Abstract
Objective
To determine how out-of-pocket costs for adjuvant endocrine therapy (AET) medication affects adherence among newly diagnosed breast cancer survivors with private health insurance who initiate therapy.
Methods
We examined medical and pharmacy claims for the 1-year period after initiating AET using the Truven Health Analytics MarketScan® database. Adherence was defined as ≥80% proportion of days covered (PDC). Mean out-of-pocket costs for AET fill were measured as the sum of copayments, coinsurance, and deductibles and adjusted to 30-day amounts. Using a multivariable logistic regression model we calculated adjusted risk ratios controlling for age, comorbidities, type of surgery, use of chemotherapy and/or radiation therapy, average out-of-pocket costs for other services, and pharmacy use characteristics.
Results
Of the 6,863 women 64 years and younger who were diagnosed with breast cancer and initiated AET, 73.9% were adherent (PDC≥80%). A total of 19% of patients had less than $5 monthly out-of-pocket costs for AET, 30% had $5–$9.99, 17% had $10–14.99, 10% had $15–19.99, and 25% had $20 or greater. Patients with out-of-pocket costs for AET between $10–14.99, $15–$19.99, and greater than $20 were 6–8% less likely to be adherent compared to patients paying less than $5.00, after controlling for covariates (p<0.05). Out-of-pocket costs for inpatient, outpatient, and other pharmacy services were not associated with adherence.
Conclusion
A substantial proportion of privately insured patients are non-adherent to AET and out-of-pocket costs for AET medication are significantly associated with a greater likelihood of non-adherence.
Keywords: Adherence, Adjuvant Endocrine Therapy, Breast Cancer, Out-of-pocket costs
INTRODUCTION
Estrogen receptor-positive (ER+) breast cancer is present in two-thirds of breast cancer cases in the US.1,2 In addition to a combination of surgery, chemotherapy and/or radiation, the standard of care for ER+ breast cancer for post-menopausal women is endocrine therapy with third generation aromatase inhibitors (AI) such as exemestane, anastrozole, or letrozole for 5 years.3–6 Similarly, the National Comprehensive Cancer Network (NCCN) at the time of the study period recommended that pre-menopausal women receive treatment with tamoxifen for up to 5 years or until menopause at which time women may switch to the AIs.6
Treatment with adjuvant endocrine therapy (AET: aromatase inhibitors and/or tamoxifen) has been demonstrated to reduce the risk of subsequent breast cancer and the rate of mortality versus non-users in both clinical trials and in diverse population-based cohorts of women in clinical practice.4,7–10 Compared with non-users, the reduction in subsequent breast cancer risk ranges from 40% to 66% across the AET groups depending on the degree of adherence.9 Treatment with tamoxifen for 5 years has been shown to reduce the rate of recurrence by 39% throughout the first decade, and reduces breast cancer mortality by about one-third throughout the first 15 years.10 However, women with a prescription benefit plan who discontinued AET early, were non-adherent, had a significantly higher mortality rate compared with those who finished the full course of therapy.11 Thus, this suggests that improvements in adherence rates to AET can reduce the morbidity and mortality of women with ER+ breast cancer.
Despite the improvement in disease prognosis among patients treated with AET, approximately 21–50% of patients are non-adherent within 4–5 years.12 In a retrospective longitudinal study, only 72–81% of patients were adherent (MPR≥80%) to anastrozole after 1 year with the mean percentage of adherent patients decreasing over a 3-year period.13
While studies have examined the association between co-payments and adherence to AET, they do not include out-of-pocket costs such as the coinsurance or deductibles paid by the patient at the time of a prescription fill for AET, do not control for other out-of-pocket costs, and/or they examine women anytime during AET treatment rather than during the first year following breast cancer diagnosis and treatment.14–17 Therefore, we sought to measure 1-year adherence to AET among women diagnosed and surgically treated with breast cancer who initiate AET within the first year in a large commercially insured population and to determine, a priori, the association between combined out-of-pocket costs for AET medication and adherence.
MATERIALS AND METHODS
Data Source
We conducted a retrospective cohort study using longitudinal inpatient, outpatient, and prescription claims data from a nation-wide, employer-based, commercially insured population in the United States. We used the Truven Health Analytics MarketScan® Commercial Claims and Encounters Databases from January 1, 2007 to December 31, 2011. The MarketScan® databases capture person-specific clinical utilization, expenditures, and enrollment across inpatient, outpatient, and prescription drug services. All data were de-identified in accordance with the Health Insurance Portability and Accountability Act requirements. Study procedures were conducted in accordance with the ethical standards of the institutional review board at the University of Washington.
Sample Selection
We identified women under the age of sixty-four with at least one prescription claim for an AI, or tamoxifen between January 1, 2008 and December 31, 2010 (Figure 1). Initiation of AET was defined as no AET prescription claims for at least six months before the first claim (index claim). Next, women were included in the cohort if they were diagnosed and surgically treated for breast cancer within twelve months prior to the index claim. Although AET treatment is recommended for up to 5 years, we were interested adherence after first initiation as a critical period for intervention. Breast cancer diagnosis was defined as a diagnostic International Classification of Diseases, Ninth Revisions, Clinical Modification code (ICD-9-CM) for ductal in situ carcinoma (ICD-9-CM: 233.0), primary invasive breast cancer (ICD-9-CM: 174.0 to 174.9) or primary invasive breast cancer with axillary lymph node involvement (ICD-9-CM: 196.0–196.3, 196.5–196.6, or 196.8–196.9) in inpatient records. Surgical treatment was defined as an ICD-9-CM code or Current Procedural Terminology (CPT) code (4th Edition) for bilateral mastectomy, mastectomy, or breast conserving surgery/lumpectomy.18–20 We restricted our sample to women continuously enrolled in a health plan that included prescription drug coverage for at least six months prior to and 12 months after the index claim. We excluded women with a diagnosis of multiple primary cancers, metastatic breast cancer to distant organs, or breast cancer recurrence any time prior to initiation and during the study period.
Figure 1.
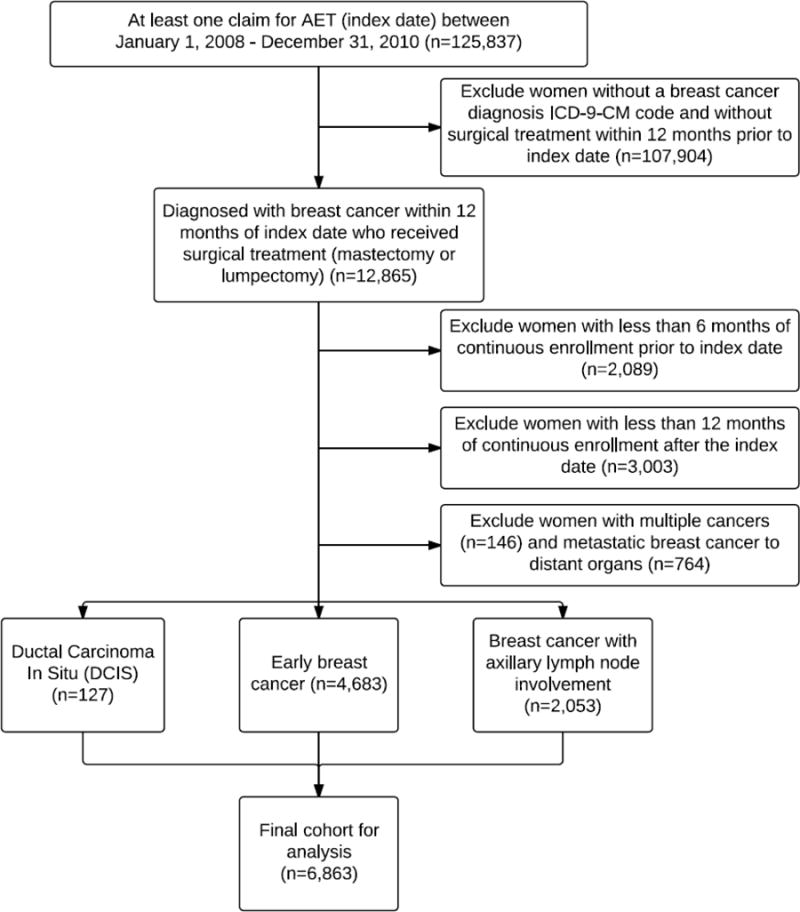
Study cohort selection and subject exclusion.
Outcome
The primary outcome was medication adherence to AET calculated using the proportion of days covered (PDC).21 We assumed women did not take more than one AET medication at a time.22 PDC was calculated based on the fill dates and the number of days’ supply for each prescription. The numerator was the total number of days covered by the prescription fill during the 12-month study period. We created coverage periods to reflect the dates that were encompassed by each prescription fill. We shifted the start date of each period so that women did not have overlapping days of coverage. We assumed that women did not take the refilled medication before exhausting the previous prescription 23. The denominator was 365, the number of days between the index claim and the end of the follow-up study period (12 months after the index claim). The ratio was multiplied by 100 to obtain the percentage of the proportion of days covered. We categorized patients as being adherent if the PDC was 80% or greater.24
Time to discontinuation
We examined the time to AET discontinuation among patients considered non-adherent (PDC<80%). We assumed that a woman discontinued AET if she went at least four consecutive months (>120 days) without medication.13 A woman was considered to discontinue treatment the month in which she filled her last prescription; therefore we could only measure up to eight months.
Out-of-pocket costs for AET
Mean out-of-pocket costs for a 30-day supply of AET medication were defined as the sum of the co-payments, deductibles, and coinsurance paid by the subject at the time that the prescription was filled. Out-of-pocket cost amounts for 60- or 90-day prescriptions were adjusted to 30-day amounts. Out-of-pocket costs for AET were categorized into quintiles of less than $4.99, $5.00 to $9.99, $10.00 to $14.99, $15.00–19.99, and more than $20.00 based on common copayment amounts in multiples of $5 and so that each category represented an equivalent dollar amount and an approximately equal distribution of patients. As a sensitivity analysis for the category cut-points we included a continuous measure of AET out-of-pocket costs to determine if out-of-pocket costs were associated with adherence.
Other out-of-pocket costs
Other out-of-pocket costs paid by the patient at the time services were rendered were calculated for inpatient and outpatient services, and pharmacy medications (excluding AET medication). Other out-of-pocket costs were calculated for each refill period and adjusted to reflect an average 30-day out-of-pocket costs from the index date until the patient no longer had AET medication on-hand, up to 365 days. Other out-of-pocket costs for 30-days were categorized into quintiles of less than $49.99, $50–99.99, $100–149.99, $150–199.99, and greater than $200 so that each category represented an equivalent dollar amount with an approximately even distribution of patients.
Co-morbidities
We used the Elixhauser index to control for comorbidities using inpatient and outpatient diagnoses (IDC-9-CM codes) during the time period prior to the index date.25 Subjects were assigned a value based on the number of comorbidities.
Additional Subject Characteristics
Subject characteristics were limited to those variables that were available in the MarketScan® Database. Age in years was evaluated categorically (less than 40, 40–49, 50–59, and 60–64 years of age) and a variable to indicate whether subjects used mail or retail pharmacy was calculated based at the index date. We created breast cancer treatment variables for surgery (bilateral mastectomy, mastectomy, or lumpectomy), and the receipt of chemotherapy and/or radiation using ICD-9-CM and CPT codes from inpatient and outpatient claims up to twelve months prior to their first AET prescription fill.18–20 We assessed whether women had an oophorectomy prior to initiating AET. We included a variable for the duration of time from surgical treatment to the index date (0–3, 4–6, 7–9, and 10–12 months). Pill burden was calculated as the count of unique drug classes filled 90 days prior to the index date. We included a variable for geographic region (Northeast, North Central, South, and West), and health plan type (health maintenance organization, preferred provider organization, other). We determined if women switched AET medication if they filled a prescription for any other AI or tamoxifen different than the index drug and were categorized as not switched, switched once, or switched 2 or more times.
Statistical Analysis
Unadjusted differences in the percentage of non-adherent (PDC<80%) and adherent (PDC≥80%) women were evaluated using the Pearson X2 test for categorical variables.
We used a multivariate logistic regression model with robust standard error estimates to calculate the odds of being adherent to AET versus being non-adherent in a one-year period. All previously described variables were included in the model and were considered to be clinically significant and potential confounders based on prior studies.14–17,26 We used the direct substitution method to determine the adjusted risk ratio (ARR) and adjusted risk difference (ARD) since adherence was not considered to be a rare event thus avoiding inappropriately reporting odds ratios, which cannot be properly interpreted as the risk of an event in this setting.27 The ARR is the ratio of the mean predicted probabilities and is a measure for the probability of adherence for each category of co-payment after controlling for potential confounders. The ARD is the difference of the mean predicted probabilities and represents differences in the absolute risk of adherence. All analyses were conducted using Stata 13.1 SE (StatCorp™ College Station, TX).
RESULTS
During the 3-year study period, 6,863 women were identified in the MarketScan databases who were 64 years and younger and initiated AET following a diagnosis and surgical treatment of early breast cancer. Among this cohort, 26.1% of patients were non-adherent because they had fewer than 80% of AET medication days covered (PDC<80%) during the first year of therapy.
Table 1 lists characteristics of the study cohort by the proportion of subjects who were adherent to AET medication (PDC≥80%). In the cohort, 59% of the patients were between 50–64 years of age. The majority had early breast cancer without lymph node involvement (68%), were enrolled in a PPO health plan (60%), filled prescriptions using a retail pharmacy (84%), received chemotherapy treatment (57%), did not have a pre-existing diagnosis of a major condition (62%), and did not switch AET during the 12 month follow-up period (84%). Half of the patients initially filled a prescription for tamoxifen (51%). The mean out-of-pocket costs for a 30 days’ supply of AET medication was $17.10 (SD 22.4) while the mean 30-day out-of-pocket costs for other prescriptions and inpatient and outpatient services was $176.50 (SD: 172.20).
Table 1.
Baseline Characteristics of Women Newly Diagnosed with Early Breast Cancer Who Initiate Adjuvant Endocrine Therapy (n=6,863)
| Total cohort | Adherent rate (PDC ≥ 80%) by category |
p-value± | ||
|---|---|---|---|---|
| % | n | % | ||
| Overall cohort | 100 | 6863 | 73.8 | |
|
|
||||
| Breast Cancer | <0.01 | |||
| DCIS | 1.9 | 127 | 69.3 | |
| Early breast cancer | 68.2 | 4683 | 73.0 | |
| Breast cancer with axillary lymph node involvement | 29.9 | 2053 | 76.3 | |
| Age, years | <0.001 | |||
| 18–39 | 8.0 | 552 | 64.3 | |
| 40–49 | 33.4 | 2292 | 71.4 | |
| 50–59 | 41.3 | 2834 | 75.7 | |
| 60–64 | 17.3 | 1185 | 79.0 | |
| Comorbidities≠ | ||||
| Hypertension | 18.1 | 1240 | 74.0 | 0.92 |
| Chronic pulmonary disease | 5.6 | 386 | 72.8 | 0.61 |
| Diabetes, uncomplicated | 4.8 | 330 | 69.7 | 0.07 |
| Hypothyroidism | 6.6 | 455 | 75.8 | 0.34 |
| Obesity | 5.1 | 350 | 75.4 | 0.51 |
| Fluid and electrolyte disorders | 2.6 | 180 | 68.3 | 0.08 |
| Deficiency anemias | 3.7 | 254 | 68.9 | 0.06 |
| Depression | 4.4 | 303 | 69.3 | 0.06 |
| Elixhauser Conditions Composite | 0.31 | |||
| 0 | 61.5 | 4219 | 74.4 | |
| 1 | 25.4 | 1744 | 73.9 | |
| 2 | 8.3 | 568 | 73.6 | |
| 3 | 3.0 | 207 | 69.6 | |
| ≥4 | 1.8 | 125 | 68.0 | |
| Surgery | 0.65 | |||
| Mastectomy | 28.6 | 1963 | 74.6 | |
| Bilateral mastectomy | 4.6 | 318 | 74.8 | |
| Breast Conservative Surgery/Lumpectomy | 66.8 | 4582 | 73.6 | |
| Time from surgical treatment to first AET fill, months | 0.12 | |||
| 0–3 | 39.2 | 2689 | 74.3 | |
| 4–6 | 30.7 | 2105 | 74.9 | |
| 7–9 | 23.1 | 1588 | 73.2 | |
| 10–12 | 7.0 | 481 | 69.9 | |
| Chemotherapy | 57.1 | 3920 | 75.1 | 0.01 |
| Radiation Therapy | 33.6 | 2303 | 74.4 | 0.50 |
| Oophorectomy | 2.2 | 152 | 72.4 | 0.67 |
| Health Plan Type | 0.12 | |||
| HMO | 18.2 | 1246 | 71.6 | |
| PPO | 60.1 | 4125 | 74.5 | |
| Other | 21.7 | 1492 | 74.3 | |
| Initiation year of AET | 0.04 | |||
| 2008 | 31.6 | 2165 | 72.8 | |
| 2009 | 34.7 | 2383 | 73.1 | |
| 2010 | 33.7 | 2315 | 75.8 | |
| Region | <0.001 | |||
| Northeast | 16.8 | 1153 | 78.8 | |
| North Central | 21.0 | 1440 | 76.5 | |
| South | 40.8 | 2801 | 70.1 | |
| West | 20.6 | 1411 | 75.1 | |
| Unknown | 0.9 | 58 | 69.0 | |
| AET drug type at index date, % (n) | <0.001 | |||
| Exemestane | 1.9 | 128 | 73.4 | |
| Anastrozole | 30.0 | 2057 | 78.0 | |
| Letrozole | 17.0 | 1166 | 72.6 | |
| Tamoxifen | 51.2 | 3512 | 72.0 | |
| Number of times AET medication switched | <0.001 | |||
| 0 | 83.9 | 5758 | 75.9 | |
| 1 | 14.1 | 966 | 63.6 | |
| 2 or more | 2.0 | 139 | 65.5 | |
| Pharmacy type at baseline | <0.001 | |||
| Retail | 84.3 | 5783 | 72.3 | |
| Mail order | 13.5 | 923 | 85.5 | |
| Unknown | 2.3 | 157 | 66.2 | |
| Number of prescriptions filled 90-days prior to the index date | <0.001 | |||
| 0 | 5.0 | 340 | 62.7 | |
| 1–5 | 56.8 | 3900 | 74.7 | |
| 6–10 | 31.6 | 2166 | 73.8 | |
| Greater than 10 | 6.7 | 457 | 76.4 | |
| Out-of-pocket costs for 30 days’ supply of AET medication, $ | <0.001 | |||
| 0–4.99 | 19.0 | 1306 | 75.7 | |
| 5.00–9.99 | 29.6 | 2029 | 76.4 | |
| 10.00–14.99 | 16.8 | 1154 | 70.3 | |
| 15.00–19.99 | 10.1 | 694 | 71.6 | |
| 20.00 or greater | 24.5 | 1680 | 73.0 | |
| Other out-of-pocket costs, 30 day average, $¥ | 0.60 | |||
| 0–49.99 | 18.9 | 1300 | 73.9 | |
| 50–99.99 | 21.0 | 1442 | 75.2 | |
| 100–149.99 | 15.0 | 1032 | 73.5 | |
| 150–199.99 | 13.3 | 910 | 74.7 | |
| 200 or greater | 31.8 | 2179 | 73.0 | |
Comorbidities presented were the most prevalent of the 30 Elixhauser conditions among members in the cohort. The Elixhauser comorbidities excludes metastatic cancer and solid tumors without metastasis since these were included as part of the exclusion criteria for cohort entry.
p-values correspond to the Pearson’s Chi square for unadjusted analysis to test for differences between adherent and non-adherent proportions for each categorical variable.
Other out-of-pocket costs include services for outpatient, inpatient, and medications (other than AET) during the follow-up period for which patients had AET medication on-hand.
In the unadjusted analysis, adherence to AET was associated with age, treatment with chemotherapy, geographical region, AET drug type, the number of times a patient switched AET medication, the number of prescription drugs filled 90-days prior to index date, and the pharmacy type that the patient used. A greater proportion of patients were less likely to be adherent to AET with increasing out-of-pocket costs for AET medication (p<0.05).
Out-of-Pocket costs for AET medication
The adjusted analysis presented in table 2 are the ARR and ARD of adherence to AET for each of the covariates. On average, copayments accounted for approximately 89% of the total out-of-pocket costs for a 30-days’ supply of AET medication, while deductibles and coinsurance accounted for 6% and 5%, respectively. We found that 30-day out-of-pocket cost for AET medication was significantly associated with the adjusted risk of adherence (p<0.001, Table 2). On average, patients with a mean monthly out-of-pocket cost for AET medication between $10–$14.99, $15.00–$19.99, and $20 or greater were 6–8% less likely to be adherent to AET compared to patients with an out-of-pocket cost of less than $4.99, after controlling for covariates (ARR: 0.93; 95% CI, 0.88 to 0.98, ARR: 0.92; 95% CI 0.85–0.98, and ARR: 0.94; 95% CI 0.89–0.99, respectively). We observed the association for each category of out-of-pocket costs for AET medication except for patients with a $5.00–$9.99 out-of-pocket cost for a 30 days’ supply of AET medication. However, a sensitivity analysis was performed classifying out-of-pocket costs for AET as a continuous variable and the results were unchanged.
Table 2.
Adjusted risk ratio (AAR) and Adjusted Risk Difference (ARD) of adherence to AET medication among women with breast cancer (n=6,863)
| Characteristics | ARR | 95% CI | ARD | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Out-of-pocket costs for 30 days’supply of AET medication, $ | ||||||||
| 0–4.99 | 1.00 | |||||||
| 5.00–9.99 | 1.01 | 0.97 | to | 1.05 | 0.01 | −0.03 | to | 0.04 |
| 10.00–14.99 | 0.93 | 0.88 | to | 0.98 | −0.05 | −0.09 | to | −0.01 |
| 15.00–19.99 | 0.92 | 0.85 | to | 0.98 | −0.06 | −0.11 | to | −0.02 |
| 20.00 or greater | 0.94 | 0.89 | to | 0.99 | −0.04 | −0.08 | to | 0.00 |
| Other out-of-pocket costs, 30 day average, $¥ | ||||||||
| 0–49.99 | 1.00 | |||||||
| 50–99.99 | 1.01 | 0.97 | to | 1.06 | 0.02 | −0.02 | to | 0.04 |
| 100–149.99 | 0.98 | 0.93 | to | 1.04 | −0.01 | −0.05 | to | 0.03 |
| 150–199.99 | 1.01 | 0.96 | to | 1.06 | 0.00 | −0.03 | to | 0.04 |
| 200 or greater | 0.99 | 0.95 | to | 1.04 | 0.00 | −0.04 | to | 0.03 |
| Breast Cancer | ||||||||
| DCIS | 1.00 | |||||||
| Early breast cancer | 1.04 | 0.94 | to | 1.16 | 0.04 | −0.05 | to | 0.11 |
| Breast cancer with axillary lymph node involvement | 1.09 | 0.99 | to | 1.19 | 0.06 | −0.01 | to | 0.13 |
| Age, years | ||||||||
| 18–39 | 1.00 | |||||||
| 40–49 | 1.08 | 1.03 | to | 1.13 | 0.06 | 0.03 | to | 0.09 |
| 50–59 | 1.15 | 1.09 | to | 1.21 | 0.10 | 0.07 | to | 0.14 |
| 60–64 | 1.17 | 1.11 | to | 1.22 | 0.12 | 0.08 | to | 0.16 |
| Health Plan Type | ||||||||
| HMO | 1 | |||||||
| PPO | 1.01 | 0.97 | to | 1.05 | 0.01 | −0.02 | to | 0.04 |
| Other | 0.99 | 0.95 | to | 1.04 | 0.01 | −0.04 | to | 0.03 |
| Initiation year of AET | ||||||||
| 2008 | 1.00 | |||||||
| 2009 | 1.00 | 0.96 | to | 1.03 | 0.00 | −0.03 | to | 0.02 |
| 2010 | 1.03 | 0.99 | to | 1.06 | 0.02 | −0.01 | to | 0.05 |
| Region | ||||||||
| Northeast | 1.00 | |||||||
| North Central | 0.96 | 0.91 | to | 1.01 | −0.03 | −0.07 | to | 0.01 |
| South | 0.89 | 0.85 | to | 0.93 | −0.08 | −0.12 | to | −0.05 |
| West | 0.94 | 0.89 | to | 0.99 | −0.04 | −0.08 | to | −0.01 |
| Unknown | 0.90 | 0.75 | to | 1.07 | −0.08 | −0.20 | to | 0.04 |
| AET drug type at index date, % (n) | ||||||||
| Exemestane | 1.00 | |||||||
| Anastrozole | 1.03 | 0.93 | to | 1.13 | 0.02 | −0.05 | to | 0.09 |
| Letrozole | 0.98 | 0.88 | to | 1.09 | −0.02 | −0.10 | to | 0.06 |
| Tamoxifen | 0.97 | 0.88 | to | 1.08 | −0.02 | −0.10 | to | 0.06 |
| Pharmacy type at baseline | ||||||||
| Retail | 1 | |||||||
| Mail order | 1.15 | 1.11 | to | 1.19 | 0.11 | 0.08 | to | 0.14 |
| Unknown | 0.94 | 0.84 | to | 1.04 | −0.05 | −0.12 | to | 0.03 |
| Number of times AET medication switched | ||||||||
| 0 | 1.00 | |||||||
| 1 | 0.84 | 0.80 | to | 0.89 | −0.12 | −0.15 | to | −0.09 |
| 2 or more | 0.83 | 0.73 | to | 0.95 | −0.12 | −0.21 | to | −0.04 |
| Surgery | ||||||||
| Mastectomy | 1.00 | |||||||
| Bilateral mastectomy | 1.01 | 0.95 | to | 1.08 | 0.01 | −0.04 | to | 0.06 |
| Breast Conservative Surgery/Lumpectomy | 0.99 | 0.96 | to | 1.02 | −0.01 | −0.03 | to | 0.02 |
| Time from surgical treatment to first AET fill, months | ||||||||
| 0–3 | 1.00 | |||||||
| 4–6 | 0.97 | 0.93 | to | 1.01 | −0.03 | −0.05 | to | 0.00 |
| 7–9 | 0.94 | 0.89 | to | 0.99 | −0.05 | −0.08 | to | −0.01 |
| 10–12 | 0.91 | 0.84 | to | 0.98 | −0.07 | −0.12 | to | −0.02 |
| Chemotherapy (yes versus no) | 1.08 | 1.04 | to | 1.12 | 0.06 | 0.03 | to | 0.09 |
| Radiation Therapy (yes versus no) | 0.99 | 0.96 | to | 1.03 | −0.01 | −0.03 | to | 0.02 |
| Oophorectomy (yes versus no) | 1.02 | 0.94 | to | 1.12 | 0.02 | −0.05 | to | 0.08 |
| Number of prescriptions filled 90-day prior to follow-up period (continuous) | 1.01 | 1.00 | to | 1.01 | 0.00 | 0.00 | to | 0.01 |
| Elixhauser Conditions Composite (continuous) | 0.98 | 0.96 | to | 0.99 | −0.02 | −0.03 | to | −0.01 |
Indicates the p-value for a test of trend using the continuous variable for mean 30-day out of pocket costs for AET medication, F-test statistic with all dummy variables
Other out-of-pocket costs include services for outpatient, inpatient, and medications (other than AET) during the follow-up period for which patients had AET medication on-hand.
Copayments, deductibles, and coinsurance accounted for 46.3%, 18.6%, and 35.2%% of 30-day out-of-pocket costs for other prescription medication, and inpatient and outpatient services (data not shown). Other out-of-pocket costs were not associated with adherence to AET medication.
Other patient characteristics
Women age 60–64 were more likely to be adherent to AET compared to women less than 39 years (ARR: 1.17, 95% CI: 1.11–1.22). Women in the geographical south (ARR: 0.89, 95% CI 0.85 to 0.93) of the US and the West (ARR: 0.94, 95% CI: 0.89 to 0.99) were significantly less likely to be adherent to AET compared to women in the Northeast. Women who used a mail pharmacy compared to a retail pharmacy for their first AET prescription fill were more likely to be adherent to AET (ARR: 1.15, 95% CI: 1.11 to 1.19). Patients were less likely to be adherent to AET for every increase in pre-existing condition that a woman had (ARR: 0.98, 95% CI: 0.96 to 0.99) and for every time she switched AET medication (2 or more times versus no switching ARR: 0.83, 95% CI: 0.73 to 0.95). Women with 10–12 months between surgical treatment and index claim were less likely to be adherent to AET compare to women with 0–3 months (ARR: 0.91, 95% CI: 0.84 to 0.98).
Time to discontinuation
We examined the time to discontinuation of AET medication among women who were considered non-adherent (PDC<80) (n=1,790). The mean PDC among non-adherent patients (PDC<80) was 50%. Figure 2 depicts the percentage of patients, by month, who went greater than 120 consecutive days without medication, considered to have discontinued AET. Among non-adherent patients, 19.1% discontinued after the first month of AET prescription fill. After 8 months, 46.7% of non-adherent patients discontinued therapy. Of the non-adherent women (PDC<80%), 53.3% were not considered to have discontinued therapy but had intermittent prescription refills throughout the 12-month study period, although not enough to have greater than 80% of medication on-hand. Patients who discontinued therapy during the study period had a mean 30-day out-of-pocket costs for AET of $19.47, compared to $18.04 for women who were non-adherent but who had not discontinued therapy.
Figure 2. Proportion of non-adherent patients who went greater than 120 consecutive days without medication on-hand, considered to have discontinued adjuvant endocrine therapy, (n=1,790).
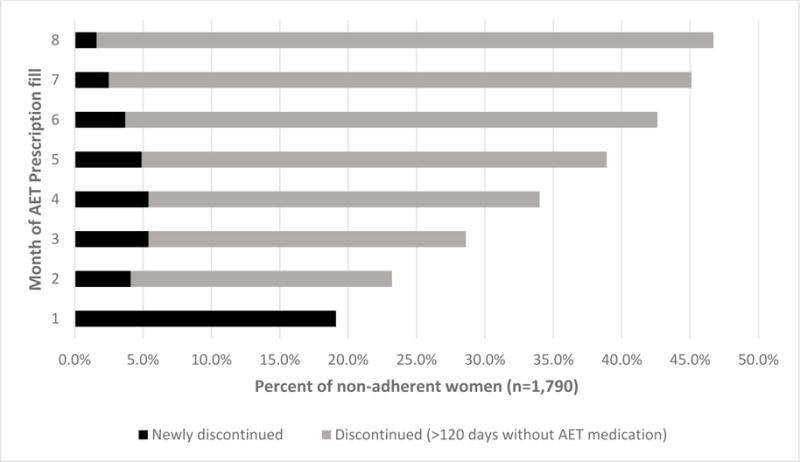
*By definition, patients were considered to have discontinued AET medication if they had greater than 120 days without medication on-hand and therefore patients could only be considered to have discontinued therapy up to the 8th month after initial prescription fill. Patients were considered to have discontinued AET medication the month that they last filled medication.
DISCUSSION
In this study, we found that 73.9% of women who initiated AET after a diagnosis of early breast cancer had filled prescriptions to have pills for at least 292 days (80%) over the first year. We observed that women with higher out-of-pocket costs for AET medication fills was significantly associated with a 6–8% lower likelihood of adherence to AET among a privately insured cohort of women with a prescription drug plan. The fact that low adherence is influenced by out-of-pocket costs for AET medication is important because low adherence to AET is associated with 10–49% higher breast cancer-specific mortality compared to women who are adherent.11,28
The finding on the association between higher out-of-pocket costs for AET and lower risk of adherence is similar to other retrospective cohort studies that have found, on average, co-payments decrease the odds of adherence to AI’s or tamoxifen.13,14,16,17 These studies, however, examined the effect of co-payments and not total out-of-pocket costs. In our study, we found that 5–6% of the total out-of-pocket costs were from coinsurance and deductibles at the time of AET prescription fill. We found that patients with a categorical out-of-pocket costs for AET medication greater than or equal to $10 was associated with a lower odds of adherence compared to patients with less than $4.99 out-of-pocket costs. In an exploratory analysis (results not shown) we observed that patients with less than $10 out-of-pocket costs for AET medication was associated with filling a prescription for tamoxifen versus an aromatase inhibitor and with filling a prescription using mail order pharmacies versus retail pharmacies. Tamoxifen drug costs tended to be cheaper than the aromatase inhibitors because a generic form of tamoxifen became available in 2002, prior to the study period. Studies suggest that the side-effects from tamoxifen may be more manageable compared to the side-effects from the aromatase inhibitors.29,30 Filling a prescription using mail order pharmacies may be more convenient and patients can often order in larger supplies (e.g. 90 days) which is more economical.31 However, despite these differences between the two groups of out-of-pocket costs, in a sensitivity analysis, a continuous variable for a $1 increase for a 30-day supply of AET medication was statistically associated with a decrease in the odds of adherence after adjusting for all other covariates.
In our study we did not find that out-of-pocket costs for other prescription pharmacy, and inpatient or outpatient services was associated with adherence. Sedjo and colleagues found that other out-of-pocket costs were associated with adherence to AET, however, out-of-pocket costs were included as the sum for the entire 12-month follow-up period even if women had discontinued filling prescriptions for AET, likely over-inflating the association with adherence to AET medication.17 In our study we calculated other out-of-pocket costs as a 30-day average limited to the time that women had filled prescriptions for AET medication. This is important since over one-quarter of the cohort were non-adherent.
Our findings on adherence rates are consistent with reports using similar claims-based methodology among insured women which found 12-month adherence to AET were ranged between 72–81% (MPR≥80%).13,15–17 We found that discontinuation among non-adherers (PDC<80%) was greatest after the first month of initiation (19%) and then decreased to 4%–7% per month, thereafter. This trend is similar to a study by Partridge and colleagues who found that the greatest percent of women who went greater than 120 days without medication was after the first month, although continued to increase thereafter.13 Additionally, we found that among non-adherers (PDC<80%), over half of the patients (53.3%) had intermittently filled prescriptions throughout the 12-month follow-up but did not go more than 120 days without medication on-hand. Thus, in addition to pharmacists and oncologists encouraging women to regularly fill prescriptions, structural factors such as automated reminder calls particularly after the first month of AET initiation may increase subsequent refills.
Similar to other research studies, we found that, on average, older women, women who used mail order versus retail pharmacies, and women with fewer comorbidities was associated with a higher probability of adherence.13,15–17 Studies have also found that patients who experience side effects from AET are more likely to be non-adherent.7,8,12,32 In our study, there was a 15% decreased probability of adherence among women who switched AET medication once compared to those that did not switch (p<0.001). In a chart review study, approximately 84% of breast cancer survivors who took anastrozole switched to another AI due to side effects.33 Thus, medication switching may partially be correlated with medication side effects. We attempted to control for side effects from initial breast cancer treatment by including the time from surgery to initiation and found that the more time that elapsed, the more the probability of adherence decreased. This is an important finding because women who allow more time to elapse may have greater side-effects from initial treatment and leads them to delay initiating AET medication.
Our study had limitations. The measure of adherence using pharmacy claims is how much medication a woman has procured over a 12-month period, but may not reflect actual medication consumption and does not reflect issues such as lost medications. Clinical and prognostic factors such as date of diagnosis, stage of disease, and ER+ status are not readily available from claims and billing data, therefore we had to develop procedural and diagnostic algorithms for these measures which may have misclassified patients. We do not know from claims if each woman in our sample had an appropriate indication for AET treatment. We assumed that if a woman filled a prescription for AET following treatment for a newly diagnosed non-metastatic breast cancer, she met NCCN treatment recommendations for AET and is likely to have ER+ breast cancer. It is possible that the index date we identified may not have been their first AET fill, although we restricted our sample to women with no evidence of recurrent breast cancer and no evidence of prior AET use. Several potential predictors such as socioeconomic factors and race/ethnicity were not available. Out-of-pocket costs for AET may be a surrogate for SES factors, however, we did not find a significant difference between other out-of-pocket costs and adherence to AET as we did with out-of-pocket costs specific to AET medication therefore limiting the plausibility of this assumption. Unmeasured factor(s) such as side effects from initial treatment and AET medication may also be a contributing factor to the findings, however our inclusion of a variable to measure time from surgery to first prescription refill and medication switching may limit the impact of treatment side effects on adherence. Finally, all of the women in the study were commercially insured in a plan that offered prescription coverage and are likely healthier and younger than the general population of breast cancer patients.
Our findings have important implications. High out-of-pocket cost for AET medication are significantly associated with non-adherence and may put patients at an increased risk of cancer recurrence and increased morbidity if they cannot access medication.4,5,9,11 Further research should focus on interventions to lower out-of-pocket costs for AET medication by exploring the role that pharmacists can play by identifying patients that belong to plans that have high cost sharing and make recommendations to select generic versus brand name drugs that are in alternate drug plan tiers.14 Even among commercially insured patients with a prescription drug plan in the United States, adherence to AET is suboptimal. Efforts to lower the out-of-pocket costs for AET medication could significantly improve adherence.
Acknowledgments
Source of Funding: This research was supported by a Ruth L. Kirschstein National Research Service Award for Individual Pre-doctoral Training grant from the National Cancer Institute (grant number F31 CA174338) for Albert Farias.
Footnotes
Conflicts of Interest: All of the authors declare no conflicts of interest.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer. 2014 Version 2.2015: www.nccn.org. Accessed May, 2015.
- 7.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 8.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 9.Haque R, Ahmed SA, Fisher A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012;1(3):318–327. doi: 10.1002/cam4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 13.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 14.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125(1):191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 18.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112(4):900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Kerner JF, Hadley J, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer. 2002;95(7):1401–1414. doi: 10.1002/cncr.10825. [DOI] [PubMed] [Google Scholar]
- 20.Srokowski TP, Fang S, Duan Z, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113(1):22–29. doi: 10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43(1):36–44. doi: 10.1345/aph.1K671. [DOI] [PubMed] [Google Scholar]
- 22.Sherman BW, Sekili A, Prakash ST, Rausch CA. Physician-specific variation in medication adherence among diabetes patients. Am J Manag Care. 2011;17(11):729–736. [PubMed] [Google Scholar]
- 23.RS L. Using arrays to calculate medication utilization. 2007 [Google Scholar]
- 24.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 30.Carlson RW, Hudis CA, Pritchard KI, Oncology NCCNBCCPGi, Inhibitors ASoCOTAotUoA, Cancer SGIECotPToEB Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Canc Netw. 2006;4(10):971–979. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 31.Valluri S, Seoane-Vazquez E, Rodriguez-Monguio R, Szeinbach SL. Drug utilization and cost in a Medicaid population: a simulation study of community vs. mail order pharmacy. BMC Health Serv Res. 2007;7:122. doi: 10.1186/1472-6963-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 33.Schwartzberg LS, Cobb P, Senecal F, et al. Initial treatment and changes in adjuvant endocrine therapy for early stage breast cancer. Breast. 2009;18(2):78–83. doi: 10.1016/j.breast.2009.01.002. [DOI] [PubMed] [Google Scholar]


