Abstract
There is debate as to whether the neurocognitive changes associated with HIV infection represent an acceleration of the typical aging process or more simply reflect a greater accentuated risk for age-related declines. We aimed to determine whether accelerated neurocognitive aging is observable in a sample of older HIV-infected individuals compared to age-matched seronegatives and older-old (i.e., aged ≥ 65) seronegative adults. Participants in a cross-sectional design included 48 HIV-seronegative (O−) and 40 HIV-positive (O+) participants between the ages of 50–65 (mean ages = 55 and 56, respectively) and 40 HIV-seronegative participants aged ≥ 65 (OO−; mean age = 74) who were comparable for other demographics. All participants were administered a brief neurocognitive battery of attention, episodic memory, speeded executive functions, and confrontation naming (i.e., Boston Naming Test). The O+ group performed more poorly than the O− group (i.e., accentuated aging), but not differently from the OO− on digit span and initial recall of a supraspan word list, consistent with an accelerating aging profile. However, the O+ group’s performance was comparable to the O− group on all other neurocognitive tests (ps > .05). These data partially support a model of accelerated neurocognitive aging in HIV-infection, which was observed in the domain of auditory verbal attention, but not in the areas of memory, language, or speeded executive functions. Future studies should examine whether HIV-infected adults over 65 evidence accelerated aging in downstream neurocognitive domains and subsequent everyday functioning outcomes.
Keywords: HIV, aging, verbal attention, neurocognitive aging, accelerated aging
While the mortality rates and life expectancies associated with HIV infection have improved due to the widespread use and effectiveness of combined antiretroviral therapies (cART), there persists a high prevalence of HIV-associated, Non-AIDS (HANA) conditions (e.g., cardiovascular disease) among persons living with HIV disease (Guaraldi et al. 2011; High et al. 2012). In parallel, there is an increasing prevalence of HIV+ adults aged 50 and over (Smit et al. 2015). Advancing age is associated with higher rates of HANA conditions among individuals living with HIV disease (Greene et al. 2013). This observation has prompted questions of whether biological changes due to HIV infection (e.g., inflammation) directly result in early onset pathophysiologies more commonly observed in older adults (accelerated aging). Alternatively, it is possible that HIV disease is associated with a higher prevalence of age-related HANA conditions that emerge at the expected age of onset (accentuated aging). In other words, accelerated aging involves direct changes that mimic and exacerbate the aging processes, such as a cohort of HIV+ persons developing congestive heart failure in their mid-30s. In contrast, an accentuated aging process would show a higher prevalence of congestive heart failure among older HIV+ adults in their mid-60s as compared to age-matched controls. While this topic remains controversial (Pathai et al. 2014), determining the extent to which HIV disease processes reflect accelerated and/or accentuated aging may allow for a better understanding of how to address HANA conditions in the context of HIV infection.
There is a host of literature showing biological cascade effects (e.g., immune dysregulation, cellular senescence) consistent with accelerated aging in diseases that affect younger and middle-aged persons. For example, age-related biological changes have been observed in sclerosis-associated fibrosis (Luckhardt and Thannickal, 2015), anxiety disorders (Perna et al. 2016), Down syndrome (Horvath et al. 2015), and schizophrenia (Czepielewski et al. 2016). In laboratory studies of HIV, accelerated aging has been observed through increased immune senescence associated with alterations in T-cell generation and gene expression (Boulias et al. 2016; Chou et al. 2013; Gianesin et al. 2016; Pathai et al. 2014) and resulting inflammation (for review, see Bhatia et al. 2011). Epigenetic studies of post-mortem brain tissues of HIV+ individuals show increased DNA methylation (Horvath and Levine 2015), suggestive of accelerated aging that may be particularly pronounced in individuals with HIV-associated neurocognitive disorders (HAND; Levine et al. 2016).
With regard to neurocognitive aging in HIV disease, the presence of both accelerated and accentuated aging are supported through a combination of indirect and direct evidence. First and foremost, age is reliably observed as an independent risk factor for HIV-associated neurocognitive dysfunction in HIV disease (Valcour et al. 2004). In fact, the rate of neurocognitive impairment is significantly higher in older compared to younger HIV-infected individuals, with a prevalence rate in older HIV+ adults estimated around 40% (Valcour et al. 2004). Among older adults, HIV and normal aging also share risk and protective factors for neurocognitive impairment, including the ApoE ε4 allele and lower cognitive reserve (Morgan et al. 2012; Valcour et al. 2004).
In the pre-cART era, Van Gorp and colleagues (1989) were the first to provide direct evidence of accelerated neurocognitive aging. In that study, younger HIV+ adults’ (mean age, 37.5 years) performance was similar to that of older seronegative adults (mean age, 70 years) on tests of learning, delayed memory, processing speed and attention, visuospatial processing, and language, suggesting that the average 40-year old with HIV had the same cognitive status as a healthy 70-year old. A careful review of more recent cART era studies suggests a similar pattern of accelerated neurocognitive aging (e.g., Woods et al. 2010; Avci et al. 2016). Across many of these studies, a stair-step effect is observed whereby age and HIV have an additive impact on neurocognitive outcomes. Specifically, younger HIV+ groups reliably perform comparably to older HIV− groups, and both of these groups perform more poorly than older HIV+ groups. For example, several papers show that the memory functioning of HIV+ individuals in their mid-30s are comparable to HIV− individuals in their mid-50s (Avci et al. 2016; Woods et al. 2010; Scott et al. 2011). While most studies have reliably shown differences between HIV+ adults and age-matched seronegative comparison subjects (potentially supporting accentuated aging), no studies in the cART era have directly examined whether older HIV+ adults – who may be at greatest risk to experience both accelerated and accentuated aging – evidence neurocognitive profiles similar to those of much older seronegative adults.
Given previous findings of accelerated neurocognitive aging in pre-cART era HIV samples (Van Gorp et al. 1989), and given other neurocognitive studies that have shown additive effects of HIV and aging on cognition, the present study sought to replicate these results using a three-group design in a well-characterized cART era HIV population. Accentuated aging as defined in the current study design would be observed if the older HIV+ group performed worse on neurocognitive domains compared to an age-matched seronegative comparison group. Not exclusively, accelerated aging in the current design is further defined as the older HIV+ group also performing no differently than older-old (aged ≥ 65) seronegative comparison participants. Therefore, the current study aims to determine whether the profile of neurocognitive performances in an older (aged 50–65) HIV+ sample is impaired relative to an age-matched seronegative comparison group (accentuated aging), and furthermore commensurate with those seen in older-old (aged ≥ 65) seronegative comparison participants (accelerated aging).
Method
Participants
A total of 128 older (i.e., ≥ 50 years of age) Caucasian participants were selected from two larger studies, one focused on prospective memory and neurocognition in HIV at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP), and the other a longitudinal study on healthy aging at UCSD and the VA San Diego Healthcare System. Participants from the UCSD HNRP study (n=40 HIV+ and n=55 HIV−) were recruited from the San Diego community and local HIV clinics. All participants were over age 50 (mean=56.3, range=50–75). In order to ensure a comparison group with a mean age more consistent with the traditional typically aging literature in support of our study aims, we also included a cohort of older individuals over 65 (n=33) from the typical aging parent study that was closely balanced with the HNRP cohorts in terms of demographic factors that may influence cognition (i.e., education, gender). These participants were recruited through newspaper advertisements and community lectures for enrollment in a longitudinal study on healthy aging at UCSD and the VA San Diego Healthcare System. Participants were stratified by age and HIV status into one of three study groups: 1) HIV seronegative aged 50–65 (older HIV−, or “O−“; n=48); 2) HIV positive aged 50–65 (older HIV+, or “O+”; n=40; and 3) “Older old” HIV negative aged ≥ 65 years (older old HIV−, or “OO−“; n=40).
General exclusion criteria for both studies included chronic neurological (e.g., stroke, seizure disorders) or psychiatric (e.g., psychosis) conditions, head injury with loss of consciousness greater than 30 minutes, and non-HIV-associated dementias. Participants within the HNRP cohorts were excluded if they met Diagnostic and Statistical Manual of Mental Disorders-IV-TR (American Psychiatric Association 2000) criteria for substance use disorders within six months of evaluation or if they tested positive on a urine toxicology screen for illicit drugs (except marijuana) on the day of testing. They were also excluded if they had an estimated verbal IQ score (VIQ) below 70 on the Wechsler Test of Adult Reading (WTAR; Psychological Corporation 2001). By nature of the inclusion/exclusion criteria of the healthy aging parent study, none of the OO− participants had lifetime histories of alcohol or other substance dependence. Based on a comprehensive neuromedical and neuropsychiatric evaluation conducted as part of the healthy aging study, none of the elderly individuals included in this study met criteria for mild neurocognitive impairment (MCI) or dementia.
Demographic, psychiatric, medical, and HIV disease characteristics for the study participants are presented in Table 1. As shown in Table 1, approximately 57.5% (n=23) of our HIV+ group had AIDS, and 92.5% (n=37) were taking antiretroviral medications. Their median duration of HIV infection was 17.9 (8.1, 22.3) years. Their median nadir and current CD4 counts were 183.5 (interquartile range: 90.0, 270.75) and 590.5 (interquartile range: 432.0, 771.5), respectively. Approximately 85.0% (n=34) had undetectable viral load in plasma. By design, both the older HIV negative and older HIV-positive groups (O− and O+) were significantly younger (mean age [SD] = 55.9 [4.2] and 54.9 [3.9], respectively) than the “older old” HIV-negative (OO−) group (mean age [SD] = 73.6 [6.1]; ps < .01), though they were comparable to each other (p > 0.10). Compared to the OO− and O+ groups, the O− group had a significantly higher rate of lifetime alcohol dependence (p = .005). Compared to the OO− group, the O− and O+ groups had a significantly higher rate of lifetime non-alcohol substance dependence (p < .001) and a significantly lower rate of Hepatitis C virus infection (p = .003). Rates of substance use dependence histories for alcohol/non-alcohol were 39/50% for the O− group, 15/45% for the O+ group, and 0/2.5 for the OO− group. On average, our study participants had 15 years of education and approximately 62% of the entire sample was male. There were no group differences in sex or sex differences on the neuropsychological tasks both across the entire cohort and within the HIV+ sample (ps > .10).
Table 1.
Demographic, psychiatric, medical, and HIV disease characteristics of the four study groups: Old HIV− (O−), Old HIV+ (O+), and Older Old HIV− (OO−).
| Variable | O− (n = 48) | O+ (n = 40) | OO− (n = 40) | p-value* | Group |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age (years) | 55.9 (4.2) | 54.9 (3.9) | 73.6 (6.1) | <.001 | OO− > O− = O+ |
| Education (years) | 14.7 (2.4) | 15.1 (2.1) | 15.4 (2.4) | .269 | ----- |
| Gender (% male) | 66.7% | 67.5% | 50.0% | .188 | ----- |
| Ethnicity (% Caucasian) | 100.0% | 100.0% | 100.0% | ----- | ----- |
| Psychiatric Characteristics | |||||
| Depression Elevated1 (%) | 10.9 | 25.6 | 30.0 | .136 | ----- |
| Geriatric Depression Scale | ----- | ----- | 3.89 (4.4) | ----- | ----- |
| POMS Depression/Dejection | 8.3 (9.3) | 12.9 (12.4) | ----- | .109 | ----- |
| POMS Total | 52.6 (24.2) | 63.4 (33.7) | ----- | .213 | ----- |
| LT Alcohol Dependence (%) | 39.1% | 15.0% | 0.0% | .005 | O− > O+, OO− |
| LT Non-Alcohol Substance Dependence (%) | 50.0% | 45.0% | 2.5% | < .001 | O−, O+ > OO− |
| Medical Characteristics | 41.3% | ||||
| Diabetes mellitus (%) | 8.3% | 7.5% | 6.5% | .953 | ----- |
| Hepatitis C Virus (%) | 10.4% | 20.0% | 0.0% | .003 | OO− > O− = O+ |
| LT Hypertension (%) | ----- | 29.0% | ----- | ----- | ----- |
| LT Hyperlipidemia (%) | ----- | 22.6% | ----- | ----- | ----- |
| HIV Disease Characteristics | |||||
| HAND2 (%) | ----- | 9.3% | ----- | ----- | ----- |
| Duration of infection (years)3 | ----- | 17.9 (8.1, 22.3) | ----- | ----- | ----- |
| CD4 nadir (cells/μl)3 | ----- | 183.5 (90.0, 270.8) | ----- | ----- | ----- |
| CD4 current (cells/μl)3 | ----- | 590.5 (432.0, 771.5) | ----- | ----- | ----- |
| Plasma VL (% undetectable) | ----- | 85.0% | ----- | ----- | ----- |
| CSF VL (% undetectable) 4 | ----- | 85.2% | ----- | ----- | ----- |
| AIDS (%) | ----- | 57.5% | ----- | ----- | ----- |
| ARVs (% on) | ----- | 92.5% | ----- | ----- | ----- |
Note. Data represent means (SD) unless otherwise noted.
p-value reflects omnibus group difference.
O− and O+ groups completed the POMS Depression/Dejection scale (elevated ≥ 1.5 SD, OO− group completed the Geriatric Depression Scale-short form (elevated ≥5);
HIV-associated neurocognitive disorder;
median (interquartile range);
n=27;
POMS = Profile of Mood States; POMS Total = POMS total mood disturbance score; LT = Lifetime; HAND = HIV-associated neurocognitive disorders; CD4 = cluster of differentiation 4; VL = viral load; CSF = cerebrospinal fluid; AIDS = Acquired Immune Deficiency Syndrome; ARVs = antiretrovirals.
Materials and Procedure
The procedures involved in this study were approved by the human subjects institutional review boards at UCSD and the VA San Diego Healthcare System. Each participant provided written, informed consent, and was administered a study-specific comprehensive neuromedical and neuropsychological evaluation. HIV serostatus was determined by enzyme-linked immunosorbent assays and confirmed by a Western blot test.
As part of their respective neuropsychological test batteries, participants within both studies were administered the Digit Span subtest of the Weschler Memory Scale 3rd edition (WMS-III; Psychological Corporation, 1997), the Trail Making Test (TMT) Parts A and B (Army Individual Test Battery, 1944), the Boston Naming Test (BNT; Goodglass et al. 2001), and the California Verbal Learning Test 2nd edition (CVLT-II; Delis et al. 2000). Raw scores for Digit Span (Total Correct), TMT (TMT A and B Total Time), BNT (Total Correct), and CVLT-II (Trial 1, Learning Total Trials 1–5, Long Delay Free Recall) were used to examine differences in neurocognitive test performance between the groups. Since the purpose of the current study was to examine possible accelerated aging effects (e.g., O+ performances that are commensurate with OO− performances), normative adjustments for age could have potentially shown this pattern even in the absence of an effect of accelerated aging (and better explained our findings), and thus raw scores were deemed the best metric to use for this study’s primary analyses. Since none of the variables listed in Table 1 were associated with any of the neurocognitive outcomes, no covariates were used for the study’s primary analyses. Raw scores were converted to population-based z-scores derived from the entire study sample (n=128) for use in the equivalence test analyses (see Data Analysis section below).
For the O+ and O− groups, current mood symptoms were assessed using the Profile of Mood States (POMS; McNair et al. 1981), which is a 65-item, self-report measure of current (i.e. the week prior to evaluation) affective distress. Each participant was asked to rate various mood symptoms (e.g., “unhappy”) on a five-point Likert-type scale ranging from 0 (i.e., “not at all”) to 4 (i.e., “extremely”), which were used to derive five subscale scores (e.g., POMS Depression subscale) and a total mood score (i.e., POMS Total Mood Disturbance). The healthy aging study participants (OO+ group) were administered the Geriatric Depression Scale (GDS; Yesavage et al. 1983), which is a 30-item self-report measure used to assess depression symptoms in the elderly. Participants rate each item yes or no, which is scored as either 0 (no) or 1 (yes) points. The total number of points is summed for the total score. Participants were classified as depressed or not depressed using the POMS or GDS using previously validated cutpoints (POMS elevated ≥ 1.5 SD of normative depressive/dejection scale scores, Patterson et al. 2006; GDS elevated ≥ 10, Yesavage et al. 1983).
Statistical analyses were conducted using a JMP software package (version 11.0) and the critical alpha was set at p < .05. Outliers greater than 4 standard deviations from the population mean were excluded from analyses. Since the majority of the neuropsychological variables (except CVLT-II Trials 1–5, for which ANOVA and Tukey-Kramer HSD post-hocs were used) were non-normally distributed as determined by Shapiro-Wilk tests of normality at a critical alpha of .05, primary analyses were conducted using Wilcoxon Rank Sums tests (ANOVA and Tukey-Kramer HSD post-hocs were used for CVLT-II Trials 1–5). We tested for equivalence/non-equivalence using a three-step process by examining: (1) group differences, (2) effect sizes, and (3) equivalence tests. Primary analyses for group differences included comparing performances between the three study groups on the overlapping neurocognitive measures, for which Z-ratio, p values, and Cliff’s d effect-size values can be found in Table 2. Next, equivalence tests were conducted using the Two One-Sided Tests (TOST) approach to further explore any null findings between the O+ and OO− groups using a threshold difference of 0.75, for which smaller differences in sample-based z-scores were considered to be practically equivalent and significant p values for both upper and lower thresholds indicating practical equivalence. We elected to utilize a threshold of 0.75 since sample-based z scores of 0.75 on either side of a respective group’s score parallels the 1.5 SD cutoff commonly used in clinical and research settings to designate impairment (e.g., Heaton et al., 2004). Finally, we examined group differences using linear regressions (i.e., using dummy codes for the 3 groups) while controlling for demographic variables (i.e., gender, education).
Table 2.
Pair-wise Wilcoxon rank-sum comparisons, effect sizes, and equivalence testing values for the Older Old HIV− (OO−), Older HIV− (O−), and Older HIV+ (O+) groups on neurocognitive measures.
| Variable | Z | p | Cliff’s d | TOST upper, lower t-ratiob | TOST upper, lower p | |
|---|---|---|---|---|---|---|
| Digit Span Total | OO− vs. O+ | 0.31 | .759 | 0.10 | −2.99, 3.81 | .002, <.001 |
| O− vs O+ | 2.30* | .022 | 0.40 | ----- | ----- | |
| CVLT-II Trial 1 | OO− vs. O+ | 1.40 | .340 | 0.18 | −1.77, 5.35 | .040, <.001 |
| O− vs O+ | 2.12* | .034 | 0.41 | ----- | ----- | |
| CVLT-II Trials 1–5 | OO− vs. O+ | 0.93 | .622 | 0.12 | −2.41, 4.44 | .009, < .001 |
| O− vs O+ | 1.72 | .196 | 0.21 | −5.26, 1.91 | <.001, .029 | |
| CVLT-II LDFRa | OO− vs. O+ | ----- | ----- | ----- | ----- | ----- |
| O− vs O+ | ----- | ----- | ----- | ----- | ----- | |
| Trail Making Test Part A | OO− vs. O+ | 2.05* | .041 | 0.39 | ----- | ----- |
| O− vs O+ | 0.04 | .972 | 0.01 | −3.50, 3.48 | <.001, < .001 | |
| Trail Making Test Part B | OO− vs. O+ | 1.84 | .065 | 0.12 | −4.62, 1.92 | < .001, .029 |
| O− vs O+ | 0.46 | .646 | 0.13 | −4.19, 2.65 | < .001, .005 | |
| Boston Naming Testa | OO− vs. O+ | ----- | ----- | ----- | ----- | ----- |
| O− vs O+ | ----- | ----- | ----- | ----- | ----- |
Note.
p < .05;
CVLT-II = California Verbal Learning Test-Second Edition; TOST = Two One-Sided Tests
Mean-level omnibus tests showed no significant differences across the 3 groups
TOST values are presented only for tests in which post-hocs indicated no significant difference between the groups
Results
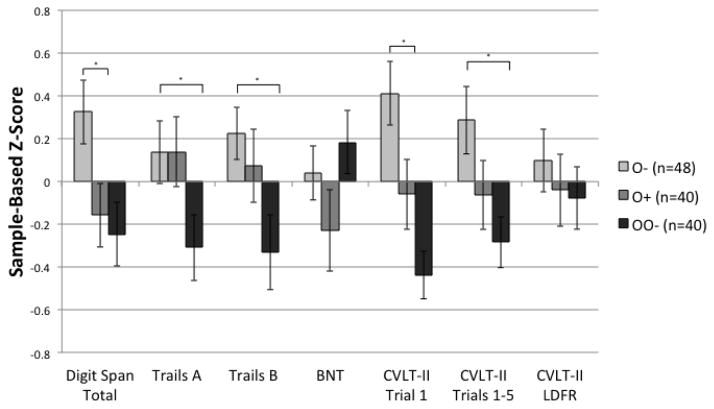
Figure 1 shows sample-based z-scores for all neuropsychological tasks across the three age groups. Mean-level comparisons of raw scores on neuropsychological tests showed that Digit Span total scores differed across the three groups (χ2[2] = 8.31, p = .016). As shown in Table 2, post hoc pair-wise Wilcoxon rank-sum tests revealed that while the O+ and OO− groups performed significantly more poorly than the O− group (p < .05), they did not differ in their performance relative to each other (p = .759). Two One-Sided Tests revealed that the O+ and OO− groups evidenced practical equivalence for performances on Digit Span Total (ps < .05). Performances on Trial 1 of the CVLT-II significantly differed across the three groups (χ2[2] = 13.95, p < .001) such the O+ and OO− performed significantly more poorly than the O− group (ps < .05), while the O+ and OO− groups did not differ (p = .340). Two One-Sided Tests revealed that the O+ and OO− groups evidenced practical equivalence for performances on CVLT-II Trial 1 (p < .05). Performances on Trail Making Test A significantly differed across the three groups (χ2[2] = 6.24, p = .044) such that the O− and the O+ group performed significantly better than the OO− group (ps < .05), while the O+ and O− group did not differ (p = .972). Performances on TMT B significantly differed across the three groups (χ2[2] = 6.45, p = .040) such that the O− performed significantly better than the OO− group (p = .014), and neither group differed from the O+ group (ps > .05). Overall performance on Trials 1–5 on the CVLT-II significantly differed across the three groups (F[2,125] = 3.86, p = .024) such that the O− group performed significantly better than the OO− group (p = .007) but neither group differed from the O+ group (ps > .05). There were no differences across the three groups in performance on Boston Naming Test or CVLT-II Long Delay Free Recall (ps > .10).
Figure 1.
Sample-based mean z-scores on neuropsychological tasks among older seronegative (O−), older HIV+ (O+), and older-old seronegative (OO−) groups. Error bars represent standard errors.
Note. * p < .05; BNT = Boston Naming Test; CVLT-II = California Verbal Learning Test-Second Edition
In order to determine whether the observed effects and null results were due to group differences on demographic variables (i.e., education, gender), we ran regression analyses to compare neurocognitive performance across the three age/HIV groups while controlling for education and gender. For Digit Span, the overall model reached statistical significance (F[4, 121] = 3.05, p = .020, R2 adjusted = .06). Within this model, Tukey HSD pairwise least square means tests revealed that compared to the O− group, the O+ group (β = 2.48, p = .039) and the OO− group (β = 3.03, p = .008) had significantly poorer performances on Digit Span. However, the O+ and OO− groups did not differ on Digit Span (β = 0.63, p = .805). Education was a significant independent predictor in this model at the level of a trend (β = 1.80, p = .074). Gender was not a significant independent predictor of Digit Span performance (β = 0.75, p = .457). For CVLT-II Trial 1, the overall model reached statistical significance (F[4, 123] = 5.42, p < .001, R2 adjusted = .12). Compared to the O− group, the O+ group (β = 2.42, p = .045) and the OO− group (β = 4.52, p < .001) had significantly poorer performances on CVLT-II Trial 1. The O+ and OO− groups did not differ on performance of the CVLT-II Trial 1 (β = 2.10, p = .095). Gender was a significant independent predictor in this model at the level of a trend (β = 1.85, p = .067). Education was not a significant independent predictor of CVLT-II Trial 1 performances (β = 1.05, p = .298).
Discussion
There is growing concern as to whether HIV infection confers risk for premature neurocognitive impairment relative to seronegative adults (accentuated aging), and whether such processes might further result in neurocognitive changes similar to that of much older seronegative adults (accelerated aging). Given previous findings of biological accelerated aging in HIV, as well as pre-cART era neurocognitive studies showing preliminary findings of accelerated neurocognitive aging (Van Gorp et al. 1989), we expected to see evidence of both accentuated (i.e., higher prevalence rates of neurocognitive impairment compared to age-matched seronegative controls) and accelerated neurocognitive aging (i.e., neurocognitive impairment rates that are similar to those of much older seronegative adults) due to HIV infection. Our results indicate that not only are Older HIV+ adults impaired in basic auditory attention compared to age-matched seronegative adults (accentuated aging), but that Older HIV+ adults also are impaired on auditory attention measures to a degree that is commensurate with much older seronegative adults in their 70s (accelerated aging). The significant differences suggestive of accentuated and accelerated aging were associated with medium effect sizes (d = .4 for Digit Span and Trial 1 CVLT-II), while the equivalence of the Older HIV+ and Older Old HIV− groups were associated with very small effect sizes in these same areas (d = .1 for Digit Span and Trial 1 CVLT-II). Of note, these findings could not be better explained by other demographic, disease, or comorbid factors. Interestingly, however, the effects observed do not appear to manifest in other neurocognitive domains including memory, language, or speeded executive functions.
This evidence of both accelerated and accentuated aging of auditory attention in HIV disease are consistent with an earlier qualitative review suggesting that aging with HIV may lead to impairment in more basic cognitive processes (Hardy and Vance 2009). In this case, the data suggest that basic auditory attention is susceptible to the effects of HIV disease (accentuated aging) and that such effects are consistent with the level and pattern of functioning observed in much older healthy adults (accelerated aging). This study utilized clinical measures of attention (i.e., digit span and single trial word-list learning,) that are non-specific tasks involving other overlapping domains (e.g., speed of information processing) and thus may not offer insights into the neural or precise neurocognitive mechanisms underlying accelerated/accentuated cognitive aging in HIV. As such, future studies may study this domain more carefully and experimentally using detailed, theoretically-informed tasks designed to assess underlying cognitive networks of attention that might be susceptible to HIV (e.g., Hinkin et al. 2000; Martin et al. 1992), and show a pattern of accelerated aging. Tasks that are grounded in theoretical models of attention, such as those proposed by Dennis and colleagues (2008), Knudsen (2007), Posner and Rothbart (2007), or Wang and Fan (2007), may provide such insights. For example, the attention network task (ANT; Fan et al. 2002) was developed using the theoretical framework of Posner and Petersen (1990) to specifically assess the alerting (e.g., sustained attention), orienting (e.g., shifting attention), and the executive (e.g., response inhibition, monitoring, voluntary control of attention) networks. Based on our current findings of higher-order processes (e.g., executive functions, delayed memory) showing no pattern of accelerated aging, such future studies with the ANT may expect to find that in HIV infection, basic attention networks (e.g., alerting, orienting) may evidence accelerated aging while higher-order attention networks (e.g., executive) may remain relatively intact.
As mentioned above, we did not observe an accelerated/accentuated aging profile in the other neurocognitive domains assessed (i.e., episodic memory, executive functions, and language). One possible explanation for these null findings is that older adults are successfully able to compensate for basic auditory attention deficits when faced with more challenging tasks (e.g., by successfully leveraging repetition of the word list on the CVLT-II). Another possible explanation is measurement challenges, given the fairly cursory way in which these higher-order domains were assessed. For example, we utilized only Trails B as a measure of executive functions, which is a broad domain that also includes constructs such as novel problem-solving, abstraction, planning, etc. that were not assessed herein and may have revealed deficits. Nevertheless, the present data call into question why changes associated with either accentuated or accelerated aging were not also found in higher-order neurocognitive functions, which are the more commonly reported problems by individuals with HIV (e.g., Heaton et al. 2011; Woods et al. 2009). Future studies could include a broader assessment of other higher-order domains (e.g., delayed memory, prospective memory, abstraction, problem-solving) to determine if such domains might also evidence patterns of accelerated neurocognitive aging.
A broader implication of the current data includes whether HIV+ adults will continue to experience accelerated aging and to what extent such processes might continue as HIV+ adults age into their 60s and 70s. In a recent study from our laboratory (Sheppard et al. 2015) HIV+ adults aged 60 and older were nearly 7 times more likely to be neurocognitively impaired as compared to HIV+ individuals aged 50–60. Thus, it will be critical as individuals with HIV disease continue to age to determine whether the presently observed accelerated aging pattern in basic verbal attention might be an indicator for broader accelerated aging in HAND rates, and/or manifest in downstream neurocognitive (e.g., delayed memory, prospective memory, problem-solving) or everyday functioning (e.g., medication management) outcomes. Indeed, previous studies have shown such stair-step additive effects on prospective memory (Woods et al. 2010) and global cognition (Moore et al. 2014), but future studies should specifically conduct equivalence tests as presently utilized—or utilize multivariate methods such structural equation modeling—in order to confirm accelerated aging patterns on these outcomes in HIV. Furthermore, longitudinal studies will be critical to determine whether HIV+ individuals who evidence an accelerated aging profile in their 50s and 60s are at an increased risk for more severe impairments as they age into their later 60s and 70s.
This study is the first cART era study to directly compare the neurocognitive performance of older HIV+ adults to that of an age-matched seronegative group and an older-old seronegative group. A limitation of the current study is the cross-sectional design, which precluded us from examining possible accelerated neurocognitive aging processes over time, as well as relatively small samples (n range 40–48), which should be increased for future studies of accelerated aging in HIV. While the alcohol and substance dependence histories were unrelated to any neuropsychological outcome variables, lifetime non-alcohol substance dependence, since higher rates were observed in the O− and O+ groups compared to the OO− groups, should be examined by future studies as a possible contributor to an overall effect of accelerated aging (Gongvatana et al. 2014; Iudicello et al. 2012). One notable characteristic of our HIV+ sample is the rates of individuals prescribed ARV regimens (92.5%), which is comparable to rates reported by Centers for Disease Control (2013) for patients under care, but nevertheless may not be generalizable to persons not engaged or retained in care, who may be at even higher risk for impairment. Another limitation of the current study is the lack of a younger seronegative group (aged 20s or 30s) to determine whether the O+ group showed accentuated aging compared to the O− group, or whether the O+ group simply showed a deficit to all younger seronegative age groups. Inclusion of younger HIV−/HIV+ and a healthy control group (as opposed to a comparison sample with broadly comparable non-HIV comorbidities as currently used) in future work investigating accelerated aging may give insight into how accelerated aging might be observable across the lifespan. Nevertheless, as the HIV+ population continues to age, future studies should also utilize similar group comparisons using O− and O+ groups in their 60s, which is when typically aging individuals would be expected to evidence age-related cognitive changes, thus providing stronger evidence for an effect of accentuated aging. Such investigations would allow for a determination of whether the aging HIV+ population experiences higher rates of neurocognitive impairment in their 60s and 70s (i.e., accentuated aging). While the present study provides modest support for a pattern of accelerated neurocognitive aging in the context of HIV disease, these findings need to be explored using neuroimaging techniques and other biomarkers, as it is possible that the pattern of older HIV+ adults evidencing similar performance to much younger seronegative individuals may also be observed in brain structure, functioning, or pathology. For example, candidate mechanisms for explaining the higher observed rates of neurocognitive impairment due to older age in HIV infection include more rapid disease progression (Goetz et al. 2001), increased neuropathological burden (Gelman and Schuenke 2004), immunological changes (Valcour et al. 2004), neurochemical changes affecting glial activation and neuronal integrity in frontal systems (Ernst and Chang 2004), and smaller frontal and temporal cortical volumes (Jernigan et al. 2005; Pfefferbaum et al. 2014). However, future studies should determine whether such changes do support a model of accelerated neurocognitive aging.
Acknowledgments
The San Diego HIV Neurobehavioral Research Program [HNRP] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H., Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., Jennifer E. Iudicello, Ph.D., David J. Moore, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.); Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Marizela Verduzco and P. Katie Riggs for their help with study management and Donald Franklin and Stephanie Corkran for their help with data processing. This study was supported by NIH grants R01-MH073419, T32-DA31098, L30-DA0321202, P30-MH62512 and K24-AG026431.
Footnotes
Conflict of Interest
The authors, David P. Sheppard, Jennifer E. Iudicello, Erin E. Morgan, Rujvi Kamat, Lindsay R. Clark, Gunes Avci, Mark W. Bondi, Steven Paul Woods, and The HIV Neurobehavioral Research Program (HNRP) Group declare that they have no conflict of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Avci G, Loft S, Sheppard DP, et al. The effects of HIV disease and older age on laboratory-based, naturalistic, and self-perceived symptoms of prospective memory: does retrieval cue type and delay interval matter? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23:716–743. doi: 10.1080/13825585.2016.1161001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J Neurovirol. 2012;18:247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- Boulias K, Lieberman J, Greer EL. An Epigenetic Clock Measures Accelerated Aging in Treated HIV Infection. Mol Cell. 2016;62:153–155. doi: 10.1016/j.molcel.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. 2013. [Google Scholar]
- Chou JP, Ramirez CM, Wu JE, Effros RB. Accelerated aging in HIV/AIDS: novel biomarkers of senescent human CD8+ T cells. PLoS ONE. 2013;8:e64702. doi: 10.1371/journal.pone.0064702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepielewski LS, Massuda R, Panizzutti B, et al. Telomere length in subjects with schizophrenia, their unaffected siblings and healthy controls: Evidence of accelerated aging. Schizophr Res. 2016;174:39–42. doi: 10.1016/j.schres.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. 2. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- Dennis M, Sinopoli KJ, Fletcher JM, Schachar R. Puppets, robots, critics, and actors within a taxonomy of attention for developmental disorders. J Int Neuropsychol Soc. 2008;14:673–690. doi: 10.1017/S1355617708080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. AIDS. 2004;18(Suppl 1):S61–67. [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, et al. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- Gianesin K, Noguera-Julian A, Zanchetta M, et al. Premature aging and immune senescence in HIV-infected children. AIDS. 2016;30:1363–1373. doi: 10.1097/QAD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15:1576–1579. doi: 10.1097/00002030-200108170-00017. [DOI] [PubMed] [Google Scholar]
- Gongvatana, Morgan, Iudicello, Letendre, et al. A history of alchohol dependence augments HIV-associated neurocognitive deficits in persons aged 60 and older. J Neurovirol. 2014;20:505–513. doi: 10.1007/s13365-014-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston diagnostic aphasia examination. 3. Philadelphia: Lippincott WilliamsWilkins; 2001. [Google Scholar]
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309:1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Vance DE. The neuropsychology of HIV/AIDS in older adults. Neuropsychol Rev. 2009;19:263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ. Dual task performance in HIV-1 infection. J Clin Exp Neuropsychol. 2000;22:16–24. doi: 10.1076/1380-3395(200002)22:1;1-8;FT016. [DOI] [PubMed] [Google Scholar]
- Horvath S, Garagnani P, Bacalini MG, et al. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Morgan EE, Gongvatana A, Letendre SL, et al. Detrimental impact of remote methamphetamine dependence on neurocognitive and everyday functioning in older but not younger HIV+ adults: evidence for a legacy effect? J Neurovirol. 2014;20:85–98. doi: 10.1007/s13365-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Quach A, Moore DJ, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22:366–375. doi: 10.1007/s13365-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhardt TR, Thannickal VJ. Systemic sclerosis-associated fibrosis: an accelerated aging phenotype? Curr Opin Rheumatol. 2015;27:571–576. doi: 10.1097/BOR.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Sorensen DJ, Robertson LC, et al. Spatial attention in HIV-1 infection: a preliminary report. J Neuropsychiatry Clin Neurosci. 1992;4:288–293. doi: 10.1176/jnp.4.3.288. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, et al. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav. 2014;18:1186–1197. doi: 10.1007/s10461-014-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS Behav. 2012;16:2279–2285. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69:833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Young C, Woods SP, et al. Screening for major depression in persons with HIV infection: the concurrent predictive validity of the Profile of Mood States Depression-Dejection Scale. Int J Methods Psychiatr Res. 2006;15:75–82. doi: 10.1002/mpr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna G, Iannone G, Alciati A, Caldirola D. Are Anxiety Disorders Associated with Accelerated Aging? A Focus on Neuroprogression. Neural Plast. 2016;2016:8457612. doi: 10.1155/2016/8457612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35:1755–1768. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Manual for the Wechsler Test of Adult Reading (WTAR) San Antonio: Author; 2001. [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Author; 1997. [Google Scholar]
- Scott JC, Woods SP, Carey CL, et al. Neurocognitive consequences of HIV infection in older adults: an evaluation of the “cortical” hypothesis. AIDS Behav. 2011;15:1187–1196. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, et al. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol. 2015;21:576–584. doi: 10.1007/s13365-015-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004a;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004b;18(Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp WG, Mitrushina M, Cummings JL, Satz P, Modesitt J. Normal Aging and the Subcortical Encephalopathy of AIDS. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1989;2(1):5–20. [Google Scholar]
- Wang H, Fan J. Human attentional networks: a connectionist model. J Cogn Neurosci. 2007;19:1678–1689. doi: 10.1162/jocn.2007.19.10.1678. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, et al. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. J Clin Exp Neuropsychol. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]