Abstract
Mononuclear cells play key roles in the pathogenic mechanisms leading to HIV associated neurocognitive disorders (HAND). We examined the association between HIV DNA within peripheral blood mononuclear cell (PBMC) subsets and HAND in Nigeria. PBMCs were collected at baseline from 36 antiretroviral naïve participants. CD14+ cells and T&B lymphocyte fractions were isolated by, respectively, positive and negative magnetic bead separation. Total HIV DNA within CD14+ and T&B cells were separately quantified using real-time PCR assay targeting HIV LTR-gag and cell input numbers determined by CCR5 copies/sample. Utilizing demographically adjusted T scores obtained from a 7-domain neuropsychological test battery, cognitive status was determined by the global deficit score (GDS) approach, with a GDS of ≥0.5 indicating cognitive impairment. In a linear regression adjusting for plasma HIV RNA, CD4 and lymphocyte count, Beck’s depression score, and years of education, there was 0.04 lower log10 HIV DNA copies within T&B lymphocytes per unit increase in global T score (p= 0.02). Adjusting for the same variables in a logistic regression, the odds of cognitive impairment were 6.2 times greater per log10 increase in HIV DNA within T&B lymphocytes (p= 0.048). The association between cognitive impairment and HIV DNA within CD14+ monocytes did not reach statistical significance. In this pretreatment cohort with mild cognitive dysfunction we found a strong association between levels of HIV DNA within the lymphocyte subset and HAND independent of plasma HIV RNA. These findings likely reflect the neurologic impact of a larger HIV reservoir and active viral replication.
Keywords: HIV DNA, mononuclear cells, neurocognitive disorders, Nigeria
Background
Despite substantial reduction in the incidence of severe forms of HIV associated neurocognitive disorders (HAND) in the era of combination antiretroviral therapy (cART), milder forms persist with high prevalence (Heaton et al. 2010). Such high level persistence has been attributed to multiple factors, including early irreversible insult to the central nervous system (CNS) during infection, the so called legacy effect, as well as low level ongoing viral replication in the CNS despite suppressed viremia (Heaton et al. 2010; Burdo et al. 2013; Chen et al. 2014).
The HIV virus enters the CNS mainly through trafficking of infected mononuclear cells (Gonzalez-Scarano and Martin-Garcia 2005). Cells of the monocyte-macrophage lineage, including dendritic cells and microglia, as well as T cells constitute an important reservoir of replication competent stock of virus that maintains chronicity of infection (Chun et al. 1998; Crowe and Sonza 2000; Gibellini et al. 2008; Palmer et al. 2011). Persistent presence of the HIV virus within the CNS likely perpetuates the pathogenic processes involved in HAND.
Levels of plasma HIV RNA and HIV DNA within peripheral blood mononuclear cells (PBMCs) have both been shown to correlate with HIV disease progression, morbidity and mortality (Mellors et al. 1996; Rouzioux et al. 2005). However, studies have not consistently demonstrated a significant association between plasma HIV RNA, the traditional marker for viral replication, and HAND, particularly among patients on cART (Heaton et al. 2011). It is therefore important to identify other viral characteristics with better predictive significance for HAND. This will improve understanding of HAND pathogenesis and potentially pave the way for the development of diagnostic and therapeutic interventions.
Emerging evidence suggests that the level of HIV DNA within PBMCs may correlate with HAND severity among both treatment-naïve and ART-suppressed individuals (Shiramizu et al. 2009; Valcour et al. 2013). These studies, conducted among populations with either HIV-1 subtype B or CRF01_AE as dominant strain, found significant associations between HAND and HIV DNA levels within unfractionated PBMCs, as well as within the monocyte subset (CD14+ cells). In this report, we examined the association between HAND and levels of HIV DNA separately within peripheral blood lymphocytes (T&B) and CD14+ cells among a pretreatment cohort in Nigeria where HIV-1 subtypes G and CRF02_AG predominate.
Methods
Design
This was a prevalent case-control study, with frequency matching by CD4 count and viral load categories, implemented among a subset of participants at baseline from a prospective cohort study of HAND conducted in Abuja Nigeria between 2011 and 2014.
Parent Cohort Description and Assessments
A total of 216 HIV infected and 114 HIV uninfected participants were enrolled consecutively between 2011 and 2013 from HIV counseling and testing centers at two tertiary facilities, the National Hospital (NHA) and the University of Abuja Teaching Hospital (UATH), both in Abuja, Nigeria. All individuals were ≥18 years of age, able to communicate in English, antiretroviral naïve, and had no history of active tuberculosis, syphilis or other infections. The participants also had no evidence of active central nervous system (CNS) or systemic disease based on clinical assessment that does not include spinal fluid analysis or brain imaging. Similarly, there was no history of significant head trauma, current or history of alcohol abuse, use of other mind-altering substances, or evidence of substance use on urine toxicology screening. Prospective participants were also excluded if they had previous diagnosis of a learning disability or psychiatric disorder. Demographic and clinical information were obtained using standardized questionnaires, and participants were administered a thorough general medical assessment and a comprehensive neuropsychological testing. Blood samples were analyzed for routine clinical testing and specific mononuclear cell-associated HIV DNA levels. Informed consent was obtained from the study participants independently or with the assistance of a family member. All study procedures were approved by University of Maryland Baltimore, Partners, NHA, and UATH Institutional Review Boards.
Neuropsychological assessment
A detailed standardized 22-test neuropsychological battery was administered to all study participants by an examiner who was blinded to the HIV serological status of the participant. Details of these are described in our other reports (Akolo et al. 2014; Royal et al. 2016). In brief, the following ability domains and individual tests within the domains were examined: speed of information processing (WAIS-III Digit Symbol, WAIS-III Symbol Search, Color Trails Test 1 and Trail Making Test A); attention/working memory (Paced Auditory Serial Addition Task, and WMS-III Spatial Span); executive function (Color Trails Test 2 and Stroop Color and Word Test), learning (Hopkins Verbal Learning Test – Revised [HVLT-R] total learning and the Brief Visuospatial Memory Test – Revised [BVMT-R] total learning); memory (HVLT-R delayed recall; BVMT-R delayed recall); verbal fluency (Letter (Word Sound Fluency; Category Fluency: Nouns (animals) and Category Fluency: Verbs [actions]); motor speed and dexterity (Grooved Pegboard Test, Finger Tapping Test and Timed Gait). Participants were screened for effort using the Hiscock Digit Memory Test. Information regarding the presence and severity of symptoms of depression were collected using the Beck Depression Inventory (Beck 1996).
Raw test scores from the neuropsychological tests were converted to scaled scores based on the scores of the HIV uninfected controls, which were then used to generate standardized T-scores for each test (with mean of 50 and standard deviation [SD] of 10), adjusted for age, gender, and education. Deficit scores (DS) ranging from 0 to 5 (0=no deficit and 5=severe deficit) were created for each test from the T-scores: T-score ≥40= DS score of 0; T-score 35–39= DS score of 1; T-score 30–34= DS score of 2; T-score 25–29= DS score of 3; T-score 20–24= DS of 4; T-score <20= DS score of 5. The T scores and deficit scores for individual tests were averaged to generate a mean T score and deficit score for each of the 7 domains, and across all tests to calculate a global T score and global deficit score (GDS) respectively. A global deficit score of 0.5 or more was defined as neurocognitive impairment (Carey et al. 2004; Blackstone et al. 2012).
Clinical laboratory studies
Whole blood from participants was used for confirmatory determination of HIV-1 serological status, measurement of plasma viral load (limit of detection: 20 copies/ml) and CD4+ T cell count performed at the Institute of Human Virology, Nigeria-supported Training Laboratory located at the Asokoro District Hospital, Abuja.
Analysis of Cell-Associated HIV DNA Levels
Patient Sample Selection
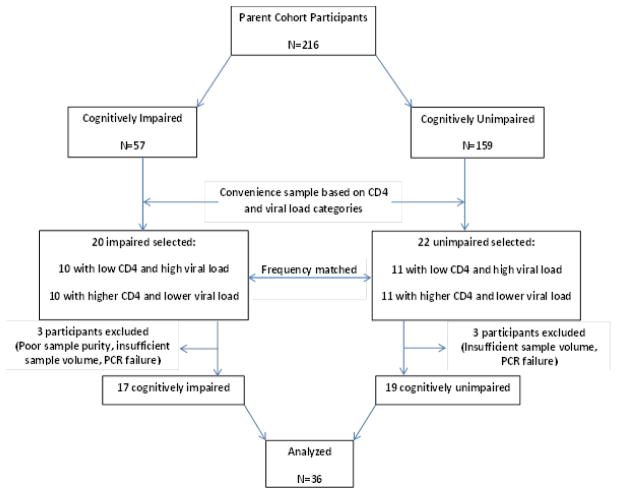
A convenience sample of 42 HIV infected individuals was selected from the parent cohort. In selecting this sample, twenty participants with impairment (cases) were frequency-matched to twenty-two unimpaired participants (controls) with similar CD4/viral load distribution. The matching categories were as follows: low CD4/high viral load and higher CD4/lower viral load (CD4 and viral load cut offs: 200 cells/mm3 and 4.5 log10 copies/ml, respectively). Samples from six individuals were subsequently excluded due to poor sample purity (n=1) as determined by flow cytometry, insufficient sample volume (n=3), and quantitative PCR assay failure (n=2). Therefore, a total of 36 HIV infected ART-naïve participants (17 neurocognitively impaired and 19 unimpaired) from the parent cohort, were included for the analyses in this study (Fig 1).
Fig. 1.
Flow diagram showing study participants and sample selection. Details are provided in the text. N: number of participants; PCR: polymerase chain reaction
Mononuclear cell purification
Peripheral blood samples were collected in Acid Citrate Dextrose (ACD) tubes and PBMC cells were separated from plasma on Ficoll-Paque density gradients. The cells were fractionated using MACS MicroBead Technology with an AutoMACS separator. PBMCs were first incubated with CD15− and CD56-conjugated magnetic microbeads to label granulocytes and natural killer cells. After magnetically removing the labeled cells, the flow-through fraction was labeled with CD14 microbeads. The magnetically labeled CD14+ cells (CD14+/CD16− and CD14+/CD16+ monocytes) were then separated and stored. The flow-through, containing T cells, B cells and, potentially CD14lowCD16+ monocytes, was then labeled with CD16 microbeads to remove the remaining CD16+ mononuclear cells, and the depleted T&B cell fractions were then also stored for subsequent analyses. The purity of the samples was assessed by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences). CD14+ cells were identified after staining PBMCs with CD4-FITC, CD14-PE, CD3-PerPCP, and CD16-APC antibodies (BD Biosciences). The monocyte purity of samples from the cohort was 84–98%.
DNA extraction and polymerase chain reaction assays
DNA from each cell fraction was extracted using the QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA). Total HIV DNA from the CD14+ and T&B cell fractions was quantified using real-time PCR assay targeting a conserved region of HIV LTR-gag (Malnati et al. 2008) as previously described (Li et al. 2016). The assay was customized with primers and HIV DNA standards specific for subtype G and CRF02_AG sequences. The results were normalized by the cell input numbers quantified by the number of CCR5 copies/sample and no DNA negative controls were included with each experiment. The quantity of DNA extracted from the CD16+ cell fraction was too small to perform any analysis.
Statistical Analysis
Demographic and clinical characteristics were compared between participants with and without cognitive impairment (cases and controls) using chi-square, Wilcoxon and t tests. A paired t test was used to compare levels of HIV DNA within the T&B lymphocytes and CD14+ cells. Multivariable linear regression models were fit to assess the association of global and individual cognitive domain T scores as well as global deficit scores (GDS) with levels of cell-associated HIV DNA for the lymphocyte and monocyte subsets, adjusting for CD4 count, plasma HIV RNA, monocyte or lymphocyte count respectively, Beck’s depression score, and years of education. Similarly, multivariable logistic regression analysis was done to assess the odds of cognitive impairment per log10 change in HIV DNA levels within the monocyte and T&B lymphocyte subsets adjusting for the same variables. All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc.).
Results
Demographic Characteristics
The median age of participants was 32 years. Women constituted about two-thirds of this sub-sample, which is the same proportion observed for the parent cohort. Age and gender did not differ by impairment status. Individuals with impairment had a higher median number of years of education compared to the unimpaired (p= 0.03) [Table 1].
Table 1.
Demographic and Immuno-virologic Characteristics
| All N=36 |
Impaired N=17 |
Unimpaired N=19 |
p value | |
|---|---|---|---|---|
| Age (years), Median (Q1,Q3) | 32 (26.5, 35.5) | 33 (27, 37) | 31 (26, 35) | 0.32w |
| Gender, Female n (%) | 24 (66.7) | 11 (64.7) | 13 (68.4) | 0.81c |
| Education (years), Median (Q1,Q3) | 13 (12, 15.5) | 14 (12, 16) | 12 (6, 14) | 0.03w |
| CD4 cell count/μL, Median (Q1,Q3) | 347 (128, 556) | 282 (127, 527) | 365 (128, 574) | 0.66w |
| Log10 Plasma HIV RNA copies/ml, Mean (SD) | 4.6 (0.69) | 4.6 (0.71) | 4.5 (0.68) | 0.44t |
| Log10 HIV DNA copies/106 CD14+ cells, Mean (SD) | 2.58 (0.92) | 2.61 (1) | 2.55 (0.86) | 0.85t |
| Log10 HIV DNA copies/106 T&B Cells, Mean (SD) | 3.54 (0.63) | 3.79 (0.61) | 3.31 (0.58) | 0.02t |
| Monocyte count x 109/L, Median (Q1,Q3) | 0.59 (0.38, 0.73) | 0.61 (0.41, 0.81) | 0.59 (0.33, 0.73) | 0.72w |
| Lymphocyte count x 109/L, Median (Q1,Q3) | 2 (1.5, 2.3) | 2 (1.4, 2.5) | 2.1(1.5, 2.3) | 0.93w |
| Beck’s Depression Score | 6 (2, 14) | 8 (5, 14) | 4 (1, 11) | 0.12w |
Wilcoxon
Chisquare
t test
Q1: 25th percentile; Q3: 75th percentile; SD: standard deviation; N: number of participants
Immunologic and Virologic Characteristics
The median CD4 cell count was 347 cells/mm3, while mean plasma HIV RNA viral load was 4.6 log10 copies/ml, and both measures did not differ by impairment status as expected due to the matched sampling. Median count for total monocytes and lymphocytes also did not differ between the impaired and unimpaired [Table 1].
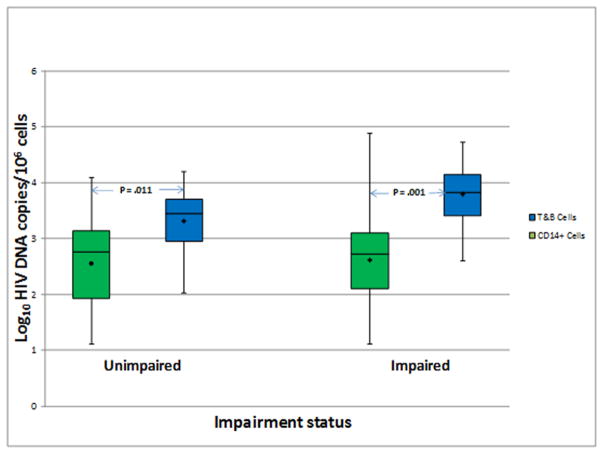
The level of log10 copies of HIV DNA/106 cells within the lymphocyte (T&B) subset was significantly higher than the level in the CD14+ cells among both impaired and unimpaired participants (Mean difference [standard error]: 1.12 [0.28] and 0.68 [0.24]; p= 0.001 and 0.011, respectively) [Fig 2]. While the difference between the subsets among those with impairment appeared larger than the difference among the unimpaired, this did not reach statistical significance (p= 0.24).
Fig. 2.
Analysis of HIV DNA copies within CD14+ and T&B cell subsets for unimpaired and impaired study participants. Mean copies of HIV DNA (Log10/106 cells) were compared between these subsets using paired t-tests. The mean difference [standard error] for the samples from subjects with impairment was 1.12 [0.28] and 0.68 [0.24] for the samples from unimpaired individuals. See text for additional details related to the analysis results.
Association of HIV DNA Levels within PBMC subsets with Global and Domain-Specific Cognitive Scores
In multivariable linear regression models, adjusting for plasma HIV RNA, CD4 and lymphocyte counts, Beck’s depression score, and years of education, there was a 0.04 decrease in log10 HIV DNA copies/106 cells per unit increase in global T score (higher score associated with better cognition) [p= 0.02], and a corresponding 0.39 increase in log10 HIV DNA copies/106 cells per unit increase in GDS (higher score associated with poorer cognition) [p= 0.135] for the T&B lymphocytes [Table 2]. For the individual cognitive domains, there was a similar significant decrease in HIV DNA log10 copies within the T&B lymphocyte subset per unit increase in T scores for memory, verbal fluency and motor speed/dexterity as well as a marginally significant decrease for the speed of information processing domain. The observed significant decrease in HIV DNA log10 copies/106 cells ranged from 0.02 (for memory) to 0.04 (for motor speed/dexterity), consistent with lower cognitive score as HIV DNA burden increased (p= 0.045 and p= 0.016 respectively) [Table 2].
Table 2.
Multivariable Linear Regression (Log10 HIV DNA Level vs Global/Domain Scores)
| Cognitive Domain | T&B Cells | CD14+ Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| N | B | SE | * p value | N | B | SE | * p value | |
| Global | ||||||||
| Global T score | 36 | −0.04 | 0.02 | 0.020 | 36 | −0.02 | 0.03 | 0.530 |
| GDS | 36 | 0.39 | 0.25 | 0.135 | 36 | 0.38 | 0.43 | 0.390 |
| Domain | ||||||||
| Speed of Information Processing | 36 | −0.02 | 0.01 | 0.071 | 36 | −0.02 | 0.02 | 0.201 |
| Attention/Working Memory | 36 | −0.01 | 0.01 | 0.306 | 36 | −0.01 | 0.02 | 0.716 |
| Executive Function | 36 | −0.01 | 0.02 | 0.388 | 36 | 0.00 | 0.03 | 0.897 |
| Learning | 36 | −0.02 | 0.01 | 0.127 | 36 | −0.02 | 0.02 | 0.446 |
| Memory | 36 | −0.02 | 0.01 | 0.045 | 36 | 0.00 | 0.02 | 0.983 |
| Verbal Fluency | 36 | −0.03 | 0.01 | 0.030 | 36 | −0.01 | 0.02 | 0.602 |
| Motor Speed and Dexterity | 36 | −0.04 | 0.01 | 0.016 | 36 | −0.00 | 0.03 | 0.928 |
Adjusted for Plasma HIV RNA, CD4, Lymphocyte/Monocyte Count, Beck’s depression score and Years of education
GDS: global deficit score; β: mean change in log10 HIV DNA per unit score change; N: number of participants; SE: standard error
Paralleling the pattern in T&B lymphocytes, levels of HIV DNA within CD14+ cells generally decreased as T scores increased; however, these changes were not statistically significant for global or any cognitive domain score [Table 2].
Association between Cognitive Impairment and HIV DNA Levels within PBMC subsets
In a multivariable logistic regression, adjusting for plasma HIV RNA, CD4 and lymphocyte counts, Beck’s depression score, and years of education, the odds of neurocognitive impairment were 6.2 times higher per log10 increase in HIV DNA within T&B lymphocytes (OR: 6.2 [95% CI, 1.01–37.4]; p= 0.048). For the CD14+ cells, adjusting for similar variables, there was a 70% higher odds of cognitive impairment per log10 increase in HIV DNA, but this was not statistically significant (OR: 1.7 [95% CI, 0.5–5.4]; p= 0.391) [Table 3].
Table 3.
Multivariable Logistic Regression (Impairment vs Log10 HIV DNA)
| PBMC Subset | N | OR (95% CI) | * p value |
|---|---|---|---|
| A. T&B Cells | 36 | 6.2 (1.01–37.4) | 0.048 |
| B. CD14+ Cells | 36 | 1.7 (0.5–5.4) | 0.391 |
Adjusted for Plasma HIV RNA, CD4, Lymphocyte/Monocyte Count, Beck’s depression score and Years of education
OR: odds ratio; CI: confidence interval; N: number of participants
PBMC: peripheral blood mononuclear cell
Discussion
In this study of pretreatment patients, we found a significant association between levels of HIV DNA within peripheral blood lymphocytes (T&B) and neurocognitive impairment, independent of plasma HIV RNA and CD4 count. Participants in this cohort had either mild or moderate impairment, with none determined to have HIV associated dementia (HAD). Other studies have demonstrated a similar association within unfractionated PBMCs (Shiramizu et al. 2005; Shiramizu et al. 2009; Oliveira et al. 2015) and within the monocyte subset (CD14+ cells) (Valcour et al. 2009; Valcour et al. 2013).
To our knowledge, this is the first demonstration of such an association for the composite T and B lymphocyte cellular compartment. It is likely that the results reflect virus that is present in the CD4+ T cell pool, the predominant fraction of the lymphocyte compartment and preferential target of the HIV virus. In the activated state, CD4+ T cells support efficient viral replication, and in the resting state permit establishment of a latent reservoir (Douek et al. 2002; Nickle et al. 2003; Gibellini et al. 2008). B cells constitute a small proportion of the lymphocyte subset, and although they have been shown to be infected with HIV in vitro, this has not been replicated in vivo (Moir et al. 1999). Instead, HIV has been found to bind to the surface of B cells with levels of bound virus reflecting levels of plasma viremia (Moir et al. 2000). Therefore, the measures of cell-associated HIV DNA in this study probably reflect viral infection of T cells, and, since CD8+ T cells are relatively resistant to infection, the source of the HIV DNA is likely from CD4+ T cells (Psallidopoulos et al. 1989; Livingstone et al. 1996).
As expected, we found a significantly higher level of HIV DNA within lymphocytes as compared to the monocytes among both impaired and unimpaired participants, indicating a significantly larger reservoir for viral replication and persistence in the lymphocyte subset. This reservoir, known to be established early at the onset of infection with the virus (Strain et al. 2003; Rouzioux et al. 2005), may play an important role in HAND pathogenesis by directly trafficking virus into the CNS. This possibility is supported by studies where unique variants of T cell derived virus have been detected in the nervous system (Schnell et al. 2011). The early establishment of the reservoir prior to initiation of treatment could explain the reason for the persistence of HAND in the era of cART, since the latent reservoir is shielded from the effect of antiretroviral drugs (Chun et al. 1998; Ibanez et al. 1999).
Our results compare with those of similar studies that looked at total PBMCs and its subsets. A study among cART naive Thai patients (Valcour et al. 2013), reported a strong association between HAND and levels of HIV DNA within CD14+ cells, but not for unfractionated PBMCs. Another study among predominantly treatment experienced Hawaii patients found significant correlation between levels of HIV DNA within total PBMCs and severity of HAND (Shiramizu et al. 2009). Furthermore, other studies from Hawaii and Thailand (Shiramizu et al. 2005; Shiramizu et al. 2006) reported significant associations between HIV dementia and HIV DNA levels within total PBMCs among treatment experienced and naïve individuals respectively. Similarly, a strong positive correlation between HIV DNA within unfractionated PBMCs and GDS was demonstrated among older adults on suppressive treatment in San Diego (Oliveira et al. 2015).
Overall, these studies show that levels of HIV DNA within total PBMCs or its constituent subsets correlate with HAND severity. Differences between the studies regarding whether total PBMCs or a subset showed statistical significance may be attributed to the limited power in most of these studies. For instance, although we did not find a statistically significant association for the CD14+ subset, we clearly saw a pattern tending in a direction that was consistent with higher likelihood of HAND as HIV DNA levels increased. It has been suggested that a dynamic equilibrium may exist between levels of HIV DNA in peripheral blood lymphocytes and monocytes, because of a positive correlation observed between them, possibly due to reciprocal infectious re-feeding by these cell populations (Garbuglia et al. 2004; Gibellini et al. 2008). However, it is also possible that levels of HIV DNA within individual subsets may reflect different HIV disease stage, HIV-1 subtype, patterns of HAND severity, as well as relationship with comorbidities and treatment status. Whereas the Thailand and Hawaii studies (Shiramizu et al. 2005; Valcour et al. 2013) had significant proportion of participants with HAD and reported associations within CD14+ cells, our study had no participants with HAD and only found significant associations for the lymphocyte subset. This may be related to known differences between early HIV disease and more advanced stages with respect to shift in cell tropism. T cell tropic virus found in the CSF has been shown to be associated with pleocytosis (Schnell et al. 2011), a feature indicating earlier stages of HIV infection (Price et al. 2014). As HIV disease progresses with resultant depletion of CD4+ T lymphocytes, there is a concomitant expansion and increased turnover of peripheral monocytes, including CD14+/CD16+ subsets known to be associated with increased susceptibility to HIV infection (Ellery et al. 2007; Burdo et al. 2013). Nonetheless, there is clearly a need for larger studies with adequate power to further explore this as well as examine the effects of HIV DNA (total and integrated) within each PBMC subset (CD14+, CD16+, CD4+, activated, resting, naïve and memory etc.) in HAND pathogenesis.
In this study, we measured total HIV DNA levels, which consisted of episomal (linear and circular) and integrated proviral DNA. All 3 forms of HIV DNA (unintegrated linear, unintegrated circular, and integrated proviral DNA) have been detected in brain tissues of patients having AIDS dementia complex, with the unintegrated form occurring at a considerably higher level (Pang et al. 1990). Although unintegrated DNA comprises the bulk of the HIV DNA among untreated individuals, it does not contribute substantially to the maintenance of productive infection or to the latent viral reservoir (Sloan and Wainberg 2011). However, total HIV DNA has been shown to correlate with HIV disease progression, morbidity and mortality (Rouzioux et al. 2005; Williams et al. 2014). Furthermore, it was demonstrated that levels of total HIV DNA show strong positive correlation with levels of integrated/proviral DNA within PBMCs among both treatment experienced and naïve individuals (Buzon et al. 2010; Williams et al. 2014). Therefore, while integrated HIV DNA is a more reliable marker of the peripheral blood viral reservoir (Mexas et al. 2012), total HIV DNA within PBMCs, reflecting both reservoir capacity and ongoing replication (Graf and O’Doherty 2013), may be a valuable predictor of disease progression and HAND. Hence, the associations observed in this study likely represent the influence of a larger viral reservoir and more active viral replication. Additional studies are needed to evaluate the effects of the HIV reservoir in patients on suppressive antiretroviral therapy.
The predominance of female participants in this study has the potential to limit the generalizability of our findings because of the known association between gender and cognitive performance, also reported for the parent cohort of this study (Royal et al. 2016). Although gender effects may potentially drive some of our findings, and will need to be explored further in larger studies, our analyses for this subset of participants show no gender difference with respect to impairment status. There was also no statistical evidence of interaction with gender in the association between HIV DNA levels and cognitive performance (results not shown). Furthermore, the findings from the Hawaii study (Shiramizu et al. 2005), which comprised of predominantly male participants, may be a further indication that gender probably has no major impact on these findings.
This study has additional limitations. We had a limited sample size for an elaborate exploration of patient characteristics. This may also partly account for our inability to observe a statistically significant association for the CD14+ subset. As a conveniently selected sub-sample, we assessed the extent to which it represented the parent cohort. Comparing demographic (age, gender and education) and clinical characteristics (CD4, plasma HIV RNA, hemoglobin, and depression score) between the study sample and the rest of the parent cohort participants, as well as between our selected controls (unimpaired) and the unimpaired in the parent cohort, we found no significant differences, apart from lower median age among the study sample. Therefore, our selected sample, and more importantly, the control set (unimpaired) in the sample, does not appear to suffer from significant selection bias. Another limitation was the lack of participants with the most severe form of HAND, HAD, in this cohort. This limited our ability to show HIV DNA correlations for the whole spectrum of HAND, and might be related to the failure to attain statistical significance for the CD14+ subset.
There are key strengths to this study. First, we excluded prospective participants with other medical conditions known to be associated with cognitive impairment, thereby enabling an un-confounded assessment of HAND. Second, we used the GDS method to determine cognitive impairment status for our selection of impaired and unimpaired participants. The GDS approach has the advantage of reducing both type 1 and type 2 errors in classifying participants by impairment status (Carey et al. 2004), in addition to being a relatively more stringent methodology (Blackstone et al. 2012). This is expected to improve the validity of assessment for impairment status. Third, this study, to our knowledge, is the first to report the association between HIV DNA within PBMCs and HAND in Africa, where there is a disproportionately higher disease burden, as well as among populations with predominant HIV-1 subtypes other than CRF01_AE or B.
Conclusion
In this study, we found a significant association between HIV DNA within peripheral blood lymphocytes (T&B) and HAND among treatment naïve patients with mild to moderate impairment. This is consistent with other reports indicating an important role for the peripheral blood viral reservoir in HAND pathogenesis and persistence. Larger studies are required to further characterize the specific roles of HIV DNA within PBMC components, in relation to HAND occurrence and severity, treatment status, comorbidities, and inflammatory responses. Ultimately, there is a glaring need for therapeutic and preventative strategies that address this peripheral reservoir as well as virus hidden within sanctuary sites.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grant #R01 MH086356 (to William A. Blattner and Walter Royal, III) and by National Institutes of Health Fogarty/AIDS International Training and Research Program grant #2D43TW001041-14 (training support to Jibreel Jumare).
Footnotes
Conflict of interest: The authors have no conflicts of interest to report
This work was presented in part at the 17th Annual International Meeting of the Institute of Human Virology, 27th–30th September 2015, Baltimore, Maryland, USA (Abstract P-D15).
References
- Akolo C, Royal W, III, Cherner M, Okwuasaba K, Eyzaguirre L, Adebiyi R, Umlauf A, Hendrix T, Johnson J, Abimiku Ae, Blattner W. Neurocognitive impairment associated with predominantly early stage HIV infection in Abuja, Nigeria. Journal of neurovirology. 2014;20(4):380–387. doi: 10.1007/s13365-014-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Ellis RJ, Atkinson JH, Grant I, Heaton RK. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254(1):102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chen MF, Gill AJ, Kolson DL. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS. 2014;9(6):559–564. doi: 10.1097/COH.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95(15):8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SM, Sonza S. HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. J Leukoc Biol. 2000;68(3):345–350. [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Garbuglia AR, Calcaterra S, D’Offizi G, Topino S, Narciso P, Lillo F, Girardi E, Capobianchi MR. HIV-1 DNA burden dynamics in CD4 T cells and monocytes in patients undergoing a transient therapy interruption. J Med Virol. 2004;74(3):373–381. doi: 10.1002/jmv.20188. [DOI] [PubMed] [Google Scholar]
- Gibellini D, Borderi M, De Crignis E, Cicola R, Cimatti L, Vitone F, Chiodo F, Re MC. HIV-1 DNA load analysis in peripheral blood lymphocytes and monocytes from naive and HAART-treated individuals. J Infect. 2008;56(3):219–225. doi: 10.1016/j.jinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Graf EH, O’Doherty U. Quantitation of integrated proviral DNA in viral reservoirs. Curr Opin HIV AIDS. 2013;8(2):100–105. doi: 10.1097/COH.0b013e32835d8132. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Puig T, Elias J, Clotet B, Ruiz L, Martinez MA. Quantification of integrated and total HIV-1 DNA after long-term highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13(9):1045–1049. doi: 10.1097/00002030-199906180-00007. [DOI] [PubMed] [Google Scholar]
- Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, Kuritzkes DR, Lederman MM, Para M, Gandhi RT. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30(3):343–353. doi: 10.1097/QAD.0000000000000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone WJ, Moore M, Innes D, Bell JE, Simmonds P. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. Edinburgh Heterosexual Transmission Study Group. Lancet. 1996;348(9028):649–654. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- Malnati MS, Scarlatti G, Gatto F, Salvatori F, Cassina G, Rutigliano T, Volpi R, Lusso P. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protocols. 2008;3(7):1240–1248. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272(5265):1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Mexas AM, Graf EH, Pace MJ, Yu JJ, Papasavvas E, Azzoni L, Busch MP, Di Mascio M, Foulkes AS, Migueles SA, Montaner LJ, O’Doherty U. Concurrent Measures Of Total And Integrated HIV DNA Monitor Reservoirs And Ongoing Replication In Eradication Trials. AIDS (London, England) 2012;26(18):2295–2306. doi: 10.1097/QAD.0b013e32835a5c2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Lapointe R, Malaspina A, Ostrowski M, Cole CE, Chun TW, Adelsberger J, Baseler M, Hwu P, Fauci AS. CD40-Mediated induction of CD4 and CXCR4 on B lymphocytes correlates with restricted susceptibility to human immunodeficiency virus type 1 infection: potential role of B lymphocytes as a viral reservoir. J Virol. 1999;73(10):7972–7980. doi: 10.1128/jvi.73.10.7972-7980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Malaspina A, Li Y, Chun TW, Lowe T, Adelsberger J, Baseler M, Ehler LA, Liu S, Davey RT, Jr, Mican JA, Fauci AS. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J Exp Med. 2000;192(5):637–646. doi: 10.1084/jem.192.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle DC, Jensen MA, Shriner D, Brodie SJ, Frenkel LM, Mittler JE, Mullins JI. Evolutionary indicators of human immunodeficiency virus type 1 reservoirs and compartments. J Virol. 2003;77(9):5540–5546. doi: 10.1128/JVI.77.9.5540-5546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MFd, Murrell B, Pérez-Santiago J, Vargas M, Ellis RJ, Letendre S, Grant I, Smith DM, Woods SP, Gianella S. Circulating HIV DNA Correlates With Neurocognitive Impairment in Older HIV-infected Adults on Suppressive ART. Scientific Reports. 2015;5:17094. doi: 10.1038/srep17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011;270(6):550–560. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- Pang S, Koyanagi Y, Miles S, Wiley C, Vinters HV, Chen IS. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Price RW, Spudich SS, Peterson J, Joseph S, Fuchs D, Zetterberg H, Gisslen M, Swanstrom R. Evolving character of chronic central nervous system HIV infection. Semin Neurol. 2014;34(1):7–13. doi: 10.1055/s-0034-1372337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psallidopoulos MC, Schnittman SM, Thompson LM, 3rd, Baseler M, Fauci AS, Lane HC, Salzman NP. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzioux C, Hubert JB, Burgard M, Deveau C, Goujard C, Bary M, Sereni D, Viard JP, Delfraissy JF, Meyer L. Early levels of HIV-1 DNA in peripheral blood mononuclear cells are predictive of disease progression independently of HIV-1 RNA levels and CD4+ T cell counts. J Infect Dis. 2005;192(1):46–55. doi: 10.1086/430610. [DOI] [PubMed] [Google Scholar]
- Royal W, 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PloS one. 2016;11(2) doi: 10.1371/journal.pone.0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):6. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19(1):45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Ratto-Kim S, Sithinamsuwan P, Nidhinandana S, Thitivichianlert S, Watt G, deSouza M, Chuenchitra T, Sukwit S, Chitpatima S, Robertson K, Paul R, Shikuma C, Valcour V. HIV DNA and dementia in treatment-naive HIV-1-infected individuals in Bangkok, Thailand. Int J Med Sci. 2006;4(1):13–18. doi: 10.7150/ijms.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Williams AE, Shikuma C, Valcour V. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. The Journal of neuropsychiatry and clinical neurosciences. 2009;21(1):68–74. doi: 10.1176/appi.neuropsych.21.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RD, Wainberg MA. The role of unintegrated DNA in HIV infection. Retrovirology. 2011;8(52):1742–4690. doi: 10.1186/1742-4690-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Gunthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, Opravil M, Levine H, Bacheler L, Spina CA, Richman DD, Wong JK. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A. 2003;100(8):4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, Shikuma C, Liang CY, Jirajariyavej S, Sithinamsuwan P, Tipsuk S, Clifford DB, Paul R, Fletcher JL, Marovich MA, Slike BM, DeGruttola V, Shiramizu B. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PloS one. 2013;8(7):e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J, Siangphoe U, Kim JH, de Souza M, Degruttola V, Paul RH, Shikuma CM. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72(11):992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, Kinloch S, Cooper D, Schechter M, Tambussi G, Fidler S, Carrington M, Babiker A, Weber J, Koelsch KK, Kelleher AD, Phillips RE, Frater J. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;12(3):03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]