Abstract
Introduction
Anxiety, depression and insomnia are each common and frequently co-occur in Parkinson’s disease (PD) patients. Yet, little is known about their temporal relationship. In this study, we tested two hypotheses: i) insomnia predicts an increase in symptoms of depression or anxiety and ii) anxiety or depression at baseline predicts the occurrence of insomnia in PD patients six months later.
Methods
We used longitudinal data from an ongoing prospective cohort study in early-stage, still medication-naïve PD patients. Primary outcome measures were: anxiety symptoms (measured with the State-Trait Anxiety Inventory score), depressive symptoms (measured with the 15-item Geriatric Depression Scale score) and insomnia (defined as a score ≥ 2 on item 1.7 of the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale). We performed linear and logistic regression analyses, correcting for baseline value of the respective outcome variable.
Results
We included 361 subjects. The was not an association between baseline insomnia and subsequent anxiety (B(SE)=1.75 (1.81), p=0.33) and depression (B(SE)=0.49 (0.31), p=0.11). However, baseline symptoms of both anxiety (B(SE)=0.02 (0.01), p=0.001) and depression (B(SE)=0.15 (0.05), p<0.01) independently predicted insomnia at follow-up.
Conclusion
Insomnia is not a risk factor for subsequent symptoms of affective disorders, but symptoms of anxiety and depression predict future insomnia in unmedicated, early-stage PD patients. This suggests that affective symptoms are a risk factor or prodrome for the development of insomnia in PD, and therefore a target for prevention research.
Keywords: insomnia, anxiety, depression, Parkinson’s disease, longitudinal, risk factor
INTRODUCTION
Parkinson’s disease (PD) is accompanied by many non-motor symptoms, including sleep disorders, anxiety and depression. PD patients report that non-motor symptoms have an even higher negative impact on quality of life than the motor symptoms [1, 2]. Affective disorders have a major impact on daily functioning and are associated with more severe cognitive impairment and motor symptoms [3–6]. Insomnia, including complaints of an insufficient amount of sleep due to difficulties falling asleep, sleep fragmentation or early awakening, occurs in up to 40% of PD patients [7, 8]. Clinically relevant symptoms of depression occur in 35% [9] and of anxiety in approximately 25% of PD patients [10]. These prevalence rates are substantially higher than in age-matched controls or the general population [8, 11, 12]. Co-occurrence of these symptoms in PD patients is common; in approximately 40% of cases, anxiety co-exists with depression [13].
Despite the frequent occurrence and negative impact of anxiety, depression and insomnia in PD patients, little is known about their course and interaction. In non-PD samples, the relationship between insomnia and affective disorders (i.e. anxiety and depression) has been studied extensively, leading to different hypotheses. The first hypothesis is that insomnia precedes depression and anxiety, and therefore can be viewed as a risk factor or a prodromal symptom of affective disorders [14–17]. Alternatively, affective disorders may cause a disturbance of sleep [15, 18]. In PD patients, an association between insomnia and both depression [8, 19–21] and anxiety [21] has been found in cross-sectional studies, but such investigations do not allow for determination of temporal order.
To our knowledge, no longitudinal study has examined the association between sleep disturbances and affective disorders in PD patients. A better understanding of the temporal evolution of these symptoms might provide a starting point for preventive strategies. We therefore analysed the relationship between insomnia and symptoms of depression and anxiety using data of an on-going prospective cohort study in early-stage, medication-naïve PD patients. We selected this study sample for two reasons. Firstly, clinical and research findings suggest that treatment with dopaminergic agents can affect sleep and mood in PD patients. Administration of levodopa suppresses REM sleep and lengthens REM sleep latency, while long-term treatment with dopamine agonists is associated with excessive daytime sleepiness and “sleep attacks” in PD patients [22]. Research and clinical findings also suggest that dopamine agonists may improve depressive symptoms [23]. Selection of a study sample of medication-naïve PD patients thus enabled us to assess the association between sleep and mood in PD, without the inference of treatment with antiparkinsonian agents. Secondly, research on starting points for prevention of insomnia and affective disorders should be aimed at early-stage PD patients, since the prevalence of these disorders increases with disease duration [7, 24].
In this study, we performed a bidirectional analysis of the relationship between insomnia and symptoms of depression and anxiety in PD patients, in order to test two hypotheses: i) insomnia at baseline predicts an increase in symptoms of depression and/or anxiety over time and ii) the severity of anxiety or depression at baseline predicts the subsequent occurrence of insomnia in PD patients.
METHODS
Design and study procedures
We used data from the Parkinson’s Progression Markers Initiative (PPMI) [13], a prospective cohort study that collects longitudinal data to assess disease progression in PD patients. The study received ethical approval from the institutional board at each site. In the first year of participation in PPMI, assessments take place every three months. For this sub-study, we used the data that were collected at baseline (T0) and after approximately six months of follow-up (T1), since depression and anxiety measures were not performed at the initial three-month follow-up visit.
Study population
The study population consisted of early-stage, medication-naïve PD patients. For details on the inclusion criteria, we refer to the study protocol on the PPMI website: www.ppmi-info.org. All subjects provided written informed consent.
In some subjects, PD medication needed to be initiated within six months from baseline. In this case, the subject was asked to come in for an advanced assessment instead of the regular follow-up assessment, so a final PD medication-free assessment could be performed. We excluded subjects in whom antiparkinsonian agents were initiated within three months from baseline, or between three and six months from baseline without having a medication-free assessment before T1. Finally, we excluded subjects with missing data for any of the primary outcome measures at T1.
Sample size
PPMI finished enrolment of 423 subjects in April 2013. Six-month follow-up data was collected in 402 subjects. As a rule of thumb, the number of subjects needs to be ten times the number of covariates added to a prediction model. Since we added a maximum of 8 covariates to the prediction model (see section ‘Outcome measures’ below), we expected this sample size to be sufficiently powered.
Outcome measures
The primary outcome measures of our study were: anxiety, measured as the total score of the State-Trait Anxiety Inventory (STAI) [25], depressive symptoms, measured as the total score of the 15-item Geriatric Depression Scale (GDS-15) [26], and insomnia, defined as a score ≥ 2 on item 1.7 of the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [27].
The STAI is a self-report questionnaire, which contains a ‘State’ subscale, designed to measure a temporary state of anxiety, and a ‘Trait’ subscale that measures a more persistent pattern of anxiety [25]. Clinically relevant anxiety in geriatric patients is defined as a STAI State score >54 [28]. The GDS-15 is a 15 –item self-report questionnaire regarding depressive symptoms [28]. In patients with PD, a score ≥5 is indicative of clinically relevant symptoms of depression [29–31]. Insomnia was rated on item 1.7 of the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [27]. Item 1.7 is a single question: “Over the past week, have you had trouble going to sleep at night or staying asleep through the night? Consider how rested you felt after waking up in the morning.” Although this is a one-item insomnia rating, it appears to be quite reliable: in a validation study by Gallagher et al (2012) [32], item 1.7 of the MDS-UPDRS had a strong positive correlation with the total score of the Pittsburgh Sleep Quality Index and Scales for Outcomes in Parkinson’s disease – Sleep, which are both recommended to assess sleep problems by the Task Force on Rating Scales for PD of the Movement Disorder Society [33]. In this study, we considered a MDS-UPDRS 1.7 score ≥2, indicating mild to moderate sleep problems that cause difficulties getting a full night of sleep, to be suggestive of insomnia.
Statistical analyses
All analyses were performed with IBM SPSS Statistics 22 (Armonk, NY, USA).
Demographics and clinical characteristics of the research population at baseline, and values of the outcome variables after approximately six months of follow-up, were summarized using percentages or mean scores with standard deviations. Correlations between continuous variables at baseline were calculated using Pearson’s correlation coefficients.
We used a linear regression analysis to assess whether the presence of insomnia at baseline predicted the severity of depressive symptoms after six months of follow-up. We corrected for baseline severity of depression by adding the baseline GDS-15 score to the model. Finally, we examined whether sex, age, severity of motor symptoms (measured with the MDS-UPDRS section III [27] score), global cognitive function (assessed with the Montreal Cognitive Assessment (MoCA) [34] score), and the use of antidepressants or anxiolytic/hypnotic agents were confounding variables. Significant confounding was defined as a change in the regression coefficient of 10% or more. To assess whether sleep problems at baseline predicted anxiety after six months of follow-up, this analysis was repeated with the STAI total score as the dependent variable. To evaluate the inverse temporal relationship between these variables, we performed a logistic regression analysis with the presence of insomnia (0 = insomnia not present, 1 = insomnia present) as the dependent variable, and either severity of depression or anxiety at baseline as independent variables.
Alpha was set at p <0.05. We present the crude model (Model 1), the model adjusted for baseline value of the outcome variable (Model 2). For the final linear regression models we calculated the regression coefficient (B) with standard error (SE), standardized regression coefficient (β), 95% confidence interval and p-value. For the multinomial regression analyses we will report the odds ratio (OR) with its 95% confidence interval and p-value.
Assumptions for regression analyses (normality and homoscedasticity of residuals) were checked. Multicollinearity was evaluated using the variance inflation factor (VIF). As a sensitivity analysis, we assessed whether there were significant differences in demographic and clinical characteristics at baseline between the included and excluded participants, using a t-test for continuous variables and Chi2-test for categorical variables.
RESULTS
Subjects
A flow chart of the study is presented in Figure 1. The total sample size of this sub-study consisted of 361 subjects.
Figure 1.
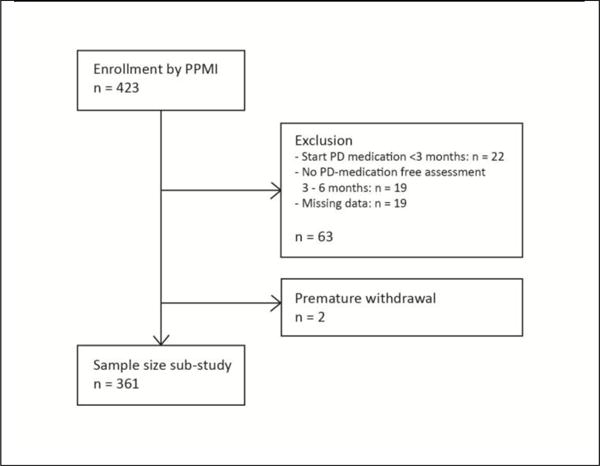
Flow chart
As a sensitivity analysis, we compared the proportion of males and females, mean age, and mean total baseline score of the STAI, GDS-15, MDS-UPDRS section III and MDS-UPDRS item 1.7 for subjects that were included and excluded. There were no significant differences these groups.
Demographic and clinical characteristics of the study population are presented in Table 1. The prevalence of insomnia at baseline was 23.1%. Clinically relevant symptoms of anxiety and depression were present in 3.6% and 14.2%, respectively.
Table 1.
Demographic and clinical characteristics of the study population at baseline (n = 361)
| % (n) | Mean (SD) | Range | |
|---|---|---|---|
|
| |||
| Female | 33.5% (121) | ||
|
| |||
| Age (yrs) | 61.8 (9.6) | 34 – 85 | |
|
| |||
| MDS-UPDRS III score | 21.0 (8.7) | 4 – 51 | |
|
| |||
| H&Y stage | |||
| Stage 1 | 42.2% (153) | ||
| Stage 2 | 57.1% (206) | ||
| Stage 3 | 0.6% (2) | ||
|
| |||
| MoCA total score | 27.1 (2.4) | 17 – 30 | |
|
| |||
| STAI total score | 64.9 (18.4) | 40 – 137 | |
| STAI State score | 32.7 (10.3) | 20 – 76 | |
| STAI Trait score | 32.2 (9.4) | 20 – 63 | |
| Clinically relevant symptoms of anxiety | 3.6% (13) | ||
|
| |||
| GDS-15 total score | 2.3 (2.5) | 0 – 14 | |
| Clinically relevant symptoms of depression | 14.2% (51) | ||
|
| |||
| Clinically relevant symptoms of insomnia | 23.1% (83) | ||
| Rating of sleep problems on MDS-UPDRS item 1.7: | |||
| Normal | 46.9% (198) | ||
| Slight | 29.6% (125) | ||
| Mild | 14.7% (62) | ||
| Moderate | 6.2% (26) | ||
| Severe | 2.6% (11) | ||
MDS-UPDRS-III= Movement Disorders Society - Unified Parkinson’s Disease Rating Scale, section III: Motor examination; H&Y = Hoehn & Yahr; MoCA = Montreal Cognitive Assessment; GDS-15 = 15-item Geriatric Depression Scale; STAI = State-Trait Anxiety Inventory
At baseline, the GDS-15 and STAI score had a moderate positive correlation (r=0.65, p<0.01). The MDS-UPDRS III score had a weak positive correlation with the STAI score (r=0.12, p<0.01) and a weak negative correlation with the MoCA score (r=−0.12, p<0.05). There were no significant correlation between the other baseline measures.
At follow-up, the prevalence of subjects with insomnia was 25.8%, with clinically relevant symptoms of anxiety and depression occurring in 4.2% and 18.6%. The mean STAI total score at follow-up was 66.0±19.7, the mean GDS-15 score 2.7±3.1. Thus, the frequency of insomnia and severity of affective symptoms increased slightly over the six-month period.
Relationship between insomnia and symptoms of depression and anxiety
Histograms demonstrated a positively skewed distribution of the residuals of the continuous outcome measures. As log transformation of the data only slightly improved normality of the distribution of the residuals of the STAI total score, we decided to use the original data for the analyses to maintain interpretability of results. Homoscedasticity was confirmed. The VIF ranged from 1.06 to 1.08, indicating non-collinearity of the data.
Results of the regression analyses are presented in Tables 2a and 2b. Baseline insomnia was not associated with symptoms of anxiety (B(SE)=1.75 (1.81), p=0.33) or depression (B(SE)=0.49 (0.31), p=0.11) at follow-up, after correcting the model for baseline severity of these affective symptoms (Table 2a). However, when looking at the reverse relationships, baseline symptoms of both anxiety (B(SE)=0.02 (0.01), p=0.001) and depression (B(SE)=0.15 (0.05), p<0.01) were significantly associated with presence of insomnia after six months of follow-up (see Table 2b).
Table 2a.
Results of the linear regression analysis with the STAI and GDS-15 total score at T1 as dependent variable, and the presence of insomnia at T0 as independent variable.
| Dependent variable → | T1 STAI total score | T1 GDS-15 total score | ||||||
|---|---|---|---|---|---|---|---|---|
| Independent variable ↓ | B (SE) | β | 95% CI | p-value | B (SE) | β | 95% CI | p-value |
|
| ||||||||
| Model 1: | ||||||||
| (Constant) | 63.67 (1.16) | – | – | – | 2.39 (0.18) | – | – | – |
| T0 Insomnia | 10.46 (2.42) | 0.22 | [5.71, 15.20] | <0.001 | 1.52 (0.38) | 0.21 | [0.78, 2.26] | <0.001 |
|
| ||||||||
| Model 2: | ||||||||
| (Constant) | 16.68 (2.70) | – | – | – | 0.90 (0.18) | – | – | – |
| T0 insomnia | 1.75 (1.81) | 0.04 | [−1.80, 5.30] | 0.33 | 0.49 (0.31) | 0.07 | [−0.12, 1.10] | 0.11 |
| T0 dependent variable | 0.76 (0.04) | 0.70 | [0.68, 0.84] | <0.001 | 0.75 (0.05) | 0.61 | [0.65, 0.85] | <0.001 |
B = regression coefficient, SE = standard error, β = standardized regression coefficient, 95% CI = 95% confidence interval
Table 2b.
Results of the logistic regression analysis with presence of insomnia at T1 as dependent variable and STAI and GDS-15 total score at T0 as independent variable.
| Dependent variable → | T1 insomnia | Dependent variable → | T1 insomnia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent variable ↓ | B (SE) | OR | 95% CI of OR | p-value | Independent variable ↓ | B (SE) | OR | 95% CI of OR | p-value |
|
| |||||||||
| Model 1: | Model 1: | ||||||||
| (Constant) | −3.34 (0.48) | – | – | (Constant) | −1.58 (0.18) | – | – | – | |
| T0 STAI total score | 0.03 (0.01) | 1.04 | [1.01, 1.05] | <0.001 | T0 GDS-15 total score | 0.21 (0.05) | 1.23 | <0.001 | |
|
| |||||||||
| Model 2: | Model 2: | ||||||||
| (Constant) | −3.40 (0.54) | – | – | – | (Constant) | −2.17 (0.22) | – | – | – |
| T0 STAI total score | 0.02 (0.01) | 1.03 | [1.01, 1.04] | 0.001 | T0 GDS-15 total score | 0.15 (0.05) | 1.16 | [1.05, 1.29] | <0.001 |
| T0 insomnia | 2.31 (0.30) | 10.10 | [5.62, 18.16] | <0.001 | T0 insomnia | 2.37 (0.30) | 10.70 | [5.99, 19.11] | <0.01 |
B = regression coefficient, SE = standard error, OR= odds ratio, 95% CI = 95% confidence interval
The use of anxiolytic/hypnotic medication at baseline was a positive confounder in the association between baseline insomnia and depression at follow-up. In the other associations, no significant confounding occurred for sex, age, severity of motor symptoms, global cognitive functioning, the use of antidepressants or the use of anxiolytic/hypnotic agents (data not shown).
DISCUSSION
We performed a bidirectional analysis on the relationship between insomnia and symptoms of depression and anxiety using data from the first six months of a prospective cohort study in early-stage, medication-naïve PD patients.
When testing our first hypothesis, we found that insomnia did not predict a change in affective symptoms over time; the association between baseline insomnia and both depression and anxiety at follow-up was no longer significant after correction for baseline severity of these affective symptoms. While longitudinal studies in PD patients on the association of insomnia and affective disorders are lacking, our findings are in line with a study in the general population, demonstrating that insomnia usually appears at the same time or after the onset of an anxiety disorder, rather than preceding it [15]. Our finding that insomnia did not predict depression contrasts with multiple studies in the general population, where insomnia was found to be a risk factor for the development of a depressive disorder [16, 18, 35, 36]. This difference may be explained by the fact that our study participants had relatively light mood disturbances; the prevalence rate of clinically relevant symptoms of depression at follow-up was 18.6%, while the prevalence is 35% in PD samples that include subjects with a more advanced disease stage [9].
When testing our second hypothesis, we found that baseline symptoms of both anxiety and depression predicted the occurrence of insomnia six months later. This is in agreement with epidemiological studies in the general population, where affective disorders are found to precede insomnia [15, 18]. Various explanatory models for this association have been proposed, including behavioral, psychological and physiological factors. Specifically, acute insomnia is generally viewed as a result of an acute stressor in an individual that is prone to sleep disturbances due to trait factors, like a tendency to ruminate or a genetically determined dysfunction in sleep-wake regulating circuitries [37, 38]. According to the behavioral model, maladaptive coping with sleep problems, like daytime napping and prolongation of time spent in bed, can contribute to the development of chronic insomnia [39]. Moreover, conditioned arousal may act as a perpetuating factor, in which the sleep environment becomes a stimulus for arousal instead of rest [39]. Conditioned arousal consists of cognitive and physiological symptoms. Cognitive or “cortical” arousal is experienced subjectively as increased cognitive activity (e.g. worrying about sleep problems and their consequences), and is objectified as increased fast frequencies on the sleep electroencephalogram (EEG) [38]. Signs of physiological arousal include differences found in neuroimaging studies, as well as deviations of neuroendocrine and neuroimmunological variables, in subjects with insomnia before and after treatment, or compared to good sleepers (for an overview, see the review by Riemann et al. 2010) [38]. Espie (2002) hypothesizes that the interaction between cognitive and physiological arousal is mediated by heightened emotionality [40], i.e. affect-laden cognitions are more likely to interfere with sleep than neutral thoughts. In a synthesis of these models, insomnia can be viewed as a result of the interaction between increased emotional, cognitive and physiological arousal, or a psychobiological state of hyperarousal [37, 38].
Previous cross-sectional studies in PD patients with insomnia do not allow us to draw conclusions on the causal relationship between affective symptoms and insomnia [8, 19–21]. However, one can imagine that having a chronic, progressive disease, which results in increasing disability over time, can cause negative cognitions and emotions, leading to psychological and physiological arousal and subsequent sleep problems. Our findings suggest that even subclinical affective symptoms (e.g. worrying or feeling tense after recently getting a diagnosis of PD), can interfere with sleep in PD patients, and contribute to the development of insomnia.
Insomnia, depression and anxiety often co-occur in PD [8, 19–21], and insomnia is a symptom of both depression and some anxiety disorders, for instance generalized anxiety disorder [41]. This may raise the question whether the associations we found can be explained by the fact that insomnia is an integral part of affective disorders. However, since we used the GDS-15 and the STAI, which do not contain items on disturbed sleep [26, 28], there is no overlap between the measures used for insomnia and affective symptoms. Moreover, we corrected for the presence of insomnia at baseline in the analyses on the association between baseline depression and insomnia at follow-up. We therefore feel fairly confident that our results indicate that affective symptoms should be viewed as an independent risk factor for the development of insomnia in PD.
To our knowledge, this is the first study to examine the longitudinal, bidirectional relationship between sleep disturbances and affective disorders in PD patients. A strength of this study is that we studied a cohort of PD patients that were not yet using antiparkinsonian medication, ruling out interference of dopaminergic treatment in the association between sleep and affective symptoms. On the other hand, the selection of a study sample of medication-naïve, early stage PD patients with relatively short follow-up also limits the generalizability of our results to the entire PD population. In PD patients, the occurrence of sleep disturbances, depression and anxiety increases with progression of the disease [7, 24]. Indeed, the prevalence rates of clinically relevant affective symptoms and insomnia in our study sample were relatively low compared to other PD samples [7–11]. The fact that the majority of our subjects had subclinical symptoms makes it difficult to draw definite clinical conclusions from the study. Replication of our findings, preferably in a sample of PD patients that has been followed for a longer time and therefore, on average, developed more severe affective symptoms and insomnia, is therefore needed. Our results do, however, indicate that clinicians should be alert to the development of insomnia in PD subjects reporting affective symptoms. Possibly, early treatment of anxiety and depression might prevent the development of insomnia in PD patients.
In conclusion, we found that symptoms of anxiety and depression predict future onset of insomnia, while our study results do not support the hypothesis that insomnia is a risk factor for mood disorders in early-stage PD patients. Our findings suggest that affective symptoms may be a risk factor for the development of insomnia in PD, which provides a target for research on preventive measures for insomnia.
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding
PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramidal, Roche, Servier, UCB and Golub Capital. For this sub-study, the researchers did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Competing interests
None
Authors’ contributions
All authors made substantial contributions the design of the study, critical revision of the manuscript for important intellectual content, and final approval of the version to be submitted. SR was responsible for analysis and interpretation of the data and drafting the article.
References
- 1.Karlsen KHTE, Årsland D, Larsen JP. Health related quality of life in Parkinson’s disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69:584–89. doi: 10.1136/jnnp.69.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsen KHLJ, Tandberg E, et al. The influence of clinical and demographic variables on quality of life in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;66:431–35. doi: 10.1136/jnnp.66.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes TARH, Mindham RH, et al. Mortality in Parkinson’s disease and its association with dementia and depression. Acta neurologica Scandinavica. 2004;110(2):118–23. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 4.Starkstein SEMH, Leiguarda R, et al. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1992;55(5):377–82. doi: 10.1136/jnnp.55.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemers ERSA, Quaid K, et al. Anxiety and motor performance in Parkinson’s disease. Mov Disord. 1993;8(4):501–06. doi: 10.1002/mds.870080415. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez AJ-JF, Garcia-Ruiz P, et al. “Panic attacks” in Parkinson’s disease. A long-term complication of levodopa therapy. Acta Neurol Scand. 1993;87(1):225–33. [PubMed] [Google Scholar]
- 7.Kumar SBM, Behari M. Sleep disorders in PD. Mov Disord. 2002;17(4):775–81. doi: 10.1002/mds.10167. [DOI] [PubMed] [Google Scholar]
- 8.Tandberg ELJ, Karlsen K. A community-based study of sleep disorders in patients with PD. Mov Disord. 1998;13(6):895–99. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 9.Reijnders JEU, Weber W, Aarsland D, Leentjens A. A systematic review of prevalence studies of depression in Parkinson’s disease. Movement Disorders. 2008;23(2):183–89. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- 10.Broen MPNN, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2016;29 doi: 10.1002/mds.26643. [DOI] [PubMed] [Google Scholar]
- 11.Richard I. Anxiety disorders in Parkinson’s disease. Adv Neurol. 2005;96:42–55. [PubMed] [Google Scholar]
- 12.Nilsson FMKL, Sørensen TM, Andersen PK, Bolwig TG. Major depressive disorder in Parkinson’s disease: a register-based study. Acta Psychiatr Scand. 2002;106:202–11. doi: 10.1034/j.1600-0447.2002.02229.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamanishi T, Tachibana H, Oguru M, et al. Anxiety and depression in patients with Parkinson’s disease. Internal medicine (Tokyo, Japan) 2013;52(5):539–45. doi: 10.2169/internalmedicine.52.8617. [DOI] [PubMed] [Google Scholar]
- 14.Roberts R, Shema S, Kaplan G, et al. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157:81–88. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon M, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of psychiatric research. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 16.Ford D, Kamerow D. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262(11):1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 17.Eaton W, Badawi M, Melton B. Prodromes and precursors: epidemiological data for primary prevention of disorders with slow onset. Am J Psychiatry. 1995;152:967–72. doi: 10.1176/ajp.152.7.967. [DOI] [PubMed] [Google Scholar]
- 18.Johnson E, Roth T, Breslau N. The association fo insomnia with anxiety disorders and depression: Exploration of the direction of risk. Journal of psychiatric research. 2006;40:700–08. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Caap-Ahlgren M, Dehlin O. Insomnia and depressive symptoms in patients with Parkinson’s disease.: relationship to health-related quality of life. An interview study of patients living at home. Archives of Gerontology and Geriatrics. 2001;32:23–33. doi: 10.1016/s0167-4943(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 20.Borek L, Kohn R, Friedmann J. Mood and sleep in Parkinson’s disease. J Clin Psychiatry. 2006;67(6):958–63. doi: 10.4088/jcp.v67n0613. [DOI] [PubMed] [Google Scholar]
- 21.Ratti P, Nègre-Pagès L, Pérez-Lloret S, et al. Subjective sleep dysfunction and insomnia symptoms in Parkinson’s disease: Insights from a cross-sectional evaluation of the French CoPark cohort. Parkinsonism & related disorders. 2015;21(11):1323–29. doi: 10.1016/j.parkreldis.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Borreguero D, Larrosa O, Bravo M. Parkinson’s disease and sleep. Sleep Med Rev. 2003;7(2):115–29. doi: 10.1053/smrv.2002.0229. [DOI] [PubMed] [Google Scholar]
- 23.Leentjens A. The role of dopamine agonists in the treatment of depression in patients with Parkinson’s disease: a systematic review. Drugs. 2011;71(3):273–86. doi: 10.2165/11585380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Aarsland D, Palhagen S, Ballard C, et al. Depression in Parkinson’s disease - epidemiology, mechanisms and management. Nat Rev Neurol. 2012;8:35–47. doi: 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 25.Spielberger C, editor. Manual for the State-Trait Anxiety Inventory STAI (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- 26.Yesavage J, Brink T, Rose T, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 28.Kvaal K, Ulstein I, Nordhus I, et al. The Spielberger State-Trait Anxiety Inventory (STAI): the state scale in detecting mental disorders in geriatric patients. Int J Ger Psychiatry. 2005;20:629–34. doi: 10.1002/gps.1330. [DOI] [PubMed] [Google Scholar]
- 29.Baillon S, Dennis M, Lo N, et al. Screening for depression in Parkinson’s disease: the performance of two screening questions. Age and aging. 2014;43(2):200–05. doi: 10.1093/ageing/aft152. [DOI] [PubMed] [Google Scholar]
- 30.Thompson A, Liu H, Hays R, et al. Diagnostic accuracy and agreement across three depression assessment measures for Parkinson’s disease. Parkinsonism & related disorders. 2011;17:40–45. doi: 10.1016/j.parkreldis.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weintraub D, Oehlberg K, Katz I, et al. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14(2):169–75. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher D, Goetz C, Stebbins G, et al. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson’s disease. Mov Disord. 2012;27(1):79–83. doi: 10.1002/mds.23939. [DOI] [PubMed] [Google Scholar]
- 33.Högl B, Arnulf I, Comella C, et al. Scales to Assess Sleep Impairment in Parkinson’s Disease: Critique and Recommendations. Mov Disord. 2010;25(16):2704–16. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 35.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43:445–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;2008:443–49. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Baglioni C, Spiegelhalder K, Lombardo C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14:227–38. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Spielman A, Caruso L, Glovinsky P. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 40.Espie C. Development, persistene and treatment of sleep disorders in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 41.Association AP. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. 4th, text revision ed. [Google Scholar]


