Abstract
This study investigated the association of HIV infection and cocaine dependence with cerebral white matter integrity using diffusion tensor imaging (DTI). 135 participants stratified by HIV and cocaine status (26 HIV+/COC+, 37 HIV+/COC−, 37 HIV−/COC+, and 35 HIV−/COC−) completed a comprehensive substance abuse assessment, neuropsychological testing, and MRI with DTI. Among HIV+ participants, all were receiving HIV care and 46% had an AIDS diagnosis. All COC+ participants were current users and met criteria for cocaine use disorder. We used tract-based spatial statistics (TBSS) to assess the relation of HIV and cocaine to fractional anisotropy (FA) and mean diffusivity (MD). In whole-brain analyses, HIV+ participants had significantly reduced FA and increased MD compared to HIV− participants. The relation of HIV and FA was widespread throughout the brain, whereas the HIV-related MD effects were restricted to the corpus callosum and thalamus. There were no significant cocaine or HIV-by-cocaine effects. These DTI metrics correlated significantly with duration of HIV disease, nadir CD4+ cell count, and AIDS diagnosis, as well as some measures of neuropsychological functioning. These results suggest that HIV is related to white matter integrity throughout the brain, and that HIV-related effects are more pronounced with increasing duration of infection and greater immune compromise. We found no evidence for independent effects of cocaine dependence on white matter integrity, and cocaine dependence did not appear to exacerbate the effects of HIV.
Keywords: HIV infection, white matter, diffusion tensor imaging (DTI), magnetic resonance imaging (MRI), cocaine, neurocognitive function
Introduction
It is well established that HIV attacks the central nervous system through several indirect mechanisms (Navia et al, 1986; Valcour et al, 2011). Early in the course of infection, HIV can infect monocytes and cross the blood-brain barrier, initiating an inflammatory cascade that involves perivascular macrophages, microglia, and astrocytes, eventually leading to pathogenesis (Lindl et al, 2010). HIV can also trigger axonal degeneration and neuronal loss by shedding neurotoxic viral proteins such as gp120 and Tat (Mocchetti et al, 2014). Even in the era of combination antiretroviral therapy, HIV-associated neurocognitive impairments are common (Spudich, 2013). For example, a multi-site study of >1500 HIV-infected persons in the United States reported a 52% prevalence of neurocognitive impairment (Heaton et al, 2010). Neurocognitive impairment is most prevalent among those with advanced HIV disease, but high rates of mild impairment are present at all disease stages (Dawes et al, 2008; Heaton et al, 2010).
Diffusion tensor imaging (DTI) is a non-invasive neuroimaging technique used to examine white matter integrity by sensitizing the MRI signal to the diffusion of water molecules within the brain (Johansen-Berg and Behrens, 2013; Le Bihan and Johansen-Berg, 2012). Physiologically, DTI metrics are influenced by the degree of myelination, microstructural features such as fiber diameter and density, and macrostructural features such as intravoxel fiber-tract coherence. The most commonly reported metrics are fractional anisotropy (FA), a normalized scalar value between 0 (perfectly isotropic) and 1 (perfectly anisotropic), and mean diffusivity (MD), the average diffusion in all directions. Typically, reduced FA and increased MD indicate compromised white matter integrity (Chanraud et al, 2010; Madden et al, 2012a) and are strongly associated with neurocognitive deficits (Bosch et al, 2012; Madden et al, 2012b; Schouten et al, 2011).
Previous DTI studies have found fairly broad effects of HIV throughout the brain. The majority reported significantly reduced FA and/or elevated MD among HIV-infected compared to HIV-uninfected persons in the corpus callosum (Chang et al, 2008; Chen et al, 2009; Du et al, 2012; Hoare et al, 2011; Müller-Oehring et al, 2010; Pfefferbaum et al, 2009; Thurnher et al, 2005; Wang et al, 2016; Wright et al, 2015; Wu et al, 2006; Zhu et al, 2013). This is likely symptomatic of a general decline in white matter integrity, as FA reduction and/or MD elevation among HIV-infected persons have also been reported in several other regions, including the cingulate cortex, frontal, parietal, temporal, and occipital white matter (Chang et al, 2008; Chen et al, 2009; Pomara et al, 2001; Zhu et al, 2013). However, the role of common co-morbidities in exacerbating these effects has not been adequately examined.
An important comorbidity is the abuse of stimulant drugs such as cocaine, which is disproportionately prevalent among HIV-infected persons in high-income countries (Bing et al, 2001; Garin Escriva et al, 2014; Mimiaga et al, 2013; Pence et al, 2008; Siconolfi et al, 2013). In a recent study of >3,000 patients receiving HIV care in four American cities, 8.5% reported crack-cocaine use in the past 3 months (Mimiaga et al, 2013). Both in vitro and in vivo studies have shown that cocaine has numerous neurotoxic effects. Cocaine can disrupt the blood-brain barrier, increase HIV replication in monocytic cells, macrophages, and astrocytes, and upregulate dendritic proteins implicated in the facilitation of HIV infection, and it can potentiate the effects of HIV-associated proteins through increased glial activation, oxidative stress, and neurotoxicity (Gandhi et al, 2010; Gaskill et al, 2009; Nath, 2010; Purohit et al, 2011). A recent meta-analysis concluded that cocaine users perform worse than controls on a range of neuropsychological tests (Spronk et al, 2013). Moreover, prior studies have demonstrated that chronic cocaine use is associated with greater neurocognitive impairment in HIV-infected persons (Meade et al, 2011; Meade et al, 2015; Nath et al, 2001). DTI studies provide preliminary evidence that chronic cocaine use is associated with reduced FA, particularly in the corpus callosum and frontal and parietal lobes, but cocaine does not appear to affect MD (Kelly et al, 2011; Lane et al, 2010; Lim et al, 2002; Lim et al, 2008; Ma et al, 2009; Ma et al, 2015; Moeller et al, 2005; Moeller et al, 2007; Romero et al, 2010). A recent study compared HIV-infected stimulant users to healthy controls and found white matter deficits in four fronto-temporal white matter tracts (Tang et al, 2015). However, without comparison groups, it is not possible to determine the unique effects of stimulant abuse versus HIV infection. While it is well established that cocaine affects HIV neuropathology, it is not known if cocaine accelerates damage to white matter in the context of HIV infection.
The current study used DTI to elucidate the independent and interactive effects of HIV and cocaine on white matter in the brain, and to examine the association between white matter integrity and neurocognitive impairment. Specifically, we compared DTI metrics (FA and MD) across four groups of adults stratified by HIV and cocaine status. Based on existing literature, we hypothesized that HIV would have broad independent effects on white matter integrity, and that co-occurring HIV and cocaine would increase the brain’s vulnerability to degeneration, particularly in FA in the corpus callosum. We expected that impaired white matter integrity – as potentially driven both by HIV status and by cocaine use – would be associated with greater neurocognitive impairment.
Methods
Participants
Data was collected from 135 individuals as part of two neuroimaging studies examining the effects of HIV infection and cocaine dependence. There were 4 groups: HIV-positive cocaine users (HIV+/COC+), HIV-positive non-cocaine users (HIV+/COC−), HIV-negative cocaine users (HIV−/COC+), and HIV-negative non-cocaine users (HIV−/COC−). All HIV+ participants were engaged in HIV care for >3 months. For HIV− individuals, an OraQuick© rapid HIV test confirmed HIV status. The COC+ groups met the following criteria: ≥4 days of past-month cocaine use or cocaine-positive urinalysis, ≥1 year of regular cocaine use, and lifetime cocaine dependence. Current alcohol and marijuana dependence were permitted if cocaine dependence was the principal diagnosis. The COC− groups met the following criteria: no lifetime cocaine use disorder, no history of regular cocaine use, 0 days of cocaine use in the past year, and cocaine-negative urinalysis. Past alcohol and marijuana dependence in full sustained remission were permitted. In all groups, alcohol, marijuana, and nicotine use were permitted. For all other drugs, individuals were excluded for lifetime dependence, history of regular use, and/or a positive drug screen (except for prescribed medications). Additional exclusion criteria were: English non-fluency or illiteracy; <8th grade education; severe learning disability with functional impairment; serious neurological disorders; acute opportunistic brain infections or a history of such infections without return to normal cognition; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; indicators of severe mental illness; and impaired mental status. These exclusions are consistent with current guidelines for classifying contributing or confounding conditions to HIV-associated neurocognitive disorders (Antinori et al, 2007). In addition, participants could have no MRI contraindications.
Procedures
Participants were recruited from the Raleigh-Durham area between October 2011 and May 2015 via advertisements in local newspapers and websites, flyers and brochures at community-based organizations and infectious diseases clinics, and participant referrals. After a telephone screen, interested individuals completed a comprehensive in-person screening that included assessment of psychiatric, substance abuse, and medical histories and urine drug and pregnancy testing, as described previously (Meade et al, 2015). Participants also provided a release of information for research staff to obtain their medical record and abstract HIV clinical variables, including years since HIV diagnosis, most recent and nadir CD4 cell count, most recent HIV viral load, and history of an AIDS diagnosis. Eligible participants returned to complete a neurobehavioral assessment and an MRI brain scan. Study procedures were approved by the institutional review boards at Duke University Health System and University of North Carolina at Chapel Hill.
Neurobehavioral assessment
Neurocognitive functioning was assessed across seven domains: processing speed (Trail Making Test Part A (Reitan and Wolfson, 1993)), verbal learning (Hopkins Verbal Learning Test – Revised, HVLT-R, immediate trials (Brandt and Benedict, 2001)), verbal memory (HVLT-R delayed trial (Brandt and Benedict, 2001)), executive functioning (Stroop Color and Word Test interference score (Golden, 1978); Trail Making Test Part B (Reitan and Wolfson, 1993)), verbal fluency (FAS letter fluency and category fluency (Benton et al, 1983)), working memory (Paced Auditory Serial Addition Task- 50 or 100 (Diehr et al, 2003); NAB Digits Forward/Digits Backward Test (Stern and White, 2009)), and motor functioning (Grooved Pegboard Test dominant and non-dominant (Klove, 1963)). Raw scores were converted to standardized T-scores using up-to-date published norms. Domain scores were computed by taking the mean T-scores of the tests comprising each respective domain.
MRI data acquisition
All scans were performed using a 3T GE scanner with an 8-channel head coil. DTI images were acquired in the axial plane using a single shot spin-echo diffusion sensitized EPI sequence (30 directions, 2mm3, flip angle 90°, FOV= 25.6 cm, matrix= 128×128, interleaved slices of 2.0 mm thickness). Additional parameters differed slightly between protocol 1 (b-value= 0 and 900 s/mm2, TR/TE= 10,000/79.4ms, 73 slices) and protocol 2 (b-value= 0 and 800 s/mm2, TR/TE= 8,000/81.4ms, 67 slices). All groups were represented in protocol 1 (n= 68; 13 HIV+/COC+, 19 HIV+/COC−, 18 HIV−/COC+, and 18 HIV−/COC−) and protocol 2 (n= 67; 13 HIV+/COC+, 18 HIV+/COC−, 19 HIV−/COC+, and 17 HIV−/COC− ), with no significant difference across protocols on the proportion in each study [χ2(3)=.075, p=.995]. Protocol was controlled for in all group comparisons.
DTI data processing and analysis
After an initial visual check of each diffusion weighted image, one participant was excluded due to incomplete spatial coverage. Each participant’s diffusion weighted image was run through the DTIPrep pipeline, which includes image dimension checking, slice-wise checking, interlace-wise checking, baseline averaging, gradient-wise checking, head motion correction, and eddy current correction (Oguz et al, 2014). Images from three participants were excluded because of motion-related artifacts. An additional visual check confirmed uniform distribution of the remaining gradient directions.
All data were processed using FMRIB Software Library (FSL) (Smith et al, 2004). First, text files containing gradient directions and b-values were extracted for each corrected image. Then, a binary brain mask was created for the data using the Brain Extraction Tool (BET) with a threshold of 0.3 (Smith, 2002). Finally, the corrected diffusion weighted image, BET binary brain mask, gradient directions, and b-values were input into DTIFIT Reconstruct Diffusion Tensors, creating a separate image for each diffusion tensor for each participant.
Voxelwise statistical analysis of FA and MD data was carried out using TBSS in FSL (Smith et al, 2006). To remove likely outliers from the diffusion tensor fitting, FA images were slightly eroded and end slices zeroed. Nonlinear registration was run on all participants’ FA data, aligning them to a 1mm3 standard space (FMRIB58_FA standard-space target image). The target image and all participants’ FA images were transformed into MNI152 standard-space. A mean FA skeleton was produced, and each participant’s aligned FA data was projected onto this skeleton. Next, the FA nonlinear registration was applied to each participants’ MD data, which was then merged into a 4D file and projected onto the original mean FA skeleton, resulting in 4D projected MD data. FSL’s GLM was used to create a two-factor design modelling the main effects of HIV and cocaine with age, gender, education, and protocol added as covariates. This design was then tested using FMRIB Randomise, a statistical approach that corrects for multiple comparisons using permutation testing (Nichols and Holmes, 2002). We used 10,000 permutations with threshold-free cluster enhancement to estimate the null distribution.
Whole-brain values were extracted in MNI152 standard space using the mean FA mask. To quantify more specific effects of HIV and cocaine, mean FA and MD values for each region were obtained using region-of-interest (ROI) masks created in FSLView using the MNI structural atlas (9 anatomical structural regions) and the JHU ICBM-DTI-81 white matter labels atlas (3 sections of the corpus callosum) (Grabner et al, 2006; Mori et al, 2008). Each mask was binarized and then multiplied by the mean FA skeleton mask to create skeletonized ROI masks for each region. These masks were then multiplied by each participant’s mean FA or MD data in MNI152 standard-space, producing mean FA and MD values within each ROI. For ease of interpretation, left and right masks were combined for bilaterally defined regions.
Statistical Analyses
Descriptive statistics were used to characterize the sample. Group differences on demographic and HIV characteristics were examined using one-way analyses of variance (ANOVA), chi-square, and independent samples t-tests. Group differences on whole-brain and ROI measures of FA and MD were examined using 2 (HIV+/HIV−) × 2 (COC+/COC−) between-subjects general linear model analyses. Age (in years), gender (male/female), education (in years), and DTI acquisition protocol were covaried in all group comparisons. To examine the impact of clinical measures within HIV+ participants, whole-brain FA and MD were correlated with current and nadir CD4 cell count and duration of HIV (years since HIV diagnosis) using Pearson correlations, and were compared between participants with and without an AIDS diagnosis and current unsuppressed HIV viral load (≥50 copies/mL) using t-tests. Nadir CD4 cell counts were square-root transformed to improve normality. Finally, domain T-scores were correlated with whole-brain FA and MD in the full sample. All of these analyses were conducted using SPSS 22.0.
Results
Sample characteristics
The final sample included 135 adults. Table 1 summarizes the sample characteristics across the study groups. Overall, the majority of participants identified as male (67%) and African-American (81%). They ranged in age from 22 to 55 years (M= 43.52, SD= 8.40), and most (90%) had at least a high school education. There were significant group differences in age and education, with COC+ participants being older [t(133)= 3.03, p= .003] and having fewer years of education [t(133)= 3.44, p= .001] than COC− participants, but there were no other demographic differences between groups.
Table 1.
Sample characteristics of the four study groups (N=135)
| HIV-negative | HIV-positive | Statistic | |||
|---|---|---|---|---|---|
| Coc− (N=35) | Coc+ (N=37) | Coc− (N=37) | Coc+ (N=26) | ||
| General characteristics | |||||
| Male-identified, % a | 62.9% | 64.9%a | 73.0% | 65.4% | χ2(3)=.964 |
| Age in years, M (SD) | 41.29 (9.43) | 45.54 (6.19) | 41.76 (8.53) | 46.15 (8.50) | F(3,134)=3.074* |
| Education in years, M (SD) | 13.97 (2.42) | 12.57 (2.53) | 13.97 (2.08) | 12.42 (2.98) | F(3,134)=3.914* |
| Race, % | χ2(6)=11.443 | ||||
| African American | 68.6% | 81.1% | 78.4% | 100.0% | |
| Caucasian | 20.0% | 13.5% | 18.9% | 0.0% | |
| Other/Mixed | 11.4% | 5.4% | 2.7% | 0.0% | |
| Hispanic ethnicity, % | 2.9% | 5.4% | 2.7% | 0.0% | χ2(3)=1.572 |
| Neurocognitive impairment, % | 54.3% | 64.9% | 75.7% | 65.4% | χ2(3)=3.629 |
| HIV characteristics | |||||
| Years since HIV diagnosis, M (SD) | - | - | 9.65 (7.56) | 14.12 (8.96) | t(61)=2.138* |
| Nadir CD4 cell count, Mdn (Q1, Q3) b | - | - | 220 (112, 445) | 92 (24, 244) | U=305* |
| Current CD4 cell count, Mdn (Q1, Q3) b | - | - | 598 (336, 790) | 414 (233, 772) | U=406 |
| Suppressed HIV viral load (<50 copies/mL), % | - | - | 75.7% | 73.1% | χ2(1)=0.054 |
| AIDS diagnosis, % | - | - | 35.1% | 61.5% | χ2(1)=4.285* |
| Cocaine characteristics | |||||
| Days of cocaine use in past 30, M (SD) | - | 10.03 (7.03) | - | 10.65 (9.11) | t(61)=0.308 |
| Years of cocaine use, M (SD) | - | 17.84 (7.86) | - | 16.27 (7.80) | t(61)=0.783 |
| Current cocaine dependence diagnosis, % | - | 94.6% | - | 92.3% | χ2(1)=0.134 |
p < .05
p < .01
p < .001
One male-to-female (MTF) trans woman
Data unavailable for one HIV+/COC− participant
Cocaine users reported using cocaine for an average of 17.19 years (SD= 7.81), and they had used on an average of 10.29 days (SD = 7.89) out of the past 30. All HIV+ participants were receiving HIV care and were on antiretroviral medications. Compared to COC− participants, COC+ participants had been diagnosed with HIV for longer, were more likely to have an AIDS diagnosis, and had a lower nadir CD4 cell count. Among cocaine users, there were no group differences in cocaine use characteristics between HIV+ and HIV− participants.
Group comparisons on DTI metrics
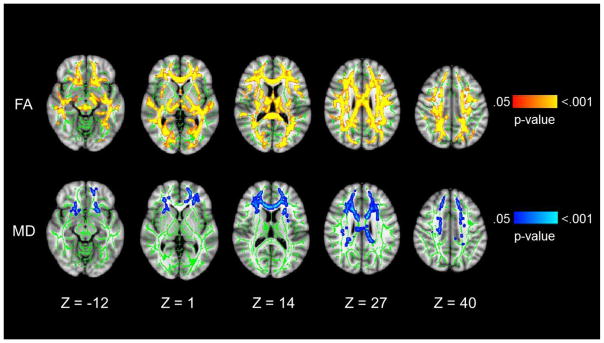
Compared to HIV− participants, HIV+ participants had widespread white matter abnormalities, with significantly reduced FA and elevated MD in the whole-brain analyses that controlled for multiple comparisons. Figure 1 illustrates the tracts in which differences were observed. Table 2 shows the FA and MD values for each of the 4 groups and presents the results of the ANCOVAs. For FA, the main effects of HIV were widespread, with significant group differences in all segments of the corpus callosum and all anatomical regions except the striatum (caudate and putamen). For MD, the HIV effects were restricted to the body and genu of the corpus callosum and the thalamus. While there were no significant main effects of cocaine or a HIV-by-cocaine interaction effect, the HIV+/COC+ group had the lowest FA and highest MD values of the study groups.
Fig. 1. Main effects of HIV on FA and MD.
Radiological presentation of regions with significantly lower FA (top row) and higher MD (bottom row) in HIV-positive compared to. HIV-negative participants (p<.05). Green represents the mean FA skeleton, red-yellow indicates regions with significantly lower FA, and blue-light blue indicates regions with significantly higher MD. P-values associated with each color range are shown to the right. Cocaine status, age, education, gender, and scanning protocol were covaried
Table 2.
Fractional anisotropy (FA) and mean diffusivity (MD) values across the study groups in the whole brain and by region (N=135)
| HIV-negative | HIV-positive | ANOVA F-values | |||||
|---|---|---|---|---|---|---|---|
| Coc−(N=35) | Coc+(N=37) | Coc−(N=37) | Coc+(N=26) | Main effect HIV | Main effect Cocaine | Interaction effect | |
| FA values | |||||||
| Whole brain | 0.482(0.003) | 0.484(0.003) | 0.475(0.003) | 0.473(0.003) | 8.670** | 0.007 | 0.349 |
| Body of corpus callosum | 0.736(0.005) | 0.741(0.005) | 0.726(0.005) | 0.729(0.006) | 4.798* | 0.481 | 0.051 |
| Genu of corpus callosum | 0.757(0.005) | 0.766(0.005) | 0.747(0.005) | 0.750(0.006) | 6.539* | 1.269 | 0.425 |
| Splenium of corpus callosum | 0.848(0.004) | 0.851(0.004) | 0.842(0.004) | 0.838(0.004) | 6.339* | 0.069 | 0.712 |
| Caudate | 0.505(0.004) | 0.504(0.003) | 0.499(0.003) | 0.498(0.004) | 2.505 | 0.048 | 0.010 |
| Cerebellum | 0.417(0.002) | 0.417(0.002) | 0.412(0.002) | 0.411(0.003) | 4.938* | 0.081 | 0.003 |
| Frontal lobe | 0.459(0.003) | 0.460(0.003) | 0.451(0.003) | 0.451(0.004) | 7.081** | 0.055 | 0.036 |
| Insula | 0.423(0.003) | 0.424(0.003) | 0.415(0.003) | 0.414(0.004) | 8.094** | 0.043 | 0.139 |
| Occipital | 0.424(0.003) | 0.426(0.003) | 0.418(0.003) | 0.412(0.004) | 9.261** | 0.216 | 1.620 |
| Parietal | 0.485(0.003) | 0.489(0.003) | 0.481(0.003) | 0.478(0.004) | 5.413* | 0.046 | 0.958 |
| Putamen | 0.492(0.003) | 0.491(0.003) | 0.487(0.003) | 0.486(0.004) | 2.358 | 0.037 | 0.000 |
| Temporal | 0.421(0.003) | 0.423(0.003) | 0.415(0.003) | 0.410(0.003) | 8.955** | 0.134 | 1.565 |
| Thalamus | 0.490(0.003) | 0.492(0.003) | 0.483(0.003) | 0.484(0.003) | 6.493* | 0.190 | 0.027 |
| MD values (10−3 mm2/s) | |||||||
| Whole brain | 0.793(0.004) | 0.792(0.004) | 0.799(0.004) | 0.805(0.005) | 4.621* | 0.248 | 0.492 |
| Body of corpus callosum | 0.849(0.006) | 0.844(0.006) | 0.863(0.006) | 0.861(0.007) | 6.443* | 0.335 | 0.046 |
| Genu of corpus callosum | 0.824(0.006) | 0.818(0.006) | 0.840(0.006) | 0.833(0.007) | 5.850* | 0.955 | 0.000 |
| Splenium of corpus callosum | 0.726(0.006) | 0.729(0.005) | 0.737(0.005) | 0.732(0.006) | 1.404 | 0.000 | 0.507 |
| Caudate | 0.807(0.005) | 0.810(0.005) | 0.814(0.005) | 0.816(0.006) | 1.365 | 0.170 | 0.000 |
| Cerebellum | 0.703(0.005) | 0.709(0.004) | 0.712(0.004) | 0.712(0.005) | 1.906 | 0.315 | 0.403 |
| Frontal lobe | 0.800(0.005) | 0.798(0.005) | 0.804(0.005) | 0.809(0.006) | 2.628 | 0.120 | 0.570 |
| Insula | 0.824(0.005) | 0.822(0.005) | 0.832(0.005) | 0.832(0.006) | 3.574 | 0.060 | 0.042 |
| Occipital | 0.762(0.005) | 0.760(0.005) | 0.761(0.005) | 0.778(0.005) | 3.251 | 2.027 | 3.638 |
| Parietal | 0.772(0.005) | 0.771(0.005) | 0.774(0.005) | 0.782(0.005) | 2.027 | 0.354 | 0.958 |
| Putamen | 0.796(0.005) | 0.799(0.005) | 0.804(0.005) | 0.803(0.006) | 1.605 | 0.000 | 0.120 |
| Temporal | 0.819(0.005) | 0.816(0.005) | 0.825(0.005) | 0.828(0.006) | 3.112 | 0.000 | 0.456 |
| Thalamus | 0.839(0.008) | 0.841(0.008) | 0.858(0.008) | 0.862(0.009) | 6.692* | 0.151 | 0.036 |
p < .05
p < .01
All models controlled for age, gender, education, and protocol. Estimated marginal means and standard error values are presented.
Since the HIV+/COC+ group had greater systemic HIV disease, we conducted a post-hoc ANCOVA among the HIV+ participants. After controlling for nadir CD4 cell count (in addition to other covariates), there was still no significant effect of cocaine on whole-brain FA or MD.
Associations between DTI metrics and clinical measures
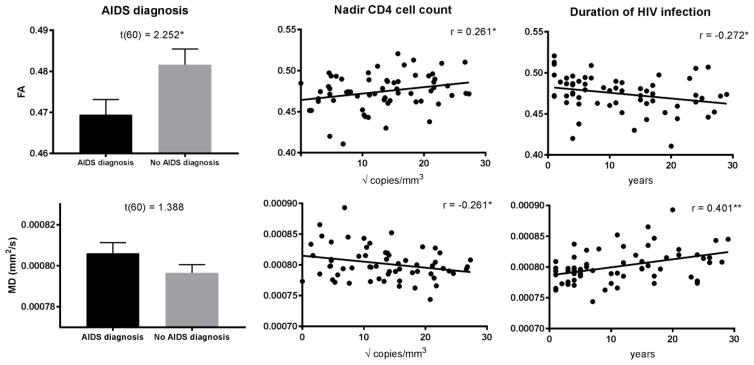
As shown in Figure 2, whole-brain FA was negatively correlated with years since HIV diagnosis and nadir CD4 cell count, and it was significantly lower among those with an AIDS diagnosis. MD was positively correlated with years since HIV diagnosis and negatively correlated with nadir CD4 cell count. There was no relationship between DTI metrics and current viral load or CD4 cell count. For neurocognitive functioning, whole-brain FA was positively correlated with executive functioning (r= .202, p= .019), and MD was negatively correlated with motor functioning (r= −.200, p=.020). However, neither of these correlations remained significant after correcting for multiple comparisons to account for the number of cognitive domains examined.
Fig. 2. Associations between DTI metrics and HIV characteristics.
Comparison of whole brain FA and MD (mean ± standard error) among HIV+ participants with and without an AIDS diagnosis, and correlations between whole-brain FA/MD and nadir CD4 cell count and duration of HIV infection. Note: nadir CD4 cell counts were square-root transformed to improve normality
Discussion
This is the first DTI study to examine the role of cocaine dependence on white matter integrity in the context of HIV infection. Our results contribute to a growing body of research demonstrating that HIV is associated with compromised white matter integrity throughout the brain (Bing et al, 2001; Chang et al, 2008; Chen et al, 2009; Du et al, 2012; Hoare et al, 2011; Müller-Oehring et al, 2010; Pfefferbaum et al, 2009; Pomara et al, 2001; Thurnher et al, 2005; Wright et al, 2015; Wu et al, 2006; Zhu et al, 2013). Specifically, HIV was associated with widespread decreases in FA, but more regionally specific increases in MD within the corpus callosum and thalamus. In contrast, we found no support for our hypothesis that cocaine dependence exacerbates the effects of HIV on white matter integrity. Lastly, while DTI metrics were associated with neurocognitive functioning in the expected direction, these correlations were small.
Among HIV+ participants, we found that a lower nadir CD4 count and longer duration of HIV infection were associated with compromised white matter integrity. Multiple studies have now reported that nadir CD4 cell count and an AIDS diagnosis are strong predictors of HIV-associated neurocognitive impairment (Ellis et al, 2011; Heaton et al, 2010; Munoz-Moreno et al, 2008; Pfefferbaum et al, 2009; Valcour et al, 2006), and advanced HIV disease progression has also been correlated with white matter degradation in some studies (Cohen et al, 2010; Hua et al, 2013; Jernigan et al, 2011; Wright et al, 2015; Zhu et al, 2013). In contrast, current markers of HIV systemic disease were not related to white matter integrity, suggesting that a history of severe immune suppression may underlie white matter compromise rather than current disease status. The data suggest that HIV may disrupt the spatial structure (axonal orientation) of the white matter tracts throughout the brain, but HIV-associated increases in diffusivity are more localized. While the precise biological mechanism underlying these differences is unknown, they may be explained by increased blood-brain barrier permeability, neuro-inflammatory cascades, and subsequent neural damage in HIV (Gray et al, 1996). Together, these findings support the conclusion that advanced immunosuppression in HIV can lead to irreversible neural injury.
Our primary finding is that the observed associations between white matter integrity and HIV infection were comparable among individuals with and without cocaine dependence. Given evidence that cocaine users have reduced FA in parts of the corpus callosum and frontal and parietal regions (Lane et al, 2010; Lim et al, 2002; Lim et al, 2008; Ma et al, 2009; Ma et al, 2015; Moeller et al, 2005; Moeller et al, 2007; Romero et al, 2010), we expected that HIV-positive persons would be vulnerable to additional white matter alterations in the face of chronic cocaine exposure. Prior studies have yielded inconsistencies in terms of which regions are affected by cocaine, possibly due to small sample sizes and varied analytic strategies (Lane et al, 2010; Lim et al, 2002; Lim et al, 2008; Ma et al, 2009; Ma et al, 2015; Moeller et al, 2005; Moeller et al, 2007; Romero et al, 2010), and at least one study has reported no effects (Kelly et al, 2011). Since our study was powered to detect medium effects, it is possible that we missed cocaine effects if they were small. However, with 63 cocaine users and 72 non-cocaine users, this is among the largest DTI studies to examine the effects of cocaine. Furthermore, we defined our groups to isolate the effects of cocaine: all of the cocaine users met diagnostic criteria for cocaine use disorder with cocaine as their primary drug of abuse, most had used cocaine regularly for many years, and – with the exception of alcohol, marijuana, and nicotine – they could not be using other substances. Several of the prior studies that found cocaine effects on white matter integrity permitted other drug use, including opiate and sedative use disorders, in their cocaine-using groups (Lane et al, 2010; Lim et al, 2002; Lim et al, 2008; Ma et al, 2009; Moeller et al, 2005; Moeller et al, 2007). A recent study found that FA reduction correlated negatively with number of substances used (Kaag et al, 2016). Thus, it is possible that prior findings reflected the broader effects of poly-substance use on white matter, rather than the specific effects of cocaine. Finally, our analyses controlled for sociodemographic factors like education, which tend to be lower in chronic cocaine users. These factors have not been consistently controlled for in prior analyses, despite having important effects on brain structure (Gianaros et al, 2013; Leonard et al, 2015; Noble et al, 2012; Noble et al, 2013).
The present study has several important strengths, including a robust sample size, a case-controlled design, and fairly well-matched comparison groups. However, there are also several limitations that warrant discussion. First, despite our attempts to match the groups, the HIV+ participants were older and had fewer years of education. To address this potential confound, both factors were controlled for in all analyses. In addition, cocaine users had been diagnosed with HIV for longer, were more likely to have an AIDS diagnosis, and had a lower nadir CD4 cell count, but this is expected to have contributed to cocaine effects, which we did not observe. More importantly, results did not change when we controlled for these HIV clinical variables in a post-hoc analysis among the HIV+ group. Lastly, the cross-sectional design prevents us from making inferences about causation. Future studies should examine these effects longitudinally.
Conclusion
In sum, our results suggest that cocaine dependence is not associated with greater white matter degradation among HIV-infected persons. While we did not detect a deleterious effect of cocaine on white matter, cocaine has been associated with a host of adverse health outcomes in HIV-infected persons, including faster HIV disease progression and higher AIDS-related mortality (Baum et al, 2009; Cook et al, 2008; Rafie et al, 2010). Indeed, the cocaine users in this sample had evidence of greater systemic HIV disease, including a lower nadir CD4 count, which was independently associated with compromised white matter integrity. Thus, cocaine users are at high risk for HIV-associated alterations in white matter integrity, independent of the impact of substance abuse on neurocognitive functioning. This study underscores the important of linking and retaining drug users in HIV care to improve overall health, including the prevention of neurological disease.
Acknowledgments
This study was funded by grants K23-DA028660, R21-DA036450, T32-AI007392, R01-AG039684, and R03-033828-02S1 from the United States National Institutes of Health. We are grateful to the UNC Center for AIDS Research (P30-AI50410) for its assistance with patient recruitment. The NIH had no further role in study design, data collection, analysis and interpretation of data, writing the report, or in the decision to submit the paper for publication. We thank all the individuals who participated in this study.
CSM designed the original project and secured grant funding; DMC, CSM, SLT, SAH, and NC conceptualized the current study; DMC, CSM, and SLT conducted the analyses, with guidance from DJM, NC, and SAH; DMC and CSM drafted the manuscript and SLT wrote additional sections; and all authors contributed to and have approved the final manuscript.
There are no conflicts of interest in this study.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. 3. AJA Associates; Iowa City, IA: 1983. [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junqué C, Solé-Padullés C, Peña-Gómez C, Bargalló N, Molinuevo JL, Bartrés-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test -- Revised Professional Manual. Psychological Assessment Resources, Inc; Lutz, FL: 2001. [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. Journal of Neuroimmune Pharmacology. 2008;3:265–74. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan E, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. NeuroImage. 2009;47:1154–62. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, Grant I, Heaton RK. Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol. 2008;30:613–26. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK. The 50 and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT): demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. J Clin Exp Neuropsychol. 2003;25:571–85. doi: 10.1076/jcen.25.4.571.13876. [DOI] [PubMed] [Google Scholar]
- Du H, Wu Y, Ochs R, Edelman RR, Epstein LG, McArthur J, Ragin AB. A comparative evaluation of quantitative neuroimaging measurements of brain status in HIV infection. Psychiatry Research: Neuroimaging. 2012;203:95–99. doi: 10.1016/j.pscychresns.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. Nadir CD4 is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N, Saiyed Z, Napuri J, Samikkannu T, Reddy PB, Agudelo M, Khatavkar P, Saxena S, Nair MN. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Garin Escriva N, Velasco Muñoz C, Thomas De Pourcq J, Lopez Garcia B, Maria Haro Abad J, Antonia Mangues Bafalluy M, Trilla Garcia A. Recreational drugs and HIV in Europe: current use of recreational drugs and principal HIV guidelines related recommendations. J Int AIDS Soc. 2014;17:19831. doi: 10.7448/IAS.17.4.19831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human Immunodeficiency Virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. The American Journal of Pathology. 2009;175:1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013;23:2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Stoelting; Chicago, IL: 1978. [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric Atlasing and Model Based Segmentation: An Application to the Hippocampus in Older Adults. In: Larsen R, Nielsen M, Sporring J, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2006. Springer; Berlin Heidelberg: 2006. pp. 58–66. [DOI] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Belli J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–12. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Fouche JP, Spottiswoode B, Sorsdahl K, Combrinck M, Stein DJ, Paul RH, Joska JA. White-matter damage in Clade C HIV-positive subjects: a diffusion tensor imaging study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:308–15. doi: 10.1176/jnp.23.3.jnp308. [DOI] [PubMed] [Google Scholar]
- Hua X, Boyle CP, Harezlak J, Tate DF, Yiannoutsos CT, Cohen R, Schifitto G, Gongvatana A, Zhong J, Zhu T, Taylor MJ, Campbell TB, Daar ES, Alger JR, Singer E, Buchthal S, Toga AW, Navia B, Thompson PM. Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatmentpatients on stable treatment. NeuroImage: Clinical. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE. Diffusion MRI: from quantitative measurement to in vivo neuroanatomy. 2. Elsevier; San Diego, CA: 2013. [Google Scholar]
- Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. 2016 doi: 10.1111/adb.12375. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Grooved Pegboard. Lafayette Instruments; Lafayette, IN: 1963. [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS ONE. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Johansen-Berg H. Diffusion MRI at 25: exploring brain tissue structure and function. NeuroImage. 2012;61:324–341. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JA, Mackey AP, Finn AS, Gabrieli JDE. Differential effects of socioeconomic status on working and procedural memory systems. Frontiers in Human Neuroscience. 2015:9. doi: 10.3389/fnhum.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. Journal of Neuroimmune Pharmacology. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Keyser-Marcus L, Ramesh D, Narayana PA, Merchant RE, Moeller FG, Cifu DX. Altered white matter in cocaine-dependent subjects with traumatic brain injury: a diffusion tensor imaging study. Drug Alcohol Depend. 2015;151:128–134. doi: 10.1016/j.drugalcdep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N-k, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012a;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012b;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med. 2011;34:128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Towe SL, Skalski LM, Robertson KR. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015;149:128–135. doi: 10.1016/j.drugalcdep.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM, Schumacher JE, Mathews WC, Mayer KH. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health. 2013;103:1457–67. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Esposito G, Turner SR, Taraballi F, Tasciotti E, Paige M, Avdoshina V. Human immunodeficiency virus-associated dementia: a link between accumulation of viral proteins and neuronal degeneration. Current trends in neurology. 2014;8:71–85. [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Research: Neuroimaging. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Callosal degradation in HIV-1 infection predicts hierarchical perception: a DTI study. Neuropsychologia. 2010;48:1133–1143. doi: 10.1016/j.neuropsychologia.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, Perez-Alvarez N, Molto J, Gomez G, Clotet B. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–7. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Annals NY Academy of Sciences. 2010;1187:122–8. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K, Grieve S, Korgaonkar M, Engelhardt L, Griffith E, Williams L, Brickman A. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012:6. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci. 2013;16:653–664. doi: 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Johnson HJ, Styner M. DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics. 2014;8:4. doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Thielman NM, Whetten K, Ostermann J, Kumar V, Mugavero MJ. Coping strategies and patterns of alcohol and drug use among HIV-infected patients in the United States Southeast. Aids Patient Care STDS. 2008;22:869–877. doi: 10.1089/apc.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–85. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–10. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Rafie C, Campa A, Smith S, Huffman F, Newman F, Baum MK. Cocaine reduces thymic endocrine function: another mechanism for fccelerated HIV disease progression. AIDS Res Hum Retroviruses. 2010 doi: 10.1089/aid.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2. Neuropsycholgy Press; Tucson, AZ: 1993. [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Research: Neuroimaging. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25:561–75. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- Siconolfi DE, Halkitis PN, Barton SC, Kingdon MJ, Perez-Figueroa RE, Arias-Martinez V, Karpiak S, Brennan-Ing M. Psychosocial and demographic correlates of drug use in a sample of HIV-positive adults ages 50 and older. Prev Sci. 2013;14:618–627. doi: 10.1007/s11121-012-0338-6. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Supplement 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Spudich S. HIV and neurocognitive dysfunction. Curr HIV AIDS Rep. 2013;10:235–243. doi: 10.1007/s11904-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, White T. NAB Digits Forward/Digits Backward Test: Professional Manual. Psychological Assessment Resources, Inc. (PAR); Lutz, FL: 2009. [Google Scholar]
- Tang VM, Lang DJ, Giesbrecht CJ, Panenka WJ, Willi T, Procyshyn RM, Vila-Rodriguez F, Jenkins W, Lecomte T, Boyda HN, Aleksic A, MacEwan GW, Honer WG, Barr AM. White matter deficits assessed by diffusion tensor imaging and cognitive dysfunction in psychostimulant users with comorbid human immunodeficiency virus infection. BMC Research Notes. 2015;8:1–15. doi: 10.1186/s13104-015-1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. Am J Neuroradiol. 2005;26:2275–81. [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Paul R, Shikuma C, Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- Wang B, Liu Z, Liu J, Tang Z, Li H, Tian J. Gray and white matter alterations in early HIV-infected patients: combined voxel-based morphometry and tract-based spatial statistics. J Magn Reson Imaging. 2016;43:1474–1483. doi: 10.1002/jmri.25100. [DOI] [PubMed] [Google Scholar]
- Wright PW, Vaida FF, Fernández RJ, Rutlin J, Price RW, Lee E, Peterson J, Fuchs D, Shimony JS, Robertson KR, Walter R, Meyerhoff DJ, Spudich S, Ances BM. Cerebral white matter integrity during primary HIV infection. AIDS. 2015;29:433–442. doi: 10.1097/QAD.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. Am J Neuroradiol. 2006;27:656–60. [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, Ombao H, Navia B, Cohen R, Schifitto G. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol. 2013;19:10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]