Highlight
Changes in Arabidopsis cell wall composition, predominantly in pectic polymers, influence penetration and establishment of Colletotrichum higginsianum hyphae in host leaves during initial biotrophy.
Keywords: Carbohydrate metabolism, cell wall, glycome profiling, hemibiotrophy, host–pathogen interaction, indolic glucosinolates, pectin, penetration resistance, plant defense, starch deficiency.
Abstract
Penetration resistance represents the first level of plant defense against phytopathogenic fungi. Here, we report that the starch-deficient Arabidopsis thaliana phosphoglucomutase (pgm) mutant has impaired penetration resistance against the hemibiotrophic fungus Colletotrichum higginsianum. We could not determine any changes in leaf cutin and epicuticular wax composition or indolic glucosinolate levels, but detected complex alterations in the cell wall monosaccharide composition of pgm. Notably, other mutants deficient in starch biosynthesis (adg1) or mobilization (sex1) had similarly affected cell wall composition and penetration resistance. Glycome profiling analysis showed that both overall cell wall polysaccharide extractability and relative extractability of specific pectin and xylan epitopes were affected in pgm, suggesting extensive structural changes in pgm cell walls. Screening of mutants with alterations in content or modification of specific cell wall monosaccharides indicated an important function of pectic polymers for penetration resistance and hyphal growth of C. higginsianum during the biotrophic interaction phase. While mutants with affected pectic rhamnogalacturonan-I (mur8) were hypersusceptible, penetration frequency and morphology of fungal hyphae were impaired on pmr5 pmr6 mutants with increased pectin levels. Our results reveal a strong impact of starch metabolism on cell wall composition and suggest a link between carbohydrate availability, cell wall pectin and penetration resistance.
Introduction
Penetration of the host leaf tissue by phytopathogenic fungi can occur either through stomatal pores or by breaching epidermal cell walls by combined action of enzymatic degradation and physicomechanical force. The subsequent establishment of hyphae inside the host tissue enables the pathogen to access nutrients of the invaded host tissue. The plant cuticle and cell wall represent the first line of structural physicochemical barriers that oppose fungal penetration.
The cuticle represents the outermost hydrophobic layer of aerial plant organs and can roughly be divided into two distinct layers. The cuticle proper, containing the lipid polyester layer, cutin, is dominated by crosslinked hydroxylated C16 and C18 fatty acids with embedded intracuticular waxes. The cutin layer is covered by epicuticular wax crystals composed of very-long-chain (>C20) fatty acid derivatives (as reviewed by Beisson et al., 2012; Nawrath et al., 2013). The chemical composition of both cutin and wax was found to play an important role in plant–fungal interactions. In recent years it was demonstrated that particular hydroxylated fatty acids (Mendoza-Mendoza et al., 2009), long-chain alcohols (Uppalapati et al., 2012) or long-chain aldehydes (Reisige et al., 2006; Hansjakob et al., 2010, 2011) in epicuticular wax are required for efficient spore differentiation and appressoria formation of (hemi)biotrophic fungal pathogens. On the other hand, cutin deficiencies can be associated with increased resistance of Arabidopsis to the fungal necrotroph Botrytis cinerea. Current models account an increased permeability of the cuticle to pathogen- or host-derived elicitors that are generated during cutin and cell wall degradation for a subsequently enhanced recognition by host cells (reviewed by Yeats and Rose, 2013). Nevertheless, contrasting correlations between cutin thickness and B. cinerea resistance have been obtained with other host plants.
Preinvasion resistance can also be accomplished by generating toxic compounds. Accumulation of the indolic glucosinolate (IG) 4-methoxy-indole-3-ylmethyl-glucosinolate (4MOI3M) and 4MOI3M cleavage by the atypical myrosinase PEN2 contribute to pre-invasion resistance against a wide range of fungal pathogens in Arabidopsis, including non-adapted powdery mildew fungi and Colletotrichum species (Lipka et al., 2005; Bednarek et al., 2009; Hiruma et al., 2010). Penetration frequency of the hemibiotrophic ascomycete C. higginsianum is only slightly increased on pen2 mutants, and therefore the role of IGs in the interaction with this adapted Colletotrichum species is as yet unclear (Huser et al., 2009). Deposition of the β(1,3)-linked glucan callose in cell wall papillae formed at attempted fungal penetration sites has been demonstrated to effectively restrict powdery mildew penetration (Ellinger et al., 2013). However, formation of callose papillae did not substantially contribute to penetration resistance against Colletotrichum higginsianum (Narusaka et al., 2004; Shimada et al., 2006; Birker et al., 2009; Huser et al., 2009).
In addition to the previously mentioned physical barriers, successful fungal penetration pegs need to penetrate the plant cell wall, a complex hydrated matrix of polysaccharides and proteins that confer mechanical stability on plant cells. In Arabidopsis, the water-insoluble scaffold of primary cell walls in expanding leaves is composed of about 14% cellulose, 24% hemicellulose and 42% pectin (Zablackis et al., 1995). Hemicelluloses are composed of neutral sugar moieties that form β(1,4)-linked polysaccharide backbones, with xyloglucan being the predominant type in primary Arabidopsis walls (Scheller and Ulvskov, 2010). Negatively charged galacturonic acid (GalA) residues are abundant in the pectic polymers xylogalacturonan, rhamnogalacturonan-I, and rhamnogalacturonan-II, while homogalacturonan is exclusively composed of GalA (Harholt et al., 2010). Hemicellulose and pectin polymers are thought to form a highly crosslinked matrix that is linked to the scaffold-forming cellulose microfibrils and plays a central role in the regulation of cell wall elasticity and rigidity (for current models on cell wall polymer interconnection, see reviews by Cosgrove and Jarvis, 2012; Peaucelle et al., 2012). The degree of pectin methylesterification (DM) is controlled by pectin methylesterase (PME) and PME inhibitor (PMEI) function and controls cell wall stiffness as demonstrated by atomic force microscopy (Peaucelle et al., 2011, 2015). However, seemingly contradictory reports exist on the effect of DM on cell wall stiffness and cell elongation, suggesting cell-specific differences (reviewed by Levesque-Tremblay et al., 2015).
Both the structural composition of the cell wall matrix and the modification of cell wall polymers have been identified as compatibility factors during the interaction of plants with fungal biotrophs and necrotrophs. Penetration pegs of most biotrophs induce limited hydrolysis of cell wall polymers. Carbohydrate-active enzyme families are expanded in fungal hemibiotrophs and necrotrophs that rely on the secretion of hydrolases to access the cell wall as a complex carbon source (e.g. Ma et al., 2010; O’Connell et al., 2012). Increased resistance of the Arabidopsis powdery mildew resistant 5 (pmr5) and pmr6 mutants towards the adapted powdery mildew fungus Golovinomyces cichoracearum is associated with increased pectin content of the cell walls (Vogel et al., 2002, 2004). PMR6 has been identified as encoding a pectate lyase-like protein (Vogel et al., 2002), while PMR5 has been characterized as a trichome birefringerence-like (TBL) protein (TBL44) that is likely to affect cell wall O-acetylation (Gille and Pauly, 2012). Congruently, reduced O-acetylation in the Arabidopsis mutant reduced wall acetylation 2 (rwa2) or Arabidopsis plants overexpressing fungal acetylesterase was associated with increased resistance to the fungal necrotroph Botrytis cinerea (Manabe et al., 2011; Pogorelko et al., 2013). There is evidence that pectinolysis by fungal polygalacturonases (PGs) plays an important role during pathogenesis of fungal necrotrophs. For instance, antisense repression of a host-borne PG inhibitor protein resulted in increased susceptibility of Arabidopsis towards B. cinerea (Ferrari et al., 2006). In turn, transgenic expression of a fungal PG strongly induced host defense responses in B. cinerea infected leaves (Ferrari et al., 2008). This might be explained with an elevated release of host-derived damage-associated molecular patterns (DAMPs) like oligogalacturonides and their subsequent recognition by wall-associated kinases that act as DAMP receptors (Ferrari et al., 2007; Kohorn and Kohorn, 2012). Arabidopsis plants with increased PMEI activity or reduced PME activity exhibited an increased resistance to B. cinerea, suggesting that a high DM either reduces pectin accessibility for PG degradation or interferes with fungal penetration through altered cell wall stiffness (Lionetti et al., 2007; Raiola et al., 2011).
Colletotrichum higginsianum is a hemibiotrophic ascomycete fungus adapted to the model plant Arabidopsis thaliana (for reviews on the life style of Colletotrichum spp. see Mendgen and Hahn, 2002; Münch et al., 2008). Leaf infections with C. higginsianum are initiated by the germination of conidia on the leaf surface. At the tip of the germ tubes, dome-shaped melanized appressoria differentiate; they accumulate sugar alcohols and build up a high turgor pressure upon the subsequent diffusion of water from outside water droplets into the appressoria. The wall of the underlying epidermal cell is subsequently pierced by a penetration peg with a combination of mechanical force and lytic enzyme activity (Bechinger et al., 1999; Deising et al., 2000). In the penetrated host epidermis cells, C. higginsianum establishes itself within 36 h post-inoculation by forming a bulbous infection vesicle that produces lobed biotrophic primary hyphae. At around 72 h post-inoculation, neighboring cells are colonized by rapidly growing, narrow-bore necrotrophic secondary hyphae, which leads to visible necrotic lesions on infected leaves.
Recently, we have shown that reduced diurnal carbon availability in genotypes with reduced starch or carboxylate turnover leads to increased susceptibility of Arabidopsis toward C. higginsianum. In addition, we observed a connection between carbon shortage and delayed defense responses in host leaves, which had most deleterious consequences during necrotrophic growth of the pathogen (Engelsdorf et al., 2013). In the present report, we demonstrate that Arabidopsis mutants with deficient starch biosynthesis or mobilization show faster penetration and establishment of biotrophic hyphae inside of host cells. We reveal complex alterations in the cell wall composition of starch-deficient phosphoglucomutase (pgm) mutants and present evidence that pectin quality and quantity can influence penetration and biotrophic establishment of C. higginsianum in Arabidopsis.
Materials and methods
Plant and fungal material and growth conditions
Arabidopsis plants were grown as described in Engelsdorf et al. (2013). Seeds for pgm (N210), adg1-1 (N3094), sex1-1 (N3093), mur4-1 (N8568), mur8-1 (N8575), mur11-1 (N8579), arad1-2 (N25046), gals1-1 (N657519), pmr5 (N6579), pmr6-3 (N66990), pmr5 pmr6-3 (N6580), pme3-1 (N400106), pme35-1 (N661699), and pmei6-2 (N475768) were obtained from Nottingham Arabidopsis Stock Centre (NASC; University of Nottingham, UK). hig1-1, myb34/myb51 and HIG1-1D seeds were kindly provided by Tamara Gigolashvili (Institute of Botany, University of Cologne; Gigolashvili et al., 2007; Frerigmann and Gigolashvili, 2014). All mutants and transformants were in the Col-0 background.
Colletotrichum higginsianum isolate MAFF 305635 (Ministry of Agriculture, Forestry and Fisheries, Japan) was grown on oat meal agar plates (5% (w/v) shredded oat meal, 1.2% (w/v) agar) for 7 d at 22 °C under illumination to promote conidia formation.
C. higginsianum infection assays
Leaf infection by C. higginsianum was performed by spray inoculation with a conidia titer of 2 × 106 conidia ml–1 as described by Voll et al. (2012).
Assessment of C. higginsianum in planta development and evaluation of susceptibility
Fungal structures were stained using trypan blue as described in Koch and Slusarenko (1990). Microscopy was performed on a Leica DMR microscope (Bensheim, Germany) with differential interference contrast optics.
Quantification of the relative genomic C. higginsianum DNA content was performed as previously described (Engelsdorf et al., 2013).
Quantification of indolic glucosinolate content
Glucosinolates were extracted and purified as described by Gigolashvili et al. (2007). Separation of desulfoglucosinolates was performed on a Dionex Ultimate 3000 HPLC system (DGP-3600MB, WPS-3000TB, PDA-3000) equipped with a Phenomenex Luna Security Guard C18 column (4.0 × 3.0 mm) and a Luna C18(2) reverse-phase column (5 µm, 250 × 4.6 mm) at 25 °C column temperature and a flow rate of 1 ml min−1 using the following gradient: 0–5 min, 0% acetonitrile (ACN); 5–30 min, 30% ACN; 30–32 min, 40% ACN; 32–36 min, 40% ACN; 36–40 min, 0% ACN; 40–50 min, 0% ACN. Peaks were quantified at 229 nm relative to an internal benzyl glucosinolate standard using the respective response factors described by Brown et al. (2003).
Analysis of epicuticular wax and cutin
For wax analysis 10–20 rosette leaves (corresponding to 10–15 cm2) of 5-week-old plants were cut and immediately immersed in chloroform for 10 s at room temperature. The resulting solution containing the cuticular waxes was spiked with 10 µg of tetracosane (Fluka) as an internal standard. The solvent was evaporated under a stream of nitrogen, and compounds containing free hydroxyl and carboxyl groups were converted into their trimethylsilyl ethers and esters, respectively, with bis-(N,N-trimethylsilyl)-trifluoroacetamide (Machery-Nagel) in pyridine for 40 min at 70 °C before GC-MS analysis. Wax constituents were identified by their electron-impact MS spectra after GC-MS analysis and quantified using an identical GC system equipped with a flame ionization detector (FID) as described previously (Kurdyukov et al., 2006).
For cuticular leaf polyester composition, wax extracted leaves were then exhaustively extracted with chloroform–methanol (1:1; v/v) with daily change of solvent over a period of 7 d to remove soluble lipids. Cutin monomers were solubilized by transesterification with 1 M methanol/HCl (Supelco) for 2 h at 80 °C in a Teflon-sealed screw-cap tube. After addition of 2 ml saturated NaCl/H2O the hydrophobic monomers were subsequently extracted three times in hexane containing 10 µg of the internal standard dotriacontane. The combined extracts were evaporated and derivatized as described above and subsequently analysed by GC-MS and GC-FID as described previously (Franke et al., 2005).
Toluidine blue staining
Toluidine blue staining was conducted as described by Bessire et al. (2007). Four droplets of a 0.025% toluidine solution were spotted onto each of nine fully expanded leaves from nine independent plants per genotype.
Cell wall preparation and analysis
Starch-free alcohol-insoluble residue (AIR) for cellulose analysis was prepared from 50 mg of Arabidopsis leaf powder by extracting twice with 80% ethanol at 80 °C followed by incubation in 90% dimethyl sulfoxide–10% ddH2O for four times at 24 h at room temperature (RT). AIR was then washed three times with ddH2O and two times with acetone. Subsequently, dried AIR was treated with 5 U of α-amylase (Sigma-Aldrich A3176) in 100 mM ammonium formate for 72 h and washed as above. Cellulose content of dried starch-free AIR was determined spectrophotometrically following the method of Updegraff (1969) and quantified in relation to glucose standards.
For the analysis of cell wall monosaccharide composition and enzymatic digestions of cell wall preparations, 30–40 mg of crushed leaves was extracted twice with 1 ml of 80% ethanol at 80 °C for 20 min and once with 1 ml acetone at RT for 5 min. Extracted leaves, i.e. crude cell wall preparations, were dried at 60°C for 10 min.
For digestion of crude cell wall preparations with arabinanase, 2 U of endo-arabinanase (Prod. Code E-EARAB, Megazyme, Bray, Ireland) in 1.4 ml 50 mM sodium acetate (pH 5.5) was added to dried cell wall pellets and the samples were incubated for 12 h at 37 °C. The reactions were stopped by the addition of 650 µl cold 95% ethanol–10 mM EDTA and the digested cell wall material was sedimented by centrifugation for 5 min at 20 000 g. For galactanase digestion, the pH of the cell wall pellets was adjusted to 4.7 with 70 µl 0.1 M KOH and 2 U of endo-1,4-β-D-galactanase (Prod. Code E_EGALN, Megazyme) was added in 1.4 ml 100 mM acetic acid prior to incubation at 40 °C for 12 h.
Water-soluble polymers were isolated as described by Reiter et al. (1997) with minor modifications. Crude cell wall fractions were prepared and dried as described above and resuspended in 1 ml ice-cold buffer I (100 mM MOPS pH 7.0, 1.5% sodium dodecyl sulfate (SDS), 5 mM sodium bisulfite) with a rotating pestle. After sonication for 5 min in an ice-water bath (Bandelin Sonopuls HD 2070 and UW 2027, 50% duty cycle, 50% power), insoluble material was pelleted at 16 000 g and 4 °C for 10 min. The pellet was washed with 1 ml of ice-cold buffer II (100 mM MOPS pH 7.0, 0.5% SDS, 3 mM sodium bisulfite) and centrifuged as above. After combination of the supernatants and the soluble fraction, the pellet was washed once with 1 ml of buffer II, three times with water and once with 1 ml of 80% ethanol. To the soluble fraction, 8 M ammonium acetate was added to a final concentration of 1 M, followed by precipitation of polymers with 5 volumes of ethanol–acetone (1:1) for 16 h at RT and centrifugation at 3200 g for 15 min.
Cell wall pellets were hydrolysed and analysed as previously described (Øbro et al., 2004). Briefly, pellets were resuspended in 1 ml of 2 M trifluoroacetic acid with a rotating pestle and hydrolysed at 120 °C for 1 h. After centrifugation at 16 000 g for 2 min, the supernatant was dried under vacuum and resuspended in water. Neutral monosaccharides and galacturonic acid were analysed by high-performance anion exchange with pulsed amperometric detection with a Dionex ICS-3000 system equipped with a CarboPac® PA20 column. To obtain base line separation of xylose and mannose, elution was started isocratically with 100% buffer C (2 mM NaOH; Fluka 72064) for 21 min. In 2 min the eluent was changed to 5% buffer A (20 mM NaOH, 1 M sodium acetate), 50% buffer B (200 mM NaOH) and 45% buffer C, in the following 19 min to 30% buffer A, 50% buffer B and 20% buffer C and then in 5 min to 50% buffer A and 50% buffer B. After 2 min of isocratic elution, the eluent was changed to 100% buffer B in 2 min and kept isocratic for 3 min to wash the column. The eluent was then changed to 100% buffer C in 3 min, followed by 13 min of re-equilibration. To obtain base-line separation of rhamnose and arabinose, elution was started isocratically with 4% buffer B and 96% buffer C for 14 min. In 2 min the eluent was changed to 2% buffer A, 48% buffer B and 50% buffer C, in the following 16 min to 30% buffer A, 50% buffer B and 20% buffer A, and then in 4 min to 50% buffer A and 50% buffer B. Washing and re-equilibration of the column was performed as above.
Glycome profiling
Cell wall extraction and glycome profiling analyses of cell wall material were carried out as previously described (Demartini et al., 2011; Pattathil et al., 2012). In brief, cell walls (AIR) were sequentially extracted with increasingly harsh reagents [50 mM ammonium oxalate, 50 mM sodium carbonate (with 0.5% sodium borohydride; pH 10), 1 M KOH (with 1% sodium borohydride) and 4 M KOH (with 1% sodium borohydride)] at a ratio of 2 ml per 10 mg of AIR. The extracts were enzyme-linked immunosorbent assay (ELISA) screened with a comprehensive suite of cell wall glycan directed monoclonal antibodies (mAbs) monitoring most major non-cellulosic plant glycans (Pattathil et al., 2012). To conduct these ELISAs cell wall extracts were coated on an equal carbohydrate basis (15 μl from 20 μg ml−1 stocks per well). Plant cell wall glycan-directed mAbs were from laboratory stocks (CCRC, JIM and MAC series) at the Complex Carbohydrate Research Center (available through CarboSource Services; http://www.carbosource.net, last accessed 16 November 2016) or were obtained from BioSupplies (Australia) (BG1, LAMP). Supporting information on mAbs (Pattathil et al., 2010) used in this study can be found in Supplementary Table S1 at JXB online and the WallMabDB (http://www.wallmabdb.net, last accessed 16 November 2016) that provides detailed information for each antibody.
Determination of soluble sugars and starch
Soluble sugars and starch were extracted and analysed as previously described by Voll et al. (2003).
Statistical analysis
Student’s t-test was performed with SigmaPlot 12 (Systat Software Inc., Chicago, IL, USA) after testing for normality (Shapiro–Wilk test) and equal variance.
Results
In planta establishment of C. higginsianum is accelerated in starch-deficient pgm mutants
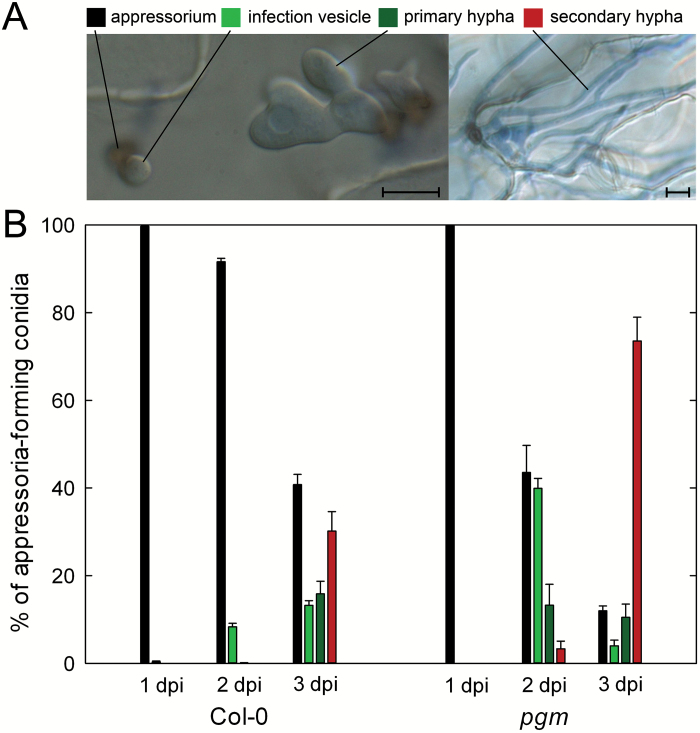
In order to analyse the impact of reduced diurnal carbohydrate availability on the early biotrophic interaction phase between Arabidopsis and C. higginsianum, fungal development was examined in leaves of the starch-deficient phosphoglucomutase (pgm) mutant. Compared with wild type, pgm mutant leaves exhibited a 10-fold accumulation of soluble sugars and showed an excessive export of sucrose from source leaves to roots (Engelsdorf et al., 2013; Brauner et al., 2014). This altered carbohydrate management leads to a 60% reduction in the diurnal turnover of carbohydrate storage in pgm source leaves and to periodic shortage for carbon towards the end of each dark phase (Gibon et al., 2004; Engelsdorf et al., 2013). Establishment of C. higginsianum was evaluated microscopically by scoring the most advanced fungal developmental stage visible at 1, 2, and 3 d post infection (dpi). After penetration by appressoria, infection vesicles (IVs) are formed first, followed by primary hyphae (PH) and secondary hyphae (SH) (Fig. 1A). At 1 dpi, appressoria had developed on both Col-0 and pgm leaves, while no fungal post-penetration structures could be observed in both genotypes. At 2 dpi, 8% of the assessed appressoria had successfully penetrated wild type leaves and differentiated IVs underneath the epidermal cell wall, whereas more than 55% of the appressoria on pgm had done so, giving rise to IVs (40%), PH (13%) and SH (3%) (Fig. 1B). Almost 80% of the appressoria on pgm leaves had formed SH at 3 dpi as compared with only 30% on wild type leaves (Fig. 1B). While the faster necrotrophic growth of C. higginsianum at 3 dpi can be explained by a reduction of induced defense responses in pgm leaves compared with wild type (Engelsdorf et al., 2013), we aimed at understanding the cause of increased fungal colonization in pgm between 1 and 2 dpi.
Fig. 1.
Colletotrichum higginsianum hyphae proliferation in pgm. Leaves of 5-week-old plants were infected with C. higginsianum, stained with trypan blue at 1, 2, and 3 d post infection (dpi) and examined by differential interference contrast microscopy. (A) Micrographs illustrating the scored fungal infection structures. Bars represent 10 µm. (B) Early fungal in planta development as given by the relative distribution of infection structures. Values are means of three biological replicates with four individual leaves each. Per replicate, the developmental status of in planta hyphae formed from 400–500 conidia was scored. The error bars represent the SE. Starting from appressoria, the most advanced infection structure was classified. The developmental order is as follows: appressoria, black bars; infection vesicles, light green bars; primary hyphae, dark green bars; secondary hyphae, red bars.
Cuticle composition is not affected in pgm
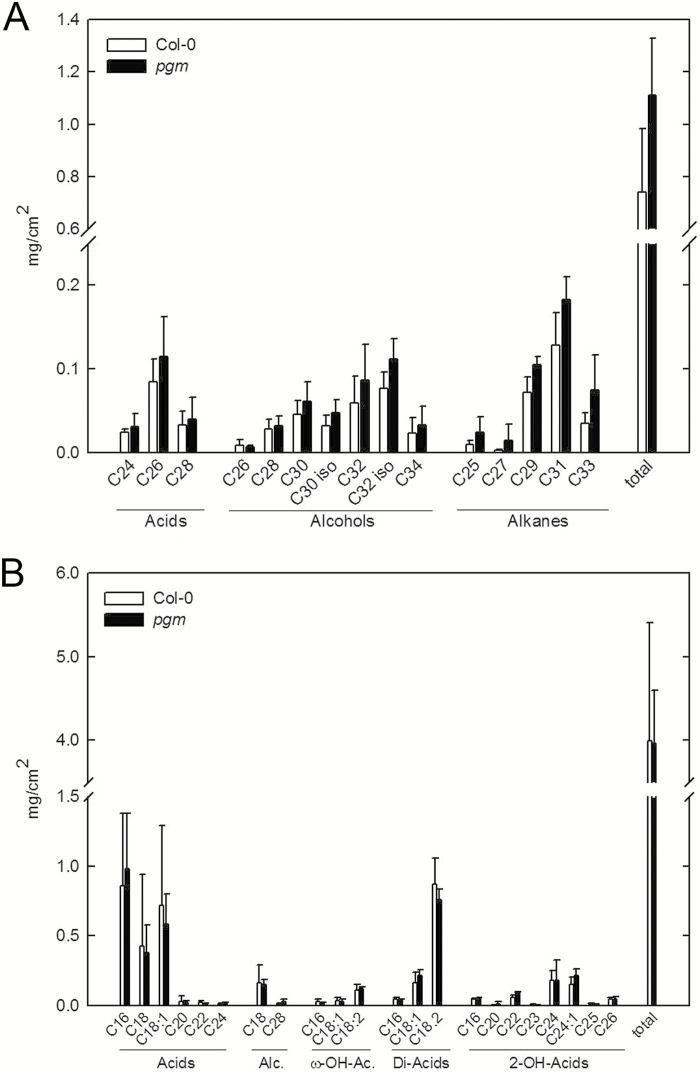
The epidermis represents the boundary tissue that gets in contact with pathogens first, and several studies have shown that the cuticle composition affects the outcome of plant–pathogen interactions (Yeats and Rose, 2013). We therefore analysed whether the composition of cuticular wax and/or cutin might account for the more rapid early development of C. higginsianum on pgm leaves. However, neither wax nor cutin composition was significantly altered in non-infected leaves of pgm mutants compared with wild type (Fig. 2). Consistently, toluidine blue staining, which indicates the permeability of the cuticle, was comparable between non-infected wild type (25.0 ± 7.2% of all inoculated sites were stained in nine independent leaves) and pgm leaves (27.8 ± 6.5% staining).
Fig. 2.
Epicuticular wax and cutin composition in pgm. Epicuticular wax (A) and cutin (B) monomer composition of fully expanded Col-0 (white bars) and pgm (black bars) leaves harvested from 5-week-old plants were determined in five biological replicates and are depicted as means ± SE. Wax constituents are shown as acids (left bracket), alcohols (middle bracket) and alkanes (right bracket). Cutin constitutents are abbreviated as follows: Alc., alcohols; ω-OH-Ac., ω-hydroxy acids; Di-Acids, dicarboxylic acids; 2-OH acids, 2-hydroxy acids. The chain lengths of the individual monomers are indicated below the bars, with the number behind the colon indicating the number of double bonds.
Indolic glucosinolate levels do not affect penetration resistance against C. higginsianum
It has previously been shown, that indolic glucosinolates (IGs), especially the PEN2 substrate 4-methoxy-indole-3-ylmethyl-glucosinolate (4MOI3M), contribute to penetration resistance against non-adapted powdery mildew fungi and non-adapted Colletotrichum species in Arabidopsis (Bednarek et al., 2009; Hiruma et al., 2010), but the role of IGs during C. higginsianum attack is yet unclear.
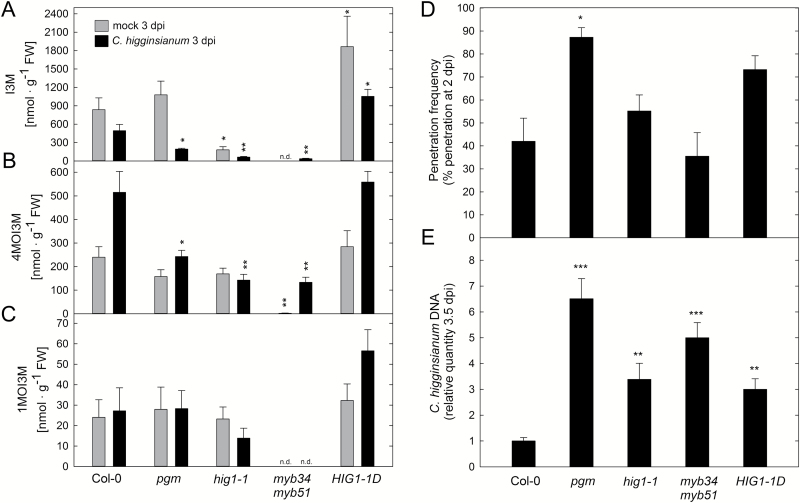
We observed that 4MOI3M contents were increased two-fold upon infection of Arabidopsis wild type with C. higginsianum (Fig. 3B). Furthermore, both indol-3-ylmethyl-glucosinolate (I3M) and 4MOI3M contents were significantly reduced in infected pgm mutant leaves compared with wild type, which prompted us to assess whether IG quantity can influence the Arabidopsis–C. higginsianum interaction. For this purpose, we analysed fungal proliferation in leaves of Arabidopsis genotypes with reduced (high indolic glucosinolate 1-1, hig1-1/myb51; myb34 myb51) and increased (HIG1-1 DOMINANT, HIG1-1D) IG content (Fig. 3A–C; Gigolashvili et al., 2007; Frerigmann and Gigolashvili, 2014). Penetration frequency at 2 dpi was not significantly altered in hig1-1, myb34 myb51 or HIG1-1D (Fig. 3D; with P=0.06 for HIG1-1D), indicating that reduced IG levels do not compromise preinvasion defense against C. higginsianum. During the necrotrophic phase at 3.5 dpi, however, fungal growth was increased in mutants with both reduced and increased IG content (Fig. 3E), suggesting that disturbance of IG metabolism interferes with postinvasion defense after fungal establishment is completed.
Fig. 3.
Analysis of indolic glucosinolate relevance in resistance to C. higginsianum. Indolic glucosinolate content was determined in 5-week-old leaves of Col-0, pgm, hig1-1, myb34 myb51, and HIG1-1D 3 dpi with C. higginsianum and mock treatment. (A–C) Indole-3-ylmethyl-glucosinolate (I3M; A), 4-methoxy-indole-3-ylmethyl-glucosinolate (4MOI3M; B), and 1-methoxy-indole-3-ylmethyl-glucosinolate (1MOI3M; C) contents were quantified in four biological replicates ±SE. (D) Penetration frequency as measured by the percentage of successful penetration events at 2 dpi. Values are means of three biological replicates ±SE. Per replicate, the penetration success of 200–300 appressoria on two leaves was scored. (E) The relative quantity of genomic C. higginsianum DNA in leaf samples was determined by qPCR at 3.5 d post-infection. Values are means of three biological replicates measured in three technical replicates ±SE. For each replicate, fully expanded leaves of two infected plants were pooled. Asterisks indicate a significant difference to Col-0 (*P<0.05; **P<0.01; ***P<0.001; Student’s t-test). n.d.: not detectable.
Cell wall monosaccharide composition and penetration resistance are affected in Arabidopsis mutants with impaired starch metabolism
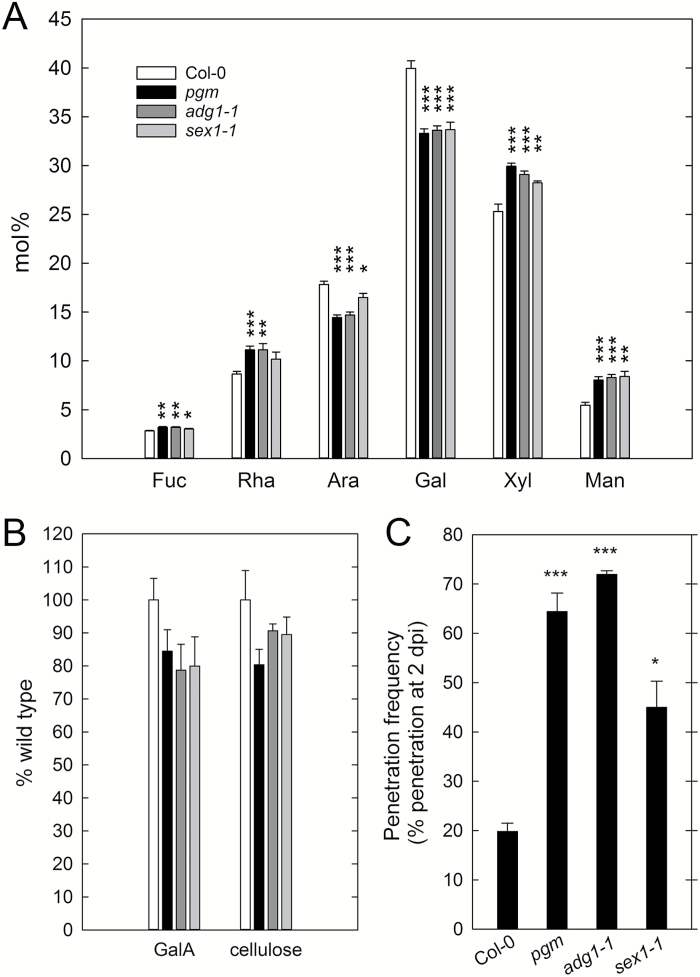
Underneath the cuticle, the plant cell wall forms the second structural barrier for fungal penetration. It has previously been shown that susceptibility to fungal biotrophs and necrotrophs is also influenced by the composition and modification of host cell walls (Vogel et al., 2002, 2004; Hernández-Blanco et al., 2007; Raiola et al., 2011; Pogorelko et al., 2013). We analysed the cell wall monosaccharide composition of pgm in comparison with the wild type. In fully expanded pgm leaves, we observed significantly reduced amounts of arabinose and galactose, while fucose, rhamnose, xylose, and mannose amounts were significantly increased compared with the wild type (Fig. 4A). Cell wall monosaccharide composition was similar in pgm and another starch-deficient mutant (ADP-glucose pyrophosphorylase 1-1, adg1-1), while a mutant affected in starch mobilization (starch excess 1-1, sex1-1) showed a slightly milder phenotype. However, galacturonic acid and cellulose contents were not significantly reduced in these genotypes (Fig. 4B). To examine whether reduced arabinose and galactose levels are caused by changes in soluble arabinogalactan proteins, we isolated water-soluble cell wall components of wild type, pgm, adg1-1, and sex1-1 leaves. The soluble fraction was mainly composed of arabinose and galactose (indicative of arabinogalactan) and showed increased fucose and decreased arabinose levels in pgm, while no differences were observed in adg1-1 and sex1-1 compared with wild type (see Supplementary Fig. S1).
Fig. 4.
Cell wall monosaccharide composition and penetration resistance of Arabidopsis mutants with impaired starch metabolism. (A) Relative content (mol%) of the neutral monosaccharides fucose (Fuc), rhamnose (Rha), arabinose (Ara), galactose (Gal), xylose (Xyl) and mannose (Man) in 5-week-old rosette leaves of Col-0, pgm, adg1-1 and sex1-1. (B) Galacturonic acid (GalA) and cellulose content in 5-week-old rosette leaves of Col-0, pgm, adg1-1 and sex1-1. Col-0, white bars; pgm, black bars; adg1-1, dark grey bars; sex1-1, light grey bars. Values are means ±SE (n=6). (C) Penetration frequency of C. higginsianum was analysed at 2 dpi in 5-week-old Col-0, pgm, adg1-1 and sex1-1 leaves. Values are means of three biological replicates ±SE. Per replicate, penetration success of hyphae formed from 260–350 conidia on two leaves was scored. Asterisks indicate a significant difference from Col-0 (*P<0.05; **P<0.01; ***P<0.001; Student’s t-test).
Next, we tested whether penetration resistance against C. higginsianum is also affected in adg1-1 and sex1-1 mutants. Consistent with the observed overall cell wall monosaccharide composition, the penetration frequency of C. higginsianum was similarly increased in pgm and adg1-1, and intermediate in sex1-1. In wild type plants, 20% of all appressoria had penetrated the leaf surface to form hyphae at 2 dpi, as compared with 64% in pgm, 72% in adg1-1, and 45% in sex1-1 (Fig. 4C). Our observations thus suggest a causal link between impaired starch metabolism, altered cell wall composition and penetration frequency of C. higginsianum.
Glycome analyses reveal significant alterations in cell wall glycan composition and extractability of starch-deficient pgm mutants
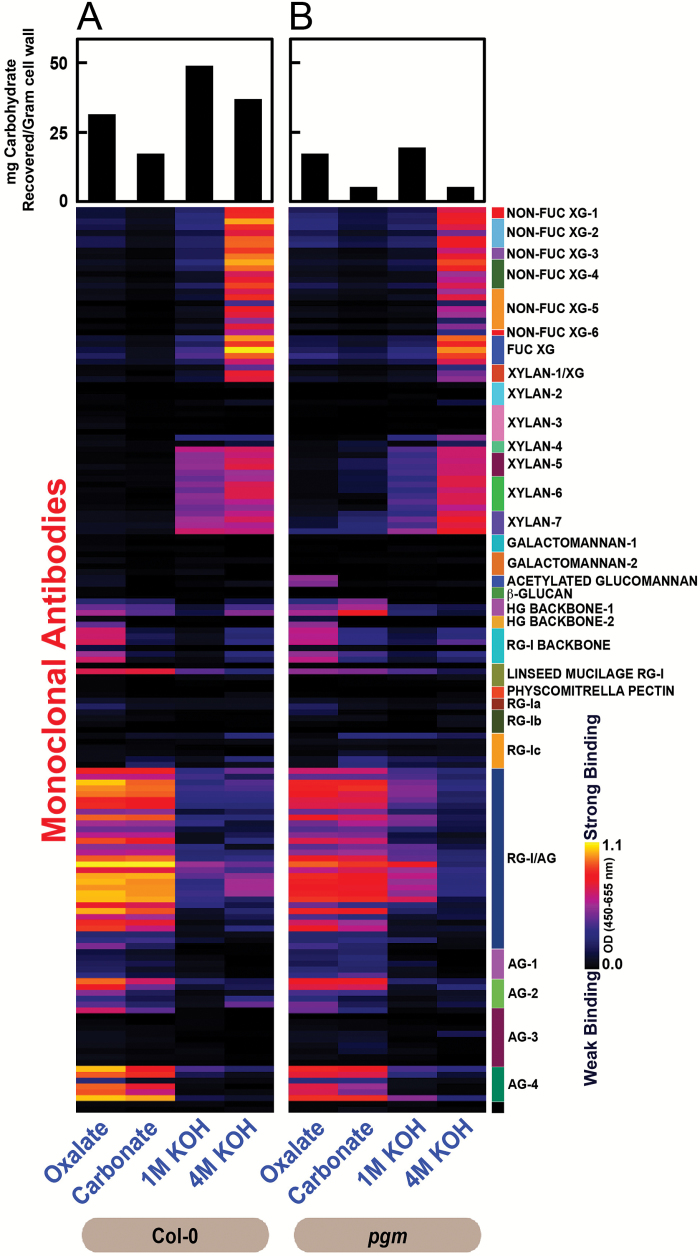
We performed glycome profiling analyses in order to elucidate differences in the extractability and overall composition of major non-cellulosic cell wall glycans between pgm and wild type leaves. Reduced amounts of carbohydrates were recovered from pgm cell wall preparations in comparison to the wild type in all extraction steps (Fig. 5, top bar charts). For ELISA analysis using cell wall glycan directed monoclonal antibodies (mAbs), equal amounts of recovered carbohydrates were coated, thus allowing for comparison of relative differences in mAb binding intensities between wild type and pgm extracts (Pattathil et al., 2012).
Fig. 5.
Glycome profiling of pgm mutants. Glycome profiling of sequential cell wall extracts prepared from rosette leaves of 5-week-old Col-0 (A) and pgm (B) plants. Samples represent pools of more than 50 fully expanded leaves collected in three independent experiments. Bar charts above the heat maps indicate the amount of carbohydrates recovered from cell wall preparations by sequential extraction with oxalate, carbonate, 1 M KOH and 4 M KOH. Extracts were analysed for abundance of cell wall glycan epitopes using 155 monoclonal antibodies (see Supplementary Table S1 and http://www.wallmabdb.net for details) whose glycan specificities are depicted on the right had side key. ELISA responses are illustrated in heat maps showing the relative binding strength of specific antibodies on a multi-color scale ranging from black (no binding) to bright yellow (strongest binding).
Extractability of pectic rhamnogalacturonan-I/arabinogalactan epitopes (indicated by the binding of RG-I/AG and AG-4 groups of mAbs) was reduced in oxalate and carbonate extracts of pgm cell wall material, while the proportion of both unsubstituted and substituted xylan epitopes (those recognized by xylan-4 to -7 groups of mAbs) as well as of homogalacturonan (HG) backbone-1 and RG-I backbone epitopes was marginally increased in the pgm carbonate extract. Xylan extractability in 1 M KOH extract was reduced in the pgm cell wall preparation, while the abundance of RG-I/AG epitopes in this 1 M KOH extract of pgm was increased compared with wild type. Taken together, these differences may indicate that RG-I/AG epitopes are more tightly linked to the pgm cell wall matrix, while xylans are more loosely linked to the pgm cell wall matrix compared with wild type. Xyloglucan extractability patterns overall remained the same in pgm and wild type, with both non-fucosylated and fucosylated xyloglucans being abundant in 4 M KOH extracts. In addition, we observed an increased abundance of acetylated glucomannan epitopes in the oxalate extract of pgm.
In summary, these studies show considerable differences in the extractability of RG-I/AG, RG-I backbone, HG backbone, xylan and acetylated glucomannan epitopes from pgm cell walls, indicating significant overall alterations in the architecture of the pgm cell wall matrix.
Comparative analysis of cell wall mutants indicates that pectin content is crucial for penetration resistance against C. higginsianum and biotrophic hyphae development
In order to gain insight into the relevance of different cell wall polymers for the susceptibility to C. higginsianum, we screened a number of cell wall mutants that (i) share particular alterations in cell wall composition with pgm, (ii) exhibit opposite changes compared with pgm or (iii) are likely to have altered cell wall stiffness. Loss of the putative arabinosyltransferase ARABINAN DEFICIENT 1 (ARAD1) and the UDP-D-xylose 4-epimerase MUR4 led to reduced arabinose content in leaf cell wall extracts (Burget and Reiter 1999; Burget et al., 2003; Harholt et al., 2006). Similarly, cell wall galactose levels were reduced in the galactosyltransferase mutant galactan synthase 1 (gals1) (Liwanag et al., 2012). Mutation of MUR8 led to a reduced cell wall rhamnose content and glycosyl linkage analysis suggested a reduced RG-I content in mur8-1 (Reiter et al., 1997; Mertz et al., 2012). A mutant of MUR11/SUPRESSOR OF ACTIN 9 (SAC9) exhibits complex alterations in monosaccharide composition, with reduced rhamnose, fucose and xylose levels and increased mannose levels, likely caused by a constitutive stress response (Reiter et al., 1997; Williams et al., 2005; Austin et al., 2011). Cell wall analysis of pmr5 pmr6 double mutants indicated reduced fucose and increased arabinose, galactose and galacturonic acid content (Vogel et al., 2004). Like in pgm mutants, the extractibility of xylan (xylan-4 to -7 mAbs) and pectin epitopes (HG and RG-I backbone mAbs) was increased in cell walls of arabinoxylan pectin arabinogalactan protein 1 (apap1) mutants (Tan et al., 2013). Finally, mutants of PME3, PME35 and PMEI6 have been analysed as alterations in DM and cell wall stiffness were expected in these mutants. Interaction phenotypes have previously been reported for pmr5, pmr6, pme3 and pme35, in different pathosystems (Vogel et al., 2002, 2004; Raiola et al., 2011; Bethke et al., 2014).
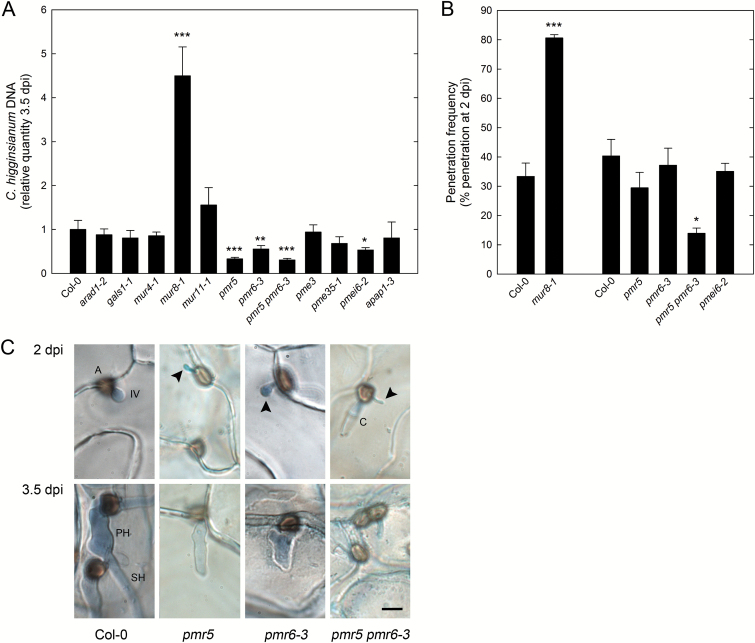
Of all mutants analysed, only mur8-1 showed a strong increase in the amount of C. higginsianum DNA at 3.5 dpi compared with wild type (Fig. 6A). Increased fungal proliferation at this late interactions stage is not linked to a defect in major carbohydrate metabolism of mur8-1 (see Supplementary Fig. S2 at JXB online). Reduced susceptibility was found for pmr5, pmr6-3, pmr5 pmr6-3 and pmei6-2 mutants. We therefore tested whether early establishment of C. higginsianum was also altered in these mutants. Penetration frequency was strongly increased in mur8-1 and decreased in pmr5 pmr6-3 (Fig. 6B). While penetration frequency was not affected in pmr5 and pmr6-3 single mutants, morphology of biotrophic hyphae formed in all pmr single and double mutants was severely altered (Fig. 6C). At 2 dpi, bulbous IVs were not observed in pmr5 and pmr5 pmr6-3, while bulbous IVs were less frequent in pmr6-3 than in wild type epidermal cells. During subsequent growth on pmr mutants, C. higginsianum hyphae either failed to branch and differentiate to SH, or IVs were shrunken. Thus, restricted fungal proliferation in pmr5, pmr6-3, and pmr5 pmr6-3 at 3.5 dpi can be seen as a consequence of impaired development of biotrophic hyphae earlier in the interaction.
Fig. 6.
Analysis of fungal proliferation, penetration efficiency and hyphal morphology on Arabidopsis cell wall mutants. (A) The relative quantity of genomic C. higginsianum DNA in leaves of 5-week-old Col-0, arad1-2, gals1-1, mur4-1, mur8-1, mur11-1, pmr5, pmr6-3, pmr5 pmr6-3, pme3, pme35-1, pmei6-2, and apap1-3 was determined by qPCR at 3.5 dpi. Values are means of three to four biological replicates measured in three technical replicates ±SE. For each replicate, fully expanded leaves of two infected plants were pooled. (B) Penetration frequency of C. higginsianum was analysed at 2 dpi in 5-week-old Col-0, mur8-1, pmr5, pmr6-3, pmr5 pmr6-3, and pmei6-2 leaves. Values are means of three biological replicates ±SE. Per replicate, penetration success of hyphae formed from >250 appressoria on two leaves was scored. Asterisks indicate a significant difference to Col-0 (*P<0.05; **P<0.01; ***P<0.001; Student’s t-test). (C) Representative pictures of early fungal development in Col-0, pmr5, pmr6-3, and pmr5 pmr6-3 leaves at 2 dpi and 3.5 dpi. C, conidium; A, appressorium; IV, infection vesicle; PH, primary hypha; SH, secondary hypha. Arrowheads point towards incompletely differentiated IVs. The bar represents 5 µm.
Altogether, these observations indicate a connection between altered pectin amount in mur8-1, pmr5, pmr6-3, and pmr5 pmr6-3 with biotrophic establishment of C. higginsianum. In turn, our results suggest that differences in pectin content or structure might affect penetration resistance in pgm. Reduced arabinose and galactose levels in the pgm cell wall as well as reduced extractability of RG-I/AG epitopes indicated that arabinan and galactan side chains of RG-I might be altered in pgm. We thus digested crude cell wall preparations of pgm, arad1-2, gals1-1 and wild type leaves with arabinanase and galactanase, to test whether enzymatic extractability of arabinose or galactose is altered in pgm compared with these lines. While an arabinanase digestion liberated 19.4 ± 3.7% of the total arabinose in pgm samples, only 9.3 ± 4.6% and 6.7 ± 3.4% of the arabinose residues could be released in wild type and arad1-2 cell wall preparations, respectively. This significant difference (P<0.01, Student’s t-test) indicates a structural difference between pgm and arad1-2 cell walls. In contrast, a galactanase digest of crude cell wall material did not reveal any difference between pgm, gals1-1, and wild type.
Discussion
In the present study, we have observed that penetration frequency and establishment of C. higginsianum hyphae were enhanced in leaves of the starch-deficient mutants pgm and adg1-1 and the starch-excess mutant sex1-1, which is hampered in starch mobilization. While these mutants suffer from nocturnal carbon limitation, the accelerated fungal development during the early interaction phase with these genotypes cannot be explained by the previously reported negative effect of reduced carbon availability on induced defense responses (Engelsdorf et al., 2013). Both salicylic acid and camalexin start to accumulate after successful penetration of host cells, when the establishment of biotrophic hyphae is already completed (Engelsdorf et al., 2013). Therefore, either preexisting (structural) defense, induced preinvasion defense or immediate post-penetration defense must account for the enhanced establishment of C. higginsianum in Arabidopsis genotypes with impaired starch metabolism.
The data obtained for the pgm mutant indicate that the cuticle composition and the diminished production of indolic glucosinolates (IGs), both major determinants of pre-invasion and non-host resistance (e.g. Bednarek et al., 2009; Sanchez-Vallet et al., 2010, Schlaeppi et al., 2010; Hansjakob et al., 2011; Uppalapati et al., 2012; Hiruma et al., 2013), do not play a major role for the earlier establishment of C. higginsianum on host leaves with impaired starch metabolism.
Regulation of indolic glucosinolate biosynthesis might contribute to postinvasion resistance against C. higginsianum
The IG 4MOI3M is supposed to serve as a substrate for the atypical myrosinase PEN2. The PEN2 reaction product is required for penetration resistance against non-adapted powdery mildews (Bednarek et al., 2009; Clay et al., 2009). The resistance of pen2-2 was significantly diminished toward two C. higginsianum pathogenicity mutants, but not toward C. higginsianum wild type (Huser et al., 2009). Therefore, the contribution of IGs to resistance against C. higginsianum seemed to be of a quantitative nature. We observed that 4MOI3M accumulated during leaf infections with C. higginsianum. I3M and 4MOI3M contents were significantly reduced in pgm leaves after infection compared with wild type, suggesting that carbon limitation in pgm disfavors carbon flux to glucosinolates. Nonetheless, a reduced capacity for IG production in the mutants hig1-1 and myb34 myb51 (Gigolashvili et al., 2007; Frerigmann and Gigolashvili, 2014) did not affect penetration frequency of C. higginsianum. Therefore, we can exclude that the reduced accumulation of I3M and 4MOI3M contributes to the expedited establishment of C. higginsianum in the pgm mutant.
At the time of extensive fungal proliferation during the necrotrophic growth phase, genotypes with both a reduction (hig1-1, myb34/51) and an increase in IG production (HIG1-1D) allowed for a more than three-fold increase in C. higginsianum colonization. However, this interaction phenotype may not exclusively be accounted for by altered IG production. The R2R3-MYB transcription factors MYB34 and MYB51 induce the cytochrome P450 monooxygenase isoforms CYP79B2 and CYP79B3, which catalyse the synthesis of indole-3-acetaldoxime (IAOx). IAOx formation from tryptophan is the committed step not only toward IG synthesis, but also toward the production of the major Arabidopsis phytoalexin camalexin, which is believed to disturb cellular integrity of fungal pathogens (Frerigmann et al., 2015). CYP79B2/B3 induction was almost absent in myb34/51/122 triple mutants, which did not differ from myb34/51 in indolic glucosinolate accumulation (Frerigmann and Gigolashvili, 2014). These findings suggest a dramatic reduction of IAOx production in myb34/51 double mutants as well. However, camalexin contents were significantly altered neither in hig1-1, myb34/51, and HIG1-1D after infection with C. higginsianum (not shown) nor in myb34/51/122 after infection with Plectosphaerella cucumerina (Frerigmann et al., 2016). Given that cruciferous species can derive other indolic phytoalexins from IAOx (Hagemeier et al., 2001; Pedras et al., 2001), it cannot be excluded that manipulation of IAOx production in hig1-1, myb34/51, and HIG1-1D affects the synthesis of indolic phytoalexins, e.g. like indole-3-carbaldehyde or indole-3-carboxylic acid derivatives (Hagemeier et al., 2001), which might play an important role for the defense against necrotrophic colonization by C. higginsianum.
Starch deficiency influences cell wall metabolism and composition
Due to the lack of starch, the transitory carbon storage capacity of the Arabidopsis pgm mutant is reduced by 66% in our growth conditions (Engelsdorf et al., 2013), making the mutant suffer from periodic carbon shortage towards the end of each dark phase, when the vacuolar sucrose reserves are depleted (Gibon et al., 2004; Engelsdorf et al., 2013). In transcriptome analyses of pgm by Thimm et al. (2004) and Bläsing et al. (2005), genes associated with cell wall metabolism were two-fold overrepresented among the downregulated genes at the end of the dark period, suggesting that cell wall metabolism is altered upon carbon shortage in pgm. The data presented here show a consistent reduction of arabinose and galactose levels and an increase in fucose, xylose and mannose levels in the cell walls of two mutants affected in starch biosynthesis (pgm, adg1-1) and one mutant affected in starch mobilization (sex1-1). Overall extractability of carbohydrates from pgm cell walls was strongly reduced. Particularly pectic epitopes (RG-I/AG and AG-4 groups of mAbs) showed reduced extractability, suggesting tighter linkage to other cell walls polymers. In turn, xylan epitopes (xylan-4 to -7 groups of mAbs) could be extracted in milder conditions, suggesting weaker linkage to other cell wall polymers. Taken together, our data indicate that pectic arabinan and galactan content is reduced in pgm cell walls, while xylan content is increased. The differences observed in pectin composition can either be explained by reduced arabinan and galactan synthesis or increased pectin degradation. Interestingly, an increased abundance of acetylated-glucomannan epitopes (recognized by mAbs, CCRC-M169 and CCRC-M170) was observed in the oxalate extract from pgm cell walls. However, additional studies are required to fully understand what structural difference(s) in pgm cell walls cause this increased abundance of these epitopes.
Pectic polysaccharides serve as carbohydrate storage in cotyledons of leguminous plants (Buckeridge et al., 2005; Gronwald et al., 2009). During mobilization of cell wall storage polysaccharides (CWSPs) in soybean cotyledons, cell wall galactose and arabinose content decrease by more than 85% (Gronwald et al., 2009), while galactan chains were shown to be the predominant CWSP in lupin cotyledons (Buckeridge et al., 2005). A study by Lee et al. (2007) demonstrated that after prolonged carbon starvation in detached Arabidopsis leaves for 2 d, the amount of all monosaccharides in pectin and hemicelluloses was substantially reduced by at least 30%, indicating an excessive mobilization of carbon from the cell wall under these extreme conditions. It seems reasonable to speculate that the degradation of cell wall polymers is restrained under in vivo conditions in intact plants. Released galactose moieties activated by galactokinase (GALK) and UDP-sugar pyrophosphorylase (USP) can be interconverted into UDP-glucose by UDP-glucose 4-epimerases (UGEs) to fuel sucrose biosynthesis in times of need (Reiter, 2008; Geserick and Tenhaken, 2013). Five UGE genes are present in Arabidopsis, of which UGE1 and UGE3 are supposed to be involved in cell wall catabolism (Barber et al., 2006). Analysis of recombinant Arabidopsis UGEs in E. coli revealed that UGE1 and UGE3 additionally have high UDP-xylose 4-epimerase activity (Kotake et al., 2009), being able to also interconvert UDP-arabinose and UDP-xylose. Similar to galactose, arabinose moieties can be activated by arabinokinase (ARA) and USP and converted into UDP-xylose by UGEs and UDP-xylose 4-epimerase (MUR4) (Reiter, 2008).
Compared with the wild type, GALK2, ARA1, UGE1, and UGE3 were upregulated in pgm mutants during the second half of the dark phase and Col-0 rosettes subjected to an extended night (see Supplementary Fig. S3; Usadel et al., 2008). In addition, accessibility of arabinose monomers to arabinanase digest was elevated in pgm cell wall preparations compared with wild type and arad1-2 mutants. Recently, Behmüller et al. (2016) suggested that ARA1 is involved in sugar signaling during arabinose feeding, thus potentially sensing flux through the salvage pathway. On the other hand, we examined gene expression levels of galactosyltransferase (GALS1–GALS3) and putative arabinosyltransferase (ARAD1 and ARAD2) genes, which are supposed to be involved in galactan and arabinan biosynthesis, in pgm mutants during the second half of the dark phase and Col-0 rosettes subjected to an extended night. GALS1 and GALS3 expression was consistently downregulated in both data sets, suggesting that galactan synthesis is attenuated upon carbon shortage, whereas ARAD1 and ARAD2 expression was unchanged compared with the respective controls (Supplementary Fig. S3; Usadel et al., 2008).
In summary, the available data indicate that galactose and arabinose moieties are mobilized and salvaged from pgm pectin during periods of nocturnal starvation while galactan synthesis is attenuated.
Pectin levels affect penetration frequency of C. higginsianum in Arabidopsis leaves
To test whether specific alterations in pgm cell walls account for the impaired penetration resistance against C. higginsianum, we screened a number of cell wall mutants for susceptibility phenotypes. As Arabidopsis mutants with a specific increase in leaf cell wall xylans have not been identified to our knowledge, we could not test the contribution of xylans in pgm. Susceptibility of the arad1-2 mutant with reduced arabinan (Harholt et al., 2006) and the gals1-1 mutant with diminished galactan side chains (Liwanag et al., 2012) was comparable to the wild type. Thus, we can rule out that increased pgm susceptibility is caused by simple deficiencies in either polymer. Susceptibility was also unaffected in mur4-1, mur11-1, pme3, pme35-1, and apap1-3 mutants, indicating that specific cell wall alterations in these mutants do not affect susceptibility to C. higginsianum either. While fungal DNA amount was reduced in pmei6-2 mutants at 3.5 dpi, penetration resistance was not affected, suggesting restricted necrotrophic growth in this mutant. Notably, the mur8-1 mutant, which has a reduced RG-I content in leaf cell walls (Mertz et al., 2012), was both hypersusceptible toward C. higginsianum and showed strongly impaired penetration resistance. Since the pectic polymer RG-I is rich in both arabinan and galactan side chains, it is tempting to assume that altered RG-I composition contributes to reduced penetration resistance in pgm as well.
On the other hand, pmr5, pmr6-3, and pmr5 pmr6-3 mutants, which exhibit increased pectin content and altered pectin modification of epidermal cell walls (Vogel et al., 2002, 2004), showed increased resistance toward C. higginsianum. While penetration resistance was not affected in pmr single mutants, penetration frequency in the double mutant was reduced to 35% of wild type. Post-invasive differentiation of infection vesicles and biotrophic hyphae was severely hampered in both pmr single and pmr double mutants. Similarly growth and integrity of E. cichoracearum epiphytic hyphae were also affected in these pmr mutants (Vogel et al., 2002, 2004). Although a partial resistance of pmr6 toward C. higginsianum had been reported previously (O’Connell and Panstruga, 2006), we have now shown that establishment of C. higginsianum is impaired on both pmr5 and pmr6 single and double mutants because of impaired hyphal expansion and differentiation. Increased pectin content in pmr mutants may simply provide a higher mechanical resistance to the invagination of the plasma membrane during formation of infection vesicles. Alternatively, an altered matrix between host and fungal cells might affect the perception of host signals by the fungus or restrict accessibility of nutrients to the fungus.
Taken together, our data suggest that Arabidopsis pectin content and composition influence both penetration by C. higginsianum and normal expansion of biotrophic C. higginsianum hyphae in host epidermal cells. Our data also strongly imply that enhanced penetration frequency of C. higginsianum in mutants with reduced starch turnover is caused by altered cell wall composition. As judged from our data, changes in pectin content or composition seem to be most relevant for altered penetration in these mutants.
Supplementary Material
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) via the priority program FOR 666 [grant number SO 300/11-2]. The generation of the CCRC series of plant cell wall glycan-directed monoclonal antibodies used in this work was supported by the NSF Plant Genome Program (DBI-0421683 and IOS-0923992).
The authors would like to acknowledge Alexandra Ammon (Department of Biochemistry, FAU Erlangen-Nuremberg) for skilled technical assistance, Tamara Gigolashvili (University of Cologne) for providing hig1-1, myb34 myb51, and HIG1-1D seeds and Nora Gigli Bisceglia (NTNU Trondheim) for critical reading of the manuscript. We greatly appreciate advisory support by Alfred Schmiedl during HPAEC-PAD analysis of cell wall monosaccharide composition (Department of Biochemistry, FAU Erlangen-Nuremberg).
References
- Austin RS, Vidaurre D, Stamatiou G, et al. 2011. Next-generation mapping of Arabidopsis genes. The Plant Journal 67, 715–725. [DOI] [PubMed] [Google Scholar]
- Barber C, Rösti J, Rawat A, Findlay K, Roberts K, Seifert GJ. 2006. Distinct properties of the five UDP-D-glucose/UDP-D-galactose 4-epimerase isoforms of Arabidopsis thaliana. Journal of Biological Chemistry 281, 17276–17285. [DOI] [PubMed] [Google Scholar]
- Bechinger C, Giebel KF, Schnell M, Leiderer P, Deising HB, Bastmeyer M. 1999. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 285, 1896–1899. [DOI] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, et al. 2009. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106. [DOI] [PubMed] [Google Scholar]
- Behmüller R, Kavkova E, Düh S, Huber CG, Tenhaken R. 2016. The role of arabinokinase in arabinose toxicity in plants. The Plant Journal 87, 376–390. [DOI] [PubMed] [Google Scholar]
- Beisson F, Li-Beisson Y, Pollard M. 2012. Solving the puzzles of cutin and suberin polymer biosynthesis. Current Opinion in Plant Biology 15, 329–337. [DOI] [PubMed] [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, et al. 2007. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. The EMBO Journal 26, 2158–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke G, Grundman RE, Sreekanta S, Truman W, Katagiri F, Glazebrook J. 2014. Arabidopsis PECTIN METHYLESTERASEs contribute to immunity against Pseudomonas syringae. Plant Physiology 164, 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birker D, Heidrich K, Takahara H, et al. 2009. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. The Plant Journal 60, 602–613. [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, et al. 2005. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. The Plant Cell 17, 3257–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner K, Hörmiller I, Nägele T, Heyer AG. 2014. Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana. The Plant Journal 79, 82–91. [DOI] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62, 471–481. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Hutcheon IS, Reid JS. 2005. The role of exo-(1–>4)-beta-galactanase in the mobilization of polysaccharides from the cotyledon cell walls of Lupinus angustifolius following germination. Annals of Botany 96, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burget EG, Reiter WD. 1999. The mur4 mutant of arabidopsis is partially defective in the de novo synthesis of uridine diphospho L-arabinose. Plant Physiology 121, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burget EG, Verma R, Mølhøj M, Reiter WD. 2003. The biosynthesis of L-arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-D-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. The Plant Cell 15, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Jarvis MC. 2012. Comparative structure and biomechanics of plant primary and secondary cell walls. Frontiers in Plant Science 3, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deising HB, Werner S, Wernitz M. 2000. The role of fungal appressoria in plant infection. Microbes and Infection 2, 1631–1641. [DOI] [PubMed] [Google Scholar]
- DeMartini JD, Pattathil S, Avci U, et al. 2011. Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy and Environmental Science, 4, 4332–4339. [Google Scholar]
- Ellinger D, Naumann M, Falter C, et al. 2013. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiology 161, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsdorf T, Horst RJ, Pröls R, et al. 2013. Reduced carbohydrate availability enhances the susceptibility of Arabidopsis toward Colletotrichum higginsianum. Plant Physiology 162, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. 2007. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiology 144, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Pontiggia D, et al. 2008. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiology 146, 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Vairo D, Cervone F, De Lorenzo G. 2006. Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Molecular Plant-Microbe Interactions 19, 931–936. [DOI] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, et al. 2005. Apoplastic polyesters in Arabidopsis surface tissues – a typical suberin and a particular cutin. Phytochemistry 66, 2643–2658. [DOI] [PubMed] [Google Scholar]
- Frerigmann H, Gigolashvili T. 2014. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Molecular Plant 7, 814–828. [DOI] [PubMed] [Google Scholar]
- Frerigmann H, Glawischnig E, Gigolashvili T. 2015. The role of MYB34, MYB51 and MYB122 in the regulation of camalexin biosynthesis in Arabidopsis thaliana. Frontiers in Plant Science 6, 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H, Piślewska-Bednarek M, Sánchez-Vallet A, et al. 2016. Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Molecular Plant 9, 682–695. [DOI] [PubMed] [Google Scholar]
- Geserick C, Tenhaken R. 2013. UDP-sugar pyrophosphorylase is essential for arabinose and xylose recycling, and is required during vegetative and reproductive growth in Arabidopsis. The Plant Journal 74, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, et al. 2004. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. The Plant Journal 39, 847–862. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Berger B, Mock HP, Müller C, Weisshaar B, Flügge UI. 2007. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. The Plant Journal 50, 886–901. [DOI] [PubMed] [Google Scholar]
- Gille S, Pauly M. 2012. O-acetylation of plant cell wall polysaccharides. Frontiers in Plant Science 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald JW, Jung HJG, Litterer LA, Somers DA. 2009. Comparison of post-germination mobilization of cell wall polysaccharides and non-cell wall carbohydrates in soybean Glycine max L. cotyledons. Journal of the Science of Food and Agriculture 89, 1981–1986. [Google Scholar]
- Hagemeier J, Schneider B, Oldham NJ, Hahlbrock K. 2001. Accumulation of soluble and wall-bound indolic metabolites in Arabidopsis thaliana leaves infected with virulent or avirulent Pseudomonas syringae pathovar tomato strains. Proceedings of the National Academy of Sciences of the United States of America 98, 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansjakob A, Bischof S, Bringmann G, Riederer M, Hildebrandt U. 2010. Very-long-chain aldehydes promote in vitro prepenetration processes of Blumeria graminis in a dose- and chain length-dependent manner. The New Phytologist 188, 1039–1054. [DOI] [PubMed] [Google Scholar]
- Hansjakob A, Riederer M, Hildebrandt U. 2011. Wax matters: absence of very-long-chain aldehydes from the leaf cuticular wax of the glossy11 mutant of maize compromises the prepenetration processes of Blumeria graminis. Plant Pathology 60, 1151–1161. [Google Scholar]
- Harholt J, Jensen JK, Sørensen SO, Orfila C, Pauly M, Scheller HV. 2006. Arabinan Deficient 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiology 140, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J, Suttangkakul A, Vibe Scheller H. 2010. Biosynthesis of pectin. Plant Physiology 153, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Blanco C, Feng DX, Hu J, et al. 2007. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. The Plant Cell 19, 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Fukunaga S, Bednarek P, et al. 2013. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proceedings of the National Academy of Sciences of the United States of America 110, 9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Onozawa-Komori M, Takahashi F, et al. 2010. Entry mode-dependent function of an indole glucosinolate pathway in Arabidopsis for nonhost resistance against anthracnose pathogens. The Plant Cell 22, 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huser A, Takahara H, Schmalenbach W, O’Connell R. 2009. Discovery of pathogenicity genes in the crucifer anthracnose fungus Colletotrichum higginsianum, using random insertional mutagenesis. Molecular Plant-Microbe Interactions 22, 143–156. [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. 1990. Arabidopsis is susceptible to infection by a downy mildew fungus. The Plant Cell 2, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kohorn SL. 2012. The cell wall-associated kinases, WAKs, as pectin receptors. Frontiers in Plant Science 3, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T, Takata R, Verma R, et al. 2009. Bifunctional cytosolic UDP-glucose 4-epimerases catalyse the interconversion between UDP-D-xylose and UDP-L-arabinose in plants. Biochemical Journal 424, 169–177. [DOI] [PubMed] [Google Scholar]
- Kurdyukov S, Faust A, Nawrath C, et al. 2006. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. The Plant Cell 18, 321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Matsumura Y, Soga K, Hoson T, Koizumi N. 2007. Glycosyl hydrolases of cell wall are induced by sugar starvation in Arabidopsis. Plant & Cell Physiology 48, 405–413. [DOI] [PubMed] [Google Scholar]
- Levesque-Tremblay G, Pelloux J, Braybrook SA, Müller K. 2015. Tuning of pectin methylesterification: consequences for cell wall biomechanics and development. Planta 242, 791–811. [DOI] [PubMed] [Google Scholar]
- Lionetti V, Raiola A, Camardella L, et al. 2007. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiology 143, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, et al. 2005. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Liwanag AJ, Ebert B, Verhertbruggen Y, et al. 2012. Pectin biosynthesis: GALS1 in Arabidopsis thaliana is a β-1,4-galactan β-1,4-galactosyltransferase. The Plant Cell 24, 5024–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, van der Does HC, Borkovich KA, et al. 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y, Nafisi M, Verhertbruggen Y, et al. 2011. Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiology 155, 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendgen K, Hahn M. 2002. Plant infection and the establishment of fungal biotrophy. Trends in Plant Science 7, 352–356. [DOI] [PubMed] [Google Scholar]
- Mendoza-Mendoza A, Berndt P, Djamei A, et al. 2009. Physical-chemical plant-derived signals induce differentiation in Ustilago maydis. Molecular Microbiology 71, 895–911. [DOI] [PubMed] [Google Scholar]
- Mertz RA, Olek AT, Carpita NC. 2012. Alterations in cell-wall glycosyl linkage structure of Arabidopsis murus mutants. Carbohydrate Polymers 89, 331–339. [DOI] [PubMed] [Google Scholar]
- Münch S, Lingner U, Floss DS, Ludwig N, Sauer N, Deising HB. 2008. The hemibiotrophic lifestyle of Colletotrichum species. Journal of Plant Physiology 165, 41–51. [DOI] [PubMed] [Google Scholar]
- Narusaka Y, Narusaka M, Park P, et al. 2004. RCH1, a locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum higginsianum. Molecular Plant-Microbe Interactions 17, 749–762. [DOI] [PubMed] [Google Scholar]
- Nawrath C, Schreiber L, Franke RB, Geldner N, Reina-Pinto JJ, Kunst L. 2013. Apoplastic diffusion barriers in Arabidopsis. The Arabidopsis Book 11, e0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øbro J, Harholt J, Scheller HV, Orfila C. 2004. Rhamnogalacturonan I in Solanum tuberosum tubers contains complex arabinogalactan structures. Phytochemistry 65, 1429–1438. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Panstruga R. 2006. Tête à tête inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytologist 171, 699–718. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Thon MR, Hacquard S, et al. 2012. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, et al. 2010. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiology 153, 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Miller JS, Hahn MG. 2012. Immunological approaches to plant cell wall and biomass characterization: Glycome profiling. Methods in Molecular Biology 908, 61–72. [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook S, Höfte H. 2012. Cell wall mechanics and growth control in plants: the role of pectins revisited. Frontiers in Plant Science 3, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H. 2011. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Current Biology 21, 1720–1726. [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Wightman R, Höfte H. 2015. The Control of growth symmetry breaking in the Arabidopsis hypocotyl. Current Biology 25, 1746–1752. [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Montaut S, Xu Y, Khan AQ, Loukaci A. 2001. Assembling the biosynthetic puzzle of crucifer metabolites, indole-3-acetaldoxime is incorporated efficiently into phytoalexins but glucobrassicin is not. Chemical Communications 17, 1572–1573. [DOI] [PubMed] [Google Scholar]
- Pogorelko G, Lionetti V, Fursova O, et al. 2013. Arabidopsis and Brachypodium distachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiology 162, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiola A, Lionetti V, Elmaghraby I, et al. 2011. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Molecular Plant-Microbe Interactions 24, 432–440. [DOI] [PubMed] [Google Scholar]
- Reisige K, Gorzelanny C, Daniels U, Moerschbacher BM. 2006. The C28 aldehyde octacosanal is a morphogenetically active component involved in host plant recognition and infection structure differentiation in the wheat stem rust fungus. Physiological and Molecular Plant Pathology 68, 33–40. [Google Scholar]
- Reiter WD. 2008. Biochemical genetics of nucleotide sugar interconversion reactions. Current Opinion in Plant Biology 11, 236–243. [DOI] [PubMed] [Google Scholar]
- Reiter WD, Chapple C, Somerville CR. 1997. Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. The Plant Journal 12, 335–345. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vallet A, Ramos B, Bednarek P, et al. 2010. Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. The Plant Journal 63, 115–127. [DOI] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. 2010. Hemicelluloses. Annual Review of Plant Biology 61, 263–289. [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Abou-Mansour E, Buchala A, Mauch F. 2010. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. The Plant Journal 62, 840–851. [DOI] [PubMed] [Google Scholar]
- Shimada C, Lipka V, O’Connell R, Okuno T, Schulze-Lefert P, Takano Y. 2006. Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Molecular Plant-Microbe Interactions 19, 270–279. [DOI] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, et al. 2013. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. The Plant Cell 25, 270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, et al. 2004. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal 37, 914–939. [DOI] [PubMed] [Google Scholar]
- Updegraff DM. 1969. Semimicro determination of cellulose in biological materials. Analytical Biochemistry 32, 420–424. [DOI] [PubMed] [Google Scholar]
- Uppalapati SR, Ishiga Y, Doraiswamy V, et al. 2012. Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. The Plant Cell 24, 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, et al. 2008. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiology 146, 1834–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC. 2002. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. The Plant Cell 14, 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Somerville CR, Somerville SC. 2004. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. The Plant Journal 40, 968–978. [DOI] [PubMed] [Google Scholar]
- Voll L, Häusler RE, Hecker R, et al. 2003. The phenotype of the Arabidopsis cue1 mutant is not simply caused by a general restriction of the shikimate pathway. The Plant Journal 36, 301–317. [DOI] [PubMed] [Google Scholar]
- Voll LM, Zell MB, Engelsdorf T, et al. 2012. Loss of cytosolic NADP-malic enzyme 2 in Arabidopsis thaliana is associated with enhanced susceptibility to Colletotrichum higginsianum. New Phytologist 195, 189–202. [DOI] [PubMed] [Google Scholar]
- Williams ME, Torabinejad J, Cohick E, et al. 2005. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiology 138, 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Rose JK. 2013. The formation and function of plant cuticles. Plant Physiology 163, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P. 1995. Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiology 107, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.