Abstract
Background and Purpose
Abnormal postural sway is associated with an increase in risk of falls but is difficult for clinicians to accurately quantify without access to laboratory equipment. Instrumenting clinical outcome measures using body-worn, movement monitors is a low-cost alternative. This is the first study to compare modified Clinical Test of Sensory Integration for Balance (i-mCTSIB) to the laboratory test of Sensory Organization Test (SOT) with dynamic posturography in a group of participants with Parkinson’s disease (PD) and subtle balance impairments. The purpose of this study was to: 1) determine the concurrent validity of i-mCTSIB with the SOT (6 and 4 conditions) and 2) compare i-mCTSIB and SOT to differentiate between individuals with and without recent falls within the previous 6 months.
Methods
This cross-sectional study examined 26 participants with idiopathic PD who had a Motor UPDRS scores of 32.7±13.5 out of 108.
Results
The Composite and Conditions 1 and 4 of i-mCTSIB and SOT scores were significantly correlated: Composite Scores r = − 0.64 (p = <.001), C1 r = −0.43 (p = 0.03), C3 r = −0.60 (˂0.01), C4 r = −0.54 (p = <.001). There was a significant difference in mean i-mCTSIB composite scores between fallers and non-fallers (p = 0.04). In contrast, the SOT composite was not significantly different between fallers and nonfallers (p = 0.31).
Discussion
The results suggest that i-mCTSIB may be a valid and clinically meaningful measure of sensory organization in persons with PD, even those with mild postural instability as measured by median H&Y score (2.0). Future research should evaluate predictive validity of i-mCTSIB for prospective falls.
Conclusion
The instrumented mCTSIB with portable, body-worn movement allows clinicians to quantify abnormal postural sway without the ceiling effects of clinical balance testing or the expense and importability of force plate technology in the SOT. Instrumenting mCTSIB may also distinguish between fallers and non-fallers.
Keywords: Balance, Falls, Measurement, Accelerometers, Body-Worn Monitors
INTRODUCTION
A primary focus of post-acute rehabilitation is on balance and falls assessment. Increased postural sway associated with aging and degenerative disease is a common indication of postural dysfunction and fall risk that can lead to morbidity, mortality, or reduced quality of life.1,2 Impairments in sensory organization for postural control can be measured by increases in body sway when sensory information for balance is altered.3 The Sensory Organization Test (SOT), which uses a sway-referencing force plate and visual surround to calculate composite equilibrium scores, is well established as the gold standard for quantifying postural sway and fall risk in the elderly.4,5 The clinical utility of the SOT has been demonstrated in many acute care and ambulatory practice settings. However, clinicians without access to this type of expensive and large laboratory equipment, such as those practicing in outpatient and post-acute settings (skilled nursing facilities, inpatient rehabilitation facilities, home health agencies, and long term acute care hospitals) cannot currently quantify postural sway.
Most clinical balance tests are performance-based, and are scored on an ordinal scale with rank- ordered values based on operationally defined properties.6 Unlike SOT which calculates scores on an interval scale, ordinal values lack equal intervals or an absolute zero, thus do not represent actual quantity.6 This limits clinical interpretation. For example, current clinical tests of sway, such as the modified Clinical Test for the Sensory Interaction on Balance (mCTSIB) is limited in that it measures how long a patient can stand in a particular sensory condition as an indirect measure of sway quantity.7 These tests are often insensitive to subtle change or to mild deficits.8 Consequently, therapists need access to portable clinical measures that are concurrently valid with gold-standard laboratory measures of balance control. Body-worn inertial sensors that include accelerometers provide balance control data on a continuous scale. These sensors have been proposed as a portable, low-cost alternative to motion analysis laboratories, force plates or dynamic posturography for measuring mobility dysfunction in older adults.2,9–12
Recent technological advancements have led to the development of small, body-worn movement monitor systems that can measure and quantify postural sway with accuracy and sensitivity similar to force plates.12–14 These portable systems provide instant motion analysis profiles that clinicians can use to identify mild abnormalities not obvious with traditional clinical testing, measure small changes due to rehabilitation, and associated risk factors such as falls.13 Recently, similar changes in postural sway were demonstrated from a traditional posturography force plate system and a body worn accelerometer during the SOT.9 As shown by Whitney et al9 in a cohort of healthy adults with no known deficits, postural sway measured by SOT (while wearing dual-axis accelerometers) correlated significantly with accelerometer peak-to-peak measures for all conditions in most trial combinations (R2= 0.05 to 0.78). It has also been shown that measures sensitive enough to detect pelvic acceleration changes as subtle as 0.001G’s are required to differentiate between less challenging mCTSIB conditions eyes open, firm surface and eyes closed, firm surface.15,16 However, the extent to which a mCITSIB test that is instrumented with a body-worn movement monitor can replace a large posturography system to measure postural sway across 4 different sensory conditions (SOT) in older adults with deficits is unknown.
The purpose of this study was to evaluate concurrent criterion-related validity of an instrumented modified Clinical Test with the Sensory Integration for Balance (i-mCTSIB) as a measure of postural control and explore its ability to differentiate fall-risk groups in community-dwelling individuals with Parkinson’s disease (PD). The SOT and recent falls (within the previous 6 months) served as criterion measures. We hypothesized that i-mCTSIB and SOT composite scores would correlate significantly with a moderate-to-good relationship. We also hypothesized that compared to SOT, i-mCTSIB may be better able to differentiate between fallers and non-fallers.
METHODS
Setting and Participants
Twenty-six participants with idiopathic PD were recruited, from a larger clinical intervention trial investigating the effectiveness of an exercise program, to participate in this cross-sectional pilot study. Therefore, the group here represents a convenience sample of participants with PD. Inclusion criteria: all people in the study were diagnosed with idiopathic PD by a movement disorders neurologist. Individuals were excluded if they were unable to ambulate unassisted, had other neurologic, cardiovascular or orthopedic problems that could impact mobility or had cognitive impairments that would limit participation in the assessment. There were 18 men (69.2%) and 8 women (30.8%) with data from inertial sensors and a self-reported history of 2 or more falls in the past 6 months (5 fallers [19.2%] and 21 non-fallers [80.8%]). Patient demographics are detailed in Table 1. The data represented in this manuscript was taken at the baseline assessment, prior to intervention. All participants were tested in their On medication state at the Oregon Health & Science University (OHSU) balance and movement disorders laboratory. All participants signed informed consent forms approved by the OHSU Institutional Review Board.
Table 1.
Participant demographics and characteristics.
| Variable | Mean (SD) | Range |
|---|---|---|
| Age, y | 66.0 (6.9) | 52–76 |
| Height, cm | 173.3 (8.8) | 160–188 |
| Weight, kg | 76.9 (12.6) | 57–118 |
| Disease chronicity, y | 5.0 (4.2) | 0–12 |
| Unified Parkinson’s Disease Rating Scale | 32.7(13.5) | 12–57 |
| Hoehn and Yahr | 2.0* | 2–3 |
| Gender | M: 18; F:8 | |
Median
Sample size
To validate that our convenience sample was not underpowered, we assigned large effect size indices as previously reported.6 The hypothesized effect size index was r = .50 for the analysis of concurrent validity and d = .80 for differences between fallers and non-fallers. The sample size needed to include at least 22 participants for validity and 20 for differences. The 26 participants in this pilot study exceeded the required sample size to achieve 80% power-efficiency.
Procedure
Participants were tested in all 6 SOT conditions per protocol.17 Participants were then instrumented and performed the 4 mCTSIB conditions. All testing occurred on the same day to control for history effects. Participants were allowed to rest as needed between tests.
Outcome Measures
Sensory Organization Test
The SOT (SMART Equitest, NeuroCom/Natus, Clackamas, OR) was used as the criterion measure, or gold standard, of postural sway using displacement of center of pressure on a force plate. The SOT composite score has shown good reliability in older adults with an intraclass correlation coefficient (ICC) of 0.66.5 Participants were tested standing under 6 sensory conditions (three 20-second trials each) in which the surface and/or visual surround are systematically modified: Condition 1 (C1) = eyes open, fixed support; Condition 2 (C2) = eyes closed, fixed support; Constant 3 (C3) = sway-referenced vision, fixed support; Condition 4 (C4) = eyes open, sway-referenced support; Condition 5 (C5) = eyes closed, sway-referenced support; and Condition 6 (C6) = sway-referenced vision, sway referenced support. For each condition, the “equilibrium score” provides a ratio between the antero-posterior (AP) peak-to-peak sway during each trial and a theoretical sway stability limit of 12.5 degrees, similar to an inverted pendulum, during each sensory condition.17 The SOT is scored on an interval scale with the highest possible score of 100 indicating no sway at all. The lowest possible score of 0 indicates the trial was stopped due to an impending fall. Higher scores are indicative of better balance (greater stability).
SOT Composite Scores
The SOT composite equilibrium score is calculated by a) independently averaging the scores for C1 and C2; and b) adding these 2 scores to the average equilibrium scores from each trial of sensory C3–6 and c) dividing that sum by the total number of trials.17 To compare similar conditions with i-mCTSIB, we also calculated a Modified SOT Composite score as the average of Conditions 1, 2, 4 and 5 (without the sway-referenced visual surround).
Instrumented Modified Clinical Test for the Sensory Interaction on Balance
The original CTSIB is a clinical version of the SOT.18,19 It uses a stop-watch to measure duration of independent standing under 6 sensory conditions, substituting a compliance foam surface for the sway-referenced surface and a visual conflict doam for the sway referenced visual surround.18 As previously reported,19,20 the CTSIB has good test-retest reliability in community-dwelling older adults. Using SOT as the criterion measure, the CTSIB has also been reported to correlate moderately with 90% sensitivity and 95% specificity.19 The CTSIB was later modified to eliminate the visual conflict doam and includes 4 increasingly challenging balance conditions: C1 = firm surface eyes open, C2 = firm surface eyes closed, C4= eyes open foam surface, and C5 eyes closed foam surface. Participants stand upright with feet together and arms across the chest for 30 seconds, first on a firm surface with and without vision, then on an Airex, medium density foam pad (18 in × 18 in × 5 in) with and without vision.18 Each condition is scored on an ordinal scale based on performance in time and sway 0–3 (0= unable, 1=˂30s, 2=30s unstable, 3=30s stable). To instrument the mCTSIB, 1 wireless tri-axial body-worn inertial sensor (Opal by APDM, Inc) was placed at L-5 with an elastic belt (Figure 1b). The system included a set of Opals with a docking station and an access point for wireless data transmission, from which the anterior-posterior (AP) and medio-lateral (ML) directions of change were used to quantify postural sway after wirelessly transmitted to a laptop using Mobility Lab software from APDM (Figure 1a). Postural sway metrics, were quantified during each stance condition.13 Although the system automatically calculates amplitude, velocity, frequency and jerkiness of postural sway in the ML direction, AP direction as well as an average of both directions, we used the amplitude measure in the AP direction (range) as the primary metric since it is equivalent to the peak-to-peak measure used to calculate the SOT equilibrium score by NeuroCom. This AP range measure, which has equal intervals and an absolute zero, is defined as the range of acceleration [m] ([m/s2]).12 In PD, the Opal range measure has been shown to be more responsive than clinical measures13,21 and have good test-retest reliability (ICC of 0.82) and significantly correlate with the clinical postural stability score r = 0.56.12
Figure 1.

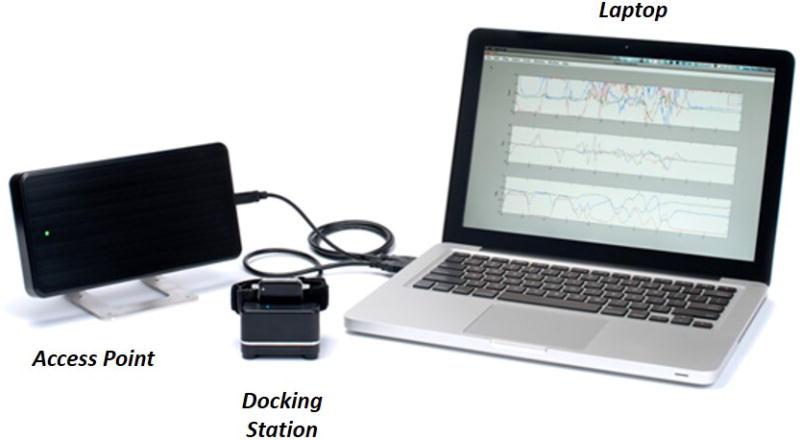
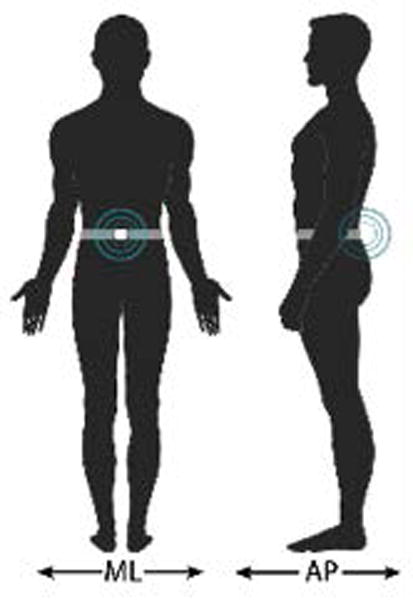
a) Body-worn movement monitor system (APDM’s Mobility Lab™) components: Inertial Sensors (e.g. accelerometers), Laptop, Access Point, and Docking Station; b) Experimental setup: instrumented modified Clinical Test for the Sensory Interaction on Balance (i-mCTSIB) Testing.
Calculating i-mCTSIB Composite Scores
Several studies have found that compared to scores from single measures, composite scores better represent the overall integrity of the balance-related mobility and fall risk identification in the elderly.2,8,22 We used previously cited approaches to calculate composite scores.3,17,23,24 To calculate i-mCTSIB composite scores, the NeuroCom SOT composite equilibrium score formula was used.17,24–26 Specifically, the scores were calculated by independently averaging scores from the 3 trials for i-mCTSIB C1 and C2. These 2 scores were then added to the average scores from C3 and C4. The sum was then divided by total trials. Higher scores are indicative of poorer balance (greater sway).
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) software, version 22 was used to analyze data. Hypotheses were tested at alpha (α) 0.05 significance level. The distribution of data were assessed using the histogram visualization and calculation of one-sample Kolmogorov-Smirnov tests for normality. Descriptive statistics of the demographics, health information, and characteristics by self-reported falls were also calculated.
Concurrent Validity
Pearson product-moment correlation (r) was used to measure the concurrent validity between i-mCTSIB and SOT outcome scores. Common criteria for evaluating correlation coefficients were used to assess the strength of the relationship between the target and reference tests (0.0 to 0.25 = little or none, 0.25 to 0.50 = fair, 0.50 to 0.75 = moderate to good, 0.75 to 1.00 = good to excellent).6 For analysis, SOT composite scores served as the criterion measure compared to i-mCTSIB composite scores. To further explore the effects of body-worn sensors on accuracy of sway detection, we examined the relationship between i-mCTSIB least (C1 and C2) and most (C3 and C4) challenging conditions against the equivalent SOT conditions. Relationship will be inverse (negative) because higher scores are indicative of poorer balance (> sway) with i-mCTSIB. Conversely, higher scores are indicative of better balance (> stability) with SOT.
We also explored the ability of the SOT and i-mCTSIB to identify individuals with subtle impairments as defined by Hoehn and Yahr (H&Y) scores.27,28 A score of 1 and 2 is indicative of mild postural instability while 3 and 4 indicates moderate postural instability, as defined by an abnormal stepping response to a backwards pull on the shoulders. Similarly, we examined the ability of SOT to identify individuals with impaired sway as defined by established cut scores (normal sway = > 69 and abnormal = ˂ 69).21 A quantitative standard for cut scores derived from inertial sensors has not yet been established.21
Fall Group Differentiation
Differences between fallers and non-fallers were measured using t-tests, and non-parametric alternatives (Mann-Whitney U-test, chi square) where appropriate. We analyzed differentiation based on the ability of the group mean scores of SOT, compared to those of i-mCTSIB, to distinguish fall-risk group (faller or non-faller). To compare tests ability to differentiate sway responses between groups, self-reported falls served as the criterion measure. To allow for comparison to previous studies, fallers were defined as individuals who have 2 or more falls in the last 6 months while non-fallers were defined as less than or equal to 1 fall.2,4,16
RESULTS
Participant Characteristics
All 26 participants completed the study. There were no significant differences between fallers’ and non-fallers’ age, gender, height, weight, or disease chronicity (Table 1). Figure 2a and 2b compares distribution of SOT and i-mCTSIB scores for participants to evaluate spread of individual scores, a score not shared by another participant. The SOT composite scores were significantly left-skewed (Kolmogorov-Smirnov test, p = <.001), with 35% (n=9) scoring within the top 20% of the test (higher score = less sway), including 1 faller and 2 with H&Y=3. Only 19% of participants (5 of 26) had an individual SOT score. In contrast, 100% of participants (26 of 26) had different i-mCTSIB scores. The i-mCTSIB test composite scores were also significantly right- skewed (Kolmogorov-Smirnov test, p = 0.03), with 19% (n=5) scoring within the top 20% of the test (lower score = less sway), none of whom were fallers. Among these 5 participants, only 1 had H&Y=3 and this person also had the highest disease duration (12 years).
Figure 2.
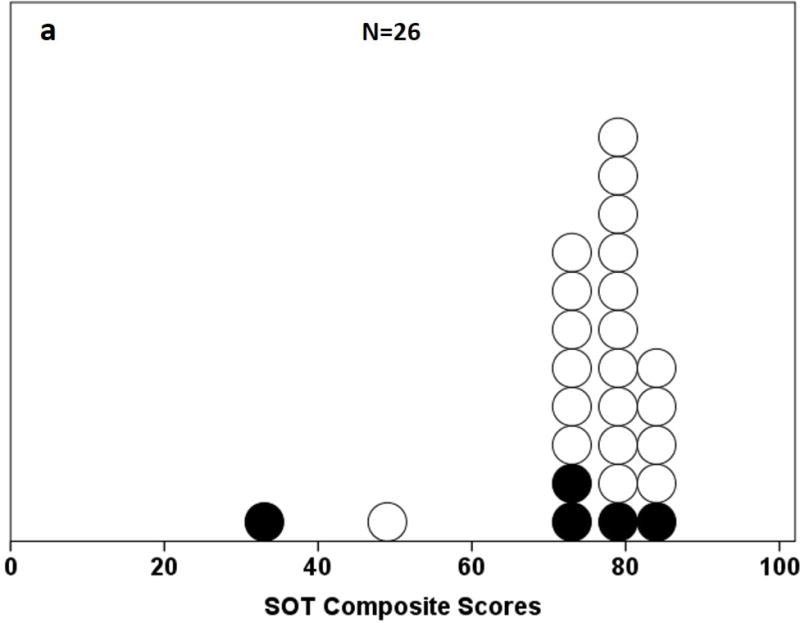
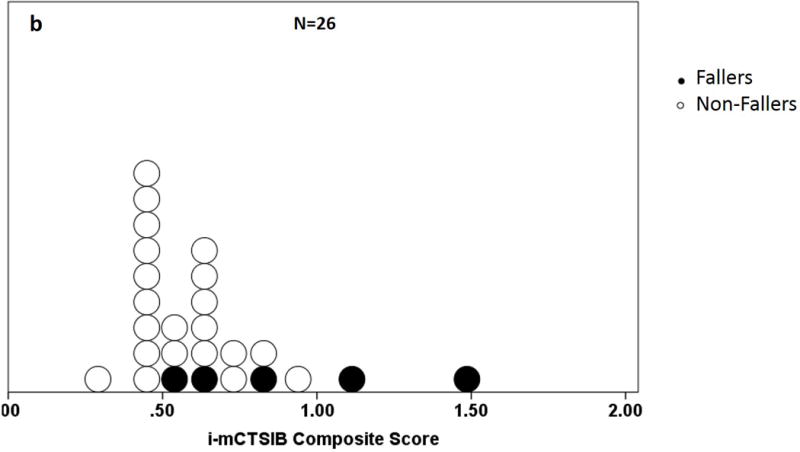
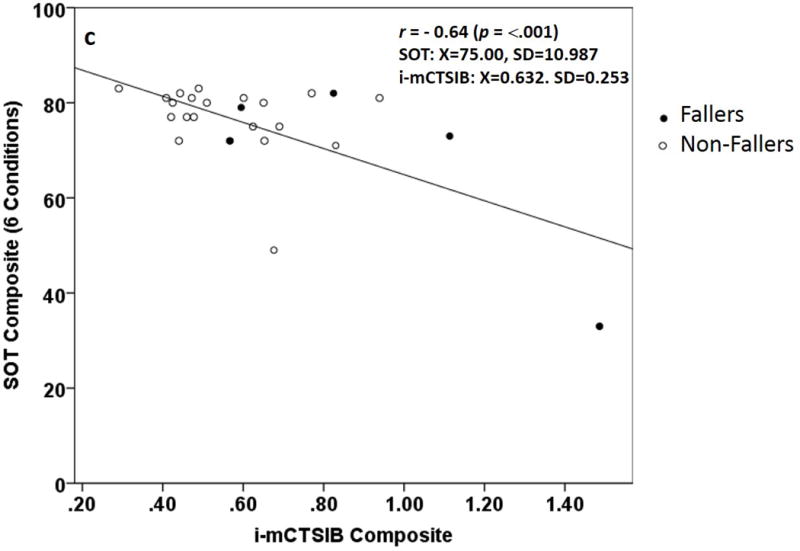
a and b. Distribution of composite scores on the a) Sensory Organization Test (SOT) and b) instrumented modified Clinical Test for the Sensory Interaction on Balance (i-mCTSIB). Shaded dots represent fallers.
c. instrumented modified Clinical Test for the Sensory Interaction on Balance (i-mCTSIB) and Sensory Organization Test (SOT) Composite score scatterplot using accelerometer data in anterior-posterior (AP). Values for Pearson (r) correlation, mean, and standard deviation are shown. Shaded dots represent fallers.
The median H&Y score was 2.0, however the range was 2–3. Approximately 73% (19 of 26) of the participants had mild postural instability (H&Y = 2) while only 27% (7 of 26) had moderate postural instability (H&Y = 3). The 5 fallers were among those with H&Y scores of 3.
Concurrent Validity: i-mCTSIB and SOT
Correlation coefficients between the SOT and i-mCTSIB in AP directions are presented in Table 2. SOT scores were significantly related to i-mCTSIB composite scores for all sway directions and almost all conditions. Significant correlations for measurements ranged from fair (r = − 0.43, p = 0.03) for C1 to good (r = − 0.64, p = <.001) for composite. There was an outlier (true score) with worst sway on SOT (composite=33±11, C4=16±14, C5=0±18) and i-mCTSIB (composite=1.4786±2.53, C3=0.82±1.41, C4=2.940±0.57). There was also an outlier (true score) with worst sway on i-mCTSIB C2=0.95±0.20 but a stable SOT C2 score of 93±3.90. This individual completed all 3 SOT trials, but was only able to complete 1 of 3 during i-mCTSIB, suggesting the trial may not reflect best performance.
Table 2.
Validity (Pearson r) of the instrumented modified Clinical Test for the Sensory Interaction on Balance (i-mCTSIB) with Sensory Organization Test (SOT) using accelerometer data in anterior-posterior (AP) direction. Mean, range, and standard deviation presented. Correlations significant at p ˂ .05 (2-tailed) represented in bold. Validity of i-mCTSIB Composite score was similar when correlated to SOT Composite scores calculated with all 6 conditions and modified Composite scores with conditions 1, 2, 4 and 5.
| AP Direction Mean (range); SD | r (p) | ||
|---|---|---|---|
| Condition | i-mCTSIB [m] ([m/s2]) | SOT | |
| Composite [Modified Composite] |
0.63 (0.29–1.49); 0.25 | 75.00 (33–83); 10.99 [76.05 (29–88);11.68] |
−0.64 (˂0.01) −0.63 (˂0.01) |
|
aEO (C1) eEO (C1) |
0.32 (0.19–0.67); 0.13 | 92.90 (88–96); 2.20 | −0.43 (<0.05) |
|
bEC (C2) fEC (C2) |
0.44 (0.19–0.95); 0.20 | 87.96 (78–95); 3.90 | −0.16 (0.45) |
|
cEOF (C3) gEOS (C4) |
0.49 (0.27–0.82); 0.14 | 80.50 (16–91); 14.24 | −0.60 (˂0.01) |
|
dECF (C4) hECS (C5) |
0.91 (0.35–2.94); 0.57 | 62.00 (0–82); 17.87 | −0.54 (˂0.01) |
i-mCTSIB Conditions
EO = eyes open, firm surface (C1)
EC = eyes closed, firm surface (C2)
EOF = eyes open, foam surface (C3)
ECF = eyes closed, foam surface (C4)
SOT Conditions
EO = eyes open, firm surface (C1)
EC = eyes closed, firm surface (C2)
EOS = eyes open, sway-referenced support (C4)
ECS = eyes closed, sway-referenced support (C5)
There was a significant inverse relationship between i-mCTSIB and SOT composite scores r = − 0.64 (p = <.001) and modified composite score r = − 0.63 (p = <.001). However, 80% (4 of 5) of the fallers with moderate postural instability were not detected by the SOT as having abnormal sway (Figure 2c). There was also a significant inverse relationship between respective i-mCTSIB and SOT conditions: C1 r = −0.43 (p = 0.03), C3 r = −0.60 (p = <.001), and C4 r = −0.54 (p = <.001). We found no relationship between i-mCTSIB C2 and SOT C2 r = −0.16 (p = 0.43).
Fall Group Differentiation: i-mCTSIB versus SOT
We compared differences in mean i-mCTSIB and SOT scores between fallers and non-fallers. There was a significant difference in mean i-mCTSIB composite scores between fallers and non-fallers (p = 0.04). In contrast, the SOT composite revealed no significant differences between groups (p = 0.31).
DISCUSSION
Validity
Clinicians without access to dynamic posturography testing (NeuroCom/Natus), need valid, alternative measures that can quantitatively identify disordered sensory organization for balance.29,30 An instrumented mCTSIB, using composite equilibrium scores, may provide a valid method to measure sensory organization in people with PD and subtle balance impairments. To our knowledge, this is the first study to compare composite equilibrium scores, comparable to the SOT, for the instrumented CTSIB. Several studies2,8,31 have suggested use of SOT composite scores as the gold standard laboratory measure of sensory organization of balance. Current findings provide preliminary validation of a method to calculate and make clinical inferences about impaired sensory organization in older adults from accelerometer-based composite scores of postural sway across several sensory conditions.
The significant correlation between i-mCTSIB and SOT composite scores is consistent with concurrent validation of accelerometer-based measures of sway and center of pressure measures from a force plate, reported in previous studies.12,9 Finding similar composite score correlations, with and without sway-referenced visual surround, agrees with previous studies, which report that measures obtained during SOT conditions 3 and 6 do not differ significantly from those obtained with eyes closed.20,29 Importantly, the relationship was influenced by an outlier with worst sway scores on SOT and i-mCTSIB. Although confirmed as true scores without error, relative extremes can significantly influence statistical description.6 This is a common delimitation of studies with small cohorts such as pilots.6 The moderate-to-good versus excellent strength of criterion-test agreement across conditions is consistent with previous reports,9,12,14 and may be related to differences in how body sway is measured between the target test (i-mCTSIB) and the criterion test (SOT).6 For instance, people do not sway like the inverted pendulum on which SOT peak-to-peak values are based. In contrast, sway strategies in the elderly with multiple chronic conditions has been shown to use hip strategy, rather than an ankle (inverted pendulum) strategy.1
Instrumented mCTSIB accelerometer-based measures also correlated well with SOT under the least (C1 = eyes open, firm surface condition) and most (C4 = eyes open, sway-referenced support and C5 = eyes closed, sway-referenced support) challenging conditions. These results concur with previous studies where strength of associations were found to increase with subject sway, thus during more challenging conditions.9,14,16 This further supports the hypothesis that body-worn inertial sensors with mCTSIB makes a significant contribution in helping clinicians detect sway during less challenging conditions in patients with subtle impairments.
Consistent with other studies, poor agreement between the SOT and accelerometry scores during the eyes closed firm surface condition requires further investigation. This finding agrees with Whitney et al,9 that the only trial combination that did not correlate significantly was SOT 3-trial average compared to accelerometer 1st trial. Similarly, poor agreement was found only when the authors9 evaluated the relationship between the least challenging conditions, which can be attributed to lower sway and variability that occurs during C1 and C2 as previously reported.2,9,16 Another possible explanation is the increased reliance on external visual information for postural control in elderly and moderate-to-severe PD reported previously,1,32,33 resulting in larger sway with eyes closed in the ML (PD) and AP (elderly) directions. The mismatch between SOT peak-to-peak (AP) and ML sway more commonly seen in PD may explain the current finding. Similarly, Whitney et al9 reported age to be a covariate with the association between peak-to-peak accelerometer and SOT during C2 and attributed the finding to changing balance strategies with increasing age, progressing from greater reliance on ankle strategy to hip strategy. This finding may be also be related to scores from a participant who was unable to complete all 3 i-mCTSIB trials, with worst sway on i-mCTSIB but stable on SOT. When the outlier was removed, the correlation increased more than 2 fold indicating that the mismatched values influenced the relationship. Future studies that include a larger sample with greater sway variability will control for these issue.
Instrumenting mCTSIB using a system of body-worn movement monitors improves scale of measure from ordinal to ratio, thus test precision, which allows clinicians to quantify sway with accuracy similar to SOT. Results from the current study suggest that i-mCTSIB may be better than the SOT in detecting postural sway in patients with mild PD and subtle balance impairments. Specifically, results showed that although i-mCTSIB correlated well with SOT, it did not have the same ceiling effects. We found different test score distributions across participants on SOT compared to i-mCTSIB. While some degree of skewness was anticipated given the mild disease severity of the sample population, i-mCTSIB scores were less skewed which can be attributed to ratio scale of measure, thus better spread of scores.6 The infinite range of i-mCTSIB scores is greater than the range of scores in the SOT (0–100) and the mCTSIB alone (0–3).17–19,25 Importantly, all participants in the current study had a different i-mCTSIB score compared to SOT in which as many as 27% (7 of 26) participants shared a score, indicating that i-mCTSIB measures a patient’s sway with a higher level of specificity. This concurs with previous findings9,26 which explain that individuals with different sway impairments and instability can share the same SOT score because the range of Center of Pressure or COP (maximum anterior-posterior lean angle) is calculated based of approximations of each person’s height.
In studies comparing accelerometers to COP, others2,9,12,16 have also shown accelerometry to be comparable to or better than with SOT, suggesting that i-mCTSIB may be able to help identify and differentiate between postural control changes over time. Whitney et al9 argue that body-worn sensors are a more accurate measure of sway than force-plates because relative acceleration and orientation of the pelvis better approximates the motion of center of mass, thus a measure of balance. This premise is corroborated by current findings that acceleration measured at the center of mass during administration of mCTSIB, improves the test’s ability to detect sway comparable to SOT. This supports the use of instrumented measures with body-worn movement monitors, that more closely approximate actual COP, to quantify sway with better precision versus tests based solely on ordinal or interval scales.6 Current results suggest that instrumenting clinical sway measures can improve clinometric properties of the test. Using i-mCTSIB may offer clinicians a lower cost and portable alternative to posturography for quantifying sensory organization in older adults, specifically when time and resources are limited. Previous studies5,9 have reported similar findings using accelerometer values from individual mCTSIB or CTSIB condition. Together, these results support concurrent validation for i-mCTSIB compared to SOT to help quantify sensory-related postural sway impairments.
Differentiation
Compared to SOT, the instrumented mCTSIB may be better able to differentiate between fallers and non-fallers. Recent history of falls has been shown to indicate risk of future falls and functional decline in elderly.22,34 The preliminary findings of the current study suggests that i-mCTSIB composite scores may be discriminate for identifying fallers with mild postural instability. This further establishes criterion validity and supports the potential use of i-mCTSIB composite scores to augment other clinical information to make determinations related to fall risk in older adults. The difference in scale of measure may have contributed to the significant finding. The SOT uses an interval scale ranging from 0–100 while i-mCTSIB is on a ratio scale with infinite end range. Therefore i-mCTSIB is able to measure extreme amounts of sway with more precision than SOT, thus improved accuracy in fall group discrimination.6 This agrees with previous findings that postural sway, measured by movement monitors and force-plates, can differentiate falls.8,15,16 Rose et al35 found that mean composite scores of 4 SOT conditions (C1, C2, C4, C5) differentiated between fallers and non-fallers in older persons.35 The current study is the first to reproduce a similar finding with instrumented mCTSIB. In the current study however, analysis concerning falls prediction was not possible because of the small number of participants with more than 2 falls.
Similarly, others2,4 have reported a significant difference in loss of balance in SOT scores between multiple-fallers (≥ 2 falls) and single-fallers. Based on a similar sample (N=17); 12 fallers and 5 non-fallers), Sullivan et al16 also found that accelerometers were able to distinguish between fall group and between all mCTSIB conditions with the exception C1 and C2.15 However, results of their study should be compared to current findings with caution as they used the accelerometer root mean square (RMS) measure instead of Range. Recently, Mancini et al12 also found RMS to be a more sensitive time-domain accelerometer measure of sway compared to range. Limitations include small sample size and subject cohort. The current study only included a cohort of community-dwelling adults with PD who were willing to participate in a larger exercise study. Furthermore, the number of participants with more than 2 recent falls was small. Study design delimitations include retrospective nature of self-reported falls and analysis of range accelerometer measures only with emphasis on AP direction. Several studies have reported on the differences in fall risk between the ML and AP planes2,4,36,37 and the sensitivity12 of RMS accelerometer measure to postural control. For instance, despite similar fall histories, fallers with PD have been shown to present with more medio-lateral sway compared to more anterior-posterior sway in age-related fallers.1 Future research should evaluate concurrent validity of i-mCTSIB using other accelerometer metrics such as RMS, predictive validity for prospective falls, and translational utility of body-worn movement monitor technology for older adults with multiple chronic conditions, specifically those receiving care in post-acute practice settings where detectable change in mobility is subtle.
CONCLUSION
This is the first study to demonstrate validity of the instrumented mCTSIB with the SOT. Instrumentation using a system of body-worn movement monitors was shown to be an efficient, quantitative alternative in the measurement of postural control in older adults with subtle sway impairments. Instrumented mCTSIB can help clinicians’ better quantify abnormal sway using a portable system. Instrumented mCTSIB may also be better able to differentiate between fallers and non-fallers than SOT.
Instrumentation can help meet the demand for value-based rehabilitation, specifically improved outcomes at a lower cost. The degree of quantification allows therapists to detect subtle changes, thus inform therapeutic need and progression without force plates. Measuring subtle change is also accretive to person-centered care, thus motivation and participation through the continuum. This may be of particular value in post-acute rehabilitation of older adults where these factors are often barriers.
Supplementary Material
Acknowledgments
The authors thank Drs. Martina Mancini and Susan Whitney for their advice on accelerometry data analysis, NeuroCom for consultation on composite score calculations, Dr. Samuel Cheng for statistical support, and Dr. Michael Yao, MD, CMD for medical rehabilitation review and critique of manuscript.
This research is partially supported by National Institute of Aging (R37AG006457), the Kinetics Foundation, and Oregon Clinical & Translational Institute (OCTRI) (ULIRR024140).
Footnotes
Authors’ contributions:
LF provided study concept/idea/design, data analysis/interpretation, and writing. GG participated in data analysis/interpretation and manuscript revisions. LK participated in study design, data collection/interpretation, and manuscript revisions. FBH and MTB contributed to study design and manuscript revision, MB contributed to idea/concept/design. All authors were responsible for critical revisions and final approval of the manuscript. FBH provided facilities/equipment.
References
- 1.Bekkers EM, Dockx K, Heremans E, et al. The contribution of proprioceptive information to postural control in elderly and patients with Parkinson’s disease with a history of falls. Front Hum Neurosci. 2014;8:939. doi: 10.3389/fnhum.2014.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitney SL, Marchetti GF, Schade AI. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch Phys Med Rehabil. 2006;87(3):402–407. doi: 10.1016/j.apmr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buatois S, Gueguen R, Gauchard GC, Benetos A, Perrin PP. Posturography and risk of recurrent falls in healthy non-institutionalized persons aged over 65. Gerontology. 2006;52(6):345–352. doi: 10.1159/000094983. [DOI] [PubMed] [Google Scholar]
- 5.Ford-Smith CD, Wyman JF, Elswick RK, Jr, Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76(1):77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 6.Portney LG. Foundations of Clinical Research : Applications to Practice. 3rd. Upper Saddle River: Pearson/Prentice Hall; 2009. [Google Scholar]
- 7.Horak FB. Clinical measurement of postural control in adults. Phys Ther. 1987;67(12):1881–1885. doi: 10.1093/ptj/67.12.1881. [DOI] [PubMed] [Google Scholar]
- 8.Wallmann HW. Comparison of elderly nonfallers and fallers on performance measures of functional reach, sensory organization, and limits of stability. J Gerontol A Biol Sci Med Sci. 2001;56(9):M580–583. doi: 10.1093/gerona/56.9.m580. [DOI] [PubMed] [Google Scholar]
- 9.Whitney SL, Roche JL, Marchetti GF, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RY, Carlisle AJ. Detection of falls using accelerometers and mobile phone technology. Age Ageing. 2011;40(6):690–696. doi: 10.1093/ageing/afr050. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti GF, Bellanca J, Whitney SL, et al. The development of an accelerometer-based measure of human upright static anterior- posterior postural sway under various sensory conditions: test-retest reliability, scoring and preliminary validity of the Balance Accelerometry Measure (BAM) J Vestib Res. 2013;23(4–5):227–235. doi: 10.3233/VES-130490. [DOI] [PubMed] [Google Scholar]
- 12.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J Bioeng Biomed Sci. 2011;(Suppl 1):007. doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rine RM, Schubert MC, Whitney SL, et al. Vestibular function assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S25–31. doi: 10.1212/WNL.0b013e3182872c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moe-Nilssen R, Helbostad JL. Trunk accelerometry as a measure of balance control during quiet standing. Gait Posture. 2002;16(1):60–68. doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan M, Blake C, Cunningham C, Boyle G, Finucane C. Correlation of accelerometry with clinical balance tests in older fallers and non-fallers. Age Ageing. 2009;38(3):308–313. doi: 10.1093/ageing/afp009. [DOI] [PubMed] [Google Scholar]
- 17.NeuroCom International I. Clinical Justification: Computerized Dynamic Posturography. Clackamas, OR: 2005. [Google Scholar]
- 18.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66(10):1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 19.Whitney SL, Wrisley DM. The influence of footwear on timed balance scores of the modified clinical test of sensory interaction and balance. Arch Phys Med Rehabil. 2004;85(3):439–443. doi: 10.1016/j.apmr.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther. 1993;73(6):346–351. doi: 10.1093/ptj/73.6.346. discussion 351–344. [DOI] [PubMed] [Google Scholar]
- 21.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov Disord. 2013;28(11):1544–1551. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panzer VP, Wakefield DB, Hall CB, Wolfson LI. Mobility assessment: sensitivity and specificity of measurement sets in older adults. Arch Phys Med Rehabil. 2011;92(6):905–912. doi: 10.1016/j.apmr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42(4):323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhry H, Bukiet B, Ji Z, Findley T. Measurement of balance in computer posturography: Comparison of methods–A brief review. J Bodyw Mov Ther. 2011;15(1):82–91. doi: 10.1016/j.jbmt.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.NeuroCom® International I. Balance Manager Systems® Clinical Interpretation Guide. In: NeuroCom® International I, editor. Computerized Dynamic Posturography. Clackamas, OR: 2007. [Google Scholar]
- 26.Chaudhry H, Findley T, Quigley KS, et al. Measures of postural stability. J Rehabil Res Dev. 2004;41(5):713–720. doi: 10.1682/jrrd.2003.09.0140. [DOI] [PubMed] [Google Scholar]
- 27.Khallaf ME, Fayed EE. Early postural changes in individuals with idiopathic Parkinson’s disease. Parkinsons Dis. 2015;2015:369454. doi: 10.1155/2015/369454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colnat-Coulbois S, Gauchard GC, Maillard L, et al. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson’s disease. Neuroscience. 2011;193:363–369. doi: 10.1016/j.neuroscience.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Allison L. Balance Disorders. In: Sasser M, editor. Neurological Rehabilitation. 3rd. St. Lois: Mosby-Year Book, Inc; 2006. pp. 802–837. [Google Scholar]
- 30.Badke MB, Miedaner JA, Shea TA, Grove CR, Pyle GM. Effects of vestibular and balance rehabilitation on sensory organization and dizziness handicap. Ann Otol Rhinol Laryngol. 2005;114(1 Pt 1):48–54. doi: 10.1177/000348940511400109. [DOI] [PubMed] [Google Scholar]
- 31.Artuso A, Garozzo A, Contucci AM, Frenguelli A, Di Girolamo S. Role of dynamic posturography (Equitest) in the identification of feigned balance disturbances. Acta Otorhinolaryngol Ital. 2004;24(1):8–12. [PubMed] [Google Scholar]
- 32.Landers MR, Backlund A, Davenport J, Fortune J, Schuerman S, Altenburger P. Postural instability in idiopathic Parkinson’s disease: discriminating fallers from nonfallers based on standardized clinical measures. J Neurol Phys Ther. 2008;32(2):56–61. doi: 10.1097/NPT.0b013e3181761330. [DOI] [PubMed] [Google Scholar]
- 33.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36(3):471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 35.Rose DJ, Clark S. Can the control of bodily orientation be significantly improved in a group of older adults with a history of falls? J Am Geriatr Soc. 2000;48(3):275–282. doi: 10.1111/j.1532-5415.2000.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 36.Ben Achour Lebib S, Missaoui B, Miri I, Ben Salah FZ, Dziri C. Role of the Neurocom Balance Master in assessment of gait problems and risk of falling in elderly people. Ann Readapt Med Phys. 2006;49(5):210–217. doi: 10.1016/j.annrmp.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Thevathasan W, Aziz T. Predicting falls in Parkinson disease: a step in the right direction. Neurology. 2010;75(2):107–108. doi: 10.1212/WNL.0b013e3181e7b6b5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


