Abstract
Purpose
To assess short-term effects of daily worries on hypothalamic-pituitary-adrenal (HPA) axis activity and later implications for adolescents’ health symptoms. We hypothesized that heightened worry would be associated with stronger next-morning cortisol awakening response (CAR) to prepare the body for the demands of the upcoming day. Guided by biological adaptation to stress theories, we also hypothesized that dysregulated CAR would heighten associations between worries and later health symptoms, while also testing direct associations between worries and dysregulated CAR and health.
Methods
Ninety-nine late adolescents during Waves 5 and 6 of a longitudinal study reported on 26 worries for 10 days. On three of the 10 days, participants also provided morning saliva samples that were assayed for cortisol to capture the CAR. At both waves, participants reported on 22 common health symptoms.
Results
Multilevel models showed significant within-person associations between high daily worries and next-morning heightened CAR for females. Contrary to expectation, worries were inversely related to concurrent health symptoms. For the whole sample, CAR moderated the effect of worries on later health symptoms: Worries were positively associated with health symptoms in adolescents with high CAR and inversely associated with health symptoms for those with low CAR.
Conclusions
In this sample of typically developing adolescents, worries alone do not increase the risk for common health complaints and may be somewhat protective in the short run. However, high worries in the context of high CAR appear to increase the risk for health symptoms.
Keywords: Worries, Adolescence, HPA axis, Cortisol morning awakening (CAR), Daily data
Is the common admonishment, “You’ll worry yourself sick” justified or misguided with respect to adolescents’ daily worries? Worry is a complex, multidimensional process involving cognition, affect, and biologically-based stress responses typically in anticipation of future events [1]. Worry is sometimes conceptualized as trait-like, i.e., certain people are “worriers”; it is a transdiagnostic indicator of psychopathology [2] and has been linked to adults’ health symptoms including fatigue, musculoskeletal pain and gastrointestinal problems [3]. Yet, more benign views recognize worry as quite common and as motivating constructive action. Adolescents typically report multiple worries per day [4–5] and the worries often wax and wane with everyday environmental pressures from school, peers, and family. Thus, to better articulate connections between worry and health, the present study investigates if ups and downs in adolescents’ daily worries are associated with shifts in diurnal cortisol patterns via the daily cortisol awakening response (CAR). Drawing upon biological adaptation theories and focusing on day-to-day variation in worries, the current study tested whether worry prompts short-term physiological benefits but also occasions long-term costs in health.
By conceptualizing worry as a process that fluctuates across days, we can measure its short-term impact on stress physiology. The hypothalamic-pituitary-adrenal (HPA) “fight or flight” response system that generates short-term mobilization of energy, attention, heightened cardiovascular activity, and cortisol release in response to stress, also regulates diurnal patterns of cortisol activity [6]. The CAR is the morning spike in circulating cortisol that occurs approximately 30–40 minutes after awakening as preparation for the upcoming demands of the day [7–8]. Anticipated stress may affect the magnitude of the CAR [9]. For example, a comparison of adults’ CAR from four weekdays versus two weekend days showed more pronounced CAR on weekdays, particularly for those reporting high worry and work-related stress [10]. In one-day assessments of worry, undergraduates who worried or ruminated at night showed higher cortisol increases the next morning [11]. Nonetheless, despite these associations between worry and heightened CAR, little is known about within-person, day-to-day fluctuations between worry and next-day HPA activity. Thus, the first objective of this study was to investigate whether within-person variability in worry reported each evening across three days corresponds to fluctuations in adolescents’ next-day CAR. We assessed next-day CAR with the assumption that worry is the anticipation of upcoming events and hypothesized that heightened worry reported in the evening would be followed by more pronounced next-day CAR.
A frequently noted paradox of biological adaptation to stress is that short-term adaptive responses can ultimately alter the body’s responses in the long-term [12–13]. For the HPA axis, repeated and chronic activation can ultimately lead to an inefficient turning on or turning off of the stress response and, through allostasis, can result in persistently high or low basal cortisol activity [14]. Dysregulation of HPA levels—whether predominantly heightened [15] or attenuated [16]—has been linked to contemporaneous disease processes among adolescents. Although one cross-sectional study found flatter CAR linked with common health complaints including stomachaches and flu in 107 adolescents [17], chronically high cortisol also is associated with inadequate immune responses [18].
Thus, the second overall objective of this study was to investigate separate and combined effects of worry and CAR on either short-term or long-term health symptoms. To our knowledge, only one study has investigated direct effects between adolescents’ worry and health: Global worry and duration of worry across six days correlated with concurrent health complaints in 171 predominantly female adolescents [5]. Our goal here was to test whether CAR strengthens associations between worry and adolescents’ experience of health concerns. According to sensitivity to context hypotheses [19], adolescents who show heightened HPA activity in combination with worry may be more prone to negative consequences of worries on health. Consistent with findings that prolonged stress and elevated cortisol alter regulation of the immune system, insulin, and blood pressure [18], we anticipated that high daily worries may impair health in those with higher physiological arousal concomitant with worry. This hypothesized interaction between worries and CAR was tested not only for concurrent health symptoms but also delayed symptoms in young adulthood, as health consequences of dysregulated CAR may emerge over time.
The present study used daily data to capture within-person variability in worries reported on three consecutive evenings and CAR on the following mornings. Self-reported health symptoms were measured during that same assessment and approximately three years later. Assuming worry is cognitive preparation for upcoming challenges, we hypothesized that higher worry would be associated with higher CAR the next day (Hypothesis 1). With prior evidence linking worries and HPA axis to health, we hypothesized that more worries and dysregulated CAR would separately relate to more health symptoms (Hypothesis 2). However, consistent with sensitivity-to-context perspectives and evidence that repeated HPA over-activation potentially disrupts bodily systems, we hypothesized that pronounced CAR would increase the association between worries and health symptoms, specifically at the later assessment (Hypothesis 3). Investigating connections between worry, stress physiology and health is particularly important during adolescence, which is considered a critical period when effects of perceived stress become biologically embedded [20–21]. Though chronic health concerns are somewhat infrequent during adolescence, contagious diseases (e.g., colds, sore throat) are common, and headaches, sleep difficulties, and musculoskeletal problems also increase [5, 22–23].
Sex differences also are relevant due to their putative importance to key study variables at this developmental stage. Though consistent sex differences in HPA activity have not emerged [24], adolescent males sometimes show less pronounced CAR than females [25]. Some studies report that females worry more [26] whereas others report no sex differences, especially in daily worries [4–5]. Female adolescents also tend to report more health symptoms [22–23]. The present study tested sex as a moderator in associations between worries, HPA activity, and health.
Method
Overview
Data were collected during waves 5 and 6 of an ongoing longitudinal study [27] examining family relations and adolescent development. Families recruited from the Los Angeles area, through flyers, newspaper advertisements, and word-of-mouth, met the following eligibility criteria: both parents lived with the child for at least three years, family members could complete procedures in English, and the child was age 9–10 (Cohort 1) or in middle school for families joining the project at wave 3 (Cohort 2). Data collection for wave 5 occurred from 2009 to 2012 when participants were in mid-to-late adolescence (M = 18.05, SD = 1.08), and from 2014 to 2016 for wave 6 when participants were young adults (M = 22.24, SD = 23.00). On average, participation in Wave 6 occurred 4.5 years following Wave 5. At wave 5, participants completed 10 consecutive daily assessments of worry. Overlapping with the worry assessments and starting on day 2 or later, we collected three consecutive weekdays of saliva samples that were assayed for cortisol. Wave 6 participants completed an online survey that included questions about health symptoms. Procedures for both waves 5 and 6 were approved by the university IRB; we obtained parental consent and adolescent consent or assent depending upon the youth’s age.
Participants
Of the 131 adolescents who participated in some portion of wave 5, 32 did not provide saliva samples, resulting in a sample of 99 (46 females). Similar to Los Angeles demographics, 32.3% self-reported their ethnicity as Hispanic/Latino. Self-identified race was 5.1% Asian/Pacific Islander, 20.2% Black/African American, 37.4% Caucasian, 29.3% multi-racial, and 8.1% other. Families’ total annual income ranged considerably with 26% reporting < $50K, 43% $50K–$100K, and 31% > $100K. To test selective attrition, we compared the 32 participants who elected not to provide saliva versus the 99 who did; no significant differences emerged for any background variables.
In wave 6, 83 participants (43 female) reported on health symptoms. The remaining 16 either could not be contacted or chose not to participate. Comparisons between the 83 wave 6 participants versus the 16 non-participants revealed no significant differences in demographic or study variables.
Procedures
Wave 5 in-lab assessments included self-report questionnaires about health symptoms and anxiety. During the lab visit, they received training in the online Qualtrics-based survey, which they then received via email each day at 5pm for 10 consecutive days. Within these 10 days of daily reports, participants selected three consecutive weekdays for cortisol collections; at least one day of worry assessment preceded the first day of saliva collection.
On each of the three days of saliva collection, participants provided five saliva samples prompted by a pre-set alarm clock. The CAR is captured in three early morning samples—at awakening, 20min post-awakening, and 40min post-awakening. The alarm for the awakening samples was set no later than 8am. Participants collected saliva samples on their own using oral swabs that were frozen at the participants’ homes after collection and then frozen at −20 degrees Celsius in the lab. Participants were instructed not to consume caffeine or alcohol for 24 hours before beginning saliva sampling and not to eat, drink, exercise, or brush their teeth for 30 minutes preceding any collection. Samples were batch-shipped on dry ice to Salimetrics, LLC for assay. The last sample from each day was also assayed for cotinine [28].
Compliance
Of a possible total of 891 morning cortisol samples (3 per day for each of 3 days for 99 participants), 861 (96.6%) samples were usable; 30 samples could not be assayed due to insufficient saliva quantity or missed sample collection (9 awakening samples, 8 second samples, and 13 third samples). Participants reported the times of their saliva collections through a brief questionnaire completed during the 2-min saliva collection. Deviation from the 20 and 40-minute interval sampling schedule exceeded 10 minutes on only 11 of the 297 days of collection (3.4%). Comparing all models with and without these 11 days showed no differences so we included all days. In a sub-sample of 25 participants, we assessed compliance with the timing of saliva collection with a Medication Event Monitoring System (MEMS) cap that recorded when the saliva collection vial was opened. Participant-reported sampling times and MEMs cap-recorded sampling times were highly consistent (mean difference = 5.15 minutes, median difference = 1.93). Compliance with the daily surveys was relatively high; we received 849 of the 990 possible daily reports (86%). We statistically accounted for missing data in the analyses using full information maximum likelihood estimation [29].
Measures
CAR
We utilized the three morning samples to calculate the area under the curve with respect to increase (AUCi) [30]. CAR was normally distributed and did not require log transformation. The intraclass correlation was .26 (within-individual variance was 0.02; between-individual variance was 0.07) [31].
Daily worries
We developed a 26-item daily worry scale [4] that includes wide-ranging items representing typical adolescent worries [32]. The most common daily worries were “academic achievements,” “money problems,” and “appearance.” Following the prompt, “Today, how much did you worry about…”, participants rated each worry on a 4-point scale ranging from 0 (not at all) to 3 (a lot). The worry score represents the mean across all items. Respondents endorsed an average of 4.45 different worries each day. Within-day reliability was .89. The intraclass correlation was .61 (within-individual variance was 0.05; between-individual variance was 0.08).
Health symptoms
Table 1 lists the 22 items used to capture common health symptoms for adolescents and young adults, including the number of symptoms endorsed at wave 6. Table 2 provides means and SDs at waves 5 and 6. Participants reported whether they had experienced each symptom (“yes” or “no”). To link these health data with other measurements, we collected symptom occurrence over the previous 3 months at wave 5 and over the past year at wave 6. Total health symptoms ranged from 2–21 for both waves 5 and 6.
Table 1.
Percent of participants reporting wave 6 health symptoms within the past year
| Item | Percent reporting ‘yes’ | ||
|---|---|---|---|
| ‘Over the past year, did you experience the following?’ | Total Sample (n = 83) |
Male (n = 40) |
Female (n = 43) |
| Mild Headaches | 78.0 | 74.4 | 81.4 |
| Congestion/Stuffy Nose | 71.6 | 66.7 | 76.2 |
| Sore Throat | 70.4 | 71.8 | 69.0 |
| Muscle Aches/Pains | 68.3 | 69.2 | 67.4 |
| Trouble Sleeping | 64.2 | 69.2 | 61.5 |
| Coughs | 60.5 | 66.7 | 57.1 |
| Stomachaches | 59.8 | 51.3 | 67.4 |
| Allergies | 52.4 | 51.3 | 53.5 |
| Diarrhea | 50.6 | 48.7 | 52.4 |
| Constipation | 35.8 | 28.2 | 43.6 |
| Severe Headaches | 38.3 | 23.1 | 51.3 |
| Flu Symptoms | 38.7 | 41.0 | 35.9 |
| Vomiting | 28.4 | 30.8 | 25.6 |
| Skin Rashes | 19.8 | 15.4 | 23.1 |
| Shortness of Breath | 21.0 | 20.5 | 21.4 |
| Dizziness/Fainting | 20.0 | 15.4 | 24.4 |
| Infection | 19.0 | 15.4 | 21.5 |
| Rapid/Irregular Pulse | 14.8 | 7.7 | 20.5 |
| Strep Throat | 11.3 | 15.4 | 7.3 |
| Asthma Symptoms | 10.3 | 7.5 | 11.3 |
| Severe Chest Pain | 11.0 | 7.7 | 14.0 |
| High Blood Pressure | 5.1 | 5.3 | 4.9 |
Table 2.
Descriptive statistics and correlations for person-level averages of all study variables and covariates
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Worriesa | — | ||||||||||
| 2. CARb | −.06 | — | |||||||||
| 3. Healthc - wave 5 | .05 | .06 | — | ||||||||
| 4. Healthc - wave 6 | −.09 | −.03 | .40*** | — | |||||||
| 5. Age | .03 | −.04 | .03 | −.05 | — | ||||||
| 6. Anxiety | .30* | .20 | .48** | .18 | −.04 | — | |||||
| 7. Cotinineb | −.10 | −.06 | −.10 | .09 | −.03 | .14 | — | ||||
| 8. Time of first cortisolb | .15 | .00 | −.05 | .08 | .30* | .08 | .15 | — | |||
| 9. Hours of sleepb | −.09 | .04 | .12 | −.08 | −.03 | −.04 | .00 | .15 | — | ||
| 10. Medication used (no = 0) | .08 | .16 | .31* | .17 | −.12 | .17 | −.12 | .14 | .15 | — | |
| 11. Sexd (female = 0) | .02 | −.12 | −.15 | −.12 | .08 | .04 | .15 | −.02 | −.09 | 1.68 | — |
|
| |||||||||||
| M (SD) |
M (SD) |
M (SD) |
M (SD) |
M (SD) |
M (SD) |
M (SD) |
M (SD) |
M (SD) |
% | % | |
|
| |||||||||||
| Total sample | 0.32 (0.27) |
0.05 (0.16) |
8.66 (4.32) |
8.57 (4.43) |
18.06 (1.09) |
0.25 (0.32) |
4.76 (21.62) |
7.39 (0.96) |
7.31 (1.71) |
22.4% — |
— |
| Females | 0.33 (0.23) |
0.07 (0.19) |
8.95 (4.05) |
9.07 (4.43) |
18.13 (1.12) |
0.24 (0.33) |
1.59 (6.50) |
7.33 (0.08) |
7.34 (169) |
28.3% — |
46% |
| Males | 0.31 (0.30) |
0.03 (0.13) |
8.37 (3.87) |
8.16 (4.51) |
17.96 (1.05) |
0.26 (0·31) |
7.81 (28.62) |
7.41 (0.95) |
7.29 (1.73) |
17.3% — |
54% |
Average score across 10 days;
Average score across 3 days;
Number of health symptoms;
χ2 is calculated for associations between two categorical variables. N = 99 except for descriptive statistics and correlations presented for wave 6 health symptoms (N = 83). CAR = Cortisol awakening response. All variables were assessed at wave 5, except for health symptoms, which were assessed at both waves 5 and wave 6.
p < .05.
p < .01.
p < .001.
Covariates
The following covariates were tested in initial analyses: (a) sex; (b) global anxiety at wave 5 assessed through the SCL-90 [33]; (c) age at Wave 5; (d) time of the first cortisol sample; (e) cotinine; (f) hours of sleep each night, and (g) use of medications in wave 5. The medications reported included oral contraceptive (6 participants), asthma medications (4 participants), thyroid medication (1 participant), and ADHD medication (2 participants).
Analytic Strategy
To test the within-person daily associations between worries and next-day CAR (HO1), we used multilevel path analysis [Mplus Version 7; 34]. Lagged associations examining across-day worry-cortisol links statistically adjusted for associations between worries and CAR on the same day and for autoregressive effects. To test predictions regarding wave 5 and wave 6 health symptoms (HO2), we used standard multilevel models that incorporated all 10 days of worries and 3 days of CAR, adjusting for wave 5 anxiety and sex in all models, and for wave 5 health symptoms when predicting wave 6 health symptoms. Other covariates did not change the significance, magnitude, or direction of the findings, and were subsequently dropped.
Results
Descriptive Statistics and Correlations
Table 2 presents between-person Ms and SDs for all study measures and the correlations among them. Male versus female comparisons on all variables produced no significant differences (all p >.13). Neither worries nor CAR correlated with concurrent (wave 5) or later (wave 6) health symptoms, although worries were correlated with anxiety. Wave 5 health symptoms were correlated with anxiety, medication use, and wave 6 health symptoms.
Hypothesis 1: Daily Worries and Morning Cortisol
With the whole sample, worries did not predict the next-day CAR, B = 0.05, SE = 0.10, p = .62. However, the cross-level interaction indicated a significant moderation effect for sex, B = −0.34, SE = 0.16, p = .04. In partial support of HO1, the association between worries and next-day CAR was positive and significant for females, B = 0.31, SE = 0.14, p = .03, but non-significant for males, B = −0.06, SE = 0.05, p = .30. Figure 1 depicts females and males’ CAR following days with low reported worries (below the median) or high reported worries (above the median).
Figure 1.
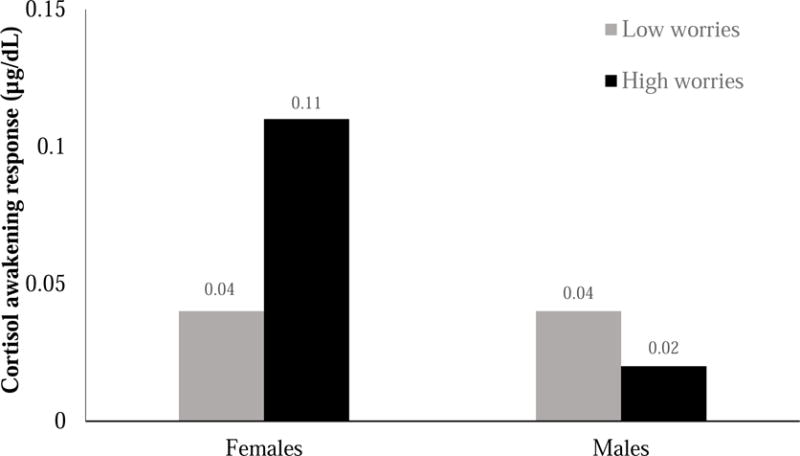
Cortisol awakening response (CAR) for females and males who reported high worries (above the median) versus low worries (below the median) on the previous day.
Hypotheses 2 & 3: Separate and Combined Effects of Worries and CAR on Concurrent and Later Health Symptoms
Table 3 presents results of multilevel models testing the direct effects of worries and CAR on health symptoms (HO2), as well as the interaction between worries and CAR on wave 5 and wave 6 health symptoms (HO3). There were no significant 3-way interactions with sex for wave 5 or 6 (p > .49) so results include the entire sample.
Table 3.
Separate and combined effects of worries and cortisol awakening response (CAR) on health symptoms
| Wave 5 health symptoms (n = 99) |
Wave 6 health symptoms (n = 83) |
|||||
|---|---|---|---|---|---|---|
| Hypothesis 3: Main effects and the interaction term | ||||||
| B | SE | p | B | SE | p | |
| Worries | −.14 | .07 | .04 | −.22 | .10 | .03 |
| CAR | .06 | .08 | .50 | −.06 | .13 | .67 |
| Sex | −.17 | .07 | .03 | −.10 | .09 | .24 |
| Covariates | ||||||
| Wave 5 health symptoms | – | – | – | .52 | .11 | <. 001 |
| Wave 5 anxiety | .55 | .13 | <. 001 | .15 | .11 | .17 |
| Hypothesis 3: Main effects and the interaction term | ||||||
| B | SE | p | B | SE | p | |
| Worries | −.14 | .06 | .006 | −.08 | .09 | .37 |
| CAR | −.10 | .11 | .36 | −.10 | .13 | .42 |
| Sex | −.16 | .07 | .03 | −.09 | .09 | .33 |
| Worries × CAR | −.16 | .20 | .41 | .41 | .20 | .04 |
| Covariates | ||||||
| Wave 5 health symptoms | – | – | – | .52 | .12 | <. 001 |
| Wave 5 anxiety | .52 | .13 | <. 001 | .11 | .11 | .33 |
Worries = Worries across ten days. B = standardized coefficient; SE = standard errors. CAR = Cortisol awakening response. R2 for wave 6 symptoms = .48; R2 for wave 5 symptoms = .37.
Contrary to HO2, that worry would be associated with more health symptoms, worries showed a significant inverse relationship with health symptoms at both waves. Also, the main effect for sex indicated that females reported more health symptoms at wave 5, but not wave 6. Contrary to HO2, no significant main effects emerged for CAR for wave 5 or wave 6 health symptoms.
Supporting HO3, the interaction between worries and CAR was significant for wave 6 health symptoms. Figure 2 illustrates simple slopes for worries and health symptoms at high (+1SD), average (M), and low (−1SD) CAR. In adolescents with high CAR, more worry was associated with more health symptoms, b = 20.25, SE = 10.20, p = .04. In contrast, in adolescents with low CAR, worry was associated with fewer health symptoms, b = −17.60, SE = 6.85, p = .01. At mean levels of CAR, worry was not related to health symptoms, b = 6.34, SE = 4.25, p = .13. The interaction between worries and CAR was non-significant for concurrent wave 5 health symptoms.
Figure 2.
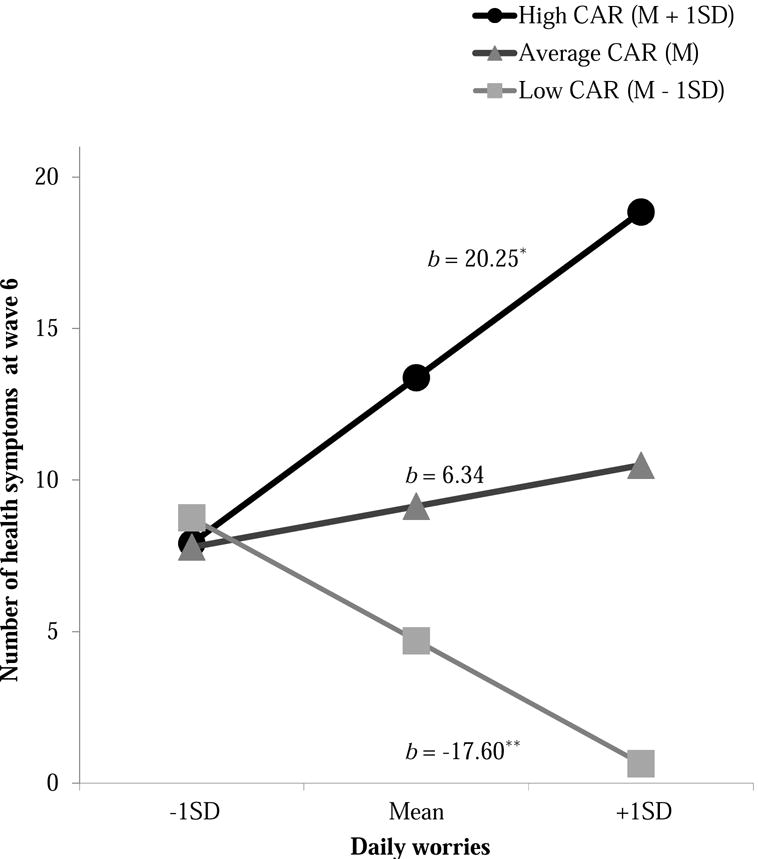
Association between daily worries and health symptoms for adolescents with high, average, and low cortisol awakening response (CAR). M = Mean. SD = Standard deviation. Coefficients represent simple slopes. b = unstandardized coefficients.
* p < .05. **p < .01. ***p < .001.
Discussion
This study used daily reports of worry and daily morning cortisol to investigate links between adolescents’ worry, HPA activity, and health symptoms. As anticipated, more pronounced increases in morning cortisol followed days with high worries, although this finding emerged only for females (HO1). Immediate and long-range implications for health symptoms were mixed. Contrary to HO2, worries were inversely associated with concurrent health symptoms. This association, however, was moderated by CAR. Adolescents who reported high everyday worries and showed pronounced CAR were at-risk for health complaints in young adulthood (HO3). Nevertheless, despite recognized risks of attenuated CAR, high worries in the context of low CAR were associated with fewer later health symptoms. Thus, for adolescents with high worries, a nonresponsive CAR may protect against common health complaints.
In line with prior findings with adults [11], reporting more worries than usual was associated with females’ steeper CAR the following morning. Our approach of modeling next-day CAR following three consecutive days of worry highlights the value of examining within-person variability. The significant within-person associations between worries and next-day CAR show that short-term upturns in worry, regardless of typical levels, are associated with physiological boosts. This daily association for females is similar to a prior study [35] where worry was associated with higher CAR for adult females but not males. Likewise, cortisol trajectories in girls ages 9–18 were more responsive to daily life influences [36]. Sex differences in the present study are not related to greater worries overall as females and males reported similar levels of daily worries. However, there may be differences in how males and females experience worries. For example, it is possible that females’ worry motivates actions to resolve a worrisome situation, as demonstrated in a recent study of college students showing associations between stress and cortisol elevations only when students took steps to resolve problems [37].
In contrast to the focus on within-person variability in worry and next-day CAR, HOs 2 and 3 examine between-person differences in worries, CAR, and total health symptoms. The unexpected finding that greater worry was associated with fewer concurrent and future health symptoms merits reconsideration of the concept of worry. Though worry generally has negative connotations, it possibly serves preventive functions with adolescents: Adolescents who worry more might be less likely to engage in risk-taking behaviors (substance use, risky sex) and more likely to engage in health-promoting behaviors (exercise, good diet). It is also notable that worry shows associations despite controlling for anxiety, highlighting the state-like more than trait-like qualities of worry.
The significant interaction between worries and CAR on later health adds to a growing literature on the HPA axis and biological adaptation to context. As noted elsewhere [12–14], repeated HPA activation in response to stress potentially results in chronically elevated or attenuated HPA patterns. In line with allostatic load theories, the heighted CAR, a physiological indicator of responsiveness, may incur undetectable wear and tear, rendering adolescents vulnerable to later negative health outcomes. As demonstrated here, the combination of high worries and high CAR may not be sustainable given its link to health symptoms. An intriguing finding of the present study, however, is that low CAR in combination with high worries is associated with fewer health ailments. That is, despite the putative benefit of upward CAR, worriers with heightened CAR may be upregulated physiologically in unhealthy ways, perhaps due to concomitant activation of the sympathetic nervous system [38]. Alternatively, consistent with differential sensitivity to context theories, decoupling worry from being in high-gear biologically may be adaptive.
More generally, these findings raise questions about the meaning of worry in a sample of typically developing adolescents without internalizing disorders. For some, worries may represent a cognitive state that prompts action and varies across days; for others, worry may be a relatively stable occurrence [2]. As with any assessment of worry—whether one or multiple items—participants may ascribe personalized meanings to the concept of “worry.” For some, worry may signal that there is something to accomplish or get through; for others, worry may represent ruminations that hinder action. Moreover, since worry surrounding chronic, uncontrollable stressors tends to incur higher physiological and health-related costs [39], future work should examine the specific nature of worries.
Several limitations deserve note. First, without assessing CAR at wave 6, we cannot know whether patterns of heightened or attenuated CAR recalibrate over time and how those changes impact health symptoms. Second, without assessing worry at wave 6, we do not know if health outcomes reflect prior or current worries. Third, although we assume that heightened CAR prompts more active coping later that day, we do not know how CAR is manifest in future behavior. Fourth, we collected cortisol only on weekdays to limit sources of variance; however, including weekends would offer a more complete picture of these findings. It also would be useful to have a measure of overall life adversity, which influences diurnal cortisol [40].
Nonetheless, our study is one of the first to take a biopsychological approach to investigating the health ramifications of adolescents’ everyday worries. Strengths of the study include the prospective design, the within-person examination of worry-CAR linkage, and the detailed examination of health outcomes in late adolescence and young adulthood. Moreover, the results provide a new, important perspective on adolescents’ worry; for the majority of adolescents, worries do not appear to increase risk for common health complaints and may even be protective. However, we identified an at-risk group of adolescents who show pronounced CARs along with high worries. Heightened cortisol as a short-term response to worry might carry hidden long-term costs, including greater health symptoms. These positive and negative implications of adolescents’ everyday worries highlight the need for better discernment about when everyday worry warrants intervention to minimize later physical health concerns.
IMPLICATIONS AND CONTRIBUTION.
Daily worries are common among adolescents but little is known about their health implications. In this study, females’ worries relate to next-day heightened cortisol awakening response (CAR), an index of physiological preparation to meet upcoming demands. High-worriers who had high CAR also showed increased risk for future health problems.
Acknowledgments
This work has been supported by NIH-NICHD Grants R01 HD 046807 and R21 HD 072170 (Margolin, PI), the David and Lucile Packard Foundation Grant 00-12802 (Margolin, PI), the American Association for University Women Fellowship, and the Israeli Council for Higher Education (Arbel, PI).
We thank our USC Family Studies Project colleagues as well as the families who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Reout Arbel, Department of Psychology, University of Southern California.
Lauren Spies Shapiro, Department of Psychology, University of Southern California.
Adela C. Timmons, Department of Psychology, University of Southern California
Ilana Kellerman Moss, Department of Psychology, University of Southern California.
Gayla Margolin, Department of Psychology, University of Southern California.
References
- 1.Borkovec TD, Ray WJ, Stober J. Worry: A cognitive phenomenon intimately linked to affective, physiological, and interpersonal behavioral processes. Cognit Ther Res. 1998;22:561–76. doi: 10.1023/A:1018790003416. [DOI] [Google Scholar]
- 2.Ehring T, Watkins ER. Repetitive negative thinking as a transdiagnostic process. Int J Cogn Ther. 2008;1:192–205. doi: 10.1521/ijct.2008.1.3.192. [DOI] [Google Scholar]
- 3.Verkuil B, Brosschot JF, Gebhardt WA, Thayer JF. When worries make you sick: a review of perseverative cognition, the default stress response and somatic health. J Exp Psychopathol. 2010;1:87–118. doi: 10.5127/jep.009110. [DOI] [Google Scholar]
- 4.Arbel R, Perrone L, Margolin G. Adolescents’ daily worries and risky behaviors: the buffering role of support seeking. J Clin Child Adolesc Psychol. 2016;3:1–12. doi: 10.1080/15374416.2016.1169536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosschot JF, Van Der Doef M. Daily worrying and somatic health complaints: testing the effectiveness of a simple worry reduction intervention. Psychol Health. 2006;21:19–31. doi: 10.1080/14768320500105346. [DOI] [Google Scholar]
- 6.Rotenberg S, McGrath JJ. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol Psychol. 2016;117:16–25. doi: 10.1016/j.biopsycho.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Law R, Hucklebridge F, Thorn L, et al. State variation in the cortisol awakening response. Stress. 2013;16:483–92. doi: 10.3109/10253890.2013.817552. [DOI] [PubMed] [Google Scholar]
- 9.McHale SM, Blocklin MK, Walter KN, et al. The role of daily activities in youths’ stress physiology. J Adolesc Health. 2012;51:623–28. doi: 10.1016/j.jadohealth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–14. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- 11.Zoccola PM, Dickerson SS, Yim IS. Trait and state perseverative cognition and the cortisol awakening response. Psychoneuroendocrinology. 2011;36:592–5. doi: 10.1016/j.psyneuen.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 13.Shirtcliff EA, Peres JC, Dismukes AR, et al. Riding the physiological roller coaster: adaptive significance of cortisol stress reactivity to social contexts. J Pers Disord. 2014;28:40–51. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–6. doi: 10.1016/j.iac.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Dreger LC, Kozyrskyj AL, HayGlass KT, et al. Lower cortisol levels in children with asthma exposed to recurrent maternal distress from birth. J Allergy Clin Immunol. 2010;125:116–22. doi: 10.1016/j.jaci.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Knack JM, Jensen-Campbell LA, Baum A. Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain Cogn. 2011;77:183–90. doi: 10.1016/j.bandc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Janicki-Deverts D, Doyle W, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. PNAS. 2012;109:5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis BJ, Boyce WT. Biological sensitivity to context. Curr Dir Psychol Sci. 2008;17:183–7. doi: 10.1073/pnas.1118355109. [DOI] [Google Scholar]
- 20.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–9. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Wiklund M, Malmgren-Olsson EB, Öhman A, Bergström E, Fjellman-Wiklund A. Subjective health complaints in older adolescents are related to perceived stress, anxiety and gender–a cross-sectional school study in Northern Sweden. BMC public health. 2012;12:1–13. doi: 10.1186/1471-2458-12-993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haugland S, Bente W. Subjective health complaints in adolescence—reliability and validity of survey methods. J Adolesc. 2001;24:611–24. doi: 10.1006/jado.2000.0393. [DOI] [PubMed] [Google Scholar]
- 24.Bouma EM, Riese H, Ormel J, et al. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009:34884–93. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Marsman R, Swinkels SH, Rosmalen JG, et al. HPA-axis activity and externalizing behavior problems in early adolescents from the general population: the role of comorbidity and gender: the TRAILS study. Psychoneuroendocrinology. 2008;33:789–98. doi: 10.1016/j.psyneuen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Fialko L, Bolton D, Perrin S. Applicability of a cognitive model of worry to children and adolescents. Behav Res Ther. 2012;50:341–9. doi: 10.1016/j.brat.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Margolin G, Vickerman KA, Oliver PH, Gordis EB. Violence exposure in multiple interpersonal domains: Cumulative and differential effects. J Adolesc Health. 2010;47:198–205. doi: 10.1016/j.jadohealth.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granger DA, Kivlighan KT, Fortunato C, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92:583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147. doi: 10.1037//1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- 30.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 31.Hruschka DJ, Kohrt BA, Worthman CM. Estimating between-and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;31(30):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Brown SL, Teufel JA, Birch DA, Kancherla V. Gender, age, and behavior differences in early adolescent worry. J Sch Health. 2006;76:430–437. doi: 10.1111/j.1746-1561.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis LR, Lipman RS, Covi L. The SCL-90: An outpatient psychiatric rating scale— preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 34.Muthé LK, Muthén BO. Mplus version 7 user’s guide. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 35.Gustafsson K, Lindfors P, Aronsson G, Lundberg U. Relationships between self-rating of recovery from work and morning salivary cortisol. J Occup Health. 2008;50:24–30. doi: 10.1539/joh.50.24. [DOI] [PubMed] [Google Scholar]
- 36.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–7. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 37.Sladek MR, Doane LD, Luecken LJ, Eisenberg N. Perceived stress, coping, and cortisol reactivity in daily life: A study of adolescents during the first year of college. Biol Psychol. 2016;117:8–15. doi: 10.1016/j.biopsycho.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ottaviani C, Thayer JF, Verkuil B, et al. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychol Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- 39.Chen E, Miller GE. “Shift-and-persist” strategies why low socioeconomic status isn’t always bad for health. Perspect Psychol Sci. 2012;7:135–58. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peckins MK, Dockray S, Eckenrode JL, et al. The longitudinal impact of exposure to violence on cortisol reactivity in adolescents. J Adolesc Health. 2012;51:366–72. doi: 10.1016/j.jadohealth.2012.0. [DOI] [PMC free article] [PubMed] [Google Scholar]


