Abstract
Deposition rates of atmospheric nitrogenous pollutants to forests in the San Bernardino Mountains range east of Los Angeles, California, are the highest reported in North America. Acidic soils from the west end of the range are N-saturated and have elevated rates of N-mineralization, nitrification, and nitrate leaching. We assessed the impact of this heavy nitrogen load on autotrophic ammonia-oxidizing communities by investigating their composition, abundance, and activity. Analysis of 177 cloned β-Proteobacteria ammonia oxidizer 16S rRNA genes from highly to moderately N-impacted soils revealed similar levels of species composition; all of the soils supported the previously characterized Nitrosospira clusters 2, 3, and 4. Ammonia oxidizer abundance measured by quantitative PCR was also similar among the soils. However, rates of potential nitrification activity were greater for N-saturated soils than for soils collected from a less impacted site, but autotrophic (i.e., acetylene-sensitive) activity was low in all soils examined. N-saturated soils incubated for 30 days with ammonium accumulated additional soluble ammonium, whereas less-N-impacted soils had a net loss of ammonium. Lastly, nitrite production by cultivated Nitrosospira multiformis, an autotrophic ammonia-oxidizing bacterium adapted to relatively high ammonium concentrations, was significantly inhibited in pH-controlled slurries of sterilized soils amended with ammonium despite the maintenance of optimal ammonia-oxidizing conditions. Together, these results showed that factors other than autotrophic ammonia oxidizers contributed to high nitrification rates in these N-impacted forest soils and, unlike many other environments, differences in nitrogen content and soil pH did not favor particular autotrophic ammonia oxidizer groups.
To date, only one study has examined the composition and role of autotrophic ammonia-oxidizing bacteria (AOB) in nitrogen cycling within coniferous forest soils of western North America (37). The present study examined autotrophic AOB in mixed-conifer forest soils in the San Bernardino Mountains of southern California within the Greater Los Angeles air basin. These forests chronically receive high inputs of atmospheric nitrogen deposition and elevated ozone exposure (17, 36). A strong gradient of these pollutants has been characterized along a 42-km transect across the ridge of the mountains from west at Camp Paivika (CP) to east at Barton Flats (BF). Incidentally, atmospheric nitrogen deposition measurements at CP are the highest reported in North America and can be in excess of 90 kg ha−1 year−1 (15). In contrast, mean nitrogen deposition at BF, the least impacted site, is ca. 5 to 9 kg ha−1 year−1 (16). Long-term deposition of nitrogenous pollutants has resulted in nitrogen saturation in CP soils, a condition that occurs when available nitrogen is in excess of total biotic demand and is no longer limiting (17). Relative to low-pollution sites in the San Bernardino Mountains, symptoms of nitrogen saturation at CP included higher rates of soil nitrate leaching well below the root zone, higher nitrate concentrations in stream and runoff waters, higher emissions of gaseous nitrogen oxides, soil acidification, and higher rates of N-mineralization and nitrification (1, 17).
It has been hypothesized that microbial activity, nitrification in particular, contributes to acidification and nitrate accumulation in soils at CP (13-15, 17, 27). High nitrification rates were demonstrated in laboratory incubations of CP soils without nutrient amendment, which accumulated nitrate at a rate of 1.0 mg kg of soil−1 day−1 and depleted the residual soil ammonium within 1 week (17). In contrast, soils from the eastern side of the pollution gradient at BF had slower rates of nitrification and took five times longer to deplete the residual ammonium. Higher nitrification rates at CP were also inferred from enrichments in the pool of 15NH4+-N in CP soils relative to that in soils from a study site 10 km down gradient (27). Furthermore, water and soils from other N-impacted forested ecosystems had depleted [15N]nitrate pools, suggesting that nitrification activity is commonly responsible for elevated nitrate concentrations in these systems (7, 8). It is widely thought that nitrification in acidic soils relies on the activities of autotrophic AOB despite their sensitivity to low pH when cultivated (11).
Autotrophic AOB are comprised of tightly clustered lineages within the β-subgroup and a lineage within the γ-subgroup of the Proteobacteria (26). The application of molecular fingerprinting techniques using 16S rRNA genes has significantly increased our understanding of the global ecology and distribution of autotrophic AOB (29). In terrestrial ecosystems, the dominant autotrophic AOB include species of Nitrosomonas and Nitrosospira, which can be further divided into nine distinct taxonomic clusters (43, 46). A number of studies have clearly demonstrated that specific environmental switches or factors, such as ammonia availability, organic matter content, and pH, select for particular clusters. For example, agricultural and forest soils tend to be dominated by Nitrosospira clusters 2, 3, and 4 (6, 21, 28, 30, 32, 35, 41, 45), whereas soils with high ammonia content are generally dominated by Nitrosospira cluster 1 or 3 and representatives of Nitrosomonas spp. (3, 31, 32, 38). Nitrosospira cluster 4 is typically dominant in unimproved soils with low ammonia content, including coniferous forest soils in Oregon (31, 32, 37). Sequences affiliated with Nitrosospira cluster 2 have often been associated with acidic soils (33, 45). For example, a recent study of autotrophic AOB in an acidic N-saturated forest soil revealed numerous gene clones from Nitrosospira cluster 2 that were close relatives to the acid-tolerant cultivar, Nitrosospira sp. strain AHB1 (33). In the present study, we used similar molecular approaches with 16S rRNA genes combined with activity measurements to characterize the role of autotrophic AOB populations in N-saturated, acidic, mixed-conifer forest soils.
We hypothesized that the composition, abundance, and activity of autotrophic AOB were positively correlated to the degree of nitrogen saturation in the soils. The San Bernardino Mountain forest soils are unique in that they are exposed to unusually high levels of nitrogen deposition under a Mediterranean climate. The soils are relatively unweathered and naturally high in base cations compared to more mesic forested areas (13). As a result of the Mediterranean climate, dry nitrogen deposition plays a dominant role during the long dry summers, in contrast to much of northern Europe and northeastern North America, where a larger fraction of nitrogen deposition occurs as acidic precipitation (5). Furthermore, deposition of nitrogen in fog is also substantial in the San Bernardino Mountains, particularly in the spring and fall (18). These forest soils are also unique in that nitrate concentrations at the most N-impacted site are considerably higher than in any other soil previously reported in the literature (16, 17). To our knowledge, there have been no other autotrophic AOB ecology studies conducted in forest soils impacted by chronic, dry, nitrogen deposition under a Mediterranean climate.
MATERIALS AND METHODS
Study area, sample collection, and soil chemistry.
The sampling transect was located along the well-documented gradient of nitrogen and ozone pollution in a mixed conifer forest in the San Bernardino Mountain range ca. 90 km east of downtown Los Angeles, California (14, 36). We chose locations along the transect for sample collection based on previously collected nitrogen deposition data, similarity of forest canopy, and likeness of soil textural properties. Mean quantities of atmospheric nitrate and ammonium deposition to the forest floor were measured from atmospheric collectors continuously maintained at the sites (16). The soils were classified previously as coarse-loamy mesic Ultic Haploxerolls of the Shaver series and were dark brown in color (2). Soil samples (250 g each) were collected aseptically from CP, Strawberry Peak (SP), and Dogwood (DW) on three different dates: March 2002, September 2002, and February 2003. At each sampling date and at each site, six soil samples were taken at least 3 m from the base of six designated ponderosa pine trees beneath the litter layer and to a soil depth of ca. 10 cm. Composite soil samples made for each site on each collection date were sieved to 2 mm. Subsamples were stored at −80°C for subsequent nucleic acid extractions. Other subsamples (six replicates per site) were mixed 1:10 (wt/vol) with a 2 M KCl solution, centrifuged to remove particles, and measured for nitrate and ammonium on an autoanalyzer (TRAACS 2000; Bran & Lubbe). Soil pH was measured from a 1:2 mixture of soil (six replicates per site) with 0.01 M CaCl2.
DNA extraction and generation of 16S rRNA gene clone libraries.
DNA was extracted directly from the soil using the UltraClean DNA extraction kit (MOBio, Solona Beach, Calif.) and vortex mixing as per the manufacturer's instructions. Due to the presence of contaminating humic material, the DNA was purified further using an additional column from the kit, and the resulting product was diluted 1:10 with deionized, UV-irradiated water. For positive controls, genomic DNA was extracted from pure cultures of Nitrosomonas europaea and Nitrosospira multiformis using a genomic DNA isolation kit according to the manufacturer's instructions (Bio-Rad, Hercules, Calif.). PCR of 16S rRNA genes using soil DNA as template was performed with βAMOf 161f and βAMOr1301 primers (34) at annealing temperatures of 55 and 62.5°C. PCR products from each reaction mixture were separately cloned into Escherichia coli TOPO10 cells by using the TA-TOPO PCR cloning kit according to the manufacturer's instructions (Invitrogen, Carlsbad, Calif.).
Clone library analysis.
Insert-containing colonies from each clone library were randomly selected and screened using a second PCR with internal primers that target β-Proteobacteria AOB (CTO189f and CTO654r) (30) at annealing temperatures of 55 or 62.5°C in correspondence with annealing temperatures used to generate the clone libraries. The clone libraries created and screened at 55°C ensured inclusion of abundant autotrophic AOB gene sequences with mismatches to the CTO primer set, i.e., some Nitrosomonas spp. (30). Alternatively, the clone libraries created and screened at 62.5°C ensured greater specificity, mainly for Nitrosospira spp., that exactly matched the CTO primer set. Clones yielding positive amplification were classified by gene sequencing and/or oligonucleotide hybridization. Complete 1.1-kb 16S rRNA gene sequences were determined by bidirectional sequencing with primers from the cloning vector, using an automated capillary DNA sequencer (model 377; Applied Biosystems). The sequences were compiled using STADEN software (12) and manually aligned to their nearest relatives using ARB (1999 release plus manual uploading of closely related sequences based on BLAST results) (47). No chimeric sequences were identified using the online program CHECK_CHIMERA (Ribosomal Database Project [RDP]). Phylogenetic inferences were calculated using a neighbor-joining algorithm with Kimura two-parameter genetic distances (2:1 transition/transversion ratio). Neighbor-joining and maximum parsimony bootstrap values were calculated separately from 1,000 resampled data sets using PHYLIP (Phylogeny Inference Package). Clone libraries created and screened using PCR annealing temperatures of 55°C mostly contained gene sequences unrelated to β-Proteobacteria AOB (90% of 600 clones) and included no representative Nitrosomonas spp. and few relatives of Nitrosospira spp. (data not shown). Therefore, our analysis focused on clone libraries generated and screened with PCR annealing temperatures of 62.5°C to characterize dominant Nitrosospira spp. in the soils.
For oligonucleotide hybridization, purified plasmid (1 μg) was transferred onto a Zeta-Probe membrane (Bio-Rad) by using a Mini-Fold I dot blot system (Schleicher & Schuell, Keene, N.H.). Prehybridization and hybridization of membranes with 32P-end-labeled (kit from Promega, Madison, Wis.) oligonucleotides specific for Nitrosospira cluster 2, 3, or 4 (45) were carried out in Z-Hybe buffer (1 mM EDTA, 0.25 M Na2HPO4 [pH 7.2], 7% sodium dodecyl sulfate) at 45°C overnight. Membranes were rinsed free of unbound probe by washing twice (15 min each) with wash buffer (0.2× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA; pH 7.7]-0.1% sodium dodecyl sulfate) at room temperature followed by one stringency wash at 2 to 3°C lower than the empirically determined melting temperature for each oligonucleotide, i.e., 42, 50, and 48°C for Nitrosospira clusters 2, 3, and 4, respectively. Membranes were placed onto phosphor storage screens for 1 h and analyzed using a Typhoon PhosphorImager and ImageQuant software (Amersham, Piscataway, N.J.).
qPCR.
Quantitative PCR (qPCR) was carried out using a previously developed primer and probe set (22). The specificities of the primers and probes were verified using the Probe Match program and the RDP database (http://rdp.cme.msu.edu/html/). The forward primer set, CTO189a/b/c, was the most highly conserved and recognized the majority of β-Proteobacteria AOB, having only a single mismatch that detected Azoarcus spp., whereas the less-specific Taqman probe and reverse primer exactly matched several β-Proteobacteria 16S rRNA genes outside of the autotrophic AOB group. The annealing temperature of the qPCR assay was optimized using both N. multiformis and N. europaea genomic DNA on the gradient function of the iCycler (Bio-Rad) to maximize specificity of the reaction and ensure detection of all dominant β-Proteobacteria AOB (i.e., Nitrosospira spp.) in the soils. Although the optimal annealing temperature was higher than the highest calculated melting temperature for the primers, the possibility remained, as with all PCR-based methods, for nonspecific amplification of 16S rRNA genes unrelated to the β-Proteobacteria AOB. However, the approach of combining specific and nonspecific probes and primers for qPCR has been successfully utilized in other microbial ecology studies (20, 48).
Due to the extreme sensitivity of the qPCR and interference by humic material, soil DNA extracted from a single MOBio Ultra-Clean DNA column was further purified by gel filtration on Sephadex G-100 columns (Island Scientific). Total DNA was carefully quantitated immediately prior to performing qPCR by measuring the intensity of ethidium bromide-stained bands on agarose gels against a dilution series of a 1-kb ladder (Promega), using a Gel Doc documentation system and Quantity One software (Bio-Rad). Quantification of autotrophic AOB abundance in each sample was determined in replicate reaction mixtures against a standard curve of 150 fg to 1.5 ng of N. multiformis genomic DNA. Standard curves generated mean correlation coefficients, PCR efficiency, and slopes of 0.93 ± 5 (mean ± coefficient of variation [CV]), 96% ± 3%, and −3.41 ± 2, respectively. The detection limit of the assay was 500 fg; ca. 200 N. multiformis cells, assuming an average genome mass of 2.3 fg and a single copy of the 16S rRNA gene per genome, as in N. europaea (10), or ca. 0.001% of total extracted DNA from the soils. Three to four separate soil DNA preparations were analyzed in triplicate from each site. To rule out PCR inhibition by humic material in extracted soil DNA, a known concentration of genomic DNA from N. multiformis was mixed with one replicate soil DNA preparation for each experiment. Due to the generally low numbers of autotrophic AOB in the soil DNA preparations, some reactions failed to generate a threshold cycle even though the control sample spiked with N. multiformis DNA amplified with high efficiency (>80%). For these reactions, we assumed that the lower limit of detection was an accurate representation of the autotrophic AOB population and included this number in calculations of the mean percentage of autotrophic AOB in total extracted soil DNA.
Potential nitrification activity of native soils.
Six replicate, composite, sieved, soil samples (5 g, air-dried weight) for each site were placed into glass bottles (125 ml), washed with sodium phosphate buffer (1 mM; pH 7.2), and collected by centrifugation to remove soluble nitrate. The washed soils were resuspended in 50 ml of the same buffer containing NH4Cl (1.5 mM). The slurries were shaken continuously in the dark at 25°C for 3 to 5 days. The pH was adjusted to 7 with NaOH every day over the course of the experiment. Half of the samples were treated with 1% (vol/vol) acetylene to inactivate autotrophic ammonia oxidation activity (24). Aliquots of slurry (2 ml) were taken at 24, 48, 72 and, for September 2002 samples, 115 h. The aliquots were centrifuged, and the supernatants were analyzed for nitrite plus nitrate ions (Rapid Flow analyzer; Alpkem). Headspace samples were analyzed on a gas chromatograph (TCD molecular sieve column; Shimadzu, Kyoto, Japan) to monitor oxygen and acetylene concentrations at the end of each experiment. In addition to the forest soils, slurries of highly drained loamy sand collected from a fertilized turf grass ecosystem (collected from the Coachella Valley in California) with high nitrification activity were monitored for nitrite plus nitrate production as a positive control.
Ammonia consumption in native soils.
Ammonia concentrations were measured at time zero and after 30 days (TRAACS 2000) for triplicate (50 g, air-dried weight) composite soil samples collected from each site in September 2002. The samples were initially amended with 200 mg of (NH4)2SO4 kg−1 and maintained at field capacity moisture in foil-covered glass flasks.
Activity of N. multiformis inoculated into sterile soils.
Soils collected from CP and DW were sterilized by gamma irradiation in an effort to maintain organic matter structure and composition. Accusand (Unimin Corporation, New Canaan, Conn.), a commercially available silica quartz matrix containing no organic matter, was sterilized by autoclave. Six replicate samples of each soil and sand (5 g, air-dried weight) were washed of free nitrate, as described above, placed aseptically into glass bottles, and inoculated with 5 × 107 N. multiformis cells (grown to mid-log phase in American Type Culture Collection medium 929) for a final concentration of 107 cells g of dry soil−1 plus sodium phosphate buffer (50 ml) and NH4Cl (1.5 mM). Three of the replicate samples were treated with acetylene to inhibit autotrophic AOB activity as described above. The slurries were shaken continuously in the dark at 25°C for 3 days while the pH was maintained at 7 with NaOH. Samples (1 ml) were taken at 2, 4, 8, 16, 24, 48, and 72 h, centrifuged, and analyzed colorimetrically for nitrite using NIT Schezchrome reagent as per the manufacturer's instructions (Polysciences, Warrington, Pa.). Uninoculated, sterilized CP and DW soils and Accusand slurries were prepared, treated, and sampled as above to serve as negative controls.
Statistics.
Analysis of variance (ANOVA) was conducted at α = 0.05 to determine significant differences between soil chemical characteristics (i.e., pH, nitrate, and ammonium) among the three sites (n = 6). Significance of the qPCR results among the sites was tested using both parametric and one-way nonparametric (Kruskal-Wallis) ANOVAs, either with or without outlying values at 95% confidence intervals. No significant differences were found among sites by using either test with or without outlying values. Reported numbers do not include outliers. Standard linear regressions were used to estimate the mean potential nitrification activity (PNA) over the 3- to 5-day time courses. The errors associated with PNA rates were calculated by regression analysis, using the mean from each data set and standard deviations from the means. ANOVA was conducted to determine the significance between rates of PNA for each site.
Nucleotide sequence accession number.
Sequence data obtained in this study were deposited in the GenBank database under accession numbers AY293074 to AY293115.
RESULTS
Confirmation of nitrogen saturation gradient.
Soil samples were analyzed from the most N-impacted site at CP, to an intermediate site at SP, to a moderately impacted site at DW (Table 1). Soil pH, nitrate, and ammonium concentrations for soils collected in March 2002 were within previously reported ranges (Table 1) (17, 18). Low soil pH at CP and significantly higher pH at DW (P = 0.009) demonstrated the continuity of the N-saturation gradient from west to east along the mountain range, as did trends in the concentrations of retained soil nitrate and ammonium (Table 1). Although differences in soil ammonium concentrations were not significantly different between the sites (P = 0.230), DW soils retained significantly less nitrate than CP or SP soils (P < 0.001).
TABLE 1.
Rates of atmospheric N deposition and chemical parameters of forest soils
| Study site | Rate of annual atmospheric N depositiona
|
Soil chemistry
|
|||
|---|---|---|---|---|---|
| NO3−-N | NH4+-N | pHb | NO3−-Nc | NH4+-Nc | |
| CP | 82.9 | 66.0 | 3.4 (0.1) | 72 (46) | 1.1 (15) |
| SP | 45.1 | 39.9 | 3.9 (0.4) | 27 (29) | 3.0 (2) |
| DW | 40.7d | 41.3d | 4.7 (0.3) | 2.6 (4) | 2.6 (2) |
Average atmospheric throughfall deposition of NO3−-N or NH4+-N under mature ponderosa pine canopies (in kilograms per hectare per year) for November 2001 to November 2002.
Determined from soil extracted with CaCl2 as described in Materials and Methods. Values in parentheses are standard deviations for n = 6.
Milligrams of NO3−-N or NH4+-N per kilogram of dry soil. Values in parentheses are standard deviations for n = 6. Samples were collected in March 2002 as described in Materials and Methods.
Nitrogen deposition data are from Heaps Peak, a monitoring site 6.3 km east of DW.
Composition of autotrophic AOB community.
Previous studies showing high nitrification rates in this ecosystem (17, 27) prompted our investigation of the composition of autotrophic AOB populations by using 16S rRNA gene clone libraries. We first PCR amplified and cloned 1.1-kb fragments (34) of 16S rRNA genes from CP, SP, and DW soils sampled in March 2002 and then amplified the cloned products with more specific internal primers to obtain a high proportion of AOB gene clones (30). The proportion of cloned genes conferring positive reamplification with the internal primers from two separate soil DNA extractions ranged from 20 to 50% at CP, 70 to 80% at SP, and 50 to 80% at DW.
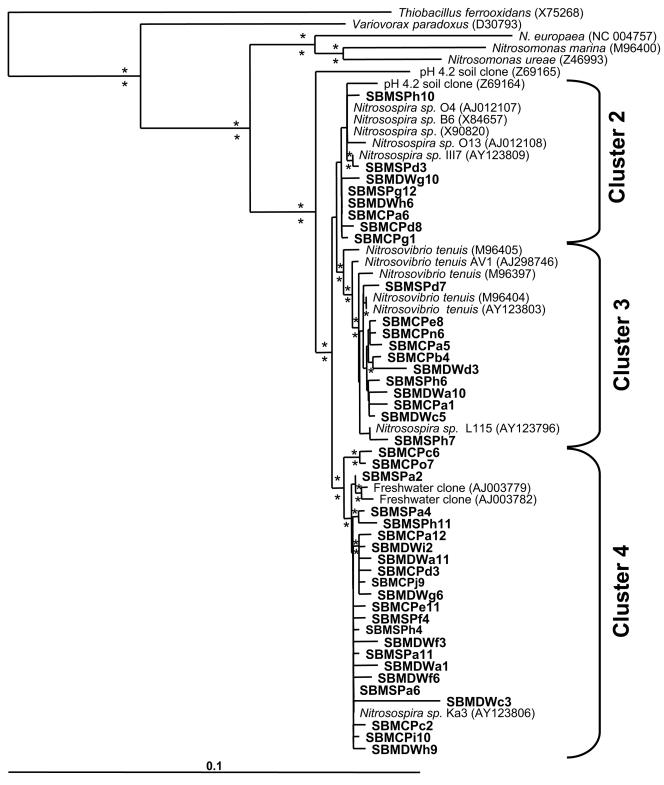
One hundred eighty-four clones that produced a PCR product from both primer sets were selected at random for additional classification by direct sequencing and/or oligonucleotide hybridization. Forty-two gene sequences (1.1 kb) shared 98 to 100% identity to cultivated Nitrosospira isolates from soils and affiliated with cluster 2, 3, or 4 (Fig. 1). More than half of all the sequences were most similar to Nitrosospira cluster 4 isolate Ka3. None of the sequences clustered with Nitrosomonas or Nitrosomonas-like species. Oligonucleotide hybridization with probes specific to Nitrosospira clusters 2, 3, and 4 (45) allowed classification of the remaining clones. The combination of gene sequence and oligonucleotide hybridization revealed the relative distribution of Nitrosospira clusters among gene clones from the three sampled sites (Table 2). Only 20% of the clones analyzed were related to Nitrosospira cluster 2, which contains acid-tolerant isolates, and were evenly distributed among the three sites despite significant differences in soil pH. There appeared to be an equal distribution of cluster 3- and 4-related sequences in CP soils, suggesting a possible trend towards cluster 3-like sequences compared to clone distributions of the other two soils. The seven gene clones (ca. 4% of the total) unrelated to Nitrosospira clustered outside of the AOB group but within the β-Proteobacteria as determined by full or partial sequences (data not shown).
FIG. 1.
Phylogenetic positions of cloned 1.1-kb 16S rRNA gene sequences. This phylogenetic tree was rooted with a 16S rRNA gene sequence for E. coli and was constructed using the neighbor-joining method and Kimura two-parameter model for nucleotide change. A mask of 733 nucleotides, including all nonambiguously aligned positions, was included. Bootstrap values over 75 (from 1,000 replications) generated using the maximum parsimony method (above) and neighbor-joining method (below) are indicated at the nodes (*). Italicized names are sequences from bacterial isolates, whereas nonitalicized names are cloned environmental gene sequences obtained from the GenBank database.
TABLE 2.
Affiliation of cloned 16S rRNA genes with Nitrosospira spp. gene clustersa
| Study site | No. of clones
|
||||
|---|---|---|---|---|---|
| Totalb | Cluster 4 (%)c | Cluster 3 (%) | Cluster 2 (%) | Non-AOBd | |
| CP | 53 | 24 (45.3) | 23 (43.4) | 5 (9.4) | 1 (1.9) |
| SP | 73 | 42 (57.5) | 16 (21.9) | 11 (15.1) | 4 (5.5) |
| DW | 58 | 37 (67.3) | 15 (27.3) | 4 (7.3) | 2 (3.4) |
Cloned PCR products were hybridized with oligonucleotides corresponding to the three dominant terrestrial clusters of Nitrosospira spp. and/or directly sequenced.
Total number of clones derived from each soil sample conferring a positive PCR amplification signal with internal primers as described in Materials and Methods.
Values in parentheses represent the percentage of the total number of gene clones for that site.
Non-Nitrosospira species were all within the β-Proteobacteria as determined by partial or complete sequencing as described in Materials and Methods.
Quantification of autotrophic AOB community.
Due to the limited number of cloned genes examined per soil and the nonquantitative nature of clone library construction and analysis, statistical differences in autotrophic AOB population compositions among the three sites could not be inferred. Thus, we investigated the abundance of autotrophic AOB as a possible mechanism for differences in nitrification rates previously measured in CP versus DW soils (17, 27). The qPCR measurements had very wide ranges, with most values closest to the detection limit of the assay (ca. 0.001% of the total soil DNA) and revealed no statistical differences in abundances of autotrophic AOB across the three sites (Table 3).
TABLE 3.
Abundance of autotrophic AOB in each soil as determined by qPCR
| Study site | % of totala
|
Total no. of reactions: no. BDLb
|
||
|---|---|---|---|---|
| Mar. 2002 | Feb. 2003 | Mar. 2002 | Feb. 2003 | |
| CP | ||||
| Mean | 0.39 | 0.11 | 12:4 | 10:3 |
| SD | 0.68 | 0.11 | ||
| Range | 0.001-2.2 | 0.001-0.3 | ||
| SP | ||||
| Mean | 0.03 | 0.01 | 12:3 | 9:3 |
| SD | 0.05 | 0.01 | ||
| Range | 0.001-0.11 | 0.001-0.03 | ||
| DW | ||||
| Mean | 0.10 | 0.13 | 8:0 | 11:1 |
| SD | 0.19 | 0.14 | ||
| Range | 0.004-0.54 | 0.003-0.45 | ||
Data represent the percentages of genomic autotrophic AOB DNA out of the total extracted soil DNA (nanograms of autotrophic AOB DNA per nonogram of soil DNA) based on standard curves generated with N. multiformis genomic DNA as described in Materials and Methods. Only reactions in which control samples spiked with N. multiformis DNA had >80% recovery and also correlated to standard curves with >90% amplification efficiency are reported. Outliers are not included in the data set.
BDL, below detection limit. Values for these samples are included in the mean and range values reported for each site as 0.001% of the total soil DNA, i.e., the lower limit of detection.
Potential nitrification activity in the soils.
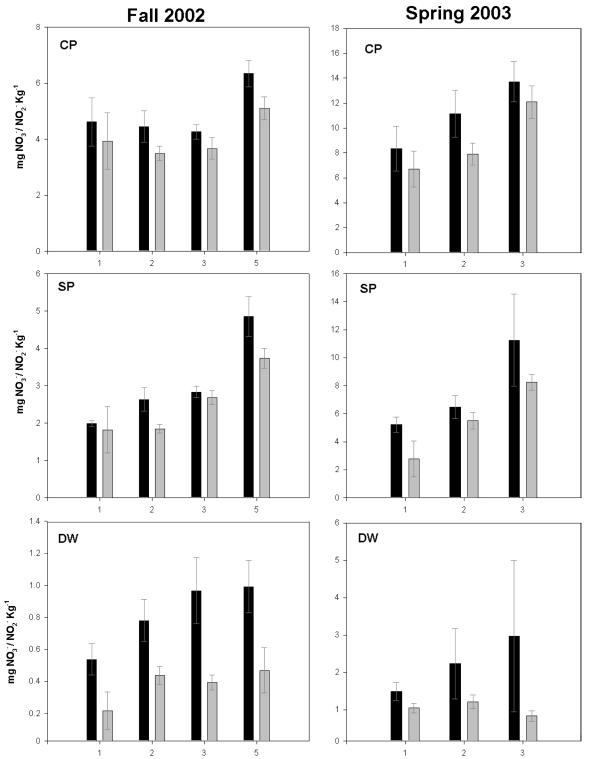
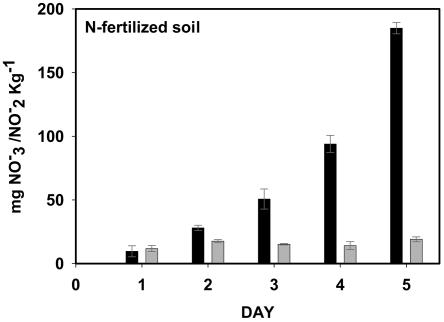
Since the population composition and abundances of autotrophic AOB were similar among the sites, we directly measured the specific contribution of autotrophic AOB to PNA in each soil. PNA was determined for soils collected in both September 2002 and February 2003. The soils were washed to remove 70 to 90% of the associated nitrate, and the remaining amounts were ca. 4, 2, and 0.5 mg · kg of dry soil−1 for CP, SP, and DW soils collected in September 2002 and 6.2, 3, and 0.8 mg · kg of dry soil−1 for the same soils collected in February 2003. Optimal nitrification conditions for autotrophic AOB, i.e., plentiful ammonia by controlling solution pH at 7 and oxygen availability by constant shaking, were maintained in the slurries. The activity of autotrophic AOB was inactivated in replicate soil slurries by the inclusion of acetylene. Nitrite plus nitrate accumulation from acetylene-treated and untreated slurries was measured over 3 to 5 days to obtain PNA rates (Fig. 2). Significantly more nitrite plus nitrate accumulated over time in CP and SP slurries compared to that in DW slurries (ca. 4- to 10-fold difference), both with and without acetylene treatment (comparing means of rates: September 2002, P = 0.016; February 2003, P = 0.005). The majority (80 to 90%) of the acetylene was retained in the treated vials, indicating that it was not limiting the inhibition of autotrophic AOB activity (data not shown). As a positive control for autotrophic AOB activity in a field soil, slurries of sandy soils sampled from an N-fertilized, irrigated, turf-covered ecosystem produced nitrite plus nitrate at over 10 times the rate of any of the forest soils and produced no nitrite or nitrate in the presence of acetylene (Fig. 3).
FIG. 2.
Daily accumulation of soluble NO2−-N plus NO3−-N in washed and buffered slurries of CP, SP, and DW soils collected in September 2002 and February 2003. Incubations without (black bars) and with (gray bars) acetylene are shown. Error bars represent 1 standard deviation from the mean (n = 3).
FIG. 3.
Daily accumulation of soluble NO2−-N plus NO3−-N in a slurry of fertilized, irrigated, sandy soil. Incubations without (black bars) and with (gray bars) acetylene are shown. Error bars represent 1 standard deviation from the mean (n = 3).
The contribution of autotrophic AOB to total PNA was calculated by subtracting the rate of PNA in acetylene-treated slurries from that of untreated slurries (Table 4; Fig. 2). The contribution of autotrophic AOB to overall nitrification rates was low and not significantly different among the three soils collected at either sampling date; however, over both sampling dates the average relative contribution of autotrophic AOB to PNA was 14, 20, and 83% for CP, SP, and DW soils, respectively. The change in ammonium content in CP, SP, and DW soils amended with ammonium at native pH and the field moisture content were also measured (Table 4). The accumulation of ammonium in CP and SP soils indicated higher rates of N-mineralization over ammonia oxidation, whereas DW soil had a net loss of ammonium. These results suggested that inorganic ammonia was not necessarily the substrate for PNA observed in CP or SP slurries, but was a likely substrate in DW slurries. This assumption is consistent with the relative contribution of autotrophic AOB to overall nitrification activity in the three soils.
TABLE 4.
PNA rates in soil slurries and change in ammonium concentrations in static soils
| Study site | PNA rates in soil slurriesa
|
ΔNH4+-Nb | |||||
|---|---|---|---|---|---|---|---|
| Sep. 2002
|
Feb. 2003
|
||||||
| − Acetylene | + Acetylene | AOB PNAc | − Acetylene | + Acetylene | AOB PNAc | ||
| CP | 0.46 (0.09) | 0.33 (0.10) | 0.13 | 2.68 (0.09) | 2.68 (0.07) | 0.004 | 3.30 (2.1) |
| SP | 0.71 (0.09) | 0.51 (0.05) | 0.20 | 3.02 (1.35) | 2.73 (0.20) | 0.29 | 2.90 (0.6) |
| DW | 0.12 (0.02) | 0.05 (0.01) | 0.06 | 0.74 (0.34) | −0.11 (0.06) | 0.85 | −0.90 (0.4) |
Rate of accumulation (milligrams of soluble NO2−-N plus NO3−-N·kg of soil−1 day−1) with standard deviations in parentheses as determined from linear regressions from means of triplicate experiments shown in Fig. 2. Rates were determined over 5 days (Sept. 2002) or 3 days (Feb. 2003) in pH-controlled soil slurries containing ammonium and with or without acetylene as described in Materials and Methods.
Change in milligrams of NH4+-N·kg of soil −1 day−1, with standard deviation in parentheses. Soils three replicates) collected in Sept. 2002 were incubated for 30 days with 200 mg of NH4+ and maintained at field capacity moisture, as described in Materials and Methods.
Contribution by autotrophic AOB to PNA rates in milligrams of soluble NO2−-N plus NO3−-N·kg of soil−1 day−1. Rates were calculated as the difference between the PNA in slurries without acetylene minus the PNA in slurries with acetylene.
Inhibition of N. multiformis activity by soils.
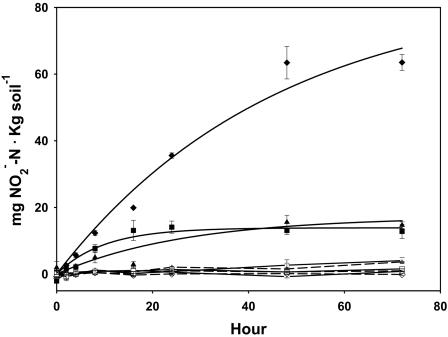
The small contribution of autotrophic AOB to nitrifying activity in the soil slurries prompted examination of potential inhibitory factors in the soils. Slurries of sterile CP and DW soils and a positive control of silica sand were inoculated with cultured cells of N. multiformis and ammonium. The rate of nitrite accumulation was measured in these slurries over 72 h while maintaining optimal nitrifying conditions (Fig. 4). The ammonia-oxidizing activities of N. multiformis cells in both CP and DW soil slurries were inhibited fivefold relative to their activities in the sand slurries. Uninoculated and acetylene-treated inoculated controls did not accumulate nitrite over the course of the incubations. Continued availability of oxygen and neutral pH were verified over the course of the experiment, and thus NH3 was not limiting. Together, these data clearly demonstrated that factors in the soils other than low pH severely hampered ammonia-oxidizing activity of a representative Nitrosospira cluster 3 isolate.
FIG. 4.
Daily accumulation of soluble NO2− by cultivated cells of N. multiformis inoculated onto sterilized, washed, and buffered soil slurries from CP (▴), DW (▪), and silica sand (⧫). Incubations containing acetylene (open symbols) and uninoculated slurries (open symbols with dashed lines) are shown. Error bars represent 1 standard deviation from the mean (n = 3).
DISCUSSION
The present study is the first to investigate the composition, abundance, and activity of autotrophic AOB populations in a U.S. forest soil heavily impacted by atmospheric N deposition. We showed that differences in soil nitrogen content and pH did not select for particular species of autotrophic AOB, nor was there an enrichment of autotrophic AOB numbers in response to increased nitrogen concentrations. We also demonstrated that potential nitrification activity was not dominated by the activity of autotrophic AOB, but rather was a product of other microorganisms or processes thriving in the N-saturated soils.
The stability of the nitrogen pollution gradient from N-saturated to less-impacted soils was demonstrated by higher soil nitrate and ammonium concentrations and lower pH values in CP soils than soils 10 km down gradient at DW (Table 1). Although soils at SP and DW received similar amounts of atmospheric N-deposition, SP soils were intermediate between CP and DW soils in pH and retained nitrate concentrations. Previous studies of soils along this pollution gradient suggested that rates of nitrification were positively correlated with rates and amounts of N-deposition (13-15, 17, 27). Because autotrophic AOB are thought to catalyze the first step of nitrification in most acidic forest soils (11), we assessed their relative role in soils at three sites along the N-deposition gradient.
We hypothesized that acid-tolerant AOB (Nitrosospira cluster 2) or those generally found in high ammonium content soils (Nitrosospira cluster 3) would preferably occupy the highly N-impacted site at CP relative to the less impacted site at DW. Sequencing and oligonucleotide probing of cloned 16 rRNA genes extracted from CP, SP, and DW soils revealed the presence of only Nitrosospira-related AOB, consistent with previous reports of the ubiquity of Nitrosospira spp. in terrestrial environments (Fig. 1 and Table 2) (3, 4, 6, 29, 37, 41, 49). The CTO PCR primer set used in this and many other studies contains mismatches to multiple Nitrosomonas spp., which could potentially misrepresent actual compositions of AOB populations in gene clone libraries (30). We ensured that gene sequences related only to Nitrosospira spp., and none related to Nitrosomonas spp., were detected in the forest soils by generating and screening gene clone libraries at both stringent and permissive annealing temperatures. The majority of AOB gene sequences from all of the soils were related to Nitrosospira cluster 4 isolate Ka3 (Fig. 1). Interestingly, the 16S rRNA gene sequence of Ka3 is nearly identical to that of Nitrosospira Ka4, the dominant group isolated from Oregon forest soils, the only other coniferous forest soil in the western United States examined to date for autotrophic AOB community composition (37). The dominance of similar gene sequences from two soils with such different physicochemical properties indicates a biogeographical linkage for this lineage of autotrophic AOB. Similar distributions of clones from Nitrosospira clusters 2, 3, and 4 were found among the three sites, indicating that differences in pH and levels of soil nitrate and ammonium did not overtly influence the composition of the community (Table 2). This result is in contrast to other studies of N-impacted soils where Nitrosospira cluster 2 dominated acidic soils (31, 33, 45, 46) and cluster 3 dominated neutral pH soils (3, 6, 49). Although pH assumedly plays a major role in selecting for autotrophic AOB due to its effect on ammonia availability, several studies including the present one indicate that acidic soils can support the presence of several Nitrosospira species, and sometimes Nitrosomonas species, rather than selecting specifically for acid-tolerant clusters (3, 6, 9, 49).
Most probable number (MPN) approaches were used in a previous study to enumerate autotrophic AOB populations in CP and DW soils (17). The reported numbers of autotrophic AOB per gram of soil collected in 1993 and 1994 had considerably variable ranges: 350 ± 76 and 801 ± 918 at CP and 263 ± 33 and 311 ± 306 at DW, respectively, such that statistical differences in numbers of bacteria between the sites were not supported. Thus, we chose to estimate the abundance of autotrophic AOB at each site by real-time qPCR with a previously developed probe and primer set for 16S rRNA genes (22). As with MPN values, the qPCR values obtained for autotrophic AOB abundances were not significantly different across the N-saturation gradient, suggesting that these populations were not preferentially enriched in soils with higher N content (Table 3). The range of values obtained from the qPCR assay was wide, which is common when enumerating target molecules near the assay detection limit (19, 48). However, even with the large reported ranges, the mean abundances of autotrophic AOB were congruent with abundances reported in other soil environments (22, 23). Furthermore, other studies have shown that molecular methods, such as competitive PCR, estimate autotrophic AOB populations at 10 to 100 times higher than MPN methods, similar to our findings (17, 42). The discrepancy in abundance estimates between molecular and cultivation-dependent methods is likely due to the detection of both active and inactive populations of bacteria with molecular techniques, versus detecting only those that grow under experimental conditions with the MPN approach. The detection limit of our qPCR assay was similar to that of another study that used qPCR to enumerate genes extracted from soils (ca. 104 cells g of soil−1; i.e., 0.1 to 0.001%, assuming the estimated 107 to 109 cells g of soil−1 (25, 50).
Although the composition and abundance of autotrophic AOB populations were not significantly different across the N-saturation gradient, the PNA rates were significantly higher in the CP and SP soils than in the DW soils, whereas the potential contribution of autotrophic AOB was highest in the DW soils (Fig. 2 and Table 4). Together, our observations indicate that other microbial populations or other processes besides those involving autotrophic AOB were likely responsible for relatively high PNA rates in CP and SP soils, whereas low PNA rates in the less N-impacted DW soil were predominantly catalyzed by autotrophic AOB. Low autotrophic AOB activities were also observed in mixed-conifer forest soils in the Sierra Nevada Mountains in California, where nitrification activity was only partially inhibited by acetylene (40). This study used 15N pool dilution techniques to demonstrate that inorganic ammonia was not the substrate for nitrate accumulation in mature forest soils.
Besides autotrophic AOB, acidic soils can support the activity of heterotrophic nitrifiers (11). Heterotrophic nitrifiers include bacteria and fungi that oxidize both organic and inorganic ammonia while utilizing organic carbon as an energy source. Evidence for heterotrophic nitrification in CP and SP soils is supported by the accumulation of nitrite plus nitrate in the presence of acetylene in ammonium-amended soil slurries (Fig. 2 and Table 4) and the increase in soluble ammonium concentrations in ammonium-amended static soil incubations (Table 4). These data indicate that the nitrifying community produced nitrate at the expense of organic rather than inorganic sources of ammonia. We are confident that we accurately determined PNA rates in the present study because of the following: (i) the average rates of PNA were similar to those observed by Fenn and colleagues for the same soils sampled in 1994 (17), (ii) the rates of PNA were mostly higher than those measured in other acidic forest soils (33, 37, 40), and (iii) acetylene completely inhibited a very high rate of PNA in a fertilized sandy soil control (Fig. 3). The inhibition of ammonia oxidation by N. multiformis when inoculated into sterile CP and DW soils further explained the low contribution of autotrophic AOB to rates of PNA in these soils, although the actual mechanism of inhibition was not characterized (Fig. 4). Apparently, the soils are not ideal environments even for an autotrophic AOB species adapted to relatively high ammonium concentrations. This inhibition may have been caused by monoterpenes or other compounds that slow nitrification activity in forest soils (39).
The inferred dominance of heterotrophic over autotrophic nitrification activity in N-saturated, low-pH forest soils is similar to that reported in another forest soil in California (40, 44), but in sharp contrast to other N-impacted soils where autotrophic AOB activities dominate (11, 32, 33, 41, 46). The ecology of heterotrophic nitrifiers has been highly understudied in the field of microbial ecology. The present study uncovered a unique N-impacted environment where heterotrophic nitrification apparently dominates over autotrophic nitrification. Furthermore, this study expanded the biogeographical range for Nitrosospira gene clusters 2, 3, and 4 to heavily N-impacted forest soils under a Mediterranean climate.
Acknowledgments
This research was supported through USDA-NRI grant no. 2002-35107-12244 and Kearney Foundation of Soil Science grant no. 2001-04 to L. Y. Stein.
We thank Elisha Wakefield and Diane Alexander for assistance with soil chemistry analyses.
REFERENCES
- 1.Aber, J. D., P. Nadelhoffer, P. Steudler, and J. M. Melillo. 1989. Nitrogen saturation in northern forest ecosystems. BioScience 39:378-386. [Google Scholar]
- 2.Arkley, R. J. 1981. Soil-moisture use by mixed conifer forest in a summer-dry climate. Soil Sci. Soc. Am. J. 45:423-427. [Google Scholar]
- 3.Avrahami, S., and R. Conrad. 2003. Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl. Environ. Microbiol. 69:6152-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bothe, H., G. Jost, M. Schloter, B. B. Ward, and K.-P. Witzel. 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 24:673-690. [DOI] [PubMed] [Google Scholar]
- 5.Bouwman, A. F., D. P. Van Vuuren, R. G. Derwent, and M. Posch. 2002. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut. 141:349-382. [Google Scholar]
- 6.Bruns, M. A., J. R. Stephen, G. A. Kowalchuk, J. I. Prosser, and E. A. Paul. 1999. Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in native, tilled, and successional soils. Appl. Environ. Microbiol. 65:2994-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, D. A., and C. Kendall. 2002. Analysis of δ15N and δ15O to differentiate NO3− sources in runoff at two watersheds in the Catskill Mountains of New York. Water Resour. Res. 38:1051. [Google Scholar]
- 8.Campbell, D. H., C. Kendall, C. C. Y. Chang, S. R. Silva, and K. A. Tonnesen. 2002. Pathways for nitrate release from an alpine watershed: determination using δ15N and δ18O. Water Resour. Res. 38:1052. [Google Scholar]
- 9.Carnol, M., G. A. Kowalchuk, and W. De Boer. 2002. Nitrosomonas europaea-like bacteria detected as the dominant β-subclass Proteobacteria ammonia oxidisers in reference and limed acid forest soils. Soil Biol. Biochem. 34:1047-1050. [Google Scholar]
- 10.Chain, P., J. Lamerdin, F. Larimer, W. Regala, V. Lao, M. Land, L. Hauser, A. B. Hooper, M. G. Klotz, J. M. Norton, L. A. Sayavedra-Soto, D. M. Arciero, N. G. Hommes, M. R. Whittaker, and D. J. Arp. 2003. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 185:2759-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dear, S., and R. Staden. 1991. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 19:3907-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Boer, W., and G. A. Kowalchuk. 2001. Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol. Biochem. 33:853-866. [Google Scholar]
- 13.Fenn, M. E., J. S. Baron, E. B. Allen, H. M. Rueth, K. R. Nydick, L. Geise, W. D. Bowman, J. O. Sickman, T. Meixner, D. W. Johnson, and P. Neitlich. 2003. Ecological effects of nitrogen deposition in the western United States. BioScience 53:404-420. [Google Scholar]
- 14.Fenn, M. E., and A. Bytnerowicz. 1993. Dry deposition of nitrogen and sulfur to ponderosa and Jeffrey pine in the San Bernardino National Forest in southern California. Environ. Pollut. 81:277-285. [DOI] [PubMed] [Google Scholar]
- 15.Fenn, M. E., R. Haeuber, G. S. Tonnesen, J. S. Baron, S. Grossman-Clarke, D. Hope, D. A. Jaffe, S. Copeland, L. Geiser, H. M. Rueth, and J. O. Sickman. 2003. Nitrogen emissions, deposition, and monitoring in the western United States. BioScience 53:391-403. [Google Scholar]
- 16.Fenn, M. E., M. A. Poth, and M. J. Arbaugh. 2002. A throughfall collection method using mixed bed ion exchange resin columns. Sci. World J. 2:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenn, M. E., M. A. Poth, and D. W. Johnson. 1996. Evidence for nitrogen saturation in the San Bernardino Mountains in southern California. For. Ecol. Manage. 82:211-230. [Google Scholar]
- 18.Fenn, M. E., M. A. Poth, S. L. Schilling, and D. B. Grainger. 2000. Throughfall and fog deposition of nitrogen and sulfur at an N-limited and N-saturated site in the San Bernardino Mountains, southern California. Can. J. For. Res. 30:1476-1488. [Google Scholar]
- 19.Gruntzig, V., S. C. Nold, J. Z. Zhou, and J. M. Tiedje. 2001. Pseudomonas stutzeri nitrite reductase gene abundance in environmental samples measured by real-time PCR. Appl. Environ. Microbiol. 67:760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms, G., A. C. Layton, H. M. Dionisi, I. R. Gregory, V. M. Garrett, S. A. Hawkins, K. G. Robinson, and G. S. Sayler. 2003. Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ. Sci. Technol. 37:343-351. [DOI] [PubMed] [Google Scholar]
- 21.Hastings, R. C., C. Butler, I. Singleton, J. R. Saunders, and A. J. McCarthy. 2000. Analysis of ammonia-oxidizing bacteria populations in acid forest soil during conditions of moisture limitation. Lett. Appl. Microbiol. 30:14-18. [DOI] [PubMed] [Google Scholar]
- 22.Hermansson, A., and P.-E. Lindgren. 2001. Quantification of ammonia-oxidizing bacteria in arable soil by real-time PCR. Appl. Environ. Microbiol. 67:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesselsoe, M., K. K. Brandt, and J. Sorensen. 2001. Quantification of ammonia oxidizing bacteria in soil using microcolony technique combined with fluorescence in situ hybridization (MCFU-FISH). FEMS Microbiol. Ecol. 38:87-95. [Google Scholar]
- 24.Hyman, M. R., and P. M. Wood. 1985. Suicidal inactivation and labeling of ammonia monooxygenase by acetylene. Biochem. J. 227:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb, S., C. Knief, S. Stubner, and R. Conrad. 2003. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 69:2423-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koops, H.-P., U. Purkhold, A. Pommerening-Röser, G. Timmermann, and M. Wagner. 2003. The lithotrophic ammonia-oxidizing bacteria. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://link.springer-ny.com/link/service/books/10125/.
- 27.Korontzi, S., S. A. Macko, I. C. Anderson, and M. A. Poth. 2000. A stable isotopic study to determine carbon and nitrogen cycling in a disturbed southern Californian ecosystem. Glob. Biogeochem. Cyc. 14:177-188. [Google Scholar]
- 28.Kowalchuk, G. A., P. L. E. Bodelier, G. H. J. Heilig, J. R. Stephen, and H. J. Laanbroek. 1998. Community analysis of ammonia-oxidising bacteria, in relation to oxygen availability in soils and root-oxygenated sediments, with PCR, DGGE and oligonucleotide probe hybridisation. FEMS Microbiol. Ecol. 27:1489-1497. [Google Scholar]
- 29.Kowalchuk, G. A., and J. R. Stephen. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbiol. 55:485-529. [DOI] [PubMed] [Google Scholar]
- 30.Kowalchuk, G. A., J. R. Stephen, W. DeBoer, J. I. Prosser, T. M. Embley, and J. W. Woldendorp. 1997. Analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalchuk, G. A., A. W. Stienstra, G. H. J. Heilig, J. Stephen, and J. W. Woldendrop. 2000. Changes in the community structure of ammonia-oxidizing bacteria during secondary succession of calcareous grasslands. Environ. Microbiol. 2:99-110. [DOI] [PubMed] [Google Scholar]
- 32.Kowalchuk, G. A., A. W. Stienstra, G. H. J. Heilig, J. R. Stephen, and J. W. Woldendrop. 2000. Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol. Ecol. 31:207-215. [DOI] [PubMed] [Google Scholar]
- 33.Laverman, A. M., A. G. C. L. Speksnijder, M. Braster, G. A. Kowalchuk, H. A. Verhoef, and H. W. van Verseveld. 2001. Spatiotemporal stability of an ammonia-oxidizing community in a nitrogen-saturated forest soil. Microb. Ecol. 42:35-45. [DOI] [PubMed] [Google Scholar]
- 34.McCaig, A. E., T. M. Embley, and J. I. Prosser. 1994. Molecular analysis of enrichment cultures of marine ammonia oxidisers. FEMS Microbiol. Lett. 120:363-368. [DOI] [PubMed] [Google Scholar]
- 35.Mendum, T. A., R. E. Sockett, and P. R. Hirsch. 1999. Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the β subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl. Environ. Microbiol. 65:4155-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, P. R., O. C. Taylor, and M. P. Poe. 1986. Air Pollut. Control Assoc. Annu. Meet., p. 86-39.2.
- 37.Mintie, A. T., R. S. Heichen, K. Cromack, D. D. Myrold, and P. J. Bottomley. 2003. Ammonia-oxidizing bacteria along meadow-to-forest transects in the Oregon Cascade Mountains. Appl. Environ. Microbiol. 69:3129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolaisen, M. H., and N. B. Ramsing. 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J. Microbiol. Methods 50:189-203. [DOI] [PubMed] [Google Scholar]
- 39.Paavolainen, L., V. Kitunen, and A. Smolander. 1998. Inhibition of nitrification in forest soil by monoterpenes. Plant Soil 205:147-154. [Google Scholar]
- 40.Pedersen, H., K. A. Dunkin, and M. K. Firestone. 1999. The relative importance of autotrophic and heterotrophic nitrification in a conifer forest soil as measured by 15N tracer and pool dilution techniques. Biogeochemistry 44:135-150. [Google Scholar]
- 41.Phillips, C. J., D. Harris, S. L. Dollhopf, K. L. Gross, J. I. Prosser, and E. A. Paul. 2000. Effects of agronomic treatments on structure and function of ammonia-oxidizing communities. Appl. Environ. Microbiol. 66:5410-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips, C. J., E. A. Paul, and J. I. Prosser. 2000. Quantitative analysis of ammonia oxidising bacteria using competitive PCR. FEMS Microbiol. Ecol. 32:167-175. [DOI] [PubMed] [Google Scholar]
- 43.Purkhold, U., A. Pommerening-Röser, S. Juretschko, M. C. Schmid, H.-P. Koops, and M. Wagner. 2000. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl. Environ. Microbiol. 66:5368-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schimel, J. P., M. K. Firestone, and K. S. Killham. 1984. Identification of heterotrophic nitrification in a Sierran forest soil. Appl. Environ. Microbiol. 48:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephen, J. R., G. A. Kowalchuk, M.-A. V. Bruns, A. E. McCaig, C. J. Phillips, T. M. Embley, and J. I. Prosser. 1998. Analysis of β-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl. Environ. Microbiol. 64:4147-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephen, J. R., A. E. McCaig, Z. Smith, J. I. Prosser, and T. M. Embley. 1996. Molecular diversity of soil and marine 16S rRNA gene sequences related to the β-subgroup ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 62:4147-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strunk, O., and W. Ludwig. 1999. ARB: a software environment for sequence data. Technical University of Munich, Munich, Germany.
- 48.Suzuki, M. T., L. T. Taylor, and E. F. DeLong. 2000. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 66:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster, G., T. M. Embley, and J. I. Prosser. 2002. Grassland management regimens reduce small-scale heterogeneity and species diversity of β-proteobacterial ammonia oxidizer populations. Appl. Environ. Microbiol. 68:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]