Highlight
Plants use the alternative oxidase to modulate the rate of mitochondrial respiration in the light, maintaining chloroplast energy balance and hence optimizing photosynthesis under varied growth conditions.
Keywords: Chloroplast energy balance, drought stress, excitation pressure, growth irradiance, light-harvesting complex II protein, mitochondrial alternative oxidase, photosynthesis, respiration.
Abstract
Mitochondria have a non-energy-conserving alternative oxidase (AOX) proposed to support photosynthesis, perhaps by promoting energy balance under varying growth conditions. To investigate this, wild-type (WT) Nicotiana tabacum were compared with AOX knockdown and overexpression lines. In addition, the amount of AOX protein in WT plants was compared with that of chloroplast light-harvesting complex II (LHCB2), whose amount is known to respond to chloroplast energy status. With increased growth irradiance, WT leaves maintained higher rates of respiration in the light (RL), but no differences in RL or photosynthesis were seen between the WT and transgenic lines, suggesting that, under non-stress conditions, AOX was not critical for leaf metabolism, regardless of growth irradiance. However, under drought, the AOX amount became an important determinant of RL, which in turn was an important determinant of chloroplast energy balance (measured as photosystem II excitation pressure, EP), and photosynthetic performance. In the WT, the AOX amount increased and the LHCB2 amount decreased with increased growth irradiance or drought severity. These changes in protein amounts correlated strongly, in opposing ways, with growth EP. This suggests that a signal deriving from the photosynthetic electron transport chain status coordinately controls the amounts of AOX and LHCB2, which then both contribute to maintaining chloroplast energy balance, particularly under stress conditions.
Introduction
Respiration in the mitochondrion and photosynthesis in the chloroplast share important carbon and energy intermediates, and hence it is thought that these pathways must act in a coordinated manner to optimize energy metabolism in the leaf cell (Hoefnagel et al., 1998; Gardestrӧm et al., 2002; Noctor et al., 2007; Noguchi and Yoshida, 2008; Nunes-Nesi et al., 2008; Tcherkez et al., 2012; Gardestrӧm and Igamberdiev, 2016). For example, an imbalance of energy intermediates in one organelle might be offset by compensatory metabolic changes in the other organelle. Numerous studies have investigated the potential role of the non-energy-conserving alternative oxidase (AOX) pathway of mitochondrial electron transport in optimizing photosynthesis (see below). Unlike electron flow from ubiquinol to cytochrome oxidase, electron flow from ubiquinol to AOX is not proton-pumping and hence is not limited by the rate of ATP turnover. The maximum capacity of the AOX pathway to consume electrons is dependent upon developmental and environmental cues, and the actual partitioning of electrons to AOX is subject to sophisticated biochemical controls (Vanlerberghe and Ordog, 2002; Finnegan et al., 2004; Clifton et al., 2006; Van Aken et al., 2009; Moore et al., 2013).
Important pioneering studies used AOX inhibitors such as salicylhydroxamic acid to examine how the absence of AOX activity would impact on photosynthetic performance (Raghavendra and Padmasree, 2003). In recent years, the availability of AOX knockdown/knockout plants, particularly in the model species Arabidopsis thaliana, have greatly aided such work. The majority of these more recent studies have used immediate and short-term (usually a few hours) increases in irradiance (high light stress) as a means to rapidly challenge chloroplast energy balance and then evaluate the importance of AOX in correcting such imbalances (Zhang et al., 2010; Florez-Sarasa et al., 2011; Yoshida et al., 2011a, b; Vishwakarma et al., 2014; Watanabe et al., 2016). In general, these studies have concluded that the absence of AOX does result in some perturbation of photosynthetic metabolism following short-term increases in irradiance. One study showed that this was associated with an exaggerated increase in reduction state of the plastoquinone (PQ) and ubiquinone pools in the AOX knockdown relative to the wild-type (WT), following the shift to higher irradiance (Yoshida et al., 2011a). Such a result suggests that AOX activity not only impacts on the mitochondrion, but on the chloroplast as well.
Other studies and types of analyses also suggest that AOX respiration has a role in optimizing leaf metabolism in response to changes in irradiance. For example, the short-term shifts to higher irradiance have been shown to rapidly increase the amount of leaf AOX transcript and/or protein in different plant species (Yoshida et al., 2007, 2008, 2011a; Dinakar et al., 2010; Wallstrӧm et al., 2014). It has also been shown, in the shade species Alocasia odora, that upon a shift to higher irradiance, existing AOX protein is converted from an oxidized inactive to a reduced active form (Noguchi et al., 2005). De-etiolation is also associated with an increase in the AOX1a transcript in both Arabidopsis (Rosso et al., 2009; Zhang et al., 2016) and wheat (Garmash et al., 2015). AOX transcript amounts also display a diurnal pattern, with the highest amounts occurring early in the light period (Dutilleul et al., 2003; Cvetkovska and Vanlerberghe, 2012).
Fewer studies have compared AOX transcript and/or protein amounts following long-term growth and development at different irradiances. The AOX protein amount did not differ between Cucumis sativus plants grown at a photosynthetic photon flux density (PPFD) of 150 and 400 µmol photons m–2 s–1 (Florez-Sarasa et al., 2009) or between Arabidopsis plants grown at 80 and 350 PPFD (Yoshida et al., 2011a). However, other studies reported that AOX transcript/protein and/or capacity were higher following growth and development at higher irradiances (Bartoli et al., 2006; Florez-Sarasa et al., 2011; Igamberdiev et al., 2014; Shabnam et al., 2015). Such findings are in keeping with analytical modelling of metabolism, which predicts the increased involvement of non-energy-conserving pathways at higher irradiances (Buckley and Adams, 2011; Cheung et al., 2015). These models predict that, with increasing irradiance, increased AOX respiration is required to progressively increase the mitochondrial consumption of excess reductant while also progressively decreasing the mitochondrial contribution to ATP synthesis, being compensated instead by ATP synthesis in the chloroplast. In planta, the steady-state activities of AOX and cytochrome oxidase (i.e. their respective rates of oxygen consumption) can only be determined using an oxygen isotope discrimination technique (Guy et al., 1989; Robinson et al., 1995). However, this technique has thus far only been developed for respiration measurements in the dark, owing to the complexities of leaf oxygen exchange in the light during photosynthesis. Hence, the use of AOX transgenic/mutant plants remains an essential means to evaluate the role of AOX respiration during photosynthesis.
As outlined above, many studies have used AOX knockdown/knockout plants to evaluate whether AOX respiration is necessary to support leaf metabolism following a short-term increase in irradiance. Surprisingly, however, few studies have utilized such plants to evaluate whether higher irradiances in the long-term (i.e. higher growth irradiances) necessitate higher AOX to optimally support metabolism. To our knowledge, the only such studies are those of Yoshida and colleagues, comparing photosynthesis of wild-type and aox1a knockout Arabidopsis plants (Yoshida et al., 2011a, b). Following long-term growth at low to medium irradiances (40 to 350 PPFD), these authors found little if any differences in photosynthesis between the WT and aox1a. Again, however, differences in photosynthetic performance were evident between the plant lines following short-term high light stress treatments. The Yoshida et al. (2011b) study reported no difference in the rate of respiration in the dark (RD) in the mutant. This corresponds with several other studies reporting no change (or even an increase) in RD in this mutant compared to the WT under varied growth conditions (Giraud et al., 2008; Watanabe et al., 2008; Strodtkӧtter et al., 2009; Florez-Sarasa et al., 2011; Gandin et al., 2012; Vishwakarma et al., 2014; Kühn et al., 2015). To our knowledge, rates of respiration in the light (RL) in this mutant have not yet been reported. In another study, it was reported that knockdown of an AOX2 gene in soybean reduced the light-saturated rate of photosynthesis, which was attributed to a biochemical, as opposed to stomatal, limitation of photosynthesis (Chai et al., 2010). However, this study did not compare plants across a range of growth irradiances. These plants exhibited no change in RD, while RL was not examined. Hence, further studies are clearly required to establish whether AOX respiration is important only for response to short-term irradiance shifts or whether it also has an important role in the longer-term acclimation of leaf metabolism to different growth irradiances.
Both laboratory and field studies have shown that the amount of AOX protein in leaf tissue responds dynamically to seasonal and environmental parameters such as irradiance, temperature, and water availability (Taylor et al., 2002; Armstrong et al., 2008; Searle and Turnbull, 2011; Searle et al., 2011a, b; Vanlerberghe, 2013; Dahal and Vanlerberghe, 2017). Transcriptome and other studies have shown that AOX gene(s) are amongst the most dynamic of the nuclear genes encoding mitochondrial components in response to numerous stress treatments (Clifton et al., 2006). Significant progress is being made to identify the molecular components that control AOX gene expression (Ng et al., 2014). However, despite these important advances, there remains limited insight into the cellular and metabolic conditions that drive the dynamic changes in AOX protein amount seen under different growth conditions.
Here, we have compared respiration and photosynthesis of WT tobacco to that of AOX knockdown and overexpression plants across a wide range of growth irradiances, and under both optimal and abiotic stress (drought) conditions. We provide evidence that AOX respiration is necessary to promote chloroplast energy balance and to optimize photosynthesis, particularly under conditions that combine high growth irradiance and abiotic stress. We also show that differences in AOX protein amount across a wide range of growth conditions correlate strongly with differences in chloroplast energy balance, suggesting that the AOX respiration capacity of leaves is tightly linked to chloroplast metabolic status.
Materials and methods
Plant materials and growth conditions
Tobacco (Nicotiana tabacum L. cv Petit Havana SR1) was used for all experiments. Transgenic plant lines with elevated levels of AOX protein (B7, B8) due to the presence of an AOX1a transgene driven by a constitutive 35S promoter, or suppressed levels of AOX protein (RI9, RI29) due to the presence of an AOX1a RNA interference construct have been described previously (Wang et al., 2011, Wang and Vanlerberghe, 2013; Cvetkovska et al., 2014). Seeds were germinated in vermiculite for 16 d, and seedlings were subsequently transplanted individually to 10-cm plastic pots, each containing equal amounts of a general purpose growing medium that consisted of four parts soil (Pro-mix BX, Premier Horticulture, Rivière-du-Loup, QC, Canada) and one part vermiculite. The plants were then raised in controlled-environment growth chambers (Models PGR-15 and PGC-20, Conviron, Winnipeg, MB, Canada) with a 16-h photoperiod, temperature of 28/22 ºC (light/dark), relative humidity of 60%, and photosynthetic photon flux density (PPFD) of either 150, 400, or 700 µmol photons m–2 s–1. Plants in the growth chambers were irrigated with 1/10th-strength Hoagland’s solution daily for 19 d (400 and 700 PPFD-grown plants) or 21 d (150 PPFD-grown plants), producing plants of approximately equal size across the growth irradiances. Then, water was withheld from plants for up to an additional 8 d. All analyses were performed on the fully developed 5th leaves of ‘well-watered’ plants, analyzed at 1 d following their last irrigation, or ‘drought-stressed’ plants, analyzed at 2 to 8 d following their last irrigation.
Gas exchange and Chl a fluorescence
Leaf CO2 exchange and Chl a fluorescence from photosystem II (PSII) were measured simultaneously at 4 to 5 h into the light period using a portable system (GFS-3000, Heinz Walz GmbH, Effeltrich, Germany). Light was provided through red and blue LEDs (Model 3055-FL, Heinz Walz GmbH). Gas flow rate was set to 750 µmol s–1 and impeller (fan) speed to step 7. Photosynthetic parameters were measured at both the growth irradiance of the plant (150, 400, or 700 PPFD) and at a saturating irradiance of 1600 PPFD.
Gas exchange data were used to calculate the net CO2 assimilation rate (A) and stomatal conductance (gs) (von Caemmerer and Farquhar, 1981; Farquhar and Sharkey, 1982). Throughout this paper, respiration rate refers to rates of non-photorespiratory CO2 efflux. Respiration in the dark (RD) was estimated following a 30-min pre-incubation in the dark. Respiration in the light (RL) was estimated by the Kok method (Kok, 1948), as described previously (Dahal et al., 2014). In brief, A was measured at eight irradiances between 0 and 120 PPFD, which generated a Kok break-point of approximately 20 PPFD. RL was then estimated by extrapolating to 0 PPFD the linear relationship between A and PPFD over the range of 20 to 120 PPFD. Over this irradiance range, lines of best-fit had typical r2 values in the WT of 0.997 (150 PPFD-grown plants), 0.988 (400 PPFD-grown), and 0.986 (700 PPFD-grown).
Chlorphyll fluorescence analyses were done following a dark adaptation period of at least 30 min. Minimum fluorescence (Fo), maximal fluorescence in the dark-adapted leaf (Fm) or light-adapted leaf (Fm′), steady state fluorescence in the light-adapted leaf (Fs), and minimal fluorescence in the light-adapted leaf (Fo′) were determined, as described previously (Maxwell and Johnson, 2000). The maximal quantum yield of PSII was calculated as: Fv/Fm = (Fm – Fo)/Fm, while the effective quantum yield (operating efficiency) of PSII was calculated as: ΦPSII = (Fm′ – Fs)/Fm′ (Genty et al., 1989). The rate of linear electron transport was calculated as: ETR = (ΦPSII) × (PPFD) × (0.84) × (0.5), where 0.84 and 0.5 represent estimates that leaves absorb 84% of incident photons and that 50% of these are absorbed by PSII (Yamori et al., 2011). Photochemical energy quenching (qP or qL) was calculated using either the puddle model [qP = (Fm′ – Fs)/(Fm′ – Fo′)] or the lake model [qL = (Fm′ – Fs)/(Fm′ – Fo′) × Fo′/Fs] (Kramer et al., 2004). The fraction of closed (reduced) PSII reaction centres, also known as the excitation pressure (EP), was then calculated as either 1 – qP or 1 – qL. The puddle model and the lake model generated similar results, so only EP data based on the puddle model (i.e. 1 – qP) is reported here. Non-photochemical energy quenching (NPQ), a measure of heat dissipation of absorbed light energy, was calculated as: NPQ = (Fm – Fm′)/Fm′ (Maxwell and Johnson, 2000).
Protein and transcript analyses
Following respiration and photosynthesis measurements, leaves were harvested and ground to a fine powder using liquid N2 and a mortar and pestle. Leaf protein was then extracted as described previously (Busch et al., 2007), and protein concentration of the extracts was determined by a modified Lowry method (Larson et al., 1986). Immunoblot analyses were then performed as described previously (Dahal et al., 2014) using primary antibodies (Agrisera, Vännäs, Sweden) raised against LHCB2 (a nuclear-encoded Chl a/b binding protein of PSII) and AOX. The signals were quantified using an image analysis system (Chemidoc XRS+ with IMAGE LAB software v.3.0; BioRad Laboratories, Mississauga, ON, Canada).
Total RNA was extracted from frozen leaf tissue using TRIzol reagent (Life Technologies) by the method described by Vanessa et al. (2008), and then treated with RNase-free DNase I (Life Technologies). The extracted RNA had A260/A280 and A260/A230 ratios >2. Comparative quantification of gene transcripts was performed using reverse transcription quantitative polymerase chain reaction (RT-qPCR). Gene-specific primers [EF-1α (AF120093), 5′-GGTACTGTCCCTGGTTGGTCG-3′, 5′-TGAAGAGCTT CGTGGTGCAT-3′; and AOX1a (X79768), 5′-GACAACATA CACGGAGAGTGGAGTC-3′, 5′-GTGGGTTACTTGGAAGAA GAGGC-3′] were designed using the NCBI primer design resources (www.ncbi.nlm.nih.gov). First-strand cDNA was synthesized from 1 µg of total RNA using SuperScript II RT (Life Technologies). qPCR was performed using a SYBR Green Jumpstart Taq ReadyMix (Sigma-Aldrich, Oakville, ON, Canada). Amplification (with three technical replicates) was monitored on a PTC-200 DNA Engine Thermal Cycler (Bio-Rad Laboratories) using the program: 3 min at 95 °C, followed by 39 cycles of amplification with 10 s of denaturation at 95 °C, 15 s of annealing at 59 °C and 30 s of extension at 72 °C. Amplification efficiency was 100–110% for each gene. Comparative quantification was by the ΔΔCt method, with EF-1α as the normalizer gene.
Other methods
Leaf water status was determined by measuring relative water content (RWC), as described previously (Wang and Vanlerberghe, 2013). Statistical analyses were conducted using PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
In well-watered tobacco, leaf AOX amount has little impact on photosynthetic and respiratory rates, regardless of growth irradiance
Tobacco plants were raised to similar size at either low (150 PPFD), medium (400 PPFD), or high (700 PPFD) irradiance. In the WT plants, the amount of leaf AOX1a transcript increased with growth irradiance. AOX1a transcript was 1.7-fold higher at 400 PPFD and 2.5-fold higher at 700 PPFD, compared with the 150 PPFD-grown plants (see Supplementary Fig. S1 at JXB online).
To determine if AOX was critical in supporting leaf metabolism, particularly at the higher growth irradiances, we compared respiration and photosynthesis of well-watered WT plants to that of two AOX knockdowns (RI9, RI29) and two AOX overexpressors (B7, B8), following long-term growth and development at either low, medium, or high irradiance. Table 1 summarizes the results of this comparison. In the WT plants, both respiration rates in the dark (RD) and light (RL) increased with growth irradiance. Relative to 150 PPFD-grown plants, RD increased 1.2-fold at 400 PPFD and 1.6-fold at 700 PPFD, while RL increased 1.6-fold at 400 PPFD and 2.5-fold at 700 PPFD. However, neither RD nor RL differed across the five plant lines differing in AOX protein amount, regardless of growth irradiance (Table 1).
Table 1.
Effect of growth irradiance on respiration and photosynthesis in well-watered WT tobacco, AOX overexpressors (B8, B7) and AOX knockdowns (RI9, RI29). Data are the average ±SE of three to five independent experiments. Within an irradiance, values not sharing a common superscript letter are significantly different from one another (P<0.05). In data sets without superscript letters, there are no significant differences between plant lines. See text for further details.
| Growth irradiance (µmol photons m –2 s –1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 PPFD | 400 PPFD | 700 PPFD | |||||||||||||
| B8 | B7 | WT | RI9 | RI29 | B8 | B7 | WT | RI9 | RI29 | B8 | B7 | WT | RI9 | RI29 | |
| F v/Fm | 0.82 ± 0.02 | 0.80 ± 0.01 | 0.82 ± 0.01 | 0.81 ± 0.02 | 0.80 ± 0.02 | 0.81 ± 0.01 | 0.80 ± 0.02 | 0.79 ± 0.01 | 0.82 ± 0.01 | 0.82 ± 0.01 | 0.80 ± 0.02 | 0.79 ± 0.01 | 0.81 ± 0.02 | 0.80 ± 0.01 | 0.82 ± 0.02 |
| R D (µmol CO2 m–2 s–1) | 0.72 ± 0.04 | 0.77 ± 0.02 | 0.83 ± 0.04 | 0.87 ± 0.03 | 0.78 ± 0.05 | 0.97 ± 0.06 | 0.93 ± 0.08 | 0.99 ± 0.05 | 1.10 ± 0.06 | 0.89 ± 0.08 | 1.25 ± 0.07 | 1.48 ± 0.15 | 1.32 ± 0.10 | 1.19 ± 0.09 | 1.35 ± 0.06 |
| R L (µmol CO2 m–2 s–1) | 0.64 ± 0.04 | 0.61 ± 0.05 | 0.72 ± 0.03 | 0.69 ± 0.05 | 0.64 ± 0.05 | 1.05 ± 0.07 | 1.09 ± 0.09 | 1.17 ± 0.12 | 1.07 ± 0.09 | 1.16 ± 0.08 | 1.72 ± 0.2 | 1.93 ± 0.14 | 1.81 ± 0.16 | 1.67 ± 0.13 | 1.79 ± 0.18 |
| Measured at growth PPFD | |||||||||||||||
| A net (µmol CO2 m–2 s–1) | 3.09 ± 0.28 | 3.37 ± 0.47 | 3.49 ± 0.24 | 3.65 ± 0.27 | 3.73 ± 0.32 | 7.40 ± 0.56 | 7.35 ± 0.15 | 7.75 ± 0.54 | 7.26 ± 0.28 | 6.91 ± 0.72 | 9.63 ± 0.88 | 10.13 ± 0.47 | 10.19 ± 0.48 | 9.37 ± 0.37 | 8.71 ± 0.60 |
| g snet (mol CO2 m–2 s–1) | 0.066 ± 0.003 | 0.062 ± 0.012 | 0.065 ± 0.008 | 0.069 ± 0.005 | 0.067 ± 0.004 | 0.087 ± 0.036 | 0.098 ± 0.020 | 0.072 ± 0.014 | 0.76 ± 0.05 | 0.068 ± 0.016 | 0.171 ± 0.031 | 0.156 ± 0.027 | 0.181 ± 0.16 | 0.149 ± 0.023 | 0.165 ± 0.038 |
| Φ PSIInet | 0.73 ± 0.14 | 0.71 ± 0.11 | 0.76 ± 0.08 | 0.74 ± 0.12 | 0.70 ± 0.15 | 0.49 ± 0.09 | 0.54 ± 0.06 | 0.51 ± 0.08 | 0.47 ± 0.10 | 0.52 ± 0.05 | 0.38 ± 0.04 | 0.34 ± 0.05 | 0.37 ± 0.02 | 0.39 ± 0.02 | 0.33 ± 0.04 |
| ETRnet (µmol e– m–2 s–1) | 45 ± 1.2 | 47 ± 1.7 | 41 ± 2.29 | 45 ± 1.3 | 42 ± 2.7 | 80 ± 3.1 | 77 ± 5.3 | 90 ± 8.6 | 78 ± 5.7 | 82 ± 1.5 | 105 ± 2.4 | 110 ± 5.2 | 120 ± 9.1 | 116 ± 9.1 | 115 ± 4.4 |
| NPQnet | 0.19 ± 0.03 | 0.20 ± 0.02 | 0.20 ± 0.03 | 0.17 ± 0.02 | 0.18 ± 0.03 | 0.77 ± 0.10 | 0.78 ± 0.10 | 0.79 ± 0.11 | 0.76 ± 0.05 | 0.82 ± 0.06 | 1.23 ± 0.21 | 1.06 ± 0.17 | 1.20 ± 0.16 | 1.24 ± 0.15 | 1.35 ± 0.17 |
| Measured at 1600 PPFD | |||||||||||||||
| A sat (µmol CO2 m–2 s–1) | 10.72 ± 0.44ab | 11.13 ± 0.48ab | 11.55 ± 0.44a | 10.71 ± 0.70ab | 9.45 ± 0.40b | 11.87 ± 1.04 | 12.42 ± 0.82 | 12.94 ± 0.39 | 10.70 ± 0.89 | 12.08 ± 0.42 | 13.17 ± 0.56ab | 14.49 ± 0.74a | 13.51 ± 0.41a | 12.92 ± 0.34ab | 11.29 ± 0.52b |
| g ssat (mol CO2 m–2 s–1) | 0.166 ± 0.01ab | 0.178 ± 0.021a | 0.183 ± 0.019a | 0.147 ± 0.01bc | 0.139 ± 0.006c | 0.171 ± 0.014b | 0.203 ± 0.003a | 0.212 ± 0.006a | 0.170 ± 0.004b | 0.166 ± 0.013b | 0.226 ± 0.007 | 0.218 ± 0.033 | 0.232 ± 0.025 | 0.213 ± 0.008 | 0.225 ± 0.016 |
| Φ PSIIsat | 0.214 ± 0.008 | 0.203 ± 0.005 | 0.219 ± 0.016 | 0.192 ± 0.010 | 0.227 ± 0.009 | 0.223 ± 0.021 | 0.235 ± 0.008 | 0.201 ± 0.015 | 0.209 ± 0.062 | 0.216 ± 0.053 | 0.202 ± 0.010 | 0.189 ± 0.021 | 0.182 ± 0.017 | 0.166 ± 0.051 | 0.179 ± 0.043 |
| ETRsat (µmol e– m–2 s–1) | 152 ± 10.3 | 147 ± 8.3 | 154 ± 6.2 | 143 ± 6.7 | 158 ± 4.1 | 143 ± 5.5 | 141 ± 9.8 | 151 ± 9.6 | 156 ± 7.8 | 148 ± 11.7 | 136 ± 12.9 | 130 ± 14.3 | 121 ± 0.71 | 136 ± 6.6 | 128 ± 6.7 |
| NPQsat | 2.08 ± 0.15 | 1.89 ± 0.08 | 1.97 ± 0.10 | 1.88 ± 0.09 | 1.96 ± 0.08 | 1.77 ± 0.11 | 1.71 ± 0.05 | 1.83 ± 0.17 | 1.70 ± 0.09 | 1.72 ± 0.16 | 1.53 ± 0.11 | 1.66 ± 0.10 | 1.55 ± 0.08 | 1.61 ± 0.15 | 1.68 ± 0.11 |
In examining photosynthesis, measurements were taken at both the growth irradiance (150, 400, or 700 PPFD) and at a saturating irradiance of 1600 PPFD (Table 1). For WT plants, the CO2 assimilation rate (A) measured at growth irradiance (termed Anet) increased from 3.5 µmol CO2 m–2 s–1 in 150 PPFD-grown plants to 7.8 µmol CO2 m–2 s–1 (400 PPFD-grown) and 10.2 µmol CO2 m–2 s–1 (700 PPFD-grown). However, no differences were observed across the five plant lines. When measured at growth irradiance, there were also no differences in stomatal conductance (gsnet), photosystem II (PSII) operating efficiency (ФPSIInet), linear electron transport rate (ETRnet), or non-photochemical quenching (NPQnet) across the five plant lines, regardless of growth irradiance (Table 1).
For WT plants, the A measured at saturating irradiance (termed Asat) increased slightly with each increase in growth irradiance [from 11.6 µmol CO2 m–2 s–1 (150 PPFD-grown), to 12.9 µmol CO2 m–2 s–1 (400 PPFD-grown), to 13.5 µmol CO2 m–2 s–1 (700 PPFD-grown)]. Again, there were no differences in Asat across the plant lines, except for a slightly lower rate in one of the knockdown lines grown at 150 or 700 PPFD (Table 1). This probably relates to our previous report that knockdown of AOX can impact on stomatal function when photosynthesis of well-watered plants is measured at saturating irradiance (Cvetkovska et al., 2014). In keeping with this, gssat also differed slightly in both knockdowns for plants grown at 150 or 400 PPFD. Nonetheless, there were no differences in other key photosynthetic parameters (ФPSIIsat, ETRsat, NPQsat) across the plant lines, regardless of growth irradiance. In summary, AOX amount (knockdown or overexpression) was found to have little if any impact on photosynthetic performance across a wide range of growth and measurement irradiances in well-watered plants (Table 1).
In drought-stressed tobacco, AOX amount is an important determinant of photosynthetic and respiratory rates, particularly during growth at higher irradiances
We previously established that tobacco AOX is critical to maintain respiration and photosynthesis during drought (Dahal et al., 2014, 2015). These previous studies were done at a low growth irradiance of 150 PPFD. To further evaluate whether AOX respiration takes on added importance at higher irradiances, plants grown at different irradiances (150, 400, and 700 PPFD) were subjected to drought. Our hypothesis was that the importance of AOX in maintaining respiration and photosynthesis during drought would be exaggerated at higher growth irradiances.
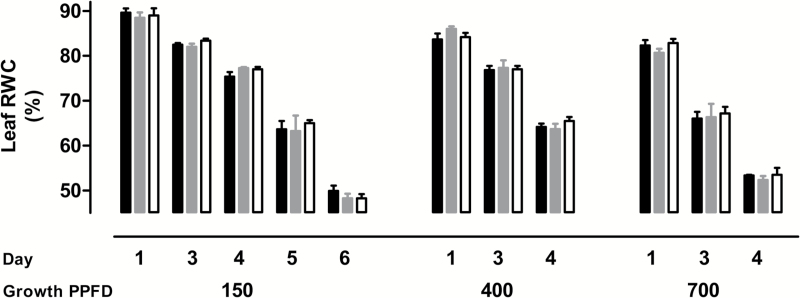
On Day 0, water was withheld from plants growing at 150, 400, or 700 PPFD. Plant water status was then assessed over time by examining leaf relative water content (RWC) (Fig. 1). Day 1 plants (i.e. one day following their last watering) were defined as well-watered, when WT plants had a RWC of approximately 89% (150 PPFD-grown), 86% (400 PPFD-grown), or 81% (700 PPFD-grown). Further declines in RWC over time occurred more rapidly at higher growth irradiance. For example, on Day 4, the RWC of the WT leaf was 77% in 150 PPFD-grown plants, 64% in 400 PPFD-grown plants, and only 52% in 700 PPFD-grown plants (Fig. 1). AOX amount had no influence on RWC. On all days and at all growth irradiances, there were no significant differences in leaf RWC between the five plant lines (Fig. 1). Hence, differences in respiration and photosynthesis between plant lines (see below) cannot be attributed to differences in leaf water status between lines.
Fig. 1.
Leaf RWC at different growth irradiances (150, 400, or 700 PPFD) and at different times following the withholding of water. ‘Day’ represents the number of days since water was withheld from well-watered plants. Data are shown for WT plants (gray bars), AOX overexpressors (black bars), and AOX knockdowns (white bars). In each independent experiment, data from two overexpressors (B7 and B8) that acted similarly were averaged, and data from two knockdowns (RI9 and RI29) that acted similarly were averaged. The data shown are the average ±SE of three to five independent experiments.
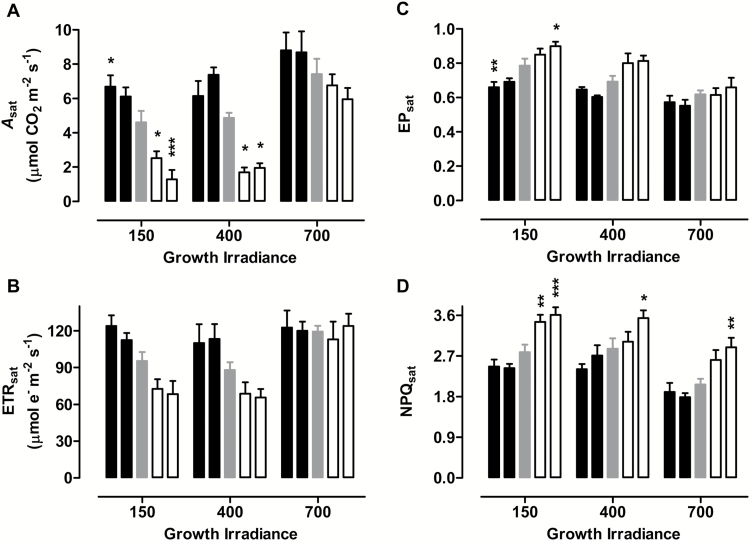
The response of photosynthesis and respiration to increasing leaf water deficit was compared across the plant lines and at each of the three growth irradiances. Since the water status of plants at different irradiances declined at different rates (as noted above), these comparisons were done as a function of leaf RWC. At high RWC (>80%), there was little if any difference in Asat, ETRsat, EPsat [the proportion of closed (reduced) PSII reaction centres, known as excitation pressure (EP) and measured as the fluorescence parameter 1 – qP)], or NPQsat across the plant lines, regardless of growth irradiance (see Supplementary Fig. S2). However, with further declines in RWC, differences emerged between lines. For 150 PPFD-grown plants, Asat and ETRsat were enhanced in AOX overexpressors and reduced in AOX knockdowns relative to the WT, while EPsat and NPQsat were enhanced in knockdowns and reduced in overexpressors relative to WT (Supplementary Fig. S2A–D). This corresponds with our previous work at 150 PPFD (Dahal et al., 2014, 2015). A similar pattern across plant lines was also evident in the 400 PPFD- and 700 PPFD-grown plants experiencing low RWC’s and measured at saturating irradiance (Supplementary Fig. S2E–L). Importantly, the magnitude of the differences between plant lines was similar, regardless of whether the plants were experiencing the low RWC at low, medium, or high growth irradiance. This conclusion is most easily illustrated and summarized by Fig. 2, in which plants at different growth irradiances are compared at the similar, and moderately low, RWC of 63% to 66%. This analysis shows that the differences in photosynthetic parameters between plant lines grown at 150 PPFD were not exaggerated at the higher growth irradiances. In fact, 700-PPFD grown plants showed the least differences in photosynthetic parameters across plant lines (Fig. 2).
Fig. 2.
Effect of growth irradiance on photosynthesis during moderate drought in WT tobacco and transgenic lines with altered amounts of AOX protein. (A) Asat. (B) ETRsat. (C) EPsat. (D) NPQsat. Plants were grown at 150, 400, or 700 PPFD under well-watered conditions for 19 to 21 days, followed by water being withheld from the plants until they were experiencing a leaf RWC of 63–66%, at which time each of the photosynthetic parameters was measured at saturating irradiance (1600 PPFD). Data are shown for WT plants (gray bars), AOX overexpressors (left to right the two black bars are B8 and B7, respectively), and AOX knockdowns (left to right the two white bars are RI9 and RI29, respectively). Data are the average ±SE of three to five independent experiments. Within an irradiance, values significantly different from the WT are indicated: *P<0.05; **P<0.01; ***P<0.001.
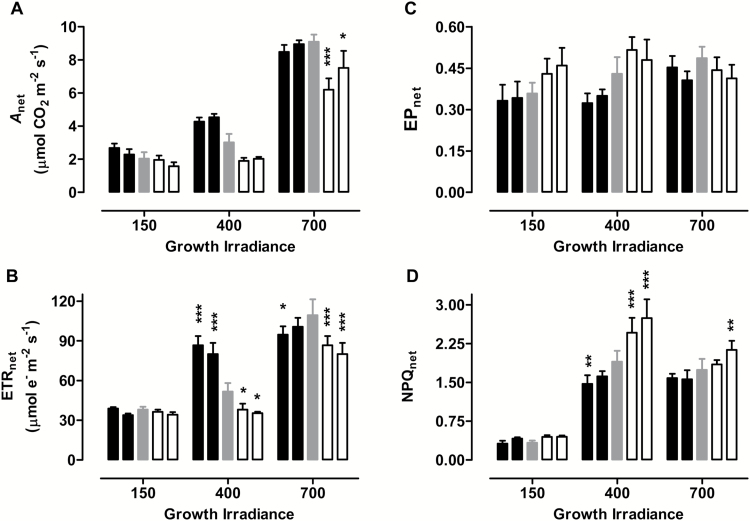
The above analysis was repeated, but with measurements done at the growth irradiance rather than a saturating irradiance. Now, for 150 PPFD-grown plants there were only modest differences in photosynthetic parameters between the plant lines at lower RWCs (see Supplementary Fig. S3A–D). Under these measurement conditions, the higher growth irradiances (400 and 700 PPFD) combined with low RWC resulted in greater differences in photosynthesis between the plant lines (Supplementary Fig. S3E–L). Again, this conclusion is most easily illustrated and summarized by Fig. 3, in which plants at different growth irradiances are compared at the similar, and moderately low, RWC of 63 to 66%. This analysis shows that the differences in photosynthetic parameters between plant lines grown at 150 PPFD and experiencing moderate drought are exaggerated at higher growth irradiances. This is particularly the case for Anet, ETRnet, and NPQnet (Fig. 3).
Fig. 3.
Effect of growth irradiance on photosynthesis during moderate drought in WT tobacco and transgenic lines with altered amounts of AOX protein. (A) Anet. (B) ETRnet. (C) EPnet. (D) NPQnet. Plants were grown at 150, 400, or 700 PPFD under well-watered conditions for 19 to 21 days, followed by water being withheld from the plants until they were experiencing a leaf RWC of 63–66%, at which time each of the photosynthetic parameters was measured at the growth irradiance (150, 400, or 700 PPFD). Data are shown for WT plants (gray bars), AOX overexpressors (left to right the two black bars are B8 and B7, respectively) and AOX knockdowns (left to right the two white bars are RI9 and RI29, respectively). Data are the average ±SE of three to five independent experiments. Within an irradiance, values significantly different from the WT are indicated: *P<0.05; **P<0.01; ***P<0.001.
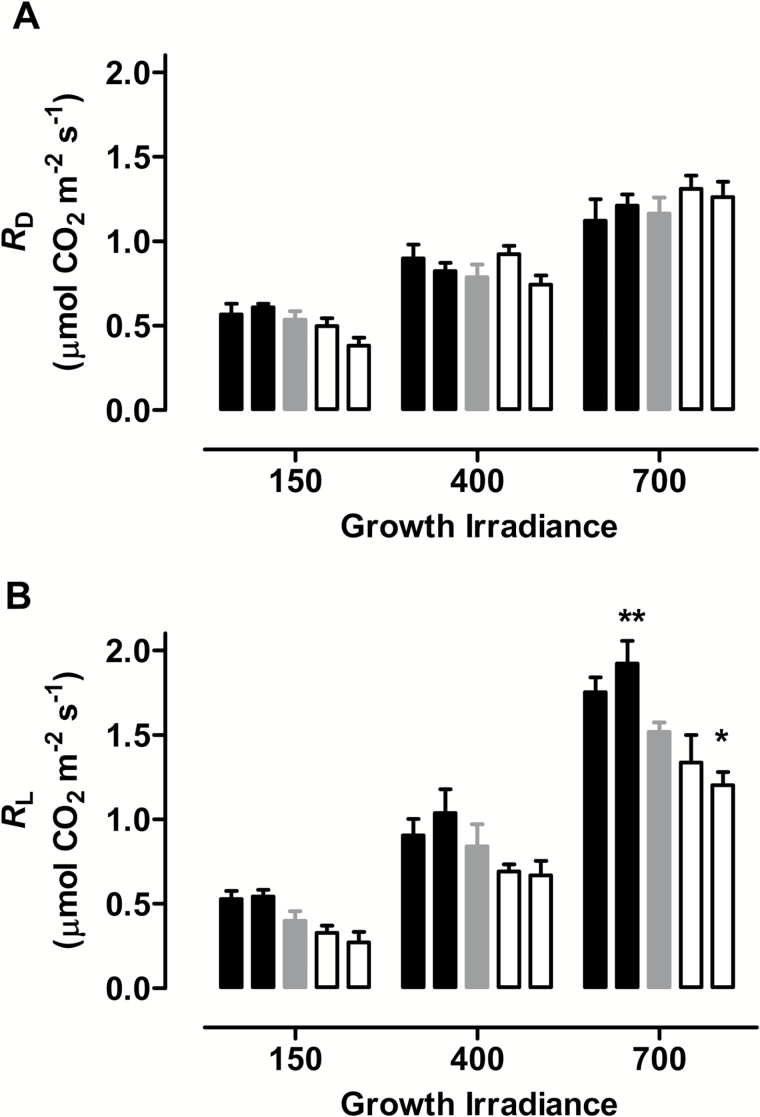
At high RWC (>80%), there was little difference in RD or RL across the plant lines, regardless of growth irradiance (see Supplementary Fig. S4 at JXB online). With further declines in RWC, a modest decrease in RD occurred similarly across the plant lines, regardless of growth irradiance. RL also declined with RWC but this response differed across plant lines and growth irradiances. Particularly at the highest growth irradiance, the decline in RL was greatest in the AOX knockdown lines and least in the AOX overexpression lines. Again, these conclusions are most easily illustrated and summarized by Fig. 4, in which plants at different growth irradiances are compared at the similar, and moderately low, RWC of 63 to 66%. This analysis shows that there are no differences in RD across plant lines (Fig. 4A) but that there are differences in RL across lines at the higher growth irradiances, particularly 700 PPFD (Fig. 4B). Overall, these results indicate that, under drought, the amount of AOX preferentially impacted the level of RL (over that of RD), with this effect being most pronounced at higher growth irradiances (Fig. 4). Knockdown of AOX compromised RL relative to the WT, while overexpression enhanced RL relative to the WT (Fig. 4B).
Fig. 4.
Effect of growth irradiance on respiration during moderate drought in WT tobacco and transgenic lines with altered amounts of AOX protein. (A) RD. (B) RL. Plants were grown at 150, 400, or 700 PPFD under well-watered conditions for 19 to 21 days, followed by water being withheld from the plants until they were experiencing a leaf RWC of 63–66%, at which time the measurements were taken. Data are shown for WT plants (gray bars), AOX overexpressors (left to right the two black bars are B8 and B7, respectively) and AOX knockdowns (left to right the two white bars are RI9 and RI29, respectively). Data are the average ±SE of three to five independent experiments. Within an irradiance, values significantly different from the WT are indicated: *P<0.05; **P<0.01; ***P<0.001.
We also examined the ratio of RL to RD across the plant lines and growth conditions. In both well-watered plants (see Supplementary Fig. S5A) and drought-stressed plants (Fig. S5B), growth at higher irradiance increased the RL/RD ratio. In well-watered plants, this ratio was similar across plant lines, regardless of growth irradiance. However, in plants experiencing moderate drought (63 to 66% RWC) at high growth irradiance (400 and 700 PPFD), the RL/RD ratio tended to be highest in the AOX overexpressors and lowest in the knockdowns, with WT plants showing an intermediate response. These differences were not statistically significant but do suggest that AOX amount is an important determinant of the RL/RD ratio in plants grown at high-irradiance and experiencing moderate drought.
In the tobacco leaf, differences in AOX protein amount across growth conditions correlates strongly with differences in the reduction state of the photosynthetic electron transport chain
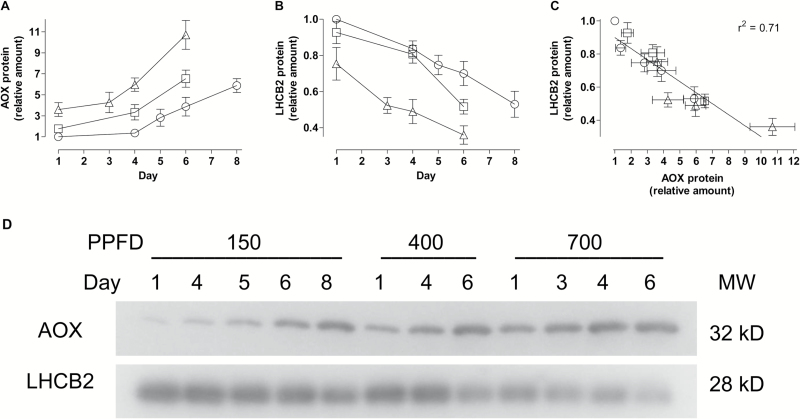
Using WT tobacco, we examined more closely the relationship between leaf AOX protein amount and growth irradiance. This included comparison with a light-harvesting complex II protein (LHCB2) whose abundance is known to respond dynamically to irradiance (Bailey et al., 2001). In well-watered plants (Day 1), the AOX protein amount increased with increases in growth irradiance (Fig. 5A), while the LHCB2 protein amount declined with increases in growth irradiance (Fig 5B). Compared to 150 PPFD-grown plants, 400 PPFD- and 700 PPFD-grown plants had 1.8-fold and 3.6-fold higher AOX protein amount, respectively. On the other hand, 400 PPFD- and 700 PPFD-grown plants had only 93% and 75%, respectively, of the LHCB2 protein amount present in 150 PPFD-grown plants. The opposite response of these two proteins was also evident following the imposition of drought. In response to increasing drought severity (Days 1–8), the AOX protein amount increased while the LHCB2 protein amount declined, with these trends seen at all three growth irradiances (Fig. 5A, B). A plot of LHCB2 protein amount versus AOX protein amount shows a strong inverse relationship between these two proteins across this wide range of growth irradiances and leaf water status (Fig. 5C).
Fig. 5.
Leaf protein amounts. (A) AOX protein amount. (B) LHCB2 protein amount. (C) Plot of LHCB2 protein amount versus AOX protein amount. (D) Representative immunoblots. WT tobacco plants were grown at an irradiance of 150 PPFD (circles), 400 PPFD (squares), or 700 PPFD (triangles) under well-watered conditions for 19 to 21 days. AOX and LHCB2 protein amounts were subsequently determined at different times for up to 8 days following a final watering of the plants on Day 0. Protein amounts in (A) and (B) are relative to that of the well-watered (Day 1) plants grown at 150 PPFD, which was set to 1. Data are the average ±SE of three independent experiments. Data in (C) are derived from that in (A) and (B).
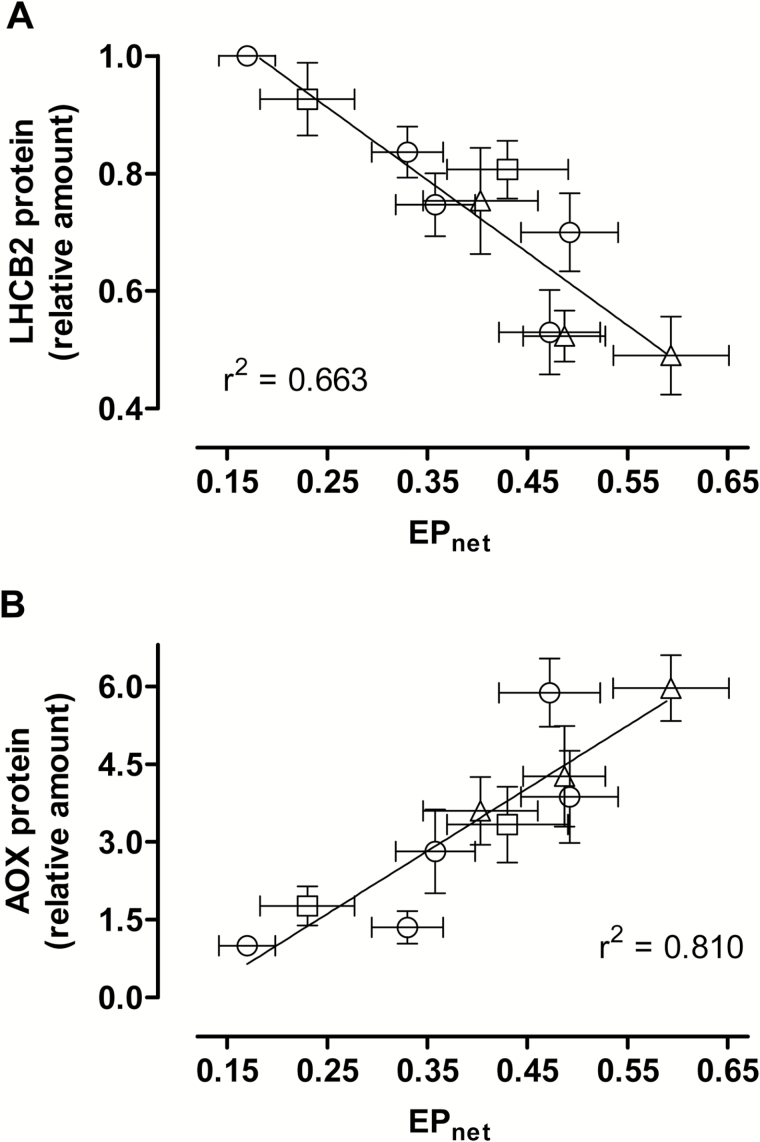
Current models suggest that LHCB2 gene expression and protein amount is responsive to signal(s) reflecting the chloroplast energy balance (Dall’Osto et al., 2015; Wobbe et al., 2016). One such indicator of energy balance is EP. High EP (i.e. a high proportion of closed PSII reaction centres) indicates an imbalance between light energy absorption and the downstream processes that utilize or dissipate that energy. High EP will also manifest itself as an increase in the reduction state of the PQ pool, since plastoquinol oxidation is typically the rate-limiting step in the photosynthetic electron transport chain (Huner et al., 1998; Rosso et al., 2009; Wobbe et al., 2016). We again grew plants at the three different growth irradiances and measured the steady-state growth EP (EPnet) being experienced by them under both well-watered conditions and in response to different severities of drought (leaf RWCs ranging from 89% to 40%). These same plants were then used to examine both LHCB2 and AOX protein amount. This analysis revealed that LHCB2 protein amount showed a strong negative relationship with EPnet (Fig. 6A) while AOX protein amount showed a strong positive relationship with EPnet (Fig. 6B) across this wide range of growth irradiances and leaf water status.
Fig. 6.
Leaf AOX and LHCB2 protein amounts and their relation to EPnet in WT tobacco. (A) The negative relationship between LHCB2 protein amount and EPnet. (B) The positive relationship between AOX protein amount and EPnet. Plants were grown at 150 PPFD (circles), 400 PPFD (squares), or 700 PPFD (triangles) under well-watered conditions for 19 to 21 days, followed by water being withheld from the plants for up to an additional 8 days. At different times following the water being withheld, AOX and LHCB2 protein amounts, as well as the EPnet being experienced by the plant were determined. All protein amounts are relative to that of well-watered plants grown at 150 PPFD, which was set to 1. All data are the average ±SE of three independent experiments.
Transgenic plants with altered AOX amount display aberrant levels of RL and an altered reduction state of the photosynthetic electron transport chain
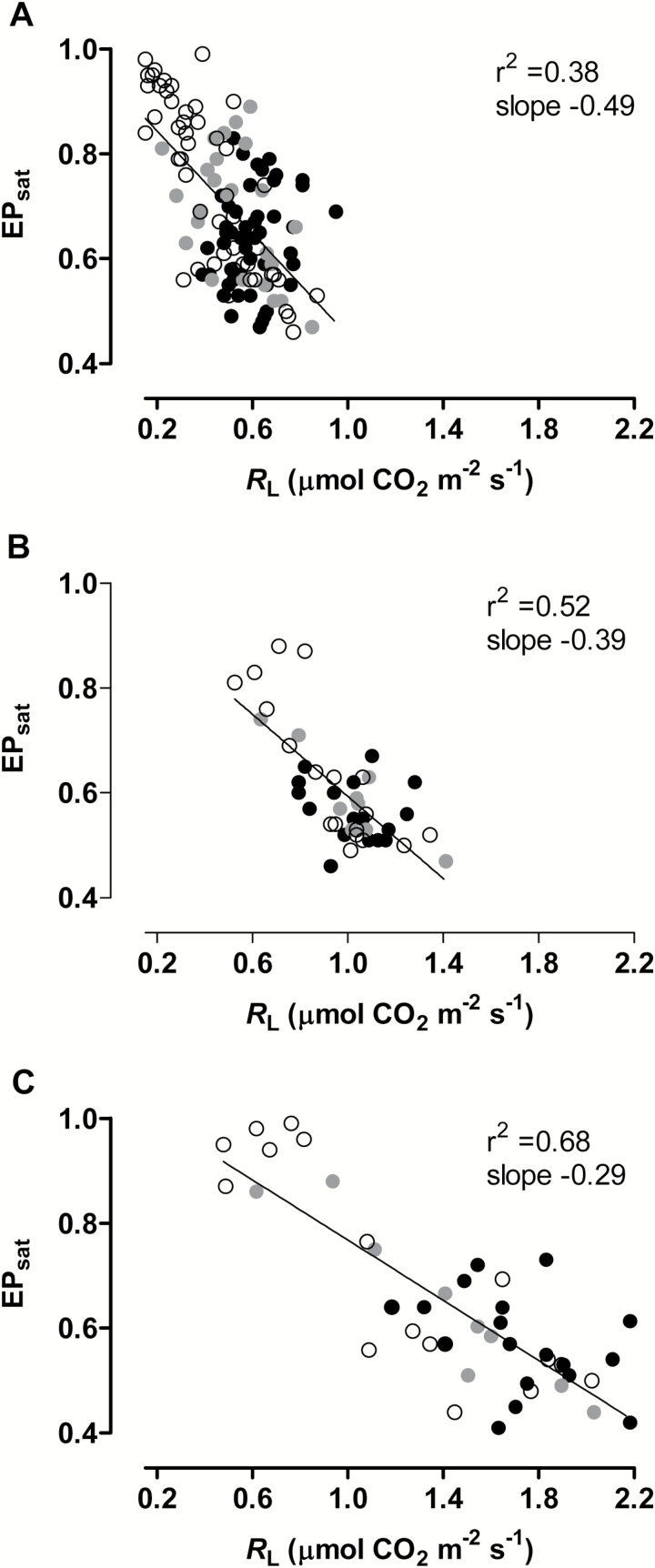
In WT tobacco, the AOX protein amount correlated strongly with EPnet (Fig. 6B), suggesting that AOX (like LHCB2) may be an important player in the control of chloroplast energy balance. To test this hypothesis, we used the WT, AOX knockdown, and AOX overexpression plants to examine the interplay between RL and chloroplast energy balance at saturating irradiance, using plants grown under the same wide range of growth irradiances and water availabilities as before (see above). In well-watered plants, EPsat varied over only a relatively narrow range (of about 0.41 to 0.62), regardless of growth irradiance or plant line (see Supplementary Fig. S6). As seen before (Table 1), RL was responsive to growth irradiance, with higher growth irradiances resulting in higher RL, but the AOX protein amount across the plant lines apparently had little influence over RL (Supplementary Fig. S6, Table 1). Hence, in well-watered plants, the amount of AOX and RL had little apparent influence over the chloroplast energy balance (EPsat), even at high growth and measurement irradiances. However, the results were quite different when data from drought-stressed plants were included in this analysis (Fig. 7). Now, the range of values measured was much greater for both EPsat and RL and, within each growth irradiance, these parameters were inversely correlated with one another. Further, the knockdown lines were responsible for most of the lowest RL and hence highest EPsat values, while the overexpression lines were responsible for most of the highest RL and hence lowest EPsat values. These data indicate that AOX amount is an important determinant of RL during drought, which in turn is an important determinant of chloroplast energy balance. We have previously established this relationship for low-irradiance-grown plants (150 PPFD) during drought (Dahal and Vanlerberghe, 2017) and the current work indicates that this relationship during drought can be seen in plants grown at a wide range of irradiances (150, 400, and 700 PPFD). However, combining all of the data across both growth irradiance and drought severity (i.e. combining all of the data in Fig. 7A–C) does not generate a strong correlation between RL and EPsat. This is because the quantitative relationship between these parameters (i.e. the value of RL necessary to maintain any given EPsat) clearly differs between growth irradiances (i.e. the slopes of the line of best fit differ between Fig. 7A, B and C).
Fig. 7.
Changes in RL and EPsat as a function of growth irradiance and leaf water status in WT tobacco and transgenic tobacco lines with altered amounts of AOX protein. Plants were grown at 150 PPFD (A), 400 PPFD (B), or 700 PPFD (C) under well-watered conditions for 19 to 21 days, followed by water being withheld from the plants for up to an additional 6 days. At different times following the water being withheld, leaf RL and EPsat were determined. Data are shown for WT plants (gray circles), AOX overexpressors (black circles; half are B7, half are B8) and AOX knockdowns (white circles; half are RI9, half are RI29). The data points are compiled from three to five independent experiments.
Discussion
We compared WT tobacco plants to AOX knockdown and overexpression plants to examine whether AOX is critical in maintaining leaf respiration and photosynthesis. This comparison was done for plants grown at low, medium, and high irradiance since it has been observed in tobacco (this study) and other species (see Introduction) that plants maintain higher amounts of AOX protein at higher growth irradiance. Under optimal growth conditions (well-watered, ample nutrients), there were no differences in respiration rate (RD or RL) or photosynthetic performance (measured at either the growth or a saturating irradiance) across the tobacco lines differing in AOX amount, regardless of whether the plants were grown at low, medium, or high irradiance (Table 1). Respiration rates (both RD and RL) did increase with growth irradiance, as has been seen in many other studies (e.g. Bartoli et al., 2006; Wright et al., 2006). Nonetheless, AOX respiration was not critical in maintaining leaf metabolism, even at the highest growth irradiance.
To our knowledge, in only one other plant species has the energy metabolism of AOX knockdown and/or overexpression plants following growth and development at different irradiances been examined. Yoshida et al. (2011a, b) compared WT Arabidopsis to an aox1a knockout following growth at low to medium irradiances (40 to 350 PPFD) and, similar to the current study, found little if any impact of AOX amount on photosynthetic performance. So why do tobacco and other species maintain higher AOX protein amounts at higher growth irradiances if it is not necessary to maintain respiration and photosynthesis? One possibility is that AOX does in fact make a greater contribution to respiration at higher growth irradiances but that, under the conditions examined, the cytochrome pathway was able to compensate for the altered AOX amount in the transgenics, resulting in little overall change in respiration rate, and little consequence for photosynthesis. For example, the amount of photoinhibition resulting from a shift to high irradiance was greater in the Arabidopsis aox1a mutant compared to the WT, but only under conditions in which the capacity of the cytochrome pathway was being simultaneously reduced using antimycin A (Watanabe et al., 2016). These results indicate that a functional mitochondrial electron transport chain (ETC) is necessary to prevent photoinhibition but do not clarify the specific importance of AOX respiration in this process. Another study compared photoinhibition across five plant species given a 2-h high irradiance treatment and found no correlation between the amount of photoinhibition and the activity of AOX during a post-treatment dark period, measured using the isotope discrimination technique (Florez-Sarasa et al., 2016). It is also possible that the higher AOX protein amount seen at higher irradiances in the current study is required to support metabolism that is unrelated to photosynthesis. Another possibility relates to a hypothesis put forward by Rasmusson and co-workers who suggested that the amount of AOX protein must be sufficient to support its ‘peak activity’, which might be significantly higher than its usual activity (Rasmusson et al., 2009). For example, it has been suggested that high AOX activity may be necessary during the photosynthetic induction period following darkness (Gardestrӧm and Igamberdiev, 2016). An energy imbalance could develop during this period since the energy-generating thylakoid reactions of photosynthesis become engaged more quickly that the energy-consuming Calvin cycle reactions. The isotope discrimination technique has shown that AOX usually operates at less than its maximal capacity (at least in the dark), suggesting that AOX amount is poised to allow a rapid increase in its activity if required (Millar et al., 1998; Guy and Vanlerberghe, 2005; Ribas-Carbo et al., 2005; Gomez-Casanovas et al., 2007; Armstrong et al., 2008).
Drought stress limits Calvin cycle activity since stomatal closure, intended to reduce leaf water loss, also restricts CO2 diffusion into the leaf (Flexas et al., 2004; Pinheiro and Chaves, 2011). Under such conditions, rates of ATP and NADPH generation by the photosynthetic ETC may outpace their rates of consumption by the Calvin cycle, potentially resulting in an energy imbalance in the chloroplast. It has been shown in several species that drought can also result in a biochemical limitation of photosynthesis that reduces A to rates below those simply due to restricted CO2 availability. This biochemical limitation involves the loss of key photosynthetic components, in particular chloroplast ATP synthase (Tezara et al., 1999; Kohzuma et al., 2009; Hoshiyasu et al., 2013). The specific metabolic conditions and signaling cascades that are responsible for these losses remain largely unknown but energy imbalances in the chloroplast have been suggested to be an important underlying factor (Lawlor and Tezara, 2009; Schӧttler and Tόth, 2014). Previously, we established that WT tobacco plants began to experience this biochemical limitation at a leaf RWC of about 65%. However, in AOX knockdown plants a lower stress severity threshold (RWC of about 75–80%) already induced this limitation, while in AOX overexpression plants a much higher stress threshold (RWC below about 55%) was necessary to induce this limitation. These results are consistent with a model whereby the non-energy-conserving nature of AOX respiration can act to alleviate chloroplast energy imbalances, hence increasing the threshold level of drought stress necessary to induce the biochemical limitations of photosynthesis (Dahal et al., 2014, 2015; Dahal and Vanlerberghe, 2017).
In the current study, we found no evidence that AOX is critical in maintaining leaf metabolism at higher growth irradiances, despite the higher amounts of AOX transcript and protein in such plants. However, higher growth irradiances might exacerbate drought-induced chloroplast energy imbalances. We therefore evaluated whether the role of AOX in maintaining photosynthetic performance during drought was exaggerated at higher growth irradiances. Interestingly, the results of this analysis were dependent upon whether photosynthetic performance was evaluated at a saturating irradiance (1600 PPFD) or at the growth irradiance. Measured at our saturating irradiance, the magnitude of the differences in photosynthetic performance across the plant lines under moderate drought (63–66% RWC) was similar regardless of growth irradiance. If anything, the differences across plant lines tended to be slightly greater for plants grown at the lowest irradiance (150 PPFD). However, these results are difficult to interpret for the following reason. Plants acclimated to growth at 700 PPFD are likely to be inherently better equipped to maintain photosynthetic performance at saturating irradiance (1600 PPFD) than plants acclimated to 150 PPFD, irrespective of AOX amount, due to a wide range of acclimation processes (Schӧttler and Tόth, 2014; Tikkanen and Aro, 2014; Dietz, 2015; Wobbe et al., 2016). As one indication of this, well-watered WT plants grown at 700 PPFD maintain a lower EPsat and NPQsat than those grown at 150 PPFD. Hence, at the saturating irradiance we used, any defects in photosynthetic performance under drought and owing to lack of AOX may be offset, in the higher-irradiance-grown plants, by an inherent capacity to better maintain energy balance and photosynthetic performance at 1600 PPFD.
For the reasons discussed above, a less ambiguous means to evaluate the importance of AOX under drought across growth irradiances is to evaluate photosynthetic performance at the growth irradiance. When photosynthesis was evaluated at the growth irradiance, then little if any difference was seen in photosynthetic performance across the plant lines grown at 150 PPFD and experiencing moderate drought. In the higher irradiance-grown plants, however, there were now clear differences in photosynthetic performance across the plant lines when experiencing moderate drought. Importantly, these results were mirrored by differences in RL. The differences in RL across plant lines during drought were most evident in the plants grown at the higher irradiances. Overall, our conclusion is that the importance of AOX in maintaining respiration and photosynthesis during drought is amplified at higher growth irradiance. This probably relates to the overall much higher values of RL seen in plants grown at higher irradiances (see below).
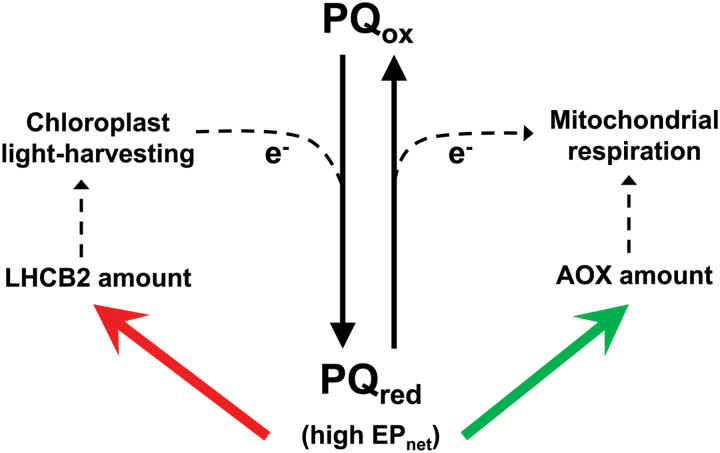
Remarkably, across a wide range of growth conditions resulting from differences in both growth irradiance and drought severity, there was a strong negative correlation in WT leaf tissue between LHCB2 and AOX protein amounts, suggesting that the abundance of these chloroplast and mitochondrial-localized components was being controlled in a coordinated and opposing manner. The abundance of many photosynthetic components is known to be responsive to changes in chloroplast energy balance (Bräutigam et al., 2009; Dall’Osto et al., 2015; Wobbe et al., 2016). Indeed, we found a strong negative correlation between the steady-state reduction state of the PQ pool, as measured by EPnet, and the LHCB2 protein amount. This suggests that acclimation to a high PQ-pool reduction state includes lowering the LHCB2 protein amount, presumably to lower the rates of light energy absorption that contribute to over-reduction (Fig. 8). A decline in LHCB2 at higher growth irradiance has also been reported in Arabidopsis (Bailey et al., 2001). On the other hand, the AOX protein amount correlated positively with EPnet. That is, the leaf acclimated to a high reduction state of the PQ pool by increasing the AOX protein amount (Fig. 8). The clearly opposing manner in which LHCB2 and AOX protein amounts responded to the redox status of the chloroplast ETC is precisely what might be expected if one of these components was contributing to the reduction of the PQ pool, as LHCB2 clearly does, while the other was contributing to the oxidation of the PQ pool, as we suggest to be an important role for AOX (see more below) (Fig. 8).
Fig. 8.
A working model for the coordinated regulation of chloroplast and mitochondrial proteins that contribute toward chloroplast energy balance. In response to growth conditions that promote a high steady-state growth excitation pressure (EPnet), plants decrease (red arrow) chloroplast components such as LHCB2 that promote energy absorption, and increase (green arrow) mitochondrial components such as AOX that promote energy dissipation. These coordinated adjustments promote energy balance in the chloroplast by reducing electron flow into the PQ pool and increasing electron flow out of the PQ pool. This figure is highly simplified to highlight just those proteins quantified in this study (LHCB2, AOX). Many other processes also contribute toward chloroplast energy balance in response to growth conditions. PQox, oxidized plastoquinone pool; PQred, reduced plastoquinone pool.
Based on the above finding, we suggest that signal(s) deriving from the photosynthetic ETC status may play a role in controlling leaf AOX protein abundance, similar to models in which the abundance of photosynthetic components, such as light-harvesting components, are hypothesized to be controlled (Bräutigam et al., 2009; Dall’Osto et al., 2015; Wobbe et al., 2016). Interestingly, the transcription factor ABI4 has been identified as a regulator of AOX expression (Giraud et al., 2009), but is also well recognized to control the expression of photosynthetic components through chloroplast retrograde pathways (Leόn et al., 2013). Speculatively, ABI4 could represent a common molecular link allowing both AOX and LHCB2 amounts to be coordinately controlled by signals deriving from the photosynthetic ETC status.
If changes in the AOX protein amount are indeed an important acclimation supporting chloroplast energy balance, then the AOX knockdowns and overexpressors might be expected to display aberrant EPs, particularly when measured at saturating irradiance (i.e. EPsat). Within each growth irradiance, there was a strong negative correlation across plant lines and drought severity treatments between the level of RL and EPsat. Further, AOX knockdowns tended to maintain higher EPsat and lower RL than the WT, while overexpressors tended to maintain lower EPsat and higher RL than the WT. These data clearly show the importance of AOX in lowering the reduction state of the chloroplast ETC by influencing the level of RL. A recent study reported increases in the ratio of RL/RD in Quercus ilex in response to long-term drought in the field, further supporting the apparent importance of RL during this abiotic stress (Sperlich et al., 2016). On the other hand, other greenhouse and field studies have reported decreases in the RL/RD ratio in response to drought (Ayub et al., 2011; Crous et al., 2012). We also saw a small decrease in RL/RD ratio in WT tobacco in response to drought, regardless of growth irradiance. Since specific levels of RL and RD appear to be dependent upon many factors, including species, tissue, age, and environment (Atkin et al., 2000; Wright et al., 2006; Tcherkez et al., 2008), we are cautious about making any broad or explicit conclusions about changes in the RL/RD ratio.
Within each growth irradiance, RL and EPsat were tightly correlated across a wide range of drought severities. However, the quantitative relationship between EPsat and RL differed across the growth irradiances, such that at higher irradiance the control of chloroplast energy balance during drought required concomitantly higher RL. We interpret this as an example of long-term coarse control versus short-term fine control of chloroplast energy balance by respiration. In the long-term, growth at higher irradiance has been shown to increase the capacity of respiration through changes in gene expression and protein amount (Noguchi et al., 1996, 2001, 2005). This allows for the higher respiratory activity typical of growth at higher irradiances (Bartoli et al., 2006; Wright et al., 2006). This higher respiratory activity increases the provision of carbon skeletons and energy intermediates that are necessary to support the higher growth rates typical of these conditions (Noguchi, 2005). Tobacco clearly respired faster at higher growth irradiances, particularly in the light, but also in the dark. Hence, the long-term coarse control of respiratory activity across growth irradiances fundamentally changed the quantitative relationship between RL and the chloroplast energy balance (EPsat). Such coarse control is probably a primary explanation for the higher AOX transcript and protein amounts seen in tobacco at higher growth irradiance, although we found no evidence that this additional AOX was an a priori requirement to support respiration under these conditions. On the other hand, the relatively shorter-term (several days) progressive increase in drought stress did not alter, within a growth irradiance, the quantitative relationship between RL and chloroplast energy balance. We view this as a short-term fine control, where a progressively increasing severity of stress and hence energy imbalance in the chloroplast is being offset by instantaneous higher RL, which is clearly AOX-dependent. This short-term fine control probably involves the activation of available AOX protein through established biochemical controls (Vanlerberghe et al., 1995). However, this shorter-term control is also associated over time with increased AOX protein and, interestingly, with declines in maximal cytochrome oxidase activity (Dahal and Vanlerberghe, 2017). Hence, the fine control in response to stress is primarily acting to rebalance the partitioning of electrons between the two respiratory branches. This rebalancing, in turn, acts to ease energy imbalance in the chloroplast. This is because, at any given rate of ATP turnover, AOX respiration consumes more electrons than the cytochrome pathway. Depending upon the actual rate of mitochondrial electron transport, AOX could be used to increase the rate of pyridine nucleotide turnover and/or to reduce the rate of ATP generation, depending upon what scenario might best correct the chloroplast energy imbalance. An additional potential consequence of AOX activity in the light (though not yet investigated) is that by influencing the mitochondrial redox state, it may influence the mix of complete versus partial tricarboxylic acid (TCA) cycle activity in the light. If lower than normal AOX activity in the light increases the matrix NAD(P)H/NADP+ ratio, this could favor partial TCA cycle activity (see models outlined in Hurry et al., 2005) and hence a reduction of TCA cycle CO2 efflux (i.e. lower RL). Conversely, if higher than normal AOX activity in the light lowers the matrix NAD(P)H/NAD(P)+ ratio, this could favor complete TCA cycle activity, hence increasing TCA cycle CO2 efflux (i.e. higher RL).
In summary, while growth irradiance acted primarily as a strong determinant of overall respiratory capacity and activity in both light and dark, drought stress acted primarily as a strong determinant of the partitioning of electrons between the cytochrome pathway and AOX, especially in the light. Further, while higher growth irradiances do result in higher AOX protein amounts in tobacco, as in other species, we found no evidence that this additional AOX is necessary to maintain photosynthetic performance under optimal growth conditions. On the other hand, with the imposition of drought stress this additional AOX was beneficial in optimizing photosynthesis.
Author contributions
GCV planned the research; KD and GCV planned and designed the experiments; KD, GDM and NAA performed the experiments; KD and GCV analyzed the data; and GCV wrote the paper.
Supplementary Material
Acknowledgements
Financial support for this work was provided by the Natural Sciences and Engineering Research Council of Canada to GCV.
Glossary
Abbreviations:
- A
net CO2 assimilation rate
- AOX
alternative oxidase
- EP
excitation pressure
- ETC
electron transport chain
- ETR
linear electron transport rate
- gs
stomatal conductance
- LHCB2
light-harvesting complex II protein
- NPQ
non-photochemical quenching
- PPFD
photosynthetic photon flux density
- PSII
photosystem II
- ΦPSII
effective quantum yield of PSII
- PQ
plastoquinone
- qP
photochemical energy quenching
- RD
respiration rate in the dark
- RL
respiration rate in the light
- RWC
relative water content
- TCA
tricarboxylic acid
- WT
wild-type.
References
- Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL. 2000. Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiology 122, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AF, Badger MR, Day DA, Barthet MM, Smith PM, Millar AH, Whelan J, Atkin OK. 2008. Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration. Plant, Cell & Environment 31, 1156–1169. [DOI] [PubMed] [Google Scholar]
- Ayub G, Smith RA, Tissue DT, Atkin OK. 2011. Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial-age atmospheric CO2 and growth temperature. The New Phytologist 190, 1003–1018. [DOI] [PubMed] [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. 2001. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213, 794–801. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH. 2006. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. Journal of Experimental Botany 57, 1621–1631. [DOI] [PubMed] [Google Scholar]
- Bräutigam K, Dietzel L, Kleine T, et al. 2009. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. The Plant Cell 21, 2715–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Adams MA. 2011. An analytical model of non-photorespiratory CO2 release in the light and dark in leaves of C3 species based on stoichiometric flux balance. Plant, Cell & Environment 34, 89–112. [DOI] [PubMed] [Google Scholar]
- Busch F, Hüner NPA, Ensminger I. 2007. Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer Jack pine. Plant Physiology 143, 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T-T, Simmonds D, Day DA, Colmer TD, Finnegan PM. 2010. Photosynthetic performance and fertility are repressed in GmAOX2b antisense soybean. Plant Physiology 152, 1638–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CYM, Ratcliffe RG, Sweetlove LJ. 2015. A method of accounting for enzyme costs in flux balance analysis reveals alternative pathways and metabolite stores in an illuminated Arabidopsis leaf. Plant Physiology 169, 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton R, Millar AH, Whelan J. 2006. Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochimica et Biophysica Acta 1757, 730–741. [DOI] [PubMed] [Google Scholar]
- Crous KY, Zaragoza-Castells J, Ellsworth DS, Duursma RA, Lӧw M, Tissue DT, Atkin OK. 2012. Light inhibition of leaf respiration in field-grown Eucalyptus saligna in whole-tree chambers under elevated CO2 and summer drought. Plant, Cell & Environment 35, 966–981. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Dahal K, Alber NA, Jin C, Cheung M, Vanlerberghe GC. 2014. Knockdown of mitochondrial alternative oxidase induces the ‘stress state’ of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytologist 203, 449–461. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M, Vanlerberghe GC. 2012. Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant, Cell & Environment 35, 1121–1136. [DOI] [PubMed] [Google Scholar]
- Dahal K, Martyn GD, Vanlerberghe GC. 2015. Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytologist 208, 382–395. [DOI] [PubMed] [Google Scholar]
- Dahal K, Vanlerberghe GC. 2017. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytologist, in press. doi:10.1111/nph.14169. [DOI] [PubMed] [Google Scholar]
- Dahal K, Wang J, Martyn GD, Rahimy F, Vanlerberghe GC. 2014. Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiology 166, 1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L, Bressan M, Bassi R. 2015. Biogenesis of light harvesting proteins. Biochimica et Biophysica Acta 1847, 861–871. [DOI] [PubMed] [Google Scholar]
- Dietz K-J. 2015. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. Journal of Experimental Botany 66, 2401–2414. [DOI] [PubMed] [Google Scholar]
- Dinakar C, Raghavendra AS, Padmasree K. 2010. Importance of AOX pathway in optimizing photosynthesis under high light stress: role of pyruvate and malate in activating AOX. Physiologia Plantarum 139, 13–26. [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R. 2003. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. The Plant Cell 15, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345. [Google Scholar]
- Finnegan PM, Soole KL, Umbach AL. 2004. Alternative mitochondrial electron transport proteins in higher plants. In: Day DA, Millar H, Whelan J, eds. Plant mitochondria: from genome to function. The Netherlands: Kluwer Academic Publishers, 163–230. [Google Scholar]
- Flexas J, Bota J, Cornic G, Sharkey TD. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology 6, 269–279. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Flexas J, Rasmusson AG, Umbach AL, Siedow JN, Ribas-Carbo M. 2011. In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant, Cell & Environment 34, 1373–1383. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Ostaszewska M, Galle A, Flexas J, Rychter AM. 2009. Changes of alternative oxidase activity, capacity and protein content in leaves of Cucumis sativus wild-type and MSC16 mutant grown under different light intensities. Physiologia Plantarum 137, 419–426. [DOI] [PubMed] [Google Scholar]
- Florez-Sarasa I, Ribas-Carbo M, Fernández Del-Saz N, Schwahn K, Nikoloski Z, Fernie AR, Flexas J. 2016. Unravelling the in vivo regulation and metabolic role of the alternative oxidase pathway in C3 species under photoinhibitory conditions. New Phytologist 212, 66–79. [DOI] [PubMed] [Google Scholar]
- Gandin A, Duffes C, Day DA, Cousins AB. 2012. The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO2 in Arabidopsis thaliana. Plant and Cell Physiology 53, 1627–1637. [DOI] [PubMed] [Google Scholar]
- Gardestrӧm P, Igamberdiev AU. 2016. The origin of cytosolic ATP in photosynthetic cells. Physiologia Plantarum 157, 367–379. [DOI] [PubMed] [Google Scholar]
- Gardestrӧm P, Igamberdiev AU, Raghavendra AS. 2002. Mitochondrial functions in the light and significance to carbon-nitrogen interactions. In: Foyer CH, Noctor G, eds. Advances in photosynthesis and respiration, volume 12. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. The Netherlands: Kluwer Academic Publishers, 151–172. [Google Scholar]
- Garmash EV, Grabelnych OI, Velegzhaninov IO, Borovik OA, Dalke IV, Voinikov VK, Golovko TK. 2015. Light regulation of mitochondrial alternative oxidase pathway during greening of etiolated wheat seedlings. Journal of Plant Physiology 174, 75–84. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92. [Google Scholar]
- Giraud E, Ho LHM, Clifton R, et al. 2008. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiology 147, 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Van Aken O, Ho LHM, Whelan J. 2009. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiology 150, 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casanovas N, Blanc-Betes E, Gonzalez-Meler MA, Azcon-Bieto J. 2007. Changes in respiratory mitochondrial machinery and cytochrome and alternative pathway activities in response to energy demand underlie the acclimation of respiration to elevated CO2 in the invasive Opuntia ficus-indica. Plant Physiology 145, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC. 1989. Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177, 483–491. [DOI] [PubMed] [Google Scholar]
- Guy RD, Vanlerberghe GC. 2005. Partitioning of respiratory electrons in the dark in leaves of transgenic tobacco with modified levels of alternative oxidase. Physiologia Plantarum 100, 165–170. [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. 1998. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochimica et Biophysica Acta 1366, 235–255. [Google Scholar]
- Hoshiyasu S, Kohzuma K, Yoshida K, Fujiwara M, Fukao Y, Yokota A, Akashi K. 2013. Potential involvement of N-terminal acetylation in the quantitative regulation of the ε subunit of chloroplast ATP synthase udner drought stress. Bioscience, Biotechnology, and Biochemistry 77, 998–1007. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. 1998. Energy balance and acclimation to light and cold. Trends in Plant Science 3, 224–230. [Google Scholar]
- Hurry V, Igamberdiev AU, Keerberg O, Pärnik T, Atkin OK, Zaragoza-Castells J, Gardestrӧm P. 2005. Respiration in photosynthetic cells: gas exchange components, interactions with photorespiration and the operation of mitochondria in the light. In: Lambers H, Ribas-Carbo M, eds. Advances in photosynthesis and respiration, volume 18. Plant respiration: from ecosystem to cell. Dordrecht, The Netherlands: Springer, 43–61. [Google Scholar]
- Igamberdiev AU, Eprintsev AT, Fedorin DN, Popov VN. 2014. Phytochrome-mediated regulation of plant respiration and photorespiration. Plant, Cell & Environment 37, 290–299. [DOI] [PubMed] [Google Scholar]
- Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage TN, Yokota A, Kramer DM. 2009. The long-term response of the photosynthetic proton circuit to drought. Plant, Cell & Environment 32, 209–219. [DOI] [PubMed] [Google Scholar]
- Kok B. 1948. A critical consideration of the quantum yield of Chlorella-photosynthesis. Enzymologia 13, 1–56. [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE. 2004. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research 79, 209–218. [DOI] [PubMed] [Google Scholar]
- Kühn K, Yin G, Duncan O, et al. 2015. Decreasing electron flux through the cytochrome and/or alternative respiratory pathways triggers common and distinct cellular responses dependent on growth conditions. Plant Physiology 167, 228–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E, Howlett B, Jagendork A. 1986. Artificial reductant enhancement of the Lowry method for protein determination. Analytical Biochemistry 155, 243–248. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103, 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leόn P, Gregorio J, Cordoba E. 2013. ABI4 and its role in chloroplast retrograde communication. Frontiers in Plant Science 3, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51, 659–668. [DOI] [PubMed] [Google Scholar]
- Millar AH, Atkin OK, Menz I, Henry B, Farquahar G, Day DA. 1998. Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiology 117, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Shiba T, Young L, Harada S, Kita K, Ito K. 2013. Unraveling the heater: new insights into the structure of the alternative oxidase. Annual Review of Plant Biology 64, 637–663. [DOI] [PubMed] [Google Scholar]
- Ng S, De Clercq I, Van Aken O, Law SR, Ivanova A, Willems P, Giraud E, Van Breusegem F, Whelan J. 2014. Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth, development, and stress. Molecular Plant 7, 1075–1093. [DOI] [PubMed] [Google Scholar]
- Noctor G, De Paepe R, Foyer CH. 2007. Mitochondrial redox biology and homeostasis in plants. Trends in Plant Science 12, 125–134. [DOI] [PubMed] [Google Scholar]
- Noguchi K. 2005. Effects of light intensity and carbohydrate status on leaf and root respiration. In: Lambers H, Ribas-Carbo M, eds. Advances in photosynthesis and respiration, volume 18. Plant respiration: from ecosystem to cell. Dordrecht, The Netherlands: Springer, 63–83. [Google Scholar]
- Noguchi K, Go C-S, Terashima I, Ueda S, Yoshinari T. 2001. Activities of the cyanide-resistant respiratory pathway in leaves of sun and shade species. Australian Journal of Plant Physiology 28, 27–35. [Google Scholar]
- Noguchi K, Sonoike K, Terashima I. 1996. Acclimation of respiratory properties of leaves of Spinacia oleracea L., a sun species, and of Alocasia macrorrhiza (L.), a shade species, to changes in growth irradiance. Plant and Cell Physiology 37, 377–384. [Google Scholar]
- Noguchi K, Taylor NL, Millar AH, Lambers H, Day DA. 2005. Response of mitochondria to light intensity in the leaves of sun and shade species. Plant, Cell & Environment 28, 760–771. [Google Scholar]
- Noguchi K, Yoshida K. 2008. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8, 87–99. [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Sulpice R, Gibon Y, Fernie AR. 2008. The enigmatic contribution of mitochondrial function in photosynthesis. Journal of Experimental Botany 59, 1675–1684. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62, 869–882. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K. 2003. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends in Plant Science 8, 546–553. [DOI] [PubMed] [Google Scholar]
- Rasmusson AG, Fernie AR, van Dongen JT. 2009. Alternative oxidase: a defense against metabolic fluctuations? Physiologia Plantarum 137, 371–382. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, et al. 2005. Effects of water stress on respiration in soybean leaves. Plant Physiology 139, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA. 1995. Beyond SHAM and cyanide: Opportunities for studying the alternative oxidase in plant respiration using oxygen isotope discrimination. Australian Journal of Plant Physiology 22, 487–496. [Google Scholar]
- Rosso D, Bode R, Li W, et al. 2009. Photosynthetic redox imbalance governs leaf sectoring in the Arabidopsis thaliana variegation mutants immutans, spotty, var1, and var2. The Plant Cell 21, 3473–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schӧttler MA, Tόth SZ. 2014. Photosynthetic complex stoichiometry dynamics in higher plants: environmental acclimation and photosynthetic flux control. Frontiers in Plant Science 5, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SY, Bitterman DS, Thomas S, Griffin KL, Atkin OK, Turnbull MH. 2011a Respiratory alternative oxidase responds to both low- and high-temperature stress in Quercus rubra leaves along an urban-rural gradient in New York. Functional Ecology 25, 1007–1017. [Google Scholar]
- Searle SY, Thomas S, Griffin KL, Horton T, Kornfeld A, Yakir D, Hurry V, Turnbull MH. 2011b Leaf respiration and alternative oxidase in field-grown alpine grasses respond to natural changes in temperature and light. New Phytologist 189, 1027–1039. [DOI] [PubMed] [Google Scholar]
- Searle SY, Turnbull MH. 2011. Seasonal variation of leaf respiration and the alternative pathway in field-grown Populus × canadensis. Physiologia Plantarum 141, 332–342. [DOI] [PubMed] [Google Scholar]
- Shabnam N, Sharmila P, Sharma A, Strasser RJ, Govindjee, Pardha-Saradhi P. 2015. Mitochondrial electron transport protects floating leaves of long leaf pondweed (Potamogeton nodosus Poir) against photoinhibition: comparison with submerged leaves. Photosynthesis Research 125, 305–319. [DOI] [PubMed] [Google Scholar]
- Sperlich D, Barbeta A, Ogaya R, Sabaté S, Penuelas J. 2016. Balance between carbon gain and loss under long-term drought: impacts on foliar respiration and photosynthesis in Quercus ilex L. Journal of Experimental Botany 67, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strodtkӧtter I, Padmasree K, Dinakar C, et al. 2009. Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Molecular Plant 2, 284–297. [DOI] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Millar AH. 2002. Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. Journal of Biological Chemistry 277, 42663–42668. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Bligny R, Gout E, Mahé A, Hodges M, Cornic G. 2008. Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proceedings of the National Academy of Sciences, USA 105, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Boex-Fontvieille E, Mahé A, Hodges M. 2012. Respiratory carbon fluxes in leaves. Current Opinion in Plant Biology 15, 308–314. [DOI] [PubMed] [Google Scholar]
- Tezara WS, Mitchell VJ, Driscoll SP, Lawlor DW. 1999. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401, 914–917. [Google Scholar]
- Tikkanen M, Aro E-M. 2014. Integrative regulatory network of plant thylakoid energy transduction. Trends in Plant Science 19, 10–17. [DOI] [PubMed] [Google Scholar]
- Van Aken O, Giraud E, Clifton R, Whelan J. 2009. Alternative oxidase: a target and regulator of stress responses. Physiologia Plantarum 137, 354–361. [DOI] [PubMed] [Google Scholar]
- Vanessa DRF, Angela PT, Mariana CO, Pio C. 2008. RNA isolation method for polysaccharide rice algae: agar producing Gracilaria tenuistipitata (Rhodophyta). Journal of Applied Phycology 20, 9–12. [Google Scholar]
- Vanlerberghe GC. 2013. Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Sciences 14, 6805–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L. 1995. Alternative oxidase activity in tobacco leaf mitochondria. Dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiology 109, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Ordog SH. 2002. Alternative oxidase: integrating carbon metabolism and electron transport in plant respiration. In: Foyer CH, Noctor G, eds. Advances in photosynthesis and respiration, volume 12. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. The Netherlands: Kluwer Academic Publishers, 173–191. [Google Scholar]
- Vishwakarma A, Bashyam L, Senthilkumaran B, Scheibe R, Padmasree K. 2014. Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana. Plant Physiology and Biochemistry 81, 44–53. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- Wallstrӧm SV, Florez-Sarasa I, Araújo WL, et al. 2014. Suppression of NDA-type alternative mitochondrial NAD(P)H dehydrogenases in Arabidopsis thaliana modifies growth and metabolism, but not high light stimulation of mitochondrial electron transport. Plant and Cell Physiology 55, 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC. 2011. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiologia Plantarum 142, 339–351. [DOI] [PubMed] [Google Scholar]
- Wang J, Vanlerberghe GC. 2013. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiologia Plantarum 149, 461–473. [DOI] [PubMed] [Google Scholar]
- Watanabe CK, Hachiya T, Terashima I, Noguchi K. 2008. The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an upregulation of antioxidant defense systems in Arabidopsis thaliana leaves. Plant, Cell & Environment 31, 1190–1202. [DOI] [PubMed] [Google Scholar]
- Watanabe CKA, Yamori W, Takahashi S, Terashima I, Noguchi K. 2016. Mitochondrial alternative pathway-associated photoprotection of photosystem II is related to the photorespiratory pathway. Plant and Cell Physiology 57, 1426–1431. [DOI] [PubMed] [Google Scholar]
- Wobbe L, Bassi R, Kruse O. 2016. Multi-level light capture control in plants and green algae. Trends in Plant Science 21, 55–68. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Atkin OK, Lusk CH, Tjoelker MG, Westoby M. 2006. Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytologist 169, 309–319. [DOI] [PubMed] [Google Scholar]
- Yamori W, Sakata N, Suzuki Y, Shikanai T, Makino A. 2011. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenases (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. The Plant Journal 68, 966–976. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Terashima I, Noguchi K. 2007. Upregulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant and Cell Physiology 48, 606–614. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I, Noguchi K. 2011. a. Distinct responses of the mitochondrial electron transport chain to long- and short-term high-light environments in Arabidopsis thaliana. Plant, Cell & Environment 34, 618–628. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Kato Y, Sakamoto W, Noguchi K. 2008. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2. Plant and Cell Physiology 49, 592–603. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe CK, Terashima I, Noguchi K. 2011b Physiological impact of mitochondrial alternative oxidase on photosynthesis and growth in Arabidopsis thaliana. Plant, Cell & Environment 34, 1890–1899. [DOI] [PubMed] [Google Scholar]
- Zhang D-W, Xu F, Zhang Z-W, Chan Y-E, Du J-B, Jia S-D, Yuan S, Lin H-H. 2010. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant, Cell & Environment 33, 2121–2131. [DOI] [PubMed] [Google Scholar]
- Zhang D-W, Yuan S, Xu F, Zhu F, Yuan M, Ye H-X, Guo H-Q, Lv X, Yin Y, Lin H-H. 2016. Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant, Cell & Environment 39, 12–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.