Highlight
A study of ERECTA cytoplasmic domain predicts a primary phosphorylation site in its activation segment and indicates that signal transduction by ERECTA differs in specific developmental processes.
Keywords: Activation segment, ERECTA, kinase domain, LRR-RLK, phosphorylation, receptor kinase, signal transduction, site-directed mutagenesis, structure–functional analysis
Abstract
In plants, extracellular signals are primarily sensed by plasma membrane-localized receptor-like kinases (RLKs). ERECTA is a leucine-rich repeat RLK that together with its paralogs ERECTA-like 1 (ERL1) and ERL2 regulates multiple aspects of plant development. ERECTA forms complexes with a range of co-receptors and senses secreted cysteine-rich small proteins from the EPF/EPFL family. Currently the mechanism of the cytoplasmic domain activation and transmission of the signal by ERECTA is unclear. To gain a better understanding we performed a structure–function analysis by introducing altered ERECTA genes into erecta and erecta erl1 erl2 mutants. These experiments indicated that ERECTA’s ability to phosphorylate is functionally significant, and that while the cytoplasmic juxtamembrane domain is important for ERECTA function, the C-terminal tail is not. An analysis of multiple putative phosphorylation sites identified four amino acids in the activation segment of the kinase domain as functionally important. Homology of those residues to functionally significant amino acids in multiple other plant RLKs emphasizes similarities in RLK function. Specifically, our data predicts Thr812 as a primary site of phosphor-activation and potential inhibitory phosphorylation of Tyr815 and Tyr820. In addition, our experiments suggest that there are differences in the molecular mechanism of ERECTA function during regulation of stomata development and in elongation of above-ground organs.
Introduction
Intercellular communications are essential for development of multicellular organisms where cell proliferation and differentiation must be cooperative and structured to attain a desired shape and function. Plants especially rely on intercellular communications as cell behavior is often position-dependent. To detect extracellular signals, plant cells have a large group of receptor-like kinases (RLKs). These receptors possess a structurally diverse extracellular ligand-sensing domain, a single-pass transmembrane domain, and a cytoplasmic serine/threonine/tyrosine kinase domain.
The ERECTA family (ERf) RLKs appeared early during land plant evolution and are involved in the regulation of multiple developmental processes (Villagarcia et al., 2012, Shpak, 2013). During embryogenesis they stimulate cotyledon elongation (Chen and Shpak, 2014). Post-embryonically, ERfs promote growth of all above-ground organs (Shpak et al., 2004). ERfs have been demonstrated to regulate stomata formation, the function of the shoot apical meristem (SAM), and the development of flowers (Shpak, 2013). In angiosperms, ERf consists of two or more genes, with Arabidopsis having three: ERECTA (ER), ERECTA-like 1 (ERL1) and ERECTA-like 2 (ERL2) (Shpak et al., 2004, Villagarcia et al., 2012). Although all three genes regulate above-ground organ elongation, they exhibit unequal redundancy. While erecta mutants have compact inflorescences due to shorter internodes and pedicels, single mutations in erl1 and erl2 confer no detectable phenotype (Torii et al., 1996; Shpak et al., 2004). Loss of all three genes leads to severe dwarfism (Shpak et al., 2004). The reduced growth of above-ground plant organs in ERf mutants is associated with a decrease in the cell proliferation rate (Shpak et al., 2003, 2004). An analysis of pedicel growth suggested that ERECTA accelerates the elongation of cells along the proximo-distal axis and shortens the duration of the cell cycle (Bundy et al., 2012). The asymmetric redundancy of ERf receptors is also evident during stomata development. In the initial stage of the stomata development process, ERfs synergistically inhibit differentiation of protodermal cells into meristemoid mother cells. ERECTA plays a major role during this process, as an increased number of asymmetric cell divisions has been observed only in the erecta single mutant (Shpak et al., 2005). Once meristemoids are formed, ERL1 and ERL2 inhibit their differentiation into guard mother cells (Shpak et al., 2005). In the SAM, ERfs seem to be equally redundant; the receptors synergistically inhibit meristem enlargement, promote leaf initiation, and contribute to establishment of phyllotaxy (Chen et al., 2013; Uchida et al., 2013). Finally, ERfs play an important role in the regulation of ovule and early anther development (Pillitteri et al., 2007; Hord et al., 2008; Bemis et al., 2013). The er erl1 erl2 mutant is sterile with compromised male and female fertility (Shpak et al., 2004).
ERECTA, ERL1, and ERL2 receptors form homo- and heterodimers (Lee et al., 2012). They also make complexes with SOMATIC EMBRYOGENESIS RECEPTOR KINASEs (SERKs) and with the transmembrane receptor-like protein TOO MANY MOUTHS (TMM) (Lee et al., 2012; Meng et al., 2015). The activity of ERf receptors is regulated by a family of secreted cysteine-rich small proteins from the EPF/EPFL family, which can function as agonists or antagonists (Shimada et al., 2011). A MAP kinase cascade consisting of YODA, MKK4, MKK5, MPK3, and MPK6 functions downstream (Bergmann et al., 2004; Wang et al., 2007; Meng et al., 2012). Changes in the structure of receptor complexes upon ligand binding and the mechanism of signal transmission from ERfs to YODA are currently not clear.
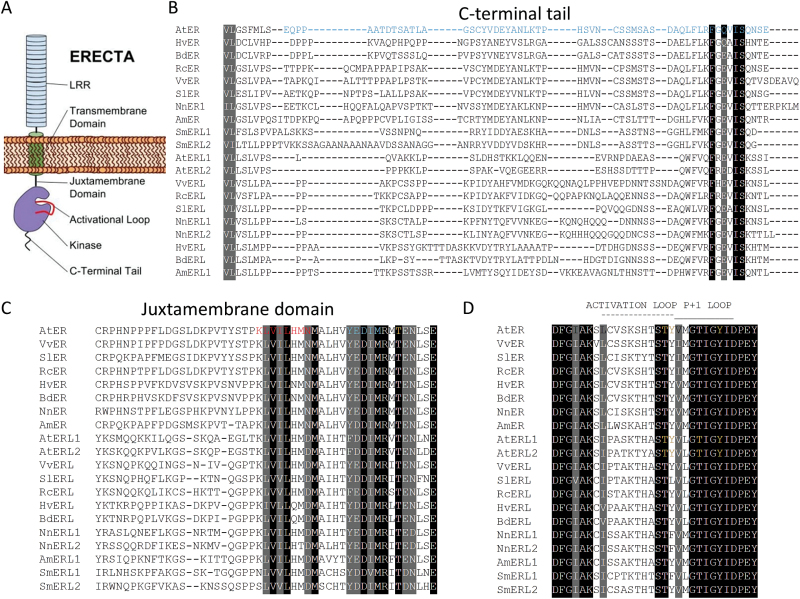
ERf protein structure consists of an extracellular leucine-rich domain (LRR), a single-span transmembrane domain, and a cytoplasmic Ser/Thr kinase domain flanked by a juxtamembrane domain (JMD) and a C-terminal tail (Fig. 1A). Previously, it was shown that the cytoplasmic segment of ERECTA is functionally important as its deletion leads to a dominant negative phenotype (Shpak et al., 2003). To gain a better understanding of how ERECTA activates downstream signaling, we performed a structure–function analysis of this domain. These studies demonstrated that the cytoplasmic JMD is important for ERECTA function, but the C-terminal tail is not. Our experiments further confirmed that ERECTA is a functional kinase and suggested that Thr807, Thr812, Tyr815, and Tyr820 in the activation segment of the kinase domain are functionally important. Based on our results, we hypothesize that phosphorylation of ERECTA at Thr812 might have a stimulatory effect on receptor activity, and at Tyr815 and Tyr820 an inhibitory effect. Our experiments also indicated that the molecular mechanism of ERECTA function is different during regulation of stomata development and in elongation of above-ground organs.
Fig. 1.
Comparison of amino acid sequences of ERECTA family proteins in different species. (A) Domain structure of the ERECTA receptor. (B–D) MAFFT alignment of the predicted amino acid sequences of ERECTA family genes from Arabidopsis thaliana (At), Vitis vinifera (Vv), Solanum lycopersicum (Sl), Ricinus communis (Rc), Hordeum vulgare (Hv), Brachypodium distachyon (Bd), Nelumbo nucifera (Nn), Amborella trichopoda (Am), and Selaginella moellendorffii (Sm). Residues that are identical among the sequences are shown with a black background, and those that are similar among the sequences are shown with a gray background. (B) The C-terminus. The blue residues have been deleted in pPZK110 and in pPZK111. (C) The juxtamembrane domain. The red residues have been deleted in pPZK104, the blue residues in pPZK105. Threonine in yellow has been substituted with Ala in pPZK102. (D) The activation loop. The predicted phosphorylation sites according to the Arabidopsis Protein Phosphorylation Site Database (PhosPht) are in yellow.
Materials and methods
Plant material and growth conditions
The Arabidopsis ecotype Columbia (Col) was used as the wild-type (WT). The er-105 and er-105 erl1-2 erl2-1 mutants have been described previously (Torii et al., 1996; Shpak et al., 2004). Plants were grown on a soil mixture of a 1:1 ratio of Promix PGX (Premier Horticulture Inc.) and Vermiculite (Pametto Vermiculite Co.) and were supplemented with Miracle-Gro (Scotts) and approximately 3.5mg cm–3 of Osmocoat 15-9-12 (Scotts). All plants were grown at 20 °C under long-day conditions (18 h light/6 h dark).
Generation of transgenic plants
In all plasmids except pPZK111 the substitutions/deletions were introduced into the genomic ERECTA-RLUC sequence by overlap extension PCR using pESH427 as a template (Karve et al., 2011). The amplified fragments were digested with PstI, inserted into pESH427, and sequenced. The constructs carry the endogenous ERECTA promoter and the 35S terminator. The pPZK111 was generated by overlap extension PCR using pKUT196 as a template (Godiard et al., 2003). The amplified fragment was digested with PstI, inserted into pKUT196, and sequenced. This construct carries the endogenous ERECTA promoter and terminator. The backbone of all plasmids is the vector pPZP222. The plasmids were introduced into Agrobacterium tumefaciens strain GV3101/pMP90 by electroporation, and into Arabidopsis er-105 and er-105 erl1-2/+ erl2-1 plants by vacuum infiltration. The transgenic plants were selected based on gentamicin resistance and the number of rescued lines has been quantified based on general plant morphology (Supplementary Tables S1 and S2). The er-105 erl1-2 erl2-1 mutants were selected based on kanamycin resistance and the homozygous status of the erl1-2 mutation was confirmed by PCR with the primers erl1g3659 (GAGCTTGGACATATAATC), erl1g4411.rc (CCGGAGAGATTGTTGAAGG), and JL202 (CATTTTATAATAACGCTGCGGACATCTAC). In addition, for transgenic lines transformed with pPZK102, pPZK110, and pPZK111 constructs, the homozygous status of the erl1-2 mutation was confirmed by analysis of kanamycin resistance in the progeny. The quantitative phenotypic analysis of er erl1 erl2 plants transformed with the described constructs has been done in T3 generation once their genetic status was established.
Measurement of Renilla luciferase activity
ERECTA-RLUC protein expression was measured by monitoring Renilla luciferase activity with a 20/20n single-tube luminometer in T1 inflorescences or in T2 8-d-old seedlings using the Renilla Luciferase Reporter Assay (Promega). The protein concentration in each sample was determined using the Bradford assay.
Analysis of mutant phenotypes
Measurements of stomata index and clustering were done on the abaxial side of cotyledons from 17-d-old seedlings using differential interference contrast (DIC) microscopy. For DIC, seedlings were incubated in a solution of 9:1 ethanol:acetic acid overnight, rehydrated with an ethanol series to 50% (v/v) ethanol, and then cleared in a mixture of 8:1:1 chloral hydrate:distilled water:glycerol.
Immunoblot analysis
The crude microsomal proteins were isolated from 11-d-old WT and T2 T807D seedlings (~0.4g per sample) using a method described by Zhang et al. (2011). The last step of this method, an enrichment for plasma membrane proteins, was omitted. Immunoblot analysis was performed as previously described with minor modifications (Shpak et al., 2003). Proteins were run on 8% or 10% SDS-PAGE. Primary anti-BAK1 polyclonal antibodies (Agrisera) were used at a dilution of 1:5000 followed by the secondary HRP Conjugated Goat Anti-Rabbit IgG antibody (Agrisera) at a dilution of 1:10 000. Primary anti-Rluc monoclonal antibodies (Millipore; clone 5B11.2) were used at a dilution of 1:5000 followed by the secondary HRP Conjugated Goat Anti-Mouse IgG antibody at a dilution of 1:7500. The detection of HRP was performed with a SuperSignal West Pico Rabbit IgG detection kit (Pierce).
Sequence alignment
Full-length amino acid sequences of ERECTA family proteins from different species were retrieved from the NCBI database and aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Arabidopsis Genome Initiative
Arabidopsis Genome Initiative numbers for the genes discussed here are as follows: ER (At2g26330), ERL1 (At5g62230), and ERL2 (At5g07180).
Results
The juxtamembrane domain (JMD) is important for ERECTA function, but the C-terminal tail is not
The activity of a RLK’s kinase domain is often modulated by the flanking regions: the JMD and the C-terminal tail. In some receptors those regions inhibit kinase function, in others they are essential for the enzymatic activity (Wang et al., 2005b; Oh et al., 2009b, 2014). Phosphorylation of residues within these regions can often alter their function. For example, phosphorylation of Ser and Thr residues in the BRI1 C-terminal tail disables its inhibitory role (Wang et al., 2005b).
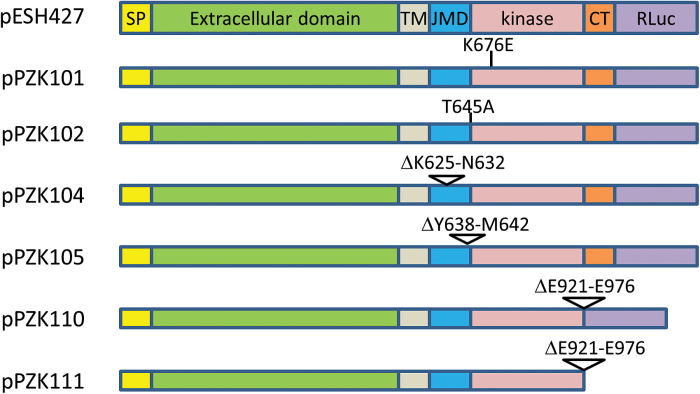
To examine whether regions flanking the ERECTA kinase domain have specific function, we created multiple constructs with modified genomic ERECTA sequences under the control of the native promoter (Fig. 2). With the exception of one (pPZK111), all constructs contained Renilla Luciferase (RLUC) at the C-terminus of the receptor to monitor the level of protein expression. The luciferase assay is a fast, reliable, and relatively cheap method to measure protein levels. Most significantly, it reflects the protein concentration in Arabidopsis extracts (Ramos et al., 2001; Subramanian et al., 2006). Protein titration assays and immunoblot analysis confirmed that RLUC activity reflects the level of ERECTA-RLUC accumulation in transgenic seedlings (Supplementary Fig. S1).
Fig. 2.
Schematic diagram of modifications introduced into the ERECTA protein. Triangles indicate deletions and lines indicate point mutations. SP, signal peptide; TM, transmembrane domain; JMD, juxtamembrane domain; CT, C-terminal tail; RLuc, Renilla Luciferase. In the constructs the genomic sequence of ERECTA is under the control of its native promoter and the 35S terminator. On the left are the names of the plasmids.
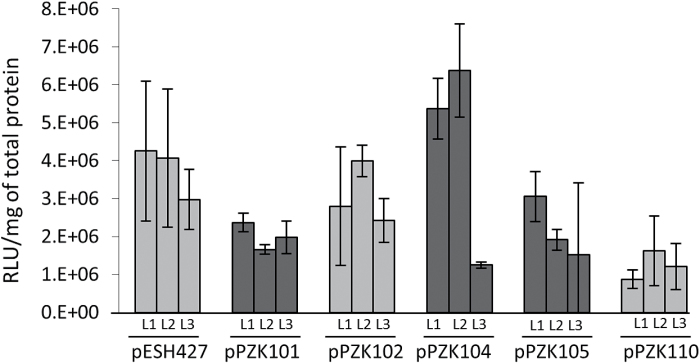
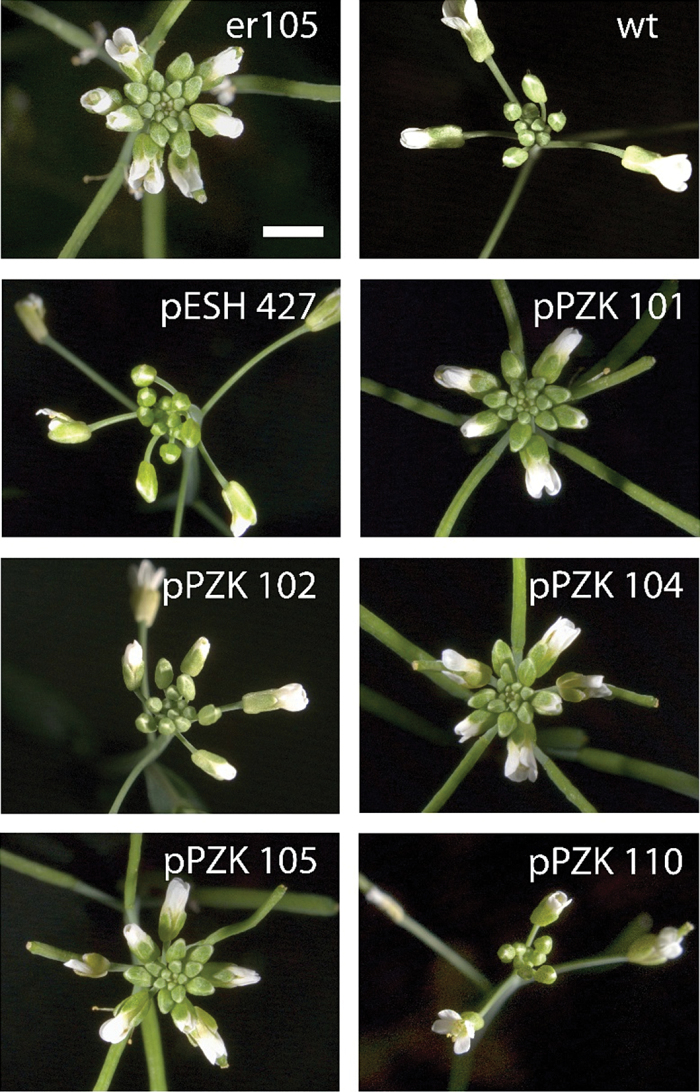
The unmodified ERECTA fused to RLUC (construct pESH 427) was used as a positive control. The constructs were transformed into er-105 and into er erl1/+ erl2 mutants and multiple independent transgenic lines were analyzed. Interestingly, we observed a decreased frequency of complementation in the T1 generation for constructs containing RLUC (Supplementary Tables S1 and S2). In our earlier experiments, the genomic ERECTA (pKUT196) rescued 100% of transgenic er-105 plants in the T1 generation (Karve et al., 2011), while this time only 27% of T1 plants were rescued by ERECTA-RLUC (pESH427). Similarly, ΔE921-E976 ERECTA (pPZK111) rescued 58% of T1 er-105 plants while ΔE921-E976 ERECTA-RLUC (pPZK110) rescued only 16% (Supplementary Table S1). As a result, the frequency of complementation in the T1 generation has not been used as a measure of a construct functionality. Instead, for each construct we analyzed multiple T1 plants with the goal of finding three to four independent transgenic lines with relatively similar protein expression. While analysis of protein expression detected a variation in the amount of ERECTA produced in different transgenic lines, the general ability of a construct to rescue the ERECTA phenotype did not correlate with the level of protein expression in selected lines (Fig. 3). For example, expression of ERECTA in non-complemented pPZK101, pPZK104, and pPZK105 transgenic lines is equal to or higher than that in complemented pPZK110 lines. Thus, we concluded that the inability of constructs to rescue er-105, er erl2, and er erl1/+ erl2 mutants was due to modification of ERECTA structure and not to poor expression of the protein.
Fig. 3.
ERECTA-RLUC is expressed in the majority of transgenic lines. The level of ERECTA-RLUC expression was determined by measuring luciferase activity per milligram of total protein in inflorescences of T1 transgenic plants. RLU indicates relative light units. The mean of three biological replicates is plotted; error bars represent the SD. Three independent transgenic lines (L1–L3) were analyzed. The lines that rescue the er-105 phenotype are in light grey and the lines that do not are in dark grey.
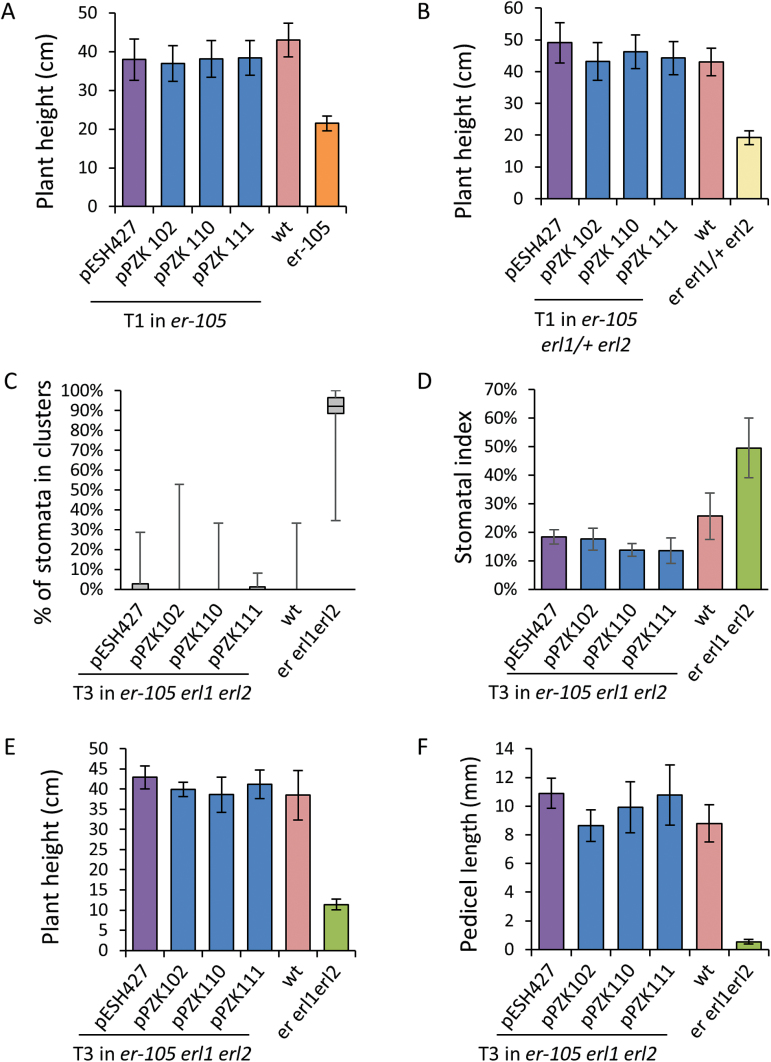
An analysis of ERf sequences from a broad variety of angiosperms suggests low conservation of the C-terminal tail except for a short stretch of amino acid residues at the very end (Fig. 1B). Two constructs (pPZK110 and pPZK111) were created to examine the role of the C-terminal tail in ERECTA function. In both constructs the last 56 amino acids of ERECTA were deleted; in pPZK110, ERECTA was fused with RLUC and in pPZK111 it was not (Fig 2). We were concerned that RLUC at the C-terminus might interfere with receptor function and that its presence could conceal any possible increased activity of ERECTA without the C-terminus tail. However, both constructs rescued inflorescence structure and plant height of er-105 and er erl1/+ erl2, similar to the positive control pESH427 (Fig. 4; Fig. 5A, B; Supplementary Table S1). In addition, pPZK110 and pPZK111 fully rescued stomata development, plant height, and pedicel length phenotypes of the er erl1 erl2 mutants (Fig. 5C–F). And while the stomatal index in the pPZK110 and pPZK111 er erl1 erl2 lines was reduced below wild-type levels, it was not statistically significantly different from that in the pESH427 line and therefore this decrease cannot be due to the absence of the C-terminus tail (Fig. 5D). Thus, the ERECTA C-terminus seems to be dispensable for regulation of plant architecture and stomata formation.
Fig. 4.
Inflorescence architecture reflects functionality of modified ERECTA receptors. Representative images of inflorescence apices of the wild-type (wt), er-105, and selected transgenic lines. All constructs were transformed into er-105. Scale bar =3 mm.
Fig. 5.
Deletion of the C-terminus domain (pPZK110 and pPZK111) or a point mutation in the JMD (T645A; pPZK 102) does not alter ERECTA’s ability to regulate stomata development or above-ground organ elongation. (A, B) Height of mature plants (A, n=11–34; B, n=8–29). (C–F) Constructs were transformed into er erl1/+erl2 mutants and transgenic er erl1 erl2 plants were analyzed in the T3 generation. In (C) the median is indicated as a thick horizontal line, upper and lower quartiles are represented by the top and the bottom of the boxes, and the vertical lines designate the maximum and the minimum. Epidermal phenotypes were analyzed on the abaxial side of 17-d-old cotyledons (n=8–13). (E.) Height of mature plants (n=9–18). (F) Length of mature pedicels on the main stem (n=80; eight measurements per stem). In (A, B, D–F) values are means ± SD.
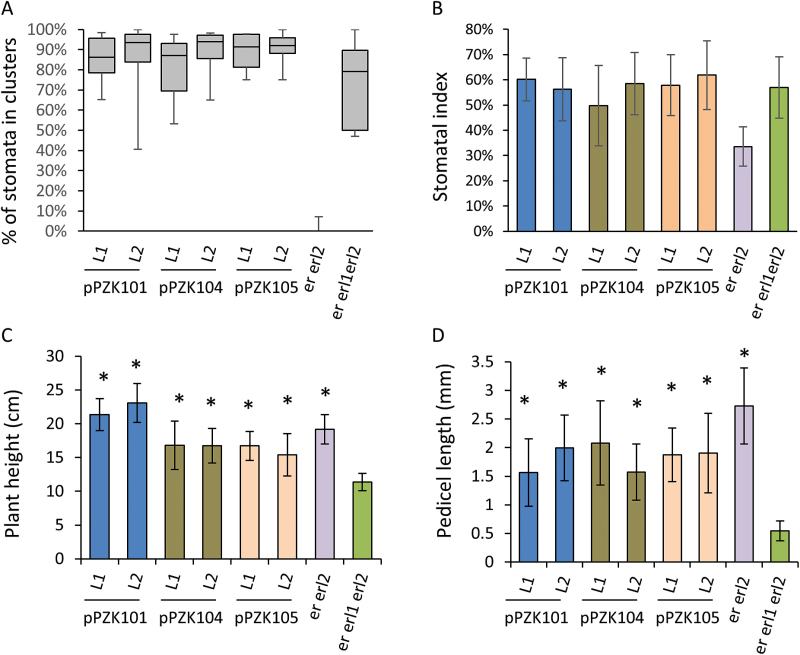
The JMD of the ERf receptors is 46–49 amino acids long. Comparison of this domain in different species revealed low conservation of the N-terminal half and high conservation of the C-terminal half (Fig. 1C). Several secondary structure prediction programs suggested the presence of a β-sheet and an α-helix in the conserved region of the JMD (Supplementary Fig. S2). Two constructs were created: one with eight residues deleted in the region of a potential β-sheet (pPZK104), and another with five residues deleted in the region of a potential α-helix (pPZK105) (Fig. 2). While ERECTA containing these modifications was expressed, it did not rescue the elongation phenotype of above-ground organs in the er-105 mutant (Fig. 3, Fig. 4). The deletions in the JMD also abolished the ability of ERECTA to inhibit stomata formation and to regulate stomata spacing (Fig. 6A, B).
Fig. 6.
While substitution of the conserved lysine residue (K676E) in the ATP binding site of the ERECTA kinase domain (pPZK101) or deletion of short JMD segments (pPZK104 and pPZK105) disrupt ability of ERECTA to rescue stomatal phenotypes of er erl1 erl2, those constructs partially rescue elongation of above-ground organs. Constructs were transformed into er erl1/+ erl2 mutants and two independent transgenic lines in the er erl1 erl2 background were analyzed in the T3 generation. In (A) the median is indicated as a thick horizontal line, upper and lower quartiles are represented by the top and the bottom of the boxes, and the vertical lines designate the maximum and the minimum. (A, B) Epidermal phenotypes were analyzed on the abaxial side of 17-d-old cotyledons (n=8–16). (B–D) Values are means ± SD. (C) Height of mature plants (n=12–21). (D). Lengths of mature pedicels on the main stem (n=80; eight measurements per stem). (C, D) Values significantly different from er erl1 erl2 (P<0.00001) are indicated by asterisks.
The phosphorylation and de-phosphorylation of JMD residues often regulates activity of RLKs, impinging on their enzymatic function or their ability to interact with downstream targets (Aifa et al., 2006; Heiss et al., 2006; Thiel and Carpenter, 2007; Chen et al., 2010). In Erf, the JMD contains only one conserved Thr (Thr645) and no conserved Ser or Tyr residues (Fig. 1C). This Thr is conserved not only in ERf but also in many other PELLE/RLK kinases (Supplementary Fig. S3). In Pto and XA21, this Thr plays an important biological function and is essential for their autophosphorylation (Sessa et al., 2000a; Chen et al. 2010). The Arabidopsis thaliana phosphorylation site database PhosPhAt predicts phosphorylation of Thr645 (Durek et al., 2010). To test whether Thr645 is important for ERECTA function, this residue was substituted with Ala in the construct pPZK102 (Fig. 2). This substitution did not alter ERECTA functionality and the construct rescued organ elongation defects in er-105 (Fig. 4, Fig. 5A, Supplementary Table S1), er-105 erl1/+ erl2 (Fig. 5B), and er-105 erl1 erl2 (Fig. 5E, F, Supplementary Table S1). In addition, the pPZK102 construct fully rescued stomata formation defects in the er erl1erl2 mutant (Fig. 5C, D). These data suggest that Thr645 is not essential for ERECTA function. While our data suggest that the JMD is essential for ERECTA functionality, we were unable to identify critical phosphorylation sites in this region.
Importance of the kinase domain for ERECTA function
In vitro ERECTA is a weak kinase (Lease et al., 2001, Meng et al., 2015). The phenotypes of several mutants with substitutions and deletions in the ERECTA kinase domain suggest that the ability to phosphorylate might be important for ERECTA function (Lease et al., 2001). Alternatively, these mutations might lead to receptor instability or change ERECTA’s capacity to bind co-receptors or downstream targets. The mutations are in the different α-helixes of the kinase domain and the exact function of those amino acids is not known. To further test whether the ability to phosphorylate is important for ERECTA function, a conserved lysine in the ATP-binding domain was replaced with a glutamate (K676E; pPZK101; Fig. 2). We identified several pPZK101 transgenic lines in the er-105 background with sufficient expression of ERECTA (Fig. 3). However, organ elongation defects were not rescued in those lines (Fig. 4). The pPZK101 construct was also unable to rescue epidermal phenotypes in the er-105 erl1 erl2 mutant (Fig. 5C, D). Thus, ERECTA is likely an active kinase in vivo and its ability to phosphorylate has functional significance.
The PhosPhAt database predicts multiple phosphorylation sites in the kinase domains of ERECTA, ERL1, and ERL2. Based on these predictions and evolutionary conservation, two residues were selected for alanine substitutions preventing phosphorylation: T823, a residue at the end of λEF helix, and T906, a residue in the αI helix. T823 of ERECTA is homologous to T872 of the receptor-like kinase HAESA, a residue phosphorylated in vitro and contributing to enzymatic activity of HAESA in vitro (Taylor et al., 2016). However, these substitutions did not disrupt functionality of ERECTA (Supplementary Fig. S4).
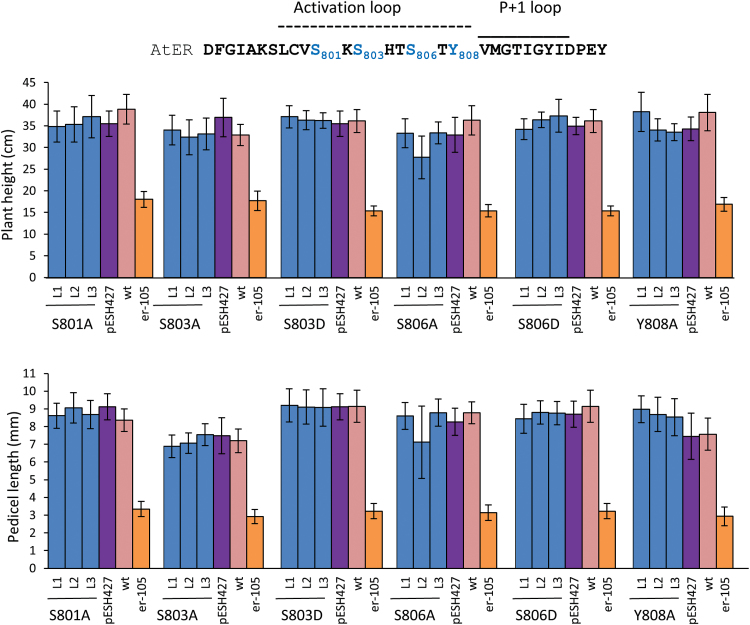
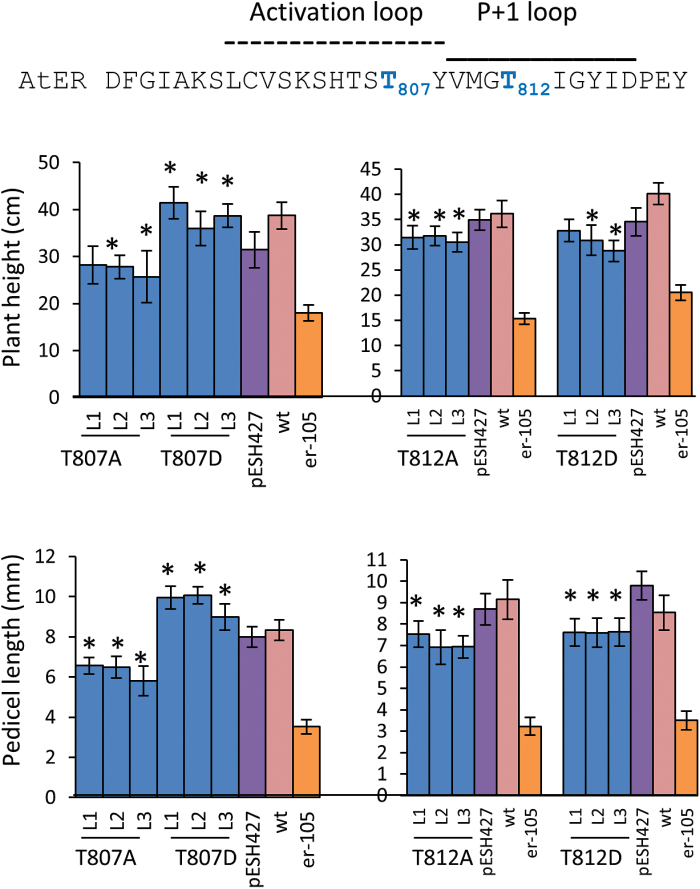
Next, we analyzed multiple Ser/Thr/Tyr in the activation segment by substituting them to Ala or as a phosphomimic to Asp. Alanine and aspartate substitution of Ser801, Ser803, Ser806, and Tyr808 in the activation loop did not have any effect on ERECTA function in control of organ elongation (Fig. 7). Substitutions of Thr812 to Ala and to Asp slightly, but statistically significantly, reduced functionality of ERECTA (Fig. 8). ERECTA with these substitutions was not able to fully rescue elongation defects of pedicels and stems when transformed into the er-105 mutant. These substitutions did not alter the expression level of ERECTA (Supplementary Fig. S5). Interestingly, these two substitutions had a very similar negative impact on ERECTA function, and the phenotype of plants expressing T812A and T812D did not differ statistically. Therefore, phosphorylation of Thr812 is unlikely to play a major role in the activation of ERECTA.
Fig. 7.
Site-directed mutagenesis of four potential phosphorylation sites in the activation loop of ERECTA suggests that these residues are not critical for ERECTA function. To determine ERECTA functionality, the constructs were transformed into er-105, and the height of mature plants (n=9–18) and the length of pedicels on the main stem (n=40; eight measurements per stem) were measured. Error bars represent ±SD. Three independent transgenic lines (L1–L3) were analyzed in the T2 generation. The mutated residues are in blue in the sequence at the top.
Fig. 8.
Site-directed mutagenesis of two conserved threonines in the activation segment impairs ERECTA function. To determine ERECTA functionality, the constructs were transformed into er-105, and the height of mature plants (n=9–18) and the length of pedicels on the main stem (n=40; eight measurements per stem) were measured. Error bars represent ±SD. Three independent transgenic lines (L1–L3) were analyzed in the T2 generation. The mutated residues are in blue in the sequence at the top. The transgenic line values significantly different from pESH427 (P<0.005) are indicated by asterisks.
Another residue that is important for ERECTA function is Thr807. The T807A substitution substantially reduced functionality of ERECTA, while organ elongation in plants expressing ERECTA with T807D substitution was similar to the wild-type or even greater (Fig. 8). Based on these data, we speculate that phosphorylation of Thr807 might have a positive impact on ERECTA function.
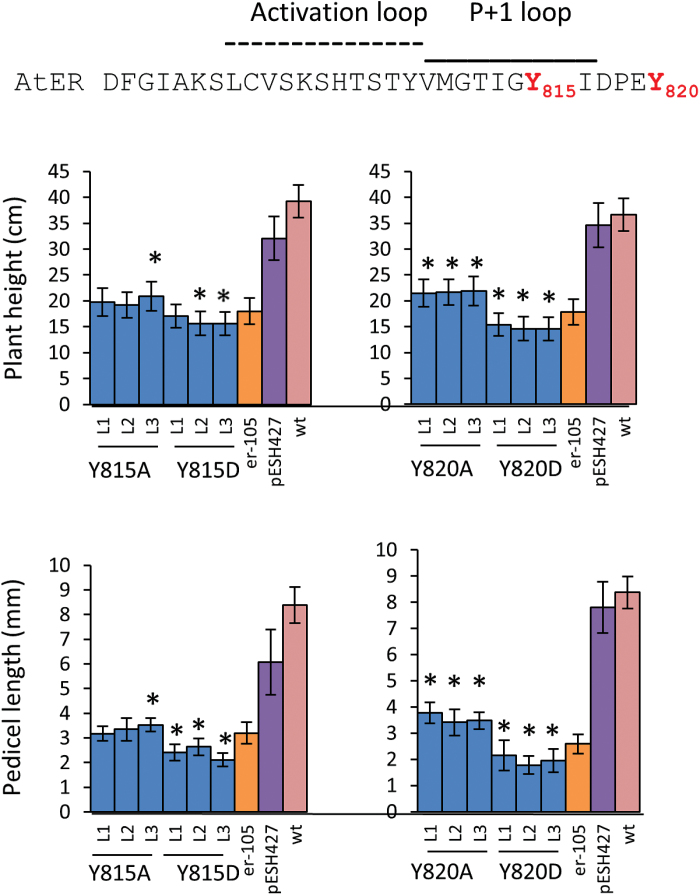
Finally, we observed that two Tyr substitutions had a very strong impact on plant growth. Both Y815A and Y820A strongly reduced ERECTA functionality, but were statistically different from er-105, suggesting that with those substitutions ERECTA retained a very low level of functionality (Fig. 9). Interestingly, substitutions of these Tyr to Asp resulted in a dominant negative phenotype (Fig. 9). Plants expressing ERECTA with Y815D or with Y820D were statistically shorter compared to er-105. This result suggests that these two Tyr are critical for ERECTA functionality and their phosphorylation might have a negative impact on ERECTA function.
Fig. 9.
Site-directed mutagenesis of two conserved tyrosines in the activation segment of ERECTA suggests a negative role of their potential phosphorylation. To determine ERECTA functionality, the constructs were transformed into er-105, and the height of mature plants (n=9–18) and the length of pedicels on the main stem (n=40; eight measurements per stem) were measured. Error bars represent ±SD. Three independent transgenic lines (L1–L3) were analyzed in the T2 generation. The mutated residues are in red in the sequence at the top. The transgenic line values significantly different from er-105 (P<0.005) are indicated by asterisks.
Distinct signaling mechanisms of ERECTA in multiple developmental pathways
While deletions in the JMD (pPZK 104 and pPZK 105) or disruption of the kinase activity by the K676E substitution (pPZK 101) destroyed ERECTA’s ability to regulate stomata development and fertility in er erl1 erl2 or to rescue er, er erl2, and er erl1/+ erl2 morphogenetic defects, these constructs were able to partially rescue stem and pedicel elongation in the er erl1 erl2 mutant (Fig. 6C, D; Fig. 10). In Fig. 6C, D the plant height and pedicel length are compared in fully mature plants. Because er erl1 erl2 grows slower and for a longer period of time, the plants are of different ages. If we compare plants of similar age as in Fig. 10, the ability of pPZK101, pPZK 104, and pPZK105 to partially rescue the er erl1 erl2 mutant becomes even more obvious. We speculate that in the er and er erl2 backgrounds the ability of pPZK101, pPZK 104, and pPZK 105 to alter organ elongation is not evident due to much stronger impact of ERL1 and ERL2 on plant growth. Taken together, these results suggest that the signal transduction by ERECTA is different in organ elongation versus control of stomata formation and development of flower organs.
Fig. 10.
pPZK101, pPZK104, and pPZK105 constructs can partially rescue elongation of above-ground organs in the er erl1 erl2 mutant. Representative 6-week-old plants from left to right: er erl1 erl2; T3 pPZK101, pPZK104, and pPZK105 in er erl1erl2 background; er erl2.
Discussion
Considerable progress has been made recently in understanding the composition of receptor–ligand complexes formed by ERf in the plasma membrane (Lee et al. 2012, 2015). However, the mechanism of the cytoplasmic domain activation and transmission of the signal by ERf is still unclear. To explore the significance of ERECTA’s kinase domain and the mechanism of its activation, we used a structure–function approach.
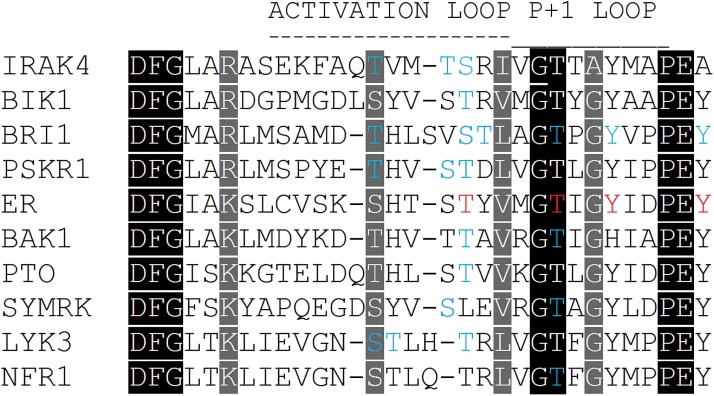
ERECTA is a RD kinase, which means that it has a conserved arginine (R) immediately preceding an aspartate (D) in the catalytic loop. Previous research has established that ERECTA is a weak kinase in vitro (Lease et al., 2001; Meng et al., 2015). Accordingly, we observed that ERECTA with substitution of the conserved lysine in the ATP-binding domain is unable to rescue the majority of developmental defects in mutants, and therefore the ability to phosphorylate is essential for ERECTA function. While inactive kinases adopt a variety of distinct conformations, their activation often depends on a change in the structure of the activation segment, which in the RD kinases is the primary site of regulatory phosphorylation (Johnson et al., 1996). The activation segment is variable in length and sequence but it is restricted by highly conserved DGF and APE motifs, and in Ser/Thr kinases it almost always contains a characteristic GlyThr or GlySer dipeptide motif (Fig. 11). In Arabidopsis, more than 99% of RD LRR RLKs have a Thr or Ser in this motif (Wang et al., 2005a). In the active state, the hydroxyl group of the threonine or serine from the GlyThr/Ser motif forms hydrogen bonds with the catalytic aspartate of the HRD motif and the lysine one nucleotide behind this motif (Nolen et al., 2004). In IRAK4 and many other mammalian Ser/Thr kinases this Thr is important for the kinase activity, but is not a major phosphorylation site (Wang et al., 2009; Bayliss et al., 2012). Alanine or phosphomimetic substitutions of homologous Thr/Ser in plant kinases such as SERK1 (T468A, T468E), BRI1 (T1049A), SYMRK (T760A), BAK1 (T455A, T455D, T455E), BIK1 (T242A), ACR4 (T681A, T681D), HAESA (S861A), and PSKR1 (T899A) lead to loss of kinase function (Shah et al., 2001; Wang et al., 2005a, 2008; Yoshida and Parniske, 2005; Laluk et al., 2011; Meyer et al., 2011; Taylor et al., 2013; Hartmann et al., 2015). In BRI1, FLS2 (T1040A), BAK1, BIK1, and HAESA these substitutions were shown to decrease functionality of receptors in planta (Wang et al., 2005a, 2008; Robatzek et al., 2006; Laluk et al., 2011; Taylor et al., 2016). While our work demonstrates that both alanine or phosphomimetic substitutions of Thr812 alter functionality of ERECTA, the effect is surprisingly small. In this respect ERECTA resembles the RD receptor-like kinases PSKR1 and FERONIA where substitution of homologous S701 and T899, respectively, does not disrupt receptor function (Hartmann et al., 2015; Kessler et al., 2015).
Fig. 11.
Alignment of the activation segments from plant receptor-like kinases ER, BAK1, LYK3, NFR1, BRI1, and PSKR1, plant kinases BIK1 and PTO, and human kinase IRAK4. Functionally significant amino acids are in red. Amino acids that are phosphorylated (either in vitro or in vivo) and functionally significant in planta or essential for the enzymatic activity are in blue. Residues that are identical among the sequences are given a black background, and those that are similar among the sequences are given a gray background.
Thr/Ser residues preceding the GlyThr motif are the primary phosphorylation sites that are essential for the activation of mammalian Ser/Thr kinases (Nolen et al., 2004; Bayliss et al., 2012). For example, the kinase activity of IRAK4 is regulated by the autophosphorylation of three sites, Thr342, Thr345, and Ser346, located in front of the GlyThr motif (Cheng et al., 2007). Based on structural studies, phosphorylation of Thr345 is responsible for the activation of IRAK4 kinase while phosphorylation of Thr342 and Ser346 might stabilize the activation loop in the active state (Kuglstatter et al., 2007). Current literature suggests that residues homologous to Thr345 or Ser346 in IRAK4 are likely to be the primary phosphorylation sites in plant Ser/Thr kinases. Thus, Thr237 of BIK1 is necessary for full kinase activity and is a major phosphorylation site in response to flg22 (Lu et al., 2010; Laluk et al., 2011). In LYK3, T475A substitution leads to decreased kinase activity (Klaus-Heisen et al., 2011). The equivalent Thr233 in Pti1 is the major site of autophosphorylation and phosphorylation by Pto kinase (Sessa et al., 2000b). In BAK1, Thr450 is phosphorylated and T450A substitution reduces functionality of the receptor (Wang et al., 2008). Crystal structure confirmed the significance of T450 phosphorylation for enzymatic activity of BAK1 (Yan et al., 2012). Ser1044 is phosphorylated in BRI1 and, out of all Ser/Thr to Ala substitutions in the activation segment, the S1044A substitution has the most severe negative impact on BRI1 functionality (Wang et al., 2005a, 2016). S856 in the activation segment of HAESA is a phosphorylation site that positively regulates kinase activity and contributes to the functionality of the receptor (Taylor et al., 2016). Thr807 of ERECTA is homologous to Ser346 of IRAK4 (Fig. 11). Therefore, it is not surprising that out of all Ser/Thr substitutions in the activation segment of ERECTA only T807A substitution significantly reduces receptor functionality, and ERECTA with a T807D substitution is fully functional. Based upon this data, we speculate that Thr807 is the primary phosphorylation site in the activation segment of ERECTA. Future structural and biochemical studies will be essential to confirm this hypothesis. In general, our findings resemble those obtained for HAESA where substitutions of only two Ser/The residues in the activation segment disrupt receptor function (Taylor et al., 2016). Those residues are homologous to Thr807 and Thr812 of ERECTA, although in ERECTA the substitution of T812 has a weaker effect on the receptor function.
Plant receptor-like kinases have dual specificity, phosphorylating both Ser/Thr and Tyr residues. Two tyrosine residues in the BRI1 P+1 loop, Tyr1052 and Tyr1057, have been shown in vivo to play an important role in BR signaling and their phosphorylation is predicted to have a negative impact on the kinase activity (Oh et al., 2009a, 2009b). Residues homologous to Tyr1057 of BRI1 have been shown to be critical for the function of other receptor-like kinases. Thus, Tyr463 of BAK1 is essential to its catalytic activity (Oh et al., 2010). Tyr250 in the activation segment of BIK1 can be autophosphorylated or transphosphorylated by BAK1 and is important for BIK1 function in plant defenses (Lin et al., 2014). Consistent with this, we observed that substitutions of homologous Tyr residues in the P+1 loop of ERECTA, Tyr815 and Tyr820, drastically reduced functionality of the receptor. As substitutions to Ala were less severe than substitutions to Asp, we hypothesize that phosphorylation of those residues could lead to inhibition of ERECTA function.
Phosphorylation events in the JMD and the C-terminal regions often alter activity of receptor-like kinases (Wang et al., 2005b; Oh et al. 2009b, 2014). However, this might not be the case for ERECTA: no changes in ERECTA functionality were observed after the deletion of the C-terminus. Thr645 is the only conserved Thr/Ser/Tyr in the JMD, yet its substitution had no effect on ERECTA function. The receptor-like kinase BAK1 associates with multiple receptor-like kinases including ERECTA family receptors (Meng et al., 2015). When BAK1 associates with BRI1 it increases its activity by phosphorylating the JMD and the C-terminus (Wang et al., 2008). The role of BAK1 during interaction with ERECTA is likely to be different as phosphorylation of ERECTA kinase’s flanking regions is not likely to be significant for its function.
A majority of the RLK/Pelle kinases have an N-terminal extension in front of the N-terminal lobe of the kinase domain (Lei et al., 2005; Wang et al., 2006). The N-terminal extension is often an integral part of the overall fold of kinase and is essential for its activity. For example, the N-terminal extension is required for BRI1 enzymatic activity (Oh et al., 2012). While there is no sequence similarity between the N-terminal extensions of various kinases, there is some similarity of structure. The crystal structure of IRAK4 revealed a short β strand and an α-helix in the N-terminal extension region while those of BRI1 and BAK1 suggested the existence of an α-helix (Wang et al., 2006; Yan et al., 2012; Bojar et al., 2014). Homology modeling of LYK3 predicted an α helix in the N-terminal extension region (Klaus-Heisen et al., 2011). Four different programs (JPRED 4, NETSURFP, PSIPRED, and I-TASSER) predicted the existence of a short β strand and an α-helix in the N-terminal extension region of ERECTA. While we were unable to identify functionally significant putative phosphorylation sites in the JMD, our work determined that this domain is significant for ERECTA function. Two deletions in that region led to a functionally inactive receptor. This may be due to disruption of the N-terminal extension structure and, as a result, inactivation of ERECTA’s enzymatic function.
Our structure–function analysis indicates that ERECTA function differs in the specific developmental processes in which it participates. The kinase function is absolutely essential for ERECTA’s ability to regulate stomata formation and flower structure. Simultaneously, the kinase-dead ERECTA is able to partially rescue stem and pedicel elongation defects in the er erl1 erl2 background. These results suggest that there are distinct signaling requirements for ERECTA in different developmental processes and imply that ERECTA might transmit the signal to downstream targets in different ways. The receptor-like kinases BAK1 and SCRAMBLED have also been shown to control multiple pathways using distinct signaling mechanisms with different requirements for their kinase domain function (Oh et al., 2010; Kwak et al., 2014). In addition, we observed that kinase-dead ERECTA and ERECTA without the cytoplasmic domain (Δkinase) function very differently. The Δkinase ERECTA confers dominant negative effects, probably titrating positive regulators of the signaling pathway through the extracellular domain (Shpak et al., 2003). The kinase-dead ERECTA is partially functional in regulation of organ elongation, which hypothetically could occur through titration of negative regulators by the kinase domain.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (IOS-0843340 to EDS). We thank Olivia Seay, Miguel Rodriguez, and Emily Miller for technical assistance. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors is EDS.
References
- Aifa S, Frikha F, Miled N, Johansen K, Lundström I, Svensson SP. 2006. Phosphorylation of Thr654 but not Thr669 within the juxtamembrane domain of the EGF receptor inhibits calmodulin binding. Biochemical and Biophysical Research Communications 347, 381–387. [DOI] [PubMed] [Google Scholar]
- Bayliss R, Fry A, Haq T, Yeoh S. 2012. On the molecular mechanisms of mitotic kinase activation. Open Biology 2, 120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis SM, Lee JS, Shpak ED, Torii KU. 2013. Regulation of floral patterning and organ identity by Arabidopsis ERECTA-family receptor kinase genes. Journal of Experimental Botany 64, 5323–5333. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. 2004. Stomatal development and pattern controlled by a MAPKK kinase. Science 304, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Bojar D, Martinez J, Santiago J, Rybin V, Bayliss R, Hothorn M. 2014. Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. The Plant Journal 78, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy MGR, Thompson OA, Sieger MT, Shpak ED. 2012. Patterns of cell division, cell differentiation and cell elongation in epidermis and cortex of Arabidopsis pedicels in the wild type and in erecta. PLoS ONE 7, e46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Shpak ED. 2014. ERECTA family genes regulate development of cotyledons during embryogenesis. FEBS Letters 588, 3912–3917. [DOI] [PubMed] [Google Scholar]
- Chen MK, Wilson RL, Palme K, Ditengou FA, Shpak ED. 2013. ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordia. Plant Physiology 162, 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chern M, Canlas PE, Jiang C, Ruan D, Cao P, Ronald PC. 2010. A conserved threonine residue in the juxtamembrane domain of the XA21 pattern recognition receptor is critical for kinase autophosphorylation and XA21-mediated immunity. The Journal of Biological Chemistry 285, 10454–10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Addona T, Keshishian H, et al. 2007. Regulation of IRAK-4 kinase activity via autophosphorylation within its activation loop. Biochemical and Biophysical Research Communications 352, 609–616. [DOI] [PubMed] [Google Scholar]
- Durek P, Schmidt R, Heazlewood JL, Jones A, MacLean D, Nagel A, Kersten B, Schulze WX. 2010. PhosPhAt: the Arabidopsis thaliana phosphorylation site database. An update. Nucleic Acids Research 38, D828–D834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiard L, Sauviac L, Torii KU, Grenon O, Mangin B, Grimsley NH, Marco Y. 2003. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. The Plant Journal 36, 353–365. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Linke D, Bönniger C, Tholey A, Sauter M. 2015. Conserved phosphorylation sites in the activation loop of the Arabidopsis phytosulfokine receptor PSKR1 differentially affect kinase and receptor activity. The Biochemical Journal 472, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss E, Masson K, Sundberg C, Pedersen M, Sun J, Bengtsson S, Rönnstrand L. 2006. Identification of Y589 and Y599 in the juxtamembrane domain of Flt3 as ligand-induced autophosphorylation sites involved in binding of Src family kinases and the protein tyrosine phosphatase SHP2. Blood 108, 1542–1550. [DOI] [PubMed] [Google Scholar]
- Hord CL, Sun YJ, Pillitteri LJ, Torii KU, Wang H, Zhang S, Ma H. 2008. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Molecular Plant 1, 645–658. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Noble ME, Owen DJ. 1996. Active and inactive protein kinases: structural basis for regulation. Cell 85, 149–158. [DOI] [PubMed] [Google Scholar]
- Karve R, Liu W, Willet SG, Torii KU, Shpak ED. 2011. The presence of multiple introns is essential for ERECTA expression in Arabidopsis. RNA 17, 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SA, Lindner H, Jones DS, Grossniklaus U. 2015. Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. EMBO Reports 16, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus-Heisen D, Nurisso A, Pietraszewska-Bogiel A, et al. 2011. Structure-function similarities between a plant receptor-like kinase and the human interleukin-1 receptor-associated kinase-4. The Journal of Biological Chemistry 286, 11202–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuglstatter A, Villaseñor AG, Shaw D, Lee SW, Tsing S, Niu L, Song KW, Barnett JW, Browner MF. 2007. Cutting edge: IL-1 receptor-associated kinase 4 structures reveal novel features and multiple conformations. Journal of Immunology 178, 2641–2645. [DOI] [PubMed] [Google Scholar]
- Kwak SH, Woo S, Lee MM, Schiefelbein J. 2014. Distinct signaling mechanisms in multiple developmental pathways by the SCRAMBLED receptor of Arabidopsis. Plant Physiology 166, 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laluk K, Luo H, Chai M, Dhawan R, Lai Z, Mengiste T. 2011. Biochemical and genetic requirements for function of the immune response regulator BOTRYTIS-INDUCED KINASE1 in plant growth, ethylene signaling, and PAMP-triggered immunity in Arabidopsis. The Plant Cell 23, 2831–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease KA, Lau NY, Schuster RA, Torii KU, Walker JC. 2001. Receptor serine/threonine protein kinases in signalling: analysis of the erecta receptor-like kinase of Arabidopsis thaliana. New Phytologist 151, 133–143. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hnilova M, Maes M, Lin YC, Putarjunan A, Han SK, Avila J, Torii KU. 2015. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU. 2012. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes & Development 26, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. 2005. The active conformation of the PAK1 kinase domain. Structure 13, 769–778. [DOI] [PubMed] [Google Scholar]
- Lin W, Li B, Lu D, Chen S, Zhu N, He P, Shan L. 2014. Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proceedings of the National Academy of Sciences, USA 111, 3632–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. 2010. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proceedings of the National Academy of Sciences, USA 107, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L. 2015. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Current Biology 25, 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang H, He Y, Liu Y, Walker JC, Torii KU, Zhang S. 2012. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. The Plant Cell 24, 4948–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Lichti CF, Townsend RR, Rao AG. 2011. Identification of in vitro autophosphorylation sites and effects of phosphorylation on the Arabidopsis CRINKLY4 (ACR4) receptor-like kinase intracellular domain: insights into conformation, oligomerization, and activity. Biochemistry 50, 2170–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B, Taylor S, Ghosh G. 2004. Regulation of protein kinases; controlling activity through activation segment conformation. Molecular Cell 15, 661–675. [DOI] [PubMed] [Google Scholar]
- Oh MH, Clouse SD, Huber SC. 2009a. Tyrosine phosphorylation in brassinosteroid signaling. Plant Signaling & Behavior 4, 1182–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Clouse SD, Huber SC. 2012. Tyrosine phosphorylation of the BRI1 receptor kinase occurs via a post-translational modification and is activated by the juxtamembrane domain. Frontiers in Plant Science 3, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Kota U, Goshe MB, Clouse SD, Huber SC. 2009b. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Kim SY, Wu X, Clouse SD, Huber SC. 2014. The Carboxy-terminus of BAK1 regulates kinase activity and is required for normal growth of Arabidopsis. Frontiers in Plant Science 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oh MH, Wang X, Wu X, Zhao Y, Clouse SD, Huber SC. 2010. Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proceedings of the National Academy of Sciences, USA 107, 17827–17832. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU. 2007. Haploinsufficiency after successive loss of signaling reveals a role for ERECTA-family genes in Arabidopsis ovule development. Development 134, 3099–3109. [DOI] [PubMed] [Google Scholar]
- Ramos JA, Zenser N, Leyser O, Callis J. 2001. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. The Plant Cell 13, 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. 2006. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes & Development 20, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, D’Ascenzo M, Martin GB. 2000a. Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto-Pto-mediated hypersensitive response. The EMBO Journal 19, 2257–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, D’Ascenzo M, Martin GB. 2000b. The major site of the pti1 kinase phosphorylated by the pto kinase is located in the activation domain and is required for pto–pti1 physical interaction. European Journal of Biochemistry 267, 171–178. [DOI] [PubMed] [Google Scholar]
- Shah K, Vervoort J, de Vries SC. 2001. Role of threonines in the Arabidopsis thaliana somatic embryogenesis receptor kinase 1 activation loop in phosphorylation. The Journal of Biological Chemistry 276, 41263–41269. [DOI] [PubMed] [Google Scholar]
- Shimada T, Sugano SS, Hara-Nishimura I. 2011. Positive and negative peptide signals control stomatal density. Cellular and Molecular Life Sciences 68, 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED. 2013. Diverse roles of ERECTA family genes in plant development. Journal of Integrative Plant Biology 55, 1238–1250. [DOI] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. 2004. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. 2003. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. The Plant Cell 15, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. 2005. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309, 290–293. [DOI] [PubMed] [Google Scholar]
- Subramanian C, Woo J, Cai X, Xu X, Servick S, Johnson CH, Nebenführ A, von Arnim AG. 2006. A suite of tools and application notes for in vivo protein interaction assays using bioluminescence resonance energy transfer (BRET). The Plant Journal 48, 138–152. [DOI] [PubMed] [Google Scholar]
- Taylor I, Seitz K, Bennewitz S, Walker JC. 2013. A simple in vitro method to measure autophosphorylation of protein kinases. Plant Methods 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I, Wang Y, Seitz K, Baer J, Bennewitz S, Mooney BP, Walker JC. 2016. Analysis of phosphorylation of the receptor-like protein kinase HAESA during Arabidopsis floral abscission. PLoS ONE 11, e0147203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KW, Carpenter G. 2007. Epidermal growth factor receptor juxtamembrane region regulates allosteric tyrosine kinase activation. Proceedings of the National Academy of Sciences, USA 104, 19238–19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. The Plant Cell 8, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Shimada M, Tasaka M. 2013. ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant & Cell Physiology 54, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagarcia H, Morin AC, Shpak ED, Khodakovskaya MV. 2012. Modification of tomato growth by expression of truncated ERECTA protein from Arabidopsis thaliana. Journal of Experimental Botany 63, 6493–6504. [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. 2007. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. The Plant Cell 19, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang S, Gan S, Wang X, Liu J, Wang X. 2016. Role of specific phosphorylation sites of Arabidopsis brassinosteroid-insensitive 1 receptor kinase in plant growth and development. Journal of Plant Growth Regulation 35, 755–769. [Google Scholar]
- Wang X, Goshe MB, Soderblom EJ, Phinney BS, Kuchar JA, Li J, Asami T, Yoshida S, Huber SC, Clouse SD. 2005a. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. The Plant Cell 17, 1685–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD. 2008. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Developmental Cell 15, 220–235. [DOI] [PubMed] [Google Scholar]
- Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J. 2005b. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Developmental Cell 8, 855–865. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu J, Sudom A, Ayres M, Li S, Wesche H, Powers JP, Walker NP. 2006. Crystal structures of IRAK-4 kinase in complex with inhibitors: a serine/threonine kinase with tyrosine as a gatekeeper. Structure 14, 1835–1844. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wesche H, Stevens T, Walker N, Yeh WC. 2009. IRAK-4 inhibitors for inflammation. Current Topics in Medicinal Chemistry 9, 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Ma Y, Liu D, et al. 2012. Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Research 22, 1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Parniske M. 2005. Regulation of plant symbiosis receptor kinase through serine and threonine phosphorylation. The Journal of Biological Chemistry 280, 9203–9209. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Peck SC. 2011. Simplified enrichment of plasma membrane proteins for proteomic analyses in Arabidopsis thaliana. Proteomics 11, 1780–1788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.