Abstract
Background
Xenogeneic islet transplantation is an emerging therapeutic option for diabetic patients. However, immunological tolerance to xenogeneic islets remains a challenge.
Methods
The current study used a pig-to-mouse discordant xenogeneic islet transplant model to examine anti-donor xenogeneic immune responses during early and late rejection, and to determine experimental therapeutic interventions that promote durable pig islet xenograft survival.
Results
We found that during early acute rejection of pig islet xenografts, the rejecting hosts exhibited a heavy graft infiltration with B220+ B cells and a robust anti-pig antibody production. In addition, early donor-stimulated IL-17 production, but not IFN-γ production, dominated during early acute rejection. Recipient treatment with donor apoptotic 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide-treated splenocytes (ECDI-SP) significantly inhibited anti-donor IL-17 response, and when combined with B cell depletion and a short course of rapamycin led to survival of pig islet xenografts beyond 100 days in ~65% recipients. Interestingly, treated recipients in this model experienced late rejection between 100 – 200 days posttransplant, which coincided with B cell reconstitution and an ensuing emergence of a robust anti-donor IFN-γ, but not IL-17, response.
Conclusions
These findings reveal that early and late rejection of pig islet xenografts may be dominated by different immune responses, and that maintenance of long-term xenogeneic tolerance will require strategies that target the temporal sequence of anti-xenogeneic immune responses.
INTRODUCTION
Xenogeneic islet transplantation has long been investigated as a future therapeutic option for diabetic patients. Pig islets may be ideal for xenogeneic islet transplantation due in part to the biochemical compatibility between porcine and human insulin, and to the potential availability of large numbers of donor pigs through relatively short turn-around farming strategies. An additional theoretical advantage of pig islets is their potential resistance to recurrence of autoimmunity directed against human β cells(1).
Antibody responses have constituted a major barrier in transplants between phylogenetically distant (discordant) species, such as pig-to-human transplantation, and result in hyperacute rejection due to preformed xenogeneic antibodies. With recent advances in the identification of carbohydrate xenoantigens(2), and genetic engineering that enables elimination of such xenoantigens(3), prevention of hyperacute rejection may now be achieved.
However, T cell mediated xenogeneic immune responses are vigorous and more difficult to control than those towards alloantigens(4). Currently, xenogeneic islet transplant requires aggressive immunosuppression, rendering the risk-benefit profile unfavorable to justify its substitution for daily insulin. Xenogeneic T cell responses to pig islets can be triggered by both direct and indirect antigen presentation(5). Once activated, T cells can mediate graft destruction by direct cytotoxicity(6), or by differentiation to cytokine-producing T helper (Th) cells that provide B cell help for class switching and antibody production, or by activating innate cells such as macrophages and NK cells that participate in xenograft rejection(7, 8). Both Th1 and Th2 cytokines, such as IFN-γ and IL-4, have been reported in xenogeneic rejection (9–11). However, the role of IL-17 in xenogeneic rejection has not been studied. Recently, this cytokine has been implicated in allograft rejection(12), especially during early rejection response as shown in human heart transplant recipients(13) where it promotes leukocyte trafficking(14), induces B cell differentiation and antibody production(15), and enhances graft fibrosis (16, 17).
In an attempt to develop strategies for tolerance induction for xenogeneic islet transplantation, we utilized our effective strategy for tolerogenic alloantigen delivery via apoptotic ethylenecarbodiimide (ECDI)-fixed donor cells(18–20), and modified it to apply to tolerogenic xenoantigen delivery for xenogeneic islet transplantation. Silent clearance of apoptotic cells exerts potent immune-regulatory effects(21, 22). Consequently, infusion of ECDI-fixed donor splenocytes (ECDI-SP) effectively induces donor-specific tolerance(19, 23–25). In murine models, ECDI-SP induce tolerance to islet allografts(18, 19, 26, 27), and when combined with short-term rapamycin or anti-CD20, also to heart allografts(20, 28, 29) and to concordant (rat-to-mouse) islet xenografts(30), respectively. More importantly, a first-in-human clinical trial using ECDI-fixed peptide-coupled autologous peripheral blood mononuclear cells has recently been conducted in patients with multiple sclerosis, demonstrating the clinical feasibility, tolerability and safety of this novel tolerogenic strategy(31).
In the current study, we used a discordant pig-to-mouse xenogeneic islet transplant model to study the mechanisms of early and late rejection of pig islet xenografts in mice, and to test the efficacy of pig ECDI-SP in inducing discordant xenogeneic tolerance.
MATERIALS AND METHODS
Animals and induction of diabetes
Male C57BL/6 (B6), BALB/c mice, and B6.Foxp3-DTR/eGFP mice, all 7–10 weeks old, were purchased from The Jackson Laboratory. Donor pigs were retired wild-type breeders aged 18 months or older. Pig splenocytes and 6–9 day-cultured islets were provided by the Schultz Diabetes Institute, University of Minnesota. Diabetes was induced by injection of 200mg/kg streptozotocin (Sigma) and confirmed by blood glucose >250mg/dL on 2 consecutive days. All studies were approved by Northwestern University and the University of Minnesota ACUC.
Islet transplantation
3,000 pig islet equivalents (IEQ), 200 BALB/c or B6 islets were transplanted under the kidney capsule of diabetic B6 mice. Rejection was diagnosed when blood glucose was >250mg/dL for 2 consecutive days.
Transplant recipient treatment
ECDI-treated pig splenocytes were prepared as described(18). 108 ECDI-SP in 200μl PBS were injected on days −7 and +1, with day 0 being the day of islet transplantation. In recipients treated with anti-CD20, 250 μg of anti-CD20 (clone 5D2; Genentech) was administrated i.v. on days −9 and 0 in recipients receiving pig ECDI-SP, or day 0 in recipients not receiving pig ECDI-SP. The additional dose of anti-CD20 on day −9 for pig ECDI-SP-treated recipients was given to inhibit the induction of anti-xenogeneic antibodies by xenogeneic ECDI-SP itself as we have shown previously(30). In selected groups, rapamycin (rapa, 1mg/kg daily) was administrated i.p. from day −8 to +10. Anti-IL-17A (clone 17F3; BioXCell) or mouse IgG1 (clone MOPC-21; BioXCell) was administrated at 100 μg/day i.p. from day 0 to 13. Anti-GM-CSFR (clone CAM3003) was injected at 600 μg/2 days i.p. from day 0 to 20 based on published report using this antibody(32). Anti-CD25 (clone PC-61, BioXCell) was injected at 0.5 mg/dose on day −9 and −7. Diphtheria toxin (Sigma) was dissolved in dH2O, and injected i.p. at 10 μg/kg in triple therapy-treated and pig islet-transplanted B6.Foxp3-DTR/EGFP mice on days −9, −8, −6, −4, −2 and 0, with day 0 being the day of pig islet transplantation.
Mouse anti-pig antibody measurement
Sera were obtained from recipients 14–21 days after rejection. Sera were heat-inactivated at 56 °C for 30min, followed by incubation with donor pig splenocytes on ice for 1hr. After washing, pig splenocytes were stained with FITC-conjugated rat anti-mouse IgM (clone R6-60.2), IgG1 (clone A85-1), IgG2a (clone R19-15), IgG2b (clone R12-3), or IgG3 (clone R40-82) mAbs (all from BD Biosciences) and analyzed by FACS. Sera from naïve B6 served as negative control.
ELISPOT assays
Splenic T cells were purified (>90% purity) by anti-CD90.2 microbeads (Miltenyi Biotec). The non-T cells were used as antigen-presenting cells (APCs). ELISPOT plates were coated with capturing antibodies to IL-17A or IFN-γ (BD Biosciences). For direct stimulation, T cells (2–5×105 cells) were co-cultured with irradiated donor pig splenocytes (4×105cells). For indirect stimulation, T cells (2–5×105) were co-cultured with irradiated B6 APC (5×105) pulsed with donor pig splenocyte lysate (from 4×105 cells). For direct + indirect stimulation, T cells (2–5×105) were co-cultured with irradiated B6 APC (5×105) and irradiated donor pig splenocytes (4×105 cells). 24–48 hrs later, cytokine-expressing cells were detected by an ELISPOT plate reader.
Intracellular staining
T cells were co-cultured as above for 5 days. Immediately before harvesting, cells were further stimulated with leukocyte activation cocktail (eBioscience) for 4 hours followed by staining with vFluor 450-anti-mCD4 (RM4-5, TONBO) and PercpCy5.5-anti-mCD8 (53–6.7, TONBO), fixed and permeabilized, and stained with PE-anti-mIL-17A (eBio17B8, eBioscience) or PE-Cy7-anti-mIFN-γ (XMG1.2, TONBO).
Delayed-type hypersensitivity (DTH) assay
DTH assays were performed as described (18). Briefly, the ear thickness of mice was measured with a Mitutoyo engineer’s micrometer (Schlesinger’s Tools) immediately before injection of 107 pig splenocyte lysate in 10 μl PBS into the dorsal surface of the ear. 24 hours later, the ear thickness was measured and increase over baseline was determined.
Real time PCR
Xenografts were peeled off from the kidney capsule of recipients 10–14 days after transplantation. Total RNAs were isolated using RNeasy kit (Qiagen) and cDNA was synthesized using a high-capacity cDNA archive kit (Lifetechnologies/Applied Biosystems). Expression levels of IL-17A (Assay ID: Mm00439618_m1), RORγC (Assay ID: Mm01261022_m1) and IFN-γ (Assay ID: Mm01168134_m1) mRNA were quantified using TaqMan Universal PCR master mix (Lifetechnologies/Applied Biosystems). GAPDH was used as an endogenous reference. Data was analyzed using ABI 7500 Sequence Detection software.
Histology
Frozen sections (10 μm) of islet grafts were blocked with 10% donkey serum (Sigma-Aldrich), stained with rat anti-mouse B220 mAb (1:250, rIgG2a κ, clone RA3–6B2; eBiosciences), guinea pig anti-insulin pAb (1:200, DAKO #A0564), or rat anti-mouse Foxp3 mAb (1:200, rIgG2a, clone FJK-16; eBiosciences), and visualized with donkey anti-rat DyLight 594 (1:500; Jackson ImmunoResearch) and/or donkey anti-guinea pig Alexa 488 (1:250, Jackson ImmunoResearch). DAPI staining was concurrently performed. Images were visualized using Leica DM5000 B microscope, acquired with a QImaging Retiga 4000r camera, and analyzed with Image Pro 6.2 software.
Statistical analysis
Graft survival was compared using Kaplan-Meier survival curves with log-rank test. Statistical significance between 2 groups was determined by Wilcoxon nonparametric tests or unpaired t tests. Data representing more than 2 groups were analyzed with one-way ANOVA. All statistical analyses were performed using GraphPad Prism 5 software.
RESULTS
B cells play a critical role during early rejection of pig islet xenografts in mice
A discordant pig-to-C57BL/6 (B6) mouse xenogeneic islet transplant model was used. In this model, un-treated B6 recipients rejected pig islets between day 7 and 26 posttransplantation (Fig. 1A). We first examined the histology of the rejecting pig islet xenografts. To our great surprise, we observed prominent graft-infiltrating B cells around the time of acute rejection (day 14 posttransplant, Fig. 1B). This is in sharp contrast to rejecting BALB/c islet allografts in which B cell infiltration was minimal (Supplementary Fig. 1). Recipients that rejected their islet xenografts also exhibited prominent anti-pig antibody production, particularly of IgG1, IgG2a and IgG2b isotypes (Fig. 1C). Consequently, when recipient were depleted of B cells at the time of transplant, a significant prolongation of pig islet xenograft survival was observed (Fig. 1D). Collectively, these findings suggest that B cells play an important role in early acute rejection of discordant pig islet xenografts in mice.
FIG. 1. B cells play a prominent role in the early rejection of pig islet xenografts.
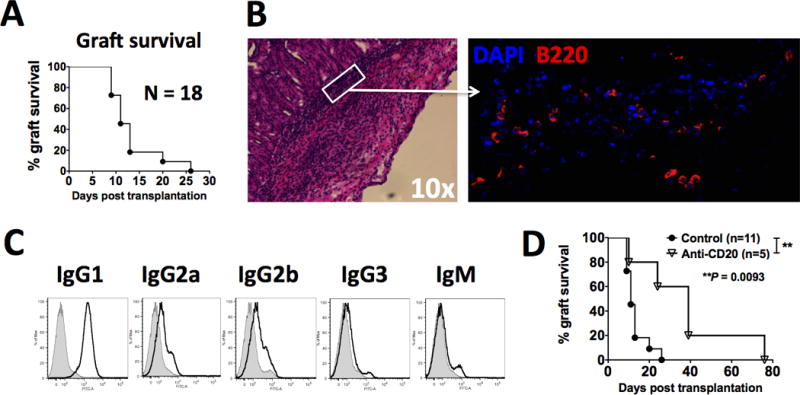
3,000 IEQ of porcine islets were transplanted underneath the kidney capsule of diabetic B6 recipients. A: Porcine islet graft survival in un-manipulated diabetic B6 recipients (N=18). B: B220+ B cell infiltration during acute rejection of pig islet xenografts. Pig islet xenografts were retrieved on day 14 posttransplantation, processed and stained as described in Materials and Methods. Left panel: hematoxylin and eosin staining, 10×; Right panel: immunofluorescent staining, blue = DAPI, red = B220. Data shown is representative of 3–5 pig islet xenografts examined. C: Anti-pig xenogeneic antibodies in recipients that have rejected the pig islet xenograft. Sera from recipient mice were obtained 20 days after confirmed graft rejection and examined for anti-pig xenogeneic antibodies by FACS analysis (line histogram) as described in Materials and Methods, and compared with those from naïve mice (shaded histogram). Results shown are representative of those from 5 mice. D: Pig islet xenograft survival in diabetic B6 recipients either with or without B cell depleting antibody anti-CD20. Recipient treatment with anti-CD20 is described in Materials and Methods. “Control”: mice transplanted with pig islet xenografts in the absence anti-CD20 treatment; “Anti-CD20”: mice transplanted with pig islet xenografts in the presence of anti-CD20 treatment. **P = 0.0093.
IL-17 is the predominant anti-donor T cell cytokine response during early rejection of pig islet xenografts in mice
It has been shown that B cells can effectively prime Th17 immune response (33–35). Given that B cells play a critical role in early acute rejection of pig islet xenografts in mice (Fig. 1), we next examined if T cell IL-17 response was induced during acute rejection. As shown in Fig. 2A (open bars), T cells from hosts rejecting pig islet xenografts showed a robust donor-stimulated IL-17 response measured by ELISPOT assays compared with naïve mice. Surprisingly, these T cells did not exhibit a significant IFN-γ response. This is in sharp contrast to the T cell response from hosts rejecting islet allografts (BALB/c → B6) in which a robust anti-donor IFN-γ, but only minimal IL-17, response was observed (Fig. 2A, filled bars). We next determined if IL-17 was also produced at the site of the rejecting pig islet xenografts. As shown in Fig. 2B, rejecting xenogeneic islets exhibited a markedly elevated level of IL-17A mRNA, but not IFN-γ mRNA. In contrast, rejecting allogeneic islets exhibited an elevated level of IFN-γ mRNA, but not IL-17A mRNA. We verified in a second murine recipient strain BALB/c that intra-graft IL-17-producing cells were also present in rejecting pig islet xenografts (Supplementary Fig. 2), confirming that this response is common to both Th1 (B6) and Th2 (BALB/c) biased recipient strains. Lastly, we determined if neutralizing IL-17A had any beneficial effect on the survival of islet xenografts. As shown in Fig. 2C, neutralization of IL-17A at the time of transplant allowed a transient prolongation of pig islet xenograft survival. IL-17-producing cells have also been shown to secrete GM-CSF to mediate Th17-driven inflammation in disease models such as experimental autoimmune encephalitis(36). Therefore, we next tested if blocking GM-CSF was also protective to pig islet xenografts. As shown in Fig. 1C, recipient treated with a course of GM-CSF receptor blocking antibody (anti-GM-CSFR) also exhibited a significant prolongation of pig islet xenograft survival. Collectively, these findings suggest a non-redundant role of IL-17 during early rejection of pig islet xenografts. However, the rather transient protection provided by anti-IL17 or anti-GM-CSFR indicates that other parallel IL-17-independent mechanisms also exist.
FIG. 2. IL-17 plays a prominent role in early rejection of pig islet xenografts.

A: Direct + indirect donor-stimulated splenic T cell IL-17 and IFN-γ responses measured by ELISPOT as described in Materials and Methods on day 14 posttransplantation. Naïve: un-transplanted B6 mice; Xeno: pig→B6 islet transplant recipients; Allo: BALB/c→B6 islet transplant recipients. B: Quantitative PCR for IL-17 and IFN-γ mRNA level from transplanted pig islet xenografts retrieved on day 14 posttransplantation. Expression levels were normalized to GAPDH and shown as “Relative expression”. Xeno: pig→B6 islet grafts; Allo: BALB/c→B6 islet grafts; Syn: B6→B6 islet grafts. C: Effect of neutralization of IL-17A (anti-IL-17A from day 0 – day 13 posttransplantation as described in Materials and Methods) or blocking of GM-CSF (anti-GM-CSFR every other day from day 0 – day 20 as described in Materials and Methods) on pig islet xenograft survival in diabetic B6 mice. Results shown in A and B are average of 3 independent experiments.
Anti-donor IL-17 response is induced by both direct and indirect donor stimulation, and is seen in both CD4 and CD8 compartments
To identify which stimulatory pathway (direct vs. indirect) is predominantly driving the xenogeneic IL-17 response, we co-cultured T cells from above rejecting B6 hosts either with irradiated donor pig splenocytes alone (direct pathway) or with irradiated syngeneic B6 splenocytes pulsed with donor pig cell lysates (indirect pathway), and measured IL-17 production by ELISPOT. As shown in Fig. 3A, both direct and indirect stimulation of T cells from rejecting hosts resulted in robust IL-17 responses. To determine which T cell compartment produced IL-17, we performed intracellular staining of the above-stimulated T cells. As shown in Fig. 3B, both CD4 and CD8 T cells were able to produce IL-17 upon either direct or indirect donor stimulation.
FIG. 3. The anti-donor IL-17 response seen in pig islet xenograft recipients is induced by both direct and indirect donor stimulation, and is in both the CD4 and CD8 compartments.
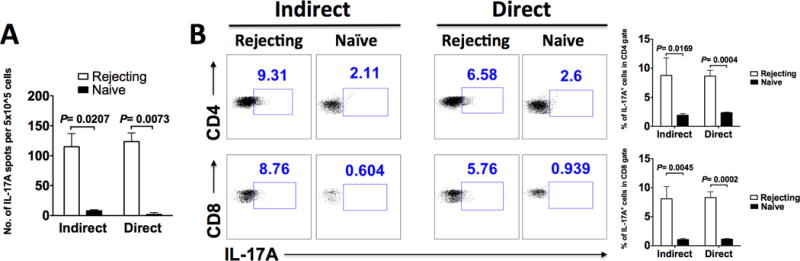
Indirect versus direct donor stimulation was set up as described in Materials and Methods on day 14 posttransplantation. A: Indirect versus direct donor-stimulated splenic T cell IL-17 response measured by ELISPOT. Results shown are the average of 3 independent experiments. B: Indirect versus direct donor-stimulated splenic T cell IL-17 response measured by intracellular staining for IL-17A and FACS analysis, gating on CD4 and CD8 T cells. The right bar graphs show the average results of 3 independent experiments. For both A and B: Naïve = un-transplanted B6 mice.
B cell depletion in combination with donor ECDI-SP significantly prolong pig islet xenograft survival in mice
Because B cell depletion alone only led to a transient graft protection (Fig. 1C), we next searched for a combinatorial regimen with additional tolerance-promoting therapies to achieve long-term pig islet xenograft protection. Previously, we have demonstrated that peri-transplant infusion of donor ECDI-SP induces robust allogeneic donor-specific tolerance via parallel mechanisms including induction of T cell anergy, deletion and regulation (18, 20, 30). We therefore tested the tolerance efficacy of donor ECDI-SP in the pig-to-mouse model. We first used a pig-to-mouse delayed-type hypersensitivity (DTH) model. We immunized mice with pig splenocyte lysates mixed with complete Freund’s adjuvant (CFA) on day 0, either with or without i.v. injection of 108 same-donor pig ECDI-SP on day −7 and +1, and measured donor-stimulated cytokine response on day 14. As shown in Fig. 4A, pig ECDI-SP treatment inhibited host anti-pig IL-17 response by ~50% compared with untreated controls. Corresponding to this inhibition, pig ECDI-SP treatment also significantly inhibited host DTH response (Fig. 4B). We next combined B cell depletion with pig ECDI-SP treatment (dual therapy) in the same model, and observed that this combination led to a remarkable further suppression of host anti-pig IL-17 response (Fig. 4A), indicating that B cells play a significant role in the priming of the early host anti-pig IL-17 response.
FIG. 4. Donor pig ECDI-SP in combination with anti-CD20 and a short course of rapamycin results in the reduction anti-donor IL-17A and anti-donor DTH response, and sustained protection of pig islet xenografts in mice.
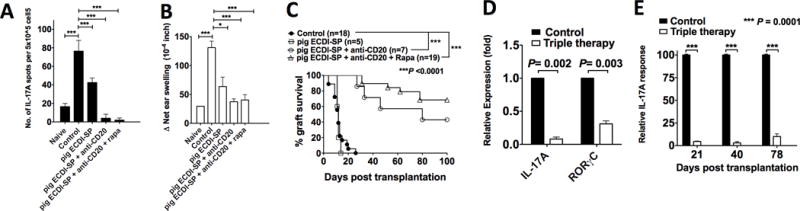
For A and B: B6 mice were immunized with pig splenocyte lysates mixed in complete Freund’s adjuvant (CFA) on day 0, and treated with pig ECDI-SP, anti-CD20, and rapamycin as described in Materials and Methods. A: Direct + indirect donor-stimulated splenic T cell IL-17 response measured by ELISPOT on day 14 post immunization. B: DTH response measured as described in Materials and Methods. “Naïve”: un-treated mice; “Control”: mice immunized in the absence of any treatment. Results shown are the average of 3 independent experiments. C: Pig islet xenograft survival in diabetic B6 recipients treated with pig ECDI-SP, anti-CD20, and rapamycin as described in Materials and Methods. “Control”: mice transplanted with pig islet xenografts in the absence of any treatment. For D and E: B6 mice were transplanted with pig islet xenografts on day 0, and treated with pig ECDI-SP, anti-CD20, and rapamycin as described in Materials and Methods. D: Quantitative PCR for IL-17A and RORγC mRNA level in transplanted pig islet xenografts retrieved on day 14 posttransplantation. Expression levels were normalized to GAPDH and shown as “Relative expression”. “Control”: pig islet xenografts from un-treated control recipients; “Triple therapy”: pig islet xenografts from ECDI-SP + anti-CD20 + rapamycin treated recipients. Results shown are the average of 3 grafts examined per group. E: Indirect donor-stimulated splenic T cell IL-17 response measured by ELISPOT on days 21, 40, and 78 posttransplantation. Results shown are the average from 3 mice per group per time point examined. “Control”: un-treated recipients; “Triple therapy”: ECDI-SP + anti-CD20 + rapamycin treated recipients.
Next, we tested the efficacy of the dual therapy in the pig-to-mouse islet transplant model. As shown in Fig. 4C, donor ECDI-SP alone did not prolong pig islet xenograft survival. However, when combined with B cell depletion, the dual therapy substantially prolonged pig islet xenograft survival beyond that seen with anti-CD20 alone (Fig. 1C), leading to ~40% graft survival beyond 100 days (Fig. 4C). Lastly, based on our published report showing a synergistic graft-protective effect between donor ECDI-SP and rapamycin(20), we further added a short course of peri-transplant rapamycin to the dual therapy. The resulting triple therapy resulted in a further increase of long-term (>100 days) graft survival rate to ~65% (Fig. 4C).
In recipients treated with the above triple therapy, we also quantified IL-17 production in the islet xenografts and the spleen. Harvested on day 14 posttransplantation, pig islet xenografts from triple therapy treated recipients exhibited markedly suppressed IL-17A and RORγC mRNA levels compared with those from control un-treated recipients (Fig. 4D). Next, splenic T cells from transplant recipients were harvested on day 21, 40 and 78 posttransplant, and the magnitude of IL-17 response by indirect donor stimulation was measured. As shown in Fig. 4E, triple therapy treatment robustly suppressed host anti-pig IL-17 response early on (day 21 posttransplantation), and persisted to over 78 days posttransplant. Collectively, these data suggest that pig ECDI-SP, when combined with transient B cell depletion and rapamycin, results in a significant and sustained inhibition of host anti-pig IL-17 response, and a significant prolongation of pig islet xenograft survival.
Foxp3+ regulatory T cells play an obligatory role in the protection of pig islet xenografts in recipients treated with the triple therapy
In recipients with islet xenograft survival >100 days, retrieval of the transplanted pig islet xenograft precipitated immediate hyperglycemia (data nor shown). Retrieved long-term functioning pig islet xenografts showed well-preserved islet structure (Fig. 5A). Clusters of lymphocytes were seen surrounding the intact islets without infiltrating into and destructing the islets. These clusters of lymphocytes were highly enriched for Foxp3+ cells (Fig. 5A). To determine if Foxp3+ regulatory T cells (Treg) were important for graft protection, we performed 2 lines of experiments. First, we injected recipients with anti-CD25 (clone PC-61) to deplete CD25+ cells around the time of transplantation. As shown in Fig. 5B left panel, depletion of CD25+ cells significantly impaired graft protection in recipients treated with the triple therapy. To more specifically deplete Foxp3+ Treg, we used Foxp3-DTR/eGFP mice as recipients. Recipients were treated with DT to deplete Foxp3+ Treg at the time of transplantation. As shown in Fig. 5B right panel, depletion of Foxp3+ Treg resulted in rapid rejection of pig islet xenografts in recipients treated with the triple therapy. Of note, pig islets are likely resistant to direct DT toxicity, as (1) the pig islets in DT-treated recipients survived for over 10 days after the last dose of DT (Fig. 5B); and (2) published literature support that pig cells are resistant to DT unless DT is guided to the targeted cells by ligand-receptor binding via DT fusion proteins (37). Collectively, these data suggests that Treg play a critical role in promoting and maintaining pig islet xenograft survival seen in recipients treated with the triple therapy.
FIG. 5. Foxp3+ Treg play an obligatory role in protecting pig islet xenografts in triple therapy-treated (pig ECDI-SP + anti-CD20 + rapamycin) diabetic B6 mice.
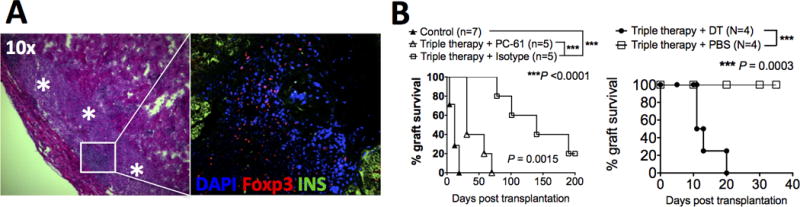
A: Retrieved long-term protected (> 100 days) pig islet xenografts show intact islet architecture by H&E (left panel, asterisks indicate intact islets) and positive Foxp3 and insulin staining by immunofluorescence (right panel). Pig islet xenografts were retrieved after 100 days posttransplant from euglycemic recipients, processed and stained as described in Materials and Methods. Blue = DAPI, red = Foxp3, green = insulin; magnification: 40×. Data shown is representative of two pig islet xenografts examined. B: Depletion of Treg at the time of triple therapy treatment abrogated graft protection in pig islet xenograft recipients. Left panel: depletion of Treg by PC-61 treatment in B6 mice: PC-61 or isotype was given i.p. as described in Materials and Methods. Right panel: depletion of Treg by diphtheria toxin (DT) treatment in B6.Foxp3-DTR/eGFP mice: DT or PBS was given i.p. as described in Materials and Methods. Triple therapy treatment with pig ECDI-SP, anti-CD20, and rapamycin was described in Materials and Methods. Control: untreated B6 recipients.
Late rejection of pig islet xenografts is associated with heavy B cell graft infiltration and anti-donor IFN-γ response
In our cohort of triple therapy treated recipients, we observed that a significant portion of them (~80%) experienced late rejection between day 100 and 200 post-transplantation (Fig. 6A).
FIG. 6. Late rejection of pig islet xenografts is associated with B cell graft infiltration and an emergence of anti-donor IFN-γ response.
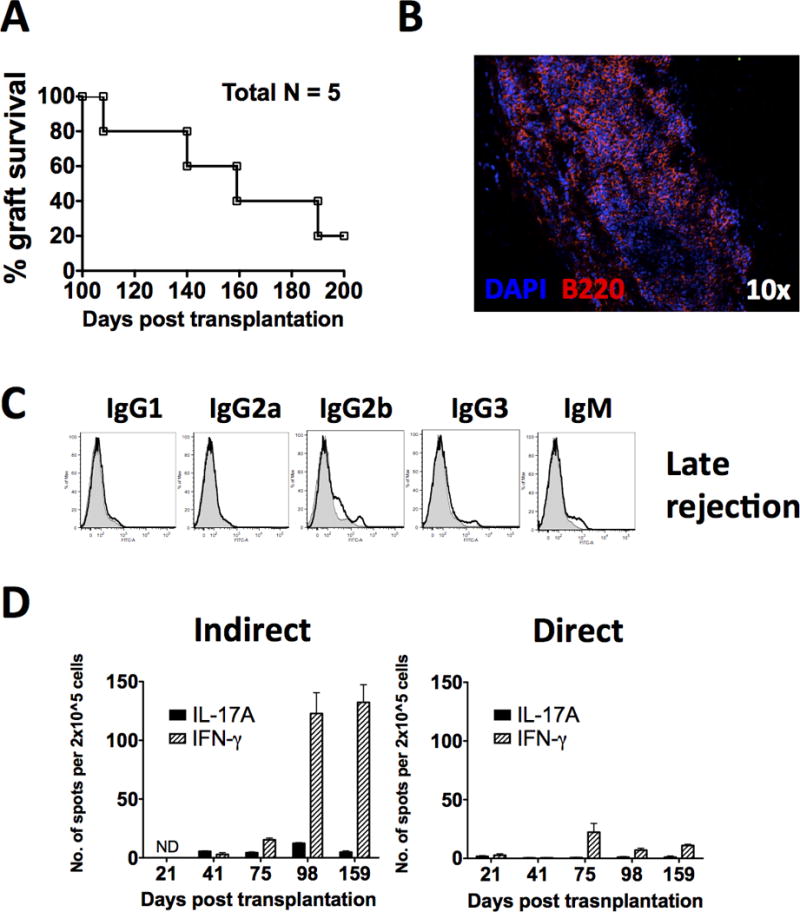
A: Late rejection is observed between 100 – 200 days posttransplantation in a high percentage of islet xenograft recipients initially treated with ECDI-SP + anti-CD20 + rapamycin and showed > 100 days of islet xenograft survival. B: B220+ B cell infiltration during late rejection of pig islet xenografts. Pig islet xenografts were retrieved either at the time of or shortly after rejection was confirmed, processed and stained as described in Materials and Methods. Blue = DAPI, red = B220, magnification: 10×. Data shown is representative of 3–5 pig islet xenografts examined. C: Anti-pig xenogeneic antibodies in recipients that have rejected the pig islet xenograft between 100 – 200 days. Sera from recipient mice undergoing rejection were obtained 20 days after confirmed graft rejection and examined for anti-pig xenogeneic antibodies by FACS analysis (line histogram) as described in Materials and Methods, and compared with those from naïve mice (shaded histogram). Results shown are representative of 5 mice examined. D: Indirect versus direct donor-stimulated splenic T cell IL-17 and IFN-γ responses measured by ELISPOT on days 21, 41, 75, 98, and 159 posttransplantation. Results shown are the average from 3 mice per time point examined.
We first examined the histology of late rejecting islet xenografts around the time of rejection. To our great surprise, these grafts were aggressively infiltrated with a large number of B220+ B cells (Fig. 6B), presumably following B cell reconstitution after anti-CD20 depletion.
We next measured anti-pig antibodies in these recipients following late rejection. Sera were collected 20 days after confirmed graft rejection. As shown in Fig. 6C, there was minimal anti-pig antibody production in all Ig subclasses in these recipients. This is in sharp contrast to untreated recipients that rejected their islet xenografts early on (Fig. 1C), in which robust anti-pig antibodies could be detected. These data indicate that returning B cells participate in late rejection of islet xenografts at the site of the graft in a capacity that is beyond their role in promoting antibody production.
Lastly, we examined the anti-donor T cell response during late rejection. Splenic T cells from triple therapy treated recipients were harvested on day 98 and 159 posttransplant, and examined for donor-stimulated cytokine production. As shown in Fig. 6D, T cells from triple therapy treated recipients on day 98 and day 159 showed a marked increase in anti-pig IFN-γ response compared to that seen early on (day 21, 41, and 75 posttransplant), while anti-pig IL-17 response remained suppressed throughout. This increase of anti-pig IFN-γ response was primarily observed with indirect (Fig. 6D, left bar graph) but not direct (Fig. 6D, right bar graph) donor stimulation, consistent with the notion that indirect immune response predominates as donor passenger leukocytes dissipate.
DISCUSSION
In the current study, we show that during early rejection of pig islet xenografts in mice, direct and indirect anti-donor IL-17 responses as well as xenogeneic antibody production play an important role, and that B cells likely contribute to the priming of both. In contrast, late rejection seen in triple therapy treated recipients is associated with indirect anti-donor IFN-γ response preceded by B cell reconstitution and aggressive graft B cell infiltration, but a complete absence of xenogeneic antibody production.
The crucial role of B cells in early acute rejection of islet xenografts was unequivocally demonstrated by B cell depletion that resulted in substantial graft prolongation (Fig. 1D). Based on the current study, the specific functions of B cells in xenogeneic rejection appear to be pleiotropic and time-dependent. During early acute rejection, B cells likely contribute to the priming of IL-17-producing T cells and to the differentiation of xenogeneic antibody-producing plasma cells. It has been shown that B cell antigen presentation by B1 B cells can effectively promote Th17 differentiation(33–35). Reciprocally, Th17 cells are effective B cell helpers and can induce B cell proliferation in vitro and trigger their class switching in vivo(15). In our model, it is conceivable that the induced xenogeneic IL-17 response feeds back to promote B cell proliferation and differentiation. Consequently, a positive feedback loop between B cells and Th17 cells is established to effectively promote early acute rejection of islet xenografts. A rather different picture of the role of B cells emerges during late rejection in recipients initially protected by the pig ECDI-SP + anti-CD20 + rapamycin triple therapy. First, late rejection appears to be exclusively cell-mediated, as xenogeneic antibodies remain undetectable during and after rejection of islet xenografts (Fig. 6C). Secondly, late rejection appears to be invariably associated with an extremely aggressive B cell infiltration in the graft (Fig. 6B). Thirdly, following B cell reconstitution, there is an ensuing emergence of indirect xenogeneic IFN-γ response (Fig. 6D) preceding the late rejection. It is conceivable that the newly emerged B cells directly acquire xenogeneic antigens in the graft and prime indirect anti-donor IFN-γ response. Graft-infiltrating B cells may also directly prime intra-graft cytotoxic T lymphocytes(38), leading to in situ graft destruction. Future studies are necessary to elucidate the subtypes and functionality of the reconstituted B cells, specifically of graft-infiltrating B cells. Such an understanding will determine the choice of B cell-targeting therapies that will most effectively prevent late rejection.
During early acute rejection, the observed IL-17 response in the xenogeneic pig-to-mouse islet transplant model is in sharp contrast to that seen in full MHC-mismatched allogeneic mouse-to-mouse islet transplant model in which IFN-γ response dominates (Fig. 2A). Determining specific xenogeneic signals that trigger implicated APCs (eg B cells) to promote T cell IL-17, rather than IFN-γ, production will be beyond the scope of the current study. One possibility is xenogeneic carbohydrate antigens. Carbohydrate antigens have been shown to induce strong IL-17 production and exacerbate IL-17 mediated diseases such as psoriasis(39). This characteristic may explain the observed advantage of xenogeneic cancer cell-based vaccines over autologous or allogeneic vaccines(40). Several possible pathways may be triggered by carbohydrate ligands, leading to enhanced IL-17 production. For example, carbohydrate ligands may conjugate with endogenous TLR ligands and enhance their immunostimulatory activity on TLRs(41, 42), leading to enhanced inflammation conducive for IL-17 production. Alternatively, carbohydrate may directly interact with lectin receptors and license lectin-bearing APCs to differentiate effector cells towards IL-17 production(43–45). While literature on carbohydrate-mediated skewing of Th17 differentiation is predominantly from models of infection(46, 47), it has recently been shown that such a skewing may also play a role in transplantation rejection such as graft-versus-host disease (GVHD)(48). Recognition of xenogeneic carbohydrates by lectin receptors has been described in discordant xenogeneic transplantation models to promote phagocytosis of xenogeneic (human) erythrocytes and platelets by porcine macrophages(49, 50). Interestingly, lectin receptor on CD4 T cells has also been associated with a propensity of their differentiation towards Th17 cells(51) and an increased risk for GVHD(52). Future dissection of the nature of xenoantigens in this process (eg α-gal versus non-α-gal carbohydrates, swine leukocyte antigens (SLA) versus non-SLAs), as well as determining the signaling requirements leading to xenogeneic IL-17 response will be imperative to shed light on potential therapeutic targets.
We have previously shown that donor ECDI-SP induce robust donor-specific T cell tolerance in models of allogeneic transplantation by parallel mechanisms including deletion, anergy and regulation(19). Others have also shown in models of autoimmunity and allergic diseases that ECDI-SP therapy can tolerize antigen-driven Th1, Th2 and Th17 responses (21). The ability of inhibiting donor-stimulated T cell responses by donor ECDI-SP is recapitulated in the current pig-to-mouse islet transplant model during early graft protection. Thus, the surge of late donor-stimulated IFN-γ T cell response preceding late rejection was surprising. Further examination of the escape mechanisms of Th1 response in this model will be necessary to best select therapeutic targets to preserve long-term tolerance.
The current study used a pig-to-B6 xenogeneic islet transplant model. To exclude a strain-dependent effect, we performed similar experiments in BALB/c (Th2-biased) recipients, and confirmed that in a pig-to-BALB/c xenogeneic islet transplant model, similar IL-17 production during acute rejection was also observed, and that islet xenografts were similarly protected by pig ECDI-SP + anti-CD20 + rapamycin triple therapy (Supplementary Fig. 2). These results support the conclusion that our findings have a broader relevance that is not just restricted to Th1-biased (B6) recipients. Based on these studies and as a prelude to human studies, we are currently rigorously testing this tolerance protocol in a pig-to-monkey islet transplant model, the results of which will be summarized separately. Given the highly promising clinical translatability of this approach(31), we believe that results obtained from our current study in this specific model demonstrating early and late immunological profiles will be highly valuable in guiding future designs of human tolerance protocols based on this specific approach.
In summary, our studies demonstrate that pig ECDI-SP in combination with initial B cell depletion and a short course of rapamycin is highly effective in protecting pig islet xenografts beyond 100 days. A better understanding of the nature of B cells during their reconstitution and the escape mechanisms of the late Th1 response will likely provide the key to identifying effective therapeutic targets for durable xenogeneic tolerance to pig islets.
Supplementary Material
Acknowledgments
We wish to acknowledge the Northwestern University Interdepartmental Immunobiology Flow Cytometry Core Facility and the Mouse Histology and Phenotyping Laboratory for their support of this work.
Funding
This work was supported by grants from the Juvenile Diabetes Research Foundation Strategic Research Agreement Grant 17-2013-495 (X.L., B.J.H., S.D.M.) and NIH/NIAID U01-AI102463 (B.J.H, X.L., S.D.M.).
ABBREVIATIONS
- DTH
delayed-type hypersensitivity
- ECDI
1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide
- ELISPOT
Enzyme-Linked ImmunoSpot
- IFN-γ
interferon gamma
- IEQ
islet equivalent
- MNCs
mononuclear cells
- SP
splenocytes
- TLR
toll-like receptor
Footnotes
Author contributions
H.K.K., S.W., S.D.M., A.D., L.Z., X.L. designed research; H.K.K., S.W., X.Z., A.S., A.D., J.M.R., S.D.M., L.Z., W.S-P., X.D., S.D.M. performed experiments; H.K.K., S.W., A.D., X.D, X.C., B.J.H., S.D.M., X.L. analyzed data; H.K.K, X.L. wrote the manuscript, B.J.H, S.D.M., E.B.T. reviewed and edited the manuscript.
The authors of this manuscript declare no conflicts of interest.
References
- 1.Koulmanda M, Qipo A, Smith RN, Auchincloss H., Jr Pig islet xenografts are resistant to autoimmune destruction by non-obese diabetic recipients after anti-CD4 treatment. Xenotransplantation. 2003;10(2):178–84. doi: 10.1034/j.1399-3089.2003.02040.x. [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Park MR, Kwon DN, Kang MH, Oh M, Han JW, Cho SG, Park C, Kim DK, Song H, et al. Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity. J Biomed Biotechnol. 2011;2011(560850) doi: 10.1155/2011/560850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Stewart JM, Gunthart M, Hawthorne WJ, Salvaris EJ, O’Connell PJ, Nottle MB, d’Apice AJ, Cowan PJ, Kearns-Jonker M. Xenoantibody response to porcine islet cell transplantation using GTKO, CD55, CD59, and fucosyltransferase multiple transgenic donors. Xenotransplantation. 2014;21(3):244–53. doi: 10.1111/xen.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol. 2009;21(2):81–6. doi: 10.1016/j.trim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunological reviews. 2014;258(1):241–58. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi S, Feng X, Wang Y, Kay TW, Wang Y, O’Connell PJ. CD4+ cells play a major role in xenogeneic human anti-pig cytotoxicity through the Fas/Fas ligand lytic pathway. Transplantation. 1999;67(3):435–43. doi: 10.1097/00007890-199902150-00017. [DOI] [PubMed] [Google Scholar]
- 7.Yi S, Feng X, Hawthorne WJ, Patel AT, Walters SN, O’Connell PJ. CD4+ T cells initiate pancreatic islet xenograft rejection via an interferon-gamma-dependent recruitment of macrophages and natural killer cells. Transplantation. 2002;73(3):437–46. doi: 10.1097/00007890-200202150-00019. [DOI] [PubMed] [Google Scholar]
- 8.Lin ML, Zhan Y, Nutt SL, Brady J, Wojtasiak M, Brooks AG, Lew AM. NK cells promote peritoneal xenograft rejection through an IFN-gamma-dependent mechanism. Xenotransplantation. 2006;13(6):536–46. doi: 10.1111/j.1399-3089.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee RS, Yamada K, Womer KL, Pillsbury EP, Allison KS, Marolewski AE, Geng D, Thall AD, Arn JS, Sachs DH, et al. Blockade of CD28-B7, but not CD40-CD154, prevents costimulation of allogeneic porcine and xenogeneic human anti-porcine T cell responses. J Immunol. 2000;164(6):3434–44. doi: 10.4049/jimmunol.164.6.3434. [DOI] [PubMed] [Google Scholar]
- 10.Mulley WR, Wee JL, Christiansen D, Milland J, Ierino FL, Sandrin MS. Lentiviral expression of CTLA4Ig inhibits primed xenogeneic lymphocyte proliferation and cytokine responses. Xenotransplantation. 2006;13(3):248–52. doi: 10.1111/j.1399-3089.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh NP, Guo L, Mhoyan A, Shirwan H. Predominant expression of Th2 cytokines and interferon-gamma in xenogeneic cardiac grafts undergoing acute vascular rejection. Transplantation. 2003;75(5):586–90. doi: 10.1097/01.TP.0000052594.83318.68. [DOI] [PubMed] [Google Scholar]
- 12.Abadja F, Sarraj B, Ansari MJ. Significance of T helper 17 immunity in transplantation. Curr Opin Organ Transplant. 2012;17(1):8–14. doi: 10.1097/MOT.0b013e32834ef4e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Besouw NM, Caliskan K, Peeters AM, Klepper M, Dieterich M, Maat LP, Weimar W, Manintveld OC, Baan CC. Interleukin-17-producing CD4(+) cells home to the graft early after human heart transplantation. J Heart Lung Transplant. 2015;34(7):933–40. doi: 10.1016/j.healun.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177(3):1265–73. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107(32):14292–7. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–44. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–94. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, Houlihan JL, Miller SD, Zhang ZJ, Luo X. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189(2):804–12. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, Wang JJ, Zhang Z, Luo X. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant. 2012;12(11):2920–9. doi: 10.1111/j.1600-6143.2012.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy DP, Bryant J, Galvin JP, Miller SD, Luo X. Tempering allorecognition to induce transplant tolerance with chemically modified apoptotic donor cells. Am J Transplant. 2015;15(6):1475–83. doi: 10.1111/ajt.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morelli AE, Larregina AT. Concise Review: Mechanisms behind apoptotic cell-based therapies against transplant rejection and graft versus host disease. Stem Cells. 2016 doi: 10.1002/stem.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, et al. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes Is Induced by PD-L1+ and IL-10-Producing Splenic Macrophages and Maintained by T Regulatory Cells. J Immunol. 2011;187(5):2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation. J Immunol. 2010;185(6):3326–36. doi: 10.4049/jimmunol.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(4):2212–20. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 26.Kheradmand T, Wang S, Gibly RF, Zhang X, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, et al. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials. 2011;32(20):4517–24. doi: 10.1016/j.biomaterials.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, Miller SD, Luo X. Preemptive Tolerogenic Delivery of Donor Antigens for Permanent Allogeneic Islet Graft Protection. Cell Transplant. 2014 doi: 10.3727/096368914X681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant J, Lerret NM, Wang JJ, Kang HK, Tasch J, Zhang Z, Luo X. Preemptive Donor Apoptotic Cell Infusions Induce IFN-gamma-Producing Myeloid-Derived Suppressor Cells for Cardiac Allograft Protection. J Immunol. 2014;192(12):6092–101. doi: 10.4049/jimmunol.1302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, Mathews CE, Earp HS, Matsushima G, Wang B, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205(1):219–32. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Tasch J, Kheradmand T, Ulaszek J, Ely S, Zhang X, Hering BJ, Miller SD, Luo X. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes. 2013;62(9):3143–50. doi: 10.2337/db12-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, et al. Antigen-specific tolerance by autologous myelin Peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(188):188ra75. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botelho FM, Nikota JK, Bauer C, Davis NH, Cohen ES, Anderson IK, Coyle AJ, Kolbeck R, Humbles AA, Stampfli MR, et al. A mouse GM-CSF receptor antibody attenuates neutrophilia in mice exposed to cigarette smoke. Eur Respir J. 2011;38(2):285–94. doi: 10.1183/09031936.00076210. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Euro J Immunol. 2007;37(9):2400–4. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 34.Weber MS, Prod’homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68(3):369–83. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, Tumang J, Gao W, Rothstein TL. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60(12):3734–43. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kara EE, McKenzie DR, Bastow CR, Gregor CE, Fenix KA, Ogunniyi AD, Paton JC, Mack M, Pombal DR, Seillet C, et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat Commun. 2015;6(8644) doi: 10.1038/ncomms9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peraino JS, Schenk M, Li G, Zhang H, Farkash EA, Sachs DH, Huang CA, Duran-Struuck R, Wang Z. Development of a diphtheria toxin-based recombinant porcine IL-2 fusion toxin for depleting porcine CD25+ cells. J Immunol Methods. 2013:398–399. 33–43. doi: 10.1016/j.jim.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham KL, Krishnamurthy B, Fynch S, Ayala-Perez R, Slattery RM, Santamaria P, Thomas HE, Kay TW. Intra-islet proliferation of cytotoxic T lymphocytes contributes to insulitis progression. Euro J Immunol. 2012;42(7):1717–22. doi: 10.1002/eji.201242435. [DOI] [PubMed] [Google Scholar]
- 39.Khmaladze I, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, Lundqvist K, Holmdahl M, Nandakumar KS, Holmdahl R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci U S A. 2014;111(35):E3669–78. doi: 10.1073/pnas.1405798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seledtsov VI, Goncharov AG, Seledtsova GV. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum Vaccin Immunother. 2015;11(4):851–69. doi: 10.1080/21645515.2015.1009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinchi H, Crain B, Yao S, Chan M, Zhang SS, Ahmadiiveli A, Suda Y, Hayashi T, Cottam HB, Carson DA. Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides. Bioconjug Chem. 2015;26(8):1713–23. doi: 10.1021/acs.bioconjchem.5b00285. [DOI] [PubMed] [Google Scholar]
- 42.Lei W, Browning JD, Jr, Eichen PA, Lu CH, Mossine VV, Rottinghaus GE, Folk WR, Sun GY, Lubahn DB, Fritsche KL. Immuno-stimulatory activity of a polysaccharide-enriched fraction of Sutherlandia frutescens occurs by the toll-like receptor-4 signaling pathway. J Ethnopharmacol. 2015;172:247–53. doi: 10.1016/j.jep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 44.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Euro J Immunol. 2008;38(12):3274–81. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loures FV, Araujo EF, Feriotti C, Bazan SB, Costa TA, Brown GD, Calich VL. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary Paracoccidioidomycosis. J Infect Dis. 2014;210(5):762–73. doi: 10.1093/infdis/jiu136. [DOI] [PubMed] [Google Scholar]
- 46.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell host & microbe. 2009;5(4):329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182(8):4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uryu H, Hashimoto D, Kato K, Hayase E, Matsuoka S, Ogasawara R, Takahashi S, Maeda Y, Iwasaki H, Miyamoto T, et al. alpha-Mannan induces Th17-mediated pulmonary graft-versus-host disease in mice. Blood. 2015;125(19):3014–23. doi: 10.1182/blood-2014-12-615781. [DOI] [PubMed] [Google Scholar]
- 49.Brock LG, Delputte PL, Waldman JP, Nauwynck HJ, Rees MA. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant. 2012;12(12):3272–82. doi: 10.1111/j.1600-6143.2012.04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bongoni AK, Kiermeir D, Denoyelle J, Jenni H, Burlak C, Seebach JD, Vogelin E, Constantinescu MA, Rieben R. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplantation. 2015;99(4):693–701. doi: 10.1097/TP.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 51.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206(3):525–34. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SE, Lim JY, Yoon JH, Shin SH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, et al. CD161(+) T cells as predictive markers for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(3):421–8. doi: 10.1016/j.bbmt.2014.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


