Abstract
During meiotic prophase cohesin complexes mediate cohesion between sister chromatids and promote pairing and synapsis of homologous chromosomes. Precisely how the activity of cohesin is controlled to promote these events is not fully understood. In metazoans, cohesion establishment between sister chromatids during mitotic divisions is accompanied by recruitment of the cohesion-stabilizing protein Sororin. During somatic cell division cycles, Sororin is recruited in response to DNA replication-dependent modification of the cohesin complex by Esco acetyltransferases. How Sororin is recruited and acts in meiosis is less clear. Here we have surveyed the chromosomal localization of Sororin and its relationship to the meiotic cohesins and other chromatin modifiers with the objective of determining how Sororin contributes to meiotic chromosome dynamics. We show that Sororin localizes to the cores of meiotic chromosomes in a manner that is dependent on synapsis and the synaptonemal complex protein SYCP1. In contrast, cohesin, with which Sororin interacts in mitotic cells, shows axial enrichment on meiotic chromosomes even in the absence of synapsis between homologs. Using high-resolution microscopy, we show that Sororin is localized to the central region of the synaptonemal complex. These results indicate that Sororin regulation during meiosis is distinct from its regulation in mitotic cells, and may suggest that it interacts with a distinctly different partner to ensure proper chromosome dynamics in meiosis.
Introduction
The pairing and synapsis of homologous chromosomes during the first meiotic prophase is essential for meiotic recombination and proper disjunction of chromosomes during gametogenesis. Failures in recombination result in non-disjunction, precocious sister chromatid separation and the formation of aneuploid gametes (reviewed in (Hunter 2015)). The absence of synapsis results in meiotic failure and apoptosis. Synapsis of homologous chromosomes is facilitated by the assembly and stabilization of a proteinaceous structure between homologous chromosome pairs, called the synaptonemal complex (SC). The SC is assembled in a stepwise fashion with the complete complex having a tripartite structure consisting of lateral elements, transverse filaments, and finally, the central element present on the mature SC (reviewed in (Cahoon and Hawley 2016)). Axial element proteins, including SYCP3 and SYCP2 in mice, begin to assemble on the chromosome axes in leptonema, before pairing and synapsis of homologous chromosomes are evident (Lammers et al. 1994; Dobson et al. 1994; Offenberg et al. 1998). Later, in zygonema, the axial elements begin to zipper together to form the tripartite SC (reviewed in (Handel and Schimenti 2010)) Once the mature SC has formed the axial elements are referred to as lateral elements and the structure that bridges the lateral elements is referred to as the central region. The central region includes the central element formed by the SYCE1, SYCE2, SYCE3 and TEX12 proteins joined to the lateral elements by transverse filaments that include the SYCP1 protein (Meuwissen et al. 1992; Costa et al. 2005; Hamer et al. 2008; Schramm et al. 2011). The SC is fully assembled in pachynema. Cells remain in pachynema until checkpoints that monitor recombination and synapsis are satisfied. Upon exit from the pachytene stage, the SC disassembles as the meiotic cell progresses through diplonema. Diplonema is identified cytologically by the removal of the central element; in immunolabelling studies, SYCP1 is lost from chromosomal arms at this stage (Meuwissen et al. 1992). Finally, after diplonema the cells enter diakinesis, a stage prior to metaphase I in which chromosome condensation is completed, and homologs remain associated only through their centromeres and sites of recombination (chiasmata).
The appearance of SC components on chromosome axes is preceded by axial enrichment of cohesin ((Eijpe et al. 2000); reviewed in Rankin(Rankin 2015)). Cohesin is a protein complex that tethers sister chromatids together along their length during both mitotic and meiotic cell divisions. Sister chromatid cohesion in somatic cells is essential for mitotic progression, accurate chromosome segregation, and certain kinds of DNA repair (reviewed in (Nasmyth and Haering 2009)). In somatic cells, the core cohesin complex is composed of four subunits: SMC1, SMC3, RAD21, and either SA-1 or SA-2. During meiotic cell divisions, in addition to the mitotic form of the cohesin complex, meiosis-specific isoforms of several of the cohesin subunits are expressed and incorporated into distinct complexes (reviewed in (Rankin 2015)). In mammals, these include one of two meiosis-specific isoforms of RAD21, or the “kleisin” subunit: REC8 or RAD21L (Lee et al. 2003; Bannister et al. 2004; Ishiguro et al. 2011; Gutiérrez-Caballero et al. 2011). In addition to the kleisin subunits, in meiotic complexes the core cohesin SMC subunit SMC1α is replaced with the related SMC1β (Revenkova et al. 2004). Finally, a meiosis-specific form of the Stromal Antigen (SA) or “stromalin” subunit called STAG3 is thought to be the predominant SA isoform in all mammalian meiotic cohesins (Pezzi et al. 2000; Prieto et al. 2001; Fukuda et al. 2010; Lee and Hirano 2011).
Mutations in genes encoding meiosis-specific cohesin subunits confirm that these isoforms are essential for normal chromosome axis formation, synapsis, and double-strand break formation and repair (Xu et al. 2004; Bannister et al. 2004; Xu et al. 2005; Fukuda et al. 2010; Winters et al. 2014; Ward et al. 2016). The unique phenotypes and patterns of association of the different meiotic cohesin complexes with chromosome axes during pairing and synapsis in meiotic prophase also suggest non-overlapping roles in meiotic chromosome dynamics (reviewed in (Rankin 2015)). The meiosis-specific kleisin subunits, REC8 and RAD21L, are found in foci or stripes along chromosome axes beginning during the leptotene-zygotene stage, and are required for normal axis formation (Eijpe et al. 2003; Lee et al. 2003; Bannister et al. 2004; Xu et al. 2005; Lee and Hirano 2011; Ishiguro et al. 2011; Gutiérrez-Caballero et al. 2011). Similarly, in the absence of the meiotic SA subunit STAG3, axis formation is severely compromised and the REC8 and RAD21L kleisins are destabilized (Fukuda et al. 2014; Winters et al. 2014). The meiosis-specific SMC1β subunit is required for normal axis formation, chromosome loop size, and recombination in meiotic prophase I (Revenkova et al. 2004; Novak et al. 2008; Biswas et al. 2013). The analysis of compound meiotic cohesin mutants confirms both that STAG3-containing cohesins are essential for normal axis formation, and that STAG3 has functional interactions with both the REC8 and RAD21L kleisins (Ward et al. 2016).
Although cohesin in its diverse forms is known to be essential for normal meiotic chromosome dynamics, the roles of cohesin regulators, such as Sororin, PDS5, and the ESCO enzymes during mammalian meiosis are less clear. In somatic cells, PDS5 associates with cohesin after it is loaded on chromatin (Sumara et al. 2000). The cohesin-stabilizing protein Sororin interacts with PDS5 and is recruited to chromatin in response to DNA replication (Rankin et al. 2005; Song et al. 2012). This association requires modification of the cohesin complex by the acetyltransferase ESCO2 (Lafont et al. 2010). Sororin competes with the cohesion destabilizing protein WAPL for PDS5 binding, and retention of Sororin at the centromere ensures that centromeric cohesion is maintained in metaphase (Gandhi et al. 2006; Kueng et al. 2006; Nishiyama et al. 2010; Liu et al. 2012; Ouyang et al. 2016). Collectively then, PDS5, Sororin, WAPL and ESCO2 control the stability of the interaction between cohesin and chromatin, ensuring that cohesin tethers sister chromatids together at the proper time and place on the chromosomes of mitotically dividing cells. But what is the role of these cohesin regulatory proteins in meiosis? In budding yeast meiosis, Pds5 helps to prevent inter-sister recombination events, and thus is essential for proper chromosome segregation (Zhang et al. 2005; Jin et al. 2009). Mammals express two orthologs of PDS5, called PDS5A and PDS5B. Pds5B is highly expressed in the testis, and loss of PDS5B expression at this site results in reduced proliferation of primordial germ cells (Zhang et al. 2007). Therefore, the precise role of PDS5 in meiotic prophase is not clear. In fission yeast, the Eso1 acetyltransferase, which is homologous to the vertebrate ESCO1 and ESCO2 enzymes, is required for monopolar attachment of sister kinetochores in meiosis I, consistent with a model in which Eso1 promotes sister chromatid cohesion through Rec8-containing cohesin (Kagami et al. 2011).
The role of Sororin in meiotic prophase is poorly understood, and the degree to which analogies can be drawn to its role in mitotic cohesion is not clear. Here, we have immunolocalized Sororin in mouse spermatocytes during meiotic prophase and tested the dependency of Sororin localization on several meiosis-specific cohesin subunits. The timing with which Sororin loads on meiotic chromosomes and its patterns of localization are different from what has been observed in mitotic cells and suggest Sororin may have roles in synapsis that are distinct from its role in regulating mitotic cohesin. Our results are consistent with and extend upon recent studies showing that Sororin is expressed in meiotic cells and localizes to synapsed meiotic chromosomes (Gómez et al. 2016).
Results
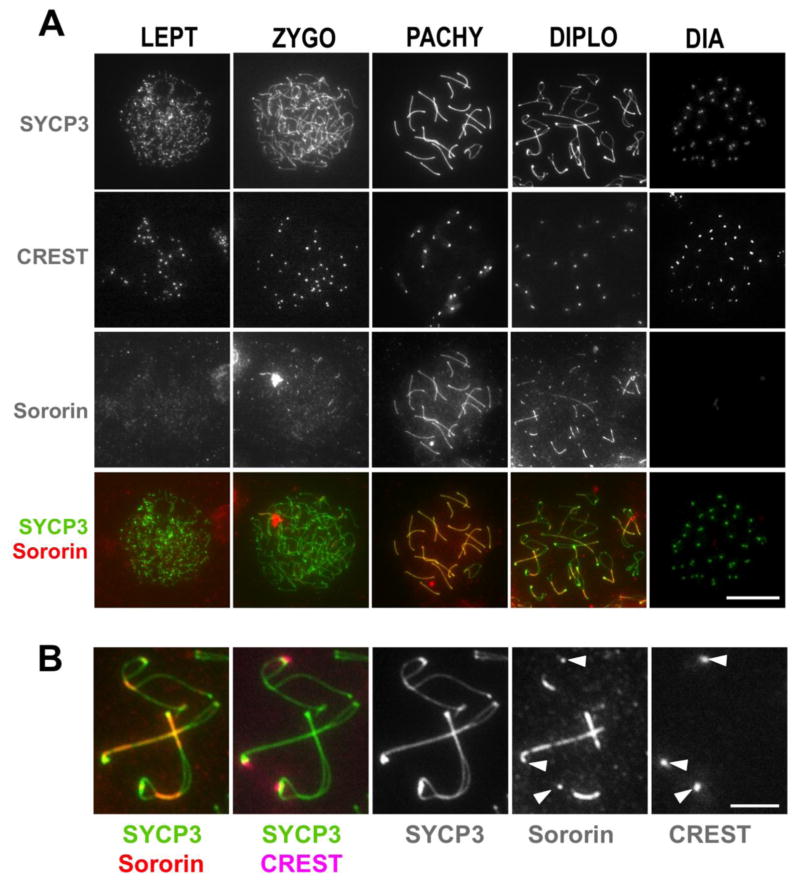
Sororin is enriched on synapsed regions of homologous chromosomes
To explore possible roles of Sororin in meiotic chromosome dynamics, surface chromosome spreads were prepared from B57BL/6 mice testes and immunostained with an anti-Sororin antibody described previously (Wu et al. 2011), after confirming that the antibody recognizes mouse Sororin (Fig. S1). The chromosome spreads were also immunostained with anti-SYCP3, an axial/lateral element (LE) protein, CREST (Calcinosis, Reynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia) serum to mark centromeres, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) which allowed us to assign to each spread a stage in meiotic prophase. In these samples, Sororin enrichment could be seen on the meiotic chromosome cores (Fig. 1). The enrichment of Sororin at cores was not evident in early stages of meiotic prophase, such as leptonema, but rather was seen only along synapsed regions of the chromosomes in zygonema, pachynema, and into diplonema. Short linear stretches of Sororin staining could first be detected in zygonema, (Fig, 1A, second column). Sororin staining was most strongly enriched on fully synapsed chromosomes in mid-pachynema. The enrichment of Sororin on the chromosome cores persisted into the diplotene stage, where it could be seen associated both with remaining synapsed regions, and at centromeres, as evidenced by colocalization of the Sororin signal with CREST staining (Fig. 1B). In diakinesis when chromosomes were fully desynapsed, Sororin enrichment at the centromeres was no longer detectable. It is possible that the epitope recognized by our antibody may be masked in the inner centromere at this stage in meiosis, as Sororin localization to the centromeres in diakinesis was recently reported (Gómez et al. 2016).
Figure 1. Sororin is enriched on cores of synapsed chromosomes.
Chromosome spreads from spermatocytes of wild-type mice were immunostained with antibodies to the indicated proteins. A. Various stages in meiotic prophase, as indicated by SYCP3 staining, were co-stained with CREST serum (to localize centromeres) and anti-Sororin antibodies. LEPT: leptonema; ZYGO: zygonema; PACHY: pachynema; DIPLO: diplonema, and DIA: diakinesis. Scale bar: 20μm. B. Enlargement of region from diplotene sample shown above, showing staining for SYCP3, Sororin, and CREST serum. Centromeric staining for Sororin and CREST is indicated by the white arrowheads. Scale bar: 5μm.
Because Sororin is thought to stabilize interactions of the cohesin complex with chromatin, we also stained spreads with antibodies against the core cohesin subunit SMC3, which is a constituent of all known cohesin complexes in meiotic and somatic cells. As expected, SMC3 showed clear enrichment at the chromosome axes, beginning in leptonema (Fig. 2). This staining continues through pachynema and persisted into diplonema following chromosome desynapsis. These observation are consistent with a number of previous studies indicating that cohesin is enriched on meiotic chromosome axes prior to pairing and synapsis (Eijpe et al. 2000; Pelttari et al. 2001; Eijpe et al. 2003). We conclude from the data in Figures 1 and 2 that cohesin is enriched at the chromosome cores well before Sororin staining is detectable. While axial enrichment of SMC3 was evident in leptonema, Sororin enrichment or recruitment occurred only at regions of chromosome synapsis as they began to appear in zygonema, and remained at all synapsed regions as meiotic prophase progressed.
Figure 2. SMC3 staining is distinctly different from Sororin.
Chromosome spreads from spermatocytes of wild-type mice were immunostained with antibodies to the indicated proteins. The SMC3 subunit of cohesin shows axial enrichment prior to synapsis, as seen in leptonema sample at left. This axial staining persists through pachynema and following desynapsis in diplonema. In diakinesis centromere staining is detected. Scale bar = 20 μM
Sororin enrichment at chromosome cores correlates with the formation of a mature SC
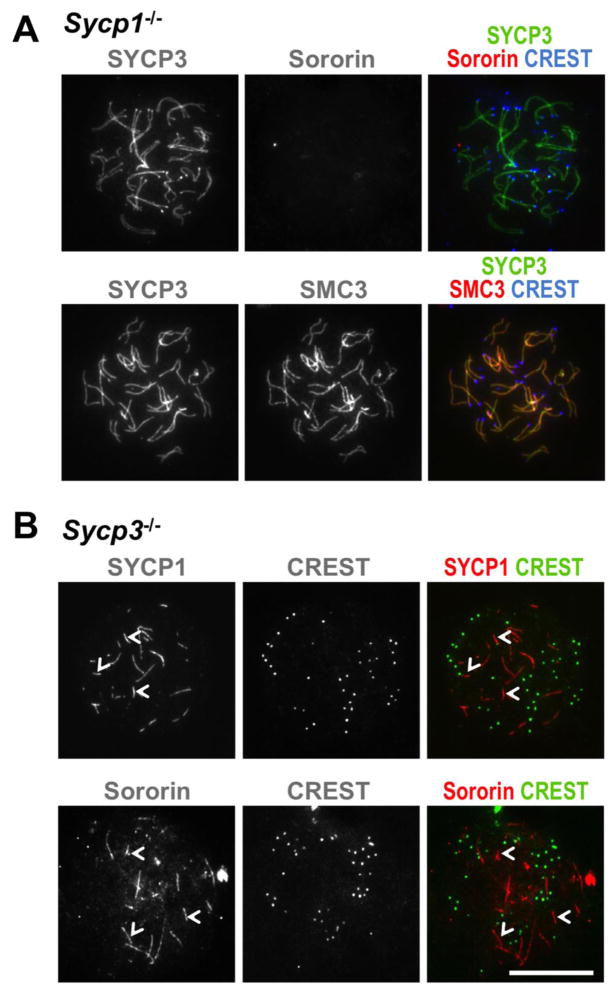
We noted in the above experiments that the enrichment of Sororin on chromosome cores correlated with the appearance of SYCP1, an SC transverse filament protein and marker of mature SC formation (Handel and Schimenti 2010). To elucidate further the requirements for enrichment of Sororin at chromosome cores and the role of SC assembly in this process, we investigated the localization of Sororin in spermatocytes from mice that do not express SYCP1. In the absence of SYCP1, chromosomes condense, close juxtaposition of the homologs is observed, axial structures form that are enriched in cohesin and SYCP3, but homologous chromosomes fail to synapse (de Vries 2005). In contrast to samples from wild-type animals, spermatocyte spreads from Sycp1−/− mice stained with anti-Sororin antibody showed no obvious enrichment of Sororin protein on particular chromosome structures (Fig. 3A). Because SYCP1 is required for synapsis and because previously we had observed a correlation between sites of Sororin enrichment and regions of chromosome synapsis, we conclude that Sororin binding at the chromosome cores is both coincident with and dependent upon synapsis. Alternatively, Sororin recruitment may depend on the presence of the SYCP1 protein per se.
Figure 3. Synaptonemal complex mutants affect Sororin localization.
Spermatocytes spreads from animals with the indicated genotypes were immunostained with antibodies as shown. A. Spreads prepared from Sycp1−/− spermatocytes were probed with antibodies to both SYCP3 and Sororin. In these samples no clear staining for Sororin was detected. B. Spreads prepared from Sycp3−/− spermatocytes were probed with CREST serum, to identify centromeres, and either anti-Sororin or anti-SYCP1 antibodies. Short linear Sororin-positive tracks (white arrowheads) are similar to those seen with anti-SYCP1 antibody from identical preparations. Scale bar = 20 μm
Sororin interacts with the cohesin complex in mitotic cells to stabilize its interaction with chromatin (Rankin et al. 2005; Nishiyama et al. 2010). Therefore, we compared Sororin localization in Sycp1−/− spermatocyte spreads to that of cohesin. Using an antibody against the universal cohesin subunit SMC3, we found, as described previously (Eijpe et al. 2000; de Vries 2005), that cohesin consistently localized to axial structures of paired, unsynapsed chromosomes in the Sycp1−/− spreads, co-localizing with the AE/LE protein SYCP3 (Fig. 3A). These data confirm that cohesin does not require assembly of the SC for axial enrichment, whereas Sororin does, and are consistent with a model in which axial enrichment of cohesin precedes both synapsis and Sororin recruitment to chromosome cores.
The axial/lateral element protein SYCP3 begins to assemble onto chromosome cores in leptonema, and forms part of the lateral element of the mature SC (Parra et al. 2004). In spermatocytes lacking SYCP3, SC assembly is abnormal, and synapsis is largely prevented though homologous pairing still occurs (Yuan et al. 2000; Kolas et al. 2004). In spite of these significant defects, short axial stretches enriched in SYCP1 can be identified in the spreads from mice lacking SYCP3, suggesting partial or incomplete attempts at synapsis (Yuan et al. 2000; Pelttari et al. 2001). To further investigate the role of individual SC components in Sororin recruitment, we stained spreads from Sycp3−/− spermatocytes with anti-Sororin antibody. In these samples we found that Sororin was enriched in short linear stretches in a manner reminiscent of SYCP1 staining in the same mutant (Fig. 3B). Although we were unable to co-stain for both Sororin and SYCP1 (available primary antibodies were both made in the same host species), the similarity in staining patterns is entirely consistent with a model in which both Sororin and SYCP1 localize to short tracks of pseudo-synapsis seen in Sycp3−/− animals.
Meiotic cohesin subunits and Sororin recruitment
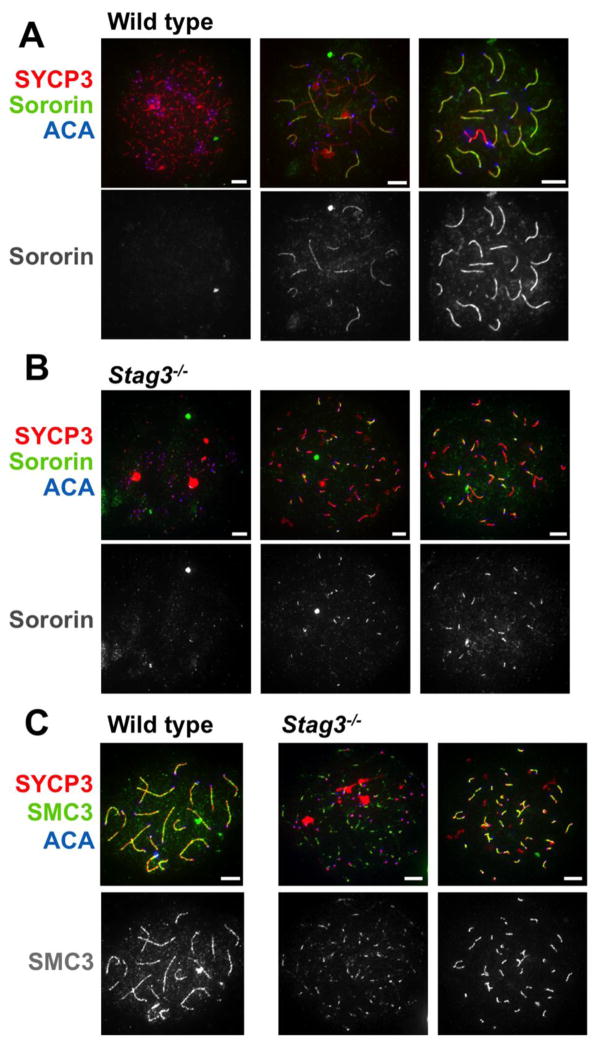
Meiotic cohesin complexes comprised of specific meiotic cohesin subunits show unique spatio-temporal localization on the chromosome axes during meiotic prophase and are essential to normal meiotic progression. To investigate the relationship between Sororin enrichment on chromosome cores and meiotic cohesin, we stained spermatocyte chromosome spreads from mice that do not express various meiotic cohesin subunits with anti-Sororin antibodies. We began with spreads from mice that have compromised expression of the meiosis-specific stromal antigen (SA) subunit STAG3. In the absence of STAG3, synapsis of homologous chromosomes largely fails, and meiosis progresses only to an early zygotene-like stage, in which short linear tracks of SC components are observed (Fukuda et al. 2014; Winters et al. 2014; Hopkins et al. 2014). These tracks may represent abortive attempts at synapsis and at least partial SC assembly, as they contain both SYCP3 and SYCP1 (Hopkins et al. 2014). In chromosome spreads from Stag3−/− spermatocytes stained with anti-Sororin antibody, some of the SYCP3-positive tracks were also positive for Sororin (Fig. 4). In this mutant, the SMC3 signal generally co-localized with the complete length of SYCP3 axes (Fig. 4C and (Hopkins et al. 2014)), whereas Sororin only partially overlapped with the SYCP3 axes. This pattern is reminiscent of the partial SYCP1 signal observed in the Stag3 mutant (Hopkins et al. 2014), and is consistent with our observations in spreads from wild-type animals, in which Sororin enrichment on chromosome axes was dependent upon synapsis or at least SYCP1 assembly (Fig. 1).
Figure 4. Sororin and meiotic cohesin.
Spreads prepared from wild-type, or Stag3−/− animals were immunostained with antibodies against Sororin and SYCP3. CREST serum immunofluorescence was used to identify centromeres. Linear tracks of Sororin, evident in the wild-type samples (A.) were not detected in the Stag3−/− spreads (B.). Instead short linear tracks that colocalize with SYCP3 were frequently found (white arrowheads). C. The cohesin subunit SMC3 is localized to linear tracks that encompass the length of the chromosome axes in wild-type spermatocytes (left). In Stag3−/− spermatocytes short tracks of SMC3 of varying lengths are seen, the longer of which colocalize with the lateral element protein SYCP3 (right). In Scale bar = 5μm
Because synapsis and SC assembly are severely compromised in the absence of STAG3 protein, it was difficult to ascribe specific stages of meiotic prophase to individual spreads from Stag3−/− spermatocytes. However, different levels of recruitment of SC proteins could be seen in individual spreads, and the length of tracks of Sororin staining appeared to correlate with the extent of the SYCP3 staining, with longer Sororin tracks coinciding with longer SYCP3 tracks. In all cases the tracks of Sororin staining were less extensive than those of SYCP3, and not all SYCP3-positive tracks showed clear evidence of Sororin localization. We conclude from this experiment that Sororin is recruited to a portion of the SC-like assemblies that form in the absence of STAG3.
Sororin and the meiotic kleisins
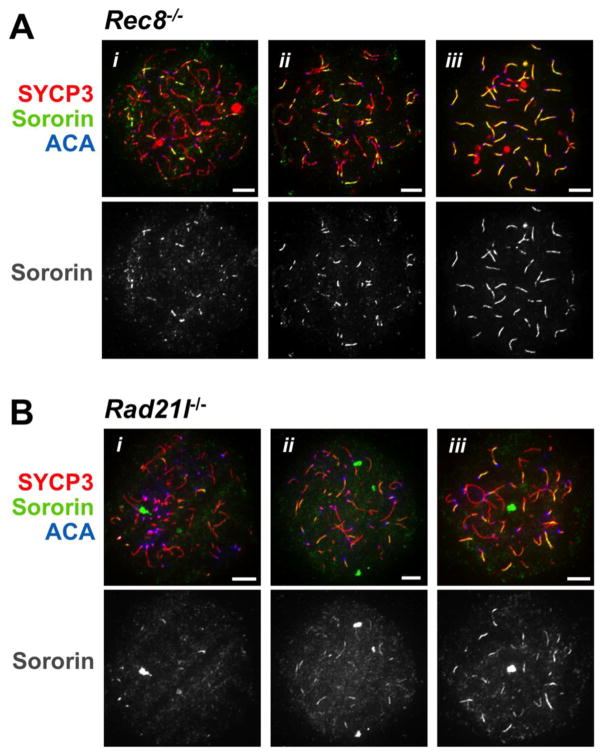
In addition to the meiosis-specific stromalin subunit STAG3, meiotic cohesin complexes also contain one of two meiosis-specific kleisin subunits: REC8 or RAD21L. In the absence of REC8, synapsis and recombination between homologous chromosomes are severely compromised, although chromosome pairing is evident (Bannister et al. 2004; Xu et al. 2005). Chromosomes in spreads from spermatocytes lacking REC8 have well-developed axes that can be detected by localization of SYCP3. Some partial synapsis may occur between homologs, and SC-like structures develop between sister chromatids (Xu et al. 2005). To further elucidate the relationship between meiotic cohesin and the axial enrichment of Sororin, we stained chromosomes spreads from Rec8−/− spermatocytes with anti-Sororin antibodies. In these preparations, several distinct staining patterns were observed. Sororin was detected at chromosome cores, and this staining overlapped with staining for SYCP3 (Fig. 5A). There was significant variability in the staining for Sororin: in some spreads, Sororin staining was limited to short patches or tracks (Fig. 5A, i, ii), while in others, nearly every chromosome axis was well-decorated with Sororin (Fig. 5A, iii). In all samples, Sororin staining colocalized with SYCP3 staining. The heterogeneity in extent of Sororin staining may reflect the non-homogeneous meiotic phenotype of Rec8−/− spermatocytes, in which up to five distinct zygotene-like stages can be distinguished in the adult testes based on patterns of SYCP3-positive staining (Xu et al. 2005). Our results indicate that in the absence of REC8 the signals that lead to axial enrichment of Sororin still develop, in spite of clearly abnormal chromosome structure and dynamics. Additionally, Sororin staining was most extensive in samples that had a mature, pachynema-like degree of chromosome compaction (Fig. 5A, panel iii). We interpret this to suggest that Sororin may be enriched on sister chromatids that have developed near continuous SYCP1 staining previously reported in Rec8−/− spermatocytes (Xu et al. 2005).
Figure 5. Sororin and meiotic kleisin subunits.
Spermatocyte chromosome spreads prepared from mice that do not express the indicated kleisin subunits were immunostained for Sororin and SYCP3. A. Shown are representative spreads from Rec8−/− animals. In these samples Sororin can be seen to decorate the length of chromosome axes of unsynapsed sister chromatid pairs. This staining is coincident with the staining for the AE/LE protein SYCP3. B. Spreads prepared from animals that do not express RAD21L were immunostained for Sororin and SYCP3. As In the Rec8−/− mutant, Sororin is coincident with the SYCP3-positive tracks, but is less extensive. Scale bar = 5μm
We also examined the role of RAD21L kleisin in Sororin axial enrichment. In the absence of RAD21L, spermatocytes show defects in synapsis and arrest in a zygotene-like state, in which SYCP3 is found in short axial tracks and SYCP1 can frequently be detected on small stretches within these tracks (Herrán et al. 2011). When spermatocyte spreads from Rad21l−/− animals were immunostained for Sororin, short linear tracks of Sororin staining were seen (Fig. 5B). As in the spreads from the Rec8−/− spermatocytes, these tracks coincided with the tracks of SYCP3 staining, but were less extensive, rarely encompassing the entire length (Fig. 5B, panels i-iii).
We conclude from these experiments that neither of the meiotic kleisins is strictly required for Sororin to load on the chromosome cores, and that Sororin enrichment depends instead upon development of SC-like structures. Although chromosome dynamics are greatly disrupted in mice lacking either kleisin subunit, in both cases linear tracks of Sororin were seen associated with chromosome cores that showed at least partial SC assembly.
Sororin localizes to central region of synapsed chromosomes
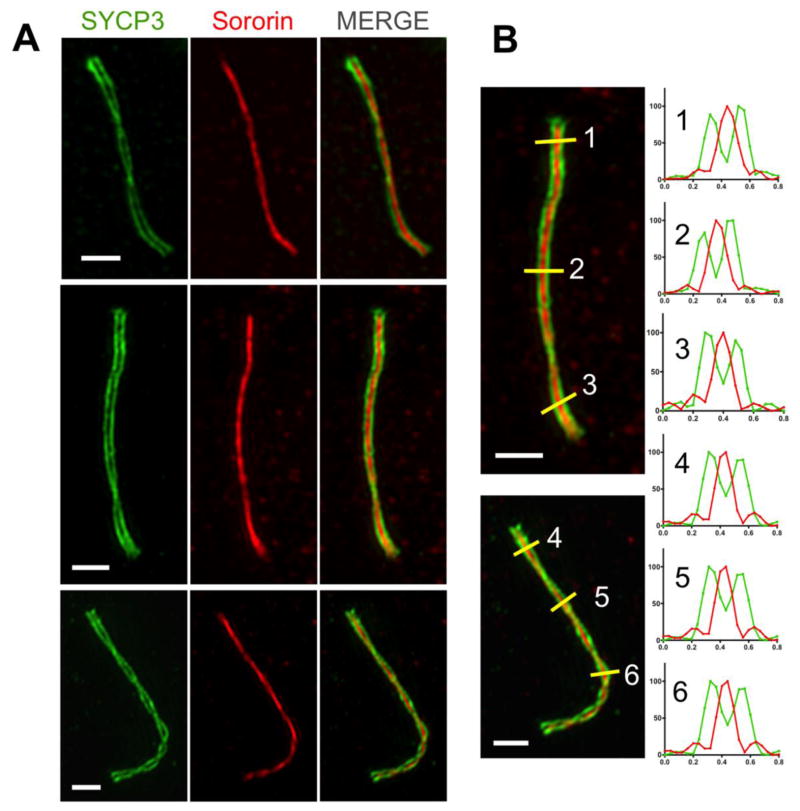
We have shown that Sororin localization to chromosome cores is dependent on the assembly of SC proteins. In particular the transverse filament protein SYCP1, a marker of mature SC assembly, seems to be critical. These results suggested the possibility that Sororin might assemble in the central region of synapsed meiotic chromosomes. This indeed has been suggested recently based on mutant analysis, but was not formally demonstrated (Gómez et al. 2016). To determine the precise relationship between the location of SC proteins and enrichment of Sororin on the chromosome cores, we used three-dimensional structured illumination microscopy (SIM). Spermatocyte chromosome spreads from wild-type mice were costained for Sororin and the AE/LE protein SYCP3. Strikingly, the Sororin signal was limited to the central regions of synapsed chromosomes (Fig. 6). Condensed pachytene chromosomes were easily identified by fully aligned paired tracks of SYCP3 signals (Fig. 6A). Some chromosomes were relatively straight while others formed a helical twist (Fig. 6A, bottom example). In the twisted chromosomes, Sororin staining was most evident along the entire chromosome length, and could be seen between lines of SYCP3 staining in the regions that lay flat relative to the slide.
Figure 6. Sororin localizes to the central region of synapsed chromosomes.
Structured illumination microscopy was used to investigate the relationship between Sororin and the lateral elements of synapsed chromosomes. A. Three examples of individual pachytene bivalents stained for SYCP3 (green) and Sororin (red). B. The spatial relationship between SYCP3 and Sororin was determined by measuring intensity profiles in both channels over three regions on each of two chromosomes from two different spreads (1–6, yellow lines). The intensity profiles of the yellow lines are graphed at right, with the green and red traces representing SYCP3 and Sororin, respectively. Each trace is normalized to an intensity range of 0–100. Note that a single peak of Sororin signal lies between two peaks of SYCP3 signal. Scale bars: 1 micron.
To confirm that Sororin localization was limited to the central region, we performed image analysis, quantitating the signal strength of both Sororin and SYCP3 relative to each other. These analyses confirmed that Sororin was localized to the region between the peaks of SYCP3 signals (Fig. 6B). In all cases where two signals of SYCP3 staining could be resolved, a single peak of Sororin signal was evident between them. These results indicate that the majority of detectable Sororin is localized to the central region of the SC in synapsed chromosome pairs.
Discussion
Sororin binds meiotic chromosomes at regions of synapsis
We have investigated the localization of Sororin on chromosomes during meiotic prophase. Our results are summarized in the cartoon shown in Figure 7. During mitotic cell divisions, the binding of Sororin to cohesin on chromosomes occurs concurrently with DNA replication and represents a stage during the cell cycle during which cohesin is stabilized on chromatin (Gerlich et al. 2006). A recent study demonstrated that Sororin accumulates on meiotic chromosomes in prophase (Gómez et al. 2016). To explore the possibility that a Sororin-dependent cohesin stabilization event occurs during meiotic chromosome development, we investigated the localization of Sororin on chromosomes during meiotic prophase. Interestingly, Sororin shows a localization pattern distinct from that seen for meiotic cohesin, which was previously shown to colocalize with SYCP3 (Agostinho et al. 2016). The results indicate that Sororin is enriched on chromosome cores at regions of synapsis between homologous chromosomes, with dynamics apparently related to formation of the tripartite SC complex, rather than the axial enrichment of cohesin. Furthermore, the time at which Sororin enrichment is seen is not explained by loading of the Sororin protein in response to DNA replication. This result was quite surprising as the cohesin complex, the only known interacting partner of Sororin, is enriched at the AE/LE of chromosomes well before homolog synapsis. Furthermore, our observations cannot be explained by localization of the cohesin accessory factor and immediate binding partner of Sororin, PDS5. PDS5 is localized to axial cores of meiotic chromosomes well before synapsis (Fukuda and Höög 2010), our unpublished observations).
Figure 7. Summary of Sororin localization in wild type and mutant primary spermatocytes.
Schematic illustrations depicting the localization of Sororin in spermatocytes in various genetic backgrounds. A. Wild-type: Observed localization of Sororin to chromosome cores during meiotic progression, which is depicted from left to right. L = leptonema, Z = zygonema, P = pachynema, DIP = diplonema, DIA = diakinesis. Linear enrichment of Sororin is seen from late zygonema and peaks during pachynema at regions of chromosome synapsis. Shorter tracks of enrichment are seen in diplonema at regions that remain synapsed, as well as at centromeres (not shown). (*) We include an asterisk to indicate that this precise localization of Sororin on top of SYCP1 is in all cases speculative. B. Sycp1−/−: Staining for Sororin is not detected in spreads from animals that do not express SYCP1, though axial SYCP3 is clearly present. C. Sycp3−/− or Stag3−/−: Short tracks of Sororin staining were seen in both of these backgrounds. These tracks, which colocalized with SYCP3 in the Stag3−/− samples, likely coincide with partial synaptic events that have previously been observed and characterized (Hopkins et al. 2014; Ward et al. 2016). D. Rec8−/−: Long tracks of Sororin staining were seen in primary spermatocyte chromatin spreads from animals that do not express the REC8 kleisin. These likely represent pseudo-synapsis between sister chromatids, as homolog synapsis fails in this mutant.
Sororin associates with the central element of the SC
We have shown that Sororin is localized to the central region of synapsed chromosomes. In partially synapsed chromosomes, for example during desynapsis in diplotene, Sororin is enriched only on regions that remain synapsed and at the centromeres, which remain paired. Collectively, our data suggest that synapsis, or at least the presence of SC central region components, is a prerequisite for Sororin binding. We were interested to note that in spreads from Rec8−/− mice Sororin was found on chromosome cores, in spite of the fact that synapsis between homologs is clearly compromised in this mutant. However, although REC8 cohesin prevents intra-sister synapsis, it is not essential for synapsis-like events to occur (Bannister et al. 2004; Xu et al. 2005). In the absence of REC8 the ability to distinguish between homolog and sister is apparently lost. This, in combination with decreased homolog pairing, results in ectopic synapsis and SC assembly between sister chromatids. Our data indicate that this pseudo-synapsis is sufficient to promote the Sororin binding. Similarly, in the Stag3−/− spreads, in which synapsis is thought to occur at least in part between sister chromatids (Hopkins et al. 2014), tracks of Sororin assembly were also evident. The correlation between SC assembly and Sororin binding was borne out in our analysis of spreads from mice lacking the transverse filament protein SYCP1, which show a complete failure in synapsis, and no Sororin staining. These striking results confirm that axial cohesin enrichment is not sufficient to recruit Sororin, as axial staining for SMC3 is robust in the absence of SYCP1. Also consistent with a requirement for SC assembly, short tracks of Sororin were seen in spreads with partial SC assembly, such as in the Sycp3 or Rad21l mutant backgrounds. Finally, like Sororin, SYCP1 is retained at centromeres in diplotene cells, where association between homologs persists even when the chromosome arms have desynapsed, (Bisig et al. 2012; Qiao et al. 2012). We have shown that Sororin not only depends on SC development for chromosome binding, but that it is also localized to the central region of synapsed chromosome pairs. The results suggest that Sororin may bind proteins other than cohesin, such as central element components of the SC. Alternatively, Sororin may interact with only a minor subset of cohesin complexes to promote synapsis, recombination, and centromere pairing between homologous chromosomes.
During preparation of this manuscript a similar report was published, in which it was shown that Sororin is recruited to regions of chromosome synapsis in meiosis (Gómez et al. 2016). Here we have shown unambiguously that this occurs at the central region by utilizing high-resolution microscopy. We have shown that single tracks of Sororin lie between lateral elements. The results we present here are broadly consistent with the data presented by Gomez et al. but provide critical localization information not present in that previous report. While Gomez et al. demonstrated that central element formation was essential for Sororin recruitment to the chromosome cores, they did not show the precise localization of Sororin (Gómez et al. 2016). Our data rule out the possibility that Sororin is recruited laterally, but in response to signals downstream of central element formation or maturation.
Signals that promote enrichment of Sororin on chromosome cores
What leads to enrichment of Sororin on synapsed chromosomes during meiotic prophase? We know of no other cohesion protein that shows the same pattern of localization. In mitotic cells, Sororin is recruited to interact with cohesin and thus associates with chromatin in response to DNA replication (Lafont et al. 2010; Nishiyama et al. 2010; Song et al. 2012). This interaction is promoted by Esco2-dependent modification of cohesin and requires active DNA replication and interaction of Esco2 with the replication machinery. In the absence of DNA replication the SMC3 subunit of cohesin is acetylated by ESCO2 but Sororin is not recruited (Song et al. 2012). The data we present here suggest that in meiosis Sororin recruitment to chromosome axes occurs much later than pre-meiotic S phase, and thus must occur through a different mechanism. Pre-meiotic S phase, during which REC8 and RAD21L cohesin are loaded onto chromatin, occurs at a stage before which we detect specific Sororin staining. Indeed, cohesin clearly shows axial enrichment even under conditions in which Sororin staining is undetectable, such as in Sycp1−/− spermatocytes. It is possible that Sororin is diffusely recruited to chromatin during pre-meiotic S, and subsequently translocated to the chromosome cores during synapsis. This recruitment of Sororin to chromosome cores well after DNA replication is not the only example of post-replication binding of cohesion factors during meiotic prophase. Several recent studies indicate that cohesion continues to develop during meiotic prophase, well after the time at which DNA replication is complete. For example, RAD21 in mammals, and COH3/4 in nematodes are recruited to chromosome axes after pairing and the initiation of recombination (Lee and Hirano 2011; Severson and Meyer 2014). Like these meiosis-specific cohesin proteins, Sororin may be recruited at a specific stage in meiotic progression to ensure stabilization of specific chromosome interactions.
We imagine two likely explanations for why Sororin shows a distinctly different localization pattern than cohesin and PDS5 on meiotic chromosomes. In the first model, Sororin might interact only with a minor sub-pool of cohesin, perhaps to stabilize cohesin-dependent events at this time and place. The enrichment of Sororin on pachytene chromosomes may reflect a stabilization of the interaction between homologous chromosomes through this minor pool of cohesin. In fact, Sororin may interact specifically with cohesin complexes that are recruited following synapsis, such as those containing the mitotic kleisin subunit RAD21 (Lee and Hirano 2011). An intriguing alternative model is that Sororin has a different interacting partner in meiosis than it does in mitotic cells. The dependence of Sororin binding on the transverse filament protein SYCP1, as well as the localization of Sororin to the central region of synapsed chromosomes, are consistent with a model in which Sororin interacts directly with the SYCP1 protein itself or another component found in the central region, perhaps to promote the structural stability of the SC. This idea provides an interesting topic for biochemical investigation. Recently it was found that Sororin still binds meiotic chromosomes in the absence of the central element protein SYCE3, so this protein is not a likely candidate (Gómez et al. 2016). In addition, because neither SYCE1, nor SYCE2, nor TEX12 load onto chromosome cores in the absence of SYCE3 (Fraune et al. 2012), we can also rule these out as immediate interacting partners of Sororin. We note that the molecular basis for the interaction between Sororin and the cohesion protein PDS5 has recently been described, and may provide a useful analogy for investigation of a potential Sororin-SC protein interaction (Ouyang et al. 2016). The identification of the meiosis-specific partners of Sororin will provide important information about how Sororin contributes to meiotic chromosome dynamics.
Materials and Methods
Mice
The Stag3 mouse mutant strain used in this study has previously been described (Hopkins et al. 2014; Ward et al. 2016). Briefly, the Stag3 allele was created by integration of the SB-cHS4core-SB-Tyro-WPRE-FUGW lentiposon transgene (LV2229) in intron 8 of Stag3 (http://www.mmrrc.org/catalog/sds.php?mmrrc_id=36275) in an FVB background. The Rec8 (B6;129S4-Rec8mei8) mutant mice used in our study has previously been described (Bannister et al. 2004). The Rad21l mouse mutant strain (B6N(Cg)-Rad21ltm1b(KOMP)Wtsi/2J) used in this study was previously described (Ward et al. 2016). Sycp1 and Sycp3 mutant mice have been described previously (Yuan et al. 2000; de Vries 2005).
Antibodies
| Antigen | Host | Source | Catalog # | Dilution | Reference |
|---|---|---|---|---|---|
| Sororin | rabbit | Rankin lab | - | 1 to 150 | (Wu et al. 2011) |
| CREST | human | Antibodies Inc. | 15–235 | 1 to 50 | |
| SYCP3 | mouse | Santa Cruz | SC-74569 | 1 to 50 | |
| SYCP3 | chicken | Pezza lab | - | ||
| SMC3 | rabbit | Rankin lab | - | 1 to 100 | (Song et al. 2012) |
| SYCP1 | rabbit | Novus | NB300-229 | 1 to 150 | |
| PDS5B | rabbit | Bethyl | A300–538A | 1:1000 (immunoblot) |
Immunofluorescence
Established experimental approaches were used for the visualization of chromosomes in surface spreads (Peters et al. 1997). Incubations with primary antibodies were carried out for 1 h at room temperature in PBS plus 2% BSA. All secondary antibodies were from Jackson Immunoresearch Laboratories and were used at a dilution of 1:300. Slides were subsequently counterstained for 3 minutes with 2 μg/ml DAPI containing Vectashield mounting solution (Vector Laboratories) and sealed with nail varnish.
Microscopy
Broad field epifluorescence images were collected using a Roper CoolSNAP HQ2 camera on a Zeiss Axio Imager 7.1 with a 100×/1.4 numerical aperture (NA) objective or a Zeiss Axioplan 2ie fitted with a 63×/1.4 NA objective microscope. Images were processed and analyzed using AxioVision software. Structured illumination images were collected on a GE Deltavision OMXSR Super resolution microscope using 3D SIM imaging. The images were captured at 1024×1024 on liquid cooled sCMOS cameras. The objective was an Olympus 60X Plan APO with a N.A. of 1.42. SIM images were analyzed using ImageJ, and data were plotted using Prism (GraphPad Software, La Jolla).
Protein samples
For rapid lysis, the internal cell mass from a single adult testis was extruded into hot sample buffer and boiled immediately. For cell fractionation, approximately 107 cells were isolated from macerated adult testes, and subjected to fractionation as described (Méndez and Stillman 2000). Plasmids expressing either GFP-mSororin, or GFP alone, under the control of the CMV promoter were introduced by transient transfection into cultured HeLa cells using Lipofectamine 2000 (Invitrogen). Cells were lysed in sample buffer and boiled.
Ethics statement
All mice were bred at Johns Hopkins University (JHU, Baltimore, MD) or at Oklahoma Medical Research Foundation (OMRF, Oklahoma City, OK) under standard conditions in accordance with the National Institutes of Health and U.S. Department of Agriculture criteria and protocols for their care and use were approved by the Institutional Animal Care and Use Committees (IACUC) of JHU and OMRF.
Supplementary Material
A. Adult testes were fractionated and assayed for the indicated proteins by immunoblot. PDS5B, SMC3, SYCP1, and SYCP3 all were enriched in the chromatin fraction. Mouse Sororin levels were below detectable limits in these samples by immunoblot, and therefore this antibody was tested by transient expression in cultured human somatic cells, as shown in (B.). B. A human cultured cell line was transfected with a plasmid encoding a GFP-mSororin fusion protein. This protein was detectable with our anti-hSororin antibody as a band at the appropriate molecular weight.
Acknowledgments
We are grateful to Christer Hoog for providing the Sycp1 mutant mouse John Schimenti for providing Sycp3 and Rec8 mutant mice, and Dean Dawson for careful review of the manuscript. We are also grateful the excellent technical support of Ben Fowler and Julie Crane of the OMRF Imaging Core in the collection of 3D SIM images. The OMXSR Super resolution microscope was recently purchased with generous support of the Oklahoma Center for Adult Stem Cell Research.
List of abbreviations
- SC
synaptonemal complex
- SA
stromal antigen
- AE
axial element
- LE
lateral element
References cited
- Agostinho A, Manneberg O, van Schendel R, et al. High density of REC8 constrains sister chromatid axes and prevents illegitimate synaptonemal complex formation. EMBO Reports. 2016;17:901–913. doi: 10.15252/embr.201642030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. doi: 10.1002/gene.20085. [DOI] [PubMed] [Google Scholar]
- Bisig CG, Guiraldelli MF, Kouznetsova A, et al. Synaptonemal complex components persist at centromeres and are required for homologous centromere pairing in mouse spermatocytes. PLoS Genet. 2012;8:e1002701. doi: 10.1371/journal.pgen.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas U, Wetzker C, Lange J, et al. Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions. PLoS Genet. 2013;9:e1003985. doi: 10.1371/journal.pgen.1003985.s012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon CK, Hawley RS. Regulating the construction and demolition of the synaptonemal complex. Nature Publishing Group. 2016;23:369–377. doi: 10.1038/nsmb.3208. [DOI] [PubMed] [Google Scholar]
- Costa Y, Speed R, Ollinger R, et al. Two novel proteins recruited by synaptonemal complex protein 1 (SYCP1) are at the centre of meiosis. J Cell Sci. 2005;118:2755–2762. doi: 10.1242/jcs.02402. [DOI] [PubMed] [Google Scholar]
- de Vries FAT. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005;19:1376–1389. doi: 10.1101/gad.329705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, et al. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci. 1994;107( Pt 10):2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Heyting C, Gross B, Jessberger R. Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci. 2000;113( Pt 4):673–682. doi: 10.1242/jcs.113.4.673. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, et al. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol. 2003;160:657–670. doi: 10.1083/jcb.200212080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraune J, Schramm S, Alsheimer M, Benavente R. The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Experimental Cell Research. 2012;318:1340–1346. doi: 10.1016/j.yexcr.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Daniel K, Wojtasz L, et al. A novel mammalian HORMA domain-containing protein, HORMAD1, preferentially associates with unsynapsed meiotic chromosomes. Experimental Cell Research. 2010;316:158–171. doi: 10.1016/j.yexcr.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Fukuda N, Agostinho A, et al. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 2014;33:1243–1255. doi: 10.1002/embj.201387329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Höög C. The Mouse Cohesin-Associated Protein PDS5B Is Expressed in Testicular Cells and Is Associated with the Meiotic Chromosome Axes. Genes (Basel) 2010;1:484–494. doi: 10.3390/genes1030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T. Human Wapl Is a Cohesin-Binding Protein that Promotes Sister-Chromatid Resolution in Mitotic Prophase. Current Biology. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich D, Koch B, Dupeux F, et al. Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr Biol. 2006;16:1571–1578. doi: 10.1016/j.cub.2006.06.068. [DOI] [PubMed] [Google Scholar]
- Gómez R, Felipe-Medina N, Ruiz-Torres M, et al. Sororin loads to the synaptonemal complex central region independently of meiotic cohesin complexes. EMBO Reports. 2016;17:695–707. doi: 10.15252/embr.201541060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Caballero C, Herrán Y, Sánchez-Martín M, et al. Identification and molecular characterization of the mammalian α-kleisin RAD21L. Cell Cycle. 2011;10:1477–1487. doi: 10.4161/cc.10.9.15515. [DOI] [PubMed] [Google Scholar]
- Hamer G, Wang H, Bolcun-Filas E, et al. Progression of meiotic recombination requires structural maturation of the central element of the synaptonemal complex. J Cell Sci. 2008;121:2445–2451. doi: 10.1242/jcs.033233. [DOI] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Herrán Y, Gutiérrez-Caballero C, Sánchez-Martín M, et al. The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J. 2011;30:3091–3105. doi: 10.1038/emboj.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J, Hwang G, Jacob J, et al. Meiosis-Specific Cohesin Component, Stag3 Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes. PLoS Genet. 2014;10:e1004413. doi: 10.1371/journal.pgen.1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. Meiotic Recombination: The Essence of Heredity. Cold Spring Harb Perspect Biol. 2015 doi: 10.1101/cshperspect.a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K-I, Kim J, Fujiyama-Nakamura S, et al. A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Reports. 2011;12:267–275. doi: 10.1038/embor.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Guacci V, Yu H-G. Pds5 is required for homologue pairing and inhibits synapsis of sister chromatids during yeast meiosis. J Cell Biol. 2009;186:713–725. doi: 10.1083/jcb.200810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami A, Sakuno T, Yamagishi Y, et al. Acetylation regulates monopolar attachment at multiple levels during meiosis I in fission yeast. EMBO Reports. 2011;12:1189–1195. doi: 10.1038/embor.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolas NK, Yuan L, Höög C, et al. Male mouse meiotic chromosome cores deficient in structural proteins SYCP3 and SYCP2 align by homology but fail to synapse and have possible impaired specificity of chromatin loop attachment. Cytogenet Genome Res. 2004;105:182–188. doi: 10.1159/000078188. [DOI] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, et al. Wapl Controls the Dynamic Association of Cohesin with Chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lafont AL, Song J, Rankin S. Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc Natl Acad Sci USA. 2010;107:20364–20369. doi: 10.1073/pnas.1011069107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Offenberg HH, van Aalderen M, et al. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 1994;14:1137–1146. doi: 10.1128/mcb.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hirano T. RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol. 2011;192:263–276. doi: 10.1083/jcb.201008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Iwai T, Yokota T, Yamashita M. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci. 2003;116:2781–2790. doi: 10.1242/jcs.00495. [DOI] [PubMed] [Google Scholar]
- Liu H, Rankin S, Yu H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol. 2012;15:40–49. doi: 10.1038/ncb2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen RL, Offenberg HH, Dietrich AJ, et al. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992;11:5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Ladurner R, Schmitz J, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143:737–749. doi: 10.1016/j.cell.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Novak I, Wang H, Revenkova E, et al. Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol. 2008;180:83–90. doi: 10.1083/jcb.200706136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenberg HH, Schalk JA, Meuwissen RL, et al. SCP2: a major protein component of the axial elements of synaptonemal complexes of the rat. Nucleic Acids Research. 1998;26:2572–2579. doi: 10.1093/nar/26.11.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Zheng G, Tomchick DR, et al. Structural Basis and IP6 Requirement for Pds5-Dependent Cohesin Dynamics. Mol Cell. 2016;62:248–259. doi: 10.1016/j.molcel.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MT, Viera A, Gómez R, et al. Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci. 2004;117:1221–1234. doi: 10.1242/jcs.00947. [DOI] [PubMed] [Google Scholar]
- Pelttari J, Hoja MR, Yuan L, et al. A meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol Cell Biol. 2001;21:5667–5677. doi: 10.1128/MCB.21.16.5667-5677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–68. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
- Pezzi N, Prieto I, Kremer L, et al. STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3-related genes flanking the Williams-Beuren syndrome deletion. FASEB J. 2000;14:581–592. doi: 10.1096/fasebj.14.3.581. [DOI] [PubMed] [Google Scholar]
- Prieto I, Suja JA, Pezzi N, et al. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol. 2001;3:761–766. doi: 10.1038/35087082. [DOI] [PubMed] [Google Scholar]
- Qiao H, Chen JK, Reynolds A, et al. Interplay between synaptonemal complex, homologous recombination, and centromeres during mammalian meiosis. PLoS Genet. 2012;8:e1002790. doi: 10.1371/journal.pgen.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. Complex elaboration: making sense of meiotic cohesin dynamics. FEBS J. 2015 doi: 10.1111/febs.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18:185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting C, et al. Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol. 2004;6:555–562. doi: 10.1038/ncb1135. [DOI] [PubMed] [Google Scholar]
- Schramm S, Fraune J, Naumann R, et al. A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLoS Genet. 2011;7:e1002088. doi: 10.1371/journal.pgen.1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, Meyer BJ. Divergent kleisin subunits of cohesin specify mechanisms to tether and release meiotic chromosomes. Elife. 2014;3:e03467. doi: 10.7554/eLife.03467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lafont A, Chen J, et al. Cohesin Acetylation Promotes Sister Chromatid Cohesion Only in Association with the Replication Machinery. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.400192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Gieffers C, et al. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol. 2000;151:749–762. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Hopkins J, Mckay M, et al. Genetic Interactions Between the Meiosis-Specific Cohesin Components, STAG3, REC8, and RAD21L. G3 (Bethesda) 2016;6:1713–1724. doi: 10.1534/g3.116.029462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters T, McNicoll F, Jessberger R. Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. EMBO J. 2014;33:1256–1270. doi: 10.1002/embj.201387330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FM, Nguyen JV, Rankin S. A Conserved Motif at the C Terminus of Sororin Is Required for Sister Chromatid Cohesion. Journal of Biological Chemistry. 2011;286:3579–3586. doi: 10.1074/jbc.M110.196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley M, Verschoor S, et al. A new role for the mitotic RAD21/SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Reports. 2004;5:378–384. doi: 10.1038/sj.embor.7400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WD, et al. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8:949–961. doi: 10.1016/j.devcel.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Zhao J, et al. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83. doi: 10.1016/s1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- Zhang B, Jain S, Song H, et al. Mice lacking sister chromatid cohesion protein PDS5B exhibit developmental abnormalities reminiscent of Cornelia de Lange syndrome. Development. 2007;134:3191–3201. doi: 10.1242/dev.005884. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ren Q, Yang H, et al. Budding yeast PDS5 plays an important role in meiosis and is required for sister chromatid cohesion. Mol Microbiol. 2005;56:670–680. doi: 10.1111/j.1365-2958.2005.04582.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Adult testes were fractionated and assayed for the indicated proteins by immunoblot. PDS5B, SMC3, SYCP1, and SYCP3 all were enriched in the chromatin fraction. Mouse Sororin levels were below detectable limits in these samples by immunoblot, and therefore this antibody was tested by transient expression in cultured human somatic cells, as shown in (B.). B. A human cultured cell line was transfected with a plasmid encoding a GFP-mSororin fusion protein. This protein was detectable with our anti-hSororin antibody as a band at the appropriate molecular weight.