Highlight
Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression in a free air CO2 enrichment experiment under simulated future climate conditions with combination of elevated CO2 and elevated temperature.
Keywords: Elevated CO2; elevated temperature; free air CO2 enrichment; Glycine max; sedoheptulose-1,7-bisphosphatase; Soy-T-FACE.
Abstract
Predictions suggest that current crop production needs to double by 2050 to meet global food and energy demands. Based on theory and experimental studies, overexpression of the photosynthetic enzyme sedoheptulose-1,7-bisphosphatase (SBPase) is expected to enhance C3 crop photosynthesis and yields. Here we test how expression of the cyanobacterial, bifunctional fructose-1,6/sedoheptulose-1,7-bisphosphatase (FBP/SBPase) affects carbon assimilation and seed yield (SY) in a major crop (soybean, Glycine max). For three growing seasons, wild-type (WT) and FBP/SBPase-expressing (FS) plants were grown in the field under ambient (400 μmol mol−1) and elevated (600 μmol mol−1) CO2 concentrations [CO2] and under ambient and elevated temperatures (+2.7 °C during daytime, +3.4 °C at night) at the SoyFACE research site. Across treatments, FS plants had significantly higher carbon assimilation (4–14%), Vc,max (5–8%), and Jmax (4–8%). Under ambient [CO2], elevated temperature led to significant reductions of SY of both genotypes by 19–31%. However, under elevated [CO2] and elevated temperature, FS plants maintained SY levels, while the WT showed significant reductions between 11% and 22% compared with plants under elevated [CO2] alone. These results show that the manipulation of the photosynthetic carbon reduction cycle can mitigate the effects of future high CO2 and high temperature environments on soybean yield.
Introduction
Crop productivity may have to increase by 60–110% over 2005 levels by 2050 (Tilman et al., 2011; Alexandratos and Bruinsma, 2012; OECD/FAO, 2012) to meet growing global food and energy demand. At the same time, atmospheric CO2 concentrations [CO2] are predicted to reach 550 μmol mol−1 by 2050 (IPCC, 2013) and this increase will be accompanied by an increase in terrestrial surface air temperatures of between 1 °C and 6 °C relative to 1961–1990, depending on geographic location (Rowlands et al., 2012). Thus, approaches to improve crop yields need to take global climate change and the predicted future environmental conditions into account.
An apparent major opportunity to increase crop yields in the future is via improving photosynthetic efficiency. Various approaches to achieve this goal have been proposed (Zhu et al., 2010; Blankenship et al., 2011; Raines, 2011; Ort et al., 2015). Particularly promising for future climatic and atmospheric conditions is increasing the rate of ribulose-1,5-bisphosphate (RuBP) regeneration (Raines, 2006, 2011). Under current atmospheric conditions, C3 photosynthesis (A) is mostly limited by the capacity for carboxylation by Rubisco, while under future elevated [CO2] and higher temperatures, the leaf photosynthesis model of carbon uptake and assimilation (Farquhar et al., 1980; von Caemmerer and Farquhar, 1981; von Caemmerer, 2000) predicts that limitation will shift towards the regeneration capacity of RuBP (Long et al., 2004). In the absence of other changes, rising temperature would increase the activity of Rubisco, but also lower its specificity for CO2 relative to O2. On balance, however, this will narrow the range of intercellular [CO2] under which Rubisco is limiting, and lower the [CO2] at which RuBP regeneration becomes limiting. In theory, the advantage of an increased capacity for RuBP regeneration would therefore be greatest under conditions of combined elevation of temperature and [CO2]. Ideally, future crops will be co-adapted to conditions of elevated [CO2] and higher temperatures. Thus, theoretically, enhancing RuBP regeneration capacity would be an effective strategy to adapt A to the higher atmospheric [CO2] and temperatures expected as climate change progresses.
The rate of RuBP regeneration can be limited by electron transport rates or by key enzymes in the photosynthetic carbon reduction (PCR) cycle. Using a complete dynamic model of photosynthetic carbon metabolism coupled to an evolutionary algorithm, Zhu et al. (2007) showed that optimizing the distribution of resources among PCR cycle enzymes is predicted to increase photosynthetic rates in CO2-enriched atmospheres. They predicted that levels of sedoheptulose-1,7-bisphosphatase (SBPase; EC 3.1.3.37) were suboptimal for maximizing A in the current atmosphere and even more suboptimal in future elevated CO2 atmospheres. SBPase is unique to the PCR cycle and catalyzes the dephosphorylation of sedoheptulose-1,7-bisphosphate to sedoheptulose-7-phosphate at the branch point of RuBP regeneration and carbon export (Raines et al., 2000). In theory, an increase in the RuBP regeneration capacity through increased activity of SBPase, assuming no ATP or NADPH limitation, would lead to higher A under conditions where RuBP regeneration becomes limiting, namely under high [CO2] and higher temperatures, as noted earlier.
Theory and model predictions are also supported by experimental evidence. Flux control analysis with SBPase antisense tobacco has shown that the enzyme can exert a strong control on A (Harrison et al., 1998; Raines, 2003), and overexpression of SBPase in tobacco increased both A and biomass (Lefebvre et al., 2005; Rosenthal et al., 2011; Simkin et al., 2015). Increases in A and growth were also observed when a cyanobacterial bifunctional form of the enzyme (fructose-1,6/sedoheptulose-1,7-bisphosphatase, FBP/SBPase) was expressed in tobacco (Miyagawa et al., 2001). The bifunctional FBP/SBPase has the same function as the two separate enzymes in higher plants, but has the advantage of being less prone to transgene silencing (Miyagawa et al., 2001).
However, species and growth conditions may impact the above responses. Rice overexpressing SBPase did not show an enhancement of A or growth, but maintained A and growth rate under salt (Feng et al., 2007a) and heat (Feng et al., 2007b) stress conditions, relative to the wild-type (WT) plants. Lefebvre et al. (2005) observed that tobacco plants overexpressing SBPase did not show increases in A or yield under low light levels and short-day conditions. Differences between WT and SBPase-overexpressing plants were also dependent on the developmental stage (Lefebvre et al., 2005; Rosenthal et al., 2011). Overall, these results suggest that overexpression of SBPase or the expression of a bifunctional FBP/SBPase can lead to immediate improvements in A and plant growth; however, the response may vary among species and environmental conditions. None of the studies tested the effect of combined elevation of temperature and [CO2], where an increased capacity for RuBP regeneration would be most beneficial, as explained above. Thus, it is as yet unclear if overexpression of SBPase could be a potential means of improving A and yield in crop species in the field and under predicted future growth conditions.
The interaction between increased SBPase activity and future growth conditions was tested in the present study using soybean (Glycine max), a major crop species and the most widely grown legume worldwide (Ainsworth et al., 2012). Soybean production increased from 27 Mt in 1961 to 308 Mt in 2014 (FAOSTAT 2016, http://faostat3.fao.org/, last accessed 19 October 2016) and is predicted to increase to 371 Mt by 2030 (Masuda amd Goldsmith, 2009). Soybean production since the year 2000 has nearly doubled, but this has been mainly achieved through increased land area for soybean cultivation (+60%), while soybean yields increased by only 19% (FAOSTAT 2016, http://faostat3.fao.org/, last accessed 19 October 2016). Given the limited availability of farmland and the negative environmental impacts of conversion of natural ecosystems, increases in soybean yield per area are a necessity for future sustainable increases in production. Experiments with soybean under elevated [CO2] and elevated temperatures indicate that CO2 alone will not increase yields in the future. In a field study, Ruiz-Vera et al. (2013) observed that in a warmer than average year for the Midwestern USA, elevated [CO2] was not able to mitigate the yield losses attributed to warming. This observation further emphasizes the importance of adapting crops to warmer and CO2-enriched environmental conditions.
To investigate if increased SBPase activity will increase yield in soybean under future climate, we grew WT and FBP/SBPase-expressing (FS) plants side by side under ambient and elevated [CO2] (400 μmol mol−1 and 600 μmol mol−1, respectively) combined with canopy heating of +2.7 °C during the day and +3.4 °C at night. The full factorial experiment was replicated over three growing seasons (2013–2015) at the SoyFACE research facility in central Illinois, USA. We hypothesized that expression of the cyanobacterial FBP/SBPase bifunctional enzyme would increase RuBP regeneration capacity (Jmax) and thus A under environmental conditions that favor RuBP regeneration-limited A (high light, elevated [CO2], warmer temperatures), and that the increases in A would be reflected in higher yields.
Materials and methods
Site description and experimental set-up
Soybean [Glycine max (L.) Merr. cv. ‘Thorne’] WT and FS plants derived from the same cultivar were grown in a complete block design (n=4) in the Soybean Temperature by Free Air CO2 Enrichment (Soy-T-FACE) experiment at the SoyFACE field site near Urbana-Champaign, IL, USA (40°2'30.49''N, 88°13'58.80''W, 230 m above sea level) during the 2013, 2014, and 2015 growing seasons. The experiment consisted of four blocks, each containing one ambient and one elevated [CO2] plot. Within each plot was nested an unheated and a heated subplot. Each subplot was further divided with plantings of the WT and FS lines. Seeds were planted by hand at 5 cm intervals in 38 cm rows. Eight 11 m long rows, four of WT and four of FS, were planted next to each other in each of the eight plots. Planting and harvest dates are given in Table 1. The ambient [CO2] plots were at ~400 μmol mol−1 and the elevated plots were fumigated to ~600 μmol mol−1 using free air CO2 enrichment (FACE) technology (Miglietta et al., 2001). The heated subplots were each equipped with an infrared heater array, as described in detail previously (Ruiz-Vera et al., 2013), installed at 1.0–.2 m above the canopy on a telescopic mast system (Ruiz-Vera et al., 2015). Using a proportional–integral–derivative (PID) feedback control system, we warmed the crop canopy to a target elevation of +3.5 °C above that of the canopy temperature in the unheated subplot. The target temperature increase was based on the low-response model predictions for surface temperature in the Midwest in 2050 (Rowlands et al., 2012).
Table 1.
Planting and harvest dates and climatic parameters in the study years
Canopy temperature (in °C) is averaged for the time period from canopy closure (V5, fifth node stage) to R7 (beginning of maturity) developmental stages (Ritchie et al., 1993) on the control (c) and heated (h) plots under ambient (400 μmol m−2 s−1) and elevated (600 μmol m−2 s−1) [CO2] with periods of rain excluded for the calculations. ΔCanopy temperature (in °C) is the difference between heated and control plots within each CO2 treatment. Day is averaged from 6:00 h to 18:00 h and night from 18:00 h to 6:00 h.
| Year | 2013 | 2014 | 2015 |
|---|---|---|---|
| ø Annual air temperature (°C) | 10.7 | 9.8 | 11.6 |
| ø Air temperature June–October (°C) | 20.1 | 19.5 | 20.4 |
| Annual sum precipitation (mm) | 845 | 1012 | 1114 |
| Sum precipitation June–October (mm) | 363 | 525 | 536 |
| ø Canopy temperature (°C) c/h | |||
| Day: ambient [CO2] | 25.7/28.6 | 23.3/26.2 | 24.7/27.2 |
| Day: elevated [CO2] | 26.6/29.3 | 23.7/26.3 | 25.0/27.6 |
| Night: ambient [CO2] | 17.4/20.8 | 15.4/18.9 | 17.3/20.7 |
| Night: elevated [CO2] | 17.7/21.1 | 15.7/19.1 | 17.1/20.5 |
| ø ΔCanopy temperature (°C) ±SD | |||
| Day: ambient [CO2] | 3.0 ± 1.0 | 2.8 ± 1.0 | 2.5 ± 1.0 |
| Day: elevated [CO2] | 2.8 ± 0.8 | 2.7 ± 1.0 | 2.6 ± 1.1 |
| Night: ambient [CO2] | 3.4 ± 0.7 | 3.4 ± 0.4 | 3.4 ± 0.4 |
| Night: elevated [CO2] | 3.4 ± 0.6 | 3.4 ± 0.4 | 3.4 ± 0.5 |
| Planting date (DOY) | 158 | 169/170 | 156 |
| Canopy closure V5 (DOY) | 191 | 206 | 190 |
| Beginning maturity R7 (DOY) | 261 | 265 | 260 |
| Harvest date, DOY (treatment) | 275 | 281 (Ah, Eh) 292 (Ac, Ec) |
274 (Ac) 275 (Ec, Eh) 279 (Ec, Eh) 280 (Ah, Ec, Eh) |
Ø, average; DOY, day of year.
During the day (6:00 h to 18:00 h), and with rainy days excluded, mean temperature differences between the subplots were between 0.5 °C and 1.0 °C lower than the target set point (Table 1), resulting in an average temperature increase of +2.7 °C. During the night, the average temperature difference was +3.4 °C. The heated subplot diameter was 3.5 m, resulting in an effective heated subplot area of 9.6 m2. Canopy temperature in each subplot was measured by infra-red radiometers (SI-111, Apogee Instruments, Logan, UT, USA) connected to data-loggers (CR1000 Micrologger, Campbell Scientific, Logan, UT, USA). Canopy temperature measurements were collected every 5 s to control the heater output and 10 min mean values were stored. The actual mean season canopy temperatures for the four treatments in the three seasons are given in Table 1. The canopy was heated continuously day and night from the VC (cotyledons expanded, Ritchie et al., 1993) growth stage until harvest. Heaters were programmed to reduce energy output during precipitation events using a rain detector (Model 260-2590 Precipitation Detector, Nova Lynx Corporation, Grass Valley, CA, USA), as the target temperature difference cannot be maintained during precipitation. The treatments are hereafter referred to as ‘Ac’ (ambient [CO2], control temperature), ‘Ah’ (ambient [CO2]+heated), ‘Ec’ (elevated [CO2], control temperature), and ‘Eh’ (elevated [CO2]+heated).
Weather conditions
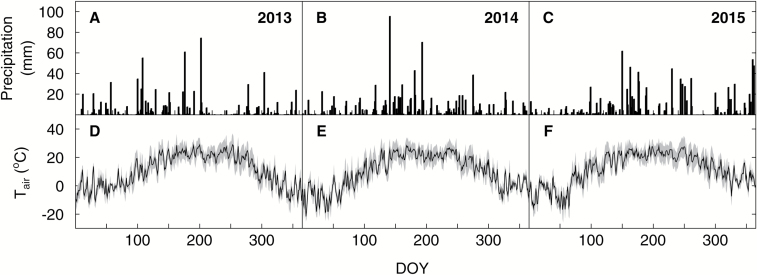
Weather data for all three seasons were available from nearby weather stations. The air temperature and precipitation data (Fig. 1) were obtained from Willard Airport (http://mrcc.isws.illinois.edu/CLIMATE/, last accessed 2 August 2016), 1 km west of the site, and the photosynthetically active radiation (PAR; μmol photons m−2 s−1) data from the University of Illinois Energy Farm (http://www.energybiosciencesinstitute.org/content/ebi-weather, last accessed 22 June 2016), 2 km to the east. Air temperature during the June–October growing season was close to the 20 year average (19.9 °C) in all three years. In comparison with the 20 year averages of the annual sum of precipitation (922 mm) and the sum of precipitation during the June–October growing season (446 mm), precipitation was slightly lower in 2013 and higher in 2014 and 2015 (Table 1). PAR was very similar in all three growing seasons, with maximum/mean values of 2190/737 μmol m−2 s−1 (2013), 2388/721 μmol m−2 s−1 (2014), and 2236/724 μmol m−2 s−1 (2015).
Fig. 1.
Precipitation and air temperature as recorded at Willard Airport Weather Station (http://mrcc.isws.illinois.edu/CLIMATE/, last accessed 2 August 2016) near the experimental site in 2013, 2014, and 2015. The sum of precipitation (in mm) is shown for individual days in (A) 2013, (B) 2014, and (C) 2015. Average daily air temperature (black line) and daily minimum and maximum temperature (gray shaded area) (in °C) is shown for (D) 2013, (E) 2014, and (F) 2015.
Plant material
Glycine max (L.) Merr. cv. ‘Thorne’ was previously transformed (Bihmidine, 2012) with the cyanobacterial gene FBP1 (Synechococcus, strain PCC7942) using the Agrobacterium-mediated method (Hinchee et al., 1988; Clemente, 1997) to test the effect of FBP1 on soybean leaf A. The FBP1 gene encodes the FBPase/SBPase bifunctional enzyme, which has the same enzymatic function as the two individual enzymes present in higher plants. The FBP1 cassette contains the FBP1 gene under the control of the Peanut chlorotic streak caulimovirus (pcisvflt36) promoter, coupled with the tobacco etch translational enhancer element, and attached to the pea Rubisco small subunit transit peptide for transport of the enzyme across the plastid membrane (see Supplementary Fig. S1 at JXB online). The transgene cassette was subcloned into the binary vector pPTN200 which harbors a Pnos bar cassette for selection. Greenhouse and field phenotyping and molecular characterizations are described in Bihmidine (2012).
Due to spatial constraints of the experimental area and statistical considerations, we compared a single transgenic line with the WT. We used the same homozygous line (480-8) selected previously (Bihmidine, 2012; Hay, 2012), as it showed consistent increases of photosynthetic rates over WT plants. The present study was performed using T7, T8, and T9 populations derived from homozygous T3 and T4 populations of this line. T5 and T6 populations were multiplied in the field in 2011 and 2012 to provide enough seed for the start of the 3 year experiment. WT and FS plants were grown side by side, and only seeds harvested from the control treatment were used for planting in the following growing season. The presence of the transgene was reconfirmed in these subsequent generations using quantitative reverse transcription–PCR (data not shown) coupled with western blotting to test for the encoded protein.
Protein extraction and western blotting
The level of bifunctional FBP/SBPase protein was assessed in fully expanded leaves that were sampled in the field at noon on the same days when in situ gas exchange measurements were collected. Additionally, the levels of transketolase were assessed as a loading control, and levels of native SBPase were assessed to check if expression of the bifunctional enzyme affected levels of the native enzyme. Three leaflets from three different plants in each treatment were cut and immediately plunged into liquid nitrogen. Leaflets were taken from the most recently fully expanded leaves at the top of the canopy.
Samples were stored at −80 °C before grinding in liquid nitrogen, and the resulting powder was subsequently stored at −80 °C until analysis. Subsamples of 100 μl of powder were mixed with 600 μl of protein extraction buffer [50 mM HEPES-NaOH (pH 8.2); 5 mM MgCl2; 1 mM EDTA; 1 mM EGTA; 10% glycerol; 0.1% Triton X-100; 2 mM benzamidine; 2 mM aminocaproic acid; 0.5 mM phenylmethylsulphonyl fluoride; 10 mM DTT] using a pre-cooled mortar and pestle at 4 °C. The resulting suspension was clarified at 10000 g for 2 min at 4 °C, and the supernatant was transferred to separate tubes for protein quantification and western blotting. Protein quantification was assessed by Bradford Assay (Bradford Reagent B6916, Sigma-Aldrich). A 200 μl aliquot of 313 mM Tris–HCl (pH 6.8), 10% SDS, 25% glycerol, 25% 2-mercaptoethanol was added to the protein samples for western blotting, boiled, and diluted to a uniform protein concentration. Samples were loaded on an equal protein basis (10 μg of soluble protein) on a 4% stacking gel, separated using 10% (w/v) SDS–PAGE, and transferred using a Mini Trans-Blot cell (Bio-Rad) to a nitrocellulose blotting membrane (Amersham Protran). The membrane was blocked in 6% (w/v) skim milk (made from Marvel Original dried skimmed milk powder) in phosphate-buffered saline (PBS) and incubated with a primary antibody (bifunctional FBPase/SBPase, dilution 1:500; native SBPase, dilution 1:2000; or transketolase (TK), dilution 1:5000) in 3% skim milk in PBS at 4 °C overnight. SBPase polyclonal antibodies were raised in rabbits against Arabidopsis SBPase, and TK antibodies were raised against tobacco plastid TK as described in Henkes et al. (2001). Bifunctional FBP/SBPase polyclonal antibodies were raised against DRPRHKELIQEIRNAG-[C]-amide (Cambridge Research Biochemicals, Cleveland, UK). The membrane was rinsed with 300 ml of PBST, then placed on a shaker table and washed for 60 min in PBST (fresh PBST replaced every 20 min). Goat anti-rabbit antibody conjugated to horseradish peroxidase (Invitrogen, Thermo Fisher Scientific, Catalog#: 31466) was used at a 1:2500 dilution in 3% skim milk in PBS with 0.05% Tween-20 (PBST) and the membrane incubated for 1.5 h at 4 °C. After incubation, the membrane was placed on a shaker table and washed for 30 min in PBST (fresh PBST replaced every 10 min). Proteins were detected using the enhanced chemiluminescence detection reagent (Pierce ECL Western Blotting Substrate, ThermoFisher).
Above-ground biomass, seed yield, harvest index, and growth parameters
After full maturity [R8, 95% of pods have reached mature pod color (Ritchie et al., 1993)] and dry-down was complete, plants were harvested by hand to determine yield and total biomass. Above-ground biomass (AGB; g m−2), seed yield (SY; g m−2), harvest index (HI=SY/AGB; unitless), 200 seed weight (200 SW, g), and growth parameters (number of plants, mean plant height, number of nodes and pods per plant) were determined in all years. AGB comprises stems and pods only, as leaves have senesced and fallen by the time of harvest. In all years, plants were cut from a 1 m length of row from the undisturbed middle two rows of each genotype subplot to determine the number of plants, mean plant height, number of nodes, and pods per plant, AGB, and HI. Pods and stems were separated by hand and dried at 65 °C to constant weight. After determination of stem and pod weight, the seeds were separated from the pods using a belt thresher and SY was determined to calculate HI. These seeds were also used to obtain individual seed weight (SW) by counting and weighing 200 seeds of each genotype in each subplot. In 2014 and 2015, we additionally harvested all plants along a total row length of 2.2 m to obtain a more robust estimate of SY, and this was added to SY from the 1 m row for statistical analysis. SY and AGB data were converted from g m−1 to g m−2.
In situ gas exchange measurements
Gas exchange measurements were conducted on 2 (2013), 5 (2014), and 4 (2015) d during the growing seasons (Table 2), covering vegetative and reproductive stages from V3 to V5 (Third to Fifth-Node Stage) to R6 (Full Seed) based on the soybean development classifications of Ritchie et al. (1993). Measurements on DOY (day of year) 255 in 2014 were excluded from the analysis as leaves were starting to senesce. Leaf-level gas exchange systems (LI-6400XT; LI-COR Inc., Lincoln, NE, USA) coupled with the LI-6400-40 Leaf Chamber Fluorometer (LI-COR, Inc.) were used for these measurements. Measurements were conducted on plants in the two outer rows. The diurnal course of the photosynthetic carbon assimilation rate (A, μmol m−2 s−1) was determined on the middle leaflet of the youngest fully expanded trifoliate on 2–3 plants per genotype on each subplot at three time points between 9:00 h and 17:00 h. Conditions in the leaf chamber (block temperature, reference [CO2], PAR) were set at the beginning of each time point, based on ambient conditions. Block temperature was set according to air temperature reported from a nearby weather station and increased by the target temperature difference of +3.5 °C for the heated treatments. The reference [CO2] was set to 410 μmol mol−1 and 610 μmol mol−1 for the ambient and elevated [CO2] plots in order to match plot treatment conditions within the leaf chamber after the leaves lowered the [CO2]. Ambient PAR was measured immediately before each time point using the LI-190 installed on the LI-6400. The output of the chamber light-emitting diodes (LEDs) was set to deliver the same PAR and was maintained at these values throughout the course of all measurements at that time point. Relative humidity (RH) in the leaf chamber was not controlled directly, but was targeted to be kept between 50% and 70% during the measurement, which was achieved for 90% of the data. Environmental conditions during the in situ measurements are presented in Table 2.
Table 2.
Day of year (DOY) and time points (9:00 h, 12:00 h, and 15:00 h) on which in situ gas exchange measurements were conducted in the three growing seasons and the corresponding developmental stages and environmental conditions (air temperature, Tair; photosynthetic active radiation, PAR)
| DOY | Growth stage | Tair (°C) control/heated | PAR (μmol photons m−2 s−1) | ||||
|---|---|---|---|---|---|---|---|
| 9:00 | 12:00 | 15:00 | 9:00 | 12:00 | 15:00 | ||
| 2013 | |||||||
| 192 | ~V3 | 22.0/25.5 | 25.0/28.5 | 25.0/28.5 | 1100 | 2000 | 1400 |
| 241 | R5/R6 | 22.0/25.5 | 26.0/29.5 | 28.5/32.0 | 250 | 900 | 1700 |
| 2014 | |||||||
| 199 | V4/V5 | 20.0/23.5 | 27.0/30.5 | 24.0/27.5 | 1000 | 500 | 1550 |
| 218 | R1/R2 | 22.0/25.5 | 24.5/28.0 | 25.5/29.0 | 600 | 500 | 400 |
| 227 | R3 | 18.0/21.5 | 20.5/24.0 | 24.5/28.0 | 1000 | 1500 | 850 |
| 238 | R5 | 25.5/29.0 | 30.0/33.5 | 32.0/35.5 | 1150 | 1800 | 1000 |
| 255 | R6 | 13.0/16.5 | 15.0/18.5 | 15.0/18.5 | 400 | 600 | 500 |
| 2015 | |||||||
| 184 | V3/V4 | 19.0/22.5 | 23.0/26.5 | 22.0/25.5 | 1000 | 930 | 390 |
| 196 | R1 | 18.0/21.5 | 23.0/26.5 | 26.0/29.5 | 600 | 2000 | 1500 |
| 216 | R4 | 21.0/24.5 | 26.0/29.5 | 27.0/30.5 | 1000 | 1800 | 1200 |
| 237 | R6 | 16.0/19.5 | 21.0/24.5 | 22.0/25.5 | 1200 | 1800 | 1650 |
| Mean ±SE | ns | 19.1 ± 0.9 | 22.6 ± 1.5 | 23.9 ± 1.3 | 815 ± 103 | 500 ± 212 | 1066 ± 180 |
| sign. | 25.5 | 25.6 ± 2 | 28 ± 4 | 1150 | 1190 ± 270 | 1275 ± 275 | |
Bold font indicates that significant differences (P<0.1) in photosynthetic rate (A) between genotypes were observed at these points in time.
Mean values for Tair and PAR where no significant (ns) or significant (sign.) differences were observed are given at the bottom of the table. When differences were significant, A was always higher for the FS plants.
V3, V4, V5, third, fourth, and fifth node stage; R1, beginning bloom; R2, full bloom; R3, beginning pod, R4, full pod; R5, beginning seed; R6, full seed (Ritchie et al., 1993)
In 2013 each of the four LI-6400 systems was randomly assigned to one block and the measurements conducted on the subplots in randomized order. In 2014 and 2015, two teams, each equipped with two LI-6400 gas exchange systems assigned at random, conducted the measurements in parallel per block, with one instrument assigned to the heated subplot and the other to the unheated subplot. At each time point, instruments stayed assigned to the same temperature treatment within a block but were switched on transition to the next block to avoid confounding any undetected instrument difference with a treatment. All measurements for a given time point were completed within 1.5–2 h.
A– C i curves: measurements and model fitting
The maximum carboxylation rate of Rubisco (Vc,max) and Jmax were determined from A–Ci curve measurements of A at varying intercellular [CO2] following the protocol of Bernacchi et al. (2005) on leaves that were collected in the field pre-dawn. A–Ci curve measurements were conducted within 2 d before or after the in situ gas exchange measurements. Measurements on DOY 255 in 2014 were excluded from the analysis as leaves were starting to senesce. The petiole of the youngest fully expanded leaf from two plants per subplot was cut close to the stem and then immediately re-cut in water. Leaves were transported to the lab in an opaque box and were kept at 20 °C and under low-light conditions (PAR <10 μmol photons m−2 s−1) until measurement. Leaves were exposed to high light (1000 μmol m−2 s−1 PAR) for 10–15 min before clamping the leaf cuvette onto the middle leaflet. Conditions in the leaf cuvette were set to reflect the treatment [CO2] (410 μmol mol−1 or 610 μmol mol−1), the light level was set to 1500 μmol m−2 s−1, leaf temperature was controlled at 25 °C (±0.3 °C SD), and mean vapor pressure deficit during the measurements was 1.4 kPa (±0.2 kPa SD). After ~5 min, the leaf had reached steady-state A and the A–Ci curve autoprogram was initiated. [CO2] was decreased stepwise to 50 μmol mol−1 or 100 μmol mol−1, then increased back to the starting value (410 μmol mol−1 or 610 μmol mol−1) and then stepwise up to 1100 μmol mol−1 or 1500 μmol mol−1, depending on the CO2 treatment and developmental stage. Measurements of A were recorded at each [CO2] set point after stability was reached. Vc,max@25 °C and Jmax@25 °C were derived from a curve-fitting procedure of the underlying biochemical models (Farquhar et al., 1980; von Caemmerer, 2000), following the procedures described by Long and Bernacchi (2003).
Statistical analysis
Gas exchange and yield data were analyzed using a mixed-model analysis of variance (PROC MIXED, SAS 9.4), taking into account the split-plot design of the experiment. The analyses were conducted separately for individual years. The model for the diurnal gas exchange data was analyzed separately by time of day (morning, midday, afternoon) and included the fixed factors [CO2] (ambient, elevated), temperature (control, heated), genotype (WT, FS), and day of the measurement (day of year, DOY), which was included as a repeated measure. Block was included as a random factor. The model for end of season yield data included the fixed factors [CO2], temperature, and genotype, and block as random factor. Significant differences between least square means for a priori determined comparisons were analyzed using post-hoc tests (LSMEANS, SAS 9.4). Probability for statistical significance was set at P<0.1 a priori to reduce the possibility of type II errors.
Results
Characterization of WT and FBP/SBPase-expressing plants by western blotting
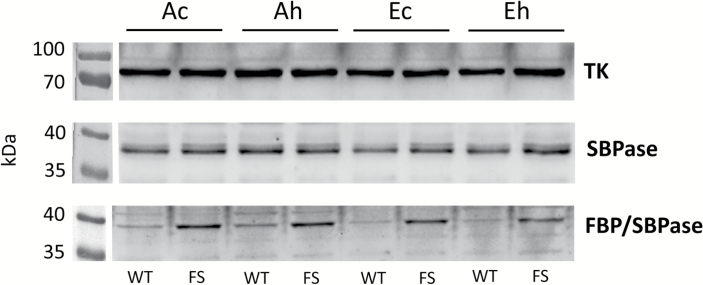
Western blotting showed that the bifunctional FBP/SBPase was present in the transgenic plants and appeared to have little effect on native SPBase levels (Fig. 2). Bands matched the expected sizes of the polypeptides of ~72 kDa for TK, ~36 kDa for SBPase, and ~38 kDa for bifunctional FBP/SBPase. The FBP/SBPase antibody showed cross-reactivity and non-specific binding, but the strongest bands were clearly only observed for the expected size of the bifunctional FBP/SBPase protein in the transgenic plants. Levels of expression did not appear to vary based on treatment (Fig. 2).
Fig. 2.
Western blot analysis of leaf protein extracts from wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants. Proteins were extracted from 2014 leaf tissue samples (R5, beginning seed developmental stage) of plants grown under the ‘Ac’ (400 μmol mol−1 [CO2], control temperature), ‘Ah’ (400 μmol mol−1 [CO2], heated +3.5 °C), ‘Ec’ (600 μmol mol−1 [CO2], control temperature), and ‘Eh’ (600 μmol mol−1 [CO2], heated +3.5 °C) treatments. A total of three leaflets (from three different plants) were combined per sample. The blot was probed at the same time with polyclonal antibodies raised against transketolase (TK) and native SBPase, and was reprobed using a polyclonal antibody raised against bifunctional FBP/SBPase after the blot was stripped. Gels were loaded on an equal protein basis. Blot marker images were cropped and pasted onto the corresponding chemiluminescence images using the editing function of the FusionCapt Advance software.
FS plants had higher photosynthesis than WT plants, especially under high light and high temperature conditions
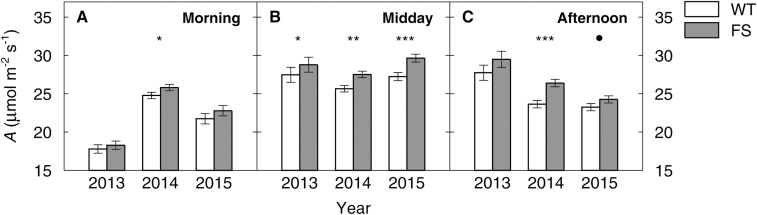
In situ gas exchange measurements showed no significant difference in A between genotypes during the morning (Fig. 3A), with the exception of 2014 where FS plants had 11% and 14% higher A than the WT in the Ac and the Ec treatment on DOY 238 (Supplementary Fig. S2). During midday measurements, FS plants always had a higher A than the WT by 5% (2013), 7% (2014), and 9% (2015) (Fig. 3B). There was a significant DOY×genotype interaction for midday A in all years (Supplementary Table S1). FS plants tended to have higher midday A than WT plants during the middle of the seasons, but no significant differences were observed early and late in the seasons (Supplementary Fig. S2). During afternoon measurements, FS plants had a 12% (2014) and 4% (2015) higher A than the WT, but no significant differences were observed for 2013 (Fig. 3C). For a summary of the ANOVA statistics, see Supplementary Table S1.
Fig. 3.
Average photosynthetic rates (A, μmol m−2 s−1) of the wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants in comparison. Morning (A), midday (B), and afternoon (C) in situ gas exchange measurements were pooled for all four treatments (ambient/elevated CO2, control/heated plots) and for all sampling days in the respective years. Temperature, reference [CO2], and light (photosynthetic active radiation, PAR) in the LI-6400XT leaf chamber were set to match ambient conditions at the beginning of the measurements and the values were maintained throughout the course of all measurements at the respective time point. Relative humidity (RH) was kept between 50% and 70% during the measurements. Environmental conditions during the in situ measurements are presented in Table 2. Error bars are ±SE of the LS means estimate as derived from a repeated measures ANOVA (Supplementary Table S1). Symbols mark significant differences between WT (empty bars) and FS (gray bars) (• <0.1, * <0.05, ** <0.01, *** <0.001). Note that the y-axis does not start at zero.
Environmental conditions on the days of in situ gas exchange measurements varied greatly, with PAR ranging from as low as 250 μmol m−2 s−1 up to 2000 μmol m−2 s−1 and air temperature from 13 °C to 32 °C (Table 2). A of FS plants was significantly higher than A of WT plants on certain days, but the response was not observed for all time points and treatments. Light levels and air temperature tended to be higher at the times when significant differences between FS and WT plants (ΔA=AFS−AWT) were observed (Table 2). Multiple regression analysis (ΔA=Tair+PAR) with the pooled data for all seasons and developmental stages revealed slight increases of ΔA with PAR (1.4 μmol CO2 m−2 s−1/1000 μmol photons m−2 s−1, P<0.01) and Tair (0.8 μmol CO2 m−2 s−1/10 °C, P<0.1), but overall variance was high (R2=0.1, P<0.001) (Supplementary Fig. S3).
FS plants had higher Vc,max and Jmax, especially during late reproductive stages
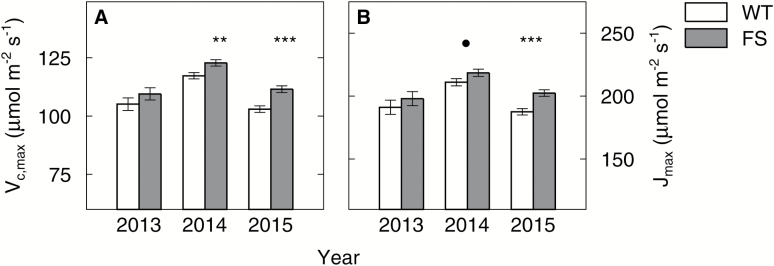
There was a significant main effect of genotype on Vc,max (Fig. 4A) and Jmax (Fig. 4B) with significantly higher values for FS plants than WT plants in 2014 (Vc,max+5.5 μmol m−2 s−1, P<0.01; Jmax+7.5 μmol m−2 s−1, P<0.1) and 2015 (Vc,max+8.6 μmol m−2 s−1, P<0.001; Jmax+14.9 μmol m−2 s−1, P<0.001). The differences in 2013 were not significant, but the trend toward higher values for the FS plants was still present. Higher values of Vc,max and Jmax of FS plants in comparison with the WT were particularly observed during the second half of the growing seasons. The biggest differences occurred at the R5/R6 developmental stage, but were not consistently observed under all treatments (Supplementry Fig. S4).
Fig. 4.
Mean Vc,max and Jmax of the wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants in comparison. Vc,max (A) and Jmax (B) were derived from A–Ci curves conducted at a leaf temperature of 25 °C and the data were pooled for all four treatments (ambient/elevated CO2, control/heated plots) and for all sampling days in the respective years. Error bars are ±SE of the LS means estimate as derived from a repeated measures ANOVA. Symbols mark significant differences between the WT (empty bars) and FS (gray bars) (• <0.1, ** <0.01 *** <0.001). Note that the y-axis does not start at zero.
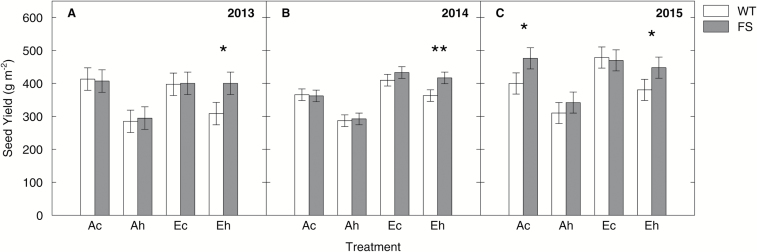
FS plants maintained seed yield under the elevated [CO2] and heat treatment while WT plants had significantly reduced seed yield
There was a significant main effect of genotype on SY in 2014 and 2015 (Table 3), and pairwise comparisons of genotypes within treatments showed a significant difference under the Eh treatment in all years (Fig. 5). This was related to significant reductions between 11% and 22% of WT SY under the Eh treatment compared with the Ec treatment, while SY of FS plants was unchanged. In 2015 (Fig. 5C), FS plants also had higher yield under the Ac treatment in comparison with the WT. Also in 2015, there was a significant interaction of genotype with [CO2] and temperature on SY (Table 3), consistent with the hypothesis that the FS plants would have a particular advantage under conditions of combined elevation of temperature and [CO2]. Both WT and FS plants had generally higher SY under the elevated [CO2] treatments in comparison with ambient [CO2] in 2014 and 2015 (P<0.05) but not in 2013 (Table 3; Fig. 5A). In all three growing seasons, elevated temperature significantly decreased SY by between 11% and 31%, depending on year and [CO2], with the exception of the FS plants in the Eh treatment, where no significant decline was observed (Fig. 5).
Table 3.
Seasonal complete block ANOVA of total seed yield (SY), weight of 200 seeds (SW), above-ground biomass (AGB), stem and branches (ST+BN), harvest index (HI), number of pods per plant (POD), stem height (STH), number of nodes per plant (NODE) for the main effects [CO2] (400 μmol mol−1, 600 μmol mol−1), temperature ‘Temp.’ (control, heat), and genotype (WT, FS) and their interaction terms
| Parameter, unit | [CO2] | Temp. | Genotype | [CO2]× Temp. |
[CO2]× Genotype |
Temp.× Genotype |
[CO2]× Temp.× Geno type |
|
|---|---|---|---|---|---|---|---|---|
| df | 3 | 6 | 12 | 6 | 12 | 12 | 12 | |
| 2013 | ||||||||
| SY | g m−2 | ns | 0.032 | ns | ns | ns | ns | ns |
| SW | g | ns | 0.001 | ns | 0.024 | ns | ns | ns |
| AGB | g m−2 | 0.099 | 0.070 | ns | ns | ns | ns | ns |
| ST+BN | g m−2 | 0.009 | 0.042 | 0.020 | ns | ns | ns | ns |
| HI | 0.066 | 0.005 | 0.008 | 0.078 | ns | ns | ns | |
| POD | No. per plant | ns | ns | 0.015 | ns | ns | ns | ns |
| STH | m | 0.014 | 0.003 | 0.047 | ns | 0.031 | ns | ns |
| NODE | No. per plant | ns | ns | ns | ns | 0.021 | ns | ns |
| 2014 | ||||||||
| SY | g m−2 | 0.014 | 0.001 | 0.011 | 0.065 | 0.015 | ns | ns |
| SW | g | ns | ns | 0.085 | ns | ns | ns | ns |
| AGB | g m−2 | 0.034 | 0.026 | 0.058 | 0.004 | ns | ns | ns |
| ST+BN | g m−2 | 0.029 | 0.030 | ns | 0.080 | ns | ns | ns |
| HI | ns | 0.097 | ns | ns | ns | ns | ns | |
| POD | No. per plant | 0.053 | 0.066 | ns | ns | ns | ns | ns |
| STH | m | 0.082 | 0.001 | 0.024 | ns | ns | 0.085 | ns |
| NODE | No. per plant | ns | 0.002 | 0.028 | ns | ns | ns | ns |
| 2015 | ||||||||
| SY | g m−2 | 0.024 | 0.001 | 0.014 | ns | ns | ns | 0.061 |
| SW | g | ns | ns | ns | ns | ns | ns | ns |
| AGB | g m−2 | 0.008 | 0.001 | ns | 0.017 | ns | ns | 0.002 |
| ST+BN | g m−2 | 0.008 | 0.036 | <0.0001 | 0.036 | ns | 0.018 | 0.006 |
| HI | ns | 0.002 | 0.001 | ns | ns | 0.049 | ns | |
| POD | No. per plant | 0.009 | 0.006 | 0.007 | ns | 0.037 | ns | 0.043 |
| STH | m | 0.004 | ns | <0.0001 | ns | ns | ns | ns |
| NODE | No. per plant | 0.013 | ns | 0.053 | ns | ns | 0.094 | ns |
Only interaction terms with significant effects are listed in the table.
Values in the table are P-values; significance was set as P<0.1.
df, degrees of freedom; ns, not significant.
Fig. 5.
Total seed yield (g m−2) of the wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants in comparison in the three growing seasons. Plants were grown under the ‘Ac’ (400 μmol mol−1 [CO2], control temperature), ‘Ah’ (400 μmol mol−1 [CO2], heated +3.5 °C), ‘Ec’ (600 μmol mol−1 [CO2], control temperature), and ‘Eh’ (600 μmol mol−1 [CO2], heated +3.5 °C) treatments at the Soy-T-FACE experiment. Plants were harvested along a total row length of 1 m in 2013 (A) and of 3.2 m in 2014 (B) and 2015 (C), and seed yield was converted into g m−2. Error bars are ±SE of the LS means estimate as derived from the complete block ANOVA (Table 3) Symbols mark significant differences between the WT (empty bars) and FS (gray bars) (* <0.05, ** <0.01).
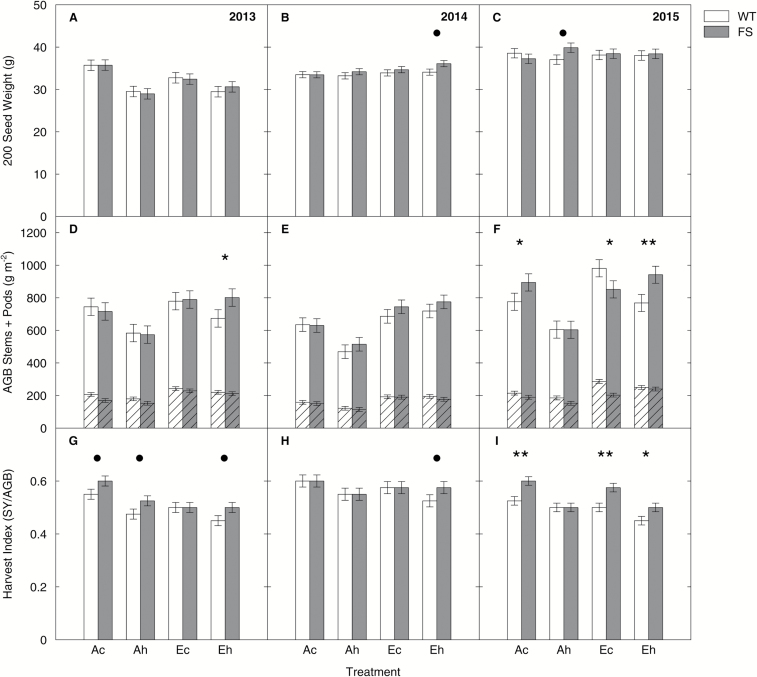
Genotype did not have consistent effects on 200 seed weight, above-ground biomass, or harvest index
There were no differences in 200 seed weight (SW) between genotypes with the exception of a slightly bigger SW of FS plants in comparison with the WT (P<0.1) in the Eh treatment in 2014 (Fig. 6B) and the Ah treatment in 2015 (Fig. 6C). Significant differences in pairwise comparisons for AGB between genotypes within treatments were found for the Eh treatment in 2013 (FS+19%, P<0.05, Fig. 6D) and the Ac (FS+15%, P<0.05), Ec (FS−13%, P<0.05), and Eh (FS+23%, P<0.01) treatment in 2015 (Fig. 6F). The high AGB of the WT under the Ec treatment in 2015 was related to a very high stem weight in 2015 (Fig. 6F). Over all treatments, WT plants showed a lower HI than FS plants in 2013 (−7%, P<0.01) and 2015 (−9%, P<0.001) but not in 2014 (Table 3; Fig. 6G-I).
Fig. 6.
Seed weight, above-ground biomass, and harvest index of the wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants in comparison in the three growing seasons. Seed weight of 200 seeds (in g), above-ground biomass (AGB, in g m−2) comprised of stems and branches (dashed area) and pods (undashed area), and harvest index [HI; seed yield (SY)/AGB] were derived from plants grown under the ‘Ac’ (400 μmol mol−1 [CO2], control temperature), ‘Ah’ (400 μmol mol−1 [CO2], heated +3.5 °C), ‘Ec’ (600 μmol mol−1 [CO2], control temperature), and ‘Eh’ (600 μmol mol−1 [CO2], heated to +3.5 °C) treatments at the Soy-T-FACE experiment. Data are from a representative sampling along 1 m length of a middle row within each subplot in the three growing seasons. Error bars are ±SE of the LS means estimate as derived from the complete block ANOVA (Table 3). Symbols mark significant differences between the WT (empty bars) and FS (gray bars) (• <0.1, * <0.05, ** <0.01).
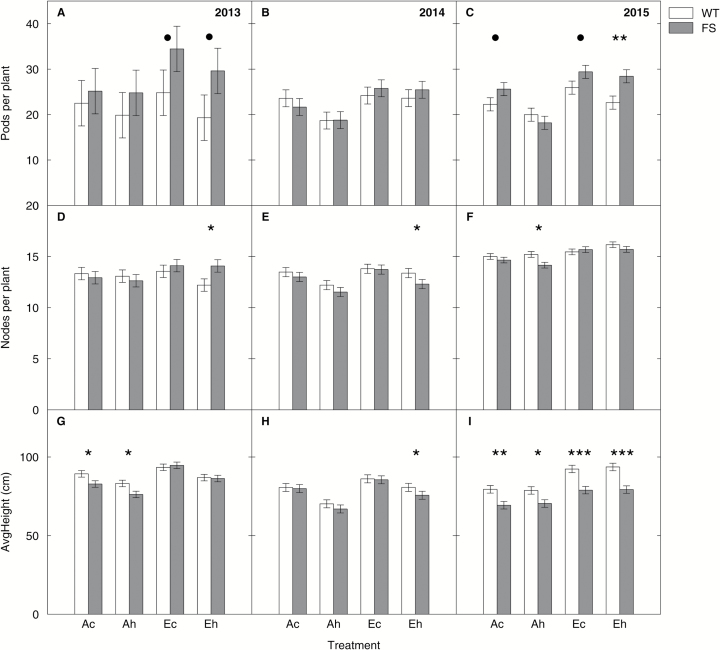
FS plants were shorter and tended to have more pods per plant than the WT, but did not differ consistently in the number of nodes per plant at the time of harvest
A significant main effect of genotype (Table 3) on the number of pods per plant was observed in 2013 (Fig. 7A) and 2015 (Fig. 7C), with on average 7 (2013, P<0.05) and 3 (2015, P<0.01) more pods on the FS plants. There was a significant main effect of genotype on number of nodes in 2014 (Fig. 7E, P<0.05) and 2015 (Fig. 7F, P<0.1) but pairwise comparisons between genotypes showed no clear treatment effect. A significant main effect of genotype on plant height (Fig. 7G–I) was observed in all three years, with FS plants being on average smaller than WT plants (2013, −3.2 cm, P<0.05; 2014, −2.4 cm, P<0.05; 2015, −11.6 cm, P<0.001).
Fig. 7.
Pods per plant, nodes per plant, and average height of the wild-type (WT) and bifunctional FBP/SBPase-expressing (FS) plants in comparison in the three growing seasons. Data were derived from plants grown under the ‘Ac’ (400 μmol mol−1 [CO2], control temperature), ‘Ah’ (400 μmol mol−1 [CO2], heated +3.5 °C), ‘Ec’ (600 μmol mol−1 [CO2], control temperature), and ‘Eh’ (600 μmol mol−1 [CO2], heated +3.5 °C) treatments at the Soy-T-FACE experiment. Data are from a representative sampling along 1 m length of a middle row within each subplot in the three growing seasons. Error bars are ±SE of the LS means estimate as derived from the complete block ANOVA (Table 3). Symbols mark significant differences between the WT (empty bars) and FS (gray bars) (• <0.1, * <0.05. ** <0.01 *** <0.001).
Discussion
The objective of this research was to determine the potential for soybean overexpressing SBPase to be better adapted to growth in a future high CO2 atmosphere and warmer climate as compared with current conditions. This study builds upon previous research showing that SBPase overexpression leads to higher A and higher biomass production in tobacco (Miyagawa et al., 2001; Lefebvre et al., 2005; Rosenthal et al., 2011; Simkin et al., 2015), which we now extend to show an effect on yield in a major food crop and under simulated climate change conditions in the field. We hypothesized that expressing a cyanobacterial, bifunctional FBP/SBPase in soybean would lead to higher A and higher yield, particularly under conditions that favor RuBP regeneration limitation, namely future elevation of [CO2] and temperature. This hypothesis is supported by the consistent observation across all three years that the transgenic plants in the Eh treatment were able to maintain SY at the Ec treatment levels, while WT plants showed significant losses in SY (Fig. 5). This suggests that expression of FBP/SBPase in soybean may help to prevent yield losses in the likely scenario where [CO2] and temperatures will increase together under future climate conditions.
Studies with tobacco plants showed that either overexpression of SBPase or expression of the bifunctional FBP/SBPase enzyme generally enhanced A of the transgenic plants in comparison with the WT under both ambient (Miyagawa et al., 2001; Lefebvre et al., 2005) and elevated (Rosenthal et al., 2011) [CO2]. Similarly, we observed that FS plants tended to have higher photosynthetic rates, but the significant genotype×DOY interactions suggested an effect of environmental conditions and/or developmental stage on the difference between FS and WT A. Our in situ gas exchange measurements throughout the three seasons covered a wide range of light levels and temperatures, with light levels ranging from 250 μmol photons m−2 s−1 to 2000 μmol photons m−2 s−1 and temperatures ranging from 13 °C to 35.5 °C. Because SBPase is only potentially limiting when A is RuBP limited, a positive effect of SBPase on A was only expected under conditions consistent with RuBP regeneration limitation. Higher A of FS plants in comparison with the WT predominantly occurred during peak light and higher temperatures, and this observation is consistent with other studies. SBPase-overexpressing tobacco plants had up to 12% higher midday A when grown in a controlled-environment greenhouse under high light (Lefebvre et al., 2005) and up to 14% higher midday A when grown in the field (Rosenthal et al., 2011) in comparison with WT tobacco plants. However, tobacco plants overexpressing SBPase grown under low light conditions and short-day conditions in the greenhouse in the winter did not show increases in growth and A relative to WT tobacco plants (Lefebvre et al., 2005).
In addition to the expected increase in Jmax, Vc,max was also increased in the FS plants (Fig. 4), indicating an increase in SBPase activity having a pleiotropic effect on the activity of Rubisco in vivo. Although the increase in Vc,max was lower than the increase in Jmax, it would result in some increase in A even at low [CO2]. This may explain why higher A was not only observed under the Eh treatment as hypothesized (Supplementary Fig. S2). This effect on Vc,max was also observed by Lefebvre et al. (2005) for tobacco overexpressing SBPase grown in a controlled-environment greenhouse (light levels of 600–1600 μmol photons m−2 s−1), but Rosenthal et al. (2011) did not find any differences in Vc,max between field-grown WT and SBPase-overexpressing tobacco plants. The Rubisco activation state may be influenced by a mechanism described by Rokka et al. (2001) and observed by Feng et al. (2007a, b) in rice plants overexpressing SBPase, which maintained A and growth rate under heat and osmotic stress and recovered faster relative to WT plants. Feng et al. (2007a, b) suggest that overexpression of SBPase prevented the sequestration of Rubisco activase (RCA) to the thylakoid membranes, thus keeping the Rubisco activation state high. This may help explain the observed consistent yield of the FS plants under the high temperature and elevated [CO2]treatment, but it is unclear why this proposed mechanism, if it occurred, was not beneficial in the heat treatment under ambient [CO2].
The measured rates of A in our experiment did not always differ under high light and high temperature conditions, indicating that factors beyond temperature and light may play a role. Rosenthal et al. (2011) only observed significant differences in A during the vegetative stage and not during the reproductive stage in the field. Under controlled environment conditions, Lefebvre et al. (2005) also found an effect primarily during early growth for tobacco overexpressing SBPase, while the tobacco plants expressing FBP/SBPase exhibited higher differences in growth rates in older plants (Miyagawa et al., 2001). The field-grown, FBP/SBPase-expressing soybean plants in our study exhibited greater differences in A with maturation. We observed no differences in A during the early vegetative developmental stages (V3–V5) even under high light levels of up to 2000 μmol m−2 s−1. Differences here compared with previous work might result from the use of different species (tobacco versus soybean), the type of promoter used [Cauliflower mosaic virus (CaMV) 35S versus Peanut chlorotic streak caulimovirus (PCISV)], differences between the SBPase and FBP/SBPase enzyme, and/or the growth conditions (field versus controlled environment).
Higher A in the tobacco plants (over)expressing either SBPase or the bifunctional FBP/SBPase enzyme translated into higher biomass in all three previous studies (Miyagawa et al., 2001; Lefebvre et al., 2005; Rosenthal et al., 2011). Here, AGB of the FS soybeans did not show consistent increases over the WT, but as leaf biomass was not included in this measure, our results cannot be compared directly with those from tobacco. Lefebvre et al. (2005) also reported a significant increase in stem height of the SBPase-overexpressing tobacco plants, while stem height of our FS soybean plants did not differ (2013 and 2015) from the WT or was even significantly smaller (2015). However, for grain crops such as soybean, increases in SY instead of total AGB are of agronomic interest. Assuming a constant carbon (C) concentration, a higher SY requires either more C to be fixed or that more of the fixed C is partitioned into seeds. In the first case, a correlation between the rate of A and SY would be expected. Modeling (Zhu et al., 2007) and previous empirical studies (Harrison et al., 1998; Miyagawa et al., 2001; Lefebvre et al., 2005; Rosenthal et al., 2011) suggest that overexpression of SBPase increases C flow through the PCR cycle, thus in theory making more C available for seed production. However, despite our observation of higher A of FS soybean plants throughout all seasons particularly under high light and high temperature conditions (Table 2, Supplementary Fig. S2), significant differences in yield between FS and WT plants were only observed on the Eh treatment.
These differences in the responses of A and yield of the transgenic plants in comparison with the WT between years and within seasons highlight the complexity of relating these traits under variable environmental conditions. Increases in A do not necessarily translate to higher biomass or yield (Long et al., 2006) and increased allocation of carbon may have occurred to plant organs that were not measured in this experiment (roots, root nodules, leaves) or may have been offset by enhanced respiration. Under the combined heat and elevated [CO2] treatment (Eh), FS plants maintained SY at the same level as under the Ec (control temperature and elevated [CO2]) treatment, while WT SY was reduced by 11% to 22% in the three years. This difference was related to a higher number of pods of FS plants in 2013 and 2015 and a higher seed weight in 2014, indicating differences in allocation between the WT and FS plants. Accordingly, HI was also increased significantly for the FS plants. The observed consistent reduction of WT SY under heat treatment in our three season long experiment was similar to the effect reported by Ruiz-Vera et al. (2013) for another soybean cultivar (‘Pioneer 93B15’) in the 2011 season, suggesting that the yield loss under heat stress may not only be restricted to cultivar ‘Thorne’, which was used in our study.
In conclusion, we show that expression of the cyanobacterial, bifunctional FBP/SBPase generally leads to higher A in field-grown soybean and prevents yield losses under high [CO2] and high temperature, conditions that are expected for the near future. These findings are the first to show with a major food crop under open-air field conditions that manipulation of the PCR cycle can be used to mitigate the effects of global increases in temperature on yield under future elevated CO2 conditions.
Supplementary Material
Acknowledgements
The authors would like to thank David Drag, the SoyFACE technical staff (Brad Dalsing, Kannan Puthuval, Chris Montes, Chad Lantz), and undergraduate students for help with the field experiment. The Raines Lab (Christine Raines, Andrew Simkin, Patricia Lopez, Stuart Fisk, Kenny Brown) is thanked for help with western blotting. This work was supported in part by an appointment to the Agricultural Research Service (ARS) Postdoctoral Research Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy and the US Department of Agriculture. ORISE is managed by ORAU under DOE contract number DE-AC05-06OR23100. This material is based upon work that is supported by the National Institute of Food and Agriculture, US Department of Agriculture, under award number 2014-67013-21783. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA, ARS, DOE, or ORAU/ORISE.
Glossary
Abbreviations:
- A
net rate of CO2 uptake per unit leaf area (μmol m−2 s−1)
- Ac
ambient CO2, control temperature treatment
- [CO2]
CO2 concentration
- Ah
ambient CO2, heated treatment
- AGB
above-ground biomass
- Ec
elevated CO2, control temperature treatment
- Eh
elevated CO2, heated treatment
- FBP/SBPase
fructose-1,6/sedoheptulose-1,7-bisphosphatase
- FS plants
bifunctional FBP/SBPase-expressing plants
- HI
harvest index
- Jmax
RuBP regeneration capacity (μmol m−2 s−1)
- PAR
photosynthetic active radiation (μmol m−2 s−1)
- PCR
photosynthetic carbon reduction
- RuBP
ribulose-1,5-bisphosphate
- SBPase
sedoheptulose-1,7-bisphosphatase
- SW
seed weight
- SY
seed yield
- Vc,max
maximum carboxylation rate of Rubisco (μmol m−2 s−1)
- WT
wild type.
References
- Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP. 2012. Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant, Cell and Environment 35, 38–52. [DOI] [PubMed] [Google Scholar]
- Alexandratos N, Bruinsma J. 2012. World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12-03. Rome: FAO. [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP. 2005. The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220, 434–446. [DOI] [PubMed] [Google Scholar]
- Bihmidine S. 2012. Crop improvement through biotechnology: targeting drought resistance and photosynthesis . Dissertation, University of Nebraska. [Google Scholar]
- Blankenship RE, Tiede DM, Barber J, et al. 2011. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809. [DOI] [PubMed] [Google Scholar]
- Clemente TE. 1997. Agrobacterium-mediated transformation. Journal of Experimental Botany 48, 151–155. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Feng L, Han Y, Liu G, et al. 2007a Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis and growth under salt stress in transgenic rice plants. Functional Plant Biology 34, 822–834. [DOI] [PubMed] [Google Scholar]
- Feng L, Wang K, Li Y, et al. 2007b Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Reports 26, 1635–1646. [DOI] [PubMed] [Google Scholar]
- Harrison EP, Willingham NM, Lloyd JC, Raines CA. 1998. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 204, 27–36. [Google Scholar]
- Hay WT. 2012. Engineering cyanobacterial genes into Glycine max (Soybean) leads to increased photosynthesis and productivity. Dissertation, University of Illinois at Urbana-Champaign. [Google Scholar]
- Henkes S, Sonnewald U, Badur R, Flachmann R, Stitt M. 2001. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. The Plant Cell 13, 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchee MAW, Connor-Ward DV, Newell CA, et al. 1988. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Nature Biotechnology 6, 915–922. [Google Scholar]
- IPCC 2013. Annex II: climate system scenario tables. In: Prather M, Flato G, Friedlingstein Pet al. , eds. In: Climate change 2013: the physical science basis. contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, USA: Cambridge University Press. [Google Scholar]
- Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M. 2005. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiology 138, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. 2004. Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology 55, 591–628. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. 2006. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54, 2393–2401. [DOI] [PubMed] [Google Scholar]
- Masuda T, Goldsmith PD. 2009. World soybean production: area harvested, yield, and long-term projections. International Food and Agribusiness Management Review 12, 143–161. [Google Scholar]
- Miglietta F, Peressotti A, Vaccari FP, Zaldei A, DeAngelis P, Scarascia-Mugnozza G. 2001. Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytologist 150, 465–476. [Google Scholar]
- Miyagawa Y, Tamoi M, Shigeoka S. 2001. Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nature Biotechnology 19, 965–969. [DOI] [PubMed] [Google Scholar]
- OECD/FAO 2012. OECD-FAO Agricultural Outlook 2012–2021 OECD Publishing and FAO; http://dx.doi.org/10.1787/agr_outlook-2012-en, last accessed 15 August 2016. [Google Scholar]
- Ort DR, Merchant SS, Alric J, et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112, 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines CA. 2003. The Calvin cycle revisited. Photosynthesis Research 75, 1–10. [DOI] [PubMed] [Google Scholar]
- Raines CA. 2006. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant, Cell and Environment 29, 331–339. [DOI] [PubMed] [Google Scholar]
- Raines CA. 2011. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiology 155, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines CA, Harrison EP, Olcer H, Lloyd JC. 2000. Investigating the role of the thiol-regulated enzyme sedoheptulose-1,7-bisphosphatase in the control of photosynthesis. Physiologia Plantarum 110, 303–308. [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO, Herman JC, Lupkes SJ. 1993. H ow a soybean plant develops. Special Report No 53. Ames, IA: Iowa State University of Science and Technology. [Google Scholar]
- Rokka A, Zhang L, Aro EM. 2001. Rubisco activase: an enzyme with a temperature-dependent dual function? The Plant Journal 25, 463–471. [DOI] [PubMed] [Google Scholar]
- Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR. 2011. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biology 11, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands DJ, Frame DJ, Ackerley D, et al. 2012. Broad range of 2050 warming from an observationally constrained large climate model ensemble. Nature Geoscience 5, 256–260. [Google Scholar]
- Ruiz-Vera UM, Siebers M, Gray SB, et al. 2013. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiology 162, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Vera UM, Siebers MH, Drag DW, Ort DR, Bernacchi CJ. 2015. Canopy warming caused photosynthetic acclimation and reduced seed yield in maize grown at ambient and elevated [CO2]. Global Change Biology 21, 4237–4249. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, McAusland L, Headland LR, Lawson T, Raines CA. 2015. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. Journal of Experimental Botany 66, 4075–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences, USA 108, 20260–20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Canberra, Australia: CSIRO Publishing. [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- Zhu XG, de Sturler E, Long SP. 2007. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiology 145, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.