Abstract
The human sense of smell decreases with age, and a poor sense of smell are among the most important prodromal symptoms of several neurodegenerative diseases. Recent evidence further suggests a racial difference in the sense of smell among U.S. older adults. However, no genome-wide association study (GWAS) on the sense of smell has been conducted in African-Americans (AAs). We performed the first genome-wide meta-analysis of the sense of smell among 1,979 AAs and 6,582 European-Americans (EAs) from three U.S. aging cohorts. In the AA population, we identified nine novel regions (KLF4-ACTL7B, RAPGEF2-FSTL5, TCF4-LOC100505474, PCDH10, KIAA1751, MYO5B, MIR320B1-CD2, NR5A2-LINC00862, SALL1-C16orf97) that were associated with the sense of smell (P < 5 × 10−8). Many of these regions have been previously linked to neuropsychiatric (schizophrenia or epilepsy) or neurodegenerative (Parkinson’s or Alzheimer’s disease) diseases associated with a decreased sense of smell. In the EA population, we identified two novel loci in or near RASGRP1 and ANXA2P3 associated with sense of smell. In conclusion, this study identified several ancestry-specific loci that are associated with the sense of smell in older adults. While these findings need independent confirmation, they may lead to novel insights into the biology of the sense of smell in older adults and its relationships to neuropsychological and neurodegenerative diseases.
Keywords: GWAS, the sense of smell, African-American
INTRODUCTION
The human sense of the smell is a complex trait that affects the safety, nutrition, quality of life and psychosocial function of human beings [1,2]. Poor sense of smell is common among older adults and independently predicts overall mortality [3]. Further, accumulating evidence suggests that poor sense of smell is one of the earliest prodromal symptoms for main neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) [4–6]. Epidemiological studies have consistently shown that the sense of smell decreases with age and is worse in men than in women [7]. Recent evidence from the U.S. further suggests that African-Americans (AAs) have a poorer sense of smell than European-Americans (EAs), and that this racial difference cannot be explained by socioeconomic factors, cognition, physical or mental health, or several other health behaviors [7].
Few studies have investigated the genetic basis for the sense of smell among older adults. Initial studies implicated genetic variants at human olfactory receptors (ORs) and ApoE ε4 allele [8,9]. Recently, we performed a genome-wide meta-analysis on the sense of smell using HapMap 3 imputation reference panel among 6,252 older EAs [10]. Although no SNP reached genome wide significance, our results suggested that MAPT (microtubule-associated protein tau) may play a role in regulating the sense of smell in older EAs, providing a potential genetic link between the sense of smell and neurodegeneration. Previous studies have almost exclusively been conducted among individuals with European ancestry. Despite the higher prevalence of anosmia among older adults of African ancestry, we are not aware of any studies that specifically investigate the genetic basis of the sense of smell among populations of African ancestry. Therefore, we conducted the first genome-wide meta-analysis on the sense of smell among AAs and updated the results for EAs using 1000 Genomes imputed variants.
METHODS
Study populations
The current study included 1,979 AAs and 6,582 EAs from three well-characterized U.S. aging cohorts: the Atherosclerosis Risk in Communities (ARIC) study, the Health, Aging, and Body Composition (Health ABC) study, and the Rush Religious Orders Study and Memory and Aging Project (ROS/MAP). Details of these cohorts have been described elsewhere [11–14]. Briefly, the ARIC study is an ongoing longitudinal study that was established in 1987–1989 to investigate risk factors for cardiovascular diseases [11]. The Health ABC study is a prospective study established in 1997–1998 to investigate risk factors for disability and functional decline among older adults [12]. The ROS and MAP studies are longitudinal cohorts established in the mid-1990s to investigate aging and AD incidence and progression among adults 65 years or older [14,13]. The total analytic sample included 1,053 AAs and 3,985 EAs from ARIC, 769 AAs and 1,311 EAs from Health ABC, 157 AAs and 1,286 EAs from ROS/MAP, all with valid data on genotyping and the smell identification test. Individual study protocols were approved by the respective Institutional Review Boards and all study participants provided written consent.
Smell Identification Test
The sense of smell was measured using a validated smell identification test at one of the cohorts’ clinical evaluation centers during 2011–2013 for ARIC, 1999–2000 for Health ABC and 2001–2013 for ROS/MAP. The ARIC study used the 12-item Sniffin’ Sticks test (Burghart, Wedel, Germany) [15] to evaluate the sense of smell and the Health ABC and ROS/MAP studies used the 12-item Brief Smell Identification Test (B-SIT, Sensonics, Haddon Heights, NJ, USA) [16]. Both tests assess participants’ ability to smell and correctly identify 12 daily odorants, although the exact odorants are somewhat variable. In Sniffin’ Sticks, each odorant is concealed in a felt-tip pen, while in B-SIT each odorant is concealed on a page of a booklet. In both tests, the participants were instructed to smell and identify the correct odorant from four possible answers in a multiple choice format. One point was given for each correct answer, with a total score ranging from 0–12. The score distributions were comparable across cohorts both in AAs and in EAs (Supplementary Figure 1).
All cohorts also assessed global cognitive function during the visit in which the sense of smell was evaluated. The ARIC and ROS/MAP studies used the Mini-Mental State Examination (MMSE) that has a maximal score of 30 and the Health ABC study used the Modified Mini Mental Status Examination (MMMSE) with a maximal score of 100. Each cohort also determined ApoE genotypes by genotyping two ApoE variants at codons 130 and 176 (formerly 112 and 158) separately.
Genotyping, quality control and imputation
Genotyping was performed independently by each cohort using either Affymetrix or Illumina platforms. For the ROS/MAP study, the genotyping data were generated in two batches for both AAs and EAs. Among ROS/MAP AAs, one batch was from the Alzheimer's Disease Genetics Consortium (ADGC; n=86) and one batch was from the Children’s Hospital of Philadelphia (CHOP; n=71). Among ROS/MAP EAs, one batch was genotyped using Affymetrix (n=998) and one batch using Illumina (n=288) (Supplementary Table 1). Each cohort applied standardized quality-control filters for call rate, Hardy-Weinberg equilibrium P-value and other measures to exclude individuals and genotyped SNPs before imputation [17,18]. Imputation to 1000 Genomes Phase 1 reference panel was performed by individual cohorts using IMPUTE2, miniMac2, or Beagle. Details of genotyping and imputations are provided in Supplementary Table 1.
Association Analysis
Each cohort conducted race-specific GWAS analyses using either R, FAST [19] or PLINK [20] software using a standardized protocol. Sense of smell was defined as the natural log of the sense of smell score plus 1 to account for the few participants who had a score of “0” [21]. Variants were defined as allele dosages based on an additive model. Linear regression was then used to model sense of smell in relation to variants adjusting for age, gender, study site, cognitive function, ApoE ε4 allele (carrier vs. non-carrier) and the first ten principal components (PCAs). After excluding INDELs and variants with either an imputation quality score below 0.3 or a minor allele count (MAC = 2*MAF*sample size) below 20 from individual cohorts, our meta-analysis included 15,719,983 SNPs in AAs and 12,972,359 SNPs in EAs.
Effect estimates and standard errors from individual datasets were meta-analyzed using fixed-effect inverse variance weighting in METAL [22]. Genomic control was applied for each cohort before meta-analysis. The primary analyses were race-specific, but we also conducted multi-ancestry analyses using METASOFT [23] with the Han and Eskin random-effects (RE2) model to combine the race-specific meta-analysis results from METAL.
We used quantile-quantile (Q-Q) plots and the genomic inflation factor (λ) to detect potential population stratification in each cohort as well as in the meta-analyses. We defined genome-wide significance as P-value < 5 × 10−8. False discovery rate (FDR) was also applied for the adjustment of multiple comparisons [24]. We used R software (version 3.0.2) to generate Q-Q and Manhattan plots and LocusZoom 1.1 to generate regional plots.
Gene Set/Pathway Enrichment Analysis
To explore potential biological pathways that are associated with the sense of smell, we applied the improved gene set enrichment analysis for GWAS (i-GSEA4GWAS v2, http://gsea4gwas-v2.psych.ac.cn/) by mapping SNPs to the nearest gene (± 100 kb). If multiple genes were located within the range of a given SNP, the closest gene was selected and assigned the association gene-wise P value. All SNP P values were log-transformed before generating the gene list. SNP label permutation was performed to reduce bias from larger loci having disproportionately larger numbers of SNPs. Enriched pathways were derived from multiple sources such as the KEGG and Gene Ontology database. The P values for pathways/gene sets were adjusted for multiple testing with a FDR < 0.05 as statistically significant.
Functional Prediction Analyses
To explore the functional relevance of the top SNPs from this GWAS analysis, we used the publicly available RegulomeDB (http://regulomedb.org/) [25] database to assign each variant an ordinal functional score. These calculations were based on a synthesis of regulatory data derived from ENCODE and other sources as well as computational predictions and manual annotations [25].
RNA-Seq Transcript Expression Profiling
We further conducted eQTL analysis to interpret the GWAS findings at the transcript level. RNA expression data were generated from frozen postmortem dorsolateral prefrontal cortex brain tissues of 447 ROS/MAP participants. cDNA libraries were compiled on the Broad Institute’s Genomics Platform using the strand specific dUTP method with poly-A selection [26]. Sequencing was completed on the Illumina HiSeq with 75M reads of at least 101 base pair each. Final data were generated using Bowtie alignments and RSEM assemblies.
RESULTS
Population characteristics by race groups and individual cohorts are presented in Table 1. Overall, AAs had poorer sense of smell and cognition than EAs, and were more likely to carry the ApoE ε4 allele. Across the cohorts, the smell score decreased with age and was positively associated with overall cognition in both race groups. In the EA population, poorer sense of smell was linked to male gender and the ApoE ε4 allele, while in AAs, these associations were not consistently observed across cohorts (Table 2).
Table 1.
Population characteristics of participating cohorts
| Race | Characteristics | ARIC | Health ABC | ROS/MAP ADGC |
ROS/MAP CHOP |
|
|---|---|---|---|---|---|---|
| AA | N | 1053 | 769 | 86 | 71 | |
|
Age at assessment (years), mean ± SD |
74.95 ± 5.09 | 75.44 ± 2.83 | 81.75 ± 5.51 | 72.37 ± 7.69 | ||
| Men,% | 351 (33.33) | 326 (42.39) | 38 (43.18) | 11 (15.49) | ||
|
Smell identification score, mean ± SD |
7.97 ± 2.61 | 8.71 ± 2.54 | 8.68 ± 2.39 | 8.97 ± 2.01 | ||
| Cognitive score, mean ± SD | ||||||
| MMSE | 25.17 ± 3.77 | - | 27.77 ± 2.19 | 27.93 ± 2.29 | ||
| MMMSE | - | 85.32 ± 10.54 | - | - | ||
| APOE genotypes, (%) | ||||||
| 22 | 16 (1.56) | 8 (1.04) | 1 (1.14) | 0 (0) | ||
| 23 | 145 (14.13) | 123 (15.99) | 15 (17.05) | 8 (11.27) | ||
| 24 | 54 (5.26) | 40 (5.20) | 7 (7.95) | 6 (8.45) | ||
| 33 | 474 (46.20) | 362 (47.07) | 46 (52.27) | 33 (46.48) | ||
| 34 | 298 (29.04) | 214 (27.83) | 15 (17.05) | 23 (32.39) | ||
| 44 | 39 (3.80) | 22 (2.86) | 3 (3.41) | 1 (1.41) | ||
| ARIC | Health ABC |
ROS/MAP batch 1 |
ROS/MAP batch 2 |
|||
| EA | N | 3985 | 1311 | 998 | 288 | |
|
Age at assessment (years), mean ± SD |
75.89 ± 5.18 | 75.69 ± 2.82 | 82.29 ± 6.71 | 81.46 ± 7.02 | ||
| Men,% | 1751 (43.94) | 691 (52.71) | 277 (27.76) | 72 (25.00) | ||
|
Smell identification score, mean ± SD |
9.50 ± 2.30 | 9.51 ± 2.12 | 8.54 ± 2.48 | 9.02 ± 2.55 | ||
| Cognitive score, mean ± SD | ||||||
| MMSE | 27.89 ± 2.28 | - | 27.94 ± 2.52 | 27.83 ± 2.72 | ||
| MMMSE | - | 92.88 ± 5.92 | - | - | ||
| APOE genotypes, (%) | ||||||
| 22 | 27 (0.71) | 9 (0.69) | 6 (0.60) | 2 (0.69) | ||
| 23 | 515 (13.52) | 164 (12.51) | 153 (15.33) | 37 (12.85) | ||
| 24 | 87 (2.28) | 21 (1.60) | 17 (1.70) | 4 (1.39) | ||
| 33 | 2307 (60.58) | 822 (62.70) | 617 (61.82) | 189 (65.63) | ||
| 34 | 817 (21.45) | 278 (21.21) | 189 (18.94) | 52 (18.06) | ||
| 44 | 55 (1.44) | 17 (1.30) | 16 (1.60) | 4 (1.39) | ||
AA: African-Americans; EA: European-Americans; ARIC: the Atherosclerosis Risk in Communities study; Health ABC: the Health, Aging, and Body Composition study; ROS/MAP: the Rush Religious Orders Study (ROS) and Memory and Aging Project (MAP); MMSE: Mini-Mental State Examination, range of possible score 0–30; MMMSE: Modified Mini Mental Status Examination, range of possible score 0–100; SD: standard deviation.
For ApoE, numbers do not add up to total due to missing values.
Table 2.
β coefficient and 95% confidence intervals for covariates in relation to the sense of smell in African-Americans (AAs) and European-Americans (EAs) a
| Race | Characteristics | ARIC | Health ABC | ROS/MAP ADGC |
ROS/MAP CHOP |
|
|---|---|---|---|---|---|---|
| AA | Age in years | −0.014 (−0.019 ~ −0.010) | −0.010 (−0.019 ~ −0.002) | −0.012 (−0.024, −0.000) | −0.007 (−0.014, −0.000) | |
| Gender | ||||||
| Female | Reference | Reference | Reference | Reference | ||
| Male | −0.093 (−0.139 ~ −0.046) | −0.083 (−0.129 ~ −0.038) | 0.082 (−0.050, 0.215) | 0.106 (−0.047, 0.258) | ||
| MMSE/MMMSE | 0.036 (0.030 ~ 0.042) | 0.011 (0.009 ~ 0.014) | 0.019 (−0.009, 0.047) | 0.012 (−0.013, 0.037) | ||
| APOE status | ||||||
| non-ε4 (22/23/33) | Reference | Reference | Reference | Reference | ||
| ε4 (24/34/44) | −0.001 (−0.017 ~ 0.045) | 0.059 (0.012 ~ 0.106) | 0.051 (−0.005, 0.107) | −0.080 (−0.193, 0.032) | ||
| ARIC | Health ABC |
ROS/MAP batch 1 |
ROS/MAP batch 2 |
|||
| EA | Age in years | −0.008 (−0.010 ~ −0.006) | −0.010 (−0.014 ~ −0.005) | −0.012 (−0.014, −0.009) | −0.015 (−0.020, −0.009) | |
| Gender | ||||||
| Female | Reference | Reference | Reference | Reference | ||
| Male | −0.057 (−0.077 ~ −0.038) | −0.068 (−0.094 ~ −0.042) | −0.104 (−0.146, −0.062) | −0.017 (−0.105, 0.071) | ||
| MMSE/MMMSE | 0.029 (0.024−0.033) | 0.007 (0.005 ~ 0.010) | 0.037 (0.029, 0.044) | 0.040 (0.025, 0.055) | ||
| APOE status | ||||||
| non-ε4 (22/23/33) | Reference | Reference | Reference | Reference | ||
| ε4 (24/34/44) | −0.038 (−0.059 ~ −0.016) | −0.039 (−0.069 ~ −0.008) | −0.074 (−0.120, −0.029) | −0.135 (−0.228, −0.042) | ||
Covariates were mutually adjusted.
AA: African-Americans; EA: European-Americans; ARIC: the Atherosclerosis Risk in Communities study; HABC: the Health, Aging, and Body Composition study; ROS/MAP: the Rush Religious Orders Study (ROS) and Memory and Aging Project (MAP) MMSE: Mini-Mental State Examination; MMMSE: Modified Mini-Mental Status Examination; ApoE: Apolipoprotein E.
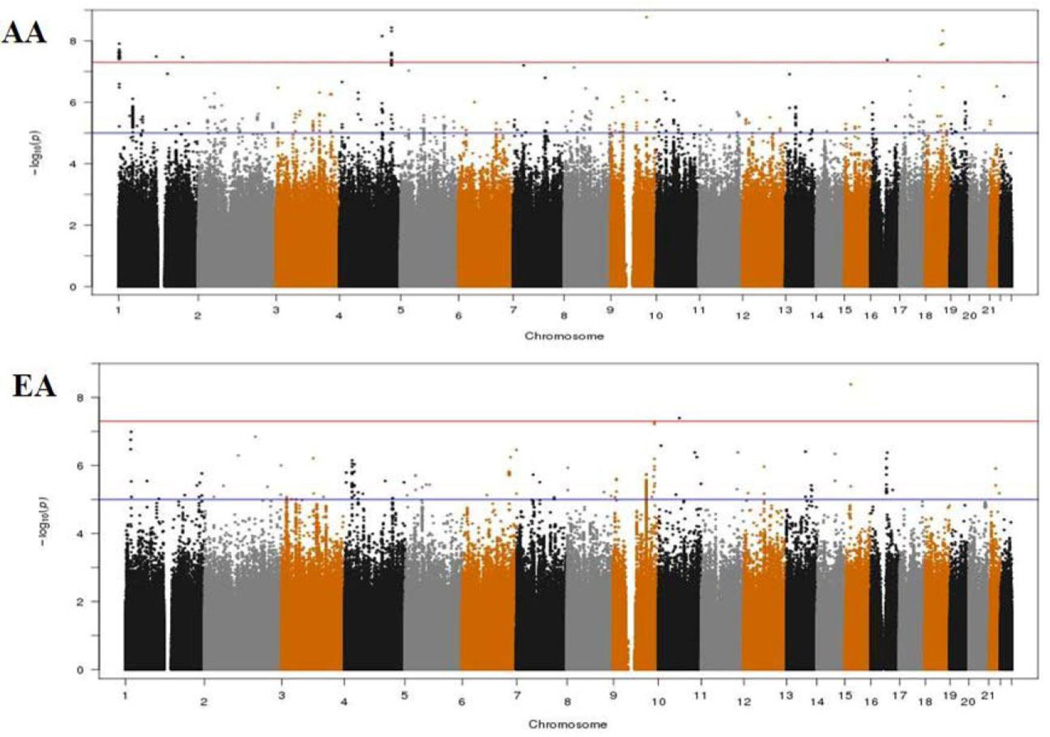
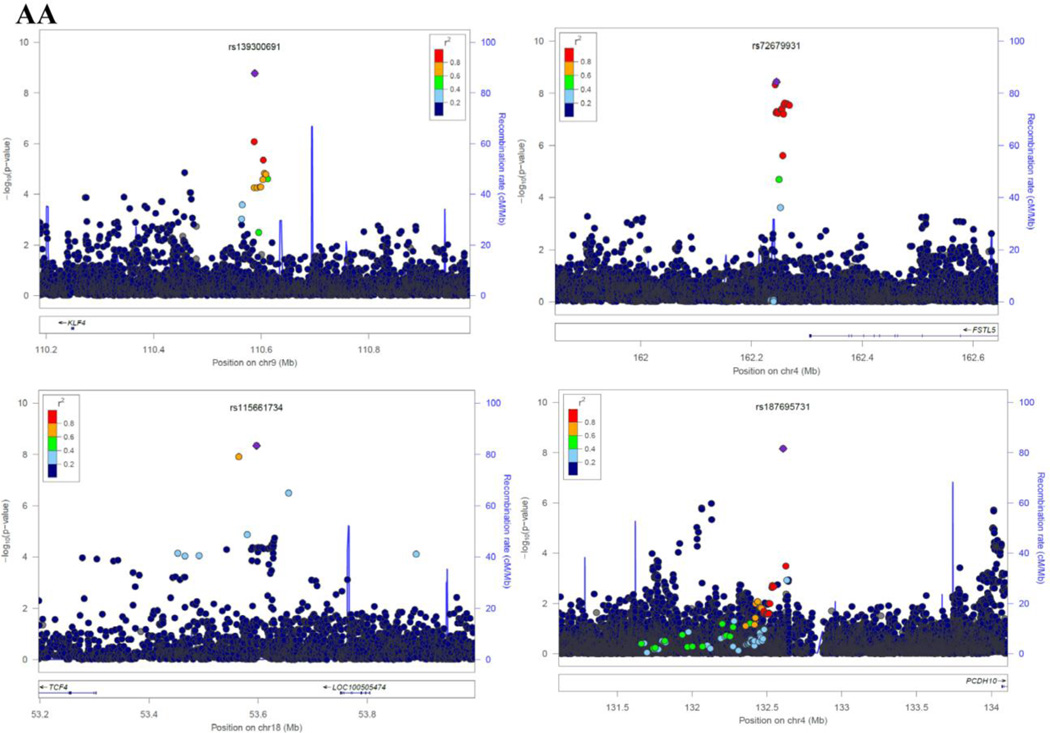
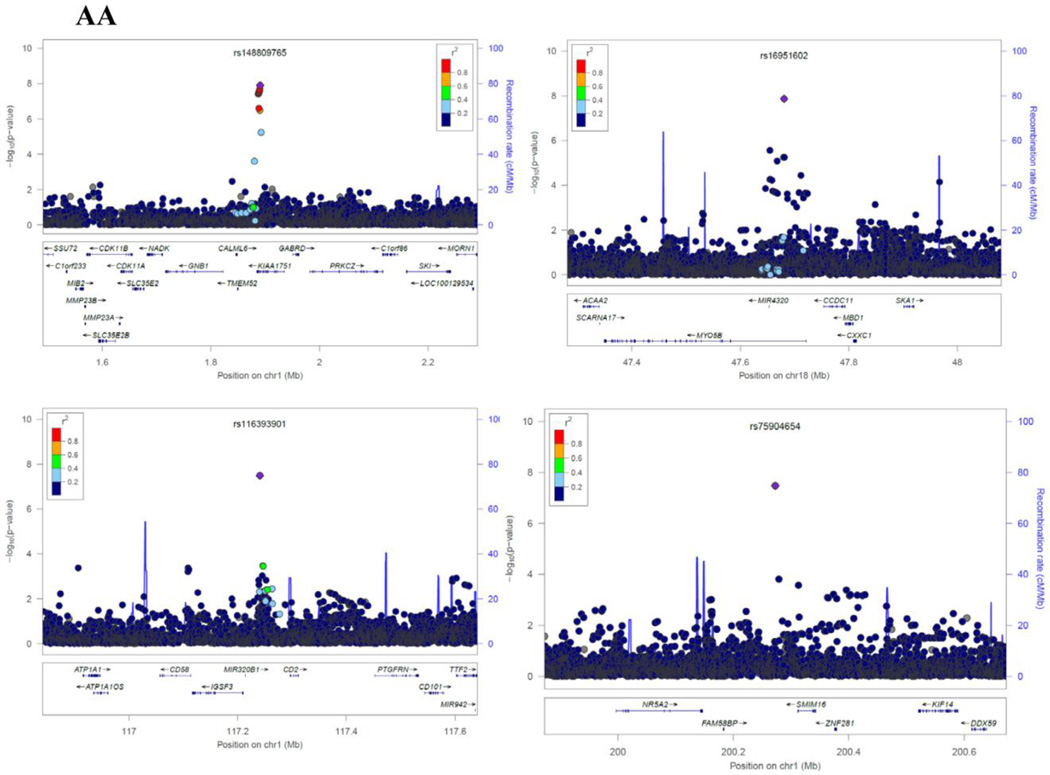
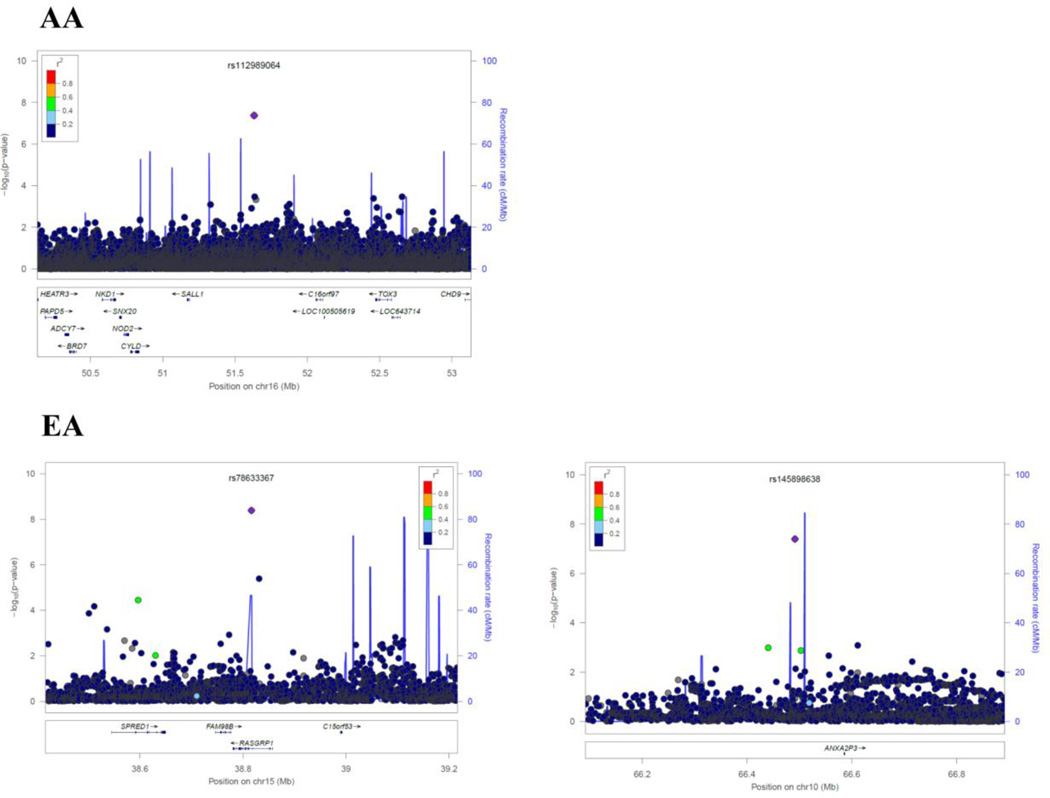
As evidenced by the Q-Q plots for both individual GWAS analyses and the meta-analyses (Supplementary Figure 2), we did not observe evidence for hidden substructure or cryptic relatedness in race-specific or multi-ancestry analyses (λ ranged from 0.995 to 1.01). Results of the genome-wide meta-analysis are summarized in the Manhattan plots by race (Figure 1). In the AA population, a total of 37 SNPs achieved genome-wide significance (Pmeta < 5 × 10−8). After examining LD among these SNPs, we identified 9 SNPs that had the lowest P-value in each LD block (r2 ≥ 0.8), with the Pmeta ranging from 4.21 × 10−8 (rs112989064 at 16q12.1) to 1.69 × 10−9 (rs139300691 at 9q31.2) (Table 3, Supplementary Table 2 and Figure 2). In the EA population, only two SNPs, rs78633367 (Pmeta = 4.14 × 10−9) at 15q14 and rs145898638 (Pmeta = 4.04 × 10−8) at 10q21.3 reached genome-wide significance. All of these top SNPs had a FDR value less than 0.02. For all the 11 SNPs, we repeated the meta-analyses using random-effects model and obtained similar results (data now shown).
Figure 1.
Manhattan plots of the genome-wide meta-analysis on the sense of smell in African-Americans (AAs) and European-Americans (EAs). The red line indicates the genome-wide significance threshold (5 × 10−8) and the blue line indicates the suggestive threshold (1 × 10−5).
Table 3.
Significant SNPs (Pmeta< 5 × 10−8) from the genome-wide meta-analysis on the sense of smell in African-Americans (AAs) and European-Americans (EAs)
| Race | SNP | Chr | Gene | BP | Effect Allele |
Other Allele |
rsq a | MAF | Effect size b |
s.e. | P_meta |
I2 for hetero |
P for Cochran’s Q |
FDR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | rs139300691 | 9q31.2 | KLF4-ACTL7B | 110587938 | A | G | 0.80 | 0.02 | −0.30 | 0.05 | 1.69×10−9 | 41.60 | 0.19 | 0.015 |
| rs72679931 | 4q32.2 | RAPGEF2-FSTL5 | 162245253 | A | T | 0.62 | 0.01 | −0.35 | 0.06 | 3.73×10−9 | 63.10 | 0.04 | 0.013 | |
| rs115661734 | 18q21.2 | TCF4-LOC100505474 | 53597122 | T | G | 0.95 | 0.01 | −0.29 | 0.05 | 4.67×10−9 | 70.10 | 0.07 | 0.013 | |
| rs187695731 | 4q28.3 | NONE-PCDH10 | 132608408 | T | C | 0.74 | 0.02 | 0.29 | 0.05 | 7.02×10−9 | 0.00 | 0.49 | 0.015 | |
| rs148809765 | 1P36.33 | KIAA1751 | 1890756 | T | C | 0.49 | 0.01 | −0.35 | 0.06 | 1.24×10−8 | 83.70 | 0.01 | 0.015 | |
| rs16951602 | 18q21.1 | MYO5B | 47680617 | A | G | 0.75 | 0.03 | 0.21 | 0.04 | 1.35×10−8 | 0.00 | 0.70 | 0.015 | |
| rs116393901 | 1p13.1 | MIR320B1-CD2 | 117241009 | A | G | 0.90 | 0.01 | 0.28 | 0.05 | 3.25×10−8 | 63.40 | 0.10 | 0.015 | |
| rs75904654 | 1q32.1 | NR5A2-LINC00862 | 200272654 | A | T | 0.46 | 0.01 | 0.36 | 0.07 | 3.39×10−8 | 31.00 | 0.23 | 0.015 | |
| rs112989064 | 16q12.1 | SALL1-C16orf97 | 51631845 | T | C | 0.68 | 0.02 | −0.28 | 0.05 | 4.21×10−8 | 0.00 | 0.91 | 0.015 | |
| EA | rs78633367 | 15q14 | RASGRP1 | 38816290 | T | C | 0.56 | 0.02 | 0.16 | 0.03 | 4.14×10−9 | 61.80 | 0.11 | 0.002 |
| rs145898638 | 10q21.3 | REEP3-ANXA2P3 | 66491421 | T | G | 0.70 | 0.01 | −0.18 | 0.03 | 4.04×10−8 | 0.00 | 0.85 | 0.006 |
Chr.: Chromosome. MAF: minor allele frequency. s.e.: standard error. FDR: false discovery rate.
Adjusted for age, gender, study center, cognitive function, ApoE genotype, and the first ten principal components.
mean imputation quality score.
Effect size in ln(smell score+1) per allele change.
Figure 2.
Regional Plots of each SNP identified in African-Americans (AAs) and European-Americans (EAs). Results (−log10 P) are shown for SNPs in the region flanking 400 kb on either side of SNP (except for rs187695731 and rs112989064 which spans ±1500 kb). The marker SNP are shown in purple and the r2 values of the rest of the SNPs are indicated by different colors. The genes within the region of interest are annotated, with arrows indicating transcription direction.
These top SNPs appeared to be race-specific (Supplementary Table 3); the Pmeta of all top SNPs was > 0.1 in the other race group with the exception of rs145898638 (Pmeta = 0.04 in AAs and 4.04×10−8 in EAs). In addition, we conducted multi-ancestry meta-analysis using the RE2 model in METASOFT to account for sample heterogeneity and to identify genetic variants that might affect the sense of smell in both race groups. The most significant SNP was rs193020892 (Poverall = 2.37× 10−8) at 9q33.3 (Supplementary Table 4).
Using the RegulomeDB database, four of the top SNPs (rs16951602, rs116393901, rs78633367 and rs145898638) mapped to regulatory regions. Two additional top SNPs (rs72679931 and rs115661734) were in strong LD with SNPs (rs72679948 and rs115356618, respectively, Supplementary Table 2) that were also in regulatory regions. These variants showed evidence of transcription factor biding sites, DNase I hypersensitivity peaks and/or spliced human ESTs regions. Among significant SNPs, we were able to perform eQTL analysis for only 2 SNPs (rs72679931 and rs16951602) which had genotyping data available. However, we did not observe any significant associations between these 2 SNPs and nearby gene expression levels using RNA-seq data of 447 postmortem frontal-cortex tissues from the ROS/MAP study (data not shown).
We further performed gene set/pathway enrichment analyses using i-GSEA4GWAS server to identify plausible biological pathways that are associated with the sense of smell separately in AAs and EAs. We queried approximately 2,000 gene sets, including canonical pathways integrated from KEGG, BioCarta and Gene Ontology (GO) functional categories. Using a false discovery rate (FDR) of 0.05, we identified 37 enriched pathways in AAs and 2 in EAs (Supplementary Table 5). Although the overrepresented gene sets encompassed a range of biological functions, many are involved in neuron generation, neuron/neurite development, neurogenesis, neurotrophin signaling, cell projection, and neuron differentiation. Other prominent pathways included BAD phosphorylation, apoptosis, immunity and cellular morphogenesis.
In our previous GWAS study in EAs, we reported 13 SNPs that showed suggestive evidence for an association with the sense of smell (Pmeta < 10−5); we therefore compared the results of these 13 SNPs with our current analysis. Among these 13 SNPs, 11 were included in the current analysis (Supplementary Table 6). Results were similar in the EA population, while in the AA population, the Pmeta was > 0.1 for all SNPs.
Finally, we conducted a post-hoc analysis to examine associations between PD- and AD-related susceptibility SNPs and the sense of smell. These top SNPs for PD and AD were identified primarily from GWAS analysis in European ancestries and GWAS data among AAs are very limited. Of the 35 top SNPs listed at http://www.pdgene.org/ and http://www.alzgene.org/, only rs76904798 near LRRK2 in EAs (Pmeta = 5.57× 10−5) reached statistical significance after accounting for Bonferroni correction (P < 0.05/35=1.43 × 10−3). In AAs, none of the SNPs were statistically significant (data not shown).
DISCUSSION
In this study, we conducted the first GWAS analysis of the sense of smell in older AA adults, and updated the findings of our previous GWAS study in EAs. Poor sense of smell has been shown to be a prodromal symptom for major neurodegenerative diseases such as PD and AD among individuals of European ancestry, but such data are largely lacking for adults of African ancestry. AAs have poorer sense of smell and higher risk for AD, but lower risk for PD compared with EAs [7,27–29]. Therefore, investigating the risk factors for the sense of smell separately in AAs and EAs may not only provide insights into the potentially race-specific etiologies of poor sense of smell but also their relationships to neurodegenerative diseases.
In AAs, we identified nine regions that might be associated with the sense of smell. Two of these regions had multiple SNPs that reached genome-wide significance: 4q32.2 represented by rs72679931 and 1p36.33 by rs148809765. The SNP rs72679931 is located between Rap Guanine Nucleotide Exchange Factor (GEF) 2 (RAPGEF2) and Follistatin-Like 5 (FSTL5). The cdk5-mediated phosphorylation of RAPGEF2 has been demonstrated to control neuronal migration in developing cerebral cortex [30]. Mutation of RAPGEF2 in mice can affect the formation of the cerebral cortex and reduce the threshold for the induction of epileptic seizure; in addition, copy number variants of RAPGEF2 in humans may confer higher risk of familial schizophrenia [31]. In adult mouse brains, FSTL5 is selectively expressed at high levels in the olfactory system, hippocampal CA3 area, and granular cell layer of the cerebellum [32]. In humans, FSTL5 has been associated with the prognosis of medulloblastoma, and it is highly expressed in a specific cell population during neuronal development [33,34]. The SNP rs148809765 is located in the intron region of KIAA1751 and upstream of gamma-aminobutyric acid (GABA) A receptor, delta (GABRD). KIAA1751 is known as cilia and flagella associated protein 74 (CFAP74). Although little is known about the gene function of CFAP74, it’s well documented that cilia are critical for proper olfactory function [35]. GABRD encodes a subunit of the ligand-gated chloride channel for GABA, the major inhibitory neurotransmitter in mammalian brain [36]. GABRD is highly expressed in the dentate gyrus subfield of the hippocampus where it helps to regulate neuronal activity and may be involved in the susceptibility of schizophrenia and epilepsy [37,38], both of which are associated with altered sense of smell [39–41].
Among the other loci identified in AAs, only one SNP at each locus reached genome-wide significance. The most significant SNP, rs139300691 at 9q31.2, is located 336 kb upstream of the gene Kruppel-like factor 4 (KLF4), a transcript factor expressed in neural stem cells with expression recently linked to increased oxidative stress and cell death in PD in vitro [42]. Additional top SNPs in AAs were mapped at or near protocadherin 10 (PCDH10), transcription factor 4 (TCF4), myosin VB (MYO5B) and CD2. These genes were reported to be essential in neuronal activity, normal brain development, memory and cognition [43–45]. Genetic variants in these genes have been linked to neuropsychiatric or neurodegenerative diseases that are often associated with olfactory dysfunction, for example, deletions of PCDH10 with autism-spectrum disorders, TCF4 with schizophrenia, CD2-associated protein with late-onset AD [43,46,45,47]. Of interest, KLF4 has been confirmed to directly interact with TCF4 in vitro [48], suggesting our findings may have some biological connections that yet to be found.
Only two SNPs in EAs reached genome-wide significance. The SNP rs78633367 is located in the intron region of RAS guanyl releasing protein 1 (RASGRP1). Interestingly, RASGRP1 is expressed in neurons and is associated with the expression of LRRK2, a susceptibility gene for PD [49]. In our post-hoc analyses of top susceptible loci for PD, we found that a SNP near LRRK2 (rs76904798) was associated with the sense of smell in EAs, and this SNP was also ranked among the top 1,000 SNPs in the GWAS analysis of EAs. In addition, we and others have previously shown that the ApoE ε4, the most important genetic contributor to sporadic AD [50], and that the MAPT, a potential susceptibility gene for sporadic PD [51], are also related to the sense of smell in EAs. Interestingly, in our samples, these associations were only found in EAs but not AAs, raising the possibility that the genetic risk profiles for the sense of smell may be different by race. Our observations also highlight the need to investigate neurodegeneration and related symptoms specifically among individuals of African ancestry.
Our study has a number of limitations. First, we did not have an independent confirmation which is often required for GWAS studies. This was primarily due to the lack of studies among older adults with both GWAS and the sense of smell data; this is particularly true for African Americans. For this reason, we chose to meta-analyze all data to maximize the statistical power to identify signals that can be investigated in future independent studies. Further, most variants we identified are relatively rare and some have modest imputation score, which again necessitates confirmation from future studies. Finally, the human sense of smell is complex and the smell identification screening tests may not capture the nature and complexity of olfactory impairment. For example, our data could not differentiate primary olfactory deficit from secondary changes due to dysfunction in other brain areas to which the olfactory structures are connected [52,53].
Nevertheless, by meta-analyzing GWAS results from three cohorts, we achieved the largest and most comprehensive genetic analysis on the sense of smell among older adults to date. Once our results are independently confirmed, they may provide genetic insights into the sense of smell among older adults which is relevant to neuropsychological and neurodegenerative diseases, and suggests potential racial difference in the genetic susceptibility of the poor sense of smell.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study, Health ABC study, and the ROS/MAP for their important contributions.
The study was supported by the intramural research program of NIH (Z01 ES101986), the National Institute of Environmental Health Sciences.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694, with the ARIC carotid MRI examination funded by U01HL075572-01; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. Additional support for this project includes grants R01-HL093029 and R01-NS087541.
The Health ABC Study research was supported by in part by the Intramural Research Program of the NIH, National Institute on Aging (NIA) and by NIA contracts N01AG62101, N01AG62103, and N01AG62106. The genome-wide association study was funded by NIA grant 1R01AG032098-01A1 to Wake Forest University Health Sciences and genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C.
The Religious Orders Study and Rush Memory and Aging Project were supported by grants from the National Institutes of Aging [R01 AG30146, P30 AG10161, R01 AG17917, R01 AG15819], the Illinois Department of Public Health, and the Translational Genomics Research Institute.
JMP was supported by the National Institute on Aging (K23 AG036762), the McHugh Otolaryngology Research Fund, the American Geriatrics Society, The Center on the Demography and Economics of Aging, and the Institute of Translational Medicine at The University of Chicago (KL2RR025000 and UL1RR024999).
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB., Jr Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Archives of otolaryngology--head & neck surgery. 1991;117(5):519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chemical senses. 2011;36(1):63–67. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PloS one. 2014;9(10):e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Annals of neurology. 2004;56(2):173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 5.Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson's disease. Annals of neurology. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. doi:NYAS04013 [pii] 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(3):323–329. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiology of aging. 2010;31(4):567–577. doi: 10.1016/j.neurobiolaging.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Menashe I, Man O, Lancet D, Gilad Y. Different noses for different people. Nature genetics. 2003;34(2):143–144. doi: 10.1038/ng1160. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Yang J, Tranah G, Franceschini N, Parimi N, Alkorta-Aranburu G, Xu Z, Alonso A, Cummings SR, Fornage M, Huang X, Kritchevsky S, Liu Y, London S, Niu L, Wilson RS, De Jager PL, Yu L, Singleton AB, Harris T, Mosley TH, Jr, Pinto JM, Bennett DA, Chen H. Genome-wide Meta-analysis on the Sense of Smell Among US Older Adults. Medicine (Baltimore) 2015;94(47):e1892. doi: 10.1097/MD.0000000000001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 12.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG Health ABCS. Fetuin-A and incident diabetes mellitus in older persons. JAMA : the journal of the American Medical Association. 2008;300(2):182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research. 2012;9(6):628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 15.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110(10):976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- 16.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) The Laryngoscope. 1996;106(3 Pt 1):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, Schabath MB, Couper DJ, Brusselle GG, Psaty BM, van Duijn CM, Rotter JI, Uitterlinden AG, Hofman A, Punjabi NM, Rivadeneira F, Morrison AC, Enright PL, North KE, Heckbert SR, Lumley T, Stricker BH, O'Connor GT, London SJ. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nature genetics. 2010;42(1):45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Q, Menzaghi C, Smith S, Liang L, de Rekeneire N, Garcia ME, Lohman KK, Miljkovic I, Strotmeyer ES, Cummings SR, Kanaya AM, Tylavsky FA, Satterfield S, Ding J, Rimm EB, Trischitta V, Hu FB, Liu Y, Qi L. Genome-wide association analysis identifies TYW3/CRYZ and NDST4 loci associated with circulating resistin levels. Human molecular genetics. 2012;21(21):4774–4780. doi: 10.1093/hmg/dds300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanda P, Huang H, Arking DE, Bader JS. Fast association tests for genes with FAST. PloS one. 2013;8(7):e68585. doi: 10.1371/journal.pone.0068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312(7038):1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. American journal of human genetics. 2011;88(5):586–598. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Y Benjamini YH. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 25.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22(9):1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adiconis X, Borges-Rivera D, Satija R, DeLuca DS, Busby MA, Berlin AM, Sivachenko A, Thompson DA, Wysoker A, Fennell T, Gnirke A, Pochet N, Regev A, Levin JZ. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nature methods. 2013;10(7):623–629. doi: 10.1038/nmeth.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okubadejo NU, Bower JH, Rocca WA, Maraganore DM. Parkinson's disease in Africa: A systematic review of epidemiologic and genetic studies. Movement disorders : official journal of the Movement Disorder Society. 2006;21(12):2150–2156. doi: 10.1002/mds.21153. [DOI] [PubMed] [Google Scholar]
- 29.Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S Social B, Diversity Research Workgroup of the A, s A. Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2008;4(4):305–309. doi: 10.1016/j.jalz.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Ye T, Ip JP, Fu AK, Ip NY. Cdk5-mediated phosphorylation of RapGEF2 controls neuronal migration in the developing cerebral cortex. Nature communications. 2014;5:4826. doi: 10.1038/ncomms5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, Woodroffe A, Rodriguez-Murillo L, Roos JL, van Rensburg EJ, Abecasis GR, Gogos JA, Karayiorgou M. Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16746–16751. doi: 10.1073/pnas.0908584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuda T, Sakuma C, Nagaoka A, Yamagishi T, Ueda S, Nagase T, Yaginuma H. Follistatin-like 5 is expressed in restricted areas of the adult mouse brain: Implications for its function in the olfactory system. Congenit Anom (Kyoto) 2014;54(1):63–66. doi: 10.1111/cga.12022. [DOI] [PubMed] [Google Scholar]
- 33.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M, Sturm D, Witt H, Haag D, Toedt G, Wittmann A, Schottler A, von Bueren AO, von Deimling A, Rutkowski S, Scheurlen W, Kulozik AE, Taylor MD, Lichter P, Pfister SM. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(29):3852–3861. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 34.Masuda T, Kai N, Sakuma C, Kobayashi K, Koga H, Yaginuma H. Laser capture microdissection and cDNA array analysis for identification of mouse KIAA/FLJ genes differentially expressed in the embryonic dorsal spinal cord. Brain research. 2009;1249:61–67. doi: 10.1016/j.brainres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chemical senses. 2009;34(5):451–464. doi: 10.1093/chemse/bjp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Windpassinger C, Kroisel PM, Wagner K, Petek E. The human gamma-aminobutyric acid A receptor delta (GABRD) gene: molecular characterisation and tissue-specific expression. Gene. 2002;292(1–2):25–31. doi: 10.1016/s0378-1119(02)00649-2. [DOI] [PubMed] [Google Scholar]
- 37.Rukova B, Staneva R, Hadjidekova S, Stamenov G, Milanova t, Toncheva D. Genome-wide methylation profiling of schizophrenia. Balkan journal of medical genetics : BJMG. 2014;17(2):15–23. doi: 10.2478/bjmg-2014-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald RL, Kang JQ, Gallagher MJ. GABAA Receptor Subunit Mutations and Genetic Epilepsies. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 39.Rupp CI. Olfactory function and schizophrenia: an update. Curr Opin Psychiatry. 2010;23(2):97–102. doi: 10.1097/YCO.0b013e328336643f. [DOI] [PubMed] [Google Scholar]
- 40.Desai M, Agadi JB, Karthik N, Praveenkumar S, Netto AB. Olfactory abnormalities in temporal lobe epilepsy. J Clin Neurosci. 2015;22(10):1614–1618. doi: 10.1016/j.jocn.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, Borgmann-Winter KE, Kohler CG, Gur RE, Turetsky BI. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophrenia bulletin. 2014;40(1):50–59. doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Wang X, Yi X, Wang Y, Liu Q, Ge R. Induction of KLF4 contributes to the neurotoxicity of MPP + in M17 cells: a new implication in Parkinson's disease. Journal of molecular neuroscience : MN. 2013;51(1):109–117. doi: 10.1007/s12031-013-9961-3. [DOI] [PubMed] [Google Scholar]
- 43.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Genetic R, Outcome in P. Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galle SA, Courchesne V, Mottron L, Frasnelli J. Olfaction in the autism spectrum. Perception. 2013;42(3):341–355. doi: 10.1068/p7337. [DOI] [PubMed] [Google Scholar]
- 47.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer's disease. Acta neuropathologica. 2012;124(3):305–323. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans PM, Chen X, Zhang W, Liu C. KLF4 interacts with beta-catenin/TCF4 and blocks p300/CBP recruitment by beta-catenin. Molecular and cellular biology. 2010;30(2):372–381. doi: 10.1128/MCB.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandemakers W, Snellinx A, O'Neill MJ, de Strooper B. LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiology of disease. 2012;48(3):582–593. doi: 10.1016/j.nbd.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Sadigh-Eteghad S, Talebi M, Farhoudi M. Association of apolipoprotein E epsilon 4 allele with sporadic late onset Alzheimer`s disease. A meta-analysis. Neurosciences. 2012;17(4):321–326. [PubMed] [Google Scholar]
- 51.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson's Disease Genomics C, Parkinson's Study Group Parkinson's Research: The Organized GI, and Me, GenePd, NeuroGenetics Research C, Hussman Institute of Human G, Ashkenazi Jewish Dataset I, Cohorts for H, Aging Research in Genetic E, North American Brain Expression C, United Kingdom Brain Expression C, Greek Parkinson's Disease C, Alzheimer Genetic Analysis G. Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nature genetics. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clin Anat. 2014;27(1):54–60. doi: 10.1002/ca.22338. [DOI] [PubMed] [Google Scholar]
- 53.Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev. 2005;50(2):287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.