Abstract
A significant portion of the key biological functions of αKlotho (αKL) and its cognate ligand Fibroblast growth factor-23 (FGF23) have been revealed through the study of rare diseases of mineral metabolism. These findings have far reaching implications for common disorders such as chronic kidney disease-mineral bone disorder (CKD-MBD). αKL’s predominant effect on mineral homeostasis is through its actions in the kidney as a co-receptor for FGF23, however emerging data has shed light on its capacity to act as a circulating factor through the cleavage of the transmembrane form of αKL (‘mKL’) to produce ‘cleaved KL’ or ‘cKL’. This review summarizes new findings from studies using extended delivery of cKL to mouse models with phenotypes reflecting those arising in CKD-MBD.
Keywords: hyperphosphatemia, hypophosphatemia, cKL, FGF23, phosphate, vascular calcification
Introduction
Phosphate metabolism is a complex process involving endocrine feedback among multiple tissues including bone, kidney, and intestine. Disturbances in this homeostatic network can cause severe manifestations including altered bone structure and function in hypophosphatemic diseases, as well as vascular calcification and secondary bone disease in situations of hyperphosphatemia. Studying disorders of phosphate handling arising from single gene defects in pathways involving FGF23 and its co-receptor, αKL, have revealed important physiological and pathophysiological information for common diseases. Additionally, observations both from the clinic and translational research in the field of mineral metabolism have further elucidated roles of cKL, the cleaved circulating form of αKL, supporting that different forms of αKL may have wider ranging, and previously unappreciated targets.
FGF23 and Klotho in Phosphate Homeostasis
Phosphate homeostasis is a finely tuned process involving endocrine feedback loops arising between the skeleton, intestine, and kidneys. FGF23 is produced in bone in response to an increase in serum phosphate or 1,25(OH)2 vitamin D (1,25D)1, 2. FGF23, acting through αKL, decreases phosphate reabsorption3 via down-regulation of the renal proximal tubule type-II sodium phosphate co-transporters NPT2a and NPT2c3, 4. FGF23 also effects kidney 1,25D production and consequently calcium-phosphate equilibrium. Indeed, vitamin D 1α-hydroxylase (Cyp27b1) expression is suppressed by FGF23, while simultaneously stimulating the catabolic vitamin D 24-hydroxylase (Cyp24a1), thus inhibiting the synthesis of active 1,25D2, 3. Consequently, an increase in bioactive FGF23 (‘intact’ or ‘iFGF23’) results in the hallmark endocrine effects of parallel reductions in serum phosphate and 1,25D concentrations.
The biological connection between FGF23 and αKL was first observed through the phenotypic similarities of their respective knockout mice5, 6. Both the Fgf23-null and αKL-null mouse models are characterized by a shortened lifespan, growth retardation, ectopic calcification, and osteoporosis5, 6. The importance of renal αKL was subsequently demonstrated in different models of tissue-specific and cell-specific deletions of αKL7,8. The kidney has been established as the principle organ mediating αKL effects as shown by a complete knockout of renal αKL that fully mimicked the phenotypes of global αKL-null mice7. Additionally, a mouse model with a 70% reduction in renal distal tubular αKL expression resulted in a vast increase in serum FGF23 and exposed a linear relationship between residual renal αKL, serum phosphate, and FGF23 concentrations8. This study also uncovered that a critical level of αKL is necessary to preserve FGF23 signaling. Until recently αKL’s role in the proximal tubule was undefined, but recent data support that αKL may have a more limited function in this segment9. In this regard, the generation of three different proximal tubule-specific αKL conditional knockout mice revealed that mice with deletion of αKL from the proximal nephron were only mildly hyperphosphatemic or normophopshatemic. However, when challenged with high phosphate drinking water the mice became hyperphosphatemic9. Therefore, the deletion of αKL from either distal or proximal tubules effects phosphate handling, with evidence supporting that distal tubule plays a substantial role in FGF23-mediated phosphate metabolism. The nature of the interplay between the tubule segments remains to be determined.
The αKL gene is comprised of five exons that encodes a type 1 single-pass transmembrane protein10, 11. αKL is detectable in a variety of tissues and cell types, with abundant expression in the kidney and parathyroid glands, as well as brain choroid plexus12. While this review will focus on αKL, the other Klotho family members include β- and γ-Klotho which are also type 1 single-pass transmembrane proteins13. β-Klotho is predominantly expressed in the liver, but is also found in the kidney, gut, and spleen and mediates the activity of FGF19 and FGF2113, 14. γ-Klotho is expressed in the kidney and skin and has undefined functions13, 15. Several isoforms of the αKL protein exist: the ‘membrane’ bound (‘mKL’) form is a 130 kD glycoprotein comprised of a large extracellular domain that directly interacts with FGF23, in addition to a short intracellular region that is not capable of signaling in isolation10. The mKL form fosters FGF23 signaling via the recruitment of canonical FGF receptors (FGFRs)16. Findings support the interactions between FGFR1c and αKL for renal phosphate handling using conditional-null mice17, and other studies suggest FGF23-αKL signaling can also be mediated through FGFR3 and FGFR418, which may play a role in vitamin D regulation19. A circulating form of αKL referred to as ‘cut-‘ or ‘cleaved-KL’ (‘cKL’) is produced by the cleavage of mKL near the transmembrane domain by membrane-bound secretases of the ADAM and BACE families, specifically ADAM10, ADAM17, and BACE120. This cleavage event results in production of the cKL form (110 kDa)10 that enters the blood, CSF, and urine21, 22.
cKL in Rare and Common Diseases
Two rare Mendelian diseases of dysfunctional phosphate metabolism involving αKL under- and over-expression have brought to light important relationships in phosphate handling. Hyperphosphatemic familial tumoral calcinosis (hFTC) is an autosomal recessive disorder in which patients present with hyperphosphatemia and ectopic calcifications. Forms of hFTC are caused by mutations in FGF2323–25 or GALNT326–28. In a single case of hFTC, a 13-year-old girl was found to have a homozygous missense mutation in the αKLOTHO gene. Specifically, an H193R mutation within the extracellular KL-1 FGF23 binding domain located in a catalytic cleft was responsible for attenuated production of αKL when expressed in vitro29. The patient exhibited biochemistries of hyperphosphatemia, hypercalcemia, along with elevated PTH and FGF23. Consistent with kidney resistance to FGF23, she had strikingly elevated iFGF23 and cFGF23, unlike FGF23- and GALNT3-mediated TC which results in reduced iFGF2329. This patient’s hyperphosphatemia and increased 1,25D likely resulted in the prevailing elevated iFGF23 through positive feedback. Hyperparathyroidism was also observed in this patient. Unlike the αKL-null mouse model and other forms of hFTC, the H193R mutation patient exhibits high PTH in the face of high 1,25D that would normally suppress PTH production. Whether this finding is due to species-specific phosphate handling or the molecular nature of the missense mutation and its effect on proteins that interact with αKL remains unclear.
The second case related to alterations in αKL was an infant that presented due to poor growth and increasing skull size in addition to moderately bowed legs. After ruling out mutations in FGF23, DMP1, PHEX, and FGFR1 it was found the girl harbored a balanced chromosomal translocation (t9:13) in proximity to the αKLOTHO gene30. Analysis of serum biochemistries revealed severe hypophosphatemia, elevated FGF23 with inappropriately normal 1,25D, and radiographs confirmed rachitic changes of the growth plate at her knees and wrists. It was determined through examination of the patient’s serum that the translocation caused elevated cKL30, supporting the surprising hypothesis that increased cKL can potently drive FGF23 expression and cause hypophosphatemia, a biological situation that is normally suppressive of FGF23 production.
To determine the molecular mechanisms underlying the role of cKL in bone FGF23 expression, adeno-associated virus 2/8 carrying cKL (AAV-cKL) was delivered to wild type (WT) mice for 4–8 weeks31. AAV-cKL treatment resulted in pharmacologic levels of blood cKL and markedly increased iFGF23. The mice also had hypophosphatemia, hypocalcemia, and hyperparathyroidism, matching the biochemical profile of the translocation patient31. Interestingly, the sustained cKL delivery resulted in a 150-fold increase in bone Fgf23 mRNA, which would explain, despite hypophosphatemia, the increased serum iFGF23 concentrations. In addition, Egr1 and c-fos, both targets of the MAPK signaling pathway, were up regulated in bone. Skeletal changes in mice treated with AAV-cKL were significant, including reduced bone mineral density and bone mineral content of the distal and medial femora. Severe osteomalacia, a characteristic of marked hypophosphatemia, was observed in AAV-cKL bones as evident by increased non-mineralized tissue along with widened growth plates31. Parallel in vitro studies demonstrated that cKL-FGFR1c signaling was initiated in the presence of FGF2331. Of note, other studies have found relationships between FGFR activity and increased FGF23 expression, as some patients with osteoglophonic dysplasia (OGD) carrying activating FGFR1c mutations increase serum iFGF2332, 33, and FGF2 over expression increases bone FGF23 production34. These studies collectively support the concept that cKL-mediated FGF23 regulation may play a physiological role in fine-tuning the control of the circulating levels of iFGF23 in a feedback loop between bone and kidney.
CKD has become increasingly prevalent; the current lifetime risk of this disease is 59%35. As the kidneys become unable to control blood phosphate, late-stage CKD-MBD is associated with increased serum FGF23, hyperphosphatemia and vascular calcification, and recently has been connected to renal αKL deficiency36–38. To address the question whether providing cKL to a model of CKD-MBD would be therapeutically beneficial, AAV-cKL was administered to mouse models with phenotypes that parallel those of patients. To test cKL’s pharmacological effects the db/db-eNOS−/− mouse model of diabetic nephropathy (DN) was used39. The db/db-eNOS−/− mouse model is characterized by a loss of activity of the leptin receptor and disruption of eNOS causing mice to develop exceedingly high blood glucose, and progressive renal damage including glomerular and interstitial fibrosis40, 41. Since DN is currently the leading cause of CKD-MBD42, 43, identifying novel pathways for pharmacological interventions are needed. cKL delivery to db/db-eNOS−/− mice resulted in a reduction in serum phosphate with increased FGF23 despite no improvements in renal function or pathology39. Although the molecular mechanisms remain to be determined, these results support that at pharmacologic levels cKL may reduce serum phosphate during compromised renal function.
The αKL-null mouse model manifests a number of CKD-MBD phenotypes including elevated serum phosphate and severe aortic calcification5, 44 due to the inability to arbitrate efficient FGF23-dependent signaling in target tissues. The αKL-null mice also provide a biological platform for examining cKL effects that are independent of all other αKL isoforms. Therefore AAV-cKL was provided to αKL-null mice for four weeks39. Interestingly, the prevailing hyperphosphatemia in αKL-null mice was significantly reduced in parallel with further FGF23 increases above the elevated baseline FGF23 levels39. In WT mice cKL delivery increased serum PTH and suppressed serum 1,25D, consistent with a reduction in the renal 1α-OHase (Cyp27b1) and an increase in the 24-OHase (Cyp24a1) mRNAs. Administration of cKL to αKL-null mice normalized their previously high 1,25D. In addition, acute cKL administration was capable of reducing Npt2a in αKL-null mice39. Consistent with these results, it was previously shown that a decrease in Npt2a expression on the proximal tubule apical membrane may result from its direct interactions with cKL, resulting in a suppression of phosphate reuptake45.
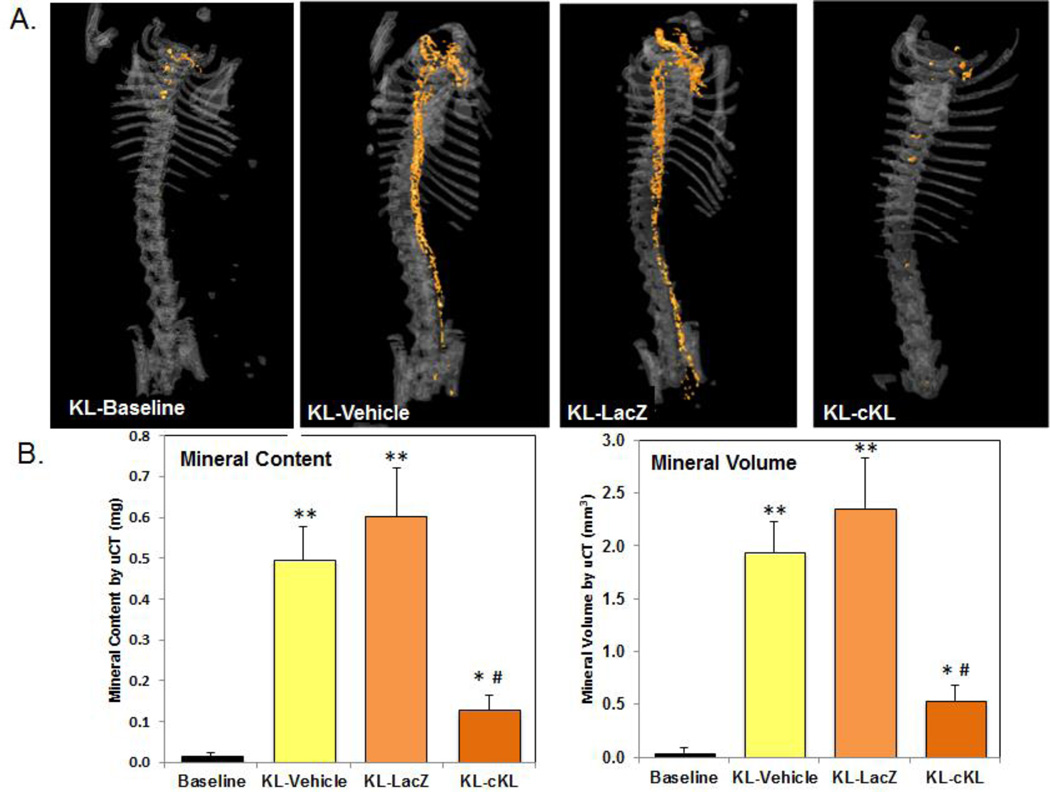
A significant consequence of CKD-MBD is vascular calcification (VC), dramatically increasing the odds of sudden death in patients46–48. Consistent with their hyperphosphatemia, αKL-null mice exhibit severe aortic VC. Previous studies testing a variety of treatment paradigms demonstrated cKL effects on lessening VCs but failed to significantly correct the elevated serum phosphate in αKL-null mice49, 50. By eight weeks of age αKL-null mice display calcification extending from the aortic arch to the bifurcation of the aorta (Figure 1A). As assessed by μCT, four-week administration of cKL led to a 74–78% reduction in total aortic mineral content and 72–77% reduction in mineral volume compared to control groups of vehicle- and AAV-LacZ injected mice (Figure 1B). These results support the previously reported in vitro effects of recombinant cKL on vasculature44, however distinguishing the direct versus indirect actions of sustained cKL delivery should be the basis of future work. Additionally, stable, long term delivery of cKL by viral vector led to a reduction in serum phosphate that was not observed by in a uninephrectomized transgenic model of mKL overexpression44. Whether sustained cKL expression overcomes potential compensatory mechanisms will be an important line of study. Collectively, these observations support that targeting cKL-mediated pathways may prevent disease phenotypes that include hyperphosphatemia and its downstream manifestations.
Figure 1. AAV-cKL effects on aortic calcification.
(A) Representative uCT images of aortic calcification (orange colorization) from αKL-null (KL) mice at baseline (four weeks of age), AAV-cKL treated, as well as vehicle and AAV-LacZ controls (treated from four weeks of age for four additional weeks). cKL administration was associated with a visually marked reduction in aortic mineralization versus KL-vehicle and KL-LacZ treated mice. (B) Mineral content and mineral volume of whole aortae were quantified and determined to be significantly elevated in controls (**p<0.01 and *p<0.05 vs baseline), whereas in cKL-treated mice mineral content and volume were significantly reduced (#p<0.005). [From: Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. JASN (2016).]
cKL actions on bone
The mechanisms by which increases in serum and bone FGF23 occurred in the αKLOTHO translocation patient and during sustained AAV-cKL delivery to normal and αKL-null mice are unknown. To this end, in vitro studies were conducted in the osteoblastic cell line UMR-106 to examine whether cKL and FGF23 were capable and/or necessary to activate FGFR-dependent pathways in bone to stimulate FGF23 production. During combination treatment of recombinant cKL and FGF23, Egr1 and Fgf23 mRNA expression increased, whereas neither cKL nor FGF23 alone were capable of inducing a change in FGF23 mRNA expression39. Similarly, an increase in p-ERK1/2 was only stimulated by combination treatment of cKL and FGF23, and inhibition of either MEK or FGFR signaling ablated the effects of cKL and FGF23 treatment. To explore the molecular mechanisms underlying the increase of FGF23, a UMR-106 cell line lacking FGFR1 was generated by CRISPR/Cas9 targeting39. FGFR1 deletion in this system ablated the cell responses to cKL and FGF23 as well as FGFR1c agonist antibody treatment39. In sum, these studies support that bone cells, in the presence of FGF23, are a target of FGFR-dependent cKL signaling, likely explaining the elevated FGF23 during the hypophosphatemia associated with elevated serum cKL.
Summary and future questions
Certainly, important questions remain with regard to the physiological and pharmacological functions of cKL, and defining the full scope of cKL targets is chief among them. We have shown extended cKL delivery capable of reducing serum phosphate in normal mice and in two different models that manifest common disease phenotypes of CKD-MBD. However, cKL did not alleviate other key characteristics of CKD-MBD such as hyperparathyroidism and progressively declining renal function. Future studies are needed to test the ability of cKL to reduce vascular calcification in additional models of CKD-MBD. Further, whether sustained delivery of cKL directly targets pathways that control phosphate handling, as well as the molecular nature of the interactions with FGFRs to modify mineral metabolism, remain unclear. Taken together, this recent work supports the need for expansion of studies to determine the cKL-mediated events that can be targeted for therapeutic intervention.
Highlights.
Many of the key biological functions of αKlotho (αKL) and its cognate ligand Fibroblast growth factor-23 (FGF23) have been found through the study of rare diseases of mineral metabolism.
Emerging data has shed new light on the capacity of the ‘cleaved KL’ or ‘cKL’ form of transmembrane αKL to act as a circulating factor.
This review highlights new findings from studies using extended delivery of cKL to mouse models with phenotypes reflecting those arising in CKD-MBD.
Acknowledgments
LMO and RCS are employees of Eli Lilly & Co. KEW received royalties from Kyowa Hakko Kirin Co., Ltd. for licensing of the FGF23 gene.
The authors would like to acknowledge support by NIH grants DK063934, DK95784, and AR070329 (KEW), an AHA postdoctoral fellowship (JMH); and a Showalter Scholar award supported through the Ralph W. and Grace M. Showalter Research Trust Fund (KEW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The other authors declare no conflicts.
References
- 1.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation. J Endocrinol. 2010;207:67–75. doi: 10.1677/JOE-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. The Journal of clinical investigation. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. Journal of the American Society of Nephrology : JASN. 2014;25:2169–2175. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. Journal of the American Society of Nephrology : JASN. 2012;23:1641–1651. doi: 10.1681/ASN.2012010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ide N, Olauson H, Sato T, Densmore MJ, Wang H, Hanai J, Larsson TE, Lanske B. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney international. 2016;90:348–362. doi: 10.1016/j.kint.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochemical and biophysical research communications. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 11.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS letters. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 12.German DC, Khobahy I, Pastor J, Kuro OM, Liu X. Nuclear localization of Klotho in brain: an anti-aging protein. Neurobiology of aging. 2012;33:1483, e1425–e1430. doi: 10.1016/j.neurobiolaging.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annual review of physiology. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahata K, Mori K, Arai H, Koide S, Ogawa Y, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nabeshima Y, Nakao K. Molecular cloning and expression of a novel klotho-related protein. Journal of molecular medicine. 2000;78:389–394. doi: 10.1007/s001090000131. [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mechanisms of development. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 16.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro OM, Mohammadi M. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 18.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. The Journal of biological chemistry. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. American journal of physiology Endocrinology and metabolism. 2011;300:E508–E517. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney international. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS letters. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 23.Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. The Journal of clinical endocrinology and metabolism. 2005;90:5523–5527. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- 24.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Human molecular genetics. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 25.Chefetz I, Heller R, Galli-Tsinopoulou A, Richard G, Wollnik B, Indelman M, Koerber F, Topaz O, Bergman R, Sprecher E, Schoenau E. A novel homozygous missense mutation in FGF23 causes Familial Tumoral Calcinosis associated with disseminated visceral calcification. Human genetics. 2005;118:261–266. doi: 10.1007/s00439-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. The Journal of clinical endocrinology and metabolism. 2005;90:2420–2423. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- 27.Specktor P, Cooper JG, Indelman M, Sprecher E. Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. Journal of human genetics. 2006;51:487–490. doi: 10.1007/s10038-006-0377-6. [DOI] [PubMed] [Google Scholar]
- 28.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nature genetics. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. The Journal of clinical investigation. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith RC, O'Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122:4710–4715. doi: 10.1172/JCI64986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrow EG, Davis SI, Mooney SD, Beighton P, Mascarenhas L, Gutierrez YR, Pitukcheewanont P, White KE. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. American journal of medical genetics Part A. 2006;140:537–539. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- 33.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. American journal of human genetics. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Esliger A, Hurley MM. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:35–45. doi: 10.1002/jbmr.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Group MS, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. Journal of the American Society of Nephrology : JASN. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 37.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. The New England journal of medicine. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hum JM, O'Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. JASN. 2016 doi: 10.1681/ASN.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. Journal of the American Society of Nephrology : JASN. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan S, Reddick RL, Musi N, Horn DA, Yan B, Prihoda TJ, Natarajan M, Abboud-Werner SL. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Laboratory investigation; a journal of technical methods and pathology. 2008;88:515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 42.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney international. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh B, Brojen T, Banerjee S, Singh N, Singh S, Sharma OP, Prakash J. The high prevalence of chronic kidney disease-mineral bone disorders: A hospital-based cross-sectional study. Indian journal of nephrology. 2012;22:285–291. doi: 10.4103/0971-4065.101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 47.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 48.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. The New England journal of medicine. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 49.Leibrock CB, Alesutan I, Voelkl J, Michael D, Castor T, Kohlhofer U, Quintanilla-Martinez L, Kubler L, Mannheim JG, Pichler BJ, Rosenblatt KP, Kuro OM, Lang F. Acetazolamide sensitive tissue calcification and aging of klotho-hypomorphic mice. Journal of molecular medicine. 2015 doi: 10.1007/s00109-015-1331-x. [DOI] [PubMed] [Google Scholar]
- 50.Leibrock CB, Alesutan I, Voelkl J, Michael D, Castor T, Kohlhofer U, Quintanilla-Martinez L, Kubler L, Mannheim JG, Pichler BJ, Rosenblatt KP, Kuro-o M, Lang F. Acetazolamide sensitive tissue calcification and aging of klotho-hypomorphic mice. Journal of molecular medicine. 2016;94:95–106. doi: 10.1007/s00109-015-1331-x. [DOI] [PubMed] [Google Scholar]