Abstract
Myocardial inflammatory responses to endotoxemia are enhanced in old mice, which results in worse cardiac dysfunction. Anti-inflammatory cytokine interleukin (IL)-37 has a broad effect on innate immunoresponses. We hypothesized that IL-37 suppresses myocardial inflammatory responses to protect cardiac function during endotoxemia in old mice. Old (20–24 month) wild-type (WT), and IL-37 transgenic (IL-37tg) mice were treated with lipopolysaccharide (LPS, 0.5 mg/kg, iv) or normal saline (0.1 ml/mouse, iv). Six hours later, left ventricle (LV) function was assessed using a pressure-volume microcatheter. Levels of monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 in plasma and myocardial tissue, as well as mononuclear cell density in the myocardium, were examined. Cardiac microvascular endothelial cells isolated from WT and IL-37tg mice were treated with LPS (0.2 μg/ml) for 0.5–24 hours. Nuclear factor-kappa B (NF-κB) p65 phosphorylation was examined by immunoblotting, and MCP-1 levels in cell culture supernatant was determined using enzyme-linked immunosorbent assay. LV dysfunction in old WT endotoxemic mice was accompanied by up-regulated MCP-1, myocardial accumulation of mononuclear cells and production of TNF-α, IL-1β and IL-6. Expression of IL-37 suppressed myocardial inflammatory responses to endotoxemia in old mice, resulting in improved LV function. Treatment of old WT endotoxemic mice with recombinant IL-37 also improved LV function. In vitro experiments revealed that cardiac microvascular endothelial cells from IL-37tg mice had attenuated NF-κB activation and MCP-1 production following LPS stimulation. In conclusion, IL-37 is potent to suppress myocardial inflammation and protects against cardiac dysfunction during endotoxemia in old mice.
Keywords: Endotoxemia, Cardiac function, Aging, IL-37, Cardiac microvascular endothelial cells
1. Introduction
Major surgery and trauma can cause endotoxemia [1]. Excessive production of pro-inflammatory mediators caused by bacterial lipopolysaccharide (LPS) frequently leads to cardiac dysfunction. We and others have observed that LPS induces cardiac contractile depression through up-regulation of myocardial production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [2–4]. Importantly, morbidity and mortality due to the systemic inflammatory response syndrome associated with trauma or sepsis are significantly increased in the elderly [5, 6].
Old animals are found to display enhanced systemic and myocardial inflammatory responses to LPS [4, 7]. We previously reported that enhanced monocyte chemoattractant protein-1 (MCP-1) production in old endotoxemic mice has a critical role in mononuclear cell accumulation and cytokine production in the myocardium and contributes to the mechanism of exaggerated cardiac contractile depression. It appears that the MCP-1-mediated myocardial inflammation, i.e. mononuclear cell infiltration and production of cardiodepressant cytokines, plays an important role in cardiac contractile depression in old endotoxemic animals, and suppression of MCP-1 production and/or the MCP-1-mediated myocardial inflammation may have therapeutic potential for preservation of cardiac function in the elderly during endotoxemia.
Nuclear factor-kappa B (NF-κB) is a master transcription factor that mediates cellular inflammatory responses, including the expression of chemokines and cytokines [8]. Toll-like receptor (TLR) 4 on cell surfaces is activated in response to LPS and interacts with intracellular adaptors to activate NF-κB. Activated NF-κB translocates to the nucleus to initiate the transcription of pro-inflammatory genes [9]. Thus, NF-κB plays is critical in mediating LPS-induced cytokine production and cardiac dysfunction [10–12].
IL-37 is a member of the IL-1 family of cytokines. It is expressed in humans, and its expression has been identified in most cell types, including monocytes, dendritic cells, endothelial cells and epithelial cells, and acts as a natural regulator of the inflammatory responses [13]. IL-37 has repressive effects on LPS-stimulated cells, such as macrophages and endothelial cells, which implies that it may interfere with the TLR4 signaling pathway. In vitro studies show that expression of IL-37 in macrophages or epithelial cells dampens constitutive or induced production of pro-inflammatory cytokines, such as IL-1α, IL-1β, TNF-α, and macrophage inflammatory protein (MIP)-2 [14]. In vivo studies indicate that expression IL-37 in mice protects against chemical-induced colitis [15], and metabolic syndrome [16]. Further, mice that express IL-37 transgenic gene exhibit reduced lung, kidney and liver injury during endotoxemic shock [14]. However, it remains unclear whether IL-37 suppresses myocardial inflammatory responses in old animals subjected to endotoxemia. In addition, the effect of IL-37 on cardiac dysfunction caused by endotoxemia has not been determined.
In the present study, we tested the hypothesis that IL-37 suppresses the inflammatory responses in old endotoxemic mice to protect cardiac function against endotoxemic depression. We examined: 1) whether old IL-37 transgenic (IL-37tg) mice have better cardiac function during endotoxemia, 2) whether expression of IL-37 results in reduced MCP-1 production in old mice during endotoxemia and attenuated myocardial mononuclear cell accumulation and cytokine production, 3) whether IL-37 suppresses LPS-induced NF-κB activation and 4) whether recombinant IL-37 can protect old mice against endotoxemic cardiac dysfunction.
2. Materials and Methods
2.1. Animals and treatment
Adult (3–4 month old) male C57BL/6 (wild-type, WT) mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). Male WT mice of 20–24 months old were obtained from the National Institute on Aging (Bethesda, Maryland, USA). Old (20–24 month) male IL-37tg mice were from our colonies at University of Colorado Denver Anschutz Medical Campus. IL-37tg mice are C57BL/6 background [14]. They are fertile and display normal growth, behavior and lifespan. No any unique abnormality in organ/tissue has been observed in old IL-37tg mice.
All animals in this study were acclimated for at least 14 days before the experiments in an animal facility with a 12:12-hour light-dark cycle and free access to water and regular chow diet. This study was approved by the Animal Care and Research Committee of the University of Colorado Denver, and it conforms to the Guide for the Care and Use of Laboratory Animals (National Research Council, revised 1996).
LPS (0.5 mg/kg; from Escherichia coli, serotype 0127:B8; Sigma Chemical Co, Saint Louis, Missouri, USA) or sterile normal saline (0.1 ml/mouse, iv) was administered to old WT and IL-37tg mice through a tail vein. An additional group of old WT mice were treated with recombinant IL-37 (rIL-37, 0.05 mg/kg, iv: R&D System, Minneapolis, Minnesota, USA) 30 minutes after injection of LPS. Six hours after injection of LPS or saline, cardiac function was assessed. Then, blood were collected for analysis of cytokines. Myocardial tissue specimens were prepared for evaluation of mononuclear cell accumulation and cytokine levels.
2.2. Measurement of cardiac function
Left ventricle (LV) hemodynamics was analyzed at 6 hours after LPS treatment as described previously [17]. Briefly, mice were anesthetized with pentobarbital sodium (50 mg/kg, ip; Vortech Pharmaceuticals, Dearborn, Michigan, USA) and anticoagulated using heparin (1,000 units/kg, ip; Elkins-Sinn, Cherry Hill, New Jersey, USA). Animals were laid supine on a heating pad and the core body temperature was kept at 37±0.5°C. A microcatheter (1F; Millar Instruments, Houston, Texas, USA) was introduced into the LV through the right common carotid artery. LV pressure-volume loops were recorded using the MPVS-400 system. LV pressure, LV volume, heart rates, and related function parameters were analyzed with the aid of PVAN software (Millar Instruments, Houston, Texas, USA).
2.3. Isolation and culture of cardiac microvascular endothelial cells
Microvascular endothelial cells were isolated from hearts of adult (3–4 month old) mice as described previously [18]. Briefly, epicardial mesothelial cells and endocardial endothelial cells in heart tissue were devitalized by immersing beating hearts in ice-cold calcium-free phosphate-buffered saline (PBS), and then dipping them into 70% ethanol. Ventricular tissue was cut into fine pieces, and digested in norminally calcium-free Hank’s balanced salt solution (HBSS) supplemented with collagenase II (1.0 mg/ml), glucose (2.0 mg/ml), taurine (2.5 mg/ml), bovine serum albumin (BSA, 0.1%), and MgCl2 (1.4 mM). Then, the tissue was digested in HBSS containing 0.125% trypsin, 0.1 mM EDTA and 2.0 mg/ml glucose. Spinning the solution at 500 rpm for 5 min to remove the tissue debris and remaining myocytes. Endothelial cells were collected by centrifuging the supernatant at 1,200 rpm (4°C) for 8 min. Cells were seeded in 24-well plates and cultured at 37°C for 2 h in endothelial cell growth medium (EBM-2 from Lonza, Walkersville, Maryland, USA). Non-attached cells were removed. When culture became 90% confluence, LPS was added to the medium (final concentrations 0.2 μg/ml).
2.4. Immunoblotting
For analysis of myocardial TLR4 levels, myocardial homogenate was mixed with a sample buffer (10% glycerol, 2% SDS, 100 mmol/L Tris-HCl, pH 6.8, 0.02% bromophenol blue; Bio-Rad Laboratories Inc, Hercules, California, USA). For analysis of intercellular adhesion molecule (ICAM)-1 levels and NF-κB p65 phosphorylation in cardiac microvascular endothelial cells, the cells were lysed with the same sample buffer. The following primary antibodies were used to detect the different proteins on Western blots: TLR4 (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), phospho-NF-κB p65 (Cell Signaling Inc., Beverly, Massachusetts, USA), total NF-κB p65 (Cell Signaling Inc., Beverly, Massachusetts, USA), ICAM-1 (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), or GAPDH (Cell Signaling, Inc. Beverly, Massachusetts, USA). Membranes were exposed on X-ray films. NIH Image J software was used to analyze the area and density of protein bands.
2.5. Immunofluorescence staining
Heart tissues embedded in the optimal cutting temperature (OCT) were cut into 5 μm thick sections, and then treated with a solution of 30% acetone and 70% methanol for 5 min, washed with PBS and fixed with 4% paraformaldehyde. Sections were incubated with a rabbit polyclonal antibody against CD68 (a biomarker of mononuclear cells), followed by Cy3-tagged goat anti-rabbit IgG (imaged on the red channel). Nuclei were stained with bis-benzimide (DAPI, imaged on the blue channel). Glycoproteins on cell surfaces were stained with Alexa 488-tagged wheat germ agglutinin (imaged on the green channel). A Leica DMRXA digital microscope (Leica Mikroskopie and System GmbH, Wetzlar, Germany) was used for microscopy. Two sections of each heart were viewed and analyzed by a blinded viewer who is a team member without any acknowledge of the treatment.
2.6. ELISA
Commercial ELISA kits (R & D Systems, Minneapolis, MN) were utilized to quantify MCP-1, TNF-α, IL-1β and IL-6 in plasma and myocardial tissue homogenates, and MCP-1 in the supernatant of cardiac microvascular endothelial cell culture. Samples and standards were prepared according to manufacturer’s instructions. Absorbance of standards and samples were determined spectrophotometrically at 450 nm, using a microplate reader (Biotek, Winooski, VT). Results were plotted against the linear portion of the standard curve.
2.7. Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using StatView software (Abacus Concepts, Calabasas, CA, USA). One-tailed analysis of variance (ANOVA) with Fisher post-hoc test was used to analyze differences among experimental groups, and differences were confirmed using the Mann-Whitney U-test. Statistical significance was defined as P≤0.05 (95% confidence interval).
3. Results
3.1. Old IL-37tg mice and old WT mice treated with rIL-37 have better cardiac function during endotoxemia
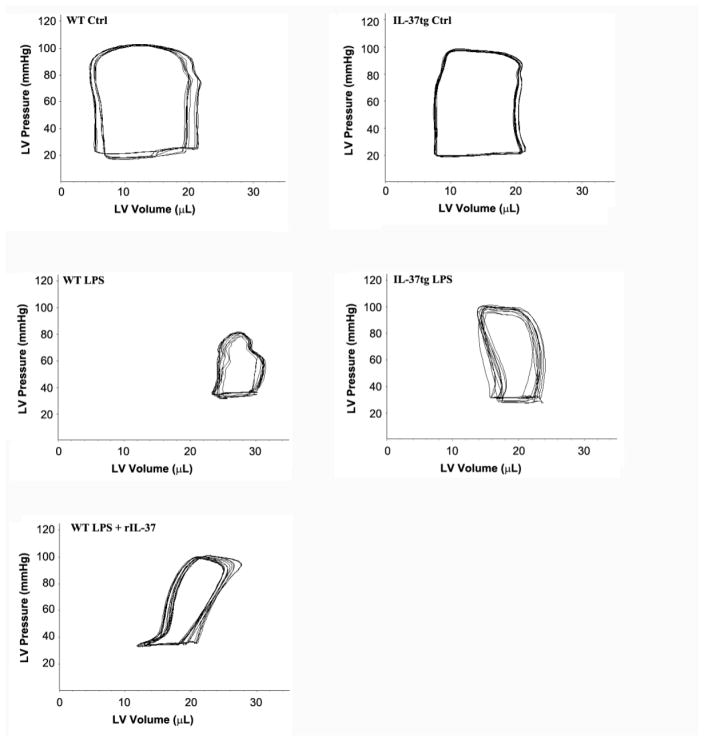
There was no significant difference in LV function between old WT mice and old IL-37tg mice following treatment with normal saline (Table 1 and Figure 1). Whereas LPS caused markedly decreases in LV contractile indices in both WT and IL-37tg mice, old IL-37tg mice displayed significantly improved developed pressure, ejection fraction and cardiac output (Table 1). Administration of recombinant IL-37 following injection of LPS had similar beneficial effects on LV function (Table 1). Compared to WT old mice treated with LPS, cardiac output increased by 46.8% (P<0.05) and 37.5% (P<0.05) in IL-37tg old mice treated with LPS and WT old mice treated with LPS+IL-37, respectively. However, expression of IL-37 and administration of recombinant IL-37 had no effect on LPS-induced tachycardia (Table 1). Thus, the improvement in cardiac output is primarily due to an improvement in contractility. Indeed, developed pressure increased by 42.9% (P<0.05) in IL-37tg old mice treated with LPS, and by 38.9% (P<0.05) in WT old mice treated with LPS+IL-37.
Table 1.
Left ventricle function parameters
| Parameter | WT Ctrl | WT LPS | IL-37tg Ctrl | IL-37tg LPS | WT LPS + rIL-37 |
|---|---|---|---|---|---|
| Heart rate (bpm) | 430 ± 36 | 528 ± 56* | 437 ± 29 | 520 ± 73# | 510 ± 61* |
| Developed pressure (mmHg) | 78.7 ± 7.8 | 47.0 ± 3.6* | 80.0 ± 11 | 67.2 ± 7.4#† | 65.3 ± 9.8† |
| End-systolic volume (μl) | 8.0 ± 5.5 | 24.5 ± 2.9* | 9.1 ± 2.2 | 14.9 ± 2.4#† | 16.5 ± 2.8*† |
| End-diastolic volume (μl) | 20.2 ± 3.9 | 31.1 ± 6.1* | 20.6 ± 3.1 | 24.2 ± 2.9 | 25.1 ± 4.4 |
| Ejection Fraction (%) | 60.4 ± 9.0 | 21.2 ± 4.6* | 55.8 ± 12 | 38.4 ± 5.3#† | 34.3 ± 6.1*† |
| Cardiac output (ml/min) | 5.3 ± 0.9 | 3.2 ± 0.3* | 5.1 ± 1.2 | 4.7 ± 0.4† | 4.4 ± 0.8† |
WT and IL-37tg old mice were treated with lipopolysaccharide (LPS, 0.5 mg/kg, iv) or normal saline. In a separate group of WT old mice, recombinant IL-37 (rIL-37, 0.05 mg/kg, iv) was administered 30 minutes after injection of LPS. WT old mice had reduced left ventricle (LV) function, including the developed pressure, ejection fraction and cardiac output, at 6 hours after treatment with LPS. IL-37tg old mice displayed better LV function after treatment with LPS in comparison to WT old mice. Treatment with recombinant IL-37 also improved LV function in WT old mice exposed to LPS. Data are expressed as mean ± SD. n=6 mice in each group; one-tailed ANOVA;
P<0.05 vs. WT old control (Ctrl);
P<0.05 vs. IL-37tg old control;
P<0.05 vs. WT old mice treated with LPS alone.
Figure 1. IL-37tg old mice and WT old mice treated with recombinant IL-37 are resistant to endotoxemic cardiac depression.
WT and IL-37tg old mice were treated with lipopolysaccharide (LPS, 0.5 mg/kg, iv) or normal saline. A group of WT old mice were treated with LPS and then with recombinant IL-37 (rIL-37, 0.05 mg/kg, iv) 30 minutes later. Representative pressure-volume loops show that WT old mice had reduced left ventricle (LV) function at 6 hours after treatment with LPS alone. Expression of IL-37 (IL-37tg) or administration of rIL-37 improved LV function in old mice exposed to LPS.
3.2. Old WT and IL-37tg mice have comparable levels of TLR4 in the myocardium
We analyzed myocardial TLR4 levels in mice treated with normal saline or LPS in order to understand whether expression of IL-37 alters myocardial TLR4 levels at baseline and/or during endotoxemia. The immunoblotting results presented in Figure 2 show comparable myocardial TLR4 levels in old WT mice and old IL-37tg mice in the absence or presence of LPS treatment. Thus, the improved cardiac function in old IL-37tg mice during endotoxemia is not due to lower myocardial TLR4 levels before or after they are exposed to LPS.
Figure 2. Old WT mice and old IL-37tg mice have comparable levels of TLR4 in the myocardium.
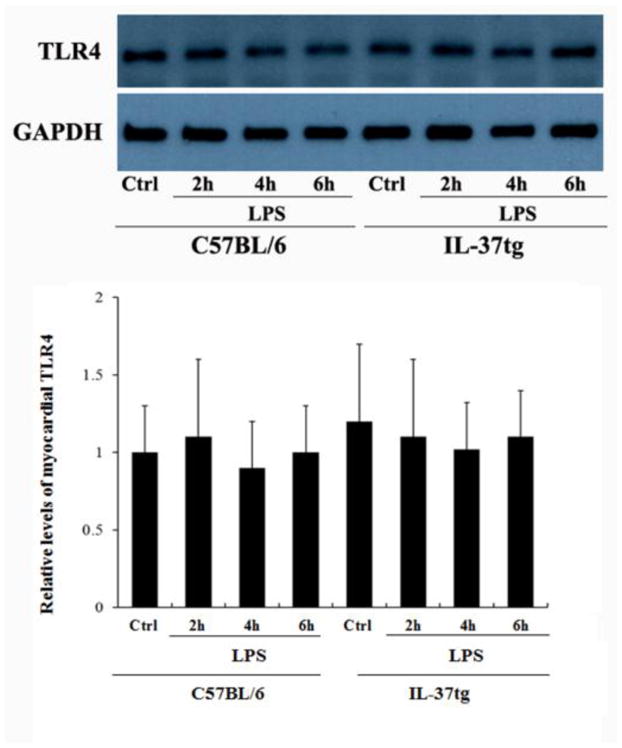
WT and IL-37tg old mice were treated with lipopolysaccharide (LPS, 0.5mg/kg, iv) or normal saline. Levels of TLR4 in myocardium tissue were analyzed with immunoblotting at 2, 4, and 6 hours after treatment. In comparison to WT old mice, IL-37tg old mice had comparable levels of TLR4 in the myocardium with or without a treatment with LPS. Data are expressed as mean ± SD. n=3.
3.3. Expression of IL-37 suppresses MCP-1 production and reduces myocardial mononuclear cell accumulation during endotoxemia
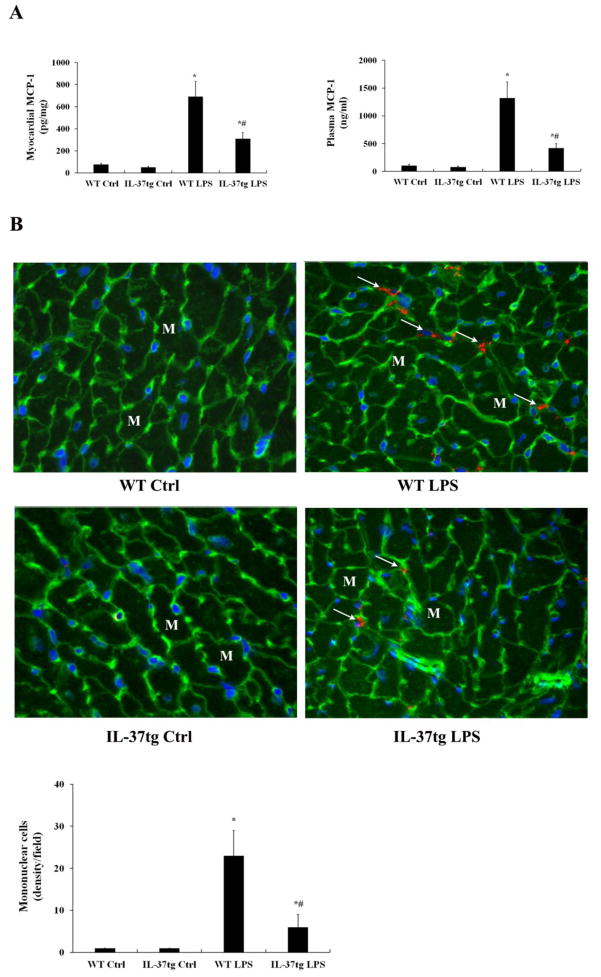
As enhanced MCP-1 production and mononuclear cell accumulation in the myocardium play important roles in the exaggerated cardiac dysfunction in old endotoxemic mice [4], we compared MCP-1 levels and myocardial mononuclear cell density in old WT mice and old IL-37tg mice. As shown in Figure 3A, MCP-1 levels in plasma and myocardial tissue after LPS treatment were significantly lower in old IL-37tg mice in comparison to old WT mice (420±32 pg/ml vs. 1318 pg/ml±128 pg/ml and 310±23 pg/mg vs. 692±56 pg/mg, both P<0.05). Similarly, old IL-37tg mice had lower densities of mononuclear cells in the myocardium (7/field vs. 23/filed in old WT mice, P<0.05; Figure 3B). Thus, expression of IL-37 suppresses MCP-1 production and reduces myocardial mononuclear cell accumulation in old mice.
Figure 3. IL-37tg old mice produce lower levels of MCP-1 and have attenuated mononuclear cell accumulation in the myocardium.
WT and IL-37tg old mice were treated with lipopolysaccharide (LPS, 0.5 mg/kg, iv) or normal saline. Levels of MCP-1 in plasma and the myocardium were analyzed by ELISA 6 hours after treatment. Mononuclear cells in the myocardium were detected by immunofluorescence staining. A. In comparison with WT old mice treated with LPS, IL-37tg old mice had lower levels of MCP-1 in both plasma and myocardial tissue after treatment with LPS. B. IL-37tg old mice had lower densities of mononuclear cells (arrows) in the myocardium after treatment with LPS. Data are expressed as mean ± SD. n=6 mice in each group; one-tailed ANOVA; *P<0.05 vs. corresponding control (ctrl); #P<0.05 vs. WT old mice treated with LPS. M=myocyte, initial amplification 40× objective.
3.4. Expression of IL-37 reduces cytokine production in old mice during endotoxemia
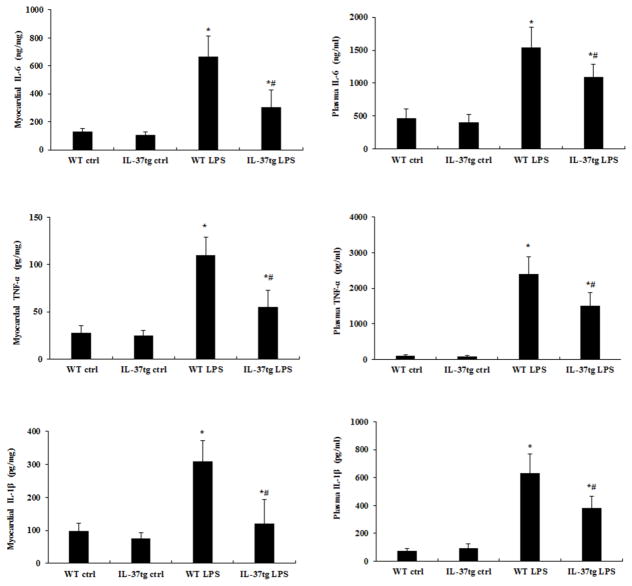
Mononuclear cells are the main sources of myocardial cytokines [19, 20]. We analyzed myocardial cytokine levels in order to confirm the outcome of reduced mononuclear cell accumulation in old IL-37tg mice. In old WT mice, LPS treatment caused marked elevations in TNF-α, IL-1β and IL-6 in both plasma and myocardial tissue (Figure 4). In old IL-37tg mice, however, the levels of all of the three cytokines were reduced in both plasma and myocardial tissue (Figure 4). It is noteworthy that the myocardium of old IL-37tg mice had significantly lower levels of TNF-α (55±10 pg/mg vs. 110±8 pg/mg, P<0.05), IL-1β (120±30 pg/mg vs. 310±26 pg/mg, P<0.05) and IL-6 (305±50 ng/mg vs. 667.5±60 ng/mg, P<0.05). The results indicate that reduced myocardial mononuclear cell accumulation in old IL-37tg mice results in lower levels of cardiodepressant cytokines in the myocardium.
Figure 4. IL-37tg old mice produce lower levels of cardiodepressant cytokines during endotoxemia.
IL-37tg and WT old mice were treated with lipopolysaccharide (LPS, 0.5 mg/kg, iv) or normal saline. Levels of IL-1β, TNF-α, and IL-6 in the myocardium and plasma were analyzed by ELISA 6 hours after LPS treatment. Levels of IL-1β (A), TNF-α (B), and IL-6 (C) in the myocardium and plasma were lower in IL-37tg mice. Data is expressed as mean ± SD. n=6 mice in each group; one-tailed ANOVA; *P<0.05 vs. corresponding control (Ctrl); #P<0.05 vs. WT mice treated with LPS.
3.5. IL-37 suppresses LPS-induced MCP-1 and ICAM-1 production and NF-κB activation in cardiac microvascular endothelial cells
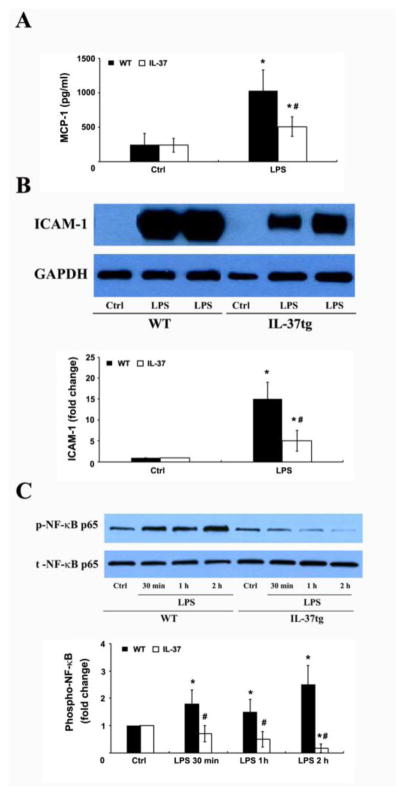
To confirm that IL-37 suppresses MCP-1 and ICAM-1 production in cardiac cells, we examined the effect of IL-37 on ICAM-1 levels in cell lysate and MCP-1 levels in culture supernatant of cardiac microvascular cells exposed to LPS. Figure 5A and 5B show that LPS increased MCP-1 and ICAM-1 production in cells isolated from WT mice. The effect of LPS on MCP-1 and ICAM-1 production was markedly reduced in cells isolated from IL-37tg mice. Moreover, the reduced MCP-1 and ICAM-1 production in IL-37tg cells correlated to markedly attenuated NF-κB p65 phosphorylation (Figure 5C). Together, these results show that IL-37 suppresses LPS-induced NF-κB activation, MCP-1 and ICAM-1 production in cardiac cells.
Figure 5. IL-37 suppresses LPS-induced MCP-1 and ICAM-1 production, and NF-κB activation in cardiac microvascular endothelial cells.
Cardiac microvascular endothelial cells isolated from WT and IL-37tg mice were cultured in monolayer and treated with lipopolysaccharide (LPS, 0.2 μg/ml) for 30 minutes to 24 hours. MCP-1 levels in culture supernatant and ICAM-1 in cell lysate were determined at 24 hours. Phosphorylated NF-κB p65 in cell lysate was analyzed at 0.5–2 hours. MCP-1 (A) and ICAM-1 (B) levels were lower in cells from IL-37tg mice compared with the cells from WT mice. NF-κB phosphorylation (C) was essentially absent after LPS stimulation in the cells from IL-37tg mice. Data are expressed as mean ± SD. n=4 experiments using separate isolates; one-tailed ANOVA; *P<0.05 vs. corresponding control (ctrl); #P<0.05 vs. WT cells treated with LPS.
4. Discussion
In a previous study, we demonstrated that old mice have enhanced myocardial inflammatory responses to endotoxemia, which leads to exaggerated cardiac dysfunction [4]. Neutralizing MCP-1 improves cardiac function in old endotoxemic mice by reducing mononuclear cell accumulation in the myocardium and associated cytokine production. In the present study, we observed that old IL-37tg mice have better cardiac function during endotoxemia compared to old WT mice, and recombinant IL-37 administered to old WT mice after injection of LPS also improve cardiac function. Improved cardiac function in old IL-37tg mice is associated with reduced MCP-1 production, and reduced myocardial mononuclear cell accumulation and cytokine production. The attenuated myocardial inflammatory responses are not due to an impact of IL-37 on myocardial TLR4 levels in the baseline or during endotoxemia. The results of in vitro experiments on cardiac microvascular endothelial cells indicate that IL-37 suppresses NF-κB activation to reduce MCP-1 production. Together, the data obtained in the present study demonstrate that IL-37 suppresses the inflammatory responses to preserve cardiac function in old endotoxemic mice.
IL-37 is found in humans, but is absent in rodents, and an anti-inflammatory function of this cytokine has been demonstrated in a variety of cell types from different species, including rodents [14, 21, 22]. Endogenous IL-37 is localized in intracellular and extracellular compartments. Extracellular IL-37 utilizes IL-18 receptor α-chain to exert its effect on cells [23]. In addition, intracellular IL-37 can translocate to the nucleus to down-regulate pro-inflammatory cytokine expression [24]. IL-37 broadly inhibits innate immune responses in vitro and in vivo [25]. Recombinant IL-37 has been shown to reduce hepatocyte injury caused by ischemia and reperfusion [26]. In addition, recombinant IL-37 protects mice against acute lung injury and reduces neutrophil infiltration following intracellular-tracheal instillation of Apergillus fumigatus [27]. Mesenchymal stromal cells transfected with IL-37 has been shown to protect mice against colitis by increasing numbers of myeloid-derived suppressor and T-regulatory cells and decreasing numbers of CD4+ cells [28].
In the present study, we observed that both old IL-37tg mice and old WT mice treated with recombinant IL-37 have improved cardiac function during endotoxemia. Moreover, expression IL-37 and administration of recombinant IL-37 have comparable effect in ameliorating endotoxemic cardiac dysfunction. It is noteworthy that recombinant IL-37 may exert an effect primarily via an extracellular fashion whereas IL-37 expressed by transgene should have both intracellular and extracellular actions. The results of the present study indicate that exogenously extracellular IL-37 can protect hearts of old mice equally well as endogenous IL-37. As administration of IL-37 30 minutes after injection of LPS has a beneficial effect, it appears that IL-37 is capable of exerting a beneficial effect even if it is administered after certain pro-inflammatory signaling event, such as TLR4 activation, occurs.
However, the beneficial effects of IL-37 on cardiac functional resistance to endotoxemic depression is not due to an effect on myocardial TLR4 levels since TLR4 levels in the myocardium of IL-37tg mice are comparable to those of WT mice in the condition of treatment with either normal saline or LPS. Previous studies found that LPS up-regulates TLR4 expression in epithelial and endothelial cells [29–31]. The results of the present study show no change in TLR4 protein levels in myocardial tissue at 2–6 hours after injection of LPS. The difference between our observation and previous findings could be due to the differences in the time course of experiment and/or tissue/cell type examined.
MCP-1, a member of the C-C chemokine family, functions as a chemotactic factor for monocytes [32]. Infiltrated mononuclear cells are important sources of tissue pro-inflammatory cytokines [20]. Several studies showed inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, cause cardiac dysfunction during endotoxemia [3, 33, 34]. In addition, pro-inflammatory cytokines have been found to be responsible for cardiac dysfunction after acute injuries caused by burn [33], sepsis [35], and myocardial ischemia and reperfusion injury [36]. We previously reported that MCP-1 plays a major role in the myocardial inflammation in old mice subjected to endotoxemia and that the exaggerated cardiac performance during endotoxemia in old mice is associated with greater levels of TNF-α, IL-1β and IL-6 in plasma and myocardium tissue [4]. In the present study, we found that old IL-37tg mice have lower density of mononuclear cells in the myocardium and lower levels of inflammatory cytokine (TNF-α, IL-1β and IL-6) in plasma and myocardium tissue. The markedly reduced myocardial cytokine response may explain the attenuated cardiac contractile depression in old IL-37tg mice during endotoxemia.
It is likely that IL-37 suppresses MCP-1 production and thereby reduces myocardial mononuclear cell accumulation and subsequent production of cardiodepressant cytokines. We observed that MCP-1 levels in both plasma and myocardial tissue are markedly lower in old IL-37tg mice subjected to endotoxemia. Moreover, lower levels of MCP-1 in old IL-37tg mice correlates with reduced mononuclear cell accumulation and reduced levels of cardiodepressant cytokine in the myocardium. In view of our previous observation that neutralizing of MCP-1 in old endotoxemic mice reduces myocardial mononuclear cell accumulation and cytokine production [4], it is reasonable to propose that IL-37 suppresses MCP-1 production to reduce myocardial mononuclear cell accumulation and subsequent production of cardiodepressant cytokines in this endotoxemia model. However, IL-37 may also exert a direct effect on mononuclear cells in the myocardium, resulting in decreased pro-inflammatory activities. Further study is needed to determine the effect of IL-37 on mononuclear cell activity and the relative role of such an effect in the mechanism of cardiac protection against endotoxemic contractile depression.
Low myocardial levels of MCP-1 could be a result of lower systemic levels of this chemokine. In order to understand whether IL-37 suppresses MCP-1 production in cardiac cells exposed to LPS, we isolated cardiac microvascular endothelial cells and performed in vitro experiments. Microvascular endothelial cells play a critical role in the overall myocardial inflammation, particularly in mediating leukocyte infiltration into the myocardium [37]. The results of in vitro experiments show that the effect of LPS on NF-κB p65 phosphorylation and MCP-1 production is markedly reduced in microvascular endothelial cells expressing IL-37. Thus, low myocardial levels of MCP-1 observed in old IL-37tg mice is at least partly due to decreased production of this chemokine by myocardial tissue.
Activation of the NF-κB complex results in inhibitor of kappa B (I-κB) degradation and subsequent release of NF-κB [38]. NF-κB then translocates to the nucleus and regulates the expression of pro-inflammatory genes, including MCP-1 [39]. Many studies show that inhibition of NF-κB reduces the release of inflammatory cytokines and inducible nitric oxide synthase (iNOS), and protects mice against lethal endotoxemia [12]. Our recent study demonstrates that IL-37 inhibits NF-κB activation induced by oxidized low-density lipoprotein in human aortic valve cells [40]. The in vitro data in the present study indicate that IL-37 represses the NF-κB response to TLR4 stimulation to modulate MCP-1 production in cardiac microvascular endothelial cells. Since microvascular endothelial cells play a critical role in regulating leukocyte infiltration into the myocardium. Suppression of MCP-1 production in such cells by IL-37 should reduce mononuclear cell accumulation and cytokine production in the myocardial tissue, and leads to attenuated cardiac contractile depression. It should be noted that IL-37 might not only affect endothelial cells, but also has an effect on other cardiac cells and immune cells. The effect of IL-37 on all of these cell may account for the attenuated overall inflammatory responses in IL-37tg mice and WT mice treated with recombinant IL-37.
This study has limitations. The old mice used in this study might have unknown disorders, such as vascular disorders, even if we examined them carefully before and after the experiment. Old WT and IL-37tg mice were from different sources. Both could be potential variables. In addition, we have not examined the levels of IL-18 receptor and IL-18 binding protein in the IL-37tg mice. Since IL-18 receptor mediates the effect of IL-37 and IL-18 binding protein can interact with IL-37, potential changes in their levels with age may alter the effectiveness of IL-37.
5. Conclusions
Transgenic expression of IL-37 and administration of recombinant IL-37 protect against cardiac dysfunction during endotoxemia in old mice. Cardiac protection by IL-37 is associated with suppression of MCP-1 production and myocardial inflammation. In addition, expression of IL-37 inhibits NF-κB activation and suppresses MCP-1 production in cardiac microvascular endothelial cells exposed to LPS. Thus, IL-37 protects hearts of old mice against endotoxemic contractile depression through suppression of the inflammatory responses. This anti-inflammatory cytokine may have therapeutic potential for protection of hearts of old subjects against cardiac dysfunction caused by endotoxemia. However, the findings in old mice should not been extrapolated to the human condition without caution.
Highlights.
IL-37 protects against cardiac dysfunction in old mice during endotoxemia.
Endotoxemic old IL-37tg mice have reduced myocardial inflammation.
IL-37 inhibits NF-κB activation in cardiac cells exposed to endotoxin.
IL-37 blocks endotoxin-induced inflammatory responses in cardiac cells.
Acknowledgments
Grant support
This study was supported in part by the National Institutes on Aging grant AG039545 (to XM) and the National Natural Science Foundation of China grant 81300123 (to JL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- IL
Interleukin
- MCP-1
monocyte chemoattractant protein-1
- TNF-α
tumor necrosis factor-α
- LPS
lipopolysaccharide
- NF-κB
Nuclear factor-kappaB
- TLR
Toll-like receptor
- MIP
macrophage inflammatory protein
Footnotes
Disclosures
The authors declare that they have no competing interests.
Authors’ contributions
XM designed the study. JL, YZ, LA and HH conducted the experiments and obtained the data. JL, YZ, DF, CA and XM analyzed and interpreted the data. JL and YZ drafted the manuscript. XM, DF and CD revised the manuscript critically for important intellectual contents. All authors have approved the final version of manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charbonney E, Tsang JY, Li Y, Klein D, Duque P, Romaschin A, Marshall JC. Endotoxemia Following Multiple Trauma: Risk Factors and Prognostic Implications. Crit Care Med. 2016;44(2):335–341. doi: 10.1097/CCM.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 2.Ao L, Song Y, Fullerton DA, Dinarello CA, Meng X. The interaction between myocardial depressant factors in endotoxemic cardiac dysfunction: role of TNF-alpha in TLR4-mediated ICAM-1 expression. Cytokine. 2007;38(3):124–129. doi: 10.1016/j.cyto.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102(22):2758–2764. doi: 10.1161/01.cir.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 4.Slimani H, Zhai Y, Yousif NG, Ao L, Zeng Q, Fullerton DA, Meng X. Enhanced monocyte chemoattractant protein-1 production in aging mice exaggerates cardiac depression during endotoxemia. Crit Care. 2014;18(5):527. doi: 10.1186/s13054-014-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Starr ME, Saito H. Sepsis in old age: review of human and animal studies. Aging Dis. 2014;5(2):126–136. doi: 10.14336/AD.2014.0500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124(10–12):1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, Seo EK, Lee KT. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 2007;30(12):2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72(3):384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Haudek SBS, Erika, Bryant Debora D. Overexpression of cardiac I-kBa prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2001;280(3):H962–H968. doi: 10.1152/ajpheart.2001.280.3.H962. [DOI] [PubMed] [Google Scholar]
- 12.Matsumori A, Nunokawa Y, Yamaki A, Yamamoto K, Hwang MW, Miyamoto T, Hara M, Nishio R, Kitaura-Inenaga K, Ono K. Suppression of cytokines and nitric oxide production, and protection against lethal endotoxemia and viral myocarditis by a new NF-kappaB inhibitor. Eur J Heart Fail. 2004;6(2):137–144. doi: 10.1016/j.ejheart.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol. 2013;25(6):466–468. doi: 10.1016/j.smim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P, Boekschoten MV, Muller M, Kersten S, Li S, Kim S, Eini H, Lewis EC, Joosten LA, Tilg H, Netea MG, Tack CJ, Dinarello CA, Stienstra R. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:4711. doi: 10.1038/ncomms5711. [DOI] [PubMed] [Google Scholar]
- 17.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansky L, Reymanova P, Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res. 2003;52(5):593–598. [PubMed] [Google Scholar]
- 20.Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34(8):959–970. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 21.Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, Kim SH. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci U S A. 2002;99(21):13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalli G, Koenders M, Kalabokis V, Kim J, Tan AC, Garlanda C, Mantovani A, Dagna L, Joosten LA, Dinarello CA. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology (Oxford) 2016;55(12):2220–2229. doi: 10.1093/rheumatology/kew325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, Kim S, Dinarello CA. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci U S A. 2015;112(8):2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Kulk N, Nold MF, Graf R, Kim SH, Reinhardt D, Dinarello CA, Bufler P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180(8):5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46(5):1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai N, Van Sweringen HL, Belizaire RM, Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ, Lentsch AB. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10(11):e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Wq, Dong K, Zhou L, Jiao Gh, Zhu Cz, Li Ww, Yu G, Wu Wt, Chen S, Sun Zn. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36(11):1377–1387. doi: 10.1038/aps.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barua RS, Sharma M, De A, Zhou J, Parashara D, Dileepan KN. Synergistic Amplification of Lipopolysaccharide-and Histamine-induced TLR4 and COX2 Expression by Cigarette Smoke Extract and Nicotine in Endothelial Cells. The FASEB Journal. 2016;30(1 Supplement):954.5. [Google Scholar]
- 30.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166(3):2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 31.Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol. 2006;79(5):989–998. doi: 10.1189/jlb.1005597. [DOI] [PubMed] [Google Scholar]
- 32.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maass DL, White J, Horton JW. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock. 2002;18(4):360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O’Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes CJ, Jr, de Assuncao MS. Myocardial dysfunction in sepsis: a large, unsolved puzzle. Crit Care Res Pract. 2012;2012:896430. doi: 10.1155/2012/896430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, Fullerton DA, Meng X. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg. 2008;85(5):1678–1685. doi: 10.1016/j.athoracsur.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 38.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh S, Dass JF. Study of pathway cross-talk interactions with NF-kappaB leading to its activation via ubiquitination or phosphorylation: A brief review. Gene. 2016;584(1):97–109. doi: 10.1016/j.gene.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Zeng Q, Song R, Fullerton DA, Ao L, Zhai Y, Li S, Ballak DB, Cleveland JC, Reece TB, McKinsey TA, Xu D, Dinarello CA, Meng X. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci U S A. 2017:201619667. doi: 10.1073/pnas.1619667114. [DOI] [PMC free article] [PubMed] [Google Scholar]