Abstract
Objectives
Anxiety diagnoses occur in 17.1% in people age 65 years and older. Individuals with anxiety may be at a higher risk of the development of probable Alzheimer’s disease (AD). Previous literature has suggested that anxiolytic medications may exacerbate the risk of AD development. This study explored anxiolytic medication as a potential moderator of AD risk in older adults.
Methods
A secondary data analysis of the National Alzheimer’s Coordinating Center Uniform Data Set was undertaken, analyzing observations from 12,083 participants with normal cognition at the first visit. Survival analysis was utilized to examine if anxiolytic medication use by those with anxiety and/or APOE ε4 moderates the hazard of AD and/or MCI development.
Results
The hazard of probable AD (HR = 3.50, [2.77 – 4.44], p <.0001) or MCI (HR = 2.13, [1.85–2.44], p <.0001) development was statistically significant for those with anxiety. This hazard was no longer statistically significant when specific anxiolytics were used. ε4 carriers experienced a statistically significant hazard of AD (HR = 1.92, [1.52–2.41], p <.001) and MCI (HR = 1.17, [1.04–1.32], p <.05) development. This effect was moderated by the use of anxiolytics.
Discussion
The results of this study suggest that anxiolytics may moderate the effect of anxiety on MCI and AD development, specifically indicating a neutralized hazard for those with ε4 carriers with anxiety.
Keywords: alzheimer’s disease, anxiolytics, anxiety, apolipoprotein ε4, mild cognitive impairment, benzodiazepine
Introduction
As the most common mood disorder category among the elderly population, anxiety diagnoses occur in 17.1% in people aged 65 years and older (Kirmizioglu et al., 2009). Individuals with anxiety may experience a higher risk of Alzheimer’s disease (AD) development (Pietrzak et al., 2015). Some researchers suggest that the use of anti-anxiety medications further exacerbates the risk of developing AD (Rosenberg, 2015a). This study explores the link between anxiety, anti-anxiety medication, and AD dementia development, exploring anxiolytic medication as a moderator of AD risk.
Risk factors of AD dementia development
Studies have suggested a possible genetic link to the development of AD dementia, which is often attributed in part to apolipoprotein e (APOE) in sporadic late-onset cases. Although APOE ε3 is the most common gene expression, White APOE ε4 carriers appear to possess the highest risk of developing AD (Alzheimer’s Association, 2015; Tang et al., 1998). This gene has commonly been associated with functional and memory impairments (Farlow et al., 2004). APOE ε4 carrier status also poses a greater risk of hippocampal atrophy in homozygous elderly carriers (Lemaître et al., 2005) and increases the risk of developing AD dementia (Alzheimer’s Association, 2015). An accumulation of amyloid beta peptides resulting in an increased quantity of amyloid plaques in the brain is hypothesized to occur in individuals who ultimately demonstrate the clinical manifestation of AD (O’Brien and Wong, 2011). Although under debate, several mechanisms, both genetic and environmental, may inhibit the process of amyloid clearance in the brain, thereby creating favorable conditions for amyloid beta accretion and deposition.
Approximately 20 to 30% of the individuals in the United States carry the APOE ε4 allele (Alzheimer’s Association, 2015). Heterozygous or homozygous ε4 carrier status is not a definitive predictor of the development of AD dementia. Advanced age (over 60 years), particularly over the age of 85, increases risk of the disease (Alzheimer’s Association, 2015). Mental health conditions such as chronic anxiety and neuroticism can have an adverse effect on cognition, especially in APOE ε4 carriers (Caselli et al., 2004; Dar-Nimrod et al., 2012; Johansson et al., 2014; Robertson et al., 2005).
Both APOE ε4 and anxiety are linked to AD, although the exact causal pathway is still unknown. Raber (2007) found that APOE ε4 may have a regulatory effect on anxiety in laboratory mice, as measured by time spent in an elevated maze with exposed arms, by mediating histamine receptor signaling and generating adrenal gland steroids. In APOE ε4 adult mice, the central nucleus of the amygdala, which is essential in the regulation of anxiety, showed pathological alterations from wildtype counterparts (Raber, 2007).
Mild cognitive impairment, AD dementia, and anxiety
Researchers have suggested that the majority of individuals diagnosed with MCI also present with neuropsychiatric symptomology, including depression, apathy, and anxiety (Palmer et al., 2007; Penna, 2013). Despite the association between neuropsychiatric symptoms, MCI, and development of dementia, it is unclear whether these symptoms are predictors of the transition from MCI to dementia (Devier et al., 2009; Edwards et al., 2009; Mah et al., 2004; Penna, 2013).
Ramakers et al. (2013) suggested that those individuals with MCI and anxiety had abnormal levels of t-tau and Aβ42 in their spinal fluid. Similar results were not observed with those experiencing depression or apathy and MCI (Ramakers et al., 2013). Palmer et al. (2007) found that the majority (over 84%) of patients diagnosed with MCI and co-occurring anxiety developed AD dementia after study follow-up. With each new symptom of anxiety, such as irritability, sleep disturbance, or uncontrollable worry, the risk of developing AD dementia doubled in individuals with MCI within a 3-year period (Palmer et al., 2007).
Studies have investigated the impact of depression on AD development (Burke et al., 2011, Bunce et al., 2012; Byers and Yaffe, 2011); however, the examination of anxiety as a predictor or risk factor associated with AD dementia remains understudied. While some studies suggest that anxiety is not a predictor of AD dementia (Devier et al., 2009), others support the link between anxiety and AD development risk. Alzheimer’s Disease Neuroimaging Initiative (ADNI) data was examined to explore anxiety as a predictor to AD dementia (Mah et al., 2004), finding an increased rate of progression from amnestic MCI to AD dementia by level of anxiety, when controlling for the effect of cognitive decline and depression. Increased anxiety levels are hypothesized to increase amyloid beta, thereby increasing the rate of cognitive decline prior to the development of AD dementia (Pietrzak et al., 2015). This may be due to oxidative stress resulting from the increased allostatic load of pathological anxiety (Goldstein, 2012; Hovatta et al., 2005; Rammal et al., 2008a; Rammal et al., 2008b). Despite the correlation between inflammation, chronic anxiety, and neurodegeneration, conflicting results pertaining to the prediction of AD development have highlighted the need for more comprehensive investigations.
Methods
In order to investigate the role of anxiolytics in moderating the risk of AD posed by anxiety independently and synergistically with APOE ε4, a secondary data analysis of the National Alzheimer’s Coordinating Center Uniform Data Set was undertaken. The variables utilized for this study included self-reported anxiety, the use of anxiolytics, and APOE genotype. Sporadic late-onset AD was an outcome of interest and is referred to as probable AD throughout this study. MCI was examined as an alternative endpoint of interest. Those with normal cognition in their first visit (n = 12,083) comprised the analytic sample. Prior to conducting any analysis, this study received approval from the Florida International University Institutional Review Board.
Apolipoprotein E was measured by the presence or absence of ε4, denoted by ε4 carrier and non-carrier. An ε4 carrier may have one or two ε4 alleles, while a non-carrier has other combinations of APOE, none of which contain ε4. The anxiolytics examined included a general category anxiolytic, alprazolam, clonazepam, lorazepam, paroxetine, and venlafaxine.
Descriptive analyses were employed for all relevant variables to determine frequencies and distributions of predictor variables and covariates. Survival analysis was utilized to test the hypothesis that anxiolytic medication use by those with anxiety and/or APOE ε4 moderates the hazard of AD development. An event was defined as a diagnosis of MCI, and secondarily, and independently, as the diagnosis of probable AD by a subject’s last observation. Time zero was equal to the subject’s first observation (visit number 1), and time was measured in days. True survival time was unknown unless a participant developed clinically observable MCI or AD by their last observation. The independent and/or synergistic effect of anxiety, ε4 carrier status, and the use of anxiety medication was examined in relation to time to the diagnosis of MCI or AD using the Cox proportional hazards model (Cox, 1972). Efron approximation was used as a technique for handling ties (Efron, 1977). To test the moderation effect of anxiolytic medication, a Cox regression model was employed for each anxiolytic category, including participants who took the medication, who did not take the medication, and who omitted their response. For each category, four models were used to examine the unadjusted main effects and adjusted main effects by different potential confounders. The main effects were examined unadjusted in the first model, and were adjusted for biological sex, age, education, race, and Hispanic origin in the second model. A further adjustment for ε4 carrier status was added in the third model, and the final model included all previous covariates with the addition of the use of AD medication. Although not displayed in the tables, depression was controlled experimentally given its status as a known comorbid disorder. Controlling for depression in the final model made no difference in the significance level of the hazard. The assumption of proportionality was examined in order to determine whether the Cox proportional hazards assumption had been met. The statistical program STATA (StataCorp, Release 14, 2015) was utilized for the analyses, and a p value <0.05 was considered statistically significant.
Results
The minimum amount of time under observation was 208 days until the first occasion that the AD diagnosis occurred, and the maximum was 3458 days (M = 1549.38; Mdn: 1456; SD: 2305.17). The mean number of visits for those with normal cognition was three, with a range of one to ten visits. There were 361 diagnoses of AD dementia by the end of the observation period among older adults who had at least an initial visit as well as a follow-up visit (analytical sample = 9138). Similarly, the minimum amount of time under observation was 171 days until the first diagnosis of MCI occurred, and the maximum was 3458 days (M = 1437.81; Mdn: 1207; SD: 2196.98). There were 1520 diagnoses of MCI by the end of the observation period among older adults who had at least an initial visit as well as a follow-up visit (analytical sample = 9184). The mean age of subjects with normal cognition at visit one was 71.05 (SD: 10.86; Mdn: 72). At visit one, 80.5% of the sample were White, 13.01% were African American, and 5.95% were from other ethnic groups. Six percent of the sample reported Hispanic origin. Almost 35% of subjects reported that their mother had been diagnosed with dementia, while 17.75% reported that their father had been diagnosed with depression. Percentages, means, and standard deviations (where applicable) are displayed in Table 1. The log-rank test for equality of survivor functions revealed statistically significant differences (p <.001) in the survival curves of those who did and did not experience anxiety.
Table 1.
Cox proportional hazards—main effects for anxiety. Outcome: probable AD
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda w/o ε4 carrier status hazard ratio (95% CI) | Main effects adjusted w/ε4 carrier status hazard ratio (95% CI) | Main effects adjustedb AD medication use hazard ratio (95% CI) |
|---|---|---|---|---|
| Anxiety | 3.50 (2.77–4.44)** | 3.22 (2.53–4.10)** | 3.12 (2.40–4.04)** | 2.28 (1.75–2.97)** |
| Use of an anxiolytic | 2.26 (1.08–4.74) | 1.94 (.917–4.11) | 1.38 (.582–3.29) | .888 (.366–2.16) |
| Did not use anxiolytic | 3.81 (2.97–4.90)** | 3.52 (2.73–4.54)** | 3.51 (2.67 – 4.61)** | 2.59 (1.96–3.42)** |
| - Alprazolam | 1.54 (.297–7.94) | 1.47 (.247–8.74) | 1.27 (.216–7.45) | .537 (.082–3.52) |
| - No Alprazolam use | 3.59 (2.83–4.56)** | 3.30 (2.58–4.20)** | 3.17 (2.44–4.12)** | 2.32 (1.77–3.03)** |
| - Clonazapam use | 1.41 (.125–15.79) | 1.23 (.035–43.70) | — | — |
| - No Clonazapam use | 3.57 (2.81–4.53)** | 3.27 (2.57–4.17)** | 3.14 (2.42–4.08)** | 2.33 (1.78–3.04)** |
| - Lorazapam use | 1.07 (.221–5.15) | 1.06 (.218–5.19) | .666 (.075–5.90) | .597 (.062–5.74) |
| - No Lorazapam use | 3.63 (2.85–4.61)** | 3.32 (2.60–4.24)** | 3.23 (2.48–4.20)** | 2.33 (1.78–3.05)** |
| - Paroxetine use | 2.63 (.454–15.19) | 3.64 (.518–25.64) | 3.54 (.450–27.74) | 3.40 (.443–26.11) |
| - No Paroxetine use | 3.53 (2.78–4.48)** | 3.24 (2.54–4.13)** | 3.11 (2.39–4.05)** | 2.25 (1.72–2.94)** |
| - Venlafaxine use | 3.88 (.773–19.50) | 2.86 (.481–17.03) | 2.29 (.383–13.75) | 3.26 (.236–45.08) |
| - No Venlafaxine use | 3.50 (2.75–4.45)** | 3.22 (2.52–4.11)** | 3.09 (2.38–4.03)** | 2.30 (1.76–3.01)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
— indicates sample size too small for analysis.
Main effects for AD development
The Kaplan–Meier plot with probable AD as an outcome is displayed in Figure 1. The hazard of probable AD development was statistically significantly higher for those reporting anxiety symptoms (HR = 3.50, 95% CI 2.77 – 4.44, p <.0001). The effect of anxiety was no longer statistically significant when an anxiolytic was in use. The use of specific generic anxiolytics produced similar results when participants with anxiety also reported taking prescription medications, including alprazolam, clonazepam, lorazepam, paroxetine, and venlafaxine. Table 2 contains the results for these models. The Kaplan–Meier plot for participants with anxiety and probable AD defined as an outcome is displayed in Figure 2.
Figure 1.
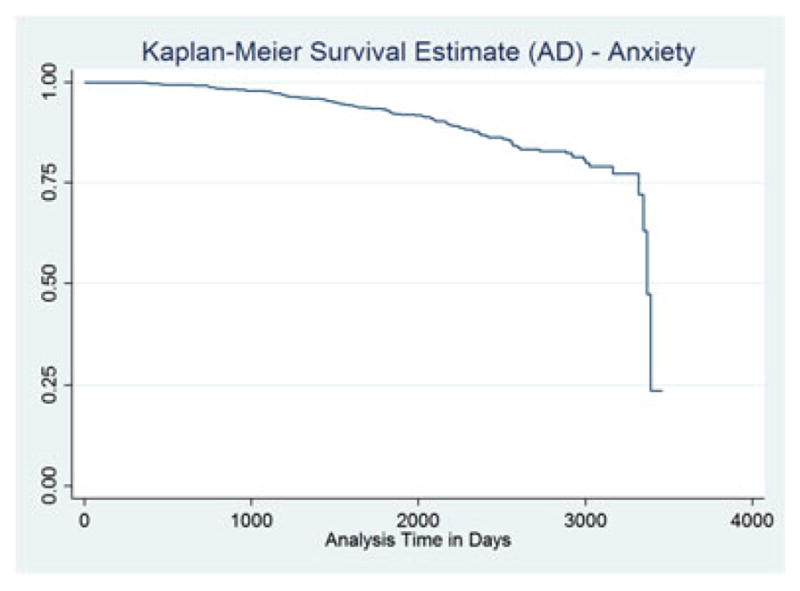
KM survival—AD.
Table 2.
Cox proportional hazards—main effects for ε4 carrier status. Outcome: probable AD
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda hazard ratio (95% CI) | Main effects adjustedb hazard ratio (95% CI) |
|---|---|---|---|
| ε4 carrier | 1.92 (1.54–2.41)** | 2.55 (2.02–3.21)** | 2.56 (2.03–3.23)** |
| Use of an anxiolytic | 1.90 (.921–3.91) | 2.35 (1.10–5.00)* | 1.79 (.837–3.85) |
| Did not use anxiolytic | 2.02 (1.58–2.58)** | 2.67 (2.07–3.43)** | 2.18 (1.69–2.82)** |
| - Alprazolam | 2.27 (.504–10.22) | 4.52 (.746–27.41) | 2.54 (.405–15.90) |
| - No Alprazolam use | 1.99 (1.57–2.51)** | 2.64 (2.07–3.36)** | 2.20 (1.72–2.81)** |
| - Clonazapam use | 4.49 (.280–71.84) | — | — |
| - No Clonazapam use | 1.99 (1.58–2.52)** | 2.65 (2.09–3.37)** | 2.21 (1.73–2.81)** |
| - Lorazapam use | 1.60 (.357–7.15) | 1.85 (.400–8.56) | 1.07 (.196–5.85) |
| - No Lorazapam use | 2.00 (1.58–2.53)** | 2.67 (2.10–3.40)** | 2.21 (1.73–2.82)** |
| - Paroxetine use | 5.48 (1.22–24.72)* | 5.70 (1.04–31.15)* | 5.88 (1.04–33.06)* |
| - No Paroxetine use | 1.96 (1.55–2.48)** | 2.62 (2.05–3.33)** | 2.15 (1.68–2.74)** |
| - Venlafaxine use | 1.18 (.216–6.48) | 1.69 (.253–11.26) | 2.51 (.273–23.04) |
| - No Venlafaxine use | 2.01 (1.59–2.55)** | 2.68 (2.11–3.41)** | 2.21 (1.73–2.83)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
— indicates sample size too small for analysis.
Figure 2.
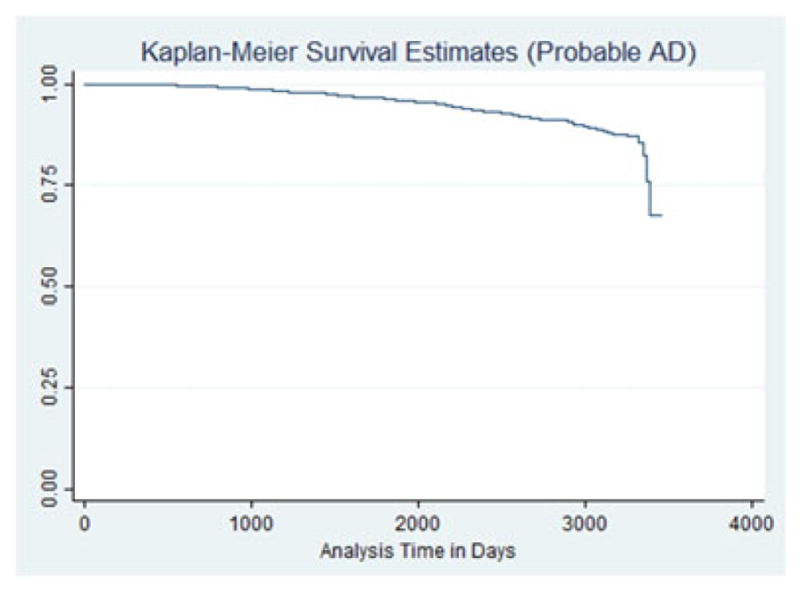
KM survival—MCI.
This main effect and anxiolytic moderation effect of ε4 carriers in relation to probable AD development was tested. ε4 carriers saw a statistically significant higher hazard of AD development (HR = 1.92, [1.52–2.41], p <.001). The hazard ratio (HR = 2.55, [2.02–3.21], p <.001) increased when adjusted for sex, age, education, race, Hispanicity, and AD medication use. This relationship was no longer significant, however, when taking into account a broad anxiolytic categorization, alprazolam, clonazepam, lorazepam, and venlafaxine use. For those ε4 carriers who took paroxetine, a statistically significant relationship to probable AD remained. These results indicated that anxiolytics appear to neutralize the risk posed by APOE ε4. The results of these models are displayed in Table 3.
Table 3.
Cox proportional hazards—additive effects for anxiety × ε4 carrier status. Outcome: probable AD
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda hazard ratio (95% CI) | Main effects adjustedb hazard ratio (95% CI) |
|---|---|---|---|
| Anxiety × ε4 carrier | 7.02 (4.98–9.89)** | 8.57 (6.05–12.14)** | 5.03 (3.50–7.22)** |
| Use of an anxiolytic | 2.78 (.791–9.80) | 2.82 (.791–10.02) | 1.47 (.397–5.44) |
| Did not use anxiolytic | 8.01 (5.60–11.47)** | 9.82 (6.82–14.14) | 5.73 (3.92–8.37)** |
| - Alprazolam | 2.44 (.215–27.68) | 4.34 (.335–56.34) | 1.20 (.072–20.25) |
| - No Alprazolam use | 7.20 (5.09–10.19)** | 8.76 (6.16–12.46)** | 5.17 (3.59–7.46)** |
| - Clonazapam use | — | — | — |
| - No Clonazapam use | 7.12 (5.05–10.04)** | 8.68 (6.12–12.29)** | 5.22 (3.63–7.49) |
| - Lorazapam use | — | — | — |
| - No Lorazapam use | 7.39 (5.23–10.42)** | 8.98 (6.33–12.73)** | 5.22 (3.63–7.51)** |
| - Paroxetine use | 16.81 (.938–301.32) | 22.83 (1.15–452.03)* | 22.33 (1.15–433.41)* |
| - No Paroxetine use | 6.89 (4.87–9.74)** | 8.44 (5.94–11.99)** | 4.89 (3.39–7.04)** |
| - Venlafaxine use | 5.57 (.884–35.12) | 5.50 (.804–37.62) | 4.74 (.412–54.45) |
| - No Venlafaxine use | 6.94 (4.89–9.84)** | 8.48 (5.94–12.09)** | 5.17 (3.58–7.47)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
— indicates sample size too small for analysis.
The additive effect of anxiety among ε4 carriers indicates a continued high risk; the hazard of which is more than double the simple sum of the two hazards alone (HR = 7.02 [4.98–9.89], p <.001). This hazard ratio increased when adjusted for sex, age, education, race, and Hispanicity (HR = 8.57 [6.05–12.14], p <.001), but decreased slightly when also adjusted for AD medication use (HR = 5.03 [3.50–7.22], p <.001). A similar pattern emerged in which the use of a general anxiolytic or alprazolam, paroxetine, or venlafaxine appeared to neutralize the previous significant hazard of AD development to the point where there was no longer a statistically significant risk. When the effect of paroxetine as a moderator was adjusted for the previously mentioned covariates, a statistically significant hazard emerged, though the sample size decreased to the point where the confidence interval greatly expanded beyond a meaningful range (Table 4).
Table 4.
Cox proportional hazards—main effects for anxiety. Outcome: MCI
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda hazard ratio (95% CI) | Main effects adjustedb w/ε4 carrier status hazard ratio (95% CI) | Main effects adjustedc AD medication use hazard ratio (95% CI) |
|---|---|---|---|---|
| Anxiety | 2.13 (1.85–2.44)** | 2.08 (1.81–2.39)** | 1.98 (1.70–2.31)** | 1.98 (1.70–2.31)** |
| Use of an anxiolytic | 2.07 (1.45–2.94)** | 1.95 (1.37–2.79)** | 2.09 (1.42–3.07)** | 1.89 (1.27–2.80)** |
| Did not use anxiolytic | 2.17 (1.87–2.52)** | 2.13 (1.83–2.48)** | 2.00 (1.69–2.37)** | 1.91 (1.61–2.26)** |
| - Alprazolam | .999 (.374–2.67) | 1.06 (.391–2.89) | 1.28 (.445–3.66) | 1.22 (.420–3.55) |
| - No Alprazolam use | 2.17 (1.89–2.49)** | 2.12 (1.84–2.44)** | 2.01 (1.72–2.35)** | 1.99 (1.71–2.33)** |
| - Clonazapam use | 2.59 (1.08–6.19)* | 2.62 (1.04–6.60)* | 3.45 (1.29–9.12)* | 2.96 (1.03–8.51)* |
| - No Clonazapam use | 2.12 (1.85–2.44)** | 2.07 (1.80–2.38)** | 1.96 (1.67–2.29)** | 1.95 (1.66–2.28)** |
| - Lorazapam use | 2.85 (1.43–5.67)* | 2.93 (1.45–5.93)* | 2.35 (1.06–5.19)* | 2.28 (1.03–5.05)* |
| - No Lorazapam use | 1.86 (1.69–2.05)** | 2.04 (1.77–2.35)** | 1.97 (1.68–2.31)** | 1.95 (1.67–2.29)** |
| - Paroxetine use | 1.54 (.519–4.58) | 1.59 (.507–4.97) | 1.84 (.545–6.21) | 1.77 (.515–6.06) |
| - No Paroxetine use | 2.15 (1.87–2.46)** | 2.10 (1.82–2.41)** | 2.00 (1.71–2.33)** | 1.98 (1.69–2.32)** |
| - Venlafaxine use | .860 (.246–3.01) | .305 (.063–1.48) | .191 (.022–1.66) | .179 (.019–1.65) |
| - No Venlafaxine use | 2.17 (1.89–2.49)** | 2.13 (1.85–2.44)** | 2.04 (1.75–2.38)** | 2.03 (1.74–2.37)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and E4 carrier status
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
MCI as the endpoint of interest
The Kaplan–Meier plot with MCI as an outcome is displayed in Figure 3. The hazard of MCI development was statistically significant higher for those reporting anxiety symptoms (HR = 2.13, 95% CI 1.85–2.44, p <.0001). The Kaplan–Meier plot for participants with anxiety and MCI defined as an outcome is displayed in Figure 4. The use of anxiolytics as a general category did not neutralize the effect of anxiety, which were virtually the same as the effect without the anxiolytic use. The effect of anxiety was no longer statistically significant when specific anxiolytics were used: alprazolam, paroxetine, and venlaxafine. Table 5 displays the results for these models.
Figure 3.
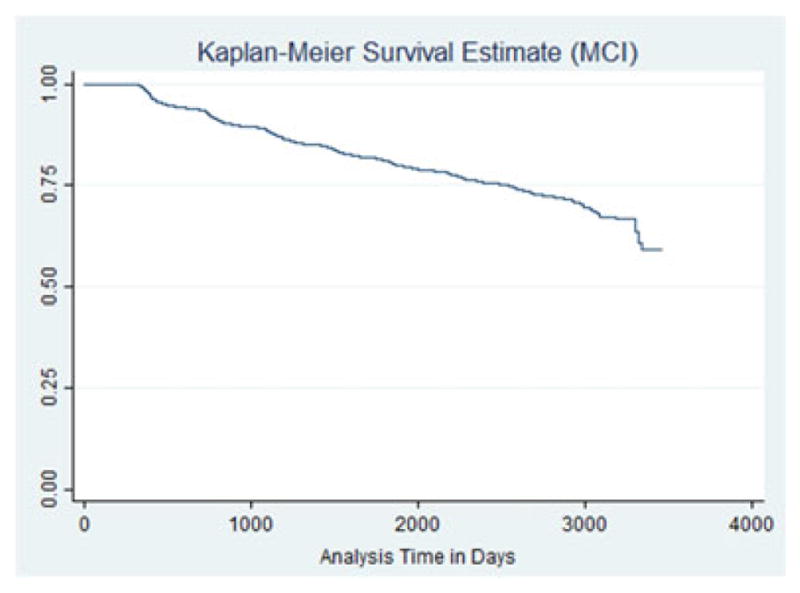
KM survival—AD as outcome among people with anxiety.
Figure 4.
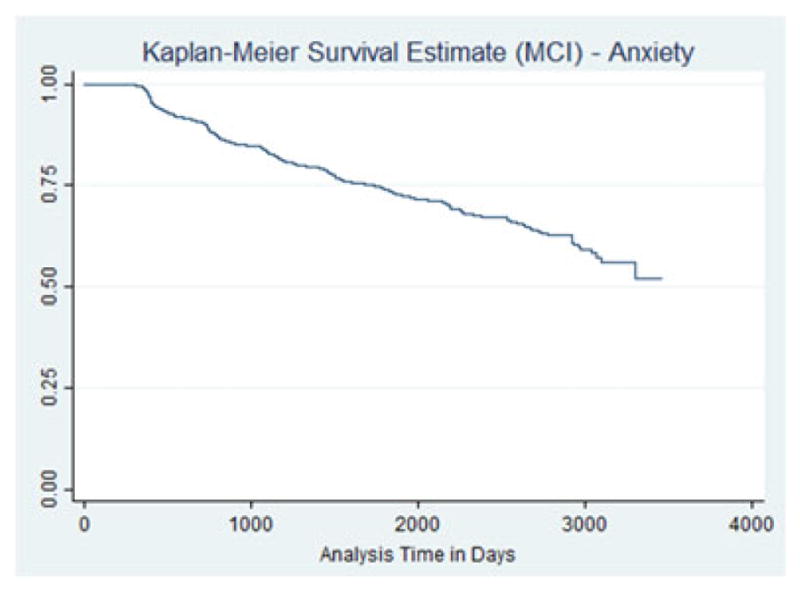
KM survival curve—outcome MCI among people with anxiety.
Table 5.
Cox proportional hazards—main effects for ε4 carrier status. Outcome: MCI
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda hazard ratio (95% CI) | Main effects adjustedb hazard ratio (95% CI) |
|---|---|---|---|
| ε4 carrier | 1.17 (1.04–1.32)* | 1.37 (1.22–1.55)** | 1.37 (1.22–1.55)** |
| Use of an anxiolytic | 1.17 (.821–1.67) | 1.36 (.949–1.96) | 1.29 (.901–1.86) |
| Did not use anxiolytic | 1.19 (1.04–1.35)* | 1.39 (1.22–1.58)** | 1.36 (1.20–1.55)** |
| - Alprazolam | 1.34 (.572–3.15) | 1.69 (.648–4.39) | 1.59 (.604–4.19) |
| - No Alprazolam use | 1.18 (1.05–1.33)* | 1.38 (1.22–1.57)** | 1.38 (1.22–1.56)** |
| - Clonazapam use | 2.01 (.788–5.15) | 2.62 (.966–7.10) | 2.02 (.688–5.91) |
| - No Clonazapam use | 1.17 (1.04–1.32)* | 1.38 (1.22–1.56)** | 1.37 (1.21–1.55)** |
| - Lorazapam use | 1.17 (.549–2.52) | 1.17 (.514–2.68) | 1.17 (.512–2.69) |
| - No Lorazapam use | 1.18 (1.05–1.34)* | 1.39 (1.21–1.57)** | 1.38 (1.22–1.56)** |
| - Paroxetine use | .964 (.351–2.65) | 1.09 (.391–3.03) | 1.07 (.383–2.98) |
| - No Paroxetine use | 1.19 (1.05–1.34)* | 1.40 (1.24–1.58)** | 1.39 (1.23–1.57)** |
| - Venlafaxine use | .344 (.078–1.53) | .358 (.068–1.88) | .215 (.039–1.19) |
| - No Venlafaxine use | 1.20 (1.06–1.35)* | 1.40 (1.24–1.58)** | 1.39 (1.23–1.58)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
This main effect and anxiolytic moderation effect among ε4 carriers in relation to MCI development was tested. ε4 carriers saw a statistically significant higher hazard of MCI development (HR = 1.17, [1.04–1.32], p <.05). The hazard ratio increased slightly when adjusted for sex, age, education, race, Hispanicity, and AD medication use (HR = 1.37 [1.22–1.55], p <.001). The relationship between ε4 carrier status and MCI development was no longer significant, however, when taking into account the intake of medications within a broad anxiolytic categorization, as well as alprazolam, clonazepam, lorazepam, paroxetine, and venlafaxine use. These results indicated that anxiolytics appear to neutralize the risk posed by APOE ε4 in terms of MCI development. The results of these models are displayed in Table 6.
Table 6.
Cox proportional hazards—additive effects for anxiety × ε4 carrier status. Outcome: MCI
| Predictor variables | Main effects (unadjusted) hazard ratio (95% CI) | Main effects adjusteda hazard ratio (95% CI) | Main effects adjustedb hazard ratio (95% CI) |
|---|---|---|---|
| Anxiety × ε4 carrier | 2.37 (1.84–3.04)** | 2.68 (2.09–3.44)** | 2.67 (2.08–3.44)** |
| Use of an anxiolytic | 3.16 (1.86–5.37)** | 3.26 (1.92–5.56)** | 2.72 (1.57–4.71)** |
| Did not use anxiolytic | 2.20 (1.66–2.93)** | 2.54 (1.91–3.39)** | 2.35 (1.76–3.15)** |
| - Alprazolam | 2.44 (.215–27.68) | 4.34 (.335–56.34) | 2.41 (.675–8.61) |
| - No Alprazolam use | 7.20 (5.09–10.19)** | 8.76 (6.16–12.46)** | 2.61 (2.02–3.38)** |
| - Clonazapam use | 7.41 (2.39–22.95)** | 8.42 (2.48–28.64)** | 6.51 (1.54–27.49)* |
| - No Clonazapam use | 2.25 (1.73–2.91)** | 2.54 (1.96–3.29)** | 2.52 (1.94–3.26)** |
| - Lorazapam use | 2.78 (.900–8.61) | 2.88 (.860–9.65) | 2.79 (.830–9.39) |
| - No Lorazapam use | 2.33 *(1.81–3.02)** | 2.64 (2.04–3.41)** | 2.60 (2.01–3.37)** |
| - Paroxetine use | 2.01 (.256–15.85) | 3.50 (.353–34.76) | 3.23 (.312–33.50) |
| - No Paroxetine use | 2.38 (1.85–3.06) | 2.70 (2.10–3.47)** | 2.67 (2.07–3.43)** |
| - Venlafaxine use | .878 (.111–6.93) | .324 (.032–3.25) | .196 (.019–2.05) |
| - No Venlafaxine use | 2.41 (1.87–309)** | 2.73 (2.12–3.51)** | 2.70 (2.10–3.48)** |
Adjusted for sex, age, education, race, and Hispanic origin.
Adjusted for sex, age, education, race, Hispanic origin, and AD medication use.
Indicates statistical significance at p <.05.
Indicates p <0.001.
The additive effect of anxiety among ε4 carriers indicated a continued high hazard of MCI development (HR = 2.37 [1.84–3.04], p <.001). The hazard ratio increased when adjusted for sex, age, education, race, and Hispanicity (HR = 2.68 [2.09–3.44] p <.001), and remained virtually unchanged when adjusted for AD medication use (HR = 2.67 [2.08–3.44], p <.001). For ε4 carriers with anxiety, the use of the variety of medications included in the general anxiolytic category offered no reduction in risk, and surprisingly raised the hazard of MCI development (HR = 3.16 (1.86–5.37), p <.001). The use of alprazolam, lorazepam, paroxetine, or venlafaxine, specifically, appeared to neutralize the previous significant effect of anxiety to the point where there was no longer a statistically significant risk. The use of clonazepam conferred a statistically significant higher hazard of MCI development among ε4 users with anxiety (HR = 7.41 [2.39–22.95], p <.001). This hazard is three times the hazard of the main itself alone, suggesting that there is something about clonazepam use that is influencing the increasing hazard. This result speaks to the previous literature on the topic of anxiolytic use and neurodegeneration, which generally indicates an increased risk because of medication itself.
Discussion
The results of this study suggest that use of anxiolytics may moderate MCI and AD development, specifically indicating a neutralized risk for those with anxiety symptoms and ε4 carriers. These results require a visitation to the classic literature, which is highly publicized, and is widely known for suggesting a correlation between anti-anxiety medicine use and AD development. Billioti de Gage et al. (2012) found a correlation between AD diagnosis and benzodiazepine use starting at least five years before the AD diagnosis. The strength of association increased with usage length; three to six months of drug prescription raising the risk of AD development by 32% and more than six months of prescription raising the risk to 84% (Billioti de Gage et al., 2012). Individuals prescribed long-acting benzodiazepines demonstrated a higher risk of AD development compared to individuals prescribed short-acting benzodiazepines (Billioti de Gage et al., 2012).
As the understanding of how anxiety relates to AD risk is still unknown, there are at least two competing explanations for the apparently neutralizing or even protective effects of anxiolytic drug use. The first is that anxiety is a prodromal symptom of AD that can appear years before AD diagnosis and may be the result of an unknown biological cause that ultimately concludes with AD diagnosis. In the second scenario anxiety is an independent disorder that, in combination with other diseases, disorders, or social problems, can exacerbate AD development.
If anxiety is an early onset symptom of AD, pharmaceutical treatment may mask the severity of other AD components, delaying identification and diagnosis. In general, symptomology of the early stages of AD includes possible difficulty with remembering events, names of individuals, and recent discussions (Alzheimer’s Association, 2015). A gradual, very slow progression with more evidence of memory impairment than intellectual difficulties are associated with late-onset AD (World Health Organization, 2016). Disorientation, agnosia, aggression, agitation, anxiety, apathy, poor judgment, confusion, with gradual difficulty with walking, swallowing, as well as speaking can also occur in the late stages of Alzheimer’s (Alzheimer’s Association, 2015; World Health Organization, 2016). An individual can display a mixed presentation with both early as well as late onset symptomology (World Health Organization, 2016).
There have been suggestions regarding the link between AD risk and the use of medications for anxiety such as benzodiazepines (Billioti de Gage et al., 2012; Rosenberg, 2015a). Rosenberg (2015b) reports use of long-acting benzodiazepine medications over an extended period may increase risk of AD development. When assessing benzodiazepine use during the prodromal phase of AD, there may be no association between the development of the disease and benzodiazepine use (Imfeld et al., 2015). Additional clinical control trials are needed to establish definitive conclusions regarding the relationship between AD development risk and benzodiazepine use (Defrancesco et al., 2015). Because of the association between anxiety and brain atrophy, anxiety may precede the onset of dementia, although this association requires further examination (Mah et al., 2004).
Anxiolytics enhance the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system (Finkelman, 1997). Researchers have suggested that GABAergic agents in medications may be responsible for reducing anxiety (Kalueff and Nutt, 2007; Lydiard, 2003). Researchers have suggested that antagonists of the glutamate receptor, N-methyl-D-aspartate (NMDA) have a potentially protective effect against neurodegeneration (Duguid and Smart, 2009; Garcia de Arriba et al., 2006; Lipton, 2004). When combined with GABA, NMDA may have positive anxiolytic-like effects (Poleszak et al., 2011; Zarrabian et al., 2016).
Several anxiolytic medications are available to address anxiety symptoms. The most popular category of anxiolytics prescribed are benzodiazepines (Cascade and Kalali, 2008). These medications increase GABA-A receptor activity, therefore reducing neuron excitability (Griffin et al., 2013). Alprazolam (Xanax, Niravam, Alprazolam Intensol, or Xanax XR,) has been commonly prescribed for many years (Fawcett and Kravits, 1982), and is classified as short-acting with high-potency (Griffin et al., 2013). Another high-potency, short-acting benzodiazepine is lorazepam (Ativan, Lorazepam Intensol; U.S. National Library of Medicine, 2014). This medication also binds to GABA-A, but with a lower affinity than alprazolam (Griffin et al., 2013). Clonazepam (Klonopin) is a high-potency benzodiazepine that acts on the GABA-A receptor agonist (Griffin et al., 2013) and works as a serotonin agonist (Chouinard et al., 1983). This medication is unique compared to other benzodiazepines as it does not bind strongly to GABA-A (Chouinard et al., 1983).
It is hypothesized that selective serotonin reuptake inhibitors (SSRIs), another class of anxiolytics, increase extracellular serotonin via the reduction of serotonin reabsorption in the presynaptic cell (Stahl, 1998), which increases synaptic cleft serotonin levels used to bind to the presynaptic receptor and stimulate the cell. This overstimulation signals the nervous system to decrease the release of serotonin (Stahl, 1998). Paroxetine (Paxil, Brisdelle, Pexeva, or Paxil CR) is a SSRI (U. S. National Library of Medicine, 2014). The medication has the highest affinity of serotonin of all SSRIs with aversion to norepinephrine and dopamine (National Center for Biotechnology Information, 2016). Venlafaxine (Effexor or Effexor XR) is a long-acting SSRI and norepinephrine reuptake inhibitor classified as specific serotonin and norepinephrine reuptake inhibitors (SNRIs; Lambert and Bourin, 2002).
Medications examined in the current study treat symptoms of anxiety, depression, and sleep difficulties. Concluding that older adults were especially sensitive to benzodiazepines and at risk of cognitive impairment, delirium, and bodily harm, the American Geriatrics Society (2015) strongly recommended that benzodiazepine use was inappropriate for older adults; the same experts also strongly recommended SSRIs as inappropriate because of an increased risk of falls (American Geriatrics Society, 2015). Many of these side effects overlap with symptoms typically associated with AD diagnosis; memory impairment, concentration issues, personality changes, sleep disturbances, and eating or weight changes. With difficulties in separating the intended treatment, the prescription medication side effects, and the symptoms of AD, a delay in diagnoses of AD may be delayed in patients prescribed psychotropic medications.
Anxiety may be a disorder that exacerbates the development of AD as part of a syndemic process. A syndemic is the synergism of two or more diseases, disorders, or social problems that result in negative health consequences that are worse than an additive effect (Singer, 2009). Anxiety, in conjunction with other biological and psychosocial factors that fatigue the body and activate a stress response system, may impact AD development. Psychopharmacological treatment of anxiety may lessen the burden on the stress response system, reducing AD development risk. While there is no current cure for AD, there are a number of treatments for its modifiable risk factors, such as anxiety. Those in the public health professions remain uniquely positioned to research and provide direct service related to assisting clients with behavioral changes, which may delay the onset of neurodegeneration.
Key points.
Although some researchers and practitioners suggest that the use of anxiolytic medications further exacerbates the risk of AD development, the current study explored anxiolytic medication as a protective moderator of AD risk in older adults.
The hazard of probable Alzheimer’s disease or mild cognitive impairment development was statistically significant for those with anxiety.
When specific anxiolytics were prescribed, the hazard of Alzheimer’s disease and mild cognitive impairment was no longer statistically significant.
ε4 carriers saw a statistically significant hazard of AD and MCI development, but this effect was moderated by the use of anxiolytics.
Acknowledgments
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Funding and disclosure
There is no funding to report.
References
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332. doi: 10.1016/j.jalz.2015.02.003. + [DOI] [PubMed] [Google Scholar]
- Bunce D, Batterham PJ, Mackinnon AJ, Christensen H. Depression, anxiety and cognition in community-dwelling adults aged 70 years and over. J Psychiatr Res. 2012;46:1662–1666. doi: 10.1016/j.jpsychires.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascade E, Kalali AH. Use of benzodiazepines in the treatment of anxiety. Psychiatry Edgmont. 2008;5:21–22. [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE. A distinctive interaction between chronic anxiety and problem solving in asymptomatic APOE e4 homozygotes. J Neuropsychiatry Clin Neurosci. 2004;16:320–329. doi: 10.1176/jnp.16.3.320. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Young SN, Annable L. Antimanic effect of clonazepam. Biol Psychiatry. 1983;18:451–466. [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972;34:89–99. [Google Scholar]
- Dar-Nimrod I, Chapman BP, Franks P, et al. Personality factors moderate the associations between apolipoprotein genotype and cognitive function as well as late onset alzheimer disease. Am J Geriatr Psychiatry. 2012;20:1026–35. doi: 10.1097/JGP.0b013e318267016b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrancesco M, Marksteiner J, Fleischhacker WW, Blasko I. Use of benzodiazepines in Alzheimer’s disease: a systematic review of literature. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devier DJ, Pelton GH, Tabert MH, et al. The impact of anxiety on conversion from mild cognitive impairment to Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;24:1335–1342. doi: 10.1002/gps.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Presynaptic NMDA receptors. In: Van Dogen AM, editor. Biology of the NMDA Receptor. CRC Press/Taylor & Francis; Boca Raton, FL: 2009. pp. 283–312. [PubMed] [Google Scholar]
- Edwards ER, Spira AP, Barnes DE, Yaffe K. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009;24:716–722. doi: 10.1002/gps.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. The efficiency of cox’s likelihood function for censored data. J Am Stat Assoc. 1977;72:557–565. [Google Scholar]
- Farlow MR, He Y, Tekin S, et al. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.WNL.0000144279.21502.B7. [DOI] [PubMed] [Google Scholar]
- Finkelman AW. Psychiatric home care. Aspen Publishers, Inc; Gaithersburg, Md: 1997. [Google Scholar]
- Garcia de Arriba S, Wegner F, Grüner K, et al. Different capacities of various NMDA receptor antagonists to prevent ischemia-induced neurodegeneration in human cultured NT2 neurons. Neurochem Int. 2006;49:466–474. doi: 10.1016/j.neuint.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Stress, allostatic load, catecholamines, and other neurotransmitters in neurodegenerative diseases. Cell Mol Neurobiol. 2012;32:661–666. doi: 10.1007/s10571-011-9780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system–mediated effects. Ochsner J. 2013;13:214–223. [PMC free article] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Imfeld P, Bodmer M, Jick SS, Meier CR. Benzodiazepine use and risk of developing alzheimer’s disease or vascular dementia: A case–control analysis. Drug Saf. 2015;38:909–919. doi: 10.1007/s40264-015-0319-3. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Duberstein PR, et al. Midlife personality and risk of Alzheimer disease and distress: A 38-year follow-up. Neurology. 2014;83:1538–1544. doi: 10.1212/WNL.0000000000000907. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;1091–4269(24):495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kirmizioglu Y, Doğan O, Kuğu N, Akyüz G. Prevalence of anxiety disorders among elderly people. Int J Geriatr Psychiatry. 2009;24:1026–1033. doi: 10.1002/gps.2215. [DOI] [PubMed] [Google Scholar]
- Lemaître H, Crivello F, Dufouil C, et al. No ε4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. NeuroImage. 2005;24:1205–1213. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64:21–27. [PubMed] [Google Scholar]
- Mah L, Binns MA, Steffens DC. Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am J Geriatr Psychiatry. 2015;23:466–476. doi: 10.1016/j.jagp.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. [accessed 7.9.16];paroxetine | C19H20FNO3 -PubChem [WWW Document] 2016 URL https://pubchem.ncbi.nlm.nih.gov/compound/paroxetine#section=Top.
- O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- Penna S. Cognitive and emotional dysfunction in mild cognitive impairment. Clin Geriatr Med. 2013;29:773–789. doi: 10.1016/j.cger.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Lim Y, Neumeister A, et al. Amyloid-β, anxiety, and cognitive decline in preclinical alzheimer disease: A multicenter, prospective cohort study. JAMA Psychiatry. 2015;72:284–291. doi: 10.1001/jamapsychiatry.2014.2476. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Socała K, Szopa A, et al. Involvement of NMDA receptor complex in the anxiolytic-like effects of chlordiazepoxide in mice. J Neural Transm. 2011;118:857–864. doi: 10.1007/s00702-011-0585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. Role of apolipoprotein E in anxiety. Neural Plast. 2007;2007:1–7. doi: 10.1155/2007/91236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers IHGB, Verhey FRJ, Scheltens P, et al. Anxiety is related to Alzheimer cerebrospinal fluid markers in subjects with mild cognitive impairment. Psychol Med. 2013;43:911–920. doi: 10.1017/S0033291712001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety level on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol. 2008a;589:173–175. doi: 10.1016/j.ejphar.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008b;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Robertson J, Curley J, Kaye J, et al. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiol Aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Rosenberg Benzodiazepine use increases alzheimer’s risk. Am J Nurs. 2015a;115:56–56. [Google Scholar]
- Rosenberg PB. Benzodiazepine exposure increases risk of Alzheimer’s disease. Evid Based Med. 2015b;20:75–75. doi: 10.1136/ebmed-2014-110117. [DOI] [PubMed] [Google Scholar]
- Singer M. Introduction to Syndemics: A Critical Systems Approach to Public and Community Health. 1. Jossey-Bass; San Francisco: 2009. [Google Scholar]
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14. StataCorp LP; College Station, TX: 2015. [Google Scholar]
- Tang M-X, Stern Y, Marder K, et al. The APOE-…4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA J Am Med Assoc. 1998;279:751. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- U. S. National Library of Medicine. [accessed 7.9.16];Paroxetine: MedlinePlus Drug Information [WWW Document] 2014 URL https://www.nlm.nih.gov/medlineplus/druginfo/meds/a698032.html.
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. World Health Organization; Geneva: 2016. [Google Scholar]
- Zarrabian S, Farahi M, Nasehi M, Zarrindast M-R. The role of CA3 GABAA receptors on anxiolytic-like behaviors and avoidance memory deficit induced by NMDA receptor antagonists. J Psychopharmacol (Oxf) 2016 doi: 10.1177/0269881115622239. 269881115622239. [DOI] [PubMed] [Google Scholar]


