A downside of immunosuppression (IS) is clearly the infections that jeopardize graft and patient survival.1,2 Reactivation of BK virus and histologically proven BK virus nephropathy (BKVN) after kidney transplantation (KT) are well described, resulting in renal graft loss in up to 15% of such patients.3 Described risk factors are lymphocyte-depleting agents, tacrolimus (Tac)–based IS, diabetes mellitus, and ureteric stents.4 In contrast to KT, only few data on BK virus viremia (BKV) after simultaneous pancreas-kidney transplantation (SPK) have been published so far.5-9
BKVN treatment consists primarily of reducing IS.3,5,8,10 However, in these patients, attempts to decrease IS bring clinicians face to face with the delicate task of preventing kidney graft failure, on one hand, and avoiding rejection of the pancreatic allograft, on the other hand. In this case report, we would like to share our experience with BKV after SPK.
CASE REPORT
After institutional review board approval, we retrospectively analyzed and identified 9 cases (4.9%) of BKV in 185 SPKs performed between 2005 and 2014. All 9 SPKs were performed according to the standard surgical technique using enteral exocrine drainage.11 Ureteral stents were implanted in 7 of 9 patients. Induction IS consisted of antithymocyte agents (n = 8) or basiliximab (n = 1) combined with 500-mg methylprednisolone (MP). Maintenance therapy consisted of gradually tapered oral prednisolone (n = 9; ie, from postoperative [p.o.] day 20: 20 mg daily reduced by 2.5 mg every 2 weeks until discontinuation), 2000-mg mycophenolate mofetil (MMF) (n = 9), and Tac (n = 8; initial trough levels 12-15 ng/mL, gradually decreased to 8 ng/mL at 9 months, to 4-6 ng/mL at 13 months, and to 3-5 ng/mL at 2 years after transplantation [Tx]) or cyclosporine A (CyA) (n = 1; initial trough levels 180-200 ng/mL, stepwise decreased to 130 ng/mL at 9 months, to 80 to 100 ng/mL at 13 months, and to 40 to 80 ng/mL at 2 years after Tx).
Of the 9 patients, 4 had initially delayed renal function; all pancreatic grafts had good primary function without any need for exogenous insulin. In renal grafts, no rejection was observed. In 2 pancreatic grafts, acute rejection was diagnosed, in both cases occurring 2 months after Tx. Diagnosis was based on laboratory values such as hyperglycemia, increase in serum amylase and lipase along with a decrease in C-peptide, and low Tac trough levels. Both acute rejections were reversed with pulsed steroids (1.5-g MP given over 3 consecutive days) and increased Tac.
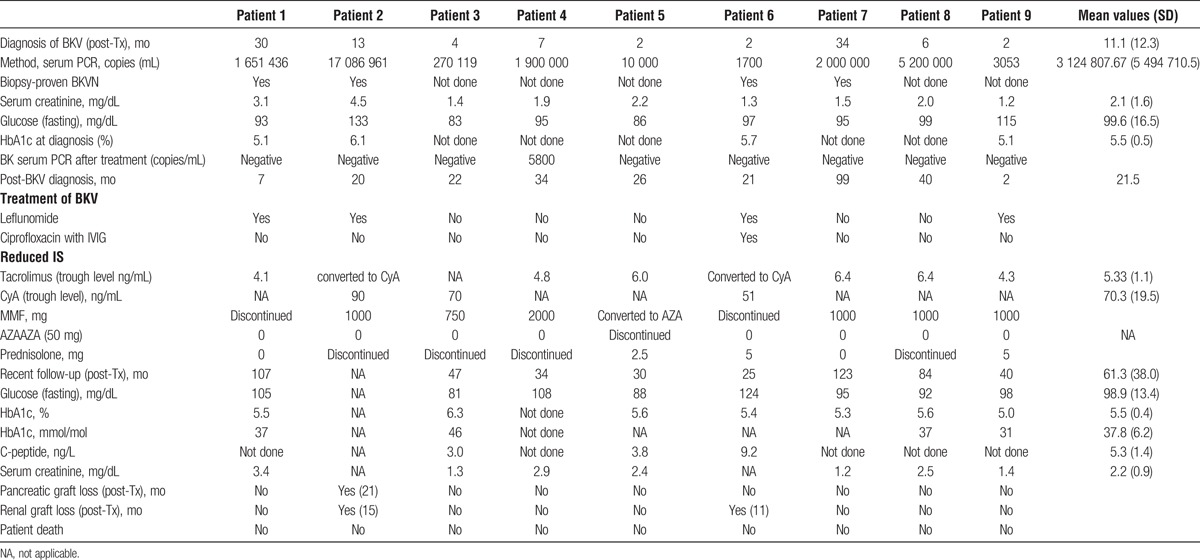
Median time from Tx to BKV diagnosis was 6 (range, 2-34) months. All BKV diagnoses were established on the basis of serum polymerase chain reaction (PCR). In 4 patients, a KT biopsy was performed showing BKVN in all 4 cases. After BKV diagnosis, treatment consisted of a 30% to 50% reduced Tac/CyA dose in all patients. In 2 patients, Tac was converted to CyA. MMF was reduced in 4 patients, discontinued in 2 patients, and converted to azathioprine (AZA) in 1 patient. Prednisolone was discontinued in 4 patients and reduced in 3 patients. Leflunomide (20 mg, without loading dose) was administered either after discontinuation of MMF (2 patients) or along with an up to 50% reduction in MMF dose. Ciprofloxacin (250 mg twice daily for 2 months) with IVIG (500 mg/kg of body weight) was administered to 1 patient after switching from MMF to leflunomide.
After a median follow-up of 43.5 (range, 25-123) months, pancreatic function remained stable in 8 (88.9%) of 9 patients (Figure 1A). Of the 9 kidney grafts, two (22.2%) were lost at month 11 and month 15 (Figure 1B). One kidney graft loss occurred 9 months after BKVN diagnosis despite having commenced all available treatment strategies, including ciprofloxacin, conversion to CyA, IVIG, and leflunomide. The other kidney was lost 2 months after BKVN diagnosis after conversion to CyA monotherapy with leflunomide and MMF dose reduction. The same patient lost his pancreas graft because of clinically diagnosed chronic rejection 6 months later; BK virus serum PCR was negative.
FIGURE 1.
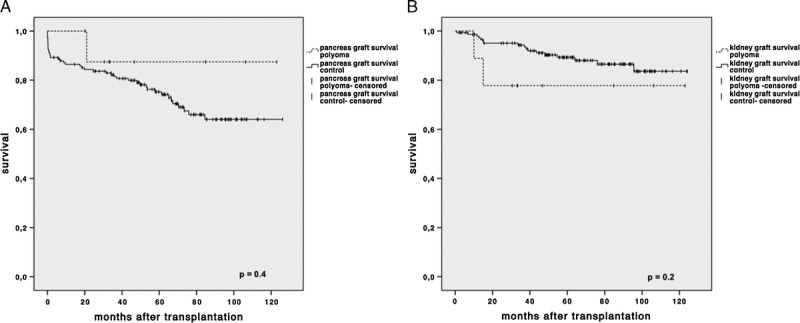
Estimated pancreas and renal graft survival. Kaplan-Meier estimates of (A) pancreas and (B) renal graft survival stratified by presence of BKV. Difference between groups was compared using the log-rank test. P ≤ 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 21.0 (Chicago, IL).
Mean serum creatinine of the surviving 7 (77.8%) kidneys was 2.1 mg/dL. Serum PCR turned negative in 8 patients after a mean of 29.8 months and significantly decreased in 1 patient (month 9). For detailed clinical data, see Tables 1 and 2.
TABLE 1.
Demographic recipient and donor data, perioperative and p.o. course, including IS preceding BKV diagnosis
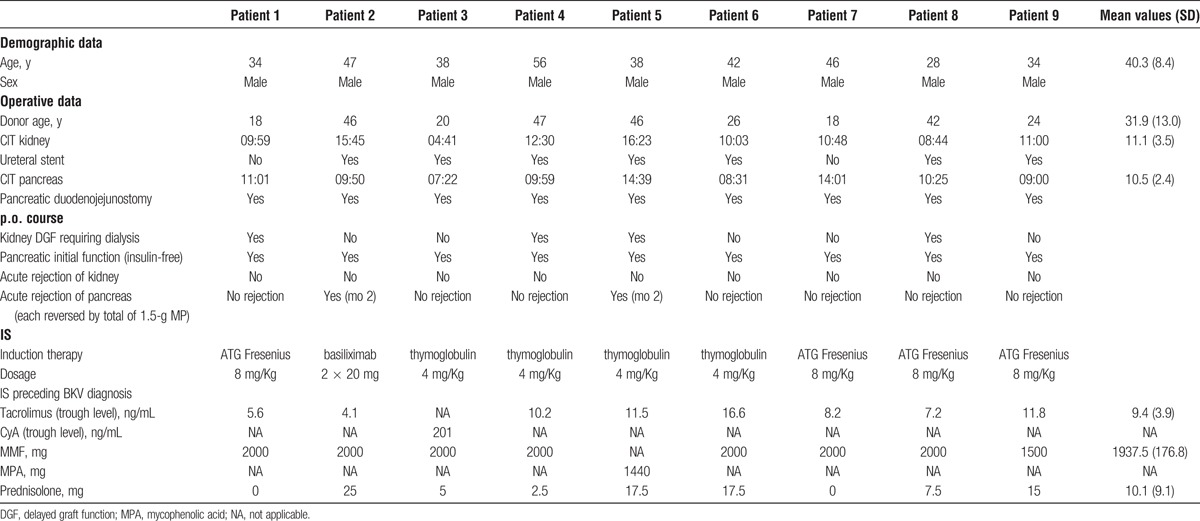
TABLE 2.
Summarized diagnoses, BKV treatment outcomes, graft loss and patient loss, pancreatic, and renal function
DISCUSSION
In our series, we experienced 2 renal graft losses of 4 biopsy-proven BKVN. One of these patients presented in our outpatient clinic with already advanced nephropathy. Despite decreasing IS and starting antibiotic treatment, renal function could not be preserved. The subsequent loss of pancreas function due to chronic rejection and the absence of BKV confirms early findings that pancreatic graft function is more likely to be affected by decreased IS than by BK virus infection.12 However, because we do not perform pancreatic graft biopsies, we cannot exclude BK virus colonization. The second patient had early replication. Out of fear of losing the pancreas, the patient was referred with only slightly reduced IS, which proved to be too mild. The patient ended up with a functioning pancreas but on hemodialysis.
The heterogeneity of our treatment approaches reflects the few available data and the low incidence of BKV after SPK over a 12-year observation period. However, the importance of promptly reducing IS as a first step in treating BKV or BKVN is of utmost importance. In our current protocol, as soon as we detect BKV, we reduce Tac/CyA trough levels by 30% to 50%, reduce MMF dosage by 50%, and minimize or discontinue steroids. According to individual risk profiles, we might convert Tac to CyA or MMF to AZA or deviate slightly from the protocol.
At our center, we do not perform protocol biopsies of the kidney. As soon as BKV is detected, we reduce IS. Kidney biopsies were performed either immediately (patient 1) or as soon as the graft deteriorated (7 months after SPK in patient 2, 11 months after SPK in patient 6, and 3 months after SPK in patient 7). Late referral and too mild reduction of IS might have formed the basis for our 2 kidney graft losses.
The usually higher maintenance IS, as compared with KT recipients, poses a difficult task for clinicians. There might be a certain reluctance to aggressively decrease IS. However, the available literature shows a fairly low number of pancreatic graft losses in SPK recipients with BKV, despite properly decreased IS.5-9 This poses the question whether we might over immunosuppress a substantial part of SPK recipients because cautious reduction of maintenance IS seems to be well tolerated by the pancreatic graft. Regular screening of BK virus could therefore be also seen as a useful tool for identifying tendencies toward IS.
Antilymphocyte agents, steroids, and Tac maintenance IS were described in an Organ Procurement and Transplantation Network analysis as potential risk factors for BKV development.13 However, the overall degree of IS, rather than the use of a specific induction or maintenance agent, might be more important.14 The Pittsburgh group, for example, reports a significantly lower BKV incidence since they started pretransplant lymphoid depletion with minimal maintenance therapy.6 Based on data associating Tac with higher BKV incidence,15 a switch to CyA is also a common strategy, even though worse pancreatic graft survival with CyA maintenance therapy has been shown.16 In our experience, both patients converted to CyA lost their renal graft. However, only 1 patient lost his pancreatic graft. This is in line with data from Elfadawy et al,7 who did not experience a higher incidence of BKVN-associated renal graft losses despite keeping recipients on Tac maintenance IS. Similarly, the risk associated with steroids has to be put into perspective because our data show the incidence of BKV in our patients to be almost identical to that in the Indianapolis group, which runs a steroid-free protocol.5 The aim of keeping maintenance IS at minimum levels seems to be the main factor for BKV prevention. Recent in vitro data suggest an inhibiting effect of mammalian target of rapamycin inhibitors on viral replication, providing a basis for future clinical trials.17 Clinical experience in this regard is less clear and points out the importance of reducing IS in general, rather than using specific immunosuppressive agents.18 We have no experience with mammalian target of rapamycin inhibitors in these patients.
The high proportion of patients with ureteral stents in this group reflects a change in our surgical standards rather than the validation of a risk factor. Since 2008, we place ureteral stents as standard practice. The 2 patients without a stent in this series were transplanted before stents were made standard. Therefore, it is difficult to draw any conclusions about the risk related to ureteral stents.
Similar to other reports,5,6 BKVN incidence is lower than in KT recipients. The reason might be the more stringent donor selection for SPK recipients, where we observe shorter cold ischemia times (CITs) and a lower incidence of delayed graft function, a proposed risk factor for BKVN.3
To our knowledge, no controlled studies concerning leflunomide/qinolones/IVIG have been performed. In our series, 2 of the 4 patients receiving leflunomide lost their kidney. Unfortunately, these 2 patients presented late at our center. In one of these patients, MMF dosage was only reduced, resulting in an inadequate reduction of the general IS. In both successfully treated patients, the decrease in IS and leflunomide was commenced at an early stage. The unusual combination of MMF reduction with leflunomide treatment in one of these patients was due to the early presence of BKV after SPK (2 months after SPK). No conclusions can be drawn.
Even though involving a relatively large case series, this study is hampered by its single-center retrospective character. The advantage might be the homogeneous study group regarding surgical procedure, donor/recipient selection, IS, and p.o. follow-up and screening. At our center, BK virus serum PCR is controlled monthly for the first 6 months, then every 3 months until year 2, and finally at least every 6 months. Preserved kidney and pancreas function in the remaining 7 patients demonstrates the importance of tight BK virus monitoring but only when combined with adequate reduction of IS as soon as BKV is diagnosed.
In conclusion, our experience is consistent with recent reports in the literature. In our opinion, the key to success consists of (1) early BK virus detection by regular surveillance and (2) immediate reduction of IS because any viral replication in the recipient of a functioning graft should be interpreted as over IS.
Footnotes
Published online 13 April, 2017.
The authors declare no funding or conflicts of interest.
C.B. and M.M. conceived the project. C.B., F.M., and C.M. performed the analysis. F.M. performed the statistics. C.B. and M.M. wrote the article. M.R., R.Ö., D.Ö., and S.S. provided expertise on analysis and interpretation of data.
REFERENCES
- 1.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. [DOI] [PubMed] [Google Scholar]
- 2.Smith SR, Butterly DW, Alexander BD, et al. Viral infections after renal transplantation. Am J Kidney Dis. 2001;37:659–676. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(suppl 4):179–188. [DOI] [PubMed] [Google Scholar]
- 4.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209–217. [DOI] [PubMed] [Google Scholar]
- 5.Mujtaba M, Fridell J, Sharfuddin A, et al. BK virus nephropathy in simultaneous pancreas kidney transplant: a potentially preventable cause of kidney allograft loss. Clin Transplant. 2012;26:E87–E93. [DOI] [PubMed] [Google Scholar]
- 6.Gupta G, Shapiro R, Thai N, et al. Low incidence of BK virus nephropathy after simultaneous kidney pancreas transplantation. Transplantation. 2006;82:382–388. [DOI] [PubMed] [Google Scholar]
- 7.Elfadawy N, Flechner SM, Schold JD, et al. Transient versus persistent BK viremia and long-term outcomes after kidney and kidney-pancreas transplantation. Clin J Am Soc Nephrol. 2014;9:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai DS, Hong J, Busuttil RW, et al. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg. 2013;148:368–373. [DOI] [PubMed] [Google Scholar]
- 9.Schachtner T, Zaks M, Kahl A, et al. Simultaneous pancreas/kidney transplant recipients present with late-onset BK polyomavirus-associated nephropathy. Nephrol Dial Transplant. 2016;31:1174–1182. [DOI] [PubMed] [Google Scholar]
- 10.Mindlova M, Boucek P, Saudek F, et al. Prevalence and risk factors of polyomavirus BK replication in simultaneous pancreas/kidney transplant recipients from a single transplant center. Clin Transplant. 2012;26:267–274. [DOI] [PubMed] [Google Scholar]
- 11.Gruessner R, Sutherland D. Pancreas transplantation. Surg Rounds. 1994;17:311–324. [Google Scholar]
- 12.Lipshutz GS, Mahanty H, Feng S, et al. BKV in simultaneous pancreas-kidney transplant recipients: a leading cause of renal graft loss in first 2 years post-transplant. Am J Transplant. 2005;5:366–373. [DOI] [PubMed] [Google Scholar]
- 13.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. [DOI] [PubMed] [Google Scholar]
- 14.Wiseman AC. Polyomavirus nephropathy: a current perspective and clinical considerations. Am J Kidney Dis. 2009;54:131–142. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saudek F, Malaise J, Boucek P, et al. Efficacy and safety of tacrolimus compared with cyclosporin microemulsion in primary SPK transplantation: 3-year results of the Euro-SPK 001 trial. Nephrol Dial Transplant. 2005;20(Suppl 2):ii3–ii10, ii62. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch HH, Yakhontova K, Lu M, et al. BK Polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant. 2016;16:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouve T, Rostaing L, Malvezzi P. Place of mTOR inhibitors in management of BKV infection after kidney transplantation. J Nephropathol. 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


