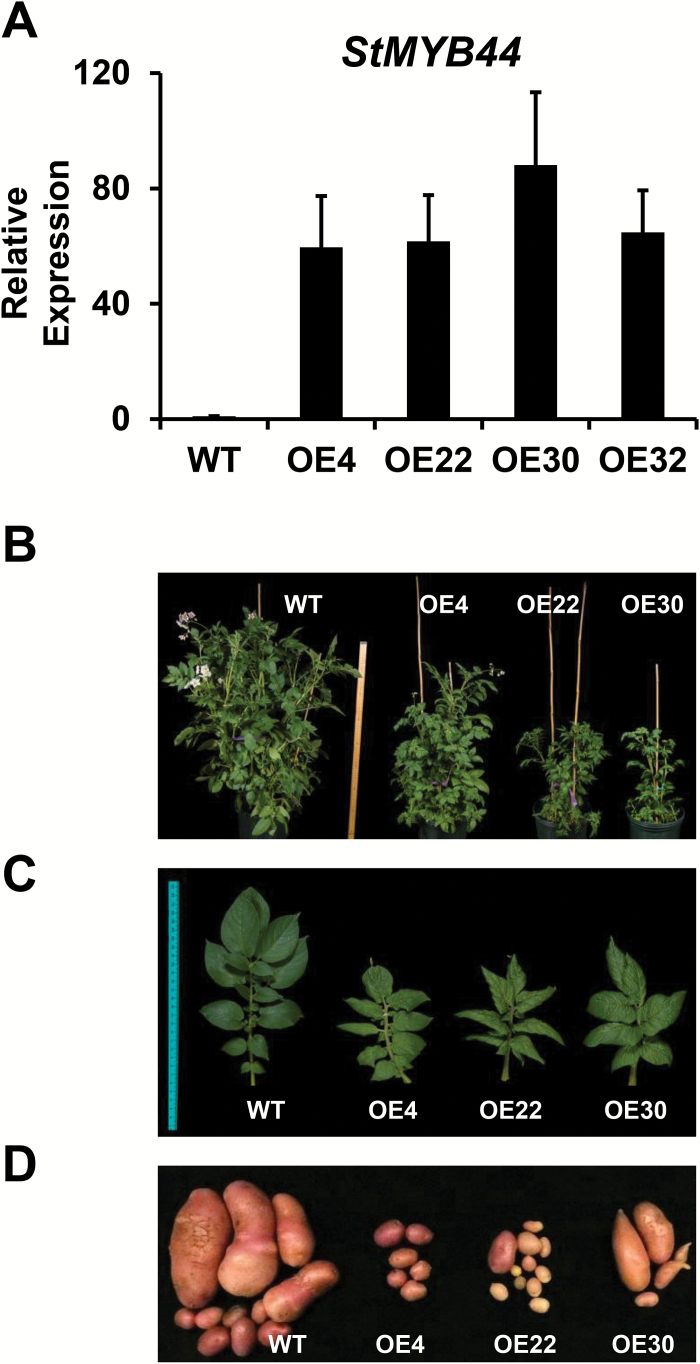
In order to reveal the physiological function of StMYB44, four transgenic potato lines with the highest expression level among 35 independent transgenic lines overexpressing StMYB44:GFP driven by a double 35S promoter were analyzed further (Fig. 5A). Under regular growth conditions in a greenhouse, the StMYB44 overexpression lines were dwarf with small and curly leaves, and produced smaller and fewer tubers, indicating that elevated expression of StMYB44 affected potato development and tuber yield (Fig. 5B–D).
Fig. 5.
Overexpression of StMYB44 in Désirée. (A) Expression of StMYB44 in the independent transgenic potato plants by qRT-PCR analysis. Actin (XM_006350963) was used as an internal control to normalize the expression of the transgene. (B) Images of transgenic potato grown in soil. Leaves (C) and tubers (D) of transgenic and wild-type (WT) potato.
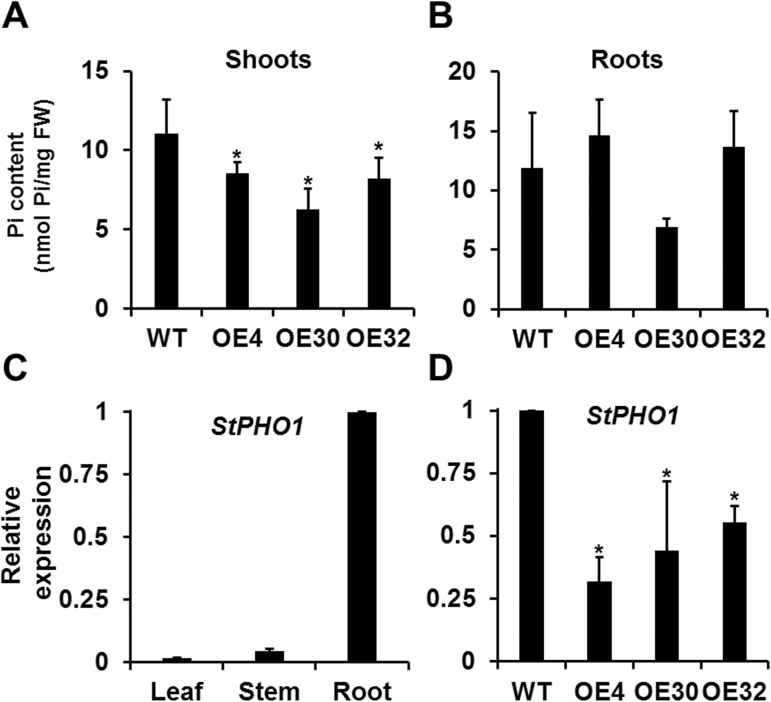
Pi levels were measured in 14-day-old wild-type and transgenic potato seedlings grown on Pi-sufficient medium (MS medium containing 1.25 mM Pi KH2PO4). The shoot Pi contents of transgenic plants ranged from 6.25 nmol mg–1 FW to 8.54 nmol mg–1 FW and that of the wild type was 11.02 nmol mg–1 FW (Fig. 6A), while no significant difference was detected between the roots of wild-type and transgenic potato plants (Fig. 6B), indicating the negative effect of StMYB44:GFP overexpression on translocation of Pi from roots to shoots.
Fig. 6.
StMYB44 overexpression leads to reduced Pi levels in shoots and down-regulation of StPHO1 in roots. (A) Pi levels in transgenic potato shoots. (B) Pi content in transgenic roots. (C) Tissue-specific expression of StPHO1. (D) Expression of StPHO1 in transgenic potato roots. Shoots and roots of wild-type (WT) and individual transgenic potato seedlings were collected for Pi content determination and gene expression analysis. The data represent means from three biological replicates. Error bars=SD. Student’s t-test was used to examine statistical significance. * indicates P<0.05.
In order to dissect the downstream genes controlled by StMYB44, we compared global gene expression profiles between two independent transgenic lines (OE22 and OE30) and wild-type seedlings by RNA-Seq analysis. A total of 80.2 million reads were obtained from nine libraries generated from three biological repeats of wild-type and two transgenic lines. Of the 174 differentially expressed genes, 52 and 122 were discovered to be up-regulated and down-regulated, respectively, over 4-fold (Tables 1, 2). The RNA-Seq analysis showed that the averaged expression of StMYB44 in the two chosen transgenic lines was increased by 44.3-fold, which is consistent with the qRT-PCR analysis of StMYB44-overexpressing lines, demonstrating the authenticity of RNA-Seq in identification of the differentially expressed genes in this study. Among the up-regulated genes in the StMYB44-overexpressing lines, Purple Acid Phosphatase 3 (PAP3; PGSC0003DMG403007838), a gene involved in the release of the phosphate from phosphate ester under phosphate starvation conditions (Bozzo et al., 2002; Y. Zhang et al., 2014), was identified. At this point, it remained unclear whether the enhanced expression of PAP3 was caused directly by the overexpression of StMYB44 or a feedback response due to reduced Pi accumulation in shoots. More interestingly, the transcript abundance of potato PHOSPHATE1 (StPHO1; PGSC0003DMG400017163) was observed to be reduced in the transgenic potato. In Arabidopsis, PHO1 is responsible for loading Pi into the xylem in roots and its translocation from root to shoot; accordingly, mutation of this gene results in reduced Pi accumulation in shoot tissues (Poirier et al., 1991; Hamburger et al., 2002).
Table 1.
Up-regulated genes in StMYB44-overexpressing lines
| Gene_id | Gene | Fold change OE22/WT | P-value | Fold change OE30/WT | P-value | Average fold change |
|---|---|---|---|---|---|---|
| PGSC0003DMG400005840 | Calcineurin B | inf | 0.00005 | inf | 0.00005 | inf |
| PGSC0003DMG400031059 | Conserved gene of unknown function | inf | 0.00005 | inf | 0.00145 | inf |
| PGSC0003DMG400025420 | Transposase | inf | 0.00005 | inf | 0.00005 | inf |
| PGSC0003DMG400003316 | Tuber-specific and sucrose- responsive element-binding factor (TSF transgene) | 30.36 | 0.00005 | 64.74 | 0.00005 | 44.34 |
| PGSC0003DMG400019773 | Sesquiterpene synthase 2 | 35.14 | 0.0001 | 18.37 | 0.00005 | 25.40 |
| PGSC0003DMG400013696 | Cytochrome P450 | 22.84 | 0.00005 | 27.68 | 0.00005 | 25.14 |
| PGSC0003DMG400016180 | Flowering locus T | 15.35 | 0.00265 | 16.33 | 0.0002 | 15.83 |
| PGSC0003DMG400010050 | Proline oxidase/dehydrogenase 1 | 8.21 | 0.00005 | 30.42 | 0.00005 | 15.80 |
| PGSC0003DMG400003954 | Conserved gene of unknown function | 15.54 | 0.00265 | 15.96 | 0.00285 | 15.75 |
| PGSC0003DMG400025628 | Pyridoxal-dependent decarboxylase, C-terminal sheet domain-containing protein | 11.64 | 0.00005 | 18.77 | 0.00005 | 14.78 |
| PGSC0003DMG400007796 | DNA-directed RNA polymerase II largest subunit | 12.22 | 0.00005 | 17.12 | 0.00005 | 14.46 |
| PGSC0003DMG400000957 | ATP-binding protein | 15.04 | 0.00005 | 13.70 | 0.00005 | 14.35 |
| PGSC0003DMG400014086 | Gene of unknown function | 22.40 | 0.0001 | 8.84 | 0.0009 | 14.07 |
| PGSC0003DMG400024452 | Pyridoxal-dependent decarboxylase, C-terminal sheet domain-containing protein | 11.26 | 0.00005 | 17.14 | 0.00005 | 13.89 |
| PGSC0003DMG400023230 | 2-Isopropylmalate synthase A | 9.54 | 0.00005 | 15.91 | 0.00005 | 12.32 |
| PGSC0003DMG400000776 | Extensin (ext) | 14.14 | 0.00005 | 8.73 | 0.00005 | 11.11 |
| PGSC0003DMG400002046 | Aspartic proteinase nepenthesin-1 | 9.29 | 0.00685 | 13.09 | 0.0005 | 11.03 |
| PGSC0003DMG400005670 | MAEWEST protein | 8.62 | 0.00075 | 13.18 | 0.00385 | 10.66 |
| PGSC0003DMG400024113 | Gene of unknown function | 11.46 | 0.0007 | 8.85 | 0.0008 | 10.07 |
| PGSC0003DMG400024602 | Conserved gene of unknown function | 8.44 | 0.0051 | 11.99 | 0.0052 | 10.06 |
| PGSC0003DMG400019274 | Indole-3-acetic acid-amido synthetase GH3.6 | 8.17 | 0.00005 | 8.93 | 0.00005 | 8.54 |
| PGSC0003DMG400031850 | 2-Hydroxyisoflavanone dehydratase | 6.69 | 0.00345 | 10.20 | 0.0068 | 8.26 |
| PGSC0003DMG400031437 | Neryl diphosphate synthase 1 | 5.78 | 0.00005 | 9.51 | 0.00005 | 7.42 |
| PGSC0003DMG400028593 | Histidine-containing phosphotransfer protein | 5.08 | 0.0048 | 9.91 | 0.00075 | 7.10 |
| PGSC0003DMG400006319 | Beta-glucosidase 01 | 4.51 | 0.00005 | 10.93 | 0.00005 | 7.02 |
| PGSC0003DMG400015173 | ATP-binding protein | 10.03 | 0.00005 | 4.87 | 0.00005 | 6.99 |
| PGSC0003DMG400015005 | Heavy metal-associated domain- containing protein | 4.37 | 0.0074 | 11.11 | 0.0005 | 6.97 |
| PGSC0003DMG402002024 | Zinc finger protein | 5.75 | 0.00005 | 8.29 | 0.00005 | 6.91 |
| PGSC0003DMG400006448 | Caffeoyl-CoA O-methyltransferase | 4.32 | 0.00005 | 10.85 | 0.00005 | 6.84 |
| PGSC0003DMG400016722 | Glutathione S-transferase | 4.21 | 0.00875 | 10.95 | 0.00005 | 6.79 |
| PGSC0003DMG400018579 | Histidine phosphotransfer protein | 5.52 | 0.00005 | 8.22 | 0.00005 | 6.74 |
| PGSC0003DMG400019293 | NAC domain-containing protein | 7.66 | 0.00145 | 5.65 | 0.00095 | 6.58 |
| PGSC0003DMG400023112 | Kinesin | 5.49 | 0.0009 | 6.93 | 0.0004 | 6.17 |
| PGSC0003DMG400010713 | Salt-responsive protein 2 | 8.54 | 0.00005 | 4.27 | 0.00005 | 6.04 |
| PGSC0003DMG400002899 | AP2/ERF domain-containing transcription factor | 7.65 | 0.0001 | 4.76 | 0.0019 | 6.03 |
| PGSC0003DMG400000493 | Carbonic anhydrase | 6.90 | 0.00005 | 5.22 | 0.00005 | 6.00 |
| PGSC0003DMG400020156 | Pectase lyase | 5.96 | 0.00005 | 5.89 | 0.00005 | 5.92 |
| PGSC0003DMG400011226 | Sodium/potassium/calcium exchanger 6 | 4.66 | 0.00005 | 7.41 | 0.00005 | 5.87 |
| PGSC0003DMG403007838 | Purple acid phosphatase 3 | 4.37 | 0.00055 | 7.87 | 0.00005 | 5.87 |
| PGSC0003DMG400003084 | Two-component response regulator ARR8 | 7.72 | 0.00005 | 4.29 | 0.00005 | 5.76 |
| PGSC0003DMG400024593 | Glycosyltransferase UGT90A7 | 6.44 | 0.00005 | 5.04 | 0.00005 | 5.70 |
| PGSC0003DMG400000730 | Transcription factor | 4.66 | 0.00005 | 6.56 | 0.00005 | 5.53 |
| PGSC0003DMG400009268 | Proteinase inhibitor | 4.07 | 0.00005 | 7.15 | 0.00005 | 5.39 |
| PGSC0003DMG400017189 | Desacetoxyvindoline 4-hydroxylase | 5.23 | 0.00035 | 5.11 | 0.00005 | 5.17 |
| PGSC0003DMG400028229 | Calcium-dependent protein kinase CDPK12 | 4.85 | 0.00005 | 5.12 | 0.00005 | 4.98 |
| PGSC0003DMG400002520 | Zinc finger protein | 4.06 | 0.00005 | 5.48 | 0.00005 | 4.72 |
| PGSC0003DMG400012977 | VQ motif-containing protein | 4.80 | 0.0067 | 4.61 | 0.0053 | 4.70 |
| PGSC0003DMG400002519 | Zinc finger protein | 4.34 | 0.00005 | 4.88 | 0.00005 | 4.61 |
| PGSC0003DMG400027212 | ATP:citrate lyase | 4.22 | 0.00015 | 4.86 | 0.00005 | 4.53 |
| PGSC0003DMG400032780 | Conserved gene of unknown function | 4.68 | 0.00005 | 4.22 | 0.00005 | 4.44 |
| PGSC0003DMG400026023 | Nuc-1 negative regulatory protein preg | 4.11 | 0.00105 | 4.73 | 0.0001 | 4.41 |
| PGSC0003DMG400025479 | PHAP2A protein | 4.02 | 0.00005 | 4.51 | 0.00005 | 4.26 |
Table 2.
Down-regulated genes in StMYB44-overexpressing lines
| Gene_id | Gene | Fold change OE22/WT | P-value | Fold change OE30/WT | P-value | Average fold change |
|---|---|---|---|---|---|---|
| PGSC0003DMG400000207 | Arabinogalactan peptide 16 | 0.00 | 0.0001 | 0.00 | 0.00005 | 0.00 |
| PGSC0003DMG400019040 | Gene of unknown function | 0.00 | 0.0001 | 0.00 | 0.00005 | 0.00 |
| PGSC0003DMG400020686 | Gene of unknown function | 0.00 | 0.00005 | 0.00 | 0.00005 | 0.00 |
| PGSC0003DMG400014767 | CND41, chloroplast nucleoid DNA- binding protein | 0.02 | 0.00265 | 0.02 | 0.00265 | 0.02 |
| PGSC0003DMG400011740 | SGA rhamnose:beta-solanine/beta- chaconine rhamnosyltransferase | 0.02 | 0.00005 | 0.02 | 0.00005 | 0.02 |
| PGSC0003DMG400004143 | SF16 protein | 0.03 | 0.00265 | 0.03 | 0.00005 | 0.03 |
| PGSC0003DMG400011334 | Phylloplanin | 0.06 | 0.00005 | 0.02 | 0.00265 | 0.04 |
| PGSC0003DMG400020677 | Conserved gene of unknown function | 0.05 | 0.0028 | 0.04 | 0.00265 | 0.04 |
| PGSC0003DMG400024770 | Conserved gene of unknown function | 0.04 | 0.00005 | 0.04 | 0.00005 | 0.04 |
| PGSC0003DMG400014104 | Patatin-2-Kuras 4 | 0.05 | 0.00005 | 0.05 | 0.00005 | 0.05 |
| PGSC0003DMG400023922 | Cytoplasmic small heat shock protein class I | 0.11 | 0.00005 | 0.03 | 0.00005 | 0.05 |
| PGSC0003DMG400030957 | Cysteine proteinase | 0.05 | 0.00265 | 0.06 | 0.00015 | 0.05 |
| PGSC0003DMG400000123 | Calcium-transporting ATPase, endoplasmic reticulum-type | 0.07 | 0.00005 | 0.06 | 0.00005 | 0.06 |
| PGSC0003DMG400006782 | Conserved gene of unknown function | 0.09 | 0.00005 | 0.05 | 0.00005 | 0.07 |
| PGSC0003DMG400000984 | 3-Oxo-5-alpha-steroid 4-dehydrogenase family protein | 0.05 | 0.0028 | 0.10 | 0.0068 | 0.07 |
| PGSC0003DMG400039214 | Arachidonic acid-induced DEA1 | 0.04 | 0.00005 | 0.14 | 0.00005 | 0.08 |
| PGSC0003DMG400010048 | Conserved gene of unknown function | 0.08 | 0.00005 | 0.08 | 0.00005 | 0.08 |
| PGSC0003DMG400011749 | UDP-galactose:solanidine galactosyltransferase | 0.05 | 0.00005 | 0.14 | 0.00005 | 0.08 |
| PGSC0003DMG402017090 | Patatin-04/09 | 0.08 | 0.00005 | 0.08 | 0.00005 | 0.08 |
| PGSC0003DMG400010067 | DNA-binding protein | 0.10 | 0.0004 | 0.07 | 0.00015 | 0.08 |
| PGSC0003DMG400011750 | Cytochrome P-450 | 0.07 | 0.00005 | 0.10 | 0.00005 | 0.08 |
| PGSC0003DMG400016458 | Multi-antimicrobial extrusion family protein | 0.06 | 0.0002 | 0.12 | 0.00005 | 0.09 |
| PGSC0003DMG400011752 | Cellulose synthase | 0.06 | 0.00005 | 0.12 | 0.00005 | 0.09 |
| PGSC0003DMG400029503 | ETAG-A3 | 0.09 | 0.00005 | 0.08 | 0.00005 | 0.09 |
| PGSC0003DMG400026404 | Fragment | 0.09 | 0.00005 | 0.08 | 0.00005 | 0.09 |
| PGSC0003DMG400000048 | Cysteine synthase | 0.08 | 0.00005 | 0.10 | 0.00005 | 0.09 |
| PGSC0003DMG400024983 | Tuber-specific and sucrose- responsive element-binding factor | 0.12 | 0.00005 | 0.08 | 0.00005 | 0.10 |
| PGSC0003DMG402008890 | Aldo-keto reductase family 4 member C10 | 0.13 | 0.00005 | 0.07 | 0.00005 | 0.10 |
| PGSC0003DMG400011751 | 2-Oxoglutarate-dependent dioxygenase | 0.09 | 0.00005 | 0.10 | 0.00005 | 0.10 |
| PGSC0003DMG400004616 | Invertase inhibitor | 0.12 | 0.0077 | 0.08 | 0.00315 | 0.10 |
| PGSC0003DMG400009033 | Myb 12 transcription factor | 0.07 | 0.00005 | 0.13 | 0.00005 | 0.10 |
| PGSC0003DMG400002495 | C2H2L domain class transcription factor | 0.11 | 0.00005 | 0.09 | 0.00005 | 0.10 |
| PGSC0003DMG400021142 | DWARF1/DIMINUTO | 0.10 | 0.00005 | 0.11 | 0.00005 | 0.10 |
| PGSC0003DMG400022933 | Auxin-induced beta-glucosidase | 0.07 | 0.00005 | 0.14 | 0.00005 | 0.10 |
| PGSC0003DMG400012797 | Short-chain dehydrogenase/ reductase family protein | 0.11 | 0.00005 | 0.10 | 0.00005 | 0.10 |
| PGSC0003DMG400018930 | Proteinase inhibitor I4, serpin | 0.10 | 0.0053 | 0.11 | 0.0007 | 0.10 |
| PGSC0003DMG400002028 | Cytoplasmic small heat shock protein class I | 0.15 | 0.00015 | 0.07 | 0.003 | 0.10 |
| PGSC0003DMG400031792 | Endo-1,4-beta-glucanase | 0.11 | 0.00005 | 0.10 | 0.00005 | 0.11 |
| PGSC0003DMG400003411 | DNA-damage-inducible protein f | 0.15 | 0.00005 | 0.08 | 0.00005 | 0.11 |
| PGSC0003DMG400011350 | OrfB protein | 0.10 | 0.00005 | 0.11 | 0.00005 | 0.11 |
| PGSC0003DMG400012763 | C-4 sterol methyl oxidase | 0.10 | 0.00005 | 0.12 | 0.00005 | 0.11 |
| PGSC0003DMG400014339 | Remorin | 0.13 | 0.00005 | 0.09 | 0.00005 | 0.11 |
| PGSC0003DMG400032817 | Squamosa promoter binding | 0.16 | 0.00005 | 0.07 | 0.00285 | 0.11 |
| PGSC0003DMG400017505 | Nam 11 | 0.13 | 0.00135 | 0.09 | 0.00435 | 0.11 |
| PGSC0003DMG400012183 | Endo-1,4-beta-glucanase | 0.11 | 0.00605 | 0.12 | 0.0008 | 0.11 |
| PGSC0003DMG401019681 | Serine-threonine protein kinase, plant-type | 0.10 | 0.00345 | 0.12 | 0.00005 | 0.11 |
| PGSC0003DMG400010215 | Cysteine protease | 0.08 | 0.00005 | 0.15 | 0.00005 | 0.11 |
| PGSC0003DMG400020777 | Gene of unknown function | 0.13 | 0.00005 | 0.10 | 0.00005 | 0.11 |
| PGSC0003DMG400014347 | PAR-1c protein | 0.19 | 0.00005 | 0.07 | 0.00005 | 0.11 |
| PGSC0003DMG400014543 | Monoglyceride lipase | 0.10 | 0.00005 | 0.13 | 0.00005 | 0.12 |
| PGSC0003DMG400023419 | Receptor kinase THESEUS 1 | 0.13 | 0.00015 | 0.11 | 0.0001 | 0.12 |
| PGSC0003DMG400000523 | Kinesin light chain | 0.24 | 0.00005 | 0.06 | 0.0002 | 0.12 |
| PGSC0003DMG400018140 | Cytochrome P450 71A4 | 0.14 | 0.0084 | 0.11 | 0.0048 | 0.12 |
| PGSC0003DMG400021814 | Conserved gene of unknown function | 0.18 | 0.0004 | 0.09 | 0.00395 | 0.12 |
| PGSC0003DMG400007552 | Conserved gene of unknown function | 0.10 | 0.00005 | 0.15 | 0.00005 | 0.13 |
| PGSC0003DMG400001544 | Conserved gene of unknown function | 0.12 | 0.0008 | 0.14 | 0.00005 | 0.13 |
| PGSC0003DMG400024362 | Anthranilate N-benzoyltransferase protein | 0.24 | 0.00005 | 0.07 | 0.00005 | 0.13 |
| PGSC0003DMG400015230 | Pectate lyase | 0.18 | 0.00005 | 0.09 | 0.00005 | 0.13 |
| PGSC0003DMG401028252 | Beta-fructofuranosidase | 0.15 | 0.00005 | 0.11 | 0.00005 | 0.13 |
| PGSC0003DMG400000719 | Sec14 cytosolic factor | 0.15 | 0.00005 | 0.12 | 0.00005 | 0.13 |
| PGSC0003DMG400005526 | Cytochrome P450 | 0.21 | 0.00005 | 0.08 | 0.00005 | 0.13 |
| PGSC0003DMG400005734 | FK506-binding protein | 0.21 | 0.00005 | 0.08 | 0.00005 | 0.13 |
| PGSC0003DMG400031763 | Conserved gene of unknown function | 0.12 | 0.00005 | 0.16 | 0.00005 | 0.14 |
| PGSC0003DMG400028622 | Acyl-protein thioesterase | 0.10 | 0.00005 | 0.18 | 0.00005 | 0.14 |
| PGSC0003DMG400019429 | Conserved gene of unknown function | 0.16 | 0.00005 | 0.12 | 0.0001 | 0.14 |
| PGSC0003DMG400030784 | Glutaredoxin family protein | 0.17 | 0.0004 | 0.11 | 0.0007 | 0.14 |
| PGSC0003DMG400012147 | Conserved gene of unknown function | 0.19 | 0.00015 | 0.10 | 0.00705 | 0.14 |
| PGSC0003DMG400006221 | Conserved gene of unknown function | 0.15 | 0.00005 | 0.14 | 0.00005 | 0.14 |
| PGSC0003DMG400001598 | Snakin-2 | 0.14 | 0.00005 | 0.15 | 0.00005 | 0.15 |
| PGSC0003DMG400027047 | UPF0497 membrane protein | 0.14 | 0.00005 | 0.15 | 0.00005 | 0.15 |
| PGSC0003DMG402003937 | P69E protein | 0.18 | 0.00395 | 0.12 | 0.00735 | 0.15 |
| PGSC0003DMG401031196 | WRKY transcription factor 16 | 0.20 | 0.00005 | 0.11 | 0.00005 | 0.15 |
| PGSC0003DMG400005633 | Conserved gene of unknown function | 0.22 | 0.00005 | 0.10 | 0.00005 | 0.15 |
| PGSC0003DMG400007621 | GAST1 protein | 0.18 | 0.00045 | 0.12 | 0.0009 | 0.15 |
| PGSC0003DMG400004493 | GATA domain class transcription factor | 0.24 | 0.00005 | 0.09 | 0.00055 | 0.15 |
| PGSC0003DMG400027937 | Conserved gene of unknown function | 0.19 | 0.00105 | 0.12 | 0.0009 | 0.15 |
| PGSC0003DMG400003848 | Sugar transporter | 0.19 | 0.00005 | 0.12 | 0.00005 | 0.15 |
| PGSC0003DMG400004009 | Phospholipase C | 0.17 | 0.00005 | 0.14 | 0.00005 | 0.15 |
| PGSC0003DMG400029937 | ZIP family metal transporter | 0.16 | 0.00005 | 0.14 | 0.00005 | 0.15 |
| PGSC0003DMG402012192 | Zinc finger protein | 0.11 | 0.00005 | 0.23 | 0.00005 | 0.16 |
| PGSC0003DMG400003626 | Lactoylglutathione lyase | 0.16 | 0.00005 | 0.16 | 0.00005 | 0.16 |
| PGSC0003DMG400018565 | Alcohol dehydrogenase | 0.13 | 0.00005 | 0.21 | 0.00005 | 0.16 |
| PGSC0003DMG400013828 | Vacoular processing enzyme 1 | 0.21 | 0.00005 | 0.13 | 0.00005 | 0.16 |
| PGSC0003DMG400025896 | Proteinase inhibitor 1 | 0.18 | 0.00005 | 0.16 | 0.00005 | 0.16 |
| PGSC0003DMG400002156 | C-4 sterol methyl oxidase 2 | 0.23 | 0.00005 | 0.12 | 0.00005 | 0.17 |
| PGSC0003DMG400018611 | Glycosyl transferase family 17 protein | 0.22 | 0.0001 | 0.13 | 0.00125 | 0.17 |
| PGSC0003DMG402008895 | Tropinone reductase 1 | 0.21 | 0.00005 | 0.14 | 0.00005 | 0.17 |
| PGSC0003DMG400010223 | Phytophthora-inhibited protease 1 | 0.24 | 0.00005 | 0.12 | 0.00005 | 0.17 |
| PGSC0003DMG400020084 | Zinc finger protein | 0.24 | 0.00005 | 0.12 | 0.00015 | 0.17 |
| PGSC0003DMG402000097 | Conserved gene of unknown function | 0.22 | 0.00205 | 0.14 | 0.00135 | 0.17 |
| PGSC0003DMG400028295 | Gene of unknown function | 0.21 | 0.00005 | 0.14 | 0.00005 | 0.17 |
| PGSC0003DMG400016977 | Diphosphoinositol polyphosphate phosphohydrolase | 0.23 | 0.0005 | 0.13 | 0.0013 | 0.17 |
| PGSC0003DMG401019343 | DNA-binding protein | 0.18 | 0.00005 | 0.17 | 0.00005 | 0.18 |
| PGSC0003DMG400014027 | Germin | 0.21 | 0.00005 | 0.14 | 0.00005 | 0.18 |
| PGSC0003DMG400014173 | Polyphosphoinositide-binding protein | 0.18 | 0.00005 | 0.18 | 0.00005 | 0.18 |
| PGSC0003DMG400017398 | Snf1-kinase beta subunit, plants | 0.18 | 0.00005 | 0.18 | 0.00005 | 0.18 |
| PGSC0003DMG400014894 | Membrane protein | 0.20 | 0.0035 | 0.16 | 0.002 | 0.18 |
| PGSC0003DMG400000715 | Conserved gene of unknown function | 0.18 | 0.0001 | 0.18 | 0.00005 | 0.18 |
| PGSC0003DMG400017163 | Xenotropic and polytropic murine leukemia virus receptor pho1 | 0.24 | 0.00005 | 0.14 | 0.00005 | 0.18 |
| PGSC0003DMG400016651 | Transcription factor RF2b | 0.23 | 0.00005 | 0.15 | 0.00005 | 0.18 |
| PGSC0003DMG400005035 | ARF GAP-like zinc finger-containing protein ZIGA3 | 0.25 | 0.00005 | 0.14 | 0.00005 | 0.19 |
| PGSC0003DMG400001529 | Acidic 27 kDa endochitinase | 0.23 | 0.00005 | 0.15 | 0.00005 | 0.19 |
| PGSC0003DMG400021603 | Hydroxyproline-rich glycoprotein (HRGP)EEYAN | 0.16 | 0.00005 | 0.22 | 0.00005 | 0.19 |
| PGSC0003DMG402027687 | Wound-inducible carboxypeptidase | 0.19 | 0.00005 | 0.19 | 0.00005 | 0.19 |
| PGSC0003DMG400009892 | Prolyl endopeptidase | 0.22 | 0.00005 | 0.16 | 0.00005 | 0.19 |
| PGSC0003DMG400015726 | Glutathione S-transferase | 0.17 | 0.0004 | 0.22 | 0.00005 | 0.19 |
| PGSC0003DMG400030172 | Aspartic proteinase oryzasin-1 | 0.20 | 0.00005 | 0.19 | 0.00005 | 0.20 |
| PGSC0003DMG400029620 | Chalcone synthase 1B | 0.19 | 0.00005 | 0.21 | 0.00005 | 0.20 |
| PGSC0003DMG400032182 | Non-specific lipid-transfer protein | 0.20 | 0.00465 | 0.20 | 0.0019 | 0.20 |
| PGSC0003DMG400007018 | 3-Phosphoshikimate 1-carboxyvinyltransferase, chloroplastic | 0.21 | 0.00005 | 0.19 | 0.00005 | 0.20 |
| PGSC0003DMG400015169 | Esterase | 0.21 | 0.00005 | 0.20 | 0.00005 | 0.21 |
| PGSC0003DMG400014093 | Flavonol synthase | 0.20 | 0.00005 | 0.22 | 0.00005 | 0.21 |
| PGSC0003DMG400021423 | Homeodomain leucine-zipper 1 | 0.23 | 0.00005 | 0.19 | 0.00005 | 0.21 |
| PGSC0003DMG400005470 | Rab GTPase activator | 0.24 | 0.00005 | 0.20 | 0.00005 | 0.22 |
| PGSC0003DMG400019110 | Chalcone synthase 2 | 0.24 | 0.00005 | 0.20 | 0.00005 | 0.22 |
| PGSC0003DMG400009959 | Ornithine decarboxylase | 0.22 | 0.00005 | 0.22 | 0.00005 | 0.22 |
| PGSC0003DMG402024767 | Pectinesterase | 0.22 | 0.00005 | 0.23 | 0.00005 | 0.22 |
| PGSC0003DMG400011502 | PEP carboxylase kinase | 0.23 | 0.00005 | 0.22 | 0.00005 | 0.23 |
| PGSC0003DMG400010034 | Photoreceptor-interacting protein | 0.22 | 0.00005 | 0.25 | 0.00005 | 0.23 |
| PGSC0003DMG400022459 | BY-2 kinesin 5 | 0.22 | 0.00005 | 0.24 | 0.00005 | 0.23 |
| PGSC0003DMG400006185 | Skp1 1 | 0.25 | 0.00005 | 0.22 | 0.00005 | 0.23 |
| PGSC0003DMG400020253 | Ribonucleoside-diphosphate reductase small chain | 0.22 | 0.00005 | 0.24 | 0.00005 | 0.23 |
Potato StPHO1, sharing 67% amino acid identity with the Arabidopsis PHO1 (Supplementary Fig. S2), is predominantly expressed in potato roots (Fig. 6C), similar to the expression pattern of PHO1 in Arabidopsis (Hamburger et al., 2002), and is expected to confer a similar Pi-translocating function in potato. qRT-PCR was used to confirm the decreased expression of StPHO1 detected by RNA-Seq, and the result showed that the expression of StPHO1 was significantly reduced in the StMYB44-overexpressing potato roots (Fig. 6D). Since PHO1 is the only identified gene with a known function related to Pi loading and translocation, the lowered Pi accumulation in the transgenic potato shoots could be attributed to the reduction of StPHO1 expression, caused by the overexpression of StMYB44, suggesting that StMYB44 negatively regulates Pi translocation from roots to shoots by specifically suppressing the expression of StPHO1.
Knock-out of StMYB44 by using the CRISPR/Cas9 system
The CRISPR/Cas9 system was employed to generate StMYB44 knock-out lines in potato (Xing et al., 2014) using the nucleotide sequence from 376 to 396 of StMYB44 mRNA as guide RNA. After Agrobacterium-mediated transformation, a total of 11 kanamycin-resistant potato lines were obtained, and PCR genotyping detected Cas9 in all transgenic lines (Supplementary Fig. S3A). A fragment of ~300 bp of StMYB44 harboring the target region was amplified by PCR and sequenced (Supplementary Fig. S3B), indicating that 9 out of 11 lines carried mutant StMYB44 alleles, with a 81.8% frequency of gene editing for this CRISPR/Cas9 system in potato. The targeted mutations ranged from 2 to 120 deleted nucleotides in all of these nine transgenic plants. In addition to deletions, insertions of nucleotide A or T were observed in four lines, C12, C17, C19, and C21, consistent with reports on other plant species (H. Zhang et al., 2014; Ma et al., 2015). Désirée is a tetraploid potato cultivar, and single nucleotide polymorphism (SNP) analysis of the cloned StMYB44 fragments indicated that it had eight alleles of StMYB44 (Supplementary Fig. S4). The sequencing results also indicated that wild-type alleles of StMYB44 were still present in C3, C12, C17, and C21, showing that not all the alleles in transgenic potato were modified. To better understand the degree of the reduced expression of StMYB44 in these lines, the expression level of wild-type StMYB44 in leaves was measured by RT-PCR. Expression of wild-type StMYB44 was hardly detected in the selected transgenic plants, while it remained high in wild-type plants (Supplementary Fig. S3C).
All StMYB44 knock-out lines displayed no visible phenotype compared with the wild type under normal conditions, except one line, C14, which grew more slowly and carried smaller leaves than the wild type. No statistically significant difference was observed in Pi content in shoots and roots between three selected StMYB44 knock-out lines and wild-type plants (Supplementary Fig. S5A, B). StPHO1 was expressed similarly in both the transgenic potato roots and wild-type roots (Supplementary Fig. S5C), implying that knock-out of StMYB44 is not enough to increase transcription of StPHO1, probably due to the presence of other negative transcription factors. Expression of StPHO1 and Pi contents were similar in C14 and the wild type (Supplementary Fig. S5), suggesting that the abnormal phenotypic change of C14 was most probably caused by an insertion in a development-related gene instead of Pi metabolism.
StMYB44 interacts with AtWRKY6 and StWRKY6 in vivo
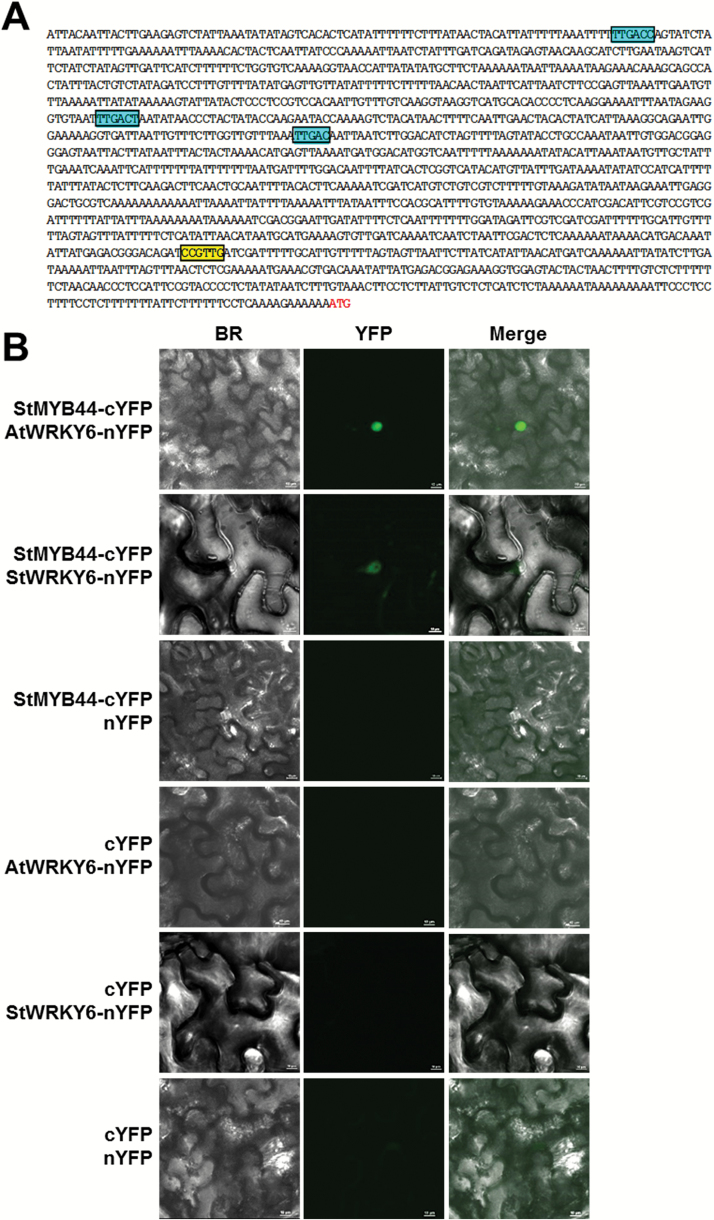
Transcription factor AtWRKY6 binds to the W-boxes in the AtPHO1 promoter and suppresses its expression in Arabidopsis (Chen et al., 2009). Three W-boxes [(T)TGAC(C/T)] were identified in the 1.5 kb StPHO1 promoter. In addition, a MYB-binding site I (MBSI) (CCGTTG), located 297 bp upstream of ATG, was identified in the potato PHO1 (Fig. 7A). EMSA showed that Arabidopsis AtMYB44 directly binds to the MBSI motif (Jung et al., 2012). It is highly possible that StMYB44 could regulate the transcription of StPHO1 by directly binding to the MBSI motif as StMYB44 shares a high amino acid identity with AtMYB44 in the DNA-binding domains (Supplementary Fig. S1). Furthermore, the juxtaposition of two kinds of cis-elements led to speculation that StMYB44 interacts with AtWRKY6 to form a protein complex. This hypothesis was tested by fusing StMYB44 to the C-terminal half of YFP (StMYB44–cYFP), and AtWRKY6 and StWRKY6 to the N-terminal half (AtWRKY6–nYFP and StWRKY6–nYFP), respectively, and introduced into tobacco leaves by agroinfiltration. Co-expression of 35S:StMYB44–cYFP and 35S:AtWRKY6–nYFP, 35S:StMYB44–cYFP, and 35S:StWRKY6–nYFP resulted in a YFP signal in the nucleus (Fig. 7B). In contrast, no YFP signal was detected when combinations of 35S:nYFP and 35S:cYFP, 35S:StMYB44–cYFP and 35S:nYFP, 35S:cYFP and 35S:AtWRKY6–nYFP, and 35S:cYFP and 35S:StWRKY6–nYFP were expressed (Fig. 7B). These results demonstrated that StMYB44 interacts with AtWRKY6 and StWRKY6 in planta.
Fig. 7.
StMYB44 interacts with AtWRKY6 and StWRKY6 in vivo. (A) Analysis of cis-elements in the StPHO1 promoter. Only the W-box (blue box) and MBSI (yellow box) are shown. (B) Interactions of StMYB44 with AtWRKY6 and StWRKY6. A suspension of Agrobacterium cells (OD600=0.05 for each strain) was infiltrated into N. benthamiana leaves. Infiltrated leaf discs were detached and examined 3 d after infiltration.
Discussion
The regulatory mechanism of Pi starvation responses in plants has been the subject of intensive investigation. A number of key genes controlling Pi homeostasis and regulation have been identified in plants, mainly in Arabidopsis and rice, where mutants or transgenic plants are easier to acquire (Rubio et al., 2001; Hamburger et al., 2002; Chen et al., 2009; Rouached et al., 2010; López-Arredondo et al., 2014). However, relatively limited information is available in species in which either a mutant collection does not exist or the generation of transgenics is more difficult. Here, the RNA-Seq approach was selected which has been widely used to study dynamic changes in gene expression in Pi-deficient plants, including Arabidopsis, white lupin, rice, and wheat (Lan et al., 2012; Oono et al., 2013a, b; O’Rourke et al., 2013; Secco et al., 2013). In the present study, a transcriptomic analysis was conducted of potato root in response to low Pi by RNA-Seq, and StMYB44, one of the transcription factor genes identified, was characterized in more detail.
Genetic responses to Pi starvation in potato roots
A total of 359 genes were identified to be Pi deficiency responsive (Supplementary Tables S1, S2). The number of differentially expressed genes is higher than that (147) in Pi-deficient maize roots at day 3 after treatment, and lower than that (967) in maize roots at day 6 after treatment, as reported in previous studies (Calderon-Vazquez et al., 2008). These genes functioned in diverse biological processes as shown by Gene Ontology (GO) analysis, including Pi homeostasis and other related metabolic processes (Fig. 1A), suggesting that Pi deficiency causes profound changes in these processes in potato roots. Common genetic responses to Pi starvation in potato and other plant species regarding Pi uptake, distribution, and signaling, lipid metabolism, carbon assimilation, and other stress pathways were observed, supporting the notion that Pi-deficient responses are largely conserved among plants (Franco-Zorrilla et al., 2004; Calderon-Vazquez et al., 2008). Interestingly, differentially expressed genes involved in two biological processes, cellular responses to fungus and nitric oxide, and negative regulation of endopeptidase activity, were also identified. The connections between Pi starvation and these biological processes were not reported in previous studies and thus could be interesting to explore in future research.
Although the focus of this study was on molecular responses to Pi starvation in potato roots, a comparison of our results with one of the previous studies in which the potato leaf was analyzed led to the identification of similar or distinct metabolic pathways between the two tissues. A few pathways involved in starch accumulation, protein degradation, lipid metabolism, and S uptake were activated, and the expression of the associated genes encoding starch synthase, phosphofructokinase, E3 ubiquitin ligase, and ubiquitin-protein ligase, plus SUT3, was found to be increased in both leaf and root tissues (Supplementary Table S1) (Hammond et al., 2011). In contrast, different responses to Pi deficiency were also observed between shoot and root in potato. For example, the patatin-encoding gene and four Phospholipase A1 (PLA1) genes were down-regulated in roots, while two Phospholipase D (PLD) genes were up-regulated in potato leaves under Pi-limiting conditions (Hammond et al., 2011). As the main tuber storage proteins, patatins also possess phospholipase A2 (PLA2) activity (Senda et al., 1996). PLDs hydrolyze structural phospholipids, while PLAs hydrolyze galactolipids more efficiently than phospholipids and are involved in auxin signaling in roots (Rietz et al., 2010; Canonne et al., 2011). The up-regulation of PLD genes in shoot and down-regulation of PLA genes in root indicated that the breakdown of phospholipids mainly occurs in the shoot while an altered auxin signaling mediated by PLAs occurss in root during Pi deficiency.
StMYB44 is a negative regulator of Pi transport from roots to shoots
The major purpose of this study was to identify the novel signaling transducers in potato in response to Pi deficiency. MYBs are among the well-characterized transcription factors regulating Pi deficiency responses. According to the phenotypic effect of either overexpression or knock-out of these MYB genes on Pi homeostasis, PHR1/OsPHR2, PSR1, AtMYB2, and OsMYB2P-1 had positive effects on Pi uptake or transport (Wykoff et al., 1999; Rubio et al., 2001; Zhou et al., 2008; Dai et al., 2012; Baek et al., 2013), whereas MYB62 negatively regulates Pi content in the shoot by reducing Pi uptake and acid phosphatase activity (Devaiah et al., 2009). Our study demonstrated that StMYB44 plays a negative role in Pi transport from root to shoot by regulating the transcription of PHO1. Genetic analysis has already demonstrated that the transcription of PHO1 is negatively regulated by the transcription factor AtWRKY6 in Arabidopsis (Chen et al., 2009). Regulation of PHO1 by StMYB44, a transcription factor from a different family from AtWRKY6, in plant roots indicated an additional regulatory mechanism of Pi transport, expanding our knowledge of the physiological functions of this gene family.
It is important to realize that the strong shoot morphological alterations in the StMYB44 overexpression lines are less likely to be caused by the reduced allocation of Pi from root to shoot. How StMYB44 mediates the growth and development of potato is worth future exploration, although current interest is focused on its involvement in Pi metabolism.
Control of PHO1 expression by multiple transcription factors
PHO1 is responsible for Pi transport from roots to shoots by loading Pi to the xylem (Hamburger et al., 2002). Transcription of PHO1 is under tight control in response to Pi availability since PHO1 was induced by Pi starvation and quickly recovered by Pi resupply in rice (Secco et al., 2013). A number of cis-elements, which can be recognized by several regulatory proteins including MYB transcription factors, in the promoter of Arabidopsis PHO1 were predicted. Similarly, the promoter region of StPHO1 was predicted to harbor several regulatory cis-elements, including binding sites for both WRKY transcription factors (W-box) and MYB transcription factors, suggesting that MYB transcription factors, such as StMYB44, could be involved in the regulation of StPHO1 expression by binding directly to its cis-elements. Moreover, it is known that not only can WRKY transcription factors physically interact with other members in the same family but they can also interact with transcription factors or regulatory proteins in other families. For example, AtWRKY6 and AtWRKY42 interacted with each other in Arabidopsis (Chen et al., 2009). HvWRKY38 interacted with Barley Prolamin-Box Binding Factor (BPBF), a non-WRKY transcription factor, to repress the expression of Amy32b in barley aleurone cells (Zou et al., 2008). These results demonstrated that these interactions could play an important role in the regulation of genes controlled by WRKY proteins, as documented previously (Chi et al., 2013).
This study showed that StMYB44 physically interacts with AtWRKY6 and StWRKY6 in vivo. To our knowledge, this is the first time that these two classes of transcription factors, WRKY and R2R3 MYB, were demonstrated to interact in the nucleus. These interactions allow us to propose that StMYB44 forms a complex with StWRKY6 in potato to regulate StPHO1 expression synergistically. Under normal conditions, expression of PHO1 is tightly controlled by the transcriptional complex to avoid overaccumulation of Pi in shoots, while upon Pi deficiency, removal of repressors StWRKY6 and/or StMYB44 leads to a lowered abundance of the transcription factor complex, facilitating the transcription of PHO1 and associated Pi transport from root to shoot. Further studies, such as functional analysis of other Pi deficiency-responsive transcription factors or identification of StMYB44-interacting proteins, would not only advance our knowledge on the regulatory mechanism of potato in response to Pi starvation, but also shed light on the selection of candidate genes that could be used for genetic enhancement of Pi deficiency tolerance in potato and other crops.
RNA-Seq data in this study have been deposited in GenBank with accession no. SRP083083.
Author contributions
XZ and CZ conceived the project and designed the experiments; XZ and MZ performed the experiments; JH and MI provided technical assistance; XZ, LL, and CZ analyzed the data and wrote the article.
Supplementary Material
Acknowledgements
We thank Dr Robert Turgeon for critical reading of the manuscript. We also thank Dr Ping Lang for the analysis of the RNA-Seq data, Drs Kashchandra Raghothama, Ajay Jain, and Chuanzao Mao for help with phosphate measurement, Dr Qi-Jun Chen for the CRISPR/Cas9 system, Dr Joyce van Eck for providing potato seedlings, and Jing Zhang for help with potato transformation. This work was funded by Purdue University as part of AgSEED Crossroads funding to support Indiana’s Agriculture and Rural Development (to CZ).
References
- Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology 8, 115–118. [Google Scholar]
- Baek D, Kim MC, Chun HJ, et al. 2013. Regulation of miR399f transcription by AtMYB2 affects phosphate starvation responses in Arabidopsis. Plant Physiology 161, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzo GG, Raghothama KG, Plaxton WC. 2002. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures. European Journal of Biochemistry 269, 6278–6286. [DOI] [PubMed] [Google Scholar]
- Calderon-Vazquez C, Ibarra-Laclette E, Caballero-Perez J, Herrera-Estrella L. 2008. Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. Journal of Experimental Botany 59, 2479–2497. [DOI] [PubMed] [Google Scholar]
- Canonne J, Froidure-Nicolas S, Rivas S. 2011. Phospholipases in action during plant defense signaling. Plant Signaling and Behavior 6, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH. 2009. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. The Plant Cell 21, 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG. 2007. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochemical Journal 405, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Yang Y, Zhou Y, Zhou J, Fan B, Yu JQ, Chen Z. 2013. Protein–protein interactions in the regulation of WRKY transcription factors. Molecular Plant 6, 287–300. [DOI] [PubMed] [Google Scholar]
- Chronis D, Chen S, Lu S, Hewezi T, Carpenter SC, Loria R, Baum TJ, Wang X. 2013. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. The Plant Journal 74, 185–196. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dai X, Wang Y, Yang A, Zhang WH. 2012. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiology 159, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG. 2007a WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiology 143, 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG. 2009. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Molecular Plant 2, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG. 2007b Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiology 145, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, González E, Bustos R, Linhares F, Leyva A, Paz-Ares J. 2004. The transcriptional control of plant responses to phosphate limitation. Journal of Experimental Botany 55, 285–293. [DOI] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y. 2002. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. The Plant Cell 14, 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, Broadley MR, Bowen HC, Spracklen WP, Hayden RM, White PJ. 2011. Gene expression changes in phosphorus deficient potato (Solanum tuberosum L.) leaves and the potential for diagnostic gene expression markers. PLoS One 6, e24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, Broadley MR, White PJ. 2004. Genetic responses to phosphorus deficiency. Annals of Botany 94, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59, 93–109. [DOI] [PubMed] [Google Scholar]
- Härtel H, Dormann P, Benning C. 2000. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proceedings of the National Academy of Sciences, USA 97, 10649–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. 2007. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology 144, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaradat MR, Feurtado JA, Huang D, Lu Y, Cutler AJ. 2013. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biology 13, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Kim YK, Oh NI, Shim JS, Seo JS, Choi YD, Nahm BH, Cheong JJ. 2012. Quadruple 9-mer-based protein binding microarray analysis confirms AACnG as the consensus nucleotide sequence sufficient for the specific binding of AtMYB44. Molecules and Cells 34, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ. 2008. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiology 146, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology 14, R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Li W, Schmidt W. 2012. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Molecular and Cellular Proteomics 11, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone A, Costa A, Consiglio F, Massarelli I, Dragonetti E, De Palma M, Grillo S. 1999. Tolerance to abiotic stresses in potato plants: a molecular approach. Potato Research 42, 333–351. [Google Scholar]
- Liu R, Chen L, Jia Z, Lü B, Shi H, Shao W, Dong H. 2011. Transcription factor AtMYB44 regulates induced expression of the ETHYLENE INSENSITIVE2 gene in Arabidopsis responding to a harpin protein. Molecular Plant-Microbe Interactions 24, 377–389. [DOI] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L. 2014. Phosphate nutrition: improving low-phosphate tolerance in crops. Annual Review of Plant Biology 65, 95–123. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q, et al. 2015. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Molecular Plant 8, 1274–1284. [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, et al. 2005. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proceedings of the National Academy of Sciences, USA 102, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods 5, 621–628. [DOI] [PubMed] [Google Scholar]
- Oono Y, Kawahara Y, Yazawa T, et al. 2013a Diversity in the complexity of phosphate starvation transcriptomes among rice cultivars based on RNA-Seq profiles. Plant Molecular Biology 83, 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Kobayashi F, Kawahara Y, Yazawa T, Handa H, Itoh T, Matsumoto T. 2013b Characterisation of the wheat (Triticum aestivum L.) transcriptome by de novo assembly for the discovery of phosphate starvation-responsive genes: gene expression in Pi-stressed wheat. BMC Genomics 14, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JA, Yang SS, Miller SS, et al. 2013. An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiology 161, 705–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. 1991. Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiology 97, 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga MI, Mateos I, Charukesi R, et al. 2014. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proceedings of the National Academy of Sciences, USA 111, 14947–14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology 50, 665–693. [DOI] [PubMed] [Google Scholar]
- Ramaiah M, Jain A, Raghothama KG. 2014. ETHYLENE RESPONSE FACTOR070 regulates root development and phosphate starvation-mediated responses. Plant Physiology 164, 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietz S, Dermendjiev G, Oppermann E, Tafesse FG, Effendi Y, Holk A, Parker JE, Teige M, Scherer GF. 2010. Roles of Arabidopsis patatin-related phospholipases A in root development are related to auxin responses and phosphate deficiency. Molecular Plant 3, 524–538. [DOI] [PubMed] [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. 2010. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Molecular Plant 3, 288–299. [DOI] [PubMed] [Google Scholar]
- Rubio V Linhares F Solano R Martín AC Iglesias J Leyva A, Paz-Ares J. 2001. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development 15, 2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco D, Jabnoune M, Walker H, Shou H, Wu P, Poirier Y, Whelan J. 2013. Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. The Plant Cell 25, 4285–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda K, Yoshioka H, Doke N, Kawakita K. 1996. A cytosolic phospholipase A2 from potato tissues appears to be patatin. Plant and Cell Physiology 37, 347–353. [DOI] [PubMed] [Google Scholar]
- Shin R, Burch AY, Huppert KA, Tiwari SB, Murphy AS, Guilfoyle TJ, Schachtman DP. 2007. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. The Plant Cell 19, 2440–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana. Current Opinion in Plant Biology 4, 447–456. [DOI] [PubMed] [Google Scholar]
- Su T, Xu Q, Zhang FC, Chen Y, Li LQ, Wu WH, Chen YF. 2015. WRKY42 modulates phosphate homeostasis through regulating phosphate translocation and acquisition in Arabidopsis. Plant Physiology 167, 1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L. 2010. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. The Plant Journal 64, 775–789. [DOI] [PubMed] [Google Scholar]
- von Arnim AG, Deng XW, Stacey MG. 1998. Cloning vectors for the expression of green fluorescent protein fusion proteins in transgenic plants. Gene 221, 35–43. [DOI] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. 2008. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. The Plant Journal 56, 505–516. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, Chen YF. 2014. Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiology 164, 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ruan W, Shi J, et al. 2014. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proceedings of the National Academy of Sciences, USA 111, 14953–14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW. 2003. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiology 132, 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K. 1999. Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proceedings of the National Academy of Sciences, USA 96, 15336–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biology 14, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P. 2005. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiology 138, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Wei P, et al. 2014. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnology Journal 12, 797–807. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Lu S, Liu D. 2014. A major root-associated acid phosphatase in Arabidopsis, AtPAP10, is regulated by both local and systemic signals under phosphate starvation. Journal of Experimental Botany 65, 6577–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Joung JG, Zheng Y, Chen YR, Liu B, Shao Y, Xiang JZ, Fei Z, Giovannoni JJ. 2011. High-throughput illumina strand-specific RNA sequencing library preparation. Cold Spring Harbor Protocols 2011, 940–949. [DOI] [PubMed] [Google Scholar]
- Zhou J, Jiao F, Wu Z, Li Y, Wang X, He X, Zhong W, Wu P. 2008. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiology 146, 1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun TH, Wang N, Ling HQ, Lu S, Li L. 2011. The cauliflower Orange gene enhances petiole elongation by suppressing expression of eukaryotic release factor 1. New Phytologist 190, 89–100. [DOI] [PubMed] [Google Scholar]
- Zhou X, Welsch R, Yang Y, Álvarez D, Riediger M, Yuan H, Fish T, Liu J, Thannhauser TW, Li L. 2015. Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proceedings of the National Academy of Sciences, USA 112, 3558–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Neuman D, Shen QJ. 2008. Interactions of two transcriptional repressors and two transcriptional activators in modulating gibberellin signaling in aleurone cells. Plant Physiology 148, 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.